Content of Selected Compounds in the Exhaust Gas of a Naturally Aspirated CI Engine Fueled with Diesel–Tire Pyrolysis Oil Blend
Abstract
1. Introduction
- Euro 7 [52]: This forthcoming standard, currently anticipated for implementation in 2029 [53], aims to further tighten emission regulations. Euro 7 is expected to encompass a broader range of pollutants, including nitrogen oxide (NOx) limits set at 60 mg/km, applicable to both petrol and diesel vehicles. Additionally, it will extend the compliance period for vehicles from 5 years or 100,000 km to 10 years or 200,000 km.
- Directive 2009/30/EC [56]: Regulates the quality of motor fuels, limiting, among other things, the permissible sulfur content of diesel oil, which has a direct impact on SOx emissions.
2. Materials and Methods
2.1. General Objectives
2.2. Engine Test Stand
2.3. Characteristics of Fuels Used in the Experiment
3. Results and Discussion
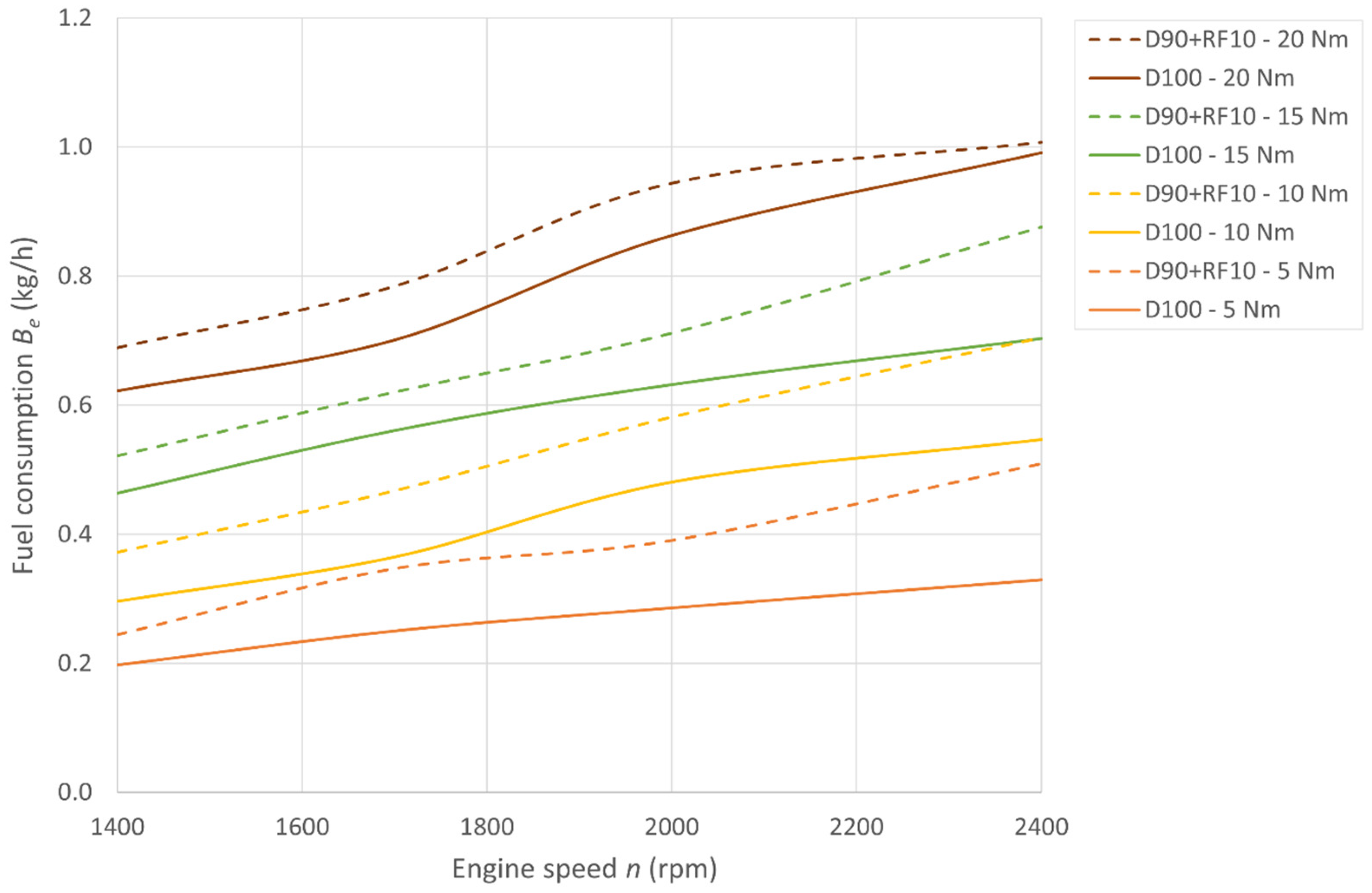
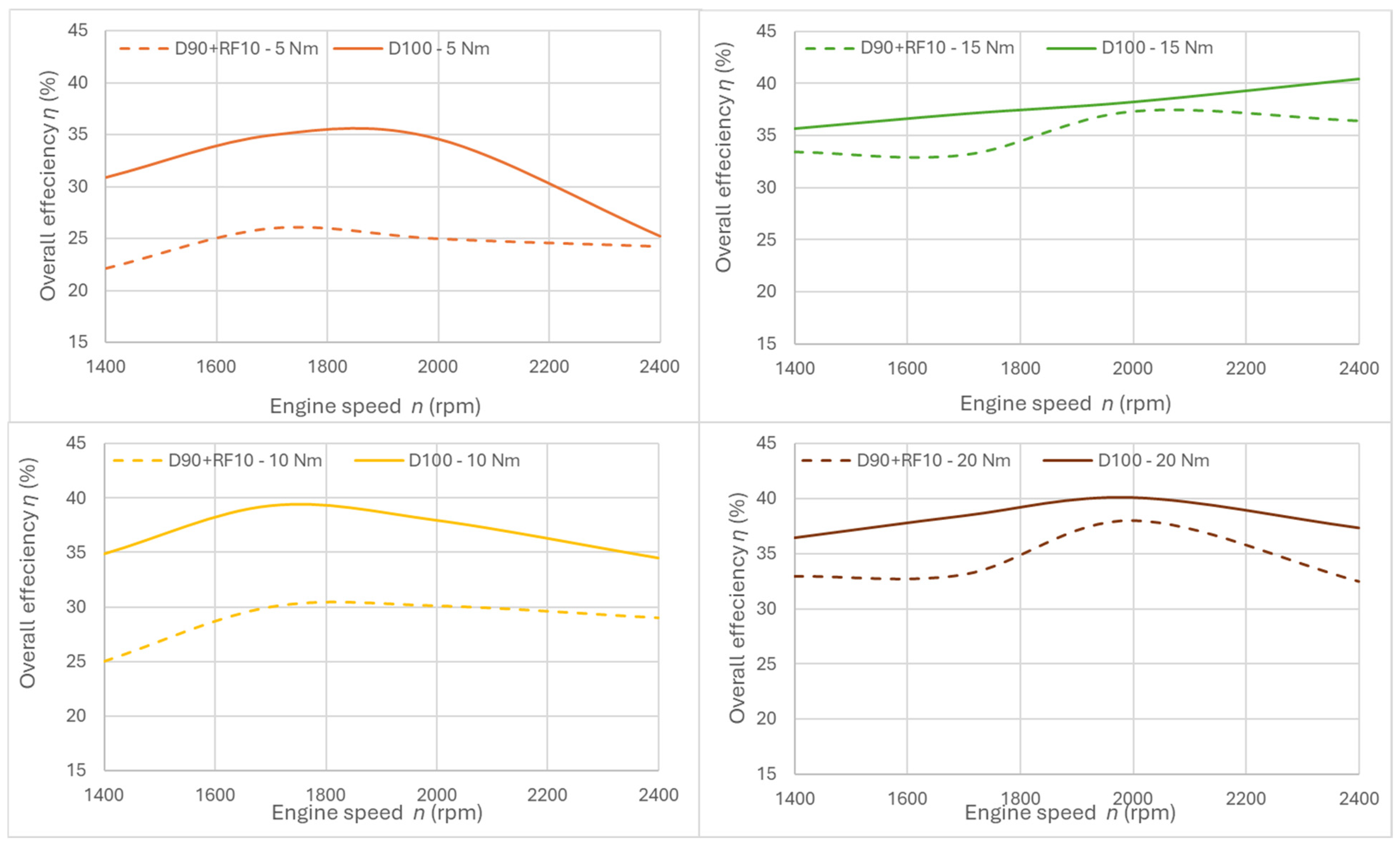
3.1. Fuel Consumption
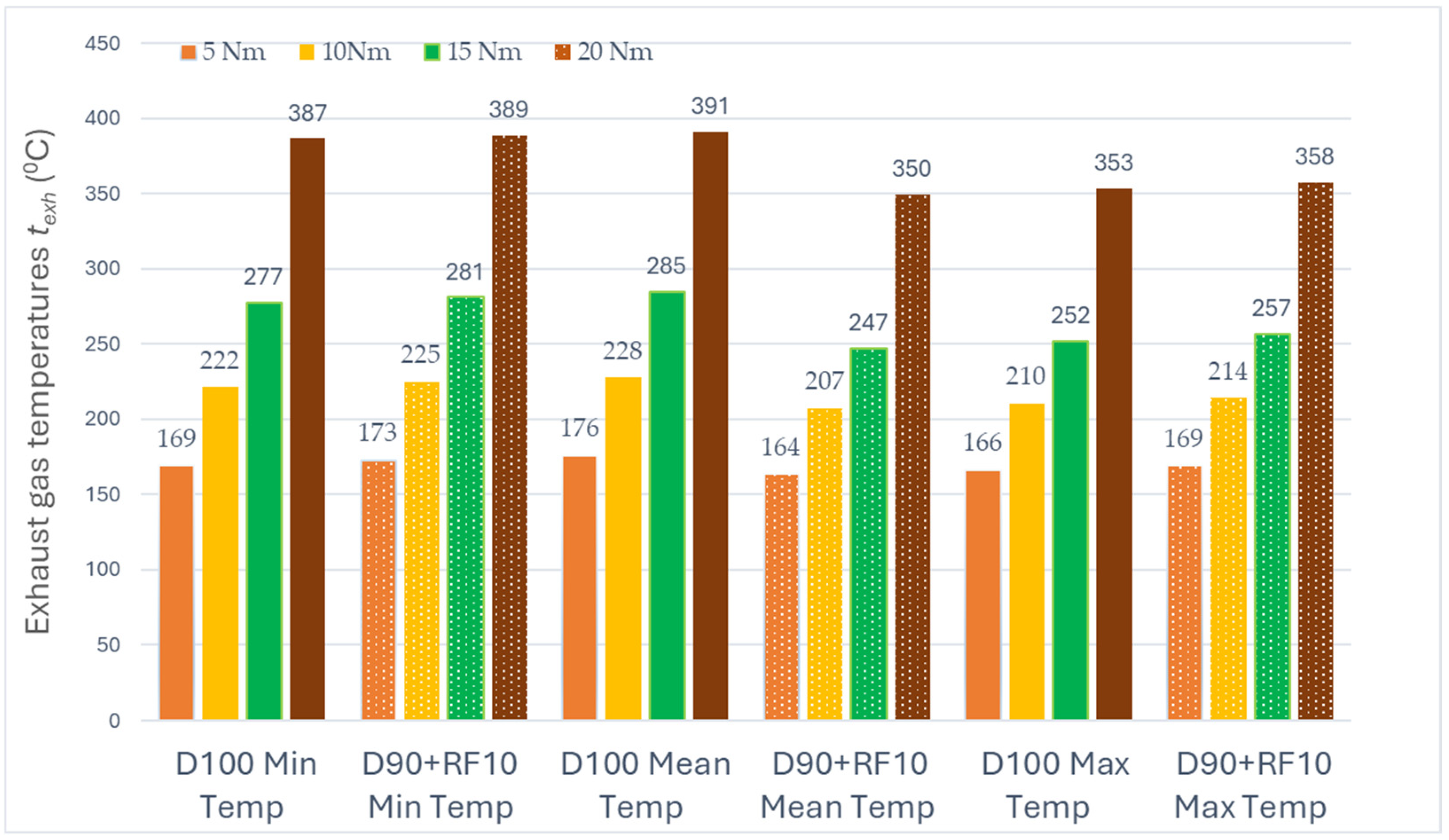
3.2. Exhaust Gas Temperature
3.3. SOx Emissions in Exhaust Gas
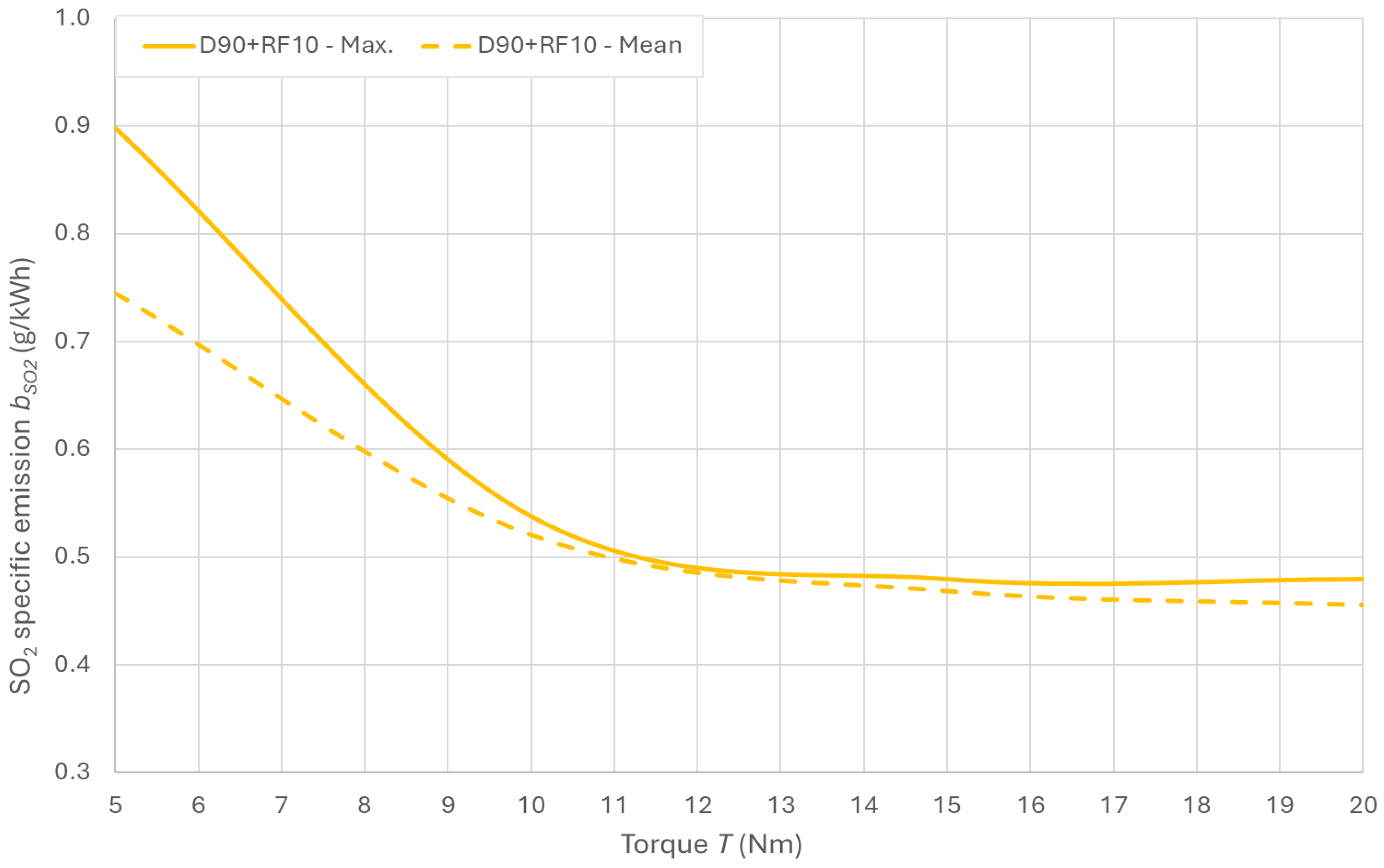
3.4. CO2 Emissions in the Exhaust Gas

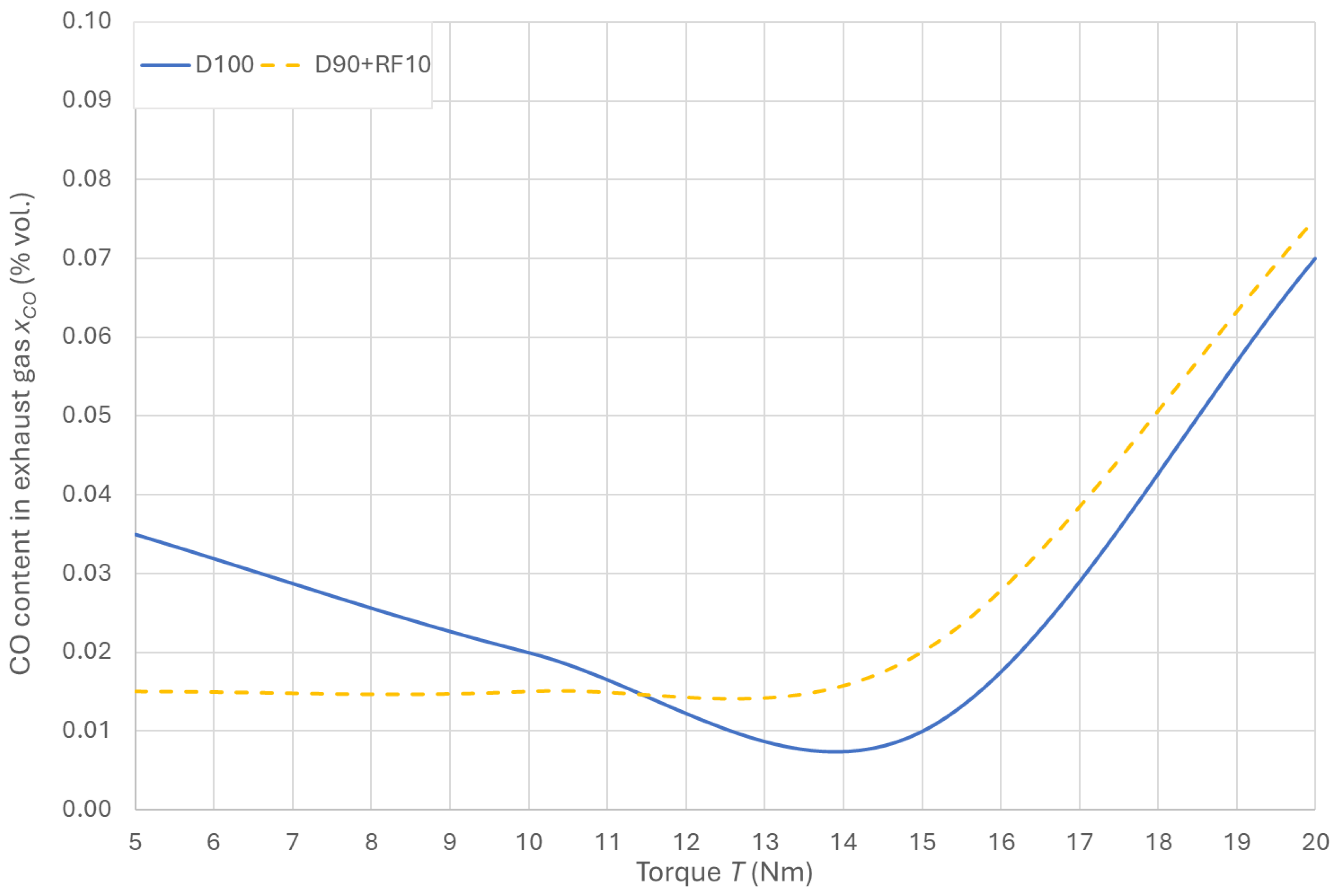
3.5. CO Emissions in the Exhaust Gas
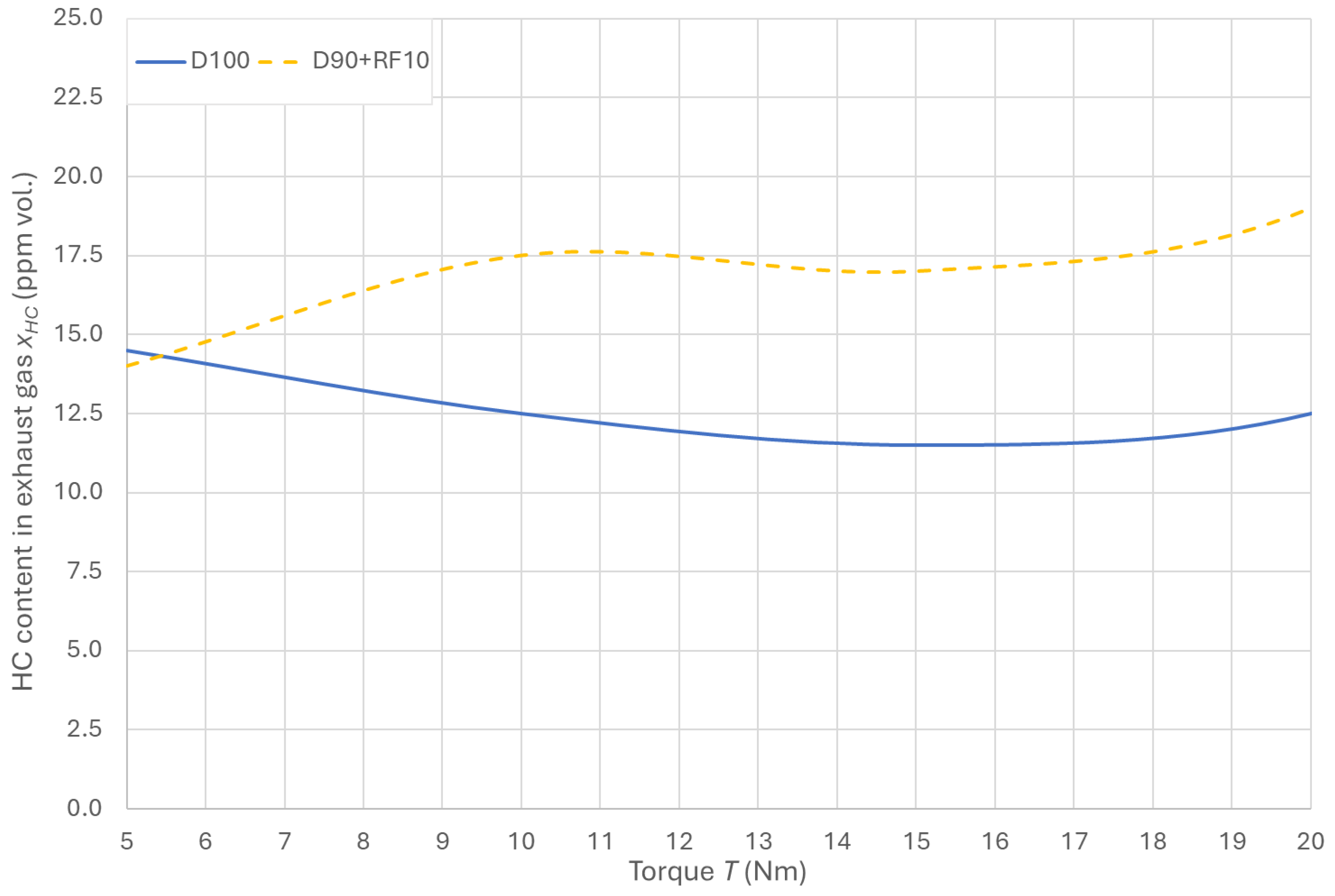
3.6. Hydrocarbon Emissions in the Exhaust Gas
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ASTM | US-based international standards organization |
| BTDC | angle before the top dead center |
| BSO2 | mass SO2 emission |
| bSO2 | specific SO2 emission |
| CO | carbon monoxide |
| CO2 | carbon dioxide |
| CS | sulfur content in the fuels under study |
| Cw | water content in the fuels under study |
| D100 | pure diesel oil without FAME admixtures |
| D90+RF10 | fuel with 90% m/m of D100 diesel and 10% m/m of pyrolytic oil (RF) |
| deSOx | flue gas desulfurization system |
| FAME | fatty acid methyl ester |
| HC | general hydrocarbon designation |
| ISO | International Organization for Standardization |
| NO | nitric oxide |
| NO2 | nitrogen dioxide |
| NOx | generic determination of NO and NO2 nitrogen oxides |
| RF | recycled fuel |
| SO2 | sulfur dioxide |
| SO3 | sulfur trioxide |
| SOx | general designation for sulfur oxides SO2 and SO3 |
| TPO | tire pyrolytic oil |
| u | uncertainty |
| W | lower heat value |
| XA | incineration residue |
| xCO | measured CO emission |
| xCO2 | measured CO2 emission |
| XCR | coking residue with 10% distillation residue |
| xHC | measured HC emission |
| XS | total sediment by hot filtration |
| ν100 | kinematic viscosity at a reference temperature of 100 °C |
| ν40 | kinematic viscosity at a reference temperature of 40 °C |
| ρ15 | density at a reference temperature of 15 °C |
References
- Formela, K.; Kurańska, M.; Barczewski, M. Recent Advances in Development of Waste-Based Polymer Materials: A Review. Polymers 2022, 14, 1050. [Google Scholar] [CrossRef] [PubMed]
