Assessing Energy Potential and Chemical Composition of Food Waste Thermodynamic Conversion Products: A Literature Review
Abstract
1. Introduction
2. Sources and Types of Food Waste
3. Materials and Methods
3.1. The Methods for Thermochemical Conversion
3.1.1. Torrefaction
3.1.2. Pyrolysis
3.1.3. Gasification
3.1.4. Hydrothermal Carbonization
3.2. Technologies Depending on FW Type
4. Results and Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Zou, C.; Zhao, Q.; Zhang, G.; Xiong, B. Energy Revolution: From a Fossil Energy Era to a New Energy Era. Nat. Gas Ind. B 2016, 3, 1–11. [Google Scholar] [CrossRef]
- Dyjakon, A.; Noszczyk, T.; Smędzik, M. The Influence of Torrefaction Temperature on Hydrophobic Properties of Waste Biomass from Food Processing. Energies 2019, 12, 4609. [Google Scholar] [CrossRef]
- Sarrion, A.; Medina-Martos, E.; Iribarren, D.; Diaz, E.; Mohedano, A.F.; Dufour, J. Life Cycle Assessment of a Novel Strategy Based on Hydrothermal Carbonization for Nutrient and Energy Recovery from Food Waste. Sci. Total Environ. 2023, 878, 163104. [Google Scholar] [CrossRef] [PubMed]
- Khoo, C.G.; Lam, M.K.; Mohamed, A.R.; Lee, K.T. Hydrochar Production from High-Ash Low-Lipid Microalgal Biomass via Hydrothermal Carbonization Effects of Operational Parameters and Products Characterization. Environ. Res. 2020, 188, 109828. [Google Scholar] [CrossRef] [PubMed]
- EIP-AGRI Focus Group. Reducing Food Loss on the Farm: Final Report; EIP AGRI Agriculture and Inovation: Brussels, Belgium, 2020. [Google Scholar]
- Definitional Framework for Food Waste—Full Report. FUSIONS Report Available in (2014). Available online: https://www.eu-fusions.org/index.php/publications (accessed on 23 February 2024).
- Bajzelj, B.; McManus, W.; Parry, A. Food Waste in Primary Production in the UK; WARP: Nevada, CA, USA, 2019. [Google Scholar] [CrossRef]
- Caldeira, C.; De Laurentiis, V.; Corrado, S.; Van Holsteijn, F.; Sala, S. Quantification of Food Waste per Product Group along the Food Supply Chain in the European Union: A Mass Flow Analysis. Resour. Conserv. Recycl. 2019, 149, 479–488. [Google Scholar] [CrossRef] [PubMed]
- Sithole, T.; Pahla, G.; Mashifana, T.; Mamvura, T.; Dragoi, E.-N.; Saravanan, A.; Sadeghifar, H. A Review of the Combined Torrefaction and Densification Technology as a Source of Renewable Energy. Alex. Eng. J. 2023, 82, 330–341. [Google Scholar] [CrossRef]
- Lu, X.; Jordan, B.; Berge, N.D. Thermal Conversion of Municipal Solid Waste via Hydrothermal Carbonization: Comparison of Carbonization Products from Current Waste Management Tehniques. Waste Manag. 2012, 32, 1353–1365. [Google Scholar] [CrossRef] [PubMed]
- Tradler, S.B.; Mayr, S.; Himmelsbach, M.; Priewasser, R.; Baumgartner, W.; Stadler, A.T. Hydrothermal Carbonization as an All-Inclusive Process for Food-Waste Conversion. Bioresour. Technol. Rep. 2018, 2, 77–83. [Google Scholar] [CrossRef]
- Pour, F.H.; Makkawi, Y.T. A Review of Post-Consumption Food Waste Management and Its Potentials for Biofuel Production. Energy Rep. 2021, 7, 7759–7784. [Google Scholar] [CrossRef]
- Socas-Rodríguez, B.; Álvarez-Rivera, G.; Valdés, A.; Ibáñez, E.; Cifuentes, A. Food By-Products and Food Wastes: Are They Safe Enough for Their Valorization? Trends Food Sci. Technol. 2021, 114, 133–147. [Google Scholar] [CrossRef]
- Scherhaufer, S.; Davis, J.; Metcalfe, P.; Gollnow, S.; Colin, F.; De Menna, F.; Vittuari, M.; Östergren, K. Environmental Assessment of the Valorisation and Recycling of Selected Food Production Side Flows. Resour. Conserv. Recycl. 2020, 161, 104921. [Google Scholar] [CrossRef]
- Esteban-Lustres, R.; Torres, M.D.; Piñeiro, B.; Enjamio, C.; Domínguez, H. Intensification and Biorefinery Approaches for the Valorization of Kitchen Wastes—A Review. Bioresour. Technol. 2022, 360, 127652. [Google Scholar] [CrossRef]
- Črnivec, I.G.O.; Korošec, M.; Maček, K.; Rac, I.; Juvančič, L.; Poklar Ulrih, N. Analiza Stanja in Vzrokov Nastajanja Odpadne Hrane v Sloveniji: Z Vlogami Ključnih Akterjev v Sloveniji v Shemi Poročanja Distribucije Presežkov Hrane; Biotehniška Fakulteta: Ljubljana, Slovenia, 2021. [Google Scholar]
