Oxidative Pyrolysis of Typical Volatile Model Compounds Under Low Oxygen Equivalence Ratios During Oxidative Pyrolysis of Biomass
Abstract
:1. Introduction
2. Experimental Section
2.1. Volatile Model Compounds
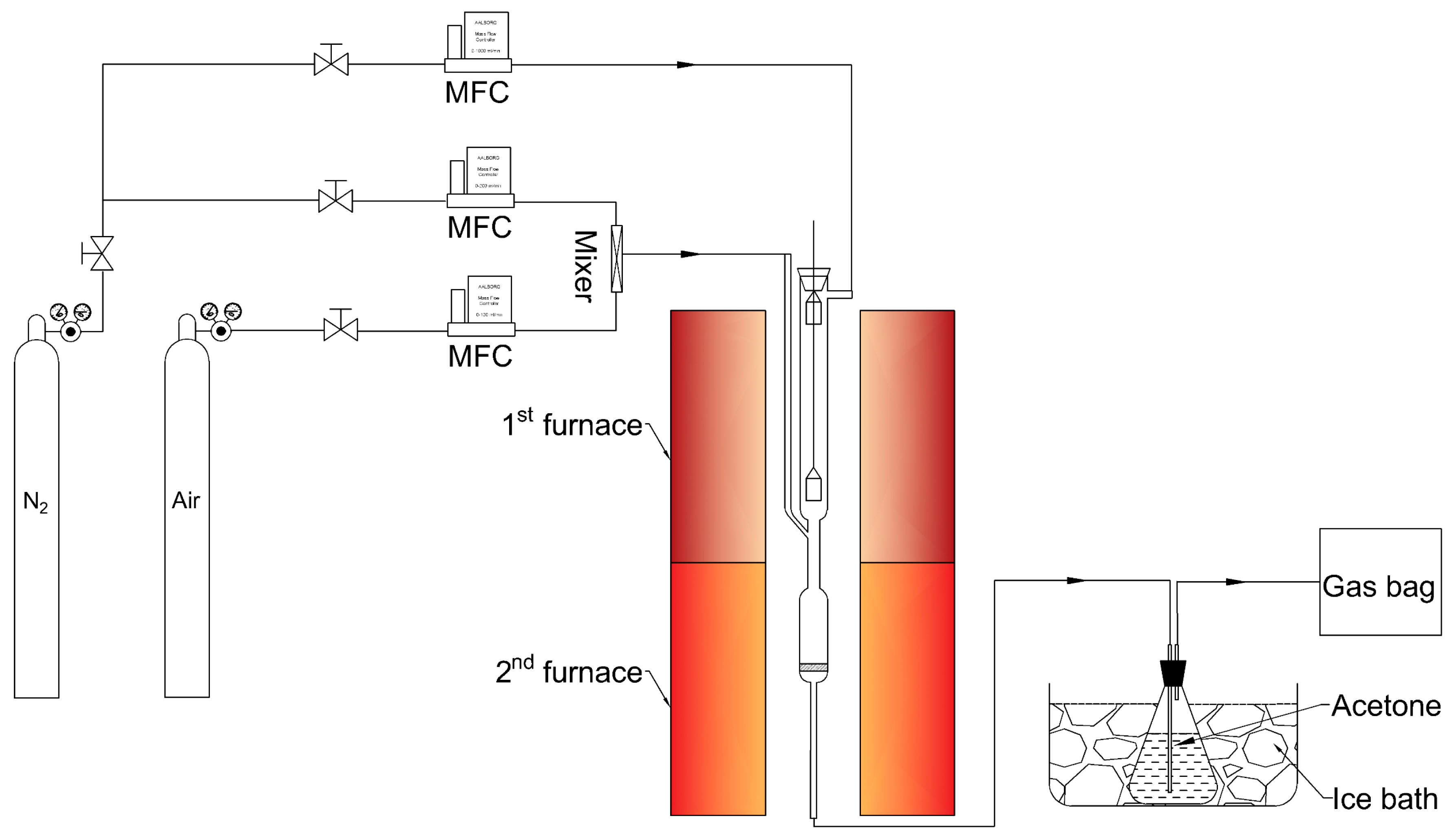
2.2. Oxidative Pyrolysis of Volatiles
2.3. Product Characterization
3. Results and Discussion
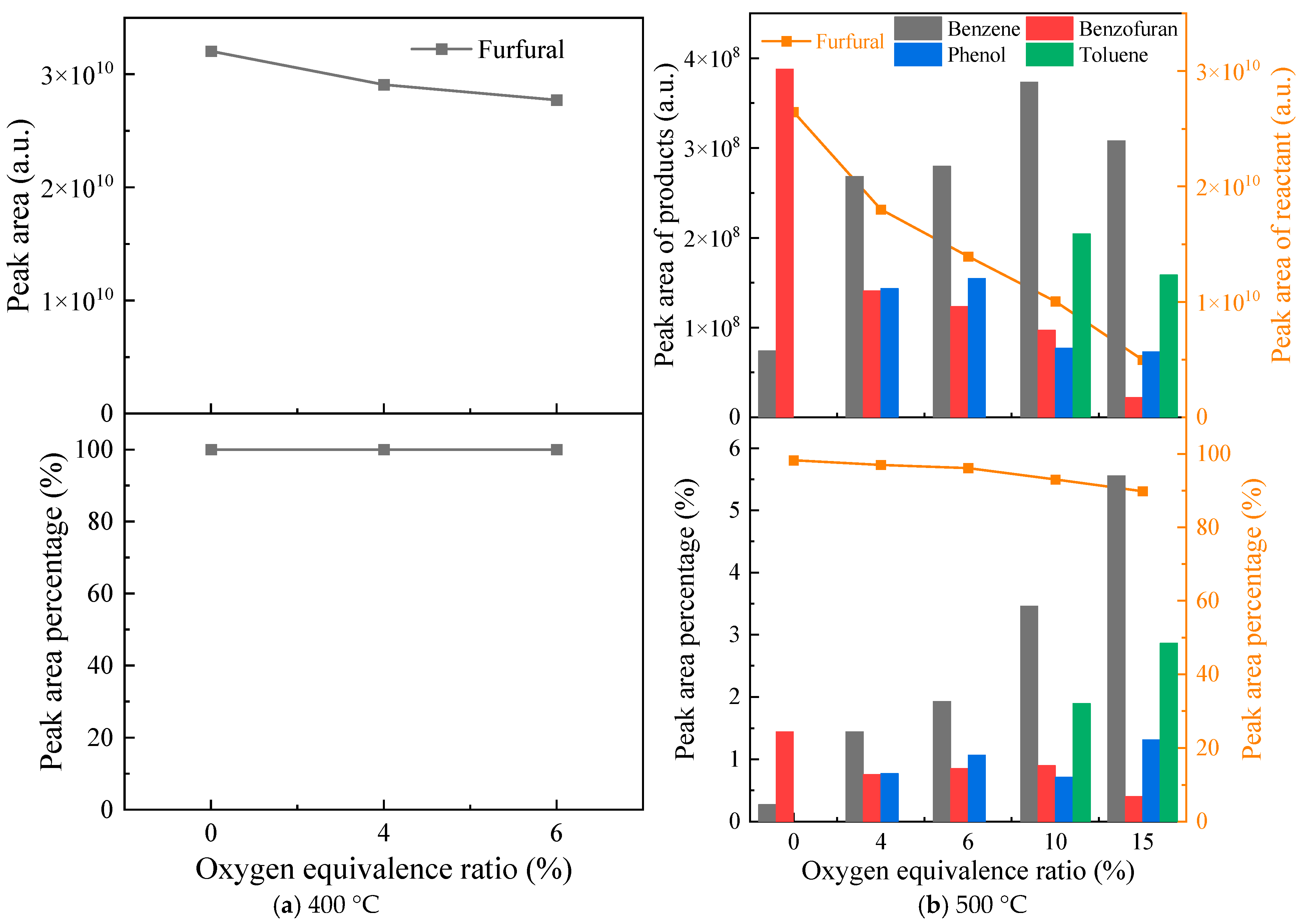
3.1. Oxidative Pyrolysis of Furfural Under Low ERs at 400 and 500 °C
3.2. Oxidative Pyrolysis of Hydroxyacetone Under Low ERs at 400 and 500 °C
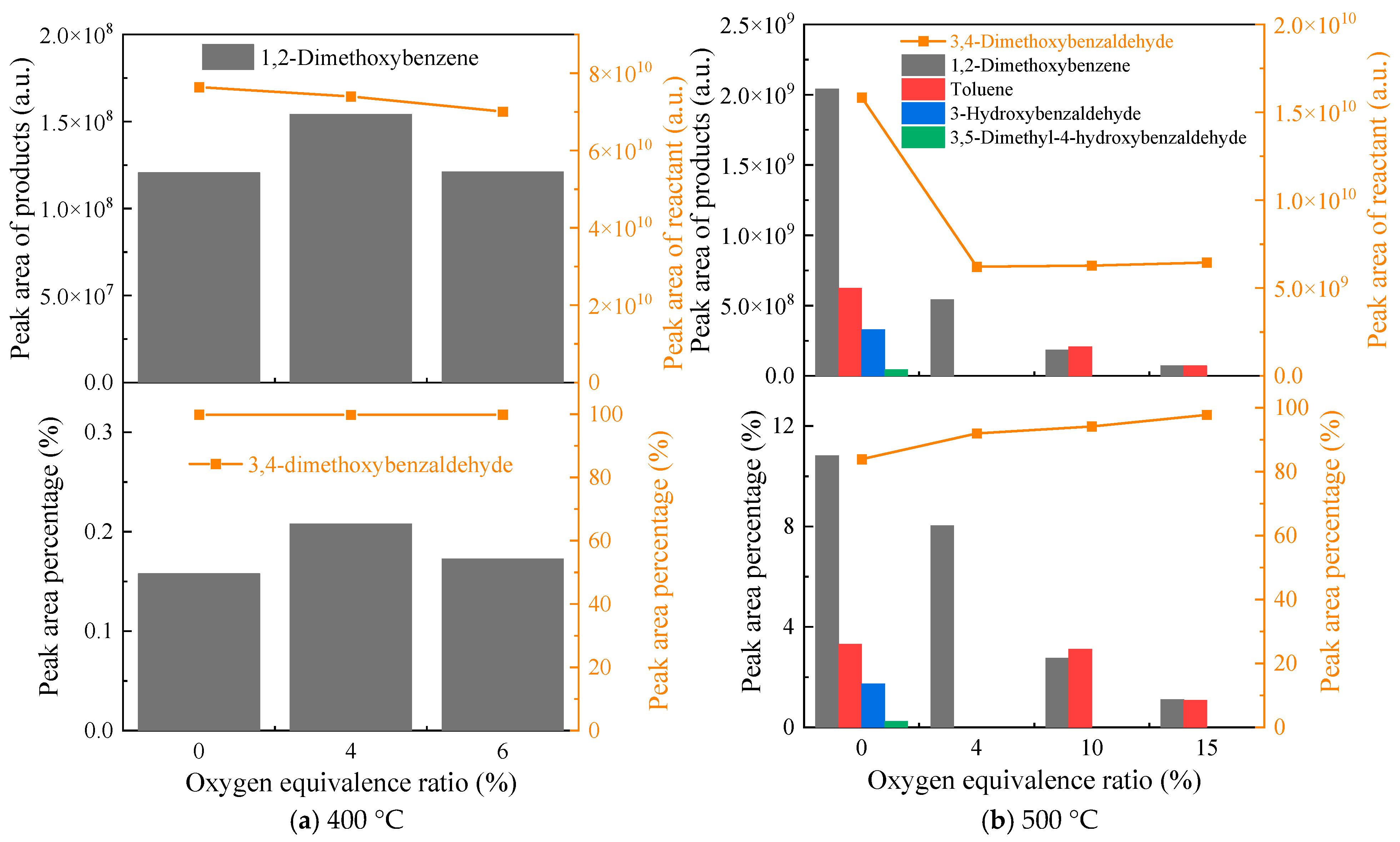
3.3. Oxidative Pyrolysis of 3,4-Dimethoxybenzaldehyde Under Low ERs at 400 and 500 °C
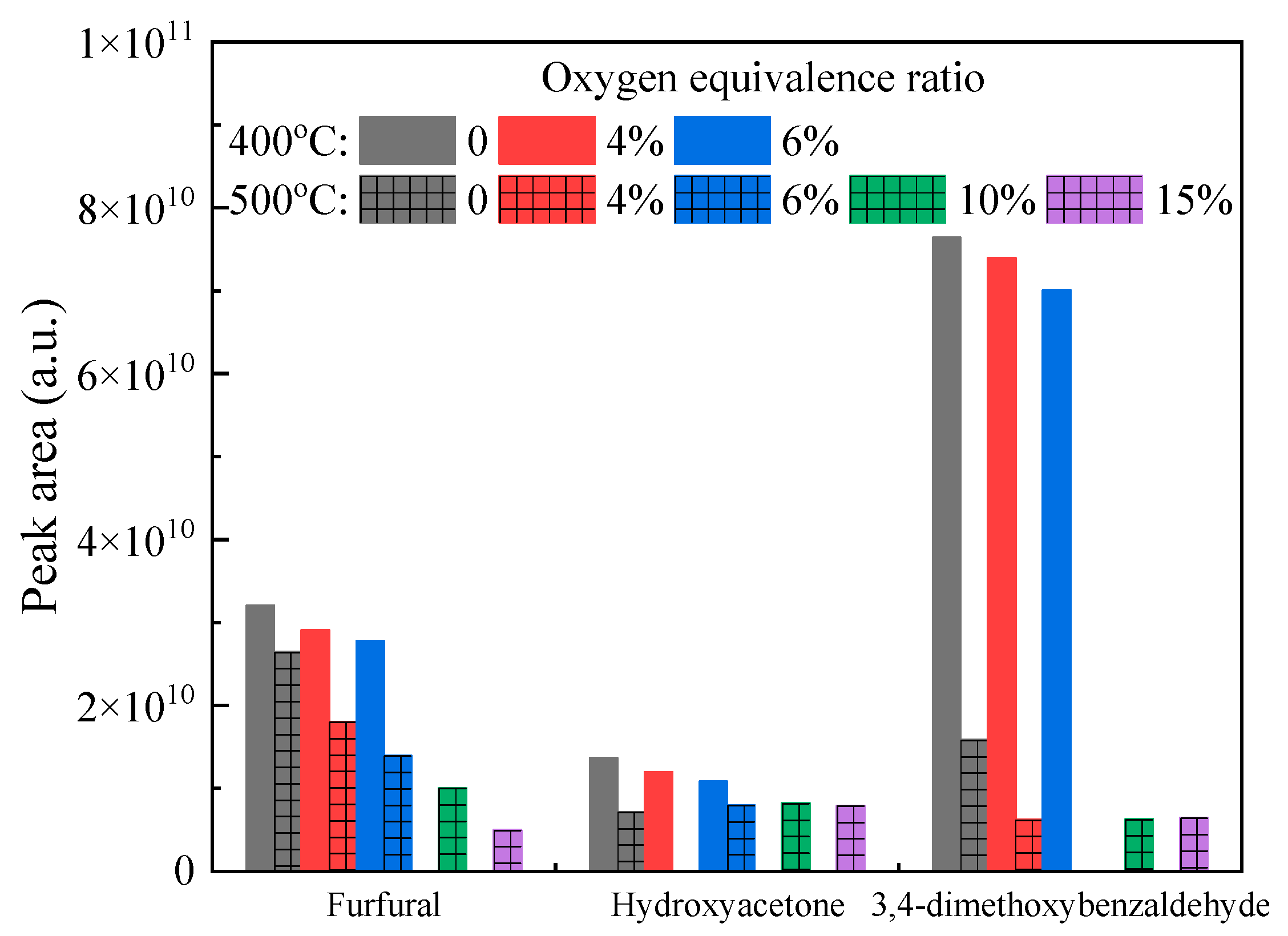
3.4. Further Discussion on Oxidative Pyrolysis of Typical Volatile Model Compounds
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Uddin, M.N.; Techato, K.; Taweekun, J.; Rahman, M.M.; Rasul, M.G.; Mahlia, T.M.I.; Ashrafur, S.M. An Overview of Recent Developments in Biomass Pyrolysis Technologies. Energies 2018, 11, 3115. [Google Scholar] [CrossRef]
- Liu, J.; Chen, X.; Chen, W.; Xia, M.; Chen, Y.; Chen, H.; Zeng, K.; Yang, H. Biomass pyrolysis mechanism for carbon-based high-value products. Proc. Combust. Inst. 2023, 39, 3157–3181. [Google Scholar] [CrossRef]
- Qiu, B.; Tao, X.; Wang, J.; Liu, Y.; Li, S.; Chu, H. Research progress in the preparation of high-quality liquid fuels and chemicals by catalytic pyrolysis of biomass: A review. Energy Convers. Manag. 2022, 261, 115647. [Google Scholar] [CrossRef]
