Ammonia-Based Clean Energy Systems: A Review of Recent Progress and Key Challenges
Abstract
1. Introduction
2. Characteristics, Synthesis, and Storage and Transport of Ammonia Fuel
2.1. Physicochemical Properties of Ammonia
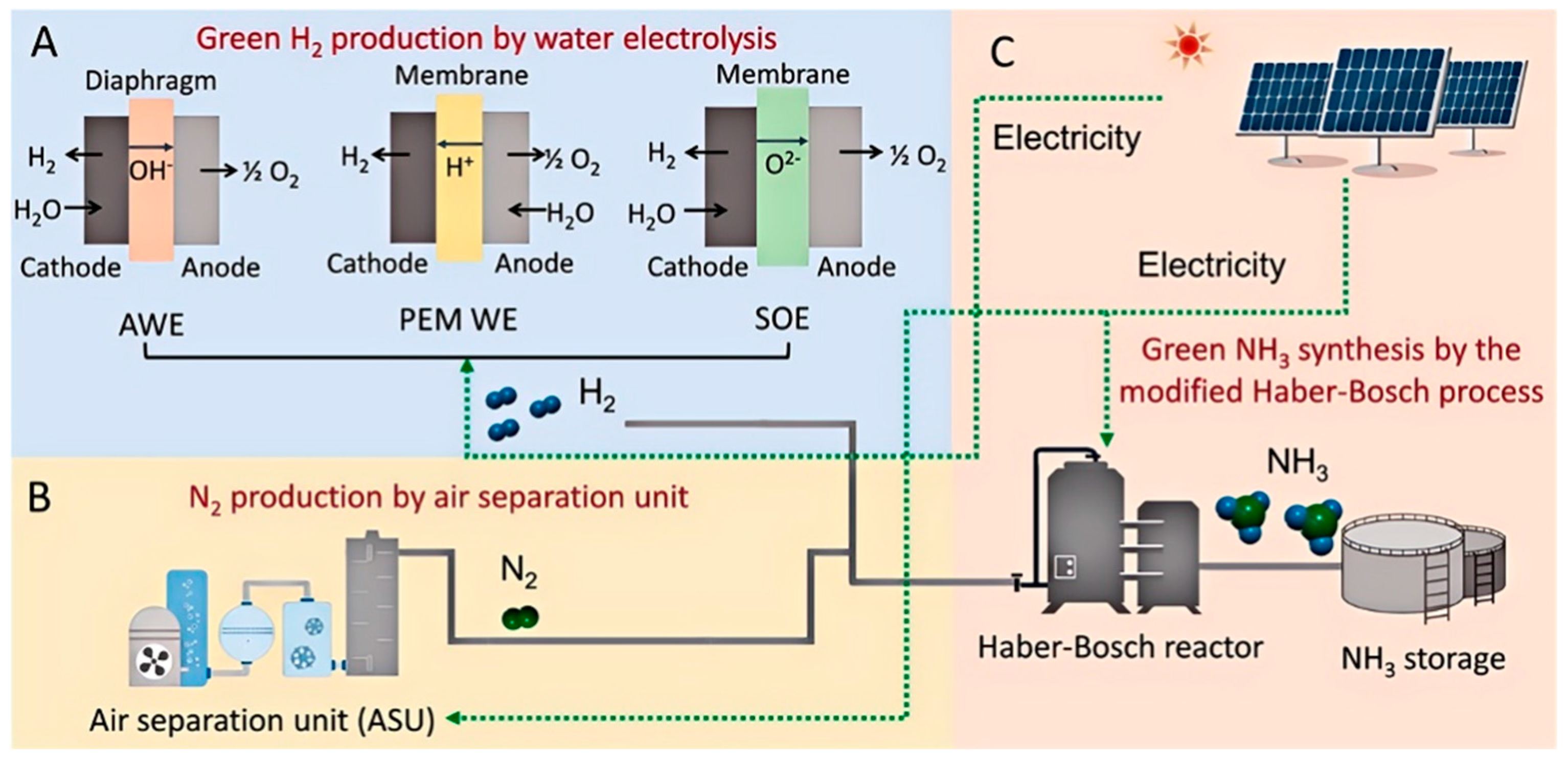
2.2. Synthesis Pathways of Ammonia
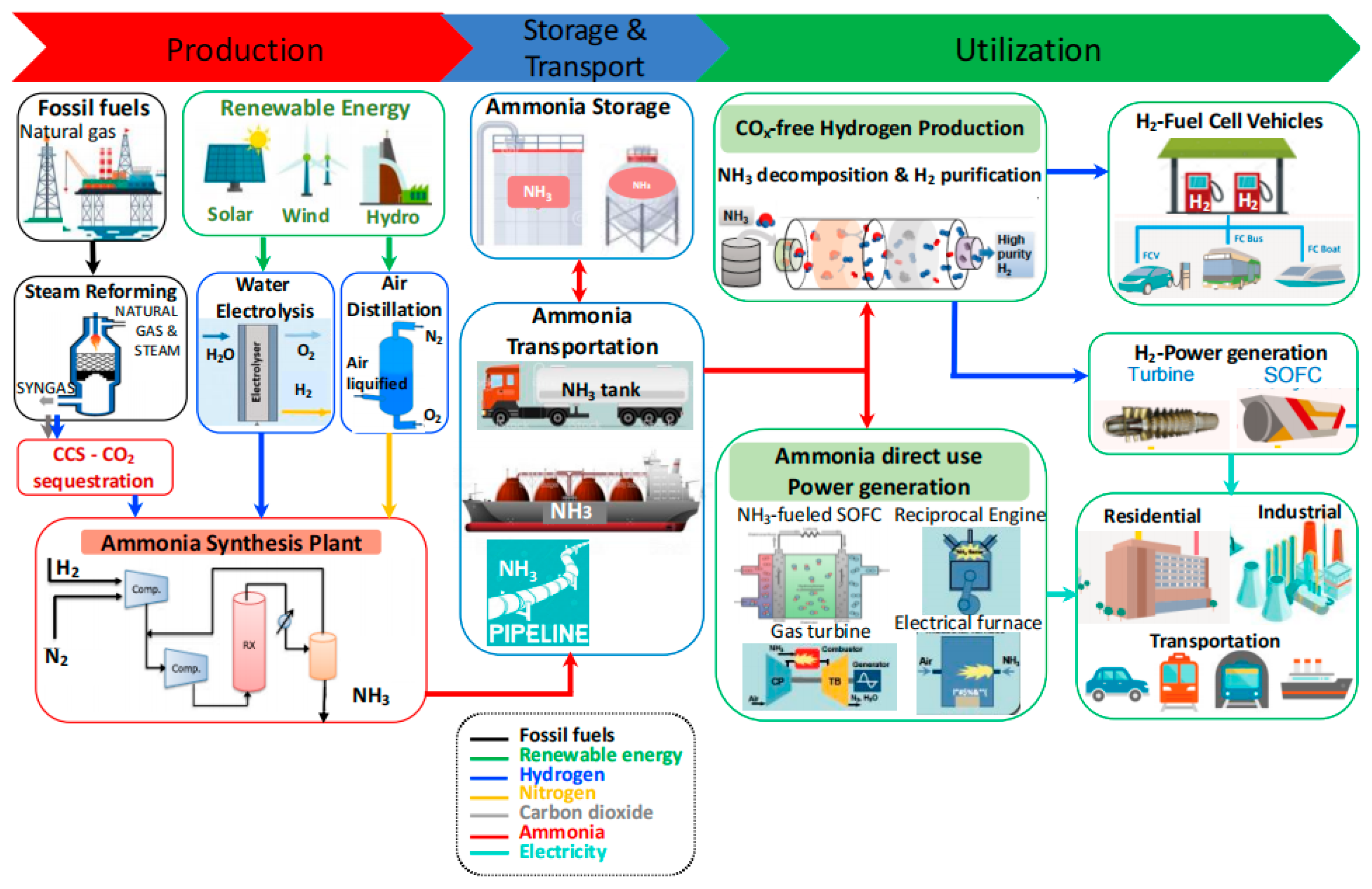
2.3. Storage and Transportation Strategies for Ammonia
2.3.1. Ammonia Storage Technologies
2.3.2. Transport Technologies for Ammonia
2.4. Section Summary
3. Combustion Mechanisms and Engineering Adaptability of Ammonia Fuel
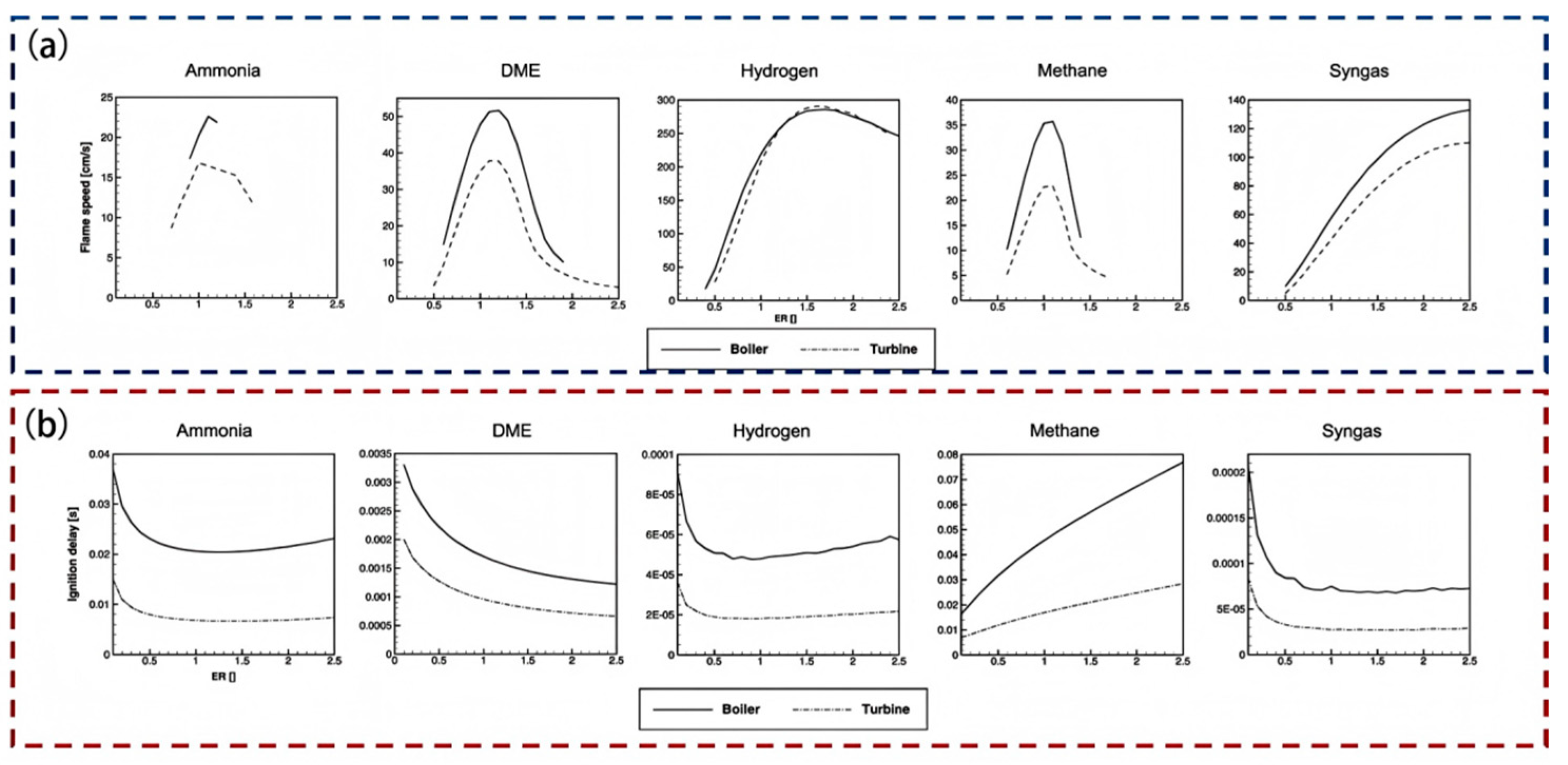
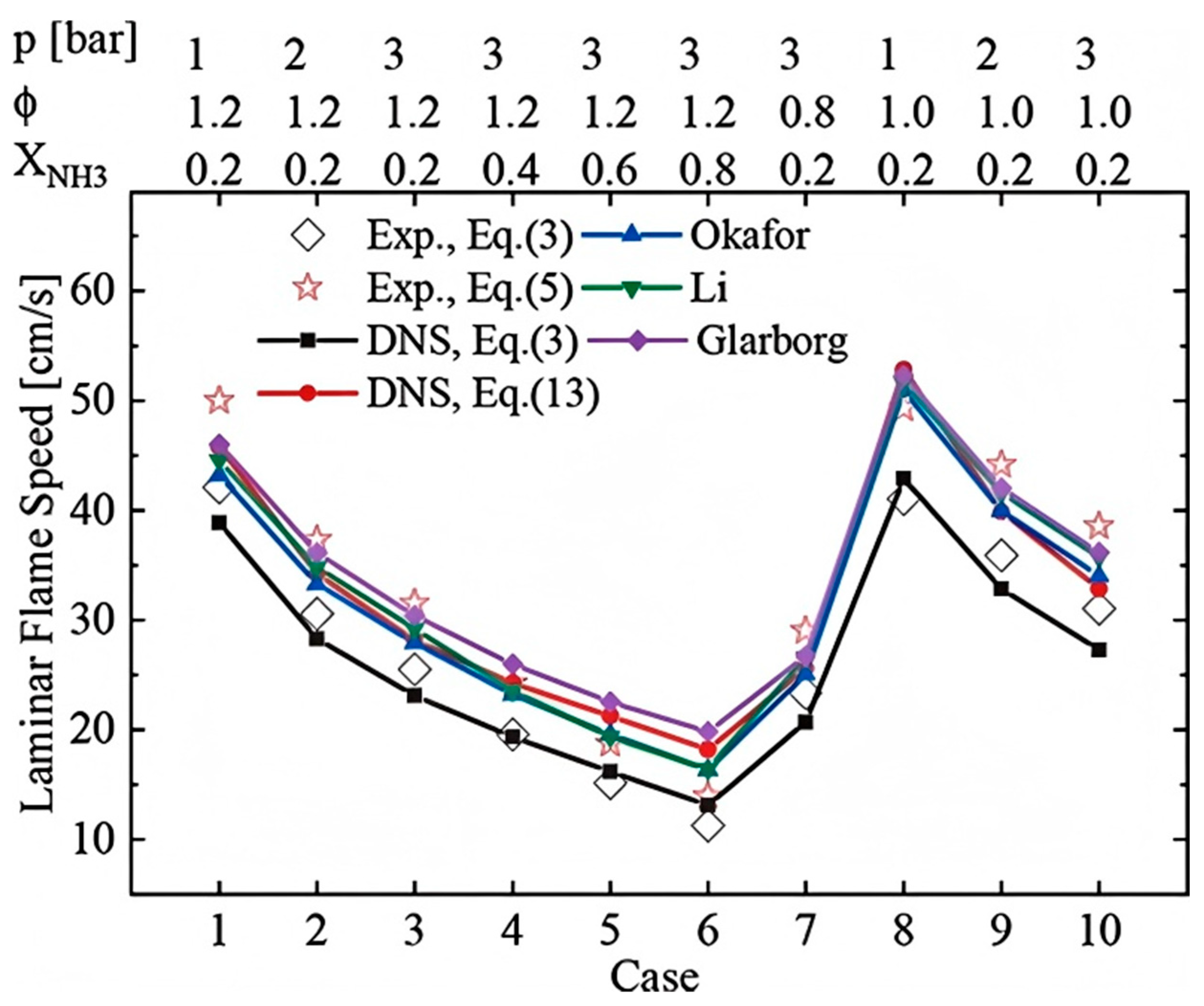
3.1. Combustion Characteristics of Ammonia Fuel
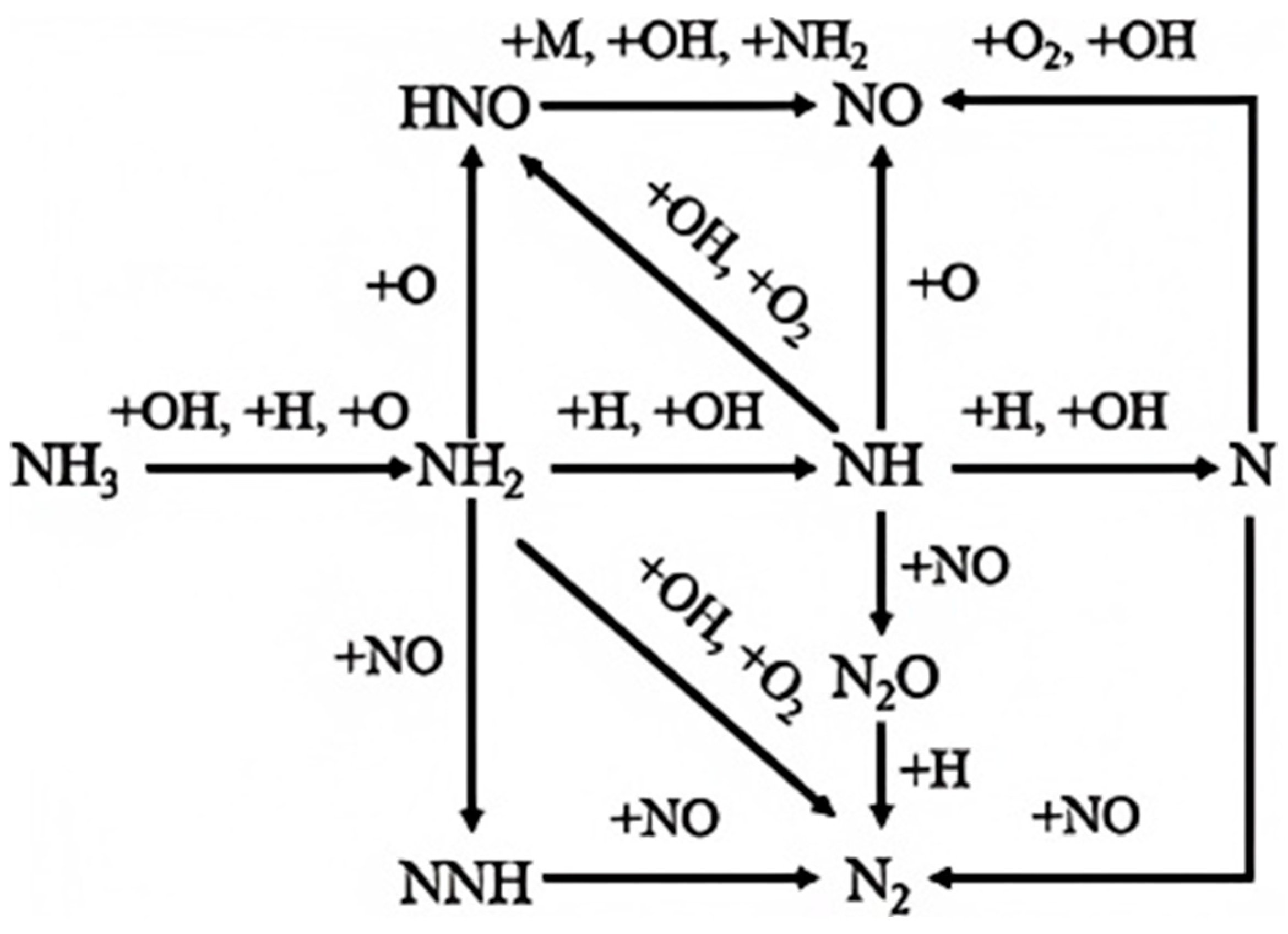
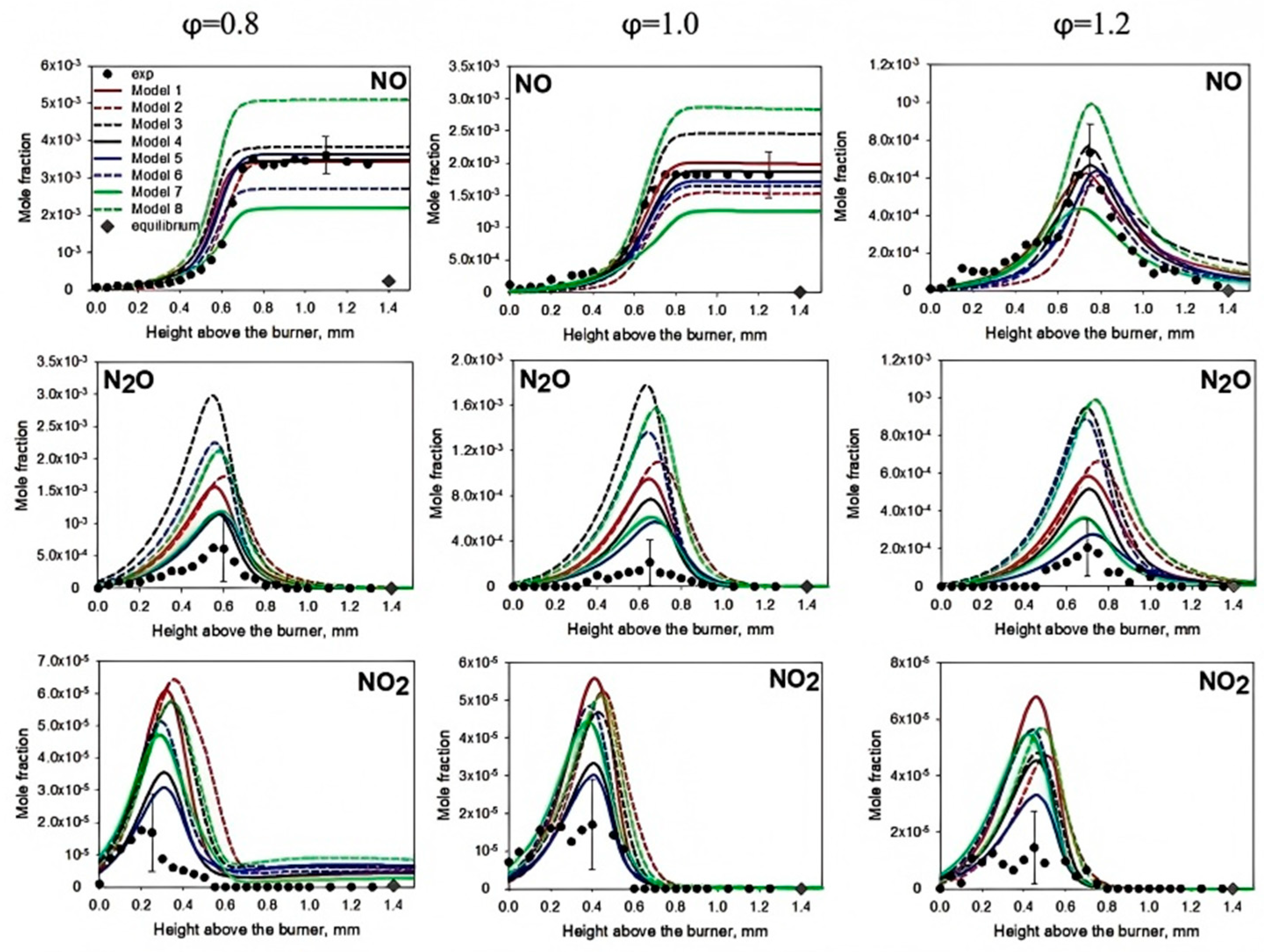
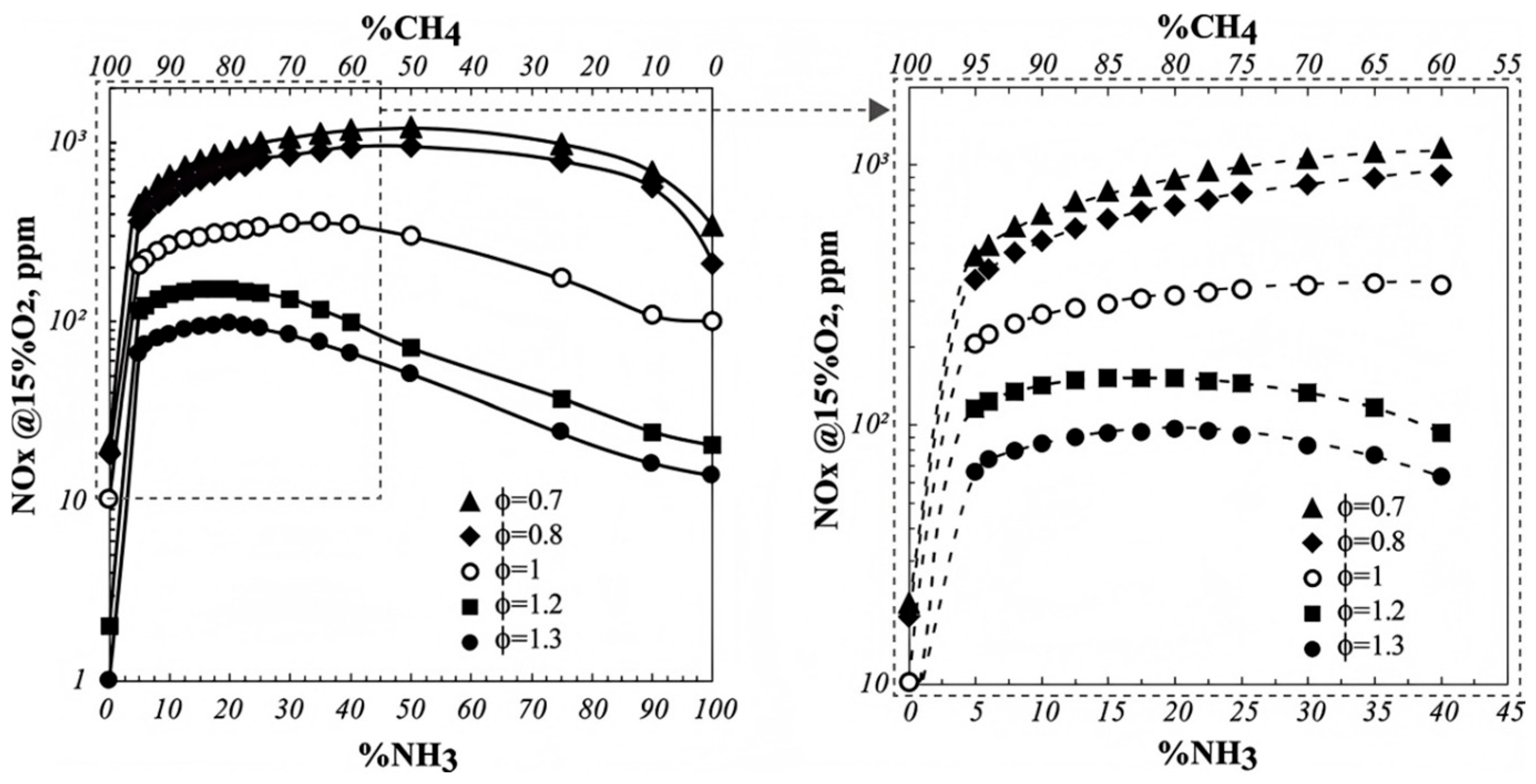
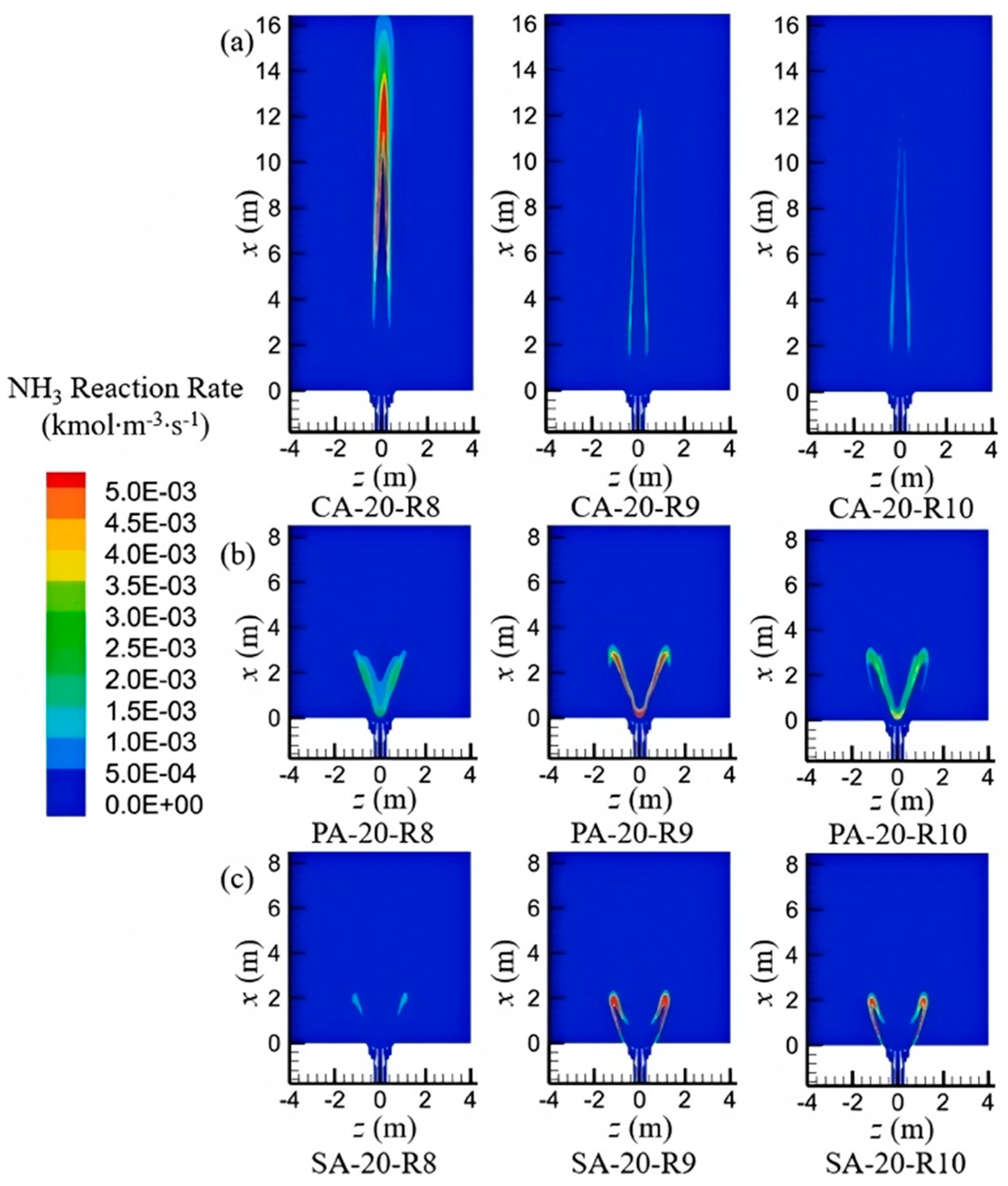
3.2. NOₓ Formation Mechanisms and Mitigation Strategies
3.3. Safety Management for Ammonia Utilization as a Fuel
3.4. Blended Combustion Strategies Involving Ammonia and Alternative Fuels
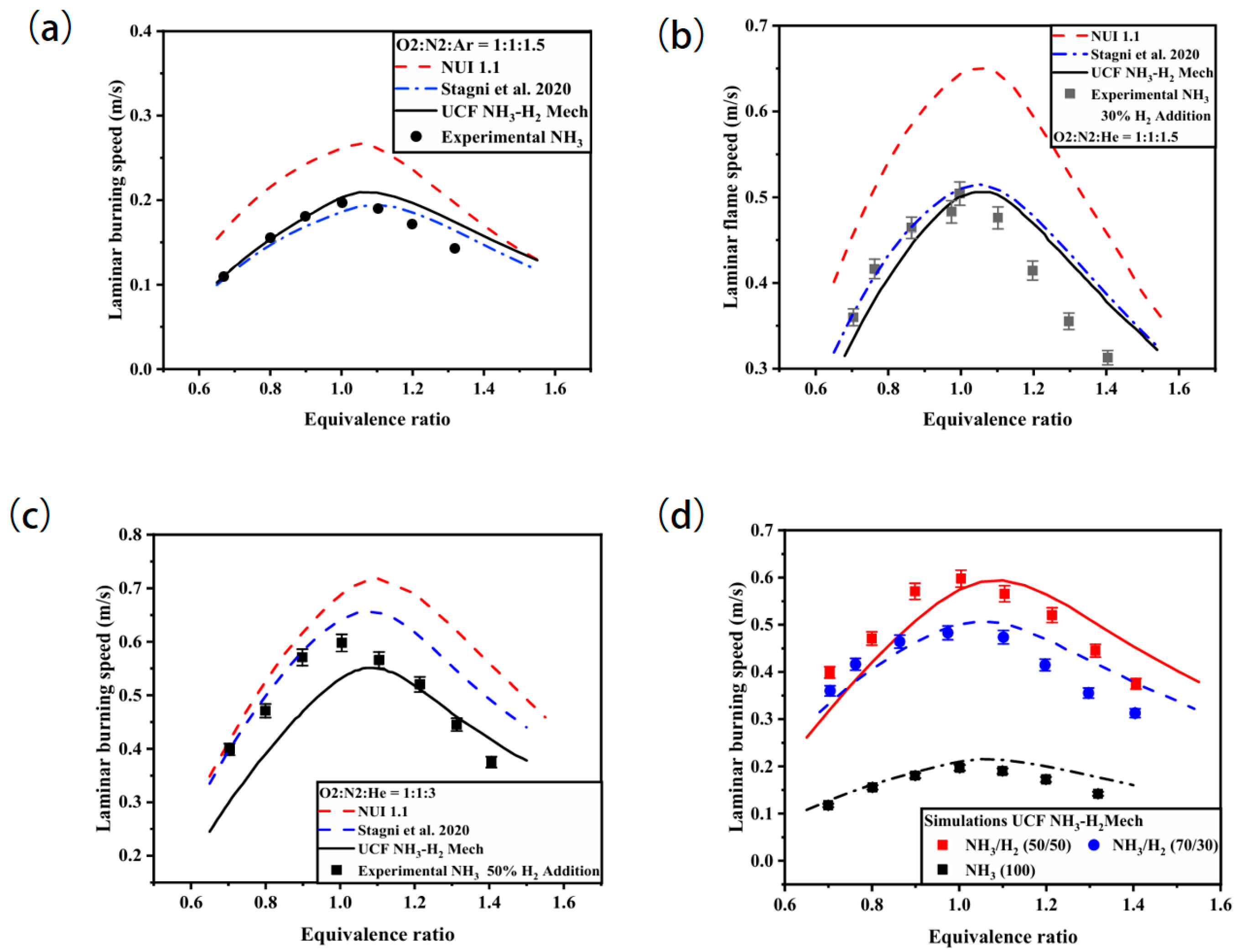
3.4.1. Ammonia–Hydrogen Blended Combustion
3.4.2. Ammonia–Methane Blended Combustion
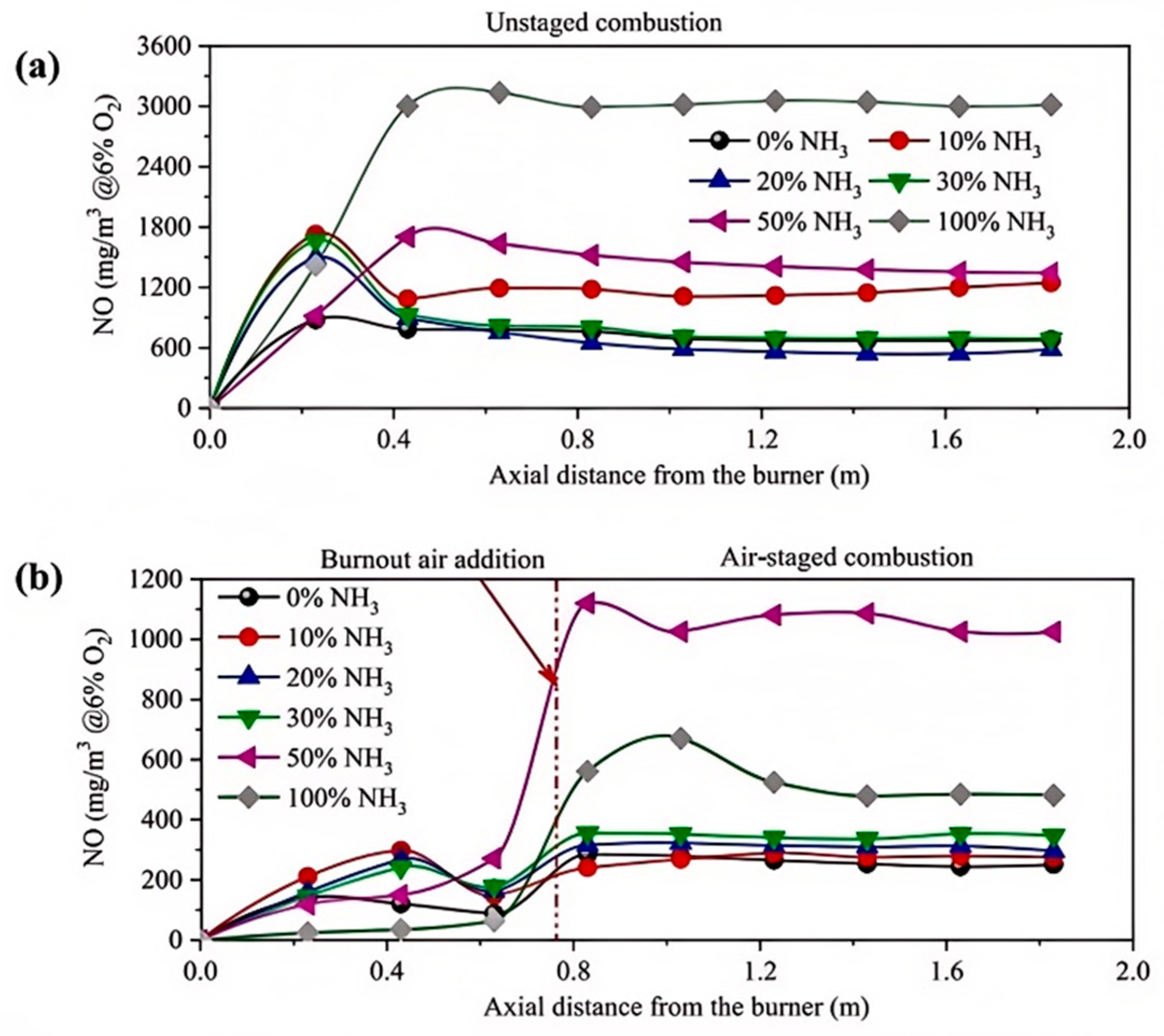
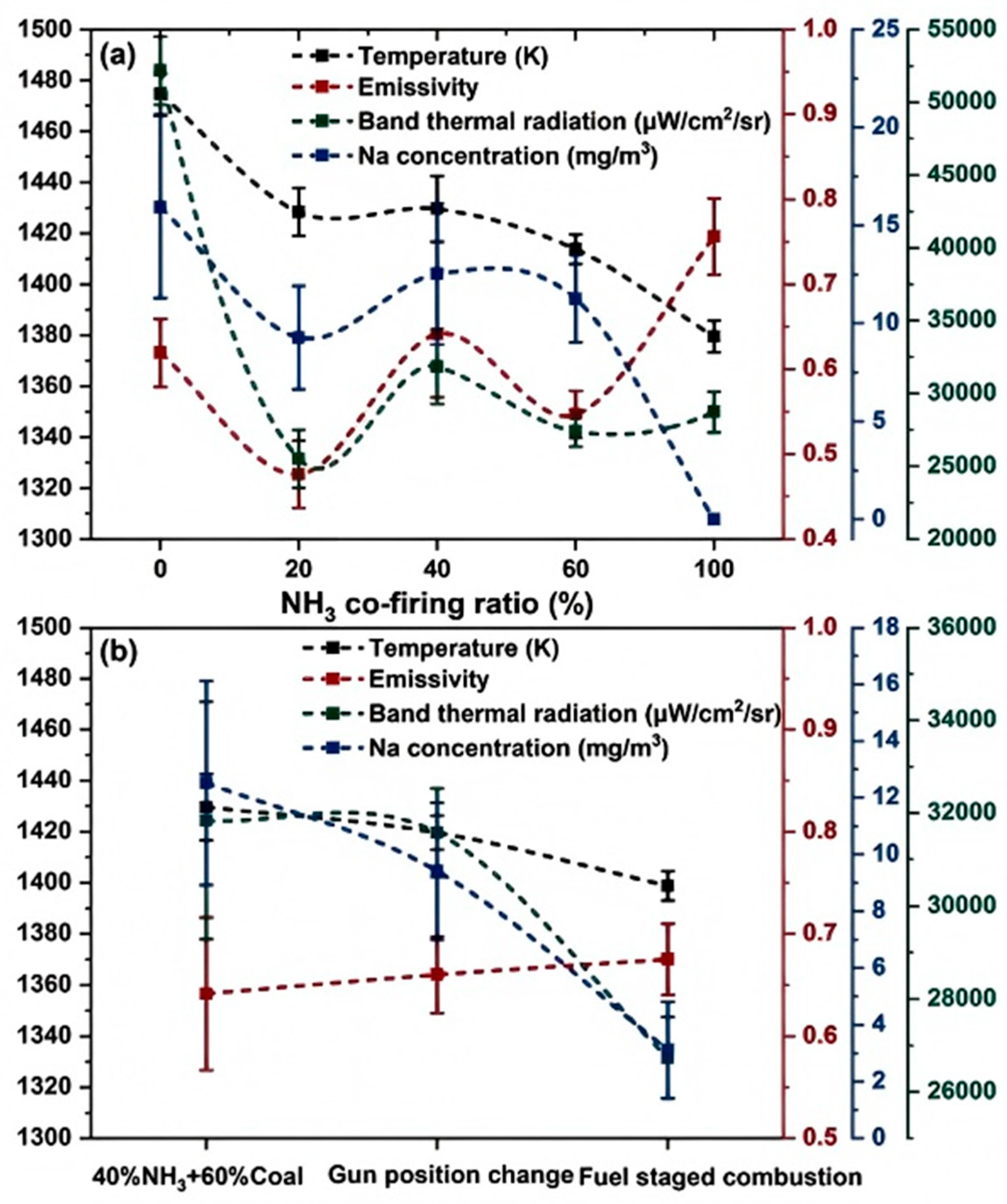
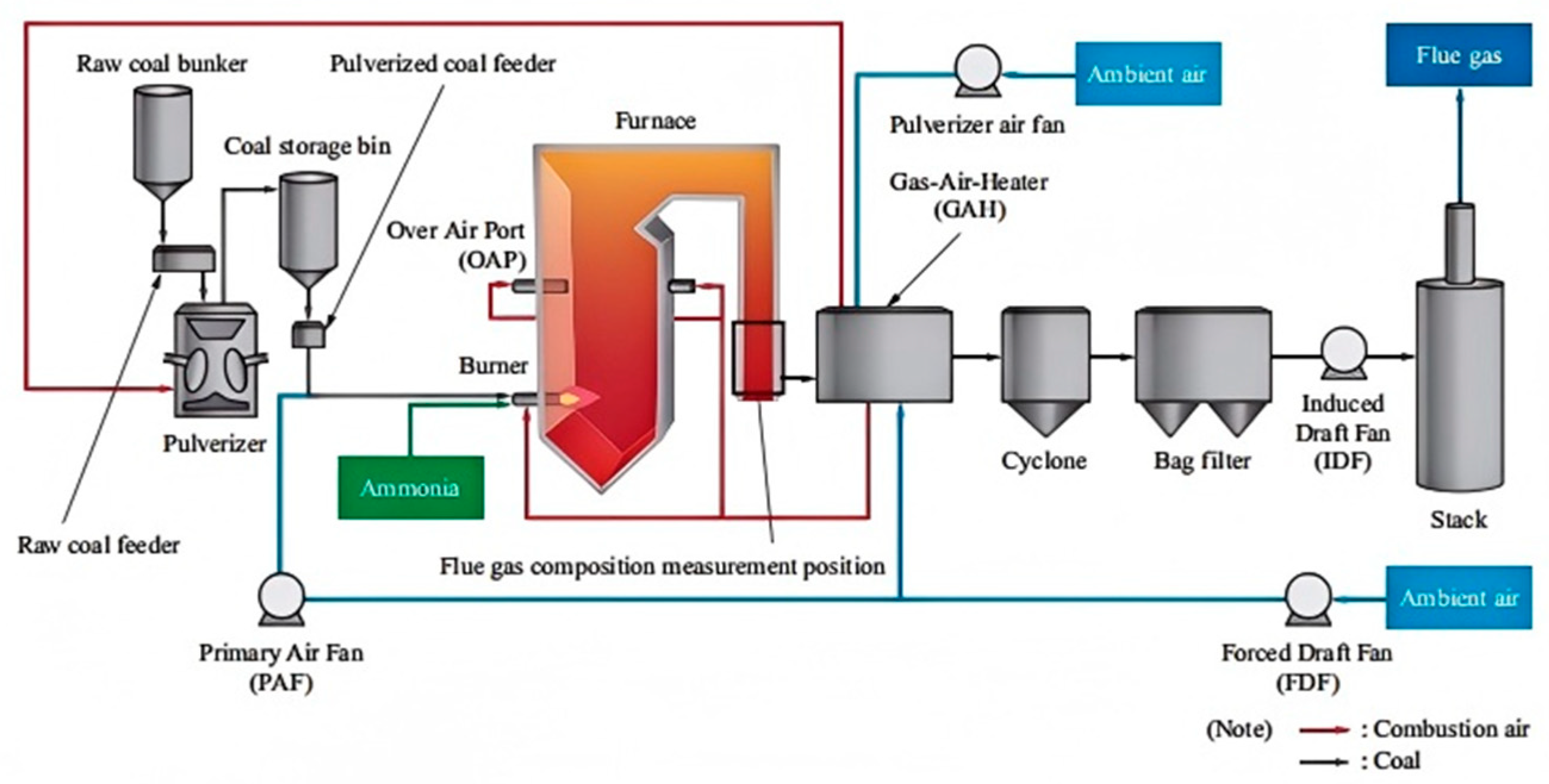
3.4.3. Ammonia–Coal Blended Combustion
3.4.4. Ammonia Blending with DME and Diesel
4. Engineering Applications of Ammonia Fuel in Representative Energy Systems
4.1. Applications and Demonstration Projects of Ammonia Fuel in Power Generation Systems
4.2. Demonstration and Pilot Projects of Ammonia Fuel in Marine Propulsion Systems
4.3. Ammonia as an Energy Carrier in Storage Systems: Chemical Storage and Fuel Cell Pathways
4.4. Section Summary
5. Future Prospects and Engineering Challenges of Ammonia-Based Clean Energy Systems
6. Summary
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Valera-Medina, A.; Xiao, H.; Owen-Jones, M.; David, W.I.; Bowen, P.J. Ammonia for power. Prog. Energy Combust. Sci. 2018, 69, 63–102. [Google Scholar] [CrossRef]
- Kobayashi, H.; Hayakawa, A.; Somarathne, K.K.A.; Okafor, E.C. Science and technology of ammonia combustion. Proc. Combust. Inst. 2019, 37, 109–133. [Google Scholar] [CrossRef]