- Saxena, S. Pyrolysis and beyond: Sustainable Valorization of Plastic Waste. Appl. Energy Combust. Sci. 2025, 21, 100311. [Google Scholar] [CrossRef]
- Produkcja Wyrobów Przemysłowych w Latach 2017–2021. Available online: https://stat.gov.pl/files/gfx/portalinformacyjny/pl/defaultaktualnosci/5477/14/4/1/produkcja_wyrobow_przemyslowych_w_latach_2017-2021.pdf (accessed on 13 February 2025).
- Bi, R.; Zhang, Y.; Jiang, X.; Yang, H.; Yan, K.; Han, M.; Li, W.; Zhong, H.; Tan, X.; Xia, L.; et al. Simulation and Techno-Economical Analysis on the Pyrolysis Process of Waste Tire. Energy 2022, 260, 125039. [Google Scholar] [CrossRef]
- Battista, M.; Gobetti, A.; Agnelli, S.; Ramorino, G. Post-Consumer Tires as a Valuable Resource: Review of Different Types of Material Recovery. Environ. Technol. Rev. 2021, 10, 1–25. [Google Scholar] [CrossRef]
- Goksal, F.P. An Economic Analysis of Scrap Tire Pyrolysis, Potential and New Opportunities. Heliyon 2022, 8, e11669. [Google Scholar] [CrossRef]
- Ćetković, J.; Lakić, S.; Žarković, M.; Vujadinović, R.; Knežević, M.; Živković, A.; Cvijović, J. Environmental Benefits of Air Emission Reduction in the Waste Tire Management Practice. Processes 2022, 10, 787. [Google Scholar] [CrossRef]
- Han, W.; Han, D.; Chen, H. Pyrolysis of Waste Tires: A Review. Polymers 2023, 15, 1604. [Google Scholar] [CrossRef]
- Akhtar, M.S.; Ali, S.; Zaman, W. Recent Advancements in Catalysts for Petroleum Refining. Catalysts 2024, 14, 841. [Google Scholar] [CrossRef]
- Onorati, A.; Payri, R.; Vaglieco, B.M.; Agarwal, A.K.; Bae, C.; Bruneaux, G.; Canakci, M.; Gavaises, M.; Günthner, M.; Hasse, C. The Role of Hydrogen for Future Internal Combustion Engines. Int. J. Engine Res. 2022, 23, 529–540. [Google Scholar] [CrossRef]
- Afash, H.; Ozarisoy, B.; Altan, H.; Budayan, C. Recycling of Tire Waste Using Pyrolysis: An Environmental Perspective. Sustainability 2023, 15, 14178. [Google Scholar] [CrossRef]
- Fazli, A.; Rodrigue, D. Recycling Waste Tires into Ground Tire Rubber (Gtr)/Rubber Compounds: A Review. J. Compos. Sci. 2020, 4, 103. [Google Scholar] [CrossRef]
- Maga, D.; Aryan, V.; Blömer, J. A Comparative Life Cycle Assessment of Tyre Recycling Using Pyrolysis Compared to Conventional End-of-Life Pathways. Resour. Conserv. Recycl. 2023, 199, 107255. [Google Scholar] [CrossRef]
- Dewang, Y.; Sharma, V.; Singla, Y.K. A Critical Review of Waste Tire Pyrolysis for Diesel Engines: Technologies, Challenges, and Future Prospects. Sustain. Mater. Technol. 2025, 43, e01291. [Google Scholar] [CrossRef]
- Pyshyev, S.; Lypko, Y.; Chervinskyy, T.; Fedevych, O.; Kułażyński, M.; Pstrowska, K. Application of Tyre Derived Pyrolysis Oil as a Fuel Component. S. Afr. J. Chem. Eng. 2023, 43, 342–347. [Google Scholar] [CrossRef]
- Gamboa, A.R.; Rocha, A.M.A.; dos Santos, L.R.; de Carvalho, J.A., Jr. Tire Pyrolysis Oil in Brazil: Potential Production and Quality of Fuel. Renew. Sustain. Energy Rev. 2020, 120, 109614. [Google Scholar] [CrossRef]
