Abstract
Heavy oils are characterized by a high content of resins and asphaltenes, which complicates refining and leads to an increase in the cost of refinery products. These components can be strongly adsorbed on the acid sites of a supported catalyst, leading to its deactivation. Currently, various salts of group 8 metals are being considered for such processes to act as catalysts during oil cracking. At the same time, the nature of the precursor often has a significant impact on the process of refining heavy oil. In this work, catalytic cracking of heavy oil from the Ashalchinskoye field using different precursors (nanodispersed catalysts formed in situ based on NiO) has been studied. The cracking was carried out at 450 °C with a catalyst content from 0.1 to 0.5 wt.%. The catalytic cracking products were analyzed via SARA, GC, XRD and SEM. Nickel acetate and nitrate promote similar yields of by-products, while formate promotes higher yields of gaseous products. Formate and nickel acetate were shown to produce 1.8 and 2.8 wt.% more light fractions than nickel nitrate. When heavy oil is cracked in the presence of Ni(NO3)2∙6H2O, the maximum decrease in sulfur content (2.12 wt.%) is observed compared to other precursors. It has been found that the composition and morphology of the resulting nickel sulfides and compaction products are influenced by the nature of the catalyst precursor. XRD and SEM analyses of coke-containing catalysts indicate the formation of Ni9S8 and Ni0.96S phases during cracking when nickel nitrate is used and the formation of NiS and Ni9S8 when nickel acetate and formate are used.
1. Introduction
The world’s growing population has resulted in a significant increase in demand for energy, leading to the continuous development of the global economy and industrialization. Historically, this demand has been met by utilizing low-viscosity crude oil resources. Due to the limited availability of these reserves, it is imperative to develop technologies that can enhance the exploration, transportation, and processing of unconventional hydrocarbons such as shale oil, bitumen, heavy oil, and extra-heavy oil. These resources account for 60–70% of the world’s proven crude oil reserves [1].
Although unconventional hydrocarbon raw materials have enormous potential, their use is limited due to their high density and viscosity [2,3]. This is because of the high content of resinous asphaltene substances (RASs), which can make up to 50 wt.% of the total. Such hydrocarbon raw materials are often characterized by a significant amount of heteroatoms, such as S, N, and O, as well as transition metals like V, Ni, Fe, Co, and Mo. Resins and asphaltenes are thermally unstable and reactive compounds, rendering traditional refining methods and catalysts ineffective [4,5,6]. RASs can adsorb to the surface of zeolite or aluminosilicate catalysts and form coke during the process, deactivating such catalyst systems [7].
In this regard, new catalysts for the processing of unconventional hydrocarbon feedstocks capable of selectively converting high-molecular-weight components with a low coke yield are being actively researched worldwide. Promising catalytic systems are in situ-formed transition metal compounds [8,9]. These catalysts are more efficient due to their smaller particle size [10,11]. Catalyst precursors can include various salts, such as nitrates, oleates, naphthenates, acetates, molybdenum, iron, nickel, cobalt formates, etc. [12,13,14,15,16]. All studies mentioned in the text report a decrease in viscosity, density, molecular weight, sulfur content, resins, and asphaltenes, as well as an increase in hydrogen content and the formation of light hydrocarbons [17,18,19,20]. Nickel-based catalytic systems are of particular interest due to their high activity in various reactions and their proven effectiveness in processing activities [21,22,23,24]. However, many questions remain regarding the influence of the anions of the studied salts on the process of upgrading heavy oils. The aim of this study was to investigate the effect of different nickel catalyst precursors on the heavy oil upgrading process.
2. Research Methods
Heavy oil from the Ashalchinskoye field was taken as the object of study (Table 1). The analyzed oil has a high sulfur content of 4.74%, as well as high levels of resins (30.8%) and asphaltenes (5.9 wt.%). Additionally, the content of aromatic hydrocarbons (AHs) is almost twice as high as that of saturated hydrocarbons (SHs), and the H/C atomic ratio is 1.52.

Table 1.
Physical and chemical characteristics of heavy oil.
The autoclave used for cracking had a volume of 12 cm3 and the heavy oil loading was 7 g. The precursor amount varied from 0.10 to 0.50 wt.% in terms of NiO. The catalyst precursor used was high-quality Ni(NO3)2∙6H2O, Ni(CH3COO)2∙4H2O, and Ni(HCOO)2∙2H2O supplied by OOO “Reakhim” (Moscow, Russia). The operating temperature was 450 °C, and the heating rate was 10 °C/min. The experiment began when the temperature inside the reactor reached 450 °C. To confirm the formation of NiO during oil cracking, the original nickel nitrate salt was calcined at 250 and 450 °C (Figure 1). It can be seen that at 450 °C (process temperature), nickel nitrate completely decomposes to form nickel oxide.
