State and Perspectives of Biomethane Production and Use—A Systematic Review
Abstract
1. Introduction
2. Materials and Methods
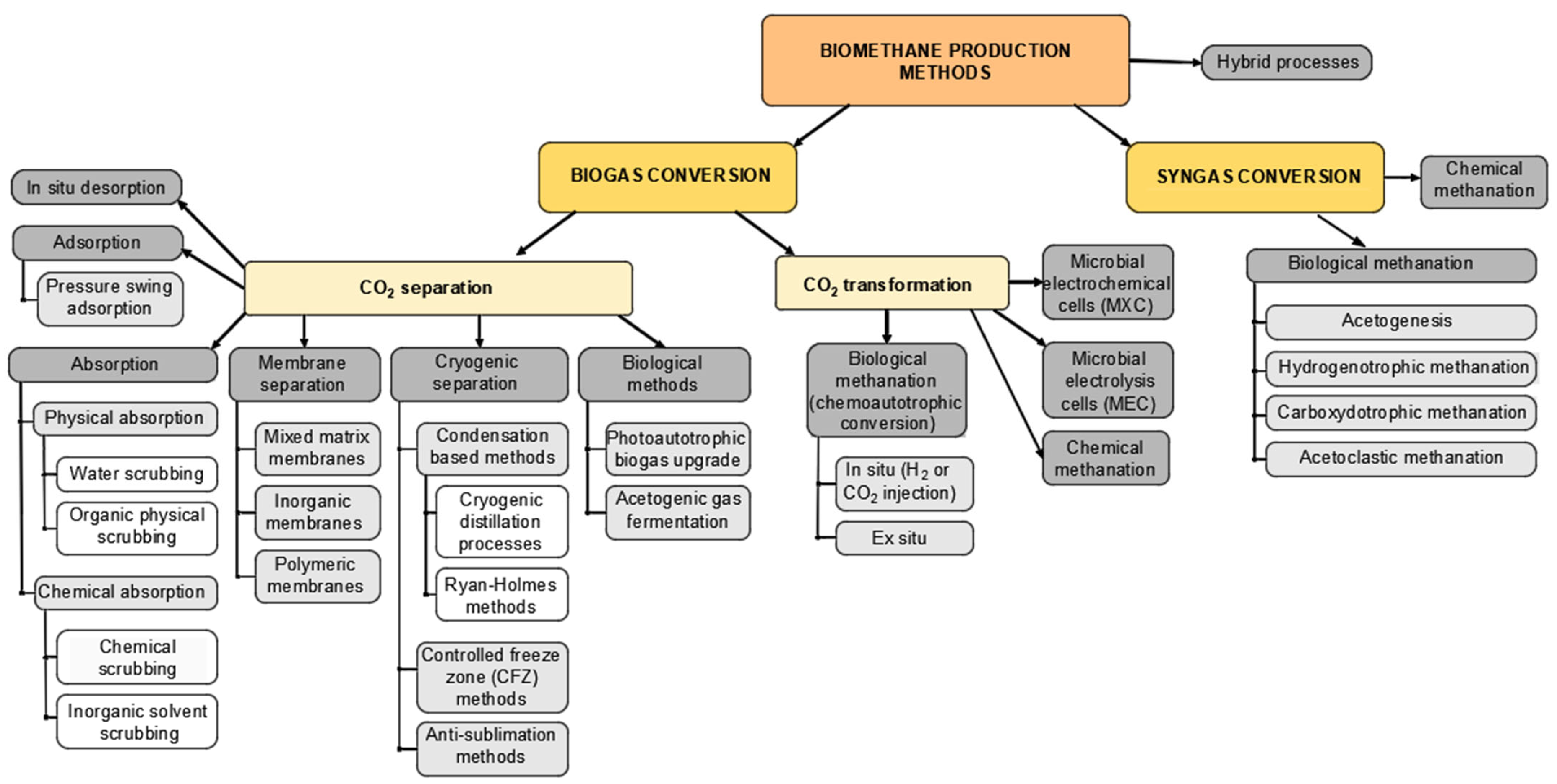
3. Biomethane Production Methods and Their Practical Application
3.1. Methods of Biomethane Production from Biogas
3.1.1. Biogas Upgrading via CO2 Separation
3.1.2. Biogas Upgrading via CO2 Transformation
3.2. Biomethane Production from Syngas
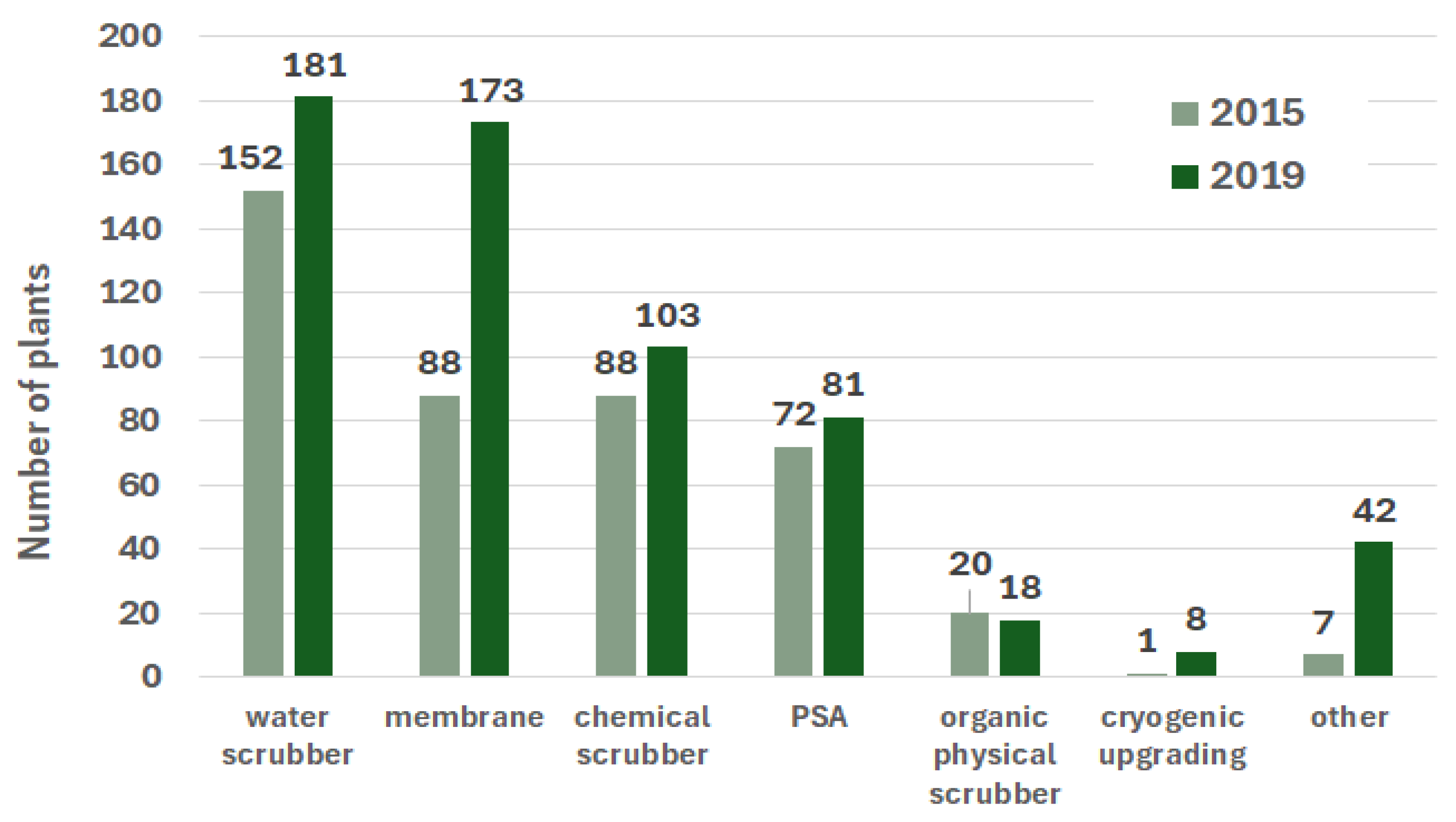
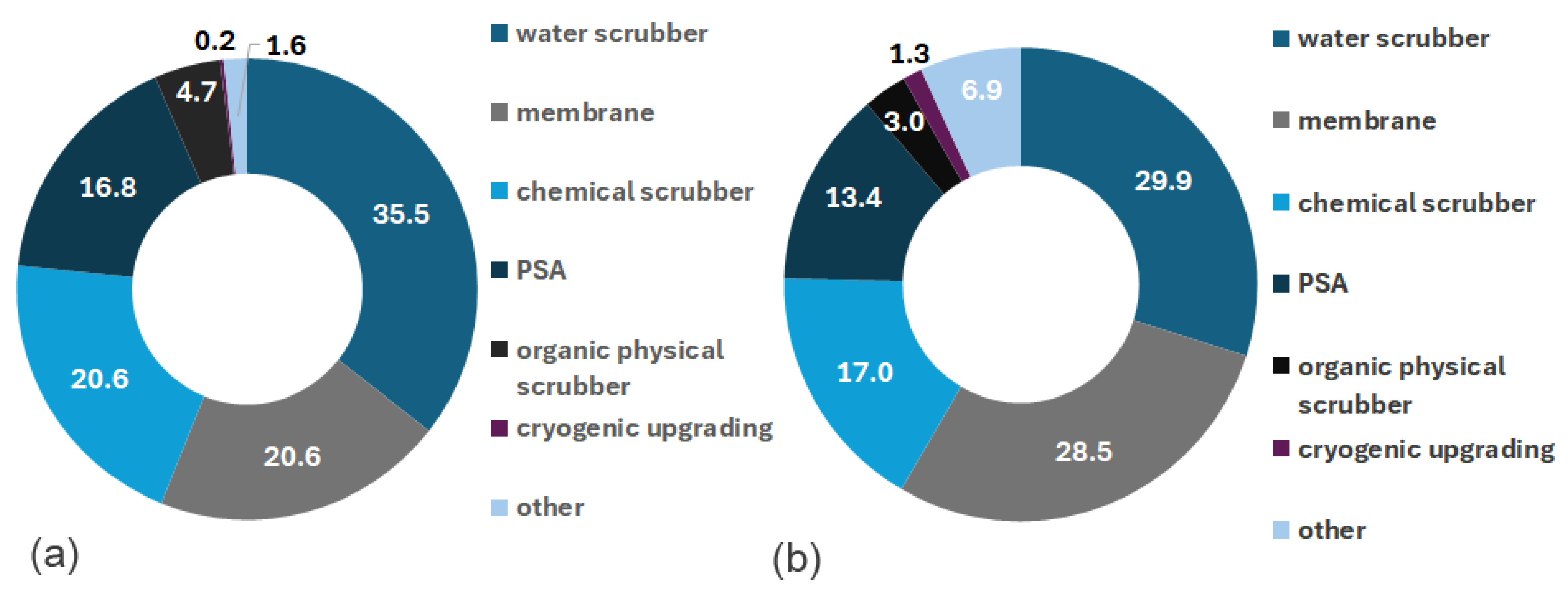
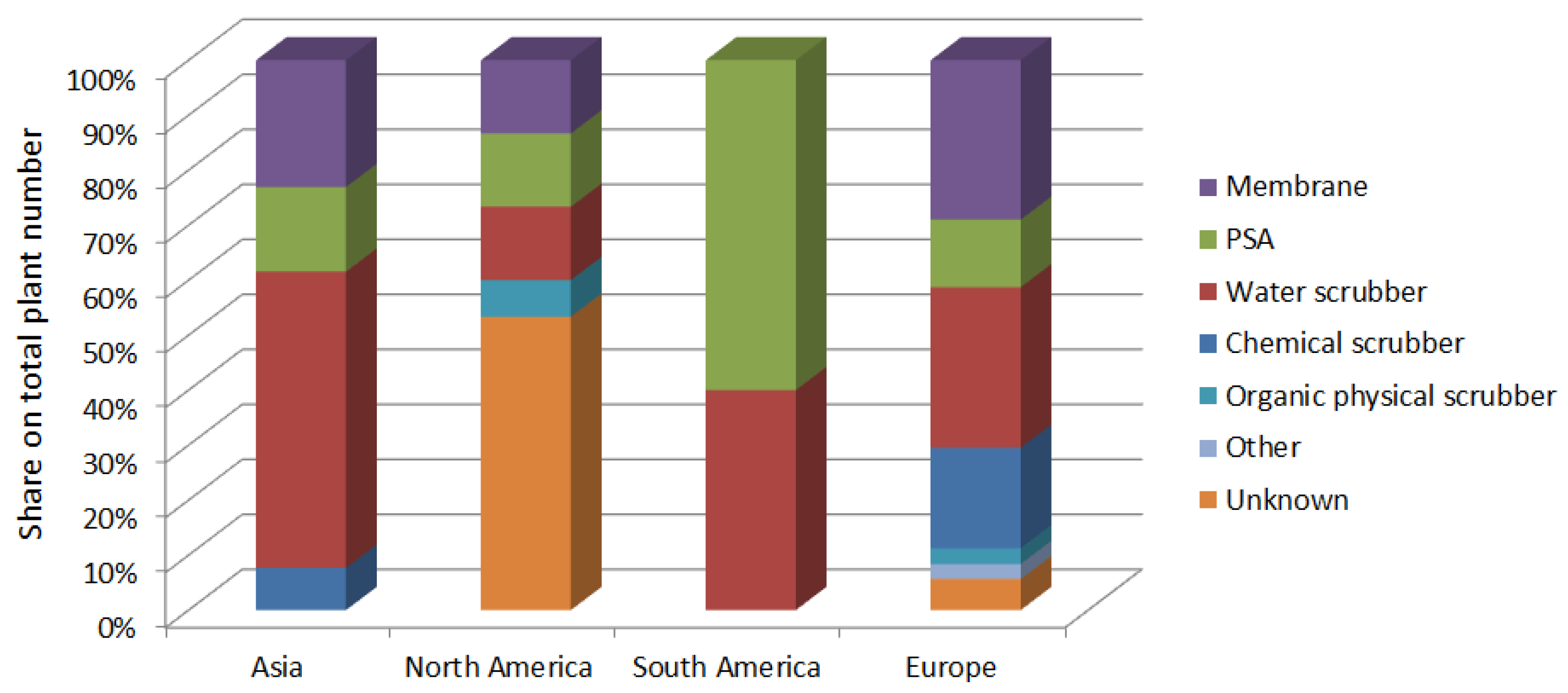
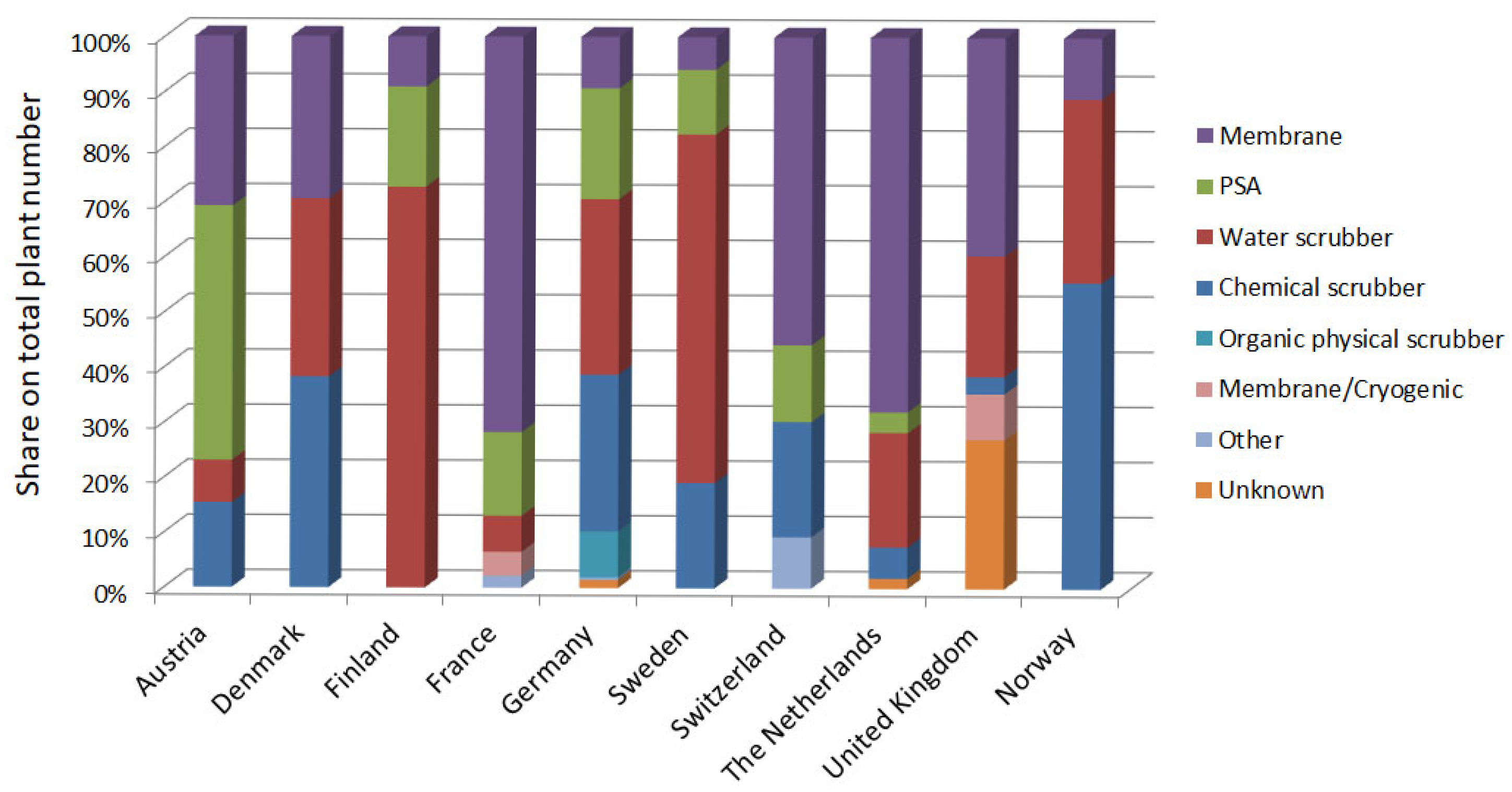
3.3. Dissemination of Methane Production Methods
4. Alternatives of Biomethane Applications
4.1. Transport Biofuel
4.2. Injection into the Gas Grid
4.3. Chemical and Biochemical Platform for Various Biorefinery Product
4.4. Raw Material for Energy Production
5. Biomethane Market Evolution and Perspectives
5.1. Current Status and Development
5.2. Prospects for the Development of Biomethane Market
6. Benefits of Biomethane and Barriers of Its Development
7. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Antar, M.; Dongmei, L.; Mahtab, N.; Ateeq, S.; Xiaomin, Z.; Donald, L.S. Biomass for a sustainable bioeconomy: An overview of world biomass production and utilization. Renew. Sustain. Energy Rev. 2021, 139, 110691. [Google Scholar]
- Osman, A.I.; Mehta, N.; Elgarahy, A.M.; Al-Hinai, A.; Al-Muhtaseb, A.A.H.; Rooney, D.W. Conversion of biomass to biofuels and life cycle assessment: A review. Environ. Chem. Lett. 2021, 19, 4075–4118. [Google Scholar] [CrossRef]
- Anderson, E.C.; Libby, W.F.; Weinhouse, S.; Reid, A.F.; Kirshenbaum, A.D.; Grosse, A.V. Natural radiocarbon from cosmic radiation. Phys. Rev. 1947, 72, 931. [Google Scholar] [CrossRef]
