Review of Electrochemical Systems for Grid Scale Power Generation and Conversion: Low- and High-Temperature Fuel Cells and Electrolysis Processes
Abstract
1. Introduction
Contributions of This Review Paper
2. Thermodynamics and Electrical Parameters of High-Temperature Solid Electrolyte Electrochemical Systems
2.1. Reversible Efficiency of an Electrochemical Energy Conversion System
2.2. Efficiency Degrading Issues
2.2.1. Electrocatalytic Activation Losses
- = activation potential (V);
- = universal gas constant (8.314 J/mol · K);
- = absolute temperature (K);
- = charge transfer coefficient;
- = number of electrons involved in the reaction;
- = Faraday’s constant (96,487 C/mol);
- = current density (A/cm2);
- = exchange current density (A/cm2).
2.2.2. Ohmic (IR) Losses
- = ohmic overpotential;
- = current density (A/cm2);
- = total ohmic resistance (Ω·cm2).
2.2.3. Mass Transport Losses
- = concentration overpotential (V);
- = universal gas constant (8.314 J/mol·K);
- = absolute temperature (K);
- = number of electrons involved in the reaction;
- = Faraday’s constant (96,487 C/mol);
- = bulk concentration of reactants;
- = concentration of reactants at the electrode surface.
2.2.4. Overall Fuel Cell Voltage
- = cell voltage;
- = reversible open circuit voltage at the Temperature used;
- = total activation overpotential;
- = total ohmic overpotential;
- = total concentration overpotential.
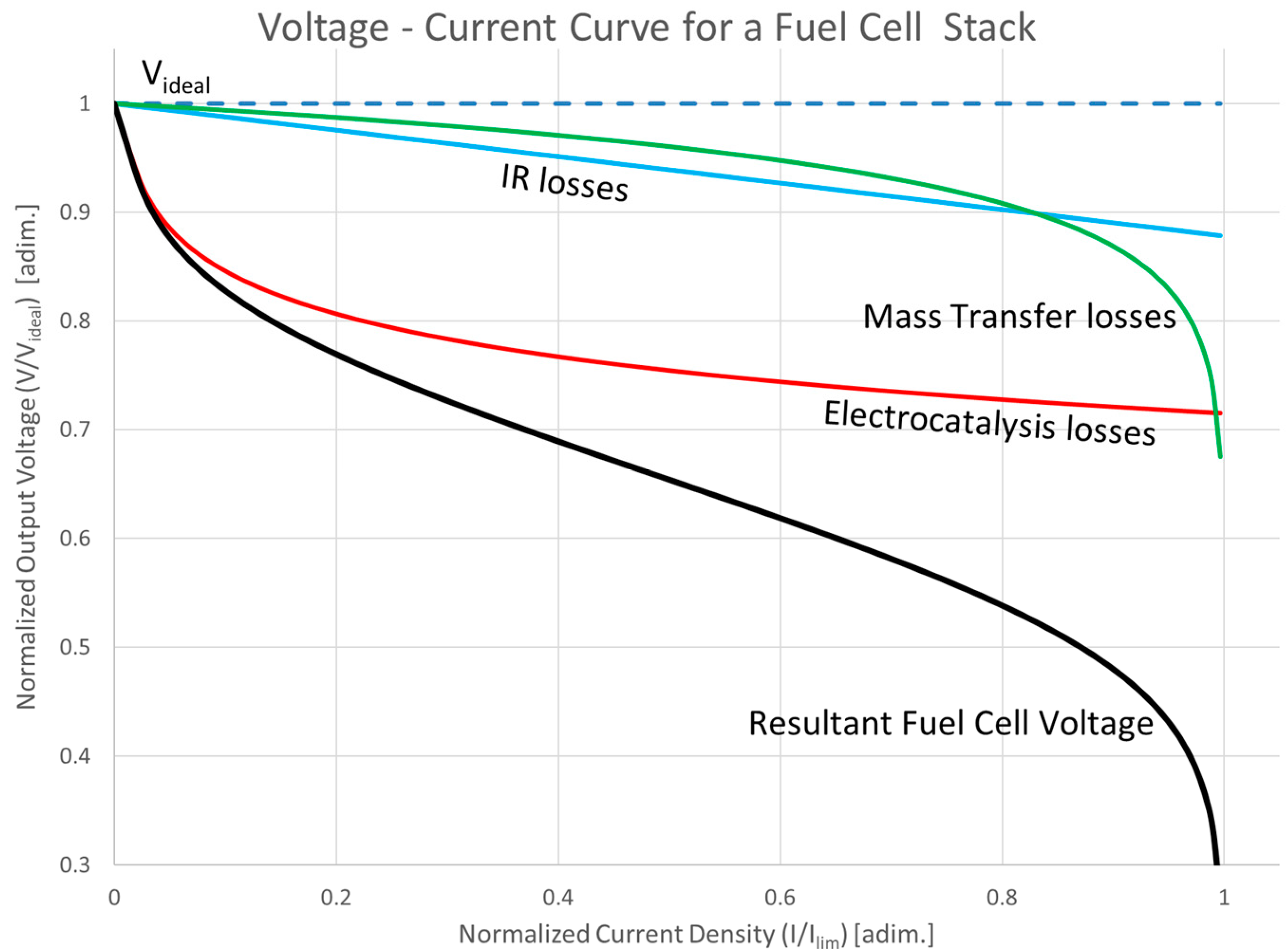
2.2.5. Fuel Utilization Ratio
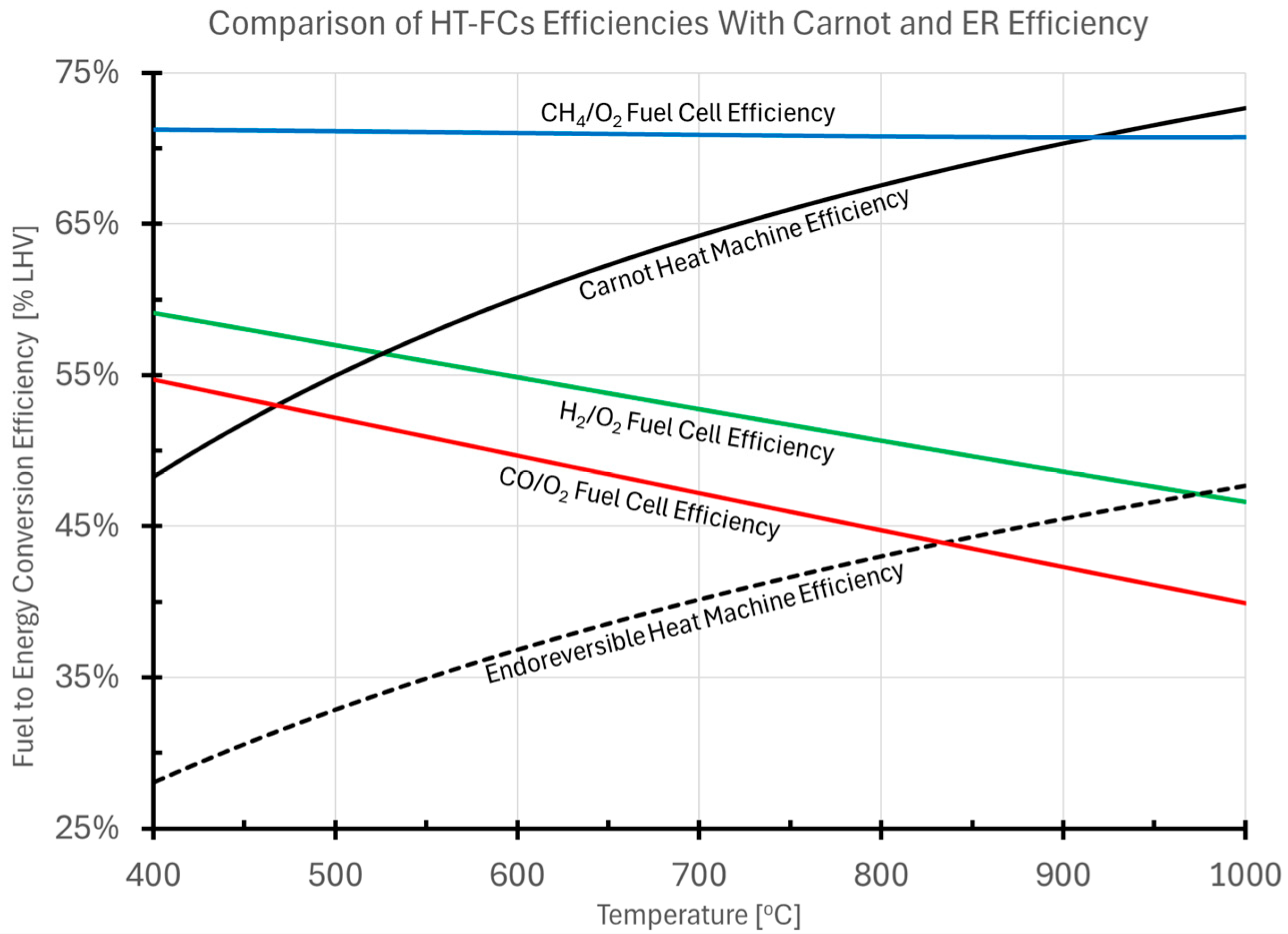
2.3. Fuel Cell Efficiency Compared to That of Heat Machines
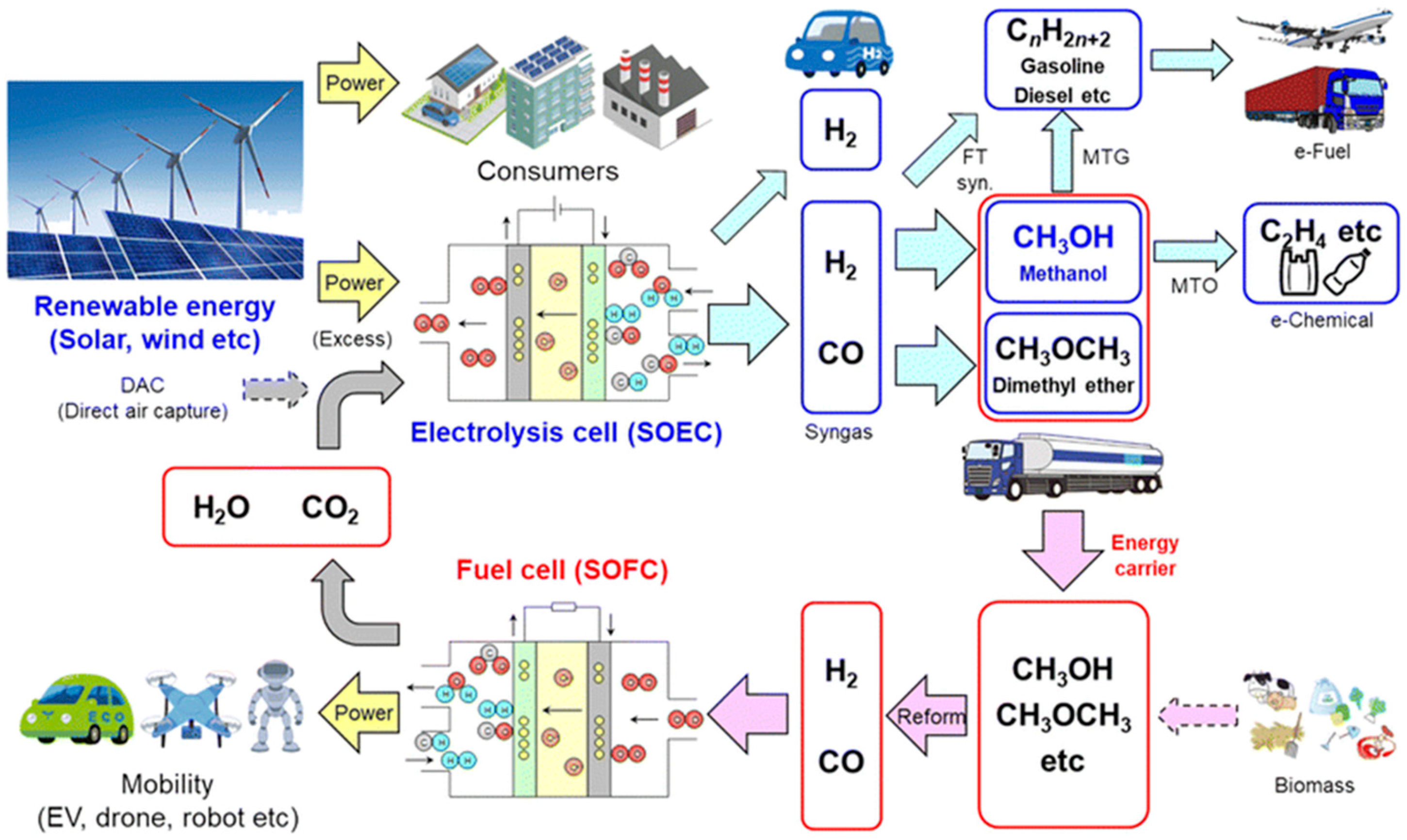
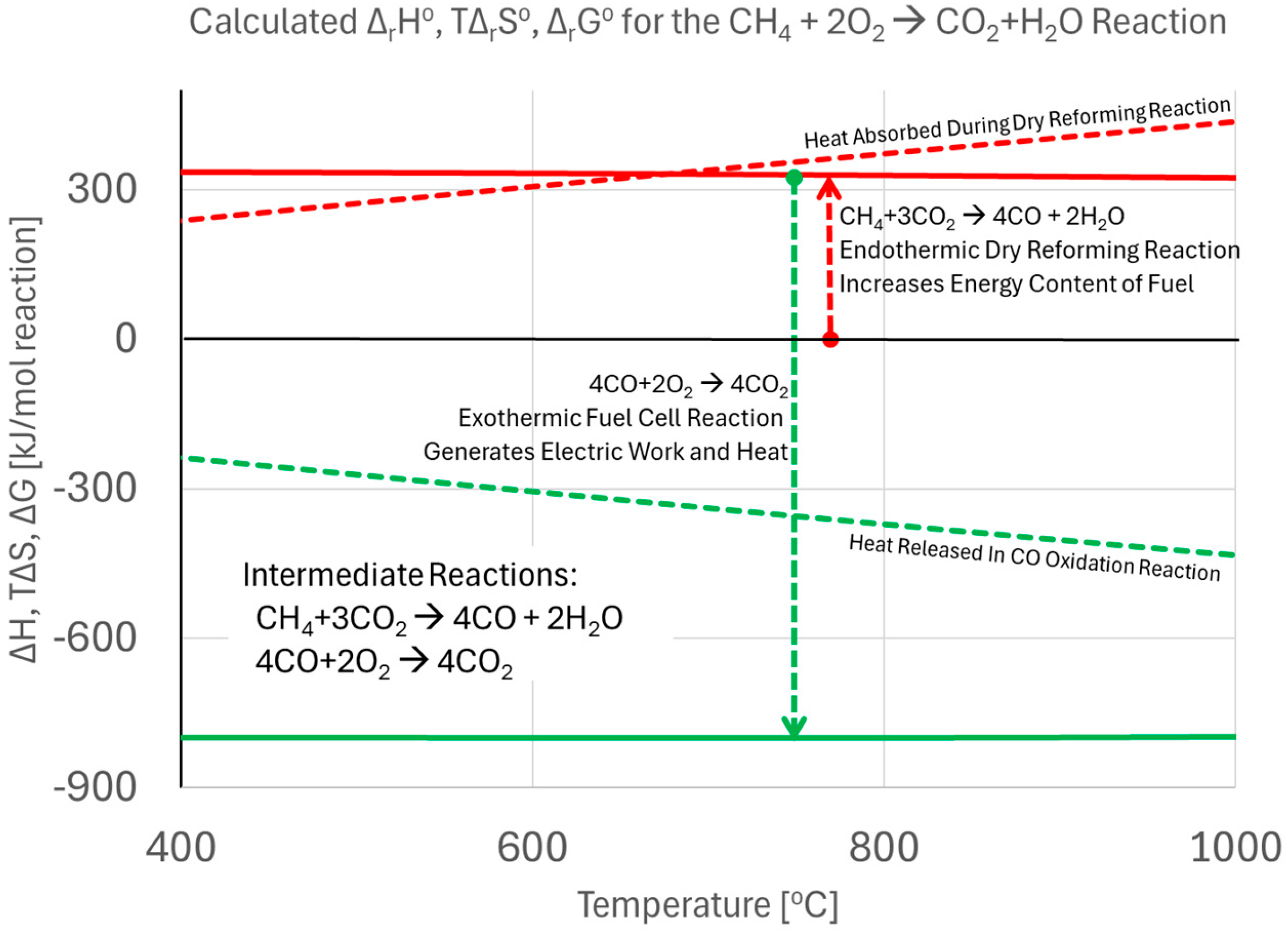
2.4. Direct Use of Hydrocarbon Fuels in Fuel Cells
2.4.1. Conventional Reforming Methods
2.4.2. Hydrocarbon Pyrolysis and Plasma Processing Methods
3. Low- and High-Temperature Fuel Cells
3.1. Low-Temperature Electrochemical Systems
3.2. High-Temperature (400~1000 °C) Electrochemical Systems
3.2.1. Molten Carbonate Fuel Cells (MCFCs)
3.2.2. Solid Oxide Fuel Cells (SOFCs)
3.2.3. Proton Conducting Electrochemical Devices
4. SOFC Materials
4.1. Electrolytes and Electrodes
4.2. Factors Influencing Electrolyte Conductivity
4.2.1. Composition
4.2.2. Microstructure