- Dimitriou, P.; Javaid, R. A review of ammonia as a compression ignition engine fuel. Int. J. Hydrogen Energy 2020, 45, 7098–7118. [Google Scholar] [CrossRef]
- Giddey, S.; Badwal, S.P.; Munnings, C.; Dolan, M. Ammonia as a renewable energy transportation media. ACS Sustain. Chem. Eng. 2017, 5, 10231–10239. [Google Scholar] [CrossRef]
- Lan, R.; Irvine, J.T.; Tao, S. Ammonia and related chemicals as potential indirect hydrogen storage materials. Int. J. Hydrogen Energy 2012, 37, 1482–1494. [Google Scholar] [CrossRef]
- Reiter, A.J.; Kong, S.-C. Combustion and emissions characteristics of compression-ignition engine using dual ammonia-diesel fuel. Fuel 2011, 90, 87–97. [Google Scholar] [CrossRef]
- Zamfirescu, C.; Dincer, I. Using ammonia as a sustainable fuel. J. Power Sources 2008, 185, 459–465. [Google Scholar] [CrossRef]
- Mørch, C.S.; Bjerre, A.; Gøttrup, M.P.; Sorenson, S.C.; Schramm, J. Ammonia/hydrogen mixtures in an SI-engine: Engine performance and analysis of a proposed fuel system. Fuel 2011, 90, 854–864. [Google Scholar] [CrossRef]
- Aziz, M.; Wijayanta, A.T.; Nandiyanto, A.B.D. Ammonia as effective hydrogen storage: A review on production, storage and utilization. Energies 2020, 13, 3062. [Google Scholar] [CrossRef]
- Li, J.; Lai, S.; Chen, D.; Wu, R.; Kobayashi, N.; Deng, L.; Huang, H. A review on combustion characteristics of ammonia as a carbon-free fuel. Front. Energy Res. 2021, 9, 760356. [Google Scholar] [CrossRef]
- Ammonia, T. Zero-Carbon Fertilizer, Fuel and Energy Store: Policy Briefing; The Royal Society: London, UK, 2020. [Google Scholar]
- Kurien, C.; Mittal, M. Review on the production and utilization of green ammonia as an alternate fuel in dual-fuel compression ignition engines. Energy Convers. Manag. 2022, 251, 114990. [Google Scholar] [CrossRef]
- Lee, B.; Winter, L.R.; Lee, H.; Lim, D.; Lim, H.; Elimelech, M. Pathways to a green ammonia future. ACS Energy Lett. 2022, 7, 3032–3038. [Google Scholar] [CrossRef]
- Sarangi, P.K.; Srivastava, R.K.; Gitanjali, J.; Sathiyan, G.; Venkatesan, G.; Kandasamy, S. Exploring cutting-edge advances in green ammonia production and storage technologies. Fuel 2024, 371, 131863. [Google Scholar] [CrossRef]
- El-Shafie, M.; Kambara, S. Recent advances in ammonia synthesis technologies: Toward future zero carbon emissions. Int. J. Hydrogen Energy 2023, 48, 11237–11273. [Google Scholar] [CrossRef]
