Study on the Influence of Temperature Distribution in Thermite Plugging Abandoned Well Technology
Abstract
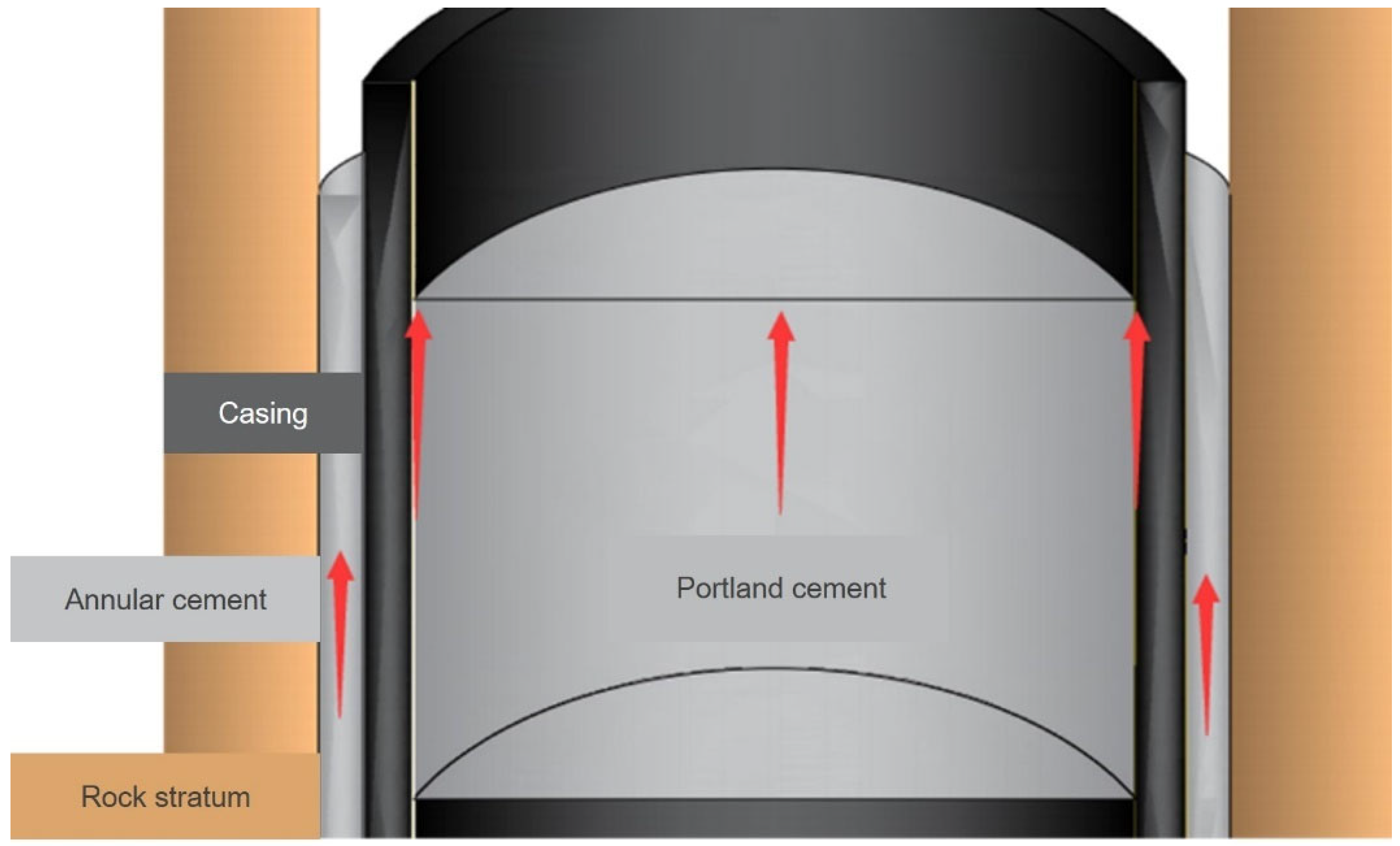
1. Introduction
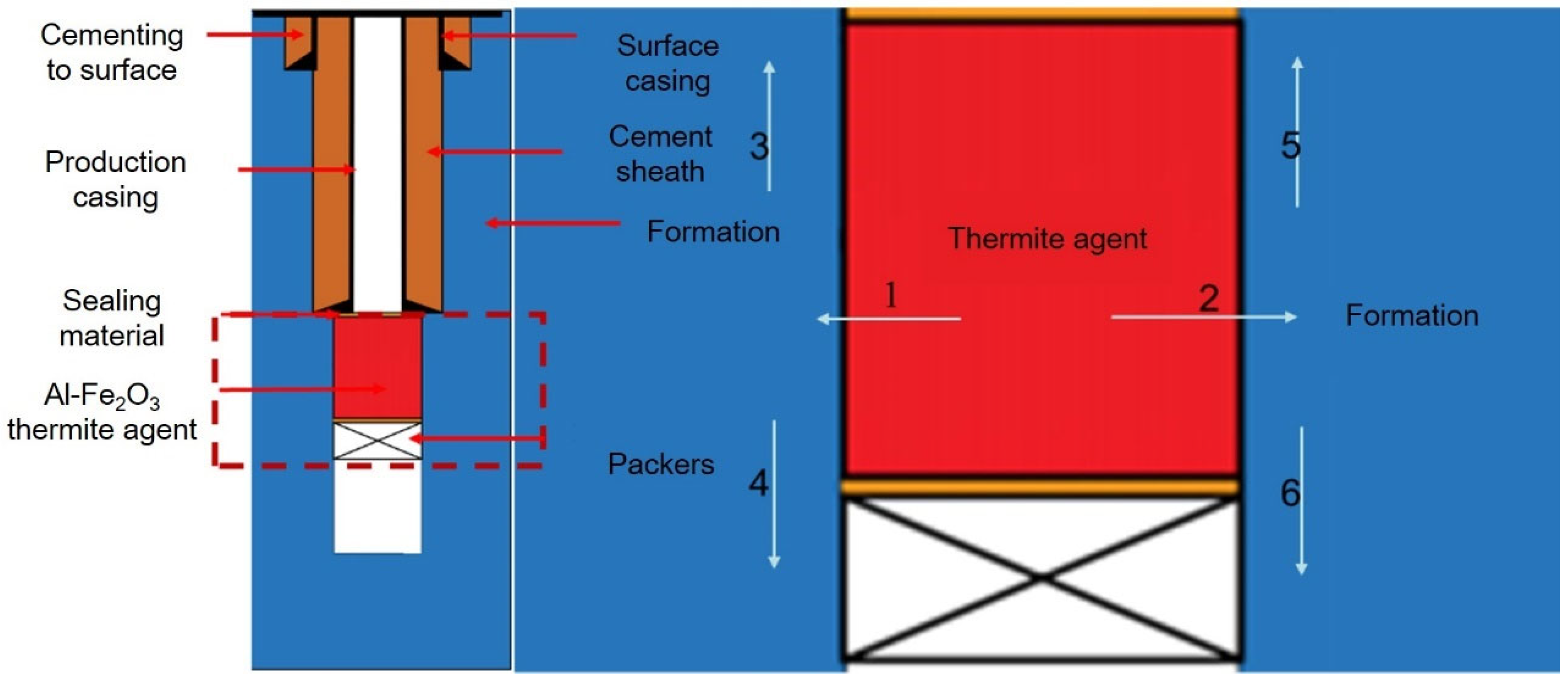
2. Aluminum Hot-Melt Model for Plugging Abandoned Wells
2.1. Basic Assumption Conditions
- (1)
- The initial mixture consists of reactants Al and Fe2O3 along with air, where air is considered an inert gas occupying void spaces within the formed porous medium;
- (2)
- The reactant mixture is considered homogeneous, and upon Al melting, the mixture becomes compacted, with any porosity eliminated;
- (3)
- Fe2O3 is the reactant controlling the reaction, and all Fe2O3 is fully consumed;
- (4)
- Thermal contact resistance at interfaces is neglected;
- (5)
- The upper and lower boundaries of the computational domain are adiabatic, while lateral boundaries remain unaffected by heat conduction;
- (6)
- The position of the packer in the study domain will not slide or shift over time.
