Anaerobic Digestion of the Halophyte Salicornia ramosissima in Co-Digestion with Swine Manure in Lab-Scale Batch and Continuous Reactor Tests
Abstract
1. Introduction
2. Materials and Methods
2.1. Biomass Feedstock and Inoculum
2.1.1. Salicornia ramosissima
2.1.2. Swine Manure
2.1.3. Inoculum
2.2. Analytical Methods
2.2.1. Total Solids and Volatile Solids
2.2.2. Elemental Analysis
2.2.3. Ammonium Nitrogen
2.2.4. pH and Volatile Fatty Acids
2.2.5. Biogas Composition
2.2.6. Kinetic Parameter Estimation
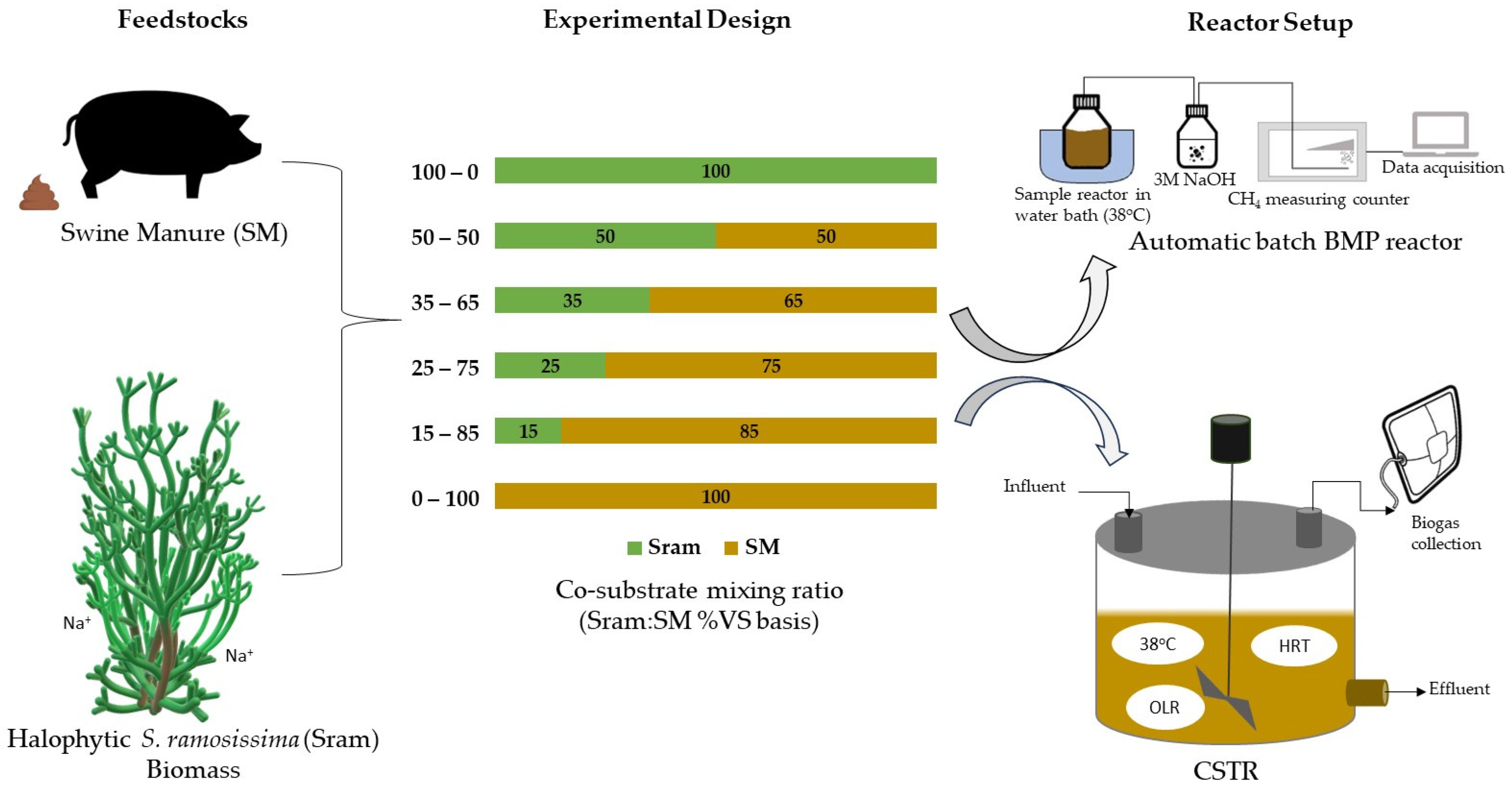
2.3. Reactor Setup and Operation
2.3.1. Batch BMP Experiments
2.3.2. Continuous Stirred Tank Reactor Experiments
3. Results and Discussion
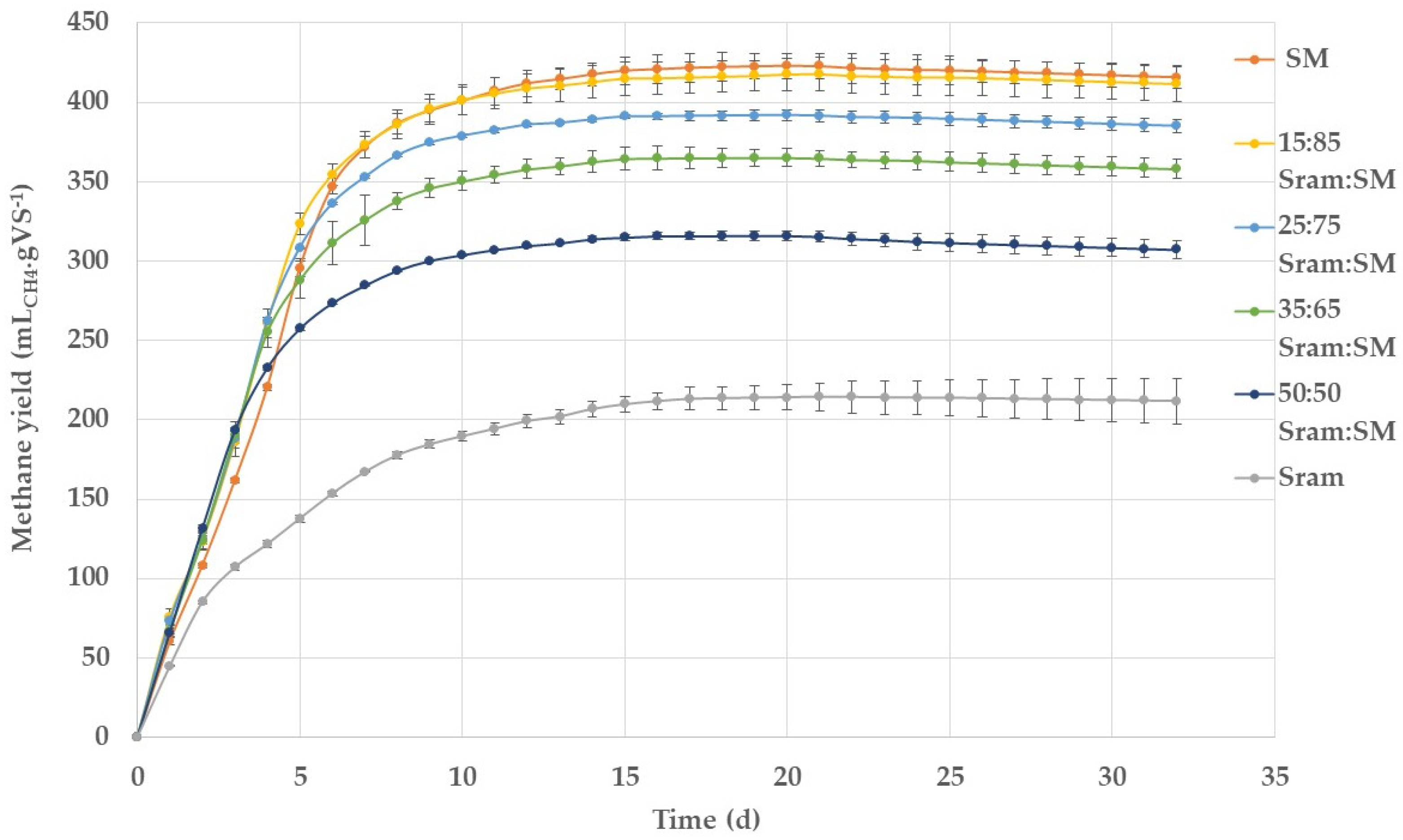
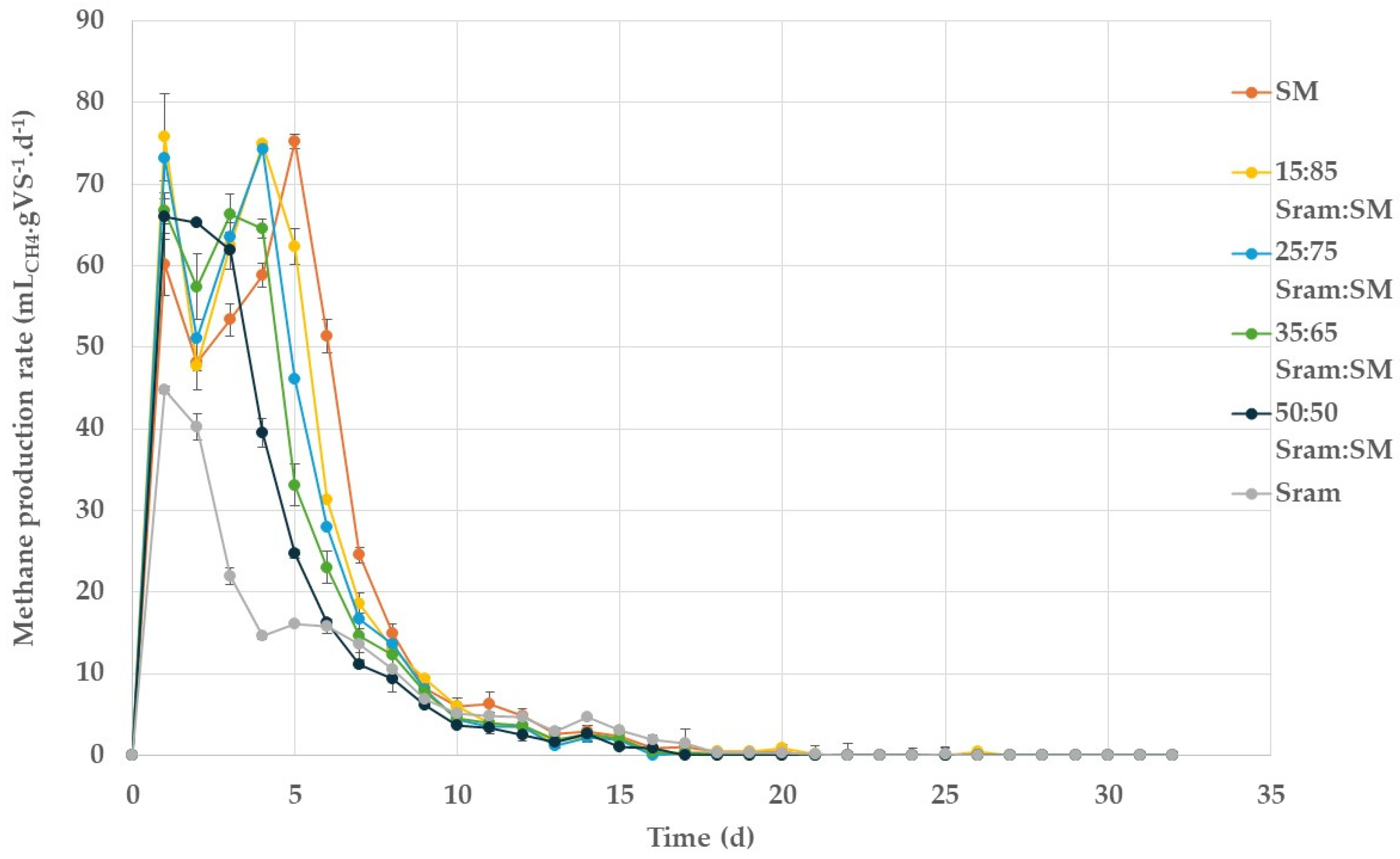
3.1. Batch BMP Experiments
3.2. Continuous Reactor Experiments
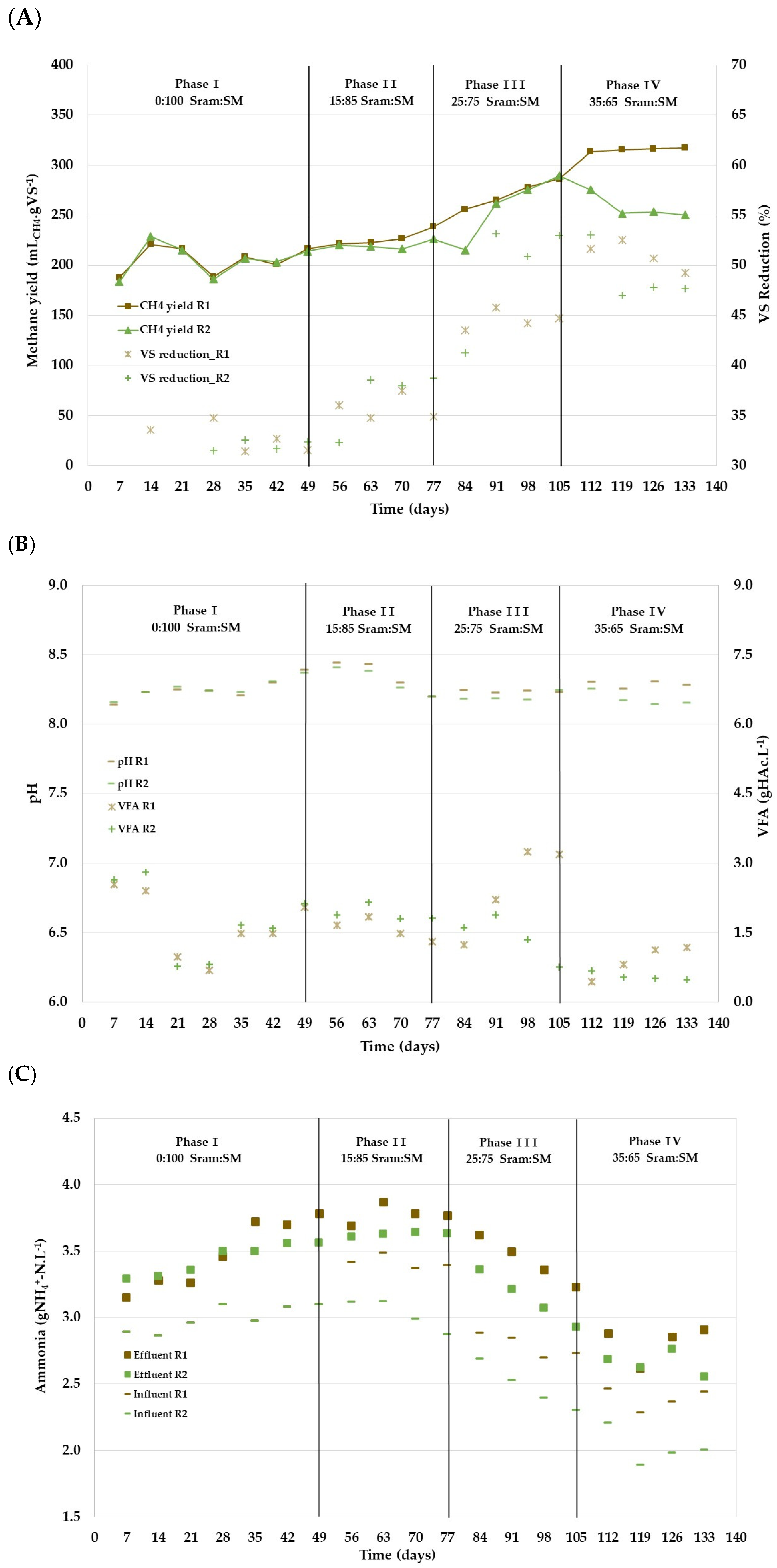
3.2.1. Phase I: Start-Up of Reactors R1 and R2 with 100% SM
3.2.2. Phase II: Increasing the Share of Sram to 15%VS in R2
3.2.3. Phase III: Increasing the Share of Sram to 25%VS in R2
3.2.4. Phase IV: Increasing the Share of Sram to 35%VS in R2
3.2.5. Overall Performance of the Continuous Reactor Process
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AD | Anaerobic Digestion |
| BMP | Biochemical Methane Potential |
| C | Carbon |
| CPI | Co-digestion Performance Index |
| CS | Corn Stover |
| CSTR | Continuous Stirred Tank Reactors |
