Pyrolysis Process, Reactors, Products, and Applications: A Review
Abstract
1. Introduction
2. Pyrolysis
2.1. Slow Pyrolysis
2.2. Intermediate Pyrolysis
2.3. Fast Pyrolysis
2.4. Catalytic Pyrolysis
3. Pyrolysis Reactors
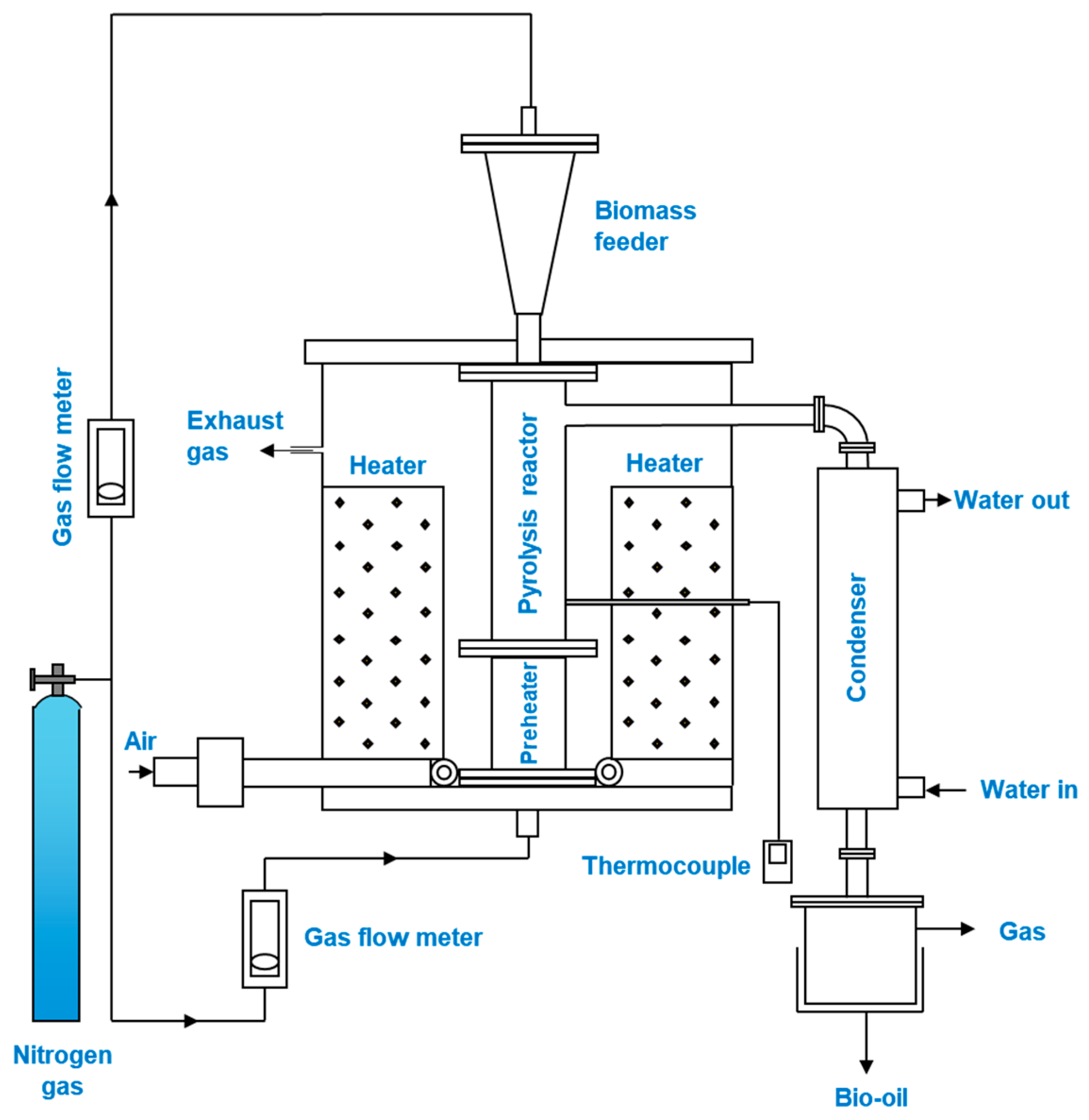
3.1. Fixed-Bed Reactor
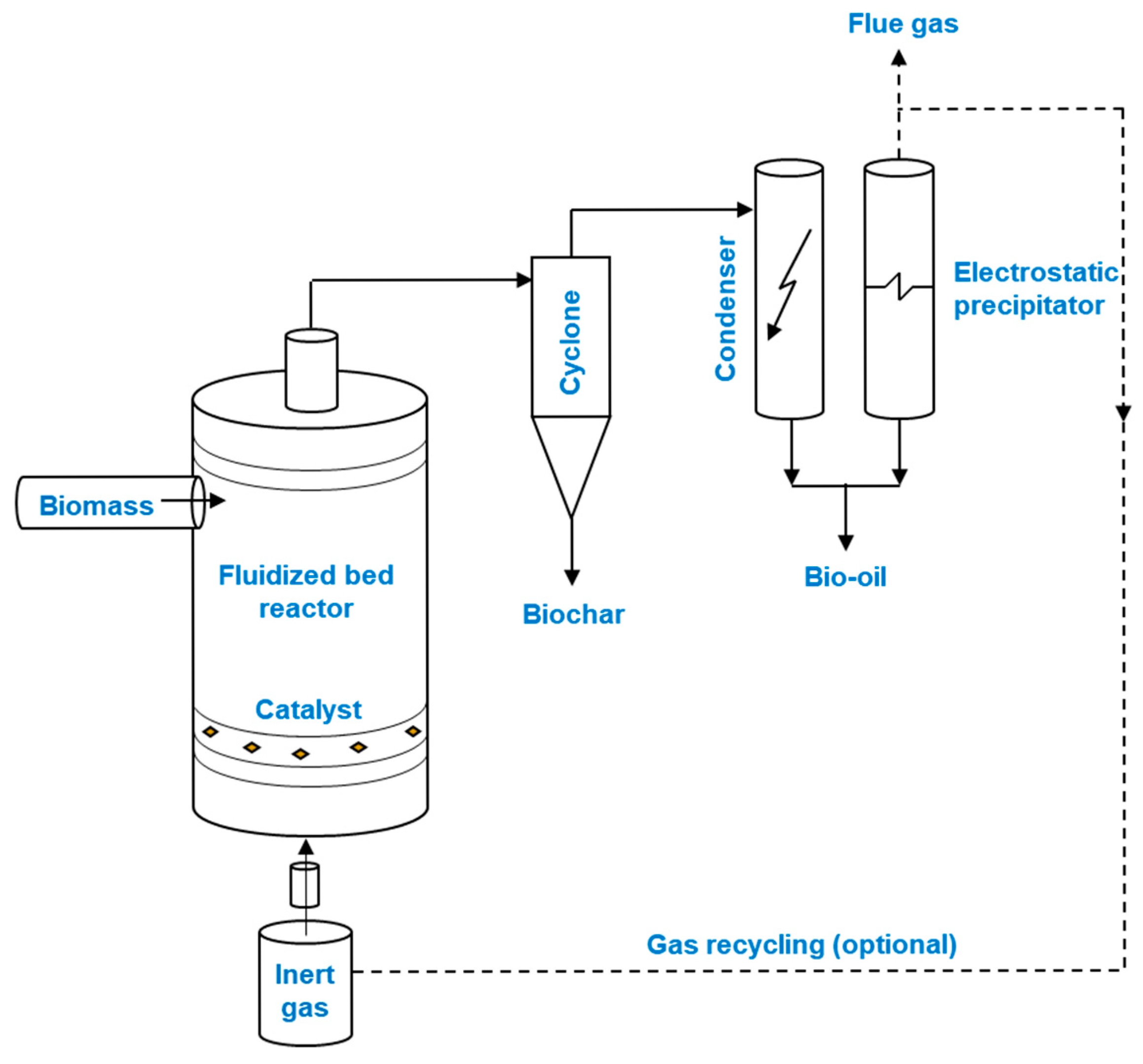
3.2. Fluidized Bed Reactor
3.3. Entrained Flow Reactor
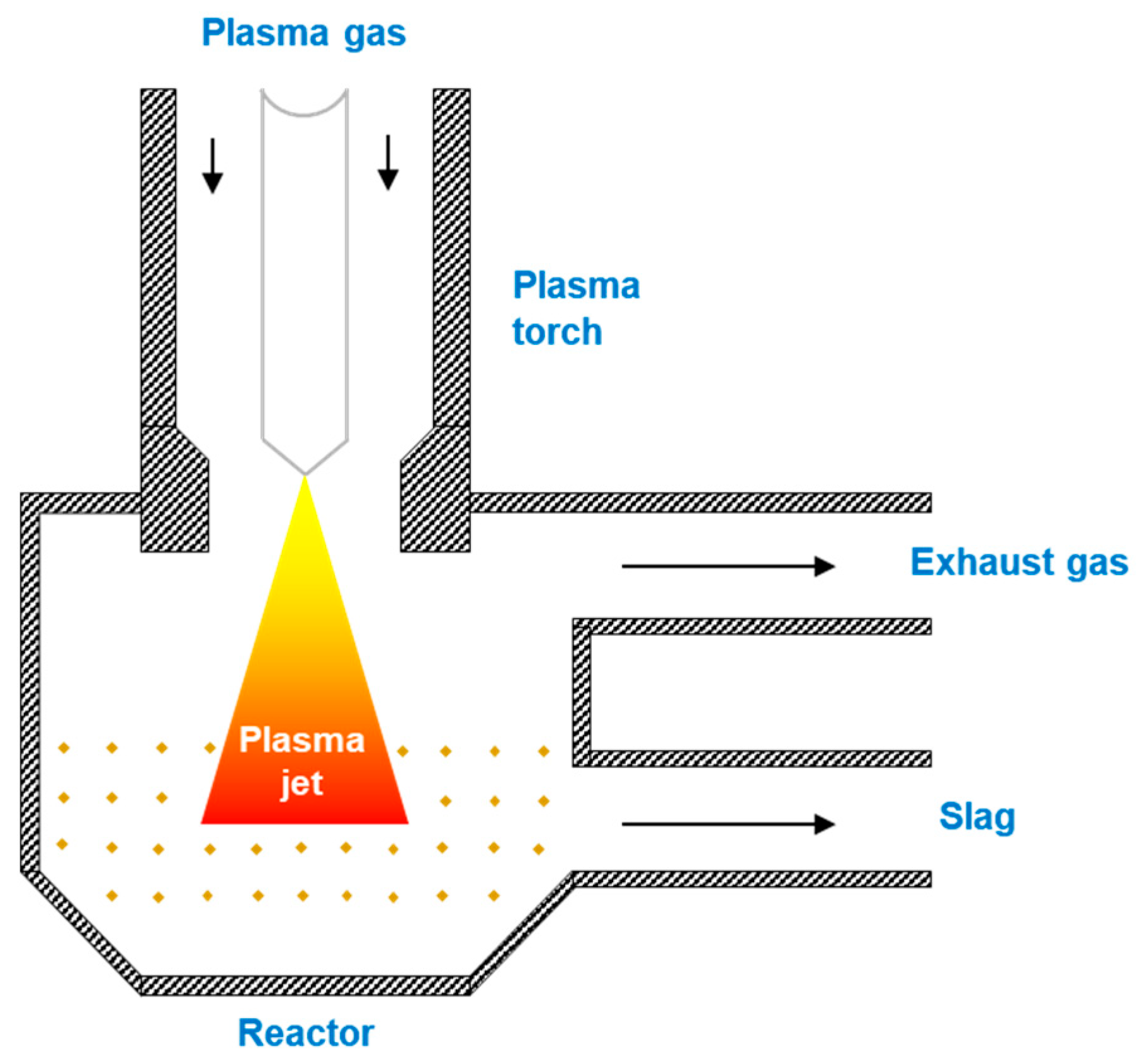
3.4. Plasma Reactor
3.5. Microwave Reactor
4. Pyrolysis Products and Applications
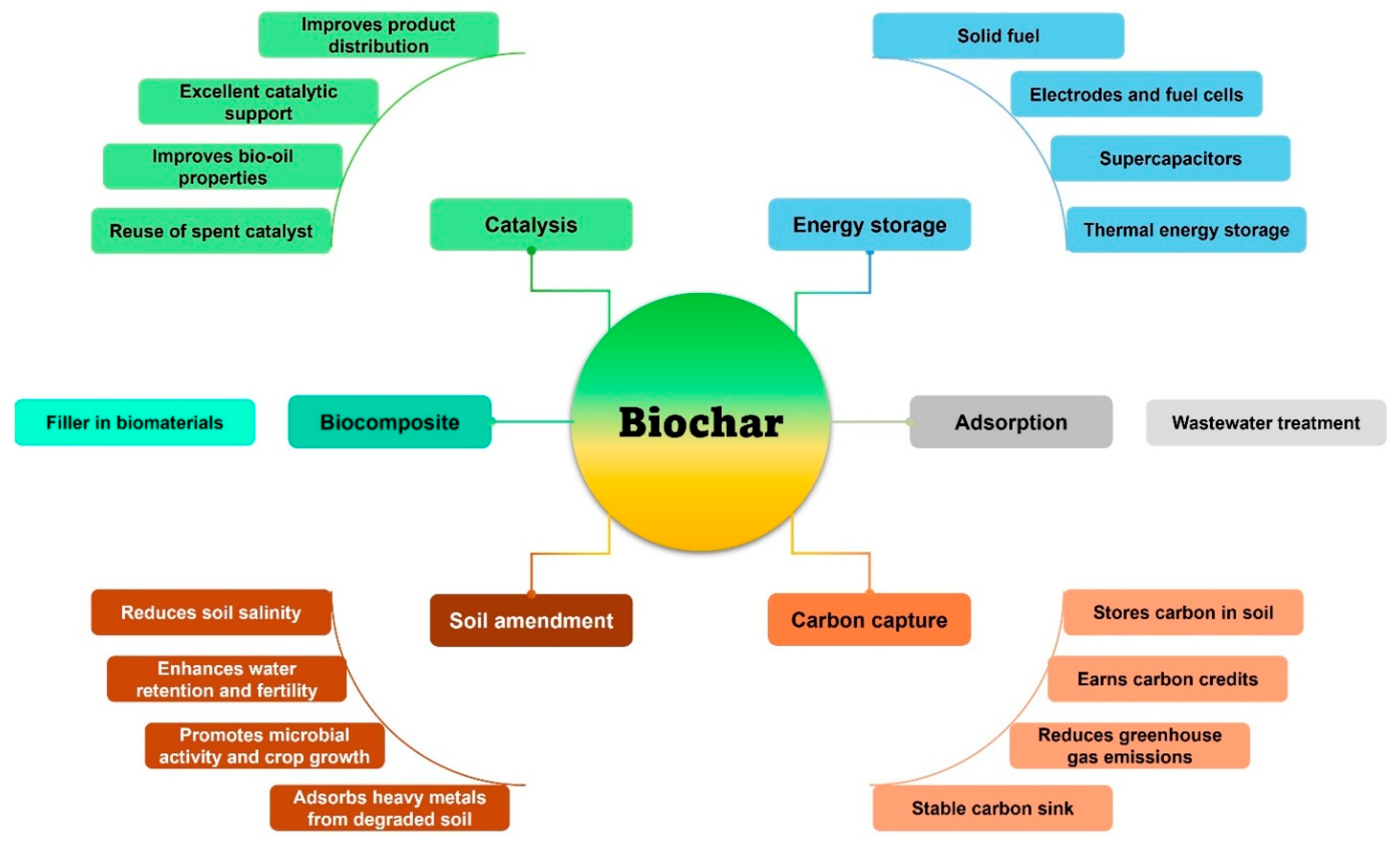
4.1. Biochar
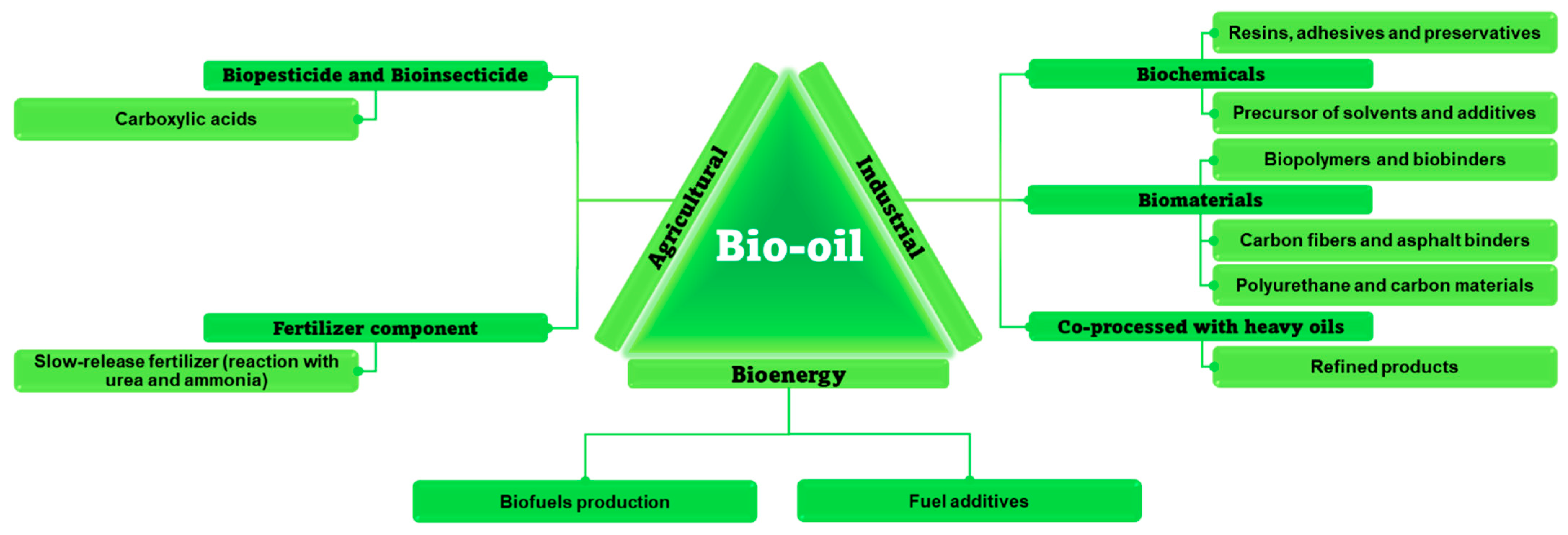
4.2. Bio-Oil
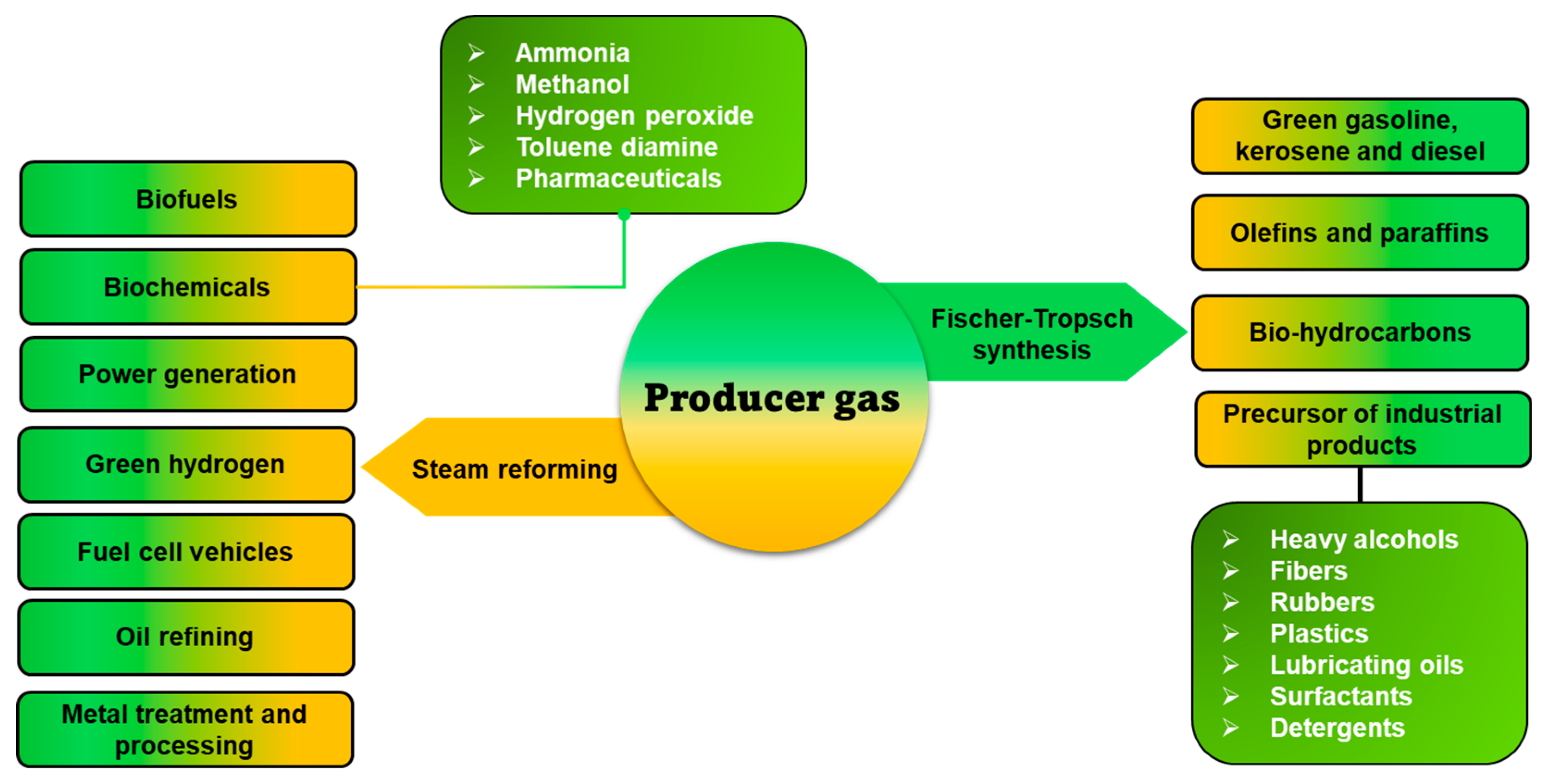
4.3. Producer Gas
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Jha, S.; Nanda, S.; Acharya, B.; Dalai, A.K. A review of thermochemical conversion of waste biomass to biofuels. Energies 2022, 15, 6352. [Google Scholar] [CrossRef]
- Gholizadeh, M.; Li, C.; Zhang, S.; Wang, Y.; Niu, S.; Li, Y.; Hu, X. Progress of the development of reactors for pyrolysis of municipal waste. Sustain. Energy Fuels 2020, 4, 5885–5915. [Google Scholar] [CrossRef]
- Our World in Data. Global Direct Primary Energy Consumption. Available online: https://ourworldindata.org/grapher/global-primary-energy (accessed on 31 May 2025).
- Thiru, S.; Kola, R.; Thimmaraju, M.K.; Dhanalakshmi, C.S.; Sharma, V.; Sakthi, P.; Maguluri, L.P.; Ranganathan, L.; Lalvani, J.I. An analytical characterization study on biofuel obtained from pyrolysis of Madhuca longifolia residues. Sci. Rep. 2024, 14, 14745. [Google Scholar] [CrossRef]
- Our World in Data. Biofuel Energy Production, 2023. Available online: https://ourworldindata.org/grapher/biofuel-production (accessed on 31 May 2025).
- Poornima, S.; Manikandan, S.; Prakash, R.; Deena, S.R.; Subbaiya, R.; Karmegam, N.; Kim, W.; Govarthanan, M. Biofuel and biochemical production through biomass transformation using advanced thermochemical and biochemical processes—A review. Fuel 2024, 372, 132204. [Google Scholar] [CrossRef]
- Nanda, S.; Berruti, F. A technical review of bioenergy and resource recovery from municipal solid waste. J. Hazard. Mater. 2021, 403, 123970. [Google Scholar] [CrossRef]
- Fahmy, T.Y.; Fahmy, Y.; Mobarak, F.; El-Sakhawy, M.; Abou-Zeid, R.E. Biomass pyrolysis: Past, present, and future. Environ. Dev. Sustain. 2020, 22, 17–32. [Google Scholar] [CrossRef]
- Patra, B.R.; Mukherjee, A.; Nanda, S.; Dalai, A.K. Biochar production, activation and adsorptive applications: A review. Environ. Chem. Lett. 2021, 19, 2237–2259. [Google Scholar] [CrossRef]
- Gogoi, D.; Kumar, M.; Lakshmi, Y.G. A comprehensive review on “pyrolysis” for energy recovery. BioEnergy Res. 2023, 16, 1417–1437. [Google Scholar] [CrossRef]
- Joshi, N.C.; Sinha, S.; Bhatnagar, P.; Nath, Y.; Negi, B.; Kumar, V.; Gururani, P. A concise review on waste biomass valorization through thermochemical conversion. Curr. Res. Microb. Sci. 2024, 6, 100237. [Google Scholar] [CrossRef]
- Patra, B.R.; Nanda, S.; Dalai, A.K.; Meda, V. Slow pyrolysis of agro-food wastes and physicochemical characterization of biofuel products. Chemosphere 2021, 285, 131431. [Google Scholar] [CrossRef]
- Wang, S.; Wan, Z.; Han, Y.; Jiao, Y.; Li, Z.; Fu, P.; Li, N.; Zhang, A.; Yi, W. A review on lignin waste valorization by catalytic pyrolysis: Catalyst, reaction system, and industrial symbiosis mode. J. Environ. Chem. Eng. 2023, 11, 109113. [Google Scholar] [CrossRef]
- Bhoi, P.R.; Ouedraogo, A.S.; Soloiu, V.; Quirino, R. Recent advances on catalysts for improving hydrocarbon compounds in bio-oil of biomass catalytic pyrolysis. Renew. Sustain. Energy Rev. 2022, 121, 109676. [Google Scholar] [CrossRef]
- Lee, X.J.; Ong, H.C.; Gan, Y.Y.; Chen, W.H.; Mahlia, T.M. State of art review on conventional and advanced pyrolysis of macroalgae and microalgae for biochar, bio-oil and bio-syngas production. Energy Convers. Manag. 2020, 210, 112707. [Google Scholar] [CrossRef]
- Aboelela, D.; Saleh, H.; Attia, A.M.; Elhenawy, Y.; Majozi, T.; Bassyouni, M. Production and characterization of bio-oil from camelthorn plant using slow pyrolysis. J. Therm. Anal. Calorim. 2024, 149, 10633–10645. [Google Scholar] [CrossRef]
- Boer, F.D.; Pignolet, L.; Valette, J.; Candelier, K.; Commandré, J.M.; Fournier, M.; Thévenon, M.F. Efficacy of slow pyrolysis liquid from sugarcane bagasse for wood protection and its leaching properties. Eur. J. Wood Wood Prod. 2024, 82, 1665–1683. [Google Scholar] [CrossRef]
- Mlonka-Mędrala, A.; Evangelopoulos, P.; Sieradzka, M.; Zajemska, M.; Magdziarz, A. Pyrolysis of agricultural waste biomass towards production of gas fuel and high–quality char: Experimental and numerical investigations. Fuel 2021, 296, 120611. [Google Scholar] [CrossRef]
- Nanda, S.; Dalai, A.K.; Berruti, F.; Kozinski, J.A. Biochar as an exceptional bioresource for energy, agronomy, carbon sequestration, activated carbon and specialty materials. Waste Biomass Valorization 2016, 7, 201–235. [Google Scholar] [CrossRef]
- Safdari, M.S.; Amini, E.; Weise, D.R.; Fletcher, T.H. Heating rate and temperature effects on pyrolysis products from live wildland fuels. Fuel 2019, 242, 295–304. [Google Scholar] [CrossRef]
- Babu, K.K.B.S.; Nataraj, M.; Tayappa, M.; Vyas, Y.; Mishra, R.K.; Acharya, B. Production of biochar from waste biomass using slow pyrolysis: Studies of the effect of pyrolysis temperature and holding time on biochar yield and properties. Mater. Sci. Energy Technol. 2024, 7, 318–334. [Google Scholar]
- del Pozo, C.; Rego, F.; Yang, Y.; Puy, N.; Bartrolí, J.; Fàbregas, E.; Bridgwater, A.V. Converting coffee silverskin to value–added products by a slow pyrolysis–based biorefinery process. Fuel Process. Technol. 2021, 214, 106708. [Google Scholar] [CrossRef]
