Performance Characteristics and Optimization of a Single-Stage Direct Air Capture Membrane System in Terms of Process Energy Intensity
Abstract
1. Introduction
2. Materials and Methods
- Z—the share of the gaseous component in the feed;
- Y—the share of the gaseous component in the permeate;
- i—gaseous component, e.g., CO2.
- -
- Increasing the feed pressure;
- -
- Lowering the permeate pressure;
- -
- Increasing the concentration of the permeate component at the membrane inlet;
- -
- Reducing the permeate concentration on the permeate side by using additional sweep gas.
- —the power requirement of the vacuum pump, kW;
- —the power requirement of the pre-fan, kW;
- —CO2 flow rate in the permeate, .
- permeate molar flux, kmol/h;
- permeate molar flux, kmol/h.
3. Results and Discussion
3.1. Effect of Changing Permeate Pressure
3.2. Effect of Changing the Membrane Surface
3.3. Effect of Changing the Permeability of Carbon Dioxide
3.4. Effect of Changing Membrane Selectivity
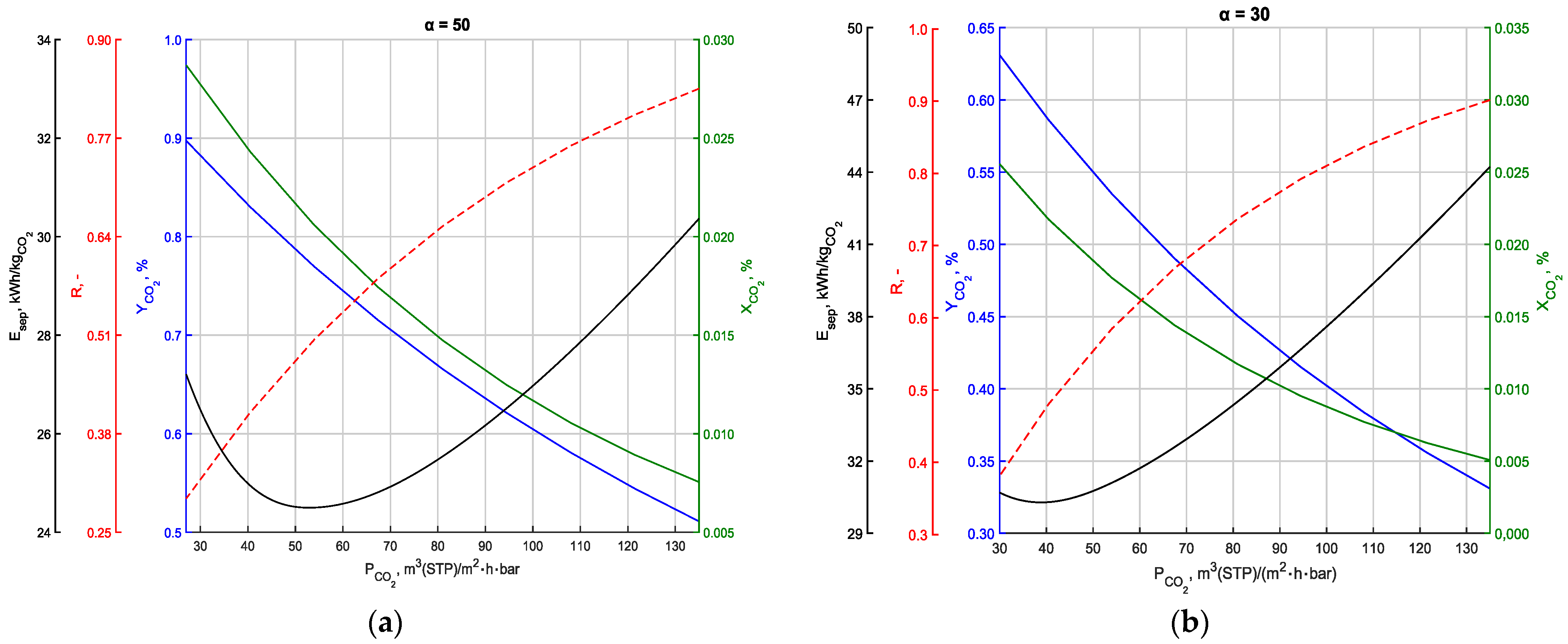
3.5. Energy Intensity of the Process
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- The Atmosphere: Getting a Handle on Carbon Dioxide. Available online: https://science.nasa.gov/earth/climate-change/greenhouse-gases/the-atmosphere-getting-a-handle-on-carbon-dioxide/ (accessed on 26 March 2024).
