Thermogravimetric Experimental Study on the Co-Combustion Characteristics of Coal and Salix
Abstract
1. Introduction
2. Material and Methods
2.1. Material
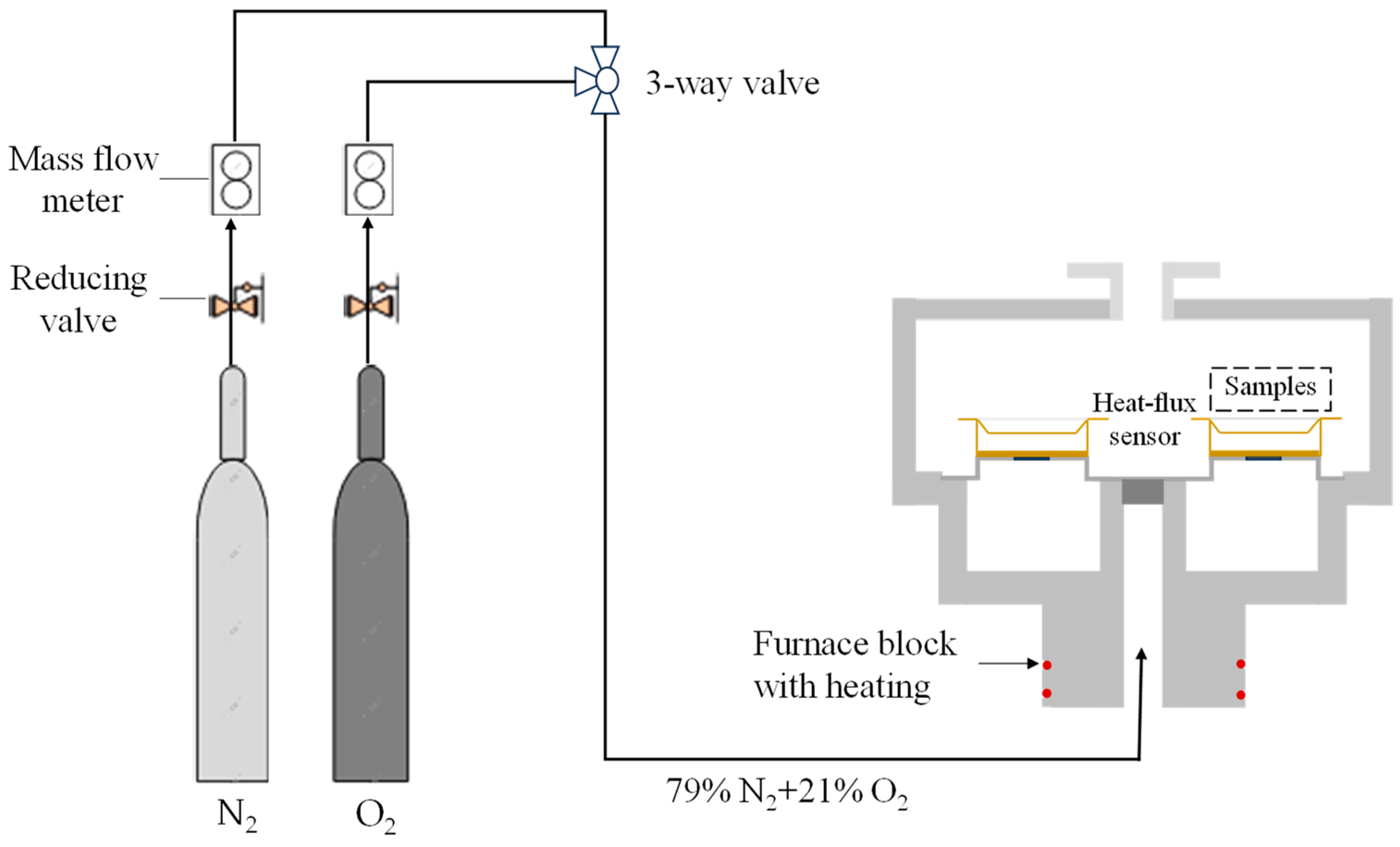
2.2. Thermogravimetric Experiment
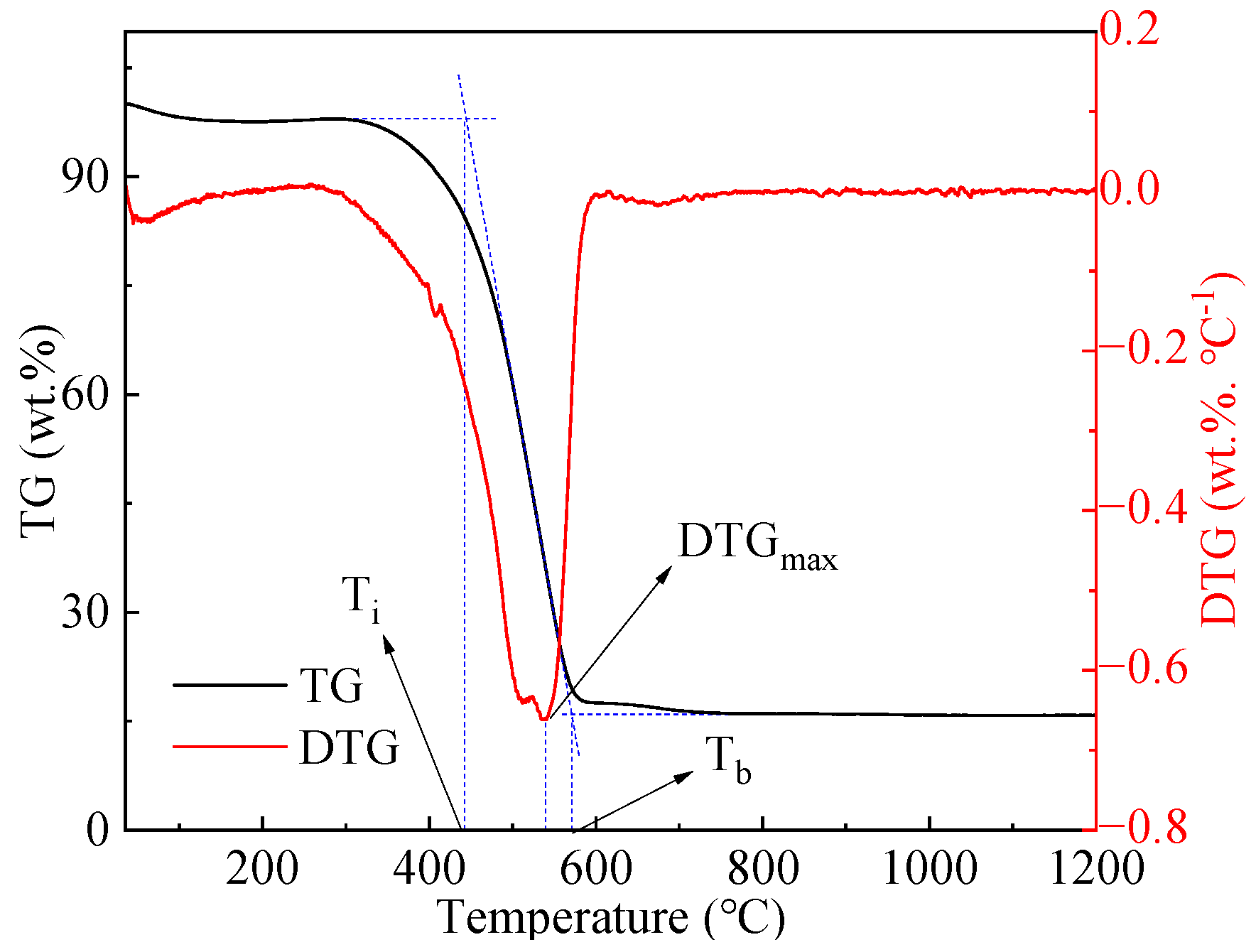
2.3. Analytical Method
3. Results and Discussion
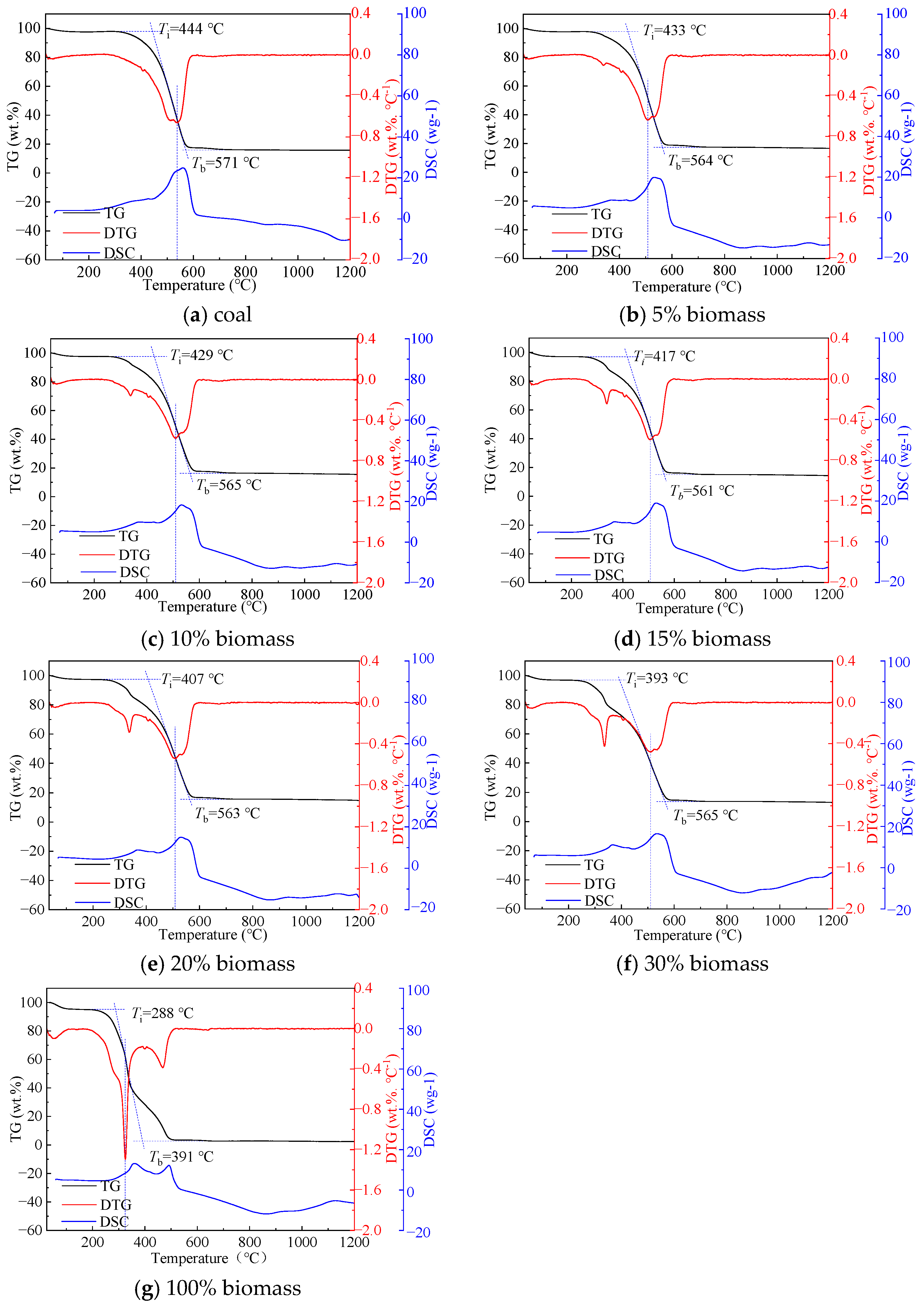
3.1. Effect of Blending Ratio
3.2. The Synergistic Effect of Blended Fuel
3.3. Kinetic Analysis
4. Conclusions
- (1)
- As the biomass blending ratio increases from 5% to 30%, both the ignition and burnout temperatures of coal-biomass blended fuels decrease, with ignition temperatures declining from 444 °C to 393 °C and burnout temperatures reducing from 571 °C to 565 °C. Simultaneously, the index of stable combustion characteristics rises, indicating that the incorporation of biomass improves the ignitability and stability of the blended fuel.
- (2)
- Thermogravimetric analysis reveals that the blending ratio of Salix significantly affects the temperature range of the fuel loss peak. At a lower biomass ratio (15%), the main loss peak is observed at 490 °C, whereas a higher ratio (100%) shifts this peak to approximately 320 °C, indicating an enhancement in combustion characteristics with biomass addition. A synergistic analysis shows that in the temperature range of 400 to 530 °C, a 15% biomass ratio most effectively promotes combustion, whereas a 30% ratio inhibits it. Above 600 °C, the inhibitory effect of combustion products becomes the dominant factor influencing the reaction rate, leading to combustion inhibition.