2.2. Heat Transfer Physical Model
- (1)
- Intense exothermic reactions of Al-Fe2O3 in the open-hole section release substantial heat under reaction kinetics;
- (2)
- The released heat, governed by thermodynamic principles, first elevates the internal temperature of the Al-Fe2O3 thermite mixture, inducing phase transitions, while simultaneously initiating heat exchange with the surrounding formation;
- (3)
- During the exothermic reaction process, the Al-Fe2O3 thermite before and after the phase change continuously exchanges heat with the surrounding strata. Meanwhile, there are also conditions for phase change in the surrounding strata, thereby interacting with the surrounding materials.
2.3. Governing Equations of the Model
2.3.1. Thermite Reaction Kinetic Equation
2.3.2. Heat Conduction Equation with Phase Change
2.3.3. Thermal–Fluid–Solid Coupling Heat Conduction Equation
2.3.4. Auxiliary Equations
- (1)
- Thermochemical Properties of Thermite Mixture
- (2)
- Thermite Melting Phase Change Model
3. Solution Method for Thermite Melting Model
3.1. Initial and Boundary Conditions
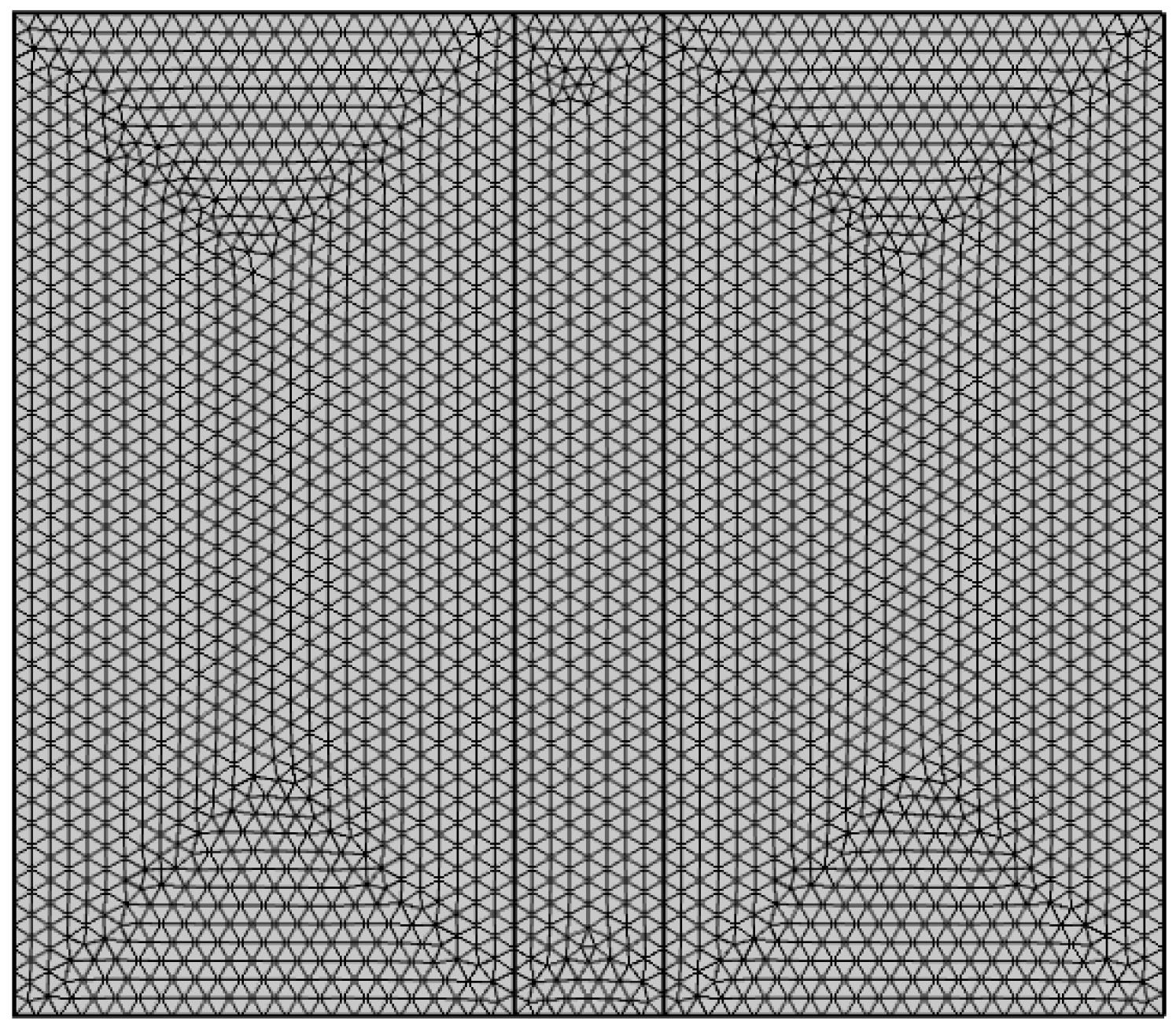
3.2. Mesh Generation and Model Solution
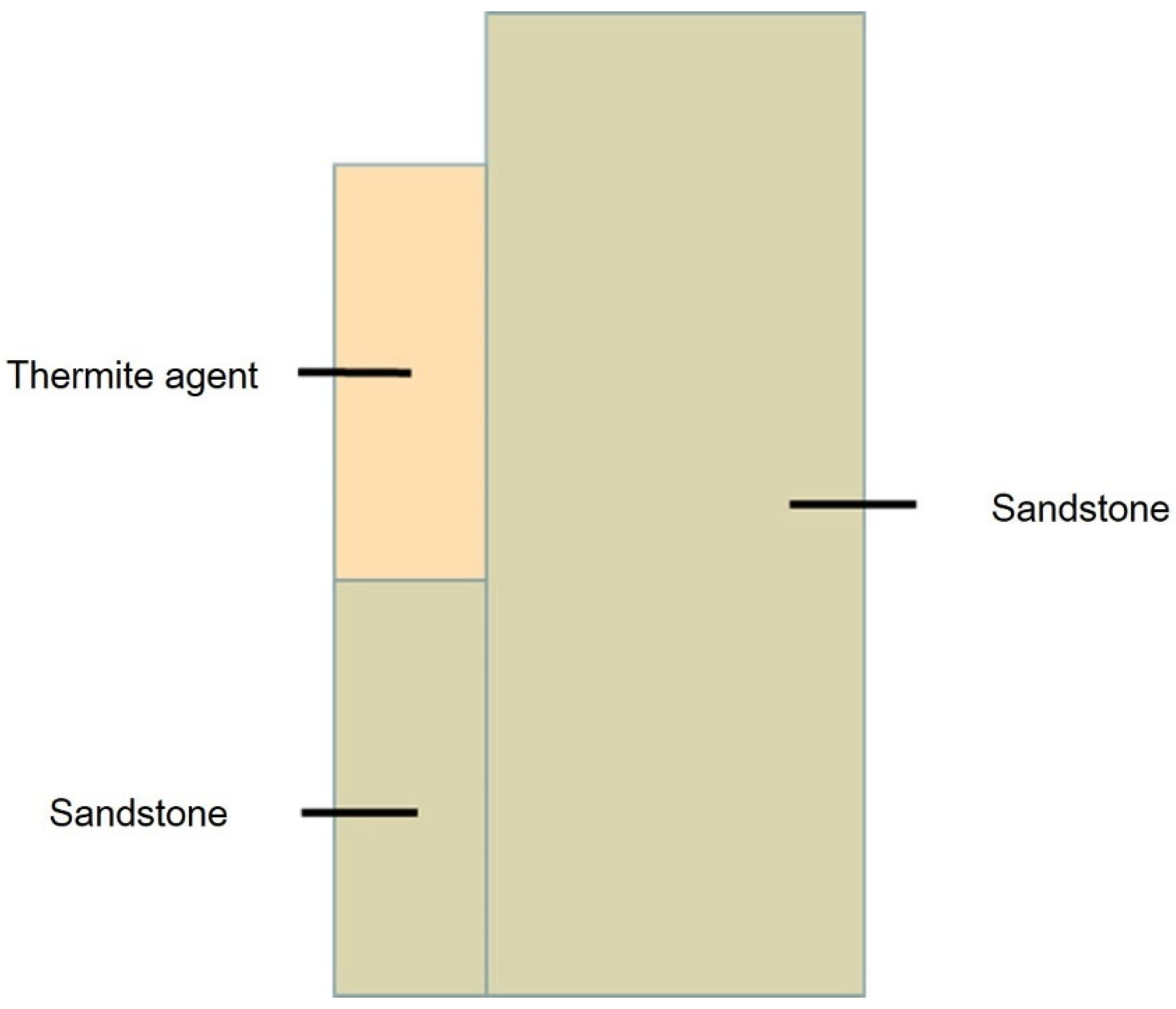
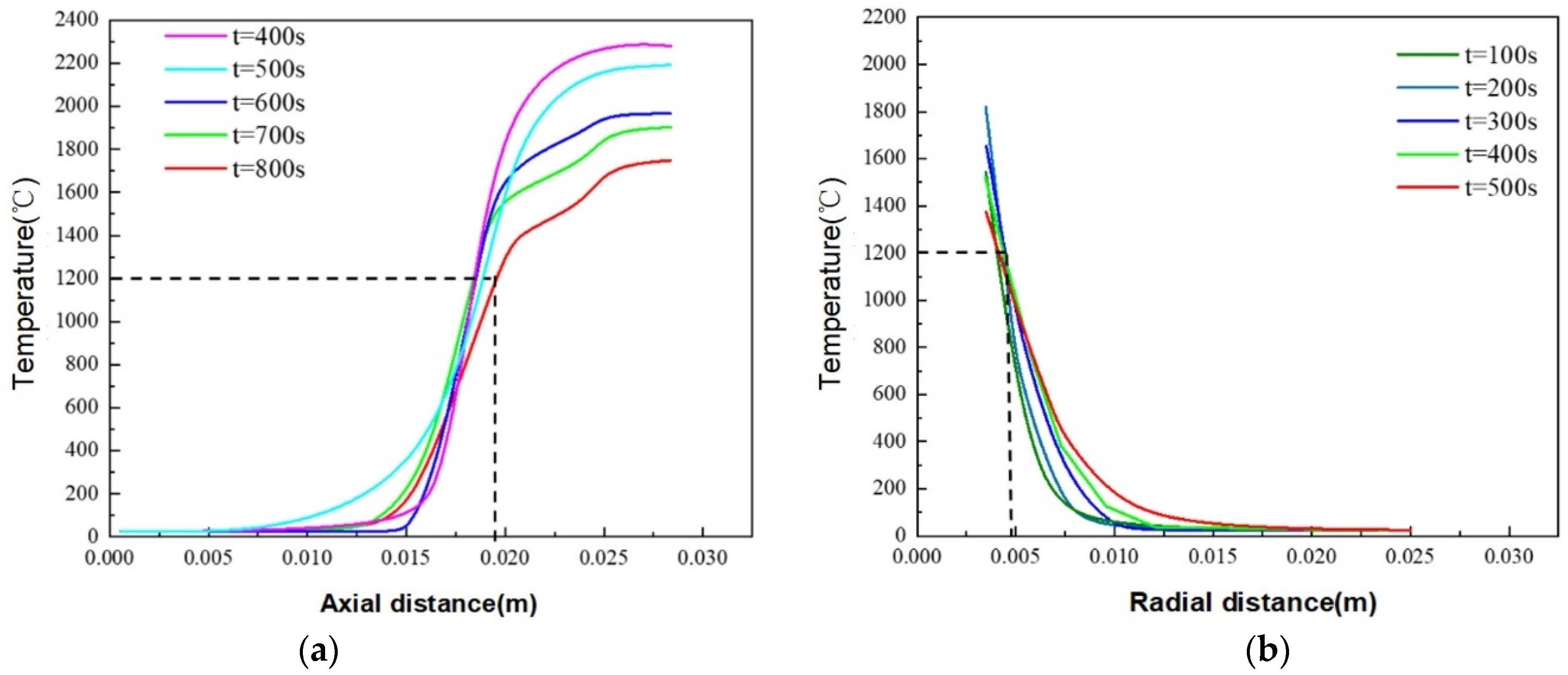
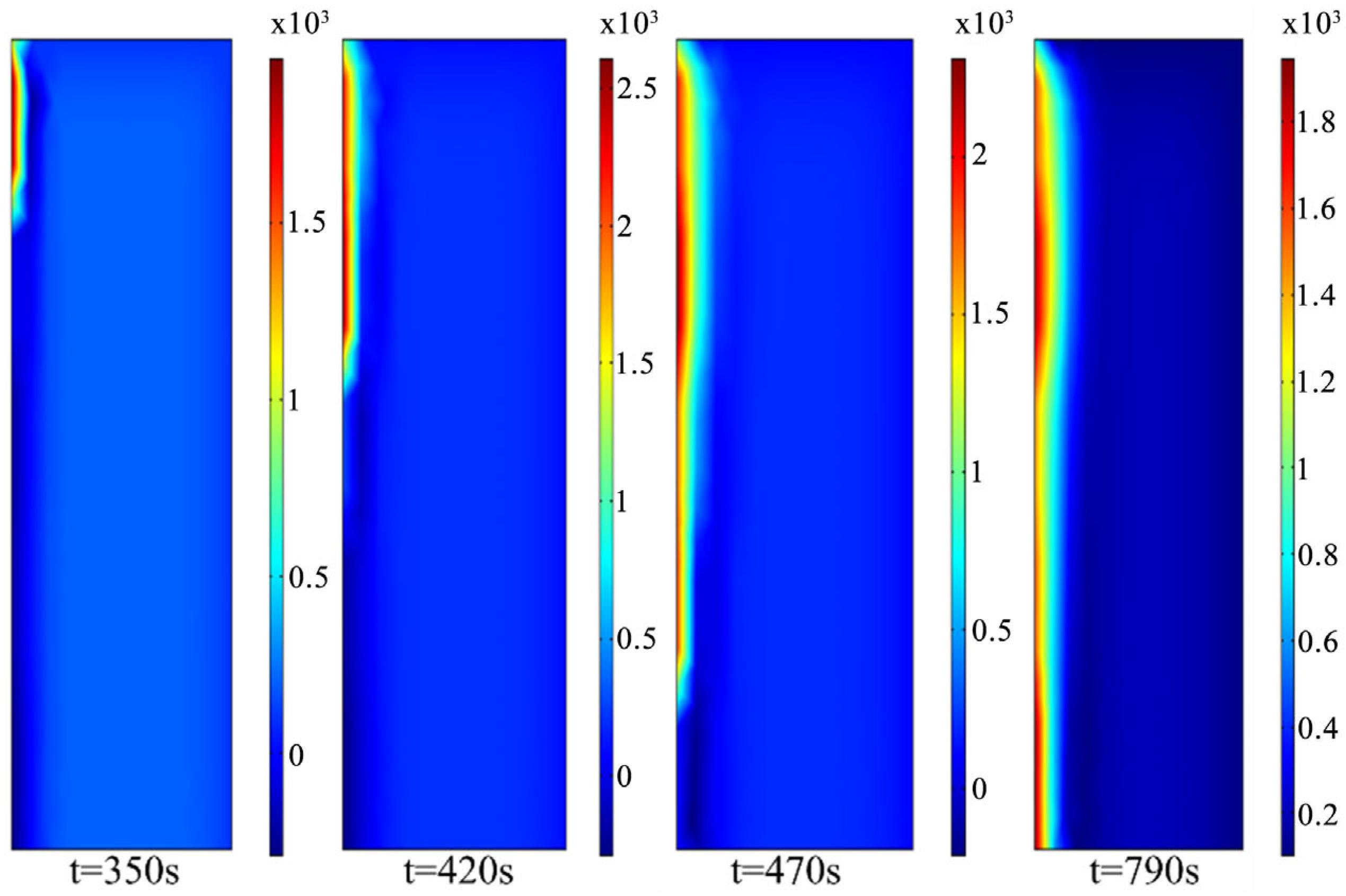
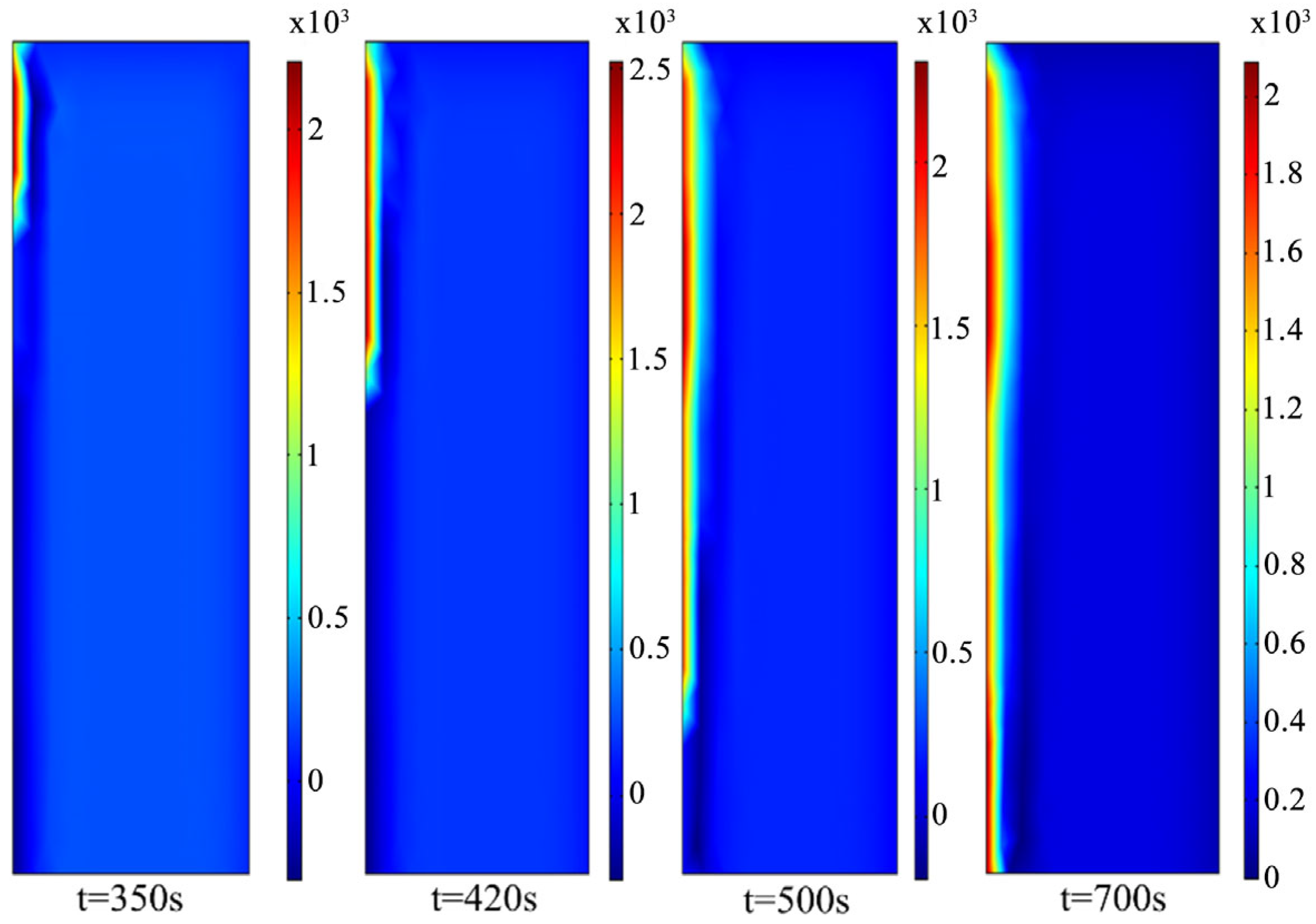
4. Thermite Melting Experiments and Model Validation
5. Analysis of Factors Influencing Temperature Distribution in Thermite Melting
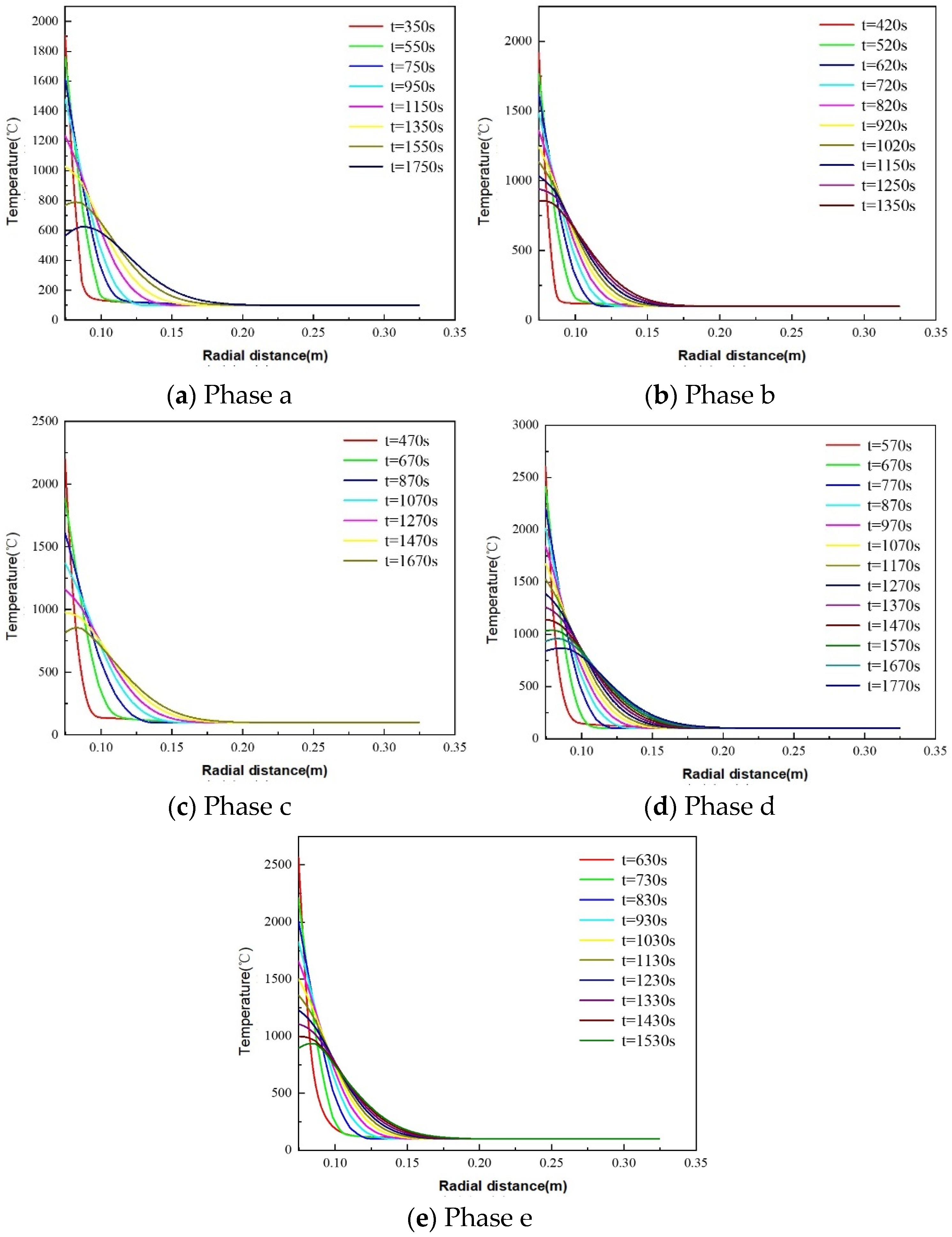
5.1. Impact of Lithology on Temperature Distribution Patterns in Thermite Melting