- Mikulski, M.; Ambrosewicz-Walacik, M.; Hunicz, J.; Nitkiewicz, S. Combustion Engine Applications of Waste Tyre Pyrolytic Oil. Prog. Energy Combust. Sci. 2021, 85, 100915. [Google Scholar] [CrossRef]
- Yaqoob, H.; Teoh, Y.H.; Jamil, M.A.; Gulzar, M. Potential of Tire Pyrolysis Oil as an Alternate Fuel for Diesel Engines: A Review. J. Energy Inst. 2021, 96, 205–221. [Google Scholar] [CrossRef]
- Karagöz, M.; Ağbulut, Ü.; Sarıdemir, S. Waste to Energy: Production of Waste Tire Pyrolysis Oil and Comprehensive Analysis of Its Usability in Diesel Engines. Fuel 2020, 275, 117844. [Google Scholar] [CrossRef]
- Chybowski, L.; Szczepanek, M.; Pusty, T.; Brożek, P.; Pełech, R.; Wieczorek, A. The Properties of Diesel Blends with Tire Pyrolysis Oil and Their Wear-Related Parameters. Energies 2025, 18, 1057. [Google Scholar] [CrossRef]
- Szwaja, M.; Chwist, M.; Szwaja, S.; Juknelevičius, R. Impact of Pyrolysis Oil Addition to Ethanol on Combustion in the Internal Combustion Spark Ignition Engine. Clean. Technol. 2021, 3, 450–461. [Google Scholar] [CrossRef]
- Auti, S.M.; Rathod, W.S. Effect of Hybrid Blends of Raw Tyre Pyrolysis Oil, Karanja Biodiesel and Diesel Fuel on Single Cylinder Four Stokes Diesel Engine. Energy Rep. 2021, 7, 2214–2220. [Google Scholar] [CrossRef]
- Aurtherson, P.B.; Nalla, B.T.; Srinivasan, K.; Mehar, K.; Devarajan, Y. Biofuel Production from Novel Prunus Domestica Kernel Oil: Process Optimization Technique. Biomass Convers. Biorefinery 2023, 13, 6249–6255. [Google Scholar] [CrossRef]
- Prajapati, A.K.; Yadav, S.; Gomey, A.K.; Choubey, A.K.; Kumar, R. Assessment of Performance and Emission Characteristics of CI Engine Using Tyre Pyrolysis Oil and Biodiesel Blends by Nano Additives: An Experimental Study. J. Energy Inst. 2024, 117, 101825. [Google Scholar] [CrossRef]
- Mello, M.; Rutto, H.; Seodigeng, T. Waste Tire Pyrolysis and Desulfurization of Tire Pyrolytic Oil (TPO)—A Review. J. Air Waste Manag. Assoc. 2023, 73, 159–177. [Google Scholar] [CrossRef]
- Hossain, M.N.; Choi, M.K.; Choi, H.S. A Review of the Desulfurization Processes Used for Waste Tire Pyrolysis Oil. Catalysts 2021, 11, 801. [Google Scholar] [CrossRef]
- Martínez, J.D. An Overview of the End-of-Life Tires Status in Some Latin American Countries: Proposing Pyrolysis for a Circular Economy. Renew. Sustain. Energy Rev. 2021, 144, 111032. [Google Scholar] [CrossRef]
- Andooz, A.; Eqbalpour, M.; Kowsari, E.; Ramakrishna, S.; Cheshmeh, Z.A. A Comprehensive Review on Pyrolysis from the Circular Economy Point of View and Its Environmental and Social Effects. J. Clean. Prod. 2023, 388, 136021. [Google Scholar] [CrossRef]
- Campuzano, F.; Martínez, J.D.; Agudelo Santamaría, A.F.; Sarathy, S.M.; Roberts, W.L. Pursuing the End-of-Life Tire Circularity: An Outlook toward the Production of Secondary Raw Materials from Tire Pyrolysis Oil. Energy Fuels 2023, 37, 8836–8866. [Google Scholar] [CrossRef]
