Demulsification Kinetics of Water-in-Oil Emulsions of Ecuadorian Crude Oil: Influence of Temperature and Salinity
Abstract
1. Introduction
2. Materials and Methods
2.1. Crude Oil
2.2. Aqueous Phase
2.3. Emulsion Preparation
2.4. Bottle Test Measurements
2.5. Interfacial Tension (IFT) Measurements
2.6. Interfacial Dilatational Rheology Measurements
2.7. Shear Rheology Measurements
2.8. Pulsed Field Gradient Nuclear Magnetic Resonance (PFG-NMR) Measurements
3. Results and Discussion
3.1. Salinity Effect
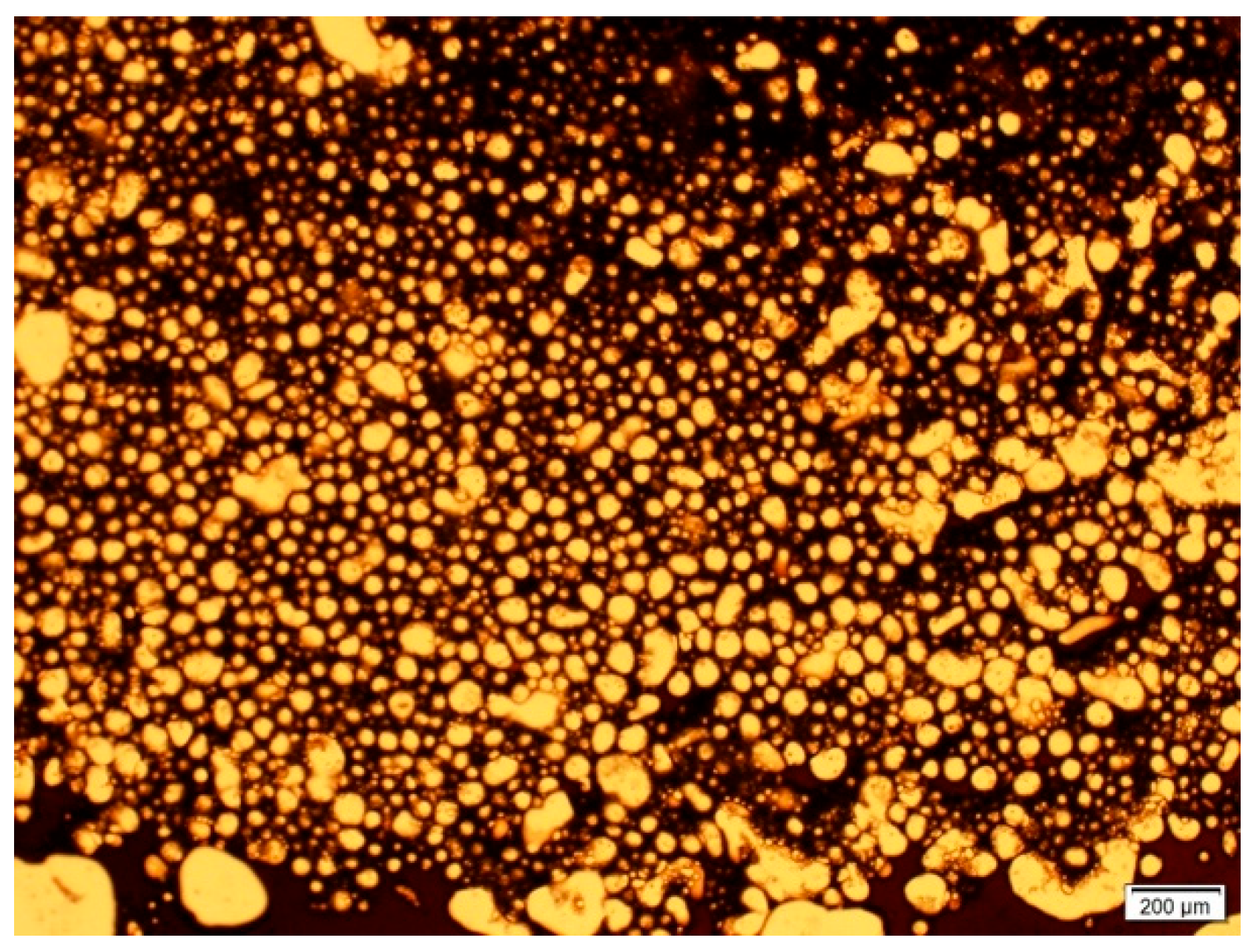