- Pires, A.P.P.; Arauzo, J.; Fonts, I.; Domine, M.E.; Fernandez Arroyo, A.; Estrella Garcia-Perez, M.; Montoya, J.; Chejne, F.; Pfromm, P.; Garcia-Perez, M. Challenges and Opportunities for Bio-oil Refining: A Review. Energy Fuels 2019, 33, 4683–4720. [Google Scholar] [CrossRef]
- Dodds, D.R.; Gross, R.A. Chemicals from Biomass. Science 2007, 318, 1250–1251. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Zhao, X.; Wang, A.; Huber, G.W.; Zhang, T. Catalytic Transformation of Lignin for the Production of Chemicals and Fuels. Chem. Rev. 2015, 115, 11559–11624. [Google Scholar] [CrossRef]
- Dai, L.; Wang, Y.; Liu, Y.; He, C.; Ruan, R.; Yu, Z.; Jiang, L.; Zeng, Z.; Wu, Q. A review on selective production of value-added chemicals via catalytic pyrolysis of lignocellulosic biomass. Sci. Total Environ. 2020, 749, 142386. [Google Scholar] [CrossRef]
- Hu, B.; Zhang, Z.-x.; Xie, W.-l.; Liu, J.; Li, Y.; Zhang, W.-m.; Fu, H.; Lu, Q. Advances on the fast pyrolysis of biomass for the selective preparation of phenolic compounds. Fuel Process. Technol. 2022, 237, 107465. [Google Scholar] [CrossRef]
- Zheng, A.; Chen, T.; Sun, J.; Jiang, L.; Wu, J.; Zhao, Z.; Huang, Z.; Zhao, K.; Wei, G.; He, F.; et al. Toward Fast Pyrolysis-Based Biorefinery: Selective Production of Platform Chemicals from Biomass by Organosolv Fractionation Coupled with Fast Pyrolysis. ACS Sustain. Chem. Eng. 2017, 5, 6507–6516. [Google Scholar] [CrossRef]