4.2.3. Processing Conditions
4.2.4. Optimization of Electrode Layers
4.2.5. Improving Layer Stability and Degradation Mechanisms
4.2.6. Recent Advancements in Solid Oxide Electrolyte Research
| Manufacturing Method | Advantage | Disadvantage |
|---|---|---|
| Thick Film (~100 μm) | ||
| Tape Casting/Screen Printing | Cost-effective Multi-layer structures and patterned layers possible | Requires homogeneous slurry Shrinkage during sintering Challenging for thinner layers |
| Extrusion | Continuous production Suitable for high volumes, uniform cross-sections, high volumetric power density (microtubular) | Limited to tubular/hollow fiber shapes Complex co-sintering of multiple layers |
| Dry Powder Pressing | Simple, high throughput, low cost for high volumes, near-net-shape capability | Difficult porosity control Challenging for thin layers |
| Transition (1~100 μm) | ||
| Sol–gel dip coating | Simple Applicable to complex shapes | Non-uniform coatings Requires repeated dips to achieve thicker films |
| Wet Powder Spraying | Simple Can achieve controlled coating thickness | Line-of-sight deposition |
| Electrodeposition | Simple Applicable to complex shapes | Limited coating thickness Poor adhesion |
| Spark Plasma Sintering | Controlling grain growth Energy efficient | limited application to manufacturing |
| Laser Reactive Deposition | Precise control of material properties High deposition rates | Technology under development Line of sight |
| Thin Film | ||
| CVD | Applicable to ceramic coatings | Thin, non-uniform coatings |
| PLD | Applicable to ceramic coatings | High cost Dependent on line-of-sight |
| RF magnetron sputtering | Applicable to ceramic coatings | High cost Dependent on line-of-sight Crack, porous coating |
| LAFAD (Large area filtered arc deposition) | Improved material properties (elimination of pore forming) | High cost Dependent on line-of-sight |
5. Conclusions
Funding
Conflicts of Interest
References
- Tucker, S. Report: 19% of New Cars are Hybrid or Electric. Kelley Blue Book. 20 September 2024. Available online: https://www.kbb.com/car-news/report-19-of-new-cars-are-hybrid-or-electric/#:~:text=The%20nation%20has%20291.1%20million,(EVs)%20made%20up%208%25 (accessed on 11 February 2025).