3.1.1. Emulsion Generation
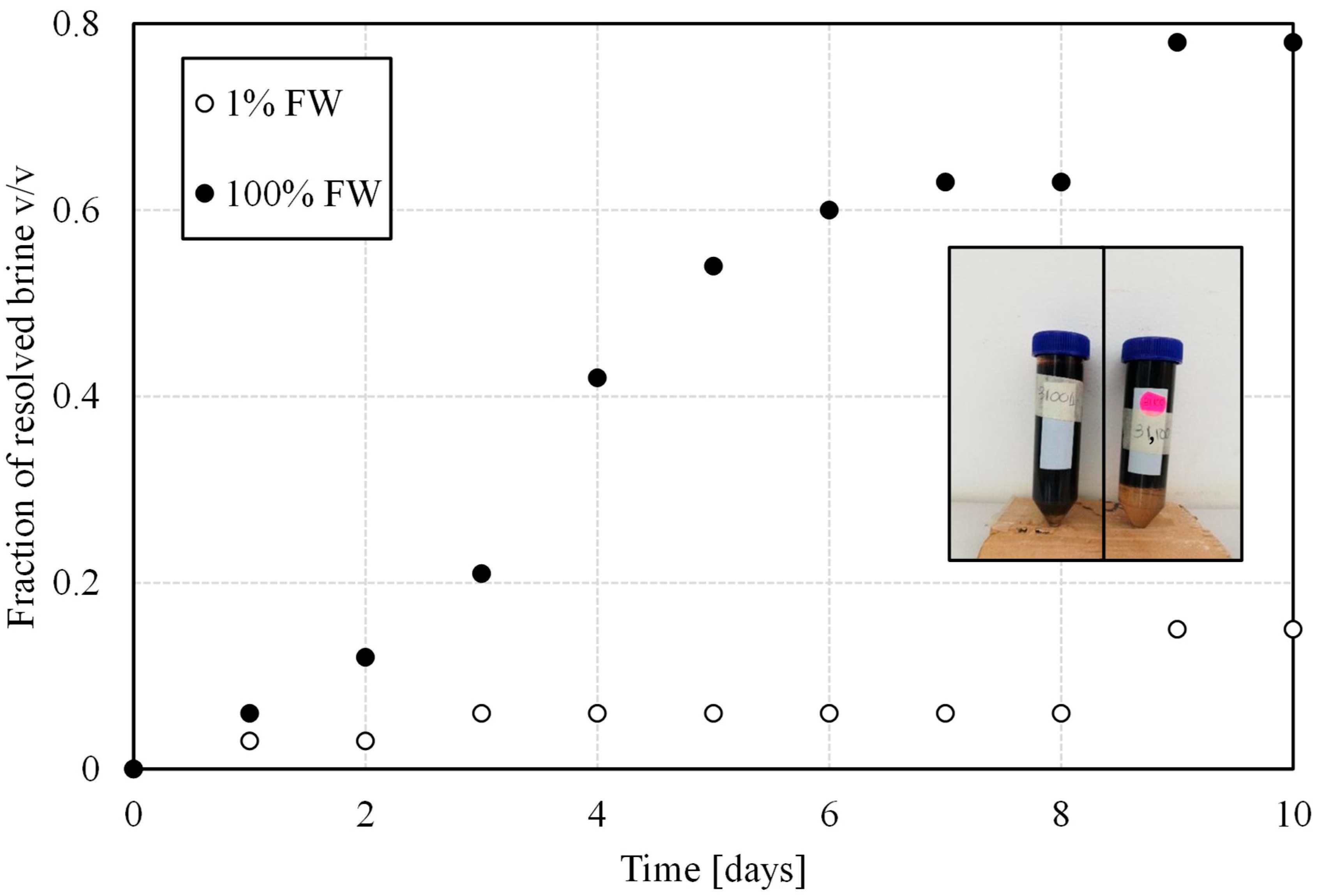
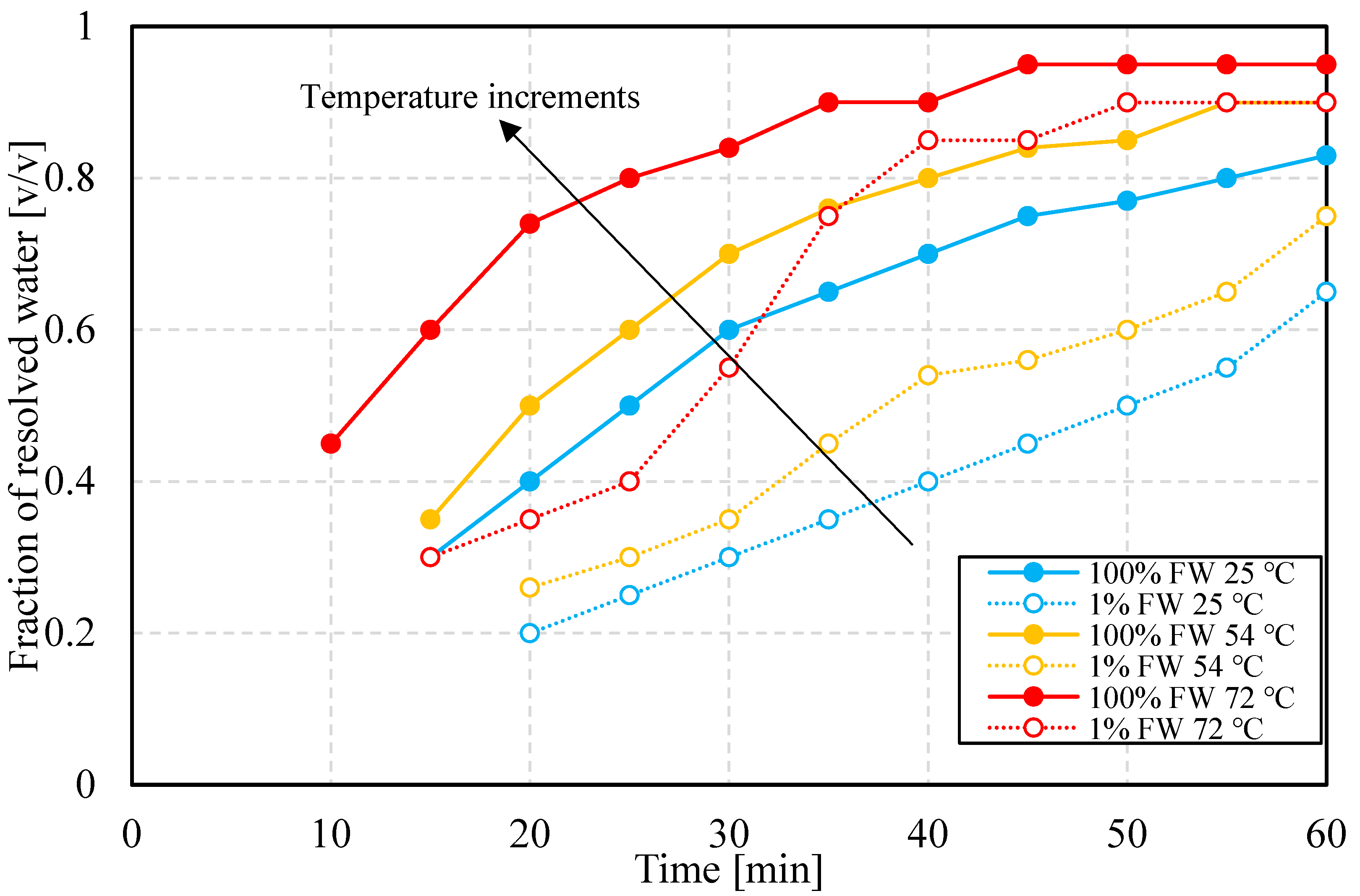
3.1.2. Bottle Tests: Salinity Effect
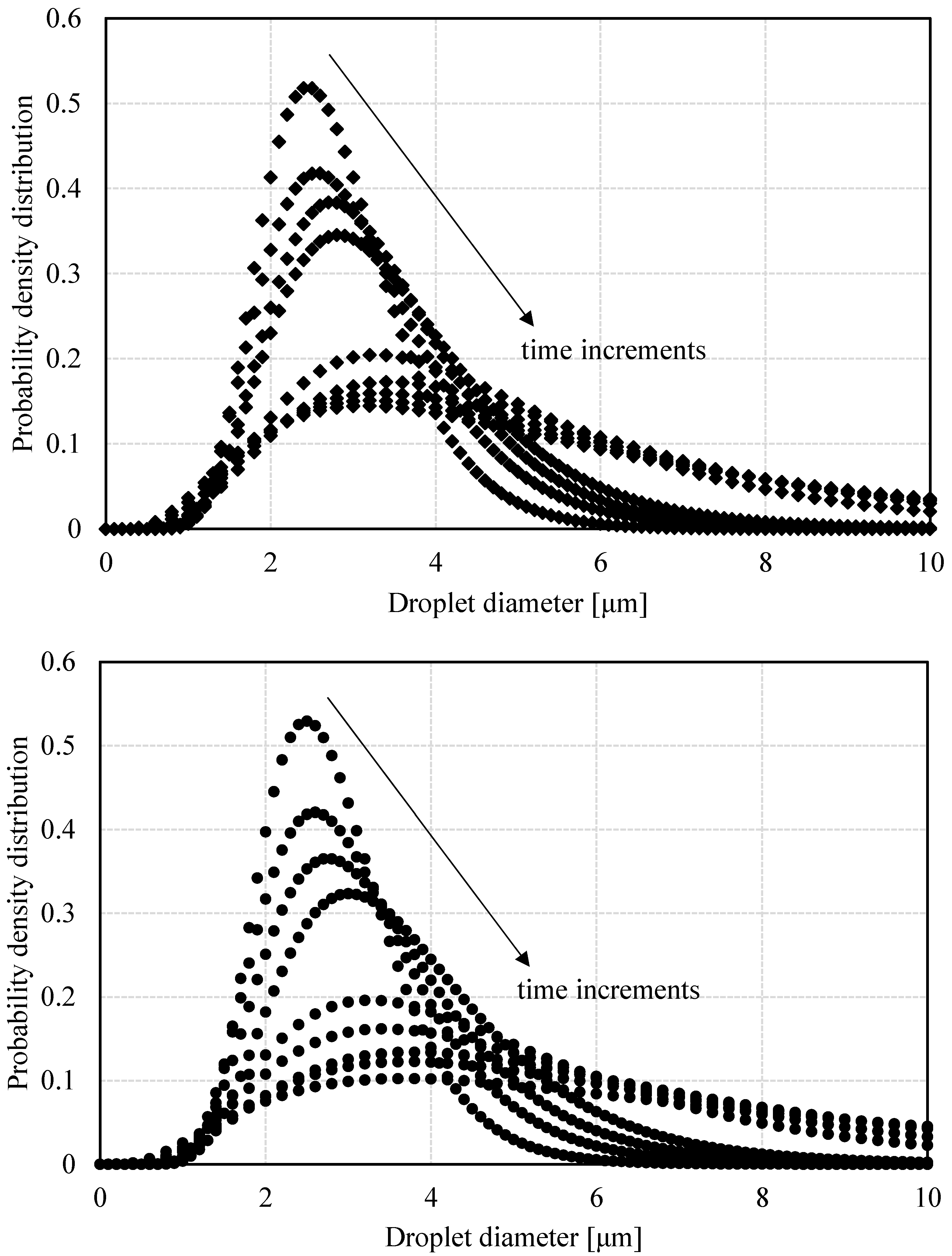
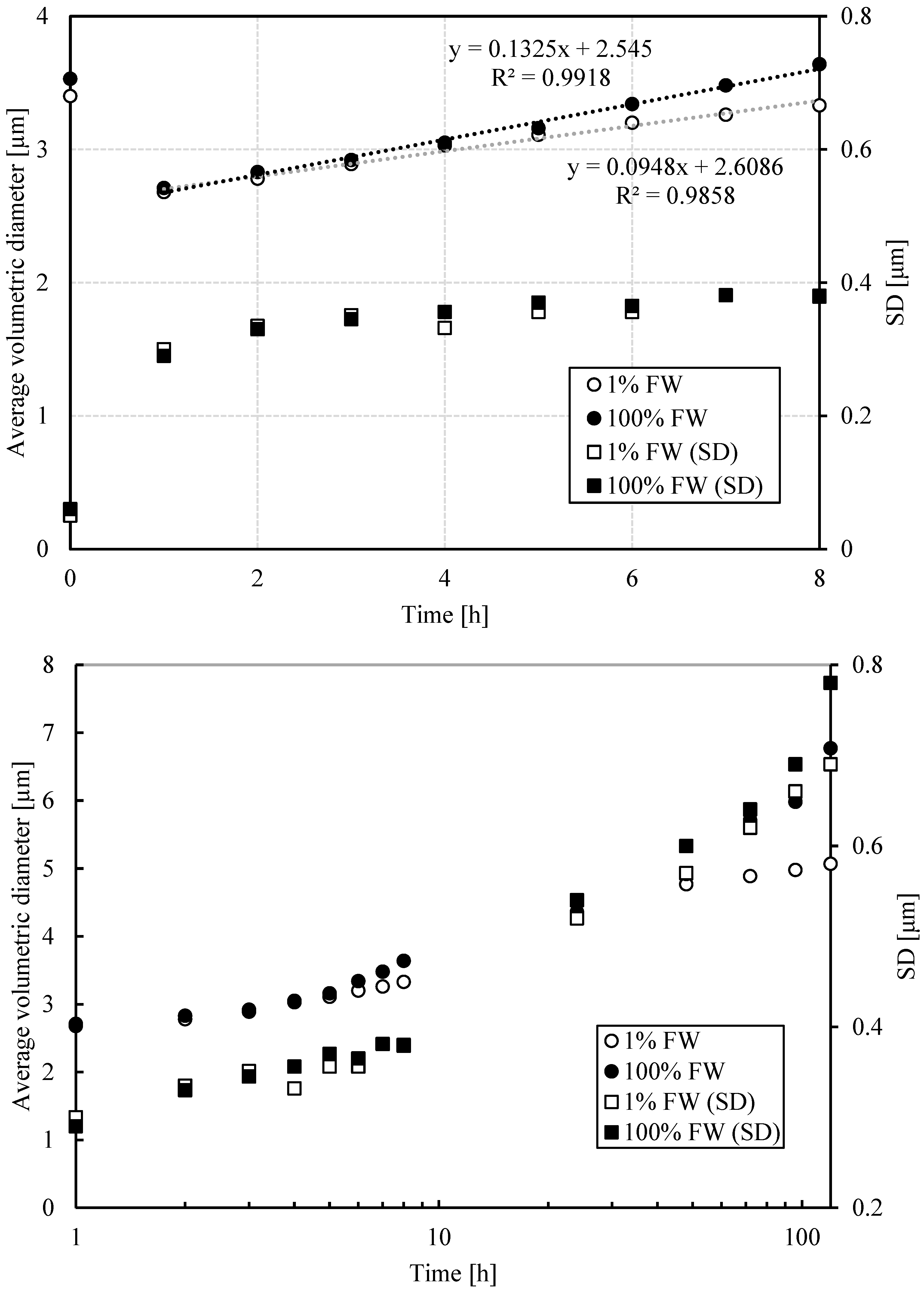
3.1.3. DSD by PFG-NMR