- (3)
- Kinetic analysis reveals that the incorporation of Salix reduces the activation energy, thereby facilitating the reaction process. With a 5% biomass addition, the activation energy is 77.42 KJ mol−1. However, as the biomass blending ratio increases to 30%, the activation energy markedly decreases to 25.38 KJ mol−1. At a 15% blending ratio, the activation energy continues to decrease with an increase in the heating rate, reaching 23.06 KJ mol−1 at a heating rate of 30 K/min. Both the increased heating rate and higher biomass ratio contribute to the reduction in activation energy, with the pre-exponential factor A also decreasing under these conditions. This results in a faster initiation of the reaction, indicating improved reaction feasibility. This study provides valuable insights into the co-combustion of coal and Salix in practical applications. Future research could focus on ash accumulation and pollutant emissions resulting from the combustion of coal and Salix.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Nomenclature
| A | Pre-exponential factor |
| Aar | Ash content as received basis |
| B | Biomass |
| C | Coal |
| Car | Carbon element as received basis |
| Ci | Flammability index |
| Stable combustion characteristic index | |
| DTGmax | Maximum burning speed |
| DTGmean | Average burning speed |
| E | Activation energy |
| FCar * | Fixed carbon as received basis |
| Har | Hydrogen element as received basis |
| Mar | Moisture as received basis |
| Nar | Nitrogen element as received basis |
| Oar | Oxygen element as received basis |
| Net calorific value as received basis | |
| R2 | Goodness of fit |
| S | Comprehensive combustion characteristics index |
| St,ar | Sulfur element as received basis |
| Tb | Burnout temperature |
| Ti | Ignition temperature |
| Weight loss of biomass | |
| Calculated rates of weight loss | |
| Weight loss of coal | |
| Experimental rates of weight loss | |
| ΔTG | Total weight loss |
| Var | Volatile as received basis |
| α | Conversion rate |
| β | Steady rate of temperature rise |
| Contents of biomass | |
| Contents of coal |
References
- Li, F.; Xu, M.; Wang, T.; Fang, Y.; Ma, M. An investigation on the fusibility characteristics of low-rank coals and biomass mixtures. Fuel 2015, 158, 884–890. [Google Scholar] [CrossRef]
- Li, C.; Suzuki, K. Resources, properties and utilization of tar. Resour. Conserv. Recycl. 2010, 54, 905–915. [Google Scholar] [CrossRef]
- Li, J.; Sun, J.; Guo, L.; Zhai, M.; Done, P. Research progress on new biomass gasification technology. Therm. Power Gener. 2016, 45, 1–6. [Google Scholar]
- Saidur, R.; Abdelaziz, E.A.; Demirbas, A.; Hossain, M.S.; Mekhilef, S. A review on biomass as a fuel for boilers. Renew. Sustain. Energy Rev. 2011, 15, 2262–2289. [Google Scholar] [CrossRef]
- Kang, Y.; Yang, Q.; Bartocci, P.; Wei, H.; Liu, S.S.; Wu, Z.; Zhou, H.; Yang, H.; Fantozzi, F.; Chen, H. Bioenergy in China: Evaluation of domestic biomass resources and the associated greenhouse gas mitigation potentials. Renew. Sustain. Energy Rev. 2020, 127, 109842. [Google Scholar] [CrossRef]
