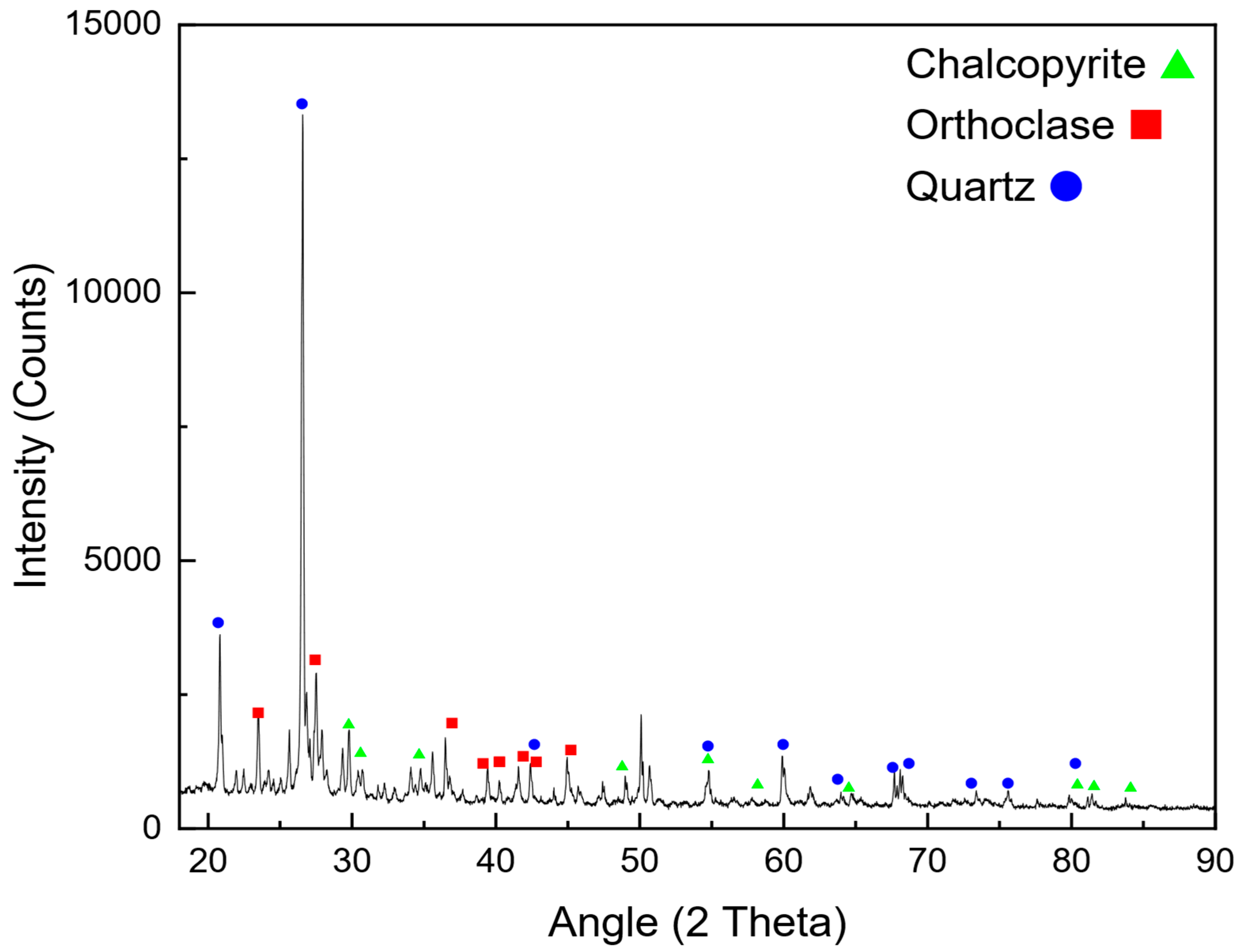
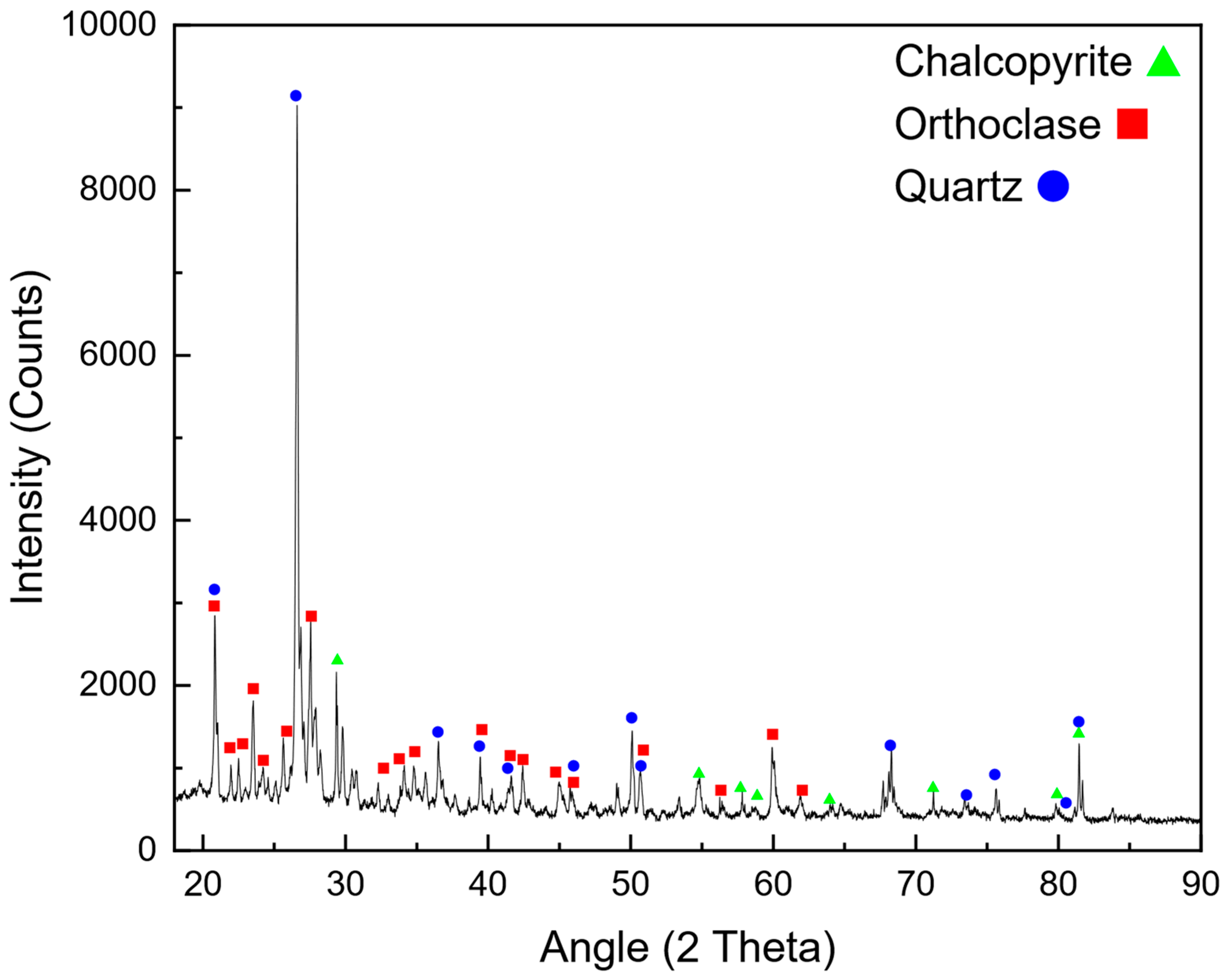
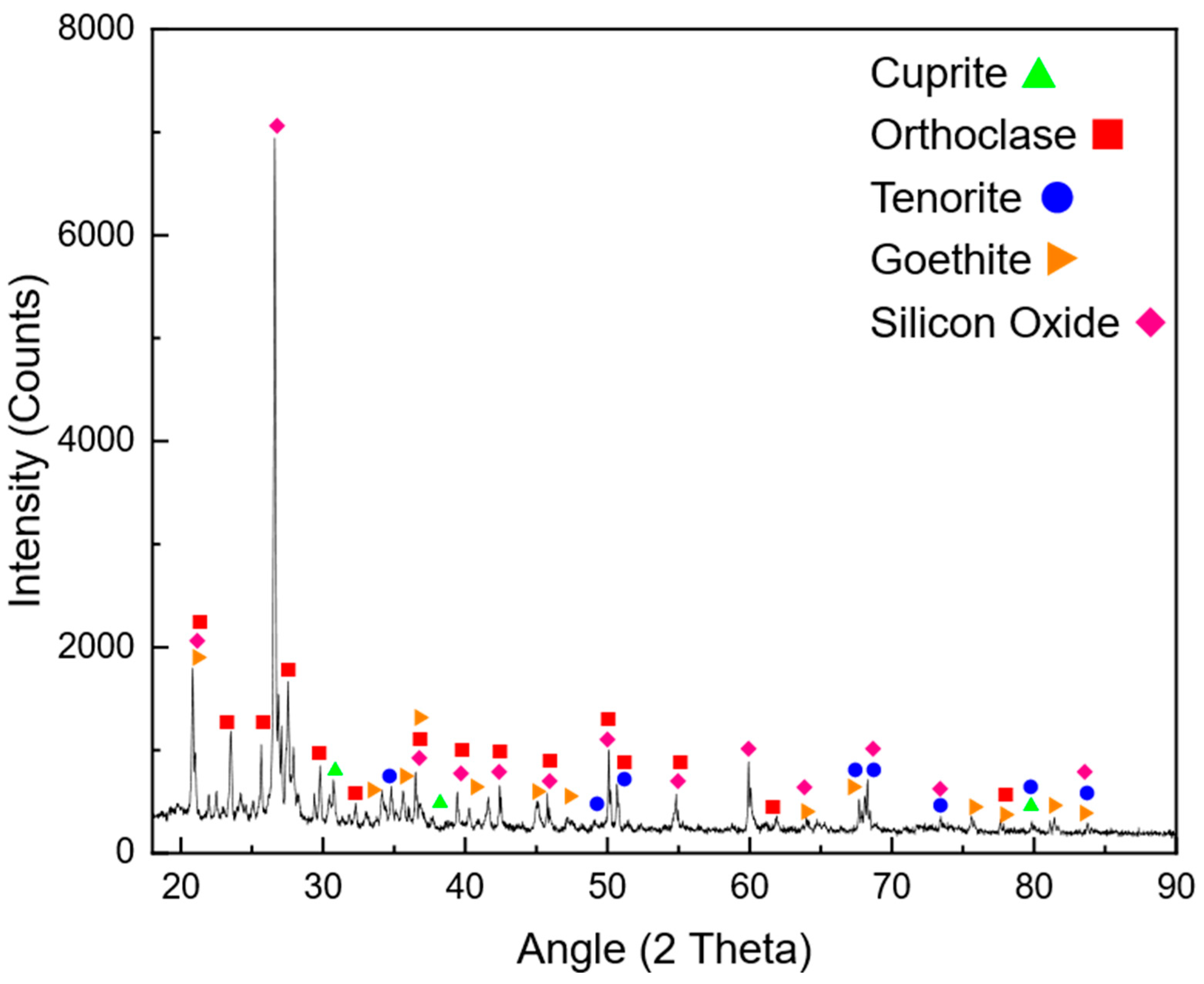
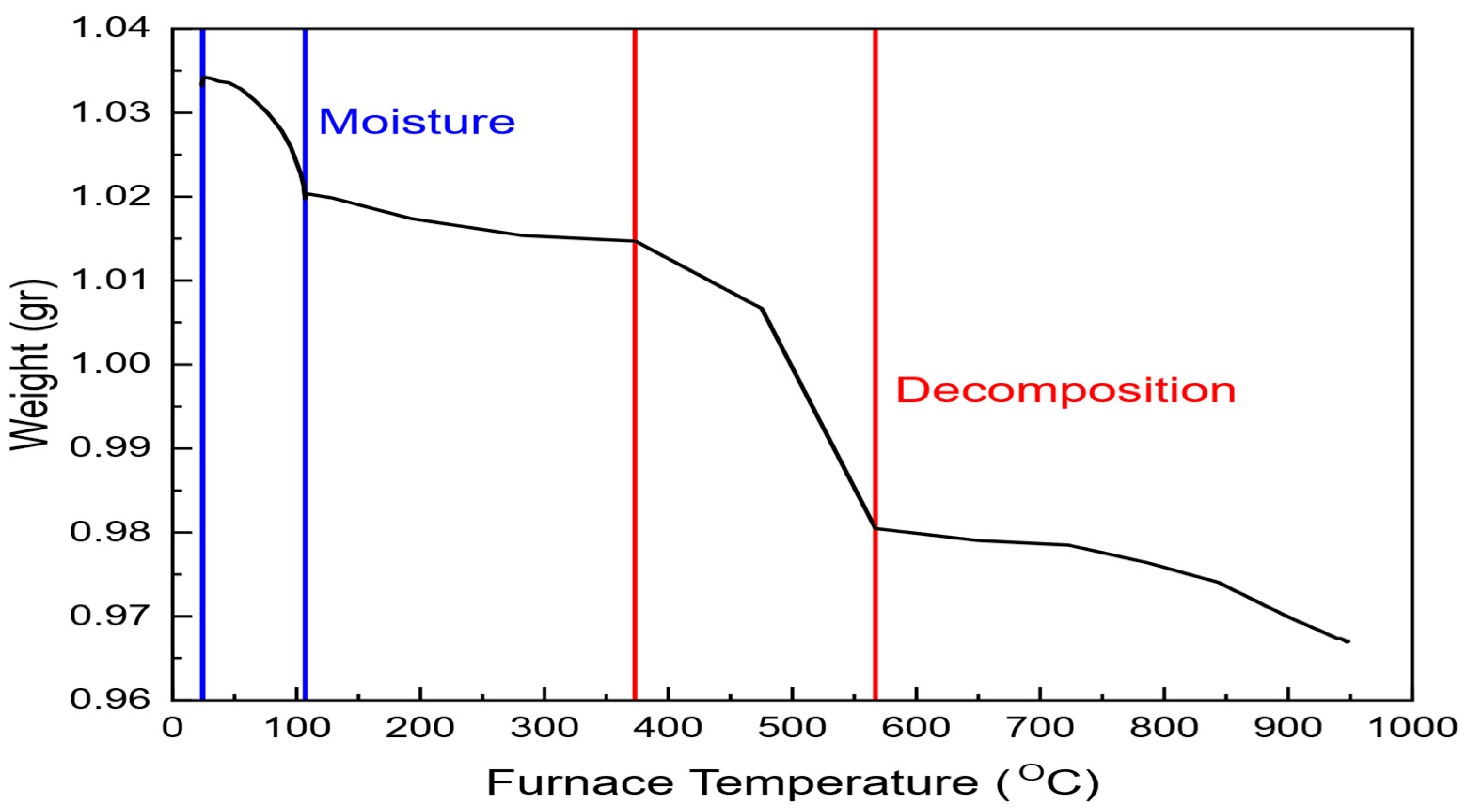
The mining industry faces significant challenges related to energy consumption, environmental impact, and operational costs. Among the various stages of mineral processing, comminution is regarded as the most energy-intensive, accounting for up to 50% of the total energy expenditure in mining operations [
1]. Ball mills, which are widely used for ore grinding, play a critical role in this process. However, traditional ball milling techniques are inherently energy-inefficient, consuming vast amounts of electricity to break down ores into finer particles. This inefficiency has driven researchers and industry professionals to seek innovative methods for reducing energy consumption while maintaining or improving grinding performance. Chalcopyrite (CuFeS
2), one of the most important copper sulfide minerals, is frequently processed through ball milling to achieve the desired liberation for downstream separation processes, such as flotation. However, its complex crystalline structure and associated hardness make its grinding particularly energy-intensive, necessitating the development of novel strategies to enhance efficiency [
