Wide-Bandgap Subcells for All-Perovskite Tandem Solar Cells: Recent Advances, Challenges, and Future Perspectives
Abstract
1. Introduction
2. Composition Engineering
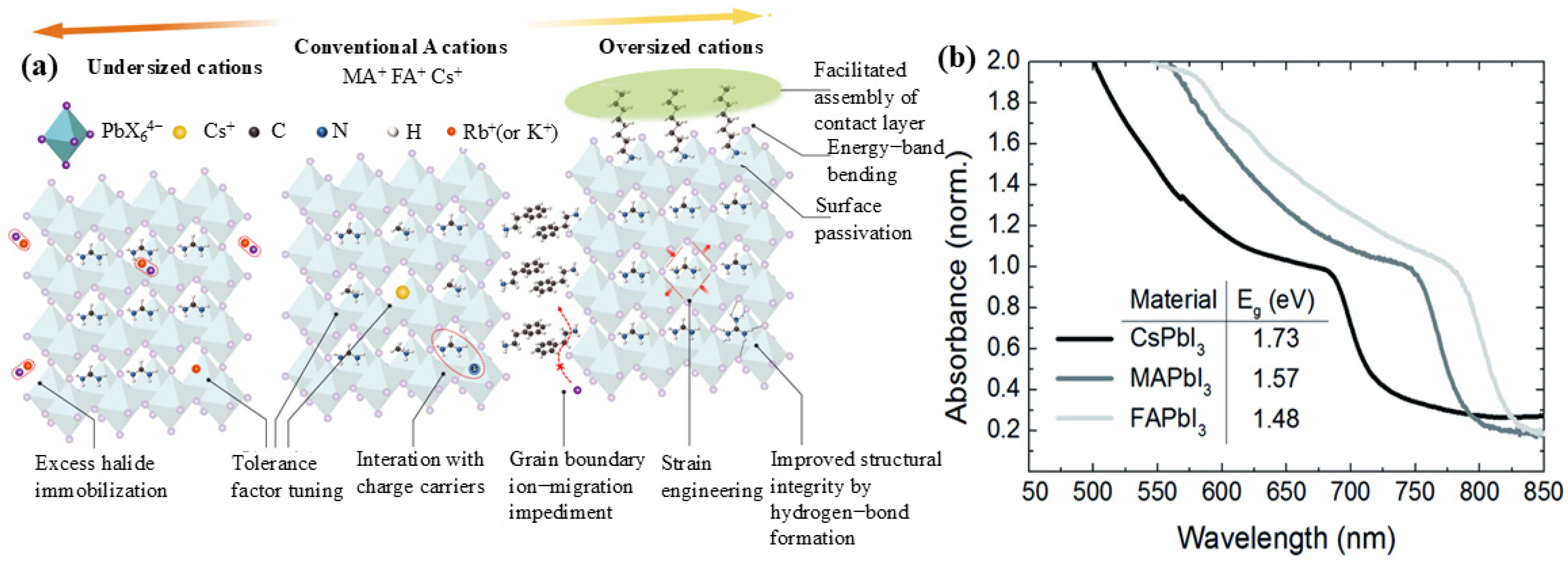
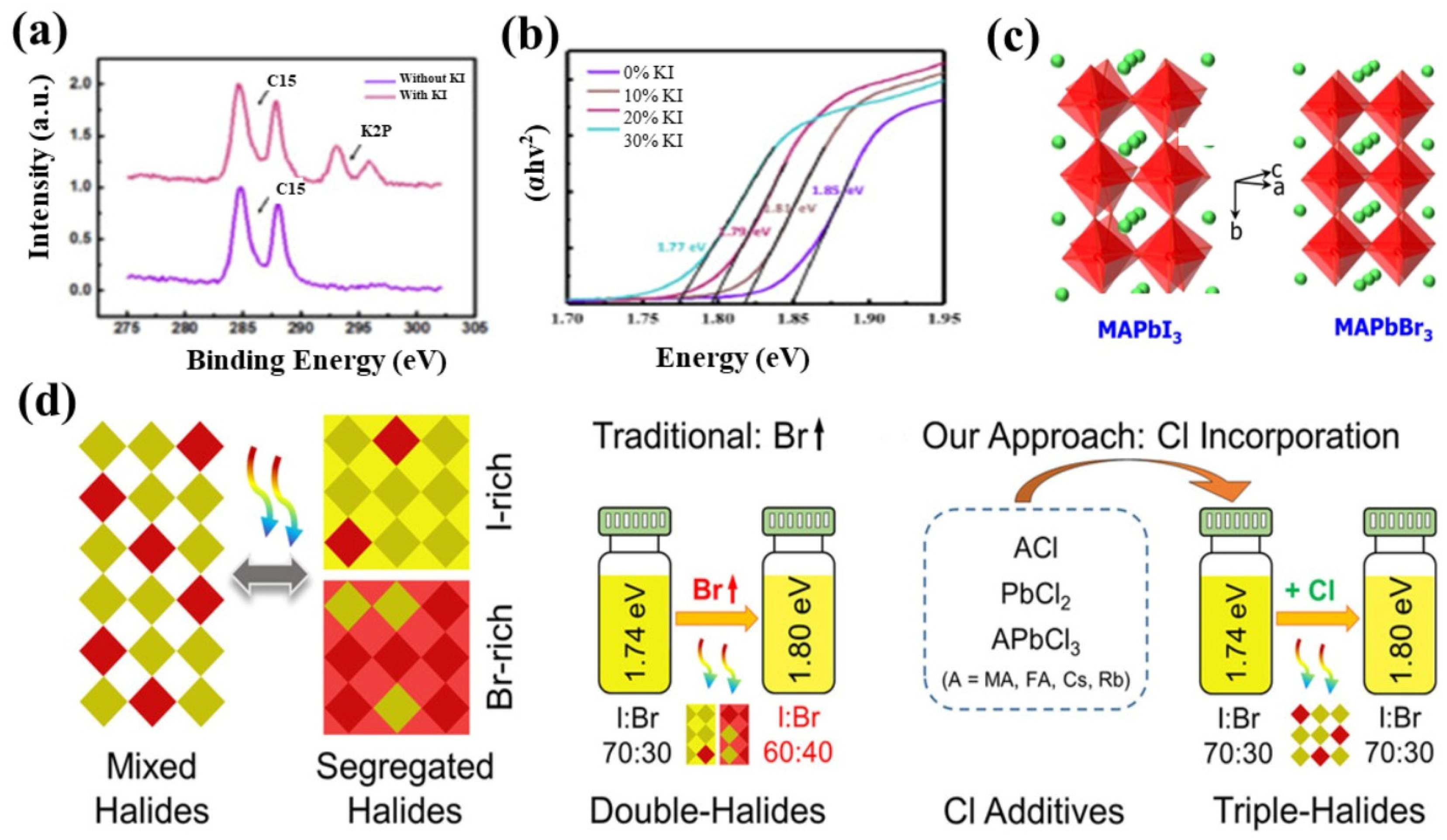
2.1. A-Site Cations, B-Site Cations, and X-Site Anions
2.2. Other Specific Additives
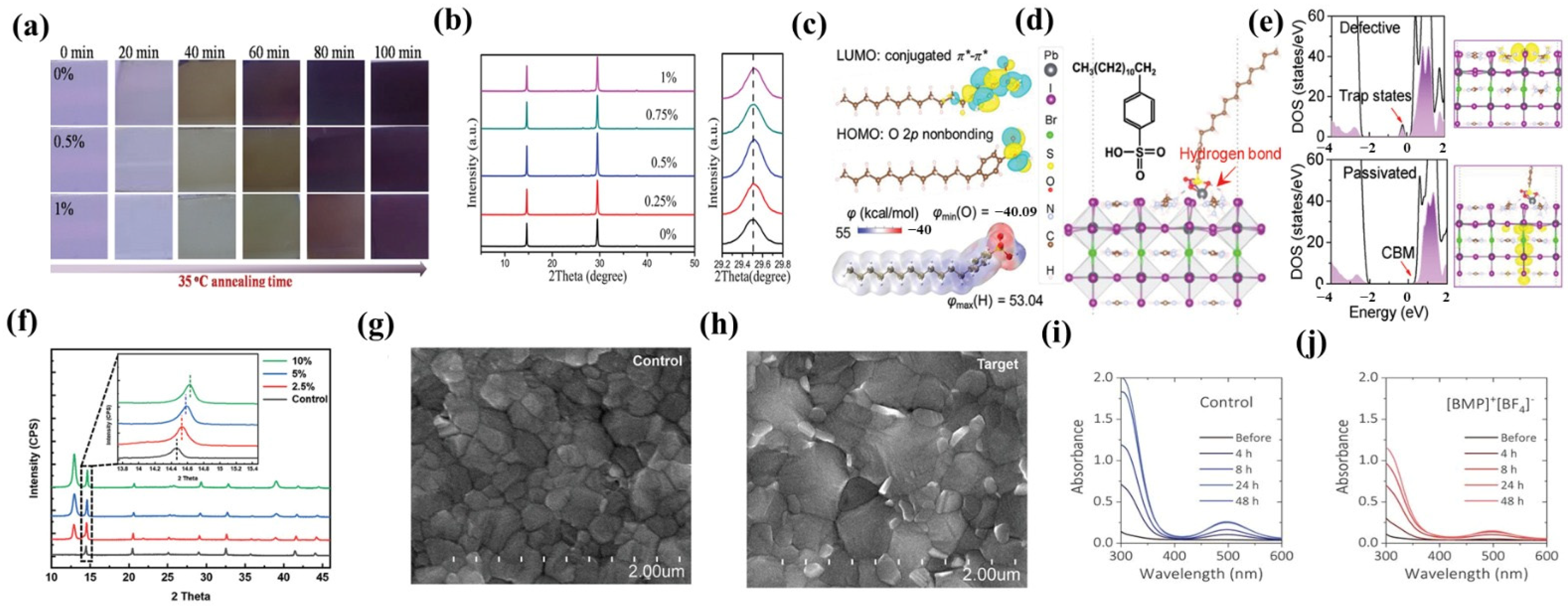
3. Interface Modification
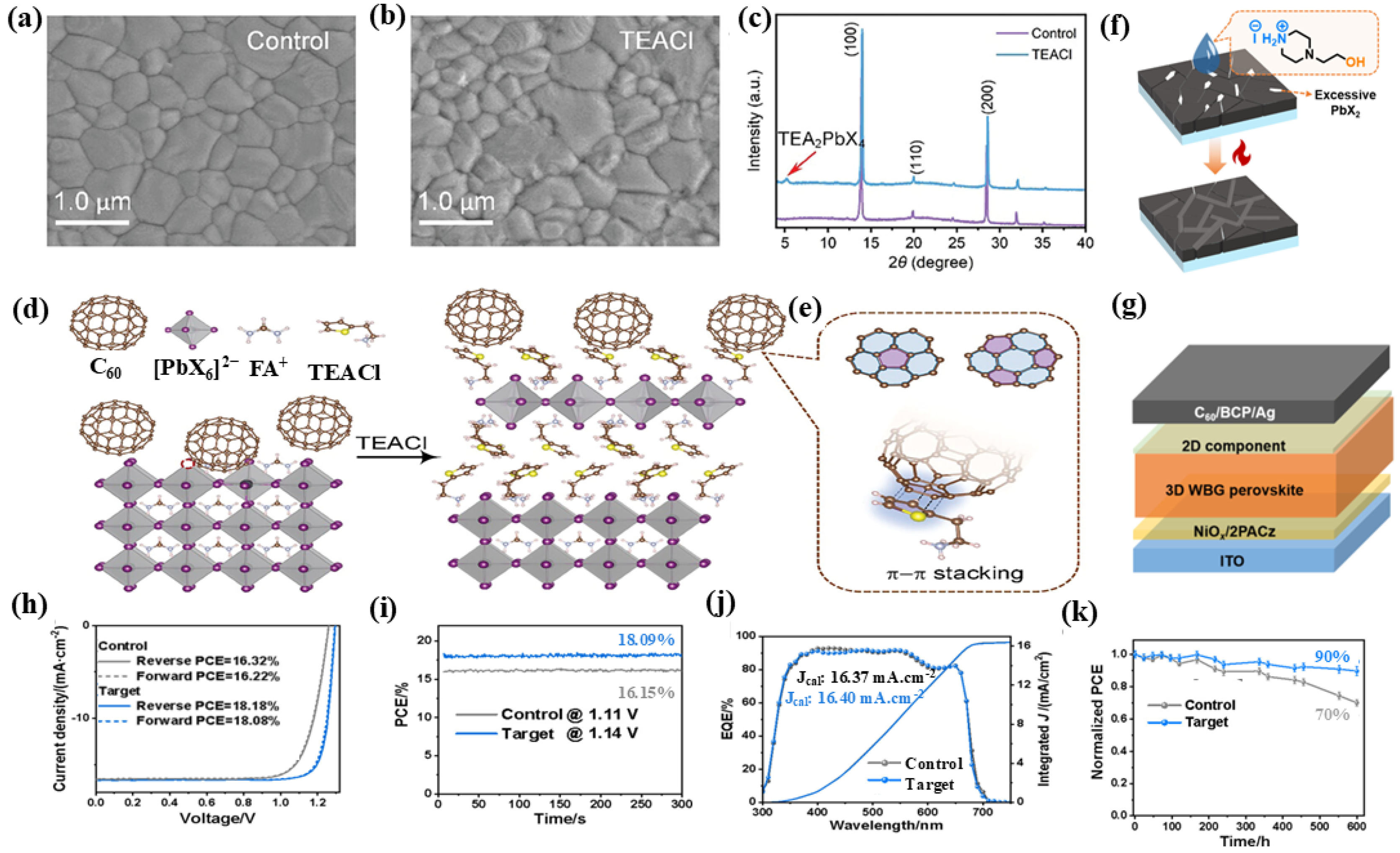
3.1. Upper Interface Treatment
3.2. Bottom Interface Modification
4. Conclusions and Outlook
- (1)
- Scalability: The large-scale commercialization of APTSCs requires advancements in scalable fabrication techniques. While spin-coating yields high efficiencies, it is unsuitable for industrial production. Alternative deposition methods such as slot-die coating, blade coating, and chemical vapor deposition (CVD) should be optimized to control film crystallization, suppress defects, and enhance reproducibility. Ink formulation and solvent engineering must also be refined for better wetting properties and drying kinetics. Beyond deposition, interface engineering is crucial for maintaining high efficiency in large-area devices. Developing robust charge transport layers with low defect densities can improve carrier extraction. Additionally, industrial encapsulation strategies are necessary to protect devices from environmental degradation. Roll-to-roll manufacturing and vacuum-based deposition should also be explored for continuous, high-yield production. Addressing these scalability challenges will enable commercialization for residential, commercial, and industrial applications.
- (2)
- Long-Term Stability: The stability of WBG perovskites remains a critical challenge due to phase segregation, ion migration, and environmental degradation. These issues lead to efficiency losses under prolonged illumination and thermal stress. Compositional engineering, such as incorporating mixed A-site cations (Cs, FA, and MA) and dopants, can help stabilize the perovskite structure. Surface passivation techniques, including SAMs and low-dimensional perovskite coatings, can further mitigate defects and suppress non-radiative recombination. Interfacial stability is equally crucial, as degradation at charge transport layers accelerates device failure. Developing chemically stable transport layers with improved energy level alignment can reduce interfacial recombination. Additionally, advanced encapsulation strategies, such as multilayer moisture barriers and UV-resistant coatings, can prolong the device’s lifespan. By addressing these stability challenges, all-perovskite tandem solar cells can achieve operational durability comparable to commercial photovoltaics.
- (3)
