Fermentation of Sugar Beet Pulp by E. coli for Enhanced Biohydrogen and Biomass Production
Abstract
1. Introduction
| Reference | Used Waste | Pretreatment | Inoculum | Fermentation Conditions | Hydrogen Yield |
|---|---|---|---|---|---|
| [18] | Hydrolyzed Sugar Beet Pulp (SBP | Enzymatic hydrolysis (saccharification with Viscozyme® and Ultraflo® Max at 45 °C, pH 5.5, 10 h) | Anaerobic sludge froma municipal wastewater treatment plant, pretreated at 80 °C for 1.5 h, pH adjusted to 5.5. Contains hydrogen-producing Clostridiales, Lactobacillales, Coriobacteriales, and methanogens (Methanosphaera sp.) | Batch: 35 °C, pH 5.5, Xo/So = 1:8 (VS-based), nitrogen purged before sealing, manually shaken daily | Batch: 279 dm3 H2 kgVS−1 (K3PO4 supplementation) |
| Semi-continuous: 35 °C, SRT = 5 days, OLR ≈ 10 gVS/m3d, pH adjusted daily | Semi-continuous: 36 dm3 H2 kgVS−1 (K3PO4 supplementation) | ||||
| [19] | Sugar Beet Pulp (SBP) | Thermal pretreatment (80 °C for 1.5 h, pH 5.5) | Anaerobic sludge from a municipal wastewater treatment plant, pretreated at 80 °C for 1 h. Contains Clostridiales, Lactobacillales, Methanobrevibacter, and Caproiciproducens | Batch: 35 °C, pH 5.5, Xo/So = 1:8 (VS-based), nitrogen purged before sealing | Batch: >200 dm3 H2 kgVS−1 (with Fe2O3, 0.1 g (dm3)−1) |
| Semi-continuous: 35 °C, SRT = 5 days, OLR ≈ 9.95 gVS/m3d | Semi-continuous: 52.11 dm3 H2 kgVS−1 (with Fe2O3) | ||||