| ESBC | Exhausted Sugar Beet Cossettes |
| H | Hydrogen |
| HAc | Acetic Acid |
| HPLC | High-Performance Liquid Chromatography |
| HRT | Hydraulic Retention Time |
| O | Oxygen |
| OLR | Organic Loading Rate |
| N | Nitrogen |
| S | Sulfur |
| SM | Swine Manure |
| Sram | S. ramosissima |
| TS | Total Solids |
| VFA | Volatile Fatty Acids |
| VS | Volatile Solids |
| WWTP | Wastewater Treatment Plant |
References
- Joshi, A.; Kanthaliya, B.; Arora, J. Halophytes: The Nonconventional Crops as Source of Biofuel Production. In Handbook of Halophytes; Springer: Berlin/Heidelberg, Germany, 2020; pp. 1–28. [Google Scholar] [CrossRef]
- Grigore, M.N.; Vicente, O. Wild Halophytes: Tools for Understanding Salt Tolerance Mechanisms of Plants and for Adapting Agriculture to Climate Change. Plants 2023, 12, 221. [Google Scholar] [CrossRef] [PubMed]
- Sharma, R.; Wungrampha, S.; Singh, V.; Pareek, A.; Sharma, M.K. Halophytes As Bioenergy Crops. Front. Plant Sci. 2016, 7, 1372. [Google Scholar] [CrossRef]
- Ventura, Y.; Sagi, M. Halophyte Crop Cultivation: The Case for Salicornia and Sarcocornia. Environ. Exp. Bot. 2013, 92, 144–153. [Google Scholar] [CrossRef]
- Taşçı Çilak, T.; Raval, S.; Fasse, S.; Thomsen, M.H.; Gottschalk, A. Modeling, simulation and techno-economic analysis of an integrated biorefinery based on halophytes. Comput. Aided Chem. Eng. 2023, 52, 2465–2470. [Google Scholar] [CrossRef]