- Guo, H.; Cui, J.; Li, J. Biomass power generation in China: Status, policies and recommendations. Energy Rep. 2022, 8, 687–696. [Google Scholar] [CrossRef]
- Antar, M.; Lyu, D.; Nazari, M.; Shah, A.; Zhou, X.; Smith, D.L. Biomass for a sustainable bioeconomy: An overview of world biomass production and utilization. Renew. Sustain. Energy Rev. 2021, 139, 110691. [Google Scholar] [CrossRef]
- Wang, H.; Fang, Y.; Zhang, T.; Liu, H.; Wang, C. Combustion characteristics and kinetics analysis of biomass. Sci. Technol. Eng. 2020, 20, 9886–9892. [Google Scholar]
- Yang, Z.; Wu, Y.; Zhang, Z.; Li, H.; Li, X.; Egorov, R.I.; Strizhak, P.A.; Gao, X. Recent advances in co-thermochemical conversions of biomass with fossil fuels focusing on the synergistic effects. Renew. Sustain. Energy Rev. 2019, 103, 384–398. [Google Scholar] [CrossRef]
- Zhao, M.; Wang, W.; Wang, Z.; Chen, L.; Ma, Z.; Wang, Q. Water use of Salix in the variably unsaturated zone of a semiarid desert region based on in-situ observation. J. Hydrol. 2020, 591, 125579. [Google Scholar] [CrossRef]
- Rönnberg-Wästljung, A.C.; Dufour, L.; Gao, J.; Hansson, P.A.; Herrmann, A.; Jebrane, M.; Johansson, A.C.; Kalita, S.; Molinder, R.; Nordh, N.E.; et al. Optimized utilization of Salix—Perspectives for the genetic improvement toward sustainable biofuel value chains. GCB Bioenergy 2022, 14, 1128–1144. [Google Scholar] [CrossRef]
- Wang, B.; Guo, J.; Song, C. Study on combustion characteristics and kinetics analysis of Salix under O2/CO2 atmosphere. Renew. Energy Resour. 2018, 36, 820–827. [Google Scholar]
- Wang, R.; Chang, S.; Cui, X.; Li, J.; Ma, L.; Kumar, A.; Nie, Y.; Cai, W. Retrofitting coal-fired power plants with biomass co-firing and carbon capture and storage for net zero carbon emission: A plant-by-plant assessment framework. GCB Bioenergy 2020, 13, 143–160. [Google Scholar] [CrossRef]
- Hariana; Putra, H.P.; Prabowo; Hilmawan, E.; Darmawan, A.; Mochida, K.; Aziz, M. Theoretical and experimental investigation of ash-related problems during coal co-firing with different types of biomass in a pulverized coal-fired boiler. Energy 2023, 269, 126784. [Google Scholar] [CrossRef]
- Trivedi, K.; Sharma, A.; Kanabar, B.K.; Arunachalam, K.D.; Gautam, S. Comparative analysis of coal and biomass for sustainable energy production: Elemental composition, combustion behavior and co-firing potential. Water Air Soil Pollut. 2024, 235, 698. [Google Scholar] [CrossRef]
- Si, F.; Zhang, H.; Feng, X.; Xu, Y.; Zhang, L.; Zhao, L.; Li, L. Thermodynamics and synergistic effects on the co-combustion of coal and biomass blends. J. Therm. Anal. Calorim. 2024, 149, 7749–7761. [Google Scholar] [CrossRef]
- Liao, X.; Singh, S.; Yang, H.; Wu, C.; Zhang, S. A thermogravimetric assessment of the tri-combustion process for coal, biomass and polyethylene. Fuel 2021, 287, 119355. [Google Scholar]
- Liu, Z.; Hu, Y.; Wang, J.; Meng, J.; Zhang, Y.; Chen, R. Study on the combustion characteristics and kinetics of water hyacinth co-combustion with anthracite. Chem. Eng. Res. Des. 2023, 200, 637–645. [Google Scholar] [CrossRef]