| [13] | Sugar Beet Pulp (SBP) Dilute acid pretreatment | Dilute acid pretreatment (H2SO4 1% v/v) | Co-culture: Escherichia coli + Saccharomyces cerevisiae | SSF: 35 °C, pH controlled, simultaneous saccharification and fermentation | SSF: 252 dm3 H2 kgVS−1 |
| [20] | Sugar Beet (various particle sizes) | Mechanical size reduction (0.1–1 cm particle sizes) | Naturally occurring microbial consortia (not specified) | Batch: various particle sizes (0.1–1 cm), 24.6 g L−1 sugar beet; optimum at 0.1 cm pH ~6, anaerobic, temp not specified | Maximum: 197.9 mL H2 g TOC−1 at 0.1 cm particle size |
| [21] | Sugar Beet Pulp (SBP) | Alkaline, Thermal-Alkaline, Microwave-Alkaline pretreatments | Anaerobic sludge (from Ankara WWTP), 1800 mg L VSS−1 | Batch: 35 °C, 175 rpm, 20 g/L COD, pH 6.0 | 115.6 mL H2 g COD−1 (alkaline pretreatment) 108.2 mL H2 g COD−1 (thermal-alkaline) 66.7 mL H2 g COD−1 (microwave-alkaline) |
2. Materials and Methods
2.1. Waste Source and Pretreatment
2.2. Bacterial Strain Cultivation and Hydrogen Production
| Strains | Genotype | Subunits Lacking | Reference |
|---|---|---|---|
| BW25113 | rrB ΔlacZ4787 HsdR514 Δ(araBAD)567Δ(rhaBAD)568 rph-1 | Wild type | [27] |
| BW25113 hyaB hybC hycA fdoG ldhA frdC aceE | BW25113 ΔhyaB ΔhybC ΔhycA ΔfdoG ΔldhA ΔfrdC ΔaceE | Large subunit of Hyd-1 and 2, repressor of FHL, α-subunit of formate dehydrogenase-N, lactate dehydrogenase | [28] |
2.3. Physicochemical Analysis
2.4. Statistical Analysis
3. Results and Discussion
3.1. Treatment Optimization and Hydrolysate Composition
3.2. Sugar Utilization and Biomass Generation
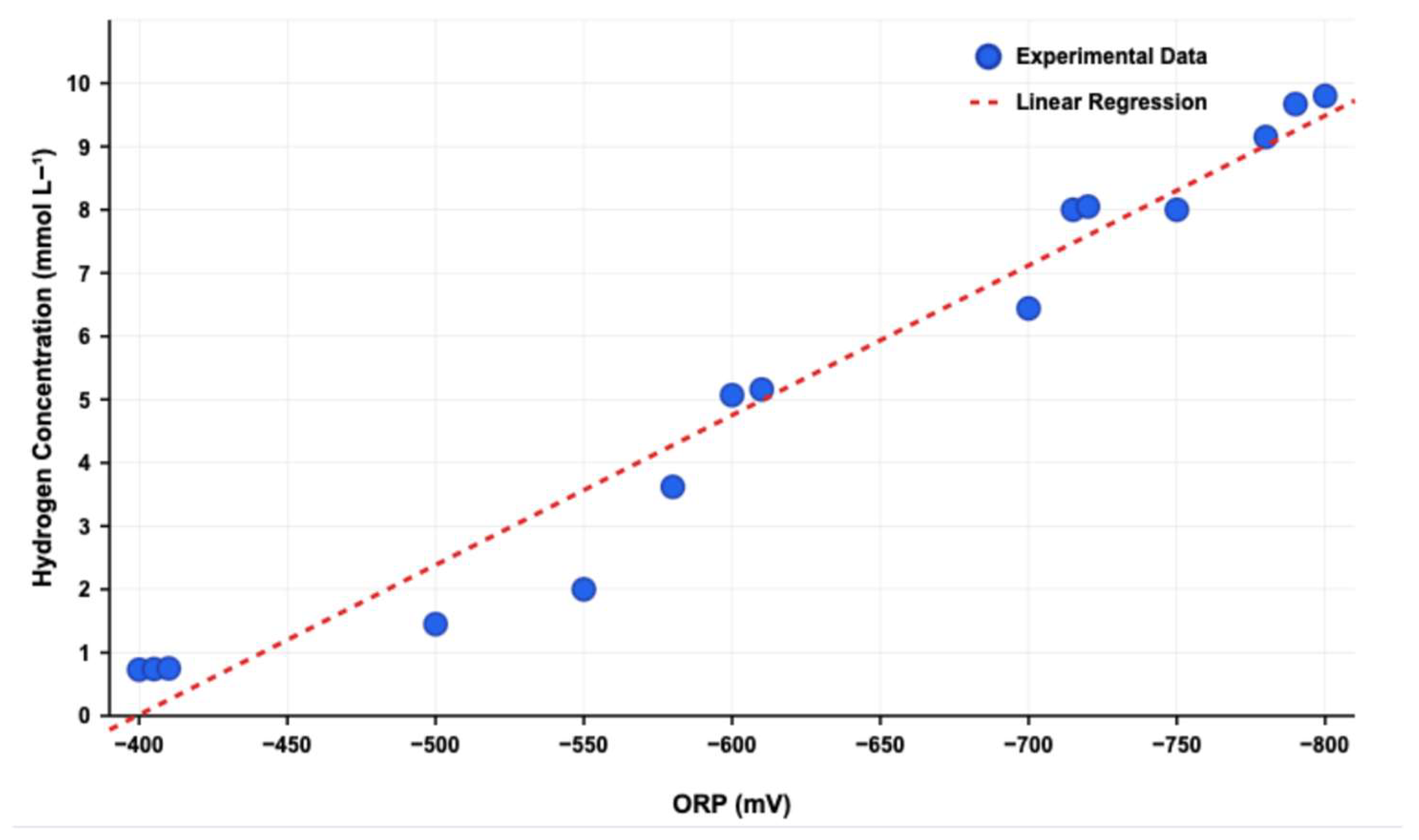