- Dresselhaus, M.S.; Thomas, I.L. Alternative energy technologies. Nature 2001, 414, 332–337. [Google Scholar] [CrossRef] [PubMed]
- Ite, A.E.; Ibok, U.J.; Ite, M.U.; Petters, S.W. Petroleum exploration and production: Past and present environmental issues in the Nigeria’s Niger Delta. Am. J. Environ. Prot. 2013, 1, 78–90. [Google Scholar] [CrossRef]
- Burmistrov, I.; Kovyneva, N.; Gorshkov, N.; Gorokhovsky, A.; Durakov, A.; Artyukhov, D.; Kiselev, N. Development of new electrode materials for thermo-electrochemical cells for waste heat harvesting. Renew. Energy Focus 2019, 29, 42–48. [Google Scholar] [CrossRef]
- Seeber, R.; Zanardi, C.; Inzelt, G. Links between electrochemical thermodynamics and kinetics. ChemTexts 2015, 1, 18. [Google Scholar] [CrossRef]
- Brouwer, J. On the role of fuel cells and hydrogen in a more sustainable and renewable energy future. Curr. Appl. Phys. 2010, 10, S9–S17. [Google Scholar] [CrossRef]
- Fang, T.; von Jouanne, A.; Agamloh, E.; Yokochi, A. Opportunities and Challenges of Fuel Cell Electric Vehicle-to-Grid (V2G) Integration. Energies 2024, 17, 5646. [Google Scholar] [CrossRef]
- Guerrero, J.M.; Blaabjerg, F.; Zhelev, T.; Hemmes, K.; Monmasson, E.; Jemei, S.; Comech, M.P.; Granadino, R.; Frau, J.I. Distributed generation: Toward a new energy paradigm. IEEE Ind. Electron. Mag. 2010, 4, 52–64. [Google Scholar] [CrossRef]
- Abbasi, T.; Abbasi, S.A. Renewable’hydrogen: Prospects and challenges. Renew. Sustain. Energy Rev. 2011, 15, 3034–3040. [Google Scholar] [CrossRef]
- Jensen, S.H.; Larsen, P.H.; Mogensen, M. Hydrogen and synthetic fuel production from renewable energy sources. Int. J. Hydrogen Energy 2007, 32, 3253–3257. [Google Scholar] [CrossRef]
- Pellow, M.A.; Emmott, C.J.; Barnhart, C.J.; Benson, S.M. Hydrogen or batteries for grid storage? A net energy analysis. Energy Environ. Sci. 2015, 8, 1938–1952. [Google Scholar] [CrossRef]
- Placke, T.; Kloepsch, R.; Dühnen, S.; Winter, M. Lithium ion, lithium metal, and alternative rechargeable battery technologies: The odyssey for high energy density. J. Solid State Electrochem. 2017, 21, 1939–1964. [Google Scholar] [CrossRef]
- Hemmes, K.; Guerrero, J.M.; Zhelev, T. Highly efficient distributed generation and high-capacity energy storage. Chem. Eng. Process. Process Intensif. 2012, 51, 18–31. [Google Scholar] [CrossRef]
- Sumi, H. Co-electrolysis SOEC and internal reforming SOFC for achieving a carbon-neutral society. RSC Sustain. 2024, 2, 1568–1579. [Google Scholar] [CrossRef]
- Ni, M.; Leung, M.K.; Leung, D.Y. Technological development of hydrogen production by solid oxide electrolyzer cell (SOEC). Int. J. Hydrogen Energy 2008, 33, 2337–2354. [Google Scholar] [CrossRef]
- Vialetto, G.; Noro, M.; Colbertaldo, P.; Rokni, M. Enhancement of energy generation efficiency in industrial facilities by SOFC–SOEC systems with additional hydrogen production. Int. J. Hydrogen Energy 2019, 44, 9608–9620. [Google Scholar] [CrossRef]
- Li, W.; Shi, Y.; Luo, Y.; Cai, N. Theoretical modeling of air electrode operating in SOFC mode and SOEC mode: The effects of microstructure and thickness. Int. J. Hydrogen Energy 2014, 39, 13738–13750. [Google Scholar] [CrossRef]
- Ai, T.-I.; Troskialina, L.; Majewski, A.; Steinberger-Wilckens, R. Methane internal reforming in solid oxide fuel cells with anode off-gas recirculation. Int. J. Hydrogen Energy 2016, 41, 553–561. [Google Scholar]
- Peters, R.; Dahl, R.; Klüttgen, U.; Palm, C.; Stolten, D. Internal reforming of methane in solid oxide fuel cell systems. J. Power Sources 2002, 106, 238–244. [Google Scholar] [CrossRef]
- Van Biert, L.; Visser, K.; Aravind, P.V. A comparison of steam reforming concepts in solid oxide fuel cell systems. Appl. Energy 2020, 264, 114748. [Google Scholar] [CrossRef]
- Peters, R.; Riensche, E.; Cremer, P. Pre-reforming of natural gas in solid oxide fuel-cell systems. J. Power Sources 2000, 86, 432–441. [Google Scholar] [CrossRef]
- Chen, H.; Yang, C.; Zhou, N.; Harun, N.F.; Tucker, D. Performance Comparison of Internal and External Reforming for Hybrid SOFC-GT Applications by Using 1D Real-Time Fuel Cell Mode. Am. Soc. Mech. Eng. 2019, 58608, V003T06A028. [Google Scholar]
- van Biert, L.; Woudstra, T.; Godjevac, M.; Visser, K.; Aravind, P.V. A thermodynamic comparison of solid oxide fuel cell-combined cycles. J. Power Sources 2018, 397, 382–396. [Google Scholar] [CrossRef]
- Haseli, Y.; Dincer, I.; Naterer, G.F. Thermodynamic modeling of a gas turbine cycle combined with a solid oxide fuel cell. Int. J. Hydrogen Energy 2008, 33, 5811–5822. [Google Scholar] [CrossRef]
- Rokni, M. Thermodynamic analysis of an integrated solid oxide fuel cell cycle with a rankine cycle. Energy Convers. Manag. 2010, 51, 2724–2732. [Google Scholar] [CrossRef]
- Rokni, M. Thermodynamic analysis of SOFC (solid oxide fuel cell)–Stirling hybrid plants using alternative fuels. Energy 2013, 61, 87–97. [Google Scholar] [CrossRef]
- Tian, Y.; Manzotti, A.; Wang, Y.; Song, Y.; Fu, X.-Z.; Chi, B.; Ciucci, F. Achieving net-zero emissions with solid oxide electrolysis cells: The power-to-X approach. J. Phys. Chem. Lett. 2023, 14, 4688–4695. [Google Scholar] [CrossRef]
- Duan, L.; Zhang, X.; Yang, Y. Exergy analysis of a novel SOFC hybrid system with zero-CO2 emission. In Advances in Gas Turbine Technology; InTech: London, UK, 2011; pp. 71–88. [Google Scholar]
- Standard Pressure. In IUPAC Compendium of Chemical Terminology, 5th ed.; International Union of Pure and Applied Chemistry: Zurich, Switzerland, 2025.