- Ritchie, H.; Rosado, P.; Roser, M. Emissions by Sector: Where Do Greenhouse Gases Come From? Available online: https://ourworldindata.org/emissions-by-sector (accessed on 21 March 2024).
- Warmuzinski, K. Harnessing Methane Emissions from Coal Mining. Process Saf. Environ. Prot. 2008, 86, 315–320. [Google Scholar] [CrossRef]
- McKinsey. Climate Risk and Decarbonization: What Every Mining CEO Needs to Know. Available online: https://www.mckinsey.com/capabilities/sustainability/our-insights/climate-risk-and-decarbonization-what-every-mining-ceo-needs-to-know (accessed on 23 April 2024).
- Azadi, M.; Northey, S.A.; Ali, S.H.; Edraki, M. Transparency on Greenhouse Gas Emissions from Mining to Enable Climate Change Mitigation. Nat. Geosci. 2020, 13, 100–104. [Google Scholar] [CrossRef]
- Sanz-Pérez, E.S.; Murdock, C.R.; Didas, S.A.; Jones, C.W. Direct Capture of CO2 from Ambient Air. Chem. Rev. 2016, 116, 11840–11876. [Google Scholar] [CrossRef]
- IEA. CO2 Emissions in 2023. 2024. Available online: https://www.iea.org/reports/co2-emissions-in-2023 (accessed on 21 March 2024).
- National Oceanic and Atmospheric Administration. Carbon Dioxide Now More than 50% Higher than Pre-Industrial Levels. Available online: https://www.noaa.gov/news-release/carbon-dioxide-now-more-than-50-higher-than-pre-industrial-levels (accessed on 24 March 2024).
- Enerdata. CO2 Emissions 2050 Forecast: World Trends from Fuel Combustion. Available online: https://eneroutlook.enerdata.net/forecast-co2-emissions-data-from-fuel-combustion.html (accessed on 23 April 2024).
- Duffour, G. Bchin Q Enerdata—Global Energy Trends—2023 Edition. A Troubled, Yet Promising Year for Energy Transition? 2022. Available online: https://www.enerdata.net/publications/reports-presentations/world-energy-trends.html (accessed on 1 April 2024).
- Greig, C.; Uden, S. The Value of CCUS in Transitions to Net-Zero Emissions. Electr. J. 2021, 34, 107004. [Google Scholar] [CrossRef]
- Gasser, T.; Guivarch, C.; Tachiiri, K.; Jones, C.D.; Ciais, P. Negative Emissions Physically Needed to Keep Global Warming below 2 °C. Nat. Commun. 2015, 6, 7958. [Google Scholar] [CrossRef]
- Fuss, S.; Canadell, J.G.; Peters, G.P.; Tavoni, M.; Andrew, R.M.; Ciais, P.; Jackson, R.B.; Jones, C.D.; Kraxner, F.; Nakicenovic, N.; et al. Betting on Negative Emissions. Nat. Clim. Chang. 2014, 4, 850–853. [Google Scholar] [CrossRef]
- Desport, L.; Selosse, S. Perspectives of CO2 Utilization as a Negative Emission Technology. Sustain. Energy Technol. Assess. 2022, 53, 102623. [Google Scholar] [CrossRef]
- IEA. Direct Air Capture—Energy System. Available online: https://www.iea.org/energy-system/carbon-capture-utilisation-and-storage/direct-air-capture (accessed on 25 March 2024).
- Okonkwo, E.C.; AlNouss, A.; Shahbaz, M.; Al-Ansari, T. Developing Integrated Direct Air Capture and Bioenergy with Carbon Capture and Storage Systems: Progress towards 2 °C and 1.5 °C Climate Goals. Energy Convers. Manag. 2023, 296, 117687. [Google Scholar] [CrossRef]
- IEA. Direct Air Capture. 2022. Available online: https://www.iea.org/reports/direct-air-capture-2022 (accessed on 25 March 2024).