- Anderson, E.C.; Libby, W.F.; Weinhouse, S.; Reid, A.F.; Kirshenbaum, A.D.; Grosse, A.V. Radiocarbon from cosmic radiation. Science 1947, 105, 576–577. [Google Scholar] [CrossRef]
- Curran, S.C. The determination of geological age by means of radioactivity. Q. Rev. Chem. Soc. 1953, 7, 1–18. [Google Scholar] [CrossRef]
- Diethorn, W. A Methane Proportional Counter System for Natural Radiocarbon Measurements; Carnegies Institute of Technology Pittsburgh: Pittsburgh, PA, USA, 1956. [Google Scholar]
- EBA. About Biogas and Biomethane. Available online: https://www.europeanbiogas.eu/about-biogas-and-biomethane/ (accessed on 20 January 2025).
- US Department of Energy. Alternatives Fuels Data Center. Available online: https://afdc.energy.gov/fuels/natural-gas-renewable (accessed on 11 March 2025).
- Golmakani, A.; Nabavi, S.A.; Wadi, B.; Manovic, V. Advances, challenges, and perspectives of biogas cleaning, upgrading, and utilisation. Fuel 2022, 317, 123085. [Google Scholar]
- IEA. Outlook for Biogas and Biomethane: Prospects for Organic Growth. An Introduction to Biogas and Biomethane; IEA: Paris, France, 2020; Available online: https://www.iea.org/reports/outlook-for-biogas-and-biomethane-prospects-for-organic-growth/an-introduction-to-biogas-and-biomethane (accessed on 27 January 2025).
- Mansson, T. Clean Vehicles with Biofuel: A State-of-the-Art Report; KFB (Swedish Transport and Communication Research): Linköping, Sweden, 1998. [Google Scholar]
- OurWorldinData. Global Direct Primary Energy Consumption. Available online: https://ourworldindata.org/grapher/global-primary-energy (accessed on 23 April 2025).
- CEDIGAZ. Global Biomethane Market—2024 Assessment Biomethane in Full Gear. Available online: https://www.cedigaz.org/global-biomethane-market-2024-assessment/ (accessed on 12 February 2025).