- Flexible Device Applications: Flexible APTSCs hold great potential for wearable electronics, portable power, and aerospace applications. However, achieving high efficiency while maintaining mechanical stability requires innovation in flexible substrates and electrode materials. Transparent polymer substrates such as PET and PEN must exhibit high thermal stability and low water permeability. Alternative flexible electrodes, such as silver nanowires and carbon-based materials, should be developed to maintain conductivity while enhancing flexibility. To improve mechanical robustness, strain-tolerant perovskite compositions and interfacial engineering strategies must be explored to prevent cracking and delamination. Advanced encapsulation, such as ultra-thin glass coatings, can enhance both mechanical durability and environmental stability. Scalable roll-to-roll and inkjet printing processes also offer cost-effective pathways for high-throughput production. These advancements will expand the application of APTSCs beyond rigid photovoltaic panels to emerging technologies.
- (4)
- Machine learning-guided material discovery: Machine learning can be cost-effectively employed to screen and design various additives and passivation materials for the WBG top subcell in APTSCs. It enables precise optimization of existing device parameters to enhance photovoltaic efficiency, while accurately predicting the stability boundaries and defect formation energy in WBG perovskites. Through multi-objective optimization algorithms, this approach effectively balances critical performance metrics, including transmittance, carrier mobility, and interfacial compatibility. Looking forward, machine learning holds the potential to integrate material discovery with device optimization into a closed-loop workflow, progressively automating the development process and advancing toward industrial-scale applications. Researchers could synergize real-time experimental feedback with cross-scale simulations to accelerate efficiency improvements in WBG top subcells for APTSCs while driving innovations in eco-friendly synthesis processes and scalable fabrication techniques.
- (5)
- Semi-transparent WBG PSCs: Semi-transparent PSCs exhibit considerable optical transmittance, allowing unabsorbed photons to pass through the device. This enables the underlying NBG subcell to harvest additional photons, thereby enhancing the PCE of the APTSCs and advancing the efficiency development of APTSCs. Future research directions could explore the implementation of semi-transparent solar cell designs for both WBG and NBG subcells. This strategy would confer unique advantages in building integrated photovoltaic applications, where such dual-functional devices could serve as power-generating architectural components, such as photovoltaic windows and curtain walls, while maintaining sufficient visible light transmission for indoor illumination requirements. This integrated approach could potentially enable building energy self-sufficiency through onsite electricity generation, simultaneously reducing operational energy consumption in modern construction.