- Wang, Y.; Wright, L.A. A Comparative Review of Alternative Fuels for the Maritime Sector: Economic, Technology, and Policy Challenges for Clean Energy Implementation. World 2021, 2, 456–481. [Google Scholar] [CrossRef]
- Cunanan, C.; Tran, M.-K.; Lee, Y.; Kwok, S.; Leung, V.; Fowler, M. A Review of Heavy-Duty Vehicle Powertrain Technologies: Diesel Engine Vehicles, Battery Electric Vehicles, and Hydrogen Fuel Cell Electric Vehicles. Clean. Technol. 2021, 3, 474–489. [Google Scholar] [CrossRef]
- Al-Enazi, A.; Okonkwo, E.C.; Bicer, Y.; Al-Ansari, T. A Review of Cleaner Alternative Fuels for Maritime Transportation. Energy Rep. 2021, 7, 1962–1985. [Google Scholar] [CrossRef]
- Wei, Y.-J.; Zhang, Y.-J.; Zhu, X.-D.; Gu, H.-M.; Zhu, Z.-Q.; Liu, S.-H.; Sun, X.-Y.; Jiang, X.-L. Effects of Diesel Hydrocarbon Components on Cetane Number and Engine Combustion and Emission Characteristics. Appl. Sci. 2022, 12, 3549. [Google Scholar] [CrossRef]
- Gnanasikamani, B.; Wan Abdullah, W.N.; Yadav, O.; Anil Kumar, S. Effect of Fuel Modification and after Treatment Techniques on Emissions Fuelled by Diesel Tyre Oil Blend. Energy Sources Part A Recovery Util. Environ. Eff. 2024, 46, 16703–16717. [Google Scholar] [CrossRef]
- Hunicz, J.; Mikulski, M.; Rybak, A. Tyre Pyrolytic Oil Blends in a State-of-the-Art Compression Ignition Engine: Towards Fuel-Optimised Combustion. Fuel 2023, 353, 129281. [Google Scholar] [CrossRef]
- Kondor, I.P.; Zöldy, M.; Mihály, D. Experimental Investigation on the Performance and Emission Characteristics of a Compression Ignition Engine Using Waste-Based Tire Pyrolysis Fuel and Diesel Fuel Blends. Energies 2021, 14, 7903. [Google Scholar] [CrossRef]
- Yaqoob, H.; Teoh, Y.H.; Sher, F.; Jamil, M.A.; Murtaza, D.; Al Qubeissi, M.; Ui Hassan, M.; Mujtaba, M.A. Current Status and Potential of Tire Pyrolysis Oil Production as an Alternative Fuel in Developing Countries. Sustainability 2021, 13, 3214. [Google Scholar] [CrossRef]
- Schwartz, N.R.; Paulsen, A.D.; Blaise, M.J.; Wagner, A.L.; Yelvington, P.E. Analysis of Emissions from Combusting Pyrolysis Products. Fuel 2020, 274, 117863. [Google Scholar] [CrossRef]
- Mikulski, M.; Hunicz, J.; Duda, K.; Kazimierski, P.; Suchocki, T.; Rybak, A. Tyre Pyrolytic Oil Fuel Blends in a Modern Compression Ignition Engine: A Comprehensive Combustion and Emissions Analysis. Fuel 2022, 320, 123869. [Google Scholar] [CrossRef]
- S, J.J.M.; Samuel, S.Y.; Sundari, K.G.; Rajamohan, S. Diesel Engine Performance and Emissions with Fuels Derived from Waste Tyres. In Advanced Technology for the Conversion of Waste Into Fuels and Chemicals; Elsevier: Amsterdam, The Netherlands, 2021; pp. 69–92. [Google Scholar]
- Bodisco, T.A.; Rahman, S.M.A.; Hossain, F.M.; Brown, R.J. On-Road NOx Emissions of a Modern Commercial Light-Duty Diesel Vehicle Using a Blend of Tyre Oil and Diesel. Energy Rep. 2019, 5, 349–356. [Google Scholar] [CrossRef]
