Study of CaSrFe0.75Co0.75Mn0.5O6-δ as an Anode in Li-Ion Battery
Abstract
1. Introduction
2. Experimental
Electrochemical Characterization of CaSr0.75Mn0.5Co0.75O6-δ
3. Results and Discussion
3.1. Crystal Structure
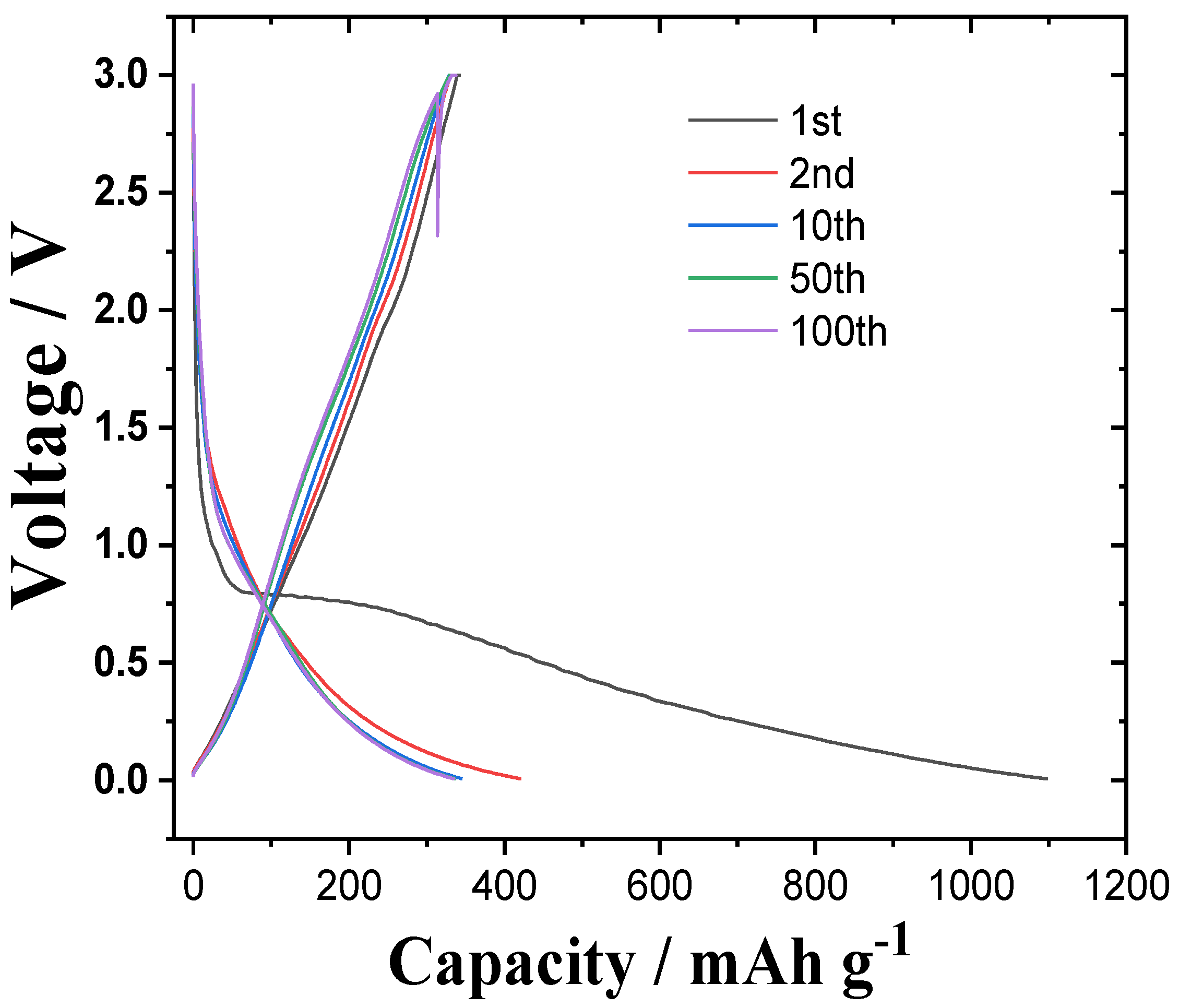
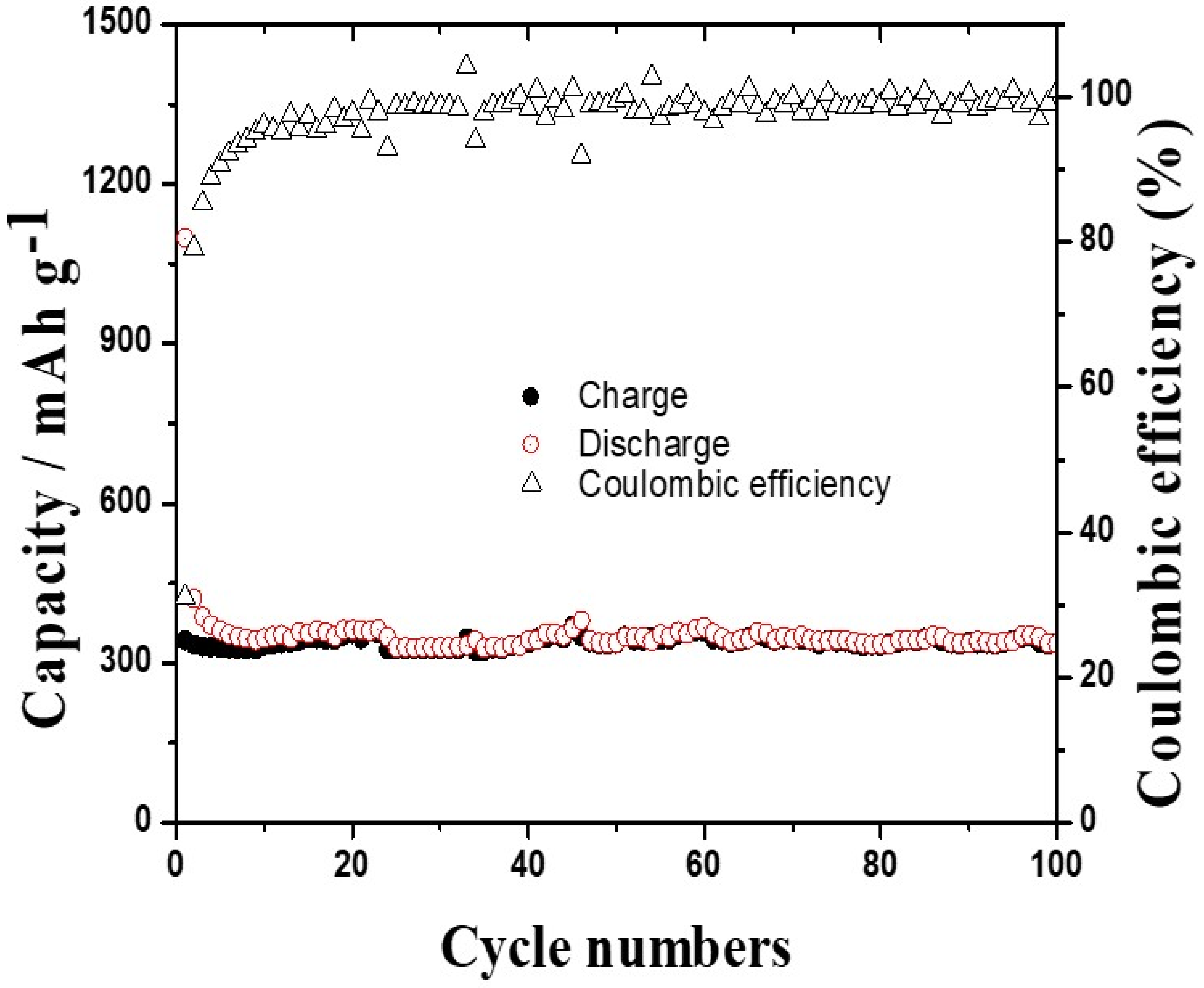
3.2. Anode Properties
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Winter, M.; Besenhard, J.O.; Spahr, M.E.; Novák, P. Insertion Electrode Materials for Rechargeable Lithium Batteries. Adv. Mater. 1998, 10, 725–763. [Google Scholar] [CrossRef]
- Sharma, N.; Shaju, K.M.; Subba Rao, G.V.; Chowdari, B.V.R. Mixed oxides Ca2Fe2O5 and Ca2Co2O5 as anode materials for Li-ion batteries. Electrochim. Acta 2004, 49, 1035–1043. [Google Scholar] [CrossRef]
- Sharma, N.; Shaju, K.M.; Subba Rao, G.V.; Chowdari, B.V.R. Sol–gel derived nano-crystalline CaSnO3 as high capacity anode material for Li-ion batteries. Electrochem. Commun. 2002, 4, 947–952. [Google Scholar] [CrossRef]
- Aurbach, D.; Nimberger, A.; Markovsky, B.; Levi, E.; Sominski, E.; Gedanken, A. Nanoparticles of SnO Produced by Sonochemistry as Anode Materials for Rechargeable Lithium Batteries. Chem. Mater. 2002, 14, 4155–4163. [Google Scholar] [CrossRef]
- Akram, M.Z.; Thapa, A.K.; Ajayi, B.P.; Atla, V.; Gong, J.R.; Sunkara, M. A new nanowire-based lithium hexaoxotungstate anode for lithium-ion batteries. Nanoscale Adv. 2019, 1, 2727–2731. [Google Scholar] [CrossRef]