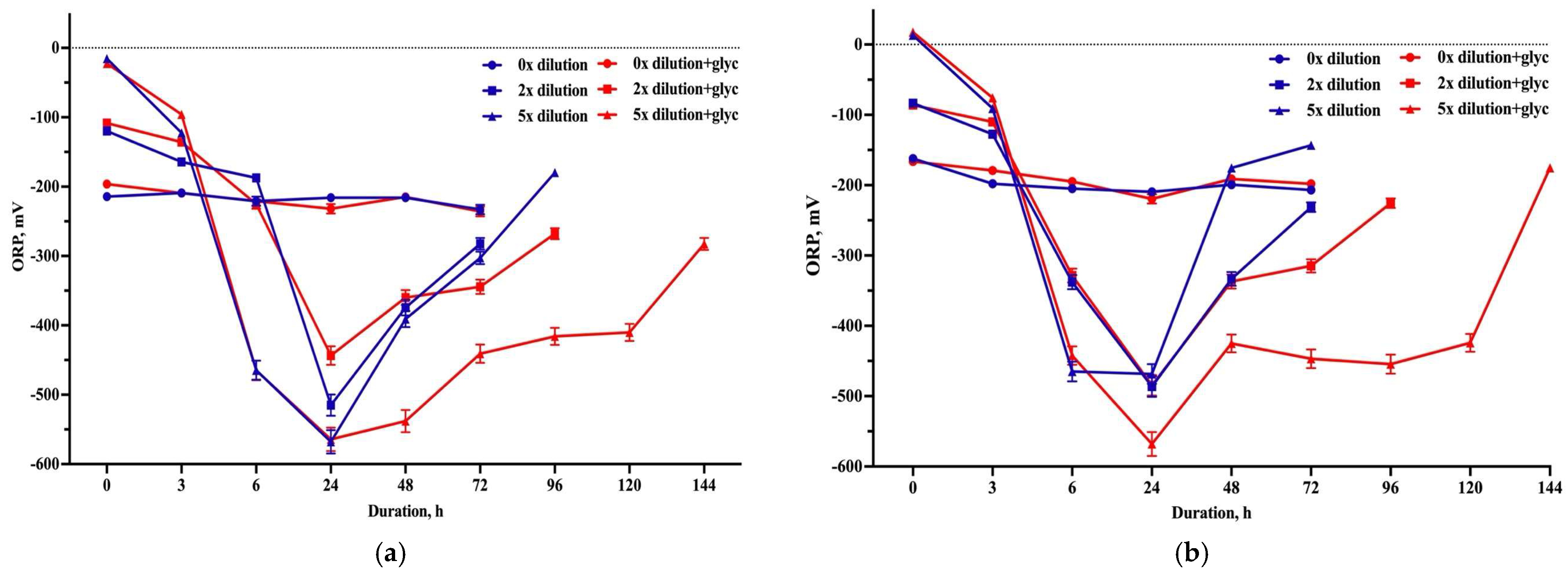
3.3. Hydrogen Production
4. Conclusions
- Undiluted hydrolysates led to minimal H2 production but maximized biomass formation, with the septuple mutant reaching 0.3 g CDW L−1, indicating a shift in metabolic activity from H2 production to growth.
- The highest H2 yield during 24th hour of fermentation was obtained in 5× diluted 30 g L−1 SBP with glycerol using the septuple mutant reaching up to 12.06 mmol H2 (g sugar)−1 and 0.28 mmol H2 (g waste)−1.
- Moderate H2 yields were obtained in 50 g L−1 SBP containing hydrolysate with glycerol in both strains. These data confirm that the combination of dilution and co-substrate is the most effective.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Correction Statement
References
- Joanna, B.; Michal, B.; Piotr, D.; Agnieszka, W.; Dorota, K.; Izabela, W. Sugar Beet Pulp as a Source of Valuable Biotechnological Products. In Advances in Biotechnology for Food Industry; Elsevier: Amsterdam, The Netherlands, 2018; pp. 359–392. [Google Scholar]
- Our World in Data. Available online: https://ourworldindata.org (accessed on 11 May 2025).
- Bonnin, E.; Ralet, M.-C.; Thibault, J.-F.; Schols, H.A. Enzymes for the Valorisation of Fruit- and Vegetable-Based Co-Products. In Handbook of Waste Management and Co-Product Recovery in Food Processing; Elsevier: Amsterdam, The Netherlands, 2009; pp. 257–285. [Google Scholar]
- Government of Kazakhstan. National Statistics Office of Kazakhstan. Available online: https://stat.gov.kz (accessed on 11 May 2025).