- Pinto, G.M.; de Souza, T.A.Z.; Coronado, C.J.R.; Flôres, L.F.V.; Chumpitaz, G.R.A.; da Silva, M.H. Experimental Investigation of the Performance and Emissions of a Diesel Engine Fuelled by Blends Containing Diesel S10, Pyrolysis Oil from Used Tires and Biodiesel from Waste Cooking Oil. Environ. Prog. Sustain. Energy 2019, 38, 13199. [Google Scholar] [CrossRef]
- Abdu Muzakkari, B.; Salihi Bayero, A.; Ibrahim Mohammed, M.; Singh, P.K.; Muhammad Jibreel, U. Waste Tyres Pyrolysis Oil (WTPO) as an Alternative Source of Fuel and Chemicals: A Review. Zast. Mater. 2024, 65, 634–644. [Google Scholar] [CrossRef]
- Yaqoob, H.; Ali, H.M.; Abbas, H.; Abid, O.; Jamil, M.A.; Ahmed, T. Performance and Emissions Characteristics of Tire Pyrolysis Oil in Diesel Engine: An Experimental Investigation. Clean. Technol. Environ. Policy 2023, 25, 3177–3187. [Google Scholar] [CrossRef]
- Polat, F. Experimental Evaluation of the Impacts of Diesel-Nanoparticles-Waste Tire Pyrolysis Oil Ternary Blends on the Combustion, Performance, and Emission Characteristics of a Diesel Engine. Process Saf. Environ. Prot. 2022, 160, 847–858. [Google Scholar] [CrossRef]
- Jakubowski, M.; Jaworski, A.; Kuszewski, H.; Balawender, K. Performance of a Diesel Engine Fueled by Blends of Diesel Fuel and Synthetic Fuel Derived from Waste Car Tires. Sustainability 2024, 16, 6404. [Google Scholar] [CrossRef]
- Wądrzyk, M.; Janus, R.; Rządzik, B.; Lewandowski, M.; Budzyń, S. Pyrolysis Oil from Scrap Tires as a Source of Fuel Components: Manufacturing, Fractionation, and Characterization. Energy Fuels 2020, 34, 5917–5928. [Google Scholar] [CrossRef]
- Pillai, S.K.; Rajamanickam, U.; Khurana, S. Impact of Diethyl Ether on Performance and Emission Characteristics of a VCR Diesel Engine Fueled by Dual Biodiesel. Energies 2023, 16, 4863. [Google Scholar] [CrossRef]
- Yaqoob, H.; Teoh, Y.H.; Jamil, M.A.; Rasheed, T.; Sher, F. An Experimental Investigation on Tribological Behaviour of Tire-Derived Pyrolysis Oil Blended with Biodiesel Fuel. Sustainability 2020, 12, 9975. [Google Scholar] [CrossRef]
- European Commission. Commission Regulation (EU) 2017/1151 of 1 June 2017 supplementing Regulation (EC) No 715/2007 on type-approval of motor vehicles with respect to emissions from light passenger and commercial vehicles (Euro 5 and Euro 6) and on access to vehicle repair and maintenance information, amending Directive 2007/46/EC, Commission Regulation (EC) No 692/2008 and Commission Regulation (EU) No 1230/2012 and repealing Commission Regulation (EC) No 692/2008. Off. J. Eur. Union 2017, L 175, 1–643. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX%3A32017R1151 (accessed on 14 May 2025).
- European Parliament & Council of the European Union. Regulation (EC) No 715/2007 of the European Parliament and of the Council of 20 June 2007 on type approval of motor vehicles with respect to emissions from light passenger and commercial vehicles (Euro 5 and Euro 6) and on access to vehicle repair and maintenance information. Off. J. Eur. Union 2007, L 171, 1–16. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX%3A32007R0715EUMonitor+7PublikacjeUE+7PublikacjeUE+7 (accessed on 14 May 2025).