- Mahmood, F.; Ali, M.; Khan, M.; Mbeugang, C.F.M.; Isa, Y.M.; Kozlov, A.; Penzik, M.; Xie, X.; Yang, H.; Zhang, S.; et al. A review of biochar production and its employment in synthesizing carbon-based materials for supercapacitors. Ind. Crops Prod. 2025, 227, 120830. [Google Scholar] [CrossRef]
- Reza, M.S.; Iskakova, Z.B.; Afroze, S.; Kuterbekov, K.; Kabyshev, A.; Bekmyrza, K.Z.; Kubenova, M.M.; Abu Bakar, M.S.; Azad, A.K.; Roy, H.; et al. Influence of Catalyst on the Yield and Quality of Bio-Oil for the Catalytic Pyrolysis of Biomass: A Comprehensive Review. Energies 2023, 16, 5547. [Google Scholar] [CrossRef]
- Lahijani, P.; Mohammadi, M.; Mohamed, A.R.; Ismail, F.; Lee, K.T.; Amini, G. Upgrading biomass-derived pyrolysis bio-oil to bio-jet fuel through catalytic cracking and hydrodeoxygenation: A review of recent progress. Energy Convers. Manag. 2022, 268, 115956. [Google Scholar] [CrossRef]
- Wang, Y.; Akbarzadeh, A.; Chong, L.; Du, J.; Tahir, N.; Awasthi, M.K. Catalytic pyrolysis of lignocellulosic biomass for bio-oil production: A review. Chemosphere 2022, 297, 134181. [Google Scholar] [CrossRef]
- Ren, X.; Ghazani, M.S.; Zhu, H.; Ao, W.; Zhang, H.; Moreside, E.; Zhu, J.; Yang, P.; Zhong, N.; Bi, X. Challenges and opportunities in microwave-assisted catalytic pyrolysis of biomass: A review. Appl. Energy 2022, 315, 118970. [Google Scholar] [CrossRef]
