Review of the Diffusion Process, Explosion Mechanism, and Detection Technology of Hydrogen and Ammonia
Abstract
:1. Introduction
2. Research Progress of Hydrogen Application Safety
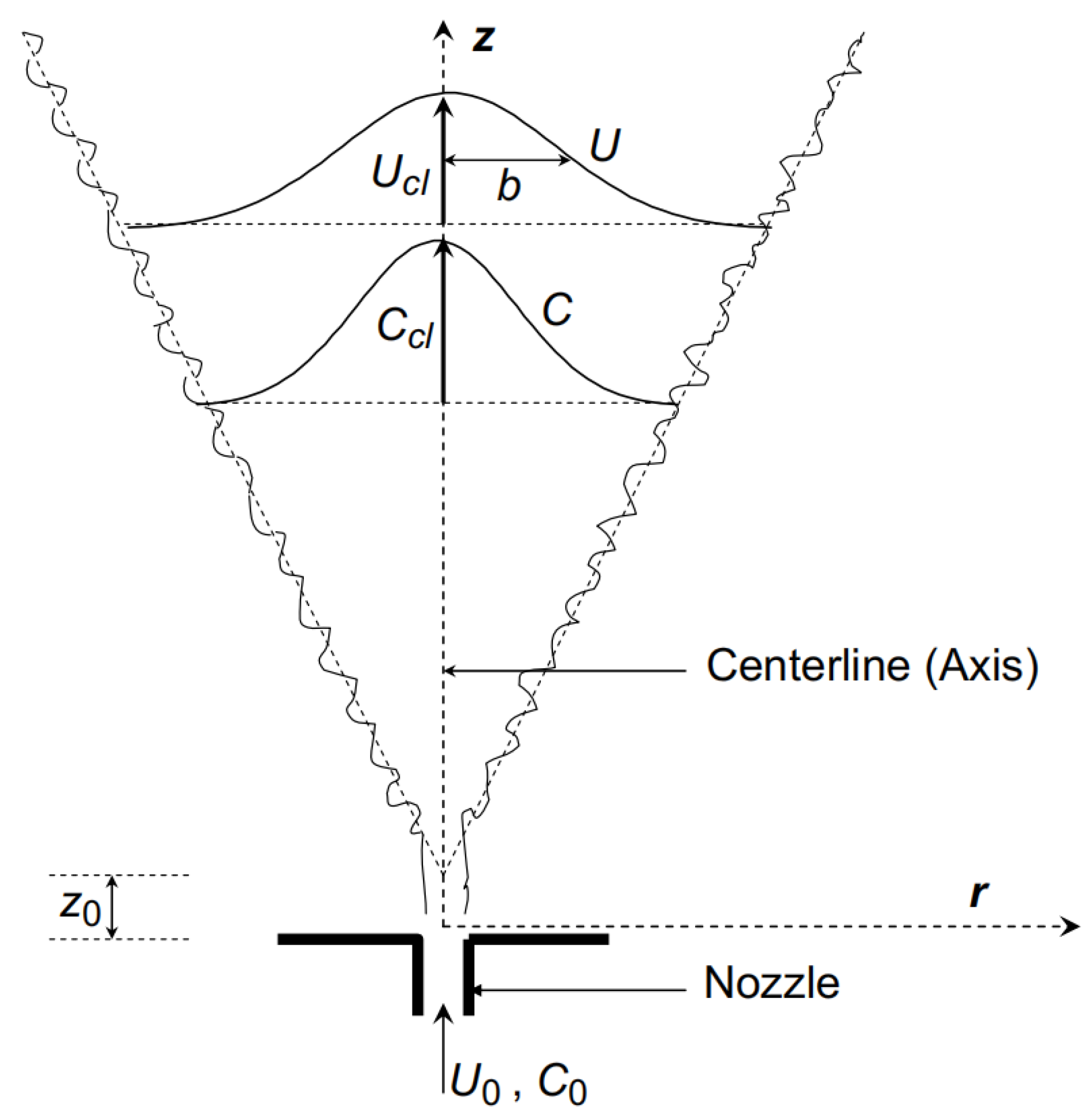
2.1. Research on Hydrogen Leakage Safety
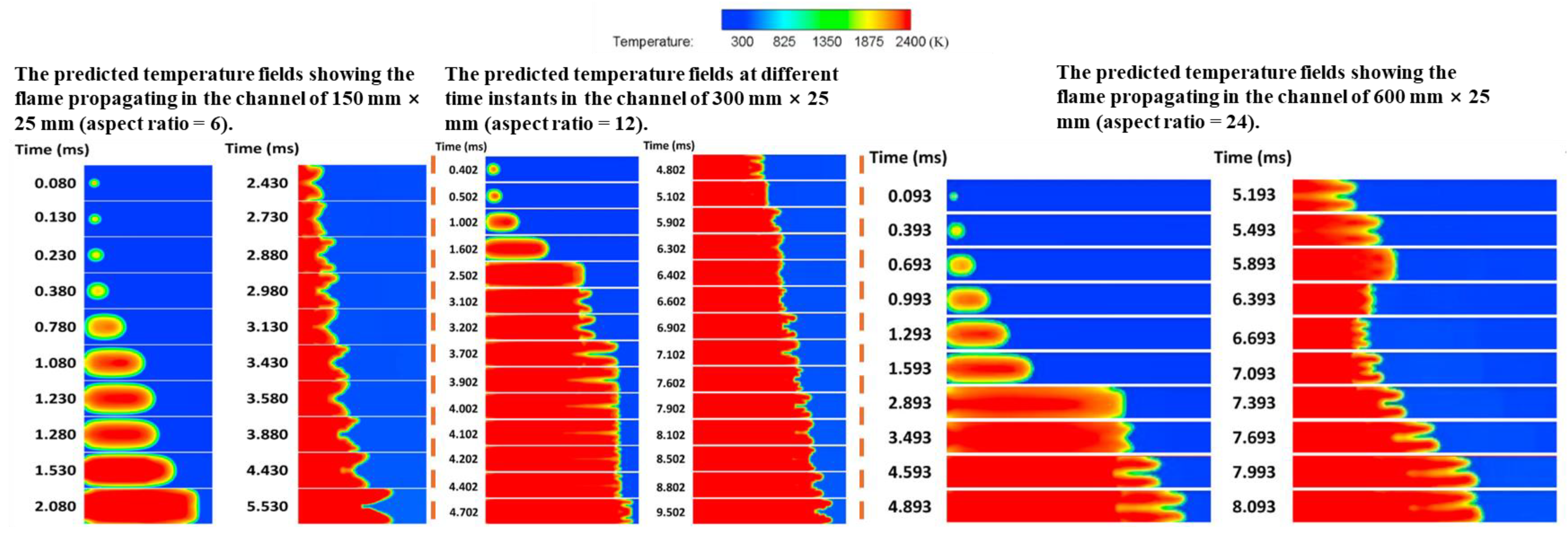
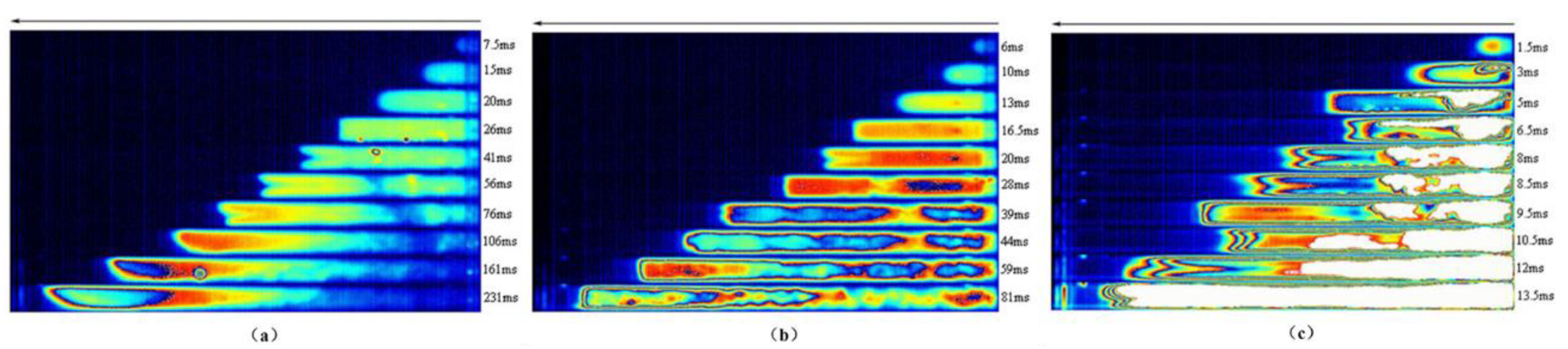
2.2. Research on the Safety of Hydrogen Explosion
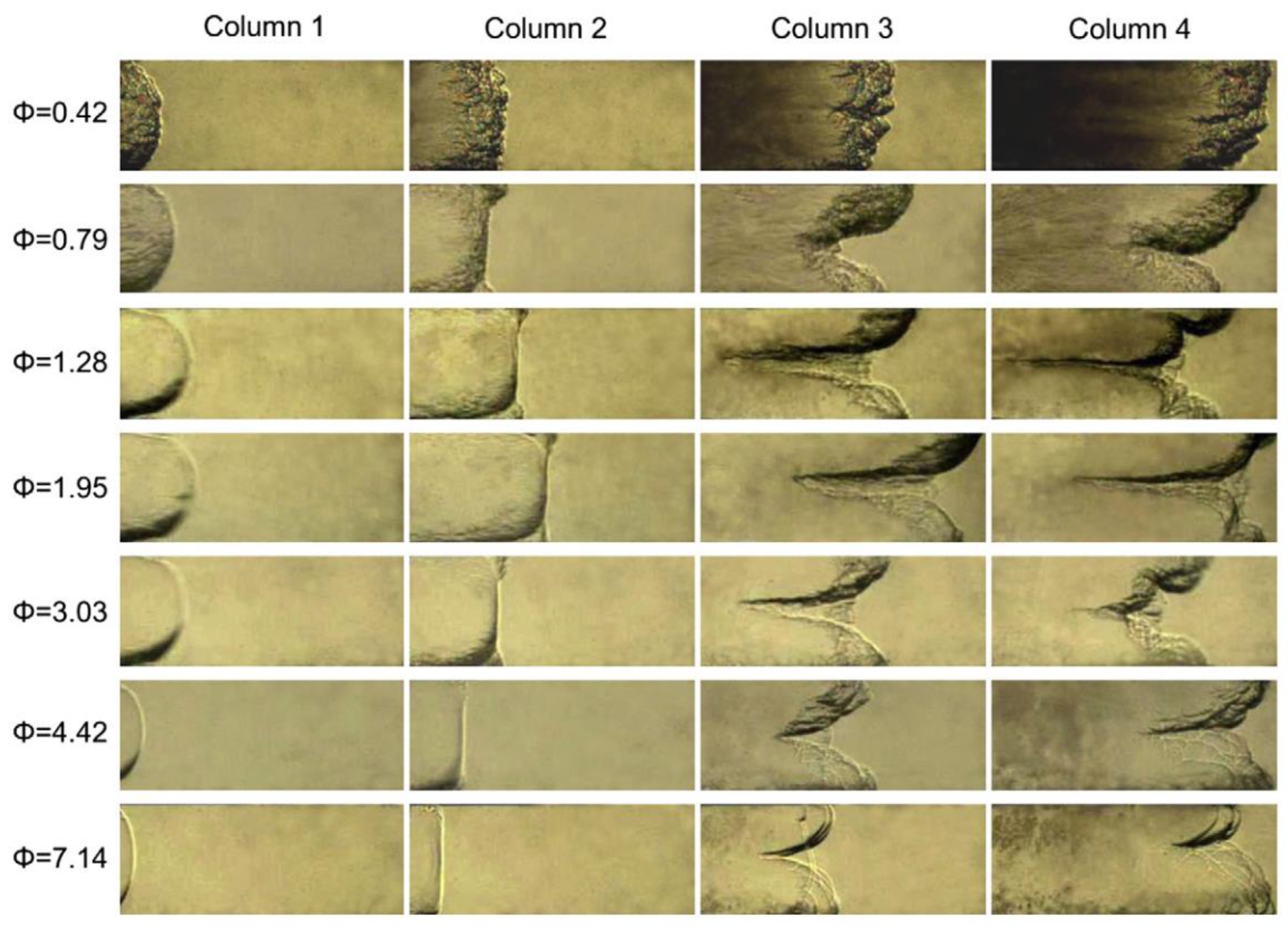
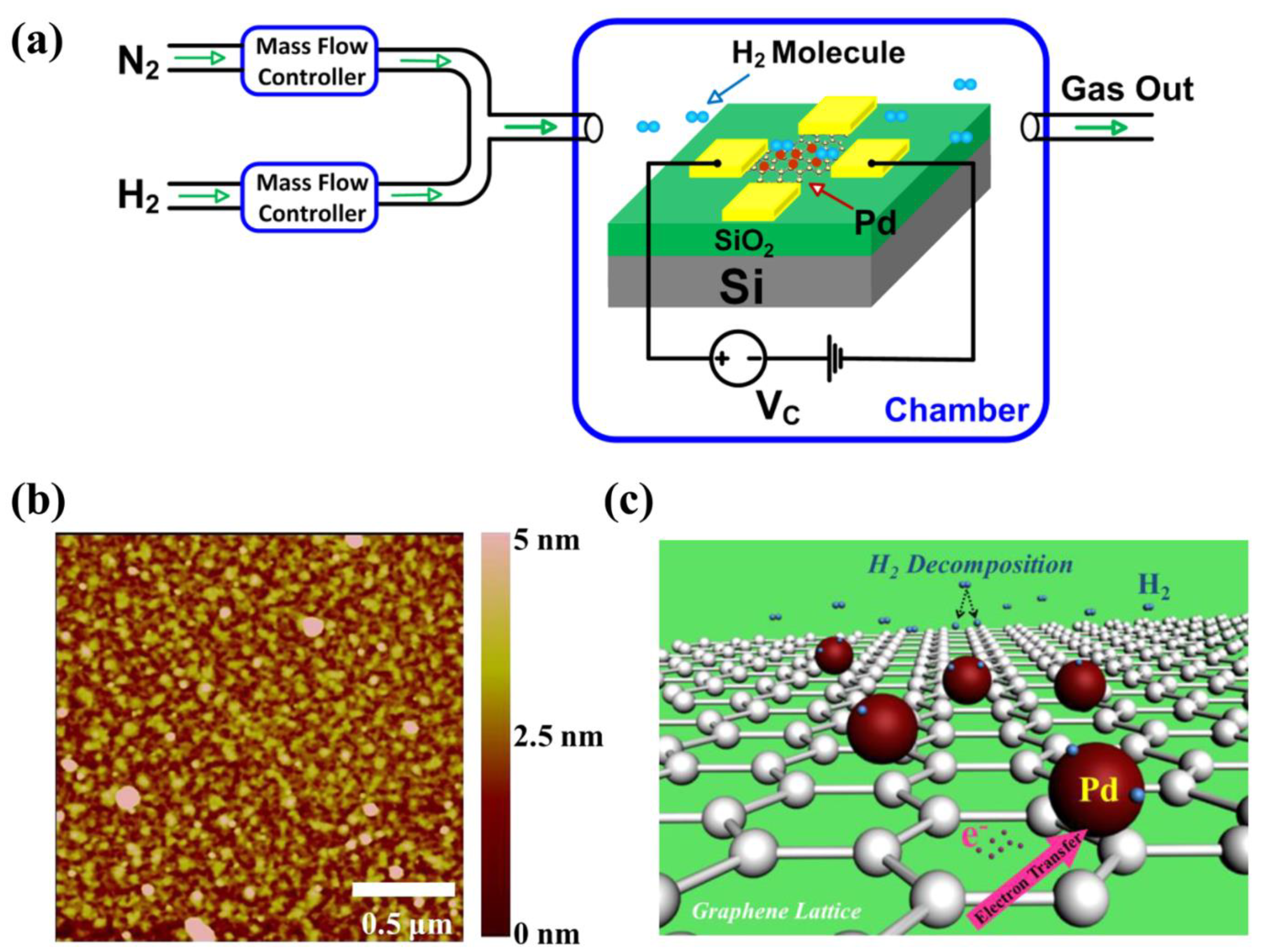
2.3. Research on Hydrogen Leakage Detection Technology
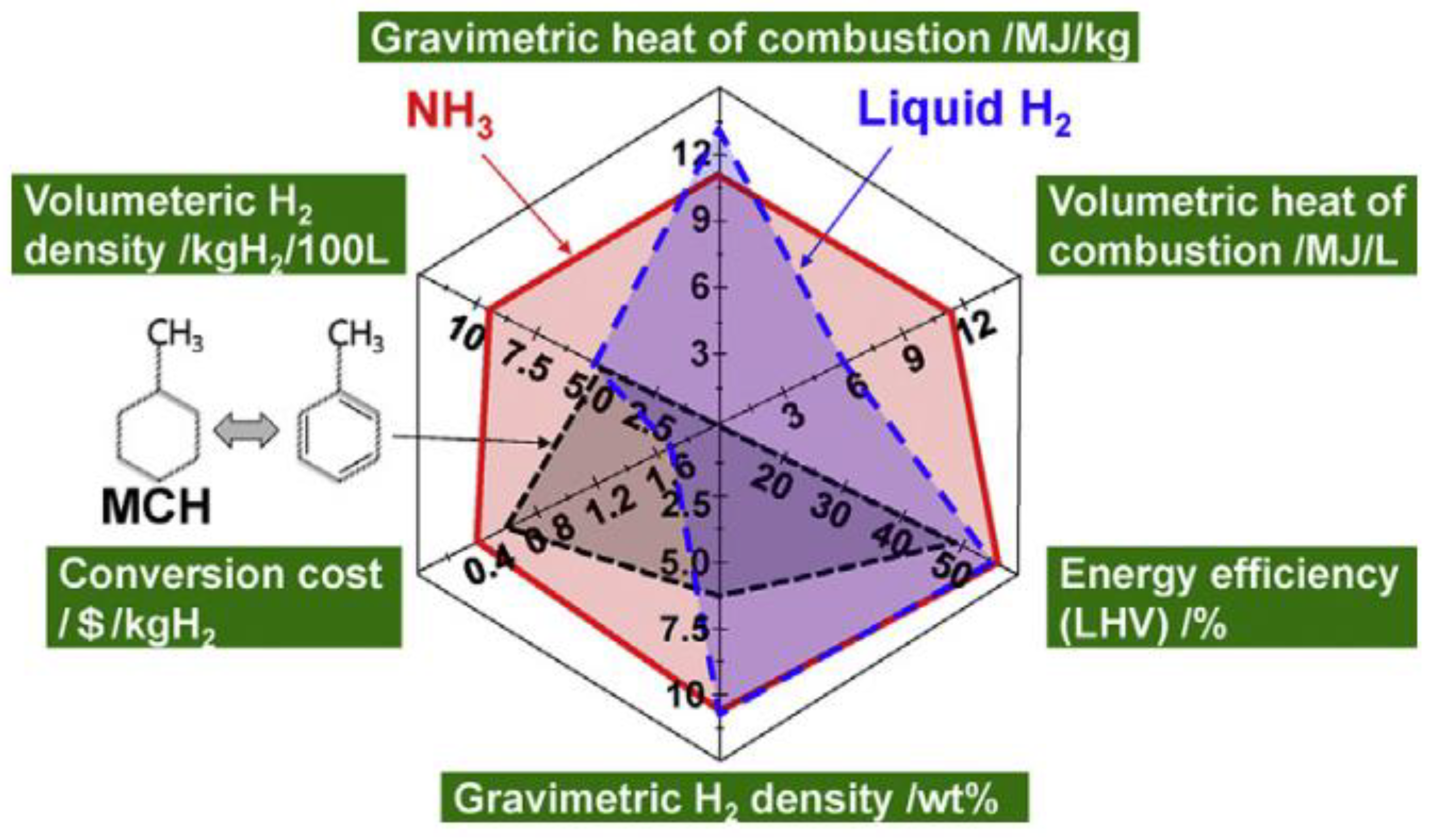