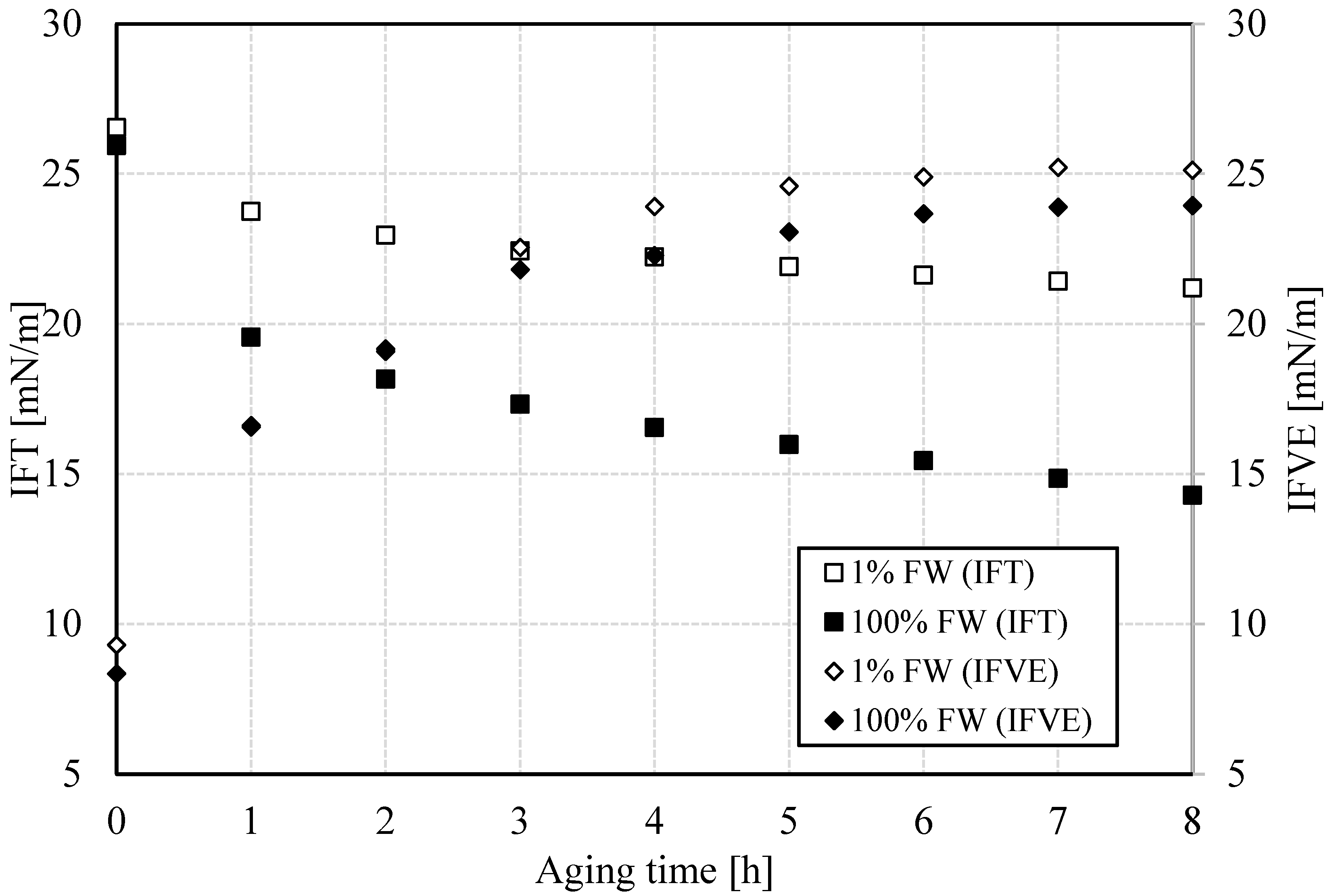
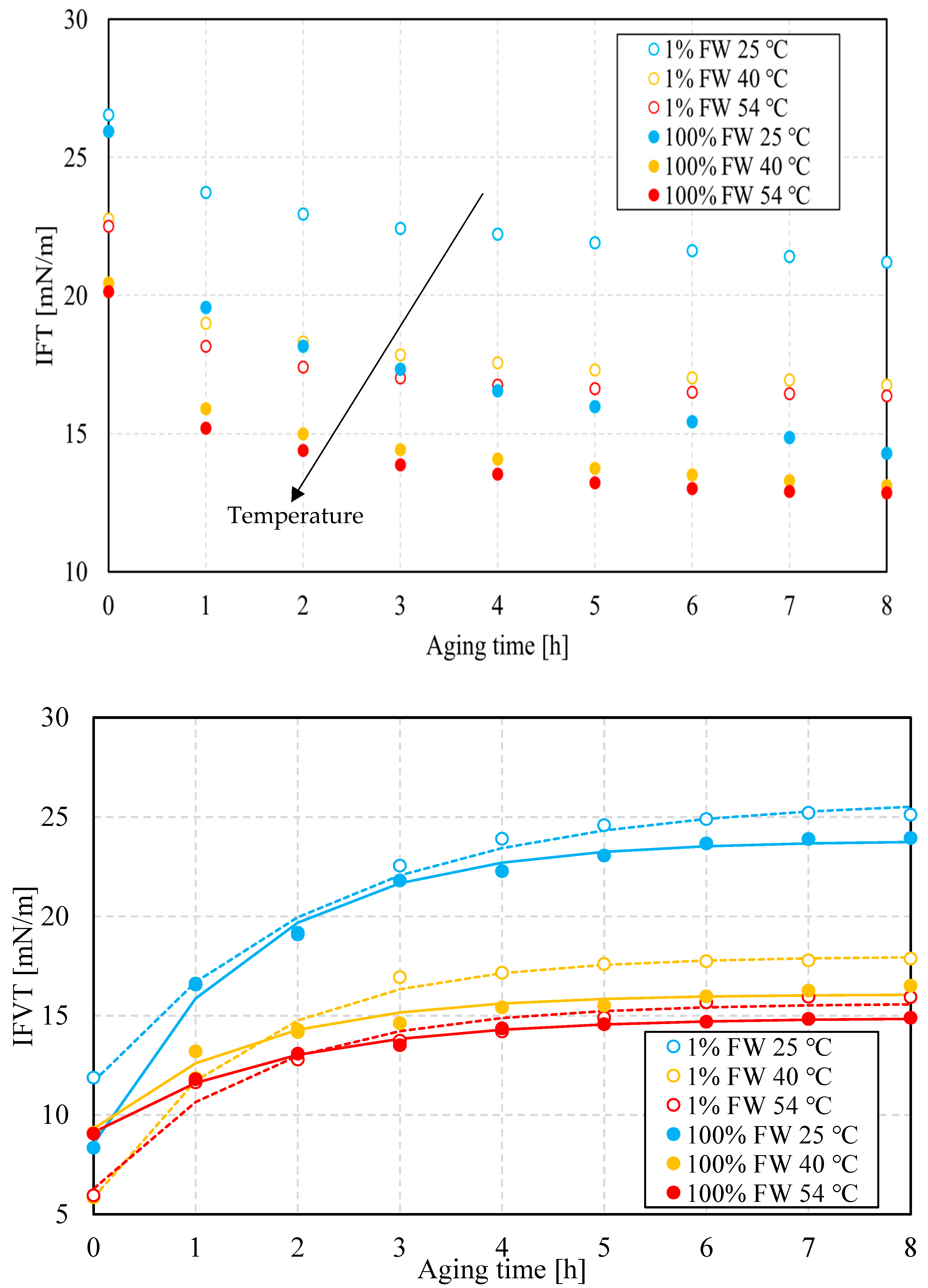
3.1.4. Interfacial Properties: Salinity Dependence
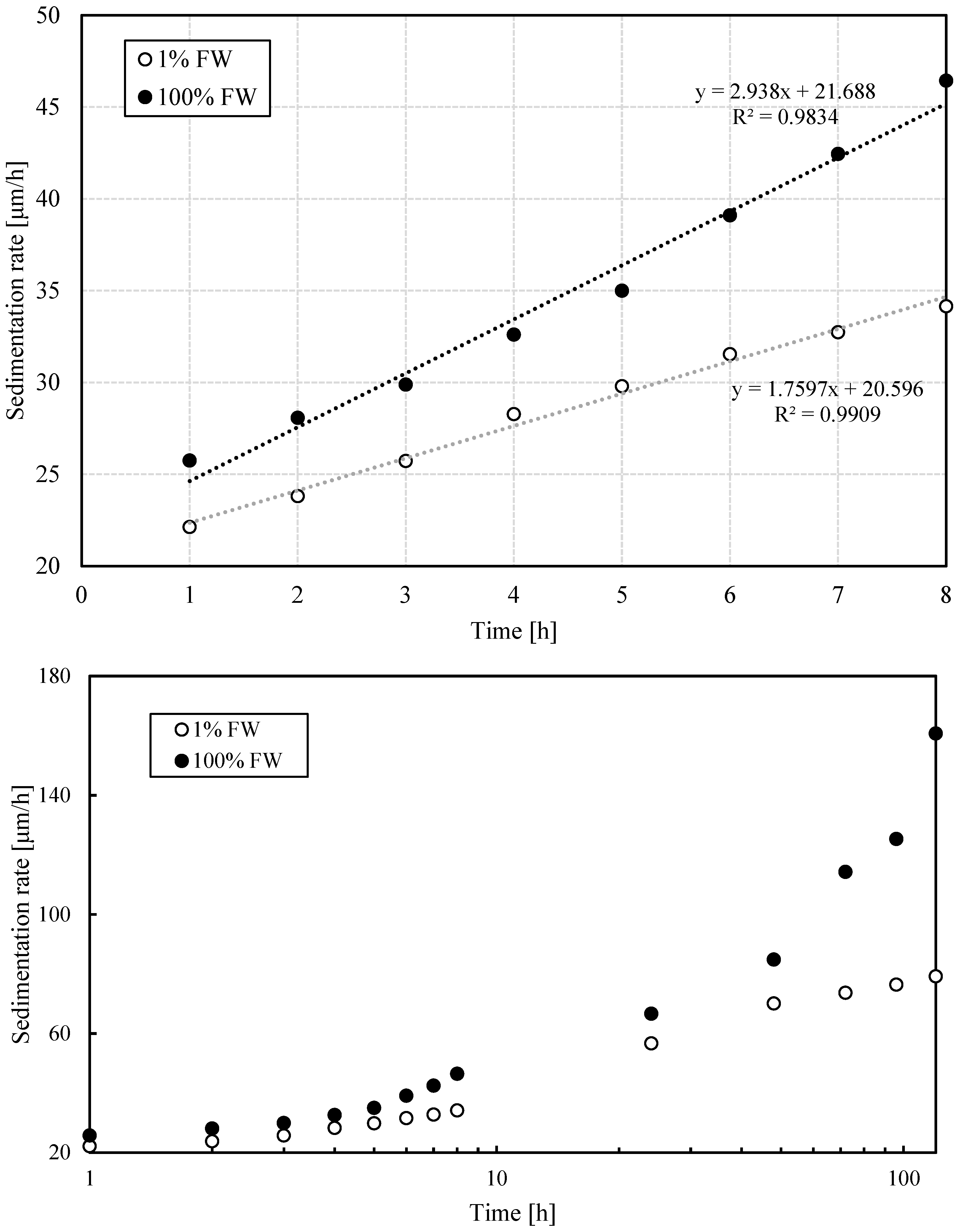
3.1.5. Estimation of Sedimentation, Flocculation and Coalescence Rates
3.2. Temperature Effect