- Xiong, Y.; Li, B.; Gu, Y.; Yan, T.; Ni, Z.; Li, S.; Zuo, J.L.; Ma, J.; Jin, Z. Photocatalytic nitrogen fixation under an ambient atmosphere using a porous coordination polymer with bridging dinitrogen anions. Nat. Chem. 2023, 15, 286–293. [Google Scholar] [CrossRef]
- Jiang, M.; Zhu, Q.; Song, X.; Gu, Y.; Zhang, P.; Li, C.; Cui, J.; Ma, J.; Tie, Z.; Jin, Z. Batch-Scale Synthesis of Nanoparticle-Agminated Three-Dimensional Porous Cu@Cu(2)O Microspheres for Highly Selective Electrocatalysis of Nitrate to Ammonia. Environ. Sci. Technol. 2022, 56, 10299–10307. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Fang, L.; Bai, Y. Harnessing Heterogeneous Interface and Oxygen Vacancy in Cu/Cu2O for Efficient Electrocatalytic Nitrate Reduction to Ammonia. Energies 2024, 17, 4467. [Google Scholar] [CrossRef]
- Jiang, M.; Han, L.; Peng, P.; Hu, Y.; Xiong, Y.; Mi, C.; Tie, Z.; Xiang, Z.; Jin, Z. Quasi-Phthalocyanine Conjugated Covalent Organic Frameworks with Nitrogen-Coordinated Transition Metal Centers for High-Efficiency Electrocatalytic Ammonia Synthesis. Nano Lett. 2022, 22, 372–379. [Google Scholar] [CrossRef]
- Smith, C.; Hill, A.K.; Torrente-Murciano, L. Current and future role of Haber–Bosch ammonia in a carbon-free energy landscape. Energy Environ. Sci. 2020, 13, 331–344. [Google Scholar] [CrossRef]
- Jiang, M.; Tao, A.; Hu, Y.; Wang, L.; Zhang, K.; Song, X.; Yan, W.; Tie, Z.; Jin, Z. Crystalline Modulation Engineering of Ru Nanoclusters for Boosting Ammonia Electrosynthesis from Dinitrogen or Nitrate. ACS Appl. Mater. Interfaces 2022, 14, 17470–17478. [Google Scholar] [CrossRef]
- Tian, F.; Zhou, N.; Chen, W.; Zhan, J.; Tang, L.; Wu, M. Progress in Green Ammonia Synthesis Technology: Catalytic Behavior of Ammonia Synthesis Catalysts. Adv. Sustain. Syst. 2024, 8, 2300618. [Google Scholar] [CrossRef]
- Vieri, H.M.; Kim, M.-C.; Badakhsh, A.; Choi, S.H. Electrochemical Synthesis of Ammonia via Nitrogen Reduction and Oxygen Evolution Reactions—A Comprehensive Review on Electrolyte-Supported Cells. Energies 2024, 17, 441. [Google Scholar] [CrossRef]