- Reddy, M.V.; Subba Rao, G.V.; Chowdari, B.V.R. Metal Oxides and Oxysalts as Anode Materials for Li Ion Batteries. Chem. Rev. 2013, 113, 5364–5457. [Google Scholar]
- Flandrois, S.; Simon, B. Carbon materials for lithium-ion rechargeable batteries. Carbon 1999, 37, 165–180. [Google Scholar] [CrossRef]
- Fu, R.; Zhou, X.; Fan, H.; Blaisdell, D.; Jagadale, A.; Zhang, X.; Xiong, R. Comparison of Lithium-Ion Anode Materials Using an Experimentally Verified Physics-Based Electrochemical Model. Energies 2017, 10, 2174. [Google Scholar] [CrossRef]
- Winter, M.; Besenhard, J.O. Electrochemical lithiation of tin and tin-based intermetallics and composites. Electrochim. Acta 1999, 45, 31–50. [Google Scholar] [CrossRef]
- Mishra, A.; Mehta, A.; Basu, S.; Malode, S.J.; Shetti, N.P.; Shukla, S.S.; Nadagouda, M.N.; Aminabhavi, T.M. Electrode materials for lithium-ion batteries. Mater. Sci. Energy Technol. 2018, 1, 182–187. [Google Scholar] [CrossRef]
- Courtney, I.A.; McKinnon, W.R.; Dahn, J.R. On the Aggregation of Tin in SnO Composite Glasses Caused by the Reversible Reaction with Lithium. J. Electrochem. Soc. 1999, 146, 59. [Google Scholar] [CrossRef]
- Mohamedi, M.; Lee, S.-J.; Takahashi, D.; Nishizawa, M.; Itoh, T.; Uchida, I. Amorphous tin oxide films: Preparation and characterization as an anode active material for lithium ion batteries. Electrochi. Acta 2001, 46, 1161–1168. [Google Scholar] [CrossRef]
- Behm, M.; Irvine, J.T.S. Influence of structure and composition upon performance of tin phosphate based negative electrodes for lithium batteries. Electrochim. Acta 2002, 47, 1727–1738. [Google Scholar] [CrossRef]