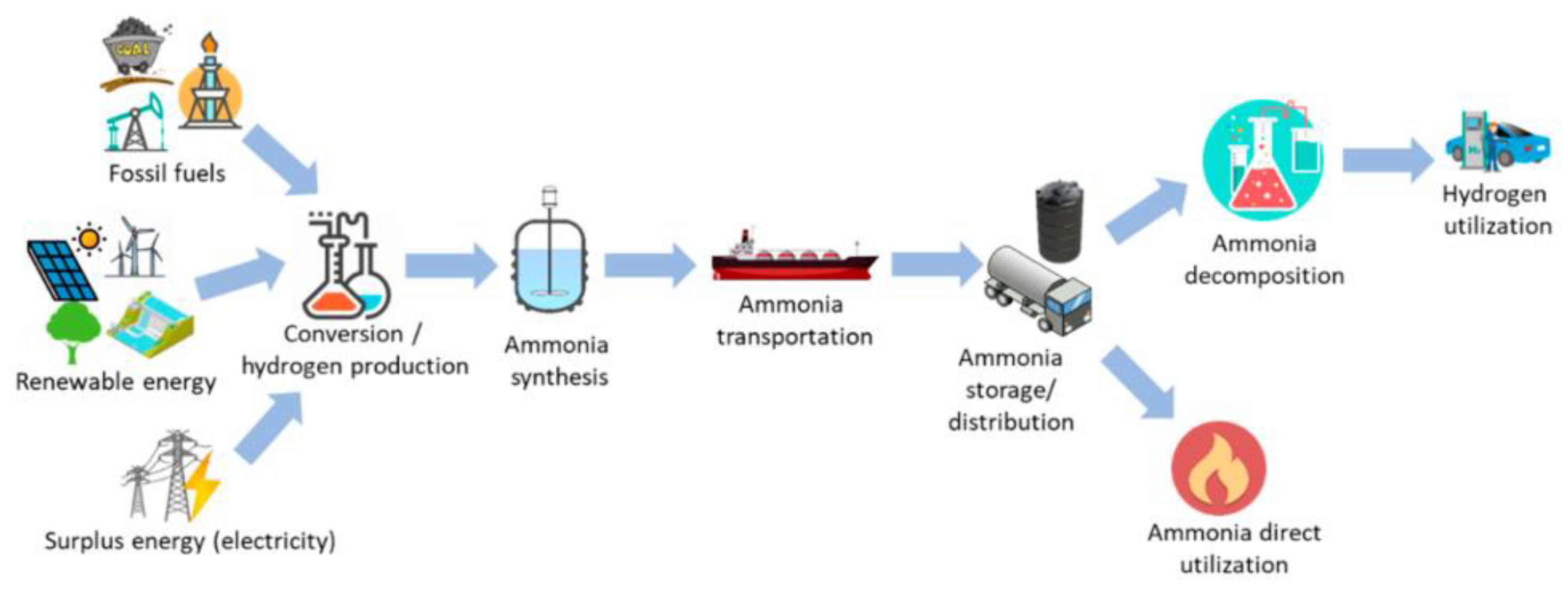
3. Research Progress of Ammonia Application Safety
3.1. Research Progress of Ammonia Application Safety
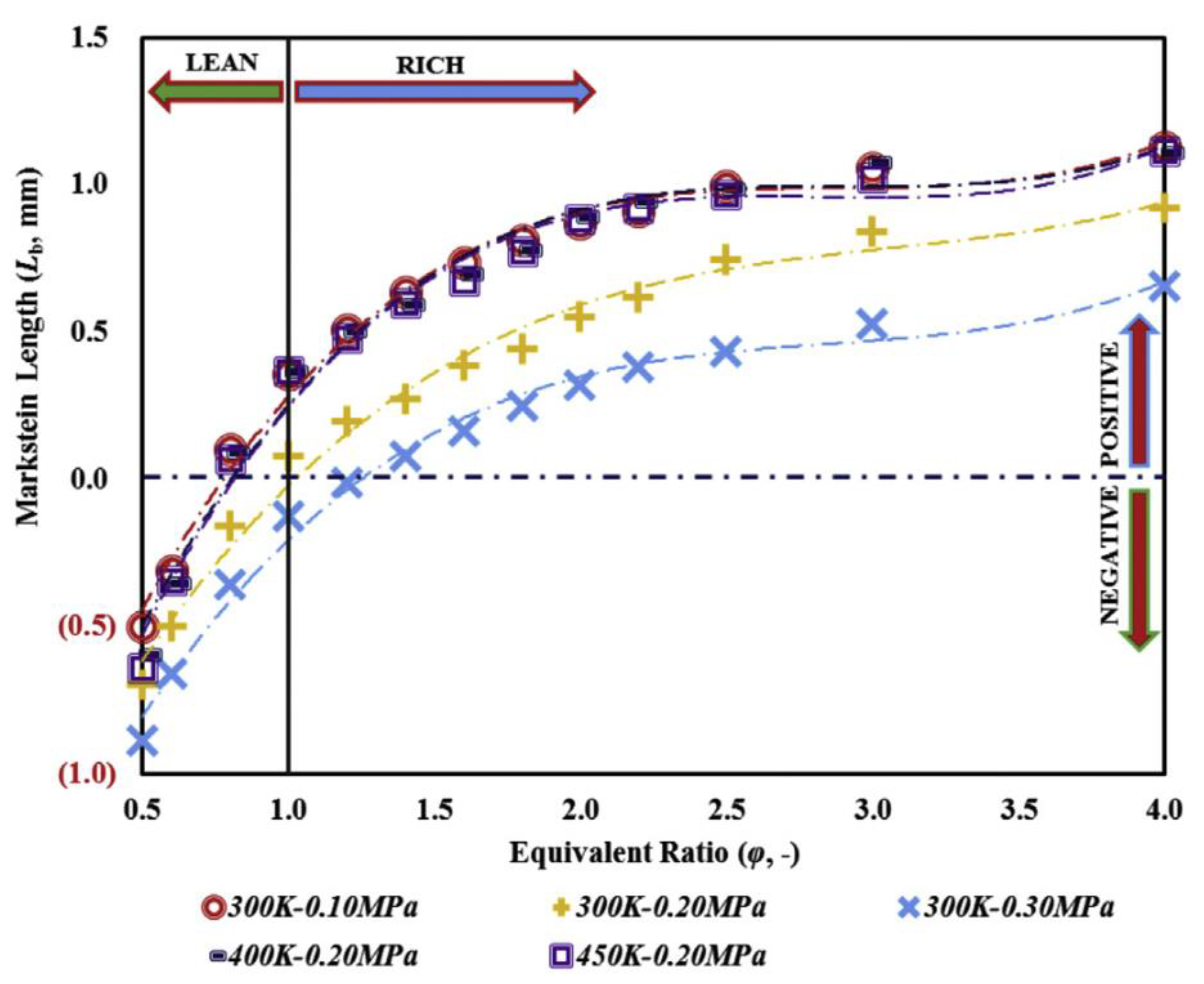
3.2. Research on the Explosion Characteristics of Ammonia Gas
4. Conclusions and Foresight
4.1. Research Progress Summary
4.2. Research Prospect
- Research on the diffusion characteristics of hydrogen–ammonia mixtures should investigate their diffusion mechanisms, with a focus on the impact of the initial environmental conditions, external parameters, obstacles, and ventilation.
