Combined Continuous Resin Adsorption and Anaerobic Digestion of Olive Mill Wastewater for Polyphenol and Energy Recovery
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Analysis
2.3. Continuous Adsorption Experiments
2.4. Column Performance
2.5. Biochemical Methane Potential (BMP) Assays
2.6. Modeling Based on the Kinetic Fractionalization of BMPs of Anaerobic Digestion of Raw and Resin Pretreated OMWW
- (i)
- At the end of the period considered for the BMP, no biodegradable matter remains. This assumption was supported by the experimental protocol. The tests were conducted over a sufficient duration (35 days), during which methane production was monitored until reaching a plateau. In other words, the degradation of the entire substrate, denoted as S, into various sub-fractions, indicated as Si, based on the corresponding compartments within the organic matter, starts immediately after the addition of the substrate to the reactor, and the slowest fraction is completely degraded at the end of the BMP.
- (ii)
- The organic matter is categorized into three sub-fractions. This is particularly important for OMWW due to its heterogeneity and phenolic content. Thus, this model structure offers improved mechanistic resolution compared to single-phase kinetic models, first-order or modified Gompertz models. In integrated treatment systems, selective removal of specific fractions (e.g., via adsorption) changes the biodegradability profile. This model allows for a more nuanced assessment of how such pretreatments redistribute organic matter and affect methane production kinetics.
- (iii)
- The degradation rate of degradation of each sub-fraction (or compartment) remains constant and follows zero-order kinetics (d/dt = − as long as Si is positive).
3. Results and Discussion
3.1. Effect of Varying Height Bed of XAD-4 Resin Column on Continuous Adsorption Efficiency of OMWW Polyphenolics Compounds
3.2. Effect of Column XAD-4 Resin Adsorption on BMP of Raw and Pretreated OMWW
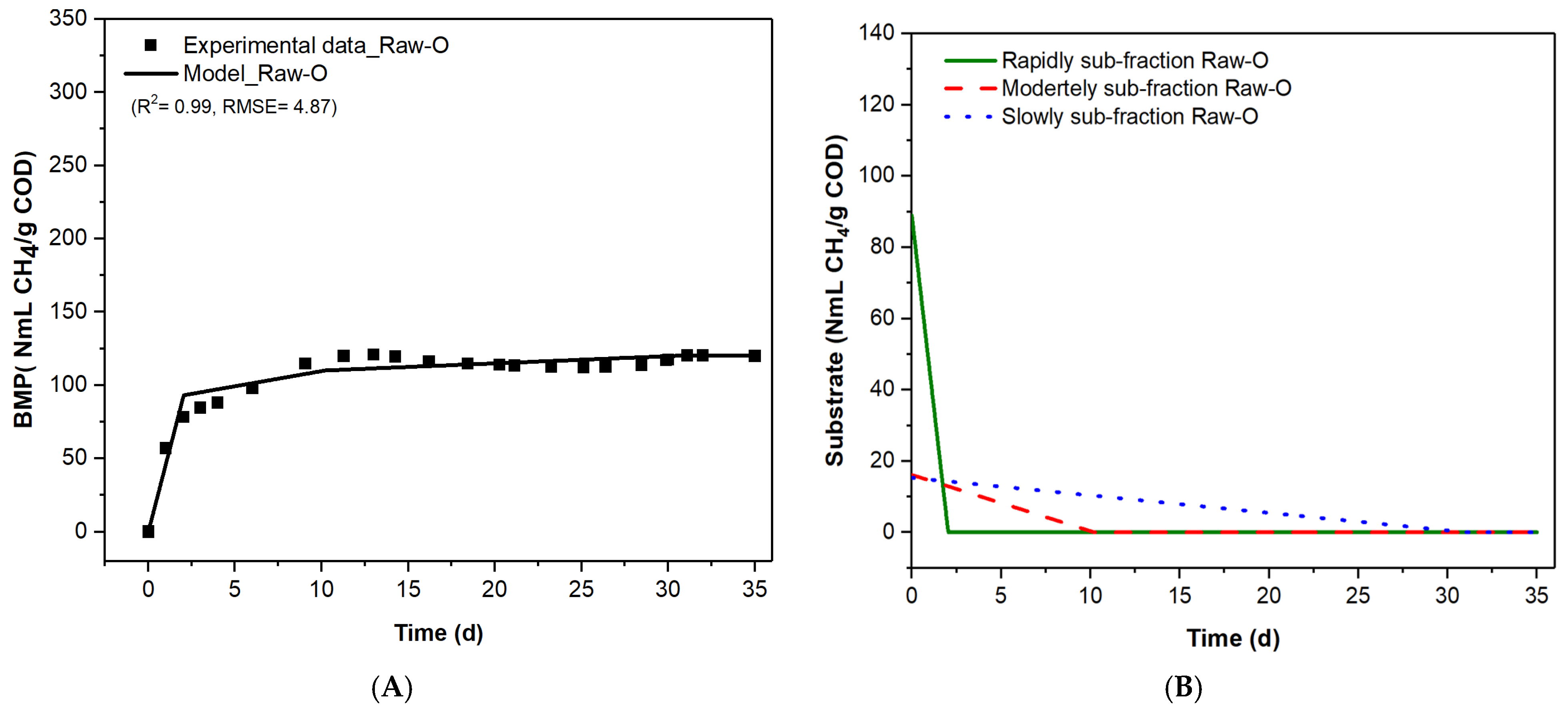
3.3. Compartmentalization of the Organic Matter Contained in Raw and Pretreated OMWW Based on the Kinetics of Anaerobic Digestion
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dahdouh, A.; Khay, I.; Le Brech, Y.; El Maakoul, A.; Bakhouya, M. Olive Oil Industry: A Review of Waste Stream Composition, Environmental Impacts, and Energy Valorization Paths. Environ. Sci. Pollut. Res. 2023, 30, 45473–45497. [Google Scholar] [CrossRef]
- Fezzani, B.; Cheikh, R. Ben Two-Phase Anaerobic Co-Digestion of Olive Mill Wastes in Semi-Continuous Digesters at Mesophilic Temperature. Bioresour. Technol. 2010, 101, 1628–1634. [Google Scholar] [CrossRef]
- International Olive Council (IOC). World Olive Oil Figures—2023/24 Season. 2024. Available online: https://www.internationaloliveoil.org/what-we-do/economic-affairs-promotion-unit/#figures (accessed on 10 January 2024).