- Mohdaly, A.A.A.; Sarhan, M.A.; Mahmoud, A.; Ramadan, M.F.; Smetanska, I. Antioxidant Efficacy of Potato Peels and Sugar Beet Pulp Extracts in Vegetable Oils Protection. Food Chem. 2010, 123, 1019–1026. [Google Scholar] [CrossRef]
- Usmani, Z.; Sharma, M.; Diwan, D.; Tripathi, M.; Whale, E.; Jayakody, L.N.; Moreau, B.; Thakur, V.K.; Tuohy, M.; Gupta, V.K. Valorization of Sugar Beet Pulp to Value-Added Products: A Review. Bioresour. Technol. 2022, 346, 126580. [Google Scholar] [CrossRef] [PubMed]
- Rana, A.K.; Gupta, V.K.; Newbold, J.; Roberts, D.; Rees, R.M.; Krishnamurthy, S.; Thakur, V.K. Sugar Beet Pulp: Resurgence and Trailblazing Journey towards a Circular Bioeconomy. Fuel 2022, 312, 122953. [Google Scholar] [CrossRef]
- Baryga, A.; Ziobro, R.; Gumul, D.; Rosicka-Kaczmarek, J.; Miśkiewicz, K. Physicochemical Properties and Evaluation of Antioxidant Potential of Sugar Beet Pulp—Preliminary Analysis for Further Use (Future Prospects). Agriculture 2023, 13, 1039. [Google Scholar] [CrossRef]
- Aljabri, M.; Alharbi, S.; Al-Qthanin, R.N.; Ismaeil, F.M.; Chen, J.; Abou-Elwafa, S.F. Recycling of Beet Sugar Byproducts and Wastes Enhances Sugar Beet Productivity and Salt Redistribution in Saline Soils. Environ. Sci. Pollut. Res. 2021, 28, 45745–45755. [Google Scholar] [CrossRef]
- Singh, R.; Kumar, R.; Sarangi, P.K.; Kovalev, A.A.; Vivekanand, V. Effect of Physical and Thermal Pretreatment of Lignocellulosic Biomass on Biohydrogen Production by Thermochemical Route: A Critical Review. Bioresour. Technol. 2023, 369, 128458. [Google Scholar] [CrossRef]
- Gong, M.; Feng, A.; Wang, L.; Wang, M.; Hu, J.; Fan, Y. Coupling of Hydrothermal Pretreatment and Supercritical Water Gasification of Sewage Sludge for Hydrogen Production. Int. J. Hydrogen Energy 2022, 47, 17914–17925. [Google Scholar] [CrossRef]
- Riaz, S.; Oluwoye, I.; Al-Abdeli, Y.M. Oxidative Torrefaction of Densified Woody Biomass: Performance, Combustion Kinetics and Thermodynamics. Renew. Energy 2022, 199, 908–918. [Google Scholar] [CrossRef]
- Puligundla, P.; Mok, C. Valorization of Sugar Beet Pulp through Biotechnological Approaches: Recent Developments. Biotechnol. Lett. 2021, 43, 1253–1263. [Google Scholar] [CrossRef]
- Bellido, C.; Infante, C.; Coca, M.; González-Benito, G.; Lucas, S.; García-Cubero, M.T. Efficient Acetone–Butanol–Ethanol Production by Clostridium Beijerinckii from Sugar Beet Pulp. Bioresour. Technol. 2015, 190, 332–338. [Google Scholar] [CrossRef]
- Vučurović, V.M.; Razmovski, R.N. Sugar Beet Pulp as Support for Saccharomyces Cerivisiae Immobilization in Bioethanol Production. Ind. Crops Prod. 2012, 39, 128–134. [Google Scholar] [CrossRef]
- Poladyan, A.; Margaryan, L.; Trchounian, K.; Trchounian, A. Biomass and Biohydrogen Production during Dark Fermentation of Escherichia coli Using Office Paper Waste and Cardboard. Int. J. Hydrogen Energy 2020, 45, 286–293. [Google Scholar] [CrossRef]
