Numerical Investigation of the Impact of Variation of Negative Electrode Porosity upon the Cycle Life of Lithium-Ion Batteries
Abstract
1. Introduction
2. Model Description and Validation
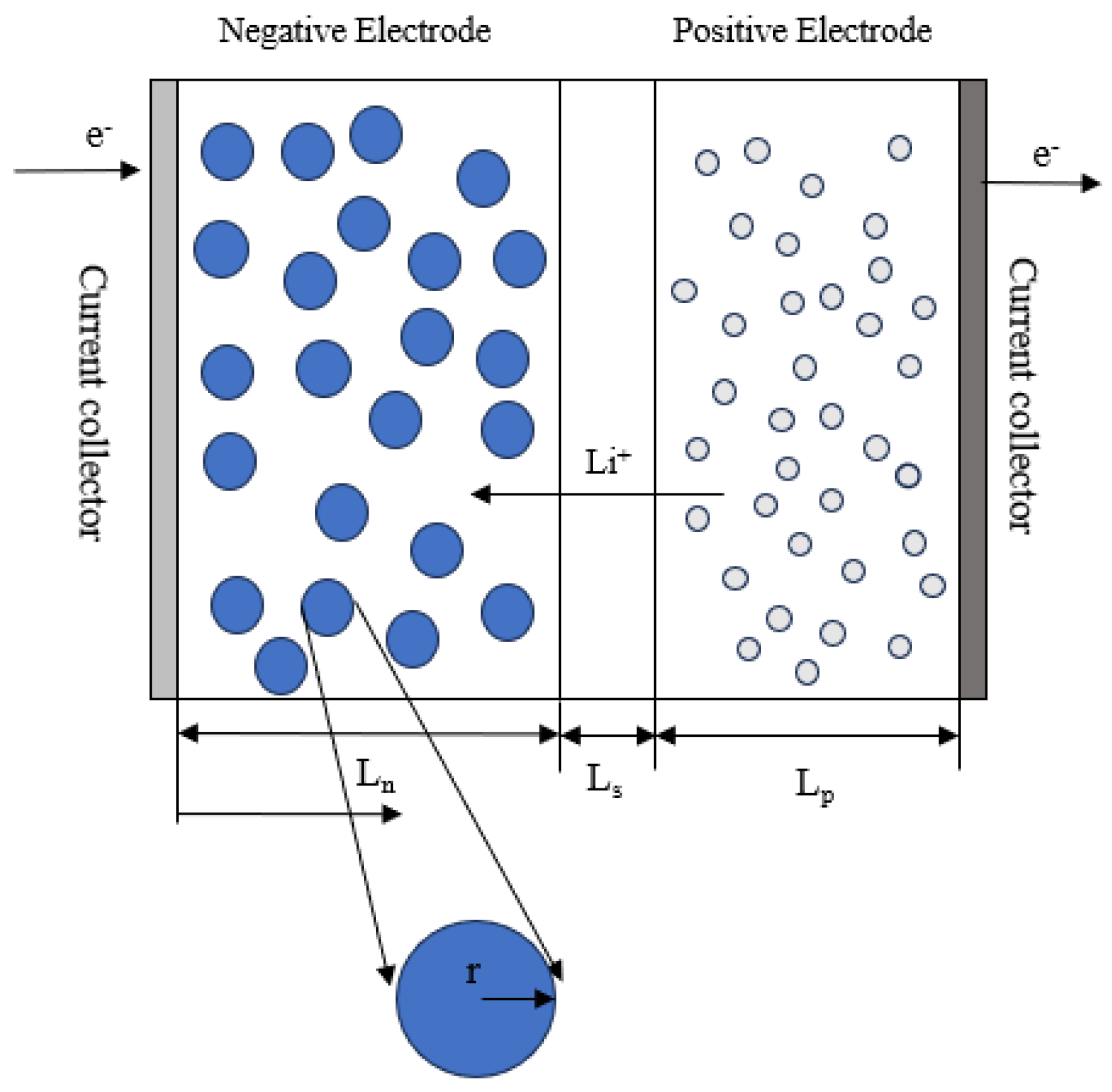
2.1. Model Description
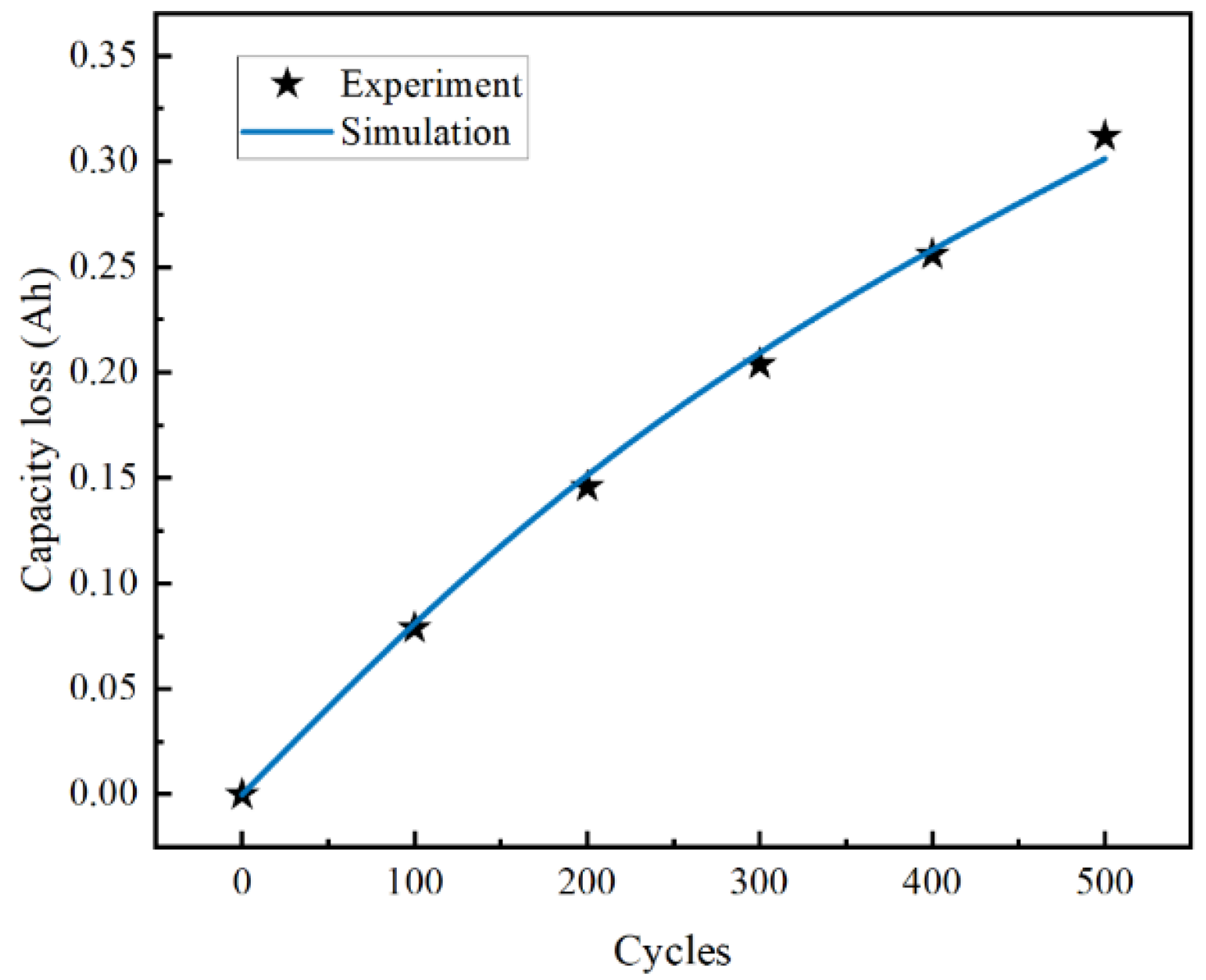
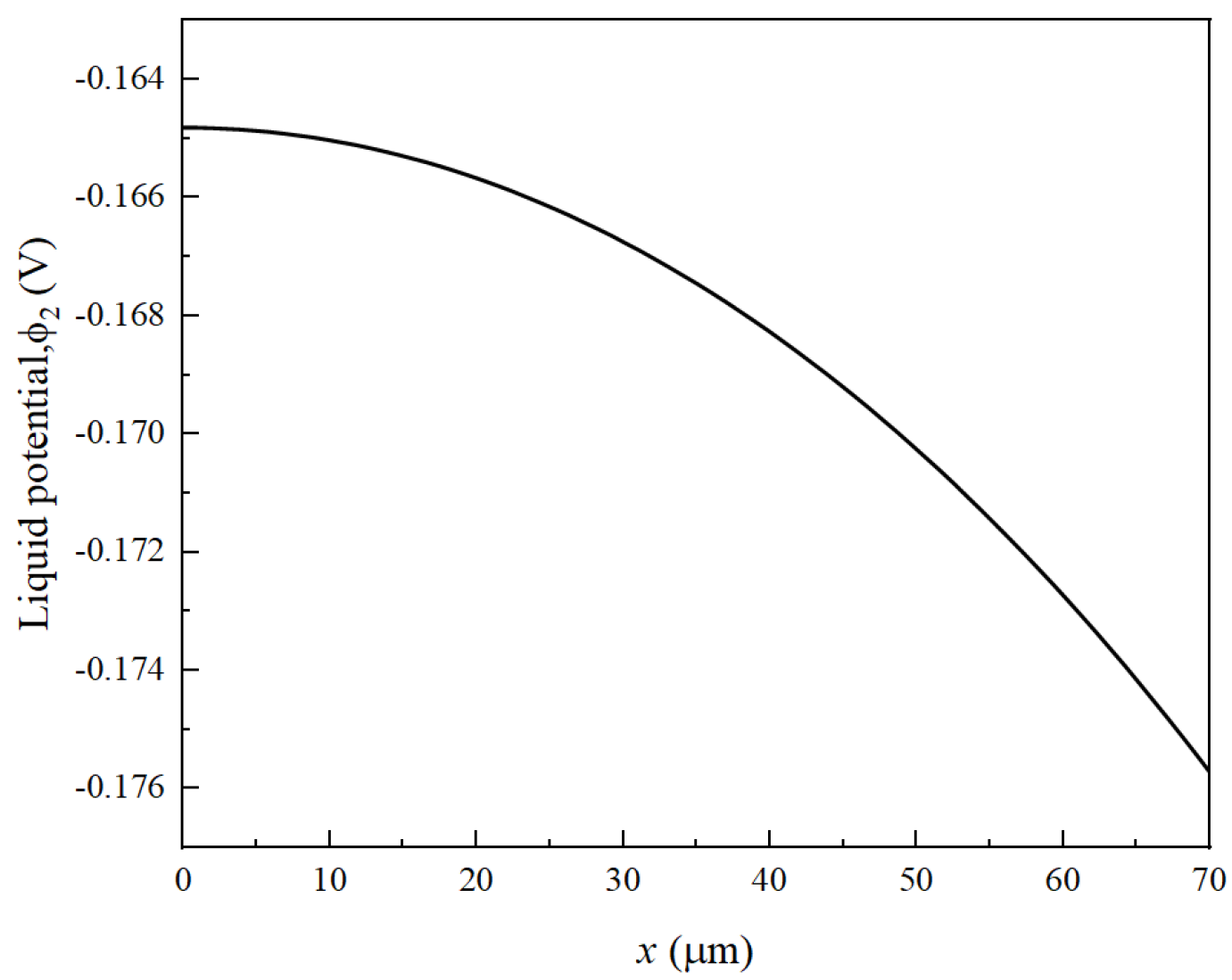
2.2. Model Validation