- Liao, X.; Zhang, S.; Wang, X.; Shao, J.; Zhang, X.; Wang, X.; Yang, H.; Chen, H. Co-combustion of wheat straw and camphor wood with coal slime: Thermal behaviour, kinetics, and gaseous pollutant emission characteristics. Energy 2021, 234, 121292. [Google Scholar]
- Guo, F.; He, Y.; Hassanpour, A.; Gardy, J.; Zhong, Z. Thermogravimetric analysis on the co-combustion of biomass pellets with lignite and bituminous coal. Energy 2020, 197, 117147. [Google Scholar] [CrossRef]
- Prayoga, M.Z.E.; Putra, H.P.; Adelia, N.; Luktyansyah, I.M.; Ifanda, I.; Prismantoko, A.; Darmawan, A.; Hartono, J.; Wirawan, S.S.; Aziz, M.; et al. Co-combustion performance of oil palm biomass with coal: Thermodynamics and kinetics analyses. J. Therm. Anal. Calorim. 2024, 149, 2873–2891. [Google Scholar] [CrossRef]
- Guo, Z.; Liu, M.; He, H.; Song, F.; Chen, X.; Guo, F.; Chen, J.; Lu, S.; Sang, S.; Wu, J. Co-pyrolysis and co-combustion characteristics of low-rank coal and waste biomass: Insights into interactions, kinetics and synergistic effects. J. Energy Inst. 2025, 118, 101918. [Google Scholar] [CrossRef]
- GB/T212-2008; Proximate Analysis of Coal. Coal Analysis Laboratory, General Research Institute of Coal Science and Team 143, Yunnan Coalfield Geological Exploration Co., State General Administration of the People’s Republic of China for Quality Supervision and Inspection and Quarantine, Standardization Administration of China: Beijing, China, 2008. (In Chinese)
- GB/T 31391-2015; Ultimate Analysis of Coal. Testing and Research Branch of the General Research Institute of Coal Science (GRIC), Shenhua Trading Group Limited, State General Administration of the People’s Republic of China for Quality Supervision and Inspection and Quarantine, Standardization Administration of China: Beijing, China, 2015. (In Chinese)
- GB/T213-2008; Determination of Calorific Value of Coal. Coal Analysis Laboratory, General Research Institute of Coal Science, State General Administration of the People’s Republic of China for Quality Supervision and Inspection and Quarantine, Standardization Administration of China: Beijing, China, 2008. (In Chinese)
- Qian, X.; Lee, S.; Chandrasekaran, R.; Yang, Y.; Caballes, M.; Alamu, O.; Chen, G. Electricity evaluation and emission characteristics of poultry litter co-combustion process. Appl. Sci. 2019, 9, 4116. [Google Scholar] [CrossRef]
- Abián, M.; Alzueta, M.U.; Carvalho, A.; Rabaçal, M.; Costa, M. Role of potassium and calcium on the combustion characteristics of biomass obtained from thermogravimetric experiments. Energy Fuels 2017, 31, 12238–12246. [Google Scholar] [CrossRef]
- Jia, G. Combustion characteristics and kinetic analysis of biomass pellet fuel using thermogravimetric analysis. Processes 2021, 9, 868. [Google Scholar] [CrossRef]
- Wang, Q.; Liu, L.; Chen, M.; Yu, Z.; Tang, J.; Tu, Y. Advanced orthogonal analysis of multi-factorial interactions influencing co-combustion performance and pollution reduction potential of coal gangue and biomass in oxy-fuel combustion conditions. Process Saf. Environ. Prot. 2024, 190, 1188–1201. [Google Scholar] [CrossRef]