2]. One of the conventional approaches for reducing energy consumption in ball mills involves optimizing operational parameters, such as ball size, ball filling ratio, mill speed, and pulp density. These factors directly influence the mill’s efficiency by affecting the impact forces between grinding media and the ore [
3]. Additionally, pretreatment techniques, such as crushing and pre-screening to reduce the feed size, have been widely used to minimize energy consumption in subsequent grinding stages [
1]. However, while these methods offer incremental improvements, they often fail to address the fundamental limitations of the ball milling process, particularly for harder ores like chalcopyrite.
In recent years, there has been a growing interest in the use of thermal pretreatment methods, such as microwave and conventional heating, to improve the grindability of ores and reduce energy consumption. Thermal pretreatment works by inducing thermal stresses within the mineral structure, which leads to microfractures and a reduction in ore hardness. Microwave-assisted comminution, in particular, has shown promising results in selectively heating mineral phases like chalcopyrite, which have high dielectric constants [
4]. When exposed to microwave energy, chalcopyrite absorbs the radiation more efficiently than surrounding gangue minerals, leading to differential expansion and the formation of internal cracks. These microcracks weaken the mineral structure, making it easier to grind and significantly reducing the energy required to achieve the desired particle size [
5]. By weakening the crystalline structure of chalcopyrite, microwave pretreatment has the potential to not only reduce energy consumption but also enhance mineral liberation, thereby improving downstream recovery processes, such as flotation. Several studies have explored the impact of microwave-assisted comminution on various ores, including chalcopyrite. Amankwah et al. (2005) [
5] demonstrated that microwave-treated chalcopyrite exhibited a reduction in the work index, indicating that less energy was required to grind the ore to the target size. Similarly, Kingman et al. (2000) [
4] found that microwave pretreatment could significantly improve the liberation of valuable minerals, which is critical for enhancing the efficiency of separation processes. Thermal pretreatment has also been studied for other ore types, such as ilmenite [
6], gold ores [
7], and iron ore [
8], demonstrating improved grindability and energy savings. For example, Manouchehri et al. (2008) [