3. Results and Discussion
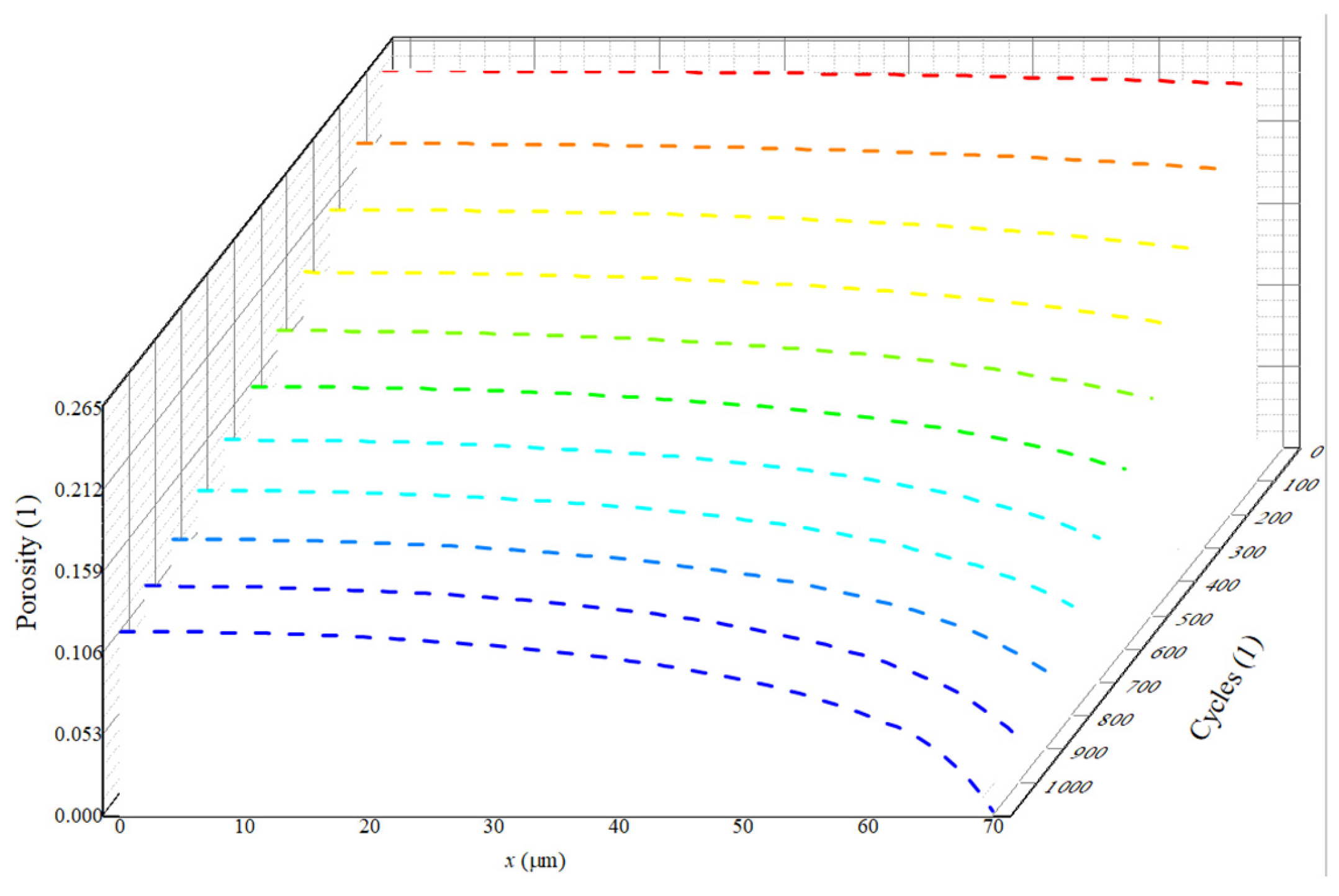
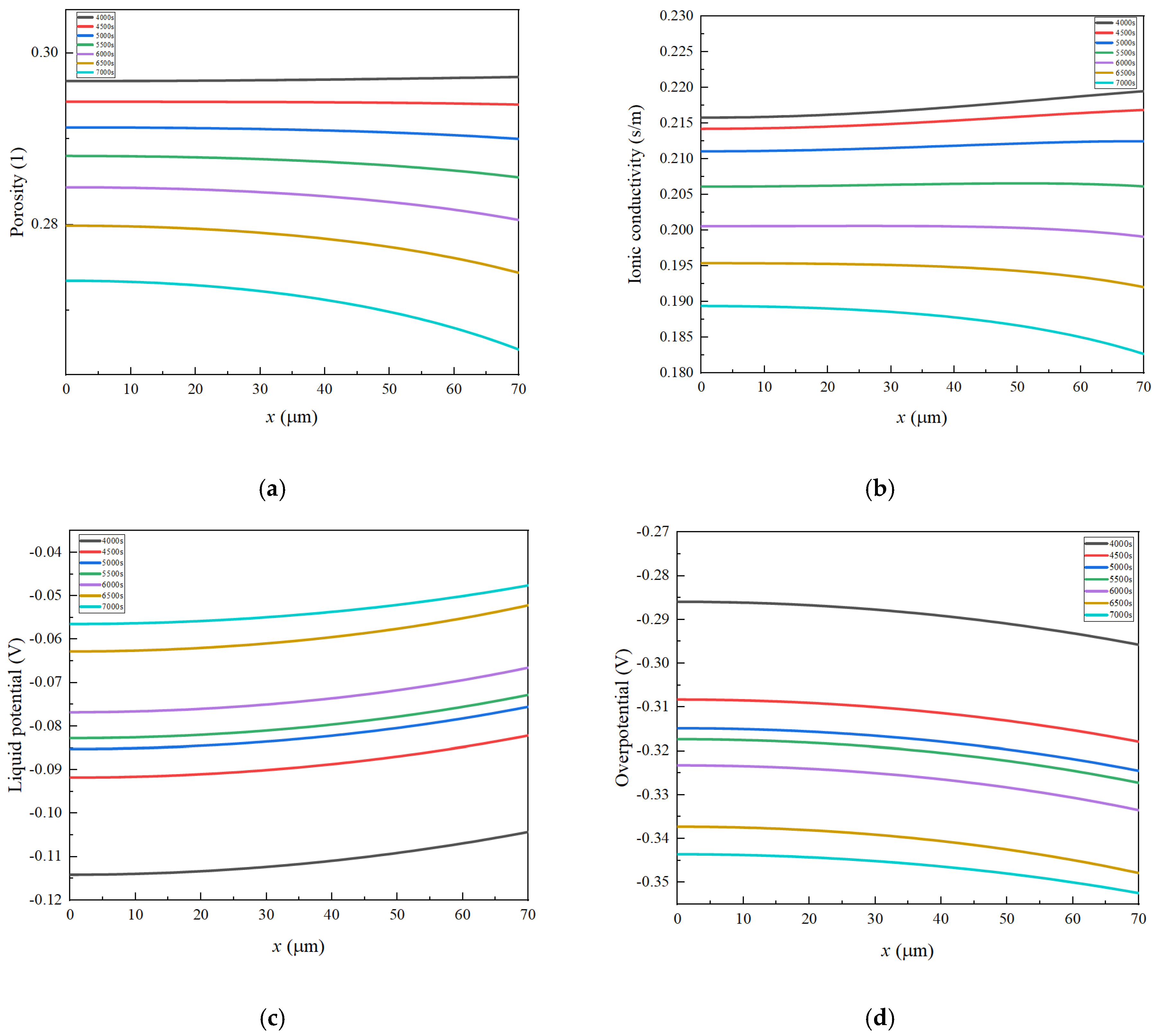
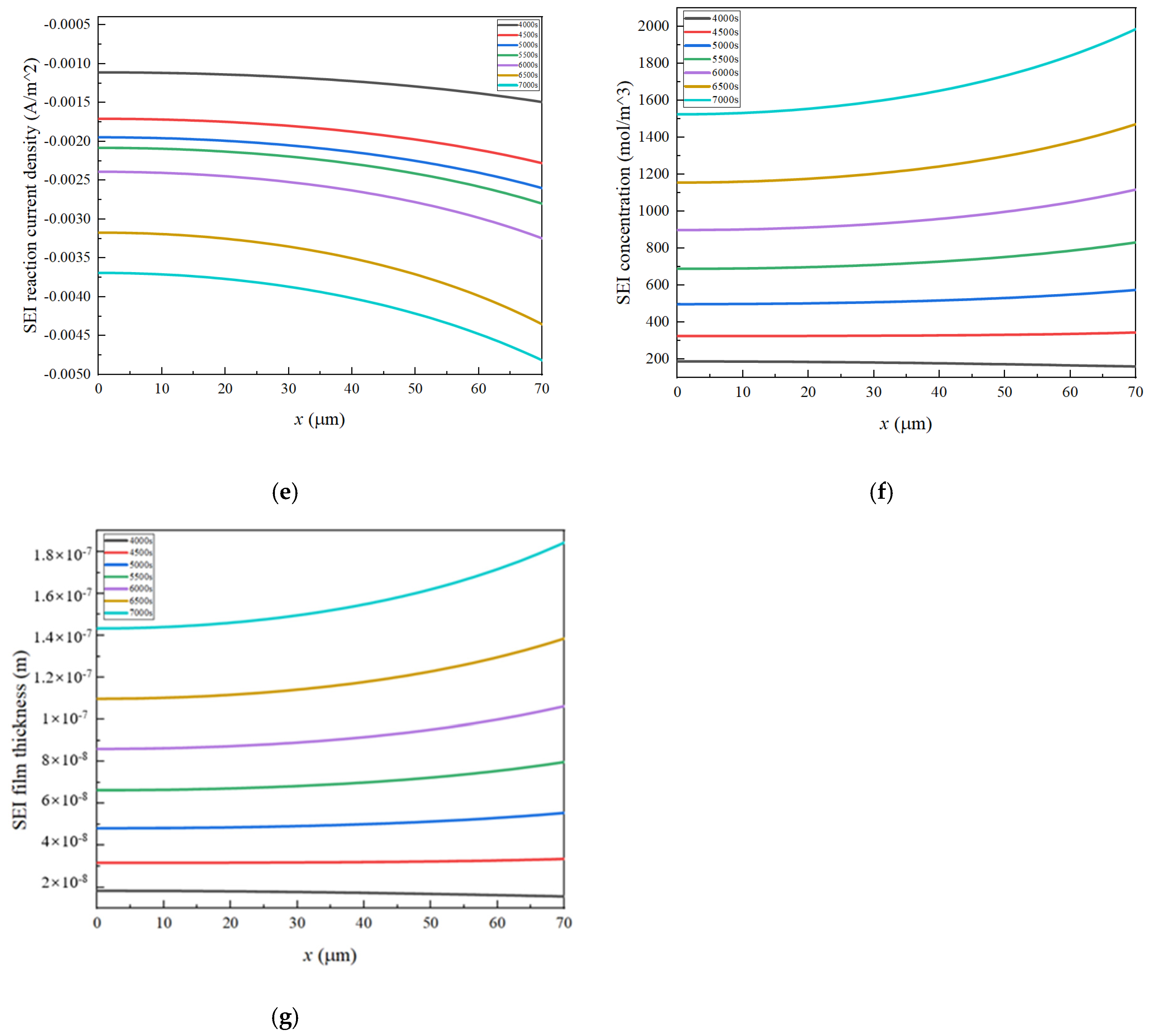
3.1. The Distribution Characteristics of Negative Electrode Porosity After a Long Cycle
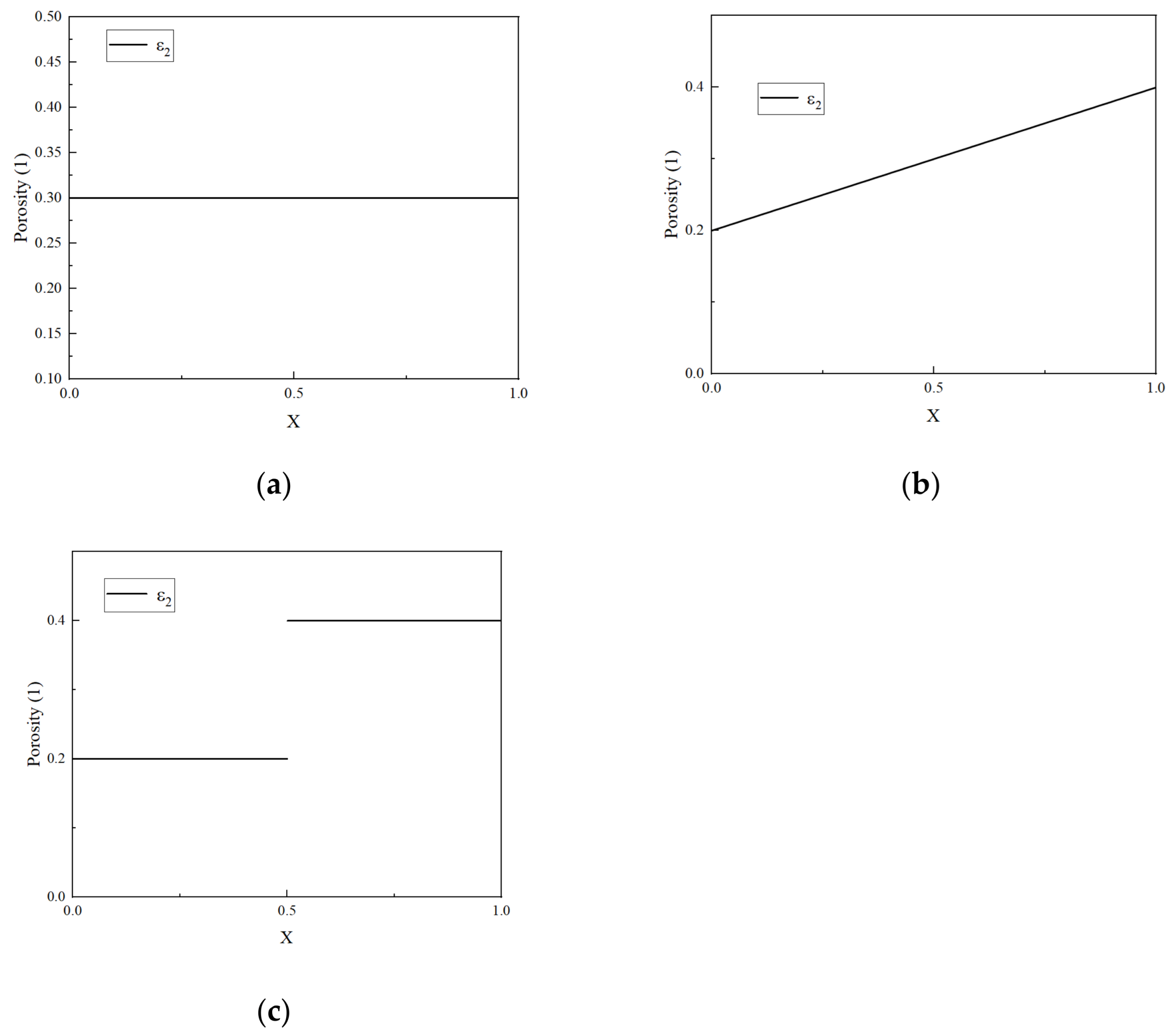
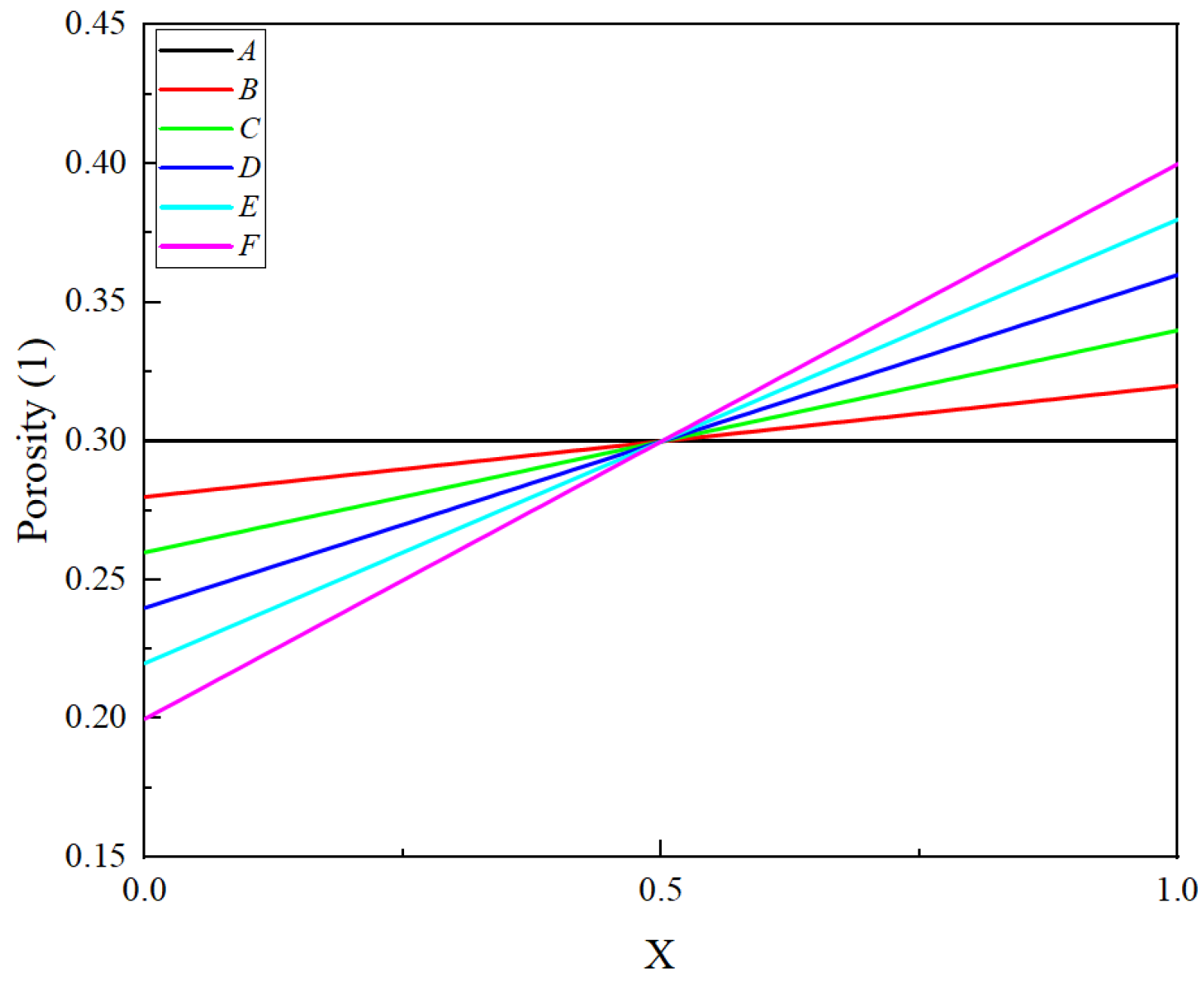
3.2. The Impact of Different Porosity Structures on the Cycle Life of LIBs
3.3. The Effect of Various Linear Porosity Structures on the Pore Clogging of the Negative Electrode
3.4. The Impact of the Linear Porosity Structure of the Negative Electrode on the Heating Behavior of the LIBs
4. Conclusions
- Porosity reduction near the separator: During charging, lithium ions diffuse from the positive electrode through the separator, leading to a reduction in porosity near the separator in the negative electrode. This reduced porosity decreases ionic conductivity, accelerates SEI growth, and promotes pore clogging. Consequently, this area experiences the most rapid decrease in porosity, impacting overall battery performance.