- European Parliament & Council of the European Union. Regulation (EU) 2024/1257 of the European Parliament and of the Council of 10 April 2024 on the type-approval of motor vehicles and engines and of systems, components and separate technical units intended for such vehicles, with respect to their emissions and battery durability (Euro 7), amending Regulations (EC) No 715/2007 and (EC) No 595/2009 and repealing Regulations (EC) No 715/2007 and (EC) No 595/2009. Off. J. Eur. Union 2024, L 1257. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX%3A32024R1257 (accessed on 14 May 2025).
- Euro 7—Od Kiedy Nowa Norma Emisji Spalin? Available online: https://elektromobilni.pl/strefa_wiedzy/euro-7-od-kiedy-nowa-norma-emisji-spalin/ (accessed on 5 May 2025).
- European Parliament & Council of the European Union. Regulation (EU) 2016/1628 of the European Parliament and of the Council of 14 September 2016 on requirements relating to gaseous and particulate pollutant emission limits and type-approval for internal combustion engines for non-road mobile machinery, amending Regulations (EU) No 1024/2012 and (EU) No 167/2013, and amending and repealing Directive 97/68/EC. Off. J. Eur. Union 2016, L 252, 53–117. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX%3A32016R1628EUMonitor+6gagnagrunnur.ees.is+6EUMonitor+6 (accessed on 14 May 2025).
- Selleri, T.; Melas, A.D.; Joshi, A.; Manara, D.; Perujo, A.; Suarez-Bertoa, R. An Overview of Lean Exhaust DeNOx Aftertreatment Technologies and NOx Emission Regulations in the European Union. Catalysts 2021, 11, 404. [Google Scholar] [CrossRef]
- Directive 2009/30/EC of the European Parliament and of the Council of 23 April 2009. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/HTML/?uri=CELEX:32009L0030 (accessed on 10 December 2024).
- Rozporządzenie Ministra Gospodarki Z Dnia 30 Kwietnia 2014 R. W Sprawie SzczegółOwych Wymagań Dla Silników Spalinowych W Zakresie Ograniczenia Emisji Zanieczyszczeń Gazowych I CząStek StałYch Przez Te Silniki. Available online: https://sip.lex.pl/akty-prawne/dzu-dziennik-ustaw/szczegolowe-wymagania-dla-silnikow-spalinowych-w-zakresie-ograniczenia-18095100 (accessed on 14 February 2025).
- UE Directive 2005/55/EC of the European Parliament and of the Council. Available online: https://eur-lex.europa.eu/eli/dir/2005/55/oj/eng (accessed on 14 February 2025).
- Regulation (EU) 2016/1628 of the European Parliament and of the Council. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX:32016R1628 (accessed on 14 February 2025).
- European Committee for Standardization EN 590:2022 Automotive Fuels—Diesel—Requirements and Test Methods. Available online: https://www.nationwidefuels.co.uk/fuel-specifications/en-590/ (accessed on 16 April 2025).