- Doiron, T. 20 °C—A short history of the standard reference temperature for industrial dimensional measurements. J. Res. Natl. Inst. Stand. Technol. 2007, 112, 1–23. [Google Scholar] [CrossRef]
- Shomate, C.H. A method for evaluating and correlating thermodynamic data. J. Phys. Chem. 1954, 58, 368–372. [Google Scholar] [CrossRef]
- Ma, M.; Shen, L.; Zhao, Z.; Guo, P.; Liu, J.; Xu, B.; Zhang, Z.; Zhang, Y.; Zhao, L.; Wang, Z. Activation methods and underlying performance boosting mechanisms within fuel cell catalyst layer. eScience 2024, 4, 100254. [Google Scholar] [CrossRef]
- Gatalo, M.; Bonastre, A.M.; Moriau, L.J.; Burdett, H.; Ruiz-Zepeda, F.; Hughes, E.; Hodgkinson, A. Importance of chemical activation and the effect of low operation voltage on the performance of Pt-alloy fuel cell electrocatalysts. ACS Appl. Energy Mater. 2022, 5, 8862–8877. [Google Scholar] [CrossRef]
- Noren, D.A.; Hoffman, M.A. Clarifying the Butler–Volmer equation and related approximations for calculating activation losses in solid oxide fuel cell models. J. Power Sources 2005, 152, 175–181. [Google Scholar] [CrossRef]
- Haji, S. Analytical modeling of PEM fuel cell i–V curve. Renew. Energy 2011, 36, 451–458. [Google Scholar] [CrossRef]
- Mennola, T.; Mikkola, M.; Noponen, M.; Hottinen, T.; Lund, P. Measurement of ohmic voltage losses in individual cells of a PEMFC stack. J. Power Sources 2002, 112, 261–272. [Google Scholar] [CrossRef]
- Sasaki, K.; Wurth, J.P.; Gschwend, R.; Gödickemeier, M.; Gauckler, L.J. Microstructure-property relations of solid oxide fuel cell cathodes and current collectors: Cathodic polarization and ohmic resistance. J. Electrochem. Soc. 1996, 143, 530. [Google Scholar] [CrossRef]
- Chan, S.H.; Xia, Z.T. Polarization effects in electrolyte/electrode-supported solid oxide fuel cells. J. Appl. Electrochem. 2002, 32, 339–347. [Google Scholar] [CrossRef]
- Chan, S.H.; Khor, K.A.; Xia, Z.T. A complete polarization model of a solid oxide fuel cell and its sensitivity to the change of cell component thickness. J. Power Sources 2001, 93, 130–140. [Google Scholar] [CrossRef]
- Marie, J.; Chenitz, R.; Chatenet, M.; Berthon-Fabry, S.; Cornet, N.; Achard, P. Highly porous PEM fuel cell cathodes based on low density carbon aerogels as Pt-support: Experimental study of the mass-transport losses. J. Power Sources 2009, 190, 423–434. [Google Scholar] [CrossRef]
- Ji, Y.; Yuan, K.; Chung, J.N.; Chen, Y.-C. Effects of transport scale on heat/mass transfer and performance optimization for solid oxide fuel cells. J. Power Sources 2006, 161, 380–391. [Google Scholar] [CrossRef]
- Wang, C.-Y. Fundamental models for fuel cell engineering. Chem. Rev. 2004, 104, 4727–4766. [Google Scholar] [CrossRef]
- Niya, S.M.R.; Hoorfar, M. Process modeling of the ohmic loss in proton exchange membrane fuel cells. Electrochim. Acta 2014, 120, 193–203. [Google Scholar] [CrossRef]
- Fang, T.; Vairin, C.; von Jouanne, A.; Agamloh, E.; Yokochi, A. Review of Fuel-Cell Electric Vehicles. Energies 2024, 17, 2160. [Google Scholar] [CrossRef]
- Arshad, A.; Ali, H.M.; Habib, A.; Bashir, M.A.; Jabbal, M.; Yan, Y. Energy and exergy analysis of fuel cells: A review. Therm. Sci. Eng. Prog. 2019, 9, 308–321. [Google Scholar] [CrossRef]
- Eveloy, V.; Karunkeyoon, W.; Rodgers, P.; Alili, A.A. Energy, exergy and economic analysis of an integrated solid oxide fuel cell–gas turbine–organic Rankine power generation system. Int. J. Hydrogen Energy 2016, 41, 13843–13858. [Google Scholar] [CrossRef]
- Esposito, M.; Kawai, R.; Lindenberg, K.; Broeck, C.V.d. Efficiency at maximum power of low-dissipation Carnot engines. Phys. Rev. Lett. 2010, 105, 150603. [Google Scholar] [CrossRef]
- Bejan, A. Entropy generation minimization: The new thermodynamics of finite-size devices and finite-time processes. J. Appl. Phys. 1996, 79, 1191–1218. [Google Scholar] [CrossRef]
- Novikov, I.I. The efficiency of atomic power stations (a review). J. Nucl. Energy 1958, 7, 125–128. [Google Scholar] [CrossRef]
- Curzon, F.L.; Ahlborn, B. Efficiency of a Carnot engine at maximum power output. Am. J. Phys. 1975, 43, 22–24. [Google Scholar] [CrossRef]
- Guo, J.; Wang, J.; Wang, Y.; Chen, J. Universal efficiency bounds of weak-dissipative thermodynamic cycles at the maximum power output. Phys. Rev. E Stat. Nonlinear Soft Matter Phys. 2013, 87, 012133. [Google Scholar] [CrossRef]
- Udagawa, J.; Aguiar, P.; Brandon, N.P. Hydrogen production through steam electrolysis: Control strategies for a cathode-supported intermediate temperature solid oxide electrolysis cell. J. Power Sources 2008, 180, 354–364. [Google Scholar] [CrossRef]
- Hauch, A. Solid Oxide Electrolysis Cells: Performance and Durability; Risø National Laboratory: Roskilde, Denmark, 2008. [Google Scholar]
- Sun, E.; Zhai, S.; Kim, D.; Gigantino, M.; Haribal, V.; Dewey, O.S.; Williams, S.M.; Wan, G.; Nelson, A.; Marin-Quiros, S.; et al. A semi-continuous process for co-production of CO2-free hydrogen and carbon nanotubes via methane pyrolysis. Cell Rep. Phys. Sci. 2023, 4, 101338. [Google Scholar] [CrossRef]
- Rojas, J.; Zhai, S.; Sun, E.; Haribal, V.; Marin-Quiros, S.; Sarkar, A.; Gupta, R.; Cargnello, M.; Chueh, W.; Majumdar, A. Technoeconomics and carbon footprint of hydrogen production. Int. J. Hydrogen Energy 2024, 49, 59–74. [Google Scholar] [CrossRef]
- Fan, Z.; Weng, W.; Zhou, J.; Gu, D.; Xiao, W. Catalytic decomposition of methane to produce hydrogen: A review. J. Energy Chem. 2021, 58, 415–430. [Google Scholar] [CrossRef]