- Accogli, R.; Tomaselli, V.; Direnzo, P.; Perrino, E.V.; Albanese, G.; Urbano, M.; Laghetti, G. Edible Halophytes and Halo-Tolerant Species in Apulia Region (Southeastern Italy): Biogeography, Traditional Food Use and Potential Sustainable Crops. Plants 2023, 12, 549. [Google Scholar] [CrossRef]
- El-Hack, M.E.A.; Samak, D.H.; Noreldin, A.E.; Arif, M.; Yaqoob, H.S.; Swelum, A.A. Towards Saving Freshwater: Halophytes as Unconventional Feedstuffs in Livestock Feed: A Review. Environ. Sci. Pollut. Res. 2018, 25, 14397–14406. [Google Scholar] [CrossRef]
- Hulkko, L.S.S.; Rocha, R.M.; Trentin, R.; Fredsgaard, M.; Chaturvedi, T.; Custódio, L.; Thomsen, M.H. Bioactive Extracts from Salicornia Ramosissima J. Woods Biorefinery as a Source of Ingredients for High-Value Industries. Plants 2023, 12, 1251. [Google Scholar] [CrossRef]
- González, R.; Peña, D.C.; Gómez, X. Anaerobic Co-Digestion of Wastes: Reviewing Current Status and Approaches for Enhancing Biogas Production. Appl. Sci. 2022, 12, 8884. [Google Scholar] [CrossRef]
- Kovačić, Đ.; Lončarić, Z.; Jović, J.; Samac, D.; Popović, B.; Tišma, M. Digestate Management and Processing Practices: A Review. Appl. Sci. 2022, 12, 9216. [Google Scholar] [CrossRef]
- Turcios, A.E.; Cayenne, A.; Uellendahl, H.; Papenbrock, J. Halophyte Plants and Their Residues as Feedstock for Biogas Production—Chances and Challenges. Appl. Sci. 2021, 11, 2746. [Google Scholar] [CrossRef]