- Ferreira, R.A.d.R.; Meireles, C.d.S.; Assunção, R.M.N.; Barrozo, M.A.S.; Soares, R.R. Optimization of the oxidative fast pyrolysis process of sugarcane straw by TGA and DSC analyses. Biomass Bioenergy 2020, 134, 105456. [Google Scholar] [CrossRef]
- Van de Velden, M.; Baeyens, J.; Brems, A.; Janssens, B.; Dewil, R. Fundamentals, kinetics and endothermicity of the biomass pyrolysis reaction. Renew. Energy 2010, 35, 232–242. [Google Scholar] [CrossRef]
- Huang, Y.; Li, B.; Liu, D.; Xie, X.; Zhang, H.; Sun, H.; Hu, X.; Zhang, S. Fundamental Advances in Biomass Autothermal/Oxidative Pyrolysis: A Review. ACS Sustain. Chem. Eng. 2020, 8, 11888–11905. [Google Scholar] [CrossRef]
- Lu, T.; Lin, D.; Isa, Y.M.; Kozlov, A.; Penzik, M.; Xie, X.; Zhang, S.; Yang, H.; Li, B. Influence of oxygen equivalence ratio on biochar oxidation characteristics under biomass oxidative pyrolysis conditions in a fluidized-bed reactor. J. Energy Inst. 2025, 119, 101995. [Google Scholar] [CrossRef]
- Brown, R.C. Heterodoxy in Fast Pyrolysis of Biomass. Energy Fuels 2020, 35, 987–1010. [Google Scholar] [CrossRef]
- Karmee, S.K.; Kumari, G.; Soni, B. Pilot scale oxidative fast pyrolysis of sawdust in a fluidized bed reactor: A biorefinery approach. Bioresour. Technol. 2020, 318, 124071. [Google Scholar] [CrossRef]