6] reported that thermal treatment improved the flotation performance and energy efficiency of ilmenite ore. In another study, Tavares et al. (2010) [
7] showed that microwave pretreatment could enhance the liberation of gold-bearing minerals in refractory ores, resulting in higher gold recovery. Moreover, Karamanev et al. (1996) [
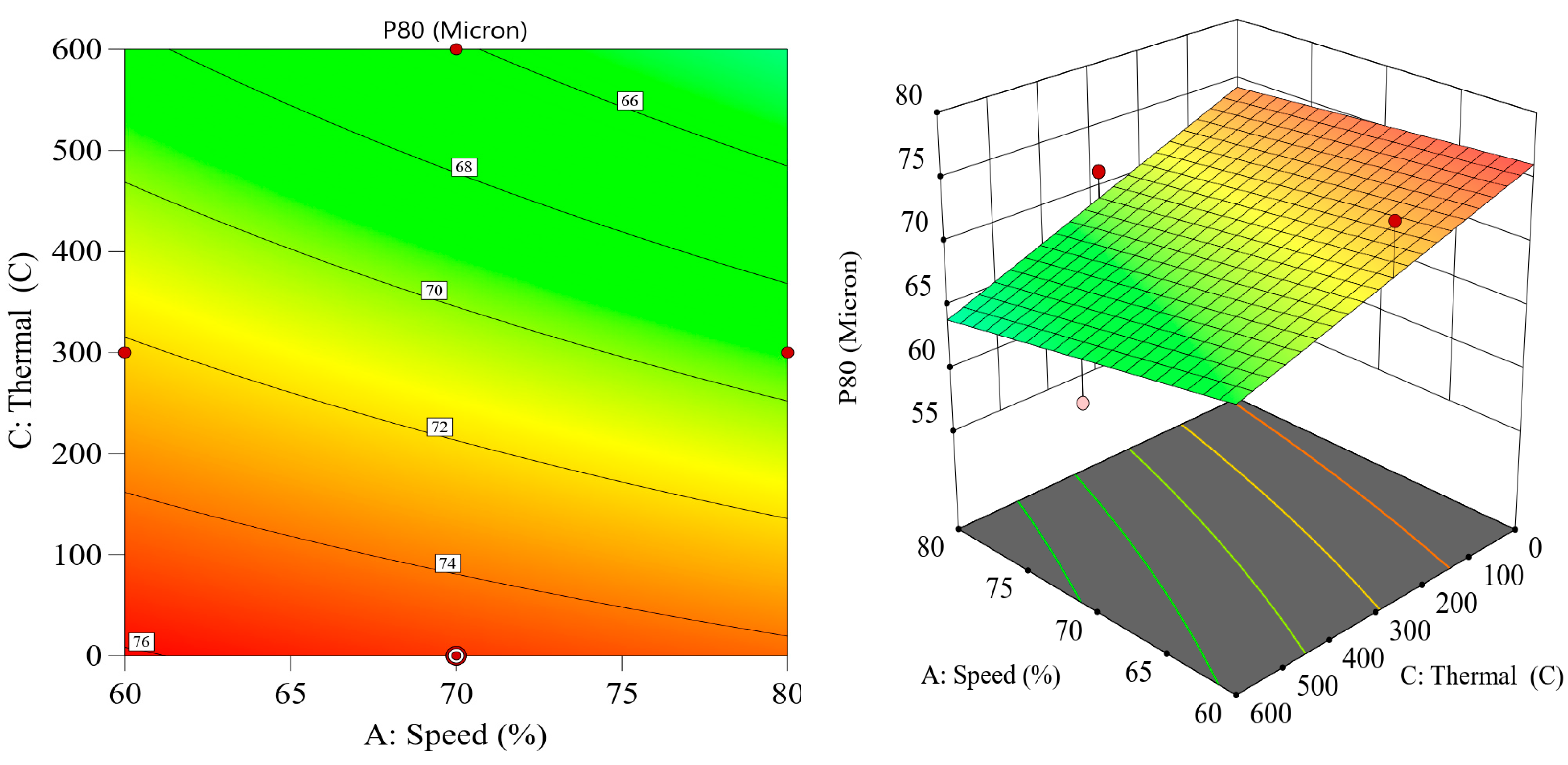
8] found that thermally pretreated iron ores displayed increased reactivity and fragmentation behavior, leading to more efficient grinding in downstream processing. These findings suggest that thermal pretreatment, and specifically microwave-assisted grinding, could play a pivotal role in reducing the energy footprint of ball milling operations. Despite the potential benefits of thermal pretreatment, there is still a need for further research to fully understand the interactions between thermal exposure and grinding performance. Key variables, such as the intensity and duration of the thermal treatment, the ball filling ratio, and mill speed, must be carefully optimized to maximize energy savings and ensure efficient grinding. Additionally, the integration of these variables into advanced experimental designs, such as the Box–Behnken design, provides a systematic approach to evaluating the effects of multiple factors on energy consumption and particle size distribution [