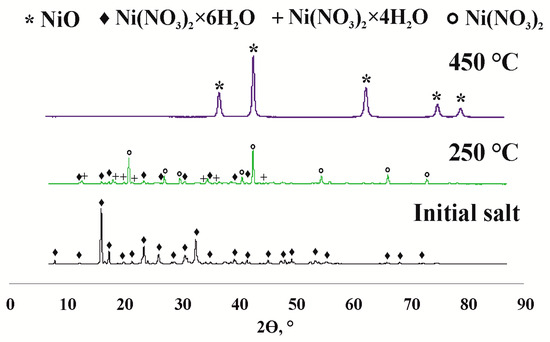
Figure 1.
Phase composition of the nickel oxide precursor (nickel nitrate).
The yield of gaseous cracking products was determined by measuring the loss in reactor mass after their removal. Gaseous products were analyzed using a Kristall-5000 gas chromatograph («Chromatec» Yoshkar-Ola, Russia) in accordance with GOST 31371.3-2008 [25]. The content of hydrogen and CO + CO2 was determined using a column filled with NaX molecular sieves. The content of hydrocarbons C1–C5 was determined using a column filled with polymeric sorbent Porapak R.
Liquid cracking products (LPs) were collected and tested using ASTM-D287 [26], ASTM D4124 [27], ASTM-D4294 [28], and ASTM-D2887 [29] methods. Saturated hydrocarbons (SHs), aromatic hydrocarbons (AHs), resins and asphaltenes were determined according to ASTM D4124 [27]. Next, the reactor was washed with chloroform, dried to remove residual solvent, and weighed. All experiments measured the yield of solid products (coke) and catalyst by determining the difference between the mass of the reactor before and after the experiment. Elemental analysis of the original oil, liquid cracking products, and coke was conducted using a Vario EL Cube CHNS analyzer («Elementar» Langenselbold, Germany). The analyzer’s absolute error did not exceed ±0.1% for each element determined. The oxygen content was estimated by subtracting the sum of the elements C, H, N, and S from 100%.
To determine the phases formed from nickel oxides during cracking, coke-containing catalysts were analyzed using X-ray phase analysis on a D8 Advance powder diffractometer («BRUKER» Karlsruhe, Germany) and scanning electron microscopy on an LEO EVO 50 («Karl Zeiss Group» Oberkochen, Germany) device coupled with an INCA Energy-250 energy-dispersive spectrometer. The sizes of coherent scattering regions (CSRs), also known as nanocrystallite sizes, were determined using the classical Debye–Scherrer formula. The annealing temperature for the compaction products of the coke-containing catalyst was determined using thermogravimetric analysis data (STA 449 F1 Jupiter (NETZSCH, Selb, Germany)).
3. Results and Discussion
Table 2 presents the material balance of Ashalchinskaya oil cracking at temperatures ranging from 350 to 450 °C and process times of 60 to 120 min, as well as the composition of the resulting liquid products. The yields of gaseous and compacted products (coke) slightly increase from 0.1 to 0.4 wt.% and from 0.7 to 1.1 wt.%, respectively, with a process duration of 60 min and an increase in temperature from 350 to 450 °C. The liquid products (LPs) exhibit a decrease in resin content from 30.8 to 20.7–27.5 wt.% and an increase in asphaltenes by 1.0 wt.% compared to the original oil. The results suggest that, under the specified conditions, the contribution of bond-breaking processes is insignificant compared to condensation reactions during cracking.

Table 2.
Material balance of heavy oil thermal cracking.
Increasing the process duration at 450 °C results in higher yields of gaseous and solid products. The maximum total yield of 6.0 wt.% is observed after 120 min of cracking. The amount of resins decreases from 20.7 to 18.0%, while the amount of asphaltenes decreases from 7.4 to 7.1 wt.%.
Based on the fractional composition data, it is evident that raising the cracking temperature from 350 to 400 °C (for a duration of 60 min) reduces the gasoline, diesel, and vacuum distillate fractions in comparison to the original oil. These fractions undergo condensation reactions, resulting in the formation of fractions that boil away above 500 °C. A slight increase in light fractions (5.3 wt.%) is observed at a cracking temperature of 450 °C and a duration of 60 min due to the destruction of the components included in the 360–500 °C and >500 °C fractions compared to the original heavy oil.
Based on the data presented in Figure 2b, it is evident that increasing the thermal cracking time from 60 to 120 min at 450 °C results in a slight increase in the content of valuable components (gasoline and diesel fractions) in the cracking products, from 37.8 to 39.9 wt.%. However, the yield of by-products increases fourfold. The changes observed in cracking products are the result of destructive transformation processes that occur via a radical reaction of the high-molecular-weight hydrocarbon components of crude oil, such as high-molecular-weight oils, resins, and partly asphaltenes, into lighter components.
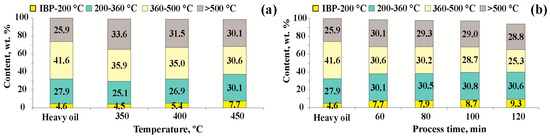
Figure 2.