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| PSCs | Perovskite solar cells |
| PCE | Power conversion efficiency |
| TSCs | Tandem solar cells |
| WBG | Wide bandgap |
| NBG | Narrow bandgap |
| APTSCs | All-perovskite tandem solar cells |
| 2-T | Two-terminal |
| 4-T | Four-terminal |
| Voc | Open-circuit voltages |
| FA+ | Formamidine |
| MA+ | Methylammonium |
| Cs+ | Cesium |
| Rb+ | Rubidium |
| CsFa | Cesium formate |
| RbSCN | Rubidium thiocyanate |
| KI | Potassium iodide |
| DMA | Dimethylamine |
| F-PEA+ | 2-(4-fluorophenyl) ethylammonium |
| MDA2+ | Methylenediammonium cations |
| w/o | Without |
| Pb2+ | Lead |
| Sn2+ | Tin |
| Cu2+ | Copper |
| I− | Iodine |
| Br− | Bromine |
| Cl− | Chlorine |
| SCN− | Thiocyanate |
| J–V | Density–voltage |
| EQE | External quantum efficiency |
| ClFA+ | Chloro-formamidinium |
| GBAC | 4-guanidinobenzoic acid hydrochloride |
| DBSA | Dodecyl-benzene-sulfonic-acid |
| HA | Histamine |
| VI | Iodine vacancies |
| TEACl | 2-thiopheneethylammonium chloride |
References
- Zhang, J.W.; Yin, X.T.; Iqbal, S.; Que, W.X. All-Perovskite Tandem Solar Cells: Rapid Development of Thin Film Photovoltaic Technology. Adv. Sustain. Syst. 2023, 7, 2300188. [Google Scholar] [CrossRef]
- Lin, R.; Wang, Y.; Lu, Q.; Tang, B.; Li, J.; Gao, H.; Gao, Y.; Li, H.; Ding, C.; Wen, J. All-perovskite tandem solar cells with 3D/3D bilayer perovskite heterojunction. Nature 2023, 620, 994–1000. [Google Scholar] [CrossRef]
- Min, H.; Lee, D.Y.; Kim, J.; Kim, G.; Lee, K.S.; Kim, J.; Paik, M.J.; Kim, Y.K.; Kim, K.S.; Kim, M.G. Perovskite solar cells with atomically coherent interlayers on SnO2 electrodes. Nature 2021, 598, 444–450. [Google Scholar] [CrossRef]
- Hou, Y.; Aydin, E.; De Bastiani, M.; Xiao, C.; Isikgor, F.H.; Xue, D.-J.; Chen, B.; Chen, H.; Bahrami, B.; Chowdhury, A.H. Efficient tandem solar cells with solution-processed perovskite on textured crystalline silicon. Science 2020, 367, 1135–1140. [Google Scholar] [CrossRef] [PubMed]
- Bairami, S.; Salimi, M.; Mirabbasi, D. A novel method for maximum power point tracking of the grid-connected three-phase solar systems based on the PV current prediction. Chin. J. Electron. 2023, 32, 353–364. [Google Scholar] [CrossRef]
- Paiano, P.; Prete, P.; Speiser, E.; Lovergine, N.; Richter, W.; Tapfer, L.; Mancini, A.M. GaAs nanowires grown by Au-catalyst-assisted MOVPE using tertiarybutylarsine as group-V precursor. J. Cryst. Growth 2007, 298, 620–624. [Google Scholar] [CrossRef]
- Persano, A.; Nabet, B.; Taurino, A.; Prete, P.; Lovergine, N.; Cola, A. Polarization anisotropy of individual core/shell GaAs/AlGaAs nanowires by photocurrent spectroscopy. Appl. Phys. Let. 2011, 98, 153106. [Google Scholar] [CrossRef]
- Cingolani, R.; Lomascolo, M.; Lovergine, N.; Dabbicco, M.; Ferrara, M.; Suemune, I. Excitonic properties of ZnSe/ZnSeS superlattices. Appl. Phys. Lett. 1994, 64, 2439–2441. [Google Scholar] [CrossRef]
- Jia, X.; Yang, D.; Zheng, D.; Chang, Z.; Liu, J.; Liu, L.; Peng, L.; Tong, Y.; Wang, K.; Liu, S. The progress and challenges of tin-lead alloyed perovskites: Toward the development of large-scale all-perovskite tandem solar cells. Chem 2025, 11, 102384. [Google Scholar] [CrossRef]
- (NREL). Best Research-Cell Efficiency Chart. Available online: https://www.nrel.gov/pv/cell-efficiency.html (accessed on 5 March 2025).
- Ehrler, B.; Alarcón-Lladó, E.; Tabernig, S.W.; Veeken, T.; Garnett, E.C.; Polman, A. Photovoltaics Reaching for the Shockley–Queisser Limit. ACS Energy Lett. 2020, 5, 3029–3033. [Google Scholar] [CrossRef]
- Hörantner, M.T.; Leijtens, T.; Ziffer, M.E.; Eperon, G.E.; Christoforo, M.G.; McGehee, M.D.; Snaith, H.J. The Potential of Multijunction Perovskite Solar Cells. ACS Energy Lett. 2017, 2, 2506–2513. [Google Scholar] [CrossRef]
- Yang, Y.; Li, F.; Chen, R.; Wang, H. Interface engineering of inverted wide-bandgap perovskite solar cells for tandem photovoltaics. Energy Mater. Devices 2024, 2, 9370031. [Google Scholar] [CrossRef]
- Lim, J.; Park, N.G.; Il Seok, S.; Saliba, M. All-perovskite tandem solar cells: From fundamentals to technological progress. Energy Environ. Sci. 2024, 17, 4390–4425. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.; Zhu, J.; Zhao, D. Progress and prospects for all-perovskite tandem solar cells. J. Energy Chem. 2025, 103, 90–96. [Google Scholar] [CrossRef]
- Saki, Z.; Byranvand, M.M.; Taghavinia, N.; Kedia, M.; Saliba, M. Solution-processed perovskite thin-films: The journey from lab- to large-scale solar cells. Energy Environ. Sci. 2021, 14, 5690–5722. [Google Scholar] [CrossRef]
- Xiao, K.; Lin, R.; Han, Q.; Hou, Y.; Qin, Z.; Nguyen, H.T.; Wen, J.; Wei, M.; Yeddu, V.; Saidaminov, M.I. All-perovskite tandem solar cells with 24.2% certified efficiency and area over 1 cm2 using surface-anchoring zwitterionic antioxidant. Nat. Energy 2020, 5, 870–880. [Google Scholar] [CrossRef]
- Lee, J.W.; Tan, S.; Seok, S.I.; Yang, Y.; Park, N.G. Rethinking the A cation in halide perovskites. Science 2022, 375, eabj1186. [Google Scholar] [CrossRef]
- Zhang, J.; Fu, J.; Wang, K.; Zhang, C.; Wang, Y.; Wang, R.; Zhao, J.; Zhong, X.; Ren, H.; Hou, G.; et al. Performance promotion strategies for wide bandgap perovskite solar cells. Sustain. Energy Fuels 2025, 9, 303–322. [Google Scholar] [CrossRef]
- Guo, Q.; Ding, Y.; Dai, Z.; Chen, Z.; Du, M.; Wang, Z.; Gao, L.; Duan, C.; Guo, Q.; Zhou, E. Multiple-cation wide-bandgap perovskite solar cells grown using cesium formate as the Cs precursor with high efficiency under sunlight and indoor illumination. Phys. Chem. Chem. Phys. 2022, 24, 17526–17534. [Google Scholar] [CrossRef]