- It is necessary to conduct research on the deflagration characteristics of hydrogen–ammonia mixtures. Based on the study of flame propagation and evolution in pipelines, it is essential to add research on deflagration characteristics and hazard assessments for scenarios where accidents may occur during the integrated application of hydrogen and ammonia.
- It is necessary to enhance the new gas concentration detection technology, improve the accuracy and speed of gas concentration detection, and be able to respond quickly to possible leakage situations.
- It is necessary to optimize the gas concentration detection technology based on schlieren imaging, clarify the jet development process after the leakage of hydrogen–ammonia mixtures, establish the attenuation formula of hydrogen–ammonia mixtures, and achieve concentration predictions.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| DTFs | Unstable distorted tulip flames |
| DDT | Deflagration-to-detonation transition |
| R–T | Rayleigh–Taylor |
| K–H | Kelvin–Helmholtz |
| D–L | Darrieus–Landau |
References
- Duro, J.A.; Padilla, E. Inequality across Countries in Energy Intensities: An Analysis of the Role of Energy Transformation and Final Energy Consumption. Energy Econ. 2011, 33, 474–479. [Google Scholar] [CrossRef]
- Michael, T. (Ed.) Evolution in the Global Energy Transformation to 2050; Energy subsidies; International Renewable Energy Agency: Abu Dhabi, United Arab Emirates, 2020; ISBN 978-92-9260-125-6. [Google Scholar]
- Vanegas Cantarero, M.M. Of Renewable Energy, Energy Democracy, and Sustainable Development: A Roadmap to Accelerate the Energy Transition in Developing Countries. Energy Res. Soc. Sci. 2020, 70, 101716. [Google Scholar] [CrossRef]
- Hafner, M.; Tagliapietra, S. (Eds.) The Geopolitics of the Global Energy Transition; Lecture Notes in Energy; Springer International Publishing: Cham, Switzerland, 2020; Volume 73, ISBN 978-3-030-39065-5. [Google Scholar]
- Blondeel, M.; Bradshaw, M.J.; Bridge, G.; Kuzemko, C. The Geopolitics of Energy System Transformation: A Review. Geogr. Compass 2021, 15, e12580. [Google Scholar] [CrossRef]
- Shu, Y.; Chen, G.; He, J.; Zhang, F. Building a New Electric Power System Based on New Energy Sources. Chin. J. Eng. Sci. 2021, 23, 61. [Google Scholar] [CrossRef]
- Dyczko, A.; Kamiński, P.; Stecuła, K.; Prostański, D.; Kopacz, M.; Kowol, D. Thermal and Mechanical Energy Storage as a Chance for Energy Transformation in Poland. Polityka Energ. Energy Policy J. 2021, 24, 43–60. [Google Scholar] [CrossRef]
- Liu, Y.; Tan, Q.; Zhang, X.; Han, J.; Guo, M. How Does Electricity Supply Mode Affect Energy-Water-Emissions Nexus in Urban Energy System? Evidence from Energy Transformation in Beijing, China. J. Clean. Prod. 2022, 366, 132892. [Google Scholar] [CrossRef]
- Cheba, K.; Bąk, I.; Szopik-Depczyńska, K.; Ioppolo, G. Directions of Green Transformation of the European Union Countries. Ecol. Indic. 2022, 136, 108601. [Google Scholar] [CrossRef]
- Veziroğlu, T.N.; Şahi, S. 21st Century’s Energy: Hydrogen Energy System. Energy Convers. Manag. 2008, 49, 1820–1831. [Google Scholar] [CrossRef]
- Jain, I.P. Hydrogen the Fuel for 21st Century. Int. J. Hydrog. Energy 2009, 34, 7368–7378. [Google Scholar] [CrossRef]
- Johnston, B.; Mayo, M.C.; Khare, A. Hydrogen: The Energy Source for the 21st Century. Technovation 2005, 25, 569–585. [Google Scholar] [CrossRef]
- Marchenko, O.V.; Solomin, S.V. The Future Energy: Hydrogen versus Electricity. Int. J. Hydrog. Energy 2015, 40, 3801–3805. [Google Scholar] [CrossRef]
- Singla, M.K.; Nijhawan, P.; Oberoi, A.S. Hydrogen Fuel and Fuel Cell Technology for Cleaner Future: A Review. Environ. Sci. Pollut. Res. 2021, 28, 15607–15626. [Google Scholar] [CrossRef] [PubMed]
- Stępień, Z. A Comprehensive Overview of Hydrogen-Fueled Internal Combustion Engines: Achievements and Future Challenges. Energies 2021, 14, 6504. [Google Scholar] [CrossRef]
- Jiang, Z.; Gao, X. Quantification and Evaluation of China’s Hydrogen Automobile Industry Policy. Int. J. Hydrog. Energy 2024, 56, 757–768. [Google Scholar] [CrossRef]
- Wei, Q.S.; Zhang, X.; Oh, B.S. The Effect of Driving Cycles and H2 Production Pathways on the Lifecycle Analysis of Hydrogen Fuel Cell Vehicle: A Case Study in South Korea. Int. J. Hydrog. Energy 2021, 46, 7622–7633. [Google Scholar] [CrossRef]
- Medisetty, V.M.; Kumar, R.; Ahmadi, M.H.; Vo, D.-V.N.; Ochoa, A.A.V.; Solanki, R. Overview on the Current Status of Hydrogen Energy Research and Development in India. Chem. Eng. Technol. 2020, 43, 613–624. [Google Scholar] [CrossRef]
- Khan, U.; Yamamoto, T.; Sato, H. Consumer Preferences for Hydrogen Fuel Cell Vehicles in Japan. Transp. Res. Part Transp. Environ. 2020, 87, 102542. [Google Scholar] [CrossRef]
- Apostolou, D.; Welcher, S.N. Prospects of the Hydrogen-Based Mobility in the Private Vehicle Market. a Social Perspective in Denmark. Int. J. Hydrog. Energy 2021, 46, 6885–6900. [Google Scholar] [CrossRef]
- Tarhan, C.; Çil, M.A. A Study on Hydrogen, the Clean Energy of the Future: Hydrogen Storage Methods. J. Energy Storage 2021, 40, 102676. [Google Scholar] [CrossRef]
- Usman, M.R. Hydrogen Storage Methods: Review and Current Status. Renew. Sustain. Energy Rev. 2022, 167, 112743. [Google Scholar] [CrossRef]
- Pu, L.; Yu, H.; Dai, M.; He, Y.; Sun, R.; Yan, T. Research Progress and Application of High-Pressure Hydrogen and Liquid Hydrogen in Storage and Transportation. Chin. Sci. Bull. 2022, 67, 2172–2191. [Google Scholar] [CrossRef]
- Lebedeva, O.; Kultin, D.; Kalenchuk, A.; Kustov, L. Advances and Prospects in Electrocatalytic Hydrogenation of Aromatic Hydrocarbons for Synthesis of “Loaded” Liquid Organic Hydrogen Carriers. Curr. Opin. Electrochem. 2023, 38, 101207. [Google Scholar] [CrossRef]
- Kojima, Y.; Yamaguchi, M. Ammonia as a Hydrogen Energy Carrier. Int. J. Hydrog. Energy 2022, 47, 22832–22839. [Google Scholar] [CrossRef]
- Aziz, M.; Wijayanta, A.T.; Nandiyanto, A.B.D. Ammonia as Effective Hydrogen Storage: A Review on Production, Storage and Utilization. Energies 2020, 13, 3062. [Google Scholar] [CrossRef]
- Klerke, A.; Christensen, C.H.; Nørskov, J.K.; Vegge, T. Ammonia for Hydrogen Storage: Challenges and Opportunities. J. Mater. Chem. 2008, 18, 2304. [Google Scholar] [CrossRef]
- Kojima, Y. Safety of Ammonia as a Hydrogen Energy Carrier. Int. J. Hydrog. Energy 2024, 50, 732–739. [Google Scholar] [CrossRef]
- Makepeace, J.W.; He, T.; Weidenthaler, C.; Jensen, T.R.; Chang, F.; Vegge, T.; Ngene, P.; Kojima, Y.; De Jongh, P.E.; Chen, P.; et al. Reversible Ammonia-Based and Liquid Organic Hydrogen Carriers for High-Density Hydrogen Storage: Recent Progress. Int. J. Hydrog. Energy 2019, 44, 7746–7767. [Google Scholar] [CrossRef]
- Ulucan, T.H.; Akhade, S.A.; Ambalakatte, A.; Autrey, T.; Cairns, A.; Chen, P.; Cho, Y.W.; Gallucci, F.; Gao, W.; Grinderslev, J.B.; et al. Hydrogen Storage in Liquid Hydrogen Carriers: Recent Activities and New Trends. Prog. Energy 2023, 5, 012004. [Google Scholar] [CrossRef]
- Olabi, A.G.; Abdelkareem, M.A.; Al-Murisi, M.; Shehata, N.; Alami, A.H.; Radwan, A.; Wilberforce, T.; Chae, K.-J.; Sayed, E.T. Recent progress in green ammonia: Production, applications, assessment; barriers, and its role in achieving the sustainable development goals. Energy Convers. Manag. 2023, 277, 116594. [Google Scholar] [CrossRef]
- Mashhadimoslem, H.; Safarzadeh Khosrowshahi, M.; Delpisheh, M.; Convery, C.; Rezakazemi, M.; Aminabhavi, T.M.; Kamkar, M.; Elkamel, A. Green Ammonia to Hydrogen: Reduction and Oxidation Catalytic Processes. Chem. Eng. J. 2023, 474, 145661. [Google Scholar] [CrossRef]
- Lamb, K.E.; Dolan, M.D.; Kennedy, D.F. Ammonia for Hydrogen Storage; a Review of Catalytic Ammonia Decomposition and Hydrogen Separation and Purification. Int. J. Hydrog. Energy 2019, 44, 3580–3593. [Google Scholar] [CrossRef]
- Li, X.; Wang, Q.; Wu, S.; Hu, Z.; Bai, J. Critical Upstream Technologies for Hydrogen Energy Industry: Research Progress on Ammonia Decomposition Catalysts. Sustain. Chem. Pharm. 2024, 38, 101492. [Google Scholar] [CrossRef]
- Mukherjee, S.; Devaguptapu, S.V.; Sviripa, A.; Lund, C.R.F.; Wu, G. Low-Temperature Ammonia Decomposition Catalysts for Hydrogen Generation. Appl. Catal. B Environ. 2018, 226, 162–181. [Google Scholar] [CrossRef]
- Valera-Medina, A.; Xiao, H.; Owen-Jones, M.; David, W.I.F.; Bowen, P.J. Ammonia for Power. Prog. Energy Combust. Sci. 2018, 69, 63–102. [Google Scholar] [CrossRef]