- Selvasembian, R. Pretreatment methods for enhanced biomethane production from crop residues: Progress, challenges, and future perspectives. Sustain. Energy Technol. Assess. 2025, 75, 104269. [Google Scholar]
- Eniyan, M.C.; Edwin, M.; Nagarajan, V.A. Mild thermo-mechanical pretreatment method for improving biomethane production: Food waste disintegration and its impact on solubilization. Therm. Sci. Eng. Prog. 2025, 60, 103432. [Google Scholar] [CrossRef]
- Akshaya, K.; Selvasembian, R. Insights into the recent advances of chemical pretreatment of waste activated sludge to enhance biomethane production. J. Environ. Chem. Eng. 2024, 12, 113999. [Google Scholar]
- Grana, M.; Riboli, G.; Tatangelo, V.; Mantovani, M.; Gandolfi, I.; Turolla, A.; Ficara, E. Anaerobic valorization of sewage sludge pretreated through hydrothermal carbonization: Volatile fatty acids and biomethane production. Bioresour. Technol. 2024, 412, 131279. [Google Scholar] [CrossRef]
- Cazier, E.A.; Brethauer, S.; Bühler, P.C.; Studer, M.H.P. Steam explosion pretreatment of separated dairy cattle manure: Mass balances and effect on biomethane potential. Waste Manag. 2025, 193, 180–189. [Google Scholar] [CrossRef]
- Mahmoodi-Eshkaftaki, M.; Dalvi-Isfahan, M. Multiple exegetically optimization of ultrasonic pretreatment and substrate mixture for biohydrogen and biomethane improvement. Energy 2024, 292, 130537. [Google Scholar] [CrossRef]
- Sidabutar, R.; Trisakti, B.; Michael, M.; Vanness, V.; Alexander, V.; Natasya, Y.; Takriff, M.S. Synergistic integration of zeolite engineering and fixed-bed column design for enhanced biogas upgrading: Adsorbent synthesis, CO2/CH4 separation kinetics, and regeneration assessment. Sep. Purif. Technol. 2025, 355, 129772. [Google Scholar] [CrossRef]
- Tabar, M.A.; Maghsoudi, H.; Denayer, J.F. Hybrid membrane-vacuum pressure swing adsorption: Low-cost technology for simultaneous biomethane and carbon dioxide production from biogas. Bioresour. Technol. 2025, 422, 132241. [Google Scholar]
- Duma, Z.; Makgwane, P.R.; Masukume, M.; Swartbooi, A.; Rambau, K.; Mehlo, T.; Mavhungu, T. A comprehensive review of metal-organic frameworks sorbents and their mixed-matrix membranes composites for biogas cleaning and CO2/CH4 separation. Mater. Today Sustain. 2024, 27, 100812. [Google Scholar] [CrossRef]
- Braga-Nan, L.; Trably, E.; Santa-Catalina, G.; Bernet, N.; Delgènes, J.P.; Escudié, R. Microbial adaptation to H2 improves the conversion of volatile fatty acids to methane during in situ biomethanation even in CO2-depleted conditions. Bioresour. Technol. 2025, 429, 132494. [Google Scholar] [CrossRef]
- Mahieux, M.; Aemig, Q.; Richard, C.; Delgenès, J.P.; Juge, M.; Trably, E.; Escudié, R. Improved organic matter biodegradation through pulsed H2 injections during in situ biomethanation. Bioresour. Technol. 2024, 407, 131101. [Google Scholar] [CrossRef]
- Chan, H.L.; Xu, H.; Zhou, Y. External ceramic membrane contactor for in-situ H2 assisted biogas upgrading. Bioresour. Technol. 2024, 406, 130981. [Google Scholar]
- Bardi, M.J.; Müller, F.; Polag, D.; Habtu, N.G.; Koch, K. The intriguing effect of CO2 enrichment in anaerobic digestion. Bioresour. Technol. 2025, 416, 131743. [Google Scholar]
- Castel, L.; Cazaudehore, G.; Beigbeder, J.B.; Guyoneaud, R.; Peyrelasse, C. Pilot-scale study of CO2 enrichment effects on anaerobic digestion performance. Chem. Eng. Sci. 2025, 304, 121070. [Google Scholar] [CrossRef]
- Andronikou, M.; Christoforou, P.; Constantinou, D.; Charalambous, P.; Samanides, C.G.; Karachaliou, P.; Vyrides, I. Critical role of bicarbonate in Zero-Valent iron for hydrogen generation and biogas upgrading in anaerobic digestion. Bioresour. Technol. 2025, 426, 132236. [Google Scholar] [CrossRef] [PubMed]
- Chicaiza-Ortiz, C.; Beihan, Z.; Zhang, J.; He, Y.; Wah, T.Y. Carbon Dioxide-Driven anaerobic digestion with Zero-Valent iron for enhanced biomethanation of food waste. Bioresour. Technol. 2025, 430, 132529. [Google Scholar] [CrossRef] [PubMed]
- Chicaiza-Ortiz, C.; Zhang, P.; Zhang, J.; Zhang, T.; Yang, Q.; He, Y. CO2-enhanced methane production by integration of bamboo biochar during anaerobic co-digestion. J. Environ. Manag. 2025, 373, 123603. [Google Scholar] [CrossRef]
- Eze, J.I.; Agbo, K. Maximizing the potentials of biogas through upgrading. Am. J. Sci. Res. 2010, 1, 604–609. [Google Scholar] [CrossRef]
- Ryckebosch, E.; Drouillon, M.; Veruaeren, H. Techniques for transformation of biogas to biomethane. Biomass Bioenergy 2011, 35, 1633–1645. [Google Scholar] [CrossRef]
- Bauer, F.; Hulteberg, C.; Persson, T.; Tamm, D. Biogas Upgrading—Review of Commercial Technologies; Technical Report; Lund University: Lund, Sweden, 2013. [Google Scholar]
- Sun, Q.; Li, H.; Yan, J.; Liu, L.; Yu, Z.; Yu, X. Selection of appropriate biogas upgrading technology—A review of biogas cleaning, upgrading and utilisation. Renew. Sustain. Energy Rev. 2015, 51, 521–532. [Google Scholar] [CrossRef]
- Awe, O.W.; Zhao, Y.; Nzihou, A.; Minh, D.P.; Lyczko, N. A review of biogas utilisation, purification and upgrading technologies. Waste Biomass Valorization 2017, 8, 267–283. [Google Scholar] [CrossRef]
- Khan, I.U.; Othman, M.H.D.; Hashim, H.; Matsuura, T.; Ismail, A.F.; Rezaei-Dasht Arzhandi, M.; Wan Azelee, I. Biogas as a renewable energy fuel—A review of biogas upgrading, utilisation and storage. Energy Convers. Manag. 2017, 150, 277–294. [Google Scholar] [CrossRef]
- Angelidaki, I.; Treu, L.; Tsapekos, P.; Luo, G.; Campanaro, S.; Wenzel, H.; Kougias, P.G. Biogas upgrading and utilization: Current status and perspectives. Biotechnol. Adv. 2018, 36, 452–466. [Google Scholar] [CrossRef]
- Sarker, S.; Lamb, J.J.; Hjelme, D.R.; Lien, K.M. Overview of recent progress towards in-situ biogas upgradation techniques. Fuel 2018, 226, 686–697. [Google Scholar] [CrossRef]
- Kapoor, R.; Ghosh, P.; Kumar, M.; Vijay, V.K. Evaluation of biogas upgrading technologies and future perspectives: A review. Environ. Sci. Pollut. Res. 2019, 26, 11631–11661. [Google Scholar] [CrossRef] [PubMed]
- Adnan, A.I.; Ong, M.Y.; Nomanbhay, S.; Chew, K.W.; Show, P.L. Technologies for biogas upgrading to biomethane: A review. Bioengineering 2019, 6, 92. [Google Scholar] [CrossRef] [PubMed]
- Atelge, M.R.; Senol, H.; Djaafri, M.; Hansu, T.A.; Krisa, D.; Atabani, A.; Eskicioglu, C.; Muratçobanoğlu, H.; Unalan, S.; Kalloum, S.; et al. A critical overview of the state-of-the-art methods for biogas purification and utilization processes. Sustainability 2021, 13, 11515. [Google Scholar] [CrossRef]
- Calise, F.; Cappiello, F.L.; Cimmino, L.; d’Accadia, M.D.; Vicidomini, M. A review of the state of the art of biomethane production: Recent advancements and integration of renewable energies. Energies 2021, 14, 4895. [Google Scholar] [CrossRef]
- Mignogna, D.; Ceci, P.; Cafaro, C.; Corazzi, G.; Avino, P. Production of biogas and biomethane as renewable energy sources: A review. Appl. Sci. 2023, 13, 10219. [Google Scholar] [CrossRef]
- Karne, H.; Mahajan, U.; Ketkar, U.; Kohade, A.; Khadilkar, P.; Mishra, A. A review on biogas upgradation systems. Mater. Today Proc. 2023, 72, 775–786. [Google Scholar] [CrossRef]
- Baena-Moreno, F.M.; Le Saché, E.; Pastor-Perez, L.; Reina, T.R. Membrane-based technologies for biogas upgrading: A review. Environ. Chem. Lett. 2020, 18, 1649–1658. [Google Scholar] [CrossRef]
- Paolini, V.; Tratzi, P.; Torre, M.; Tomassetti, L.; Segreto, M.; Petracchini, F. Chapter 3—Water Scrubbing for Biogas Upgrading: Developments and Innovations. In Emerging Technologies and Biological Systems for Biogas Upgrading; Nabin, A., Ottosen, L.D.M., Vedel, M., Kofoed, W., Pant, D., Eds.; Academic Press: Cambridge, MA, USA, 2021; pp. 57–71. [Google Scholar]
- Abd, A.A.; Othman, M.R.; Helwani, Z.; Kim, J. An overview of biogas upgrading via pressure swing adsorption: Navigating through bibliometric insights towards a conceptual framework and future research pathways. Energy Convers. Manag. 2024, 306, 118268. [Google Scholar] [CrossRef]
- Muntaha, N.; Rain, M.I.; Goni, L.K.M.; Ali Shaikh, M.A.; Jamal, M.S.; Hossain, M. A review on carbon dioxide minimization in biogas upgradation technology by chemical absorption processes. ACS Omega 2022, 7, 33680–33698. [Google Scholar] [CrossRef]
- Nayeem, A.; Shariffuddin, J.H.; Yousuf, A. Chapter 3—Absorption Technology for Upgrading Biogas to Biomethane. In Applied Biotechnology Reviews, Biogas to Biomethane; Yousuf, A., Melville, L., Eds.; Woodhead Publishing: Cambridge, UK, 2024; pp. 69–84. [Google Scholar]
- Wu, L.; Wei, W.; Song, L.; Woźniak-Karczewska, M.; Chrzanowski, Ł.; Ni, B.-J. Upgrading biogas produced in anaerobic digestion: Biological removal and bioconversion of CO2 in biogas. Renew. Sustain. Energy Rev. 2021, 150, 111448. [Google Scholar] [CrossRef]
- Lóránt, B.; Tardy, G.M. Current status of biological biogas upgrading technologies. Period. Polytech. Chem. Eng. 2022, 66, 465–481. [Google Scholar] [CrossRef]
- Chatzis, A.; Gkotsis, P.; Zouboulis, A. Biological methanation (BM): A state-of-the-art review on recent research advancements and practical implementation in full-scale BM units. Energy Convers. Manag. 2024, 314, 118733. [Google Scholar] [CrossRef]
- Meena, P.K.; Pal, A.A. A comprehensive review on methane enrichment in biogas through the purification process using biomass-based adsorbents. Biomass Convers. Biorefin. 2024, 15, 8287–8309. [Google Scholar] [CrossRef]
- Rönsch, S.; Schneider, J.; Matthischke, S.; Schlüter, M.; Götz, M.; Lefebvre, J.; Prabhakaran, P.; Bajohr, S. Review on methanation—From fundamentals to current projects. Fuel 2016, 166, 276–296. [Google Scholar] [CrossRef]
- Wu, Y.; Ye, X.; Wang, Y.; Wang, L. Methane production from biomass by thermochemical conversion: A review. Catalysts 2023, 13, 771. [Google Scholar] [CrossRef]
- Khan, M.U.; Lee, J.T.E.; Bashir, M.A.; Dissanayake, P.D.; Ok, Y.S.; Tong, Y.W.; Shariati, M.A.; Wu, S.; Ahring, B.K. Current status of biogas upgrading for direct biomethane use: A review. Renew. Sustain. Energy Rev. 2021, 149, 111343. [Google Scholar] [CrossRef]
- Ahmed, S.F.; Mofijur, M.; Tarannum, K.; Chowdhury, A.T.; Rafa, N.; Nuzhat, S.; Kumar, S.; Vo, D.-V.N.; Lichtfouse, E.; Mahlia, T.M.I. Biogas upgrading, economy and utilization: A review. Environ. Chem. Lett. 2021, 19, 4137–4164. [Google Scholar] [CrossRef]
- Mallouppas, G.; Yfantis, E.A.; Ioannou, C.; Paradeisiotis, A.; Ktoris, A. Application of biogas and biomethane as maritime fuels: A review of research, technology development, innovation proposals, and market potentials. Energies 2023, 16, 2066. [Google Scholar] [CrossRef]
- Scarlat, N.; Dallemand, J.-F.; Fahl, F. Biogas: Developments and perspectives in Europe. Renew. Energy 2018, 129, 457–472. [Google Scholar] [CrossRef]
- Sulewski, P.; Ignaciuk, W.; Szymańska, M.; Wąs, A. Development of the biomethane market in Europe. Energies 2023, 16, 2001. [Google Scholar] [CrossRef]
- Schmid, C.; Horschig, T.; Pfeiffer, A.; Szarka, N.; Thrän, D. Biogas upgrading: A review of national biomethane strategies and support policies in selected countries. Energies 2019, 12, 3803. [Google Scholar] [CrossRef]
- IEA. Available online: https://www.iea.org/about/structure (accessed on 8 April 2025).
- European Network of Transmission System Operators for Gas (ENTSOG). Available online: https://ec.europa.eu/digital-building-blocks/sites/pages/viewpage.action?pageId=533365448 (accessed on 8 April 2025).