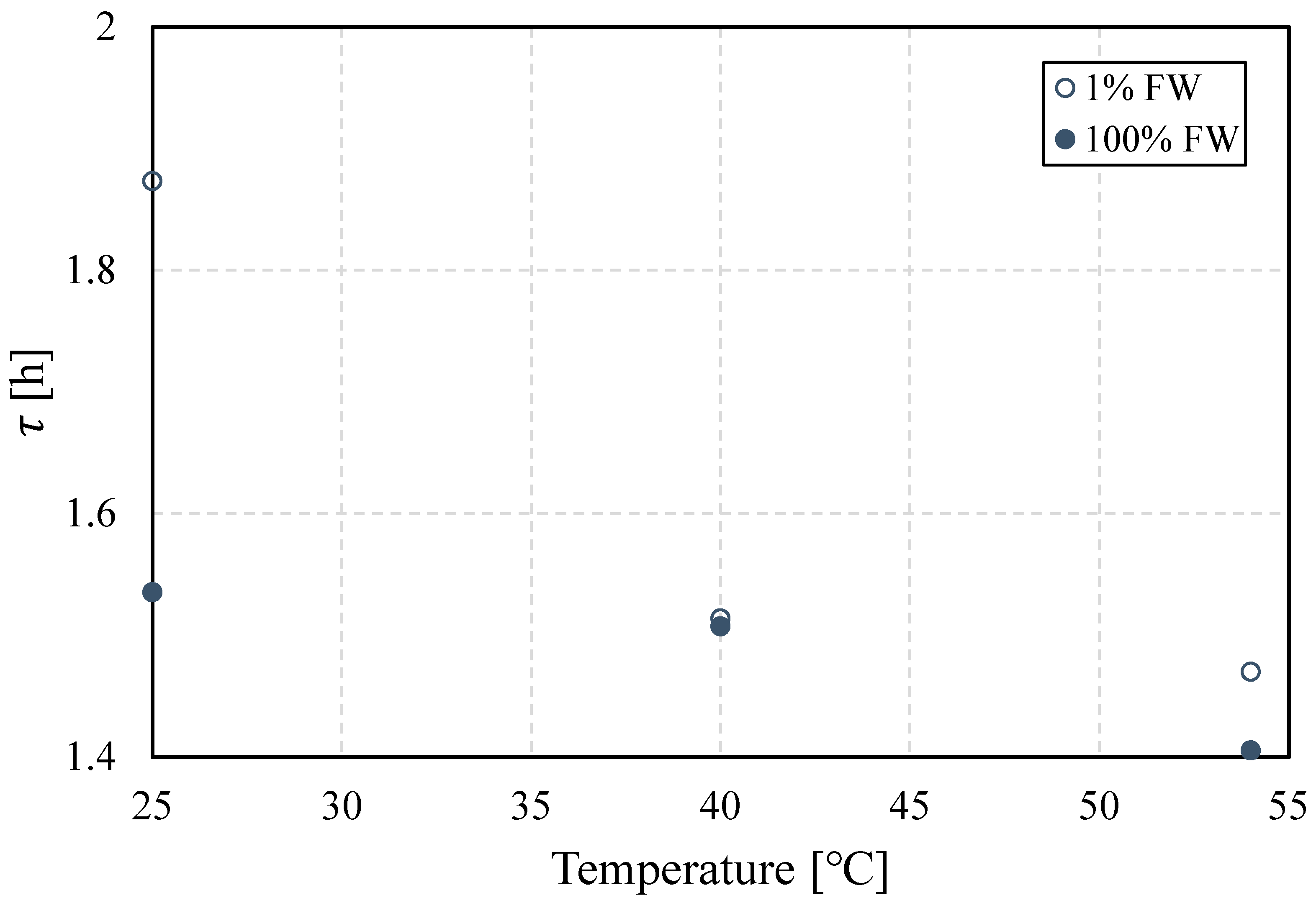
3.2.1. Bottle Tests: Temperature Dependence
3.2.2. Interfacial Properties: Temperature Effect
4. Conclusions
- A comprehensive stability analysis of W/O emulsions prepared with Sacha crude oil was conducted to elucidate emulsion breakdown controls under conditions established in this research. The resulting emulsions were more resilient at lower salinity. This is the first time that emulsions with oil originated from this basin have been analyzed in this context in the literature, to the best of our knowledge.