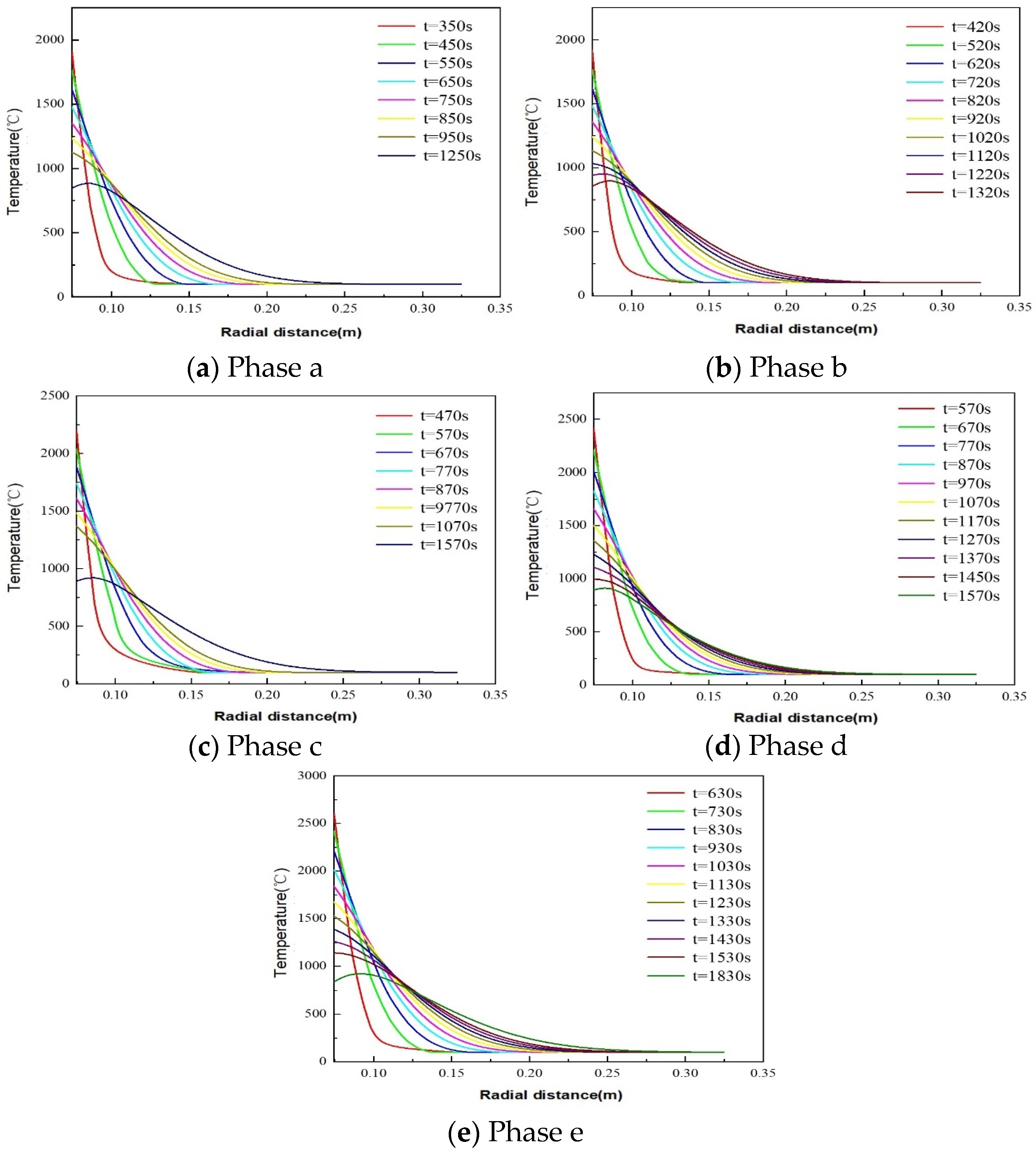
5.1.1. Thermite–Dolomite TP&A System Temperature Distribution Patterns
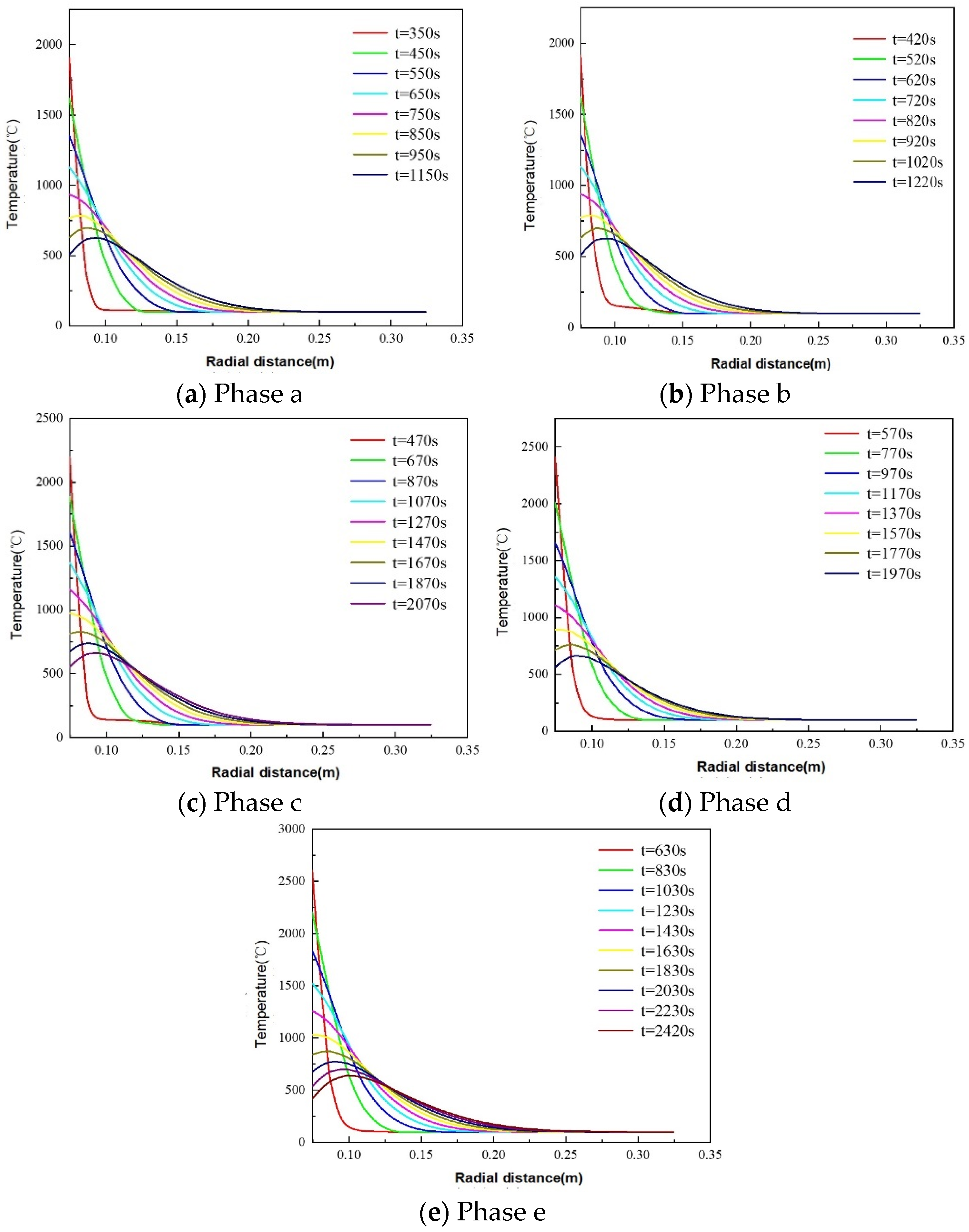
5.1.2. Thermite–Limestone TP&A System Temperature Distribution Patterns
5.1.3. Thermite–Granite TP&A System Temperature Distribution Characteristics
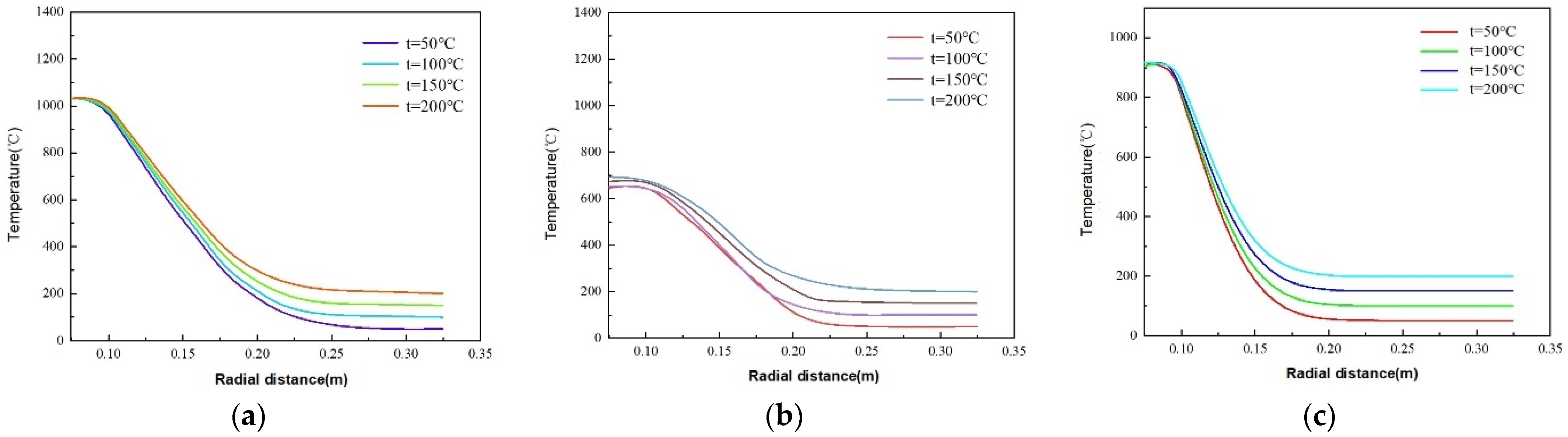
5.2. Effects of Downhole Initial Temperature on Thermite Melt Temperature Distribution Patterns
6. Conclusions
- (1)
- Unlike conventional approaches that solely employ solid heat conduction models, this study establishes a transient thermal–fluid–solid coupled model for open-hole abandoned wellbores, incorporating phase change reactions during heat transfer and integrating the kinetic equations of thermite reactions.
- (2)
- Exploratory experiments on thermite melting were conducted, demonstrating that under semi-confined conditions, the sandstone borehole diameter expanded by approximately 9.29 mm, with an axial extension of about 17.19 mm at the well bottom. In simulation studies, these values reached 9.8 mm and 18.9 mm, respectively, corresponding to error margins of 5.5% and 4.7%. These results validate the accuracy of the temperature model established in this study.
- (3)
- The maximum phase transition thicknesses for dolomite, limestone, and granite are 0.0354 m, 0.0350 m, and 0.0234 m, respectively. Thus, dolomite and limestone exhibit superior plugging efficacy. In the initial stage of the thermite reaction (stage a), limestone demonstrates enhanced thermite melting effectiveness, with its phase transition thickness exceeding that of dolomite by 59.78%. The influence of downhole initial temperature on temperature distribution in open-hole abandoned wells during thermite melting is negligible.