- Asmontas, S.; Mujahid, M. Recent Progress in Perovskite Tandem Solar Cells. Nanomaterials 2023, 13, 1886. [Google Scholar] [CrossRef]
- Li, Y.; Yao, D.-S.; Long, F.; Zhang, G.-Z.; Yu, F. Interfacial engineering for efficient and stable two-terminal perovskite-based tandem solar cells. Rare Met. 2024, 43, 5573–5624. [Google Scholar]
- Zhang, W.; Fang, J. Stable all-perovskite tandems with interfacial dipole-bridged inorganic wide-bandgap perovskite subcells. Sci. China Chem. 2023, 66, 2445–2446. [Google Scholar] [CrossRef]
- Guan, H.; Fu, S.; Chen, W.; Ke, W.; Fang, G.; Feng, W. Challenges and Perspectives toward Wide-Bandgap Perovskite Subcell in Four-terminal All-Perovskite Tandem Solar Cells. DeCarbon 2025, 8, 100098. [Google Scholar] [CrossRef]
- Deng, X.; Zhang, D.; Chen, Y.; Yang, S. Characteristic Mode Analysis: Application to Electromagnetic Radiation, Scattering, and Coupling Problems. Chin. J. Electron. 2023, 32, 663–677. [Google Scholar] [CrossRef]
- Yoon, G.W.; Jo, B.; Boonmongkolras, P.; Han, G.S.; Jung, H.S. Perovskite tandem solar cells for low earth orbit satellite power applications. Adv. Energy Mater. 2024, 15, 2400204. [Google Scholar] [CrossRef]
- Tong, J.; Jiang, Q.; Zhang, F.; Kang, S.B.; Kim, D.H.; Zhu, K. Wide-Bandgap Metal Halide Perovskites for Tandem Solar Cells. ACS Energy Lett. 2021, 6, 232–248. [Google Scholar] [CrossRef] [PubMed]
- Leijtens, T.; Bush, K.A.; Prasanna, R.; McGehee, M.D. Opportunities and challenges for tandem solar cells using metal halide perovskite semiconductors. Nat. Energy 2018, 3, 828–838. [Google Scholar] [CrossRef]
- Liu, L.; Zheng, D.; Du, M.; Liu, J.; Liu, J.; Li, Z.; Dong, X.; Xu, C.; He, Y.; Wang, K.; et al. Advancements and Challenges in Wide-Bandgap Perovskite Solar Cells: From Single Junction to Tandem Solar Cells. Sol. RRL 2024, 8, 2400359. [Google Scholar] [CrossRef]
- He, R.; Ren, S.; Chen, C.; Yi, Z.; Luo, Y.; Lai, H.; Wang, W.; Zeng, G.; Hao, X.; Wang, Y. Wide-bandgap organic–inorganic hybrid and all-inorganic perovskite solar cells and their application in all-perovskite tandem solar cells. Energy Environ. Sci. 2021, 14, 5723–5759. [Google Scholar] [CrossRef]
- Islam, M.R.; Wu, Y.; Liu, K.; Wang, Z.; Qu, S.; Wang, Z. Recent progress and future prospects for light management of all-perovskite tandem solar cells. Adv. Mater. Interfaces 2022, 9, 2101144. [Google Scholar] [CrossRef]
- Shan, H.; Li, C.; Li, X.; Li, M.; Song, Y.; Ma, S.; Xu, B. Model Parameter Extraction for InGaN/GaN Multiple Quantum Well-Based Solar Cells Using Dynamic Programming. Chin. J. Electron. 2024, 34, 412–421. [Google Scholar] [CrossRef]
- Saliba, M.; Correa-Baena, J.P.; Gratzel, M.; Hagfeldt, A.; Abate, A. Perovskite Solar Cells: From the Atomic Level to Film Quality and Device Performance. Angew Chem. Int. Edit. 2018, 57, 2554–2569. [Google Scholar] [CrossRef]
- Song, Z.; Chen, C.; Li, C.; Awni, R.A.; Zhao, D.; Yan, Y. Wide-bandgap, low-bandgap, and tandem perovskite solar cells. Semicond. Sci. Technol. 2019, 34, 093001. [Google Scholar] [CrossRef]
- Chen, M.; Kapil, G.; Li, Y.; Kamarudin, M.A.; Baranwal, A.K.; Nishimura, K.; Sahamir, S.R.; Sanehira, Y.; Li, H.; Ding, C.; et al. Large synergy effects of doping, a site substitution, and surface passivation in wide bandgap Pb-free ASnI2Br perovskite solar cells on efficiency and stability enhancement. J. Power Sources 2022, 520, 230848. [Google Scholar] [CrossRef]
- Zuo, C.; Tan, L.; Dong, H.; Chen, J.; Hao, F.; Yi, C.; Ding, L. Natural drying yields efficient perovskite solar cells. DeCarbon 2023, 2, 100020. [Google Scholar] [CrossRef]
- Dang, H.X.; Wang, K.; Ghasemi, M.; Tang, M.-C.; De Bastiani, M.; Aydin, E.; Dauzon, E.; Barrit, D.; Peng, J.; Smilgies, D.-M.; et al. Multi-cation Synergy Suppresses Phase Segregation in Mixed-Halide Perovskites. Joule 2019, 3, 1746–1764. [Google Scholar] [CrossRef]
- Jiang, Z.; Sha, Y.; Nie, L.; Xuan, X. A Beam-Steering Broadband Microstrip Antenna with High Isolation. Chin. J. Electron. 2023, 32, 325–333. [Google Scholar] [CrossRef]
- Siekmann, J.; Ravishankar, S.; Kirchartz, T. Apparent Defect Densities in Halide Perovskite Thin Films and Single Crystals. ACS Energy Lett. 2021, 6, 3244–3251. [Google Scholar] [CrossRef]
- Fang, Z.; Yan, N.; Liu, S. Modulating preferred crystal orientation for efficient and stable perovskite solar cells—From progress to perspectives. InfoMat 2022, 4, e12369. [Google Scholar] [CrossRef]
- Chen, P.; Xiao, Y.; Li, S.; Jia, X.; Luo, D.; Zhang, W.; Snaith, H.J.; Gong, Q.; Zhu, R. The Promise and Challenges of Inverted Perovskite Solar Cells. Chem. Rev. 2024, 124, 10623–10700. [Google Scholar] [CrossRef]
- Mitzi, D.B. Synthesis, structure, and properties of organic-inorganic perovskites and related materials. Prog. Inorg. Chem. 1999, 48, 1–121. [Google Scholar] [CrossRef]
- Eperon, G.E.; Stranks, S.D.; Menelaou, C.; Johnston, M.B.; Herz, L.M.; Snaith, H.J. Formamidinium lead trihalide: A broadly tunable perovskite for efficient planar heterojunction solar cells. Energy Environ. Sci. 2014, 7, 982–998. [Google Scholar] [CrossRef]
- Zhou, Y.; Jia, Y.H.; Fang, H.H.; Loi, M.A.; Xie, F.Y.; Gong, L.; Qin, M.C.; Lu, X.H.; Wong, C.P.; Zhao, N. Composition-Tuned Wide Bandgap Perovskites: From Grain Engineering to Stability and Performance Improvement. Adv. Funct. Mater. 2018, 28, 1803130. [Google Scholar] [CrossRef]