- Chernova, N.I.; Grigorenko, A.V.; Kiseleva, S.V.; Larina, O.M.; Kumar, V.; Vlaskin, M.S. Comparative evaluation of pyrolysis and hydrothermal liquefaction for obtaining biofuel from a sustainable consortium of microalgae Arthrospira platensis with heterotrophic bacteria. Processes 2022, 10, 2202. [Google Scholar] [CrossRef]
- Bhattacharjee, N.; Biswas, A.B. Pyrolysis of orange bagasse: Comparative study and parametric influence on the product yield and their characterization. J. Environ. Chem. Eng. 2019, 7, 102903. [Google Scholar] [CrossRef]
- Ferreira, M.F.; Oliveira, B.F.; Pinheiro, W.B.; Correa, N.F.; França, L.F.; Ribeiro, N.F. Generation of biofuels by slow pyrolysis of palm empty fruit bunches: Optimization of process variables and characterization of physical–chemical products. Biomass Bioenergy 2020, 140, 105707. [Google Scholar] [CrossRef]
- Baghel, P.; Sakhiya, A.K.; Kaushal, P. Influence of temperature on slow pyrolysis of Prosopis juliflora: An experimental and thermodynamic approach. Renew. Energy 2022, 185, 538–551. [Google Scholar] [CrossRef]
- Vieira, F.R.; Luna, C.M.R.; Arce, G.L.A.F.; Ávila, I. Optimization of slow pyrolysis process parameters using a fixed bed reactor for biochar yield from rice husk. Biomass Bioenergy 2020, 132, 105412. [Google Scholar] [CrossRef]
- Greco, G.; Di Stasi, C.; Rego, F.; González, B.; Manyà, J.J. Effects of slow-pyrolysis conditions on the products yields and properties and on exergy efficiency: A comprehensive assessment for wheat straw. Appl. Energy 2020, 279, 115842. [Google Scholar] [CrossRef]
- Babinszki, B.; Sebestyén, Z.; Jakab, E.; Kőhalmi, L.; Bozi, J.; Várhegyi, G.; Wang, L.; Skreiberg, Ø.; Czégény, Z. Effect of slow pyrolysis conditions on biocarbon yield and properties: Characterization of the volatiles. Bioresour. Technol. 2021, 338, 125567. [Google Scholar] [CrossRef]
- Jerzak, W.; Reinmöller, M.; Magdziarz, A. Estimation of the heat required for intermediate pyrolysis of biomass. Clean Technol. Environ. Policy 2022, 24, 3061–3075. [Google Scholar] [CrossRef]
- Pahnila, M.; Koskela, A.; Sulasalmi, P.; Fabritius, T. A review of pyrolysis technologies and the effect of process parameters on biocarbon properties. Energies 2023, 16, 6936. [Google Scholar] [CrossRef]
- Mohammed, I.Y.; Abakr, Y.A.; Kazi, F.K.; Yusuf, S.; Alshareef, I.; Chin, S.A. Pyrolysis of Napier grass in a fixed bed reactor: Effect of operating conditions on product yields and characteristics. BioResources 2015, 10, 6457–6478. [Google Scholar] [CrossRef]
- Kumar, V.K.; Hallad, S.C.; Panwar, N.L. Thermogravimetric pyrolysis investigation of pistachio shell for its potential of thermal properties, kinetics and thermodynamics. Discov. Energy 2024, 4, 7. [Google Scholar] [CrossRef]
- Kazawadi, D.; Ntalikwa, J.; Kombe, G. A review of intermediate pyrolysis as a technology of biomass conversion for coproduction of biooil and adsorption biochar. J. Renew. Energy 2021, 2021, 533780. [Google Scholar] [CrossRef]
- Ahmed, A.; Bakar, M.S.; Azad, A.K.; Sukri, R.S.; Phusunti, N. Intermediate pyrolysis of Acacia cincinnata and Acacia holosericea species for bio-oil and biochar production. Energy Convers. Manag. 2018, 176, 393–408. [Google Scholar] [CrossRef]
- Paramasivam, B.; Kasimani, R.; Rajamohan, S. Characterization of pyrolysis bio-oil derived from intermediate pyrolysis of Aegle marmelos de–oiled cake: Study on performance and emission characteristics of C.I. engine fueled with Aegle marmelos pyrolysis oil-blends. Environ. Sci. Pollut. Res. 2018, 25, 33806–33819. [Google Scholar] [CrossRef]
- Nyoni, B.; Hlangothi, S. Intermediate pyrolysis of Scenedesmus microalgae in a rotary kiln pyrolyser: Effect of temperature on bio-oil yields and composition. Biomass Convers. Biorefin. 2024, 14, 12155–12165. [Google Scholar] [CrossRef]
- Tinwala, F.; Mohanty, P.; Parmar, S.; Patel, A.; Pant, K.K. Intermediate pyrolysis of agro–industrial biomasses in bench–scale pyrolyser: Product yields and its characterization. Bioresour. Technol. 2015, 188, 258–264. [Google Scholar] [CrossRef]
- Ochieng, R.; Cerón, A.L.; Konist, A.; Sarker, S. Experimental and modeling studies of intermediate pyrolysis of wood in a laboratory–scale continuous feed retort reactor. Bioresour. Technol. Rep. 2023, 24, 101650. [Google Scholar] [CrossRef]
- Torri, I.D.; Paasikallio, V.; Faccini, C.S.; Huff, R.; Caramão, E.B.; Sacon, V.; Oasmaa, A.; Zini, C.A. Bio-oil production of softwood and hardwood forest industry residues through fast and intermediate pyrolysis and its chromatographic characterization. Bioresour. Technol. 2016, 200, 680–690. [Google Scholar] [CrossRef]
- Bouaik, H.; Tabal, A.; Barakat, A.; El Harfi, K.; Aboulkas, A. Optimal parameters and structural composition of bio-oil and biochar from intermediate pyrolysis of red algal biomass. C. R. Chim. 2021, 24, 85–99. [Google Scholar] [CrossRef]
- Ibrahim, M.D.; Abakr, Y.A.; Gan, S.; Lee, L.Y.; Thangalazhy-Gopakumar, S. Intermediate pyrolysis of Bambara groundnut shell (BGS) in various inert gases (N2, CO2, and N2/CO2). Energies 2022, 15, 8421. [Google Scholar] [CrossRef]
- Fardhyanti, D.S.; Megawati Chafidz, A.; Prasetiawan, H.; Raharjo, P.T.; Habibah, U.; Abasaeed, A.E. Production of bio-oil from sugarcane bagasse by fast pyrolysis and removal of phenolic compounds. Biomass Convers. Biorefin. 2024, 14, 217–227. [Google Scholar] [CrossRef]
- SriBala, G.; Carstensen, H.H.; Van Geem, K.M.; Marin, G.B. Measuring biomass fast pyrolysis kinetics: State of the art. Wiley Interdiscip. Rev. Energy Environ. 2019, 8, e326. [Google Scholar] [CrossRef]