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Li, J.S.; Xiao, N.W. Technical Research on Ecological Risk Assessment of Onshore Oil Exploitation; China Environmental Science Press: Beijing, China, 2013. [Google Scholar]
- Zhu, M.Y.; Cheng, T.; Li, S.F.; Pang, H.L.; Liu, C.Y. Research progress of abandonment technology in Bohai Oilfield. China Offshore Plat. 2022, 37, 92–96. [Google Scholar]
- Jiang, J.W.; Li, J.; Liu, G.H.; Lian, W.; Yang, H.W. Numerical simulation of cohesive cement-casing interface bonding failure based on cohesive unit method in abandoned wells. Drill. Complet. Fluids 2020, 37, 351–357. [Google Scholar]
- Yang, Y.B. Offshore platform abandonment technology and market prospect forecast. China Offshore Plat. 2013, 28, 4–7. [Google Scholar]
- Jia, Q.Z. Exploration and application of permanent plugging technology for abandoned oil and gas wells. Chem. Eng. Equip. 2017, 241, 165–166. [Google Scholar]
- Carroll, S.; Carey, J.W.; Dzombak, D.; Huerta, N.J.; Li, L.; Richard, T.; Um, W.; Walsh, S.D.; Zhang, L. Role of chemistry, mechanics, and transport on well integrity in CO2 storage environments. Int. J. Greenh. Gas Control 2016, 49, 149–160. [Google Scholar] [CrossRef]
- Li, Z.J.; Han, C.; Wei, A.C.; Liu, X.Y.; Yang, Y.H. Cement plug technology for abandonment of offshore UHTP exploration wells. Drill. Fluid Complet. Fluid 2019, 36, 617–621. [Google Scholar]