- Yanmar TF70 Engine Specifications. Available online: https://www.yanmar.com/in/engine/products/diesel/h_watercooled/tf70/ (accessed on 5 February 2025).
- Chybowski, L.; Szczepanek, M.; Pusty, T.; Brożek, P.; Pełech, R.; Borowski, P. Evaluation of the Ignition Properties of Fuels Based on Oil Diesel Fuel with the Addition of Pyrolytic Oil from Tires. Energies 2025, 18, 860. [Google Scholar] [CrossRef]
- ISO 12185: 2024; Crude Petroleum, Petroleum Products and Related Products—Determination of Density—Laboratory Density Meter with an Oscillating U-Tube Sensor. ISO: Geneva, Switzerland, 2024.
- ISO 3104:2023; Petroleum Products—Transparent and Opaque Liquids—Determination of Kinematic Viscosity and Calculation of Dynamic Viscosity. ISO: Geneva, Switzerland, 2023.
- PKN PN-C-04062:2018-05; Przetwory Naftowe—Oznaczanie Ciepła Spalania Paliw Ciekłych w Bombie Kalorymetrycznej i Obliczanie Wartości Opałowej z Zastosowaniem Wzorów Empirycznych. PKN: Warszawa, Poland, 2018.
- ISO 2719:2016; Determination of Flash Point—Pensky-Martens Closed Cup Method. ISO: Geneva, Switzerland, 2016.
- D7668 Standard Test Method for Determination of Derived Cetane Number (DCN) of Diesel Fuel Oils—Ignition Delay and Combustion Delay Using a Constant Volume Combustion Chamber Method. Available online: https://www.astm.org/d7668-17.html (accessed on 6 October 2024).
- ISO 12937: 2005; Petroleum Products—Determination of Water—Coulometric Karl Fischer Titration Method. ISO: Geneva, Switzerland, 2005.
- ISO 8754:2003; Petroleum Products—Determination of Sulfur Content—Energy-Dispersive X-Ray Fluorescence Spectrometry. ISO: Geneva, Switzerland, 2003.
- ISO 10370:2014; Petroleum Products—Determination of Carbon Residue—Micro Method. ISO: Geneva, Switzerland, 2014.
- ISO 6245: 2008; Petroleum Products—Determination of Ash. ISO: Geneva, Switzerland, 2008.
- ASTM 6595-17; Standard Test Method for Determination of Wear Metals and Contaminants in Used Lubricating Oils or Used Hydraulic Fluids by Rotating Disc Electrode Atomic Emission Spectrometry. ASTM: West Conshohocken, PA, USA, 2022.
- Kim, J.-H.; Kim, J.-H.; Kim, H.-S.; Kim, H.-J.; Kang, S.-H.; Ryu, J.-H.; Shim, S.-S. Reduction of NOx Emission from the Cement Industry in South Korea: A Review. Atmosphere 2022, 13, 121. [Google Scholar] [CrossRef]
- Rozporządzenie Ministra Klimatu i Środowiska z Dnia 26 Czerwca 2024 r. w Sprawie Wymagań Jakościowych Dla Paliw Ciekłych. Dziennik Ustaw Rzeczypospolitej Polskiej. 2024. Poz. 1018. Available online: https://sip.lex.pl/akty-prawne/dzu-dziennik-ustaw/wymagania-jakosciowe-dla-paliw-cieklych-22026814 (accessed on 14 May 2025).

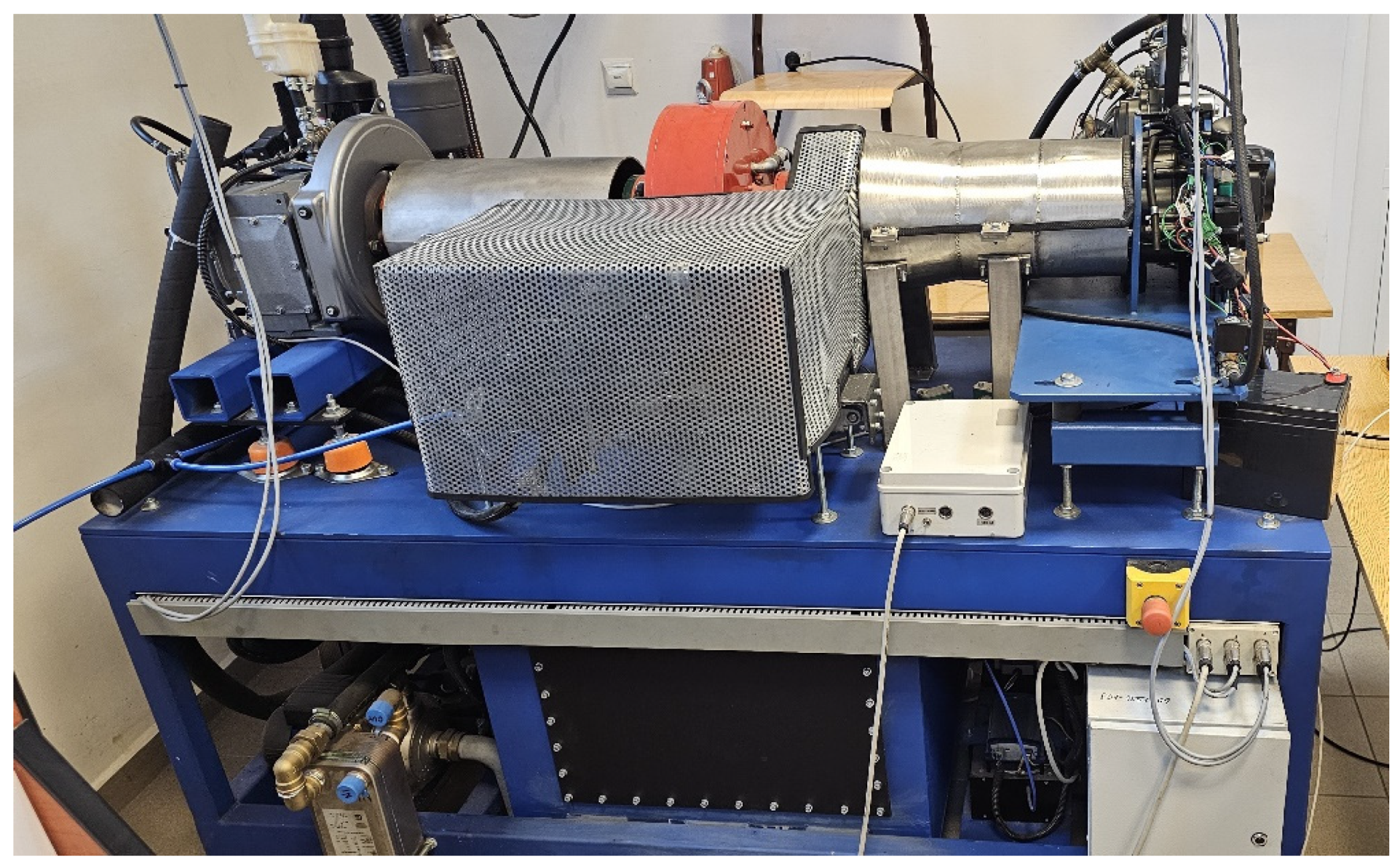


| Parameter | Value/Description | Parameter | Value/Description |
|---|---|---|---|
| Injection timing | 17 °BTDC | Dimensions length | 607.5 mm |
| Cylinder bore | 78 mm | width | 311.5 mm |
| Piston stroke | 80 mm | height | 469.0 mm |
| Displacement | 0.382 dm3 | Net weight | 67.5 kg |
| Rated continuous output @ 2400 rpm | 4.5 kW | ||
| Rated output at 1 h @ 2400 rpm | 5.2 kW | Lubrication Cooling | circulating pressurized liquid, forced |
| Maximum torque @ 1800 rpm | 24.33 Nm | ||
| Compression ratio | 18.1 | ||
| Specific fuel oil consumption | 237.93 g/kWh |
| Parameter | Unit | Measurement Standard | Diesel Oil (D100) | Diesel Oil and Recycled Oil Blend (D90+RF10) |
|---|---|---|---|---|
| Density @ 15 °C, ρ15 | kg/m3 | ISO 12185:2024 [63] | 836.4 | 846.5 |
| Kinematic viscosity @ 40 °C, ν40 | mm2/s | ISO 3104:2023 [64] | 2.728 | 2.791 |
| Kinematic viscosity @ 100 °C, ν100 | mm2/s | ISO 3104:2023 [64] | 1.173 | 1.198 |
| Lower heat value W | MJ/kg | PN-C-04062:2018-05 [65] | 45.46 | 46.68 |
| Flash point temperature tFP | °C | ISO 2719:2016 [66] | 64 | 59 |