- Emulsions, regardless of aqueous-phase salinity, are more stable at lower temperatures. This is explained by the formation of less viscoelastic water–oil interfaces, as the viscoelastic modulus results show. This in turn indicates that lower salinity favors interfacial viscoelasticity.
- The interfacial response rate is clearly a function of temperature and salinity. This can be associated with temperature-dependent processes, including the interfacial viscous environment that is temperature-dependent as well as the structure rate of the interface, as shown in the time-course analysis of the interfacial viscoelasticity and interfacial tension.
- The effectiveness of the phase-separation process at higher salinity in the aqueous phase can be explained by the increased density contrast between water and oil, which favors the sedimentation rate, accompanied by a relative increase in the coalescence rate, as the results here show.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| W/O | water-in-oil (W/O) |
| O/W | Oil-in-Water |
| DSD | droplet-size distribution |
| TD-NMR | Time-Domain Nuclear Magnetic Resonance |
| ODG | Oscillating drop generator |
| FW | Formation water |
| TDS | Total Dissolved Solids |
| IFT | Interfacial tension |
| IFVE | interfacial viscoelasticity |
| PFG-NMR | Pulsed Field Gradient Nuclear Magnetic Resonance |
| SD | Standard deviation |
| EDL | Electrical double layer |
References
- Kim, Y.H.; Wasan, D.T.; Breen, P.J. A study of dynamic interfacial mechanisms for demulsification of water-in-oil emulsions. Colloids Surf. A 1995, 95, 235–247. [Google Scholar] [CrossRef]
- Liu, J.; Liu, S.; He, Y.; Zhong, L.; Hao, T.; Liu, Y.; Wang, P.; Gao, P.; Guo, Q. Experimental study of produced fluid emulsification during water/steam flooding for heavy oil recovery. Energy Fuel 2022, 36, 14838–14851. [Google Scholar] [CrossRef]
- Maaref, S.; Ayatollahi, S. The effect of brine salinity on water-in-oil emulsion stability through droplet size distribution analysis: A case study. J. Disper. Sci. Technol. 2018, 39, 721–733. [Google Scholar] [CrossRef]
- Liu, J.; Zhong, L.; Lyu, C.; Liu, Y.; Zhang, S. W/O emulsions generated by heavy oil in porous medium and its effects in reemulsification. Fuel 2022, 310, 12344. [Google Scholar] [CrossRef]
- Alvarado, V.; Wang, X.; Moradi, M. Stability Proxies for water in oil emulsions.and implications in aqueous-based enhanced oil recovery. Energies 2011, 4, 1058–1086. [Google Scholar] [CrossRef]
- Pal, N.; Alzahid, Y.; AlSofi, A.; Ali, M.; Hoteit, H. Review on microemulsions for conformance improvement technology: Fundamentals, design considerations and perspectives. Energy Fuel 2023, 37, 858–875. [Google Scholar] [CrossRef]
- Rocha, J.; Baydak, E.; Yarranton, H.; Sztukowski, D.; Ali-Marcano, V.; Gong, L.; Shi, C.; Zeng, H. Role of aqueous phase chemistry, interfacial film properties, and surface coverage in stabilizing water-in-bitumen emulsions. Energy Fuel 2016, 30, 5240–5252. [Google Scholar] [CrossRef]
- Dimitrova, T.D.; Leal-Calderon, F.J. Forces between emulsion droplets stabilized with Tween 20 and proteins. Langmuir 1999, 15, 8813–8821. [Google Scholar] [CrossRef]
- Tian, Y.; Zhou, J.; He, C.; He, L.; Li, X.; Sui, H. The formation, stabilization, and separation of oil-water emulsions: A review. Processes 2022, 10, 738. [Google Scholar] [CrossRef]
- Zhao, F.; Tian, Z.; Yu, Z.; Shang, H.; Wu, Y.; Zhang, Y. Research status and analysis of stabilization mechanisms and demulsification methods of heavy oil emulsions. Energy Sci. Eng. 2020, 8, 4158–4175. [Google Scholar]
- Hu, Y.; Ting, Y.; Hu, J.; Hsieh, S. Techniques and methods to study functional characteristics of emulsions systems. JFDA 2017, 25, 16–26. [Google Scholar] [CrossRef]
- Dickinson, E. Strategies to control and inhibit the flocculation of protein-stabilized oil-in-water emulsions. Food Hydrocoll. 2019, 96, 209–223. [Google Scholar] [CrossRef]
- Dinh, H.; Carreras, E.; Schmitt, V.; Lequeux, F. Coalescence in concentrated emulsions: Theoretical predictions and comparision with experimental bottle test behavior. Soft Matter 2020, 16, 10301–10309. [Google Scholar] [CrossRef]
- Abdulredha, M.; Hussain, S.; Abdullah, L. Overview on petroleum emulsions, formation, influence and demulsification treatment techniques. Arab. J. Chem. 2020, 13, 3403–3428. [Google Scholar] [CrossRef]
- Russ, J.C. Image Analysis of Food Microstructure; CRC Press: Boca Raton, FL, USA, 2004. [Google Scholar]
- Alade, O.; Mahmoud, M.; Al Shehri, D.; Sultan, A. Rapid determination of emulsion stability using turbidity measurement incorporating artificial neural network (ANN): Experimental validation using video/optical microscopy and kinetic modeling. ACS Omega 2021, 6, 5910–5920. [Google Scholar] [CrossRef]
- Manickam, S.; Sivakumar, K.; Pang, C. Investigations on the generation of oil-in-water (O/W) nanoemulsions trough the combination of ultrasound and microchannel. Ultrason. Sonochem. 2020, 69, 105258. [Google Scholar] [CrossRef]
- Marques, D.; White, R. The digitalization of bottle tests nuclear magnetic resonance evaluation of emulsion stability. J. Pet. Technol. 2020, 7, 40–42. [Google Scholar] [CrossRef]
- D6560; Standard Test Method for Determination of Asphaltenes (Heptane Insolubles) in Crude Petroleum and Petroleum Products. ASTM: Conshohocken, PA, USA, 2022.