- Guiberti, T.F.; Pezzella, G.; Hayakawa, A.; Sarathy, S.M. Mini Review of Ammonia for Power and Propulsion: Advances and Perspectives. Energy Fuels 2023, 37, 14538–14555. [Google Scholar] [CrossRef]
- Tan, H.; Sang, Z.; Tian, Y.; Peng, W.; Liu, X.; Liang, J. Ammonia as a Green Carbon-Free Fuel: A Pathway to the Sustainable Energy Economy. ACS Energy Lett. 2024, 5120–5136. [Google Scholar] [CrossRef]
- Jeerh, G.; Zhang, M.; Tao, S. Recent Progress in Ammonia Fuel Cells and Their Potential Applications. J. Mater. Chem. A 2021, 9, 727–752. [Google Scholar] [CrossRef]
- Zamfirescu, C.; Dincer, I. Ammonia as a Green Fuel and Hydrogen Source for Vehicular Applications. Fuel Process. Technol. 2009, 90, 729–737. [Google Scholar] [CrossRef]
- Al-Hamed, K.H.M.; Dincer, I. A Novel Ammonia Solid Oxide Fuel Cell-Based Powering System with on-Board Hydrogen Production for Clean Locomotives. Energy 2021, 220, 119771. [Google Scholar] [CrossRef]
- Wan, Z.; Tao, Y.; Shao, J.; Zhang, Y.; You, H. Ammonia as an Effective Hydrogen Carrier and a Clean Fuel for Solid Oxide Fuel Cells. Energy Convers. Manag. 2021, 228, 113729. [Google Scholar] [CrossRef]
- Machaj, K.; Kupecki, J.; Malecha, Z.; Morawski, A.W.; Skrzypkiewicz, M.; Stanclik, M.; Chorowski, M. Ammonia as a Potential Marine Fuel: A Review. Energy Strategy Rev. 2022, 44, 100926. [Google Scholar] [CrossRef]
- Akgun, I.; Dincer, I. Development of a Smart Powering System with Ammonia Fuel Cells and Internal Combustion Engine for Submarines. Energy 2024, 294, 130747. [Google Scholar] [CrossRef]
- Cheliotis, M.; Boulougouris, E.; Trivyza, N.L.; Theotokatos, G.; Livanos, G.; Mantalos, G.; Stubos, A.; Stamatakis, E.; Venetsanos, A. Review on the Safe Use of Ammonia Fuel Cells in the Maritime Industry. Energies 2021, 14, 3023. [Google Scholar] [CrossRef]
- El-Amin, M.F. Non-Boussinesq Turbulent Buoyant Jet Resulting from Hydrogen Leakage in Air. Int. J. Hydrog. Energy 2009, 34, 7873–7882. [Google Scholar] [CrossRef]
- El-Amin, M.F.; Sun, S. Horizontal H2–Air Turbulent Buoyant Jet Resulting from Hydrogen Leakage. Int. J. Hydrog. Energy 2012, 37, 3949–3957. [Google Scholar] [CrossRef]
- Birch, A.D.; Brown, D.R.; Dodson, M.G.; Swaffield, F. The Structure and Concentration Decay of High Pressure Jets of Natural Gas. Combust. Sci. Technol. 1984, 36, 249–261. [Google Scholar] [CrossRef]
- Birch, A.D.; Hughes, D.J.; Swaffield, F. Velocity Decay of High Pressure Jets. Combust. Sci. Technol. 1987, 52, 161–171. [Google Scholar] [CrossRef]
- Ewan, B.C.R.; Moodie, K. Structure and Velocity Measurements in Underexpanded Jets. Combust. Sci. Technol. 1986, 45, 275–288. [Google Scholar] [CrossRef]
- Yüceil, K.B.; Ötügen, M.V. Scaling Parameters for Underexpanded Supersonic Jets. Phys. Fluids 2002, 14, 4206–4215. [Google Scholar] [CrossRef]
- Kang, Y.; Ma, S.; Song, B.; Xia, X.; Wu, Z.; Zhang, X.; Zhao, M. Simulation of Hydrogen Leakage Diffusion Behavior in Confined Space. Int. J. Hydrog. Energy 2023, 53, 75–85. [Google Scholar] [CrossRef]
- Wang, T.; Huang, T.; Hu, S.; Li, Y.; Yang, F.; Ouyang, M. Simulation and Risk Assessment of Hydrogen Leakage in Hydrogen Production Container. Int. J. Hydrog. Energy 2023, 48, 20096–20111. [Google Scholar] [CrossRef]
- Kang, Y.; Ma, S.; Song, B.; Xia, X.; Wu, Z.; Zhang, X.; Zhao, M. Study on the Hydrogen Leakage Diffusion Behavior by Obstacles in Confined Spaces. Fuel 2023, 358, 130110. [Google Scholar] [CrossRef]
- Zhang, T.; Jiang, Y.; Wang, S.; Pan, X.; Hua, M.; Wang, Z.; Wang, Q.; Li, Y.; Jiang, J. Numerical Study on the Flow Characteristics of Pressurized Hydrogen Leaking into the Confined Space through Different Shaped Orifices. Int. J. Hydrog. Energy 2022, 47, 35527–35539. [Google Scholar] [CrossRef]
- Shu, Z.; Liang, W.; Zheng, X.; Lei, G.; Cao, P.; Dai, W.; Qian, H. Dispersion Characteristics of Hydrogen Leakage: Comparing the Prediction Model with the Experiment. Energy 2021, 236, 121420. [Google Scholar] [CrossRef]
- Xin, J.; Duan, Q.; Jin, K.; Sun, J. A Reduced-Scale Experimental Study of Dispersion Characteristics of Hydrogen Leakage in an Underground Parking Garage. Int. J. Hydrog. Energy 2023, 48, 16936–16948. [Google Scholar] [CrossRef]
- Cerbarano, D.; Tieghi, L.; Delibra, G.; Lo Schiavo, E.; Minotti, S.; Corsini, A. Characterization of High-Pressure Hydrogen Leakages. J. Eng. Gas Turbines Power 2024, 146, 51019. [Google Scholar] [CrossRef]
- Longden, T.; Beck, F.J.; Jotzo, F.; Andrews, R.; Prasad, M. ‘Clean’ Hydrogen?—Comparing the Emissions and Costs of Fossil Fuel versus Renewable Electricity Based Hydrogen. Appl. Energy 2022, 306, 118145. [Google Scholar] [CrossRef]
- Shu, Z.; Liang, W.; Liu, F.; Lei, G.; Zheng, X.; Qian, H. Diffusion Characteristics of Liquid Hydrogen Spills in a Crossflow Field: Prediction Model and Experiment. Appl. Energy 2022, 323, 119617. [Google Scholar] [CrossRef]
- Yang, N.; Deng, J.; Wang, C.; Bai, Z.; Qu, J. High Pressure Hydrogen Leakage Diffusion: Research Progress. Int. J. Hydrog. Energy 2023, 50, 1029–1046. [Google Scholar] [CrossRef]
- Park, J.; Kang, S.; Kim, S.; Kim, H.; Kim, S.-K.; Lee, J.H. Optimizing Green Hydrogen Systems: Balancing Economic Viability and Reliability in the Face of Supply-Demand Volatility. Appl. Energy 2024, 368, 123492. [Google Scholar] [CrossRef]
- Yan, Y.; Wang, Y.; Yan, J.; Zhang, H.; Shang, W. Wind Electricity-hydrogen-Natural Gas Coupling: An Integrated Optimization Approach for Enhancing Wind Energy Accommodation and Carbon Reduction. Appl. Energy 2024, 369, 123482. [Google Scholar] [CrossRef]
- Thawani, B.; Hazael, R.; Critchley, R. Numerical Modelling of Hydrogen Leakages in Confined Spaces for Domestic Applications. Int. J. Hydrog. Energy 2024, 56, 797–806. [Google Scholar] [CrossRef]
- Xu, Q.; Chen, G.; Xie, M.; Li, X.; Zhao, Y.; Su, S.; Li, S. Experimental and Numerical Studies on Hydrogen Leakage and Dispersion Evolution Characteristics in Space with Large Aspect Ratios. J. Clean. Prod. 2024, 438, 140467. [Google Scholar] [CrossRef]
- Xie, Y.; Liu, J.; Qin, J.; Xu, Z.; Zhu, J.; Liu, G.; Yuan, H. Numerical Simulation of Hydrogen Leakage and Diffusion in a Ship Engine Room. Int. J. Hydrog. Energy 2023, 55, 42–54. [Google Scholar] [CrossRef]
- Sun, R.; Pu, L.; Yu, H.; Dai, M.; Li, Y. Modeling the Diffusion of Flammable Hydrogen Cloud under Different Liquid Hydrogen Leakage Conditions in a Hydrogen Refueling Station. Int. J. Hydrog. Energy 2022, 47, 25849–25863. [Google Scholar] [CrossRef]
- Mao, X.; Ying, R.; Yuan, Y.; Li, F.; Shen, B. Simulation and Analysis of Hydrogen Leakage and Explosion Behaviors in Various Compartments on a Hydrogen Fuel Cell Ship. Int. J. Hydrog. Energy 2020, 46, 6857–6872. [Google Scholar] [CrossRef]
- Li, F.; Yuan, Y.; Yan, X.; Malekian, R.; Li, Z. A Study on a Numerical Simulation of the Leakage and Diffusion of Hydrogen in a Fuel Cell Ship. Renew. Sustain. Energy Rev. 2018, 97, 177–185. [Google Scholar] [CrossRef]
- Shen, Y.; Lv, H.; Zheng, T.; Liu, Y.; Zhou, W.; Zhang, C. Temporal and Spatial Evolution of Hydrogen Leakage and Diffusion from Tube Fittings on Fuel Cell Vehicles under the Effect of Ambient Wind. Renew. Sustain. Energy Rev. 2023, 185, 113596. [Google Scholar] [CrossRef]
- Xie, L.; Rong, Y.; Chen, J.; Yuan, F.; Long, R. Impacts of Wind Conditions on Hydrogen Leakage during Refilling Hydrogen-Powered Vehicles. Energy Storage Sav. 2023, 2, 449–458. [Google Scholar] [CrossRef]
- Li, X.; Xu, Y.; Li, X.; Jin, Z.; Qian, J. Effect of Wind Condition on Unintended Hydrogen Release in a Hydrogen Refueling Station. Int. J. Hydrog. Energy 2020, 46, 5537–5547. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, Y.; Zhao, H.; Xiong, Y. Study on the Influence of Barriers in the Diffusion Process of Liquid Hydrogen Leakage. IOP Conf. Ser. Earth Environ. Sci. 2020, 546, 42–48. [Google Scholar] [CrossRef]
- Hou, X.; Lan, H.; Zhao, Z.; Li, J.; Hu, C.; Li, Y. Effect of Obstacle Location on Hydrogen Dispersion in a Hydrogen Fuel Cell Bus with Natural and Mechanical Ventilation. Process Saf. Environ. Prot. 2023, 171, 995–1008. [Google Scholar] [CrossRef]
- Qian, J.; Li, X.; Gao, Z.; Jin, Z. A Numerical Study of Hydrogen Leakage and Diffusion in a Hydrogen Refueling Station. Int. J. Hydrog. Energy 2020, 45, 14428–14439. [Google Scholar] [CrossRef]
- Li, Y.; Hou, X.; Wang, C.; Wang, Q.; Qi, W.; Li, J.; Zhang, X. Modeling and Analysis of Hydrogen Diffusion in an Enclosed Fuel Cell Vehicle with Obstacles. Int. J. Hydrog. Energy 2021, 47, 5745–5756. [Google Scholar] [CrossRef]