Fractional composition of liquid cracking products: (a) at a duration of 60 min and different cracking temperatures; (b) at a temperature of 450 °C and various durations.
Table 3 shows that the presence of a catalyst speeds up the destruction reactions of heavy oil components. Compared to thermal cracking, an additional 13.3 wt.% of gasoline and diesel fractions are formed. When 0.1% Ni is used, the gas and coke yield is 11.2% and 2.6 wt.%, respectively. Changing the duration of cracking with a given amount of catalyst from 60 to 100 min has a negative effect on the composition of the products. The amount of coke formed increases almost threefold, from 2.6 to 7.3 wt.%.

Table 3.
Material balance of catalytic cracking of oil in the presence of a nickel nitrate precursor.
The total yield of by-products (gas and coke) decreases from 13.8 to 10.0% when the amount of catalyst increases from 0.1 to 0.2 wt.%. However, when 0.3% catalyst is used, the content of by-products increases to 13.2 wt.%, mainly due to greater gas formation. In experiment no. 7, a minimum gas yield of 5.8% and coke yield of 3.4 wt.% are observed.
A decrease in sulfur content by more than 2 wt.% is observed in liquid cracking products due to the formation of sulfur-containing gases and the concentration and binding of sulfur in compaction products, as well as sulfidation of the catalyst surface. It is worth noting that an increase in the amount of catalyst used results in a decrease in the degree of sulfur removal, from 48.5% to 34.4% relatively.
Furthermore, liquid products resulting from catalytic cracking are considerably richer in gasoline and diesel fractions compared to those from thermal cracking (Table 3). In experiment no. 2, the yield of light fractions was 13.3 wt.% higher, for a total of 51.1 wt.%, with gasoline fractions accounting for up to 15.1 wt.%. This is because components boiling above 360 °C were destroyed. The overall amount of these constituents decreases by 21.2% and 11.2%, respectively, in comparison to the original oil, and by 10.2% and 15.4 wt.%, respectively, in comparison to thermal cracking. Experiments no. 4 and no. 7 yielded the highest amount of target products, at 55.0% and 56.0 wt.%, respectively. Notably, experiment no. 4 resulted in the greatest decrease in the content at 360–500 °C and >500 °C, with a reduction of 18.7 wt.% and 16.1 wt.%, respectively, compared to the original heavy oil.
In Figure 3 shows data on changes in the content of resin and asphaltene components in liquid cracking products with increasing durations (Figure 3a) and an increasing amount of catalyst (Figure 3b). The yield of asphaltenes decreases linearly with an increasing process duration, while the resin content increases after 80 min of cracking due to condensation reactions occurring in the chain: hydrocarbons → resins → asphaltenes → coke.
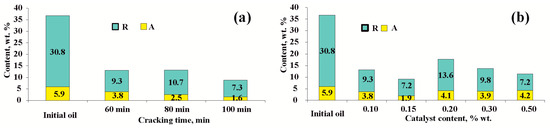
Figure 3.
The content of resin and asphaltenes was analyzed under two different conditions: (a) with a fixed amount of 0.10 wt.% catalyst and varying durations; (b) with a fixed duration of 60 min and varying amounts of catalyst.
In all experiments, the catalytic upgrading of heavy oil resulted in a decrease in the content of resins and asphaltenes compared to the original oil (Figure 3). However, the level of destruction of RASs changes nonlinearly when the amount of catalyst increases from 0.10 to 0.50 wt.%, passing through the minimum point in experiment no.4. Catalytic cracking in the presence of 0.10 and 0.15 wt.% NiO resulted in a decrease in the RAS content to 13.1 and 9.1 wt.%, respectively. When increasing the NiO content from 0.15% to 0.20%, there is a predominance of condensation processes of high-molecular-weight components over their destruction. This is evidenced by the highest resin content in the experiment with 0.20% NiO, which is equal to 13.6 wt.% Increasing the catalyst loading in experiment 5–7 does not lead to a noticeable decrease in asphaltene content compared to the original oil, and their content remains in the range of 3.9–4.2 wt.%. From the data obtained, we can conclude that the optimal amount of catalyst, at which maximum conversion of resins and asphaltenes, sulfur removal and yield of light fractions is observed, is 0.15 wt.%.
Previous experiments have demonstrated the positive effects of catalytic cracking of heavy oil in the presence of nickel oxide. The high rates of upgrading heavy oil are due to the high adsorption affinity of nickel oxide for asphaltenes, as well as the formation of O2 and NO2, which promote oxidative cracking reactions [6]. However, an undesirable effect of the increased asphaltene conversion is the condensation of compaction products, which reduces the catalyst’s activity. To reduce the amount of coke formed, one solution is to use organic nickel salts as catalyst precursors. The decomposition products of these salts can prevent the recombination of asphaltenes into coke.
