Development of an Energy-Saving Melting Reactor for Energy-Efficient Disposal of Slag Dumps
Abstract
1. Introduction
2. Method of the Research
The Novelty of the Work Is
- -
- The discovery of a new phenomenon, showing that in the melt layer, there are two reactions opposite in direction and intensity, (1)—slow reactions of the decomposition of complex components (Zn2SiO4 and ZnFe2O4) into simple molecules (ZnO, SiO2, and Fe2O3) and (2)—rapid reactions of the formation of complex components from simple molecules; the dominance of one of the two reactions affects the process’s fuel consumption;
- –
- The creation of a new physicochemical method for calculating the technological parameters of a melting reactor;
- –
- A novel approach, a combination of “ideal” mixing and “ideal” displacement patterns, enhancing the level of zinc recovery from 30% to 70%;
- –
- A combination of a melting reactor and a rotary kiln, reducing specific fuel consumption by 3–4 times compared to existing analogs;
- –
- A new mode of iron recovery from clinker, with a residual amount of 9–10%, allowing the simultaneous acquisition of two products, carbon iron and stone castings.
3. Development of a Physicochemical Method for Calculating a Melting Reactor’s Parameters
- -
- The molten bath operates in a mode close to ideal mixing. According to this assumption, the zinc concentration throughout the layer is the same and equal to the concentration at the outlet ;
- -
- The melt in the layer consists of particles of equivalent diameter, The initial concentration of particles entering the layer is
- -
- The particles of the layer exist over time τ*, after which they immediately collide, interflow, mix, and re-separate, which leads to the equalization of the concentration in the volume of the particle, and the renewal of the interfacial reaction surface;
- -
- For the entire set of particles, the lifetime τ* is the same;
- -
- Over time τ*, the melt particles are dezincified according to the law of non-stationary internal diffusion under boundary conditions of the first kind (particle surface concentration Csur = 0, since the limiting stage is the internal mass transfer). In this case, the amount of zinc removed from the particle during time τ* is determined from the following expression [23]:
4. Results of Experiments on Dump Slag
- (1)
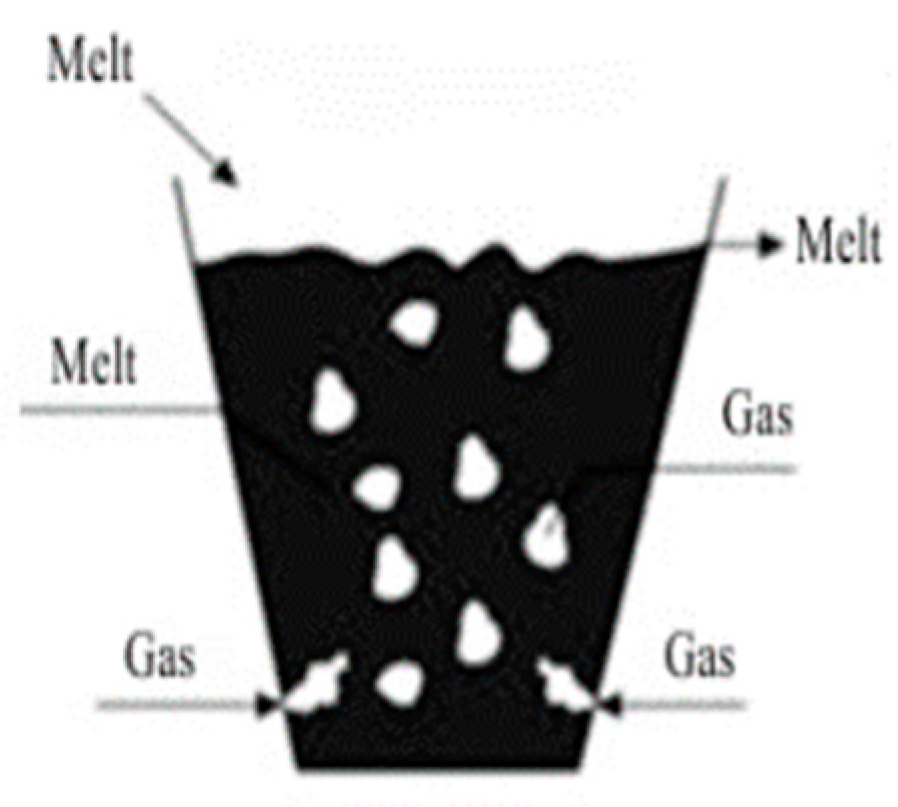
- Bubble layer mode, within the purging intensity = 0.036–0.09;
- (2)
- “Ideal” mixing mode within
- (3)
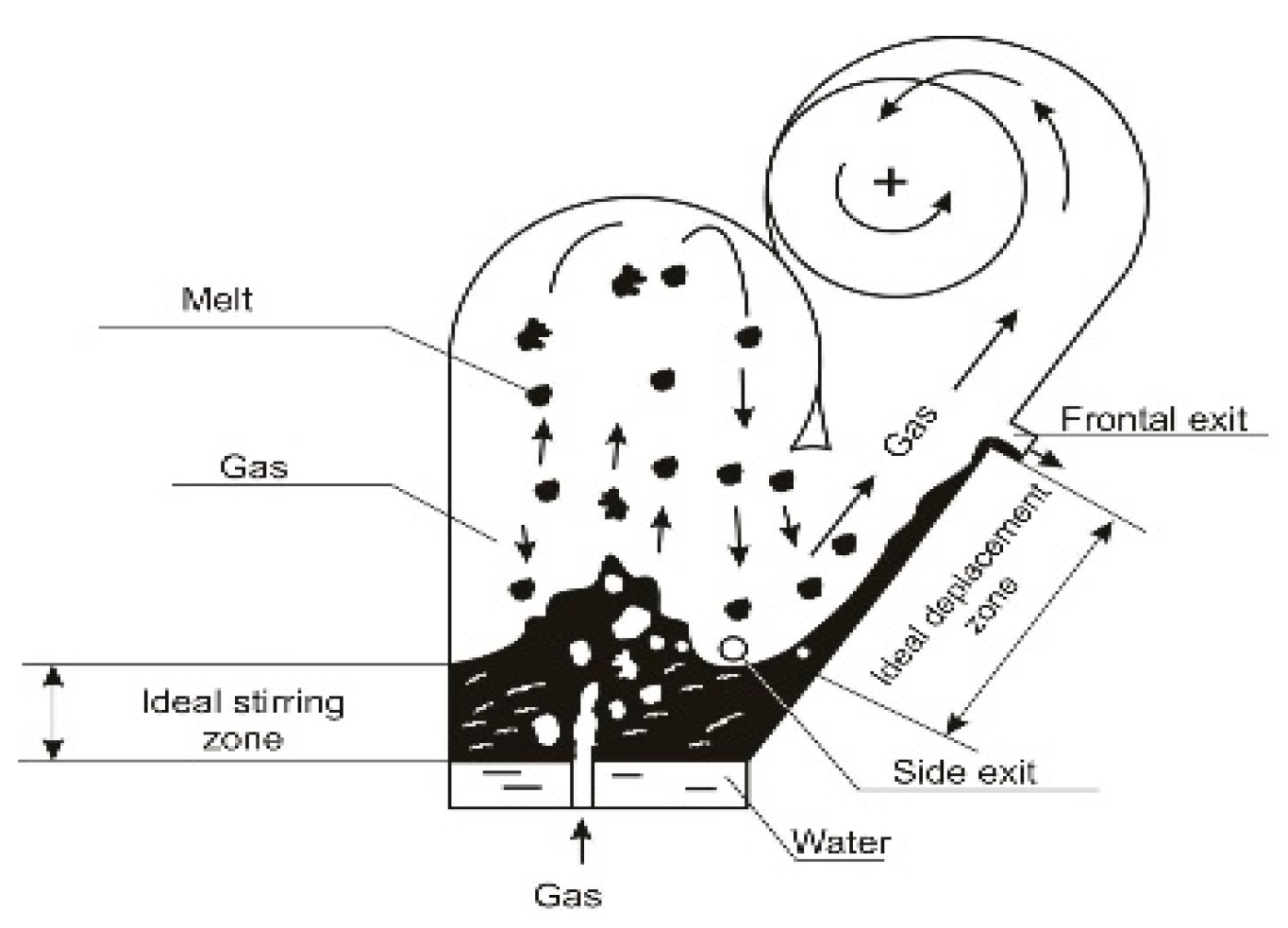
- “Ideal” displacement mode within
- (1)
- From a vertical caisson (side exit);
- (2)
- From the inclined caisson (frontal exit).