- D14011-02; Standard Test Method for Water Separability of Petroleum Oils and Synthetic Fluids. ASTM: Conshohocken, PA, USA, 2002.
- Mansoori, G.; Vazquez, D.; Shariaty-Niassar, M. Polidispersity of heavy organics in crude oils and their role in oil well fouling. J. Pet. Sci. Eng. 2007, 58, 375–390. [Google Scholar] [CrossRef]
- Khadem, B.; Parrott, A.; Nordon, A.; Sheibat-Othman, N. Low field high resolution PFG-NMR to predict the size distribution of inner droplets in double emulsions. Eur. J. Lipid Sci. Technol. 2021, 123, 2000193. [Google Scholar] [CrossRef]
- Garcia, B.; Saraji, S. Transient interfacial rheology and polar component dynamics at oil-brine interfaces. Colloids Surf. A Physicochem. Eng. Asp. 2021, 624, 126773. [Google Scholar] [CrossRef]
- Sarmas-Farfan, J.; Medina-Rodriguez, B.; Alvarado, V. Dynamic stability of a crude oil/brine interface: Effect of anion type. Fuel 2023, 335, 127002. [Google Scholar] [CrossRef]
- Song, X.; Shi, P.; Duan, M.; Fang, S.; Ma, Y. Investigation of demulsification efficiency in water-in-crude oil emulsions using dissipative particle dynamics. RSC Adv. 2015, 5, 62971. [Google Scholar] [CrossRef]
- Moradi, M.; Alvarado, V. Influence of Aqueous-Phase Ionic Strength and Composition on the Dynamics of Water–Crude Oil Interfacial Film Formation. Energy Fuel 2016, 30, 9170–9180. [Google Scholar] [CrossRef]
- Hoyer, P.; Carvalho, M.S.; Alvarado, V. Snap-off in Constricted Capillary with Elastic Interface. Phys. Fluids 2016, 28, 012104. [Google Scholar] [CrossRef]
- Hoyer, P.; Alvarado, V. Stability of liquid bridge with elastic interface. RSC Adv. 2017, 7, 49344–49352. [Google Scholar] [CrossRef]
| Crude Oil Sample | Density [g/mL] | Viscosity [cP] | Asphaltene Content [% m/m] |
|---|---|---|---|
| Campo Sacha | 0.9136 | 53.20 | 8.65 |
| Brines | Composition [ppm] | [kg/mol] | [g/mL] | pH | ||||
|---|---|---|---|---|---|---|---|---|
| NaCl | NaHCO3 | CaCl2 | MgCl2 | TDS * | IS ** | Density | ||
| 100% FW | 13,195 | 370 | 637 | 321 | 14,523 | 0.2690 | 1.0087 | 6.38 |
| 1% FW | 132 | 4 | 6 | 3 | 145 | 0.0027 | 0.9972 | 6.89 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sarmas-Farfan, J.; Diaz-Barrios, A.; Lehmann, T.E.; Alvarado, V. Demulsification Kinetics of Water-in-Oil Emulsions of Ecuadorian Crude Oil: Influence of Temperature and Salinity. Energies 2025, 18, 3115. https://doi.org/10.3390/en18123115
Sarmas-Farfan J, Diaz-Barrios A, Lehmann TE, Alvarado V. Demulsification Kinetics of Water-in-Oil Emulsions of Ecuadorian Crude Oil: Influence of Temperature and Salinity. Energies. 2025; 18(12):3115. https://doi.org/10.3390/en18123115
Chicago/Turabian StyleSarmas-Farfan, Jordy, Antonio Diaz-Barrios, Teresa E. Lehmann, and Vladimir Alvarado. 2025. "Demulsification Kinetics of Water-in-Oil Emulsions of Ecuadorian Crude Oil: Influence of Temperature and Salinity" Energies 18, no. 12: 3115. https://doi.org/10.3390/en18123115
APA StyleSarmas-Farfan, J., Diaz-Barrios, A., Lehmann, T. E., & Alvarado, V. (2025). Demulsification Kinetics of Water-in-Oil Emulsions of Ecuadorian Crude Oil: Influence of Temperature and Salinity. Energies, 18(12), 3115. https://doi.org/10.3390/en18123115