- Anania, G.; Pupo, D.M.R. Olive Oil in the Mediterranean Area: Production, Consumption and Trade; The CIHEM Watch Letter Analysis: Paris, France, 2014. [Google Scholar]
- Eder, S.; Müller, K.; Azzari, P.; Arcifa, A.; Peydayesh, M.; Nyström, L. Mass Transfer Mechanism and Equilibrium Modelling of Hydroxytyrosol Adsorption on Olive Pit–Derived Activated Carbon. Chem. Eng. J. 2021, 404, 126519. [Google Scholar] [CrossRef]
- Sierra, J.; Martí, E.; Montserrat, G.; Cruaáas, R.; Garau, M.A. Characterisation and Evolution of a Soil Affected by Olive Oil Mill Wastewater Disposal. Sci. Total Environ. 2001, 279, 207–214. [Google Scholar] [CrossRef]
- Roig, A.; Cayuela, M.L.; Sánchez-Monedero, M.A. An Overview on Olive Mill Wastes and Their Valorisation Methods. Waste Manag. 2006, 26, 960–969. [Google Scholar] [CrossRef]
- Vaz, T.; Quina, M.M.J.; Martins, R.C.; Gomes, J. Olive Mill Wastewater Treatment Strategies to Obtain Quality Water for Irrigation: A Review. Sci. Total Environ. 2024, 931, 172676. [Google Scholar] [CrossRef]
- Enaime, G.; Dababat, S.; Wichern, M.; Lübken, M. Olive Mill Wastes: From Wastes to Resources. Environ. Sci. Pollut. Res. 2024, 31, 20853–20880. [Google Scholar] [CrossRef]
- Li, H.; Jia, Y.; Zhao, H.; Wang, Z.; Hu, Z.; Meng, E.; Li, J.; Zhou, B.; Zhang, G.; Zhang, Z. The Strategy to Promote the Degradation of Phenol by Electro-Fenton: The Synergistic Effect of N-Doping and Electrode Aeration Promotes the Adsorption Capacity of Activated Carbon Cathode and Fe2+/Fe3+ Cycle. J. Environ. Chem. Eng. 2023, 11, 110736. [Google Scholar] [CrossRef]
- Agabo-García, C.; Repetto, G.; Albqmi, M.; Hodaifa, G. Evaluation of the Olive Mill Wastewater Treatment Based on Advanced Oxidation Processes (AOPs), Flocculation, and Filtration. J. Environ. Chem. Eng. 2023, 11, 109789. [Google Scholar] [CrossRef]
- Jaouad, Y.; Villain-Gambier, M.; Mandi, L.; Marrot, B.; Ouazzani, N. Comparison of Aerobic Processes for Olive Mill Wastewater Treatment. Water Sci. Technol. 2020, 81, 1914–1926. [Google Scholar] [CrossRef]
- Jamrah, A.; Al-Zghoul, T.M.; Darwish, M.M. A Comprehensive Review of Combined Processes for Olive Mill Wastewater Treatments. Case Stud. Chem. Environ. Eng. 2023, 8, 100493. [Google Scholar] [CrossRef]
- Grace Pavithra, K.; Sundar Rajan, P.; Arun, J.; Brindhadevi, K.; Hoang Le, Q.; Pugazhendhi, A. A Review on Recent Advancements in Extraction, Removal and Recovery of Phenols from Phenolic Wastewater: Challenges and Future Outlook. Environ. Res. 2023, 237, 117005. [Google Scholar] [CrossRef]
- Dich, A.; Abdelmoumene, W.; Belyagoubi, L.; Assadpour, E.; Belyagoubi Benhammou, N.; Zhang, F.; Jafari, S.M. Olive Oil Wastewater: A Comprehensive Review on Examination of Toxicity, Valorization Strategies, Composition, and Modern Management Approaches; Springer: Berlin/Heidelberg, Germany, 2025; ISBN 1135602536127. [Google Scholar]
- Zagklis, D.P.; Papageorgiou, C.S.; Paraskeva, C.A. Technoeconomic Analysis of the Recovery of Phenols from Olive Mill Wastewater through Membrane Filtration and Resin Adsorption/Desorption. Sustainability 2021, 13, 2376. [Google Scholar] [CrossRef]