- (1)
- In bubble layer mode— = 0.036–0.09, with the release of the melt from the vertical caisson (side exit), and zinc recovery does not exceed 30%;
- (2)
- In the “ideal” mixing mode— and in a mode beyond the “ideal” mixing—0.42, with the release of the melt from the vertical caisson (side exit), and the degree of zinc extraction is no more than 40%;
- (3)
- In the “ideal” mixing–“ideal” displacement mode—0.42, and only when the melt is released from the inclined caisson (frontal exit) does the zinc extraction degree reach 70%.
5. Results of Experiments on the Clinker
Discussion of Experimental Results
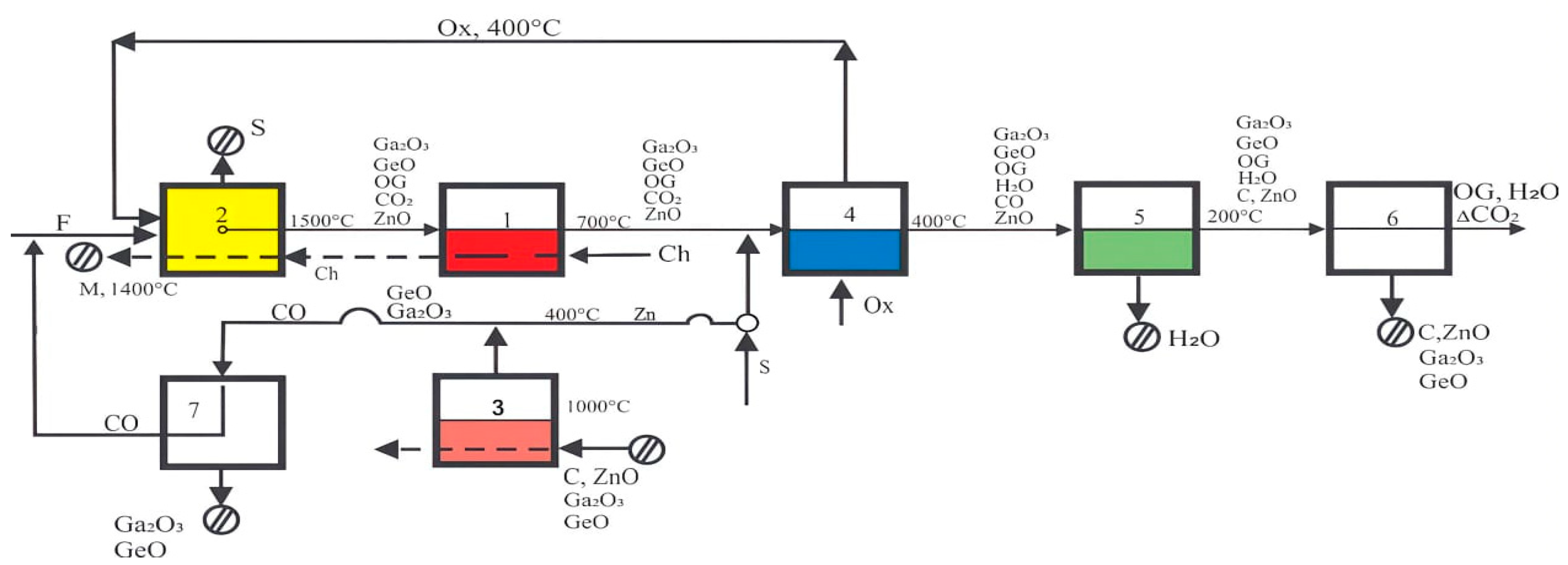
6. Development of an Environmentally Friendly Slag-Processing Thermal Diagram
7. Conclusions
- In the bubble layer mode = 0.036–0.09), the “ideal” mixing mode ( = 0.091–0.19), and beyond the “ideal” mixing mode, = 0.42), with the release of the melt from the vertical caisson, the degree of zinc extraction is no more than 40%; the latter proves that only with an increase in the intensity of purging is it impossible to increase the extraction of zinc and, accordingly, reduce the specific fuel consumption.