Based on the data presented in Table 4, the yield of by-products is similar when using nickel acetate (NiAc) and nitrate (NiNit), while the use of nickel formate (NiFor) increases the gas yield. This is likely due to the involvement of Ni(HCOO)2 decomposition products in the formation of gaseous alkanes, such as methane, via the Sabatier reaction [30,31].

Table 4.
Material balance of catalytic cracking of oil in the presence of various precursors of nickel-containing catalysts.
The fractional composition of the resulting liquid products indicates that the nature of the precursor also affects the composition of the resulting light fractions. The formation of predominantly gasoline fractions is helped by the presence of formate and acetate, as evidenced by the ibp–200/200–360 °C ratio of 0.76 and 0.59. In contrast, the experiment using nickel nitrate resulted in a ratio of 0.45. When using organic precursors of nickel oxide, trace amounts of metallic nickel may form, which can act as a catalyst for cracking and isomerization of C8–C16 alkanes [32,33,34,35,36]. The use of nickel nitrate results in a more profound destruction of the components of fractions that boil away at temperatures above 500 °C and the accumulation of predominantly vacuum distillates (360–500 °C) in liquid cracking products, compared to experiments with nickel formate and acetate. It is important to note that nickel nitrate is more effective than formate and acetate at removing sulfur from liquid products.
The data presented in Figure 4 show that the yield of resins and asphaltenes during heavy oil cracking is also influenced by the nature of the nickel catalyst precursor. Nickel nitrate reduces RAS content in liquid cracking products by 75%, formate reduces it by 63% and acetate reduces it by 61%. It is important to note that the destruction of asphaltenes in the presence of formate and acetate does not exceed 51%. The level of RAS conversion may depend on the intermediate compounds formed during the decomposition of nickel salts [32,33,34,35,37].
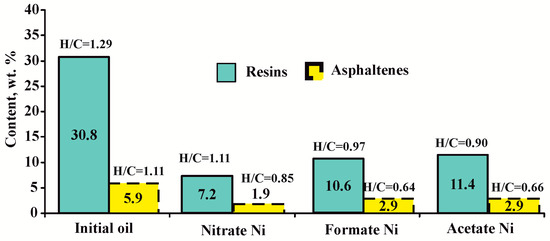
Figure 4.
Content of resins and asphaltenes in liquid cracking products depending on the catalyst precursor used.
It has been established that there is less destruction of resins and asphaltenes when using nickel acetate and formate; these components are characterized by lower values of the H/C atomic ratio compared to nickel nitrate. The high yield of gasoline fractions, in combination with this observation, may suggest that hydrogen from the resin and asphaltene components is being disproportionated to lighter fractions.
Based on the data regarding the composition of gaseous cracking products, the use of nickel nitrate results in the formation of 1.75–2.20 times more CO + CO2 compared to the use of nickel acetate and formate (Table 5). It is possible that the decomposition products of nickel nitrate oxidize the hydrocarbons in oil, which are then broken down to release carbon oxides. When nickel formate is used, high yields of C1–C3 gases are observed, while the amount of CO + CO2 decreases. This is due to the participation of decomposition products of nickel formate in the Fischer-Tropsch process [38]. On the other hand, when cracking in the presence of nickel acetate, a greater yield of C4–C5 gases is observed compared to previous experiments. The participation of acetone molecules in the formation of methane and C4–C5 gases in the presence of NiO may be the cause of this phenomenon [39]. During catalytic cracking, a sulfur content reduction occurs mainly due to its removal in the form of gaseous products. The highest yield of sulfur-containing gases was observed during cracking in the presence of nickel nitrate. This result is also due to oxidative cracking in the presence of nickel nitrate decomposition products [21].

Table 5.
Composition of gaseous products depending on the catalyst precursor.
Figure 5 displays X-ray diffraction patterns of spent Ni-containing catalysts with compaction products. Catalytic upgrading of heavy oil is accompanied by sulfidation of the decomposition products of nickel salts, resulting in the formation of non-stoichiometric nickel sulfides. When nickel nitrate is used as a catalyst precursor, the X-ray diffraction patterns show reflections of the Ni9S8 (PDF 01-078-1886) and Ni0.96S (PDF 00-050-1791) phases. The average CSR value (coherent scattering region) for Ni9S8 and Ni0.96S was 19 nm and 20 nm, respectively (Figure 5a). Cracking heavy oil in the presence of organic nickel oxide precursors results in the formation of Ni9S8 (PDF 01-078-1886) and NiS (PDF 00-002-1280) phases (Figure 5b,c). Stoichiometric nickel sulfide can form due to the presence of trace amounts of metallic nickel, which undergoes sulfidation according to the scheme outlined in Richard D. Tilley’s article [40]. The size of Ni9S8 and NiS crystallites when using nickel acetate as the precursor is comparable to the size observed in the experiment where nickel nitrate was used. The crystallites measure 17 nm and 18 nm. Nickel formate produces nickel sulfides Ni9S8 and NiS with average CSR values of 20 nm and 9 nm, respectively. The literature suggests that NixSy sulfides have catalytic properties in hydrotreating, and NiS is favorable for cleaving C-C bonds and isomerization reactions [41]. Therefore, the higher yield of fuel fractions when using NiFor and NiAc, compared to NiNit, may also be attributed to the presence of NiS. According to [20], the level of desulfurization of heavy oil is affected by the phase ratio of nickel sulfides due to phase transitions between NiSlow and NiShigh [42].