- Trchounian, A. Mechanisms for Hydrogen Production by Different Bacteria during Mixed-Acid and Photo-Fermentation and Perspectives of Hydrogen Production Biotechnology. Crit. Rev. Biotechnol. 2015, 35, 103–113. [Google Scholar] [CrossRef]
- Cieciura-Włoch, W.; Borowski, S.; Domański, J. Dark Fermentative Hydrogen Production from Hydrolyzed Sugar Beet Pulp Improved by Nitrogen and Phosphorus Supplementation. Bioresour. Technol. 2021, 340, 125622. [Google Scholar] [CrossRef]
- Cieciura-Włoch, W.; Borowski, S.; Domański, J. Dark Fermentative Hydrogen Production from Hydrolyzed Sugar Beet Pulp Improved by Iron Addition. Bioresour. Technol. 2020, 314, 123713. [Google Scholar] [CrossRef]
- Eker, S.; Erkul, B. Biohydrogen Production by Extracted Fermentation from Sugar Beet. Int. J. Hydrogen Energy 2018, 43, 10645–10654. [Google Scholar] [CrossRef]
- Ozkan, L.; Erguder, T.H.; Demirer, G.N. Effects of Pretreatment Methods on Solubilization of Beet-Pulp and Bio-Hydrogen Production Yield. Int. J. Hydrogen Energy 2011, 36, 382–389. [Google Scholar] [CrossRef]
- Vanyan, L.; Aghekyan, H.; Vassilian, A.; Poladyan, A.; Trchounian, K. Biotechnological Potential of Spent Coffee Grounds for Biohydrogen Production by Escherichia coli. Int. J. Hydrogen Energy 2024, in press. [Google Scholar] [CrossRef]
- Petrosyan, H.; Vanyan, L.; Mirzoyan, S.; Trchounian, A.; Trchounian, K. Roasted Coffee Wastes as a Substrate for Escherichia coli to Grow and Produce Hydrogen. FEMS Microbiol. Lett. 2020, 367, fnaa088. [Google Scholar] [CrossRef]
- Mirzoyan, S.; Aghekyan, H.; Vanyan, L.; Vassilian, A.; Trchounian, K. Coffee Silverskin as a Substrate for Biobased Production of Biomass and Hydrogen by Escherichia coli. Int. J. Energy Res. 2022, 46, 23110–23121. [Google Scholar] [CrossRef]
- Mirzoyan, S.; Trchounian, A.; Trchounian, K. Hydrogen Production by Escherichia coli during Anaerobic Utilization of Mixture of Lactose and Glycerol: Enhanced Rate and Yield, Prolonged Production. Int. J. Hydrogen Energy 2019, 44, 9272–9281. [Google Scholar] [CrossRef]
- Poladyan, A.; Trchounian, K.; Vassilian, A.; Trchounian, A. Hydrogen Production by Escherichia coli Using Brewery Waste: Optimal Pretreatment of Waste and Role of Different Hydrogenases. Renew. Energy 2018, 115, 931–936. [Google Scholar] [CrossRef]
- Trchounian, K.; Trchounian, A. Escherichia coli Hydrogen Gas Production from Glycerol: Effects of External Formate. Renew. Energy 2015, 83, 345–351. [Google Scholar] [CrossRef]
- Maeda, T.; Sanchez-Torres, V.; Wood, T.K. Enhanced Hydrogen Production from Glucose by Metabolically Engineered Escherichia coli. Appl. Microbiol. Biotechnol. 2007, 77, 879–890. [Google Scholar] [CrossRef]
- Gleizer, S.; Ben-Nissan, R.; Bar-On, Y.M.; Antonovsky, N.; Noor, E.; Zohar, Y.; Jona, G.; Krieger, E.; Shamshoum, M.; Bar-Even, A.; et al. Conversion of Escherichia coli to Generate All Biomass Carbon from CO2. Cell 2019, 179, 1255–1263.e12. [Google Scholar] [CrossRef]