- In the melt layer, two reactions opposite in direction and intensity were discovered, slow reactions of decomposition of complex components (Zn2SiO4, ZnFe2O4) into simple molecules (ZnO, SiO2, Fe2O3) and rapid reactions of the formation of complex components from simple molecules; since in the inclined layer of the melt, there is “ideal” displacement, the process of decomposition of complex molecules is also underway and each elementary stream in it moves parallel to each other, then the probability of the mixing of recombined molecules, ZnO, SiO2, and Fe2O3, and accordingly, the formation of complex components, Zn2SiO4 and ZnFe2O4, from them decreases, and the degree of zinc reduction according to the formula ZnO + CO = Zng+ CO2 increases. In the “ideal” mixing–“ideal” displacement mode 0.42), and only when the melt is released from the inclined surface, the zinc extraction degree reaches 70%; at the same time, the degree of zinc sublimation increases by 2.33 times, and the specific fuel consumption decreases by 2.36 times. A new phenomenon has been found by the last, which we have named a combination of “ideal” mixing and ”ideal” displacement modes.
- In traditional processes for processing zinc-rich slag, for example, by fuming or Waeltzing, the waste slag was thrown into a dump due to its unsuitability for the production of building material. In the experiments, after reducing and separating the iron, during the pouring of the melt into molds, at 1300 °C, the samples quickly solidified in ambient between 850 and 900 degrees Celsius; gold and silver particles scattered throughout the melt act as catalysts for crystallization, promoting intensive solidification of the samples; and exposure in a muffle furnace at a temperature of 900 °C for 5—10 min leads to complete volumetric crystallization of the samples. Thus, the novelty of the work is the production of a melt suitable for stone casting when clinker is depleted of iron, with a residual iron content of 9–10%; the latter also contributes to the reduction in specific fuel consumption for the process.
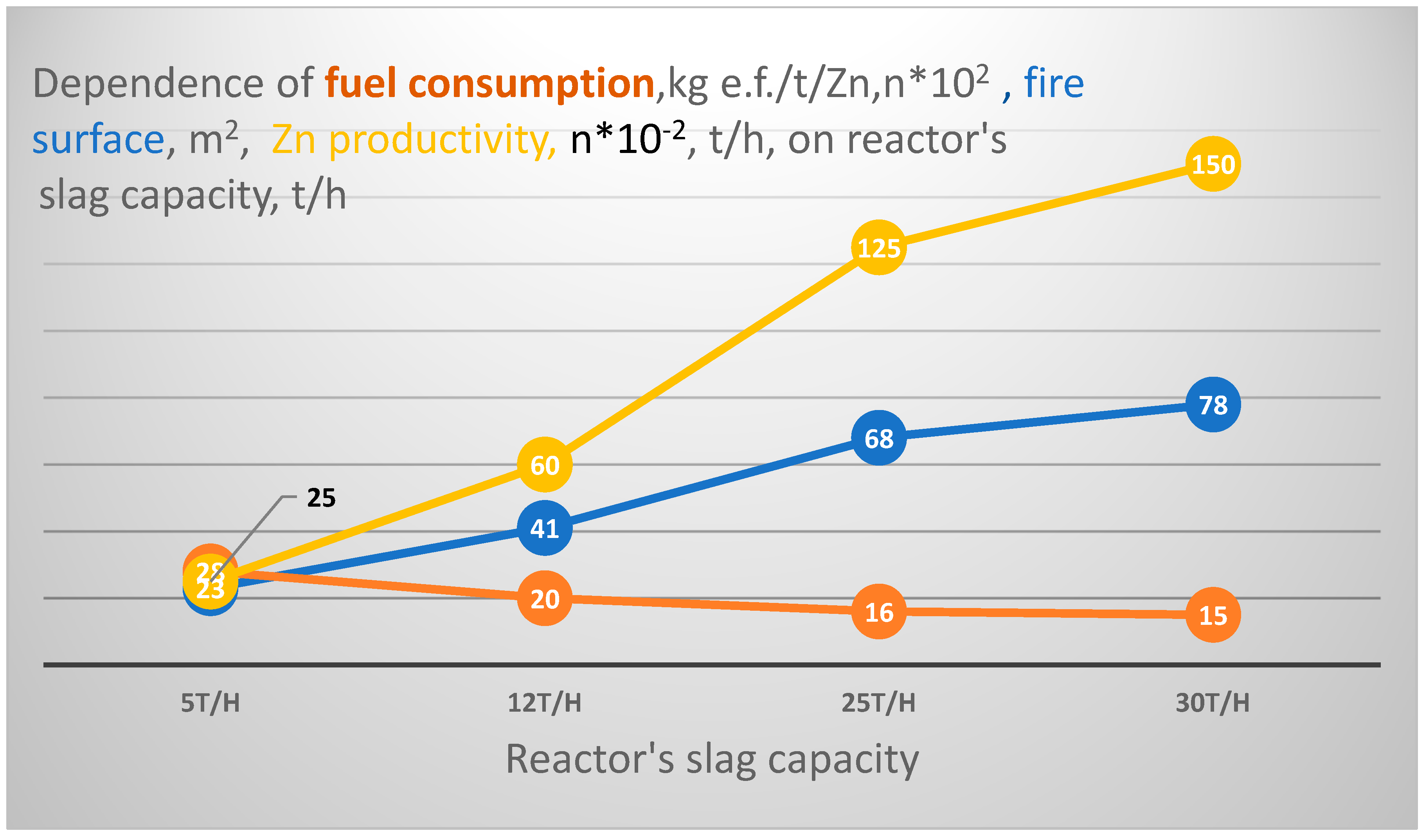
- The melting reactor based on a combination of the “ideal” mixing and “ideal” displacement methods is also new; experiments carried out for the processing of ~250 tons of “poor” slag showed the structural reliability of the unit. According to the experiment results while processing the slag, the specific fuel consumption in the reactor inversion phase would be approximately 1.5 times lower than when utilizing molten slag in the fuming furnace and 3–4 times lower than when processing granulated slag in Waelz kiln.
- Based on the zinc method neutralization of CO2, a thermal diagram of an energy-saving, waste-free, and environmentally friendly system for processing dump slags and clinker was developed. Implementation of the proposed system ensures the neutralization of carbon dioxide and nitrogen oxides by hydrogen and the binding of sulfur with the CaO, subsequently eliminating the appearance of sulfurous gases.
