Abstract
This paper presents an overview of the status and future prospects of fuel-cell electric vehicles (FC-EVs). As global concerns about emissions escalate, FC-EVs have emerged as a promising substitute for traditional internal combustion engine vehicles. This paper discusses the fundamentals of fuel-cell technology considering the major types of fuel cells that have been researched and delves into the most suitable fuel cells for FC-EV applications, including comparisons with mainstream vehicle technologies. The present state of FC-EVs, ongoing research, and the challenges and opportunities that need to be accounted for are discussed. Furthermore, the comparison between promising proton-exchange membrane fuel cell (PEMFC) and solid oxide fuel cell (SOFC) technologies used in EVs provides valuable insights into their respective strengths and challenges. By synthesizing these aspects, the paper aims to provide a comprehensive understanding and facilitate decision-making for future advancements in sustainable FC-EV transportation, thereby contributing to the realization of a cleaner, greener, and more environmentally friendly future.
1. Introduction
Energy resources can be divided into three major categories: hydrocarbons (i.e., fossil fuels), nuclear reactions, and renewable energy (e.g., wind, water, geological sources of steam or hot water, etc.) [1]. Among all of them, hydrocarbons are the most widely used energy source in our daily lives. Hydrocarbons have the benefits of easy conversion into energy by combustion, and, because the distribution infrastructure is fully developed, they are readily available to large portions of the population. However, hydrocarbon production and use can cause significant environmental damage to the atmosphere; soil and sediments; surface and groundwater; marine environments; and terrestrial ecosystems [2].
With increased public awareness and published laws for environmental protection, the need for alternative energies has increased in recent years [3,4]. The use of renewable energy for transportation could essentially eliminate the emissions to the environment caused by hydrocarbon combustion [5]. However, since most renewable energy sources are diffuse and only intermittently available, e.g., wind and solar [6], they must be aggregated, likely through the electrical grid, and an energy storage system like a battery system must be included [7]. Further, because re-energizing battery electric vehicles (i.e., EV recharging) requires large draws from the electrical grid [8], in order to manage the loads generated by EV charging, it is also necessary to include battery energy storage at the charging station itself [9,10].
Along with battery EVs, fuel-cell electric vehicles (FC-EVs) have emerged as a promising and environmentally sustainable transportation solution in the context of mounting concerns regarding energy security and air quality. If fueled using fossil hydrocarbon fuels like methane or hydrogen derived from natural gas, then, because fuel cells are not heat machines and are therefore not Carnot-limited, they will still demonstrate higher well-to-wheel efficiency [11]. If the fuel cell is fueled by hydrogen or a hydrocarbon derived from renewable energy, such as by electrolysis or by renewable biomethane, then the FC-EV would truly be emission-free. In this manner, fuel cells energized using electrofuels derived from renewable energy can overcome the intermittency of renewable energies and remove the EV recharging load intermittency.
As the global automotive industry experiences a paradigm shift from conventional internal combustion engine vehicles (ICEVs) to cleaner and more sustainable alternatives, FC-EVs have gained significant attention for their potential to address some of the limitations of battery electric vehicles (BEVs), like the range and recharge-rate challenges for zero-emission mobility.
The motivation behind FC-EVs lies in their ability to use hydrogen or biofuels as a clean energy carrier. By combining hydrogen with oxygen in a fuel cell, these vehicles produce electricity to power an electric motor, emitting only water vapor as a byproduct. Likewise, if using biofuels like biomethane, an FC-EV would add no net carbon emissions to the atmosphere. These processes can be highly efficient and hold the promise of providing zero-emission, long-range transportation solutions, all while mitigating the reliance on fossil fuels.
The very first fuel cell was invented by Sir William Grove in 1842; however, that fuel cell did not produce enough electricity to be harvested [12]. Almost a century later, in 1932, Francis Thomas Bacon finally made a fuel cell that could be useful. In the mid-1960s, Bacon developed an alkaline fuel cell that could be used by NASA’s Moon Project and resolve the issue of how to power lunar capsules in space. After that, fuel-cell research and development became very popular, and in 1993, Van Hool was able to power a full-size bus with an alkaline fuel cell [13]. The hydrogen fuel cell is an electrochemical device that converts the chemical energy stored in hydrogen gas and oxygen from the air into electricity, heat, and water through a process that involves several key fundamental principles. Understanding these principles is essential to grasp the operation and efficiency of hydrogen fuel cells in the context of FC-EVs.
Through this review, the authors aim to provide researchers in the field of FC-EVs with a primer on the types of fuel cells available and their advantages and issues, as well as an understanding of the major integration challenges to be addressed in the field. With six major types of fuel cells, we have addressed the challenges with PEMFCs and SOFCs and utilized them for personal EV applications. For PEMFCs, developing non-platinum group catalysts reduced the component cost, such as using Fe, Co, Mn, etc. [14]. For SOFCs, reducing the operation temperature to 500 °C with a metal-support cell made thermal management in mobile vehicles possible [15,16].
Contribution and Organization of This Review
FC-EV reviews in the past have predominantly centered on proton-exchange membrane fuel cell (PEMFC) development and implementation in electric vehicles. For example, Li et al. published a review focusing on hydrogen storage systems [17], while Sankir provided a detailed book review on hydrogen electric vehicles [18]. Interestingly, despite previous considerations that solid oxide fuel cells (SOFCs) were unsuitable for electric vehicle applications, Nissan demonstrated their feasibility in 2016 [19]. SOFCs are renowned for their fuel flexibility, namely their ability to use hydrocarbons as well as hydrogen as fuel. This breakthrough has broadened the possibilities for incorporating various types of fuel cells into electric vehicles and therefore providing a bridge technology if fuel-cell technology is diversified beyond PEMFCs.
This paper presents an examination of FC-EVs, with a focus on their technological underpinnings, current market status, necessary advancements for the future of the technology, and recommendations for further development in the FC-EV space. Understanding the state of FC-EVs is pivotal for evaluating their potential role in the future of sustainable transportation and for addressing the need for clean, efficient, and practical solutions to reduce greenhouse gas emissions.
In the sections that follow, we delve into the fundamental principles of fuel-cell technology, analyze the ongoing research and current challenges faced by the industry, and explore potential prospects for research and development that lie ahead. The aim of this paper is to provide a better understanding of FC-EVs and to provide insights into the opportunities and obstacles that must be addressed as we collectively strive to reshape the future of automotive mobility.
Therefore, Section 2 describes aspects of fuel-cell technologies and Section 3 discusses other advancements that are needed to enable the wide-scale adoption of the technology for automotive applications. Specifically, Section 2.1 describes the different types of fuel cells and their suitability in FC-EV applications, while Section 2.2 and Section 2.3 describe the fundamental principles of the most promising fuel cells for electric vehicle applications. Section 2.4 provides a general fuel-cell model, while Section 2.5 and Section 2.6 present battery- and hybrid-EVs, respectively, for comparison. Section 2.7 then presents FC-EV architectures. Section 3.1 summarizes some issues in well-to-wheel efficiency, Section 3.2 the need for infrastructure implementation, Section 3.3 the cost considerations, and Section 3.4 the public perception of FC-EVs.
2. Fuel-Cell Technology and Models
2.1. Types of Fuel Cells
As the name implies, hydrogen fuel cells use hydrogen as fuel and generate very little pollution (the byproduct is water), and fuel cells do not have sunlight or wind velocity constraints compared to solar or wind power; therefore, there are no geographic limitations. Even in space, NASA used fuel cells to power the space shuttle about 70 years ago, and they used the byproduct—water—to supply drinking water for the crew and conduct cabin air humidification [20]. Hydrogen fuel cells come in various types, each tailored for specific applications and operational conditions. There are six main types of fuel cells, and they are classified by the types of electrolytes used. Each operates at different temperature ranges and uses different fuels. They all have their own advantages and unique applications [20]. Understanding these different types is crucial for evaluating their advantages, limitations, and potential applications in the field of hydrogen fuel-cell technology and electric vehicle applications. For example, fuel cells that operate at higher temperatures are impractical in FC-EV applications because maintaining these temperatures in a moving vehicle requires complex and extensive thermal management systems, which would increase the vehicle weight while reducing the overall efficiency.
2.1.1. Alkaline Fuel Cells (AFCs)
Alkaline fuel cells, or AFCs, utilize an alkaline electrolyte, typically potassium hydroxide in an aqueous solution. AFCs were the first fuel cell developed and also the first fuel cell NASA used in various spacecraft. This type of fuel cell can operate in extreme conditions, including in an environment like space [21,22]. Because they take advantage of the high conductivity of OH- in aqueous solutions due to the Grotthuss transport mechanism [23], they can operate at relatively low temperatures, typically around 60–80 °C, and have efficiencies in the order of 60%. These low temperatures would greatly simplify the thermal management of a fuel cell in a vehicle, particularly the cold start issue. However, again, because they rely on the conductivity of OH-, AFCs are sensitive to carbon dioxide and other impurities and require CO and CO2-free hydrogen and CO2-free air or oxygen inputs. Finally, the reaction between hydrogen fuel and oxygen (from the air) will result in the production of water, which remains in the electrolyte solution, thereby diluting it, necessitating an electrolyte reconcentration subsystem. Because of these limitations, including the volume and weight required to achieve a suitable power output (i.e., power density and specific power), AFCs are not likely to be utilized for FC-EVs in the foreseeable future.
2.1.2. Phosphoric Acid Fuel Cells (PAFCs)
Phosphoric acid fuel cells, referred to as PAFCs, are well-developed and commercially available. As the name indicates, they employ phosphoric acid as the electrolyte, and similar to the AFCs described above, they rely on the anomalously high conductivity of H+ in aqueous solution [24]. They operate at higher temperatures, typically around 150–200 °C, with an efficiency of up to 50%. PAFCs are known for their reliability and long operating life, making them suitable for stationary power generation applications, where the use of waste heat increases overall achievable energy utilization to as high as 85% [24]. Again, similarly to AFCs, the product—water—can dilute the electrolyte, and an electrolyte reconcentration subsystem is necessary. Therefore, though useful for stationary power generation, because of their lower power density, PAFCs are not a viable fuel cell for FC-EVs.
2.1.3. Proton-Exchange Membrane Fuel Cells (PEMFCs)
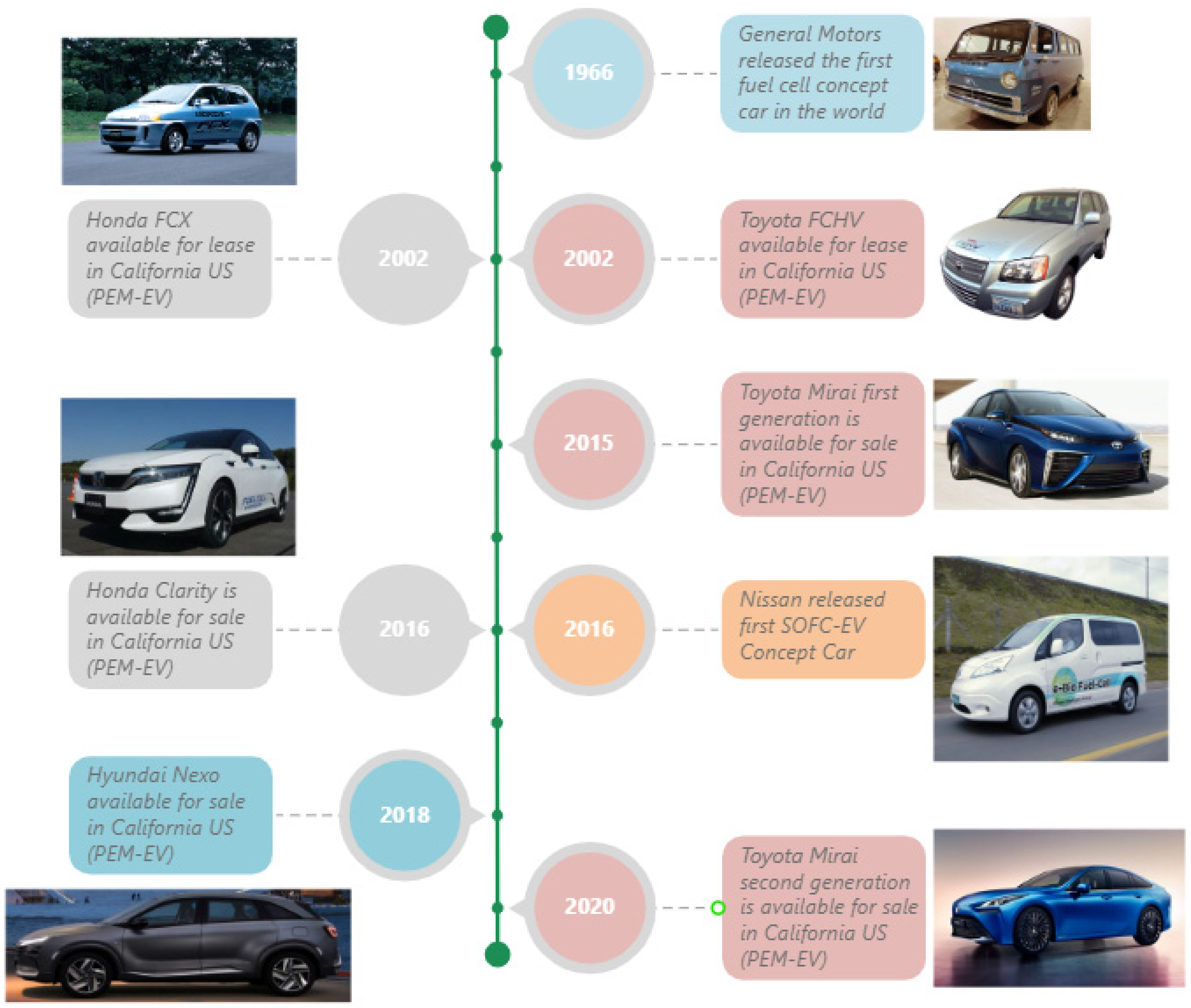
Proton-exchange membrane fuel cells, commonly known as PEMFCs, are well known to the public due to their most common application in cars and buses. PEMFCs employ a solid polymer electrolyte membrane as the proton-exchange medium. This membrane facilitates the selective transport of protons while preventing the passage of electrons. PEMFCs are well-suited for applications demanding quick response times, such as in FC-EVs. They operate at relatively low temperatures, typically around 80 °C [25], and unlike AFCs and PEMFCs, the product—water—does not affect the electrolyte. Indeed, to maintain the performance of the PEMFC, some of the produced water needs to be reintroduced into the fuel stream in a process known as water management [26]. The low operating temperature again results in rapid start-up times, and system simplicity results in high power density compared to other types of fuel cells. For this reason, many of the historic fuel-cell vehicles that have been developed by various leading automotive manufacturers around the world since the 1960s (shown in Figure 1) were based on PEMFCs.
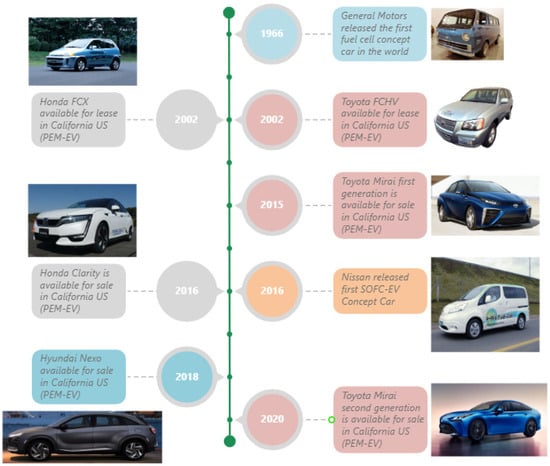
Figure 1.
Fuel-cell vehicles’ key milestones up to the year 2024 [27,28,29,30,31,32,33,34].
2.1.4. Direct Methanol Fuel Cells (DMFCs)
Direct methanol fuel cells, or DMFCs, are a variation of PEMFCs that use methanol as the fuel instead of hydrogen. In order to efficiently convert the methanol fuel, slightly higher operating temperatures are required, in the order of 120 °C, with practical energy-conversion efficiencies in the order of 40%. It should be noted that methanol is a highly toxic alcohol leading to some health and environmental risks. Therefore, though DMFCs could be particularly advantageous for portable and small-scale applications due to their compact design and liquid fuel storage, the health risks pose significant barriers to use. They have found applications in portable electronic devices, where the use of gaseous hydrogen is impractical, and the current portable electronics market is dominated by Li- or Ni-based rechargeable batteries [35]. Further, methanol will capture water from the atmosphere to produce aqueous mixtures, which are rather corrosive. Because of these perceived difficulties, DMFCs are not a current focus for vehicle applications.