- Patlolla, S.R.; Katsu, K.; Sharafian, A.; Wei, K.; Herrera, O.E.; Mérida, W. A review of methane pyrolysis technologies for hydrogen production. Renew. Sustain. Energy Rev. 2023, 181, 113323. [Google Scholar] [CrossRef]
- Stenina, I.; Yaroslavtsev, A. Modern technologies of hydrogen production. Processes 2022, 11, 56. [Google Scholar] [CrossRef]
- Moghaddam, A.L.; Hejazi, S.; Fattahi, M.; Kibria, M.G.; Thomson, M.; AlEisa, R.; Khan, M.A. Methane Pyrolysis for Hydrogen Production: Navigating the Path to a Net Zero Future. Energy Environ. Sci. 2025, 18, 2747–2790. [Google Scholar] [CrossRef]
- Msheik, M.; Rodat, S.; Abanades, S. Experimental comparison of solar methane pyrolysis in gas-phase and molten-tin bubbling tubular reactors. Energy 2022, 260, 124943. [Google Scholar] [CrossRef]
- Lei, F.; Freiberg, L.; Wang, Y.; Reddick, I.; Jovanovic, G.; Yokochi, A.; AuYeung, N. Non-catalytic ethane cracking using concentrated solar energy. Chem. Eng. J. 2019, 355, 58–64. [Google Scholar] [CrossRef]
- Wnukowski, M. Methane Pyrolysis with the use of plasma: Review of plasma reactors and process products. Energies 2023, 16, 6441. [Google Scholar] [CrossRef]
- Miao, Y.; Yokochi, A.; Jovanovic, G.; Zhang, S.; von Jouanne, A. Application-oriented non-thermal plasma in chemical reaction engineering: A review. Green Energy Resour. 2023, 1, 100004. [Google Scholar] [CrossRef]
- Prabowo, J.; Lai, L.; Chivers, B.; Burke, D.; Dinh, A.H.; Ye, L.; Wang, Y.; Wang, Y.; Wei, L.; Chen, Y. Solid carbon co-products from hydrogen production by methane pyrolysis: Current understandings and recent progress. Carbon 2024, 216, 118507. [Google Scholar] [CrossRef]
- Mekhilef, S.; Saidur, R.; Safari, A. Comparative study of different fuel cell technologies. Renew. Sustain. Energy Rev. 2012, 16, 981–989. [Google Scholar] [CrossRef]
- Warshay, M.; Prokopius, P.R. The fuel cell in space: Yesterday, today and tomorrow. In Proceedings of the Grove Anniversary (1839–1989) Fuel Cell Symposium, London, UK, 18–21 September 1989. [Google Scholar]
- Burke, K. Fuel cells for space science applications. In Proceedings of the 1st International Energy Conversion Engineering Conference (IECEC), Portsmouth, VA, USA, 17–21 August 2003; p. 5938. [Google Scholar]
- Araya, S.S.; Zhou, F.; Liso, V.; Sahlin, S.L.; Vang, J.R.; Thomas, S.; Gao, X.; Jeppesen, C.; Kær, S.K. A comprehensive review of PBI-based high temperature PEM fuel cells. Int. J. Hydrogen Energy 2016, 41, 21310–21344. [Google Scholar] [CrossRef]
- Schmidt, T.J. Durability and degradation in high-temperature polymer electrolyte fuel cells. ECS Trans. 2006, 1, 19. [Google Scholar] [CrossRef]
- Haider, R.; Wen, Y.; Ma, Z.-F.; Wilkinson, D.P.; Zhang, L.; Yuan, X.; Song, S.; Zhang, J. High temperature proton exchange membrane fuel cells: Progress in advanced materials and key technologies. Chem. Soc. Rev. 2021, 50, 1138–1187. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, K.S.; Mishler, J.; Cho, S.C.; Adroher, X.C. A review of polymer electrolyte membrane fuel cells: Technology, applications, and needs on fundamental research. Appl. Energy 2011, 88, 981–1007. [Google Scholar] [CrossRef]
- Joghee, P.; Malik, J.N.; Pylypenko, S.; O’Hayre, R. A review on direct methanol fuel cells–In the perspective of energy and sustainability. MRS Energy Sustain. 2015, 2, E3. [Google Scholar] [CrossRef]
- Okayama, H. Report of the Twentieth Meeting of the Dangerous Goods Panel. In Proceedings of the Dangerous Goods Panel (DGP) Twentieth Meeting, Montreal, QC, Canada, 24 October–4 November 2005; ICAO: Montreal, Canada, 2005. [Google Scholar]
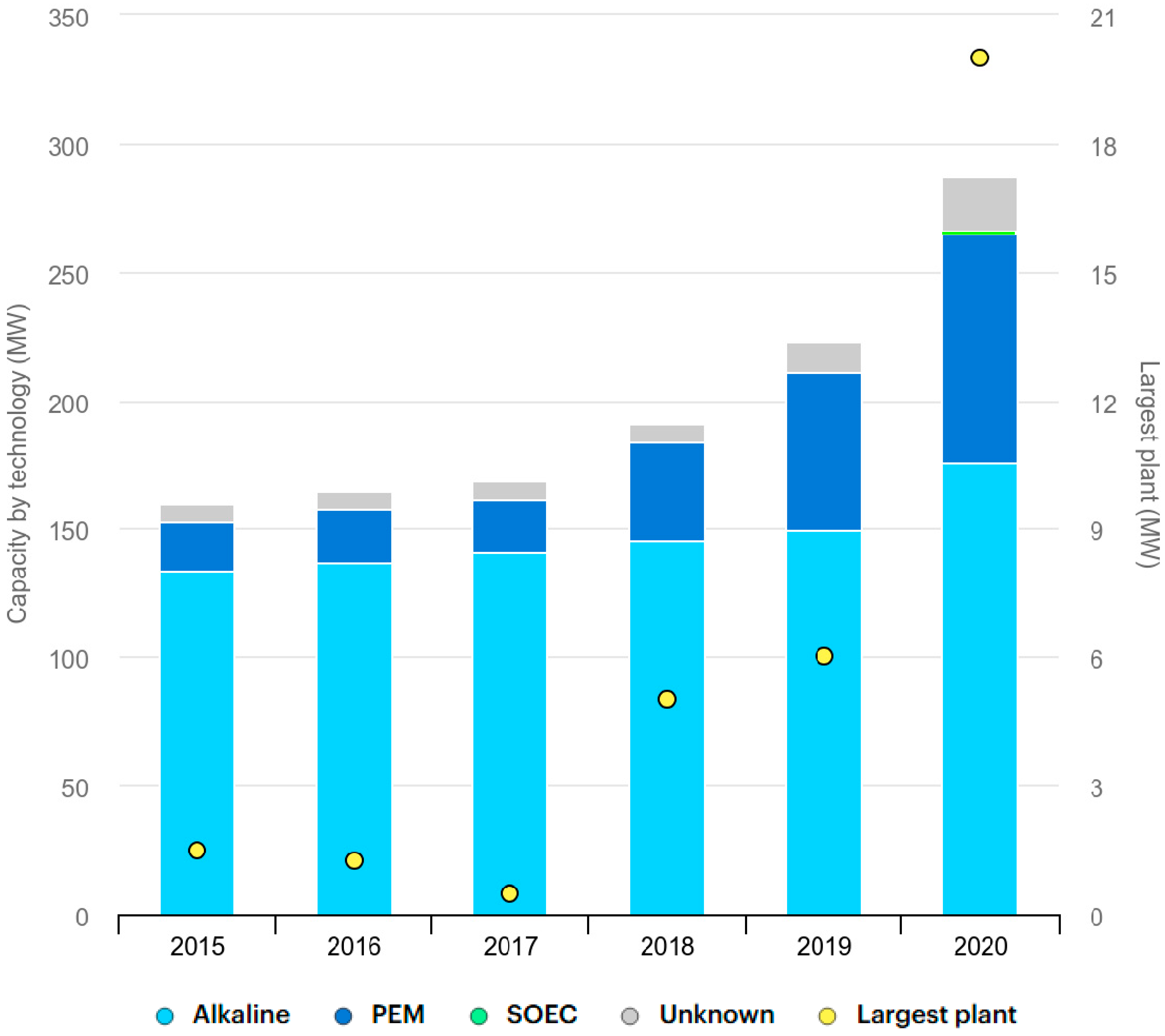
- IEA. Global Installed Electrolysis Capacity by Technology, 2015–2020. 4 November 2021. Available online: https://www.iea.org/data-and-statistics/charts/global-installed-electrolysis-capacity-by-technology-2015-2020 (accessed on 28 April 2025).
- Sebbahi, S.; Nabil, N.; Alaoui-Belghiti, A.; Laasri, S.; Rachidi, S.; Hajjaji, A. Assessment of the three most developed water electrolysis technologies: Alkaline water electrolysis, proton exchange membrane and solid-oxide electrolysis. Mater. Today Proc. 2022, 66, 140–145. [Google Scholar] [CrossRef]
- Ahmed, S.; Krumpelt, M. Hydrogen from hydrocarbon fuels for fuel cells. Int. J. Hydrogen Energy 2001, 26, 291–301. [Google Scholar] [CrossRef]
- Pérez-Fortes, M.; Mian, A.; Srikanth, S.; Wang, L.; Diethelm, S.; Varkaraki, E.; Mirabelli, I. Design of a pilot sofc system for the combined production of hydrogen and electricity under refueling station requirements. Fuel Cells 2019, 19, 389–407. [Google Scholar] [CrossRef]
- Shikhar, U.; Hemmes, K.; Woudstra, T. Exploring the possibility of using molten carbonate fuel cell for the flexible coproduction of hydrogen and power. Front. Energy Res. 2021, 9, 656490. [Google Scholar] [CrossRef]
- Kreuer, K.-D. Proton-conducting oxides. Annu. Rev. Mater. Res. 2003, 33, 333–359. [Google Scholar] [CrossRef]
- Nakamura, T.; Mizunuma, S.; Kimura, Y.; Mikami, Y.; Yamauchi, K.; Kuroha, T.; Taniguchi, N.; Tsuji, Y.; Okuyama, Y.; Amezawa, K. Energy efficiency of ionic transport through proton conducting ceramic electrolytes for energy conversion applications. J. Mater. Chem. A 2018, 6, 15771–15780. [Google Scholar] [CrossRef]
- Wikipedia. Available online: https://en.wikipedia.org/wiki/Molten_carbonate_fuel_cell (accessed on 24 March 2005).