- Li, D.; Hui, J.; Liu, S.; Ni, C.; Ni, J. Role of a Topotactic Electrochemical Reaction in a Perovskite-Type Anode for Lithium-Ion Batteries. ChemElectroChem 2017, 4, 2474–2479. [Google Scholar] [CrossRef]
- Yabuuchi, N.; Takeuchi, M.; Nakayama, M.; Shiiba, H.; Ogawa, M.; Nakayama, K.; Ohta, T.; Endo, D.; Ozaki, T.; Inamasu, T.; et al. High-capacity electrode materials for rechargeable lithium batteries: Li3NbO4-based system with cation-disordered rocksalt structure. Proc. Natl. Acad. Sci. USA 2015, 112, 7650–7655. [Google Scholar] [CrossRef]
- Sharma, Y.; Sharma, N.; Subba Rao, G.V.; Chowdari, B.V.R. Nanophase ZnCo2O4 as a High Performance Anode Material for Li-Ion Batteries. Adv. Funct. Mater. 2007, 17, 2855–2861. [Google Scholar] [CrossRef]
- Kim, S.-W.; Lee, H.-W.; Muralidharan, P.; Seo, D.-H.; Yoon, W.-S.; Kim, D.K.; Kang, K. Electrochemical performance and ex situ analysis of ZnMn2O4 nanowires as anode materials for lithium rechargeable batteries. Nano Res. 2011, 4, 505–510. [Google Scholar] [CrossRef]
- Hona, R.K.; Thapa, A.K.; Ramezanipour, F. An Anode Material for Lithium-Ion Batteries Based on Oxygen-Deficient Perovskite Sr2Fe2O6−δ. ChemistrySelect 2020, 5, 5706–5711. [Google Scholar] [CrossRef]
- Hona, R.K.; Huq, A.; Mulmi, S.; Ramezanipour, F. Transformation of Structure, Electrical Conductivity, and Magnetism in AA′Fe2O6−δ, A = Sr, Ca and A′ = Sr. Inorg. Chem. 2017, 56, 9716–9724. [Google Scholar] [CrossRef]