- Zhao, S.; Xu, W.; Chen, L. The modeling and products prediction for biomass oxidative pyrolysis based on PSO-ANN method: An artificial intelligence algorithm approach. Fuel 2022, 312, 122966. [Google Scholar] [CrossRef]
- Polin, J.P.; Peterson, C.A.; Whitmer, L.E.; Smith, R.G.; Brown, R.C. Process intensification of biomass fast pyrolysis through autothermal operation of a fluidized bed reactor. Appl. Energy 2019, 249, 276–285. [Google Scholar] [CrossRef]
- Siwal, S.S.; Zhang, Q.; Sun, C.; Thakur, S.; Gupta, V.K.; Thakur, V.K. Energy production from steam gasification processes and parameters that contemplate in biomass gasifier—A review. Bioresour. Technol. 2020, 297, 122481. [Google Scholar] [CrossRef]
- Tezer, O.; Karabag, N.; Ongen, A.; Colpan, C.O.; Ayol, A. Biomass gasification for sustainable energy production: A review. Int. J. Hydrogen Energy 2022, 47, 15419–15433. [Google Scholar] [CrossRef]
- Elwardany, M. Enhancing steam boiler efficiency through comprehensive energy and exergy analysis: A review. Process Saf. Environ. Prot. 2024, 184, 1222–1250. [Google Scholar] [CrossRef]
- Zhu, S.; Lee, S.W. Co-combustion performance of poultry wastes and natural gas in the advanced Swirling Fluidized Bed Combustor (SFBC). Waste Manag. 2005, 25, 511–518. [Google Scholar] [CrossRef]
- Kim, K.H.; Bai, X.; Rover, M.; Brown, R.C. The effect of low-concentration oxygen in sweep gas during pyrolysis of red oak using a fluidized bed reactor. Fuel 2014, 124, 49–56. [Google Scholar] [CrossRef]