- Bouaboula, H.; Chaouki, J.; Belmabkhout, Y.; Zaabout, A. Comparative Review of Direct Air Capture Technologies: From Technical, Commercial, Economic, and Environmental Aspects. Chem. Eng. J. 2024, 484, 149411. [Google Scholar] [CrossRef]
- Quang, D.V.; Milani, D.; Abu Zahra, M. A Review of Potential Routes to Zero and Negative Emission Technologies via the Integration of Renewable Energies with CO2 Capture Processes. Int. J. Greenh. Gas Control 2023, 124, 103862. [Google Scholar] [CrossRef]
- Mostafa, M.; Antonicelli, C.; Varela, C.; Barletta, D.; Zondervan, E. Capturing CO2 from the Atmosphere: Design and Analysis of a Large-Scale DAC Facility. Carbon Capture Sci. Technol. 2022, 4, 100060. [Google Scholar] [CrossRef]
- Drechsler, C.; Agar, D.W. Characteristics of DAC Operation within Integrated PtG Concepts. Int. J. Greenh. Gas Control 2021, 105, 103230. [Google Scholar] [CrossRef]
- Daggash, H.A.; Patzschke, C.F.; Heuberger, C.F.; Zhu, L.; Hellgardt, K.; Fennell, P.S.; Bhave, A.N.; Bardow, A.; MacDowell, N. Closing the Carbon Cycle to Maximise Climate Change Mitigation: Power-to-Methanol vs. Power-to-Direct Air Capture. Sustain. Energy Fuels 2018, 2, 1153–1169. [Google Scholar] [CrossRef]
- Motlaghzadeh, K.; Schweizer, V.; Craik, N.; Moreno-Cruz, J. Key Uncertainties behind Global Projections of Direct Air Capture Deployment. Appl. Energy 2023, 348, 121485. [Google Scholar] [CrossRef]