2.1.5. Molten Carbonate Fuel Cells (MCFCs)
Molten carbonate fuel cells, or MCFCs, employ molten carbonate salt mixtures as the electrolyte. To achieve sufficient conductivity of the carbonate ion through the liquid electrolyte, they operate at elevated temperatures, typically in the range of 600–700 °C.
The set of chemical reactions in an MCFC operating using hydrogen as a fuel is:
Anode: H2 + CO32− → H2O + CO2 + 2e−
Cathode: ½O2 + CO2 + 2e− → CO32−
Cell: H2 + ½O2 → H2O
Therefore, the cell requires recirculation of CO2 from the exhaust side to the oxygen side of the fuel cell while simultaneously requiring the removal of the water present in the exhaust. This requires additional equipment, increasing the overall weight of the fuel cell. Because in a chemical system, one will inevitably lose some fraction of a recirculating component, a makeup supply of CO2 should also be carried onboard, which would normally be unnecessary for a hydrocarbon-fueled MCFC. For these reasons, MCFCs are well-suited for stationary power generation due to their high efficiency of 65% when generating power alone and tolerance for impure hydrogen sources. Originally developed for natural gas or coal-based power plants, when they combine heat and power systems, the energy utilization can be as high as 85% [24]. Because of their elevated operating temperatures, use of a liquid electrolyte, and higher mass due to the reaction gases management systems, they are less viable for FC-EVs.
2.1.6. Solid Oxide Fuel Cells (SOFCs)
Solid oxide fuel cells (SOFC) have traditionally been used in power plants. Similarly to MCFCs, their electrical efficiency can reach 60%, and they can achieve energy utilization up to 85% when used in combined heat and power systems. Originally, SOFCs required temperatures in the order of 1000 °C to operate, mainly to achieve the necessary oxide ion conductivity through the electrolyte, which was originally based on yttrium-stabilized zirconia, also known as YSZ [36]. Developments in fast ion conductor oxides, like the discovery of gadolinium-cerium oxide-based electrolytes, significantly reduced the operating temperatures [37]. These oxides, coupled with recent advancements in metal-support SOFCs, mean the operating temperature is now reduced to 550 °C or even lower [15,16]. The benefit of metal-support SOFCs is that they can withstand thermal cycling and mechanical stress (e.g., vibration from transportation) and offer quick start-up times [15,16], which are critical factors for FC-EVs. More advantages of SOFCs are the broadened range of suitable fuel selections, including light hydrocarbon fuels such as methane, propane, butane, alcohols, and other organic liquids when compared to other types of fuel cells that have limited fuel (e.g., pure hydrogen) selections. This flexibility in fuel selection makes SOFCs a popular design for power plants [24]. An additional advantage of SOFCs is the use of solid-state components as opposed to other fuel cells such as PAFCs, which use liquid components.
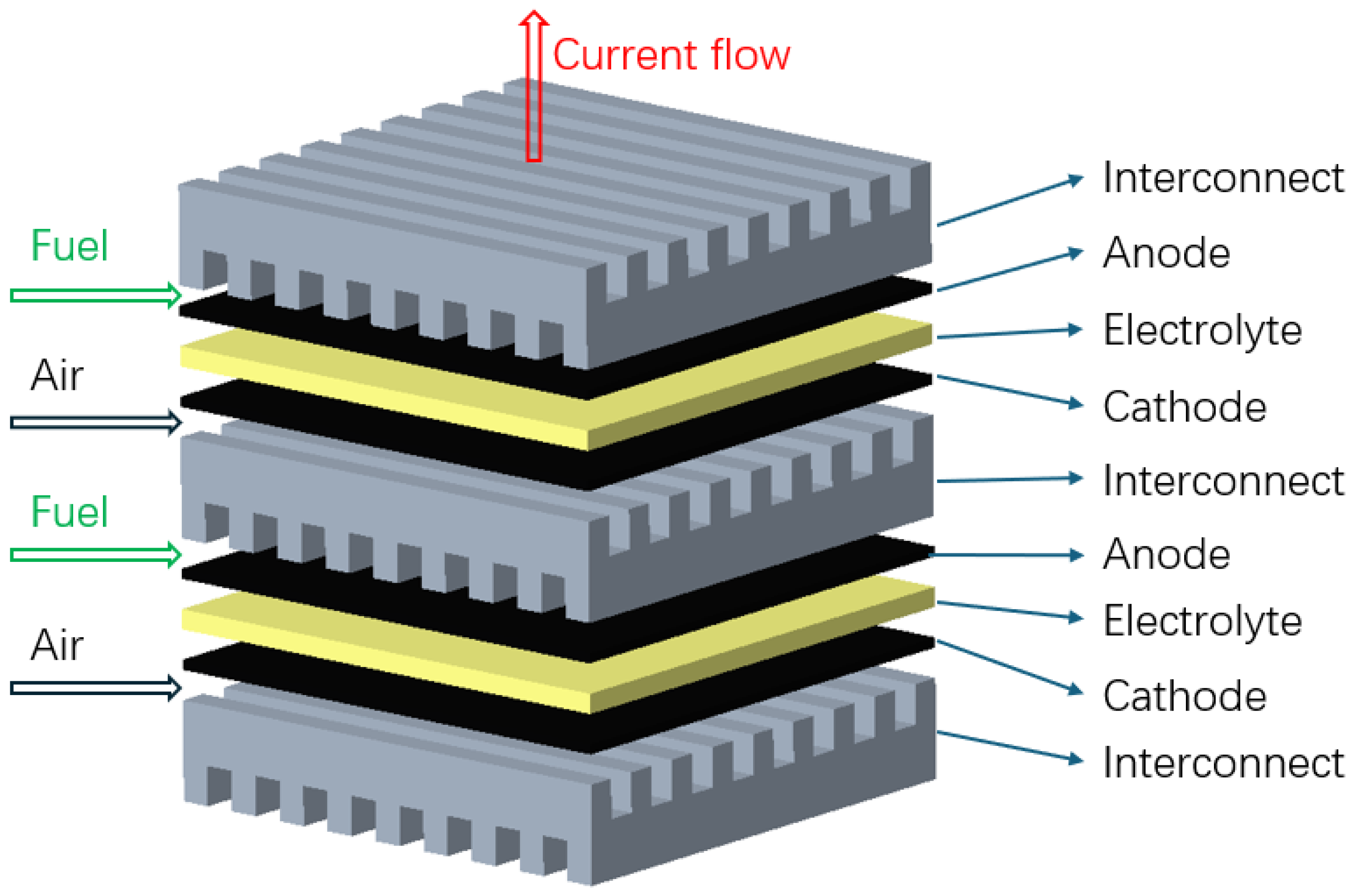
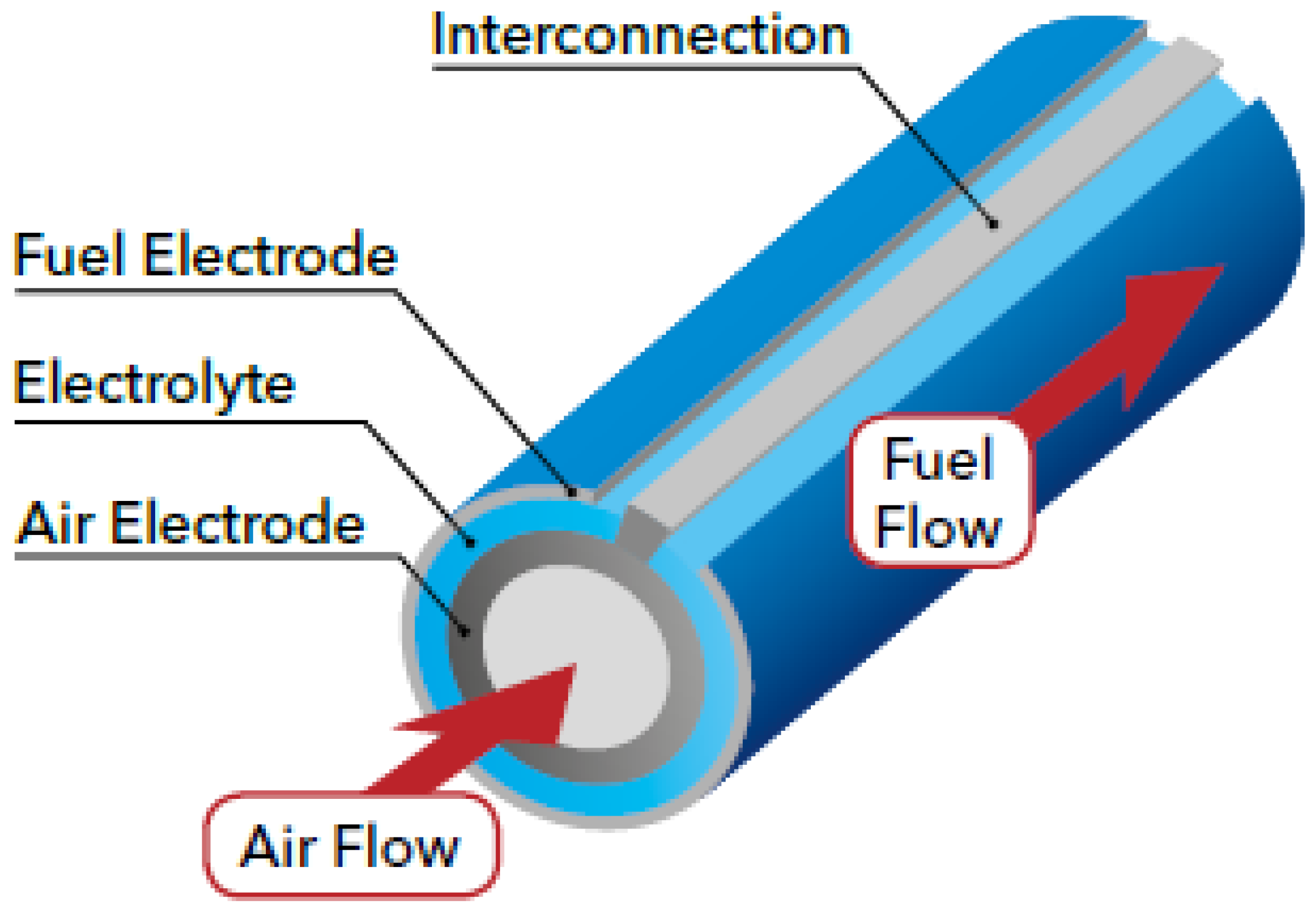
There are two commonly used design configurations for SOFCs. One is planar SOFCs, as shown in Figure 2, where the layers are stacked on top of each other. The interconnect acts as a gas channel and also as a separator for the anode and cathode side of the SOFC [38,39]. An alternative is a tubular SOFC, as shown in Figure 3, where cells are constructed in layers around the cathode, with a hollow center for airflow and fuel flows on the exterior side of the tube [38]. Tubular SOFCs were the first commercially introduced SOFCs, and they have high manufacturing costs and high electrical resistance compared to planar SOFCs. Table 1 highlights some key differences between the planar and tubular SOFCs. SOFCs are unlike other PAFCs and MCFCs due to their solid-state nature and more stable form, and they could be used for FC-EVs and are highlighted as a promising alternative.
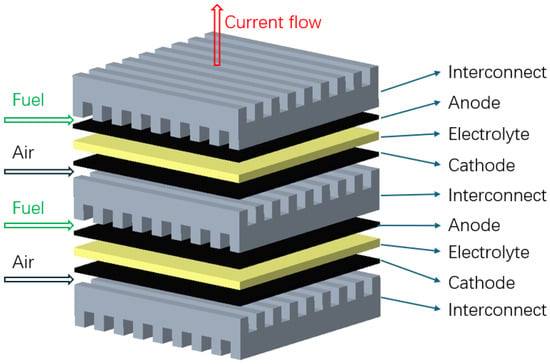
Figure 2.
Planar solid oxide fuel cell, adapted from [38].
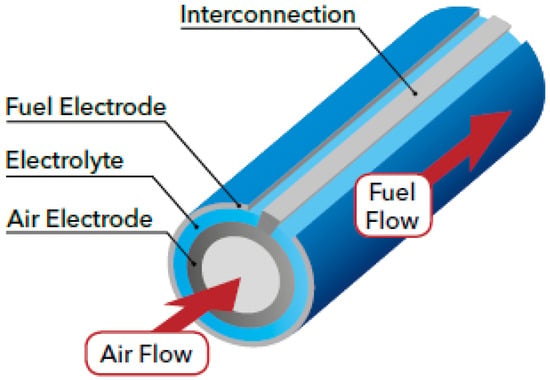
Figure 3.
Tubular solid oxide fuel cell [40].

Table 1.
Performance comparison of planar and tubular SOFCs, adapted from [38,41].
2.2. Fundamental Principles of PEMFCs
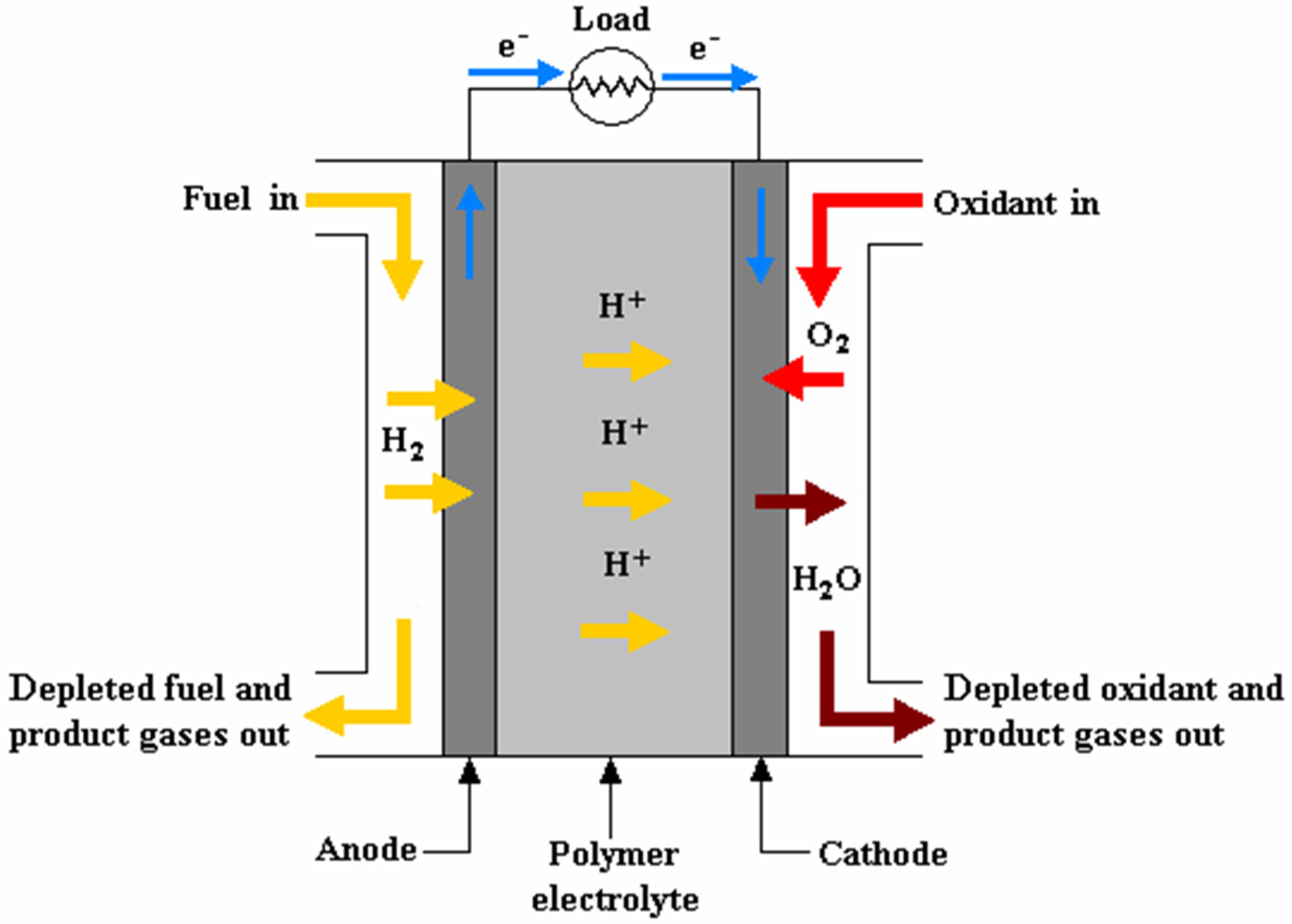
At the heart of a basic PEMFC is an electrochemical reaction that takes place within the cell’s membrane electrode assembly (MEA). This reaction can be represented as follows [42]:
Anode: H2 → 2H+ + 2e−
Cathode: 2e− + 2H+ + 0.5(O2) → H2O
Cell: H2 + 0.5(O2) → H2O
In this reaction, hydrogen molecules at the anode are split into protons and electrons. The protons migrate through the PEM to the cathode, while the electrons flow through an external circuit, creating an electric current. At the cathode, the electrons combine with oxygen molecules and protons to form water (H2O) (see Figure 4). This electrochemical process is highly efficient compared to traditional ICEs and generates electricity with only water vapor as the byproduct, making it a clean and environmentally friendly energy-conversion method.