- Stein, A.F. Verification of barriers in a suspension or abandonment phase. In Proceedings of the SPE Norway One Day Seminar, Bergen, Norway, 18 April 2018; p. 191301. [Google Scholar]
- Vrålstad, T.; Saasen, A.; Fjær, E.; Øia, T.; Ytrehus, J.D.; Khalifeh, M. Plug & abandonment of offshore wells: Ensuring long-term well integrity and cost-efficiency. J. Pet. Sci. Eng. 2019, 173, 478–491. [Google Scholar]
- de Souza, K.M.; de Lemos, M.J. Detailed numerical modeling and simulation of Fe2O3−Al thermite reaction. Propellants Explos. Pyrotech. 2021, 46, 806–824. [Google Scholar] [CrossRef]
- Pena, F.J.C.; de Lemos, M.J.S. Unsteady heat conduction with phase change applied to a novel thermal plug and abandonment process. Int. J. Therm. Sci. 2021, 170, 107155. [Google Scholar] [CrossRef]
- Pena, F.J.C.; de Lemos, M.J.S. Numerical investigation of an innovative through-tubing solution to thermal plug and abandonment of oil wells with thermite reactions. Appl. Therm. Eng. 2024, 254, 123874. [Google Scholar] [CrossRef]
- Magalhães, E.S.; de Lemos, M.J.S. A thermal study of a new oil well plugging & abandonment operation. Int. J. Therm. Sci. 2020, 155, 106421. [Google Scholar]
- de Souza, K.M.; de Lemos, M.J.S.; Ribeiro, R.R.; Marin, A.M.G. Advanced isoconversional kinetic analysis of Fe2O3-2Al thermite reaction for plug and abandonment of oil wells. Chem. Eng. J. 2023, 455, 140725. [Google Scholar] [CrossRef]
- de Andrade, G.S.; Nascimento, E.J.G.; de Lemos, M.J.S. Finite integral transform with homogenized boundary conditions solution of transient heat conduction applied to thermal plug and abandonment of oil wells. Geoenergy Sci. Eng. 2024, 233, 212530. [Google Scholar] [CrossRef]