- Paraskeva, P.; Diamadopoulos, E. Technologies for Olive Mill Wastewater (OMW) Treatment: A Review. J. Chem. Technol. Biotechnol. 2006, 81, 1475–1485. [Google Scholar] [CrossRef]
- Brémond, U.; Bertrandias, A.; Steyer, J.P.; Bernet, N.; Carrere, H. A Vision of European Biogas Sector Development towards 2030: Trends and Challenges. J. Clean. Prod. 2021, 287, 125065. [Google Scholar] [CrossRef]
- Azbar, N.; Tutuk, F.; Keskin, T. International Biodeterioration & Biodegradation Biodegradation Performance of an Anaerobic Hybrid Reactor Treating Olive Mill Effluent under Various Organic Loading Rates. Int. Biodeterior. Biodegrad. 2009, 63, 690–698. [Google Scholar] [CrossRef]
- Gelegenis, J.; Georgakakis, D.; Angelidaki, I.; Christopoulou, N.; Goumenaki, M. Optimization of biogas production from olive-oil mill wastewater, by codigesting with diluted poultry-manure. Appl. Energy 2007, 84, 646–663. [Google Scholar] [CrossRef]
- Maazoun, A.M.; Aounallah, M.K.; Hammami, S.; Damergi, C. The Potential Use of Phenolic Compounds Recovered from Olive Mill Wastewater in Food Model Systems. Eur. J. Nutr. Food Saf. 2022, 14, 39–63. [Google Scholar] [CrossRef]
- Asses, N.; Ayed, L.; Bouallagui, H.; Sayadi, S.; Hamdi, M. International Biodeterioration & Biodegradation Biodegradation of Different Molecular-Mass Polyphenols Derived from Olive Mill Wastewaters by Geotrichum Candidum. Int. Biodeterior. Biodegrad. 2009, 63, 407–413. [Google Scholar] [CrossRef]
- Obied, H.K.; Prenzler, P.D.; Konczak, I.; Rehman, A.U.; Robards, K. Chemistry and Bioactivity of Olive Biophenols in Some Antioxidant and Antiproliferative in Vitro Bioassays. Chem. Res. Toxicol. 2009, 22, 227–234. [Google Scholar] [CrossRef]
- Obied, H.K.; Allen, M.S.; Bedgood, D.R.; Prenzler, P.D.; Robards, K.; Stockmann, R. Bioactivity and Analysis of Biophenols Recovered from Olive Mill Waste. J. Agric. Food Chem. 2005, 53, 823–837. [Google Scholar] [CrossRef]
- Cabrera, F.; Serrano, A.; Torres, Á.; Rodriguez-Gutierrez, G.; Jeison, D.; Fermoso, F.G. The Accumulation of Volatile Fatty Acids and Phenols through a PH-Controlled Fermentation of Olive Mill Solid Waste. Sci. Total Environ. 2019, 657, 1501–1507. [Google Scholar] [CrossRef]
- Calabrò, P.S.; Fòlino, A.; Tamburino, V.; Zappia, G.; Zema, D.A. Increasing the Tolerance to Polyphenols of the Anaerobic Digestion of Olive Wastewater through Microbial Adaptation. Biosyst. Eng. 2018, 172, 19–28. [Google Scholar] [CrossRef]
- Monlau, F.; Sambusiti, C.; Barakat, A.; Quéméneur, M.; Trably, E.; Steyer, J.P.; Carrère, H. Do Furanic and Phenolic Compounds of Lignocellulosic and Algae Biomass Hydrolyzate Inhibit Anaerobic Mixed Cultures? A Comprehensive Review. Biotechnol. Adv. 2014, 32, 934–951. [Google Scholar] [CrossRef]
- Masmoudi, M.A.; Abid, N.; Feki, F.; Karray, F.; Chamkha, M.; Sayadi, S. Study of Olive Mill Wastewater Adsorption onto Biochar as a Pretreatment Option within a Fully Integrated Process. Euro-Mediterr. J. Environ. Integr. 2024, 9, 621–635. [Google Scholar] [CrossRef]