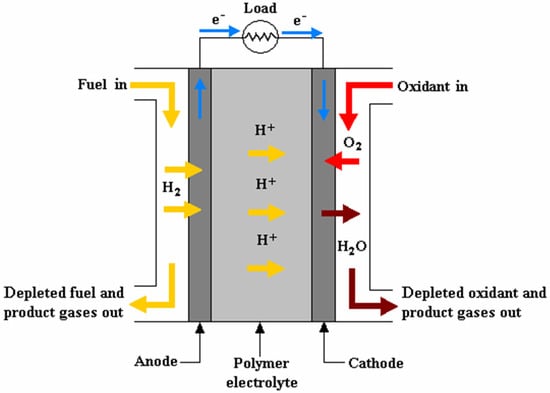
Figure 4.
Operating concept of a PEMFC [43].
2.2.1. Anode and Cathode PEMFC
The anode and cathode catalysts are exposed to highly acidic environments and oxidation. Corrosion requirements are a must when selecting materials; traditionally, platinum is the best choice. Mainly due to cost and resulting issues in competition between manufacturers in the EV market, platinum-based alloys were introduced, like PtNi and PtCo [44]. Currently, platinum-free metal/nitrogen-doped carbon catalysts using earth-abundant metals like Fe, Co, Mn, etc. [14] are under examination for use in EVs.
2.2.2. Electrolyte in PEMFC
The proton-exchange membrane is a separator between anode and cathode materials, as shown in Figure 4. They must also meet the requirements for electrolytes to have good ionic conductivity and insulate between the electrodes. The first membrane was developed by General Electric in the 1960s for space missions [45]. Currently, most membrane electrolytes used are based on Nafion, a sulfonated tetrafluoroethylene-based fluoropolymer-copolymer [46].
2.2.3. Durability of PEMFCs
The average PEMFC lifespan projected in automotive applications, which includes the effects of road vibration [47], is in the range of 3700 h, with 10% voltage degradation [48]. The purity of hydrogen is a critical factor in the durability of PEMFCs, as most hydrogen is extracted from natural gas, which may contain traces of CO in the fuel. This CO can poison Pt-based catalysts, preventing the hydrogen and oxygen from reaching the catalyst. Another significant cause of degradation is related to water management in the fuel cell (i.e., the control of humidity on the membranes). If the membrane is too dry, its ionic conductivity will be reduced. Under very wet conditions, flooding occurs, blocking the reaction gas’s reach to the reaction site [49]. Moreover, with high-power-density operation, water accumulates faster, resulting in rapid performance degradation [50]. Improving water management is crucial to overall system performance.
2.3. Fundamental Principles of SOFCs
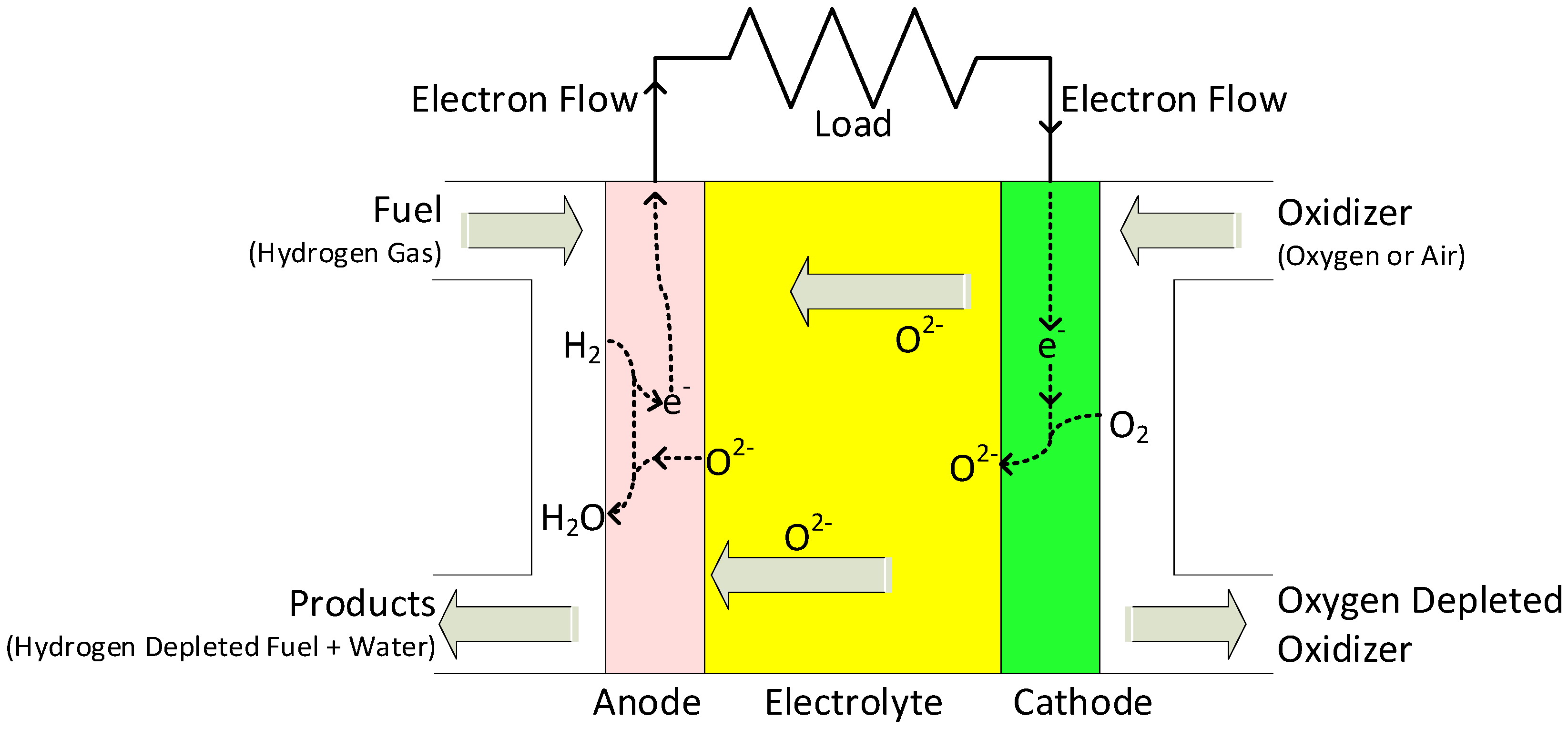
Unlike PEMFCs, SOFCs have all solid-state components. Regardless of the various shapes and designs of SOFC stacks, they all have the same components and working principles. A single SOFC consists of a porous anode and cathode on each side of the dense electrolyte. Reactions take place on each side of the electrode simultaneously. On the cathode side, where it is exposed to air, oxygen molecules are reduced to form oxygen ions (O2−). They are diffused through the electrolyte to the anode side, where the fuel (typically H2) is oxidized to form water vapor (H2O). Since this is a chemical reaction, it has an open circuit voltage of around 1.2 V, before any useful amount of current can be collected. SOFCs must be stacked in a series to produce enough voltage and power. When constructing SOFC stacks, interconnects are used as physical mounts, fuel/air manifolds, and electrical/thermal conductors for each unit cell, as shown in Figure 5 [51,52].
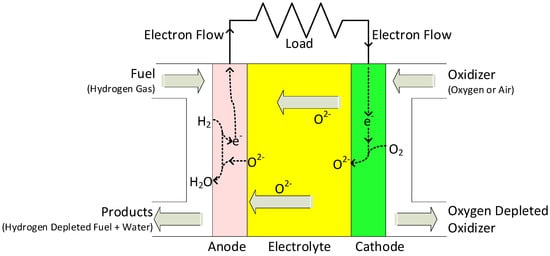
Figure 5.
Operating concept of an SOFC.
2.3.1. Anode in SOFC
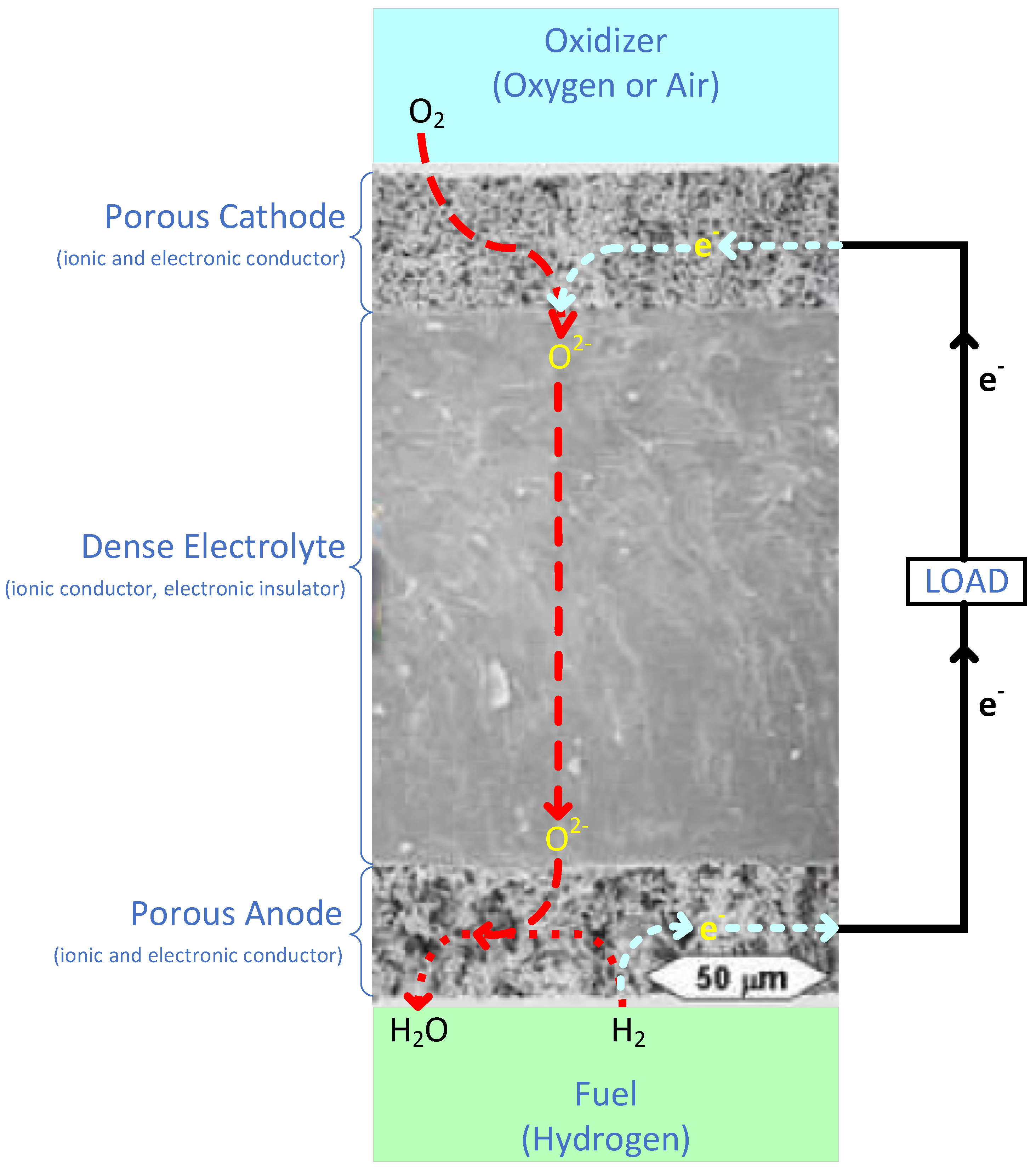
The SOFC anode is porous in order to allow (hydrogen) fuel to flow to the electrode/electrolyte interface. It is responsible for catalyzing the oxidation of the fuel at the layer where the anode and electrolyte meet, as illustrated in Figure 6. The fuel, typically hydrogen; incoming oxide ions from cathode side; and the oxidizing catalyst, all combine at this triple phase boundary (TPB) [53]. This TPB reaction can be given by Equation (7):
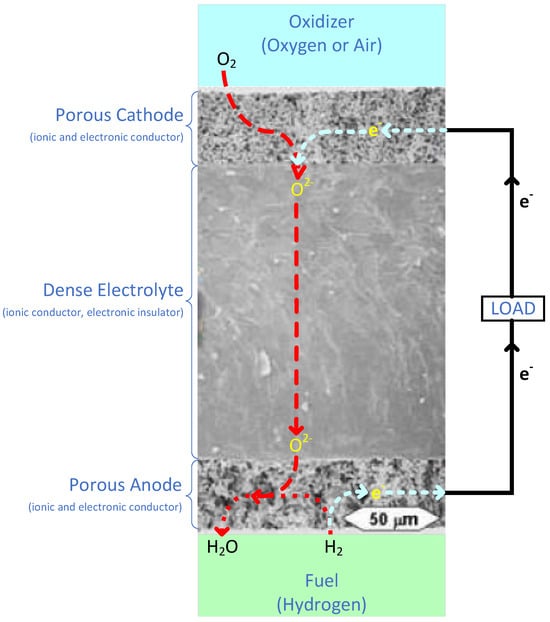
Figure 6.
Schematic of processes occurring within the air electrode, electrolyte, and fuel electrode and at their interfaces, superimposed upon an appropriate microstructure for such an electrode/electrolyte structure. Adapted from [54].
The anode must meet requirements for electrical conductivity, thermal expansion compatibility, and porosity in a reducing environment. The reducing conditions combined with electrical conductivity requirements make metals attractive candidates for anode materials. Refer to Table 2 for anode requirements.

Table 2.
Requirements for SOFC electrodes, adapted from [54].
Most commercial development of anodes has focused on Ni-YSZ, due to the availability and affordability of nickel. NiO has a well-matched thermal expansion with yttria-stabilized zirconia (YSZ); they can be co-sintered with YSZ to form cermet and then reduced in a hydrogen-rich environment during operation [55]. At high temperature operations, nickel tends to sinter and close off its porosity. These problems can also be solved by using a Ni-YSZ anode composite because YSZ can provide structural support to separate nickel particles and prevent them from sintering together at the same time as matching the thermal expansions. This could also improve the adhesion of the anode to the electrolyte. However, when hydrocarbon fuels are used, Ni-YSZ is very sensitive to carbon build-ups; also, low concentrations of sulfur species such as H2S could easily poison the anode [56,57]. This could lead to a degradation of anode performance [58,59].
To resolve these limitations, alternative anode materials have been developed based on single-phase oxides, to enhance and provide better conductivity. Usually, the transition metals are added to form a doped oxide material, e.g., lanthanum-doped ceria (LDC) [60,61].
2.3.2. Cathode in SOFC
Similar to the anode, the cathode needs to be porous in order to allow oxygen molecules to reach to the electrode/electrolyte interface, as shown in Figure 6. The cathode materials need to be stable in an oxidizing environment. The coefficient of thermal expansion (CTE) of both the electrolyte and interconnect must be matched and have excellent mixed electronic and ionic conductivity [62,63]. The reaction equation can be given as:
In the past, researchers have used platinum and other noble metals as cathodes, but due to their cost and availability, they are not ideal for commercial use. In order to ensure the sustainable development of SOFCs, there is a strong desire to develop alternative cathode materials. This has led to the development of using perovskites as cathode materials, because they are highly conductive at elevated temperatures [64]. Today, the most commonly used cathode material is lanthanum manganite (LaMnO3), a p-type perovskite. To further enhance the conductivity, it is doped with rare earth elements (e.g., Sr, Ce, Pr), with strontium being the most widely used dopant to form LSM (La1−xSrxMnO3) [65,66]. All of these perovskites have good electric conductivity, and there is limited ionic conductivity. They are well known for being compatible with YSZ (which provides the ionic conductivity in the composite cathode), and they have the advantage of adequate functionality at intermediate fuel-cell temperatures of about 700 °C [55,67].