- Aboelela, D.; Saleh, H.; Attia, A.M.; Elhenawy, Y.; Majozi, T.; Bassyouni, M. Recent advances in biomass pyrolysis processes for bioenergy production: Optimization of operating conditions. Sustainability 2023, 15, 1238. [Google Scholar] [CrossRef]
- Sotoudehniakarani, F.; Alayat, A.; McDonald, A.G. Characterization and comparison of pyrolysis products from fast pyrolysis of commercial Chlorella vulgaris and cultivated microalgae. J. Anal. Appl. Pyrolysis 2019, 139, 258–273. [Google Scholar] [CrossRef]
- Álvarez-Chávez, B.J.; Godbout, S.; Le Roux, É.; Palacios, J.H.; Raghavan, V. Bio-oil yield and quality enhancement through fast pyrolysis and fractional condensation concepts. Biofuel Res. J. 2019, 6, 1054–1064. [Google Scholar] [CrossRef]
- Alvarez, J.; Hooshdaran, B.; Cortazar, M.; Amutio, M.; Lopez, G.; Freire, F.B.; Haghshenasfard, M.; Hosseini, S.H.; Olazar, M. Valorization of citrus wastes by fast pyrolysis in a conical spouted bed reactor. Fuel 2018, 224, 111–120. [Google Scholar] [CrossRef]
- Adelawon, B.O.; Latinwo, G.K.; Eboibi, B.E.; Agbede, O.O.; Agarry, S.E. Comparison of the slow, fast, and flash pyrolysis of recycled maize-cob biomass waste, Box-Benhken process optimization and characterization studies for the thermal fast pyrolysis production of bioenergy. Chem. Eng. Commun. 2022, 209, 1246–1276. [Google Scholar] [CrossRef]
- Chukwuneke, J.L.; Ewulonu, M.C.; Chukwujike, I.C.; Okolie, P.C. Physico-chemical analysis of pyrolyzed bio-oil from Swietenia macrophylla (mahogany) wood. Heliyon 2019, 5, e01790. [Google Scholar] [CrossRef]
- Hasan, M.M.; Rasul, M.G.; Jahirul, M.I.; Khan, M.M.K. Fast pyrolysis of municipal green waste in an auger reactor: Effects of residence time and particle size on the yield and characteristics of produced oil. Energies 2024, 17, 2914. [Google Scholar] [CrossRef]
- Benamara, I.; Amara, S.; Tabet, F. Modelling and simulation of fast pyrolysis of pomace from three–phase olive mill targeting optimal yields of pyrolysis products. Biofuels 2024, 15, 349–361. [Google Scholar] [CrossRef]
- Maaoui, A.; Trabelsi, A.B.; Abdallah, A.B.; Chagtmi, R.; Lopez, G.; Cortazar, M.; Olazar, M. Assessment of pine wood biomass wastes valorization by pyrolysis with focus on fast pyrolysis biochar production. J. Energy Inst. 2023, 108, 101242. [Google Scholar] [CrossRef]
- Li, P.; Shi, X.; Wang, X.; Song, J.; Fang, S.; Bai, J.; Zhang, G.; Chang, C.; Pang, S. Bio-oil from biomass fast pyrolysis: Yields, related properties and energy consumption analysis of the pyrolysis system. J. Clean. Prod. 2021, 328, 129613. [Google Scholar] [CrossRef]
- Inayat, A.; Ahmed, A.; Tariq, R.; Waris, A.; Jamil, F.; Ahmed, S.F.; Ghenai, C.; Park, Y.K. Techno-economical evaluation of bio-oil production via biomass fast pyrolysis process: A review. Front. Energy Res. 2022, 9, 2021. [Google Scholar] [CrossRef]
- Makepa, D.C.; Chihobo, C.H.; Musademba, D. Advances in sustainable biofuel production from fast pyrolysis of lignocellulosic biomass. Biofuels 2023, 14, 529–550. [Google Scholar] [CrossRef]
- Karkach, B.; Tahiri, M.; Haibi, A.; Bouya, M.; Kifani-Sahban, F. Review on fast pyrolysis of biomass for biofuel production from date palm. Appl. Sci. 2023, 13, 10463. [Google Scholar] [CrossRef]
- Chen, W.; Tao, X.; Shi, X.; Guo, W.; Wang, Y.; Liu, B.; Yang, H. Insight into catalytic effects of alkali metal salts addition on bamboo and cellulose pyrolysis. npj Mater. Sustain. 2024, 2, 25. [Google Scholar] [CrossRef]
- Kang, K.; Nanda, S.; Hu, Y. Current trends in biochar application for catalytic conversion of biomass to biofuels. Catal. Today 2022, 404, 3–18. [Google Scholar] [CrossRef]
- Zhong, N.; Ren, X.; Cheng, L.; Yamamoto, M.; Leskinen, T.; Lommi, J.; Zhu, H.; Granstrom, T.; Saddler, J.; Bi, X. Microwave-assisted catalytic pyrolysis of commercial residual lignin with in–situ catalysts to produce homogenous bio-oil and high–yield biochar with enriched pores. Energy Convers. Manag. 2022, 295, 117620. [Google Scholar] [CrossRef]
- Kanattukara, B.V.; Singh, G.; Sarkar, P.; Chopra, A.; Singh, D.; Mondal, S.; Kapur, G.S.; Ramakumar, S.S. Catalyst-mediated pyrolysis of waste plastics: Tuning yield, composition, and nature of pyrolysis oil. Environ. Sci. Pollut. Res. 2023, 30, 64994–65010. [Google Scholar] [CrossRef]
- Wang, Y.; Akbarzadeh, A.; Chong, L.; Du, J.; Tahir, N.; Awasthi, M.K. Catalytic pyrolysis of lignocellulosic biomass for bio-oil production: A review. Chemosphere 2022, 297, 134181. [Google Scholar] [CrossRef]
- Wang, S.; Cao, B.; Liu, X.; Xu, L.; Hu, Y.; Afonaa-Mensah, S.; Abomohra, A.E.; He, Z.; Wang, Q.; Xu, S. A comparative study on the quality of bio-oil derived from green macroalga Enteromorpha clathrata over metal modified ZSM–5 catalysts. Bioresour. Technol. 2018, 256, 446–455. [Google Scholar] [CrossRef] [PubMed]
- Pattnaik, F.; Patra, B.R.; Okolie, J.A.; Nanda, S.; Dalai, A.K.; Naik, S. A review of thermocatalytic conversion of biogenic wastes into crude biofuels and biochemical precursors. Fuel 2022, 320, 123857. [Google Scholar] [CrossRef]
- Lee, H.W.; Choi, S.J.; Park, S.H.; Jeon, J.K.; Jung, S.C.; Kim, S.C.; Park, Y.K. Pyrolysis and co-pyrolysis of Laminaria japonica and polypropylene over mesoporous Al-SBA-15 catalyst. Nanoscale Res. Lett. 2014, 9, 376. [Google Scholar] [CrossRef]
- Norouzi, O.; Jafarian, S.; Safari, F.; Tavasoli, A.; Nejati, B. Promotion of hydrogen-rich gas and phenolic-rich bio-oil production from green macroalgae Cladophora glomerata via pyrolysis over its bio-char. Bioresour. Technol. 2016, 219, 643–651. [Google Scholar] [CrossRef]