- Hamdi, M. Anaerobic Digestion of Olive Mill Wastewaters. Process Biochem. 1996, 31, 105–110. [Google Scholar] [CrossRef]
- Vavouraki, A.I.; Dareioti, M.A.; Kornaros, M. Olive Mill Wastewater (OMW) Polyphenols Adsorption onto Polymeric Resins: Part I—Batch Anaerobic Digestion of OMW. Waste Biomass Valorization 2021, 12, 2271–2281. [Google Scholar] [CrossRef]
- Bovina, S.; Frascari, D.; Ragini, A.; Avolio, F.; Scarcella, G.N.; Pinelli, D. Development of a Continuous-Flow Anaerobic Co-Digestion Process of Olive Mill Wastewater and Municipal Sewage Sludge. J. Chem. Technol. Biotechnol. 2021, 96, 532–543. [Google Scholar] [CrossRef]
- Micoli, L.; Di Rauso Simeone, G.; Turco, M.; Toscano, G.; Rao, M.A. Biochar Enhances Anaerobic Digestion of Olive Mill Wastewater. Chem. Eng. Trans. 2023, 99, 85–90. [Google Scholar] [CrossRef]
- Manna, C.; D’Angelo, S.; Migliardi, V.; Loffredi, E.; Mazzoni, O.; Morrica, P.; Galletti, P.; Zappia, V. Protective Effect of the Phenolic Fraction from Virgin Olive Oils against Oxidative Stress in Human Cells. J. Agric. Food Chem. 2002, 50, 6521–6526. [Google Scholar] [CrossRef]
- Khoufi, S.; Feki, F.; Sayadi, S. Detoxification of Olive Mill Wastewater by Electrocoagulation and Detoxification of Olive Mill Wastewater by Electrocoagulation and Sedimentation Processes. J. Hazard. Mater. 2007, 142, 58–67. [Google Scholar] [CrossRef]
- Erraji, H.; Abdessadek, E.; Tallou, A.; Asehraou, A. Enhanced Biomethane Production from Olive Mill Wastewater via Co-Digestion with Cow Dung, Fruit, Vegetable, and Fish Wastes: An Experimental and Kinetic Study. Waste Biomass Valorization 2025, 1–12. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, Z.; Fang, Y.; Song, Y.; Li, J.; Feng, Y. Assessing the Long-Term Impact of Incorporating GAC and Fe&G Mediators for Enhancing Phenol Containing Simulated Wastewater Treatment in UASB Reactor. J. Hazard. Mater. 2025, 494, 138459. [Google Scholar] [CrossRef]
- Madigou, C.; Poirier, S.; Bureau, C.; Chapleur, O. Acclimation Strategy to Increase Phenol Tolerance of an Anaerobic Microbiota. Bioresour. Technol. 2016, 216, 77–86. [Google Scholar] [CrossRef]
- Tamborrino, A.; Catalano, F.; Leone, A.; Bianchi, B. A Real Case Study of a Full-Scale Anaerobic Digestion Plant Powered by Olive by-Products. Foods 2021, 10, 1946. [Google Scholar] [CrossRef]
- Rivadávia, R.; Rosa, A.P.; Nascimento, L.A.; Rocha, D.N.; Del Rei Passos, F.L.; dos Santos Renato, N.; Borges, A.C. Effects of Coagulation/Flocculation Followed by Dissolved Air Flotation on Anaerobic Digestion of Coffee Processing Wastewater. Biomass Convers. Biorefinery 2024, 15, 11645–11658. [Google Scholar] [CrossRef]
- Levén, L.; Nyberg, K.; Schnürer, A. Conversion of Phenols during Anaerobic Digestion of Organic Solid Waste—A Review of Important Microorganisms and Impact of Temperature. J. Environ. Manag. 2012, 95, S99–S103. [Google Scholar] [CrossRef]