- Rouwenhorst, K.H.R.; Krzywda, P.M.; Benes, N.E.; Mul, G.; Lefferts, L. Ammonia production technologies. In Techno-Economic Challenges of Green Ammonia as an Energy Vector; Elsevier: Amsterdam, The Netherlands, 2021; pp. 41–83. [Google Scholar]
- Khaksar, S.A.N.; Rahimpour, H.R.; Rahimpour, M.R. Ammonia storage and transportation. In Progresses in Ammonia: Science, Technology and Membranes; Elsevier: Amsterdam, The Netherlands, 2024; pp. 251–270. [Google Scholar]
- Ghavam, S.; Vahdati, M.; Wilson, I.; Styring, P. Sustainable ammonia production processes. Front. Energy Res. 2021, 9, 580808. [Google Scholar] [CrossRef]
- Chang, F.; Gao, W.; Guo, J.; Chen, P. Emerging Materials and Methods toward Ammonia-Based Energy Storage and Conversion. Adv. Mater. 2021, 33, e2005721. [Google Scholar] [CrossRef]
- Wang, Y.; Xiao, X.; Ji, Y. A Review of LCA Studies on Marine Alternative Fuels: Fuels, Methodology, Case Studies, and Recommendations. J. Mar. Sci. Eng. 2025, 13, 196. [Google Scholar] [CrossRef]
- Song, M.; Wang, Q.; Wang, Z.; Fang, Y.; Qu, W.; Gong, Z.; Feng, L. Auto-ignition characteristics and chemical reaction mechanism of ammonia/n-heptane mixtures with low n-heptane content. Fuel 2024, 364, 131011. [Google Scholar] [CrossRef]
- Kim, J.H.; Kim, T.W.; Kim, Y.H.; Kwon, O.C. Combustion characteristics of premixed ammonia-hydrogen/air swirl flames at elevated pressure. Int. J. Hydrogen Energy 2024, 74, 423–433. [Google Scholar] [CrossRef]
- Taneja, T.S.; Johnson, P.N.; Yang, S. Nanosecond pulsed plasma assisted combustion of ammonia-air mixtures: Effects on ignition delays and NOx emission. Combust. Flame 2022, 245, 112327. [Google Scholar] [CrossRef]
- Mong, G.R.; Chiong, M.-C.; Chong, C.T.; Ng, J.-H.; Mashruk, S.; Tran, M.-V.; Lee, K.M.; Samiran, N.A.; Wong, K.Y.; Valera-Medina, A. Fuel-lean ammonia/biogas combustion characteristics under the reacting swirl flow conditions. Fuel 2023, 331, 125983. [Google Scholar] [CrossRef]
- Bazooyar, B.; Coomson, G.; Manovic, V.; Nabavi, S.A. Comparative analysis of ammonia combustion for domestic applications. J. Energy Inst. 2023, 106, 101130. [Google Scholar] [CrossRef]
- Mathieu, O.; Petersen, E.L. Experimental and modeling study on the high-temperature oxidation of Ammonia and related NOx chemistry. Combust. Flame 2015, 162, 554–570. [Google Scholar] [CrossRef]