- Influence of gradient and linear porosity structures: Both gradient and linear porosity structures contribute to mitigating pore clogging, though with different effects on capacity loss. Notably, the linear porosity structure enables a more uniform distribution of SEI, leading to better management of lithium-ion transport during cycling.
- Optimization of linear porosity slope: The slope of the linear porosity structure plays a critical role in maintaining cell stability and performance. A high slope leads to premature porosity reduction near the current collector, increasing local current density and reducing conductivity. Conversely, a low slope causes early porosity depletion near the separator, affecting lithium-ion transport and overall electrochemical performance. This finding underscores the need for precise optimization of porosity gradients in battery design.
- Reduction in ohmic heating: The linear porosity structure is found to significantly reduce ohmic heating in the negative electrode after extended cycling, enhancing the thermal stability and overall efficiency of the LIBs.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Nomenclature
| area of the positive (both sides) that has opposing negative (m2) | |
| lithium-ion concentration in solid phase (mol m−3) | |
| lithium-ion concentration in electrolyte (mol m−3) | |
| maximum lithium-ion concentration (mol m−3) | |
| heat capacity (J kg−1K−1) | |
| solid-phase diffusivity (m2 s−1) | |
| solid-phase diffusivity at reference temperature (m2 s−1) | |
| reaction active energy (kJ mol−1) | |
| solid-phase current density (A m−2) | |
| liquid-phase current density (A m−2) | |
| exchange current density (A m−2) | |
| local current density (A m−2) | |
| reaction rate constant at reference temperature (m2.5mol−0.5s−1) | |
| reaction rate constant (m2.5mol−0.5s−1) | |
| thermal conductivity (Wm−1K−1) | |
| battery thickness (μm) | |
| active heat generation (W m−3) | |
| ohmic heat generation (W m−3) | |
| reaction heat generation (W m−3) | |
| characteristic radius of electrode particles (μm) | |
| initial state of charge | |
| time (s) | |
| transference number of lithium-ion species dissolved in liquid | |
| absolute temperature (K) | |
| thermodynamic, open circuit voltage (V) | |
| total weight of the battery (kg) | |
| discharge time (s) | |
| lithium-ion flux (mol m−2s−1) | |
| Faraday constant (C mol−1) | |
| transfer coefficient | |
| Overpotential (V) | |
| Bruggeman tortuosity exponent | |
| volume fraction | |
| electric potential (V) | |
| ionic conductivity (S m−1) | |
| electronic conductivity (S m−1) | |
| tortuosity | |
| thermodynamic factor relating to electrolyte activity | |
| density (kg m−3) | |
| 0 | initial or equilibrium state |
| 1 | solid phase |
| eq | equilibrium potential |
| pos | positive |
| s | separator |
| cc | current collector |
| eff | effective |
| 2 | liquid phase |
| i | pos or neg |
| neg | negative |
| dis | discharge |
| max | maximum value |
References
- Kulova, T.L.; Fateev, V.N.; Seregina, E.A.; Grigoriev, A.S. A brief review of post-lithium-ion batteries. Int. J. Electrochem. Sci. 2020, 15, 7242–7259. [Google Scholar] [CrossRef]
- Li, C.; Zhang, H.; Ding, P.; Yang, S.; Bai, Y. Deep feature extraction in lifetime prognostics of lithium-ion batteries: Advances, challenges and perspectives. Renew. Sustain. Energy Rev. 2023, 184, 113576. [Google Scholar] [CrossRef]