- To predict the characteristics of an industrial sample of the melting unit, the affine modeling method was used; recalculating fuel consumption from a pilot plant with a 1.5 t/h clinker (slag) capacity to an industrial sample with a 25 t/h capacity revealed that, under a pessimistic estimate, the investment payback period would be between 4 and 5 years.
8. Prospects
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Nomenclature
| M | flow of substance mass (kg); |
| β | mass-transfer coefficient in the melt (m/s); |
| F | total surface of mass transfer (m2); |
| ΔC | gradient of impurities concentration at the boundary of phases (kg/m3); |
| τ | dwelling time of bubbles in the melt (s); |
| θ | particle’s medium volumetric dimensionless concentration of ZnO; |
| initial and final particle concentration of the zinc in the melt; | |
| melt stirring time in the processing zone; | |
| homochrony criterion; | |
| K | reaction’s equilibrium constant, –; |
| ΔG | change in Gibbs (free) energy (kJ); |
| In/Gb | ratio of the momentum of gases in the nozzles of the purge grate to the weight of the reactor’s bath, –; |
| Wn/Wg | ratio of the nozzle and purge grate gas velocities, –; |
| Mb | mass of the reactor inversion phase bath (kg); |
| P | reactor’s capacity (kg/h). |
| Acronyms | |
| MR-RK | melting reactor-rotary kiln; |
| LPP | Leninogorsk polymetallic plant; |
| AH | air heater. |
References
- Bolatbaev, K. State, problems and reserves of polymetallic raw material beneficiation technologies. Ind. Kazakhstan 2001, 4, 91–93. [Google Scholar]
- Daukeev, S.Z. Mineral raw materials resources of Kazakhstan-opportunities for scientific and technical development. Issues of complex processing of raw materials of Kazakhstan. In Proceedings of the First International Conference, Almaty, Kazakhstan, 28 August 2003; p. 11. (457p). (In Russian). [Google Scholar]
- Cotrina-Teatino, M.A.; Marquina-Araujo, J.J. Circular economy in the mining industry: A bibliometric and systematic literature review. Resour. Policy 2025, 102, 105513. [Google Scholar] [CrossRef]
- Slobodkin, L.V.; Sannikov, Y.A.; Grinin, Y.A.; Lyamina, M.A. The Kivcet treatment of polymetallic feeds. In Proceedings of the Lead-Zinc Symposium, Pittsburgh, PA, USA, 22–25 October 2000; pp. 687–691. [Google Scholar]
- Sohn, H.Y.; Olivas-Martinez, M. Lead and zinc production. Treatise Process Metall. 2014, 3, 671–700. [Google Scholar]
- Mounsey, E.N. A review of Ausmelt technology for lead smelting. In Proceedings of the Lead-Zinc Symposium, Pittsburgh, PA, USA, 22–25 October 2000; pp. 149–169. [Google Scholar]
- Baby-Jean Robert Mungyeko, B.; Huchet, F. Rotary kiln process: An overview of physical mechanisms, models and applications. Appl. Therm. Eng. 2022, 221, 119637. [Google Scholar] [CrossRef]
- Vartiainen, A.; Ahokainen, T.B. Outotec’s smelting solutions in nonferrous metals. In Proceedings of the International Smelting Technology Symposium, 6th Advances in Sulfide Smelting Symposium (TMS), Orlando, FL, USA, 11–15 March 2012; pp. 89–97. [Google Scholar]