- Cayenne, A.; Turcios, A.E.; Thomsen, M.H.; Rocha, R.M.; Papenbrock, J.; Uellendahl, H. Halophytes as Feedstock for Biogas Production: Composition Analysis and Biomethane Potential of Salicornia spp. Plant Material from Hydroponic and Seawater Irrigation Systems. Fermentation 2022, 8, 189. [Google Scholar] [CrossRef]
- Hulkko, L.S.S.; Turcios, A.E.; Kohnen, S.; Chaturvedi, T.; Papenbrock, J.; Thomsen, M.H. Cultivation and Characterisation of Salicornia Europaea, Tripolium Pannonicum and Crithmum Maritimum Biomass for Green Biorefinery Applications. Sci. Rep. 2022, 12, 20507. [Google Scholar] [CrossRef] [PubMed]
- Turcios, A.E.; Braem, L.; Jonard, C.; Lemans, T.; Cybulska, I.; Papenbrock, J. Compositional Changes in Hydroponically Cultivated Salicornia Europaea at Different Growth Stages. Plants 2023, 12, 2472. [Google Scholar] [CrossRef]
- Siddique, M.N.I.; Wahid, Z.A. Achievements and Perspectives of Anaerobic Co-Digestion: A Review. J. Clean. Prod. 2018, 194, 359–371. [Google Scholar] [CrossRef]
- Hartmann, H.; Angelidaki, I.; Ahring, B.K. Co-digestion of the organic fraction of municipal waste with other waste types. In Biomethanization of the Organic Fraction of Municipal Solid Wastes; Mata-Alvarez, J., Ed.; IWA Publishing: London, UK, 2002; pp. 181–200. [Google Scholar]