- Joppa, L.; Luers, A.; Willmott, E.; Friedmann, S.J.; Hamburg, S.P.; Broze, R. Microsoft’s Million-Tonne CO2-Removal Purchase—Lessons for Net Zero. Nature 2021, 597, 629–632. [Google Scholar] [CrossRef]
- The Verge. Microsoft Inks Another Deal to Capture and Store Its Carbon Emissions Underground. Available online: https://www.theverge.com/2023/3/22/23651587/microsoft-climate-tech-startup-carboncapture-wyoming (accessed on 25 March 2024).
- The New York Times. Heirloom Opens First U.S. Direct Air Capture Plant. Available online: https://www.nytimes.com/2023/11/09/climate/direct-air-capture-carbon.html (accessed on 25 March 2024).
- Kiani, A.; Jiang, K.; Feron, P. Techno-Economic Assessment for CO2 Capture from Air Using a Conventional Liquid-Based Absorption Process. Front. Energy Res. 2020, 8, 517915. [Google Scholar] [CrossRef]
- Fasihi, M.; Efimova, O.; Breyer, C. Techno-Economic Assessment of CO2 Direct Air Capture Plants. J. Clean. Prod. 2019, 224, 957–980. [Google Scholar] [CrossRef]
- Young, J.; McQueen, N.; Charalambous, C.; Foteinis, S.; Hawrot, O.; Ojeda, M.; Pilorgé, H.; Andresen, J.; Psarras, P.; Renforth, P.; et al. The Cost of Direct Air Capture and Storage Can Be Reduced via Strategic Deployment but Is Unlikely to Fall below Stated Cost Targets. One Earth 2023, 6, 899–917. [Google Scholar] [CrossRef]
- Kulkarni, A.R.; Sholl, D.S. Analysis of Equilibrium-Based TSA Processes for Direct Capture of CO2 from Air. Ind. Eng. Chem. Res. 2012, 51, 8631–8645. [Google Scholar] [CrossRef]