- Hansson, R.; Holmgren, H.; Lehner, T. Recovery of recycled zinc by slag fuming at the Ronnskar Smelter. In Proceedings of the Lead-Zinc 2010, Vancouver, BC, Canada, 3–6 October 2010; pp. 1235–1244. [Google Scholar]
- Borell, M. Slag–A Resource in the Sustainable Society’, Securing the future. In Proceedings of the International Conference on Mining and the Environment, Metals and Energy Recovery, Skellefteå, Sweden, 27 June–1 July 2005; pp. 130–138. [Google Scholar]
- Zhang, Z.; Xie, F.; Wang, W. A review on lead extraction from ore and spent lead paste by hydrometallurgical processes. JOM 2024, 76, 5569–5588. [Google Scholar] [CrossRef]
- Sonule, B.B.; Kulkarni, A.N.; Kakde, N.K.; Madrewar, K.T. Comparative Analysis of Pyrometallurgy, Hydrometallurgy, and BioHydro-Metallurgy for Extraction of Metals from E-Waste. Int. J. Res. Publ. Rev. 2023, 4, 1970–1977. [Google Scholar]
- Dikhanbaev, B.; Dikhanbaev, A.; Chandima, G. Design and implementation of an energy-saving melting reactor. Int. J. Case Stud. Therm. Eng. 2021, 26, 101003. [Google Scholar] [CrossRef]
- Jaken, G.; Samal, S.; Alexander, F.; Alexandr, K.; Yergazy, Z. Overview of techniques and methods of processing the waste of stale clinkers of zinc production. Min. Informational Anal. Bull. 2024, 4, 44–55. [Google Scholar] [CrossRef]
- Cheng, Z.; Tan, Z.; Guo, Z.; Yang, J.; Wang, Q. Technologies and fundamentals of waste heat recovery from high-temperature solid granular materials. Appl. Therm. Eng. 2020, 179, 115703. [Google Scholar] [CrossRef]
- Koizhanova, A.K.; Osipovskaya, L.L.; Erdenova, M.B. Study of precious metals extraction recovery from technogenic wastes. In Proceedings of the 12th International Multidisciplinary Scientific Geo Conference DSGEM, Albena, Bulgaria, 17–23 June 2012; Volume 1, pp. 843–846. [Google Scholar]
- Chanturiya, V.A.; Shadrunova, I.V.; Orekhova, N.N.; Chalkova, N.L. Technology of zinc recovery from mine and waste dump water. Uran IPKON RAS 2011, 1, 35–39. [Google Scholar]
- Kenzhaliev, B.B.; Berkinbayeva, N.; Suleimenov, E.N. Use of conjoint reactions for extraction of metals from mineral raw materials. Eur. Sci. J. 2014, 10, 41–46. [Google Scholar]
- Dikhanbayev, B.; Dikhanbayev, A. On the problem of transition of slag fumingation from periodical to continuous regime. Complex Using Miner. Raw Mater. 2011, 2, 51–56. [Google Scholar]
- Cheng, Z.; Khaliq, A.; Blanpain, B.; Guo, M. Zn Fuming Kinetics in a Bubble-Stirred Molten Slag Bath. Metall. Mater. Trans. B Process Metall. Mater. Process. Sci. 2022, 53, 1308–1319. [Google Scholar] [CrossRef]
- Dikhanbaev, B.; Gomes, C.; Dikhanbaev, A.B. Energy Efficient System for Galena Concentrate Processing. IEEE Access 2019, 7, 23389–23395. [Google Scholar] [CrossRef]
- Dikhanbayev, B. Creation of Pilot Plant on Energy-Saving Processing of Excavated Slag; Collected Scientific Works of National Research Institute of Non-Ferrous Metals “Gintsvetmet”: Moscow, Russia, 2008; pp. 546–553. (In Russian) [Google Scholar]