- de Andrade, G.S.; Pena, F.J.C.; de Lemos, M.J.S. A new analytical method for transient heat conduction in composite disks applied to thermal plug and abandonment of oil wells. Int. Commun. Heat Mass Transf. 2024, 155, 107465. [Google Scholar] [CrossRef]
- He, L.; Chen, L.G.; Zheng, Q.C.; Song, W.J.; Wang, Y.J. Treatment Method of Abandoned Open Hole in Coal Mining Area. China Patent CN103590768B, 15 February 2017. [Google Scholar]
- Shen, W.D.; Jiang, Z.M. Engineering Thermodynamics; Higher Education Press: Beijing, China, 1982. [Google Scholar]
- Eckert, E.R.G.; Goldstein, R.J.; Ibele, W.E.; Simon, T.W.; Kuehn, T.H.; Strykowski, P.J.; Tamma, K.K.; Bar-Cohen, A.; Heberlein, J.V.R.; Davidson, J.H.; et al. Heat transfer—A review of 1996 literature. Int. J. Heat Mass Transf. 2000, 43, 1273–1371. [Google Scholar]
- Pei, H.B. Study on Reaction Mechanism and Energy Release Law of Aluminum Powder in Aluminum-Containing Explosive. Ph.D. Thesis, School of Mechanical and Electrical Engineering, Beijing Institute of Technology, Beijing, China, 2015. [Google Scholar]
- Zhao, C. Study on Energy Release Law of Nano-Thermite. Ph.D. Thesis, School of Mechanical and Electrical Engineering, Beijing Institute of Technology, Beijing, China, 2014. [Google Scholar]
- Mei, J.; Halldearn, R.D.; Xiao, P. Mechanisms of the aluminum-iron oxide thermite reaction. Scr. Mater. 1991, 41, 541–548. [Google Scholar] [CrossRef]
- Durães, L.; Campos, J.; Portugal, A. Radial combustion propagation in iron (III) oxide/aluminum thermite mixtures. Propellants Explos. Pyrotech. 2007, 31, 42–49. [Google Scholar] [CrossRef]
