Abstract
This laboratory study investigated the anaerobic co-digestion process of the halophyte S. ramosissima (Sram) together with swine manure (SM) in different mixing ratios in batch and continuous reactor experiments. In the batch experiments, a methane yield of 214 mLCH4·gVS−1 was obtained for Sram in mono-digestion. In co-digestion with SM, the methane yields were slightly higher than calculated from the yields of each substrate in mono-digestion. Also, the kinetic rate constant in the co-digestion with swine manure increased from 0.219 d−1 for mono-digested S. ramosissima to 0.318 d−1 in the co-digestion of 50:50 Sram:SM (based on VS). Two continuous 5 L lab-scale CSTR reactors were operated: one as a control (100% SM) and the other as a co-digestion reactor with an increasing VS share of Sram (15, 25, and 35%) in the feed. Both reactors were operated at an organic loading rate (OLR) of 2.5 gVS.L−1·d−1 and a hydraulic retention time (HRT) of 20 days. In the continuous process, the highest methane yield of 276 mLCH4·gVS−1 was achieved at a co-digestion VS ratio of Sram:SM 25:75, corresponding to a methane yield from the added S. ramosissima of 277 mLCH4·gVS−1. This showed successful operation of the continuous co-digestion process of S. ramosissima and swine manure, with higher methane yields of S. ramosissima than in the mono-digestion batch tests.
1. Introduction
Halophytes are a distinctive group of plant species that can optimally thrive and complete their life cycle in saline environments without hindering their biomass yields and seed production [1,2,3,4]. This makes halophyte plants suitable candidates for the cultivation of natural saline land areas and agricultural land that has become saline due to soil salinization. Notably, the genus Salicornia is one of the most productive species of halophytes, with the ability to yield as much as conventional agricultural crops [3], making it a potential candidate for the valorization of saline soil without competing for arable land. Halophytes can be either used directly as feedstock for bioenergy production or in an integrated biorefinery approach for the production of food, feed additives, high-value bioactive compounds, and bioenergy [5,6,7,8].
Anaerobic digestion (AD) is an established technology for converting various types of biomass such as agricultural crops and crop residues, animal manure, the organic fraction of municipal waste, and other organic waste streams into bioenergy and nutrient-rich fertilizers [9,10]. The utilization of agricultural crops for bioenergy production is dependent on agricultural land and water resources for cultivation. In contrast, establishing halophytic biomass for bioenergy production on marginal lands that cannot be used for crop production avoids the competition between food and energy crops. The AD of halophytic biomass for biogas production has previously been investigated, showing biogas yields similar to those from conventional energy crops [11]. Low cultivation costs, high biomass yields, and degradability in AD systems with an associated low lignin content make halophytic biomass an appropriate crop for bioenergy production. However, some precautions should be taken with the AD of halophytes as these plants may contain higher salt and sulfur concentrations, which can cause inhibition during anaerobic digestion [12]. In addition, it is essential to consider the most suitable time for harvesting or cultivation technique to be used, as the biochemical composition of these plants is dependent on such factors [12,13,14].
The anaerobic co-digestion of biomass that have adverse effects on the AD process together with other types of biomass provides one possibility to overcome the degradability limitations and, subsequently, improve biogas production. Such improvement is achieved through nutrient balancing (carbon/nitrogen (C:N) and acidity/alkalinity ratios), stimulating complementary relationships between co-substrate mixtures (synergistic effects), and diluting potential inhibitory compounds [15,16,17]. Nonetheless, their biochemical composition and co-substrate mixing ratios should be carefully considered when selecting suitable substrates for co-digestion. From this perspective, anaerobic co-digestion of halophytic biomass and liquid organic waste streams, like manure, can offer the possibility of resolving inhibitory effects by diluting the salt concentration and establishing favorable conditions for the microorganism in the AD process. For nutrient balancing, co-digestion of halophytic biomass characterized by a low nitrogen content [11] together with nitrogen-rich manure [15,16,17] can be beneficial for attaining a suitable C:N ratio and methane yields that are higher than the yields of the single substrates [18].
Anaerobic co-digestion has been extensively investigated for many different co-substrates in combination with basic substrates such as animal manure and sewage sludge [19,20]. However, the potential of using halophytic biomass as a co-substrate has yet to be explored. Previous studies investigated the co-digestion of halophytic biomass with animal manure using different mixing ratios and observed that the use of manure improved the degradability of the biomass, stimulated synergy in the co-substrate mixture, and improved biomethane yields [18,21]. Based on previous findings, that S. ramosissima is characterized by a low protein content and a high C:N ratio, co-digestion of S. ramosissima with a protein-rich substrate such as cow or swine manure has been suggested [12], forming the basis for the present study. Notably, previous studies were based on batch tests, and the results have limited information about the long-term effect in continuous digestion processes. Continuous reactor tests allow for optimization on a long-term basis with respect to feedstock mixing ratios, biogas or methane productivity, inhibitory effects, hydraulic retention time (HRT), organic loading rate (OLR), and buffering capacity. Long-term studies of the continuous co-digestion process of halophytic biomass have not been conducted yet. To fill this knowledge gap, this research aims to assess the performance and stability of the continuous co-digestion process of Salicornia ramosissima and swine manure in different mixing ratios. For this purpose, as seen in Figure 1, batch reactor tests were first conducted to determine the co-substrate ratio with the highest methane yield. Subsequently, the performance of the continuous process was studied in continuous stirred tank reactors (CSTRs) with the same mixing ratios of S. ramosissima and swine manure as in the batch experiments. The findings of this research would be beneficial to manure-based AD plants potentially located in marginal regions where these salt-tolerant plants can be cultivated and utilized as additional feedstock.
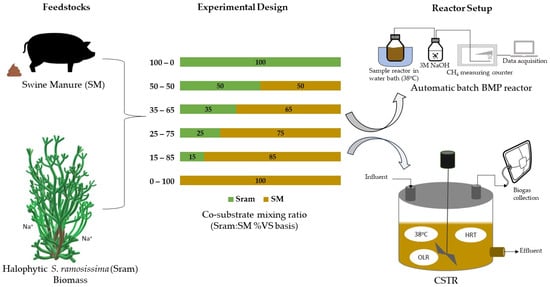
Figure 1.
Feedstocks, experimental design, and reactor setup used in this co-digestion study.
2. Materials and Methods
2.1. Biomass Feedstock and Inoculum
The characteristics of the feedstock used for the Salicornia ramosissima biomass, swine manure, and inoculum can be seen in Table 1.

Table 1.
Main characteristics of substrates and inoculum used for the batch and CSTR experiments (standard deviation of triplicates).