- Hona, R.K.; Karki, S.B.; Cao, T.; Mishra, R.; Sterbinsky, G.E.; Ramezanipour, F. Sustainable Oxide Electrocatalyst for Hydrogen- and Oxygen-Evolution Reactions. ACS Catal. 2021, 11, 14605–14614. [Google Scholar] [CrossRef]
- Sengodan, S.; Choi, S.; Jun, A.; Shin, T.H.; Ju, Y.-W.; Jeong, H.Y.; Shin, J.; Irvine, J.T.S.; Kim, G. Layered oxygen-deficient double perovskite as an efficient and stable anode for direct hydrocarbon solid oxide fuel cells. Nat. Mater. 2015, 14, 205–209. [Google Scholar] [CrossRef]
- Larson, A.C.; Dreele, R.B.V. General Structure Analysis System (GSAS). In Los Alamos National Laboratory Report LAUR; Los Alamos National Laboratory: Los Alamos, NM, USA, 1994; pp. 86–748. [Google Scholar]
- Toby, B. EXPGUI, a graphical user interface for GSAS. J. Appl. Crystallogr. 2001, 34, 210–213. [Google Scholar] [CrossRef]
- Hona, R.K.; Karki, S.B.; Ramezanipour, F. Oxide Electrocatalysts Based on Earth-Abundant Metals for Both Hydrogen- and Oxygen-Evolution Reactions. ACS Sustain. Chem. Eng. 2020, 8, 11549–11557. [Google Scholar] [CrossRef]
- Ghaffari, M.; Liu, T.; Huang, H.; Tan, O.K.; Shannon, M. Investigation of local structure effect and X-ray absorption characteristics (EXAFS) of Fe (Ti) K-edge on photocatalyst properties of SrTi(1−x)FexO(3−δ). Mater. Chem. Phys. 2012, 136, 347–357. [Google Scholar] [CrossRef]
- Davison, N.; McWhinnie, W.R.; Hooper, A. X-Ray Photoelectron Spectroscopic Study of Cobalt(II) and Nickel(II) Sorbed on Hectorite and Montmorillonite. Clays Clay Miner. 1991, 39, 22–27. [Google Scholar] [CrossRef]