- Uddin, M.; Wright, M. Anaerobic digestion fundamentals, challenges, and technological advances. Phys. Sci. Rev. 2023, 8, 2819–2837. [Google Scholar] [CrossRef]
- Ge, X.; Xu, F.; Li, Y. Solid-state anaerobic digestion of lignocellulosic biomass: Recent progress and perspectives. Bioresour. Technol. 2016, 205, 239–249. [Google Scholar] [CrossRef] [PubMed]
- Nie, E.; He, P.; Zhang, H.; Hao, L.; Shao, L.; Lü, F. How does temperature regulate anaerobic digestion? Renew. Sustain. Energy Rev. 2021, 150, 111453. [Google Scholar] [CrossRef]
- Wellinger, A.; Murphy, J.; Baxter, D. The Biogas Handbook: Science, Production and Applications; Woodhead Publishing Limited: Cambridge, UK, 2013. [Google Scholar]
- Tansel, B.; Surita, S.C. Managing siloxanes in biogas-to-energy facilities: Economic comparison of pre- vs post-combustion practices. Waste Manag. 2019, 96, 121–127. [Google Scholar] [CrossRef]
- Angelidaki, I.; Xie, L.; Luo, G.; Zhang, Y.; Oechsner, H.; Lemmer, A.; Munoz, R.; Kougias, P.G. Biogas Upgrading Current and Emerging Technologies, 2nd ed.; Elsevier Inc.: Amsterdam, The Netherlands, 2019. [Google Scholar]
- López, M.E.; Rene, E.R.; Veiga, M.C.; Kennes, C. Biogas Technologies and Cleaning Techniques. In Environmental Chemistry for a Sustainable World; Lichtfouse, E., Schwarzbauer, J., Robert, D., Eds.; Springer: Dordrecht, Germany, 2012; Volume 2, pp. 347–377. [Google Scholar]
- Sisani, E.; Cinti, G.; Discepoli, G.; Penchini, D.; Desideri, U.; Marmottini, F. Adsorptive removal of H2S in biogas conditions for high temperature fuel cell systems. Int. J. Hydrogen Energy 2014, 39, 21753–21766. [Google Scholar] [CrossRef]
- Liu, X.; Wang, B.; Dong, X.; Qiu, Y.; Meng, Q. Enhancement effect of nanofluids on the desulfurization and regeneration performance of ionic liquid-based system. J. Hazard. Mater. 2021, 419, 126394. [Google Scholar] [CrossRef]
- Lin, C.; Qin, W.; Dong, C. H2S adsorption and decomposition on the gradually reduced α-Fe2O3(001) surface: A DFT study. Appl. Surf. Sci. 2016, 387, 720–731. [Google Scholar] [CrossRef]
- Ghimire, A.; Gyawali, R.; Lens, P.N.L.; Lohani, S.P. Chapter 11—Technologies for Removal of Hydrogen Sulfide (H2S) from Biogas. In Emerging Technologies and Biological Systems for Biogas Upgrading; Nabin, A., Ottosen, L.D.M., Vedel, M., Kofoed, W., Pant, D., Eds.; Academic Press: Cambridge, MA, USA, 2021; pp. 295–320. [Google Scholar]
- Costa, C.; Cornacchia, M.; Pagliero, M.; Fabiano, B.; Vocciante, M.; Reverberi, A.P. Hydrogen sulfide adsorption by iron oxides and their polymer composites: A case-study application to biogas purification. Materials 2020, 13, 4725. [Google Scholar] [CrossRef]
- Abdirakhimov, M.; Al-Rashed, M.H.; Wójcik, J. Recent attempts on the removal of H2S from various gas mixtures using zeolites and waste-based adsorbents. Energies 2022, 15, 5391. [Google Scholar] [CrossRef]
- Hoang, T.L.G.; Doan, D.T.; Nanda, S.; Lavoie, R.; Nguyen-Tri, P. Development of metal-organic framework-based systems for H2S removal: A comprehensive review. Coord. Chem. Rev. 2025, 529, 216466. [Google Scholar] [CrossRef]
- Esser, G.; Crits, R.; Barozzino-Consiglio, G.; Daouli, A.; Maurin, G.; Filinchuk, Y.; Hermans, S.; Steenhaut, T. Appending polyamines on metal–organic frameworks as an efficient strategy for selective removal of H2S under humid conditions. ACS Appl. Eng. Mater. 2024, 2, 2619–2625. [Google Scholar] [CrossRef]
- Das, B.; Basumatary, S.; Kalita, P. Hydrogen sulphide removal from raw biogas using novel coconut husk and sugarcane bagasse composite biochar adsorbent. IOP Conf. Ser. Earth Environ. Sci. 2024, 1372, 012040. [Google Scholar] [CrossRef]
- Bora, D.; Roy, K.; Mahanta, P.; Barbora, L. Hydrogen sulfide removal from biogas using biomass-derived naturally alkaline biochars: Performance analysis and kinetics. J. Mater. Cycles Waste Manag. 2024, 26, 1544–1556. [Google Scholar] [CrossRef]
- Martin, A.D.; Ravenni, G.; Thomsen, T.P. Low-cost hydrogen sulfide removal with biochar and activated biochar. Next Res. 2025, 2, 100286. [Google Scholar] [CrossRef]
- Passalacqua, E.; Correcher, R.; Mantovani, M.; Collina, E.; Fullana, A. Use of carbon-encapsulated zero-valent iron nanoparticles from waste biomass to hydrogen sulphide wet removal. Waste Manag. Res. 2024, 43, 0734242X241273800. [Google Scholar] [CrossRef]
- Cattaneo, C.R.; Muñoz, R.; Korshin, G.V.; Naddeo, V.; Belgiorno, V.; Zarra, T. Biological desulfurization of biogas: A comprehensive review on sulfide microbial metabolism and treatment biotechnologies. Sci. Total Environ. 2023, 893, 164689. [Google Scholar] [CrossRef]
- Cano, P.I.; Almenglo, F.; Ramírez, M.; Cantero, D. Integration of a nitrification bioreactor and an anoxic biotrickling filter for simultaneous ammonium-rich water treatment and biogas desulfurization. Chemosphere 2021, 284, 131358. [Google Scholar] [CrossRef]
- Poser, M.; Duarte e Silva, L.R.; Peu, P.; Couvert, A.; Dumont, É. Cellular concrete waste: An efficient new way for H2S removal. Sep. Purif. Technol. 2023, 309, 123014. [Google Scholar] [CrossRef]
- Mohammadi, K.; Vaiskunaite, R.; Zagorskis, A. Innovative biofiltration materials for H2S removal from biogas. Environ. Health Eng. Manag. 2024, 11, 361–370. [Google Scholar] [CrossRef]
- Severi, C.A.; Pascual, C.; Perez, V.; Muñoz, R.; Lebrero, R. Pilot-scale biogas desulfurization through anoxic biofiltration. J. Hazard. Mater. 2025, 485, 136830. [Google Scholar] [CrossRef] [PubMed]
- Caliari, P.C.; Pacheco, M.J.; Ciríaco, L.F.; Lopes, A.M.C. Anodic oxidation of sulfide to sulfate: Effect of current density on the process kinetics. J. Braz. Chem. Soc. 2017, 28, 557–566. [Google Scholar] [CrossRef]
- Borgquist, S.; Villadsen, S.N.B.; Skitsi, C.; Boesgaard, K.; Abildskov, J.; Rivera-Tinoco, E.; Rasmussen, J.B.; Fosbøl, P.L. Power-to-X electroscrubbing parameter analysis for biogas desulfurization. J. Hazard. Mater. 2023, 452, 131334. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; De Schryver, P.; De Gusseme, B.; De Muynck, W.; Boon, N.; Verstraete, W. Chemical and biological technologies for hydrogen sulfide emission control in sewer systems: A review. Water Res. 2008, 42, 1–12. [Google Scholar] [CrossRef]
- Galloni, M.; Di Marcoberardino, G. Biogas upgrading technology: Conventional processes and emerging solutions analysis. Energies 2024, 17, 2907. [Google Scholar] [CrossRef]
- Grande, C.A. Biogas Upgrading by Pressure Swing Adsorption. In Biofuel’s Engineering Process Technology; Dos Santos Bernardes, M.A., Ed.; IntechOpen: London, UK, 2011. [Google Scholar]
- Abd, A.A.; Shabbani, H.J.K.; Helwani, Z.; Othman, M.R. Experimental study and static numerical optimization of scalable design of non-adiabatic and non-isothermal pressure swing adsorption for biogas upgrading. Energy 2022, 257, 124781. [Google Scholar] [CrossRef]
- Canevesi, R.; Grande, C.A. Biogas upgrading by pressure swing adsorption using zeolite 4A. Effect of purge on process performance. Sep. Purif. Technol. 2023, 309, 123015. [Google Scholar] [CrossRef]
- Chen, Y.-F.; Lin, P.-W.; Chen, W.-H.; Yen, F.-Y.; Yang, H.-S.; Chou, C.-T. Biogas upgrading by pressure swing adsorption with design of experiments. Processes 2021, 9, 1325. [Google Scholar] [CrossRef]
- Canevesi, R.L.; Borba, C.E.; Da Silva, E.A.; Grande, C.A. Towards a design of a pressure swing adsorption unit for small scale biogas upgrading. Energy Procedia 2019, 158, 848–853. [Google Scholar] [CrossRef]
- Golmakani, A.; Wadi, B.; Manović, V.; Nabavi, S.A. Comparative evaluation of PSA, PVSA, and twin PSA processes for biogas upgrading: The purity, recovery, and energy consumption dilemma. Energies 2023, 16, 6840. [Google Scholar] [CrossRef]
- Abd, A.A.; Othman, M.R.; Helwani, Z.; Kim, J. Waste to wheels: Performance comparison between pressure swing adsorption and amine-absorption technologies for upgrading biogas containing hydrogen sulfide to fuel grade standards. Energy 2023, 272, 127060. [Google Scholar] [CrossRef]
- Wantz, E.; Benizri, D.; Dietrich, N.; Hébrard, G. Rate-based modeling approach for high pressure water scrubbing with unsteady gas flowrate and multicomponent absorption applied to biogas upgrading. Appl. Energy 2022, 312, 118754. [Google Scholar] [CrossRef]
- Wantz, E.; Lemonnier, M.; Benizri, D.; Dietrich, N.; Hébrard, G. Innovative high-pressure water scrubber for biogas upgrading at farm-scale using vacuum for water regeneration. Appl. Energy 2023, 350, 121781. [Google Scholar] [CrossRef]
- Benizri, D.; Dietrich, N.; Labeyrie, P.; Hébrard, G. A compact, economic scrubber to improve farm biogas upgrading systems. Sep. Purif. Technol. 2019, 219, 169–179. [Google Scholar] [CrossRef]
- Walozi, R.; Nabuuma, B.; Sebiti, A. Application of low pressure water scrubbing technique for increasing methane content in biogas. Univers. J. Agric. Res. 2016, 4, 60–65. [Google Scholar] [CrossRef]
- Hjuler, K.; Aryal, N. Review of Biogas Upgrading; Dansk Gasteknisk Center: Horsholm, Denmark, 2017; pp. 8–9. [Google Scholar]
- Cavaignac, R.S.; Ferreira, N.L.; Guardani, R. Techno-economic and environmental process evaluation of biogas upgrading via amine scrubbing. Renew. Energy 2021, 171, 868–880. [Google Scholar] [CrossRef]
- Jabraeelzadeh, A.; Gharehghani, A.; Saray, J.A.; Andwari, A.M.; Borhani, T.N. Techno-economic analysis of biogas upgrading through amine scrubbing: A comparative study of different single amines. Fuel 2025, 381, 33662. [Google Scholar] [CrossRef]
- Morero, B.; Campanella, E.A. Simulation of the process of chemical absorption using amine solutions for biogas purification. Inf. Tecnol. 2013, 24, 25–32. [Google Scholar] [CrossRef]
- Dutcher, B.; Fan, M.; Russell, A.G. Amine-based CO2 capture technology development from the beginning of 2013—A review. ACS Appl. Mater. Interfaces 2015, 7, 2137–2148. [Google Scholar] [CrossRef]
- Nordberg, Å.; Edström, M.; Uusi-Penttilä, M.; Rasmuson, Å.C. Selective desorption of carbon dioxide from sewage sludge for in-situ methane enrichment: Enrichment experiments in pilot scale. Biomass Bioenergy 2012, 37, 196–204. [Google Scholar] [CrossRef]
- Koutsiantzi, C.; Koukovinos, K.; Liatsou, A.; Gkotsis, P.; Zouboulis, A.; Mitrakas, M.; Kikkinides, E.S. Anaerobic digestion biogas upgrading using a two-stage membrane system under pilot-scale conditions. Environ. Res. 2024, 245, 118080. [Google Scholar] [CrossRef] [PubMed]