- Jiao, M.; Zhu, H.; Huang, J.; Zhang, X. Numerical Simulation of Hydrogen Leakage and Diffusion Process of Fuel Cell Vehicle. World Electr. Veh. J. 2021, 12, 193. [Google Scholar] [CrossRef]
- Xie, Y.; Lv, N.; Wang, X.; Wu, D.; Wang, S. Thermal and Fire Characteristics of Hydrogen Jet Flames in the Tunnel at Longitudinal Ventilation Strategies. Fuel 2021, 306, 121659. [Google Scholar] [CrossRef]
- Liu, S.; He, R. Optimization of Hydrogen Sensor Placement for Hydrogen Leakage Monitoring in the Fuel Cell Truck. J. Braz. Soc. Mech. Sci. Eng. 2023, 45, 193. [Google Scholar] [CrossRef]
- Lv, H.; Shen, Y.; Zheng, T.; Zhou, W.; Ming, P.; Zhang, C. Numerical Study of Hydrogen Leakage, Diffusion, and Combustion in an Outdoor Parking Space under Different Parking Configurations. Renew. Sustain. Energy Rev. 2023, 173, 113093. [Google Scholar] [CrossRef]
- Hao, D.; Wang, X.; Zhang, Y.; Wang, R.; Chen, G.; Li, J. Experimental Study on Hydrogen Leakage and Emission of Fuel Cell Vehicles in Confined Spaces. Automot. Innov. 2020, 3, 111–122. [Google Scholar] [CrossRef]
- Wang, X.; Yi, F.; Su, Q.; Zhou, J.; Sun, Y.; Guo, W.; Shu, X. Influence of Longitudinal Wind on Hydrogen Leakage and Hydrogen Concentration Sensor Layout of Fuel Cell Vehicles. Sustainability 2023, 15, 10712. [Google Scholar] [CrossRef]
- Tamura, Y.; Takeuchi, M.; Sato, K. Effectiveness of a Blower in Reducing the Hazard of Hydrogen Leaking from a Hydrogen-Fueled Vehicle. Int. J. Hydrog. Energy 2014, 39, 20339–20349. [Google Scholar] [CrossRef]
- Xiao, H.; Wang, Q.; He, X.; Sun, J.; Shen, X. Experimental Study on the Behaviors and Shape Changes of Premixed Hydrogen–Air Flames Propagating in Horizontal Duct. Int. J. Hydrog. Energy 2011, 36, 6325–6336. [Google Scholar] [CrossRef]
- Xiao, H.; Duan, Q.; Sun, J. Premixed Flame Propagation in Hydrogen Explosions. Renew. Sustain. Energy Rev. 2018, 81, 1988–2001. [Google Scholar] [CrossRef]
- Matalon, M. The Darrieus–Landau Instability of Premixed Flames. Fluid Dyn. Res. 2018, 50, 051412. [Google Scholar] [CrossRef]
- Kwon, O.C.; Faeth, G.M. Flame/Stretch Interactions of Premixed Hydrogen-Fueled Flames: Measurements and Predictions. Combust. Flame 2001, 124, 590–610. [Google Scholar] [CrossRef]
- Shen, T.; Li, M.; Xiao, H. Propagation of Premixed Hydrogen-Air Flame Initiated by a Planar Ignition in a Closed Tube. Int. J. Hydrog. Energy 2022, 47, 4903–4915. [Google Scholar] [CrossRef]
- Yang, L.; Chen, Z. Effects of Cryogenic Temperature on Premixed Hydrogen/Air Flame Propagation in a Closed Channel. Proc. Combust. Inst. 2023, 39, 2991–2999. [Google Scholar] [CrossRef]
- Sun, Z.-Y.; Li, G.-X. Propagation Characteristics of Laminar Spherical Flames within Homogeneous Hydrogen-Air Mixtures. Energy 2016, 116, 116–127. [Google Scholar] [CrossRef]
- Pareja, J.; Burbano, H.J.; Amell, A.; Carvajal, J. Laminar Burning Velocities and Flame Stability Analysis of Hydrogen/Air Premixed Flames at Low Pressure. Int. J. Hydrog. Energy 2011, 36, 6317–6324. [Google Scholar] [CrossRef]
- Dayma, G.; Halter, F.; Dagaut, P. New Insights into the Peculiar Behavior of Laminar Burning Velocities of Hydrogen–Air Flames According to Pressure and Equivalence Ratio. Combust. Flame 2014, 161, 2235–2241. [Google Scholar] [CrossRef]
- Hu, E.; Huang, Z.; He, J.; Miao, H. Experimental and Numerical Study on Laminar Burning Velocities and Flame Instabilities of Hydrogen–Air Mixtures at Elevated Pressures and Temperatures. Int. J. Hydrog. Energy 2009, 34, 8741–8755. [Google Scholar] [CrossRef]
- Huang, Z.; Zhang, Y.; Zeng, K.; Liu, B.; Wang, Q.; Jiang, D. Measurements of Laminar Burning Velocities for Natural Gas–Hydrogen–Air Mixtures. Combust. Flame 2006, 146, 302–311. [Google Scholar] [CrossRef]
- Di Sarli, V.; Benedetto, A.D. Laminar Burning Velocity of Hydrogen–Methane/Air Premixed Flames. Int. J. Hydrog. Energy 2007, 32, 637–646. [Google Scholar] [CrossRef]
- Miao, H.; Jiao, Q.; Huang, Z.; Jiang, D. Effect of Initial Pressure on Laminar Combustion Characteristics of Hydrogen Enriched Natural Gas. Int. J. Hydrog. Energy 2008, 33, 3876–3885. [Google Scholar] [CrossRef]
- Miao, H.; Ji, M.; Jiao, Q.; Huang, Q.; Huang, Z. Laminar Burning Velocity and Markstein Length of Nitrogen Diluted Natural Gas/Hydrogen/Air Mixtures at Normal, Reduced and Elevated Pressures. Int. J. Hydrog. Energy 2009, 34, 3145–3155. [Google Scholar] [CrossRef]
- Hu, E.; Huang, Z.; He, J.; Zheng, J.; Miao, H. Measurements of Laminar Burning Velocities and Onset of Cellular Instabilities of Methane–Hydrogen–Air Flames at Elevated Pressures and Temperatures. Int. J. Hydrog. Energy 2009, 34, 5574–5584. [Google Scholar] [CrossRef]
- Tang, C.; He, J.; Huang, Z.; Jin, C.; Wang, J.; Wang, X.; Miao, H. Measurements of Laminar Burning Velocities and Markstein Lengths of Propane–Hydrogen–Air Mixtures at Elevated Pressures and Temperatures. Int. J. Hydrog. Energy 2008, 33, 7274–7285. [Google Scholar] [CrossRef]
- Shen, X.; Xu, J.; Wen, J.X. Phenomenological Characteristics of Hydrogen/Air Premixed Flame Propagation in Closed Rectangular Channels. Renew. Energy 2021, 174, 606–615. [Google Scholar] [CrossRef]
- Du, Y.; Liu, Y.; Zhou, F.; Li, L.; Zhang, J.; Zhang, Z. Propagation and Intensity of Blast Wave from Hydrogen Pipe Rupture Due to Internal Detonation. J. Loss Prev. Process Ind. 2023, 81, 104933. [Google Scholar] [CrossRef]
- Yang, Z.; Wang, Z.; Cao, X.; Chen, B.; Fan, R.; Lu, Y. Influences of Concentration Gradients and Ignition Positions on Unconfined Inhomogeneous Hydrogen Explosion. Int. J. Hydrog. Energy 2024, 50, 857–869. [Google Scholar] [CrossRef]
- Wang, X.; Li, B.; Han, B.; Jin, X.; Zhang, D.; Bi, M. Explosion of High Pressure Hydrogen Tank in Fire: Mechanism, Criterion, and Consequence Assessment. J. Energy Storage 2023, 72, 108455. [Google Scholar] [CrossRef]
- Ilbas, M.; Crayford, A.; Yilmaz, I.; Bowen, P.; Syred, N. Laminar-Burning Velocities of Hydrogen–Air and Hydrogen–Methane–Air Mixtures: An Experimental Study. Int. J. Hydrog. Energy 2006, 31, 1768–1779. [Google Scholar] [CrossRef]
- Cheng, T.S.; Chang, Y.-C.; Chao, Y.-C.; Chen, G.-B.; Li, Y.-H.; Wu, C.-Y. An Experimental and Numerical Study on Characteristics of Laminar Premixed H2/CO/CH4/Air Flames. Int. J. Hydrog. Energy 2011, 36, 13207–13217. [Google Scholar] [CrossRef]
- Zheng, K.; Yu, M.; Zheng, L.; Wen, X.; Chu, T.; Wang, L. Experimental Study on Premixed Flame Propagation of Hydrogen/Methane/Air Deflagration in Closed Ducts. Int. J. Hydrog. Energy 2017, 42, 5426–5438. [Google Scholar] [CrossRef]
- Jia, J.; Chen, Y.; Che, G.; Zhu, J.; Wang, F.; Jia, P. Experimental Study on the Explosion Characteristics of Hydrogen-Methane Premixed Gas in Complex Pipe Networks. Sci. Rep. 2021, 11, 21204. [Google Scholar] [CrossRef]
- Wang, S.; Xiao, G.; Mi, H.; Feng, Y.; Chen, J. Experimental and Numerical Study on Flame Fusion Behavior of Premixed Hydrogen/Methane Explosion with Two-Channel Obstacles. Fuel 2023, 333, 126530. [Google Scholar] [CrossRef]
- Chen, J.; Jin, K.; Duan, Q.; Sun, J. Dynamics of Premixed Hydrogen-Air Flame Propagation in the Duct with Pellets Bed. Int. J. Hydrog. Energy 2021, 46, 15780–15792. [Google Scholar] [CrossRef]
- Sheng, Z.; Yang, G.; Li, S.; Shen, Q.; Sun, H.; Jiang, Z.; Liao, J.; Wang, H. Modeling of Turbulent Deflagration Behaviors of Premixed Hydrogen-Air in Closed Space with Obstacles. Process Saf. Environ. Prot. 2022, 161, 506–519. [Google Scholar] [CrossRef]
- Qiming, X.; Guohua, C.; Qiang, Z.; Shen, S. Numerical Simulation Study and Dimensional Analysis of Hydrogen Explosion Characteristics in a Closed Rectangular Duct with Obstacles. Int. J. Hydrog. Energy 2022, 47, 39288–39301. [Google Scholar] [CrossRef]
- Sheng, Z.; Yang, G.; Gao, W.; Li, S.; Shen, Q.; Sun, H. Study on the Dynamic Process of Premixed Hydrogen-Air Deflagration Flame Propagating in a Closed Space with Obstacles. Fuel 2023, 334, 126542. [Google Scholar] [CrossRef]
- Jeon, J.; Kim, S.J. Recent Progress in Hydrogen Flammability Prediction for the Safe Energy Systems. Energies 2020, 13, 6263. [Google Scholar] [CrossRef]
- Yang, X.; Yu, M.; Han, S.; Luo, Z. Experimental Study on the Premixed Syngas-Air Explosion in Duct with Both Ends Open. Int. J. Hydrog. Energy 2021, 46, 11004–11014. [Google Scholar] [CrossRef]
- Cao, W.; Li, W.; Zhang, L.; Chen, J.; Yu, S.; Zhou, Z.; Zhang, Y.; Shen, X.; Tan, Y. Flame Characteristics of Premixed H2-Air Mixtures Explosion Venting in a Spherical Container through a Duct. Int. J. Hydrog. Energy 2021, 46, 26693–26707. [Google Scholar] [CrossRef]