- Dubarry, M.; Liaw, B.Y. Identify capacity fading mechanism in a commercial LiFePO4 cell. J. Power Sources 2009, 194, 541–549. [Google Scholar] [CrossRef]
- Schmitt, J.; Rehm, M.; Karger, A.; Jossen, A. Capacity and degradation mode estimation for lithium-ion batteries based on partial charging curves at different current rates. J. Energy Storage 2023, 59, 106517. [Google Scholar] [CrossRef]
- Wu, X.G.; Wang, W.B.; Du, J.Y. Effect of charge rate on capacity degradation of LiFePO4 power battery at low temperature. Int. J. Energy Res. 2020, 44, 1775–1785. [Google Scholar] [CrossRef]
- Liu, J.; Duan, Q.; Qi, K.; Liu, Y.; Sun, J.; Wang, Z.; Wang, Z.; Wang, Q. Capacity fading mechanisms and state of health prediction of commercial lithium-ion battery in total lifespan. J. Energy Storage 2022, 46, 103910. [Google Scholar] [CrossRef]
- Seo, G.; Ha, J.; Kim, M.; Park, J.; Lee, J.; Park, E.; Bong, S.; Lee, K.; Kwon, S.J.; Moon, S.P.; et al. Rapid determination of lithium-ion battery degradation: High C-rate LAM and calculated limiting LLI. J. Energy Chem. 2022, 67, 663–671. [Google Scholar] [CrossRef]
- Campbell, I.D.; Marzook, M.; Marinescu, M.; Offer, G.J. How observable is lithium plating? Differential voltage analysis to identify and quantify lithium plating following fast charging of cold lithium-ion batteries. J. Electrochem. Soc. 2019, 166, A725–A741. [Google Scholar] [CrossRef]
- Fedorov, R.; Maletti, S.; Heubner, C.; Michaelis, A.; Ein-Eli, Y. Molecular engineering approaches to fabricate artificial solid-electrolyte interphases on anodes for Li-ion batteries: A critical review. Adv. Energy Mater. 2021, 11, 2101173. [Google Scholar] [CrossRef]
- Sarkar, A.; Nlebedim, I.; Shrotriga, P. Performance degradation due to anodic failure mechanisms in lithium-ion batteries. J. Power Sources 2020, 502, 229145. [Google Scholar] [CrossRef]
- Keil, J.; Jossen, A. Electrochemical modeling of linear and nonlinear aging of lithium-ion cells. J. Electrochem. Soc. 2020, 167, 110535. [Google Scholar] [CrossRef]
- Zhou, F.; Bao, C. Analysis of the lithium-ion battery capacity degradation behavior with a comprehensive mathematical model. J. Power Sources 2021, 515, 230630. [Google Scholar] [CrossRef]
- Sun, F.; Manke, L. Differentiating and quantifying dead lithium. ChemElectroChem 2019, 6, 5787–5795. [Google Scholar] [CrossRef]
- Kushima, A.; So, K.P.; Su, C.; Bai, P.; Kuriyama, N.; Maebashi, T.; Fujiwara, Y.; Bazant, M.Z.; Li, J. Liquid cell transmission electron microscopy observation of lithium metal growth and dissolution: Root growth, dead lithium and lithium flotsams. Nano Energy 2017, 32, 271–279. [Google Scholar] [CrossRef]
- Samad, H.; Malik, M.S.S.; Gulzar, M. A non-linear model predictive control strategy to minimise mechanical degradation effects of lithium-ion battery. J. Energy Storage 2023, 71, 108050. [Google Scholar] [CrossRef]
- Yang, X.; Leng, Y.; Zhang, G.; Ge, S.; Wang, C. Modeling of lithium plating induced aging of lithium-ion batteries: Transition from linear to nonlinear aging. J. Power Sources 2017, 360, 28–40. [Google Scholar] [CrossRef]
- Pfrang, A.; Kersys, A.; Kriston, A.; Sauer, D.U.; Rahe, C.; Kabitz, S.; Figgemeier, E. Long-term cycling induced jelly roll deformation in commercial 18650 cells. J. Power Sources 2018, 392, 168–175. [Google Scholar] [CrossRef]
- Muller, D.; Dufaux, T.; Brike, K.P. Model-based investigation of porosity profiles in graphite anodes regarding sudden-death and second-life of lithium ion cells. Batteries 2019, 5, 49. [Google Scholar] [CrossRef]