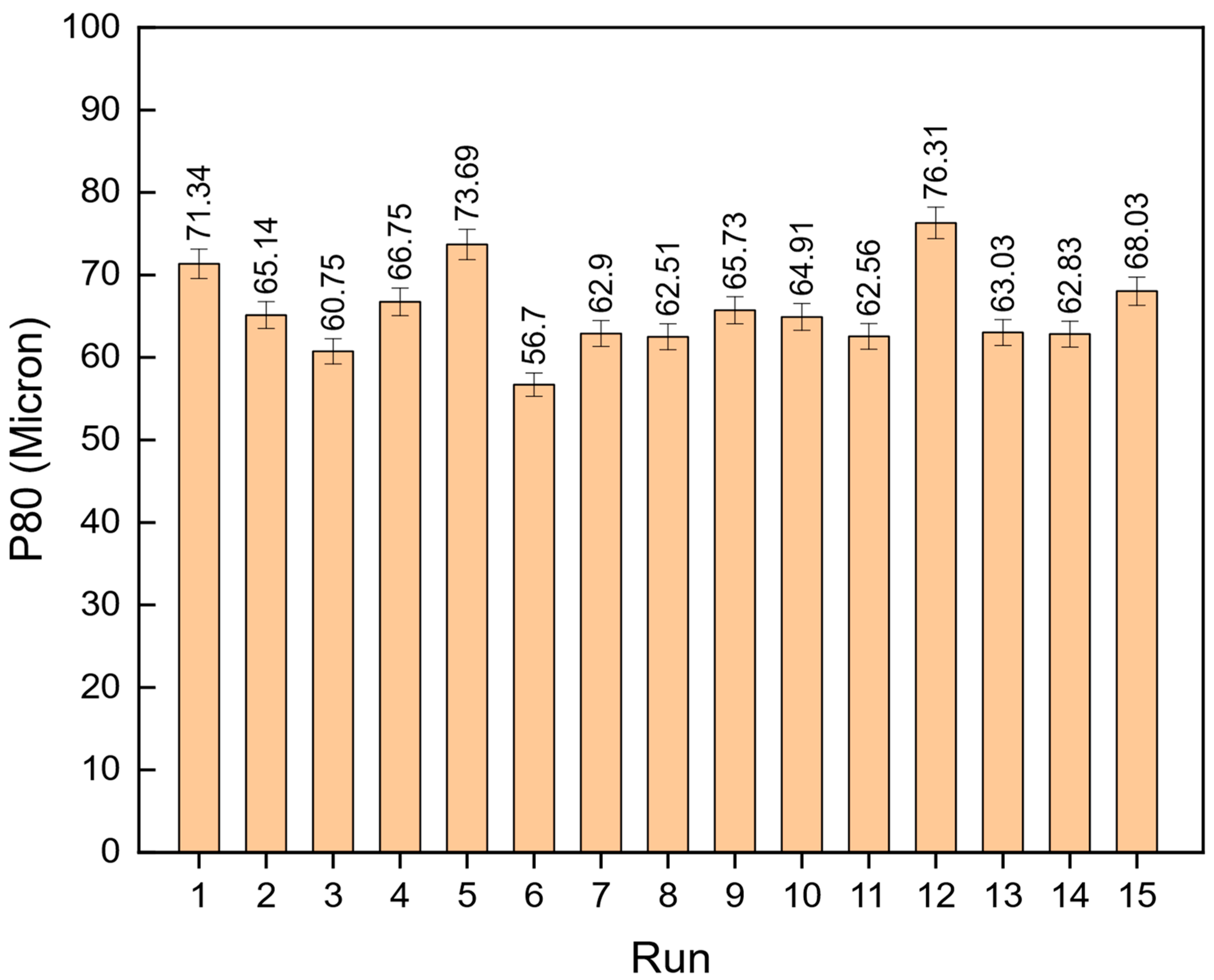
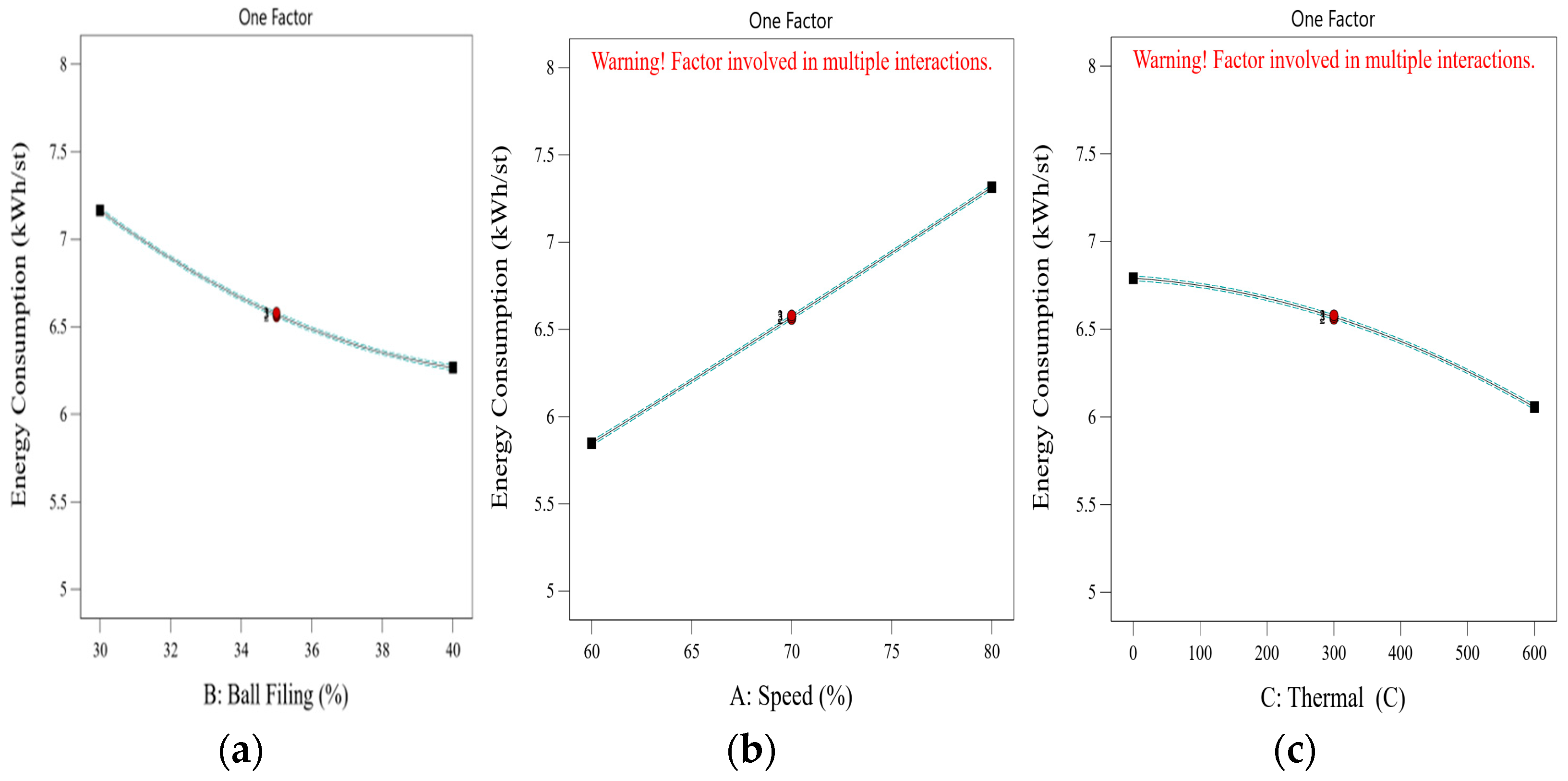
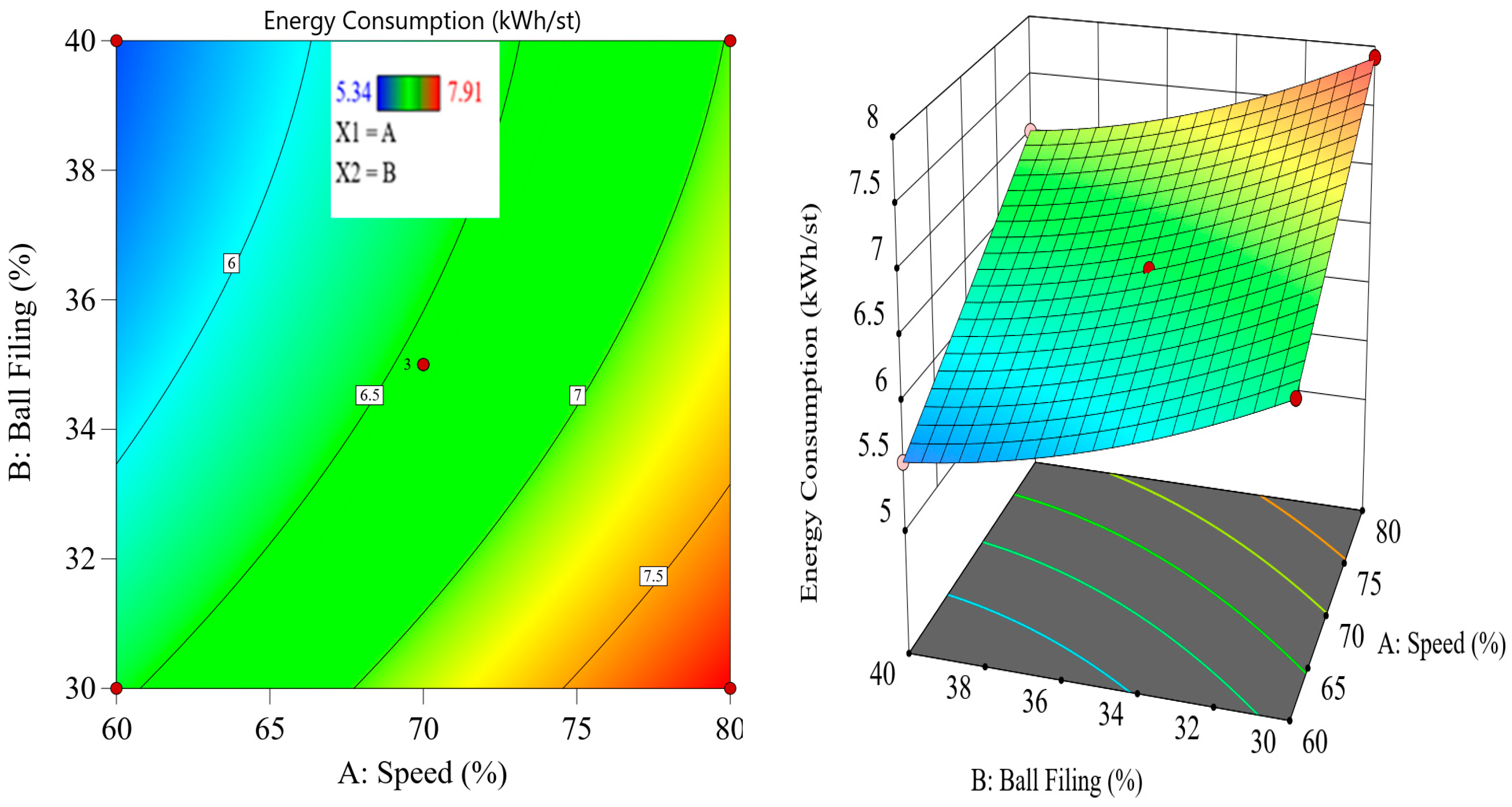
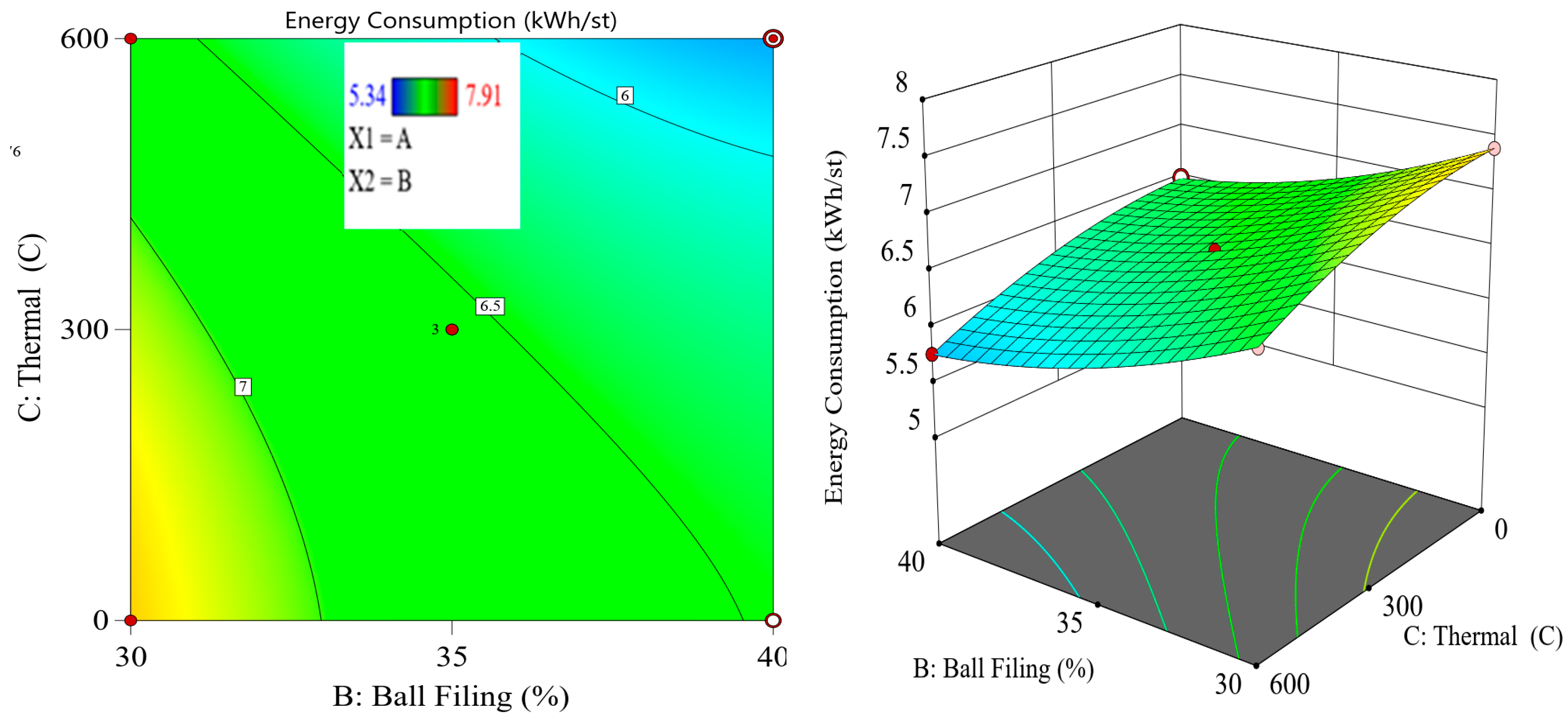
9]. This design of experiments (DoE) approach allows researchers to assess the combined influence of variables and identify optimal operating conditions for thermal pretreatment and ball milling. The importance of reducing energy consumption in comminution goes beyond operational cost savings. In the context of global efforts to reduce greenhouse gas emissions and achieve more sustainable mining practices, improving the energy efficiency of mineral processing has become a critical priority. According to Tavares et al. (2012) [
10], comminution is responsible for a significant portion of a mine’s carbon footprint, and reducing energy usage in this area could have substantial environmental benefits. By lowering the energy required for ore grinding, thermal pretreatment techniques, such as microwave irradiation, contribute to more sustainable mining practices while also reducing costs associated with electricity consumption and equipment wear. As a whole, although various thermal pretreatment techniques, such as microwave irradiation, have shown potential in enhancing ore grindability and reducing energy consumption, their practical application in industrial settings remains limited. In many cases, microwave-based methods were not fully optimized for different ore types, and their scalability and cost-effectiveness pose significant challenges. Additionally, the operational parameters—such as exposure time, intensity, and ore mineralogy—were not always systematically evaluated, resulting in inconsistent outcomes. On the other hand, several alternative pretreatment approaches involving chemical reagents or additives have raised environmental and safety concerns due to their toxicity and potential for generating hazardous byproducts. These limitations underscore the need for an effective, scalable, and environmentally responsible pretreatment method that can be seamlessly integrated into existing comminution circuits. Therefore, developing a comprehensive approach that considers the combined effects of thermal pretreatment with key operational grinding parameters—using accurate energy measurements and a statistically robust design—is essential to advancing both the efficiency and sustainability of mineral processing practices.