However, the effectiveness of the perovskite-base cathode diminishes with decreased temperature, so there is a continuous need for the development of intermediate-temperature materials for SOFCs. Therefore, an alternative high-performance cathode material at a lower temperature is desired. On the other hand, using mixed ionic electronic conductors shows improvements in performance because the size of the reactive surface area increases at the cathode/electrolyte interface, and this allows for an increase in the diffusion of oxide ions across the electrode. More research has been carried out using cobalt-containing perovskite. For example, La1−xSrxCoO3 (LSC) is a known alternative to LSM. This mixed ionic electronic conductor has faster oxygen transfer kinetics at intermediate temperatures [68,69,70,71,72]. Unfortunately, the cobalt-containing LSC has a high CTE, and it tends to react with the YSZ electrode to form insulating compounds during the sintering process, which will cause the cell degradation. Overall, LSM remains one of the most widely used cathode materials. It is mostly compatible with the YSZ electrolyte. The future development of intermediate-temperature cathode materials is necessary because any reduction in operating temperature reduces the operating costs, and this creates an opportunity for additional cost savings.
2.3.3. Electrolyte in SOFC
The electrolyte for SOFCs must demonstrate stability in both reducing and oxidizing operation environments. It must possess high ionic conductivity to allow converted oxygen ions to migrate from the cathode (air side) through the electrolyte to the anode (fuel side) in a cell. To prevent a short circuit, the electrolyte must also have low electronic conductivity to insulate between the electrodes. The electrolyte must be formed densely to stop fuel leaking to the air side of the cell, and it must be thin enough to minimize resistive losses. Lastly, it should not react with adjacent cell components. These electrolytes should be thermally and structurally stable across a wide range of temperatures [73,74].
Nernst was the first person to demonstrate a solid oxide electrolyte’s oxide-ion conductivity. However, modern SOFC function and working principles were defined by Baur and Preis. Until now, stabilized zirconia—especially yttria-stabilized zirconia, which possesses a fluorite structure—has been the most favored electrolyte for SOFCs. YSZ has demonstrated long-term stability in both reducing and oxidizing environments [73,74].
2.3.4. Interconnect in SOFC
As stated in Section 2.3, a single SOFC has an open circuit voltage of around 1.2 V. Before any useful amount of current can be collected, SOFCs must be connected together and stacked in a series to produce enough voltage and power. In order to stack the SOFCs, a physical structure and electrical connection are required; interconnects are used to serve this purpose.
Interconnects do not just serve as a physical barrier between the anode and cathode to prevent the fuel from mixing with air. They also need to have excellent electrical conductivity in an extreme environment, which means they must meet the stringent material requirements [39,75]:
- Exceptional electrical conductivity with an acceptable area-specific resistance (ASR), which is generally expected to be below 0.1 Ω cm−2;
- Thermal, mechanical, and chemical stability at evaluated operational temperature in both oxidizing and reducing environments during the lifetime of the service;
- Impermeable to oxygen and hydrogen, to prevent the direct combination of the fuel and oxidant during operation;
- The CTE matches with other cell components throughout the heat and cooling cycle to avoid unnecessary thermal stress generation and cell damage, with the generally accepted value of around (10–12) × 10−6 K−1;
- Absolutely no reaction or interdiffusion between interconnects with electrodes, electrolytes, or sealing materials exposed simultaneously in reducing or oxidizing environments at the SOFC operation temperature;
- Excellent in thermal conductivity at SOFC operational temperature to facilitate the internal reforming of hydrocarbon fuels, with the accepted value of above 5 W·m−1·K−1;
- Adequate resistance to oxidation, sulfidation, and carbon cementation;
- Adequate mechanical strength and creep resistance at the operational temperature;
- Comparably low cost of manufacturing and ease of assembly for commercial mass production.
Due to all these stringent requirements, there are not many interconnects available to choose from. Ceramic or metallic interconnects are the only options available at this time.
In recent years, researchers have made major progress toward reducing the operating temperature of SOFCs. An anode-supported SOFC can operate at as low as 800 °C. This is made possible by synthesizing thin-film electrolytes for SOFCs to operate at reduced temperatures without compromising the power density. Reduced temperatures also lead to easier fabrication and lower manufacturing costs [76,77].
Lower operating temperatures spurred the development of metallic interconnects, compared to ceramic interconnects. Metallic interconnects have many advantages over ceramic interconnects, including higher electronic and thermal conductivity, lower material and manufacturing costs, and better mechanical strength and workability. However, they do not have much to offer in the way of oxidization and corrosion resistance. They also have a relatively high risk of chemically reacting with neighboring components [78]. Earlier research on the use of metallic interconnects has focused on Ni-, Fe-, and Cr-based alloys. At high operating temperatures, these are found to form protective, but generally electrically insulating, surface oxide scales. Among all these alloys, it was found that chromium-forming alloys were the most preferable to use as interconnects due to their relatively high conductivity of Cr2O3 scales compared to other alloys [79].
2.3.5. Durability of SOFC
The average SOFC projected degradation rate is 0.2–0.5% per every 1000 h of usage [80]. The major durability issue arises with SOFCs due to metallic interconnects. They are cheaper to manufacture compared to their ceramic interconnect counterparts. However, by using chromium-forming alloys, the main issue is the Cr volatility associated with the Cr2O3 scale. Cr species vaporize and migrate to the cathode side of the SOFC. This results in the serious performance degradation of the SOFC’s cathode electrochemical activity because Cr blocks the oxygen reaction by reacting with oxygen and depositing it to the cathode [81,82]. In order to retain the advantages of using ferritic stainless steels as SOFC interconnects, several methods have been proposed and examined for preventing oxidation and chromium migration. One method is to modify the stainless-steel composition in order to increase resistance to oxidation. This leads to the development of new alloys, which are specially designed for SOFCs. One of these new interconnect alloys, the Crofer 22 APU alloy, contains about 22 wt.% Cr and small amounts of Mn, Ti, and La. The addition of Mn into this alloy forms a (Cr,Mn)3O4 spinel outer layer, which reduces Cr volatility significantly [83,84].
Special alloys developed for SOFC interconnect applications remain susceptible to Cr evaporation and the continuous growth of oxide scale during stack operation, which still leads to long-term stack performance degradation. Alternatively, other researchers are working on developing electrically conductive coatings for protecting ferritic stainless steels. They have made tremendous improvements in coatings, which share similar developmental characteristics to interconnects. The proposed requirements are as follows [75,85]:
- Thermally and chemically stable in both oxidizing and reducing environments at SOFC operating temperatures;
- Negligible reaction with neighboring components, including anodes, cathodes, electrode contact materials, seals, or substrates;
- Ability to block Cr migration or diffusion from ferritic stainless steel;
- Low ohmic resistance;
- Adequate match in CTE with ferritic stainless steel during heating and cooling cycles.
There are various protective coating materials that were developed to accommodate the use of currently available ferritic stainless steel. They could be a single metal, e.g., Ni, Co, Cu, which will be converted to an oxide (e.g., NiO, Co3O4, CuO) at high temperatures—these are relatively cheap coatings. Studies show they can block up to 99% of Cr migration. However, their CTE is much higher than the acceptable range of (10–12) × 10−6 K−1, which is the standard for most ferritic stainless steel [86,87,88]. Other protective coatings available for use include reactive element oxide coatings (reactive element oxide coatings do not prevent Cr migration from Cr-forming stainless steel, but they have shown long-term stability improvements in chromium scale adhesion [84,89]), conductive perovskite coatings, and conductive spinel coatings. (Mn,Co)3O4 spinel coatings are generally known as the most effective currently available coatings due to their high thermal and electrical conductivity. They can be easily modified to closely match the CTE of the ferritic stainless steel and ceramic component used in SOFCs [90,91]. Furthermore, they show exceptional capability in blocking chromium migration to the cathode.
2.4. General Fuel-Cell Model
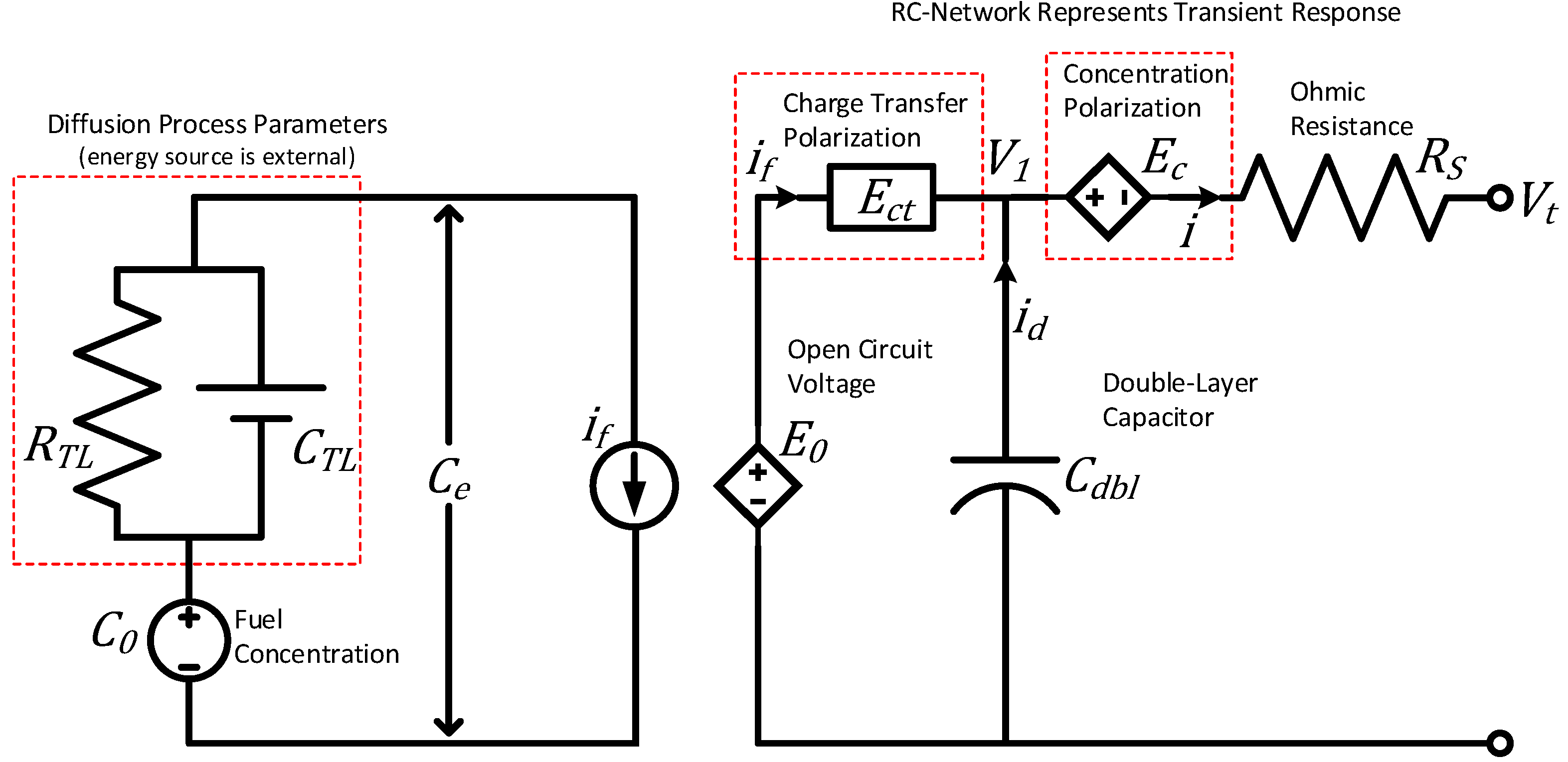
A fuel cell is an electrochemical device similar to that of a battery in that all the physical processes are essentially the same, except the energy source for a fuel cell is found externally. A lumped parameter model for fuel cells can be seen in Figure 7 [42].
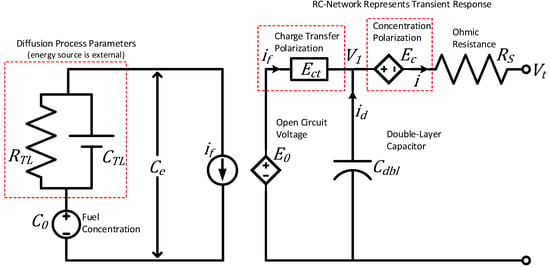
Figure 7.
Fuel-cell model based on the fundamental principles of electrochemistry, adapted from [42].
The key distinction between lumped parameter models for fuel cells and batteries lies in how they handle the diffusion process. In a battery, the electrolyte not only facilitates ion movement but also stores energy, necessitating a relatively larger volume of electrolyte. This larger volume allows for the faster response characteristics of batteries compared to fuel cells. In contrast, a fuel cell primarily utilizes the electrolyte as an ionic conductor in the diffusion process. Consequently, the electrolyte layer in a fuel cell is intentionally thin, thus minimizing resistance to ionic conduction while serving as electronic insulation between the electrodes.
The maximum electrical energy for a fuel cell operating at a constant temperature and pressure is given by the change in Gibbs free energy, as seen in Equation (9) [42].
Wel = −ΔG = nFE
The electrochemical potential, represented as the cell voltage (E), is the driving force behind the movement of electrons from the anode to the cathode. The Nernst equation (10) below relates the cell voltage to the concentrations of reactants and products, as well as temperature [42].
where , and represent the concentrations of hydrogen, oxygen, and water, respectively. This applies to the reaction written as , and therefore, the number of electrons “” involved in the reaction in Equation (10) is two.
The electrochemical potential of a hydrogen fuel cell is dependent on the operating conditions, such as hydrogen and oxygen pressures and temperature. Understanding and optimizing these parameters are essential for achieving maximum efficiency and power output in FC-EVs.