- Zhang, Y.; Mathieu, O.; Petersen, E.L.; Bourque, G.; Curran, H.J. Assessing the predictions of a NOx kinetic mechanism on recent hydrogen and syngas experimental data. Combust. Flame 2017, 182, 122–141. [Google Scholar] [CrossRef]
- Pochet, M.; Dias, V.; Moreau, B.; Foucher, F.; Jeanmart, H.; Contino, F. Experimental and numerical study, under LTC conditions, of ammonia ignition delay with and without hydrogen addition. Proc. Combust. Inst. 2019, 37, 621–629. [Google Scholar] [CrossRef]
- Joo, J.; Lee, S.; Kwon, O. Effects of ammonia substitution on combustion stability limits and NOx emissions of premixed hydrogen–air flames. Int. J. Hydrogen Energy 2012, 37, 6933–6941. [Google Scholar] [CrossRef]
- Silverman, L.; Whittenbbrger, J.; Muller, J. Physiologieal Response of Man to Ammonia in Low Concentrations. J. Ind. Hyg. Toxicol. 1949, 31, 74–78. [Google Scholar]
- Vignat, G.; Akoush, B.; Toro, E.R.; Boigné, E.; Ihme, M. Combustion of lean ammonia-hydrogen fuel blends in a porous media burner. Proc. Combust. Inst. 2023, 39, 4195–4204. [Google Scholar] [CrossRef]
- Chai, W.S.; Bao, Y.; Jin, P.; Tang, G.; Zhou, L. A review on ammonia, ammonia-hydrogen and ammonia-methane fuels. Renew. Sustain. Energy Rev. 2021, 147, 111254. [Google Scholar] [CrossRef]
- Yovino, L.; Kim, G.; Rahman, R.K.; Pierro, M.; Vasu, S.S.; Winquist, M.; Subbaraman, G.; Steele, R. Flame Speed Measurements of Ammonia–Hydrogen Mixtures for Gas-Turbines. J. Eng. Gas Turbines Power 2025, 147, 031030. [Google Scholar] [CrossRef]
- Meng, X.; Qin, M.; Liu, L.; Wei, F.; Tian, J.; Long, W.; Bi, M. Visualization and simulation study of ammonia blending with hydrogen as combustion application in lean-burn condition. Fuel 2024, 357, 129812. [Google Scholar] [CrossRef]
- Zhao, D.; Gao, W.; Li, Y.; Fu, Z.; Hua, X.; Zhang, Y. Simulation Study on Combustion Performance of Ammonia-Hydrogen Fuel Engines. Energies 2024, 17, 2337. [Google Scholar] [CrossRef]
- Bayramoğlu, K.; Bahlekeh, A.; Masera, K. Numerical investigation of the hydrogen, ammonia and methane fuel blends on the combustion emissions and performance. Int. J. Hydrogen Energy 2023, 48, 39586–39598. [Google Scholar] [CrossRef]
- Osipova, K.N.; Sarathy, S.M.; Korobeinichev, O.P.; Shmakov, A.G. Chemical structure of premixed ammonia/hydrogen flames at elevated pressures. Combust. Flame 2022, 246, 112419. [Google Scholar] [CrossRef]