- Dupin, J.C.; Gonbeau, D.; Benqlilou-Moudden, H.; Vinatier, P.; Levasseur, A. XPS analysis of new lithium cobalt oxide thin-films before and after lithium deintercalation. Thin Solid Films 2001, 384, 23–32. [Google Scholar] [CrossRef]
- Jo, K.; Lee, S.; Lee, H. Oxygen-Bonding State and Oxygen-Reduction Reaction Mechanism of Pr0.7Ca0.3Mn1−xCoxO3−d (x = 0, 0.1, 0.2, 0.3). Ceramics 2023, 6, 2386–2393. [Google Scholar] [CrossRef]
- Carreon, M.L.; Thapa, A.K.; Jasinski, J.B.; Sunkara, M.K. The Capacity and Durability of Amorphous Silicon Nanotube Thin Film Anode for Lithium Ion Battery Applications. ECS Electrochem. Lett. 2015, 4, A124. [Google Scholar] [CrossRef][Green Version]
- Lin, Y.-M.; Abel, P.R.; Heller, A.; Mullins, C.B. α-Fe2O3 Nanorods as Anode Material for Lithium Ion Batteries. J. Phys. Chem. Lett. 2011, 2, 2885–2891. [Google Scholar] [CrossRef]
- Chen, X.; Bi, Q.; Sajjad, M.; Wang, X.; Ren, Y.; Zhou, X.; Xu, W.; Liu, Z. One-Dimensional Porous Silicon Nanowires with Large Surface Area for Fast Charge–Discharge Lithium-Ion Batteries. Nanomaterials 2018, 8, 285. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, B.; Zhou, J.; Xia, R.; Chu, Y.; Huang, J. Preparation of Advanced CuO Nanowires/Functionalized Graphene Composite Anode Material for Lithium Ion Batteries. Materials 2017, 10, 72. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Zhang, X.; Tian, G.; Zhang, Q.; Knapp, M.; Ehrenberg, H.; Chen, G.; Shen, Z.; Yang, G.; Gu, L.; et al. Lithium lanthanum titanate perovskite as an anode for lithium ion batteries. Nature Commun. 2020, 11, 3490. [Google Scholar] [CrossRef] [PubMed]