2.1.1. Salicornia ramosissima
S. ramosissima biomass was cultivated at the Riasearch (RSR) facilities in Murtosa, Portugal, under greenhouse conditions irrigated with saline water at 12 g/L salinity, which was maintained using a combination of recirculating aquaculture effluents and freshwater as previously described [12]. The above-ground fraction of the fresh succulent green plant was harvested and shipped in styrofoam boxes with cold packs to Flensburg University of Applied Sciences, Germany. The biomass was cold-washed to remove inorganic material, like soil and salts, and subsequently stored at −20 °C to minimize microbial degradation. The plant material was allowed to reach room temperature before composition analysis and fermentation experiments. A salt content of 118 mg·gTS−1 was analyzed for S. ramosissima in a previous study [12].
2.1.2. Swine Manure
Swine manure was obtained from Wiesbyller Schweine KG, a pig-fattening farming company in Sollerup, Schleswig-Holstein, Germany. At this farm, swine manure is stored in a 1000 m3 tank, which is intermittently stirred and kept at ambient temperature (15 °C) before being transported as feedstock to a nearby biogas plant, Wiesbyller Biogas GmbH. The manure was collected from the storage tank and subsequently screened through a 2 mm mesh sieve to remove fibrous material and stored in containers at −8 ± 2 °C. Before fermentation experiments and compositional analysis, the manure was allowed to reach room temperature.
2.1.3. Inoculum
For the batch experiments, sewage sludge from the biogas fermenter (operated at mesophilic conditions; 38–42 °C) of the wastewater treatment plant (WWTP) in Flensburg (Kielseng 17), Germany, was used as the inoculum. For the continuous experiments, the CSTR reactors were previously inoculated with sewage sludge from the same WWTP and operated with the same swine manure for six weeks before starting the co-digestion experiments.
2.2. Analytical Methods
2.2.1. Total Solids and Volatile Solids
Total solids (TS), volatile solids (VS), and ash content were analyzed according to the DIN EN 12880 [22] and DIN EN 12879 [23] standards, respectively. The TS content was determined by drying the substrates in an oven at 105 ± 5 °C until a constant weight was achieved. The VS content was analyzed based on the weight loss after incinerating the dried sample at 550 °C for 3 h in a muffle furnace (M104; ThermoFisher Scientific Corporation, Waltham, MA, USA).
The TS and VS analysis values of the feedstock samples used in the continuous experiments were corrected according to Weissbach & Strubelt [24] to compensate for the loss of volatile compounds during the TS analysis and to avoid overestimation of the biomethane yields.
2.2.2. Elemental Analysis
The content of the elements carbon (C) and nitrogen (N) in the biomass samples was analyzed in a CHNS-O Thermo Scientific FlashSmart elemental analyzer. Before analysis, the biomass samples were placed in an oven at a constant temperature of 40 °C until a moisture content of <5% was achieved. The dried samples were then milled to a powdered form.
2.2.3. Ammonium Nitrogen
Ammonium nitrogen (NH4+-N) was analyzed using a benchtop Hach DR 3900 Laboratory visible-spectrum spectrophotometer (Hach Lange GmbH, Düsseldorf, Germany) with Radio Frequency Identification (RFID) technology. The samples were prepared by centrifugation in 50 mL centrifuge tubes for 10 min at 10,000 rpm. The supernatant was collected and diluted with de-ionized (DI) water to correspond to the measuring range of the HACH Lange cuvette tests.
2.2.4. pH and Volatile Fatty Acids
The pH of the different samples was measured using a pH benchtop meter (inoLab® pH 7110; WTW-Wissenschaftlich Technische Werkstätten GmbH, Weilheim, Germany). The volatile fatty acids (VFA) content was analyzed as the sum of acetic, propionic, butyric, and valeric acid using a Hitachi Chromaster High-Performance Liquid Chromatography (HPLC) (Hitachi High-Tech, Hitachinakashi, Ibaraki, Japan). The total VFA content was expressed as acetic acid equivalents (gHAc·L−1). Before the VFA analysis, the samples were centrifuged for 10 min at 10,000 rpm, and the supernatant was taken and diluted with DI water based on the expected acid concentration. Subsequently, the solution was filtered through a membrane filter of 0.45 μm pore size to be analyzed in the HPLC. Using 5 mM H2SO4 as the mobile phase, the HPLC was operated at 60 °C with a corresponding flow rate of 0.6 mL/min.
2.2.5. Biogas Composition
The methane content of the biogas produced in the continuous reactors was measured using a biogas analyzer VISIT 03 (Messtechnik EHEIM GmbH, Schwaigern, Germany) after the gas was collected in RESTEK Multi-Layer 18″ × 24″ polypropylene gas sampling bags of 25 L volume (Restek GmbH, Bad Homburg v. d. Höhe, Germany).
2.2.6. Kinetic Parameter Estimation
The kinetic parameters were determined for the batch experiments based on a first-order model. For first-order degradation kinetics, the measured biochemical methane potential (BMP) values are related to time t according to Equation (1), where the plot of over time will produce a linear curve with k as its slope [25,26]:
where BMPmax is the substrate’s ultimate methane yield (L-CH4·kg-VS−1), k (d−1) is the first-order kinetic rate constant, and BMP is the cumulative methane yield at a given time t (d).
After rearranging Equation (1), the BMP values can directly be modeled by Equation (2):
As recommended by [26], the BMP values were modeled in this study by simultaneous parameter estimation of k and BMPmax in Equation (2) with the help of the Solver function in the MS Excel software.
2.3. Reactor Setup and Operation
2.3.1. Batch BMP Experiments
The biochemical methane potential (BMP) of the substrates in mono- and co-digestion was measured using an Automated Methane Potential Test System II (AMPTS II; Bioprocess Control, Sweden AB) according to the standard protocol, VDI 4630 [27]. The gas outlet of each batch of the AMPTS was connected to a 3M sodium hydroxide (NaOH) solution, followed by a gas volume measuring device. Using an alkaline solution allows for the trapping of acidic gases like CO2 and H2S and results in direct measurements of the produced biomethane (CH4).
Batch experiments were conducted using different ratios of S. ramosissima and swine manure on the basis of the VS, i.e., 0:100, 15:85, 25:75, 35:65, 50:50, and 100:0 (%VS Sram:SM). Each substrate ratio was performed in triplicate in 0.5L flasks under mesophilic conditions (37 ± 2 °C) with an inoculum-to-substrate (ISR) ratio of 2. In compliance with the standard protocol [27], batch experiments were completed when the daily biogas production for each batch assay was <1 vol% of the cumulative biogas production, i.e., after 32 days. The methane production rate of the batch assays was derived from the cumulative specific biomethane yield when the yield plateaued divided by the respective digestion time.