- Gao, L.; Sun, J.; Xu, W.; Xiao, G. Catalytic pyrolysis of natural algae over Mg-Al layered double oxides/ZSM-5 (MgAl-LDO/ZSM-5) for producing bio-oil with low nitrogen content. Bioresour. Technol. 2017, 225, 293–298. [Google Scholar] [CrossRef]
- Kawale, H.D.; Kishore, N. Production of hydrocarbons from a green algae (Oscillatoria) with exploration of its fuel characteristics over different reaction atmospheres. Energy 2019, 178, 344–355. [Google Scholar] [CrossRef]
- Norouzi, O.; Tavasoli, A.; Jafarian, S.; Esmailpour, S. Catalytic upgrading of bio–products derived from pyrolysis of red macroalgae Gracilaria gracilis with a promising novel micro/mesoporous catalyst. Bioresour. Technol. 2017, 243, 1–8. [Google Scholar] [CrossRef]
- Lin, F.; Xu, M.; Ramasamy, K.K.; Li, Z.; Klinger, J.L.; Schaidle, J.A.; Wang, H. Catalyst deactivation and its mitigation during catalytic conversions of biomass. ACS Catal. 2022, 12, 13555–13599. [Google Scholar] [CrossRef]
- Grams, J.; Ruppert, A.M. Catalyst stability—Bottleneck of efficient catalytic pyrolysis. Catalysts 2021, 11, 265. [Google Scholar] [CrossRef]
- Hatta, A.H.; Jalil, A.A.; Hassan, N.S.; Hamid, M.Y.S.; Bahari, M.B.; Aziz, M.A.; Alhassan, M.; Ibrahim, N.; Jusoh, N.W.C.; Hairom, N.H.H. A comprehensive review on the advancements in catalyst regeneration strategies for enhanced reactivity in CO methanation. Mater. Today Chem. 2023, 33, 101743. [Google Scholar] [CrossRef]
- Wang, Y.; Wu, J.J. Thermochemical conversion of biomass: Potential future prospects. Renew. Sustain. Energy Rev. 2023, 187, 113754. [Google Scholar] [CrossRef]
- Marafi, M.; Stanislaus, A. Spent catalyst waste management: A review: Part I—Developments in hydroprocessing catalyst waste reduction and use. Resour. Conserv. Recycl. 2008, 52, 859–873. [Google Scholar] [CrossRef]
- Raza, M.; Inayat, A.; Ahmed, A.; Jamil, F.; Ghenai, C.; Naqvi, S.R.; Shanableh, A.; Ayoub, M.; Waris, A.; Park, Y.-K. Progress of the pyrolyzer reactors and advanced technologies for biomass pyrolysis processing. Sustainability 2021, 13, 11061. [Google Scholar] [CrossRef]
- Ore, O.T.; Adebiyi, F.M. A review on current trends and prospects in the pyrolysis of heavy oils. J. Pet. Explor. Prod. 2021, 11, 1521–1530. [Google Scholar] [CrossRef]
- Brahma, S.; Nath, B.; Basumatary, B.; Das, B.; Saikia, P.; Patir, K.; Basumatar, S. Biodiesel production from mixed oils: A sustainable approach towards industrial biofuel production. Chem. Eng. J. Adv. 2022, 10, 100284. [Google Scholar] [CrossRef]
- Mong, G.R.; Chong, C.T.; Chong, W.W.; Ng, J.H.; Ong, H.C.; Ashokkumar, V.; Tran, M.V.; Karmakar, S.; Goh, B.H.; Yasin, M.F. Progress and challenges in sustainable pyrolysis technology: Reactors, feedstocks and products. Fuel 2022, 324, 124777. [Google Scholar] [CrossRef]
- Feng, X.; Jiang, L.; Li, D.; Tian, S.; Zhu, X.; Wang, H.; He, C.; Li, K. Progress and key challenges in catalytic combustion of lean methane. J. Energy Chem. 2022, 75, 173–215. [Google Scholar] [CrossRef]
- Wang, J.; Ku, X.; Lin, J.; Yang, S. Impact of the reactor structure on biomass pyrolysis in fluidized–bed reactors: A course–grained CFD–DEM study. Energy Fuels 2021, 35, 10035–10050. [Google Scholar] [CrossRef]
- Pienihakkinen, E.; Lindfors, C.; Ohra-aho, T.; Lehtonen, J.; Granström, T.; Yamamoto, M.; Oasmaa, A. Fast pyrolysis of hydrolysis lignin in fluidized bed reactors. Energy Fuels 2021, 35, 14758–14769. [Google Scholar] [CrossRef]
- Zapater, D.; Kulkarni, S.R.; Wery, F.; Cui, M.; Herguido, J.; Menendez, M.; Heynderickx, G.J.; Van Geem, K.M.; Gascon, J.; Castano, P. Multifunctional fluidized bed reactors for process intensification. Prog. Energy Combust. Sci. 2024, 105, 101176. [Google Scholar] [CrossRef]
- Amjad, U.E.; Ishaq, M.; Rehman, H.U.; Ahmad, N.; Sherin, L.; Hussain, M.; Mustafa, M. Diesel and gasoline like fuel production with minimum styrene content from catalytic pyrolysis of polystyrene. Environ. Prog. Sustain. Energy 2021, 40, e13493. [Google Scholar] [CrossRef]
- Bieniek, A.; Sieradzka, M.; Jerzak, W.; Magdziarz, A. Fast pyrolysis of agricultural biomass in drop tube reactor for bio-oil production: Numerical calculations. J. Anal. Appl. Pyrolysis 2023, 176, 106241. [Google Scholar] [CrossRef]
- Hameed, Z.; Aslam, M.; Khan, Z.; Maqsood, K.; Atabani, A.E.; Ghauri, M.; Khurram, M.S.; Rehan, M.; Nizami, A.S. Gasification of municipal solid waste blends with biomass for energy production and resources recovery: Current status, hybrid technologies and innovative prospects. Renew. Sustain. Energy Rev. 2021, 136, 110375. [Google Scholar] [CrossRef]
- Ramani, B.; Anjum, A.; Bramer, E.; Dierkes, W.; Blume, A.; Brem, G. Flash pyrolysis of waste tires in an entrained flow reactor—An experimental study. Polymers 2024, 16, 1746. [Google Scholar] [CrossRef]
- Tang, L.; Huang, H.; Hao, H.; Zhao, K. Development of plasma pyrolysis/gasification systems for energy efficient and environmentally sound waste disposal. J. Electrost. 2013, 71, 839–847. [Google Scholar] [CrossRef]
- Li, Z.; Lin, Q.; Li, M.; Cao, J.; Liu, F.; Pan, H.; Wang, Z.; Kawi, S. Recent advances in process and catalyst for CO2 reforming of methane. Renew. Sustain. Energy Rev. 2020, 134, 110312. [Google Scholar] [CrossRef]
- Li, T.; Rehmet, C.; Cheng, Y.; Jin, Y.; Cheng, Y. Experimental comparison of methane pyrolysis in thermal plasma. Plasma Chem. Plasma Proc. 2017, 37, 1033–1049. [Google Scholar] [CrossRef]
- Lim, M.T.; Tan, E.S.; Chai, Y.H.; Chin, B.L.; Juwono, F.H.; Hisham, D.H.; Anuar, M.A. Pilot-scale flue gas pyrolysis system for organic and plastic wastes with improved liquid properties in a non–thermal plasma reactor. J. Anal. Appl. Pyrolysis 2023, 173, 106062. [Google Scholar] [CrossRef]