- Broehm, M.; Strefler, J.; Bauer, N. Techno-Economic Review of Direct Air Capture Systems for Large Scale Mitigation of Atmospheric CO2. SSRN Electron. J. 2015. [Google Scholar] [CrossRef]
- Realmonte, G.; Drouet, L.; Gambhir, A.; Glynn, J.; Hawkes, A.; Köberle, A.C.; Tavoni, M. An Inter-Model Assessment of the Role of Direct Air Capture in Deep Mitigation Pathways. Nat. Commun. 2019, 10, 3277. [Google Scholar] [CrossRef]
- Kárászová, M.; Zach, B.; Petrusová, Z.; Červenka, V.; Bobák, M.; Šyc, M.; Izák, P. Post-Combustion Carbon Capture by Membrane Separation, Review. Sep. Purif. Technol. 2020, 238, 116448. [Google Scholar] [CrossRef]
- Kotowicz, J.; Janusz-Szymańska, K.; Wiciak, G. Technologie Membranowe Wychwytu Dwutlenku Węgla Ze Spalin Dla Nadkrytycznego Bloku Węglowego; Wydawnictwo Politechniki Śląskiej: Gliwice, Poland, 2015. [Google Scholar]
- Kotowicz, J.; Chmielniak, T.; Janusz-Szymańska, K. The Influence of Membrane CO2 Separation on the Efficiency of a Coal-Fired Power Plant. Energy 2010, 35, 841–850. [Google Scholar] [CrossRef]
- Wu, H.; Li, Q.; Guo, B.; Sheng, M.; Wang, D.; Mao, S.; Ye, N.; Qiao, Z.; Kang, G.; Cao, Y.; et al. Industrial-Scale Spiral-Wound Facilitated Transport Membrane Modules for Post-Combustion CO2 Capture: Development, Investigation and Optimization. J. Membr. Sci. 2023, 670, 121368. [Google Scholar] [CrossRef]
- Gkotsis, P.; Peleka, E.; Zouboulis, A. Membrane-Based Technologies for Post-Combustion CO2 Capture from Flue Gases: Recent Progress in Commonly Employed Membrane Materials. Membranes 2023, 13, 898. [Google Scholar] [CrossRef]