- Hartmann, H.; Ahring, B.K. Anaerobic Digestion of the Organic Fraction of Municipal Solid Waste: Influence of Co-Digestion with Manure. Water Res. 2005, 39, 1543–1552. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Zheng, Z.; Yang, S.; Fang, C.; Zou, X.; Zhang, J. Improving Conversion of Spartina Alterniflora into Biogas by Co-Digestion with Cow Feces. Fuel Process. Technol. 2010, 91, 1416–1421. [Google Scholar] [CrossRef]
- Neshat, S.A.; Mohammadi, M.; Najafpour, G.D.; Lahijani, P. Anaerobic Co-Digestion of Animal Manures and Lignocellulosic Residues as a Potent Approach for Sustainable Biogas Production. Renew. Sustain. Energy Rev. 2017, 79, 308–322. [Google Scholar] [CrossRef]
- Jasińska, A.; Grosser, A.; Meers, E. Possibilities and Limitations of Anaerobic Co-Digestion of Animal Manure—A Critical Review. Energies 2023, 16, 3885. [Google Scholar] [CrossRef]
- Li, C.; Strömberg, S.; Liu, G.; Nges, I.A.; Liu, J. Assessment of Regional Biomass as Co-Substrate in the Anaerobic Digestion of Chicken Manure: Impact of Co-Digestion with Chicken Processing Waste, Seagrass and Miscanthus. Biochem. Eng. J. 2017, 118, 1–10. [Google Scholar] [CrossRef]
- DIN EN 12880; Characterization of Sludges e Determination of Dry Residue and Water Content, German Version. Beuth Verlag: Berlin, Germany, 2000.
- DIN EN 12879; Characterization of Sludges e Determination of the Loss on Ignition of Dry Mass, German Version. Beuth Verlag: Berlin, Germany, 2000.