- Heidari, A.; Ferraris, S.; Wen, J.X.; Tam, V.H.Y. Numerical Simulation of Large Scale Hydrogen Detonation. Int. J. Hydrog. Energy 2011, 36, 2538–2544. [Google Scholar] [CrossRef]
- Machniewski, P.; Molga, E. CFD Analysis of Large-Scale Hydrogen Detonation and Blast Wave Overpressure in Partially Confined Spaces. Process Saf. Environ. Prot. 2022, 158, 537–546. [Google Scholar] [CrossRef]
- Liu, K.; He, C.; Yu, Y.; Guo, C.; Lin, S.; Jiang, J. A Study of Hydrogen Leak and Explosion in Different Regions of a Hydrogen Refueling Station. Int. J. Hydrog. Energy 2023, 48, 14112–14126. [Google Scholar] [CrossRef]
- Tanaka, T.; Azuma, T.; Evans, J.; Cronin, P.; Johnson, D.; Cleaver, R. Experimental Study on Hydrogen Explosions in a Full-Scale Hydrogen Filling Station Model. Int. J. Hydrog. Energy 2007, 32, 2162–2170. [Google Scholar] [CrossRef]
- Zhou, Y.; Li, Y.; Gao, W. Experimental Research on Unconfined Hydrogen Explosion of Different Gas Scale and the Overpressure Prediction Method. Int. J. Hydrog. Energy 2023, 48, 30985–30996. [Google Scholar] [CrossRef]
- Zhou, Y.; Li, Y.; Gao, W. The Analysis on the Overpressure Propagation Characteristics of Gas Cloud Explosion. Int. J. Hydrog. Energy 2024, 64, 212–219. [Google Scholar] [CrossRef]
- Li, Y.; Xie, H.; Bi, M.; Bo, Y.; Gao, W. Effects of Cloud Size and Built-in Obstacles on Hydrogen Cloud Explosion Using Large Eddy Simulation. J. Loss Prev. Process Ind. 2022, 77, 104788. [Google Scholar] [CrossRef]
- Zhou, Y.; Huang, L.; Li, Y.; Gao, W. The Prediction Model for Explosion Overpressure in Unconfined Hydrogen Cloud Explosion. J. Loss Prev. Process Ind. 2024, 88, 105254. [Google Scholar] [CrossRef]
- Li, Y.; Bi, M.; Zhou, Y.; Jiang, H.; Huang, L.; Zhang, K.; Gao, W. Experimental and Theoretical Evaluation of Hydrogen Cloud Explosion with Built-in Obstacles. Int. J. Hydrog. Energy 2020, 45, 28007–28018. [Google Scholar] [CrossRef]
- Gill, J.; Atkinson, G.; Cowpe, E.; Phylaktou, H.; Andrews, G. Experimental Investigation of Potential Confined Ignition Sources for Vapour Cloud Explosions. Process Saf. Environ. Prot. 2020, 135, 187–206. [Google Scholar] [CrossRef]
- Lowesmith, B.J.; Hankinson, G.; Johnson, D.M. Vapour Cloud Explosions in a Long Congested Region Involving Methane/Hydrogen Mixtures. Process Saf. Environ. Prot. 2011, 89, 234–247. [Google Scholar] [CrossRef]
- Li, Y.; Bi, M.; Huang, L.; Liu, Q.; Li, B.; Ma, D.; Gao, W. Hydrogen Cloud Explosion Evaluation under Inert Gas Atmosphere. Fuel Process. Technol. 2018, 180, 96–104. [Google Scholar] [CrossRef]
- Li, Y.; Zhou, Y.; Zhang, Z.; Shang, S.; Gao, W. Experimental and Theoretical Investigation on Hydrogen Cloud Explosion Subjected to External Turbulence. Int. J. Hydrog. Energy 2023, 48, 15331–15340. [Google Scholar] [CrossRef]
- Jiang, Y.; Li, Y.; Zhou, Y.; Jiang, H.; Zhang, K.; Gao, Z.; Gao, W. Investigation on Unconfined Hydrogen Cloud Explosion with External Turbulence. Int. J. Hydrog. Energy 2022, 47, 8658–8670. [Google Scholar] [CrossRef]
- Wan, H.; Wang, C.; Zhang, Q. Thermal Energy Propagation Characteristics and Hazard Effects of Hydrogen Cloud Explosion. Appl. Therm. Eng. 2024, 257, 124396. [Google Scholar] [CrossRef]
- Koo, W.-T.; Cho, H.-J.; Kim, D.-H.; Kim, Y.H.; Shin, H.; Penner, R.M.; Kim, I.-D. Chemiresistive Hydrogen Sensors: Fundamentals, Recent Advances, and Challenges. ACS Nano 2020, 14, 14284–14322. [Google Scholar] [CrossRef]
- Ramaiyan, K.; Tsui, L.; Brosha, E.L.; Kreller, C.; Stetter, J.R.; Russ, T.; Du, W.; Peaslee, D.; Hunter, G.; Xu, J.; et al. Recent Developments in Sensor Technologies for Enabling the Hydrogen Economy. ECS Sens. Plus 2023, 2, 045601. [Google Scholar] [CrossRef]
- Shah, V.; Bhaliya, J.; Patel, G.M.; Joshi, P. Recent Advancement in Pd-Decorated Nanostructures for Its Catalytic and Chemiresistive Gas Sensing Applications: A Review. Top. Catal. 2022. [Google Scholar] [CrossRef]
- Christofides, C.; Mandelis, A. Solid-State Sensors for Trace Hydrogen Gas Detection. J. Appl. Phys. 1990, 68, R1–R30. [Google Scholar] [CrossRef]
- Scharnagl, K.; Eriksson, M.; Karthigeyan, A.; Burgmair, M.; Zimmer, M.; Eisele, I. Hydrogen Detection at High Concentrations with Stabilised Palladium. Sens. Actuators B Chem. 2001, 78, 138–143. [Google Scholar] [CrossRef]
- Patton, J.F.; Hunter, S.R.; Sepaniak, M.J.; Daskos, P.G.; Smith, D.B. Rapid Response Microsensor for Hydrogen Detection Using Nanostructured Palladium Films. Sens. Actuators Phys. 2010, 163, 464–470. [Google Scholar] [CrossRef]
- Nugroho, F.A.A.; Darmadi, I.; Cusinato, L.; Susarrey-Arce, A.; Schreuders, H.; Bannenberg, L.J.; Da Silva Fanta, A.B.; Kadkhodazadeh, S.; Wagner, J.B.; Antosiewicz, T.J.; et al. Metal–Polymer Hybrid Nanomaterials for Plasmonic Ultrafast Hydrogen Detection. Nat. Mater. 2019, 18, 489–495. [Google Scholar] [CrossRef]
- Varghese, O.K.; Mor, G.K.; Grimes, C.A.; Paulose, M.; Mukherjeeb, N. A Titania Nanotube-Array Room-Temperature Sensor for Selective Detection of Hydrogen at Low Concentrations. J. Nanosci. Nanotechnol. 2004, 4, 733–737. [Google Scholar] [CrossRef]
- Suzuki, K.; Miyazaki, H.; Yuzuriha, Y.; Maru, Y.; Izu, N. Characterization of a Novel Gas Sensor Using Sintered Ceria Nanoparticles for Hydrogen Detection in Vacuum Conditions. Sens. Actuators B Chem. 2017, 250, 617–622. [Google Scholar] [CrossRef]
- Rumiche, F.; Wang, H.H.; Hu, W.S.; Indacochea, J.E.; Wang, M.L. Anodized Aluminum Oxide (AAO) Nanowell Sensors for Hydrogen Detection. Sens. Actuators B Chem. 2008, 134, 869–877. [Google Scholar] [CrossRef]
- Hoa, N.D.; An, S.Y.; Dung, N.Q.; Van Quy, N.; Kim, D. Synthesis of P-Type Semiconducting Cupric Oxide Thin Films and Their Application to Hydrogen Detection. Sens. Actuators B Chem. 2010, 146, 239–244. [Google Scholar] [CrossRef]
- Hazra, A.; Das, S.; Kanungo, J.; Sarkar, C.K.; Basu, S. Studies on a Resistive Gas Sensor Based on Sol–Gel Grown Nanocrystalline p-TiO2 Thin Film for Fast Hydrogen Detection. Sens. Actuators B Chem. 2013, 183, 87–95. [Google Scholar] [CrossRef]
- Thathsara, T.; Harrison, C.J.; Schönauer-Kamin, D.; Mansfeld, U.; Moos, R.; Malherbe, F.M.; Hocking, R.K.; Shafiei, M. Pd Nanoparticles Decorated Hollow TiO2 Nanospheres for Highly Sensitive and Selective UV-Assisted Hydrogen Gas Sensors. ACS Appl. Energy Mater. 2024, 7, 5608–5620. [Google Scholar] [CrossRef]
- Cai, Z.; Park, S. Synthesis of Pd Nanoparticle-Decorated SnO2 Nanowires and Determination of the Optimum Quantity of Pd Nanoparticles for Highly Sensitive and Selective Hydrogen Gas Sensor. Sens. Actuators B Chem. 2020, 322, 128651. [Google Scholar] [CrossRef]
- Meng, X.; Bi, M.; Xiao, Q.; Gao, W. Ultra-Fast Response and Highly Selectivity Hydrogen Gas Sensor Based on Pd/SnO2 Nanoparticles. Int. J. Hydrog. Energy 2022, 47, 3157–3169. [Google Scholar] [CrossRef]
- Kerketta, U.; Daniel, T.T.; Yadav, V.K.S.; Paily, R.P. Room Temperature Hydrogen Gas Sensing Using Zn2 TiO4 Thin Film. IEEE Sens. Lett. 2023, 7, 1–4. [Google Scholar] [CrossRef]
- Agarwal, S.; Kumar, S.; Agrawal, H.; Moinuddin, M.G.; Kumar, M.; Sharma, S.K.; Awasthi, K. An Efficient Hydrogen Gas Sensor Based on Hierarchical Ag/ZnO Hollow Microstructures. Sens. Actuators B Chem. 2021, 346, 130510. [Google Scholar] [CrossRef]
- Nguyen, T.T.D.; Dao, D.V.; Kim, D.-S.; Lee, H.-J.; Oh, S.-Y.; Lee, I.-H.; Yu, Y.-T. Effect of Core and Surface Area toward Hydrogen Gas Sensing Performance Using pd@ZnO Core-Shell Nanoparticles. J. Colloid Interface Sci. 2021, 587, 252–259. [Google Scholar] [CrossRef]
- Kim, J.-H.; Mirzaei, A.; Osada, M.; Kim, H.W.; Kim, S.S. Hydrogen Sensing Characteristics of Pd-Decorated Ultrathin ZnO Nanosheets. Sens. Actuators B Chem. 2021, 329, 129222. [Google Scholar] [CrossRef]
- Gottam, S.R.; Tsai, C.-T.; Li, C.-Y.; Chu, S.-Y. Investigation of MoS2 Nanosheet-Coated ZnO Thin Films for Hydrogen Gas Sensing Applications and the Effect of Temperature on the Enhanced Sensor Response. J. Electrochem. Soc. 2020, 167, 087507. [Google Scholar] [CrossRef]
- Huang, L.; Zhang, Z.; Li, Z.; Chen, B.; Ma, X.; Dong, L.; Peng, L.-M. Multifunctional Graphene Sensors for Magnetic and Hydrogen Detection. ACS Appl. Mater. Interfaces 2015, 7, 9581–9588. [Google Scholar] [CrossRef]
- Sayago, I.; Terrado, E.; Aleixandre, M.; Horrillo, M.C.; Fernández, M.J.; Lozano, J.; Lafuente, E.; Maser, W.K.; Benito, A.M.; Martinez, M.T.; et al. Novel Selective Sensors Based on Carbon Nanotube Films for Hydrogen Detection. Sens. Actuators B Chem. 2007, 122, 75–80. [Google Scholar] [CrossRef]