- Carapellucci, R.; Giordano, L.; Vaccarelli, M. Study of a Natural Gas Combined Cycle with Multi-Stage Membrane Systems for CO2 Post-Combustion Capture. Energy Procedia 2015, 81, 412–421. [Google Scholar] [CrossRef]
- Merkel, T.C.; Lin, H.; Wei, X.; Baker, R. Power Plant Post-Combustion Carbon Dioxide Capture: An Opportunity for Membranes. J. Membr. Sci. 2010, 359, 126–139. [Google Scholar] [CrossRef]
- Castro-Muñoz, R.; Zamidi Ahmad, M.; Malankowska, M.; Coronas, J. A New Relevant Membrane Application: CO2 Direct Air Capture (DAC). Chem. Eng. J. 2022, 446, 137047. [Google Scholar] [CrossRef]
- Fujikawa, S.; Selyanchyn, R. Direct Air Capture by Membranes. MRS Bull. 2022, 47, 416–423. [Google Scholar] [CrossRef]
- Fujikawa, S.; Selyanchyn, R.; Kunitake, T. A New Strategy for Membrane-Based Direct Air Capture. Polym. J. 2020, 53, 111–119. [Google Scholar] [CrossRef]
- Fujikawa, S.; Ariyoshi, M.; Selyanchyn, R.; Kunitake, T. Ultra-Fast, Selective CO2 Permeation by Free-Standing Siloxane Nanomembranes. Chem. Lett. 2019, 48, 1351–1354. [Google Scholar] [CrossRef]
- Yoo, M.J.; Kim, K.H.; Lee, J.H.; Kim, T.W.; Chung, C.W.; Cho, Y.H.; Park, H.B. Ultrathin Gutter Layer for High-Performance Thin-Film Composite Membranes for CO2 Separation. J. Membr. Sci. 2018, 566, 336–345. [Google Scholar] [CrossRef]
- Bandehali, S.; Ebadi Amooghin, A.; Sanaeepur, H.; Ahmadi, R.; Fuoco, A.; Jansen, J.C.; Shirazian, S. Polymers of Intrinsic Microporosity and Thermally Rearranged Polymer Membranes for Highly Efficient Gas Separation. Sep. Purif. Technol. 2021, 278, 119513. [Google Scholar] [CrossRef]