- Zou, H.; Evrendilek, F.; Liu, J.; Buyukada, M. Combustion behaviors of pileus and stipe parts of Lentinus edodes using thermogravimetric-mass spectrometry and Fourier transform infrared spectroscopy analyses: Thermal conversion, kinetic, thermodynamic, gas emission and optimization analyses. Bioresour. Technol. 2019, 288, 121481. [Google Scholar] [CrossRef] [PubMed]
- Greenhalf, C.E.; Nowakowski, D.J.; Harms, A.B.; Titiloye, J.O.; Bridgwater, A.V. A comparative study of straw, perennial grasses and hardwoods in terms of fast pyrolysis products. Fuel 2013, 108, 216–230. [Google Scholar] [CrossRef]
- Liu, H.-P.; Liang, W.-X.; Qin, H.; Wang, Q. Synergy in co-combustion of oil shale semi-coke with torrefied cornstalk. Appl. Therm. Eng. 2016, 109, 653–662. [Google Scholar] [CrossRef]
- Dong, L.; Huang, X.; Ren, J.; Deng, L.; Da, Y. Thermogravimetric assessment and differential thermal analysis of blended fuels of coal, biomass and oil sludge. Appl. Sci. 2023, 13, 11058. [Google Scholar] [CrossRef]
- Qin, H.; Wang, W.; Liu, H.; Zhang, L.; Wang, Q.; Shi, C.; Yao, K. Thermal behavior research for co-combustion of furfural residue and oil shale semi-coke. Appl. Therm. Eng. 2017, 120, 19–25. [Google Scholar] [CrossRef]
- Kumar, A.; Mylapilli, S.V.P.; Reddy, S.N. Thermogravimetric and kinetic studies of metal (Ru/Fe) impregnated banana pseudo-stem (Musa acuminate). Bioresour. Technol. 2019, 285, 121318. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Wang, Y.; Wang, J.; Cui, S.; Li, Z.; Meng, W. Research on coal combustion catalysts for cement kiln via comprehensive evaluation method based on combustion characteristics. Case Stud. Therm. Eng. 2023, 50, 103440. [Google Scholar] [CrossRef]
- Deng, L.; Qiu, Y.; Jiang, J.; Zhu, Z.; Che, D. Co-combustion characteristics of electrolytic aluminum waste and coal. Fuel 2022, 325, 124890. [Google Scholar] [CrossRef]
- Sfakiotakis, S.; Vamvuka, D. Study of co-pyrolysis of olive kernel with waste biomass using TGA/DTG/MS. Thermochim. Acta 2018, 670, 44–54. [Google Scholar] [CrossRef]
- Al-Rumaihi, A.; Parthasarathy, P.; Fernandez, A.; Al-Ansari, T.; Mackey, H.R.; Rodriguez, R.; Mazza, G.; McKay, G. Thermal degradation characteristics and kinetic study of camel manure pyrolysis. J. Environ. Chem. Eng. 2021, 9, 106071. [Google Scholar] [CrossRef]
- Karunadasa, K.S.P.; Manoratne, C.H.; Pitawala, H.M.T.G.A.; Rajapakse, R.M.G. Thermal decomposition of calcium carbonate (calcite polymorph) as examined by in-situ high-temperature X-ray powder diffraction. J. Phys. Chem. Solids 2019, 134, 21–28. [Google Scholar] [CrossRef]
- Scaltsoyiannes, A.; Lemonidou, A. CaCO3 decomposition for calcium-looping applications: Kinetic modeling in a fixed-bed reactor. Chem. Eng. Sci. X 2020, 8, 100071. [Google Scholar] [CrossRef]
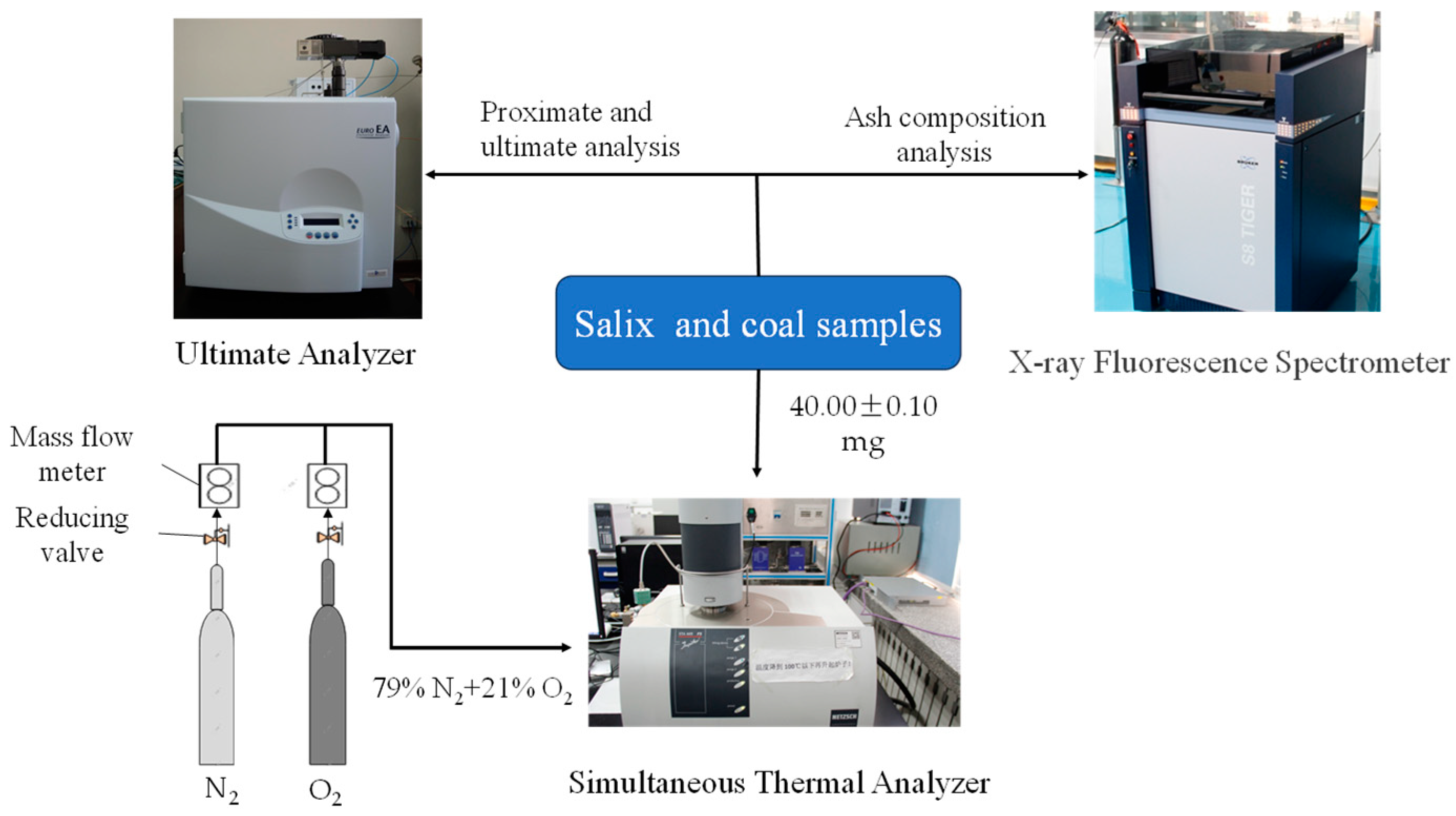

| Sample | Proximate Analysis | Ultimate Analysis | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Mar | Aar | Var | FCar * | Car | Har | Nar | Oar * | St,ar | |||
| Salix | 8.20 | 2.75 | 75.49 | 13.56 | 45.00 | 5.82 | 0.43 | 37.72 | 0.08 | 17.14 | |
| Coal | 4.50 | 18.03 | 29.66 | 47.81 | 61.17 | 3.93 | 0.79 | 10.95 | 0.63 | 23.52 | |