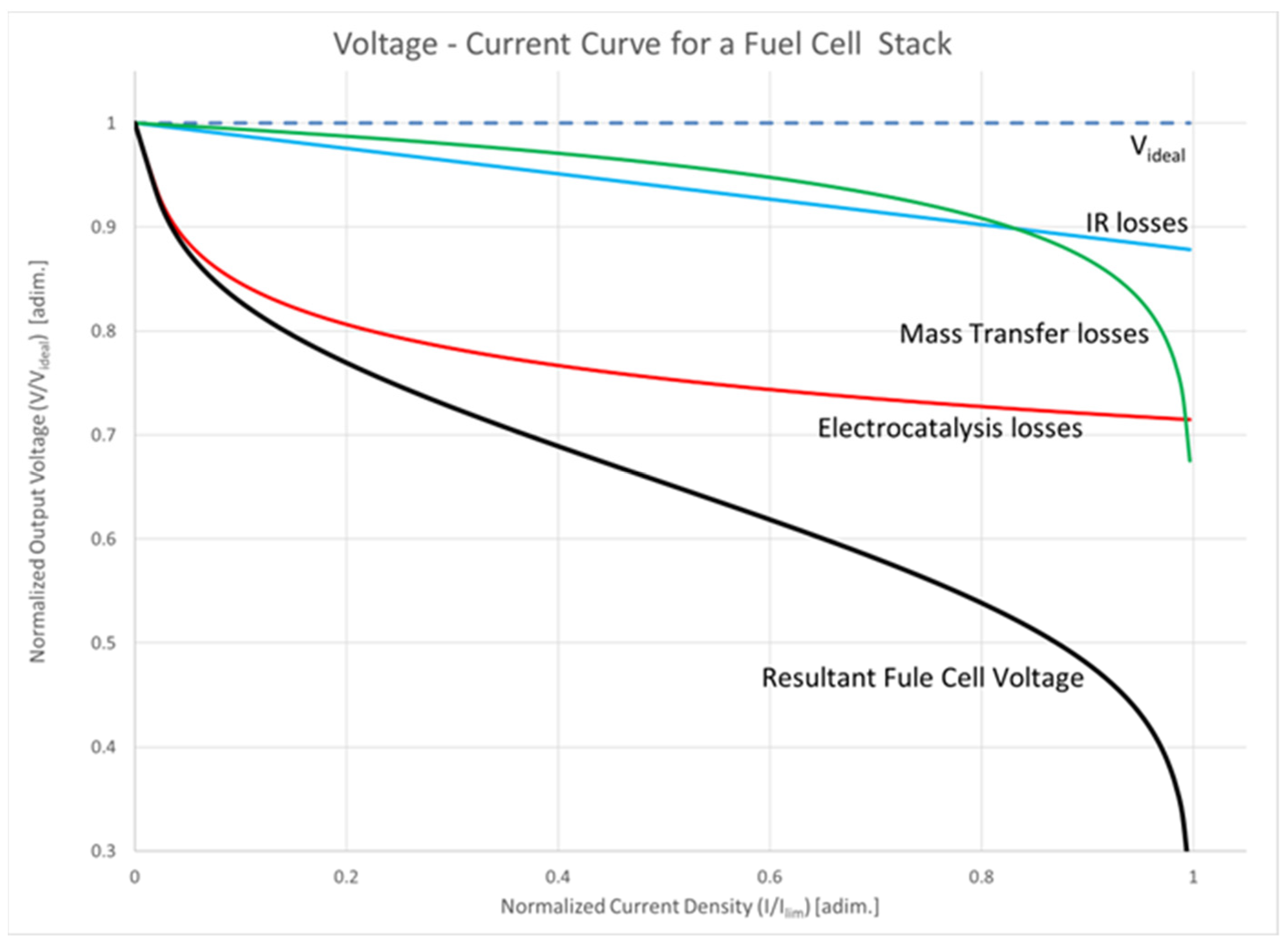
The voltage–current relationship for a hydrogen/oxygen cell is depicted in black in Figure 8. Theoretical predictions suggest that a higher potential of around 1 V per cell can be achieved, shown as the dashed top line in the figure. These levels are unattainable in practical cells due to electrocatalysis (in red in Figure 8), IR (blue line in Figure 8), and mass transfer losses (green line in Figure 8). Upon the inception of a reaction, electrocatalysis losses reduce the voltage from the idealized value. In the linear region, ohmic losses lead to a reduction in cell potential, which corresponds to the operating range of a practical fuel cell. At the limit, mass transfer within the fuel cell limits the concentration of reactants or products close to the electrodes, so the rate of reaction falls to a level that completely disrupts the output voltage of the cell, as implied by Equation (10). In normal operational conditions, therefore, the practical range of operations is the linear region in the curve. The resistive components within the cell are what impose the limits on the efficiency achievable by a fuel cell, and a critical consideration in the design of fuel-cell-powered electric and hybrid vehicles is that as the current drain increases, the cell’s operating voltage decreases. Due to the modest cell potential, multiple cells are connected in series to attain the desired voltage. A significant advantage of fuel cells lies in their reduced sensitivity to scaling, ensuring consistent overall system efficiencies across a wide power range, from kilowatts to megawatts.
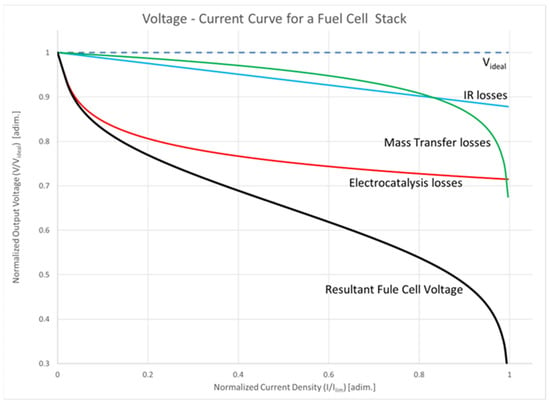
Figure 8.
Voltage–current relationship of a hydrogen/oxygen cell.
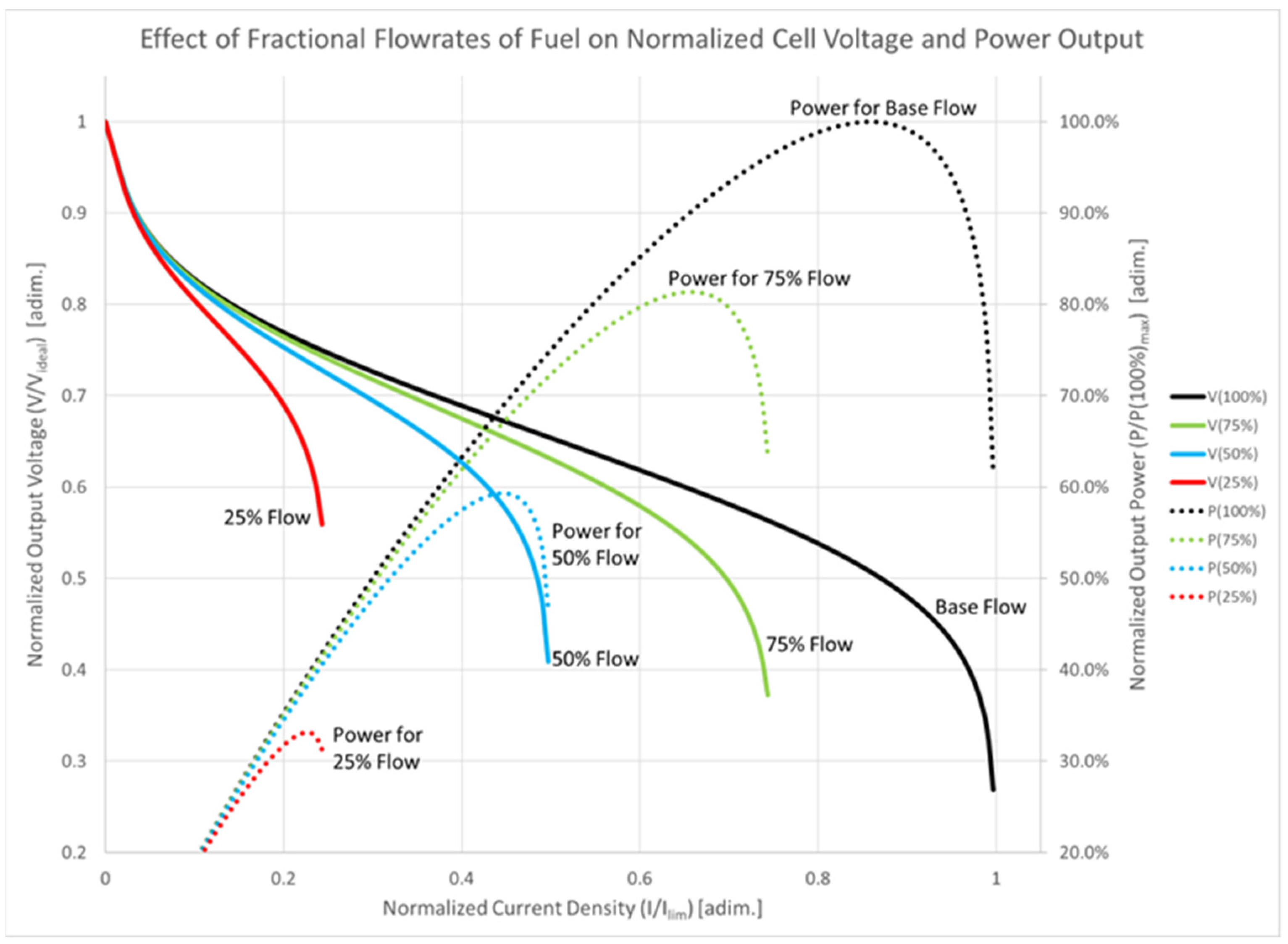
Fuel-cell performance is particularly sensitive to load variations due to its low voltage and high current output characteristics [42]. The fuel-cell controller, utilizing voltage and current feedback information, regulates the flow of hydrogen into the fuel-cell stack to achieve a reaction rate that provides the required electrical power while minimizing excess hydrogen venting [42]. Attempts to draw more power from the fuel cell without adjusting the flow rate can deplete the hydrogen concentration, leading to a reduction in output voltage and potential damage to the fuel-cell membrane [42]. When the hydrogen utilization rate approaches 100%, the cell enters a current limit mode, or concentration polarization mode, dominated by high internal losses [42]. These fuel-cell characteristics as a function of flow rate can be seen in Figure 9. The fuel-cell controller must avoid operating in the current limit regime to maintain efficient operation. The deliverability of output power from the fuel-cell stack decreases as the hydrogen flow rate is reduced, though if lower power is required for propulsion, operating the fuel cell at a reduced flow rate minimizes wasted fuel [42]. The ideal controller ensures that fuel is supplied to the cell at the same rate it is consumed, to generate the electricity needed for propulsion [42]. However, due to the fuel cell’s slow response characteristics, a reserve of energy is necessary to ensure uninterrupted operation. The byproduct of the fuel-cell reaction is water in the form of steam, which exits the cell along with any excess hydrogen [42]. The water vapor can be employed to heat the vehicle’s interior, but the vented hydrogen represents system waste.
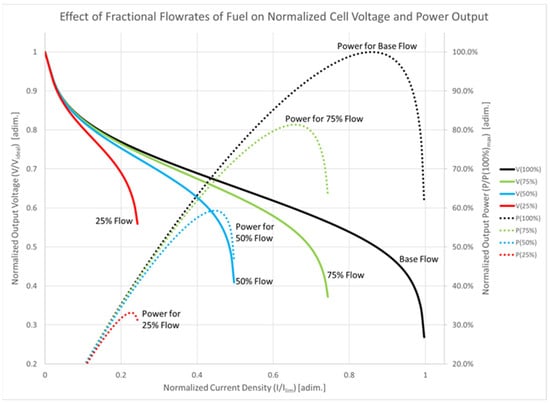
Figure 9.
Fuel-cell characteristics as a function of flow rate.
2.5. Battery Electrical Vehicles
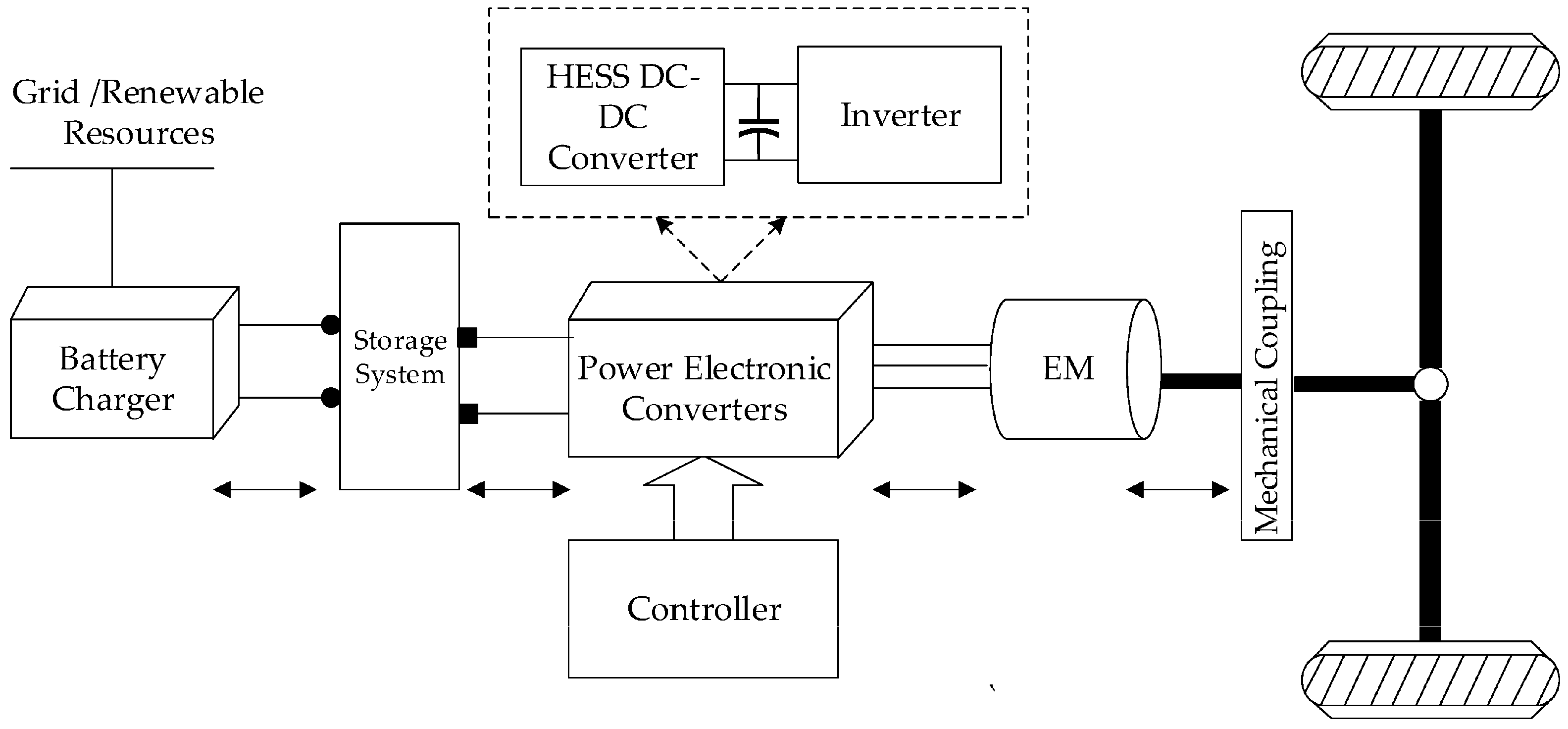
Battery electric vehicles (BEVs) have gained popularity with Tesla, and Tesla currently dominates the BEV market, which was previously dominated by Nissan [92]. In 2018, Tesla held the largest share of the BEV market with a well-established supercharger network infrastructure [92]. However, BEVs have many limitations compared to internal combustion engines. Especially during long-distance driving, charging time is of concern. Even with a Tesla Supercharger station rated at 250 kW, theoretically, it will take only 24 min to charge a full 100 kWh battery. In real-world situations, it can only sustain at the peak rate for a very short time, and then the charge wattage gradually decreases over time. Eventually, it will take about an hour to fully charge a 100 kWh battery, while filling a gasoline tank takes only a few minutes. The protection circuit design used to avoid overcharging and thermal protections may cause an unsustainable peak charging rate. Rapid charging can also damage the battery cell, reducing capacity and aging [93]. In addition, with the rapid growth of battery-powered vehicles, the current power grid is facing pressure; some supercharging stations will only charge at reduced power during peak hours, further increasing the charging time of distance travelers. It is costly to build more power-generating plants to accommodate the increase in BEVs. Figure 10 shows a block diagram of a battery-powered EV, which is similar to an FC-EV except that it only charges with the grid. FC-EVs incorporate an FC generator and a reduced battery pack size.
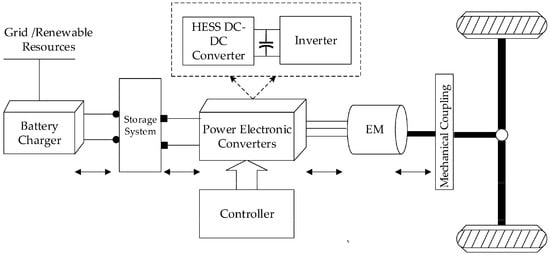
Figure 10.
Block diagram of battery electrical vehicle [94].