- Rodero, M.D.R.; Muñoz, R.; González-Sánchez, A.; Ruiz, H.A.; Quijano, G. Membrane materials for biogas purification and upgrading: Fundamentals, recent advances and challenges. J. Environ. Chem. Eng. 2024, 12, 114106. [Google Scholar] [CrossRef]
- Kelley, B.T.; Valencia, J.A.; Northrop, P.S.; Mart, C.J. Controlled Freeze Zone™ for developing sour gas reserves. Energy Procedia 2011, 4, 824–829. [Google Scholar] [CrossRef]
- Pellegrini, L.A.; De Guido, G.; Langé, S. Biogas to liquefied biomethane via cryogenic upgrading technologies. Renew. Energy 2018, 124, 75–83. [Google Scholar] [CrossRef]
- Naquash, A.; Agarwal, N.; Nizami, M.; Nga, N.N.; Aziz, M.; Lee, M. Unlocking the potential of cryogenic biogas upgrading technologies integrated with bio-LNG production: A comparative assessment. Appl. Energy 2024, 371, 123720. [Google Scholar] [CrossRef]
- Song, C.; Liu, Q.; Deng, S.; Li, H.; Kitamura, Y. Cryogenic-based CO2 capture technologies: State-of-the-art developments and current challenges. Renew. Sustain. Energy Rev. 2019, 101, 265–278. [Google Scholar] [CrossRef]
- Riva, M.; Campestrini, M.; Toubassy, J.; Clodic, D.; Stringari, P. Solid-liquid-vapor equilibrium models for cryogenic biogas upgrading. Ind. Eng. Chem. Res. 2014, 53, 17506–17514. [Google Scholar] [CrossRef]
- Vakili, S.S.; Kargari, A.; Sanaeepur, H. Chapter 12—Cryogenic-Membrane Gas Separation Hybrid Processes. In Current Trends and Future Developments on (Bio-) Membranes; Basile, A., Favvas, E.P., Eds.; Elsevier: Amsterdam, The Netherlands, 2024; pp. 349–368. [Google Scholar]
- Garlapati, V.K.; Sharma, S.; Sevda, S. Chapter 14—Photosynthetic Biogas Upgrading: An Attractive Biological Technology for Biogas Upgrading. In Emerging Technologies and Biological Systems for Biogas Upgrading; Nabin, A., Ottosen, L.D.M., Vedel, M., Kofoed, W., Pant, D., Eds.; Academic Press: Cambridge, MA, USA, 2021; pp. 383–409. [Google Scholar]
- Deviram, G.; Thangavel, M.; Susaimanickam, A.; Tharifkhan, S.A.; Devanesan, A.A.; Arivalagan, P. Applications of microalgal and cyanobacterial biomass on a way to safe, cleaner and a sustainable environment. J. Clean. Prod. 2020, 253, 119770. [Google Scholar] [CrossRef]
- Méndez, L.; García, D.; Perez, E.; Blanco, S.; Munoz, R. Photosynthetic upgrading of biogas from anaerobic digestion of mixed sludge in an outdoors algal-bacterial photobioreactor at pilot scale. J. Water Process Eng. 2022, 48, 102891. [Google Scholar] [CrossRef]
- Wang, J.; Liu, H.; Fu, B.; Xu, K.; Chen, J. Trophic link between syntrophic acetogens and homoacetogens during the anaerobic acidogenic fermentation of sewage sludge. Biochem. Eng. J. 2013, 70, 1–8. [Google Scholar] [CrossRef]
- Jepleting, A.; Mecha, A.C.; Sombei, D.; Moraa, D.; Chollom, M.N. Potential of low-cost materials for biogas purification, a review of recent developments. Renew. Sustain. Energy Rev. 2025, 210, 115152. [Google Scholar] [CrossRef]
- Imran-Masood, M.; García-Díez, E.; Usman, M.; Lodhi, B.K.; Waqas, M.; García, S. Development of a novel bio char for CO2 capture and biogas upgrade: Static and dynamic testing. J. CO2 Util. 2024, 89, 102958. [Google Scholar] [CrossRef]
- Lee, J.T.; Ok, Y.S.; Song, S.; Dissanayake, P.D.; Tian, H.; Tio, Z.K.; Tong, Y.W. Biochar utilisation in the anaerobic digestion of food waste for the creation of a circular economy via biogas upgrading and digestate treatment. Bioresour. Technol. 2021, 333, 125190. [Google Scholar] [CrossRef] [PubMed]
- Wei, H.; Deng, S.; Hu, B.; Chen, Z.; Wang, B.; Huang, J.; Yu, G. Granular bamboo-derived activated carbon for high CO2 adsorption: The dominant role of narrow micropores. ChemSusChem 2012, 5, 2354–2360. [Google Scholar] [CrossRef]
- Peng, X.; Peng, Y.-L.; Huo, M.; Zhao, J.; Ma, Q.; Liu, B.; Deng, C.; Yang, M.; Dong, B.; Sun, C.; et al. High efficient pre-combustion CO2 capture by using porous slurry formed with ZIF-8 and isoparaffin C16. Sep. Purif. Technol. 2023, 305, 122424. [Google Scholar] [CrossRef]
- Tsang, M.Y.; Convery, J.P.; Lai, B.; Cahir, J.; Erbay, Y.; Rooney, D.; Murrer, B.; James, S.L. Porous liquids as solvents for the economical separation of carbon dioxide from methane. Mater. Today 2022, 60, 9–16. [Google Scholar] [CrossRef]
- Muñoz, R.; Meier, L.; Diaz, I.; Jeison, D. A review on the state-of-the-art of physical/chemical and biological technologies for biogas upgrading. Rev. Environ. Sci. Biotechnol. 2015, 14, 727–759. [Google Scholar] [CrossRef]
- Aile, N. Biogas to Biomethane. In Technology Review; Vienna University of Technology: Vienna, Austria, 2012. [Google Scholar]
- Beil, M.; Beyrich, W. Biogas Upgrading to Biomethane. In The Biogas Handbook; Elsevier: Amsterdam, The Netherlands, 2013; pp. 342–377. [Google Scholar]
- Sahota, S.; Shah, G.; Ghosh, P.; Kapoor, R.; Sengupta, S.; Singh, P.; Vijay, V.; Sahay, A.; Vijay, V.K.; Thakur, I.S. Review of trends in biogas upgradation technologies and future perspectives. Bioresour. Technol. Rep. 2018, 1, 79–88. [Google Scholar] [CrossRef]
- Petersson, A.; Wellinger, A. Biogas Upgrading Technologies—Developments and Innovations. Available online: https://www.ieabioenergy.com/wp-content/uploads/2009/10/upgrading_rz_low_final.pdf (accessed on 10 December 2024).
- Hoye, K.; Hulteberg, C.; Svensson, M.; Jernberg, J.; Norregard, O. Biogas Upgrading-Technical Review; Energiforsk: Stockholm, Sweden, 2016. [Google Scholar]
- Nie, H.; Jiang, H.; Chong, D.; Wu, Q.; Xu, C.; Zhou, H. Comparison of water scrubbing and propylene carbonate absorption for biogas upgrading process. Energy Fuels 2013, 27, 3239–3245. [Google Scholar] [CrossRef]
- Persson, M.; Jöonsson, O.; Wellinger, A. Biogas Upgrading to Vehicle Fuel Standards and Grid Injection. Available online: https://www.ieabioenergy.com/wp-content/uploads/2007/12/upgrading_report_final.pdf (accessed on 10 December 2024).
- Ong, M.; Williams, R.; Kaffka, S. DRAFT Comparative Assessment of Technology Options for Biogas Clean-Up; University of California: Los Angeles, CA, USA, 2014. [Google Scholar]
- Yang, L.; Ge, X.; Wan, C.; Yu, F.; Li, Y. Progress and perspectives in converting biogas to transportation fuels. Renew. Sustain. Energy Rev. 2014, 40, 1133–1152. [Google Scholar] [CrossRef]
- Huertas, J.; Giraldo, N.; Izquierdo, S. Removal of H2S and CO2 from Biogas by Amine Absorption. Mass Transfer in Chemical Engineering Processes; IntechOpen: London, UK, 2011. [Google Scholar]
- Luo, G.; Angelidaki, I. Integrated biogas upgrading and hydrogen utilization in an anaerobic reactor containing enriched hydrogenotrophic methanogenic culture. Biotechnol. Bioeng. 2012, 109, 2729–2736. [Google Scholar] [CrossRef] [PubMed]
- Agneessens, L.M.; Ottosen, L.D.M.; Voigt, N.V.; Nielsen, J.L.; de Jonge, N.; Fischer, C.H.; Kofoed, M.V.W. In-situ biogas upgrading with pulse H2 additions: The relevance of methanogen adaption and inorganic carbon level. Bioresour. Technol. 2017, 233, 256–263. [Google Scholar] [CrossRef] [PubMed]
- Kadam, R.; Panwar, N.L. Recent advancement in biogas enrichment and its applications. Renew. Sustain. Energy Rev. 2017, 73, 892–903. [Google Scholar] [CrossRef]
- Calbry-Muzyka, A.S.; Schildhauer, T.J. Direct methanation of biogas-technical challenges and recent progress. Front. Energy Res. 2020, 8, 570887. [Google Scholar] [CrossRef]
- Wang, F.; Swinbourn, R.; Li, C. Shipping Australian sunshine: Liquid renewable green fuel export. Int. J. Hydrogen Energy 2023, 48, 14763–14784. [Google Scholar] [CrossRef]
- Lecker, B.; Illi, L.; Lemmer, A.; Oechsner, H. Biological hydrogen methanation—A review. Bioresour. Technol. 2017, 245, 1220–1228. [Google Scholar] [CrossRef]
- Vesselli, E.; Schweicher, J.; Bundhoo, A.; Frennet, A.; Kruse, N. Catalytic CO2 hydrogenation on nickel: Novel insight by chemical transient kinetics. J. Phys. Chem. C 2011, 115, 1255–1260. [Google Scholar] [CrossRef]
- Brooks, K.P.; Hu, J.; Zhu, H.; Kee, R.J. Methanation of carbon dioxide by hydrogen reduction using the Sabatier process in microchannel reactors. Chem. Eng. Sci. 2007, 62, 1161–1170. [Google Scholar] [CrossRef]
- Sahebdelfar, S.; Ravanchi, M.T. Carbon dioxide utilization for methane production: A thermodynamic analysis. J. Pet. Sci. Eng. 2015, 134, 14–22. [Google Scholar] [CrossRef]
- Panagiotopoulou, P.; Kondarides, D.I.; Verykios, X. Selective methanation of CO over supported noble metal catalysts: Effects of the nature of the metallic phase on catalytic performance. Appl. Catal. A Gen. 2008, 344, 45–54. [Google Scholar] [CrossRef]
- Abdel-Mageed, A.M.; Widmann, D.; Olesen, S.E.; Chorkendorff, I.; Biskupek, J.; Behm, R.J. Selective CO methanation on Ru/TiO2 catalysts: Role and influence of metal-support interactions. ACS Catal. 2015, 5, 6753–6763. [Google Scholar] [CrossRef]
- Meng, F.; Li, X.; Li, M.; Cui, X.; Li, Z. Catalytic performance of CO methanation over La-promoted Ni/Al2O3 catalyst in a slurry-bed reactor. Chem. Eng. J. 2017, 313, 1548–1555. [Google Scholar] [CrossRef]
- Qiu, M.; Tao, H.; Li, Y.; Zhang, Y. Insight into the mechanism of CO2 and CO methanation over Cu (100) and Co-modified Cu (100) surfaces: A DFT study. Appl. Surf. Sci. 2019, 495, 143457. [Google Scholar] [CrossRef]
- Ma, J.; Li, S.; Xu, B.; Chu, W.; Jiang, Q.; Liu, Y. Promotion of low-temperature Ni-based CO2 methanation catalysts by LaOx confined in mesoporous silica channels. Mol. Catal. 2025, 574, 114883. [Google Scholar] [CrossRef]
- Peebles, D.E.; Goodman, D.W.; White, J.M. Methanation of carbon dioxide on Ni (100) and the effects of surface modifiers. J. Phys. Chem. 1983, 87, 4378–4387. [Google Scholar] [CrossRef]
- Fu, S.; Angelidaki, I.; Zhang, Y. In situ biogas upgrading by CO2-to-CH4 bioconversion. Trends Biotechnol. 2021, 39, 336–347. [Google Scholar] [CrossRef]
- Habib, M.A.; Abdulrahman, G.A.Q.; Alquaity, A.B.S.; Qasem, N.A.A. Hydrogen combustion, production, and applications: A review. Alex. Eng. J. 2024, 100, 182–207. [Google Scholar] [CrossRef]
- Manna, J.; Jha, P.; Sarkhel, R.; Banerjee, C.; Tripathi, A.K.; Nouni, M.R. Opportunities for green hydrogen production in petroleum refining and ammonia synthesis industries in India. Int. J. Hydrogen Energy 2021, 46, 38212–38231. [Google Scholar] [CrossRef]
- Chehade, G.; Dincer, I. Progress in green ammonia production as potential carbon-free fuel. Fuel 2021, 299, 120845. [Google Scholar] [CrossRef]