- Han, Q.; Bae, S.H.; Sun, P.; Hsieh, Y.T.; Yang, Y.M.; Rim, Y.S.; Zhao, H.; Chen, Q.; Shi, W.; Li, G.; et al. Single Crystal Formamidinium Lead Iodide (FAPbI3): Insight into the Structural, Optical, and Electrical Properties. Adv. Mater. 2016, 28, 2253–2258. [Google Scholar] [CrossRef]
- Philippe, B.; Saliba, M.; Correa-Baena, J.-P.; Cappel, U.B.; Turren-Cruz, S.-H.; Grätzel, M.; Hagfeldt, A.; Rensmo, H. Chemical Distribution of Multiple Cation (Rb+, Cs+, MA+, and FA+) Perovskite Materials by Photoelectron Spectroscopy. Chem. Mater. 2017, 29, 3589–3596. [Google Scholar] [CrossRef]
- Zhang, B.; Liao, Y.; Tong, L.; Yang, Y.; Wang, X. Ion migration in Br-doped MAPbI3 and its inhibition mechanisms investigated via quantum dynamics simulations. Phys. Chem. Chem. Phys. 2020, 22, 7778–7786. [Google Scholar] [CrossRef] [PubMed]
- Prasanna, R.; Gold-Parker, A.; Leijtens, T.; Conings, B.; Babayigit, A.; Boyen, H.G.; Toney, M.F.; McGehee, M.D. Band Gap Tuning via Lattice Contraction and Octahedral Tilting in Perovskite Materials for Photovoltaics. J. Am. Chem. Soc. 2017, 139, 11117–11124. [Google Scholar] [CrossRef]
- Xie, Y.M.; Xu, X.; Ma, C.; Li, M.; Ma, Y.; Lee, C.S.; Tsang, S.W. Synergistic Effect of Pseudo-Halide Thiocyanate Anion and Cesium Cation on Realizing High-Performance Pinhole-Free MA-Based Wide-Band Gap Perovskites. ACS Appl. Mater. Interfaces 2019, 11, 25909–25916. [Google Scholar] [CrossRef]
- Wang, Z.; Han, Z.; Chu, X.; Zhou, H.; Yu, S.; Zhang, Q.; Xiong, Z.; Qu, Z.; Tian, H.; Wang, W.; et al. Regulation of Wide Bandgap Perovskite by Rubidium Thiocyanate for Efficient Silicon/Perovskite Tandem Solar Cells. Adv. Mater. 2024, 36, e2407681. [Google Scholar] [CrossRef]
- Duong, T.; Wu, Y.; Shen, H.; Peng, J.; Fu, X.; Jacobs, D.; Wang, E.C.; Kho, T.C.; Fong, K.C.; Stocks, M.; et al. Rubidium Multication Perovskite with Optimized Bandgap for Perovskite-Silicon Tandem with over 26% Efficiency. Adv. Energy Mater. 2017, 7, 1700228. [Google Scholar] [CrossRef]
- Sun, H.; Liu, S.; Liu, X.; Gao, Y.; Wang, J.; Shi, C.; Raza, H.; Sun, Z.; Pan, Y.; Cai, Y.; et al. Suppressed Phase Segregation with Small A-Site and Large X-Site Incorporation for Photostable Wide-Bandgap Perovskite Solar Cells. Small Methods 2024, 8, e2400067. [Google Scholar] [CrossRef] [PubMed]
- Saliba, M.; Matsui, T.; Seo, J.Y.; Domanski, K.; Correa-Baena, J.P.; Nazeeruddin, M.K.; Zakeeruddin, S.M.; Tress, W.; Abate, A.; Hagfeldt, A.; et al. Cesium-containing triple cation perovskite solar cells: Improved stability, reproducibility and high efficiency. Energy Environ. Sci. 2016, 9, 1989–1997. [Google Scholar] [CrossRef]
- Saidaminov, M.I.; Kim, J.; Jain, A.; Quintero-Bermudez, R.; Tan, H.; Long, G.; Tan, F.; Johnston, A.; Zhao, Y.; Voznyy, O.; et al. Suppression of atomic vacancies via incorporation of isovalent small ions to increase the stability of halide perovskite solar cells in ambient air. Nat. Energy 2018, 3, 648–654. [Google Scholar] [CrossRef]
- Zhao, X.; Cao, J.; Nie, T.; Liu, S.; Fang, Z. Cation Engineering for Efficient and Stable Wide-Bandgap Perovskite Solar Cells. Sol. RRL 2024, 8, 2400521. [Google Scholar] [CrossRef]
- Son, D.Y.; Kim, S.G.; Seo, J.Y.; Lee, S.H.; Shin, H.; Lee, D.; Park, N.G. Universal Approach toward Hysteresis-Free Perovskite Solar Cell via Defect Engineering. J. Am. Chem. Soc. 2018, 140, 1358–1364. [Google Scholar] [CrossRef]
- Palmstrom, A.F.; Eperon, G.E.; Leijtens, T.; Prasanna, R.; Habisreutinger, S.N.; Nemeth, W.; Gaulding, E.A.; Dunfield, S.P.; Reese, M.; Nanayakkara, S.; et al. Enabling Flexible All-Perovskite Tandem Solar Cells. Joule 2019, 3, 2193–2204. [Google Scholar] [CrossRef]
- Xie, Y.-M.; Ma, C.; Xu, X.; Li, M.; Ma, Y.; Wang, J.; Chandran, H.T.; Lee, C.-S.; Tsang, S.-W. Revealing the crystallization process and realizing uniform 1.8 eV MA-based wide-bandgap mixed-halide perovskites via solution engineering. Nano Res. 2019, 12, 1033–1039. [Google Scholar] [CrossRef]
- Zhou, J.; Wen, T.; Sun, J.; Shi, Z.; Zou, C.; Shen, Z.; Li, Y.; Wang, Y.; Lin, Y.; Yang, S.; et al. Phase-Stable Wide-Bandgap Perovskites with 2D/3D Structure for All-Perovskite Tandem Solar Cells. ACS Energy Lett. 2024, 9, 1984–1992. [Google Scholar] [CrossRef]
- Tian, L.; Bi, E.; Yavuz, I.; Deger, C.; Tian, Y.; Zhou, J.; Zhang, S.; Liu, Q.; Shen, J.; Yao, L.; et al. Divalent cation replacement strategy stabilizes wide-bandgap perovskite for Cu(In,Ga)Se2 tandem solar cells. Nat. Photonics 2025, 19, 479–485. [Google Scholar] [CrossRef]
- Tao, L.; Qiu, J.; Sun, B.; Wang, X.; Ran, X.; Song, L.; Shi, W.; Zhong, Q.; Li, P.; Zhang, H.; et al. Stability of mixed-halide wide bandgap perovskite solar cells: Strategies and progress. J. Energy Chem. 2021, 61, 395–415. [Google Scholar] [CrossRef]
- Pandey, P.; Cho, S.; Hayase, S.; Sang Cho, J.; Kang, D.-W. New strategies to develop High-Efficiency Lead-Free wide bandgap perovskite solar cells. Chem. Eng. J. 2022, 448, 137622. [Google Scholar] [CrossRef]
- Wang, K.L.; Wang, R.; Wang, Z.K.; Li, M.; Zhang, Y.; Ma, H.; Liao, L.S.; Yang, Y. Tailored Phase Transformation of CsPbI2Br Films by Copper(II) Bromide for High-Performance All-Inorganic Perovskite Solar Cells. Nano Lett. 2019, 19, 5176–5184. [Google Scholar] [CrossRef]
- Cao, Y.; Liu, H.; Gao, F.; Li, D.; Xiang, L.; Gao, J.; Gao, P.; Zhang, Y.; Li, S. Interface optimization and growth control for high efficiency wide bandgap perovskite solar cells. Surf. Interfaces 2023, 37, 102680. [Google Scholar] [CrossRef]