- Bhatt, K.P.; Patel, S.; Upadhyay, D.S.; Patel, R.N. Production of hydrogen–rich fuel gas from waste plastics using continuous plasma pyrolysis reactor. J. Environ. Manag. 2024, 356, 120446. [Google Scholar] [CrossRef]
- Putra, P.H.M.; Rozali, S.; Patah, M.F.A.; Idris, A. A review of microwave pyrolysis as a sustainable plastic waste management technique. J. Environ. Manag. 2022, 303, 132204. [Google Scholar] [CrossRef]
- Ke, L.; Zhou, N.; Wu, Q.; Zeng, Y.; Tian, X.; Zhang, J.; Fan, L.; Ruan, R.; Wang, Y. Microwave catalytic pyrolysis of biomass: A review focusing on absorbents and catalysts. npj Mater. Sustain. 2024, 2, 24. [Google Scholar] [CrossRef]
- Jahirul, M.I.; Rasul, M.G.; Schaller, D.; Khan, M.M.; Hasan, M.M.; Hazrat, M.A. Transport fuel from waste plastics pyrolysis—A review on technologies, challenges and opportunities. Energy Convers. Manag. 2022, 258, 115451. [Google Scholar] [CrossRef]
- Iturbides, R.D.; Haza, U.J.; Polaert, I. Recent technological innovations on continuous microwave assisted biomass pyrolysis and perspectives for industrial scale applications. Bioresour. Technol. Rep. 2022, 19, 101202. [Google Scholar]
- Mishra, R.K.; Mohanty, K. A review of the next–generation biochar production from waste biomass for material applications. Sci. Total Environ. 2023, 904, 167171. [Google Scholar] [CrossRef]
- Shen, C.; Song, Z.; Qiu, Z.; Gao, R. Effects of pyrolysis temperature on drinking water treatment residual biochar properties and organophosphorus pesticides adsorption. Chem. Pap. 2024, 78, 5289–5299. [Google Scholar] [CrossRef]
- Chen, D.; Xu, J.; Ling, P.; Fang, Z.; Ren, Q.; Xu, K.; Jiang, L.; Wang, Y.; Su, S.; Hu, S.; et al. Formation and evolution mechanism of persistent free radicals in biochar during biomass pyrolysis: Insights from biochar’s element composition and chemical structure. Fuel 2024, 357, 129910. [Google Scholar] [CrossRef]
- Wu, Y.; Yan, Y.; Wang, Z.; Tan, Z.; Zhou, T. Biochar application for the remediation of soil contaminated with potentially toxic elements: Current situation and challenges. J. Environ. Manag. 2024, 351, 119775. [Google Scholar] [CrossRef] [PubMed]
- Bilias, F.; Kalderis, D.; Richardson, C.; Barbayiannis, N.; Gasparatos, D. Biochar application as a soil potassium management strategy: A review. Sci. Total Environ. 2023, 858, 159782. [Google Scholar] [CrossRef]
- Jiang, Y.; Li, T.; Xu, X.; Sun, J.; Pan, G.; Cheng, K. A global assessment of the long–term effects of biochar application on crop yield. Curr. Res. Environ. Sustain. 2024, 7, 100247. [Google Scholar] [CrossRef]
- Kumar, A.; Bhattacharya, T.; Shaikh, W.A.; Roy, A. Sustainable soil management under drought stress through biochar application: Immobilizing arsenic, ameliorating soil quality, and augmenting plant growth. Environ. Res. 2024, 259, 119531. [Google Scholar] [CrossRef]
- Yadav, A.; Yadav, P.; Bojjagani, S.; Srivastava, J.K.; Raj, A. Investigation of the speciation and environmental risk of heavy metals in biochar produced from textile sludge waste by pyrolysis at different temperatures. Chemosphere 2024, 360, 142454. [Google Scholar] [CrossRef] [PubMed]
- Chi, W.; Nan, Q.; Liu, Y.; Dong, D.; Qin, Y.; Li, S.; Wu, W. Stress resistance enhancing with biochar application and promotion on crop growth. Biochar 2024, 6, 43. [Google Scholar] [CrossRef]
- Rawat, S.; Wang, C.T.; Lay, C.H.; Hotha, S.; Bhaskar, T. Sustainable biochar for advanced electrochemical/energy storage applications. J. Energy Storage 2023, 63, 107115. [Google Scholar] [CrossRef]
- Sarker, T.R.; Ethen, D.Z.; Nanda, S. Decarbonization of metallurgy and steelmaking industries using biochar: A review. Chem. Eng. Technol. 2024, 47, e202400217. [Google Scholar] [CrossRef]
- Gupta, M.; Savla, N.; Pandit, C.; Pandit, S.; Gupta, P.K.; Pant, M.; Khilari, S.; Kumar, Y.; Agarwal, D.; Nair, R.R.; et al. Use of biomass–derived biochar in wastewater treatment and power production: A promising solution for a sustainable environment. Sci. Total Environ. 2022, 825, 153892. [Google Scholar] [CrossRef]
- Wang, X.; Guo, Z.; Hu, Z.; Zhang, J. Recent advances in biochar application for water and wastewater treatment: A review. PeerJ 2020, 8, e9164. [Google Scholar] [CrossRef]
- Liu, X.; Chen, Z.; Lu, S.; Shi, X.; Qu, F.; Cheng, D.; Wei, W.; Shon, H.K.; Ni, B.J. Persistent free radicals on biochar for its catalytic capability: A review. Water Res. 2024, 250, 120999. [Google Scholar] [CrossRef]
- Chen, H.; Xu, H.; Zhu, H.; Yan, S.; Zhang, S.; Zhang, H.; Guo, X.; Hu, X.; Gao, W. A review on bioslurry fuels derived from bio-oil and biochar: Preparation, fuel properties and application. Fuel 2024, 355, 129283. [Google Scholar] [CrossRef]
- Neina, D.; Agyarko-Mintah, E. The Terra Preta Model soil for sustainable sedentary yam production in West Africa. Heliyon 2023, 9, e15896. [Google Scholar] [CrossRef]
- Shoudho, K.N.; Khan, T.H.; Ara, U.R.; Khan, M.R.; Shawon, Z.B.Z.; Hoque, M.E. Biochar in global carbon cycle: Towards sustainable development goals. Curr. Res. Green Sustain. Chem. 2024, 8, 100409. [Google Scholar] [CrossRef]
- Alhashimi, H.A.; Aktas, C.B. Life cycle environmental and economic performance of biochar compared with activated carbon: A meta-analysis. Resour. Conserv. Recycl. 2017, 118, 13–26. [Google Scholar] [CrossRef]
- Hamedani, S.R.; Kuppens, T.; Malina, R.; Bocci, E.; Colantoni, A.; Villarini, M. Life cycle assessment and environmental valuation of biochar production: Two case studies in Belgium. Energies 2019, 12, 2166. [Google Scholar] [CrossRef]