- Lykov, A.V. Theory of Heat Conduction; Moscow Higher School: Moscow, Russia, 1967; 599р. (In Russian) [Google Scholar]
- Dikhanbaev, A.B. Improving the Efficiency of Waste Disposal from Thermal Power Plants and Metallurgy. Ph.D. Thesis, Republic of Kazakhstan, Almaty, Kazakhstan, 2020; 164р. (In Russian). [Google Scholar]
- Ybray, S.; Dikhanbaev, A.; Dikhanbaev, B.; Mergalimova, A.; Georgiev, A. Development of a technology for the production of hydrogen-enriched synthesis gas with waste-free processing of Ekibastuz coal. Energy 2023, 278, 127817. [Google Scholar] [CrossRef]
- Outokumpu HSC Chemistry for Windows. Chemical Reaction and Equilibrium Software with Extensive Thermochemical Database, Version 5.1; Outokumpu: Helsinki, Finland, 2012. [Google Scholar]
- Yakymchuk, A.; Maxand, S.; Lewandowska, A. Economic Analysis of Global CO2 Emissions and Energy Consumption Based on the World Kaya Identity. Energies 2025, 18, 1661. [Google Scholar] [CrossRef]
- Ren, S.; Jiao, X.; Zheng, D.; Zhang, Y.; Xie, H.; Zhang, R. Impact of Carbon Neutrality Goals on China’s Coal Industry: Mechanisms and Evidence. Energies 2025, 18, 1672. [Google Scholar] [CrossRef]
- Łukaniszyn-Domaszewska, K.; Mazur-Włodarczyk, K.; Łukaniszyn, M. Review: Unveiling the Interrelations Between Migration, Climate Change, and Energy Transitions in the Context of Socioeconomic Disparities. Energies 2025, 18, 1625. [Google Scholar] [CrossRef]
- Lin, C.-H.; Wen, L.-C.; Lo, J.-C. Optimizing Corporate Energy Choices: A Framework for the Net-Zero Emissions Transition. Energies 2025, 18, 1582. [Google Scholar] [CrossRef]
- Jiang, X.; Lu, S. Prediction of Peak Path of Building Carbon Emissions Based on the STIRPAT Model: A Case Study of Guangzhou City. Energies 2025, 18, 1633. [Google Scholar] [CrossRef]
- Dikhanbaev, B.; Dikhanbaev, A.; Koshumbayev, M.; Ybray, S.; Mergalimova, A.; Georgiev, A. On the issue of neutralizing carbon dioxide at processing coal in boilers of thermal power plants. Energy 2024, 295, 130978. [Google Scholar] [CrossRef]




| Melt Processing Methods | P, kg/h | kg | , m3/h | , m3/h | % | % | E, % | We kWh | kg/h | |||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Bubbling melt layer 0.09 | 1300 | 670 | 0.064 | 103 | 288 | 4.3 | 3.01 | 30 | 235 | ~1500 | 17,173 |
| 2 | “Ideal” mixing layer = 0.09–0.19 | 1336 | 400 | 0.099–0.26 | 108 | 305–319 | 4.3 | 2.32 | 42 | 230 | ~1500 | 12,640 |
| 3 | Combination of the layer “ideal” mixing–“ideal” displacement” = 0.19–0.42 | 1400 | 130 | 0.26 | 105 | 319 | 4.3 | 0.8 | 70 | 230 | ~1500 | 7272 |
| Measured Value | Limit of Error | Name, Type, Serial Number | Measuring Range |
|---|---|---|---|
| Consumption of natural gas | At maximum gas flow—(±1)%, at minimum—(±2)% | Rotary gas meter, Delta, Munich, Germany, type 6250, serial number 7026904002. | From 0.8 m3/h to 400 m3/h |
| Flue gas temperature downstream of the rotary kiln, air heater, and scrubber | 0.01 of measured medium temperature | Thermoelectric converters Metran, Ekaterinburg, Russia. Type TXA-0292K, 4 pcs. | From (−400 °C) to (+1000 °C) |