| Sample | Ash Composition/% | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Fe2O3 | Al2O3 | CaO | MgO | SiO2 | TiO2 | SO3 | K2O | Na2O | MnO2 | |
| 100% C | 4.96 | 14.04 | 9.16 | 1.10 | 54.51 | 0.85 | 5.39 | 2.68 | 1.32 | 0.127 |
| 100% B | 3.27 | 5.62 | 31.94 | 3.06 | 13.22 | 0.14 | 1.85 | 7.02 | 0.52 | 0.225 |
| 5% B + 95% C | 5.52 | 16.60 | 8.71 | 1.12 | 53.52 | 0.80 | 5.45 | 2.80 | 1.38 | 0.132 |
| 10% B + 90% C | 5.44 | 16.64 | 8.82 | 1.12 | 53.20 | 0.85 | 4.94 | 2.69 | 1.28 | 0.133 |
| 15% B + 85% C | 5.60 | 16.46 | 9.61 | 1.14 | 53.65 | 0.81 | 5.04 | 2.84 | 1.26 | 0.13 |
| 20% B + 80% C | 5.36 | 16.01 | 9.83 | 1.10 | 54.53 | 0.85 | 5.50 | 2.89 | 1.19 | 0.12 |
| 30% B + 70% C | 5.60 | 14.51 | 10.84 | 1.21 | 50.58 | 0.77 | 4.75 | 3.17 | 1.24 | 0.14 |
| Sample | Ti (°C) | Tb (°C) | Dw (%·min−1·K−2) | Ci (%·min−1·K−2) | S (%2·min−2·K−3) |
|---|---|---|---|---|---|
| 100% C | 444 | 571 | 2.68 × 10−6 | 3.44 × 10−6 | 4.36 × 10−10 |
| 5% B + 95% C | 433 | 564 | 2.76 × 10−6 | 3.60 × 10−6 | 4.56 × 10−10 |
| 10% B + 90% C | 429 | 565 | 2.51 × 10−6 | 3.30 × 10−6 | 4.23 × 10−10 |
| 15% B + 85% C | 417 | 561 | 2.63 × 10−6 | 3.54 × 10−6 | 4.64 × 10−10 |
| 20% B + 80% C | 407 | 563 | 2.49 × 10−6 | 3.44 × 10−6 | 4.47 × 10−10 |
| 30% B + 70% C | 393 | 565 | 2.25 × 10−6 | 3.23 × 10−6 | 4.26 × 10−10 |
| 100% B | 288 | 391 | 1.19 × 10−5 | 1.62 × 10−5 | 3.47 × 10−9 |
| Sample | Rate (K min−1) | α | E (KJ mol−1) | A (mol−1) | R2 | f(α) |
|---|---|---|---|---|---|---|
| 100% C | 10 | 0.2~0.8 | 86.94 | 83,084.38 | 0.9949 | −ln(1 − α) |
| 5% B + 95% C | 10 | 0.2~0.8 | 77.42 | 19,963.17 | 0.9894 | −ln(1 − α) |
| 10% B + 90% C | 10 | 0.2~0.8 | 60.01 | 1093.26 | 0.98 | −ln(1 − α) |
| 15% B + 85% C | 10 | 0.2~0.8 | 47.84 | 144.06 | 0.95 | −ln(1 − α) |
| 20% B + 80% C | 10 | 0.2~0.8 | 38.81 | 29.40 | 0.94 | −ln(1 − α) |
| 30% B + 70% C | 10 | 0.2~0.8 | 25.38 | 2.53 | 0.91 | −ln(1 − α) |
| 100% B | 10 | 0.2~0.8 | 34.46 | 60.48 | 0.85 | −ln(1 − α) |
| 15% B + 85% C | 20 | 0.2~0.8 | 26.54 | 2.59 | 0.91 | −ln(1 − α) |
| 15% B + 85% C | 30 | 0.2~0.8 | 23.06 | 1.05 | 0.94 | −ln(1 − α) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, Y.; Feng, B.; Gao, L.; Guo, Z.; Ai, Y.; Sun, H.; Zhang, Y.; Pan, Z.; Mao, J.; Yan, R.; et al. Thermogravimetric Experimental Study on the Co-Combustion Characteristics of Coal and Salix. Energies 2025, 18, 56. https://doi.org/10.3390/en18010056
Ma Y, Feng B, Gao L, Guo Z, Ai Y, Sun H, Zhang Y, Pan Z, Mao J, Yan R, et al. Thermogravimetric Experimental Study on the Co-Combustion Characteristics of Coal and Salix. Energies. 2025; 18(1):56. https://doi.org/10.3390/en18010056
Chicago/Turabian StyleMa, Yinsheng, Bao Feng, Li Gao, Zhenyu Guo, Yu Ai, Haoying Sun, Yong Zhang, Zhenyan Pan, Jingwen Mao, Ruyu Yan, and et al. 2025. "Thermogravimetric Experimental Study on the Co-Combustion Characteristics of Coal and Salix" Energies 18, no. 1: 56. https://doi.org/10.3390/en18010056
APA StyleMa, Y., Feng, B., Gao, L., Guo, Z., Ai, Y., Sun, H., Zhang, Y., Pan, Z., Mao, J., Yan, R., Ye, N., & Deng, L. (2025). Thermogravimetric Experimental Study on the Co-Combustion Characteristics of Coal and Salix. Energies, 18(1), 56. https://doi.org/10.3390/en18010056