- Sahoo, K.; Upadhyay, A.; Runge, T.; Bergman, R.; Puettmann, M.; Bilek, E. Life-cycle assessment and techno-economic analysis of biochar produced from forest residues using portable systems. Int. J. Life Cycle Assess. 2021, 26, 189–213. [Google Scholar] [CrossRef]
- Osman, A.I.; Farghali, M.; Rashwan, A.K. Life cycle assessment of biochar as a green sorbent for soil remediation. Curr. Opin. Green Sustain. Chem. 2024, 46, 100882. [Google Scholar] [CrossRef]
- Okolie, J.A.; Tabat, M.E.; Gunes, B.; Epelle, E.I.; Mukherjee, A.; Nanda, S.; Dalai, A.K. A techno-economic assessment of biomethane and bioethanol production from crude glycerol through integrated hydrothermal gasification, syngas fermentation and biomethanation. Energy Convers. Manag. X 2021, 12, 100131. [Google Scholar] [CrossRef]
- Okolie, J.A.; Nanda, S.; Dalai, A.K.; Kozinski, J.A. Techno-economic evaluation and sensitivity analysis of a conceptual design for supercritical water gasification of soybean straw to produce hydrogen. Bioresour. Technol. 2021, 331, 125005. [Google Scholar] [CrossRef]
- Mohanty, A.; Ajmera, S.; Chinnam, S.; Kumar, V.; Mishra, R.K.; Acharya, B. Pyrolysis of waste oils for biofuel production: An economic and life cycle assessment. Fuel Commun. 2024, 18, 100108. [Google Scholar] [CrossRef]
- Alherbawi, M.; Parthasarathy, P.; Elkhalifa, S.; Al-Ansari, T.; McKay, G. Techno-economic and environmental analyses of the pyrolysis of food waste to produce bio-products. Heliyon 2024, 10, e27713. [Google Scholar] [CrossRef]
- Dai, L.; Wang, Y.; Liu, Y.; Ruan, R.; He, C.; Yu, Z.; Jiang, L.; Zeng, Z.; Tian, X. Integrated process of lignocellulosic biomass torrefaction and pyrolysis for upgrading bio-oil production: A state–of–the–art review. Renew. Sustain. Energy Rev. 2019, 107, 20–36. [Google Scholar] [CrossRef]
- Qing, M.; Long, Y.; Liu, L.; Yi, Y.; Li, W.; He, R.; Yin, Y.; Tian, H.; He, J.; Cheng, S.; et al. Pyrolysis of the food waste collected from catering and households under different temperatures: Assessing the evolution of char structure and bio-oil composition. J. Anal. Appl. Pyrolysis 2022, 164, 105543. [Google Scholar] [CrossRef]
- Qamar, O.A.; Jamil, F.; Hussain, M.; Ala’a, H.; Inayat, A.; Waris, A.; Akhter, P.; Park, Y.K. Feasibility-to-applications of value-added products from biomass: Current trends, challenges, and prospects. Chem. Eng. J. 2023, 454, 140240. [Google Scholar] [CrossRef]
- Panwar, N.L.; Paul, A.S. An overview of recent development in bio-oil upgrading and separation techniques. Environ. Eng. Res. 2021, 26, 200382. [Google Scholar] [CrossRef]
- Xiong, Z.; Syed-Hassan, S.S.; Xu, J.; Wang, Y.; Hu, S.; Su, S.; Zhang, S.; Xiang, J. Evolution of coke structures during the pyrolysis of bio-oil at various temperatures and heating rates. J. Anal. Appl. Pyrolysis 2018, 134, 336–342. [Google Scholar] [CrossRef]
- Dada, K.T.; Sheehan, M.; Murugavelh, S.; Antunes, E. A review on catalytic pyrolysis for high–quality bio-oil production from biomass. Biomass Convers. Biorefin. 2021, 13, 2595–2614. [Google Scholar] [CrossRef]
- Liu, R.; Rahman, M.M.; Sarker, M.; Chai, M.; Li, C.; Cai, J. A review on the catalytic pyrolysis of biomass for the bio-oil production with ZSM-5: Focus on structure. Fuel Process. Technol. 2020, 199, 106301. [Google Scholar]
- Zhao, X.; Wei, L.; Cheng, S.; Julson, J. Review of heterogeneous catalysts for catalytically upgrading vegetable oils into hydrocarbon biofuels. Catalysts 2017, 7, 83. [Google Scholar] [CrossRef]
- Rjeily, M.A.; Gennequin, C.; Pron, H.; Abi-Aad, E.; Randrianalisoa, J.H. Pyrolysis-catalytic upgrading of bio-oil and pyrolysis-catalytic steam reforming of biogas: A review. Environ. Chem. Lett. 2021, 19, 2825–2872. [Google Scholar] [CrossRef]
- Chen, Z.; Wang, D.; Yang, H.; Zhang, Y.; Li, Y.; Li, C.; Yu, J.; Gao, S. Novel application of red mud as disposal catalyst for pyrolysis and gasification of coal. Carbon Resour. Convers. 2021, 4, 10–18. [Google Scholar] [CrossRef]
- Xu, G.X.; Nanda, S.; Guo, J.J.; Song, Y.Q.; Kozinski, J.A.; Dalai, A.K.; Fang, Z. Red mud supported Ni-Cu bimetallic material for hydrothermal production of hydrogen from biomass. Ind. Crops Prod. 2024, 212, 118370. [Google Scholar] [CrossRef]
- An, X.; Tang, C.; Liu, J.; Song, X.; Huang, C. Catalytic pyrolysis of biomass using desulfurization ash: Enhanced bio-oil yields and reaction pathway modulation. Energy 2025, 330, 136817. [Google Scholar] [CrossRef]
- Lu, J.; Guo, S.; Fu, Y.; Chang, J. Catalytic upgrading of bio-oil by simultaneous esterification and alkylation with azeotropic water removal. Fuel Process. Technol. 2017, 161, 193–198. [Google Scholar] [CrossRef]
- Park, Y.K.; Ha, J.M.; Oh, S.; Lee, J. Bio-oil upgrading through hydrogen transfer reactions in supercritical solvents. Chem. Eng. J. 2021, 404, 126527. [Google Scholar] [CrossRef]
- Shao, S.; Zhang, P.; Li, X.; Yu, Y. Steam reforming of the simulated aqueous fraction of bio-oil based on pre-reforming with dolomite. Fuel 2023, 344, 128116. [Google Scholar] [CrossRef]
- Lin, B.J.; Chen, W.H.; Budzianowski, W.M.; Hsieh, C.T.; Lin, P.H. Emulsification analysis of bio-oil and diesel under various combinations of emulsifiers. Appl. Energy 2016, 178, 746–757. [Google Scholar] [CrossRef]
- Drugkar, K.; Rathod, W.; Sharma, T.; Sharma, A.; Joshi, J.; Pareek, V.K.; Ledwani, L.; Diwekar, U. Advanced separation strategies for up-gradation of bio-oil into value-added chemicals: A comprehensive review. Sep. Purif. Technol. 2022, 283, 120149. [Google Scholar] [CrossRef]