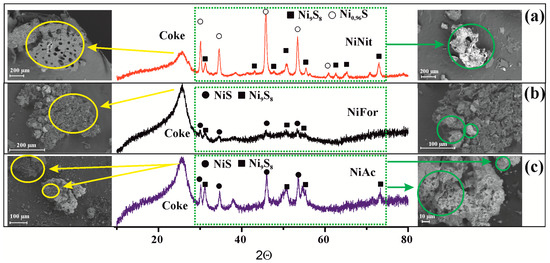
Figure 5.
X-ray diffraction and SEM images of a coke-containing catalyst using (a) nickel nitrate; (b) nickel formate; (c) nickel acetate.
The effect of the catalyst precursor on the morphology of the spent catalyst and coke was evaluated via scanning electron microscopy. The presence of a nickel nitrate precursor during the cracking of heavy oil leads to the formation of irregularly shaped particles containing Ni. In contrast, the use of nickel formate and acetate leads to the observation of submicron catalyst particles on the carbonaceous material. This suggests that the organic anion enhances the adhesion of the catalyst precursor and coke. A rise in temperature leads to the full decomposition of nickel formate and acetate, resulting in the formation of systems that resemble supported catalysts [43]. The compaction products in all cases are angular and porous particles. However, the edges of the coke produced by cracking oil in the presence of nickel nitrate exhibit fractures. According to [44], the presence of significant cracks is linked to the quick elimination of volatile substances from within the compacted particle.
To remove coke completely, the catalyst containing coke was calcined in air at a temperature of 800 °C for 6 h. The annealing temperature for the coke was determined using thermogravimetric data from the coke-containing catalyst. The data showed that the sample experienced weight loss in the temperature range of 400–800 °C (Figure 6a). X-ray diffraction of the powder formed after calcination confirmed that under the specified conditions, the coke burns out completely and nickel sulfide is converted into oxide (Figure 6b). The nickel oxide obtained was subsequently treated with nitric acid to yield the nickel nitrate salt (Figure 6c). Cumulative evidence suggests that the precursor can be regenerated and reused.
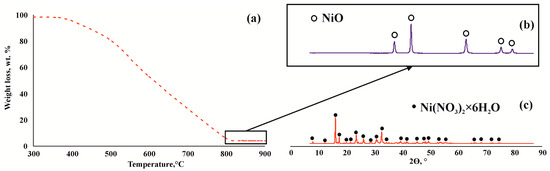
Figure 6.
TG analysis of coke-containing catalyst (NiNit precursor) (a); XRF analysis of the resulting powder after calcination of the coke-containing catalyst (b); XRF analysis of the resulting sample after treatment with nitric acid (c).
4. Conclusions
Research has shown the utility of cracking heavy crude oil from the Ashalchinskoye field via a catalyst precursor containing 0.15% by weight of nickel nitrate and heating to 450 °C for 60 min. The use of a catalyst results in an increase of 22.5 wt.% and 17.2 wt.% in the total content of gasoline and diesel fractions (ibp–360 °C) in liquid cracking products compared to original heavy oil and cracking without a catalyst, respectively. The use of this precursor promotes the destruction of high-molecular-weight components in heavy oil, including resinous asphaltene components (more than 75% relatively), of which asphaltenes are the most undesirable.
It has been shown that the nature of the catalyst precursor significantly affects the resulting cracking products. Nickel formate and acetate lead to a higher production of light fractions (by 1.8% and 2.8 wt.%, respectively, compared to nickel nitrate). However, the presence of these precursors also results in an increase in by-products of 0.2% with acetate and 1.6 wt.% with nickel formate (compared to nickel nitrate). It was found that when nickel nitrate is used, more extreme destruction of resin and asphaltene components and a higher degree of sulfur removal are observed than in the presence of acetate and formate.
It was found that the nature of the catalyst precursor affects the particle composition and morphology of the formed nickel sulfides and compaction products. When heavy oil is cracked in the presence of a precursor of nickel nitrate, irregularly shaped nickel-containing particles are formed on the surface, on which coke is deposited. When nickel formate or acetate is used, submicron catalyst particles are formed on the carbonaceous material. The formation of coke and the size and phase of the resulting nickel sulfides during cracking with heavy oil are influenced by the anion of the metal salts (precursors). Nickel nitrate salt has been found to be recoverable and reusable.
Author Contributions
Conceptualization, N.N.S.; methodology, Y.A.S.; investigation, Y.A.S. and V.R.U.; data curation, K.K.U.; writing—original draft preparation, Y.A.S. and N.N.S.; writing—review and editing, K.K.U. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Ministry of Science and Higher Education of the Russian Federation (Project No. FWRN-2021-0005).