- Azbar, N.; Bayram, A.; Filibeli, A.; Muezzinoglu, A.; Sengul, F.; Ozer, A. A Review of Waste Management Options in Olive Oil Production. Crit. Rev. Environ. Sci. Technol. 2004, 34, 209–247. [Google Scholar] [CrossRef]
- Kouas, M.; Torrijos, M.; Sousbie, P.; Harmand, J.; Sayadi, S. Modeling the Anaerobic Co-Digestion of Solid Waste: From Batch to Semi-Continuous Simulation. Bioresour. Technol. 2019, 274, 33–42. [Google Scholar] [CrossRef]
- Turkmen, N.; Sari, F.; Velioglu, Y.S. Effects of Extraction Solvents on Concentration and Antioxidant Activity of Black and Black Mate Tea Polyphenols Determined by Ferrous Tartrate and Folin-Ciocalteu Methods. Food Chem. 2006, 99, 835–841. [Google Scholar] [CrossRef]
- Elalami, D.; Carrère, H.; Abdelouahdi, K.; Oukarroum, A.; Dhiba, D.; Arji, M.; Barakat, A. Combination of Dry Milling and Separation Processes with Anaerobic Digestion of Olive Mill Solid Waste: Methane Production and Energy Efficiency. Molecules 2018, 23, 3295. [Google Scholar] [CrossRef]
- Frascari, D.; Bacca, A.E.M.; Zama, F.; Bertin, L.; Fava, F.; Pinelli, D. Olive Mill Wastewater Valorisation through Phenolic Compounds Adsorption in a Continuous Flow Column. Chem. Eng. J. 2016, 283, 293–303. [Google Scholar] [CrossRef]
- Kizito, S.; Wu, S.; Wandera, S.M.; Guo, L.; Dong, R. Evaluation of Ammonium Adsorption in Biochar-Fixed Beds for Treatment of Anaerobically Digested Swine Slurry: Experimental Optimization and Modeling. Sci. Total Environ. 2016, 563–564, 1095–1104. [Google Scholar] [CrossRef]
- Elalami, D.; Carrere, H.; Abdelouahdi, K.; Garcia-Bernet, D.; Peydecastaing, J.; Vaca-Medina, G.; Oukarroum, A.; Zeroual, Y.; Barakat, A. Mild Microwaves, Ultrasonic and Alkaline Pretreatments for Improving Methane Production: Impact on Biochemical and Structural Properties of Olive Pomace. Bioresour. Technol. 2020, 299, 122591. [Google Scholar] [CrossRef]
- Sambusiti, C.; Ficara, E.; Malpei, F.; Steyer, J.P.; Carrère, H. Influence of Alkaline Pre-Treatment Conditions on Structural Features and Methane Production from Ensiled Sorghum Forage. Chem. Eng. J. 2012, 211–212, 488–492. [Google Scholar] [CrossRef]
- Zhang, X.; Li, A.; Jiang, Z.; Zhang, Q. Adsorption of Dyes and Phenol from Water on Resin Adsorbents: Effect of Adsorbate Size and Pore Size Distribution. J. Hazard. Mater. 2006, 137, 1115–1122. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, C.; Yuan, J.; Zhang, C. Adsorption Characteristics of Adsorbent Resins and Antioxidant Capacity for Enrichment of Phenolics from Two-Phase Olive Waste. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2017, 1040, 38–46. [Google Scholar] [CrossRef]

| Parameter | Surface Area (m2/g) | Pore Diameter (nm) | Mean Pore Size (Å) | Particle Size (mm) |
|---|---|---|---|---|
| XAD-4 resin | 725 | 15 | 100 | 0.46–0.69 |
| Parameters (Unit) | Raw-O | Filtred-O |
|---|---|---|
| pH | 4.1 ± 0.1 | 4.03 ± 0.06 |
| TCOD (gO2/L) | 260 ± 6 | 243 ± 2 |