- Aristidou, A.A.; San, K.-Y.; Bennett, G.N. Improvement of Biomass Yield and Recombinant Gene Expression in Escherichia coli by Using Fructose as the Primary Carbon Source. Biotechnol. Prog. 1999, 15, 140–145. [Google Scholar] [CrossRef]
- Piskarev, I.M.; Ushkanov, V.A.; Aristova, N.A.; Likhachev, P.P.; Myslivets, T.S. Establishment of the Redox Potential of Water Saturated with Hydrogen. Biophysics 2010, 55, 13–17. [Google Scholar] [CrossRef]
- Sołowski, G.; Konkol, I.; Cenian, A. Methane and Hydrogen Production from Cotton Waste by Dark Fermentation under Anaerobic and Micro-Aerobic Conditions. Biomass Bioenergy 2020, 138, 105576. [Google Scholar] [CrossRef]
- Raposo, F.; De la Rubia, M.; Borja, R.; Alaiz, M. Assessment of a Modified and Optimised Method for Determining Chemical Oxygen Demand of Solid Substrates and Solutions with High Suspended Solid Content. Talanta 2008, 76, 448–453. [Google Scholar] [CrossRef]
- Bradstreet, R.B. Kjeldahl Method for Organic Nitrogen. Anal. Chem. 1954, 26, 185–187. [Google Scholar] [CrossRef]
- DuBois, M.; Gilles, K.A.; Hamilton, J.K.; Rebers, P.A.; Smith, F. Colorimetric Method for Determination of Sugars and Related Substances. Anal. Chem. 1956, 28, 350–356. [Google Scholar] [CrossRef]
- Javanmardi, J. Antioxidant Activity and Total Phenolic Content of Iranian Ocimum Accessions. Food Chem. 2003, 83, 547–550. [Google Scholar] [CrossRef]
- Kumaran, A.; Karunakaran, R.J. Antioxidant Activity of Cassia Auriculata Flowers. Fitoterapia 2007, 78, 46–47. [Google Scholar] [CrossRef]
- Gevorgyan, H.; Abaghyan, T.; Mirumyan, M.; Yenkoyan, K.; Trchounian, K. Propionic and Valproic Acids Have an Impact on Bacteria Viability, Proton Flux and ATPase Activity. J. Bioenerg. Biomembr. 2023, 55, 397–408. [Google Scholar] [CrossRef]
- Vanyan, L.; Trchounian, K. Glucose Concentration Is Determinant for the Functioning of Hydrogenase 1 and Hydrogenase 2 in Regulating the Proton and Potassium Fluxes in Escherichia coli at PH 7.5. Biochimie 2024, 227, 205–216. [Google Scholar] [CrossRef]
- Zheng, Y.; Cheng, Y.-S.; Yu, C.; Zhang, R.; Jenkins, B.M.; VanderGheynst, J.S. Improving the Efficiency of Enzyme Utilization for Sugar Beet Pulp Hydrolysis. Bioprocess. Biosyst. Eng. 2012, 35, 1531–1539. [Google Scholar] [CrossRef]
- Desai, T.A.; Rao, C.V. Regulation of Arabinose and Xylose Metabolism in Escherichia coli. Appl. Environ. Microbiol. 2010, 76, 1524–1532. [Google Scholar] [CrossRef]


| 30 g L−1 SBP | 50 g L−1 SBP | |
|---|---|---|
| Total phenolics (mg GAE g solids−1) | 0.116 | 0.0921 |
| Total flavonoids (mg (QE) g solids−1) | 0.1204 | 0.213 |
| COD (mg O2 L−1) | 15,461.6 | 33,332.8 |
| Total nitrogen (mg (100 mL)−1) | 15.83806 | 17.25892 |
| Total sugar (mg (mL)−1) | 6.543548 | 11.41742 |
| Total solids [%FM] | 77 | 72 |
| Volatile solids [% TS] | 26 | 31 |
| SBP g L−1 | Dilution | Glycerol | C:N Ratio Start | C:N Ratio 24 h—BW25113 | C:N Ratio—Septuple Mutant |
|---|---|---|---|---|---|
| 30 | 0× | Glyc− | 36.7 ± 1.1 | 15.9 ± 0.5 | 26.20 ± 0.79 |