2.6. Hybrid Electric Vehicles (HEVs)
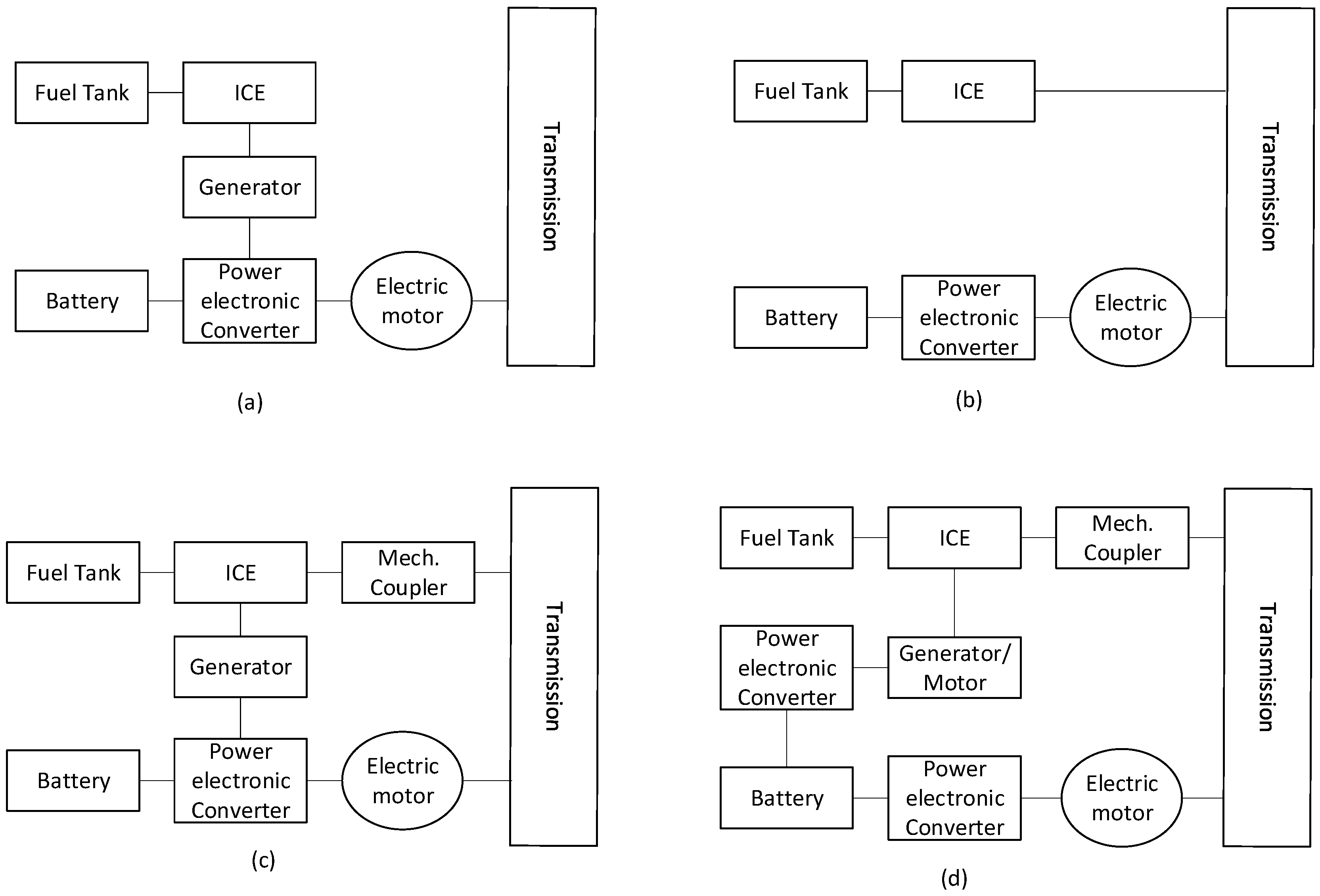
Hybrid electric vehicles became popular with the Toyota Prius, first introduced in 1997, and many generations have evolved [95]. They combine an internal combustion engine with an electric motor and a small battery pack. They have a more complex system than ICEs or BEVs. Figure 11 shows the different architectural designs in HEVs. Table 3 shows key parameters when comparing ICEs, EVs, HEVs, and FC-EVs, where MPGe is “miles per gallon of gasoline-equivalent”. The EPA calculates that 33.7 kWh (121 MJ) is equivalent to one gallon of gas [96,97,98,99].
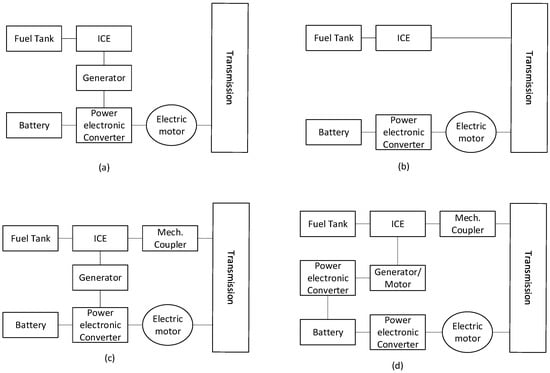
Figure 11.
Various architectures of an HEV. (a) Series hybrid (b) Parallel hybrid (c) Series–parallel hybrid (d) Complex hybrid. Adapted from [100].

Table 3.
Comparison between ICEs, EVs, HEVs, and FC-EVs [96,97,98,99].
2.7. FC-EV Architecture
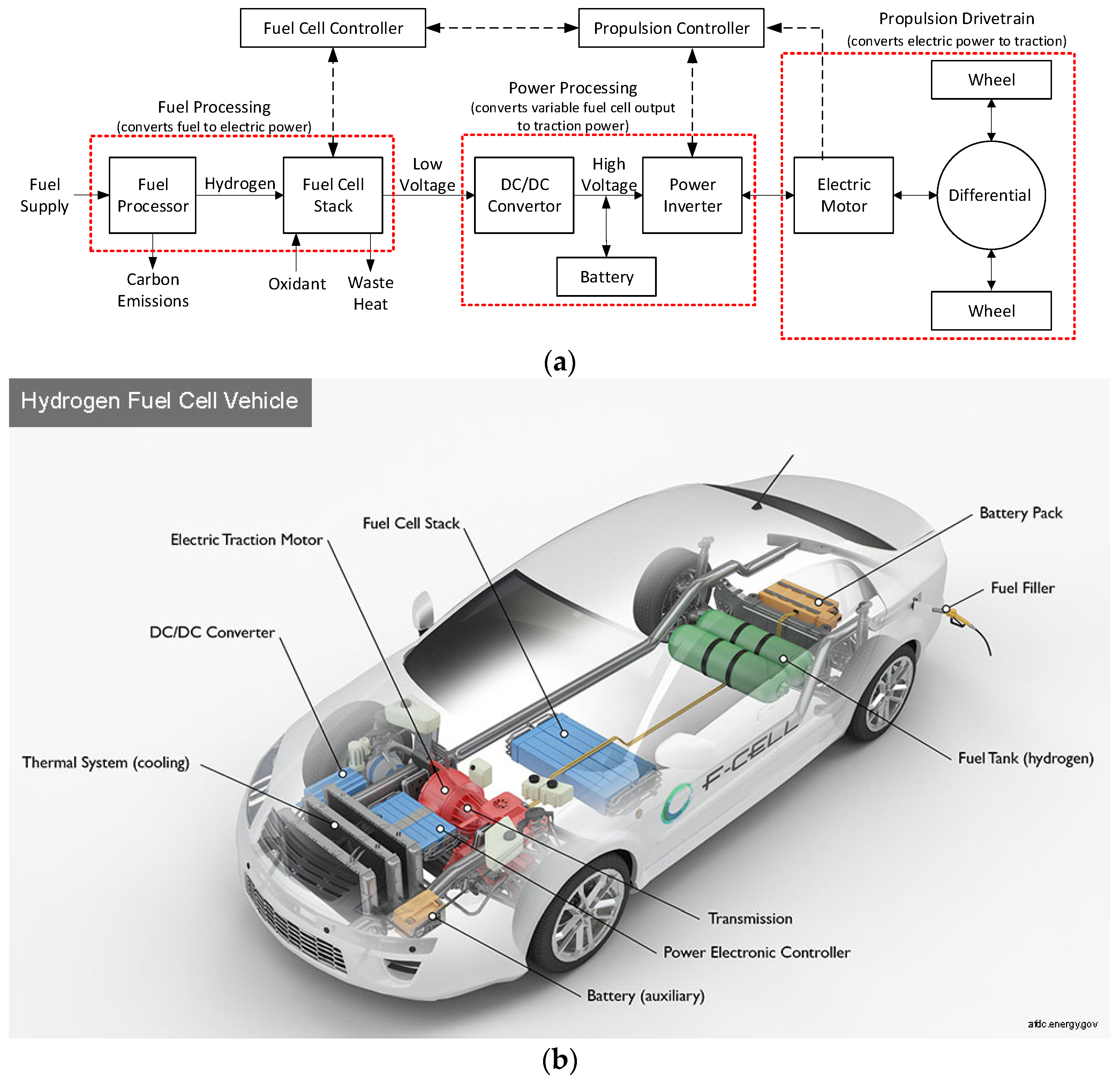
An FC-EV comprises several key components, including a fuel storage system, which may include a fuel processor for converting raw fuel into hydrogen. It also includes a fuel-cell stack and its control unit; a power processing unit and its controller; and a propulsion unit consisting of an electric machine and a drivetrain [42]. The fuel cell exhibits current source-type characteristics and operates with a low cell voltage. To achieve higher voltage levels, multiple fuel cells are stacked in series, and the output voltage is subsequently boosted to interface with a DC/AC inverter that drives an AC propulsion motor, typically used for higher-power-density applications. Given the low fuel-cell output voltage, a DC/DC converter is employed to elevate and regulate the voltage before supplying it to the electric motor.
The power electronic interface circuit connecting the fuel cell and the electric motor includes the DC/DC converter for voltage boost, a DC/AC inverter for supplying an AC motor, a microprocessor/digital signal processor for controls, and batteries/capacitors for energy storage [42]. Notably, the time constant of the fuel-cell stack is considerably slower than that of the electrical load dynamics [42]. Thus, a battery storage system is necessary to provide power during transient and overload conditions and absorb reverse energy flow resulting from regenerative braking [42]. While a high-voltage battery pack can interface directly with the high-voltage DC link, this requires a substantial number of series cells. Alternatively, a bidirectional DC/DC converter link can connect a lower-voltage battery pack to the high-voltage DC bus. The electric output of the fuel cell is directed into the lower voltage DC bus, which is also maintained by the battery pack. A block diagram of this architecture can be seen in Figure 12a,b.
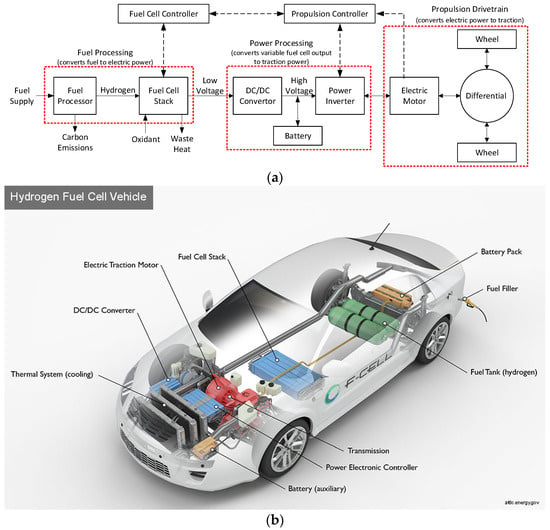
Figure 12.
(a) FC-EV block diagram architecture, adapted from [42]. (b) FC-EV schematic architecture [101].
3. Necessary Advancements
Recent advancements in fuel-cell technology have broadened the options available for incorporating fuel cells into vehicles. As discussed, traditionally, PEMFCs have been the primary choice as they are known for their low operating temperatures and compact designs. As also highlighted, SOFCs have emerged as a promising alternative, offering higher efficiency, a wider range of fuel options (including biofuels), and the advantage of not requiring expensive catalysts like platinum. Additionally, they exhibit robustness and durability, particularly under constant load conditions. While PEMFCs are praised for their quick start-up times, compactness, and suitability for variable power demands, SOFCs present an appealing option for applications prioritizing efficiency, fuel flexibility, and cost-effectiveness. As research and development progress, both technologies are being refined to address their respective strengths and challenges, contributing to the evolution of fuel-cell electric vehicles.
In an era characterized by an escalating demand for sustainable and environmentally conscious transportation solutions, FC-EVs have emerged as a promising candidate. Nevertheless, their widespread integration requires critical advancements across a spectrum of domains, encompassing technology, infrastructure, economics, and public perception. It is important to undertake a comprehensive analysis of four areas that require advancement in the FC-EV field, including (1) well-to-wheel efficiency (discussed in Section 3.1), entailing the optimization of the complete hydrogen production, transportation, and utilization process; (2) infrastructure implementation (discussed in Section 3.2), accentuating the imperative need for a robust and expansive network of refueling stations; (3) cost considerations (discussed in Section 3.3), delving into the intricacies of both production and operational financial aspects; and (4) public perception (discussed in Section 3.4), duly acknowledging the profound impact of public attitudes, awareness, and acceptance in shaping the trajectory of FC-EVs on a global scale.
3.1. Well-to-Wheel Efficiency
Well-to-wheel (WTW) efficiency in the context of FC-EVs is a critical metric that assesses the overall energy efficiency of the entire hydrogen production, distribution, and consumption process. It considers the entire lifecycle of hydrogen, from the source of energy used for its production to its utilization in a fuel-cell vehicle, providing a comprehensive view of how efficiently hydrogen energy is converted into vehicle propulsion.
When considering the numerous methods to generate, distribute, and consume hydrogen for FC-EVs, the WTW efficiency for these types of vehicles can reach 29.2% efficiency when utilizing the most efficient forms of generation, distribution, and consumption [102]. When utilizing renewable energy to produce hydrogen through water electrolysis, the WTW efficiency of FC-EVs can be anywhere between 21.9 and 29.2%, which beats the WTW efficiency of internal combustion vehicles but is dwarfed by the renewable WTW efficiency of battery electric vehicles, which is in the range of 62.9% [102]. When utilizing grid power to manufacture hydrogen by electrolysis, the WTW efficiency range significantly drops to between 8.2% and 11%, which makes FC-EVs less efficient than traditional ICE vehicles [102].
FC-EVs operate more efficiently than traditional gasoline vehicles (ICEVs) and do not emit pollutants from their tailpipes. However, using current hydrocarbon reforming technologies, producing, transporting, and refueling hydrogen requires more energy and may generate more carbon emissions when compared to gasoline. To compare the two types of vehicles, researchers conducted a well-to-wheel analysis, considering energy use and emissions [103]. Researchers looked at a specific FC-EV (the Toyota Mirai) and a gasoline car (such as the Mazda 3) using two sets of fuel consumption data: one from the EPA’s fuel economy figure and another from physical vehicle testing. The WTW results showed that when using hydrogen produced from fossil fuels like natural gas, FC-EVs use 5% to 33% less WTW fossil energy and emit 15% to 45% fewer WTW greenhouse gas emissions compared to gasoline cars [103]. However, the results depend on how electricity is sourced for hydrogen compression or liquefaction. This emphasizes the importance of considering the entire energy lifecycle when evaluating the environmental impacts of different vehicle technologies [103].
To make FC-EVs a viable option for transportation, it is imperative that researchers find more efficient means of producing hydrogen with renewable energy through water electrolysis, so that the input and output of these FC-EVs can create a net-neutral closed loop.
3.2. Infrastructure Implementation
Improvements to the infrastructure for FC-EVs are essential for their widespread adoption. First and foremost, an extensive network of hydrogen refueling stations needs to be established, both in terms of increasing their numbers and expanding their geographic coverage. As of 2019, there were only 376 hydrogen refueling stations (HRSs) worldwide, and in the United States, all HRSs are located within California [104]. By the end of 2023, there were 921 HRSs worldwide, resulting in 2.4 times (i.e., a 240% growth) more HRSs compared to the year 2019 [105]. As of 2023, 59 HRSs were operational in the United States and another 50 are currently being planned or are under construction [106]. The standardization of refueling protocols and equipment is necessary to ensure compatibility and ease of use across different FC-EV models.
To support a clean and sustainable hydrogen supply, the scaling up of green hydrogen production methods, particularly those utilizing renewable energy sources, is crucial. Efficient distribution systems, including pipelines and transportation methods, are required to deliver hydrogen to refueling stations. Lastly, integration with other energy systems, like renewable sources and electric vehicle charging networks, can foster efficient and adaptable hydrogen infrastructure. Addressing these factors collectively is essential to make FC-EVs a more practical and sustainable mode of transportation.
In terms of the ease of transporting hydrogen, it can be converted into a liquid state, and a multi-step process is typically employed. Initially, hydrogen gas undergoes compression, where its pressure is increased using specialized compressors [107]. This compression forces the hydrogen molecules closer together, raising their pressure. Following compression, the gas is subjected to cooling procedures. This cooling process, known as liquefaction, involves reducing the temperature of the compressed hydrogen to levels below its boiling point [107]. By doing so, the gas transitions into a liquid form. This liquid hydrogen is then stored in specialized cryogenic tanks designed to maintain extremely low temperatures, which are necessary to sustain its liquid state. These cryogenic tanks are heavily insulated to prevent heat transfer from the surroundings, ensuring the hydrogen remains in its liquid form. Overall, the process involves compressing hydrogen gas, cooling it to extremely low temperatures to liquefy it, and storing it in cryogenic tanks [107]. This liquid hydrogen can then be transported and utilized as a versatile and efficient energy source for various applications, including powering fuel-cell vehicles and other hydrogen-based technologies. Researchers have also explored many other methods of storing hydrogen beyond compressing it into liquid. These methods include storing it in metallic hybrids [108] and organic liquid [109].