- Shen, J.; Zhang, Q.; Tian, S.; Li, X.; Liu, J.; Tian, J. The role of hydrogen in iron and steel production: Development trends, decarbonization potentials, and economic impacts. Int. J. Hydrogen Energy 2024, 92, 1409–1422. [Google Scholar] [CrossRef]
- Zhang, K.; He, L.; Jiang, L.; Jiang, S.; Yu, R.; Lau, H.C.; Xie, C.; Chen, Z. The role of hydrogen in the energy transition of the oil and gas industry. Energy Rev. 2024, 3, 100090. [Google Scholar] [CrossRef]
- Hwang, J.; Maharjan, K.; Cho, H. A review of hydrogen utilization in power generation and transportation sectors: Achievements and future challenges. Int. J. Hydrogen Energy 2023, 48, 28629–28648. [Google Scholar] [CrossRef]
- Abe, J.O.; Popoola, A.P.I.; Ajenifuja, E.; Popoola, O.M. Hydrogen energy, economy and storage: Review and recommendation. Int. J. Hydrogen Energy 2019, 44, 15072. [Google Scholar] [CrossRef]
- Shet, S.P.; Shanmuga Priya, S.; Sudhakar, K.; Tahir, M. A review on current trends in potential use of metal-organic framework for hydrogen storage. Int. J. Hydrogen Energy 2021, 46, 11782. [Google Scholar] [CrossRef]
- Xie, L.; Xu, J.; Zhang, Y.; He, Y. Chapter 7—Biogas Upgrading. In Advances in Bioenergy; Li, Y., Khanal, S.K., Eds.; Elsevier: Amsterdam, The Netherlands, 2020; Volume 5, pp. 309–344. [Google Scholar]
- Hashemi, A.W.; Wijnsma, S.; Hillestad, M.; Austbø, B. Direct vs. indirect biogas methanation for liquefied biomethane production: A concept evaluation. Fuel 2024, 371, 131835. [Google Scholar] [CrossRef]
- Mercader, V.D.; Durán, P.; Aragüés-Aldea, P.; Francés, E.; Herguido, J.; Peña, J.A. Biogas upgrading by intensified methanation (SESaR): Reaction plus water adsorption—Desorption cycles with Ni-Fe/Al2O3 catalyst and LTA 5A zeolite. Catal. Today 2024, 433, 114667. [Google Scholar] [CrossRef]
- Enrich-Prast, A.; Machado-Silva, F.; Bastviken, D.; Crill, P.; Negrão Signori, C. Chemosynthesis. In Encyclopedia of Inland Waters, 2nd ed.; Mehner, T., Tockner, K., Eds.; Elsevier: Oxford, UK, 2022; pp. 118–135. [Google Scholar]
- Borja, R.; Rincón, B. Biogas Production. In Reference Module in Life Sciences; Elsevier: Amsterdam, The Netherlands, 2017; pp. 1–24. [Google Scholar]
- Guneratnam, A.J.; Ahern, E.; FitzGerald, J.A.; Jackson, S.A.; Xia, A.; Dobson, A.D.W.; Murphy, J.D. Study of the performance of a thermophilic biological methanation system. Bioresour. Technol. 2017, 225, 308–315. [Google Scholar] [CrossRef]
- Figeac, N.; Trably, E.; Bernet, N.; Delgenès, J.-P.; Escudié, R. Temperature and inoculum origin influence the performance of ex-situ biological hydrogen methanation. Molecules 2020, 25, 5665. [Google Scholar] [CrossRef]
- Strübing, D.; Moeller, A.B.; Mößnang, B.; Lebuhn, M.; Drewes, J.E.; Koch, K. Load change capability of an anaerobic thermophilic trickle bed reactor for dynamic H2/CO2 biomethanation. Bioresour. Technol. 2019, 289, 121735. [Google Scholar] [CrossRef]
- Feickert Fenske, C.; Md, Y.; Strübing, D.; Koch, K. Preliminary gas flow experiments identify improved gas flow conditions in a pilot-scale trickle bed reactor for H2 and CO2 biological methanation. Bioresour. Technol. 2023, 371, 128648. [Google Scholar] [CrossRef]
- Feickert Fenske, C.; Kirzeder, F.; Strübing, D.; Koch, K. Biogas upgrading in a pilot-scale trickle bed reactor—Long-term biological methanation under real application conditions. Bioresour. Technol. 2023, 376, 128868. [Google Scholar] [CrossRef] [PubMed]
- Thema, M.; Weidlich, T.; Kaul, A.; Bollmann, A.; Huber, H.; Bellack, A.; Karl, J.; Sterner, M. Optimized biological CO2-methanation with a pure culture of thermophilic methanogenic archaea in a trickle-bed reactor. Bioresour. Technol. 2021, 333, 125135. [Google Scholar] [CrossRef] [PubMed]
- Aghel, B.; Behaein, S.; Wongwises, S.; Shadloo, M.S. A review of recent progress in biogas upgrading: With emphasis on carbon capture. Biomass Bioenergy 2022, 160, 106422. [Google Scholar] [CrossRef]
- Salomoni, C.; Caputo, A.; Bonoli, M.; Francioso, O.; Rodriguez-Estrada, M.T.; Palenzona, D. Enhanced methane production in a two-phase anaerobic digestion plant, after CO2 capture and addition to organic wastes. Bioresour. Technol. 2011, 102, 6443–6448. [Google Scholar] [CrossRef]
- Muntau, M.; Lebuhn, M.; Polag, D.; Bajón-Fernández, Y.; Koch, K. Effects of CO2 enrichment on the anaerobic digestion of sewage sludge in continuously operated fermenters. Bioresour. Technol. 2021, 332, 125147. [Google Scholar] [CrossRef]
- Tao, B.; Zhang, Y.; Heaven, S.; Banks, C.J. Predicting pH rise as a control measure for integration of CO2 biomethanisation with anaerobic digestion. Appl. Energy 2020, 277, 115535. [Google Scholar] [CrossRef]
- Bajón, F.Y.; Soares, A.; Vale, P.; Koch, K.; Masse, A.L.; Cartmell, E. Enhancing the anaerobic digestion process through carbon dioxide enrichment: Initial insights into mechanisms of utilization. Environ. Technol. 2019, 40, 1744–1755. [Google Scholar] [CrossRef]
- He, Z.-W.; Zou, Z.-S.; Ren, Y.-X.; Tang, C.-C.; Zhou, A.-J.; Liu, W.; Wang, L.; Li, Z.; Wang, A. Roles of zero-valent iron in anaerobic digestion: Mechanisms, advances and perspectives. Sci. Total Environ. 2022, 852, 158420. [Google Scholar] [CrossRef]
- Yang, J.; Yang, H.; Xing, T.; Sun, Y.; Qu, B.; Li, L.; Li, Y.; Guo, Y.; Zhen, F.; Pang, Y. Insight into the mechanisms of methane production enhancement by bioaugmentation with H2/CO2 in anaerobic digestion. Int. J. Hydrogen Energy 2025, 113, 340–347. [Google Scholar] [CrossRef]
- Cheng, S.; Xing, D.; Call, D.F.; Logan, B.E. Direct biological conversion of electrical current into methane by electromethanogenesis. Environ. Sci. Technol. 2009, 43, 3953–3958. [Google Scholar] [CrossRef]
- Kokkoli, A.; Zhang, Y.; Angelidaki, I. Microbial electrochemical separation of CO2 for biogas upgrading. Bioresour. Technol. 2018, 247, 380–386. [Google Scholar] [CrossRef] [PubMed]
- Villano, M.; Aulenta, F.; Ciucci, C.; Ferri, T.; Giuliano, A.; Majone, M. Bioelectrochemical reduction of CO2 to CH4 via direct and indirect extracellular electron transfer by a hydrogenophilic methanogenic culture. Bioresour. Technol. 2010, 101, 3085–3090. [Google Scholar] [CrossRef] [PubMed]
- Ren, J.; Liu, Y.-L.; Zhao, X.-Y.; Cao, J.-P. Methanation of syngas from biomass gasification: An overview. Int. J. Hydrogen Energy 2020, 45, 4223–4243. [Google Scholar] [CrossRef]
- Motta, I.L.; Miranda, N.T.; Filho, R.M.; Maciel, M.R.W. Biomass gasification in fluidized beds: A review of biomass moisture content and operating pressure effects. Renew. Sustain. Energy Rev. 2018, 94, 998–1023. [Google Scholar] [CrossRef]
- Saleem, F.; Harris, J.; Zhang, K.; Harvey, A. Non-thermal plasma as a promising route for the removal of tar from the product gas of biomass gasification—A critical review. Chem. Eng. J. 2020, 382, 122761. [Google Scholar] [CrossRef]
- Zhang, Y.; Liang, Y.; Li, S.; Yuan, Y.; Hang, D.; Wu, Y.; Xie, H.; Brindhadev, K.; Pugazhendhi, A.; Xia, C. A review of biomass pyrolysis gas: Forming mechanisms, influencing parameters, and product application upgrades. Fuel 2023, 347, 128461. [Google Scholar] [CrossRef]
- Luo, Z. Coal-to-Liquids and Polygeneration Using Low Rank Coals. In Low-Rank Coals for Power Generation, Fuel and Chemical Production; Luo, Z., Agraniotis, M., Eds.; Woodhead Publishing: Cambridge, UK, 2017; pp. 241–268. [Google Scholar]
- Ferrari, J. Chapter 4—Renewable Fuels for Long-Term Energy Storage. In Electric Utility Resource Planning; Ferrari, J., Ed.; Elsevier: Amsterdam, The Netherlands, 2021; pp. 109–138. [Google Scholar]
- Larsson, A.; Gunnasrsson, I.; Tengberg, F. The GoBiGas Project Demonstration of the Production of Biomethane from Biomass Via Gasification.; Göteborg Energi: Göteborg, Sweden, 2018. [Google Scholar] [CrossRef]
- Grimalt-Alemany, A.; Skiadas, I.V.; Gavala, H.N. Syngas biomethanation: State of the art review and perspectives. Biofuels Bioprod. Biorefining 2018, 12, 139–158. [Google Scholar] [CrossRef]
- Paniagua, S.; Lebrero, R.; Muñoz, R. Syngas biomethanation: Current state and future perspectives. Bioresour. Technol. 2022, 358, 127436. [Google Scholar] [CrossRef]
- Schöne, C.; Rother, M. Methanogenesis from Carbon Monoxide. In Biogenesis of Hydrocarbons. Handbook of Hydrocarbon and Lipid Microbiology; Stams, A., Sousa, D., Eds.; Springer: Cham, Switzerland, 2019; pp. 123–151. [Google Scholar]
- Aryal, N.; Odde, M.; Bøgeholdt Petersen, C.; Ditlev, L.; Ottosen, M.; Vedel, M.; Kofoed, W. Methane production from syngas using a trickle-bed reactor setup. Bioresour. Technol. 2021, 333, 125183. [Google Scholar] [CrossRef]
- Neves, R.C.; Klein, B.C.; da Silva, R.J.; Rezende, M.C.A.F.; Funke, A.; Olivarez-Gómez, E.; Bonomi, A.; Maciel-Filho, R. A vision on biomass-to-liquids (BTL) thermochemical routes in integrated sugarcane biorefineries for biojet fuel production. Renew. Sust. Energ. Rev. 2020, 119, 109607. [Google Scholar] [CrossRef]
- Motola, V.; Hurtig, O.; Scarlat, N.; Buffi, M.; Georgakaki, A.; Letout, S.; Mountraki, A. Clean Energy Technology Observatory: Advanced Biofuels in the European Union—2023 Status Report on Technology Development, Trends, Value Chains and Markets; Publications Office of the European Union: Luxembourg, 2023. [Google Scholar]
- Figueras, J.; Benbelkacem, H.; Dumas, C.; Buffiere, P. Biomethanation of syngas by enriched mixed anaerobic consortium in pressurized agitated column. Bioresour. Technol. 2021, 338, 125548. [Google Scholar] [CrossRef] [PubMed]
- Hoyer, K. Biogas Upgrading—A Technical Review. Kuala Lumpur. 2016. Available online: http://sgc.camero.se/ckfinder/userfiles/files/BAPF2016Hoyer+Energiforsk.pdf (accessed on 10 February 2025).
- IEA. Task 37, New-IEA Bioenergy Task 37 Updated List of Biogas Upgrading Plants. Available online: https://www.ieabioenergy.com/blog/publications/new-iea-bioenergy-task-37-updated-list-of-biogas-upgrading-plants/ (accessed on 10 February 2025).
- The World Bank. The World by Income and Region. Available online: https://datatopics.worldbank.org/world-development-indicators/the-world-by-income-and-region.html (accessed on 15 April 2025).
- Grand View Research. Biomethane Market Size, Share & Trends Analysis Report by Source (Energy Crops, Animal Manure, Municipal Waste, Waste Water Sludge), by End Use (Construction, Industrial), by Region, and Segment Forecasts. 2024–2030. Available online: https://www.grandviewresearch.com/industry-analysis/biomethane-market-report (accessed on 28 April 2025).