A co-digestion performance index (CPI) was applied to evaluate the synergistic effects of the co-substrate mixtures [28,29,30,31,32]. The CPI was calculated based on the ratio of the experimental (BMPexp) to the calculated (BMPcalc) methane yields. BMPcalc was calculated from the methane yields of each substrate in mono-digestion and the %VS share of each substrate in the co-digestion mixture. For a CPI > 1, the effect is considered synergistic, while for a CPI < 1, it is considered an antagonistic or inhibitory effect, whereas a value of CPI = 1 indicates no effect on the methane yield in co-digestion.
2.3.2. Continuous Stirred Tank Reactor Experiments
Table 2 outlines the operation parameters during the four phases of the continuous reactor experiment. Two 5 L CSTRs (R1 and R2), each with a 3 L operating volume, were used for the continuous digestion experiments. The co-substrate mixtures were prepared thrice a week using a blender for ease of manual feeding and stored in the refrigerator at 8 ± 2 °C. The anaerobic sludge used as the inoculum for both reactors contained 3.4% TS, 2.1% VS, and an NH4+-N content of 2.9 g/L with a pH of 8.2 (Table 1).

Table 2.
CSTR operating parameters during different experimental phases.
The control reactor, R1, was used for the mono-digestion of SM, while the co-digestion reactor, R2, was used for the co-digestion of Sram:SM at varying mixing ratios. Both reactors were operated under mesophilic conditions using a recirculating temperature-controlled water bath (Lauda Alpha A6) connected to the water jacket of each reactor. The reactor content was stirred with a blade stirrer at 100 rpm. Gas-tight valves at the top of the reactors were used for manual feeding and biogas collection, while the effluent was discharged through a valve at the base. Before each feeding, an equal amount was removed from the bottom of the reactor to maintain a constant working volume of 3 L in the digester.
The influent and effluent pH was measured daily, TS and VS three times per week, VFA twice per week, and NH4+-N and the biogas composition once per week.
Both reactors operated at a constant HRT of 20 d and at the same OLR, increasing from 2.0 gVS·L−1·d−1 in phase I to 2.5 gVS·L−1·d−1 in Phase IV. After the start-up phase (I) of both reactors, with a duration of 50 days, each phase spanned a duration of 4 weeks before the co-digestion ratio of the feed was changed. The total duration of the continuous co-digestion experiments was limited to 12 weeks due to the limited availability of the same Sram material.
3. Results and Discussion
3.1. Batch BMP Experiments
In the batch BMP assays, biomethane production started immediately within the first day of digestion, without a lag phase (Figure 2), indicating that there was no pronounced inhibition for all the batch tests. Also, the pH of all the mono- and co-substrate set-ups remained constant at 8.1 ± 0.2 during the whole batch experiment period, indicating that there was no acid accumulation during the batch process. The methane yield of swine manure was 423 mLCH4·gVS−1 compared with 214 mLCH4·gVS−1 from S. ramosissima, indicating a higher content of easily degradable organic matter in swine manure and a higher content of lignocellulosic fibers in S. ramosissima. Accordingly, the methane yields in the co-digestion mixtures were lower, with a higher proportion of Sram fibers. Approximately 95% of the ultimate biomethane yield was achieved within 10 days for swine manure and for the co-digestion mixtures and 13 days for the mono-digestion of Sram fibers. For the co-digestion batches, the highest methane yield of 418 mLCH4·gVS−1 was achieved using a mixing ratio of 15:85%VS Sram:SM, and the lowest yield of 315 mLCH4·gVS−1 was obtained from at a ratio of 50:50%VS Sram:SM.
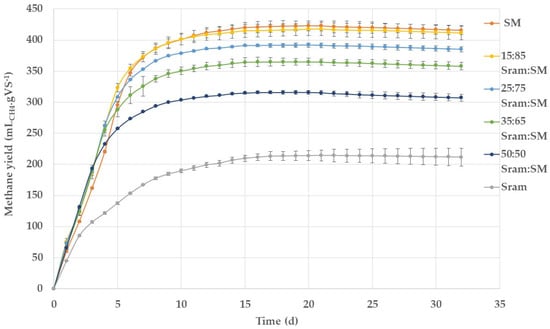
Figure 2.
Profile of specific methane yields of S. ramosissima (Sram) and swine manure (SM) in anaerobic batch tests in mono- and co-digestion at different ratios based on VS.
The biomethane yield of swine manure achieved in the batch assay was similar to previously reported studies operating under mesophilic conditions [33,34,35].
The methane yield of S. ramosissima used in this study was significantly lower than that in a previous study (293 mLCH4·gVS−1) with S. ramosissima from the same origin and harvested in the same season one year before [12]. Presumably, a variation in the cultivation conditions may have occurred, resulting in different plant life-cycle responses and causing a change in the biochemical composition [13]. Likewise, plants harvested at a later maturity stage in their life cycle are characterized by a higher lignin content and a lower fraction of bio-accessible organic material like carbohydrates and proteins [14], which correlate with lower methane yields, as analyzed previously in detail [36,37,38]. This was observed for Spartina alterniflora, which had lower methane yields [18] and was harvested from the same region but in a different season than earlier reported [39].
Comparing the experimental (BMPexp) and calculated (BMPcalc) biomethane yields in the co-digestion batch assays (Table 3), a slight synergistic effect by the addition of swine manure into the mixture with S. ramosissima was observed, as the CPI values increased from 0.99 to 1.07 with a higher proportion of swine manure in the mixture. In comparison, CPI values increased from 1.08 to 1.39 in a co-digestion study of corn stover (CS) and chicken manure (CM) when the VS ratio of cow manure was increased from CM:CS 1:2 to 2:1 [30]. A study on the co-digestion of food waste with dairy manure slurry reported CPI values ranging from 0.68 to 1.21 [32]. In the present study, the differences between BMPcalc and BMPexp are not statistically significant due to the standard deviation of the experimental BMP values. Furthermore, other studies have pointed out the limitations of BMP tests to detect synergistic effects [40,41]. Therefore, the results of the batch assay simply indicate better performance of the AD via co-digestion of the two substrates, which achieved slightly higher methane yields than from each substrate in mono-digestion.

Table 3.
Batch assay experimental and calculated BMP values (standard deviation in parentheses).