| The pressure of natural gas and blast air in front of the furnace | From (±0.25)% to (±0.5)% | Overpressure sensors Sapphire 22M-DI, Ekaterinburg, Russia, model 2140, 2 pcs. | 0~250 kPa |
| The composition of the combustion products behind the reactor, rotary kiln, air heater, and scrubber | From (±5%) to (±1%) | Manual automatic gas analyzer with display and printer, “TEMPEST 100” from Telegan, Antverpen, Belgium. | CO2-(0.0÷15%); CO-(0.0–15%); O2(0–30%)800 °C |
| Melt temperature in the melting reactor | ±3°C | Optical pyrometer “Smotrych”, Ekaterinburg, Russia. No. 6792. | From 800 °C to 5000 °C |
| 1 | Melt temperature at the exit from the tap hole, °C | 1300–1350 |
| 2 | Melt viscosity, poise | 9–10 |
| 3 | Specific heat flux through water-cooled reactor caissons, kW/m2 | 120–130 |
| 4 | Density, g/cm3 | 2.9–3.0 |
| 5 | Water absorption‚% | 0.16 |
| 6 | Compressive strength, kgf/cm2 | 2500 |
| 7 | Abrasion loss, g/cm2 | 0.11–0.23 |
| 8 | Softening temperature, °C | 1000 |
| 9 | Acid resistance in hydrochloric acid, % | 97.7 |
| 10 | Grain (crystal) size, microns | 60–150 |
| 11 | Heat resistance, heat changes | 18–20 |
| Reactions | ∆G, kJ | K | |
|---|---|---|---|
| 1 | Zn2SiO4 = 2ZnO + SiO2 | 36.203 | 0.086 |
| 2 | ZnFe2O4 = ZnO + Fe2O3 | 33.426 | 0.103 |
| 3 | ZnO + CO = Zng + CO2 | −20.959 | 4.145 |
| 4 | 2ZnO + SiO2 = Zn2SiO4 | –36.20373 | 11.662 |
| 5 | ZnO + Fe2O3 = ZnFe2O4 | –33.426 | 9.656 |
| When Producing Only Zinc Sublimates (Pessimistic Scenario) | At the Production of Zinc Sublimates and Stone-Casting Products (Optimistic Scenario) | ||||
|---|---|---|---|---|---|
| Product price , USD/y | Payback period , years | Expected net profit, USD/y | Product price , USD/y | Payback period , years | Expected net profit, USD/y |
| 10,189,274 | 4.64 | 4,466,039 | 16,186,428 | 2.24 | 9,263,762 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dikhanbaev, A.; Dikhanbaev, B.; Georgiev, A.; Ybray, S.; Baubekov, K.; Koshumbayev, M.; Zhangazy, A. Development of an Energy-Saving Melting Reactor for Energy-Efficient Disposal of Slag Dumps. Energies 2025, 18, 2548. https://doi.org/10.3390/en18102548
Dikhanbaev A, Dikhanbaev B, Georgiev A, Ybray S, Baubekov K, Koshumbayev M, Zhangazy A. Development of an Energy-Saving Melting Reactor for Energy-Efficient Disposal of Slag Dumps. Energies. 2025; 18(10):2548. https://doi.org/10.3390/en18102548
Chicago/Turabian StyleDikhanbaev, Arystan, Bayandy Dikhanbaev, Aleksandar Georgiev, Sultan Ybray, Kuat Baubekov, Marat Koshumbayev, and Alimzhan Zhangazy. 2025. "Development of an Energy-Saving Melting Reactor for Energy-Efficient Disposal of Slag Dumps" Energies 18, no. 10: 2548. https://doi.org/10.3390/en18102548
APA StyleDikhanbaev, A., Dikhanbaev, B., Georgiev, A., Ybray, S., Baubekov, K., Koshumbayev, M., & Zhangazy, A. (2025). Development of an Energy-Saving Melting Reactor for Energy-Efficient Disposal of Slag Dumps. Energies, 18(10), 2548. https://doi.org/10.3390/en18102548