- Oli, N.; Sapkota, N.; Weiner, B.R.; Morell, G.; Katiyar, R.S. Unveiling BaTiO3-SrTiO3 as Anodes for Highly Efficient and Stable Lithium-Ion Batteries. Nanomaterials 2024, 14, 1723. [Google Scholar] [CrossRef] [PubMed]

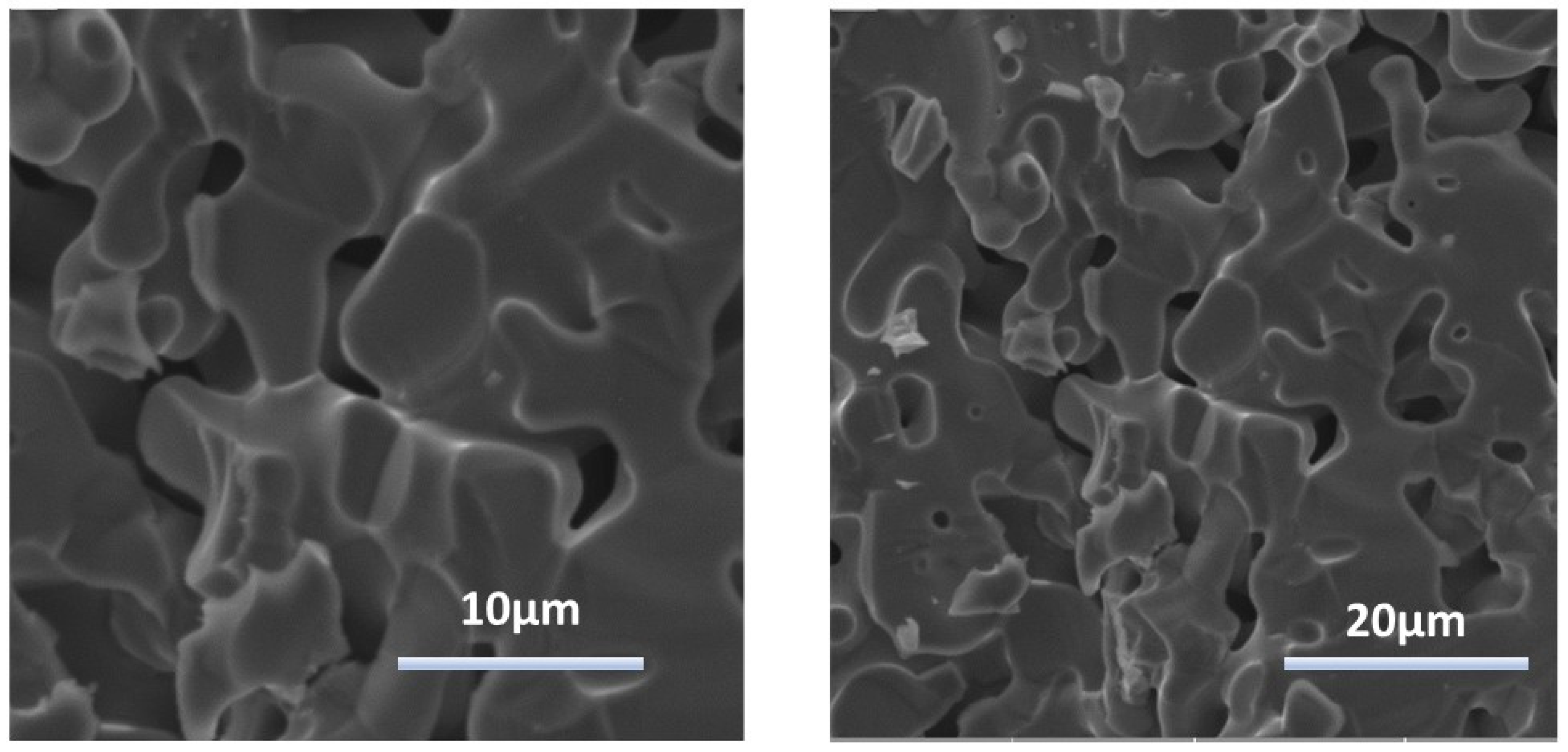
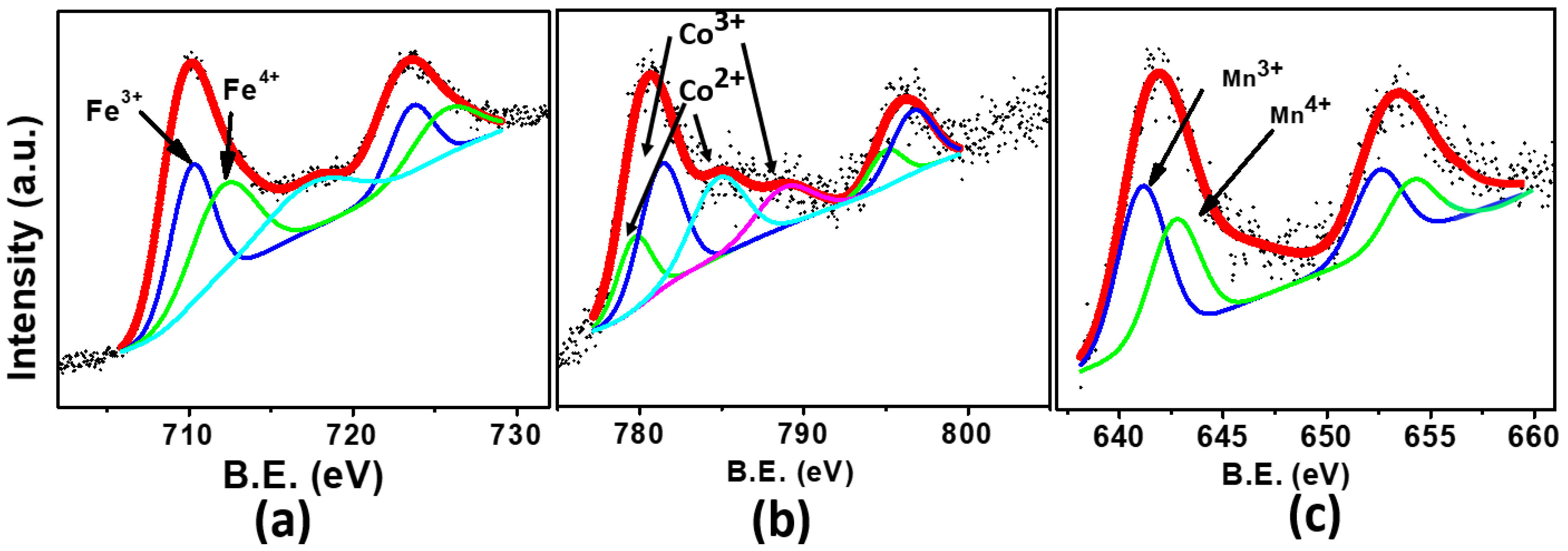


| Elements | x | y | z | Occupancy | Uiso | Multiplicity |
|---|---|---|---|---|---|---|
| Ca/Sr | 0.5 | 0.5 | 0.5 | 0.5/0.5 | 0.0132 (8) | 1 |
| Fe/Co/Mn | 0.0 | 0.0 | 0.25 | 0.375/0.375/0.250 | 0.0295(5) | 1 |
| O | 0.5 | 0 | 0 | 0.91 | 0.0487(5) | 3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Thapa, A.K.; Fogel, A.; Hona, R.K. Study of CaSrFe0.75Co0.75Mn0.5O6-δ as an Anode in Li-Ion Battery. Energies 2025, 18, 2508. https://doi.org/10.3390/en18102508
Thapa AK, Fogel A, Hona RK. Study of CaSrFe0.75Co0.75Mn0.5O6-δ as an Anode in Li-Ion Battery. Energies. 2025; 18(10):2508. https://doi.org/10.3390/en18102508
Chicago/Turabian StyleThapa, Arjun Kumar, Ariella Fogel, and Ram Krishna Hona. 2025. "Study of CaSrFe0.75Co0.75Mn0.5O6-δ as an Anode in Li-Ion Battery" Energies 18, no. 10: 2508. https://doi.org/10.3390/en18102508
APA StyleThapa, A. K., Fogel, A., & Hona, R. K. (2025). Study of CaSrFe0.75Co0.75Mn0.5O6-δ as an Anode in Li-Ion Battery. Energies, 18(10), 2508. https://doi.org/10.3390/en18102508