3.3. Cost Considerations
The cost considerations associated with FC-EVs are multifaceted, with production costs occupying a central role. The manufacturing process of PEMFC components, including the proton-exchange membrane, catalysts, and bipolar plates, represents a substantial portion of the overall production cost. Research and development efforts are aimed at advancing cost-effective materials and manufacturing techniques to reduce the expense of these critical components [110]. For SOFCs, the advancement in metal-support cells significantly reduced manufacturing costs compared to electrolyte- or anode-support cells. Reducing the operating temperature from 1000 °C to 500 °C has also lowered usage costs in maintaining a high temperature [15,16]. Additionally, economies of scale play a pivotal role in cost reduction, as larger production volumes are expected to lower unit costs. Collaborations between automakers and suppliers to streamline the supply chain and optimize production processes are imperative for achieving cost competitiveness in the FC-EV market. Beyond fuel-cell components, the cost of hydrogen storage and tank materials, as well as the assembly of the fuel-cell stack and balance of plant components, contribute significantly to production costs [110]. Enhancing production efficiency and minimizing material waste are ongoing areas of research and development [102].
Operational costs are an integral component of the cost considerations for FC-EVs, encompassing aspects such as refueling, maintenance, and overall cost of ownership. The cost of refueling, dependent on the price of hydrogen and the efficiency of the hydrogen production process, significantly influences the total cost of operating an FC-EV [111]. Overall, meticulous attention to both production and operational cost aspects is pivotal in advancing the affordability and competitiveness of FC-EVs.
SOFC vehicles offer a compelling alternative to traditional PEMFC vehicles, particularly due to their potential for lower costs. Unlike PEMFCs, which require expensive platinum catalysts, SOFCs utilize more abundant materials like ceramics as their electrolytes. This fundamental difference in materials significantly reduces manufacturing costs associated with SOFC technology. Additionally, SOFC systems can operate at higher temperatures, allowing for the direct use of hydrocarbon fuels such as natural gas or biogas without the need for costly purification processes, further lowering operational expenses. Moreover, the durability and long lifespan of SOFCs contribute to reduced maintenance costs over the vehicle’s lifetime compared to PEMFC systems. Overall, the lower cost of SOFC technology makes it an attractive option for widespread adoption in FC-EVs, promising both economic benefits and environmental sustainability.
3.4. Public Perception
Public perception of FC-EVs faces a myriad of challenges that necessitate strategic interventions for widespread acceptance and adoption. Chief among these challenges is the limited awareness and familiarity surrounding FC-EVs, as these vehicles remain relatively novel to the public [104]. Safety concerns related to hydrogen as a fuel source, although meticulously addressed through rigorous safety standards, influence perception [112]. Furthermore, the insufficient availability of hydrogen refueling infrastructure, coupled with perceived high production costs and a lack of incentives, can discourage potential FC-EV adoption [104]. Range anxiety and concerns about FC-EV driving range compared to conventional vehicles remain influential factors. Addressing these challenges requires comprehensive education, awareness campaigns, and infrastructure development to emphasize the environmental benefits, safety standards, and economic viability of FC-EVs, ultimately shaping a more favorable public perception.
4. Conclusions
Proton-exchange membrane fuel cell (PEMFC) and solid oxide fuel cell (SOFC) vehicles encounter significant challenges, impeding their widespread adoption. PEMFC vehicles confront issues primarily related to cost, durability, and infrastructure. The reliance on expensive platinum-based catalysts drives up manufacturing costs, while degradation over time due to factors like catalyst poisoning and membrane wear affects long-term viability. Additionally, the limited availability of hydrogen refueling stations poses a barrier to PEMFC vehicle proliferation. On the other hand, SOFCs do not require expensive platinum group catalysts; however, SOFC vehicles struggle with challenges concerning high operating temperatures, fuel flexibility, system complexity, and stack degradation. Operating at temperatures between 500 °C and 1000 °C, SOFCs face hurdles in thermal management and material compatibility. Ensuring efficient performance across various fuel types, simplifying system designs, and mitigating stack degradation are crucial for advancing SOFC technology. Collaborative efforts in research and development are vital to address these obstacles and accelerate the adoption of both PEMFC and SOFC vehicles as sustainable transportation solutions.
This paper presented a review of the currently utilized hydrogen fuel-cell technologies and models and emphasized the importance of addressing the critical factors that will facilitate the successful integration of FC-EVs into the contemporary transportation landscape. These factors include enhancing well-to-wheel efficiency, optimizing infrastructure implementation, streamlining cost considerations, and shaping positive public perception. FC-EVs promise a compelling avenue to achieving sustainable and environmentally conscious mobility, with the potential to revolutionize the navigation of our ever-evolving world. Nevertheless, achieving this vision requires paying meticulous attention to the intricacies of technology, infrastructure, economics, and public attitudes.
Enhancing well-to-wheel efficiency is integral to ensuring that the entire hydrogen production, transportation, and utilization process is optimized, and expanding the refueling network is also essential for convenient and widespread adoption. Simultaneously, reducing both production and operational costs is pivotal to enhancing the affordability and competitiveness of FC-EVs. Moreover, the cultivation of a positive public perception and heightened awareness is indispensable, as the ultimate success of FC-EVs largely hinges on societal acceptance. Together, these necessary changes not only represent technological advancements but also a broader societal shift toward sustainable transportation. The realization of these advancements, achieved through collaborative efforts among governments, industries, and research communities, will pave the way for a future where FC-EVs play a pivotal role in shaping a cleaner and more sustainable mobility paradigm.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Dresselhaus, M.S.; Thomas, I.L. Alternative energy technologies. Nature 2001, 414, 332–337. [Google Scholar] [CrossRef] [PubMed]
- Ite, A.E.; Ibok, U.J.; Ite, M.U.; Petters, S.W. Petroleum Exploration and Production: Past and Present Environmental Issues in the Nigeria’s Niger Delta. Am. J. Environ. Prot. 2013, 1, 78–90. [Google Scholar] [CrossRef]
- Progress Cleaning the Air and Improving People’s Health. United States Environmental Protectio Agency. Available online: https://www.epa.gov/clean-air-act-overview/progress-cleaning-air-and-improving-peoples-health (accessed on 14 April 2024).
- U.S. Public Views on Climate and Energy. Pew Research Center. Available online: https://www.pewresearch.org/science/2019/11/25/u-s-public-views-on-climate-and-energy/ (accessed on 14 April 2024).
- Erickson, L.E.; Jennings, M. Energy, Transportation, Air Quality, Climate Change, Health Nexus: Sustainable Energy is Good for Our Health. AIMS Public Health 2017, 4, 47–61. [Google Scholar] [CrossRef] [PubMed]
- Sun, C.; Negro, E.; Vezzù, K.; Pagot, G.; Cavinato, G.; Nale, A.; Bang, Y.H.; Noto, V.D. Hybrid inorganic-organic proton-conducting membranes based on SPEEK doped with WO3 nanoparticles for application in vanadium redox flow batteries. Electrochim. Acta 2019, 309, 311–325. [Google Scholar] [CrossRef]
- Brekken, T.K.A.; Yokochi, A.; Jouanne, A.V.; Yen, Z.Z.; Hapke, H.M.; Halamay, D.A. Optimal Energy Storage Sizing and Control for Wind Power Applications. IEEE Trans. Sustain. Energy 2011, 2, 69–77. [Google Scholar] [CrossRef]
- Collin, R.; Miao, Y.; Yokochi, A.; Enjeti, P.; Jouanne, A.V. Advanced Electric Vehicle Fast-Charging Technologies. Energies 2019, 12, 1839. [Google Scholar] [CrossRef]
- Adegbohun, F.; Jouanne, A.V.; Agamloh, E.; Yokochi, A. A Review of Bidirectional Charging Grid Support Applications and Battery Degradation Considerations. Energies 2024, 17, 1320. [Google Scholar] [CrossRef]
- Adegbohun, F.; Jouanne, A.V.; Agamloh, E.; Yokochi, A. Geographical Modeling of Charging Infrastructure Requirements for Heavy-Duty Electric Autonomous Truck Operations. Energies 2023, 16, 4161. [Google Scholar] [CrossRef]
- Moghbel, H.; Ganapavarapu, K.; Langari, R.; Ehsani, M. A Comparative Review of Fuel Cell Vehicles (FCVs) and Hybrid Electric Vehicles (HEVs) Part I: Performance and Parameter Characteristics, Emissions, Well-to-Wheels Efficiency and Fuel Economy, Alternative Fuels, Hybridization of FCV, and Batteries for HVs; SAE International: Warrendale, PA, USA, 2003; Volume 112, pp. 1860–1870. [Google Scholar]
- Andu, J.; Segura, F. Fuel cells: History and updating. A walk along two centuries. Renew. Sustain. Energy Rev. 2009, 13, 2309–2322. [Google Scholar]
- Fuel Cell in Transit Buses Summary. Department of Energy. Available online: https://www1.eere.energy.gov (accessed on 19 March 2024).
- Thompson, S.T.; Papageorgopoulos, D. Platinum group metal-free catalysts boost cost competitiveness of fuel cell vehicles. Nat. Catal. 2019, 2, 558–561. [Google Scholar] [CrossRef]
- Xu, H.; Han, Y.; Zhu, J.; Ni, M.; Yao, Z. Status and progress of metal-supported solid oxide fuel cell: Towards large-scale manufactory and practical applications. Energy Rev. 2024, 3, 100051. [Google Scholar] [CrossRef]
- Blennow, P.; Hjelm, J.; Klemensø, T.; Persson, Å.H.; Ramousse, S.; Mogensen, M. Planar Metal-Supported SOFC with Novel Cermet Anode. Fuel Cells 2011, 5, 661–668. [Google Scholar] [CrossRef]
- Li, M.; Bai, Y.; Zhang, C.; Song, Y.; Jiang, S.; Grouset, D.; Zhang, M. Review on the research of hydrogen storage system fast refueling in fuel cell vehicle. Int. J. Hydrog. Energy 2019, 44, 10677–10693. [Google Scholar] [CrossRef]
- Sankır, M.; Sankir, N. Hydrogen Electrical Vehicles; John Wiley & Sons: Hoboken, NJ, USA, 2023. [Google Scholar]
- Vargas, J.E.V.; Seabra, J.E.A. Fuel-cell technologies for private vehicles in Brazil: Environmental mirage or prospective romance? A comparative life cycle assessment of PEMFC and SOFC light-duty vehicles. Sci. Total Environ. 2021, 798, 149265. [Google Scholar] [CrossRef] [PubMed]
- Acres, G.; Frost, J.; Hards, G.; Potter, R.; Ralph, T.; Thompsett, D.; Burstein, G.; Hutchings, G. Electrocatalysts for fuel cells. Catal. Today 1997, 38, 393–400. [Google Scholar] [CrossRef]
- Warshay, M.; Prokopius, P.R. The Fuel Cell in Space: Yesterday, Today and Tomorrow. NASA Technical Memorandum 102366. In Proceedings of the Grove Anniversary (1839–1989) Fuel Cell Symposium, London, UK, 18–21 September 1989. [Google Scholar]
- Burke, K.A. Fuel Cells for Space Science Applications. In Proceedings of the First International Energy Conversion Engineering Conference, Portsmouth, VA, USA, 17–21 August 2003. [Google Scholar]
- Agmon, N. The Grotthuss mechanism. Chem. Phys. Lett. 1995, 244, 456–462. [Google Scholar] [CrossRef]
- Mekhilef, S.; Saidur, R.; Safari, A. Comparative study of different fuel cell technologies. Renew. Sustain. Energy Rev. 2012, 16, 981–989. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, K.S.; Mishler, J.; Cho, S.C.; Adroher, X.C. A review of polymer electrolyte membrane fuel cells: Technology, applications, and needs on fundamental research. Appl. Energy 2011, 88, 981–1007. [Google Scholar] [CrossRef]
- Jiao, K.; Li, X. Water transport in polymer electrolyte membrane fuel cells. Prog. Energy Combust. Sci. 2011, 37, 221–291. [Google Scholar] [CrossRef]
- Hydrogen Cars Now. Available online: https://www.hydrogencarsnow.com/index.php/gm-electrovan/ (accessed on 24 March 2024).
- Honda Motor Co. Available online: https://global.honda/en/newsroom/worldnews/2002/4020724.html (accessed on 24 March 2024).
- Toyota Motor Corporation. Available online: https://techinfo.toyota.com/techInfoPortal/staticcontent/en/techinfo/html/prelogin/docs/fchverg.pdf (accessed on 24 March 2024).
- Toyota Knoxville. Available online: https://www.toyotaknoxville.com/blogs/1021/toyota-debut-mirai-at-japan-auto-show/ (accessed on 24 March 2024).
- Toyota Europe. Available online: https://www.toyota-europe.com/news/2020/mirai-2020 (accessed on 24 March 2024).
- Nissan Motor Corporation. Available online: https://global.nissannews.com/en/releases/nissan-unveils-worlds-first-solid-oxide-fuel-cell-vehicle (accessed on 24 March 2024).
- Honda of Fort Worth. Available online: https://www.hondaoffortworth.com/blog/2016/may/20/2016-honda-clarity-exudes-engineering-excellence.htm (accessed on 24 March 2024).
- Hyundai Motor Company. Available online: https://www.hyundai.news/eu/models/electrified/nexo/press-kit/all-new-hyundai-nexo-design.html (accessed on 24 March 2024).
- Wee, J.-H. Which type of fuel cell is more competitive for portable application: Direct methanol fuel cells or direct borohydride fuel cells? J. Power Sources 2006, 161, 1–10. [Google Scholar] [CrossRef]
- Inoishi, A.; Sakai, T.; Ju, Y.W.; Ida, S.; Ishihara, T. Improved cycle stability of Fe-air solid state oxide rechargeable battery using LaGaO3-based oxide ion conductor. J. Power Sources 2014, 262, 310–315. [Google Scholar] [CrossRef]
- Aguadero, A.; Fawcett, L.; Taub, S.; Woolley, R.; Wu, K.-T.; Xu, N.; Kilner, J.A.; Skinner, S.J. Materials development for intermediate-temperature solid oxide. J. Mater. Sci. 2012, 47, 3925–3948. [Google Scholar] [CrossRef]
- Hossain, S.; Abdalla, A.M.; Jamain, S.N.B.; Zaini, J.H.; Azad, A.K. A review on proton conducting electrolytes for clean energy and intermediate temperature—Solid oxide fuel cells. Renew. Sustain. Energy Rev. 2017, 79, 750–764. [Google Scholar] [CrossRef]
- Zhu, W.; Deevi, S. Development of interconnect materials for solid oxide fuel cells. Mater. Sci. Eng. 2003, 348, 227–243. [Google Scholar] [CrossRef]
- Tronstad, T.; Åstrand, H.H.; Haugom, G.P.; Langfeldt, L. Study on the Use of Fuel Cells in Shipping; EMSA European Maritime Safety: Lisbon, Portugal, 2017.
- Solid Oxide Fuel Cell. Special Power Sources, 15 August 2023. Available online: https://spsources.com/a-comprehensive-comparison-of-planar-and-tubular-solid-oxide-fuel-cells/#:~:text=Lower%20Power%20Density%3A%20Tubular%20SOFCs,consuming%2C%20potentially%20increasing%20manufacturing%20costs (accessed on 20 April 2024).
- Husain, I. Electric and Hybrid Vehicles: Design Fundamentals; CRC Press: Boca Raton, FL, USA, 2010; pp. 159–169. [Google Scholar]
- Baker, R.; Zhang, J. Proton exchange membrane or polymer electrolyte membrane (pem) fuel cells. Electrochem. Encycl. 2011, 1, 11–22. [Google Scholar]
- Wang, P.; Shao, Q.; Huang, X. Updating Pt-Based Electrocatalysts for Practical Fuel Cells. Joule 2018, 2, 2511–2518. [Google Scholar] [CrossRef]
- PEM Fuel Cell History. Smithsonian Institution. Available online: https://americanhistory.si.edu/fuelcells/pem/pemmain.htm (accessed on 22 March 2024).