- Fąfara, J.-M. CFD study case of ammonia-hydrogen mixture powered methane gas microturbine combustor in the context of the temperature repartition modifications. Combust. Engines 2025, 200, 78–86. [Google Scholar] [CrossRef]
- Novella, R.; Pastor, J.; Gómez-Soriano, J.; Sánchez-Bayona, J. Numerical study on the use of ammonia/hydrogen fuel blends for automotive spark-ignition engines. Fuel 2023, 351, 128945. [Google Scholar] [CrossRef]
- Tang, G.; Jin, P.; Bao, Y.; Chai, W.S.; Zhou, L. Experimental investigation of premixed combustion limits of hydrogen and methane additives in ammonia. Int. J. Hydrogen Energy 2021, 46, 20765–20776. [Google Scholar] [CrossRef]
- Chen, J.; Jiang, X.; Qin, X.; Huang, Z. Effect of hydrogen blending on the high temperature auto-ignition of ammonia at elevated pressure. Fuel 2021, 287, 119563. [Google Scholar] [CrossRef]
- Xiao, H.; Valera-Medina, A.; Bowen, P.J. Study on premixed combustion characteristics of co-firing ammonia/methane fuels. Energy 2017, 140, 125–135. [Google Scholar] [CrossRef]
- Rocha, R.C.; Zhong, S.; Xu, L.; Bai, X.-S.; Costa, M.; Cai, X.; Kim, H.; Brackmann, C.; Li, Z.; Alden, M. Structure and laminar flame speed of an ammonia/methane/air premixed flame under varying pressure and equivalence ratio. Energy Fuels 2021, 35, 7179–7192. [Google Scholar] [CrossRef]
- Ariemma, G.B.; Sorrentino, G.; Ragucci, R.; de Joannon, M.; Sabia, P. Ammonia/Methane combustion: Stability and NOx emissions. Combust. Flame 2022, 241, 112071. [Google Scholar] [CrossRef]
- Lin, Q.; Sun, W.; Li, H.; Liu, Y.; Chen, Y.; Liu, C.; Jiang, Y.; Cheng, Y.; Ma, N.; Ya, H. Experimental study on ammonia Co-firing with coal for carbon reduction in the boiler of a 300-MW coal-fired power station. Engineering 2024, 40, 247–259. [Google Scholar] [CrossRef]
- Pu, Y.; Jia, Z.; Wang, Z.; Yao, B.; Lou, C.; Li, Y. Experimental study of combustion characteristics and ash-related issues of ammonia co-firing with high alkali pulverized coal in a 4 MW boiler. Proc. Combust. Inst. 2024, 40, 105642. [Google Scholar] [CrossRef]
- Wu, X.; Hu, F.; Ding, C.; Yang, Y.; Yang, C.; Liao, H.; Lu, K.; Li, B.; Liu, T.; Liu, C. Progress in numerical simulations and fundamental characteristics of pulverized coal co-firing with ammonia. Int. J. Hydrogen Energy 2024, 82, 740–758. [Google Scholar] [CrossRef]