- Weissbach, F.; Strubelt, C. Die Korrektur des Trockensubstanzgehaltes von Maissilagen als Substrat für Biogasanlagen [The correction of the total solids content of maize silages as substrate for biogas plants]. Landtechnik 2008, 63, 82–83. [Google Scholar]
- Angelidaki, I.; Alves, M.; Bolzonella, D.; Borzacconi, L.; Campos, J.L.; Guwy, A.J.; Kalyuzhnyi, S.; Jenicek, P.; Van Lier, J.B. Defining the Biomethane Potential (BMP) of Solid Organic Wastes and Energy Crops: A Proposed Protocol for Batch Assays. Water Sci. Technol. 2009, 59, 927–934. [Google Scholar] [CrossRef] [PubMed]
- Jensen, P.D.; Ge, H.; Batstone, D.J. Assessing the role of biochemical methane potential tests in determining anaerobic degradability rate and extent. Water Sci. Technol. 2011, 64, 880–886. [Google Scholar] [CrossRef]
- VDI 4630; Fermentation of Organic Materials: Characterisation of the Substrate, Sampling, Collection of Material Data, Fermentation Tests. Verein Deutscher Ingenieure e.V.: Düsseldorf, Germany, 2016.
- Xie, S.; Wickham, R.; Nghiem, L.D. Synergistic Effect from Anaerobic Co-Digestion of Sewage Sludge and Organic Wastes. Int. Biodeterior. Biodegrad. 2017, 116, 191–197. [Google Scholar] [CrossRef]
- Tian, P.; Gong, B.; Bi, K.; Liu, Y.; Ma, J.; Wang, X.; Ouyang, Z.; Cui, X. Anaerobic Co-Digestion of Pig Manure and Rice Straw: Optimization of Process Parameters for Enhancing Biogas Production and System Stability. Int. J. Environ. Res. Public Health 2023, 20, 804. [Google Scholar] [CrossRef]
- Yu, X.; Wang, Y.; Yan, L.; Wang, Y.; Lu, J.; Huang, Y.; Bi, S.; Wang, W. Synergistic Effects of Anaerobic Co-Digestion of Chicken Manure and Corn Stover in Batch and Continuous Modes. Fermentation 2023, 9, 666. [Google Scholar] [CrossRef]
- Kim, J.; Baek, G.; Kim, J.; Lee, C. Energy Production from Different Organic Wastes by Anaerobic Co-Digestion: Maximizing Methane Yield versus Maximizing Synergistic Effect. Renew. Energy 2019, 136, 683–690. [Google Scholar] [CrossRef]
- Ebner, J.H.; Labatut, R.A.; Lodge, J.S.; Williamson, A.A.; Trabold, T.A. Anaerobic Co-Digestion of Commercial Food Waste and Dairy Manure: Characterizing Biochemical Parameters and Synergistic Effects. Waste Manag. 2016, 52, 286–294. [Google Scholar] [CrossRef]
- Aboudi, K.; Álvarez-Gallego, C.J.; Romero-García, L.I. Improvement of Exhausted Sugar Beet Cossettes Anaerobic Digestion Process by Co-Digestion with Pig Manure. Energy Fuels 2015, 29, 754–762. [Google Scholar] [CrossRef]
- Woraruthai, T.; Jiemanukunkij, T.; Tirapanampai, C.; Weeranoppanant, N.; Chaiyen, P.; Wongnate, T. Solid-Liquid Separation through Sieve Mesh for Enhancing Biogas Production in a Swine Farm. Int. J. Energy Res. 2022, 46, 15362–15375. [Google Scholar] [CrossRef]
- Amaral, C.d.A.; Kunz, A.; Radis Steinmetz, R.L.; Scussiato, L.A.; Tápparo, D.C.; Gaspareto, T.C. Influence of Solid-Liquid Separation Strategy on Biogas Yield from a Stratified Swine Production System. J. Environ. Manag. 2016, 168, 229–235. [Google Scholar] [CrossRef]
- Mottet, A.; Francois, E.; Latrille, E.; Steyer, J.P.; Déléris, S.; Vedrenne, F.; Carrère, H. Estimating anaerobic biodegradability indicators for waste activated sludge. Chem. Eng. J. 2010, 160, 488–496. [Google Scholar] [CrossRef]
- Nielfa, A.; Cano, R.; Fdz-Polanco, M. Theoretical methane production generated by the co-digestion of organic fraction municipal solid waste and biological sludge. Biotechnol. Rep. 2015, 5, 14–21. [Google Scholar] [CrossRef] [PubMed]