- Contreras, R.R.; Almarza, J.; Rincón, L. Molten carbonate fuel cells: A technological perspective and review. Energy Sources Part A Recovery Util. Environ. Eff. 2021, 1–15. [Google Scholar] [CrossRef]
- Hu, L.; Rexed, I.; Lindbergh, G.; Lagergren, C. Electrochemical performance of reversible molten carbonate fuel cells. Int. J. Hydrogen Energy 2014, 39, 12323–12329. [Google Scholar] [CrossRef]
- Martsinchyk, A.; Szczęśniak, A.; Martsinchyk, K.; Dybiński, O.; Cinti, G.; Milewski, J.; Shuhayeu, P. Molten carbonate electrolyzer for synthetic fuel generation. J. Power Sources 2025, 628, 235741. [Google Scholar] [CrossRef]
- Audasso, E.; Kim, K.I.; Accardo, G.; Kim, H.S.; Yoon, S.P. Investigation of molten carbonate electrolysis cells performance for H2 production and CO2 capture. J. Power Sources 2022, 523, 231039. [Google Scholar] [CrossRef]
- Dicks, A.L. Fuel cells—Molten carbonate fuel cells | overview. Encycl. Electrochem. Power Sources 2009, 2, 446–453. [Google Scholar]
- Hemmes, K.; Patil, A.; Woudstra, N. Flexible coproduction of hydrogen and power using internal reforming solid oxide fuel cells system. ASME 2008, 5, 041010. [Google Scholar] [CrossRef]
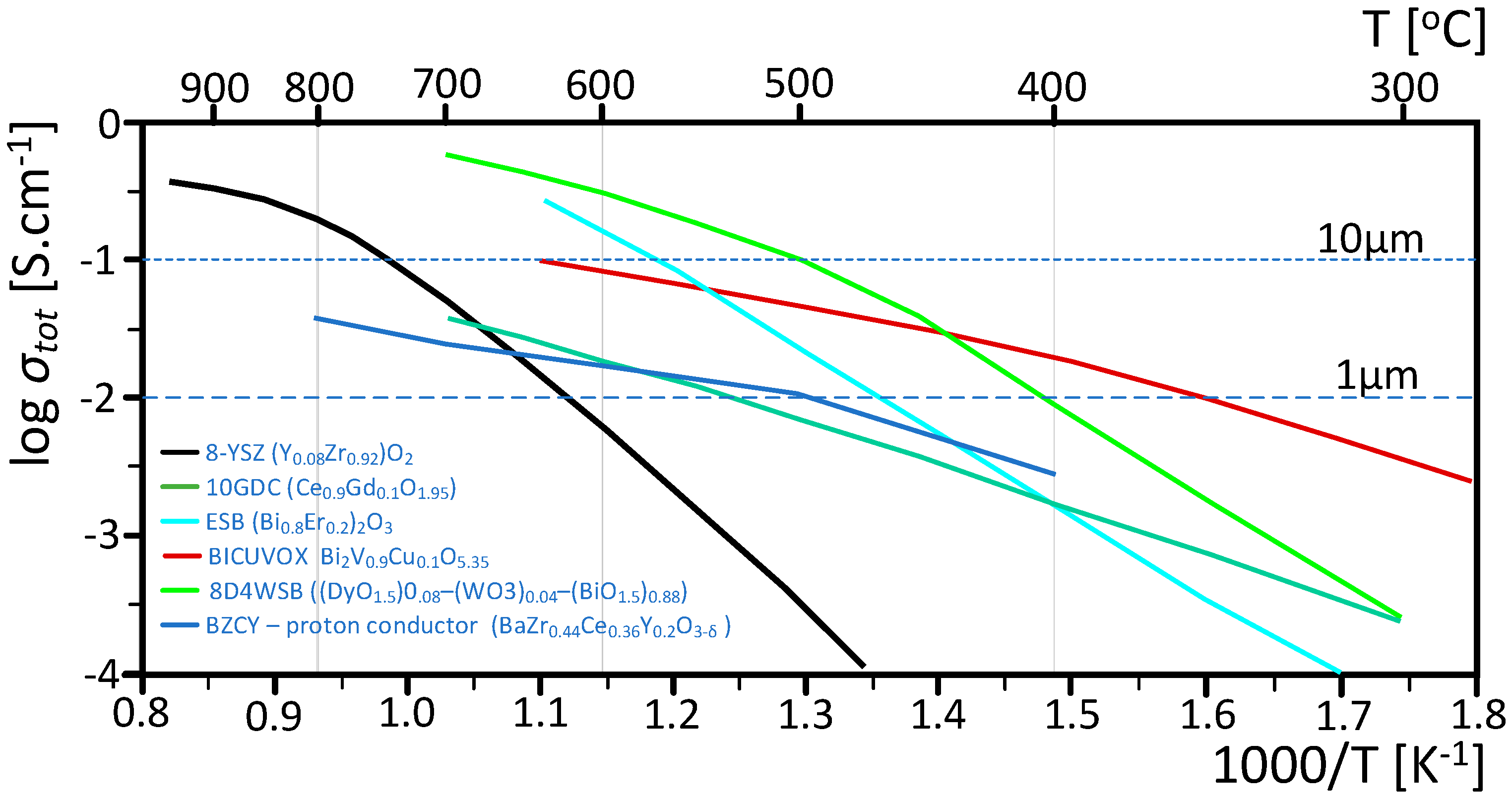
- Li, J.; Cai, Q.; Horri, B.A. Highly conductive and stable electrolytes for solid oxide electrolysis and fuel cells: Fabrication, characterisation, recent progress and challenges. Mater. Adv. 2024, 6, 39–83. [Google Scholar] [CrossRef]
- Iwahara, H.; Esaka, T.; Uchida, H.; Maeda, N. Proton conduction in sintered oxides and its application to steam electrolysis for hydrogen production. Solid State Ion. 1981, 3, 359–363. [Google Scholar] [CrossRef]
- Iwahara, H. Proton conducting ceramics and their applications. Solid State Ion. 1996, 86, 9–15. [Google Scholar] [CrossRef]
- Iwahara, H.; Uchida, H.; Morimoto, K. High Temperature Solid Electrolyte Fuel Cells Using Perovskite-Type Oxide Based on BaCeO3. J. Electrochem. Soc. 1990, 137, 462. [Google Scholar] [CrossRef]
- Duan, C.; Tong, J.; Shang, M.; Nikodemski, S.; Sanders, M.; Ricote, S.; Almansoori, A.; O’Hayre, R. Readily processed protonic ceramic fuel cells with high performance at low temperatures. Science 2015, 349, 1321–1326. [Google Scholar] [CrossRef] [PubMed]
- Minh, N.Q. Ceramic fuel cells. J. Am. Ceram. Soc. 1993, 76, 563–588. [Google Scholar] [CrossRef]
- Le, L.Q.; Hernandez, C.H.; Rodriguez, M.H.; Zhu, L.; Duan, C.; Ding, H.; O’Hayre, R.P.; Sullivan, N.P. Proton-conducting ceramic fuel cells: Scale up and stack integration. J. Power Sources 2021, 482, 228868. [Google Scholar] [CrossRef]
- Ferguson, K.; Dubois, A.; Albrecht, K.; Braun, R.J. High performance protonic ceramic fuel cell systems for distributed power generation. Energy Convers. Manag. 2021, 248, 114763. [Google Scholar] [CrossRef]
- Meisel, C.; Huang, J.D.; Kim, Y.D.; Stockburger, S.; O’Hayre, R.; Sullivan, N.P. Insights on proton-conducting ceramic electrochemical cell fabrication. J. Am. Ceram. Soc. 2025, 108, e20321. [Google Scholar] [CrossRef]
- Mu, S.; Zhao, Z.; Huang, H.; Lei, J.; Peng, F.; Xiao, H.; Brinkman, K.S.; Tong, J.J. Advanced Manufacturing of Intermediate-Temperature Protonic Ceramic Electrochemical Cells. Electrochem. Soc. Interface 2020, 29, 67. [Google Scholar] [CrossRef]
- Liu, F.; Diercks, D.; Kumar, P.; Seong, A.; Jabbar, M.H.A.; Gumeci, C.; Furuya, Y. Redesigning protonic ceramic electrochemical cells to lower the operating temperature. Sci. Adv. 2025, 11, eadq2507. [Google Scholar] [CrossRef]
- Kuzmin, A.V.; Lesnichyova, A.S.; Tropin, E.S.; Stroeva, A.Y.; Vorotnikov, V.A.; Solodyankina, D.M.; Belyakov, S.A. LaScO3-based electrolyte for protonic ceramic fuel cells: Influence of sintering additives on the transport properties and electrochemical performance. J. Power Sources 2020, 466, 228255. [Google Scholar] [CrossRef]
- Rehman, J.; Hanif, M.B.; Khan, M.Z.; Ullah, M.; Starostina, I.A.; Muhammad, M.T.; Li, Z. A Review of Proton-Conducting Electrolytes for Efficient Low-Temperature Solid Oxide Fuel Cells: Progress, Challenges, and Perspectives. Energy Fuels 2024, 38, 22637–22665. [Google Scholar] [CrossRef]
- Koh, J.-H.; Yoo, Y.-S.; Park, J.-W.; Lim, H.C. Carbon deposition and cell performance of Ni-YSZ anode support SOFC with methane fuel. Solid State Ion. 2002, 149, 157–166. [Google Scholar] [CrossRef]
- Maček, J.; Novosel, B.; Marinšek, M. Ni–YSZ SOFC anodes—Minimization of carbon deposition. J. Eur. Ceram. Soc. 2007, 27, 487–491. [Google Scholar] [CrossRef]
- Babaei, A.; Zhang, L.; Liu, E. Performance and carbon deposition over Pd nanoparticle catalyst promoted Ni/GDC anode of SOFCs in methane, methanol and ethanol fuels. Int. J. Hydrogen Energy 2012, 37, 15301–15310. [Google Scholar] [CrossRef]
- Morozan, A.; Jousselme, B.; Palacin, S. Low-platinum and platinum-free catalysts for the oxygen reduction reaction at fuel cell cathodes. Energy Environ. Sci. 2011, 4, 1238–1254. [Google Scholar] [CrossRef]
- Tsai, T.; Barnett, S.A. Effect of LSM-YSZ cathode on thin-electrolyte solid oxide fuel cell performance. Solid State Ion. 1997, 93, 207–217. [Google Scholar] [CrossRef]
- Wang, C.C.; Gholizadeh, M.; Hou, B.; Fan, X. Integrated Cr and S poisoning of a La0.6Sr0.4Co0.2Fe0.8O3−δ (LSCF) cathode for solid oxide fuel cells. RSC Adv. 2021, 11, 7–14. [Google Scholar] [CrossRef]
- Niedrig, C. Electrochemical Performance and Stability of Ba0.5Sr0.5Co0.8Fe0.2O3-δ for Oxygen Transport Membranes; KIT Scientific Publishing: Karlsruhe, Germany, 2015; Volume 28. [Google Scholar]
- Inaba, H.; Tagawa, H. Ceria-based solid electrolytes. Solid State Ion. 1996, 83, 1–16. [Google Scholar] [CrossRef]
- Arunkumar, P.; Meena, M.; Babu, K.S. A review on cerium oxide-based electrolytes for ITSOFC. Nanomater. Energy 2012, 1, 288–305. [Google Scholar] [CrossRef]
- Robinson, I.A.; Huang, Y.L.; Horlick, S.A.; Obenland, J.; Robinson, N.; Gritton, J.E.; Hussain, A.M.; Wachsman, E.D. Mitigating electronic conduction in ceria-based electrolytes via external structure design. Adv. Funct. Mater. 2024, 34, 2308123. [Google Scholar] [CrossRef]
- Xiong, Y.-P.; Kishimoto, H.; Yamaji, K.; Yoshinaga, M.; Horita, T.; Brito, M.E.; Yokokawa, H. Electronic conductivity of pure ceria. Solid State Ion. 2011, 192, 476–479. [Google Scholar] [CrossRef]
- Wang, T. Solid-State Oxide Fuel Cells. Appl. Comput. Eng. 2024, 89, 88–93. [Google Scholar] [CrossRef]
- Peng, X.-L.; Xu, B.-X. Unraveling impacts of polycrystalline microstructures on ionic conductivity of ceramic electrolytes by computational homogenization and machine learning. J. Appl. Phys. 2024, 10, 136. [Google Scholar] [CrossRef]