- Liu, M.; Chen, S.; Zhu, H.; Zhou, Z.; Xu, J. Numerical investigation of ammonia/coal co-combustion in a low NOx swirl burner. Energy 2023, 282, 128358. [Google Scholar] [CrossRef]
- Chen, C.; Wang, Z.; Zhu, R.; Tan, J.; He, Y.; Cen, K. Co-firing characteristics and fuel-N transformation of ammonia/pulverized coal binary fuel. Fuel 2023, 337, 126857. [Google Scholar] [CrossRef]
- Zha, X.; Zhang, Z.; Zhao, Z.; Yang, L.; Mao, W.; Wu, F.; Li, X.; Luo, C.; Zhang, L. Comparative study on co-firing characteristics of normal and superfine pulverized coal blended with NH3 under the MILD combustion mode. Energy 2024, 305, 132206. [Google Scholar] [CrossRef]
- Wang, X.; Fan, W.; Chen, J.; Feng, G.; Zhang, X. Experimental study and kinetic analysis of the impact of ammonia co-firing ratio on products formation characteristics in ammonia/coal co-firing process. Fuel 2022, 329, 125496. [Google Scholar] [CrossRef]
- Jin, W.; Si, F.; Cao, Y.; Yu, C.; Wang, J. Numerical research on ammonia-coal co-firing in a 1050 MW coal-fired utility boiler under ultra-low load: Effects of ammonia ratio and air staging condition. Appl. Therm. Eng. 2023, 233, 121100. [Google Scholar] [CrossRef]
- Cai, T.; Zhao, D. Enhancing and assessing ammonia-air combustion performance by blending with dimethyl ether. Renew. Sustain. Energy Rev. 2022, 156, 112003. [Google Scholar] [CrossRef]
- Cai, T.; Zhao, D. Temperature dependence of laminar burning velocity in ammonia/dimethyl ether-air premixed flames. J. Therm. Sci. 2022, 31, 189–197. [Google Scholar] [CrossRef]
- Chen, C.; Zhou, Y.; Fang, H.; Peng, X.; Jiang, L. Progress and challenges in energy storage and utilization via ammonia. Surf. Sci. Technol. 2023, 1, 13. [Google Scholar] [CrossRef]
- Nagatani, G.; Ishi, H.; Ito, T.; Ohno, E.; Okuma, Y. Development of co-firing method of pulverized coal and ammonia to reduce greenhouse gas emissions. IHI Eng. Rev. 2020, 53, 1–10. [Google Scholar]
- Xu, X.; Liu, E.; Zhu, N.; Liu, F.; Qian, F. Review of the current status of ammonia-blended hydrogen fuel engine development. Energies 2022, 15, 1023. [Google Scholar] [CrossRef]