- Tanaka, K.; Takahashi, T.; Ban, T.; Kondo, T.; Uchida, K.; Miura, N. Comparative study on the excitons in lead-halide-based perovskite-type crystals CH3NH3PbBr3 CH3NH3PbI3. Solid State Commun. 2003, 127, 619–623. [Google Scholar] [CrossRef]
- Noh, J.H.; Im, S.H.; Heo, J.H.; Mandal, T.N.; Seok, S.I. Chemical management for colorful, efficient, and stable inorganic-organic hybrid nanostructured solar cells. Nano Lett. 2013, 13, 1764–1769. [Google Scholar] [CrossRef]
- Chiang, Y.H.; Frohna, K.; Salway, H.; Abfalterer, A.; Pan, L.; Roose, B.; Anaya, M.; Stranks, S.D. Vacuum-Deposited Wide-Bandgap Perovskite for All-Perovskite Tandem Solar Cells. ACS Energy Lett. 2023, 8, 2728–2737. [Google Scholar] [CrossRef] [PubMed]
- Fang, Z.; Nie, T.; Liu, S.; Ding, J. Overcoming Phase Segregation in Wide-Bandgap Perovskites: From Progress to Perspective. Adv. Funct. Mater. 2024, 34, 2404402. [Google Scholar] [CrossRef]
- Siegrist, S.; Quintana Ceres, P.; Marrugat Arnal, V.; Kothandaraman, R.K.; Kurisinkal Pious, J.; Lai, H.; Vlnieska, V.; Tiwari, A.N.; Fu, F. Unveiling the Role of Cl Incorporation Enables Scalable MA-Free Triple-Halide Wide-Bandgap Perovskites for Slot-Die-Coated Photovoltaic Modules. Sol. RRL 2025, 8, 2949–8813. [Google Scholar] [CrossRef]
- Han, Y.; Zhao, H.; Duan, C.; Yang, S.; Yang, Z.; Liu, Z.; Liu, S. Controlled n-Doping in Air-Stable CsPbI2Br Perovskite Solar Cells with a Record Efficiency of 16.79%. Adv. Funct. Mater. 2020, 30, 1909972. [Google Scholar] [CrossRef]
- Qin, S.; Lu, C.; Jia, Z.; Wang, Y.; Li, S.; Lai, W.; Shi, P.; Wang, R.; Zhu, C.; Du, J.; et al. Constructing Monolithic Perovskite/Organic Tandem Solar Cell with Efficiency of 22.0% via Reduced Open-Circuit Voltage Loss and Broadened Absorption Spectra. Adv. Mater. 2022, 34, e2108829. [Google Scholar] [CrossRef]
- Kim, D.H.; Muzzillo, C.P.; Tong, J.; Palmstrom, A.F.; Larson, B.W.; Choi, C.; Harvey, S.P.; Glynn, S.; Whitaker, J.B.; Zhang, F.; et al. Bimolecular Additives Improve Wide-Band-Gap Perovskites for Efficient Tandem Solar Cells with CIGS. Joule 2019, 3, 1734–1745. [Google Scholar] [CrossRef]
- Zhao, Y.; Wang, C.; Ma, T.; Zhou, L.; Wu, Z.; Wang, H.; Chen, C.; Yu, Z.; Sun, W.; Wang, A. Reduced 0.418 V VOC-deficit of 1.73 eV wide-bandgap perovskite solar cells assisted by dual chlorides for efficient all-perovskite tandems. Energy Environ. Sci. 2023, 16, 2080–2089. [Google Scholar] [CrossRef]
- Guan, H.; Zhou, S.; Fu, S.; Pu, D.; Chen, X.; Ge, Y.; Wang, S.; Wang, C.; Cui, H.; Liang, J.; et al. Regulating Crystal Orientation via Ligand Anchoring Enables Efficient Wide-Bandgap Perovskite Solar Cells and Tandems. Adv. Mater. 2024, 36, e2307987. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.H.; Sakai, N.; Da, P.; Wu, J.; Sansom, H.C.; Ramadan, A.J.; Mahesh, S.; Liu, J.; Oliver, R.D.J.; Lim, J.; et al. A piperidinium salt stabilizes efficient metal-halide perovskite solar cells. Science 2020, 369, 96–102. [Google Scholar] [CrossRef] [PubMed]
- Gu, X.; Xiang, W.; Tian, Q.; Liu, S.F. Rational Surface-Defect Control via Designed Passivation for High-Efficiency Inorganic Perovskite Solar Cells. Angew. Chem. Int. Edit. 2021, 60, 23164–23170. [Google Scholar] [CrossRef]
- Li, J.; Zhu, S.; Yin, C.; Chen, C.; Yuan, J.; Zhao, H.; Gong, H.; Yang, B.; Zheng, D.; Xing, G.; et al. Homogenizing the Halogen Distribution via a Multifunctional Fluorine-Containing Additive Toward High-Performance Inverted Wide-Bandgap Perovskite Solar Cells. Adv. Funct. Mater. 2025, 2422175. [Google Scholar] [CrossRef]
- Lee, H.B.; Kumar, N.; Tyagi, B.; Ko, K.-J.; Kang, J.-W. Dimensionality and Defect Engineering Using Fluoroaromatic Cations for Efficiency and Stability Enhancement in 3D/2D Perovskite Photovoltaics. Sol. RRL 2020, 5, 2000589. [Google Scholar] [CrossRef]
- Zhang, A.; Li, M.; Dong, C.; Ye, W.; Yang, X.; Shaker, A.; Salem, M.S.; Li, Z.; Yang, J.; Li, X.; et al. pi-pi Stacking at the Perovskite/C60 Interface Enables High-Efficiency Wide-Bandgap Perovskite Solar Cells. Small 2024, 20, e2401197. [Google Scholar] [CrossRef]
- Guan, H.; Zhang, W.; Liang, J.; Wang, C.; Hu, X.; Pu, D.; Huang, L.; Ge, Y.; Cui, H.; Zou, Y.; et al. Low-Dimensional 2-thiopheneethylammonium Lead Halide Capping Layer Enables Efficient Single-Junction Methylamine-Free Wide-Bandgap and Tandem Perovskite Solar Cells. Adv. Funct. Mater. 2023, 33, 202300860. [Google Scholar] [CrossRef]
- Enkhbayar, E.; Otgontamir, N.; Kim, S.; Lee, J.; Kim, J. Understanding of Defect Passivation Effect on Wide Band Gap p-i-n Perovskite Solar Cell. ACS Appl. Mater. Interfaces 2024, 16, 35084–35094. [Google Scholar] [CrossRef]
- Wang, Y.; Lin, R.; Liu, C.; Wang, X.; Chosy, C.; Haruta, Y.; Bui, A.D.; Li, M.; Sun, H.; Zheng, X.; et al. Homogenized contact in all-perovskite tandems using tailored 2D perovskite. Nature 2024, 635, 867–873. [Google Scholar] [CrossRef]
- Wang, Z.; Kang, S.; Zhou, X.; Chen, H.; Jiang, X.; Zhang, Z.; Zheng, J.; Zhang, R.; Chen, W.; Zhang, J.; et al. Piperazine-Assisted Construction of 2D/3D Wide-Bandgap Perovskite for Realizing High-Efficiency Perovskite/Organic Tandem Solar Cells. Chin. J. Chem. 2024, 42, 1819–1827. [Google Scholar] [CrossRef]
- Hu, M.; Du, S.; Yu, Z.; Chen, G.; Liang, J.; Cai, Q.; Fang, G. Improving efficiency and stability of wide-bandgap perovskite solar cells and four-terminal tandems with iso-propylammonium 2D passivator. Chem. Eng. J. 2025, 505, 159453. [Google Scholar] [CrossRef]