- Sahoo, S.K.; Danali, S.M.; Arya, P.R. Ignition behavior of Al/Fe2O3 metastable intermolecular composites. Int. J. Eng. Res. Sci. 2017, 3, 2395–6992. [Google Scholar] [CrossRef]
- Franceschi, K.; Lugo, L.; Páez, G.; Magarelli, D. Development of a software for solving problems applied to thermodynamics. Ing. UC 2016, 23, 138–152. [Google Scholar]
- Durães, L.; Brito, P.; Campos, J.; Portugal, A. Modelling and simulation of Fe2O3/Aluminum thermite combustion: Experimental validation. Comput. Aided Chem. Eng. 2006, 21, 365–370. [Google Scholar]
- de Souza, K.M.; de Lemos, M.J.S. A new hybrid analytical/numerical method for transient heat conduction in composite hollow cylinders applied to plug and abandonment of oil wells. Int. J. Therm. Sci. 2021, 46, 106981. [Google Scholar]
- Voller, V.R.; Shadabi, L. Enthalpy methods for tracking a phase-change boundary in 2 dimensions. Int. Commun. Heat Mass Transf. 1984, 11, 239–249. [Google Scholar] [CrossRef]





| Rock Sample | Rock Dimensions (mm × mm × mm) | Central Borehole (mm) | Central Borehole Depth (mm) | Al-Fe2O3 Mass (g) | Melting Point (°C) | Experimental Containment Measures |
|---|---|---|---|---|---|---|
| Sandstone | 500 × 500 × 500 | 117.8 | 300 | 2500 | 1000 | Semi-Confined |
| Rock Type | Radius (mm) | Density (kg/m3) | Thermal Conductivity (W/m·°C) | Specific Heat Capacity (J/kg·°C) | Latent Heat (J/kg) | Melting Point (°C) | Porosity (%) |
|---|---|---|---|---|---|---|---|
| Dolomite | 0.3246 | 2860 | 5.5 | 846 | 250,000 | 1000 | 0.21 |