- Jimenez, J.; Lei, H.; Steyer, J.P.; Houot, S.; Patureau, D. Methane production and fertilizing value of organic waste: Organic matter characterization for a better prediction of valorization pathways. Bioresour. Technol. 2017, 241, 1012–1021. [Google Scholar] [CrossRef]
- Yang, S.; Li, J.; Zheng, Z.; Meng, Z. Lignocellulosic Structural Changes of Spartina Alterniflora after Anaerobic Mono- and Co-Digestion. Int. Biodeterior. Biodegrad. 2009, 63, 569–575. [Google Scholar] [CrossRef]
- Bareha, Y.; Faucher, J.-P.; Michel, M.; Houdon, M.; Vaneeckhaute, C. Evaluating the impact of substrate addition for anaerobic co-digestion on biogas production and digestate quality: The case of deinking sludge. J. Environ. Manag. 2022, 319, 115657. [Google Scholar] [CrossRef]
- Koch, K.; Hafner, S.D.; Weinrich, S.; Astals, S.; Holliger, C. Power and Limitations of Biochemical Methane Potential (BMP) Tests. Front. Energy Res. 2020, 8, 63. [Google Scholar] [CrossRef]
- Amon, T.; Amon, B.; Kryvoruchko, V.; Zollitsch, W.; Mayer, K.; Gruber, L. Biogas Production from Maize and Dairy Cattle Manure-Influence of Biomass Composition on the Methane Yield. Agric. Ecosyst. Environ. 2007, 118, 173–182. [Google Scholar] [CrossRef]
- Mao, C.; Zhang, T.; Wang, X.; Feng, Y.; Ren, G.; Yang, G. Process Performance and Methane Production Optimizing of Anaerobic Co-Digestion of Swine Manure and Corn Straw. Sci. Rep. 2017, 7, 9379. [Google Scholar] [CrossRef]
- Molinuevo-Salces, B.; Ahring, B.K.; Uellendahl, H. Optimization of the Co-Digestion of Catch Crops with Manure Using a Central Composite Design and Reactor Operation. Appl. Biochem. Biotechnol. 2015, 175, 1710–1723. [Google Scholar] [CrossRef]
- McCarty, P.L. Anaerobic Waste Treatment Fundamentals—Part Four—Process Design. Public Work 1964, 95, 95–99. [Google Scholar]
- Angelidaki, I.; Ahring, B.K. Thermophilic Anaerobic Digestion of Livestock Waste: The Effect of Ammonia. Appl. Microbiol. Biotechnol. 1993, 38, 560–564. [Google Scholar] [CrossRef]
- Yenigün, O.; Demirel, B. Ammonia Inhibition in Anaerobic Digestion: A Review. Process Biochem. 2013, 48, 901–911. [Google Scholar] [CrossRef]
- Chen, Y.; Cheng, J.J.; Creamer, K.S. Inhibition of Anaerobic Digestion Process: A Review. Bioresour. Technol. 2008, 99, 4044–4064. [Google Scholar] [CrossRef] [PubMed]
| Characteristics | Units | S. ramosissima | Swine Manure | Inoculum (Batch) | Inoculum (CSTR) |
|---|---|---|---|---|---|
| TSFM | %FM | 15.3 (0.4) | 5.1 (0.1) | 2.01 (0.1) | 3.4 (0.02) |
| VSFM | %FM | 10.0 (0.4) | 3.6 (0.1) | 1.1 (0.1) | 2.1 (0.02) |
| VSTS | %TS | 65.6 (0.9) | 71.4 (0.2) | 58.3 (0.2) | 60.4 (0.1) |
| Ash content | %TS | 34.4 (0.9) | 28.6 (0.2) | 41.8 (0.2) | 39.6 (0.1) |
| pH | - | - | 8.3 | 7.8 (0.1) | 8.1 (0.1) |
| C:N | - | 26.8 | 13.2 | - | - |
| Experimental Phase | I | II | III | IV | |
| Duration | Days 0–49 | Days 50–77 | Days 78–105 | Days 106–133 | |
| d | 50 | 28 | 28 | 28 | |
| Co-digestion Ratio R2 | %VS Sram:SM | 0:100 | 15:85 | 25:75 | 35:65 |
| OLR | gVS·L−1·d−1 | 2.0 | 2.2 | 2.5 | 2.5 |
| HRT | d | 20 | 20 | 20 | 20 |
| Mixing Ratio (%VS) | BMPexp | BMPcalc | CPI | C:N Ratio | |
|---|---|---|---|---|---|
| Sram | SM | mLCH4·gVS−1 | |||
| 0 | 100 | 422.8 (7.9) | - | - | 13.2 |
| 15 | 85 | 417.8 (10.7) | 391.7 (8.0) | 1.07 | 14.1 |
| 25 | 75 | 391.5 (3.7) | 370.9 (8.1) | 1.06 | 14.8 |
| 35 | 65 | 364.8 (5.4) | 350.0 (8.2) | 1.04 | 15.6 |
| 50 | 50 | 315.3 (3.2) | 318.7 (8.3) | 0.99 | 17.0 |