- Huo, X.; Li, Y.; Liu, W.; Huang, X.; Meng, J.; Lu, Y.; Meng, N.; Zhang, Y.; Zhao, S.; Qiao, B.; et al. Nonpolar and Ultra-long-chain Ligand to Modify the Perovskite Interface toward High-Efficiency and Stable Wide Bandgap Perovskite Solar Cells. ACS Appl. Energy Mater. 2023, 6, 1731–1740. [Google Scholar] [CrossRef]
- Chen, H.; Maxwell, A.; Li, C.; Teale, S.; Chen, B.; Zhu, T.; Ugur, E.; Harrison, G.; Grater, L.; Wang, J.; et al. Regulating surface potential maximizes voltage in all-perovskite tandems. Nature 2023, 613, 676–681. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Xu, J.; Lin, R.; Teale, S.; Li, H.; Liu, Z.; Duan, C.; Zhao, Q.; Xiao, K.; Wu, P.; et al. Inorganic wide-bandgap perovskite subcells with dipole bridge for all-perovskite tandems. Nat. Energy 2023, 8, 610–620. [Google Scholar] [CrossRef]
- Xu, T.; Xiang, W.; Yang, J.; Kubicki, D.J.; Tress, W.; Chen, T.; Fang, Z.; Liu, Y.; Liu, S. Interface Modification for Efficient and Stable Inverted Inorganic Perovskite Solar Cells. Adv. Mater. 2023, 35, e2303346. [Google Scholar] [CrossRef]
- Hu, X.; Yao, F.; Wang, C.; Cui, H.; Jia, P.; Du, S.; Zhou, S.; Guan, H.; Lin, Q.; Ke, W.; et al. Tail states suppression via surface-modification of wide-bandgap perovskites for high-efficiency all-perovskite photovoltaic tandems. Chem. Eng. J. 2024, 489, 151379. [Google Scholar] [CrossRef]
- Wang, J.; Branco, B.; Remmerswaal, W.H.M.; Hu, S.; Schipper, N.R.M.; Zardetto, V.; Bellini, L.; Daub, N.; Wienk, M.M.; Wakamiya, A.; et al. Performance and stability analysis of all-perovskite tandem photovoltaics in light-driven electrochemical water splitting. Nat. Commun. 2025, 16, 174. [Google Scholar] [CrossRef]
- Li, S.; Zheng, Z.; Ju, J.; Cheng, S.; Chen, F.; Xue, Z.; Ma, L.; Wang, Z. A Generic Strategy to Stabilize Wide Bandgap Perovskites for Efficient Tandem Solar Cells. Adv. Mater. 2024, 36, e2307701. [Google Scholar] [CrossRef]
- Luo, Y.; Zhu, J.; Yin, X.; Jiao, W.; Gao, Z.; Xu, Y.; Wang, C.; Wang, Y.; Lai, H.; Huang, H.; et al. Enhanced Efficiency and Stability of Wide-Bandgap Perovskite Solar Cells Via Molecular Modification with Piperazinium Salt. Adv. Energy Mater. 2024, 14, 202304429. [Google Scholar] [CrossRef]
- Lv, X.; Li, W.; Zhang, J.; Yang, Y.; Jia, X.; Ji, Y.; Lin, Q.; Huang, W.; Bu, T.; Ren, Z.; et al. Surface repair of wide-bandgap perovskites for high-performance all-perovskite tandem solar cells. J. Energy Chem. 2024, 93, 64–70. [Google Scholar] [CrossRef]
- Meng, X.; Liu, X.; Zhou, Q.; Liu, Z.; Chen, W. Additive and interface passivation dual synergetic strategy enables reduced voltage loss in wide-bandgap perovskite solar cells. Nano Energy 2024, 128, 109984. [Google Scholar] [CrossRef]
- Pu, D.; Zhou, S.; Guan, H.; Jia, P.; Chen, G.; Fang, H.; Fu, S.; Wang, C.; Hushvaktov, H.; Jumabaev, A.; et al. Enhancing Efficiency and Intrinsic Stability of Large-Area Blade-Coated Wide-Bandgap Perovskite Solar Cells Through Strain Release. Adv. Funct. Mater. 2024, 34, 2314349. [Google Scholar] [CrossRef]
- Zhang, K.; Liu, C.; Peng, Z.; Li, C.; Tian, J.; Li, C.; Cerrillo, J.G.; Dong, L.; Streller, F.; Späth, A.; et al. Binary cations minimize energy loss in the wide-band-gap perovskite toward efficient all-perovskite tandem solar cells. Joule 2024, 8, 2863–2882. [Google Scholar] [CrossRef]
- Yu, Y.; Xu, M.; Liu, R.; Ren, H.; Chen, J. Molecular cation passivation and bromine vacancy supplement strategy for efficient wide-bandgap perovskite solar cells. Chem. Eng. J. 2025, 507, 106339. [Google Scholar] [CrossRef]
- Wang, S.; Wang, P.; Shi, B.; Sun, C.; Sun, H.; Qi, S.; Huang, Q.; Xu, S.; Zhao, Y.; Zhang, X. Inorganic perovskite surface reconfiguration for stable inverted solar cells with 20.38% efficiency and its application in tandem devices. Adv. Mater. 2023, 35, 2300581. [Google Scholar] [CrossRef]
- Zhao, Y.; Ma, T.; Liu, T.; Zhou, L.; Wu, Z.; Chen, C.; Liu, Y.; Chen, C.; Ma, D.; Qin, L.; et al. Passivator-Assisted Close Space Annealing for High-Performance Wide-Bandgap Perovskite Solar Cells. Sol. RRL 2024, 8, 2301016. [Google Scholar] [CrossRef]
- Bi, H.; Liu, J.; Zhang, Z.; Wang, L.; Beresneviciute, R.; Tavgeniene, D.; Kapil, G.; Ding, C.; Baranwal, A.K.; Sahamir, S.R.; et al. All-Perovskite Tandem Solar Cells Approach 26.5% Efficiency by Employing Wide Bandgap Lead Perovskite Solar Cells with New Monomolecular Hole Transport Layer. ACS Energy Lett. 2023, 8, 3852–3859. [Google Scholar] [CrossRef]
- He, R.; Wang, W.; Yi, Z.; Lang, F.; Chen, C.; Luo, J.; Zhu, J.; Thiesbrummel, J.; Shah, S.; Wei, K.; et al. Improving interface quality for 1-cm2 all-perovskite tandem solar cells. Nature 2023, 618, 80–86. [Google Scholar] [CrossRef]
- Wang, W.; Liu, X.; Wang, J.; Chen, C.; Yu, J.; Zhao, D.; Tang, W. Versatile Self-Assembled Molecule Enables High-Efficiency Wide-Bandgap Perovskite Solar Cells and Organic Solar Cells. Adv. Energy Mater. 2023, 13, 2300694. [Google Scholar] [CrossRef]
- Shi, Z.-E.; Cheng, T.-H.; Lung, C.-Y.; Lin, C.-W.; Wang, C.-L.; Jiang, B.-H.; Hsiao, Y.-S.; Chen, C.-P. Achieving over 42 % indoor efficiency in wide-bandgap perovskite solar cells through optimized interfacial passivation and carrier transport. Chem. Eng. J. 2024, 498, 155512. [Google Scholar] [CrossRef]
- Yi, Z.; Wang, W.; He, R.; Zhu, J.; Jiao, W.; Luo, Y.; Xu, Y.; Wang, Y.; Zeng, Z.; Wei, K.; et al. Achieving a high open-circuit voltage of 1.339 V in 1.77 eV wide-bandgap perovskite solar cells via self-assembled monolayers. Energy Environ. Sci. 2024, 17, 202–209. [Google Scholar] [CrossRef]