| Limestone | 0.3246 | 2620 | 2.82 | 878.64 | 360,000 | 650 | 0.34 |
| Granite | 0.3246 | 2350 | 2.5 | 1380 | 396,000 | 900 | 0.36 |
| Thermite Material | Open-Hole Section Radius (mm) | Density (kg/m3) | Thermal Conductivity Coefficient (W/m·°C) | Specific Heat Capacity (J/kg·°C) | Melting Point (°C) |
|---|---|---|---|---|---|
| Al | 0.0746 | 2710 | 237 | 875 | 624 |
| Fe2O3 | 0.0746 | 5270 | 15 | 1100 | 1662 |
| Well ID | Well Type | Geothermal Gradient (°C/m) | Well Depth (m) | Borehole Size (mm) | Simulated Section Length (m) |
|---|---|---|---|---|---|
| XX1 | Vertical Well | 2.5 | 2000 | 149.2 | 0.5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, H.; Zhang, J.; Sun, R.; Li, X.; Yao, J.; Zhou, J. Study on the Influence of Temperature Distribution in Thermite Plugging Abandoned Well Technology. Energies 2025, 18, 2941. https://doi.org/10.3390/en18112941
Liu H, Zhang J, Sun R, Li X, Yao J, Zhou J. Study on the Influence of Temperature Distribution in Thermite Plugging Abandoned Well Technology. Energies. 2025; 18(11):2941. https://doi.org/10.3390/en18112941
Chicago/Turabian StyleLiu, Hao, Jie Zhang, Ruitao Sun, Xiang Li, Jiajun Yao, and Jiazheng Zhou. 2025. "Study on the Influence of Temperature Distribution in Thermite Plugging Abandoned Well Technology" Energies 18, no. 11: 2941. https://doi.org/10.3390/en18112941
APA StyleLiu, H., Zhang, J., Sun, R., Li, X., Yao, J., & Zhou, J. (2025). Study on the Influence of Temperature Distribution in Thermite Plugging Abandoned Well Technology. Energies, 18(11), 2941. https://doi.org/10.3390/en18112941