- Lachos-Perez, D.; Martins-Vieira, J.C.; Missau, J.; Anshu, K.; Siakpebru, O.K.; Thengane, S.K.; Morais, A.R.; Tanabe, E.H.; Bertuol, D.A. Review on biomass pyrolysis with a focus on bio-oil upgrading techniques. Analytica 2023, 4, 182–205. [Google Scholar] [CrossRef]
- Gupta, S.; Mondal, P.; Borugadda, V.B.; Dalai, A.K. Advances in upgradation of pyrolysis bio-oil and biochar towards improvement in bio–refinery economics: A comprehensive review. Environ. Technol. Innov. 2021, 21, 101276. [Google Scholar] [CrossRef]
- Li, H.; Mahmood, N.; Ma, Z.; Zhu, M.; Wang, J.; Zheng, J.; Yuan, Z.; Wei, Q.; Xu, C.C. Preparation and characterization of bio-polyol and bio-based flexible polyurethane foams from fast pyrolysis of wheat straw. Ind. Crops Prod. 2017, 103, 64–72. [Google Scholar] [CrossRef]
- Palmay, P.; Puente, C.; Haro, C.; Bruno, J.C.; Coronas, A. Bio oil as cutter stock in fuel oil blends for industrial applications. Energies 2023, 16, 1485. [Google Scholar] [CrossRef]
- Zhang, Z.; Fang, Y.; Yang, J.; Li, X. A comprehensive review of bio-oil, bio-binder and bio-asphalt materials: Their source, composition, preparation and performance. J. Traffic Transp. Eng. (Engl. Ed.) 2022, 9, 151–166. [Google Scholar] [CrossRef]
- Abd-Elnabi, A.D.; El-sawy, E.A.F.; El-Adawy, E.M. Insecticidal effects of the fast pyrolysis bio-oil against Spodoptera littoralis and Aphis gossypii insect pests. J. Asia-Pac. Entomol. 2024, 27, 102237. [Google Scholar] [CrossRef]
- Yang, Q.; Liu, P.; Dong, S.; Zhang, J.; Zhao, B. Combined application of organic and inorganic fertilizers mitigates ammonia and nitrous oxide emissions in a maize field. Nutr. Cycl. Agroecol. 2020, 117, 13–27. [Google Scholar] [CrossRef]
- Yek, P.N.; Chan, Y.H.; Foong, S.Y.; Mahari, W.A.; Chen, X.; Liew, R.K.; Ma, N.L.; Tsang, Y.F.; Sonne, C.; Cheng, Y.W.; et al. Co-processing plastics waste and biomass by pyrolysis-gasification: A review. Environ. Chem. Lett. 2024, 22, 171–188. [Google Scholar] [CrossRef]
- Zadeh, Z.E.; Abdulkhani, A.; Aboelazayem, O.; Saha, B. Recent insights into lignocellulosic biomass pyrolysis: A critical review on pretreatment, characterization, and products upgrading. Processes 2020, 8, 799. [Google Scholar] [CrossRef]
- Zhang, Y.; Liang, Y.; Li, S.; Yuan, Y.; Zhang, D.; Wu, Y.; Xie, H.; Brindhadevi, K.; Pugazhendhi, A.; Xia, C. A review of biomass pyrolysis gas: Forming mechanisms, influencing parameters, and product application upgrades. Fuel 2023, 347, 128461. [Google Scholar] [CrossRef]
- Afraz, M.; Muhammad, F.; Nisar, J.; Shah, A.; Munir, S.; Ali, G.; Ahmad, A. Production of value-added products from biomass waste by pyrolysis: An updated review. Waste Manag. Bull. 2024, 1, 30–40. [Google Scholar] [CrossRef]
- Xia, C.; Cai, L.; Zhang, H.; Zuo, L.; Shi, S.Q.; Lam, S.S. A review on the modeling and validation of biomass pyrolysis with a focus on product yield and composition. Biofuel Res. J. 2021, 8, 1296–1315. [Google Scholar] [CrossRef]
- Rajpoot, L.; Tagade, A.; Deshpande, G.; Verma, K.; Geed, S.R.; Patle, D.S.; Sawarkar, A.N. An overview of pyrolysis of de–oiled cakes for the production of biochar, bio-oil, and pyro–gas: Current status, challenges, and future perspective. Bioresour. Technol. Rep. 2022, 19, 101205. [Google Scholar] [CrossRef]
- Bayat, F.; Pirbazari, S.M.; Shojaei, N.; Kiani, S.; Tavasoli, A. Green catalyst innovation: Enhanced Fischer-Tropsch synthesis using potassium-promoted cobalt catalysts supported on pyrolyzed peanut shells and Cladophora glomerata modified biochars. Fuel Process. Technol. 2024, 258, 108094. [Google Scholar] [CrossRef]
- Yousefian, F.; Babatabar, M.A.; Eshaghi, M.; Poor, S.M.; Tavasoli, A. Pyrolysis of rice husk, coconut shell, and Cladophora glomerata algae and application of the produced biochars as support for cobalt catalyst in Fischer-Tropsch synthesis. Fuel Process. Technol. 2023, 247, 107818. [Google Scholar] [CrossRef]
- Nguyen-Thi, T.X.; Nguyen, P.Q.; Tran, V.D.; Ağbulut, Ü.; Nguyen, L.H.; Balasubramanian, D.; Tarelko, W.; Bandh, S.A.; Pham, N.D. Recent advances in hydrogen production from biomass waste with a focus on pyrolysis and gasification. Int. J. Hydrogen Energy 2024, 54, 127–160. [Google Scholar] [CrossRef]
- Martinelli, M.; Gnanamani, M.K.; LeViness, S.; Jacobs, G.; Shafer, W.D. An overview of Fischer–Tropsch Synthesis: XtL processes, catalysts and reactors. Appl. Catal. A Gen. 2020, 608, 117740. [Google Scholar] [CrossRef]
- dos Santos, R.G.; Alencar, A.C. Biomass-derived syngas production via gasification process and its catalytic conversion into fuels by Fischer Tropsch synthesis: A review. Int. J. Hydrogen Energy 2020, 45, 18114–18132. [Google Scholar] [CrossRef]
- de Oliveira, D.C.; Lora, E.E.; Venturini, O.J.; Maya, D.M.; Garcia-Pérez, M. Gas cleaning systems for integrating biomass gasification with Fischer-Tropsch synthesis—A review of impurity removal processes and their sequences. Renew. Sustain. Energy Rev. 2023, 172, 113047. [Google Scholar] [CrossRef]
- Jenčík, J.; Hönig, V.; Obergruber, M.; Hájek, J.; Vráblík, A.; Černý, R.; Schlehöfer, D.; Herink, T. Advanced biofuels based on Fischer-Tropsch synthesis for applications in diesel engines. Materials 2021, 14, 3077. [Google Scholar] [CrossRef]
- Chen, Y.; Wei, J.; Duyar, M.S.; Ordomsky, V.V.; Khodakov, A.Y.; Liu, J. Carbon-based catalysts for Fischer-Tropsch synthesis. Chem. Soc. Rev. 2011, 50, 2337–2366. [Google Scholar] [CrossRef]
- Aziz, M.; Darmawan, A.; Juangsa, F.B. Hydrogen production from biomasses and wastes: A technological review. Int. J. Hydrogen Energy 2021, 46, 33756–33781. [Google Scholar] [CrossRef]