The increasing methane yields correlate with a decreasing C:N ratio in the different feeds (Table 3). According to Amon et al. [42], a C:N ratio between 10 and 30 allows for a stable digestion process, while a ratio between 20 and 30 is considered optimal by Yu et al. [30]. Recent studies indicate that a lower C:N ratio can be favorable in AD and biomethane production. For example, Aboudi et al. [33] demonstrated that maximum methane yield could be achieved at a C:N ratio of 18.5 when investigating the co-digestion of exhausted sugar beet cossettes (ESBC) and pig manure, while Mao et al. [43] found that a C:N ratio of 27.1 was the most effective in the co-digestion of swine manure and corn straw. On the other hand, utilizing halophytic biomass as a substrate, Chen et al. [18] found that a C:N ratio between 16.9 and 20.4 was optimal for enhancing the methane yields from the co-digestion of S. alterniflora and cow feces. Accordingly, only the C:N ratios of the sole substrates are at the edge of the optimal range and may have an influence on the efficiency of the AD process. In comparison, a positive synergistic effect by balancing the nutrient content and the reduction/dilution of inhibitory compounds was proposed in a co-digestion study of Spartina alterniflora and cow manure [18]. Here, a methane yield of 177 mLCH4·gVS−1 was found using a mixing ratio of 75% S. alterniflora and 25% cow manure compared with a yield of 138 and 111 mLCH4·gVS−1 from the mono-digestion of S. alterniflora and cow manure, respectively.
To evaluate the kinetics of the BMP assay, the methane production rate was plotted (Figure 3), and the kinetic rate constants were determined by the first-order model (Table 4). For all the co-digestion assays, the specific methane production rate was highest on the first day, with a value of 76 and 73 mLCH4.gVS−1·d−1 for the co-digestion mixtures of 15 and 25%VS Sram, respectively, and 67 mLCH4.gVS−1·d−1 for the co-digestion mixtures of 35 and 50%VS Sram (Figure 3). Mono-digestion showed a lower methane production rate of 60 and 45 mLCH4·gVS−1·d−1 for both swine manure and S. ramosissima, respectively. Thus, the degradation rate of swine manure was also improved by the addition of S. ramosissima for co-digestion. The rate curve of mono-digested S. ramosissima fibers showed an initial peak on the first day, followed by a steady subsequent decline. Contrarily, the rate curve of the mono-digested swine manure and of the three co-digestion mixtures with a high manure share showed successive double peaks, achieving the highest methane production rate of 75 mLCH4·gVS−1·d−1 on day 5. This suggests that microorganisms require more time to develop hydrolytic enzymes to degrade some of the more complex organic matter present in manure.
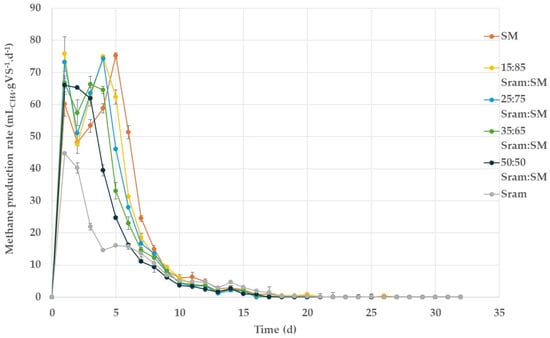
Figure 3.
Profile of specific methane production rate of batch anaerobic mono- and co-digestion of S. ramosissima (Sram) with swine manure (SM).

Table 4.
First-order model parameters for batch experiments.
The batch test data could be well fitted by the first-order model, resulting in R2 values above 0.9 (Table 4). The mono-digestion of fresh S. ramosissima showed the lowest k value of 0.219 d−1 (R2 0.998), presumably due to its lignocellulosic content, while mono-digested swine manure exhibited a k value of 0.226 (R2 0.976). For all the co-digestion set-ups, the kinetic rate constant, k, was higher than in the mono-digestion of the two substrates. This supports the synergistic effect of the co-digestion of both substrates. The relevance of the kinetic parameters for the determination of synergistic effects has been previously reported [40]. With an increasing share of S. ramosissima in the co-digestion mixture, k values were 1.2 to 1.5 and 1.1 to 1.4 times higher than those from the mono-digestion of S. ramosissima and swine manure, respectively, even though the methane yield decreased. Accordingly, the highest k value (0.318 d−1) was attained in the co-digestion of 50:50%VS Sram, although this ratio achieved the lowest final methane yield in the co-digestion set-ups. This indicates that the addition of S. ramosissima triggered the degradation kinetics of both swine manure and S. ramosissima, although S. ramosissima has a lower content of easily degradable organic constituents, resulting in lower methane yields.
3.2. Continuous Reactor Experiments
In the continuous reactor experiments, reactor R1 received solely swine manure during the entire experiment. Meanwhile, Sram was progressively added to the swine manure feed of reactor R2 in increasing ratios.
Table 5 represents the influent characteristics of the feed for R1 and R2 at each experimental phase. The variations in the characteristics of the influent used are due to the different batches of swine manure collected. The analyses show that the characteristics of the swine manure used in phases III and IV were significantly different from the manure used in phases I and II. TS and VS were significantly higher while the pH value and NH4+-N content were lower than in the manure used in phases I and II. The lower pH of the manure used in phases III and IV correlates with its higher VFA content. This may be due to microbial hydrolysis and acidification of the manure during storage prior to the experiments.

Table 5.
Average influent characteristics of the CSTRs within each experimental phase (standard deviation in parentheses).
3.2.1. Phase I: Start-Up of Reactors R1 and R2 with 100% SM
In the start-up phase (days 0–49), both reactors (R1 and R2) received only manure. As illustrated in Figure 4A, methane yields varied between 190 and 217 mLCH4·gVS−1, but the average yield (208 mLCH4·gVS−1), the CH4 content in the biogas (65%), and the VS reduction (32%) were very similar for both reactors. The average VFA concentration in R1 and R2 was initially 2.6 gHAC·L−1, then steadily decreased to about 1.8 gHAC·L−1 (Figure 4B). Accordingly, a pH increase from 8.2 to 8.4 was observed. Likewise, the ammonia content in both reactors rose from 3.3 to 3.8 gNH4+-N·L−1 in R1 and 3.3 to 3.6 gNH4+-N·L−1 in R2 (Figure 4C), indicating the hydrolysis of proteins, presumably mainly in the swine manure.