- Mauritz, K.A.; Moore, R.B. State of Understanding of Nafion. Chem. Rev. 2004, 104, 4535–4585. [Google Scholar] [CrossRef]
- Hou, Y.; Hao, D.; Shen, J.; Li, P.; Zhang, T.; Wang, H. Effect of strengthened road vibration on performance degradation of PEM fuel cell stack. Int. J. Hydrog. Energy 2016, 41, 5123–5134. [Google Scholar] [CrossRef]
- Kurtz, J.; Dinh, H.; Saur, G.; Ainscough, C. Fuel Cell Technology Status: Degradation; National Renewable Energy Laboratory: Washington, DC, USA, 2017.
- Chen, H.; Zhang, R.; Xia, Z.; Weng, Q.; Zhang, T.; Pei, P. Experimental investigation on PEM fuel cell flooding mitigation under heavy loading condition. Appl. Energy 2023, 349, 121632. [Google Scholar] [CrossRef]
- Yan, S.; Yang, M.; Sun, C.; Xu, S. Liquid Water Characteristics in the Compressed Gradient Porosity Gas Diffusion Layer of Proton Exchange Membrane Fuel Cells Using the Lattice Boltzmann Method. Energies 2023, 16, 6010. [Google Scholar] [CrossRef]
- Lewis, M.J. Synthesis and Evaluation of Electrolytically-Deposited Coatings to Protect Ferritic Stainless Steels. Master’s Thesis, Tennessee Technological University, Cookeville, TN, USA, 2011. [Google Scholar]
- Lewis, B.J. Synthesis and Evaluation of Manganese Cobalt Spinel Layers for Solid Oxide Fuel Cell Cathode-Interconnect Contact Application. Master’s Thesis, Tennessee Technological University, Cookeville, TN, USA, 2011. [Google Scholar]
- Singhal, S.C.; Kendall, K. High Temperature Solid Oxide Fuel Cells: Fundamentals, Design and Applications; Elsevier Science: Amsterdam, The Netherlands, 2003. [Google Scholar]
- Tonekabonimoghaddam, M.; Shamiri, A. Simulation and Sensitivity Analysis for Various Geometries and Optimization of Solid Oxide Fuel Cells: A Review. Eng 2021, 2, 386–415. [Google Scholar] [CrossRef]
- Jacobson, A.J. Materials for Solid Oxide Fuel Cells. Chem. Mater. 2010, 22, 660–674. [Google Scholar] [CrossRef]
- Bierschenk, D.M.; Pillai, M.R.; Lin, Y.; Barnett, S.A. Effect of Ethane and Propane in SimulatedNatural Gas on the Operation of Ni–YSZ Anode Supported SolidOxide Fuel Cells. Fuel Cells 2010, 6, 1129–1134. [Google Scholar] [CrossRef]
- Rasmussen, J.F.; Hagen, A. The effect of H2S on the performance of Ni–YSZ anodes in solid oxide fuel cells. J. Power Sources 2009, 191, 534–541. [Google Scholar] [CrossRef]
- Liu, J.; Barnett, S.A. Operation of anode-supported solid oxide fuel cells on methane and natural gas. Solid State Ion. 2003, 158, 11–16. [Google Scholar] [CrossRef]
- Kuhn, J.N.; Lakshminarayanan, N.; Ozkan, U.S. Effect of hydrogen sulfide on the catalytic activity of Ni-YSZ cermets. J. Mol. Catal. 2008, 282, 9–21. [Google Scholar] [CrossRef]
- Bi, Z.H.; Zhu, J.H. A Cu–CeO2-LDC Composite Anode for LSGM Electrolyte-Supported Solid Oxide Fuel Cells. Electrochem. Solid-State Lett. 2009, 12, B107–B111. [Google Scholar] [CrossRef]
- Fergus, J.W. Oxide anode materials for solid oxide fuel cells. Solid State Ion. 2006, 177, 1529–1541. [Google Scholar] [CrossRef]
- Tietz, F.; Mai, A.; Stöver, D. From powder properties to fuel cell performance—A holistic approach for SOFC cathode development. Solid State Ion. 2008, 179, 1509–1515. [Google Scholar] [CrossRef]
- Nielsen, J.; Jacobsen, T. SOFC cathode/YSZ—Non-stationary TPB effects. Solid State Ion. 2008, 179, 1314–1319. [Google Scholar] [CrossRef]
- Sun, C.; Hui, R.; Roller, J. Cathode materials for solid oxide fuel cells: A review. J. Solid State Electrochem. 2009, 14, 1125–1144. [Google Scholar] [CrossRef]
- Bell, R.J.; Millar, G.J.; Drennan, J. Influence of synthesis route on the catalytic properties of La Sr MnO. Solid State Ion. 2000, 131, 211–220. [Google Scholar] [CrossRef]
- Jiang, S.P. Development of lanthanum strontium manganite perovskite cathode materials of solid oxide fuel cells: A review. J. Mater. Sci. 2008, 43, 6799–6833. [Google Scholar] [CrossRef]
- Backhaus-Ricoult, M.; Adib, K.; St, T.; Luerssen, B.; Gregoratti, L.; Barinov, A. In-situ study of operating SOFC LSM/YSZ cathodes under polarization by photoelectron microscopy. Solid State Ion. 2008, 179, 891–895. [Google Scholar] [CrossRef]
- Wang, S.; Yoon, J.; Kim, G.; Huang, D.; Wang, H.; Jacobson, A.J. Electrochemical Properties of Nanocrystalline La0.5Sr0.5CoO3-x Thin Films. Chem. Mater 2010, 22, 776–782. [Google Scholar] [CrossRef]
- Uhlenbruck, S.; Tietz, F. High-temperature thermal expansion and conductivity of cobaltites: Potentials for adaptation of the thermal expansion to the demands for solid oxide fuel cells. Mater. Sci. Eng. 2004, B107, 277–282. [Google Scholar] [CrossRef]
- Yang, S.; He, T.; He, Q. Sm0.5Sr0.5CoO3 cathode material from glycine-nitrate process: Formation, characterization, and application in LaGaO3-based solid oxide fuel cells. J. Alloys Compd. 2008, 450, 400–404. [Google Scholar] [CrossRef]
- Yang, Y.L.; Chen, C.L.; Chen, S.Y.; Chu, C.W.; Jacobson, A.J. Impedance Studies of Oxygen Exchange on Dense Thin Film Electrodes of LSCO. J. Electrochem. Soc. 2000, 147, 4001–4007. [Google Scholar] [CrossRef]
- Rembelski, D.; Viricelle, J.-P.; Rieu, M.; Combemale, L. Characterization and Comparison of Different Cathode Materials for SC-SOFC: LSM, BSCF, SSC, and LSCF. Fuel Cells 2012, 12, 256–264. [Google Scholar] [CrossRef]
- Minh, N.Q. Solid oxide fuel cell technology—Features and applications. Solid State Ion. 2004, 174, 271–277. [Google Scholar] [CrossRef]
- Han, M.; Tang, X.; Yin, H.; Peng, S. Fabrication, Microstructure and Properties of a YSZ electrolyte for SOFCs. J. Power Sources 2007, 165, 757–763. [Google Scholar] [CrossRef]
- Wu, J.; Liu, X. Recent Development of SOFC Metallic Interconnect. J. Mater. Sci. Technol. 2010, 26, 293–305. [Google Scholar] [CrossRef]
- Matsuda, M.; Hosomi, T.; Murata, K.; Fukui, T.; Miyake, M. Fabrication of bilayered YSZ/SDC electrolyte film by electrophoretic deposition for reduced-temperature operating anode-supported SOFC. J. Power Sources 2007, 165, 102–107. [Google Scholar] [CrossRef]
- Huijsmans, J.; Berkel, F.V.; Christie, G. Intermediate temperature SOFC—A promise for the 21st century. J. Power Sources 1998, 71, 107–110. [Google Scholar] [CrossRef]
- Geng, S.; Zhu, J.; Lu, Z. Evaluation of several alloys for solid oxide fuel cell interconnect application. Scr. Mater. 2006, 55, 239–242. [Google Scholar] [CrossRef]
- Quadakkers, W.; Piron-Abellan, J.; Shemet, V.; Singheiser, L. Metallic interconnectors for solid oxide fuel cells—A review. Mater. High Temp. 2003, 20, 115–127. [Google Scholar]
- Seo, H.G.; Staerz, A.; Dimitrakopoulos, G.; Kim, D.; Yildiz, B.; Tuller, H.L. Degradation and recovery of solid oxide fuel cell performance by control of cathode surface acidity: Case study—Impact of Cr followed by Ca infiltration. J. Power Sources 2023, 558, 232589. [Google Scholar] [CrossRef]
- Matsuzaki, Y.; Yasuda, I. Electrochemical properties of a SOFC cathode in contact with a chromium-containing alloy separator. Solid State Ion. 2000, 132, 271–278. [Google Scholar] [CrossRef]
- Yokokawa, H.; Horita, T.; Sakai, N.; Yamaji, K.; Brito, M.; Xiong, Y.-P.; Kishimoto, H. Thermodynamic considerations on Cr poisoning in SOFC cathodes. Solid State Ion. 2006, 177, 3193–3198. [Google Scholar] [CrossRef]
- Liu, W.; Sun, X.; Stephens, E.; Khaleel, M. Life prediction of coated and uncoated metallic interconnect for solid oxide fuel cell applications. J. Power Sources 2009, 189, 1044–1050. [Google Scholar] [CrossRef]
- Simner, S.P.; Anderson, M.D.; Xia, G.-G.; Yang, Z.; Pederson, L.R.; Stevenson, J.W. SOFC Performance with Fe-Cr-Mn Alloy Interconnect. J. Electrochem. Soc. 2005, 152, A740–A745. [Google Scholar] [CrossRef]
- Huang, K.; Hou, P.Y.; Goodenough, J.B. Characterization of iron-based alloy interconnects for reduced temperature solid oxide fuel cells. Solid State Ion. 2000, 129, 237–250. [Google Scholar] [CrossRef]
- Stanislowski, M.; Froitzheim, J.; Niewolak, L.; Quadakkers, W.; Hilpert, K.; Markus, T.; Singheiser, L. Reduction of chromium vaporization from SOFC interconnectors by highly effective coatings. J. Power Sources 2007, 164, 578–589. [Google Scholar] [CrossRef]
- Ebrahimifar, H.; Zandrahimi, M. Mn coating on AISI 430 ferritic stainless steel by pack cementation method for SOFC interconnect applications. Solid State Ion. 2011, 183, 71–79. [Google Scholar] [CrossRef]
- Deng, X.; Wei, P.; Bateni, M.R.; Petric, A. Cobalt plating of high temperature stainless steel interconnects. J. Power Sources 2006, 160, 1225–1229. [Google Scholar] [CrossRef]
- Alman, D.; Johnson, C.; Collins, W.; Jablonski, P. The effect of cerium surface treated ferritic stainless steel current collectors on the performance of solid oxide fuel cells (SOFC). J. Power Sources 2007, 168, 351–355. [Google Scholar] [CrossRef]
- Yang, Z.; Xia, G.; Simner, S.P.; Stevenson, J.W. Thermal Growth and Performance of Manganese Cobaltite Spinel Protection Layers on Ferritic Stainless Steel SOFC Interconnects. J. Electrochem. Soc. 2005, 152, A1896–A1901. [Google Scholar] [CrossRef]
- Yang, Z.; Xia, G.-G.; Li, X.-H.; Stevenson, J.W. (Mn,Co)3O4 spinel coatings on ferritic stainless steels for SOFC interconnect applications. Int. J. Hydrog. Energy 2007, 32, 3648–3654. [Google Scholar] [CrossRef]
- Long, Z.; Axsen, J.; Miller, I.; Kormos, C. What does Tesla mean to car buyers? Exploring the role of automotive brand in perceptions of battery electric vehicles. Transp. Res. Part A 2019, 129, 185–204. [Google Scholar] [CrossRef]
- Wassiliadis, N.; Kriegler, J.; Gamra, K.A.; Lienkamp, M. Model-based health-awarefast charging to mitigate the risk of lithium plating and prolong the cycle life of lithium-ion batteries in electric vehicles. J. Power Sources 2023, 561, 232586. [Google Scholar] [CrossRef]
- Sangeetha, E.; Ramachandran, V. Different Topologies of Electrical Machines, Storage Systems, and Power Electronic Converters and Their Control for Battery Electric Vehicles—A Technical Review. Energies 2022, 15, 8959. [Google Scholar] [CrossRef]
- Toyota. Available online: https://www.toyotaofmelbourne.com/toyota-prius-model-timeline.html (accessed on 23 March 2024).
- Toyota Motor Sales, U.S.A., Inc. Available online: https://www.toyota.com/corolla/features/mpg_other_price/1852/1868/1866 (accessed on 24 March 2024).
- Auto Nation Toyota Corpus Christi. Available online: https://www.autonationtoyotacorpuschristi.com/showroom/2024/Toyota/bZ4X/SUV.htm (accessed on 24 March 2024).
- Toyota Motor Sales, U.S.A., Inc. Available online: https://www.toyota.com/prius/features/mpg_other_price/1223/1225/1227 (accessed on 24 March 2024).
- Toyota Motor Sales, U.S.A., Inc. Available online: https://www.toyota.com/mirai/2024/features/mpg_other_price/3002/3003 (accessed on 24 March 2024).
- Ehsani, M.; Singh, K.V.; Bansal, H.O.; Mehrjardi, R.T. State of the Art and Trends in Electric and Hybrid Electric Vehicles. Proc. IEEE 2021, 109, 967–984. [Google Scholar] [CrossRef]
- U.S. Department of Energy. Available online: https://afdc.energy.gov/vehicles/how-do-fuel-cell-electric-cars-work (accessed on 12 March 2024).
- Lu, Q.; Zhang, B.; Yang, S.; Peng, Z. Life cycle assessment on energy efficiency of hydrogen fuel cell vehicle in China. Energy 2022, 257, 124731. [Google Scholar] [CrossRef]
- Liu, X.; Reddi, K.; Elgowainy, A.; Lohse-Busch, H.; Wang, M.; Rustagi, N. Comparison of well-to-wheels energy use and emissions of a hydrogen fuel cell electric vehicle relative to a conventional gasoline-powered internal combustion engine vehicle. Int. J. Hydrogen Energy 2019, 45, 972–983. [Google Scholar] [CrossRef]
- Ajanovic, A.; Haas, R. Prospects and impediments for hydrogen and fuel cell vehicles in the transport sector. Int. J. Hydrog. Energy 2021, 46, 10049–10058. [Google Scholar] [CrossRef]
- LBST. 16th Annual Assessment of H2stations.org by LBST. LBST, 2024. Available online: https://www.h2stations.org/wp-content/uploads/2024-01-24-LBST-HRS-2023-en.pdf (accessed on 20 March 2024).
- US Department of Energy. Available online: https://afdc.energy.gov/fuels/hydrogen_stations.html (accessed on 19 March 2024).
- Abdalla, A.M.; Hossain, S.; Nisfindy, O.B.; Azad, A.T.; Dawood, M.; Azad, A.K. Hydrogen production, storage, transportation and key challenges with applications: A review. Energy Convers. Manag. 2018, 165, 602–627. [Google Scholar] [CrossRef]
- Rusman, N.; Dahari, M. A review on the current progress of metal hydrides material for solid-state hydrogen storage applications. Int. J. Hydrogen Energy 2016, 41, 12108–12126. [Google Scholar] [CrossRef]
- Chu, C.; Wu, K.; Luo, B.; Cao, Q.; Zhang, H. Hydrogen storage by liquid organic hydrogen carriers: Catalyst, renewable carrier, and technology—A review. Carbon Resour. Convers. 2023, 6, 334–351. [Google Scholar] [CrossRef]
- Li, H.; Chaoui, H.; Gualous, H. Cost Minimization Strategy for Fuel Cell Hybrid Electric Vehicles Considering Power Sources Degradation. IEEE Trans. Veh. Technol. 2020, 69, 12832–12842. [Google Scholar] [CrossRef]
- Li, Y.; Taghizadeh-Hesary, F. The economic feasibility of green hydrogen and fuel cell electric vehicles for road transport in China. Energy Policy 2022, 160, 112703. [Google Scholar] [CrossRef]
- Ringland, J.T. Safety Issues for Hydrogen-Powered Vehicles; Sandia Report; USDOE: Washington, DC, USA, 1994.
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).