- Li, B.; Song, M.; Xie, X.; Wei, J.; Xu, D.; Ding, K.; Huang, Y.; Zhang, S.; Hu, X.; Zhang, S.; et al. Oxidative fast pyrolysis of biomass in a quartz tube fluidized bed reactor: Effect of oxygen equivalence ratio. Energy 2023, 270, 126987. [Google Scholar] [CrossRef]
- Jiang, S.; Hu, X.; Wu, L.; Zhang, L.; Wang, S.; Li, T.; Xia, D.; Li, C.-Z. Oxidative pyrolysis of mallee wood biomass, cellulose and lignin. Fuel 2018, 217, 382–388. [Google Scholar] [CrossRef]
- Zhao, S.; Luo, Y.; Zhang, Y.; Long, Y. Experimental Investigation of Rice Straw and Model Compound Oxidative Pyrolysis by in Situ Diffuse Reflectance Infrared Fourier Transform and Coupled Thermogravimetry–Differential Scanning Calorimetry/Mass Spectrometry Method. Energy Fuels 2015, 29, 4361–4372. [Google Scholar] [CrossRef]
- Wang, S.R.; Dai, G.X.; Yang, H.P.; Luo, Z.Y. Lignocellulosic biomass pyrolysis mechanism: A state-of-the-art review. Prog. Energy Combust. Sci. 2017, 62, 33–86. [Google Scholar] [CrossRef]
- Li, B.; Zhao, L.; Xie, X.; Lin, D.; Xu, H.; Wang, S.; Xu, Z.; Wang, J.; Huang, Y.; Zhang, S.; et al. Volatile-char interactions during biomass pyrolysis: Effect of char preparation temperature. Energy 2021, 215, 119189. [Google Scholar] [CrossRef]