- Wang, Y.; Chang, X.W.; Li, Z.Y.; Mei, Y.N.; Zhang, Y.X.; Liu, L.; Wang, K.; Gu, H.T.; Li, L.S. Preventing sudden death of high-energy lithium-ion batteries at elevated temperature through interfacial ion-flux rectification. Adv. Funct. Mater. 2023, 33, 2208329. [Google Scholar] [CrossRef]
- Broussely, M.; Biensan, P.; Bonhomme, F.; Blanchard, P.; Herreyre, S.; Nechev, K.; Staniewicz, R.J. Main aging mechanisms in Li ion batteries. J. Power Sources 2005, 146, 90–96. [Google Scholar] [CrossRef]
- Bach, T.C.; Schuster, S.F.; Fleder, E.; Muller, J.; Brand, M.J.; Lorrmann, H.; Jossen, A.; Sextl, G. Nonlinear aging of cylindrical lithium-ion cells linked to heterogeneous compression. J. Energy Storage 2016, 5, 212–223. [Google Scholar] [CrossRef]
- Mukhopadhyay, A.; Sheldon, B.W. Deformation and stress in electrode materials for Li-ion batteries. Prog. Mater. Sci. 2014, 63, 58–116. [Google Scholar] [CrossRef]
- Lee, D.; Kim, B.; Shin, C.B.; Oh, S.-M.; Song, J.; Jang, I.-C.; Woo, J.-J. Modeling the combined effects of cyclable lithium loss and electrolyte depletion on the capacity and power fades of a lithium-ion battery. Energies 2022, 15, 7056. [Google Scholar] [CrossRef]
- Sauertieg, D.; Hanselmann, N.; Arzberger, A.; Reinshagen, H.; Ivanov, S.; Bund, A. Electrochemical-mechanical coupled modeling and parameterization of swelling and ionic transport in lithium-ion batteries. J. Power Sources 2018, 378, 235–247. [Google Scholar] [CrossRef]
- Kim, Y. Encapsulation of LiNi0.5Co0.2Mn0.3O2 with a thin inorganic electrolyte film to reduce gas evolution in the application of lithium ion batteries. Phys. Chem. Chem. Phys. 2015, 17, 6400–6405. [Google Scholar] [CrossRef]
- Niu, S.J.; Zhu, G.B.; Wu, K.; Zheng, H. The feasibility for natural graphite to replace artificial graphite in organic electrolyte with different film-forming additives. Chin. J. Chem. Eng. 2023, 56, 58–69. [Google Scholar] [CrossRef]
- Ji, Y.; Zhang, Y.; Wang, C.Y. Li-ion cell operation at low temperatures. J. Electrochem. Soc. 2013, 160, A636–A649. [Google Scholar] [CrossRef]
- Miranda, D.; Costa, C.M.; Almeida, A.M.; Lanceros-Mendez, S. Computer simulations of the influence of geometry in the performance of conventional and unconventional lithium-ion batteries. Appl. Energy 2016, 165, 318–328. [Google Scholar] [CrossRef]
- Ye, G.; Tong, W.; Liu, X.; Song, X.; Zhou, J.; Zhou, X. An analytical method for the optimization of pore network in lithium-ion battery electrodes. Chem. Eng. Res. Des. 2019, 149, 226–234. [Google Scholar] [CrossRef]
- Suthar, B.; Northrop, P.W.C.; Rife, D.; Subramanian, V.R. Effect of porosity, thickness and tortuosity on capacity fade of anode. J. Electrochem. Soc. 2015, 162, A1708–A1722. [Google Scholar] [CrossRef]
- Yu, R.; Li, P.; Wang, K.; Zhang, H. Numerical investigation on the impact of linear variation of positive electrode porosity upon the performance of lithium-ion batteries. J. Electrochem. Soc. 2023, 170, 050502. [Google Scholar] [CrossRef]
- Fuller, T.F.; Doyle, M.; Newman, J. Modeling of galvanostatic charge and discharge of the lithium/polymer/insertion cell. J. Electrochem. Soc. 1993, 140, 1526–1533. [Google Scholar] [CrossRef]
- Doyle, M.; Newman, J.; Gozdz, A.S.; Schmutz, C.N.; Tarascon, J.M. Comparison of modeling predictions with experimental data from plastic lithium ion cells. J. Electrochem. Soc. 1996, 143, 1890–1903. [Google Scholar] [CrossRef]