- Morlanés, N.; Katikaneni, S.P.; Paglieri, S.N.; Harale, A.; Solami, B.; Sarathy, S.M.; Gascon, J. A technological roadmap to the ammonia energy economy: Current state and missing technologies. Chem. Eng. J. 2021, 408, 127310. [Google Scholar] [CrossRef]
- Cheliotis, M.; Boulougouris, E.; Trivyza, N.L.; Theotokatos, G.; Livanos, G.; Mantalos, G.; Stubos, A.; Stamatakis, E.; Venetsanos, A. Review on the safe use of ammonia fuel cells in the maritime industry. Energies 2021, 14, 3023. [Google Scholar] [CrossRef]
- Braun, A.; Gierenz, N.; Braun, S.; Kubach, H.; Bernhardt, S.; Prehn, S.; Müller, M.; Engelmeier, L.; Fehlemann, L.; Steffen, M. Aspects of ammonia as green fuel for propulsion systems of inland water vessels. Energy Technol. 2025, 13, 2301648. [Google Scholar] [CrossRef]
- Zhao, Y.; Setzler, B.P.; Wang, J.; Nash, J.; Wang, T.; Xu, B.; Yan, Y. An efficient direct ammonia fuel cell for affordable carbon-neutral transportation. Joule 2019, 3, 2472–2484. [Google Scholar] [CrossRef]
- Machaj, K.; Kupecki, J.; Malecha, Z.; Morawski, A.; Skrzypkiewicz, M.; Stanclik, M.; Chorowski, M. Ammonia as a potential marine fuel: A review. Energy Strategy Rev. 2022, 44, 100926. [Google Scholar] [CrossRef]
- Al-Shetwi, A.Q.; Atawi, I.E.; Abuelrub, A.; Hannan, M. Techno-economic assessment and optimal design of hybrid power generation-based renewable energy systems. Technol. Soc. 2023, 75, 102352. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, M.; Ling, Z.; Mao, J.; Zeng, X.; Yuan, D.; Liu, M. Ammonia-Based Clean Energy Systems: A Review of Recent Progress and Key Challenges. Energies 2025, 18, 2845. https://doi.org/10.3390/en18112845
Sun M, Ling Z, Mao J, Zeng X, Yuan D, Liu M. Ammonia-Based Clean Energy Systems: A Review of Recent Progress and Key Challenges. Energies. 2025; 18(11):2845. https://doi.org/10.3390/en18112845
Chicago/Turabian StyleSun, Mengwei, Zhongqian Ling, Jiani Mao, Xianyang Zeng, Dingkun Yuan, and Maosheng Liu. 2025. "Ammonia-Based Clean Energy Systems: A Review of Recent Progress and Key Challenges" Energies 18, no. 11: 2845. https://doi.org/10.3390/en18112845
APA StyleSun, M., Ling, Z., Mao, J., Zeng, X., Yuan, D., & Liu, M. (2025). Ammonia-Based Clean Energy Systems: A Review of Recent Progress and Key Challenges. Energies, 18(11), 2845. https://doi.org/10.3390/en18112845