- Wąsiak-Maciejak, A.; Przypis, Ł.; Żuraw, W.; Rycek, K.; Janicka, P.; Ścigaj, M.; Dyk, K.; Lai, H.; Piejko, A.; Pucicki, D.; et al. Compositional and interfacial engineering for improved light stability of flexible wide-bandgap perovskite solar cells. J. Mater. Chem. A 2025, 507, 106339. [Google Scholar] [CrossRef]
- Wei, Z.; Zhou, Q.; Niu, X.; Liu, S.; Dong, Z.; Liang, H.; Chen, J.; Shi, Z.; Wang, X.; Jia, Z.; et al. Surpassing 90% Shockley–Queisser VOC limit in 1.79 eV wide-bandgap perovskite solar cells using bromine-substituted self-assembled monolayers. Energy Environ. Sci. 2025, 18, 1847–1855. [Google Scholar] [CrossRef]
- Zhu, J.; Luo, Y.; He, R.; Chen, C.; Wang, Y.; Luo, J.; Yi, Z.; Thiesbrummel, J.; Wang, C.; Lang, F.; et al. A donor–acceptor-type hole-selective contact reducing non-radiative recombination losses in both subcells towards efficient all-perovskite tandems. Nat. Energy 2023, 8, 714–724. [Google Scholar] [CrossRef]
- Singh, A.K.; Parveen, S.; Chauhan, M.S.; Patel, S.P.; Chaudhary, D.K.; Singh, P.P.; Singh, R.S.; Singh, V.K. Unlocking all-perovskite tandem solar cells to ~ 30% efficiency: A simulation and optimization approach with MPA2FPh-BT-BA as a hole selective contact. J. Opt. 2025, 54, 96–105. [Google Scholar] [CrossRef]
- Cui, H.; Huang, L.; Zhou, S.; Wang, C.; Hu, X.; Guan, H.; Wang, S.; Shao, W.; Pu, D.; Dong, K.; et al. Lead halide coordination competition at buried interfaces for low VOC-deficits in wide-bandgap perovskite solar cells. Energy Environ. Sci. 2023, 16, 5992–6002. [Google Scholar] [CrossRef]
- Bi, H.; Fujiwara, Y.; Kapil, G.; Tavgeniene, D.; Zhang, Z.; Wang, L.; Ding, C.; Sahamir, S.R.; Baranwal, A.K.; Sanehira, Y.; et al. Perovskite Solar Cells Consisting of PTAA Modified with Monomolecular Layer and Application to All-Perovskite Tandem Solar Cells with Efficiency over 25%. Adv. Funct. Mater. 2023, 33, 2300089. [Google Scholar] [CrossRef]
- Yong, J.; Lee, Y.K.; Park, H.; Muthu, S.; Shin, J.; Whang, D.R.; Kim, B.G.; Chang, D.W.; Park, H.J. Enhancement of Interfacial Properties by Indoloquinoxaline-Based Small Molecules for Highly Efficient Wide-Bandgap Perovskite Solar Cells. Adv. Funct. Mater. 2023, 34, 2312505. [Google Scholar] [CrossRef]
- Wang, R.; Liu, X.; Yan, S.; Meng, N.; Zhao, X.; Chen, Y.; Li, H.; Qaid, S.M.H.; Yang, S.; Yuan, M.; et al. Efficient wide-bandgap perovskite photovoltaics with homogeneous halogen-phase distribution. Nat. Commun. 2024, 15, 8899. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Xiao, K.; Gao, H.; Duan, C.; Zhao, S.; Wen, J.; Wang, Y.; Lin, R.; Zheng, X.; Luo, H.; et al. Scalable Solution-Processed Hybrid Electron Transport Layers for Efficient All-Perovskite Tandem Solar Modules. Adv. Mater. 2024, 36, e2308706. [Google Scholar] [CrossRef] [PubMed]


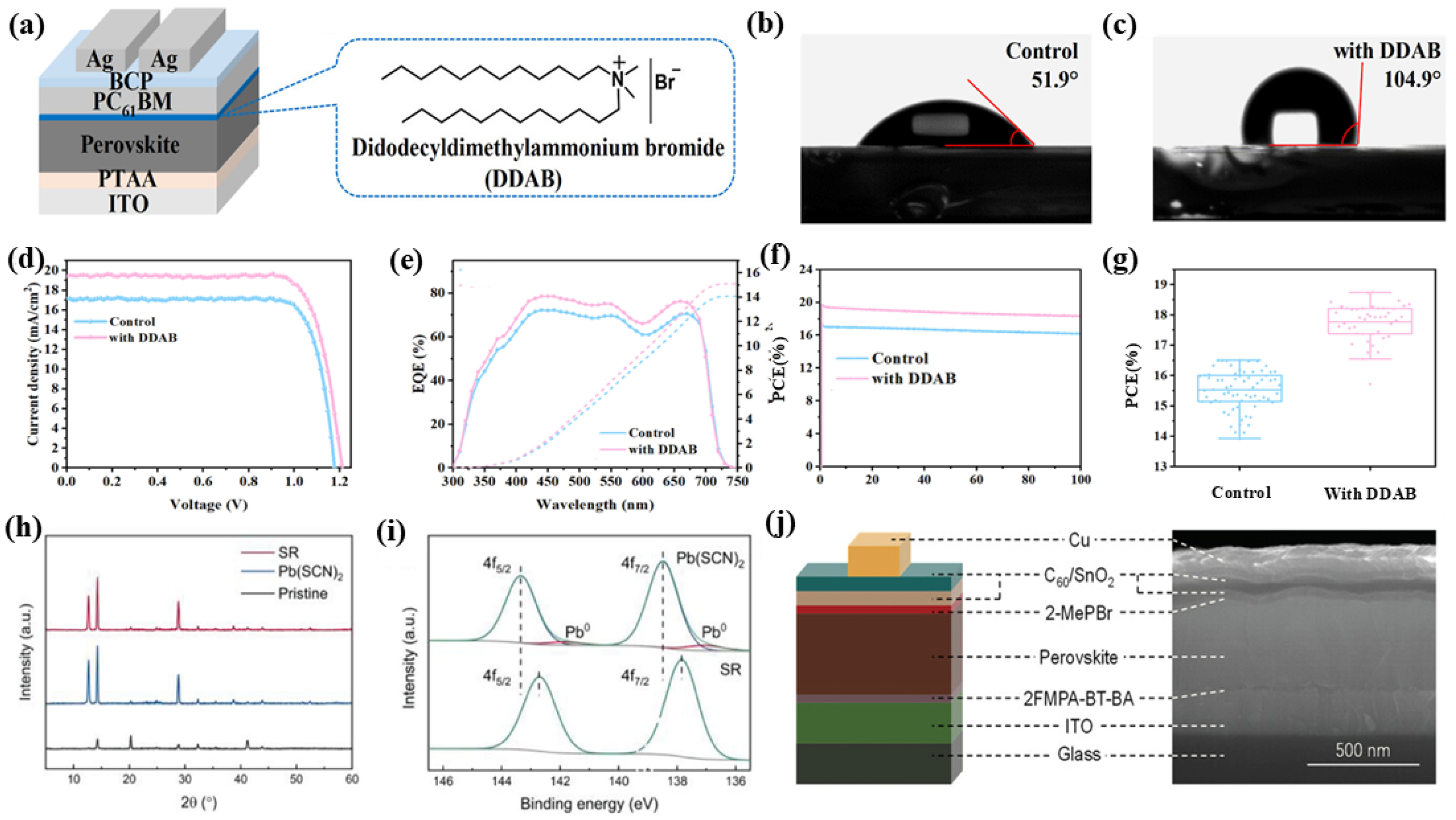

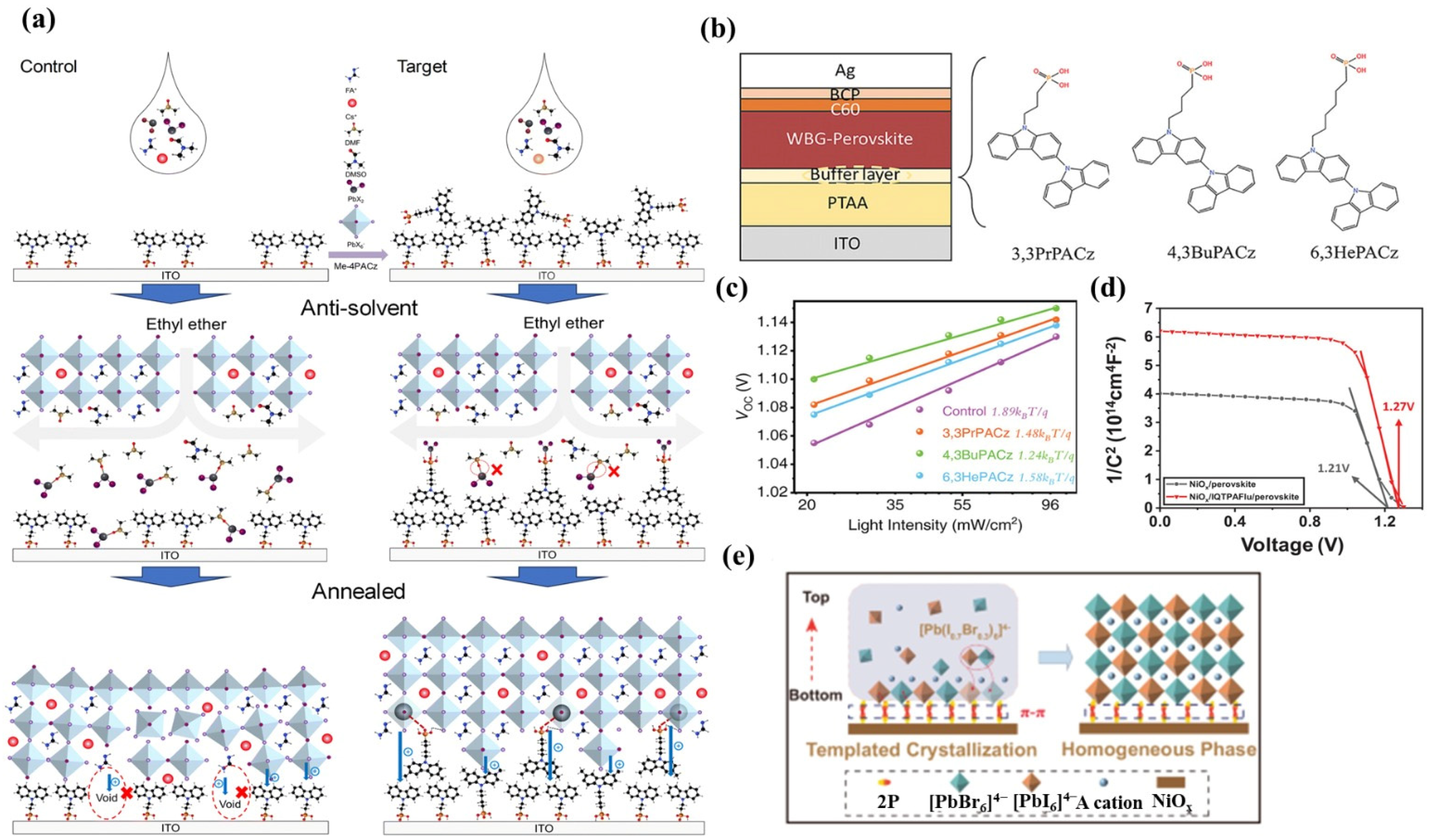
| WBG Device Structure | Material | Voc (V) | PCE of WBG PSCs (%) | PCE of APTSCs (%) | Year | Ref. |
|---|---|---|---|---|---|---|
| ITO/MeO-2PACz/FA0.75Cs0.25Pb(I0.8Br0.2)3/2D/C60/BCP/Cu | TEACl | 1.23 | 21.7 | 26.64 | 2023 | [80] |
| ITO/HTL/PVK/2D layer/C60/SnO2/Cu | TTDL | 1.35 | 20.5 | 28.5 | 2024 | [82] |
| ITO/NiOx/Me-4PACz/PVK/2D layer/PCBM/C60/SnOx/Ag | i-PAI | 1.25 | 22.4 | 31.1 | 2025 | [84] |
| ITO/NiOx/Me-4PACz/Cs0.2FA0.8Pb(I0.6Br0.4)3/PDA/C60/SnOx/Ag | PDA | 1.33 | 19.3 | 27.4 | 2023 | [86] |
| ITO/MeO-2PACz/FA0.7MA0.05Cs0.25Pb(I0.8Br0.2)3/PDAI2/C60/SnOx/Cu | PDAI2 | 1.243 | 21.48 | 28.0 | 2024 | [89] |
| ITO/Me-4PACz/PVK/SEBr/C60/BCP/Ag | SEBr | 1.28 | 22.47 | 27.1 | 2024 | [93] |
| ITO/Meo-2PACz/1.77PVK/PDADI/FAI/C60/BCP/Cu. | PDADI /FAI | 1.28 | 19.52 | 27.64 | 2024 | [95] |
| WBG Device Structure | Material | Voc(V) | PCE of WBG PSCs (%) | PCE of APTSCs (%) | Year | Ref. |
|---|---|---|---|---|---|---|
| ITO/SAMs/FA0.8Cs0.2PbI1.8Br1.2/C60/BCP/Ag | 4dp3PACz | 1.214 | 17.17 | 26.47 | 2023 | [100] |
| ITO/SAMs/PVK/C60/SnO2/Cu | 4PADCB | 1.31 | 18.46 | 27 | 2023 | [101] |
| ITO/SAMs/FA0.8Cs0.2PbI1.8Br1.2/C60/BCP/Cu | DCB-BPA | 1.339 | 18.88 | 26.9 | 2024 | [104] |
| ITO/SAMs/1.79 PVK/C60/BCP/Ag | DCB-Br-2 | 1.37 | 20.76 | 27.7 | 2025 | [106] |
| ITO/2PACz/Me-4PACz/(FA0.8Cs0.2)Pb(I0.6Br0.4)3/C60/BCP/Cu | Me-4PACz | 1.36 | 19.83 | 27.34 | 2023 | [109] |
| ITO/PTAA/MNL/ FA0.8Cs0.2PbI1.8Br1.2/C60/BCP/Ag. | MNL | 1.175 | 16.57 | 25.24 | 2023 | [110] |
| ITO/HTLs/Cs0.35FA0.65PbI1.8Br1.2/HF/Cu | HF | 1.321 | 19.0 | 27.4 | 2024 | [113] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Q.; Chai, W.; Luo, X.; Zhu, W.; Chen, D.; Zhou, L.; Xi, H.; Dong, H.; Zhang, C.; Hao, Y. Wide-Bandgap Subcells for All-Perovskite Tandem Solar Cells: Recent Advances, Challenges, and Future Perspectives. Energies 2025, 18, 2415. https://doi.org/10.3390/en18102415
Li Q, Chai W, Luo X, Zhu W, Chen D, Zhou L, Xi H, Dong H, Zhang C, Hao Y. Wide-Bandgap Subcells for All-Perovskite Tandem Solar Cells: Recent Advances, Challenges, and Future Perspectives. Energies. 2025; 18(10):2415. https://doi.org/10.3390/en18102415
Chicago/Turabian StyleLi, Qiman, Wenming Chai, Xin Luo, Weidong Zhu, Dazheng Chen, Long Zhou, He Xi, Hang Dong, Chunfu Zhang, and Yue Hao. 2025. "Wide-Bandgap Subcells for All-Perovskite Tandem Solar Cells: Recent Advances, Challenges, and Future Perspectives" Energies 18, no. 10: 2415. https://doi.org/10.3390/en18102415
APA StyleLi, Q., Chai, W., Luo, X., Zhu, W., Chen, D., Zhou, L., Xi, H., Dong, H., Zhang, C., & Hao, Y. (2025). Wide-Bandgap Subcells for All-Perovskite Tandem Solar Cells: Recent Advances, Challenges, and Future Perspectives. Energies, 18(10), 2415. https://doi.org/10.3390/en18102415