- Foong, S.Y.; Chan, Y.H.; Cheah, W.Y.; Kamaludin, N.H.; Ibrahim, T.N.; Sonne, C.; Peng, W.; Show, P.L.; Lam, S.S. Progress in waste valorization using advanced pyrolysis techniques for hydrogen and gaseous fuel production. Bioresour. Technol. 2021, 320, 124299. [Google Scholar] [CrossRef]
- Vuppaladadiyam, A.K.; Vuppaladadiyam, S.S.; Awasthi, A.; Sahoo, A.; Rehman, S.; Pant, K.K.; Murugavelh, S.; Huang, Q.; Anthony, E.; Fennel, P.; et al. Biomass pyrolysis: A review on recent advancements and green hydrogen production. Bioresour. Technol. 2022, 364, 128087. [Google Scholar] [CrossRef]
- Yuan, T.; Zhao, J.N.; Bao, N.R. Hydrogen applications: Advances in the field of medical therapy. Med. Gas. Res. 2022, 12, 99–107. [Google Scholar] [CrossRef]
- Bezergianni, S.; Dimitriadis, A.; Kikhtyanin, O.; Kubička, D. Refinery co-processing of renewable feeds. Prog. Energy Combust. Sci. 2018, 68, 29–64. [Google Scholar] [CrossRef]
- Singh, S.V.; Ming, Z.; Fennell, P.S.; Shah, N.; Anthony, E.J. Progress in biofuel production from gasification. Prog. Energy Combust. Sci. 2017, 61, 189–248. [Google Scholar]
- Mondal, P.; Dang, G.S.; Garg, M.O. Syngas production through gasification and cleanup for downstream applications—Recent developments. Fuel Process. Technol. 2011, 92, 1395–1410. [Google Scholar] [CrossRef]
- Oreggioni, G.D.; Brandani, S.; Luberti, M.; Baykan, Y.; Friedrich, D.; Ahn, H. CO2 capture from syngas by an adsorption process at a biomass gasification CHP plant: Its comparison with amine-based CO2 capture. Int. J. Greenh. Gas Control 2015, 35, 71–81. [Google Scholar] [CrossRef]


| Feedstock | Process Conditions | Biochar Yield (wt%) | Calorific Value of Biochar (MJ/kg) | Bio-Oil Yield (wt%) | Calorific Value of Bio-Oil (MJ/kg) | Producer Gas Yield (wt%) | Reference |
|---|---|---|---|---|---|---|---|
| Coconut husk waste |
| 41–29 | 21–24 | - | - | - | Babu et al. [21] |
| Coffee silverskin |
| 32–81 | 23–24 | 18–23 | - | 15–30 | del Pozo et al. [22] |
| Cyanobacteria (Arthrospira platensis) |
| 27 | - | 22 | - | 17–39 | Chernova et al. [23] |
| Food waste |
| 28–52 | 21–23 | 36 | - | 36 | Patra et al. [12] |
| Grape pomace |
| 54–94 | 23–24 | 6–15 | - | 7–25 | del Pozo et al. [22] |
| Madhuca longifolia biomass |
| 42–10 | - | 33–42 | - | 25–52 | Thiru et al. [4] |
| Orange bagasse |
| 33 | 27 | 28 | 22 | 31 | Bhattacharjee and Biswas [24] |
| Palm empty fruit bunches |
| 28 | 19 | 45 | 25 | 28 | Ferreira et al. [25] |
| Wood waste |
| 36–22 | 25–32 | - | - | - | Babu et al. [21] |
| Feedstock | Process Conditions | Biochar Yield (wt%) | Bio-Oil Yield (wt%) | Calorific Value of Bio-Oil (MJ/kg) | Producer Gas Yield (wt%) | Reference |
|---|---|---|---|---|---|---|
| Acacia cincinnata trunk |
| 32 | 53 | 24 | 15 | Ahmed et al. [35] |
| Acacia cincinnata phyllodes |
| 35 | 45 | 31 | 20 | Ahmed et al. [35] |
| Acacia holosericea phyllodes |
| 39 | 41 | 28 | 20 | Ahmed et al. [35] |
| Aegle marmelos de-oiled cake |
| 24–27 | 42–55 | 41 | 32–35 | Paramasivam et al. [36] |
| Microalgae (Scenedesmus) |
| - | 15–40 | - | - | Nyoni and Hlangothi [37] |
| Sawdust |
| 29 | 37 | 39 | 34 | Tinwala et al. [38] |
| Woody biomass |
| 29 | 44 | - | 28 | Ochieng et al. [39] |
| Feedstock | Process Conditions | Biochar Yield (wt%) | Calorific Value of Biochar (MJ/kg) | Bio-Oil Yield (wt%) | Calorific Value of Bio-Oil (MJ/kg) | Producer Gas Yield (wt%) | Reference |
|---|---|---|---|---|---|---|---|
| Algae (Chlorella vulgaris) |
| 43 | 26–32 | 48 | 27–32 | - | Sotoudehniakarani et al. [46] |
| Black spruce |
| 39 | - | 25 | - | 37 | Álvarez-Chávez et al. [47] |
| Citrus waste |
| 33–27 | 26–28 | 55 | - | 12–24 | Alvarez et al. [48] |
| Corn cob |
| 33 | - | 43 | - | 24 | Adelawon et al. [49] |
| Mahogany wood waste |
| 34 | - | 70 | 30 | 10–38 | Chukwuneke et al. [50] |
| Municipal solid waste |
| 27 | - | 40 | 25 | 33 | Hasan et al. [51] |
| Olive husk |
| 10 | - | 60 | - | 30 | Benamara et al. [52] |
| Olive wood |
| 25 | - | 30 | - | 35 | Benamara et al. [52] |
| Pinecone (Pinus halepensis) |
| 18–32 | 24–27 | - | - | - | Maaoui et al. [53] |
| Pinecone (Pinus pinea) |
| 25–32 | 22–26 | - | - | - | Maaoui et al. [53] |
| Feedstock | Process Conditions | Biochar Yield (wt%) | Bio-Oil Yield (wt%) | Producer Gas Yield (wt%) | Reference |
|---|---|---|---|---|---|
| Algae (Laminaria japonica) and polypropylene |
| 42 | 31 | 25 | Lee et al. [65] |
| Algae (Chlorella vulgaris) |
| 26 | 53 | 22 | Bhoi et al. [14] |
| Algae (Cladophora glomerata) |
| 35 | 30 | 35 | Norouzi et al. [66] |
| Cyanobacteria |
| 24 | 36 | 39 | Gao et al. [67] |
| Cyanobacteria (Oscillatoria) |
| 43 | 33 | 27 | Kawale and Kishore [68] |
| Seaweed (Enteromorpha clathrata) |
| 46 | 37 | 23 | Wang et al. [63] |
| Seaweed (Gracilaria gracilis) |
| 36 | 39 | 27 | Norouzi et al. [69] |
| Reactor | Advantages | Limitations |
|---|---|---|
| Fixed bed |
|
|
| Fluidized bed |
|
|
| Entrained flow |
|
|
| Plasma |
|
|
| Microwave |
|
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Talwar, P.; Agudelo, M.A.; Nanda, S. Pyrolysis Process, Reactors, Products, and Applications: A Review. Energies 2025, 18, 2979. https://doi.org/10.3390/en18112979
Talwar P, Agudelo MA, Nanda S. Pyrolysis Process, Reactors, Products, and Applications: A Review. Energies. 2025; 18(11):2979. https://doi.org/10.3390/en18112979
Chicago/Turabian StyleTalwar, Prakhar, Mariana Alzate Agudelo, and Sonil Nanda. 2025. "Pyrolysis Process, Reactors, Products, and Applications: A Review" Energies 18, no. 11: 2979. https://doi.org/10.3390/en18112979
APA StyleTalwar, P., Agudelo, M. A., & Nanda, S. (2025). Pyrolysis Process, Reactors, Products, and Applications: A Review. Energies, 18(11), 2979. https://doi.org/10.3390/en18112979