- Marie, M.; Yirga, F.; Alemu, G.; Azadi, H. Status of energy utilization and factors affecting rural households’ adoption of biogas technology in north-western Ethiopia. Heliyon 2021, 18, e06487. [Google Scholar] [CrossRef] [PubMed]
- Pham, C.H.; Vu, C.C.; Sommer, S.G.; Bruun, S. Factors Affecting Process Temperature and Biogas Production in Small-scale Rural Biogas Digesters in Winter in Northern Vietnam. Asian-Australas J. Anim. Sci. 2014, 27, 1050–1056. [Google Scholar] [CrossRef] [PubMed]
- Biró, K.; Woon, K.S.; Ounsaneha, W.; Rattanapan, C. A global perspective on sustainable pathways for biogas adoption. Glob. J. Environ. Sci. Manag. 2025, 11, 825–856. [Google Scholar]
- Situmeang, R.; Mazancová, J.; Roubík, H. Technological, Economic, Social and Environmental Barriers to Adoption of Small-Scale Biogas Plants: Case of Indonesia. Energies 2022, 15, 5105. [Google Scholar] [CrossRef]
- Filanowski, P. Porównanie oraz wyznaczenie wspólnych parametrów jakościowych dla biometanu zatłaczanego do sieci gazowej oraz biometanu przeznaczonego do celów transportowych bioCNG (Comparison and determination of common quality parameters for biomethane injected into the gas network and biomethane intended for bioCNG transport). Gaz. Woda I Tech. Sanit. 2022, 17, 12–15. (In Polish) [Google Scholar]
- Flemming, P.; Church, B.J. Gas. Chapter 13; In Plant Engineer’s Reference Book, 2nd ed.; Snow, D.A., Ed.; Butterworth-Heinemann: Oxford, UK, 2002; pp. 13–35. [Google Scholar]
- The Engineering ToolBox. Fuels—Higher and Lower Calorific Values. 2003. Available online: https://www.engineeringtoolbox.com/fuels-higher-calorific-values-d_169.html (accessed on 3 February 2025).
- Ronkainen, M. Biovoima Supplied Gas Transportation Containers to Iceland Ahead of Schedule. Available online: https://biovoima.com/en/news/biovoima-supplied-gas-transportation-containers-to-iceland-ahead-of-schedule (accessed on 3 February 2025).
- EN 16723-2:2017; Natural Gas and Biomethane for Use in Transport and Biomethane for Injection in the Natural Gas Network—Part 2: Automotive Fuels Specification. CEN: Brussels, Belgium, 2017.
- American Biogas Council RNG Purity Recommendation. Available online: https://americanbiogascouncil.org/resources/rng-purity-recommendation-american-biogas-council/ (accessed on 12 March 2025).
- Owusu, G.O.; Maniatis, K. Development of Standardization Processes for Biomethane Deliverable n. 1.4 GreenMeUp—Green Biomethane Market Uptake. 2024. Available online: https://www.greenmeup-project.eu/wp-content/uploads/2024/07/D1.4.pdf (accessed on 12 March 2025).
- Aisbl, M. Quality of Biomethane Required in European Countries for Injecting into Natural Gas Grid. 2024. Available online: https://www.marcogaz.org/wp-content/uploads/2024/03/Quality-of-biomethane.pdf (accessed on 11 March 2025).
- Owusu, G.O. EBA, From Plant to Grid: Navigating Biomethane Injection. 2024. Available online: https://www.europeanbiogas.eu/from-plant-to-grid-navigating-biomethane-injection/ (accessed on 12 March 2025).
- EN 16723-1:2016; Natural Gas and Biomethane for Use in Transport and Biomethane for Injection in the Natural Gas Network—Part 1: Specifications for Biomethane for Injection in the Natural Gas Network. CEN: Brussels, Belgium, 2016.
- EN 16726:2015+A1:2018; Gas Infrastructure. Quality of Gas. CEN: Brussels, Belgium, 2018.
- Rasi, S.; Lehtinen, J.; Rintala, J. Determination of organic silicon compounds in biogas from wastewater treatments plants, landfills, and co-digestion plants. Renew. Energy 2010, 35, 2666–2673. [Google Scholar] [CrossRef]
- AS4564:2020; General-Purpose Natural Gas. Standards Australia: Sydney, Australia, 2020.
- Biogasworld. Renewable Natural Gas Quality Specifications in North America. 2019. Available online: https://biogasworld.com/news/renewable-natural-gas-quality-specifications-in-north-america/ (accessed on 10 March 2025).
- Benhelal, E.; Hoseinpour, M.; Karami, R.; Mirvakili, A.; Rashid, M.I. Chapter 6—Energy and Exergy Analysis of Blue Hydrogen Production and Conversion. In Hydrogen Energy Conversion and Management; Khan, M.M.K., Azad, A.K., Oo, A.M.T., Eds.; Elsevier: Amsterdam, The Netherlands, 2024; pp. 157–207. [Google Scholar]
- Bagi, Z.; Ács, N.; Böjti, T.; Kakuk, B.; Rákhely, G.; Strang, O.; Szuhaj, M.; Wirth, R.; Kovács, K.L. Biomethane: The energy storage, platform chemical and greenhouse gas mitigation target. Anaerobe 2017, 46, 13–22. [Google Scholar] [CrossRef]
- Dincer, I.; Rosen, M.A.; Al-Zareer, M. Chemical Energy Production. In Comprehensive Energy Systems; Dincer, I., Ed.; Elsevier: Amsterdam, The Netherlands, 2018; pp. 470–520. [Google Scholar]
- Gunawardane, K. Chapter 1—Evolution of Hydrogen Energy and Its Potential Opportunities Around the Globe. In Hydrogen Energy Conversion and Management; Khan, M.M.K., Azad, A.K., Oo, A.M.T., Eds.; Elsevier: Amsterdam, The Netherlands, 2024; pp. 3–33. [Google Scholar]
- Abbas, H.F.; Wan Daud, W.M.A. Hydrogen production by methane decomposition: A review. Int. J. Hydrogen Energy 2010, 35, 1160–1190. [Google Scholar] [CrossRef]
- da Silva Pinto, R.L.; Vieira, A.C.; Scarpetta, A.; Marques, F.S.; Matos, R.M.; Bail, J.A.; Jorge, L.M.M.; Corazza, M.L.; Ramos, L.P. An overview on the production of synthetic fuels from biogas. Bioresour. Technol. Rep. 2022, 18, 101104. [Google Scholar] [CrossRef]
- Speight, J.G. Chapter 12—Synthesis Gas and the Fischer–Tropsch Process. In The Refinery of the Future, 2nd ed.; Speight, J.G., Ed.; Gulf Professional Publishing: Houston, TX, USA, 2020; pp. 427–468. [Google Scholar]
- Bezergianni, S.; Dimitriadis, A. Comparison between different types of renewable diesel. Renew. Sustain. Energy Rev. 2013, 21, 110–116. [Google Scholar] [CrossRef]
- Van Dyk, S.; Su, J.; McMillan, J.D.; Saddler, J.N. Drop-in’ Biofuels: The Key Role that Co-Processing will Play in Its Production. Task 39 Drop in Biofuels Full Report January 2019. Available online: https://www.ieabioenergy.com/wp-content/uploads/2019/09/Task-39-Drop-in-Biofuels-Full-Report-January-2019.pdf (accessed on 12 December 2024).
- Kargbo, H.; Harris, J.S.; Phan, A.N. “Drop-in” fuel production from biomass: Critical review on techno-economic feasibility and sustainability. Renew. Sustain. Energy Rev. 2021, 135, 110168. [Google Scholar] [CrossRef]
- Vasudevan, U.M.; Mai, D.H.A.; Krishna, S.; Lee, E.Y. Methanotrophs as a reservoir for bioactive secondary metabolites: Pitfalls, insights and promises. Biotechnol. Adv. 2023, 63, 108097. [Google Scholar]
- Brigham, S. Applications of polyhydroxyalkanoates in the medical industry. Int. J. Biotechnol. Wellness Ind. 2012, 1, 53–60. [Google Scholar] [CrossRef]
- Bugnicourt, E.; Cinelli, P.; Lazzeri, A.; Alvarez, V. Polyhydroxyalkanoate (PHA): Review of synthesis, characteristics, processing and potential applications in packaging. Express Polym. Lett. 2014, 8, 791–808. [Google Scholar] [CrossRef]
- Strong, P.J.; Xie, S.; Clarke, W.P. Methane as a resource: Can the methanotrophs add value? Environ. Sci. Technol. 2015, 49, 4001–4018. [Google Scholar] [CrossRef]
- Haag, N.L.; Grumaz, C.; Wiese, F.; Kirstahler, P.; Merkle, W.; Nägele, H.J.; Sohn, K.; Jungbluth, T.; Oechsner, H. Advanced green biorefining: Effects of ensiling treatments on lactic acid production, microbial activity and supplementary methane formation of grass and rye. Biomass Convers. Biorefin 2016, 6, 197–208. [Google Scholar] [CrossRef]
- Simya, O.K.; Radhakrishnan, P.; Ashok, A. Chapter 41—Engineered Nanomaterials for Energy Applications. In Micro and Nano Technologies, Handbook of Nanomaterials for Industrial Applications; Hussain, C.M., Ed.; Elsevier: Amsterdam, The Netherlands, 2018; pp. 751–767. [Google Scholar]
- Guney, M.S.; Tepe, Y. Classification and assessment of energy storage systems. Renew. Sustain. Energy Rev. 2017, 75, 1187–1197. [Google Scholar] [CrossRef]
- Wang, M.; Su, C.; Zhu, Z.; Wang, H.; Ge, L. Composite cathodes for protonic ceramic fuel cells: Rationales and materials. Compos. B: Eng. 2022, 238, 109881. [Google Scholar] [CrossRef]
- Hong, K.; Choi, M.; Bae, Y.; Min, J.; Lee, J.; Kim, D.; Bang, S.; Lee, H.-K.; Lee, W.; Hong, J. Direct methane protonic ceramic fuel cells with self-assembled Ni-Rh bimetallic catalyst. Nat. Commun. 2023, 14, 7485. [Google Scholar] [CrossRef]
- Bang, E.-S.; Kim, M.-H.; Park, S.-K. Options for methane fuel processing in PEMFC system with potential maritime applications. Energies 2022, 15, 8604. [Google Scholar] [CrossRef]
- Mustafa, L.; Ślefarski, R.; Jankowski, R. Thermodynamic analysis of gas turbine systems fueled by a CH4/H2 mixture. Sustainability 2024, 16, 531. [Google Scholar] [CrossRef]
- Reale, F.; Calabria, R.; Chiariello, F.; Pagliara, R.; Massoli, P. A micro gas turbine fuelled by methane-hydrogen blends. AMM 2012, 232, 792–796. [Google Scholar] [CrossRef]
- Yağlı, H.; Koç, Y.; Köse, Ö.; Koç, A.; Yumrutaş, R. Optimisation of simple and regenerative organic Rankine cycles using jacket water of an internal combustion engine fuelled with biogas produced from agricultural waste. Process Saf. Environ. Prot. 2021, 155, 17–31. [Google Scholar] [CrossRef]
- Benato, A.; Macor, A. Biogas engine waste heat recovery using organic Rankine cycle. Energies 2017, 10, 327. [Google Scholar] [CrossRef]
- Köse, Ö.; Koç, Y.; Yağlı, H. Is Kalina cycle or organic Rankine cycle for industrial waste heat recovery applications? A detailed performance, economic and environment based comprehensive analysis. Process Saf. Environ. Prot. 2022, 163, 421–437. [Google Scholar] [CrossRef]
- Guerron, G.; Nicolalde, J.F.; Martínez-Góme, J.; Dávila, P.; Velásquez, C. Experimental analysis of a pilot plant in Organic Rankine Cycle configuration with regenerator and thermal energy storage (TES-RORC). Energy 2024, 308, 132964. [Google Scholar] [CrossRef]
- IEA. Bioenergy Task 33: 07 2022 Sustainable Natural Gas Production Through Gasification IEA. Available online: https://www.ieabioenergy.com/wp-content/uploads/2022/08/Position-paper-Sustainable-Natural-Gas-production-through-gasification-rev.pdf (accessed on 11 March 2025).
- EBA. 22 Bcm of Biogases Were Produced in Europe in 2023. 4 December 2024. Available online: https://www.europeanbiogas.eu/22-bcm-of-biogases-were-produced-in-europe-in-2023according-to-a-new-report-released-today/ (accessed on 11 March 2025).
- Biogas in Denmark. Available online: https://ens.dk/en/energy-sources/biogas-denmark (accessed on 10 March 2025).
- IEA. Bioenergy: Countries’ Report-Update 2024, Implementation of Bioenergy in the IEA Bioenergy Member Countries, Luc Pelkmans, Technical Coordinator, IEA Bioenergy TCP, IEA Bioenergy ExCo. 2024. Available online: https://www.ieabioenergy.com/wp-content/uploads/2025/01/CountriesReport2024_final.pdf (accessed on 5 January 2025).
- EBA. European Biomethane Map 2022–2023. Available online: https://www.europeanbiogas.eu/strongnew-record-for-biomethane-production-in-europebrshows-eba-gie-biomethane-map-2022-2023-strong/ (accessed on 12 December 2024).