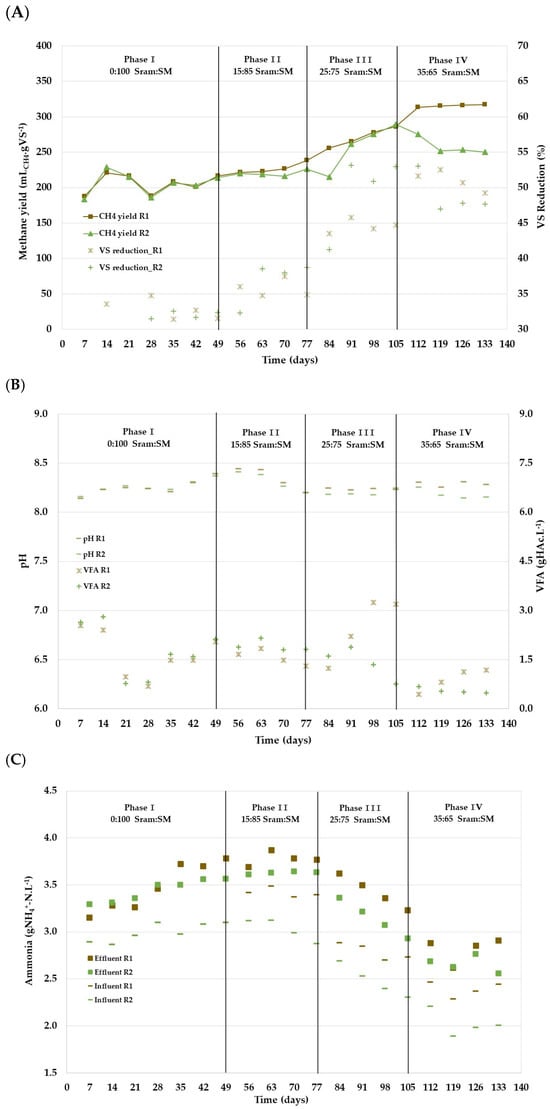
Figure 4.
Continuous reactor performance in R1 (100% SM) and R2 (co-digestion Sram:SM) throughout the experimental phases: methane yield and VS reduction (A), pH and VFA (B), and NH4+-N in the influent and effluent (C).
3.2.2. Phase II: Increasing the Share of Sram to 15%VS in R2
In phase II (days 50–77), R2 was loaded with a co-digestion feed containing 15%VS Sram and 85%VS SM. Introducing this new feed into R2 led to similar methane yields (216–226 mLCH4.gVS−1) as in the previous phase (Figure 4A). On the other hand, the methane yield of R1 increased to 222–239 mLCH4.gVS−1 at the end of phase I. The lower methane yield in R2 compared with R1 was obviously owing to the lower methane yield of the added Sram in the co-digestion. Still, both reactors showed a similar VS reduction compared with phase I, with a mean of 36%.
The VFA concentration in R1 and R2 remained relatively constant at 1.6 and 1.9 gHAC.L−1, respectively, whereas the pH value of both reactors declined to 8.2 at the end of this phase (Figure 4B). The NH4+-N concentration in the influent and effluent of R1 were higher than for R2, indicating a higher degradation of proteins in the feed of R1 with solely swine manure (Figure 4C).
3.2.3. Phase III: Increasing the Share of Sram to 25%VS in R2
During phase III (days 78–105), the share of Sram in the feed of R2 was increased to 25%VS Sram. During the initial 7 days (days 78–84), the same manure from phase II was used in both reactors. From days 85 to 105, a new batch of SM was used that had a higher VFA content (Table 5). Accordingly, the pH value of the feed dropped from 8.0 in phase II to 7.8 and 7.7 in R1 and R2 in phase III, respectively. Due to the lower NH4+-N content in the new batch of manure (Table 5), the NH4+-N concentration also decreased in the effluent of both reactors, with a significantly lower level in R2 due to the higher proportion of Sram with a lower NH4+-N content (Figure 4C).
The methane yield in both reactors increased significantly with the feeding of the new batch of manure. The methane yield in reactor R2 with a share of 25%VS Sram increased to 215–289 mLCH4·gVS−1 (Figure 4A), while methane yields of 256–286 mLCH4·gVS−1 were achieved in the control reactor R1. The similar methane yields achieved in reactor R2 compared to R1 indicate a significantly higher degradation of Sram during phase III. Accordingly, the VS degradation increased in both reactors compared with phase II, with an average of 45% in R1 and 52% in R2.
Furthermore, the VFA concentration in R1 increased from 1.6 to 2.9 gHAc·L−1, whereas the concentration decreased in R2 from 1.9 to 1.3 gHAc·L−1 (Figure 4B), indicating a more stable process in the co-digestion of Sram and SM.
3.2.4. Phase IV: Increasing the Share of Sram to 35%VS in R2
A further increase in the share of Sram in R2 to 35%VS during days 106–133 showed a considerable decrease in methane yield and VS degradation in the co-digestion compared with phase III. Specific methane yields of between 250–254 mLCH4·gVS−1 were achieved and the VS degradation decreased to an average of 48%. Conversely, in R1, methane yields of 315–317 mLCH4·gVS−1 were obtained, with a methane content of 68–70% and a higher VS reduction of 51% (Figure 4A). Moreover, the average VFA concentration stabilized at low levels in both reactors (Figure 4B), indicating that a balanced process involving acidifying and methanogenic microorganisms also occurs in R1. The decline in the methane yield and the VS reduction in R2 compared with phase III indicates that the positive co-digestion effect in phase III is no longer observed for the higher proportion of Sram in 35%VS.
3.2.5. Overall Performance of the Continuous Reactor Process
Table 6 presents the overall reactor performance for each experimental phase. In phase I (0–49), an average methane yield of 208 mLCH4·gVS−1 was achieved in both reactors from treating swine manure alone.

Table 6.
Average CSTR performance in each phase.
After introducing 15%VS S. ramosissima into R2 in phase II, the methane yield of the co-digestion mixture (220 mLCH4·gVS−1) was almost as high as the methane yield from manure alone in R1 (227 mLCH4·gVS−1). Increasing the %VS share of Sram to 25%VS in R2 in phase III produced the highest average methane yield of 276 mLCH4·gVS−1, similar to the yield obtained from the control reactor (R1). This corresponds to a 22% increase in methane yield when compared with phase II in R2. However, when reactor R2 was loaded with 35%VS S. ramosissima in the feed mixture in phase IV, the mean methane yield decreased to 252 mLCH4·gVS−1 while the control R1 reactor achieved 317 mLCH4·gVS−1 at the end of this experimental phase. The reduction in methane yield when increasing the Sram share from 25 to 35%VS Sram in the feed mixture may be attributed to the higher content of recalcitrant organic matter in Sram.