- Xi, L.; Zhang, D.; Xu, X.; Wu, Y.; Li, F.; Yao, S.; Zhu, M.; Liu, J. Interface Engineering of All-Solid-State Batteries Based on Inorganic Solid Electrolytes. ChemSusChem 2023, 16, e202202158. [Google Scholar] [CrossRef]
- Dasari, H.P.; Ahn, K.; Park, S.-Y.; Hong, J.; Kim, H.; Yoon, K.J.; Son, J.-W.; Kim, B.-K.; Lee, H.-W.; Lee, J.-H. Record-low sintering-temperature (600 °C) of solid-oxide fuel cell electrolyte. J. Alloys Compd. 2016, 672, 397–402. [Google Scholar] [CrossRef]
- Arshad, M.S.; Billing, C.; Billing, D.G.; Guan, W. Phase-assisted tailored conductivity of doped ceria electrolytes to boost SOFC performance. ACS Appl. Mater. Interfaces 2023, 15, 39396–39407. [Google Scholar] [CrossRef]
- Senthil Kumar, S.; Aruna, S.T. Hydrocarbon compatible SOFC anode catalysts and their syntheses: A review. Sustain. Chem. 2021, 2, 707–763. [Google Scholar] [CrossRef]
- Wehrle, L.; Wang, Y.; Boldrin, P.; Nigel; Brandon, P.; Deutschmann, O.; Banerjee, A. Optimizing solid oxide fuel cell performance to re-evaluate its role in the mobility sector. ACS Environ. Au 2021, 2, 42–64. [Google Scholar] [CrossRef]
- Bertei, A.; Nucci, B.; Nicolella, C. Engineered electrode microstructure for optimization of solid oxide fuel cells. Chem. Eng. Trans. 2013, 32, 2293–2298. [Google Scholar]
- Schiller, G.; Ansar, A.S.; Soysal, D. Development of Nanostructured Solid Oxide Fuel Cell Electrodes. World J. Eng. 2010, 7, 220–229. [Google Scholar]
- Park, B.-K.; Zhang, Q.; Voorhees, P.W.; Barnett, S.A. Conditions for stable operation of solid oxide electrolysis cells: Oxygen electrode effects. Energy Environ. Sci. 2019, 12, 3053–3062. [Google Scholar] [CrossRef]
- Vinchhi, P.; Khandla, M.; Chaudhary, K.; Pati, R. Recent advances on electrolyte materials for SOFC: A review. Inorg. Chem. Commun. 2023, 152, 110724. [Google Scholar] [CrossRef]
- Sohal, M.S.; O’Brien, J.E.; Stoots, C.M.; Sharma, V.I.; Yildiz, B.; Virkar, A. Degradation issues in solid oxide cells during high temperature electrolysis. ASME 2011, 9, 011017. [Google Scholar]
- Jiang, S.P. Challenges in the development of reversible solid oxide cell technologies: A mini review. Asia Pac. J. Chem. Eng. 2016, 11, 386–391. [Google Scholar] [CrossRef]
- Fabbri, E.; Pergolesi, D.; Traversa, E. Materials challenges toward proton-conducting oxide fuel cells: A critical review. Chem. Soc. Rev. 2010, 39, 4355–4369. [Google Scholar] [CrossRef]
- Chen, G.; Luo, Y.; Sun, W.; Liu, H.; Ding, Y.; Li, Y.; Geng, S.; Yu, K.; Liu, G. Electrochemical performance of a new structured low temperature SOFC with BZY electrolyte. Int. J. Hydrogen Energy 2018, 43, 12765–12772. [Google Scholar] [CrossRef]
- Fabbri, E.; Bi, L.; Pergolesi, D.; Traversa, E. Towards the next generation of solid oxide fuel cells operating below 600 °C with chemically stable proton-conducting electrolytes. Adv. Mater. 2012, 24, 195–208. [Google Scholar] [CrossRef]
- Jhuang, J.-W.; Lee, K.-R.; Chang, J.-k.; Shen, C.-T.; Lee, Y.-H.; Lee, S.-W.; Tseng, C.-J. Chemical stability and electrical and mechanical properties of BaZrxCe0.8-xY0.2O3 with CeO2 protection method. Int. J. Hydrogen Energy 2017, 42, 22259–22265. [Google Scholar] [CrossRef]
- Jaiswal, N.; Tanwar, K.; Suman, R.; Kumar, D.; Upadhyay, S.; Parkash, O. A brief review on ceria based solid electrolytes for solid oxide fuel cells. J. Alloys Compd. 2019, 781, 984–1005. [Google Scholar] [CrossRef]
- Tong, J.; Su, L.; Haraya, K.; Suda, H. Thin Pd membrane on α-Al2O3 hollow fiber substrate without any interlayer by electroless plating combined with embedding Pd catalyst in polymer template. J. Membr. Sci. 2008, 310, 93–101. [Google Scholar] [CrossRef]
- Anwar, M.; Muchtar, A.; Somalu, M.R. Effects of various co-dopants and carbonates on the properties of doped ceria-based electrolytes: A brief review. Int. J. Appl. Eng. Res. ISSN 2016, 11, 973–4562. [Google Scholar]
- Zhang, Y.; Liu, J.; Singh, M.; Hu, E.; Jiang, Z.; Raza, R.; Wang, F.; Wang, J.; Yang, F.; Zhu, B. Superionic conductivity in ceria-based heterostructure composites for low-temperature solid oxide fuel cells. Nano-Micro Lett. 2020, 12, 178. [Google Scholar] [CrossRef] [PubMed]
- Gao, Z.; Mogni, L.V.; Miller, E.C.; Railsback, J.G.; Barnett, S.A. A perspective on low-temperature solid oxide fuel cells. Energy Environ. Sci. 2016, 9, 1602–1644. [Google Scholar] [CrossRef]
- Wachsman, E.D.; Lee, K.T. Lowering the temperature of solid oxide fuel cells. Science 2011, 334, 935–939. [Google Scholar] [CrossRef]
- Fergus, J.W. Electrolytes for solid oxide fuel cells. J. Power Sources 2006, 162, 30–40. [Google Scholar] [CrossRef]
- Kan, W.H.; Samson, A.J.; Thangadurai, V. Trends in electrode development for next generation solid oxide fuel cells. J. Mater. Chem. 2016, 4, 17913–17932. [Google Scholar] [CrossRef]
- Simner, S.P.; Suarez-Sandoval, D.; Mackenzie, J.D.; Dunn, B. Synthesis, densification, and conductivity characteristics of BICUVOX oxygen-ion-conducting ceramics. J. Am. Ceram. Soc. 1997, 80, 2563–2568. [Google Scholar] [CrossRef]
- Punn, R.; Feteira, A.M.; Sinclair, D.C.; Greaves, C. Enhanced oxide ion conductivity in stabilized δ-Bi2O3. J. Am. Chem. Soc. 2006, 128, 15386–15387. [Google Scholar] [CrossRef]
- Kwon, O.H.; Choi, G.M. Electrical conductivity of thick film YSZ. Solid State Ion. 2006, 177, 3057–3062. [Google Scholar] [CrossRef]
- Jung, D.W.; Duncan, K.L.; Wachsman, E.D. Effect of total dopant concentration and dopant ratio on conductivity of (DyO1.5)x−(WO3)y−(BiO1.5)1−x−y. Acta Mater. 2010, 58, 355–363. [Google Scholar] [CrossRef]
- Kuterbekov, K.A.; Nikonov, A.V.; Bekmyrza, K.Z.; Pavzderin, N.B.; Kabyshev, A.M.; Kubenova, M.M.; Kabdrakhimova, G.D.; Aidarbekov, N. Classification of solid oxide fuel cells. Nanomaterials 2022, 12, 1059. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Xia, G.-G.; Li, X.-H.; Stevenson, J.W. (Mn, Co)3O4 spinel coatings on ferritic stainless steels for SOFC interconnect applications. Int. J. Hydrogen Energy 2007, 32, 3648–3654. [Google Scholar] [CrossRef]
- Puranen, J.; Pihlatie, M.; Lagerbom, J.; Bolelli, G.; Laakso, J.; Hyvärinen, L.; Kylmälahti, M. Post-mortem evaluation of oxidized atmospheric plasma sprayed Mn–Co–Fe oxide spinel coatings on SOFC interconnectors. Int. J. Hydrogen Energy 2014, 39, 17284–17294. [Google Scholar] [CrossRef]
- Gannon, P.; Deibert, M.; White, P.; Smith, R.; Chen, H.; Priyantha, W.; Lucas, J.; Gorokhovsky, V. Advanced PVD protective coatings for SOFC interconnects. Int. J. Hydrogen Energy 2008, 33, 3991–4000. [Google Scholar] [CrossRef]