- Wong, Y.M.; Kang, W.P.; Davidson, J.L.; Wisitsora-at, A.; Soh, K.L. A Novel Microelectronic Gas Sensor Utilizing Carbon Nanotubes for Hydrogen Gas Detection. Sens. Actuators B Chem. 2003, 93, 327–332. [Google Scholar] [CrossRef]
- Hashtroudi, H.; Kumar, R.; Savu, R.; Moshkalev, S.; Kawamura, G.; Matsuda, A.; Shafiei, M. Hydrogen Gas Sensing Properties of Microwave-Assisted 2D Hybrid Pd/rGO: Effect of Temperature, Humidity and UV Illumination. Int. J. Hydrog. Energy 2021, 46, 7653–7665. [Google Scholar] [CrossRef]
- Chu, B.H.; Nicolosi, J.; Lo, C.F.; Strupinski, W.; Pearton, S.J.; Ren, F. Effect of Coated Platinum Thickness on Hydrogen Detection Sensitivity of Graphene-Based Sensors. Electrochem. Solid-State Lett. 2011, 14, K43. [Google Scholar] [CrossRef]
- Aysha Parveen, R.; Ajay Rakkesh, R.; Durgalakshmi, D.; Balakumar, S. Graphene-Ag2S Hybrid Nanostructures: A Hybrid Gas Sensor for Room Temperature Hydrogen Sensing Application. Mater. Lett. 2021, 303, 130470. [Google Scholar] [CrossRef]
- Villatoro, J.; Monzón-Hernández, D. Fast Detection of Hydrogen with Nano Fiber Tapers Coated with Ultra Thin Palladium Layers. Opt. Express 2005, 13, 5087. [Google Scholar] [CrossRef]
- Bévenot, X.; Trouillet, A.; Veillas, C.; Gagnaire, H.; Clément, M. Hydrogen Leak Detection Using an Optical Fibre Sensor for Aerospace Applications. Sens. Actuators B Chem. 2000, 67, 57–67. [Google Scholar] [CrossRef]
- Sumida, S.; Okazaki, S.; Asakura, S.; Nakagawa, H.; Murayama, H.; Hasegawa, T. Distributed Hydrogen Determination with Fiber-Optic Sensor. Sens. Actuators B Chem. 2005, 108, 508–514. [Google Scholar] [CrossRef]
- Slaman, M.; Dam, B.; Pasturel, M.; Borsa, D.M.; Schreuders, H.; Rector, J.H.; Griessen, R. Fiber Optic Hydrogen Detectors Containing Mg-Based Metal Hydrides. Sens. Actuators B Chem. 2007, 123, 538–545. [Google Scholar] [CrossRef]
- Slaman, M. Optimization of Mg-Based Fiber Optic Hydrogen Detectors by Alloying the Catalyst. Int. J. Hydrog. Energy 2008, 33, 1084–1089. [Google Scholar] [CrossRef]
- Wei, X.; Wei, T.; Xiao, H.; Lin, Y.S. Nano-Structured Pd-Long Period Fiber Gratings Integrated Optical Sensor for Hydrogen Detection. Sens. Actuators B Chem. 2008, 134, 687–693. [Google Scholar] [CrossRef]
- Ma, G.; Li, C.; Luo, Y.; Mu, R.; Wang, L. High Sensitive and Reliable Fiber Bragg Grating Hydrogen Sensor for Fault Detection of Power Transformer. Sens. Actuators B Chem. 2012, 169, 195–198. [Google Scholar] [CrossRef]
- Ma, G.-M.; Li, C.-R.; Mu, R.-D.; Jiang, J.; Luo, Y.-T. Fiber Bragg Grating Sensor for Hydrogen Detection in Power Transformers. IEEE Trans. Dielectr. Electr. Insul. 2014, 21, 380–385. [Google Scholar] [CrossRef]
- Ngene, P.; Radeva, T.; Slaman, M.; Westerwaal, R.J.; Schreuders, H.; Dam, B. Seeing Hydrogen in Colors: Low-cost and Highly Sensitive Eye Readable Hydrogen Detectors. Adv. Funct. Mater. 2014, 24, 2374–2382. [Google Scholar] [CrossRef]
- Cai, S.; Liu, F.; Wang, R.; Xiao, Y.; Li, K.; Caucheteur, C.; Guo, T. Narrow Bandwidth Fiber-Optic Spectral Combs for Renewable Hydrogen Detection. Sci. China Inf. Sci. 2020, 63, 222401. [Google Scholar] [CrossRef]
- Ma, Y.; Liang, T.; Qiao, S.; Liu, X.; Lang, Z. Highly Sensitive and Fast Hydrogen Detection Based on Light-Induced Thermoelastic Spectroscopy. Ultrafast Sci. 2023, 3, 0024. [Google Scholar] [CrossRef]
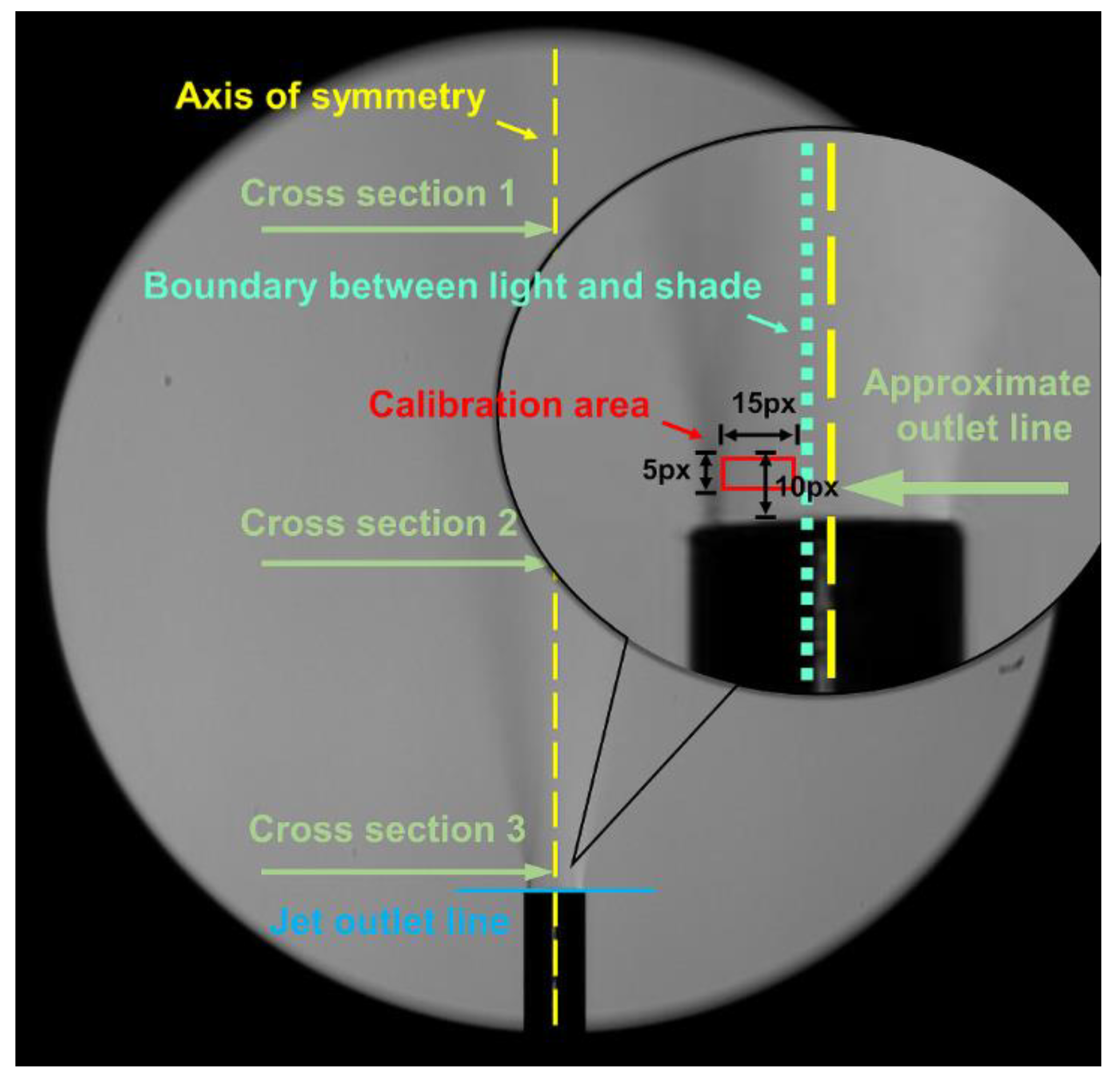
- Li, J.; Tian, Z.; Yang, Q.; Feng, L.; Su, H.; Lan, H. A New Visual Approach with the Concentration Calibration Method for the Hydrogen Leakage and Distribution Research. Fuel 2023, 346, 128132. [Google Scholar] [CrossRef]
- Zou, W.; Li, J.; Wan, X.; Jia, B. Investigation of Concentration Measurement for Hydrogen Leakage with a New Calibration Visual Approach. Int. J. Hydrog. Energy 2023, 48, 28235–28245. [Google Scholar] [CrossRef]
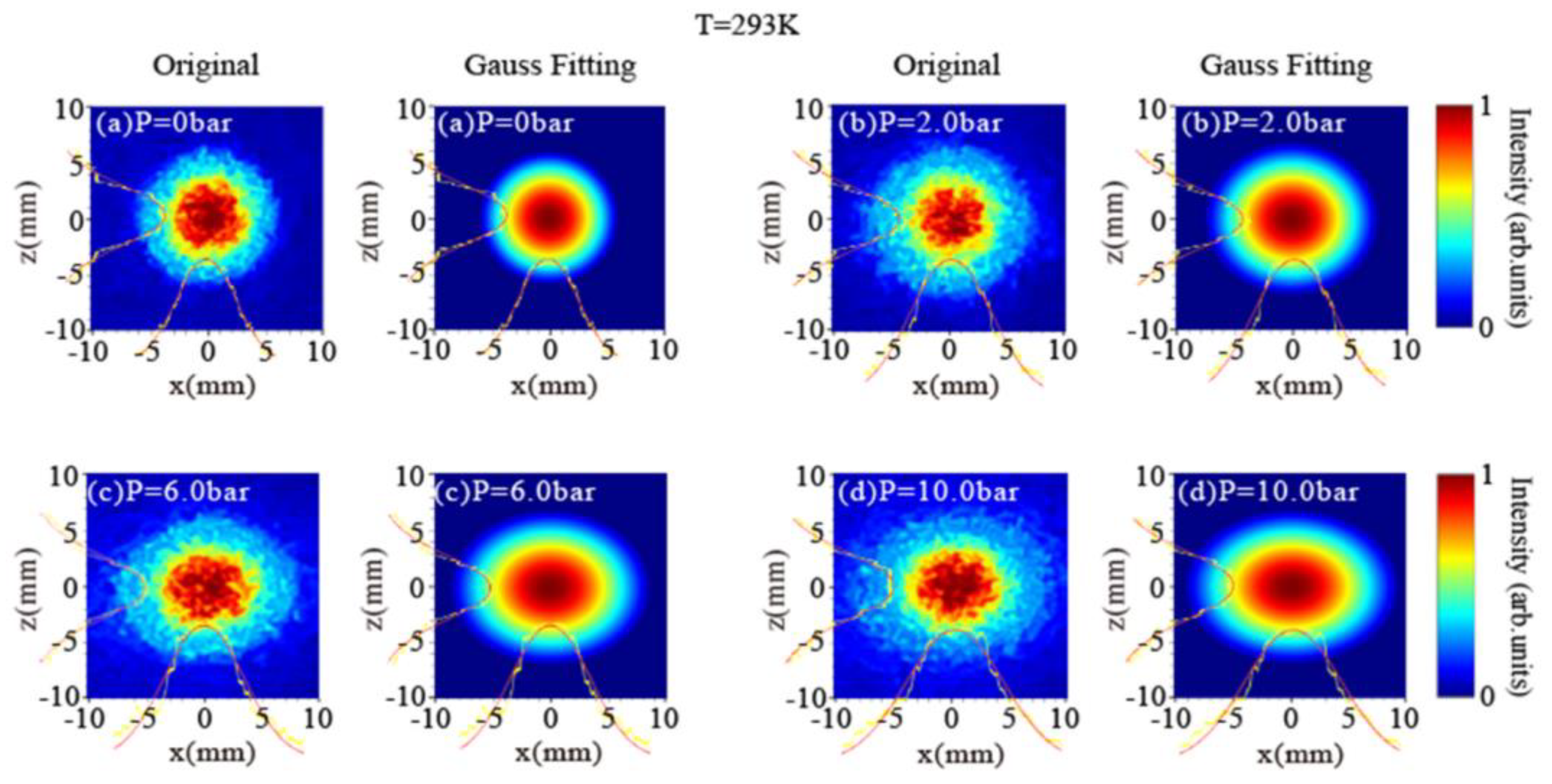
- Sun, L.; Jia, C.; Miao, Y. Visualization of Hydrogen Leak for Electro-Hydrogen Coupled System Based on Background Oriented Schlieren. Process Saf. Environ. Prot. 2023, 175, 437–446. [Google Scholar] [CrossRef]
- Miao, Y.; Jia, C.; Hua, Y.; Li, Y.; Tang, Q.; Xu, J.; Wu, D.; Zhang, X. Simultaneous Visualization of Density and Pressure in Hydrogen Leakage Based on Self-Imaging via Dual-Channel Interference. Process Saf. Environ. Prot. 2023, 177, 976–985. [Google Scholar] [CrossRef]
- Miao, Y.; Jia, C.; Zhang, X.; Li, Y.; Sun, L.; Yiao, L.; Huang, G.; Liu, H. Visualization of Hydrogen Jet Using Intensity Projection of the Laser Beam. Int. J. Hydrog. Energy 2023, 48, 34094–34104. [Google Scholar] [CrossRef]
- Cao, R. Simulation Study on Leakage and Diffusion of the Refrigerant in Ammonia Refrigeration Plant Room. Master’s Thesis, Harbin University of Commerce, Harbin, China, 2024. [Google Scholar]
- Shi, X. Diffusion Law of Ammonia Leakage in Finite Space. Master’s Thesis, College of Mechanical Engineering Zhejiang University of Technology, Hangzhou, China, 2018. [Google Scholar]
- Du, H. Study on Ammonia Leakage and Dispersion in Liquid Ammonia Refrigeration System Specific Space. Master’s Thesis, Tianjin University, Tianjin, China, 2018. [Google Scholar]
- Bouet, R.; Duplantier, S.; Salvi, O. Ammonia Large Scale Atmospheric Dispersion Experiments in Industrial Configurations. J. Loss Prev. Process Ind. 2005, 18, 512–519. [Google Scholar] [CrossRef]
- Chen, D.; Yang, L.; Ni, K. Research on Leakage and Diffusion Impact of Ammonia Leak Accident. Adv. Mater. Res. 2014, 926–930, 4298–4301. [Google Scholar] [CrossRef]
- Galeev, A.D.; Starovoytova, E.V.; Ponikarov, S.I. Numerical Simulation of the Consequences of Liquefied Ammonia Instantaneous Release Using FLUENT Software. Process Saf. Environ. Prot. 2013, 91, 191–201. [Google Scholar] [CrossRef]
- Tong, L.; Zhou, W.; Shi, X.; Chang, B.; Sun, M. Analysis of Ammonia Diffusion Process in Finite Space. China Special Equip. Saf. 2018, 34, 8–12. [Google Scholar] [CrossRef]
- Liu, D.; Wang, L.; Wang, T.; Liu, Y.; Chen, Y. Optimization of Ventilation and Alarm Setting during the Process of Ammonia Leak in Refrigeration Machinery Room Based on Numerical Simulation. J. Risk Anal. Crisis Response 2017, 7, 37. [Google Scholar] [CrossRef]
- Jia, L. Study on Leakage and Diffusion Law of Storage Tank in Ammonia Refrigeration Room. Master’s Thesis, Tianjin University of Commerce, Tianjin, China, 2021. [Google Scholar]
- Guan, W.; Wang, H.; Liu, L.; Li, J. Simulation of leakage and diffusion of liquid ammonia in tank area. J. Tianjin Univ. Technol. 2023, 39, 51–57. [Google Scholar] [CrossRef]
- Zhang, J. Study on Numerical Stimulation of Liquid Ammonia Leakage and Evacuation. Master’s Thesis, Tianjin University of Technology, Tianjin, China, 2017. [Google Scholar]
- Galeev, A.D.; Salin, A.A.; Ponikarov, S.I. Consequence Analysis of Aqueous Ammonia Spill Using Computational Fluid Dynamics. J. Loss Prev. Process Ind. 2013, 26, 628–638. [Google Scholar] [CrossRef]
- Jie, J.; Xiaoxia, C.; Xuefeng, H. Rapid Simulation and Visualization Analysis of Liquid Ammonia Tank Leakage Risk. Procedia Eng. 2014, 84, 682–688. [Google Scholar] [CrossRef]
- Tan, W.; Du, H.; Liu, L.; Su, T.; Liu, X. Experimental and Numerical Study of Ammonia Leakage and Dispersion in a Food Factory. J. Loss Prev. Process Ind. 2017, 47, 129–139. [Google Scholar] [CrossRef]
- Qi, H.; Wu, Y.; Wang, D.; Wang, Q.; Li, D. Analysis of Ammonia Leakage Diffusion Characteristics and Accident Consequence of Ammonia Transport Pipeline for Cold Storage. Environ. Protect. Sci. 2020, 46, 117–122. [Google Scholar] [CrossRef]
- Tong, L.; Zhou, W.; Shi, X.; Sun, W. Simulation analysis of ammonia leakage in confined space. Special Equip. Saf. Technol. 2017, 6, 23–25. [Google Scholar] [CrossRef]
- Wang, Z.; Men, H.; Liao, H.; Wang, D.; Guo, X.; Liu, Y.; Zhao, Z. Study on diffusion characteristics of leakage refrigerant in low pressure pipeline of ammonia refrigeration compressor. Chem. Eng. Equip. 2024, 1, 1–4. [Google Scholar] [CrossRef]
- Cheng, J.; Shen, J.; Jia, L.; Hu, K. Simulation Analysis of the Influence of Leakage Location and Flow Rate on the Leakage of Liquid Ammonia Tank. In Proceedings of the International Conference on Computational Modeling, Simulation, and Data Analysis (CMSDA 2021), Sanya, China, 3–5 December 2021; Cen, F., Bouras, C.J., Yaakob, R., Eds.; SPIE: Sanya, China, 2022; p. 88. [Google Scholar]
- Mao, S.; Zhang, X.; Wang, Y.; Shi, X. Study on leakage and diffusion characteristics of liquid ammonia horizontal storage tank in chemical park. Energy Chem. Ind. 2021, 42, 67–70. [Google Scholar] [CrossRef]