Due to the high nitrogen content in swine manure, the NH4+-N content of the influent and effluent was higher in R1 (2.4–3.5 and 2.9–3.9 gNH4+-N·L−1, respectively) than in R2 (2.0–3.1 and 2.7–3.6 gNH4+-N·L−1, respectively). The increase in NH4+-N content in the effluent compared with the influent indicates that protein degradation occurred during hydrolysis [44]. Earlier findings suggest the AD process is inhibited at any pH value when ammonium concentrations exceed 3.0 gNH4+-N·L−1 [45]. However, an adapted anaerobic process can tolerate ammonium levels of 3–4 gNH4+-N·L−1 [46] and up to 5 gNH4+-N·L−1 [47]. The relatively low methane yields from SM in R1 compared with R2 in phases II and III may be attributed to a temporary ammonia inhibition, which is supported by the significantly higher VFA levels found in R1 in phase III. The stabilization of the methane yield at a higher ammonium level and the decreasing VFA concentration in R1 in phase IV indicate that the temporary inhibition was overcome, and the methane yield is higher from swine manure alone than in co-digestion with Sram with a lower specific methane yield.
In Table 7, the biomethane yields achieved in the different co-digestion ratios in the reactor experiment (BMPCSTR) are compared to the calculated BMPcalc values, which were calculated from the biomethane yields of SM in the corresponding phases in the continuous experiments and the methane yield of mono-digested S. ramosissima in the batch test.

Table 7.
Experimental and calculated BMP values in continuous CSTR experiments (standard deviation in parentheses).
In phase II of the continuous experiments with a feed of 15:85%VS Sram:SM, a specific methane yield of 180 mLCH4·gVS−1 was calculated for S. ramosissima in this co-digestion ratio. This indicates that co-digestion with this high proportion of manure did not reach the degradation efficiency of 214 mLCH4·gVS−1 for S. ramosissima in mono-digestion in the batch experiments.
However, increasing the VS share of S. ramosissima to 25:75 Sram:SM produced considerably higher methane yields of up to 276 mLCH4·gVS−1, with a calculated methane yield of 277 mLCH4·gVS−1 for Sram fibers alone. Thus, a 29% higher methane yield was attained than in batch mono-digestion of the biomass. A further increase in the %VS share of S. ramosissima to 35%VS of Sram resulted in a lower average methane yield of 252 mLCH4·gVS−1 from the co-digestion mixture, with a methane yield of 132 mLCH4.gVS−1 for Sram; thus, no synergistic effect of the co-digestion was observed. Accordingly, the CPI value was higher than 1 only in the co-substrate mixture of 25:75%VS Sram:SM.
Overall, the continuous reactor experiments showed the benefits of anaerobic co-digestion of fresh green S. ramosissima fibers together with liquid swine manure. The highest methane yield and VS reduction were found for a co-substrate VS ratio of 25:75 Sram:SM. The beneficial aspect of the co-digestion of Sram and swine manure was attributed to the balancing of the content of slowly degradable fiber material in Sram and the high nitrogen content in swine manure [48].
4. Conclusions
Based on a previous study of the correlation between the biochemical composition and BMP of halophytes, the present study addressed the benefits of the co-digestion of S. ramosissima, which is characterized by a high C:N ratio, together with ammonia-rich swine manure. The anaerobic co-digestion process was studied in batch and continuous reactor experiments with different mixing ratios of the S. ramosissima and swine manure under mesophilic conditions. In the batch assays, no signs of microbial inhibition were observed, indicating that there were no critical salt or sulfur concentrations in S. ramosissima. All the co-substrate mixtures showed a slight positive synergistic effect, with methane yields higher than the calculated yields from the mono-digestion of the single substrates (214 mLCH4·gVS−1 for Sram and 423 mLCH4·gVS−1 for SM). Furthermore, co-digestion mixtures exhibited higher kinetic rate constants compared with the mono-digestion of the substrates.
In the continuous reactor processes at an OLR of 2.5 gVS·L−1·d−1 and an HRT of 20 days, a synergistic effect with improved methane yields of up to 276 mLCH4·gVS−1 were achieved for the 25:75%VS Sram co-digestion mixture. The significantly higher yields of S. ramosissima in the continuous co-digestion process was attributed to the balancing of the C:N ratio in halophytes and swine manure. The results show that S. ramosissima can be used as substrate for biogas plants in a 25:75%VS Sram:SM co-digestion mixture, which is equivalent to an addition of 120–173 kg Sram per ton of SM (for a VSFM of 10.0% for Sram and 3.6–5.2% for SM). This would pave the way for the sustainable valorization of marginal land for bioenergy production and nutrient recirculation.
Author Contributions
Conceptualization, A.C. and H.U.; methodology, analysis, and investigation, A.C.; writing—original draft preparation, A.C.; writing—review and editing, A.C. and H.U.; visualization, A.C. and H.U.; supervision, H.U.; project work package funding acquisition and administration, H.U. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the European Union’s Horizon 2020 research and innovation program under grant number 862834.
Data Availability Statement
The original contributions presented in this study are included in the article. Further inquiries can be directed to the corresponding author.
Acknowledgments
The authors would like to thank Riasearch in Murtosa, Portugal, for the biomass supply of S. ramosissima samples for this study.