| Total solids (g/L) | 145 ± 4 | 138 ± 2 |
| Total volatile solids (g/L) | 111 ± 3 | 104 ± 4 |
| Total phenolic compounds (g/L) | 8 ± 1 | 7 ± 2 |
| Parameters | C1-5.7 cm | C2-12.1 cm | C3-18.5 cm |
|---|---|---|---|
| Bed Volume Vb (mL) | 11.45 | 24.34 | 37.18 |
| EBCT (min) | 2.29 | 4.86 | 7.41 |
| Superficial Velocity (m/s) | 4.15·10−4 | ||
| Reynolds Number (Re) | 66 | ||
| Breakthrough Time (min) | 2.25 | 3.7 | 8.0 |
| Breakthrough Volume (mL) | 4.5 | 7.4 | 16 |
| Initial TCOD (g O2/L) | 122 ± 2 | ||
| Final TCOD (g O2/L) | 47 ± 4 | 44 ± 2 | 24 ± 2 |
| COD removal yield (%) | 61 | 64 | 80 |
| Polyphenols Initial (mg GAE/L) | 3764 ± 88 | ||
| Polyphenols Final (mg GAE/L) | 2275 ± 148 | 1824 ± 270 | 1333 ± 388 |
| Total polyphenol adsorption capacity, qtotal (mg) | 72.78 | 233.35 | 462.60 |
| Adsorption capacity (mg/g) | 29 | 47 | 47 |
| Polyphenol adsorption yield at saturation (%) | 43 | 56 | 64 |
| Time to saturation (min) | 22.5 | 55.5 | 96 |
| Samples | Initial TPhCs Concentration (mgTPhCs/L) | Methane Yield (NmL CH4/g COD) | Biodegradability (%) | Biodegradability Enhancement (%) | Methane Production (NLCH4/L of OMWW) |
|---|---|---|---|---|---|
| R-O | 44.3 ± 0.1 | 120 ± 1 | 34 | - | 31.2 |
| C1 | 78.1 ± 2.5 | 267 ± 2 | 76 | +122 | 25.1 |
| C2 | 63.2 ± 0.2 | 287 ± 2 | 82 | +138 | 25.2 |
| C3 | 95.4 ± 0.8 | 272 ± 3 | 78 | +126 | 6.5 |
| Substrate | Parameters | |||||
|---|---|---|---|---|---|---|
| S1(0) % | K1 (mL CH4/d) | S2(0) % | K2 (mL CH4/d) | S3(0) % | K3 (mL CH4/d) | |
| Raw-O | 74 | 1.82 | 13 | 0.07 | 13 | 0.02 |
| C1 | 39 | 5.49 | 43 | 20.68 | 18 | 1.38 |
| C2 | 34 | 18.08 | 34 | 9.41 | 33 | 2.68 |
| C3 | 50 | 23.86 | 13 | 2.47 | 38 | 2.92 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hakim, C.; Neffa, M.; Essadek, A.; Battimelli, A.; Escudie, R.; García-Bernet, D.; Harmand, J.; Carrère, H. Combined Continuous Resin Adsorption and Anaerobic Digestion of Olive Mill Wastewater for Polyphenol and Energy Recovery. Energies 2025, 18, 3226. https://doi.org/10.3390/en18133226
Hakim C, Neffa M, Essadek A, Battimelli A, Escudie R, García-Bernet D, Harmand J, Carrère H. Combined Continuous Resin Adsorption and Anaerobic Digestion of Olive Mill Wastewater for Polyphenol and Energy Recovery. Energies. 2025; 18(13):3226. https://doi.org/10.3390/en18133226
Chicago/Turabian StyleHakim, Chaimaa, Mounsef Neffa, Abdessadek Essadek, Audrey Battimelli, Renaud Escudie, Diana García-Bernet, Jérôme Harmand, and Hélène Carrère. 2025. "Combined Continuous Resin Adsorption and Anaerobic Digestion of Olive Mill Wastewater for Polyphenol and Energy Recovery" Energies 18, no. 13: 3226. https://doi.org/10.3390/en18133226
APA StyleHakim, C., Neffa, M., Essadek, A., Battimelli, A., Escudie, R., García-Bernet, D., Harmand, J., & Carrère, H. (2025). Combined Continuous Resin Adsorption and Anaerobic Digestion of Olive Mill Wastewater for Polyphenol and Energy Recovery. Energies, 18(13), 3226. https://doi.org/10.3390/en18133226