| 100 | 0 | 214.4 (8.7) | - | - | 26.8 |
| Mixing Ratio (%VS) | BMPmax | k | R2 | |
|---|---|---|---|---|
| Sram | SM | mLCH4·gVS−1 | d−1 | |
| 0 | 100 | 434.3 (4.1) | 0.226 | 0.976 |
| 15 | 85 | 421.1 (2.3) | 0.257 | 0.984 |
| 25 | 75 | 392.2 (2.9) | 0.271 | 0.986 |
| 35 | 65 | 366.3 (1.7) | 0.280 | 0.990 |
| 50 | 50 | 314.4 (0.7) | 0.318 | 0.995 |
| 100 | 0 | 215.0 (0.6) | 0.219 | 0.998 |
| Experimental Phase | Mixing Ratio (%VS) | TSFM | VSFM | pH | NH4+-N | VFA | ||
|---|---|---|---|---|---|---|---|---|
| Sram | SM | gTS·L−1 | gVS·L−1 | - | gNH4+-N·L−1 | gHAc·L−1 | ||
| I (day 0–49) | R1, R2 | 0 | 100 | 51.7 (0.7) | 42.9 (1.1) | 7.8 (0.1) | 3.0 (0.1) | 6.1 (0.1) |
| II (day 50–77) | R1 | 0 | 100 | 50.3 (1.0) | 41.2 (0.7) | 8.0 (0.1) | 3.4 (0.1) | 4.8 (0.9) |
| R2 | 15 | 85 | 54.1 (0.6) | 43.1 (2.2) | 8.0 (0.1) | 3.0 (0.1) | 4.2 (1.1) | |
| III (day 78–105) | R1 | 0 | 100 | 61.5 (0.6) | 51.3 (0.1) | 7.8 (0.1) | 2.8 (0.1) | 12.9 (0.2) |
| R2 | 25 | 75 | 64.9 (1.0) | 51.7 (1.3) | 7.7 (0.1) | 2.5 (0.2) | 11.7 (0.4) | |
| IV (day 106–133) | R1 | 0 | 100 | 62.5 (0.05) | 51.5 (0.3) | 7.4 (0.2) | 2.4 (0.1) | 12.6 (0.1) |
| R2 | 35 | 65 | 65.1 (0.8) | 50.0 (1.0) | 7.0 (0.1) | 2.0 (0.1) | 10.6 (0.1) | |
| Experimental Phase | CH4 Yield | CH4 Concentration | VS Reduction | pH Effluent | NH4+-N | VFA | |
|---|---|---|---|---|---|---|---|
| mLCH4·gVS−1 | % | % | - | gNH4+-N·L−1 | gHAc·L−1 | ||
| Phase I | R1 | 208.5 (7.9) | 64.6 (0.9) | 31.9 (0.7) | 8.3 (0.1) | 3.7 (0.04) | 1.7 (0.3) |
| R2 | 208.1 (5.5) | 64.9 (0.6) | 32.2 (0.5) | 8.3 (0.1) | 3.5 (0.04) | 1.8 (0.3) | |
| Phase II | R1 | 227.4 (7.8) | 65.5 (0.7) | 35.8 (1.3) | 8.3 (0.1) | 3.8 (0.1) | 1.6 (0.2) |
| R2 | 220.3 (4.3) | 63.9 (0.3) | 36.9 (3.1) | 8.3 (0.1) | 3.6 (0.1) | 1.9 (0.2) | |
| Phase III | R1 | 276.4 (10.7) | 69.2 (1.0) | 44.9 (0.8) | 8.2 (0.01) | 3.4 (0.1) | 2.9 (0.6) |
| R2 | 275.6 (13.6) | 65.1 (0.3) | 52.3 (1.2) | 8.2 (0.04) | 3.1 (0.1) | 1.3 (0.6) | |
| Phase IV | R1 | 316.5 (0.9) | 69.4 (0.9) | 50.8 (1.6) | 8.3 (0.03) | 2.8 (0.1) | 1.0 (0.2) |
| R2 | 251.8 (1.6) | 66.4 (0.1) | 47.5 (0.4) | 8.2 (0.01) | 2.7 (0.1) | 0.5 (0.03) | |
| Mixing Ratio (%VS) | BMPCSTR | BMPcalc | CPI | BMP of Sram in Co-Digestion | |
|---|---|---|---|---|---|
| Sram | SM | mLCH4·gVS−1 | mLCH4·gVS−1 | ||
| 15 | 85 | 220.3 (4.3) | 225.5 (7.9) | 0.98 | 179.9 |
| 25 | 75 | 275.6 (13.6) | 260.9 (10.2) | 1.06 | 276.8 |
| 35 | 65 | 251.8 (1.6) | 280.7 (3.6) | 0.90 | 131.7 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cayenne, A.; Uellendahl, H. Anaerobic Digestion of the Halophyte Salicornia ramosissima in Co-Digestion with Swine Manure in Lab-Scale Batch and Continuous Reactor Tests. Energies 2025, 18, 3085. https://doi.org/10.3390/en18123085
Cayenne A, Uellendahl H. Anaerobic Digestion of the Halophyte Salicornia ramosissima in Co-Digestion with Swine Manure in Lab-Scale Batch and Continuous Reactor Tests. Energies. 2025; 18(12):3085. https://doi.org/10.3390/en18123085
Chicago/Turabian StyleCayenne, Aadila, and Hinrich Uellendahl. 2025. "Anaerobic Digestion of the Halophyte Salicornia ramosissima in Co-Digestion with Swine Manure in Lab-Scale Batch and Continuous Reactor Tests" Energies 18, no. 12: 3085. https://doi.org/10.3390/en18123085
APA StyleCayenne, A., & Uellendahl, H. (2025). Anaerobic Digestion of the Halophyte Salicornia ramosissima in Co-Digestion with Swine Manure in Lab-Scale Batch and Continuous Reactor Tests. Energies, 18(12), 3085. https://doi.org/10.3390/en18123085