| Derived cetane number DCN | – | ASTM D7668(2017) [67] | 54.35 | 50.40 |
| Water content Cw | % m/m | ISO 12937:2005 [68] | 0.0020 | 0.0035 |
| Sulfur content CS | % m/m | ISO 8754:2003 [69] | 0.000 | 0.102 |
| Coke residue (with 10% distillation residue) XCR | % m/m | ISO 10370:2014-12 [70] | 0.015 | 0.054 |
| XA incineration residue | % m/m | ISO 6245:2008 [71] | 0.004 | 0.032 |
| Elemental composition | ||||
| Fe | ppm | ASTM D6595-17 [72] | 0.0 | 0.0 |
| Cr | 1.1 | 0.3 | ||
| Pb | 7.3 | 4.6 | ||
| Cu | 0.0 | 0.0 | ||
| Sn | 7.4 | 21.0 | ||
| Al | 2.5 | 0.0 | ||
| Ni | 7.6 | 7.9 | ||
| Ag | 0.5 | 0.0 | ||
| Si | 27.0 | 13.4 | ||
| B | 1.0 | 0.6 | ||
| Mg | 0.0 | 0.4 | ||
| Ba | 0.0 | 0.0 | ||
| P | 0.0 | 0.0 | ||
| Zn | 9.5 | 2.5 | ||
| Mo | 1.3 | 2.1 | ||
| Ti | 1.7 | 0.0 | ||
| V | 0.0 | 0.0 |
| Fuel | Torque T (Nm) | Engine Speed (rpm) | |||
|---|---|---|---|---|---|
| 1400 | 1600 | 2000 | 2400 | ||
| D100 | 5 | 0.032 | 0.022 | 0.069 | 0.053 |
| 10 | 0.016 | 0.092 | 0.108 | 0.030 | |
| 15 | 0.014 | 0.015 | 0.034 | 0.012 | |
| 20 | 0.006 | 0.037 | 0.017 | 0.013 | |
| D90 +RF10 | 5 | 0.114 | 0.039 | 0.062 | 0.017 |
| 10 | 0.081 | 0.018 | 0.017 | 0.017 | |
| 15 | 0.029 | 0.042 | 0.034 | 0.027 | |
| 20 | 0.015 | 0.012 | 0.010 | 0.006 | |
| Fuel | Torque T (Nm) | Engine Speed (rpm) | |||
|---|---|---|---|---|---|
| 1400 | 1600 | 2000 | 2400 | ||
| D100 | 5 | 2.671 | 3.100 | 4.378 | 4.534 |
| 10 | 3.215 | 3.526 | 4.184 | 4.388 | |
| 15 | 3.843 | 3.660 | 5.569 | 2.679 | |
| 20 | 3.099 | 1.943 | 2.105 | 1.994 | |
| D90 +RF10 | 5 | 2.200 | 3.475 | 3.035 | 4.562 |
| 10 | 3.286 | 3.502 | 3.109 | 5.698 | |
| 15 | 3.702 | 9.292 | 2.067 | 5.184 | |
| 20 | 1.715 | 1.904 | 2.737 | 11.125 | |
| Torque T (Nm) | Uncertainty u() | Uncertainty u() |
|---|---|---|
| 5 | 0.072 | 0.111 |
| 10 | 0.008 | 0.146 |
| Fuel | Torque T (Nm) | Uncertainty u(xCO2) |
|---|---|---|
| D100 | 5 | 2.671 |
| 10 | 3.215 | |
| 15 | 3.843 | |
| 20 | 3.099 | |
| D90 +RF10 | 5 | 2.200 |
| 10 | 3.286 | |
| 15 | 3.702 | |
| 20 | 1.715 |
| Fuel | Torque T (Nm) | Uncertainty u(xCO) |
|---|---|---|
| D100 | 5 | 0.005 |
| 10 | 0.010 | |
| 15 | 0.001 | |
| 20 | 0.050 | |
| D90 +RF10 | 5 | 0.005 |
| 10 | 0.005 | |
| 15 | 0.010 | |
| 20 | 0.055 |
| Fuel | Torque T (Nm) | Uncertainty u(xHC) |
|---|---|---|
| D100 | 5 | 0.500 |
| 10 | 0.500 | |
| 15 | 0.500 | |
| 20 | 0.500 | |
| D90 +RF10 | 5 | 1.000 |
| 10 | 1.500 | |
| 15 | 1.000 | |
| 20 | 1.000 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chybowski, L.; Szczepanek, M.; Kuczyński, W.; Michalska-Pożoga, I.; Pusty, T.; Brożek, P.; Pełech, R. Content of Selected Compounds in the Exhaust Gas of a Naturally Aspirated CI Engine Fueled with Diesel–Tire Pyrolysis Oil Blend. Energies 2025, 18, 2621. https://doi.org/10.3390/en18102621
Chybowski L, Szczepanek M, Kuczyński W, Michalska-Pożoga I, Pusty T, Brożek P, Pełech R. Content of Selected Compounds in the Exhaust Gas of a Naturally Aspirated CI Engine Fueled with Diesel–Tire Pyrolysis Oil Blend. Energies. 2025; 18(10):2621. https://doi.org/10.3390/en18102621
Chicago/Turabian StyleChybowski, Leszek, Marcin Szczepanek, Waldemar Kuczyński, Iwona Michalska-Pożoga, Tomasz Pusty, Piotr Brożek, and Robert Pełech. 2025. "Content of Selected Compounds in the Exhaust Gas of a Naturally Aspirated CI Engine Fueled with Diesel–Tire Pyrolysis Oil Blend" Energies 18, no. 10: 2621. https://doi.org/10.3390/en18102621
APA StyleChybowski, L., Szczepanek, M., Kuczyński, W., Michalska-Pożoga, I., Pusty, T., Brożek, P., & Pełech, R. (2025). Content of Selected Compounds in the Exhaust Gas of a Naturally Aspirated CI Engine Fueled with Diesel–Tire Pyrolysis Oil Blend. Energies, 18(10), 2621. https://doi.org/10.3390/en18102621