- Castel, C.; Bounaceur, R.; Favre, E. Membrane Processes for Direct Carbon Dioxide Capture from Air: Possibilities and Limitations. Front. Chem. Eng. 2021, 3, 668867. [Google Scholar] [CrossRef]
- Ignatusha, P.; Lin, H.; Kapuscinsky, N.; Scoles, L.; Ma, W.; Patarachao, B.; Du, N. Membrane Separation Technology in Direct Air Capture. Membranes 2024, 14, 30. [Google Scholar] [CrossRef]
- Selyanchyn, O.; Selyanchyn, R.; Fujikawa, S. Critical Role of the Molecular Interface in Double-Layered Pebax-1657/PDMS Nanomembranes for Highly Efficient CO2/N2 Gas Separation. ACS Appl. Mater. Interfaces 2020, 12, 33196–33209. [Google Scholar] [CrossRef]

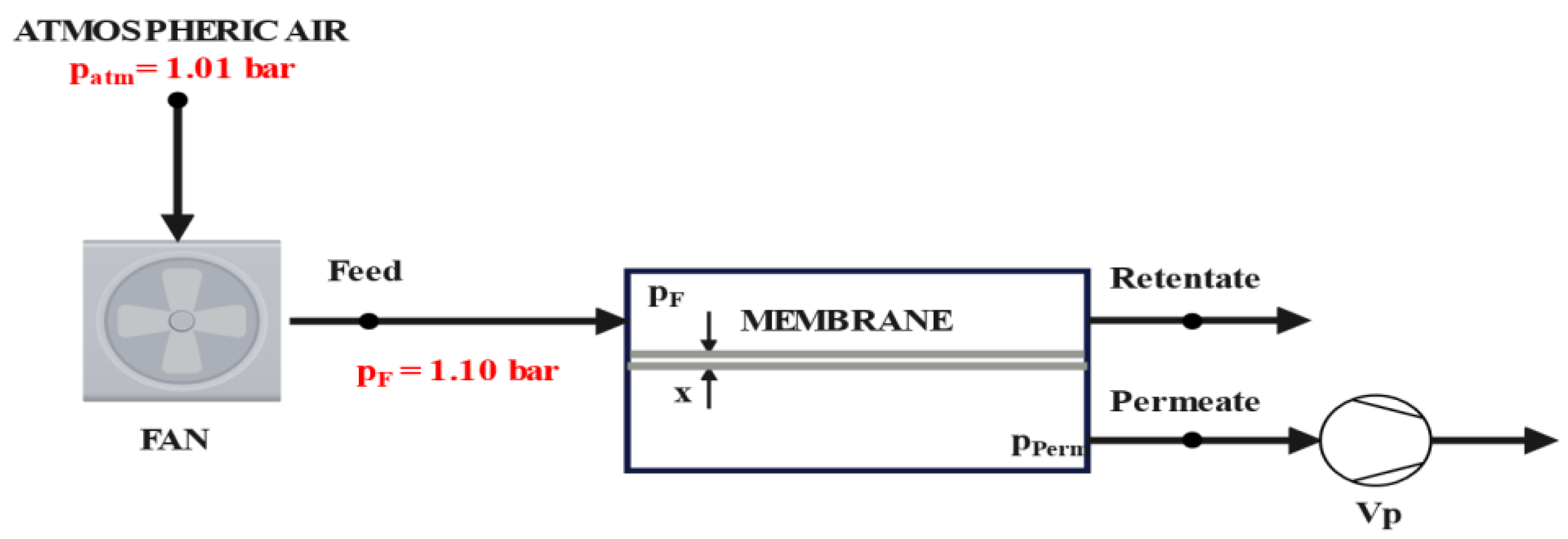
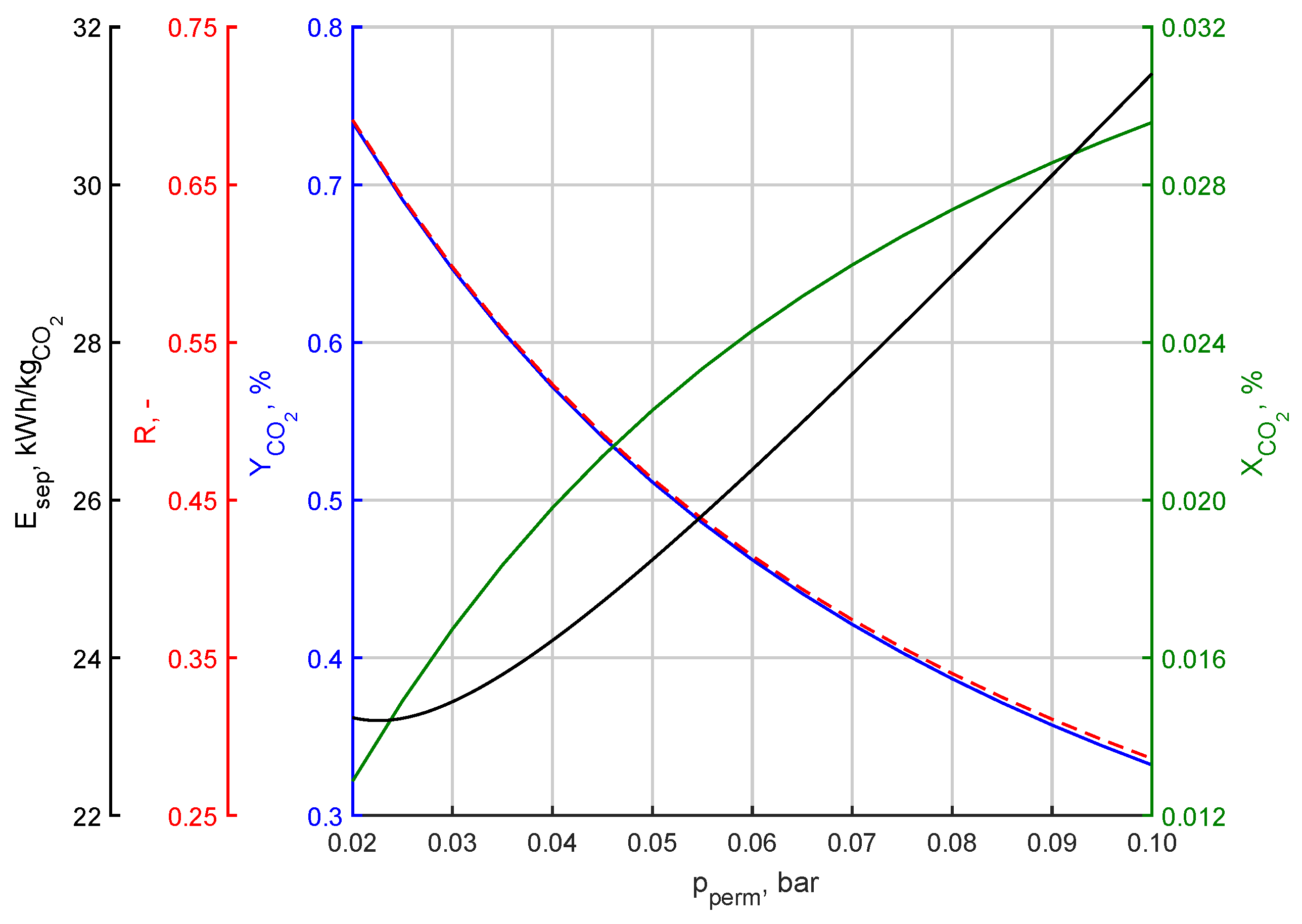
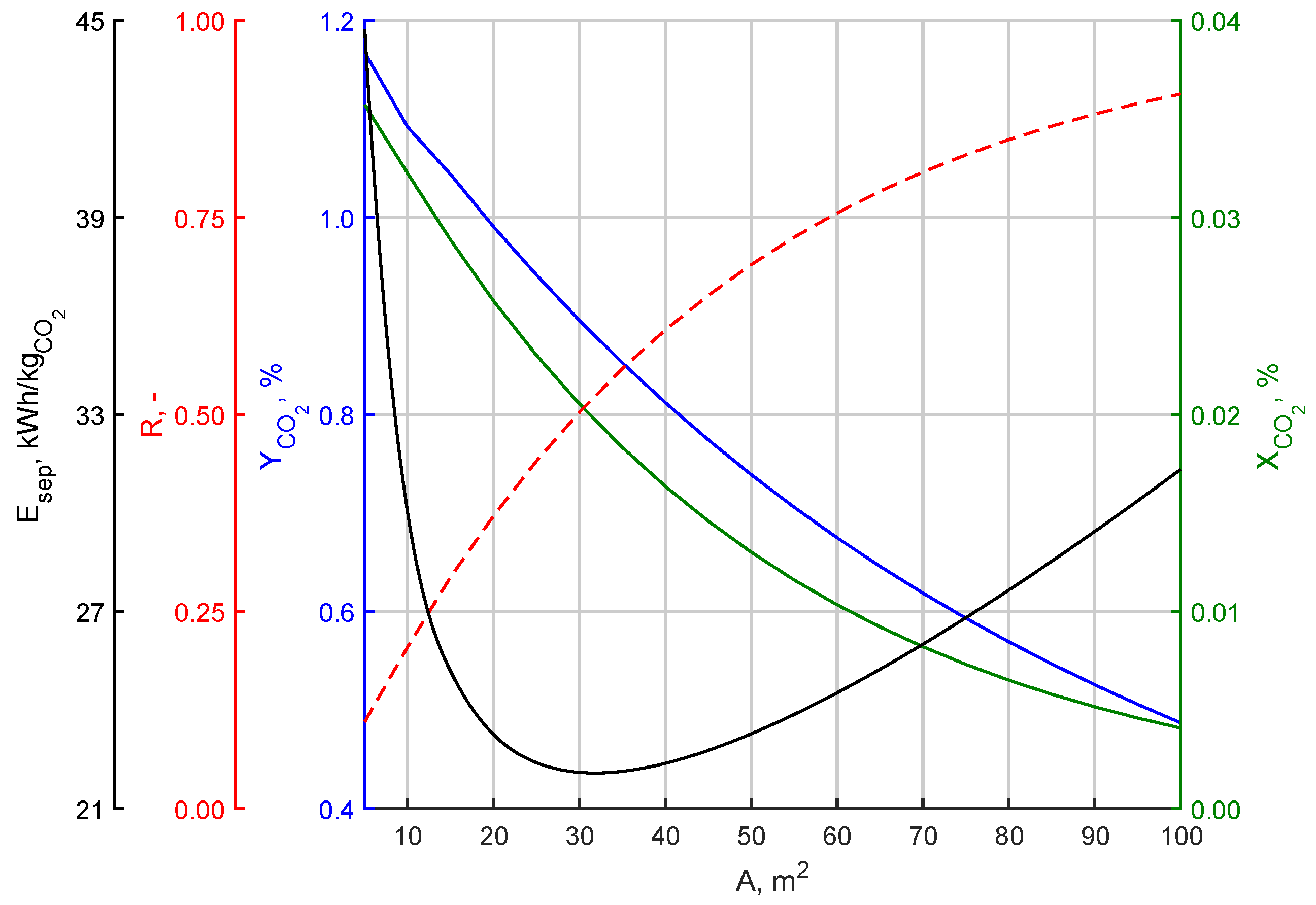
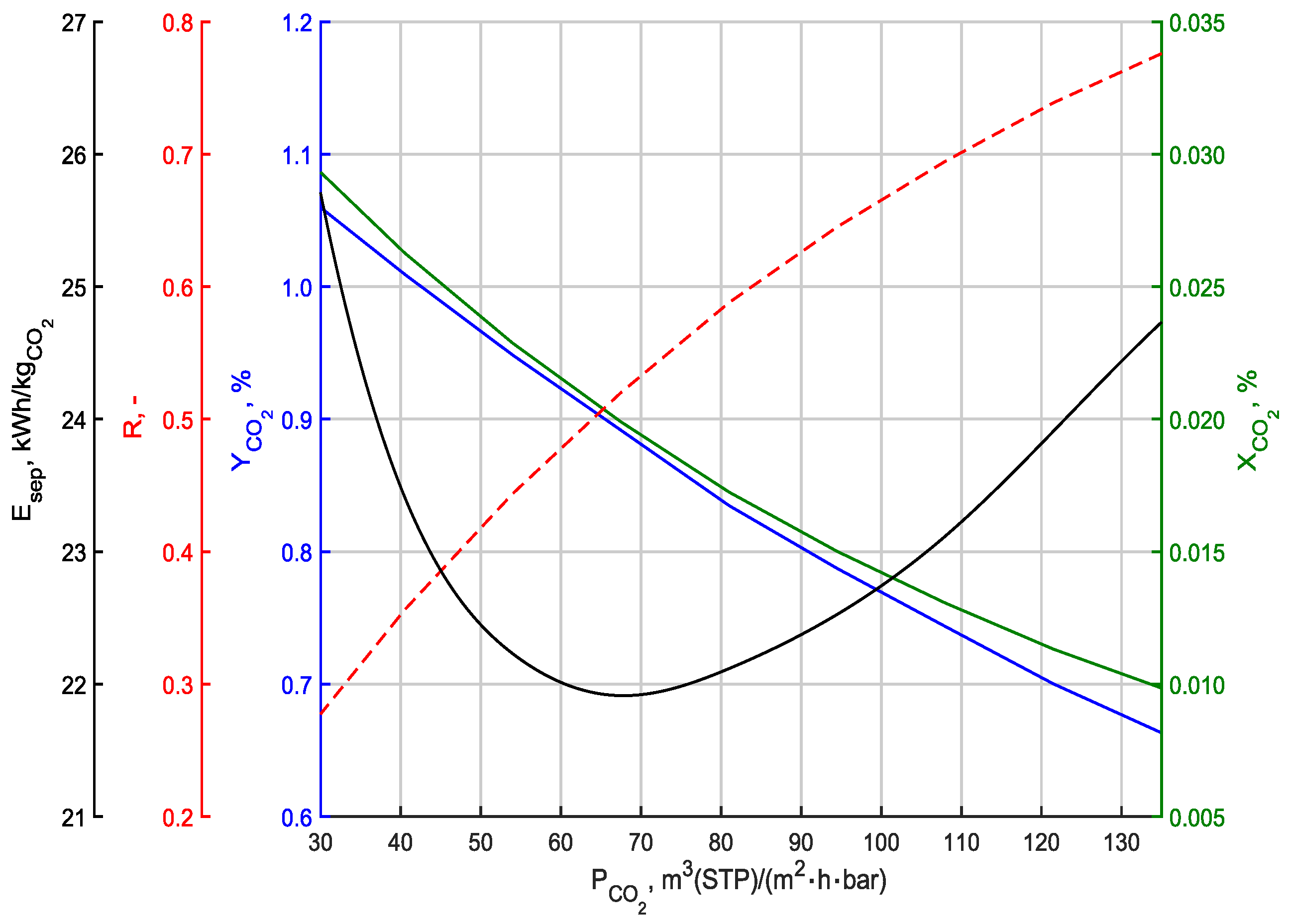


| Parameter | Symbol | Value | Unit |
|---|---|---|---|
| Composition of ambient air: | - | % | |
| -N2 | 78.08 | ||
| -O2 | 20.95 | ||
| -Ar | 0.93 | ||
| -CO2 | 0.04 | ||
| Molar flux of the feed | 100 | kmol/h | |
| Atmospheric pressure | 1.01 | bar | |
| Feed pressure | 1.10 | bar | |
| Air temperature | 298.15 | K | |
| Isentropic efficiency of pump and fan | 90 | % | |
| Mechanical efficiency of the pump and fan | 99 | % | |
| Membrane selectivity | 70 | - | |
| CO2 permeability | 108 | ||
| Membrane surface | 50 | m2 |
| L(x) | ||||
|---|---|---|---|---|