- Vasiliou, A.K.; Kim, J.H.; Ormond, T.K.; Piech, K.M.; Urness, K.N.; Scheer, A.M.; Robichaud, D.J.; Mukarakate, C.; Nimlos, M.R.; Daily, J.W.; et al. Biomass pyrolysis: Thermal decomposition mechanisms of furfural and benzaldehyde. J. Chem. Phys. 2013, 139, 142386. [Google Scholar] [CrossRef]
- Cheng, Y.-T.; Huber, G.W. Production of targeted aromatics by using Diels–Alder classes of reactions with furans and olefins over ZSM-5. Green Chem. 2012, 14, 3114–3125. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, L.; Lin, D.; Liu, D.; Xie, X.; Zhang, S.; Li, B. Oxidative Pyrolysis of Typical Volatile Model Compounds Under Low Oxygen Equivalence Ratios During Oxidative Pyrolysis of Biomass. Energies 2025, 18, 2996. https://doi.org/10.3390/en18112996
Wang L, Lin D, Liu D, Xie X, Zhang S, Li B. Oxidative Pyrolysis of Typical Volatile Model Compounds Under Low Oxygen Equivalence Ratios During Oxidative Pyrolysis of Biomass. Energies. 2025; 18(11):2996. https://doi.org/10.3390/en18112996
Chicago/Turabian StyleWang, Liying, Dan Lin, Dongjing Liu, Xing Xie, Shihong Zhang, and Bin Li. 2025. "Oxidative Pyrolysis of Typical Volatile Model Compounds Under Low Oxygen Equivalence Ratios During Oxidative Pyrolysis of Biomass" Energies 18, no. 11: 2996. https://doi.org/10.3390/en18112996
APA StyleWang, L., Lin, D., Liu, D., Xie, X., Zhang, S., & Li, B. (2025). Oxidative Pyrolysis of Typical Volatile Model Compounds Under Low Oxygen Equivalence Ratios During Oxidative Pyrolysis of Biomass. Energies, 18(11), 2996. https://doi.org/10.3390/en18112996