Conflicts of Interest
The authors declare no conflicts of interest. The funders had no role in the design of the study, in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Abbreviations
The following abbreviations are used in this manuscript:
| AD | Anaerobic Digestion |
| BMP | Biochemical Methane Potential |
| C | Carbon |
| CPI | Co-digestion Performance Index |
| CS | Corn Stover |
| CSTR | Continuous Stirred Tank Reactors |
| ESBC | Exhausted Sugar Beet Cossettes |
| H | Hydrogen |
| HAc | Acetic Acid |
| HPLC | High-Performance Liquid Chromatography |
| HRT | Hydraulic Retention Time |
| O | Oxygen |
| OLR | Organic Loading Rate |
| N | Nitrogen |
| S | Sulfur |
| SM | Swine Manure |
| Sram | S. ramosissima |
| TS | Total Solids |
| VFA | Volatile Fatty Acids |
| VS | Volatile Solids |
| WWTP | Wastewater Treatment Plant |
References
- Joshi, A.; Kanthaliya, B.; Arora, J. Halophytes: The Nonconventional Crops as Source of Biofuel Production. In Handbook of Halophytes; Springer: Berlin/Heidelberg, Germany, 2020; pp. 1–28. [Google Scholar] [CrossRef]
- Grigore, M.N.; Vicente, O. Wild Halophytes: Tools for Understanding Salt Tolerance Mechanisms of Plants and for Adapting Agriculture to Climate Change. Plants 2023, 12, 221. [Google Scholar] [CrossRef] [PubMed]
- Sharma, R.; Wungrampha, S.; Singh, V.; Pareek, A.; Sharma, M.K. Halophytes As Bioenergy Crops. Front. Plant Sci. 2016, 7, 1372. [Google Scholar] [CrossRef]
- Ventura, Y.; Sagi, M. Halophyte Crop Cultivation: The Case for Salicornia and Sarcocornia. Environ. Exp. Bot. 2013, 92, 144–153. [Google Scholar] [CrossRef]
- Taşçı Çilak, T.; Raval, S.; Fasse, S.; Thomsen, M.H.; Gottschalk, A. Modeling, simulation and techno-economic analysis of an integrated biorefinery based on halophytes. Comput. Aided Chem. Eng. 2023, 52, 2465–2470. [Google Scholar] [CrossRef]
- Accogli, R.; Tomaselli, V.; Direnzo, P.; Perrino, E.V.; Albanese, G.; Urbano, M.; Laghetti, G. Edible Halophytes and Halo-Tolerant Species in Apulia Region (Southeastern Italy): Biogeography, Traditional Food Use and Potential Sustainable Crops. Plants 2023, 12, 549. [Google Scholar] [CrossRef]
- El-Hack, M.E.A.; Samak, D.H.; Noreldin, A.E.; Arif, M.; Yaqoob, H.S.; Swelum, A.A. Towards Saving Freshwater: Halophytes as Unconventional Feedstuffs in Livestock Feed: A Review. Environ. Sci. Pollut. Res. 2018, 25, 14397–14406. [Google Scholar] [CrossRef]
- Hulkko, L.S.S.; Rocha, R.M.; Trentin, R.; Fredsgaard, M.; Chaturvedi, T.; Custódio, L.; Thomsen, M.H. Bioactive Extracts from Salicornia Ramosissima J. Woods Biorefinery as a Source of Ingredients for High-Value Industries. Plants 2023, 12, 1251. [Google Scholar] [CrossRef]
- González, R.; Peña, D.C.; Gómez, X. Anaerobic Co-Digestion of Wastes: Reviewing Current Status and Approaches for Enhancing Biogas Production. Appl. Sci. 2022, 12, 8884. [Google Scholar] [CrossRef]
- Kovačić, Đ.; Lončarić, Z.; Jović, J.; Samac, D.; Popović, B.; Tišma, M. Digestate Management and Processing Practices: A Review. Appl. Sci. 2022, 12, 9216. [Google Scholar] [CrossRef]
- Turcios, A.E.; Cayenne, A.; Uellendahl, H.; Papenbrock, J. Halophyte Plants and Their Residues as Feedstock for Biogas Production—Chances and Challenges. Appl. Sci. 2021, 11, 2746. [Google Scholar] [CrossRef]
- Cayenne, A.; Turcios, A.E.; Thomsen, M.H.; Rocha, R.M.; Papenbrock, J.; Uellendahl, H. Halophytes as Feedstock for Biogas Production: Composition Analysis and Biomethane Potential of Salicornia spp. Plant Material from Hydroponic and Seawater Irrigation Systems. Fermentation 2022, 8, 189. [Google Scholar] [CrossRef]
- Hulkko, L.S.S.; Turcios, A.E.; Kohnen, S.; Chaturvedi, T.; Papenbrock, J.; Thomsen, M.H. Cultivation and Characterisation of Salicornia Europaea, Tripolium Pannonicum and Crithmum Maritimum Biomass for Green Biorefinery Applications. Sci. Rep. 2022, 12, 20507. [Google Scholar] [CrossRef] [PubMed]
- Turcios, A.E.; Braem, L.; Jonard, C.; Lemans, T.; Cybulska, I.; Papenbrock, J. Compositional Changes in Hydroponically Cultivated Salicornia Europaea at Different Growth Stages. Plants 2023, 12, 2472. [Google Scholar] [CrossRef]
- Siddique, M.N.I.; Wahid, Z.A. Achievements and Perspectives of Anaerobic Co-Digestion: A Review. J. Clean. Prod. 2018, 194, 359–371. [Google Scholar] [CrossRef]
- Hartmann, H.; Angelidaki, I.; Ahring, B.K. Co-digestion of the organic fraction of municipal waste with other waste types. In Biomethanization of the Organic Fraction of Municipal Solid Wastes; Mata-Alvarez, J., Ed.; IWA Publishing: London, UK, 2002; pp. 181–200. [Google Scholar]
- Hartmann, H.; Ahring, B.K. Anaerobic Digestion of the Organic Fraction of Municipal Solid Waste: Influence of Co-Digestion with Manure. Water Res. 2005, 39, 1543–1552. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Zheng, Z.; Yang, S.; Fang, C.; Zou, X.; Zhang, J. Improving Conversion of Spartina Alterniflora into Biogas by Co-Digestion with Cow Feces. Fuel Process. Technol. 2010, 91, 1416–1421. [Google Scholar] [CrossRef]
- Neshat, S.A.; Mohammadi, M.; Najafpour, G.D.; Lahijani, P. Anaerobic Co-Digestion of Animal Manures and Lignocellulosic Residues as a Potent Approach for Sustainable Biogas Production. Renew. Sustain. Energy Rev. 2017, 79, 308–322. [Google Scholar] [CrossRef]
- Jasińska, A.; Grosser, A.; Meers, E. Possibilities and Limitations of Anaerobic Co-Digestion of Animal Manure—A Critical Review. Energies 2023, 16, 3885. [Google Scholar] [CrossRef]
- Li, C.; Strömberg, S.; Liu, G.; Nges, I.A.; Liu, J. Assessment of Regional Biomass as Co-Substrate in the Anaerobic Digestion of Chicken Manure: Impact of Co-Digestion with Chicken Processing Waste, Seagrass and Miscanthus. Biochem. Eng. J. 2017, 118, 1–10. [Google Scholar] [CrossRef]
- DIN EN 12880; Characterization of Sludges e Determination of Dry Residue and Water Content, German Version. Beuth Verlag: Berlin, Germany, 2000.
- DIN EN 12879; Characterization of Sludges e Determination of the Loss on Ignition of Dry Mass, German Version. Beuth Verlag: Berlin, Germany, 2000.
- Weissbach, F.; Strubelt, C. Die Korrektur des Trockensubstanzgehaltes von Maissilagen als Substrat für Biogasanlagen [The correction of the total solids content of maize silages as substrate for biogas plants]. Landtechnik 2008, 63, 82–83. [Google Scholar]
- Angelidaki, I.; Alves, M.; Bolzonella, D.; Borzacconi, L.; Campos, J.L.; Guwy, A.J.; Kalyuzhnyi, S.; Jenicek, P.; Van Lier, J.B. Defining the Biomethane Potential (BMP) of Solid Organic Wastes and Energy Crops: A Proposed Protocol for Batch Assays. Water Sci. Technol. 2009, 59, 927–934. [Google Scholar] [CrossRef] [PubMed]
- Jensen, P.D.; Ge, H.; Batstone, D.J. Assessing the role of biochemical methane potential tests in determining anaerobic degradability rate and extent. Water Sci. Technol. 2011, 64, 880–886. [Google Scholar] [CrossRef]
- VDI 4630; Fermentation of Organic Materials: Characterisation of the Substrate, Sampling, Collection of Material Data, Fermentation Tests. Verein Deutscher Ingenieure e.V.: Düsseldorf, Germany, 2016.