- EBA. European Biomethane Map 2024. Available online: https://www.europeanbiogas.eu/european-biomethane-map-2024/ (accessed on 13 March 2025).
- Global Biogas Upgrading Equipment Market Report 2024: Size, Drivers, and Top Segments, Latest Global Market Insights by the Business Research Company. Available online: https://blog.tbrc.info/2024/10/global-biogas-upgrading-equipment-market/ (accessed on 12 March 2025).
- Emprin, L.; Toop, G.; Yordanova, S.; Alberici, S.; Cihlar, J. Manual for national Biomethane Strategies, Guidehouse Netherlands B.V. 2022. Available online: https://www.europeanbiogas.eu/wp-content/uploads/2022/09/2022-Manual-for-National-Biomethane-Strategies_Gas-for-Climate.pdf (accessed on 24 April 2025).
- Statista. Available online: https://www.statista.com/statistics/263981/worldwide-energy-consumption-forecast-between-1980-and-2030/ (accessed on 24 April 2025).
- REPowerEU: Joint European Action for More Affordable, Secure and Sustainable Energy, Communication from the Commission to the European Parliament, the European Council, the Council, the European Economic and Social Committee and the Committee of the Regions Strasbourg, 8.3.2022. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=COM:2022:108:FIN (accessed on 13 March 2025).
- Molnar, G. 2024 Biomethane: A global Stocktake, IEA. Available online: https://iea.blob.core.windows.net/assets/33c29a6e-a2f2-4e20-9fa1-e057263c893a/IEA_Biomethanewebinar_GergelyMOLNAR.pdf (accessed on 13 March 2025).
- Gas Market Report, Q1-2025. Available online: https://iea.blob.core.windows.net/assets/6bd6c46d-21d7-4ae7-af9f-25dc9f8e7f3b/GasMarketReport,Q1-2025.pdf (accessed on 13 March 2025).
- ENTSOG 2050 Roadmap for Gas Grids. 2019. Available online: https://www.entsog.eu/sites/default/files/2019-12/ENTSOG%20Roadmap%202050%20for%20Gas%20Grids.pdf (accessed on 14 March 2025).
- CAN/BNQ 3672-100; Biomethane—Quality Specifications for Injection into Natural Gas Distribution and Transmission Systems QUÉBEC. CNW: Québec, Canada, 2023.
- RNG. Inflation Reduction Act (IRA) Public Law 117–169, 136 Stat. 1818. 16 August 2022. Available online: https://www.rngcoalition.com. (accessed on 11 March 2025).
- Horizon Databook: The World’s Largest Portal for Market Reports & Statistics. Available online: https://www.grandviewresearch.com/horizon/outlook/biomethane-market/china/statistics (accessed on 19 April 2025).
- Jain, S.; Words Biogas Association. Market Report Japan. Available online: http://epower.pw/wp-content/themes/epower/img/WBA-japan-4ppa4.pdf (accessed on 14 March 2025).
- Polyplastics Evonik. One of the Largest Biogas Upgrading Plants in Japan Uses SEPURAN® Green Membranes and is Running Smoothly Now. Available online: https://www.pp-evonik.com/english/news_release/press/94.html (accessed on 11 March 2025).
- Horizon Databook: The World’s Largest Portal for Market Reports & Statistics. Available online: https://www.grandviewresearch.com/horizon/outlook/biomethane-market/japan/statistics (accessed on 24 April 2025).
- Malabar Biomethane Facility. Available online: https://www.jemena.com.au/future-energy/future-gas/Malabar-Biomethane-Injection-Plant/ (accessed on 13 March 2025).
- Submission: Standards Australia Consultation on Amending AS/NZS 4564:2020 General-Purpose Natural Gas. 2023. Available online: https://apga.org.au/submissions/standards-australia-consultation-on-amendments-to-as4564 (accessed on 13 March 2025).
- Kabeyi, J.B.M.; Olanrewaju, A.O. Biomethane Production and Applications; IntechOpen: London, UK, 2024. [Google Scholar] [CrossRef]
- Hernø, T. Production of Upgraded Biogas-Optimization of Costs and Climate Impact EUDP-j.nr. 64018-0512, December 2020, Danish Gas Technology Centre. Available online: https://planenergi.eu/wp-content/uploads/2023/09/Prod_upgraded_biogas_optimization_uk_summary.pdf (accessed on 8 March 2025).
- Miltner, M.; Makaruk, A.; Harasek, M. Review on available biogas upgrading technologies and innovations towards advanced solutions. J. Clean. Prod. 2017, 161, 1329–1337. [Google Scholar] [CrossRef]
- Panoutsou, C.; Germer, S.; Karka, P.; Papadokostantakis, S.; Kroyan, Y.; Wojcieszyk, M.; Maniatis, K.; Marchand, P.; Landalv, I. Advanced biofuels to decarbonise European transport by 2030: Markets, challenges, and policies that impact their successful market uptake. Energy Strategy Rev. 2021, 34, 100633. [Google Scholar] [CrossRef]
- Noussan, M.; Negro, V.; Prussi, M.; Chiaramonti, D. The potential role of biomethane for the decarbonization of transport: An analysis of 2030 scenarios in Italy. Appl. Energy 2024, 355, 122322. [Google Scholar] [CrossRef]
- EBA. Biomethane in Transport. Available online: https://www.europeanbiogas.eu/wp-content/uploads/2019/07/Biomethane-in-transport.pdf (accessed on 8 March 2025).
- Vinardell, S.; Feickert Fenske, C.; Heimann, A.; Cortina, J.L.; Valderrama, C.; Koch, K. Exploring the potential of biological methanation for future defossilization scenarios: Techno-economic and environmental evaluation. Energy Convers. Manag. 2024, 307, 18339. [Google Scholar] [CrossRef]
- Orecchini, F.; Santiangeli, A.; Zuccari, F. Biomethane use for automobiles towards a CO2-neutral energy system. Clean. Energy 2021, 5, 124–140. [Google Scholar] [CrossRef]
- Biernat, K.; Samson-Bręk, I.; Chłopek, Z.; Owczuk, M.; Matuszewska, A. Assessment of the Environmental Impact of Using Methane Fuels to Supply Internal Combustion Engines. Energies 2021, 14, 3356. [Google Scholar] [CrossRef]
- EBA. 2025. Available online: https://www.europeanbiogas.eu/benefits/affordable-energy/ (accessed on 15 March 2025).
- Jonsson, O.; Persson, M. Biogas as Transportation Fuel, Fachtagung. 2003. Available online: https://www.bioenergy.org.nz/documents/resource/Report-Biogas-transport-sweden-2003.pdf (accessed on 13 March 2025).
- Ferella, F.; Cucchiella, F.; D’Adamo, I.; Gallucci, K. A techno-economic assessment of biogas upgrading in a developed market. J. Clean. Prod. 2019, 210, 945–957. [Google Scholar] [CrossRef]
- Budzianowski, W.M.; Brodacka, M. Biomethane storage: Evaluation of technologies, end uses, business models, and sustainability. Energy Convers. Manag. 2017, 141, 254–273. [Google Scholar] [CrossRef]
- Paturska, A.; Repele, M.; Bazbauers, G. Economic assessment of biomethane supply system based on natural gas infrastructure. Energy Procedia 2015, 72, 71–78. [Google Scholar] [CrossRef]
- Molíková, A.; Vítězová, M.; Vítěz, T.; Buriánková, I.; Huber, H.; Dengler, L.; Urbanová, I. Underground gas storage as a promising natural methane bioreactor and reservoir? J. Energy Storage 2022, 47, 103631. [Google Scholar] [CrossRef]
- Blanco, A.A.G.; Vallone, A.F.; Korili, S.A.; Gil, A.; Sapag, K. A comparative study of several microporous materials to store methane by adsorption. Microporous Mesoporous Mater. 2016, 224, 323–331. [Google Scholar] [CrossRef]
- Adlak, K.; Chandra, R.; Vijay, V.K.; Pant, K.K. Physicochemical activation and palletisation of Azadirachta indica wood carbons for increased biomethane adsorbed energy storage. J. Anal. Appl. Pyrolysis 2021, 155, 105102. [Google Scholar] [CrossRef]
- Memetova, A.; Tyagi, I.; Karri, R.R.; Kumar, V.; Tyagi, K.; Memetov, N.; Singh, K. Porous carbon-based material as a sustainable alternative for the storage of natural gas (methane) and biogas (biomethane): A review. Chem. Eng. J. 2022, 446, 137373. [Google Scholar] [CrossRef]


| Technology | Final CH4 Content [%vol.] CH4 Losses [%] | Advantages | Disadvantages | Investment Cost [€ */Year] Maintenance Cost [€ */Year] ** |
|---|---|---|---|---|
| Pressure swing adsorption (PSA) | 95–99 1.5–12.0 | Combined removal of CO2, N2, and O2 No need for chemicals Compact technology Possibility of use for a small scale Low energy demand High tolerance for impurities Fast installation Fast regeneration of sorbent | Necessity of prior H2S and H2O removal High investment and operational cost Susceptible to fouling and operating nuisances | 1,750,000 56,000 |
| Water scrubbing | 95–99 0.5–5.0 | Combined removal of CO2, H2S, and NH3 High tolerance for impurities No need for chemicals Flexibility in adjusting capacity by temperature and pressure change Ease of water regeneration | High water volume requirement Slow process High requirement for power Clogging due to bacterial growth Problems with foaming, corrosion and wastewater disposal Necessity to remove water from biomethane | 1,000,000 15,000 |
| Organic physical scrubbing | 95–99 <1.0–4.0 | Combined removal of H2O, H2S, and NH3 Possibility of removing residual CH4 by heating | High investment and operational cost High solvent cost High energy demand for solvent regeneration Expensive for small-scale operation Decrease in process efficiency due to solvent dilution in water | 1,000,000 39,000 |
| Chemical scrubbing | 97–99 <0.1–4.0 | Fast process Low operational cost Ease of solvent regeneration Complete H2S removal | High cost of amine solvents and investment costs High requirement for energy for solvent regeneration Problems with corrosion, foaming and precipitation of salts Problems with toxic for human and environment waste chemicals disposal | 2,000,000 59,000 |
| Membrane separation | 90–99 <0.5–20.0 | Environmentally friendly Fast installation Low energy requirement Compact and simple technology Combined removal of CO2, H2S, and H2O No need for chemicals High reliability Possibility of upgrading even at low gas flows | Strong recommendation for prior H2S and H2O removal High cost of membranes Requirement for multiple modules for high purity Need for membrane replacement every 1–5 years Low membrane selectivity Low efficiency in single step process Possibility of membranes congestion and contamination | 2,000,000 25,000 |
| Cryogenic separation | 97–99 <0.1–2.0 | Environmentally friendly No need for chemicals High purity CO2 production for further use Minimal additional energy requirement for liquid biomethane (LBM) production | High investment and operational costs High energy demand Temperature dependent process efficiency | nd.*** nd.*** |
| Biological upgrading | 65–100 nd.*** | Environmentally friendly Simple and inexpensive process Low maintenance costs No need for chemicals No waste | Used on a pilot scale High requirement for H2 Not suitable for high H2S concentrations Not recommended for large-scale use Strong recommendation for monitoring and controlling of operational parameters | nd.*** nd.*** |
| Parameter | Gaseous Biomethane | Compressed Biomethane (CBM) | Liquefied Biomethane (LBM) |
|---|---|---|---|
| Methane content [%vol.] | 97 | 97 | 99.995 |
| Pressure [bar] | 1.01325 | 250 | 1.03125 |
| and temperature [°C] | 15 | 15 | −162 |
| Density [kg/m3] | 0.68 | 186.88 | 424.14 |
| Energy content, NCV [MJ/m3] | 32.96 | 9063 | 21,000 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pawłowska, M.; Zdeb, M.; Bis, M.; Pawłowski, L. State and Perspectives of Biomethane Production and Use—A Systematic Review. Energies 2025, 18, 2660. https://doi.org/10.3390/en18102660
Pawłowska M, Zdeb M, Bis M, Pawłowski L. State and Perspectives of Biomethane Production and Use—A Systematic Review. Energies. 2025; 18(10):2660. https://doi.org/10.3390/en18102660
Chicago/Turabian StylePawłowska, Małgorzata, Magdalena Zdeb, Marta Bis, and Lucjan Pawłowski. 2025. "State and Perspectives of Biomethane Production and Use—A Systematic Review" Energies 18, no. 10: 2660. https://doi.org/10.3390/en18102660
APA StylePawłowska, M., Zdeb, M., Bis, M., & Pawłowski, L. (2025). State and Perspectives of Biomethane Production and Use—A Systematic Review. Energies, 18(10), 2660. https://doi.org/10.3390/en18102660