- Gorokhovsky, V.I.; Gannon, P.E.; Deibert, M.C.; Smith, R.J.; Kayani, A.; Kopczyk, M.; VanVorous, D. Deposition and evaluation of protective PVD coatings on ferritic stainless steel SOFC interconnects. J. Electrochem. Soc. 2006, 153, A1886–A1893. [Google Scholar] [CrossRef]
- Bobruk, M.; Molin, S.; Chen, M.; Brylewski, T.; Hendriksen, P.V. Sintering of MnCo2O4 coatings prepared by electrophoretic deposition. Mater. Lett. 2018, 213, 394–398. [Google Scholar] [CrossRef]
- Shaigan, N.; Qu, W.; Ivey, D.G.; Chen, W. A review of recent progress in coatings, surface modifications and alloy developments for solid oxide fuel cell ferritic stainless steel interconnects. J. Power Sources 2010, 195, 1529–1542. [Google Scholar] [CrossRef]
- Molin, S.; Jasinski, P.; Mikkelsen, L.; Zhang, W.; Chen, M.; Hendriksen, P.V. Low temperature processed MnCo2O4 and MnCo1.8Fe0.2O4 as effective protective coatings for solid oxide fuel cell interconnects at 750 °C. J. Power Sources 2016, 336, 408–418. [Google Scholar] [CrossRef]
- Molin, S.; Chen, M.; Hendriksen, P.V. Oxidation study of coated Crofer 22 APU steel in dry oxygen. J. Power Sources 2014, 251, 488–495. [Google Scholar] [CrossRef]
- Lee, K.; Yoon, B.; Kang, J.; Lee, S.; Bae, J. Evaluation of Ag-doped (MnCo)3O4 spinel as a solid oxide fuel cell metallic interconnect coating material. Int. J. Hydrogen Energy 2017, 42, 29511–29517. [Google Scholar] [CrossRef]
- Xu, Y.; Wen, Z.; Wang, S.; Wen, T. Cu doped Mn–Co spinel protective coating on ferritic stainless steels for SOFC interconnect applications. Solid State Ion. 2011, 192, 561–564. [Google Scholar] [CrossRef]
- Chen, G.; Xin, X.; Luo, T.; Liu, L.; Zhou, Y.; Yuan, C.; Lin, C.; Zhan, Z.; Wang, S. Mn1.4Co1.4Cu0.2O4 spinel protective coating on ferritic stainless steels for solid oxide fuel cell interconnect applications. J. Power Sources 2015, 278, 230–234. [Google Scholar] [CrossRef]
- Uehara, T.; Yasuda, N.; Okamoto, M.; Baba, Y. Effect of Mn–Co spinel coating for Fe–Cr ferritic alloys ZMG232L and 232J3 for solid oxide fuel cell interconnects on oxidation behavior and Cr-evaporation. J. Power Sources 2011, 196, 7251–7256. [Google Scholar] [CrossRef]
- Singh, R.; Pratap, R.; Narayanan, J.A.; Thangamani, G.; Krishnan, V.V.; Arjunan, A.; Hughes, D. Advances in additive manufacturing of fuel cells: A review of technologies, materials, and challenges. Sustain. Mater. Technol. 2025, 43, e01317. [Google Scholar] [CrossRef]
- Pesce, A.; Hornés, A.; Núñez, M.; Morata, A.; Torrell, M.; Tarancón, A. 3D printing the next generation of enhanced solid oxide fuel and electrolysis cells. J. Mater. Chem. 2020, A8, 16926–16932. [Google Scholar] [CrossRef]
- Wei, L.; Zhang, J.; Yu, F.; Zhang, W.; Meng, X.; Yang, N.; Liu, S. A novel fabrication of yttria-stabilized-zirconia dense electrolyte for solid oxide fuel cells by 3D printing technique. Int. J. Hydrogen Energy 2019, 44, 6182–6191. [Google Scholar] [CrossRef]
- Han, G.D.; Bae, K.; Kang, E.H.; Choi, H.J.; Shim, J.H. Inkjet printing for manufacturing solid oxide fuel cells. ACS Energy Lett. 2020, 5, 1586–1592. [Google Scholar] [CrossRef]
- Zouridi, L.; Garagounis, I.; Vourros, A.; Marnellos, G.E.; Binas, V. Advances in Inkjet-Printed Solid Oxide Fuel Cells. Adv. Mater. Technol. 2022, 7, 2101491. [Google Scholar] [CrossRef]
- Jang, I.; Kelsall, G.H. Fabrication of 3D NiO-YSZ structures for enhanced performance of solid oxide fuel cells and electrolysers. Electrochem. Commun. 2022, 137, 107260. [Google Scholar] [CrossRef]
- Anelli, S.; Rosa, M.; Baiutti, F.; Torrell, M.; Esposito, V.; Tarancón, A. Hybrid-3D printing of symmetric solid oxide cells by inkjet printing and robocasting. Addit. Manuf. 2022, 51, 102636. [Google Scholar] [CrossRef]
- Lamy, C. From hydrogen production by water electrolysis to its utilization in a PEM fuel cell or in a SO fuel cell: Some considerations on the energy efficiencies. Int. J. Hydrogen Energy 2016, 41, 15415–15425. [Google Scholar] [CrossRef]



| Species | |||||||
|---|---|---|---|---|---|---|---|
| Param. | H2 | O2 | CO | CH4 | H2O (g) | H2O (L) | CO2 |
| A | 33.06618 | 31.32234 | 25.56759 | 0.703029 | 30.092 | 203.606 | 24.99735 |
| B | −11.3634 | −20.2353 | 6.096130 | 108.4773 | 6.832514 | 1523.29 | 55.18696 |
| C | 11.43282 | 57.86644 | 4.054656 | 42.52157 | 6.793435 | 3196.413 | 33.69137 |
| D | −2.77287 | −36.5062 | 2.671301 | 5.862788 | −2.53448 | 2474.455 | 7.948387 |
| E | −0.15856 | −0.00737 | 0.131021 | 0.678565 | 0.082139 | 3.855326 | 0.136638 |
| F | −9.9808 | −8.90347 | 118.0089 | 76.84376 | −250.881 | 256.5478 | 403.6075 |
| G | 172.708 | 246.7945 | 227.3665 | 158.7163 | 223.3967 | 488.7163 | 228.2431 |
| H | 0 | 0 | 110.5271 | 74.87310 | −241.826 | 285.8304 | 393.5224 |
| Electrochemical System Type | Advantages | Disadvantages |
|---|---|---|
| Low Temperature: Alkaline, Phosphoric Acid, Polymer Electrolyte Membrane (PEM), Direct Methanol PEM | Simple to Operate, Fast Dynamic Response, Commercial Maturity | Lower Efficiency, Noble Metal Catalysts (e.g., Pt, Ru), Strict Fuel Purity Constraints |
| High Temperature: Molten Carbonate, Solid Oxide | High Efficiency, Heat Recovery Enables Higher Fuel Utilization, Non-Noble Metal Catalysts (e.g., Ni), Internal Hydrocarbon Fuel Processing Capability, Lower Sensitivity to Poisoning | Challenging Operational Conditions, Slower Dynamic Response, Pre-Commercial Maturity |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fang, T.; von Jouanne, A.; Yokochi, A. Review of Electrochemical Systems for Grid Scale Power Generation and Conversion: Low- and High-Temperature Fuel Cells and Electrolysis Processes. Energies 2025, 18, 2493. https://doi.org/10.3390/en18102493
Fang T, von Jouanne A, Yokochi A. Review of Electrochemical Systems for Grid Scale Power Generation and Conversion: Low- and High-Temperature Fuel Cells and Electrolysis Processes. Energies. 2025; 18(10):2493. https://doi.org/10.3390/en18102493
Chicago/Turabian StyleFang, Tingke, Annette von Jouanne, and Alex Yokochi. 2025. "Review of Electrochemical Systems for Grid Scale Power Generation and Conversion: Low- and High-Temperature Fuel Cells and Electrolysis Processes" Energies 18, no. 10: 2493. https://doi.org/10.3390/en18102493
APA StyleFang, T., von Jouanne, A., & Yokochi, A. (2025). Review of Electrochemical Systems for Grid Scale Power Generation and Conversion: Low- and High-Temperature Fuel Cells and Electrolysis Processes. Energies, 18(10), 2493. https://doi.org/10.3390/en18102493