- Pals, C.R.; Newman, J. Thermal modeling of the lithium/polymer battery: I. Discharge behavior of a single cell. J. Electrochem. Soc. 1995, 142, 3274–3281. [Google Scholar] [CrossRef]
- Pals, C.R.; Newman, J. Thermal modeling of the lithium/polymer battery: II. Temperature profiles in a cell stack. J. Electrochem. Soc. 1995, 142, 3282–3288. [Google Scholar] [CrossRef]
- Yin, L.; Björneklett, A.; Söderlund, E.; Brandell, D. Analyzing and mitigating battery ageing by self-heating through a coupled thermal-electrochemical model of cylindrical Li-ion cells. J. Energy Storage 2021, 39, 102648. [Google Scholar] [CrossRef]
- Sikha, G.; Popov, B.N.; White, R.E. Effect of porosity on the capacity fade of a lithium-ion battery. J. Electrochem. Soc. 2004, 151, A1104–A1114. [Google Scholar] [CrossRef]
- Lu, L.L.; Lu, Y.Y.; Zhu, Z.X.; Shao, J.X.; Yao, H.B.; Wang, S.; Zhang, T.W.; Ni, Y.; Wang, X.X.; Yu, S.H. Extremely fast-charging lithium ion battery enabled by dual-gradient structure design. Sci. Adv. 2022, 8, eabm6624. [Google Scholar] [CrossRef]
- Zhou, H.Y.; Gao, L.T.; Li, Y.M.; Lyu, Y.; Guo, Z.-S. Electrochemical performance of lithium-ion batteries with two-layer gradient electrode architectures. Electrochim. Acta 2024, 476, 143656. [Google Scholar] [CrossRef]
- Luo, P.; Li, P.; Ma, D.; Wang, K.; Zhang, H. A novel capacity fade model of lithium-ion cells considering the influence of stress. J. Electrochem. Soc. 2022, 169, 100535. [Google Scholar] [CrossRef]
- Zhao, R.; Liu, J.; Gu, J. The effects of electrode thickness on the electrochemical and thermal characteristics of lithium ion battery. Appl. Energy 2015, 139, 220–229. [Google Scholar] [CrossRef]



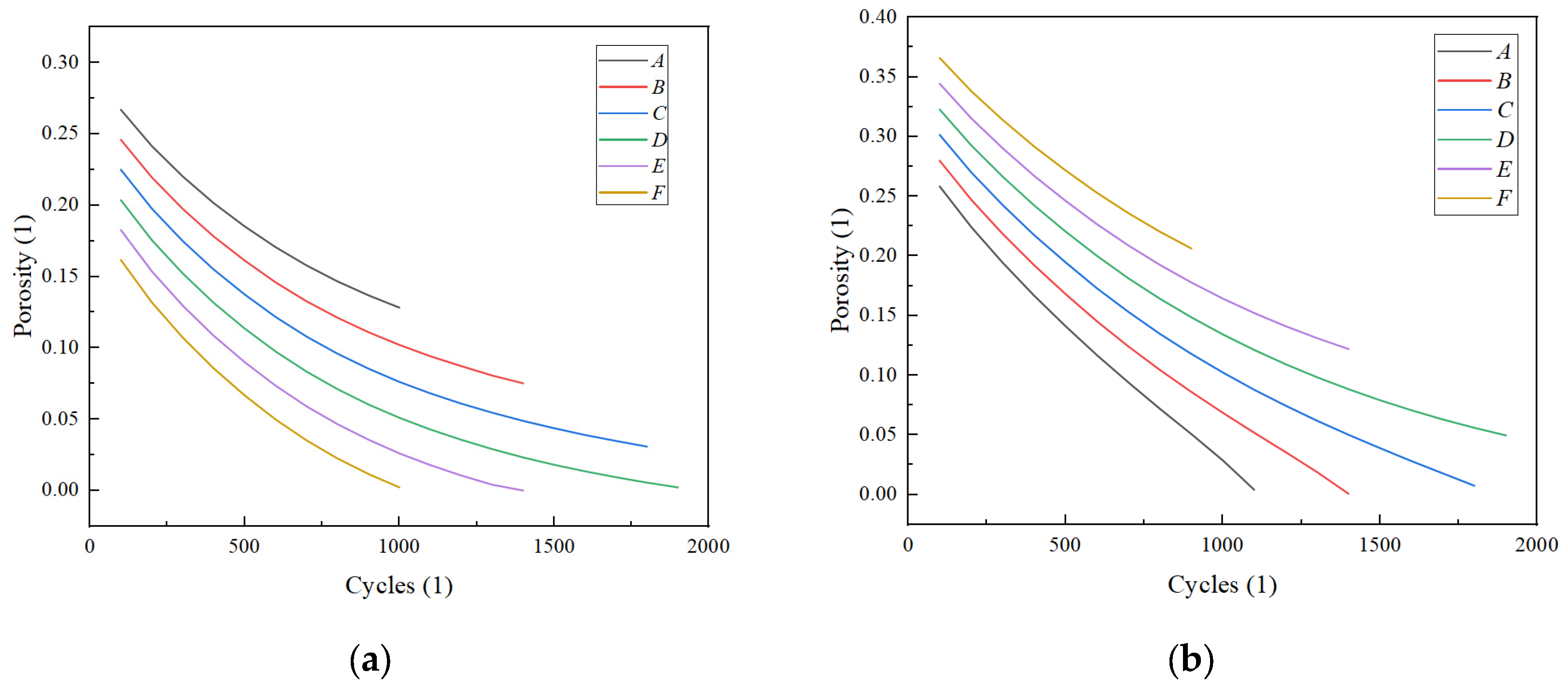
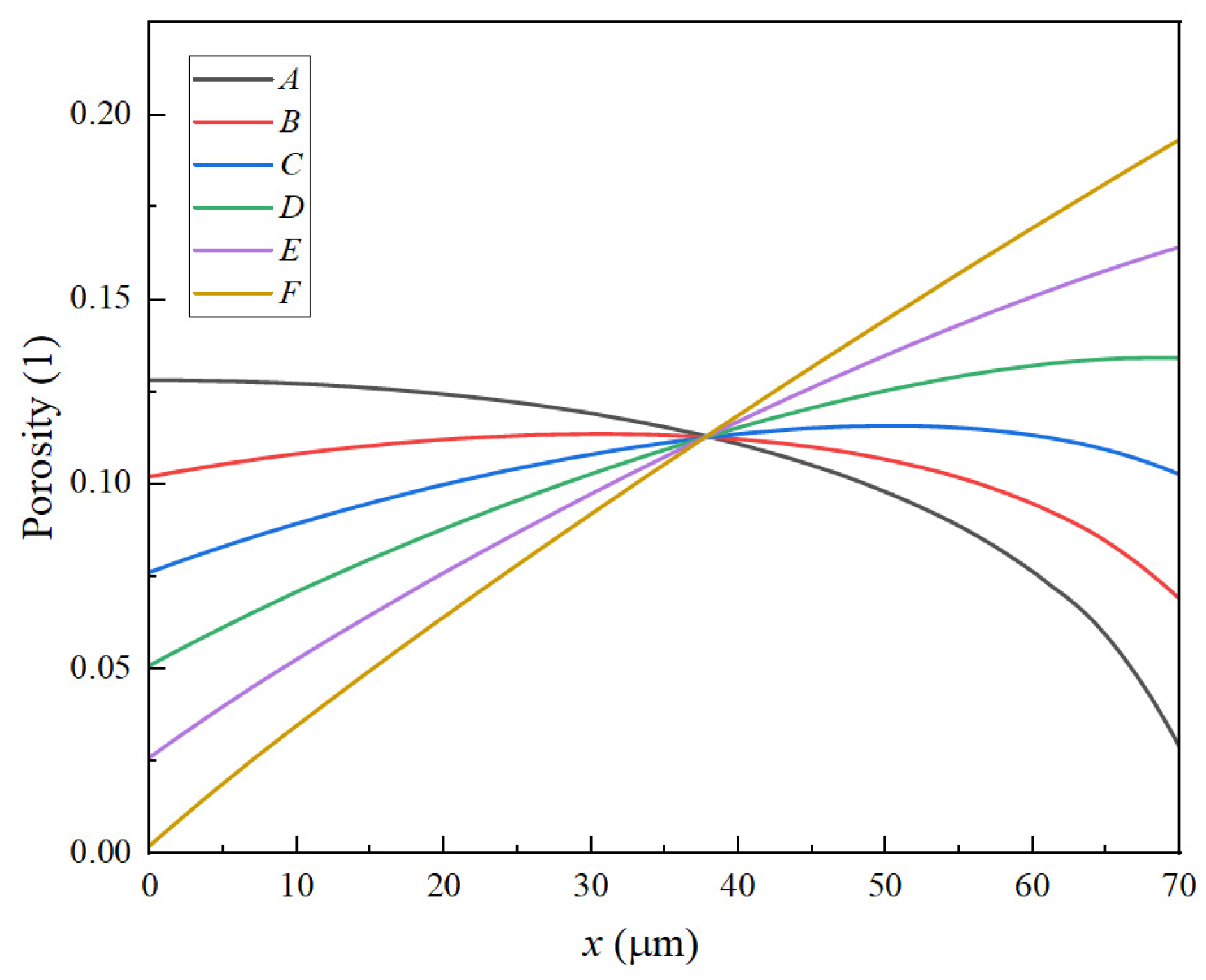
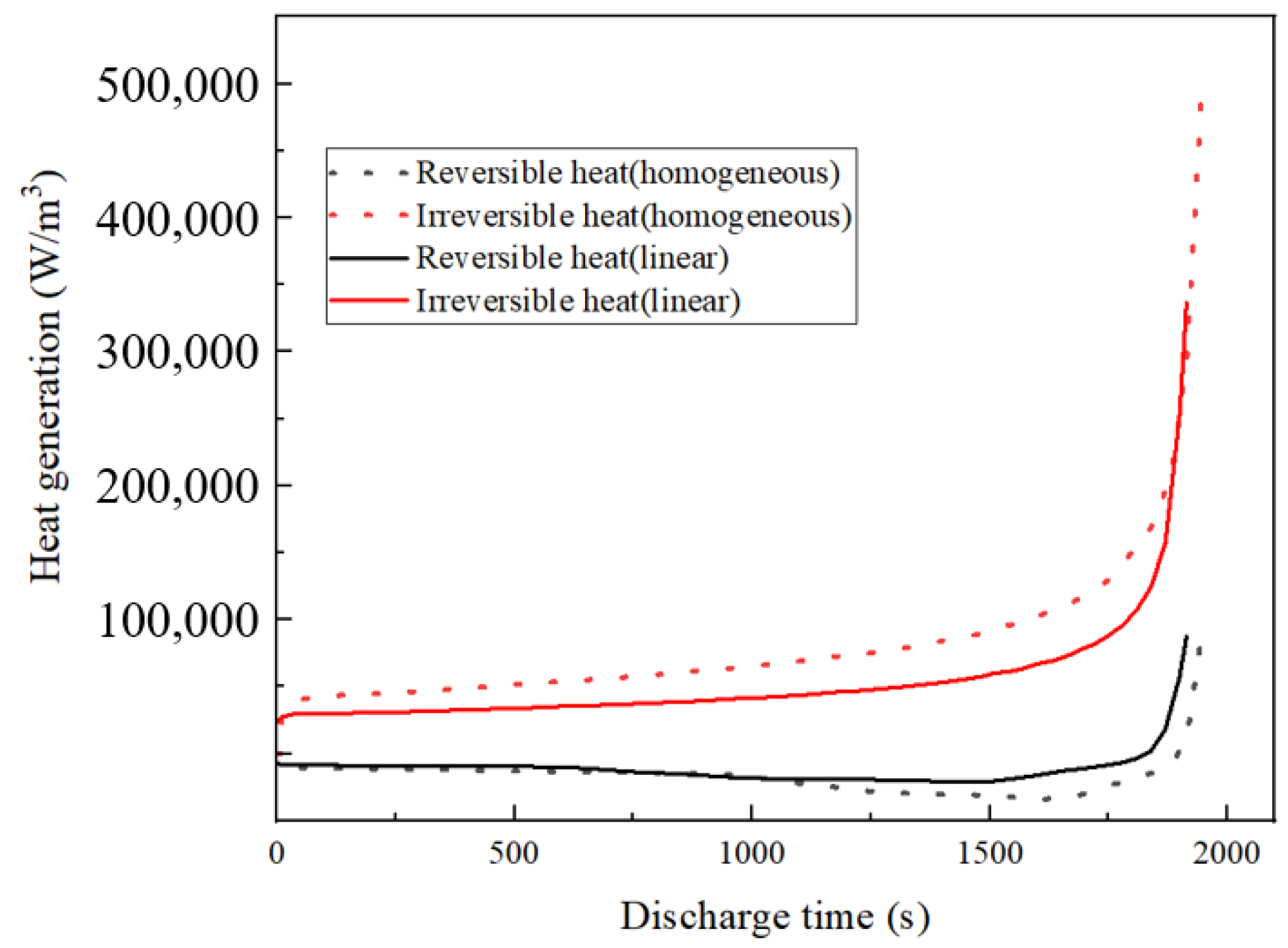
| Parameter | Cc (neg) | Neg | Sep | Pos | Cc (pos) |
|---|---|---|---|---|---|
| 0.4275 | |||||
| 0.59 | 0.61 | ||||
| 0.3 | 0.45 | 0.3 | |||
| 10 | 70 | 20 | 60 | 15 | |
| 10 | 4 | ||||
| 1000 | |||||
| 31,370 | 51,385 | ||||
| 0.8 | 0.4 | ||||
| 0.5 | 0.5 | ||||
| 1.5 | 4 | 1.5 | |||
| 50 | 25 | ||||
| 0.363 | |||||
| 96,487 | |||||
| 298.15 | |||||
| Parameters | Value |
|---|---|
| Reaction rate constant for SEI formation, (m s−1) | |
| Equilibrium potential SEI formation, (V) | 0.4 |
| Lower and upper voltage boundary for cycling, (V) | 2.75–4.2 |
| charge transfer coefficients for side reactions, | 0.5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, S.; Li, P.; Yu, R. Numerical Investigation of the Impact of Variation of Negative Electrode Porosity upon the Cycle Life of Lithium-Ion Batteries. Energies 2025, 18, 2883. https://doi.org/10.3390/en18112883
Li S, Li P, Yu R. Numerical Investigation of the Impact of Variation of Negative Electrode Porosity upon the Cycle Life of Lithium-Ion Batteries. Energies. 2025; 18(11):2883. https://doi.org/10.3390/en18112883
Chicago/Turabian StyleLi, Shuangchao, Peichao Li, and Runzhou Yu. 2025. "Numerical Investigation of the Impact of Variation of Negative Electrode Porosity upon the Cycle Life of Lithium-Ion Batteries" Energies 18, no. 11: 2883. https://doi.org/10.3390/en18112883
APA StyleLi, S., Li, P., & Yu, R. (2025). Numerical Investigation of the Impact of Variation of Negative Electrode Porosity upon the Cycle Life of Lithium-Ion Batteries. Energies, 18(11), 2883. https://doi.org/10.3390/en18112883