- Xie, S.; Wickham, R.; Nghiem, L.D. Synergistic Effect from Anaerobic Co-Digestion of Sewage Sludge and Organic Wastes. Int. Biodeterior. Biodegrad. 2017, 116, 191–197. [Google Scholar] [CrossRef]
- Tian, P.; Gong, B.; Bi, K.; Liu, Y.; Ma, J.; Wang, X.; Ouyang, Z.; Cui, X. Anaerobic Co-Digestion of Pig Manure and Rice Straw: Optimization of Process Parameters for Enhancing Biogas Production and System Stability. Int. J. Environ. Res. Public Health 2023, 20, 804. [Google Scholar] [CrossRef]
- Yu, X.; Wang, Y.; Yan, L.; Wang, Y.; Lu, J.; Huang, Y.; Bi, S.; Wang, W. Synergistic Effects of Anaerobic Co-Digestion of Chicken Manure and Corn Stover in Batch and Continuous Modes. Fermentation 2023, 9, 666. [Google Scholar] [CrossRef]
- Kim, J.; Baek, G.; Kim, J.; Lee, C. Energy Production from Different Organic Wastes by Anaerobic Co-Digestion: Maximizing Methane Yield versus Maximizing Synergistic Effect. Renew. Energy 2019, 136, 683–690. [Google Scholar] [CrossRef]
- Ebner, J.H.; Labatut, R.A.; Lodge, J.S.; Williamson, A.A.; Trabold, T.A. Anaerobic Co-Digestion of Commercial Food Waste and Dairy Manure: Characterizing Biochemical Parameters and Synergistic Effects. Waste Manag. 2016, 52, 286–294. [Google Scholar] [CrossRef]
- Aboudi, K.; Álvarez-Gallego, C.J.; Romero-García, L.I. Improvement of Exhausted Sugar Beet Cossettes Anaerobic Digestion Process by Co-Digestion with Pig Manure. Energy Fuels 2015, 29, 754–762. [Google Scholar] [CrossRef]
- Woraruthai, T.; Jiemanukunkij, T.; Tirapanampai, C.; Weeranoppanant, N.; Chaiyen, P.; Wongnate, T. Solid-Liquid Separation through Sieve Mesh for Enhancing Biogas Production in a Swine Farm. Int. J. Energy Res. 2022, 46, 15362–15375. [Google Scholar] [CrossRef]
- Amaral, C.d.A.; Kunz, A.; Radis Steinmetz, R.L.; Scussiato, L.A.; Tápparo, D.C.; Gaspareto, T.C. Influence of Solid-Liquid Separation Strategy on Biogas Yield from a Stratified Swine Production System. J. Environ. Manag. 2016, 168, 229–235. [Google Scholar] [CrossRef]
- Mottet, A.; Francois, E.; Latrille, E.; Steyer, J.P.; Déléris, S.; Vedrenne, F.; Carrère, H. Estimating anaerobic biodegradability indicators for waste activated sludge. Chem. Eng. J. 2010, 160, 488–496. [Google Scholar] [CrossRef]
- Nielfa, A.; Cano, R.; Fdz-Polanco, M. Theoretical methane production generated by the co-digestion of organic fraction municipal solid waste and biological sludge. Biotechnol. Rep. 2015, 5, 14–21. [Google Scholar] [CrossRef] [PubMed]
- Jimenez, J.; Lei, H.; Steyer, J.P.; Houot, S.; Patureau, D. Methane production and fertilizing value of organic waste: Organic matter characterization for a better prediction of valorization pathways. Bioresour. Technol. 2017, 241, 1012–1021. [Google Scholar] [CrossRef]
- Yang, S.; Li, J.; Zheng, Z.; Meng, Z. Lignocellulosic Structural Changes of Spartina Alterniflora after Anaerobic Mono- and Co-Digestion. Int. Biodeterior. Biodegrad. 2009, 63, 569–575. [Google Scholar] [CrossRef]
- Bareha, Y.; Faucher, J.-P.; Michel, M.; Houdon, M.; Vaneeckhaute, C. Evaluating the impact of substrate addition for anaerobic co-digestion on biogas production and digestate quality: The case of deinking sludge. J. Environ. Manag. 2022, 319, 115657. [Google Scholar] [CrossRef]
- Koch, K.; Hafner, S.D.; Weinrich, S.; Astals, S.; Holliger, C. Power and Limitations of Biochemical Methane Potential (BMP) Tests. Front. Energy Res. 2020, 8, 63. [Google Scholar] [CrossRef]
- Amon, T.; Amon, B.; Kryvoruchko, V.; Zollitsch, W.; Mayer, K.; Gruber, L. Biogas Production from Maize and Dairy Cattle Manure-Influence of Biomass Composition on the Methane Yield. Agric. Ecosyst. Environ. 2007, 118, 173–182. [Google Scholar] [CrossRef]
- Mao, C.; Zhang, T.; Wang, X.; Feng, Y.; Ren, G.; Yang, G. Process Performance and Methane Production Optimizing of Anaerobic Co-Digestion of Swine Manure and Corn Straw. Sci. Rep. 2017, 7, 9379. [Google Scholar] [CrossRef]
- Molinuevo-Salces, B.; Ahring, B.K.; Uellendahl, H. Optimization of the Co-Digestion of Catch Crops with Manure Using a Central Composite Design and Reactor Operation. Appl. Biochem. Biotechnol. 2015, 175, 1710–1723. [Google Scholar] [CrossRef]
- McCarty, P.L. Anaerobic Waste Treatment Fundamentals—Part Four—Process Design. Public Work 1964, 95, 95–99. [Google Scholar]
- Angelidaki, I.; Ahring, B.K. Thermophilic Anaerobic Digestion of Livestock Waste: The Effect of Ammonia. Appl. Microbiol. Biotechnol. 1993, 38, 560–564. [Google Scholar] [CrossRef]
- Yenigün, O.; Demirel, B. Ammonia Inhibition in Anaerobic Digestion: A Review. Process Biochem. 2013, 48, 901–911. [Google Scholar] [CrossRef]
- Chen, Y.; Cheng, J.J.; Creamer, K.S. Inhibition of Anaerobic Digestion Process: A Review. Bioresour. Technol. 2008, 99, 4044–4064. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).