| Glyc+ | 53.1 ± 1.6 | 49.0 ± 1.5 | 40.40 ± 1.21 | ||
| 2× | Glyc− | 21.5 ± 0.6 | 44.1 ± 1.3 | 13.00 ± 0.39 | |
| Glyc+ | 83.4 ± 2.5 | 53.8 ± 1.6 | 76.40 ± 2.29 | ||
| 5× | Glyc− | 64.6 ± 1.9 | 70.9 ± 2.1 | 20.40 ± 0.61 | |
| Glyc+ | 291.0 ± 14.7 | 161.3 ± 4.8 | 62.00 ± 1.86 | ||
| 50 | 0× | Glyc− | 72.2 ± 2.2 | 28.9 ± 0.9 | 31.00 ± 0.93 |
| Glyc+ | 117.0 ± 3.5 | 46.2 ± 1.4 | 49.20 ± 1.48 | ||
| 2× | Glyc− | 78.7 ± 2.4 | 37.7 ± 1.1 | 20.74 ± 0.62 | |
| Glyc+ | 108.0 ± 3.2 | 75.6 ± 2.3 | 71.00 ± 2.13 | ||
| 5× | Glyc− | 74.0 ± 2.2 | 7.2 ± 0.2 | 27.00 ± 0.81 | |
| Glyc+ | 166.0 ± 5.0 | 43.3 ± 1.3 | 45.70 ± 1.37 |
| Strain | Hydrolysate (SBP) | Dilution Factor | Glycerol Condition | H2 Yield (mmol/L) | H2 per g Waste Added (mmol/g Waste) | H2 per Gram Sugar (mmol/g Sugar) |
|---|---|---|---|---|---|---|
| BW25113 | 30 g L−1 | 2× | Glyc- | 0.70 ± 0.02 | 0.05 ± 0.002 | 0.289 ± 0.008 |
| Glyc+ | 0.78 ± 0.02 | 0.02 ± 0.0006 | 3.6 ± 0.108 | |||
| 5× | Glyc- | 1.49 ± 0.04 | 0.25 ± 0.007 | 1.49 ± 0.045 | ||
| Glyc+ | 1.5 ± 0.05 | 0.25 ± 0.007 | 3.78 ± 0.113 | |||
| 50 g L−1 | 2× | Glyc- | 1.36 ± 0.04 | 0.05 ± 0.003 | 0.37 ± 0.011 | |
| Glyc+ | 1.37 ± 0.04 | 0.055 ± 0.003 | 1.82 ± 0.054 | |||
| 5× | Glyc- | 2.16 ± 0.06 | 0.864 ± 0.01 | 2.126 ± 0.06 | ||
| Glyc+ | 3.57 ± 0.11 | 0.36 ± 0.02 | 4.17 ± 0.125 | |||
| Septuple mutant | 30 g L−1 | 2× | Glyc- | 1.4 ± 0.04 | 0.09 ± 0.003 | 0.7 ± 0.02 |
| Glyc+ | 1.36 ± 0.04 | 0.09 ± 0.003 | 7.23 ± 0.22 | |||
| 5× | Glyc- | 1.4 ± 0.04 | 0.23 ± 0.007 | 1.22 ± 0.036 | ||
| Glyc+ | 3.57 ± 0.11 | 0.28 ± 0.009 | 12.06 ± 0.36 | |||
| 50 g L−1 | 2× | Glyc- | 1.49 ± 0.04 | 0.06 ± 0.006 | 0.4 ± 0.012 | |
| Glyc+ | 3.54 ± 0.11 | 0.14 ± 0.01 | 5.8 ± 0.174 | |||
| 5× | Glyc- | 0.81 ± 0.02 | 0.08 ± 0.002 | 0.416 ± 0.012 | ||
| Glyc+ | 3.57 ± 0.11 | 0.36 ± 0.007 | 4.134 ± 0.12 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mikoyan, G.; Vanyan, L.; Toleugazykyzy, A.; Bekbayeva, R.; Baichiyeva, K.; Bekbayev, K.; Trchounian, K. Fermentation of Sugar Beet Pulp by E. coli for Enhanced Biohydrogen and Biomass Production. Energies 2025, 18, 2648. https://doi.org/10.3390/en18102648
Mikoyan G, Vanyan L, Toleugazykyzy A, Bekbayeva R, Baichiyeva K, Bekbayev K, Trchounian K. Fermentation of Sugar Beet Pulp by E. coli for Enhanced Biohydrogen and Biomass Production. Energies. 2025; 18(10):2648. https://doi.org/10.3390/en18102648
Chicago/Turabian StyleMikoyan, Gayane, Liana Vanyan, Akerke Toleugazykyzy, Roza Bekbayeva, Kamila Baichiyeva, Kairat Bekbayev, and Karen Trchounian. 2025. "Fermentation of Sugar Beet Pulp by E. coli for Enhanced Biohydrogen and Biomass Production" Energies 18, no. 10: 2648. https://doi.org/10.3390/en18102648
APA StyleMikoyan, G., Vanyan, L., Toleugazykyzy, A., Bekbayeva, R., Baichiyeva, K., Bekbayev, K., & Trchounian, K. (2025). Fermentation of Sugar Beet Pulp by E. coli for Enhanced Biohydrogen and Biomass Production. Energies, 18(10), 2648. https://doi.org/10.3390/en18102648