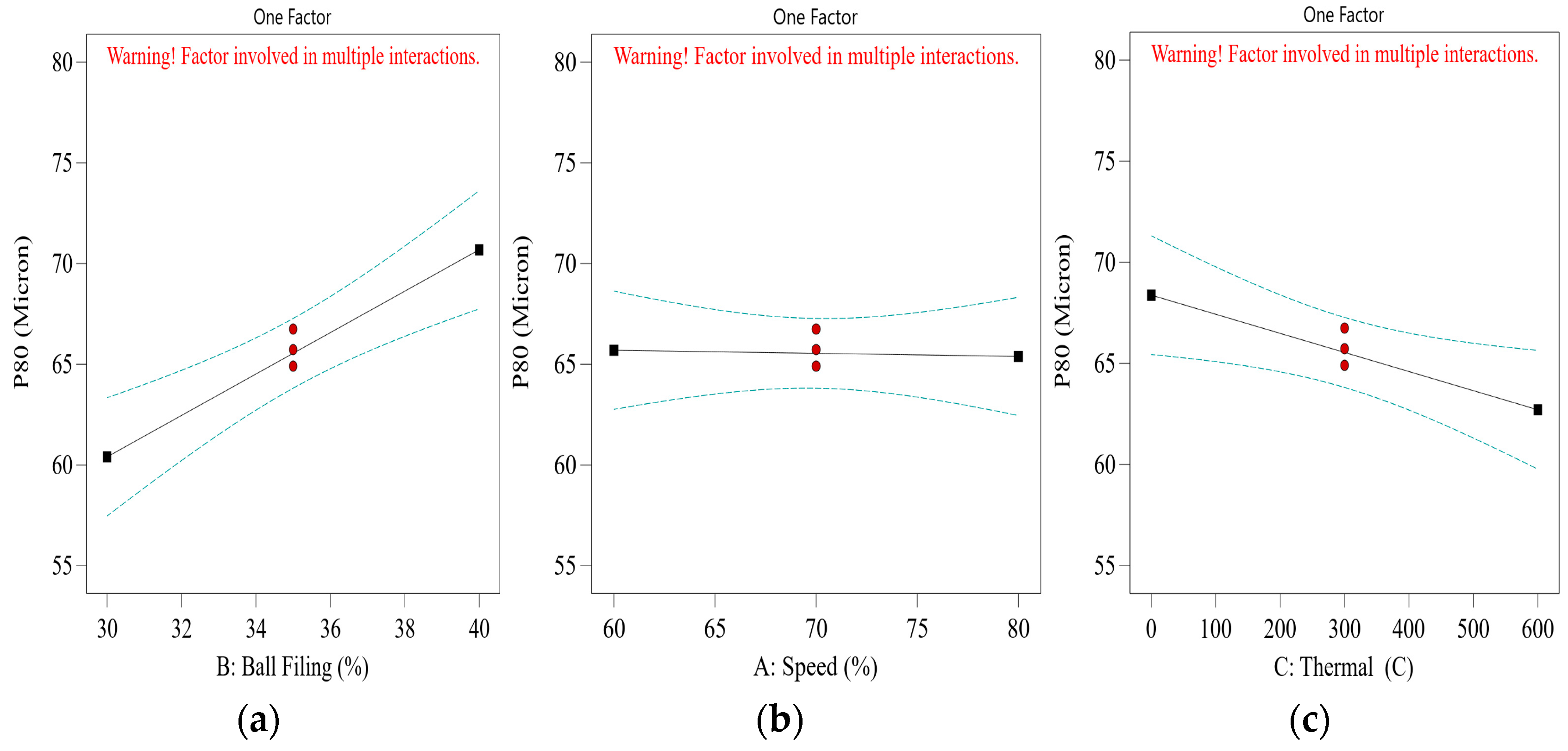
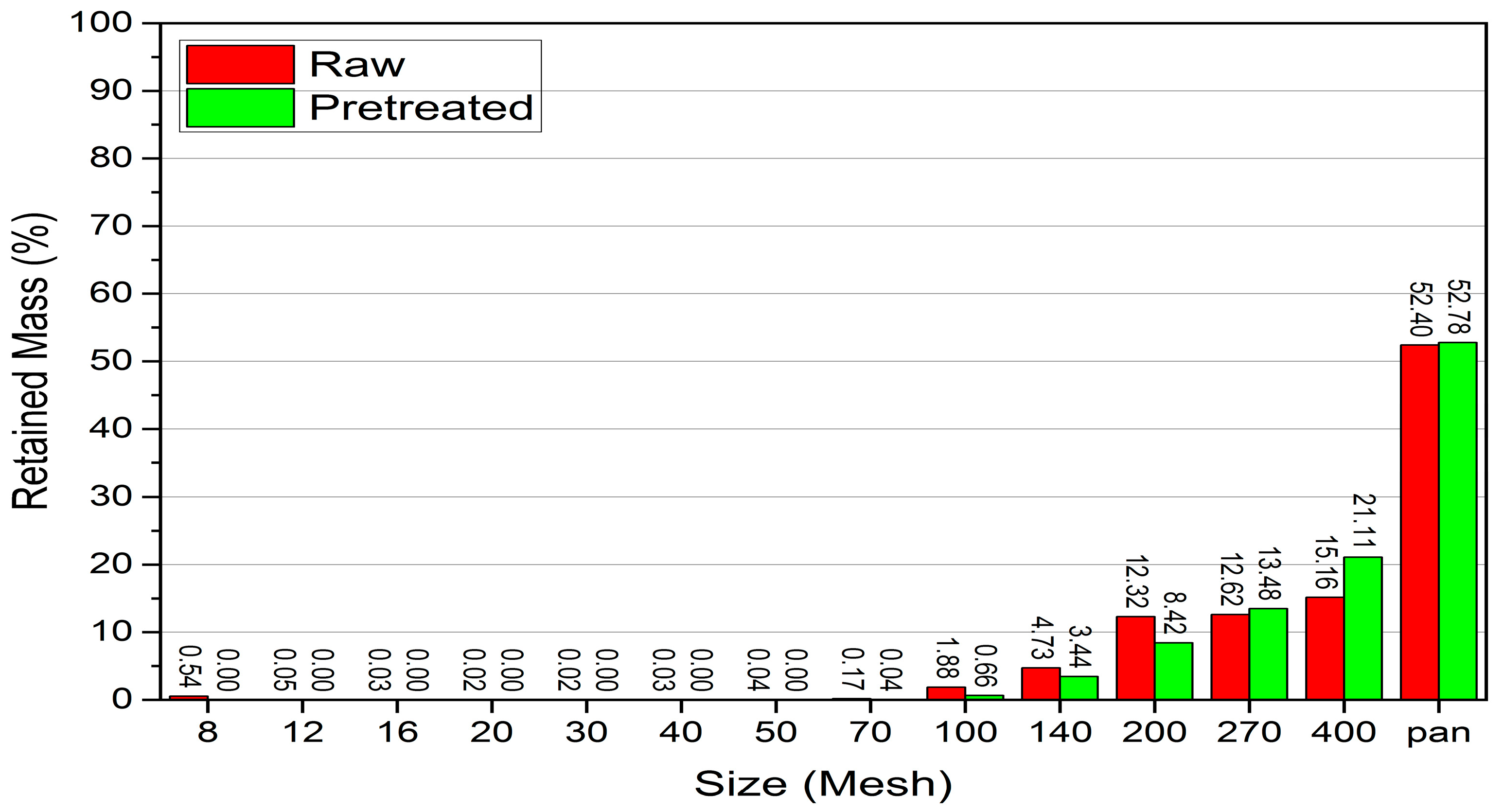
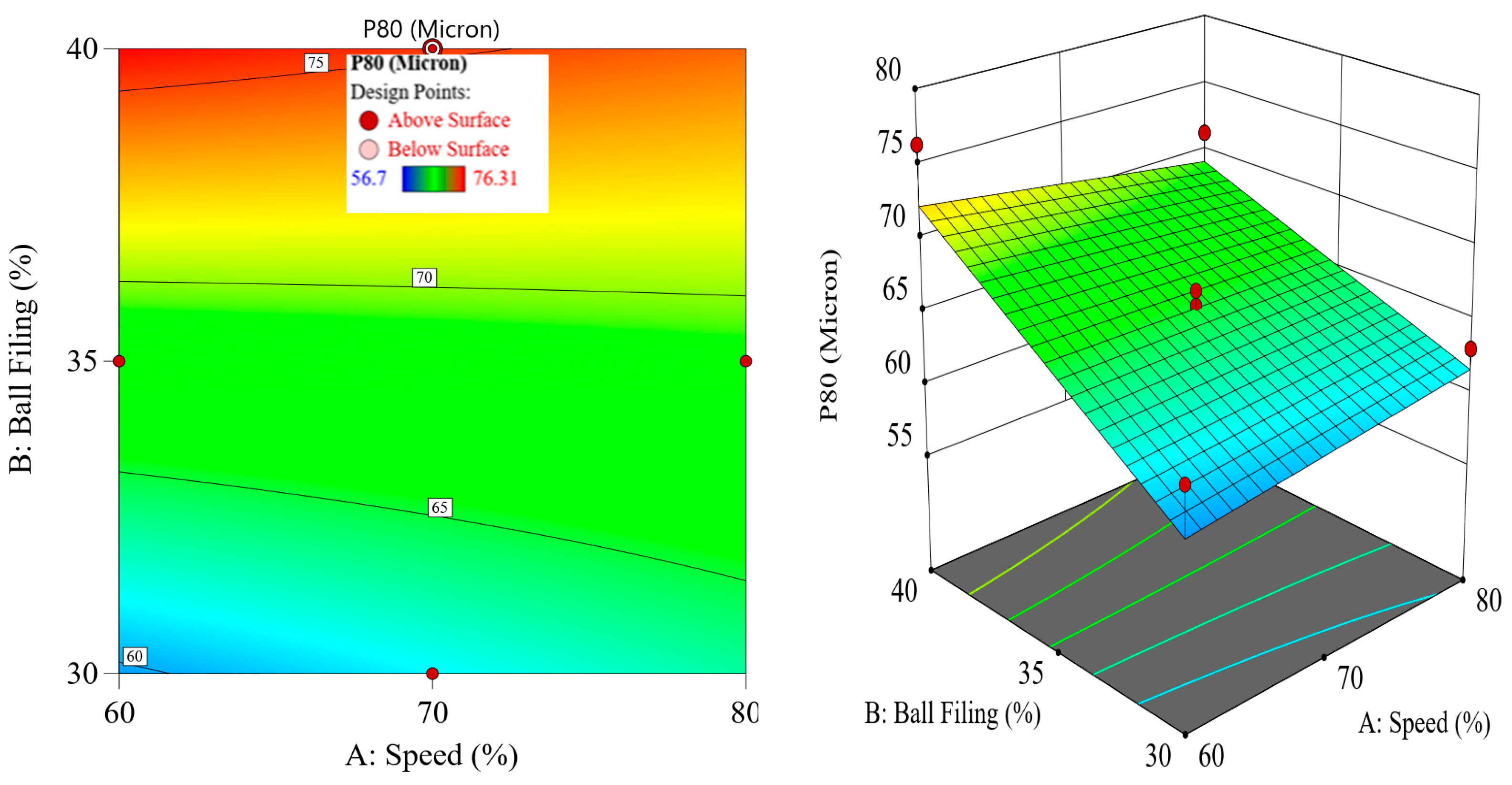
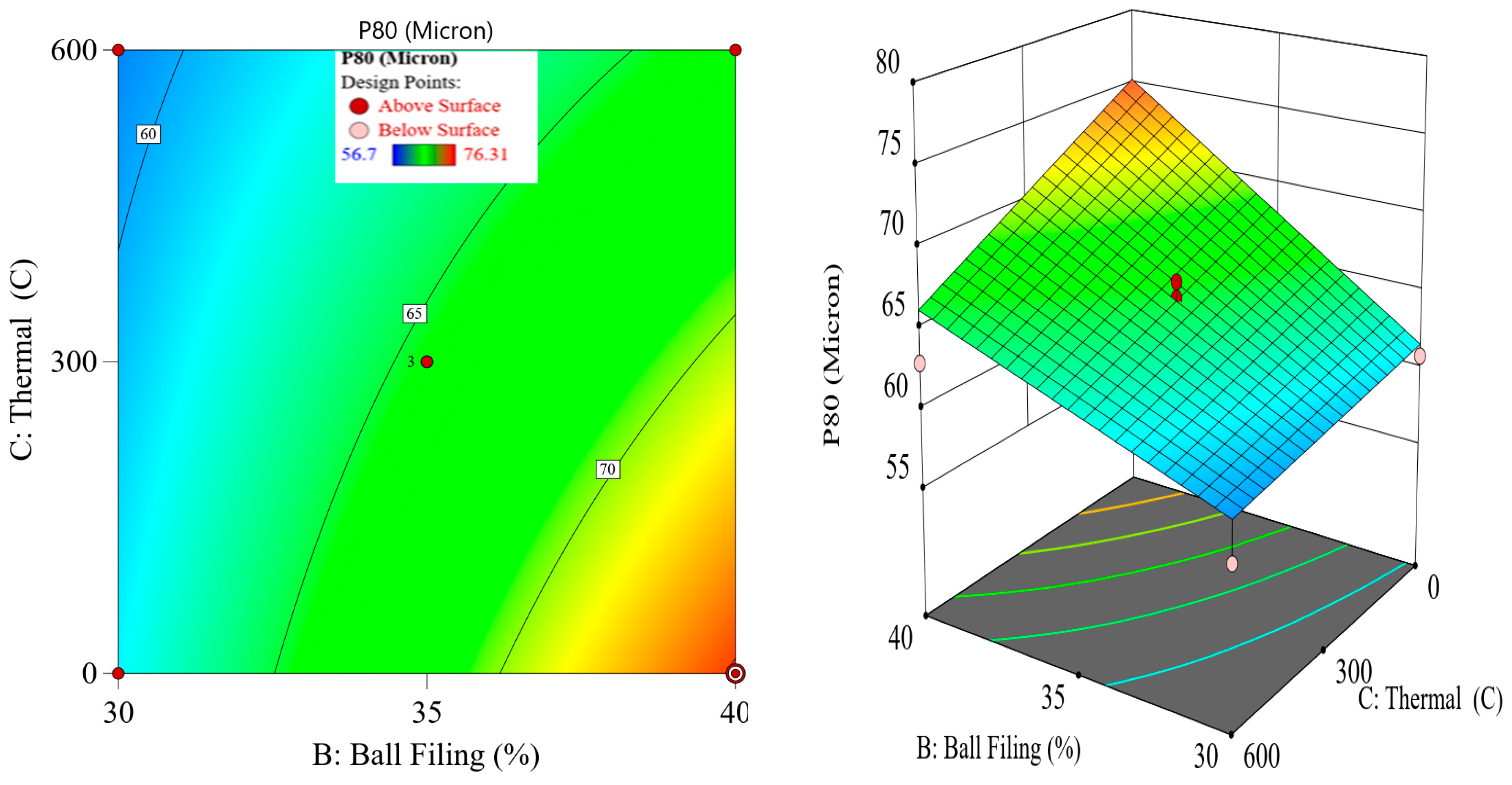
Although thermal pretreatment has been recognized as a potential method to improve mineral grinding, its effect on the energy efficiency of chalcopyrite grinding has not been systematically evaluated using accurate, real-time energy measurements. Most previous studies lacked precise quantification of energy consumption and did not consider the combined influence of key grinding parameters. Therefore, this study aims to address this gap by accurately measuring the specific energy consumption through torque, rotational speed, voltage, and current data, and by systematically analyzing the effects of thermal pretreatment along with ball filling ratio and mill speed using a Box–Behnken design. The findings offer reliable insights into optimizing grinding processes for enhanced energy efficiency in sulfide ore processing.