- Pang, B.; Liang, C.; Ma, L.; Luo, B. Numerical Simulation of Leakage and Diffusion of Liquid Ammonia Tank. In Proceedings of the 2014 Fourth International Conference on Instrumentation and Measurement, Computer, Communication and Control, Harbin, China, 18–20 September 2014; IEEE: Piscataway, NJ, USA, 2014; pp. 216–220. [Google Scholar]
- Lei, X.; Wang, J. Study on the influencing factors of ammonia diffusion in liquid ammonia storage tank leakage in chemical plant. Coal Chem. Ind. 2023, 51, 108–111. [Google Scholar] [CrossRef]
- Lhuillier, C.; Brequigny, P.; Lamoureux, N.; Contino, F.; Mounaïm-Rousselle, C. Experimental Investigation on Laminar Burning Velocities of Ammonia/Hydrogen/Air Mixtures at Elevated Temperatures. Fuel 2020, 263, 116653. [Google Scholar] [CrossRef]
- Ichikawa, A.; Hayakawa, A.; Kitagawa, Y.; Kunkuma Amila Somarathne, K.D.; Kudo, T.; Kobayashi, H. Laminar Burning Velocity and Markstein Length of Ammonia/Hydrogen/Air Premixed Flames at Elevated Pressures. Int. J. Hydrog. Energy 2015, 40, 9570–9578. [Google Scholar] [CrossRef]
- Wang, Y.; Zhou, X.; Liu, L. Theoretical Investigation of the Combustion Performance of Ammonia/Hydrogen Mixtures on a Marine Diesel Engine. Int. J. Hydrog. Energy 2021, 46, 14805–14812. [Google Scholar] [CrossRef]
- Wang, D.; Ji, C.; Wang, S.; Yang, J.; Wang, Z. Numerical Study of the Premixed Ammonia-Hydrogen Combustion under Engine-Relevant Conditions. Int. J. Hydrog. Energy 2021, 46, 2667–2683. [Google Scholar] [CrossRef]
- Cai, T.; Tang, A.; Li, C. Experimental and Kinetic Analyses on the Flame Dynamics and Stabilization of Ammonia/Hydrogen-Air Mixtures in a Micro-Planar Combustor. Chem. Eng. J. 2023, 477, 147038. [Google Scholar] [CrossRef]
- Zitouni, S.; Brequigny, P.; Mounaïm-Rousselle, C. Turbulent Flame Speed and Morphology of Pure Ammonia Flames and Blends with Methane or Hydrogen. Proc. Combust. Inst. 2023, 39, 2269–2278. [Google Scholar] [CrossRef]
- He, X.; Shu, B.; Nascimento, D.; Moshammer, K.; Costa, M.; Fernandes, R.X. Auto-Ignition Kinetics of Ammonia and Ammonia/Hydrogen Mixtures at Intermediate Temperatures and High Pressures. Combust. Flame 2019, 206, 189–200. [Google Scholar] [CrossRef]
- Chen, J.; Jiang, X.; Qin, X.; Huang, Z. Effect of Hydrogen Blending on the High Temperature Auto-Ignition of Ammonia at Elevated Pressure. Fuel 2021, 287, 119563. [Google Scholar] [CrossRef]
- Zhang, K.; Shang, S.; Li, X.; Gao, W. Lower Flammability Limits of NH3/H2 Mixtures under Different Initial Temperatures and Initial Pressures. Fuel 2023, 331, 125982. [Google Scholar] [CrossRef]
- Fernández-Tarrazo, E.; Gómez-Miguel, R.; Sánchez-Sanz, M. Minimum Ignition Energy of Hydrogen–Ammonia Blends in Air. Fuel 2023, 337, 127128. [Google Scholar] [CrossRef]
- Lhuillier, C.; Brequigny, P.; Contino, F.; Mounaïm-Rousselle, C. Experimental Study on Ammonia/Hydrogen/Air Combustion in Spark Ignition Engine Conditions. Fuel 2020, 269, 117448. [Google Scholar] [CrossRef]
- Lee, J.H.; Kim, J.H.; Park, J.H.; Kwon, O.C. Studies on Properties of Laminar Premixed Hydrogen-Added Ammonia/Air Flames for Hydrogen Production. Int. J. Hydrog. Energy 2010, 35, 1054–1064. [Google Scholar] [CrossRef]
- Cai, T.; Zhao, D.; Chan, S.H.; Shahsavari, M. Tailoring Reduced Mechanisms for Predicting Flame Propagation and Ignition Characteristics in Ammonia and Ammonia/Hydrogen Mixtures. Energy 2022, 260, 125090. [Google Scholar] [CrossRef]
- Gaucherand, J.; Laera, D.; Schulze-Netzer, C.; Poinsot, T. DNS of Turbulent Premixed Ammonia/Hydrogen Flames: The Impact of Thermo-Diffusive Effects. Flow Turbul. Combust. 2024, 112, 587–614. [Google Scholar] [CrossRef]
- Ichikawa, A.; Naito, Y.; Hayakawa, A.; Kudo, T.; Kobayashi, H. Burning Velocity and Flame Structure of CH4/NH3/Air Turbulent Premixed Flames at High Pressure. Int. J. Hydrog. Energy 2019, 44, 6991–6999. [Google Scholar] [CrossRef]
- Chen, X.; Liu, Q.; Zhao, W.; Li, R.; Zhang, Q.; Mou, Z. Experimental and Chemical Kinetic Study on the Flame Propagation Characteristics of Ammonia/Hydrogen/Air Mixtures. Fuel 2023, 334, 126509. [Google Scholar] [CrossRef]
- Mei, B.; Zhang, X.; Ma, S.; Cui, M.; Guo, H.; Cao, Z.; Li, Y. Experimental and Kinetic Modeling Investigation on the Laminar Flame Propagation of Ammonia under Oxygen Enrichment and Elevated Pressure Conditions. Combust. Flame 2019, 210, 236–246. [Google Scholar] [CrossRef]
- Liu, Q.; Chen, X.; Huang, J.; Shen, Y.; Zhang, Y.; Liu, Z. The Characteristics of Flame Propagation in Ammonia/Oxygen Mixtures. J. Hazard. Mater. 2019, 363, 187–196. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.; Huang, S.; Zhang, Z.; Li, T.; Yi, P.; Wu, D.; Chen, G. Laminar Burning Characteristics of Ammonia/Hydrogen/Air Mixtures with Laser Ignition. Int. J. Hydrog. Energy 2021, 46, 31879–31893. [Google Scholar] [CrossRef]
- Ji, C.; Wang, Z.; Wang, D.; Hou, R.; Zhang, T.; Wang, S. Experimental and Numerical Study on Premixed Partially Dissociated Ammonia Mixtures. Part I: Laminar Burning Velocity of NH3/H2/N2/Air Mixtures. Int. J. Hydrog. Energy 2022, 47, 4171–4184. [Google Scholar] [CrossRef]
- Li, Y.; Bi, M.; Li, B.; Gao, W. Explosion Behaviors of Ammonia–Air Mixtures. Combust. Sci. Technol. 2018, 190, 1804–1816. [Google Scholar] [CrossRef]
- Hayakawa, A.; Goto, T.; Mimoto, R.; Arakawa, Y.; Kudo, T.; Kobayashi, H. Laminar Burning Velocity and Markstein Length of Ammonia/Air Premixed Flames at Various Pressures. Fuel 2015, 159, 98–106. [Google Scholar] [CrossRef]
- Okafor, E.C.; Naito, Y.; Colson, S.; Ichikawa, A.; Kudo, T.; Hayakawa, A.; Kobayashi, H. Experimental and Numerical Study of the Laminar Burning Velocity of CH4–NH3–Air Premixed Flames. Combust. Flame 2018, 187, 185–198. [Google Scholar] [CrossRef]
- Xiao, H.; Chen, A.; Zhang, M.; Guo, Y.; Ying, W. Using Ammonia as Future Energy: Modelling of Reaction Mechanism for Ammonia/Hydrogen Blends. J. Phys. Conf. Ser. 2022, 2361, 12012. [Google Scholar] [CrossRef]
- Hu, X.; Luo, C.; Chen, X.; Liu, Q.; Su, M. Study on Flame Propagation and Inherent Instability of Hydrogen/Ammonia/Air Mixture. Fuel 2024, 357, 129848. [Google Scholar] [CrossRef]
- Jing, Q.; Huang, J.; Liu, Q.; Wang, D.; Chen, X.; Wang, Z.; Liu, C. The Flame Propagation Characteristics and Detonation Parameters of Ammonia/Oxygen in a Large-Scale Horizontal Tube: As a Carbon-Free Fuel and Hydrogen-Energy Carrier. Int. J. Hydrog. Energy 2021, 46, 19158–19170. [Google Scholar] [CrossRef]
- Sun, Z.; Huang, Y.; Luan, Z.; Gao, S.; You, Y. Three-Dimensional Simulation of a Rotating Detonation Engine in Ammonia/Hydrogen Mixtures and Oxygen-Enriched Air. Int. J. Hydrog. Energy 2023, 48, 4891–4905. [Google Scholar] [CrossRef]
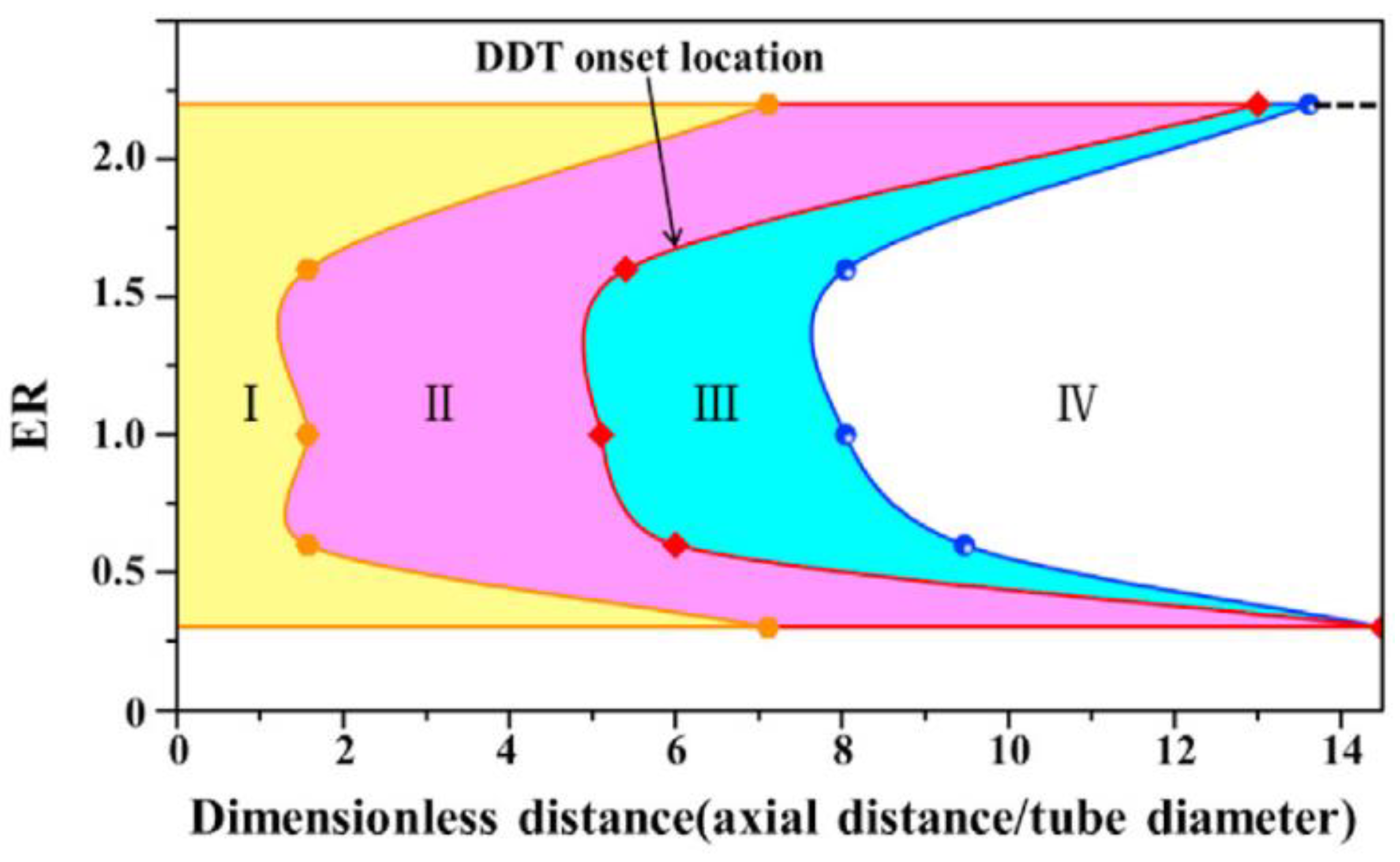
- Zhu, R.; Zhao, M.; Zhang, H. Numerical Simulation of Flame Acceleration and Deflagration-to-Detonation Transition in Ammonia-Hydrogen–Oxygen Mixtures. Int. J. Hydrog. Energy 2021, 46, 1273–1287. [Google Scholar] [CrossRef]
- Mukundakumar, N.; Bastiaans, R. DNS Study of Spherically Expanding Premixed Turbulent Ammonia-Hydrogen Flame Kernels, Effect of Equivalence Ratio and Hydrogen Content. Energies 2022, 15, 4749. [Google Scholar] [CrossRef]
- Zhu, R.; Fang, X.; Xu, C.; Zhao, M.; Zhang, H.; Davy, M. Pulsating One-Dimensional Detonation in Ammonia-Hydrogen–Air Mixtures. Int. J. Hydrog. Energy 2022, 47, 21517–21536. [Google Scholar] [CrossRef]









Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Z.; Zhang, Z.; Zhou, Y.; Ouyang, Y.; Sun, J.; Zhang, J.; Li, B.; Zhang, D.; Wang, Y.; Yao, J.; et al. Review of the Diffusion Process, Explosion Mechanism, and Detection Technology of Hydrogen and Ammonia. Energies 2025, 18, 2526. https://doi.org/10.3390/en18102526
Zhang Z, Zhang Z, Zhou Y, Ouyang Y, Sun J, Zhang J, Li B, Zhang D, Wang Y, Yao J, et al. Review of the Diffusion Process, Explosion Mechanism, and Detection Technology of Hydrogen and Ammonia. Energies. 2025; 18(10):2526. https://doi.org/10.3390/en18102526
Chicago/Turabian StyleZhang, Zilong, Zhaotong Zhang, Yuqi Zhou, Yujie Ouyang, Jiangtao Sun, Jing Zhang, Bin Li, Dan Zhang, Yongxu Wang, Jian Yao, and et al. 2025. "Review of the Diffusion Process, Explosion Mechanism, and Detection Technology of Hydrogen and Ammonia" Energies 18, no. 10: 2526. https://doi.org/10.3390/en18102526
APA StyleZhang, Z., Zhang, Z., Zhou, Y., Ouyang, Y., Sun, J., Zhang, J., Li, B., Zhang, D., Wang, Y., Yao, J., Xing, H., & Xie, L. (2025). Review of the Diffusion Process, Explosion Mechanism, and Detection Technology of Hydrogen and Ammonia. Energies, 18(10), 2526. https://doi.org/10.3390/en18102526