Data Availability Statement
Data are contained within the present article. The authors will provide additional data related to this paper upon request.
Acknowledgments
The authors would like to thank Utyaganova V.R. for the investigation of samples by the method of scanning electron microscopy using the equipment (LEO EVO 50 Zeiss device) of the ‘NANOTECH’ Collective Use Center of the Institute of Strength Physics and Materials Science of the Siberian Branch of the Russian Academy of Sciences.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Maity, S.K.; Ancheyta, J.; Marroquín, G. Catalytic Aquathermolysis Used for Viscosity Reduction of Heavy Crude Oils: A Review. Energy Fuels 2010, 24, 2809–2816. [Google Scholar] [CrossRef]
- AL-Rubaye, A.H.; Suwaid, M.A.; Al-Muntaser, A.A.; Varfolomeev, M.A.; Rakhmatullin, I.Z.; Hakimi, M.H.; Saeed, S.A. Intensification of the steam stimulation process using bimetallic oxide catalysts of MFe2O4 (M = Cu, Co, Ni) for in-situ upgrading and recovery of heavy oil. J. Pet. Explor. Prod. Technol. 2022, 12, 577–587. [Google Scholar] [CrossRef]
- Hashemi, R.; Nassar, N.N.; Pereira Almao, P. Nanoparticle technology for heavy oil in-situ upgrading and recovery enhancement: Opportunities and challenges. Appl. Energy 2014, 133, 374–387. [Google Scholar] [CrossRef]
- Li, H.; Gao, H.; Zhao, X.; Xia, Z.; Yu, B.; Sun, D. Experimental study on viscosity reduction of heavy oil with water content by synergistic effect of microwave and nano-catalyst. J. Pet. Sci. Eng. 2022, 208, 109271. [Google Scholar] [CrossRef]
- Chen, Q.; Shan, Y.; Liu, H.; Zhao, B.; Cao, J. Upgrading of Venezuela extra-heavy oil vacuum residue by two-step thermal treatment. Pet. Sci. Technol. 2020, 38, 166–169. [Google Scholar] [CrossRef]
- Kang, K.H.; Kim, G.T.; Park, S.; Seo, P.W.; Seo, H.; Lee, C.W. A review on the Mo-precursors for catalytic hydroconversion of heavy oil. J. Ind. Eng. Chem. 2019, 76, 1–16. [Google Scholar] [CrossRef]
- Ortiz-Moreno, H.; Ramírez, J.; Cuevas, R.; Marroquín, G.; Ancheyta, J. Heavy Oil Upgrading at Moderate Pressure Using Dispersed Catalysts: Effects of Temperature, Pressure and Catalytic Precursor. Fuel 2012, 100, 186–192. [Google Scholar] [CrossRef]
- Zhao, F.; Liu, Y.; Lu, N.; Xu, T.; Zhu, G.; Wang, K. A review on upgrading and viscosity reduction of heavy oil and bitumen by underground catalytic cracking. Energy Rep. 2021, 7, 4249–4272. [Google Scholar] [CrossRef]
- Kadkin, O.N.; Mikhailova, A.N.; Khafizov, N.R.; Yuan, C.; Varfolomeev, M.A. A molecular mechanics and molecular dynamics study of the structural organization of Cu(II), Ni(II), Co(II), and Fe(II) stearates as potential catalysts for in situ upgrading of heavy oil. Fuel 2022, 313, 123056. [Google Scholar] [CrossRef]
- Marei, N.N.; Nassar, N.N.; Hmoudah, M.; El-Qanni, A.; Vitale, G.; Hassan, A. Nanosize effects of NiO nanosorbcats on adsorption and catalytic thermo-oxidative decomposition of vacuum residue asphaltenes. Can. J. Chem. Eng. 2017, 95, 1864–1874. [Google Scholar] [CrossRef]
- Aliev, F.A.; Mukhamatdinov, I.I.; Sitnov, S.A.; Ziganshina, M.R.; Onishchenko, Y.V.; Sharifullin, A.V.; Vakhin, A.V. In-situ heavy oil aquathermolysis in the presence of nanodispersed catalysts based on transition metals. Processes 2021, 9, 127. [Google Scholar] [CrossRef]
- Yeletsky, P.M.; Zaikina, O.O.; Sosnin, G.A.; Kukushkin, R.G.; Yakovlev, V.A. Heavy oil cracking in the presence of steam and nanodispersed catalysts based on different metals. Fuel Process. Technol. 2020, 199, 106239. [Google Scholar] [CrossRef]