| 70 | 50 | 30 | ||
| GPU | ||||
| 50,000 | 135.0 | 1.93 | 2.70 | 4.50 |
| 45,000 | 121.5 | 1.74 | 2.43 | 4.05 |
| 40,000 | 108.0 | 1.54 | 2.16 | 3.60 |
| 35,000 | 94.5 | 1.35 | 1.89 | 3.15 |
| 30,000 | 81.0 | 1.16 | 1.62 | 2.70 |
| 25,000 | 67.5 | 0.96 | 1.35 | 2.25 |
| 20,000 | 54.0 | 0.77 | 1.08 | 1.80 |
| 15,000 | 40.5 | 0.58 | 0.81 | 1.35 |
| 10,000 | 27.0 | 0.39 | 0.54 | 0.90 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Niesporek, K.; Kotowicz, J.; Baszczeńska, O.; Maj, I. Performance Characteristics and Optimization of a Single-Stage Direct Air Capture Membrane System in Terms of Process Energy Intensity. Energies 2024, 17, 2046. https://doi.org/10.3390/en17092046
Niesporek K, Kotowicz J, Baszczeńska O, Maj I. Performance Characteristics and Optimization of a Single-Stage Direct Air Capture Membrane System in Terms of Process Energy Intensity. Energies. 2024; 17(9):2046. https://doi.org/10.3390/en17092046
Chicago/Turabian StyleNiesporek, Kamil, Janusz Kotowicz, Oliwia Baszczeńska, and Izabella Maj. 2024. "Performance Characteristics and Optimization of a Single-Stage Direct Air Capture Membrane System in Terms of Process Energy Intensity" Energies 17, no. 9: 2046. https://doi.org/10.3390/en17092046
APA StyleNiesporek, K., Kotowicz, J., Baszczeńska, O., & Maj, I. (2024). Performance Characteristics and Optimization of a Single-Stage Direct Air Capture Membrane System in Terms of Process Energy Intensity. Energies, 17(9), 2046. https://doi.org/10.3390/en17092046