- Wu, Z.; Chen, H.; Cai, X.; Gou, Q.; Jiang, L.; Chen, K.; Chen, Z.; Jiang, S. Current Status and Future Trends of In Situ Catalytic Upgrading of Extra Heavy Oil. Energies 2023, 16, 4610. [Google Scholar] [CrossRef]
- Xiong, P.; Yang, H.; Wu, P.; Liao, Y.; Tan, D.; Ma, Z.; Yan, X. Study on catalytic aquathermolysis of heavy oil by simple synthesis of highly dispersed nickel-loaded nitrogen-doped carbon catalysts. Mol. Catal. 2022, 529, 112528. [Google Scholar] [CrossRef]
- Abdelsalam, Y.I.I.; Aliev, F.A.; Mirzayev, O.O.; Sitnov, S.A.; Katnov, V.E.; Akhmetzyanova, L.A.; Mukhamatdinova, R.E.; Vakhin, A.V. Aquathermolysis of Heavy Crude Oil: Comparison Study of the Performance of Ni(CH3COO)2 and Zn(CH3COO)2 Water-Soluble Catalysts. Catalysts 2023, 13, 873. [Google Scholar] [CrossRef]
- Mukhamatdinov, I.I.; Mahmoud, A.R.; Affane, B.; Mukhamatdinova, R.E.; Sitnov, S.A.; Vakhin, A.V. Development of a Nanodispersed Catalyst Based on Iron and Nickel for In Situ Upgrading Ashal′ cha Heavy Oil. Energy Fuels 2023, 37, 13912–13927. [Google Scholar] [CrossRef]
- Khanghah, M.A.; Jafari, A. Kinetic modeling and CFD simulation of catalytic upgrading reactions: From batch to continuous reactors. J. Taiwan Inst. Chem. Eng. 2022, 134, 104254. [Google Scholar] [CrossRef]
- Mukhamatdinov, I.I.; Khaidarova, A.R.; Mukhamatdinova, R.E.; Affane, B.; Vakhin, A.V. Development of a catalyst based on mixed iron oxides for intensification the production of heavy hydrocarbon feedstocks. Fuel 2022, 312, 123005. [Google Scholar] [CrossRef]
- Liu, H.; Wang, J.; Wang, Z.; Fan, S.; Chen, K. Hydrogenation of Nickel Octaethylporphyrin over Dispersed MoS2 Catalysts Formed In Situ. ChemistrySelect 2018, 3, 4292–4297. [Google Scholar] [CrossRef]
- Urazov, K.K.; Sviridenko, N.N.; Iovik, Y.A.; Kolobova, E.N.; Grabchenko, M.V.; Kurzina, I.A.; Mukhamatdinov, I.I. Effect of hydrogen-donor of heavy crude oil catalytic aquathermolysis in the presence of a nickel-based catalyst. Catalysts 2022, 12, 1154. [Google Scholar] [CrossRef]
- Urazov, K.K.; Sviridenko, N.N. NiO based catalysts obtained “in-situ” for heavy crude oil upgrading: Effect of NiO precursor on the catalytic cracking products composition. J. Taiwan Inst. Chem. Eng. 2021, 127, 151–156. [Google Scholar] [CrossRef]
- Simão, A.; Domínguez-Álvarez, E.; Yuan, C.; Suwaid, M.A.; Varfolomeev, M.A.; Ancheyta, J.; Al-mishaal, O.F.; Kudryashov, S.I.; Afanasiev, I.S.; Antonenko, D.A.; et al. On the use of metallic nanoparticulated catalysts for in-situ oil upgrading. Fuel 2022, 313, 122677. [Google Scholar] [CrossRef]
- Grabchenko, M.; Pantaleo, G.; Puleo, F.; Kharlamova, T.S.; Zaikovskii, V.I.; Vodyankina, O.; Liotta, L.F. Design of Ni-based catalysts supported over binary La-Ce oxides: Influence of La/Ce ratio on the catalytic performances in DRM. Catal. Today 2021, 382, 71–81. [Google Scholar] [CrossRef]
- Vakhin, A.V.; Aliev, F.A.; Mukhamatdinov, I.I.; Sitnov, S.A.; Sharifullin, A.V.; Kudryashov, S.I.; Afanasiev, I.S.; Petrashov, O.V.; Nurgaliev, D.K. Catalytic Aquathermolysis of Boca de Jaruco Heavy Oil with Nickel-Based Oil-Soluble Catalyst. Processes 2020, 8, 532. [Google Scholar] [CrossRef]
- GOST 31371.3-2008; Natural gas. Determination of Composition with Defined Uncertainty by Gas Chromatography Method. Part 3. Determination of Hydrogen, Helium, Oxigen, Nitrogen, Carbon Dioxide and Hydrocarbons up to C8 Using Two Packed Columns. Standardinform: Moscow, Russia, 2008.
- ASTM-D287; Standard Test Method for API Gravity of Crude Petroleum and Petroleum Products. ASTM International: West Conshohocken, PA, USA, 2012. [CrossRef]
- ASTM D4124; Standard Test Method for Separation of Asphalt into Four Fractions. ASTM International: West Conshohocken, PA, USA, 2018. [CrossRef]
- ASTM-D4294; Standard Test Method for Sulfur in Petroleum and Petroleum Products by Energy Dispersive X-ray Fluorescence Spectrometry. ASTM International: West Conshohocken, PA, USA, 2021. [CrossRef]
- ASTM-D2887; Standard Test Method for Boiling Range Distribution of Petroleum Fractions by Gas Chromatography. ASTM International: West Conshohocken, PA, USA, 2023. [CrossRef]
- Moioli, E.; Züttel, A. A model-based comparison of Ru and Ni catalysts for the Sabatier reaction. Sustain. Energy Fuels 2020, 4, 1396–1408. [Google Scholar] [CrossRef]
- Hosseinpour, M.; Akizuki, M.; Oshima, Y.; Soltani, M. Influence of formic acid and iron oxide nanoparticles on active hydrogenation of PAHs by hot compressed water. Isotope tracing study. Fuel 2019, 254, 115675. [Google Scholar] [CrossRef]
- De Jesus, J.C.; González, I.; Quevedo, A.; Puerta, T. Thermal decomposition of nickel acetate tetrahydrate: An integrated study by TGA, QMS and XPS techniques. J. Mol. Catal. A Chem. 2005, 228, 283–291. [Google Scholar] [CrossRef]
- Puzan, A.N.; Baumer, V.N.; Lisovytskiy, D.V.; Mateychenko, P.V. Structure transformations in nickel oxalate dihydrate NiC2O4·2H2O and nickel formate dihydrate Ni(HCO2)2·2H2O during thermal decomposition. J. Solid State Chem. 2018, 266, 133–142. [Google Scholar] [CrossRef]
- Iglesia, E.; Boudart, M. Decomposition of formic acid on copper, nickel, and copper-nickel alloys: III. Catalytic decomposition on nickel and copper-nickel alloys. J. Catal. 1983, 81, 224–238. [Google Scholar] [CrossRef]
- Couttenye, R.A.; De Vila, M.H.; Suib, S.L. Decomposition of methane with an autocatalytically reduced nickel catalyst. J. Catal. 2005, 233, 317–326. [Google Scholar] [CrossRef]
- Tan, Y.; Hu, W.; Du, Y.; Li, J. Species and impacts of metal sites over bifunctional catalyst on long chain n-alkane hydroisomerization: A review. Appl. Catal. A 2021, 611, 117916. [Google Scholar] [CrossRef]
- Brockner, W.; Ehrhardt, C.; Gjikaj, M. Thermal decomposition of nickel nitrate hexahydrate, Ni(NO3)2·6H2O, in comparison to Co(NO3)2·6H2O and Ca(NO3)2·4H2O. Thermochim. Acta 2007, 456, 64–68. [Google Scholar] [CrossRef]
- Enger, B.C.; Holmen, A. Nickel and Fischer-Tropsch Synthesis. Catal. Rev. 2012, 54, 437–488. [Google Scholar] [CrossRef]
- Hussein, G.A.M.; Nohman, A.K.H.; Attyia, K.M.A. Characterization of the decomposition course of nickel acetate tetrahydrate in air. J. Therm. Anal. 1994, 42, 1155–1165. [Google Scholar] [CrossRef]
- Tilley, R.D.; Jefferson, D.A. The Synthesis of Nickel Sulfide Nanoparticles on Graphitized Carbon Supports. J. Phys. Chem. B 2002, 106, 10895–10901. [Google Scholar] [CrossRef]
- Djimasbe, R.; Varfolomeev, M.A.; Al-Muntaser, A.A.; Yuan, C.; Feoktistov, D.A.; Suwaid, M.A.; Kirgizov, A.J.; Davletshin, R.R.; Zinnatullin, A.L.; Fatou, S.D.; et al. Oil dispersed nickel-based catalyst for catalytic upgrading of heavy oil using supercritical water. Fuel 2022, 313, 122702. [Google Scholar] [CrossRef]
- Osasuyi, O.; Quang, D.V.; Basina, G.; Al Wahedi, Y.; Abu Zahra, M.R.M.; Palmisano, G.; Al-Ali, K. Reversible Metal Sulfide Transition in a Two-Step Thermochemical H2S Splitting. Ind. Eng. Chem. Res. 2022, 61, 6135–6145. [Google Scholar] [CrossRef]
- Kharat, A.N.; Pendleton, P.; Badalyan, A.; Abedini, M.; Amini, M.M. Decomposition of Nickel Formate on Sol–Gel Alumina and Characterization of Product by X-Ray Photoelectron and TOF-SIMS Spectroscopies. J. Catal. 2002, 205, 7–15. [Google Scholar] [CrossRef]
- Lewis, A.D.; Fletcher, E.G.; Fletcher, H.T. CO2 Gasification Rates of Petroleum Coke in a Pressurized Flat-Flame Burner Entrained-Flow Reactor. Energy Fuels 2014, 28, 4447–4457. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).