- Engelberth, A.S. Evaluating Economic Potential of Food Waste Valorization: Onward to a Diverse Feedstock Biorefinery. Curr. Opin. Green Sustain. Chem. 2020, 26, 100385. [Google Scholar] [CrossRef]
- Ouadi, M.; Bashir, M.A.; Speranza, L.G.; Jahangiri, H.; Hornung, A. Food and Market Waste–A Pathway to Sustainable Fuels and Waste Valorization. Energy Fuels 2019, 33, 9843–9850. [Google Scholar] [CrossRef]
- Jo, J.-H.; Kim, S.-S.; Shim, J.-W.; Lee, Y.-E.; Yoo, Y.-S. Pyrolysis Characteristics and Kinetics of Food Wastes. Energies 2017, 10, 1191. [Google Scholar] [CrossRef]
- Jouhara, H.; Ahmad, D.; Czajczyńska, D.; Ghazal, H.; Anguilano, L.; Reynolds, A.; Rutkowski, P.; Krzyżyńska, R.; Katsou, E.; Simons, S.; et al. Experimental Investigation on the Chemical Characterisation of Pyrolytic Products of Discarded Food at Temperatures up to 300 °C. Therm. Sci. Eng. Prog. 2018, 5, 579–588. [Google Scholar] [CrossRef]
- Chen, W.-H.; Lin, B.-J.; Lin, Y.-Y.; Chu, Y.-S.; Ubando, A.T.; Show, P.L.; Ong, H.C.; Chang, J.-S.; Ho, S.-H.; Culaba, A.B.; et al. Progress in Biomass Torrefaction: Principles, Applications and Challenges. Prog. Energy Combust. Sci. 2021, 82, 100887. [Google Scholar] [CrossRef]
- Brilovich Mosseri, M.; Duenyas, A.; Cohen, E.M.A.; Vitkin, E.; Steinbruch, E.; Epstein, M.; Kribus, A.; Gozin, M.; Golberg, A. Hydrothermal Liquefaction of Representative to Israel Food Waste Model. Energy Convers. Manag. X 2023, 20, 100475. [Google Scholar] [CrossRef]
- Ong, H.C.; Yu, K.L.; Chen, W.-H.; Pillejera, M.K.; Bi, X.; Tran, K.-Q.; Pétrissans, A.; Pétrissans, M. Variation of Lignocellulosic Biomass Structure from Torrefaction: A Critical Review. Renew. Sustain. Energy Rev. 2021, 152, 111698. [Google Scholar] [CrossRef]
- Chen, L.; Chen, X.; Zhao, Y.; Xie, X.; Yang, S.; Hua, D.; Wang, C.; Li, T. Effect of Torrefaction on the Physiochemical Characteristics and Pyrolysis of the Corn Stalk. Polymers 2023, 15, 4069. [Google Scholar] [CrossRef]
- Matsakas, L.; Gao, Q.; Jansson, S.; Rova, U.; Christakopoulos, P. Green Conversion of Municipal Solid Wastes into Fuels and Chemicals. Electron. J. Biotechnol. 2017, 26, 69–83. [Google Scholar] [CrossRef]
- Jouhara, H.; Ahmad, D.; Van Den Boogaert, I.; Katsou, E.; Simons, S.; Spencer, N. Pyrolysis of Domestic Based Feedstock at Temperatures up to 300 °C. Therm. Sci. Eng. Prog. 2018, 5, 117–143. [Google Scholar] [CrossRef]
- Widiyannita, A.M.; Cahyono, R.B.; Budiman, A.; Sutijan; Akiyama, T. Study of Pyrolysis of Ulin Wood Residues; AIP Publishing: New York, NY USA, 2016; p. 050004. [Google Scholar]
- Jouhara, H.; Nannou, T.K.; Anguilano, L.; Ghazal, H.; Spencer, N. Heat Pipe Based Municipal Waste Treatment Unit for Home Energy Recovery. Energy 2017, 139, 1210–1230. [Google Scholar] [CrossRef]
- Mendoza Martinez, C.L.; Sermyagina, E.; Saari, J.; Silva De Jesus, M.; Cardoso, M.; Matheus De Almeida, G.; Vakkilainen, E. Hydrothermal Carbonization of Lignocellulosic Agro-Forest Based Biomass Residues. Biomass Bioenergy 2021, 147, 106004. [Google Scholar] [CrossRef]
- Khan, M.A.; Hameed, B.H.; Siddiqui, M.R.; Alothman, Z.A.; Alsohaimi, I.H. Hydrothermal Conversion of Food Waste to Carbonaceous Solid Fuel—A Review of Recent Developments. Foods 2022, 11, 4036. [Google Scholar] [CrossRef] [PubMed]
- Shi, Z.; Liu, S.; Wang, S.; Niedzwiecki, L.; Baranowski, M.; Czerep, M.; Tang, C.; Kawi, S.; Wang, C.-H.; Jiang, J.; et al. Hydrothermal Carbonization Coupled with Pyrolysis: An Innovative Approach to Digestate Management. Green Energy Resour. 2023, 1, 100034. [Google Scholar] [CrossRef]
- Lopez, J.S.; Caldeira, C.; De Laurentiis, V.; Sala, S. Brief on Food Waste in the European Union; The European Commission’s Knowledge Centre for Bioeconomy: Brussels, Belgium, 2020. [Google Scholar]
- Ugwu, S.N.; Enweremadu, C.C. Ranking of Energy Potentials of Agro-Industrial Wastes: Bioconversion and Thermo-Conversion Approach. Energy Rep. 2020, 6, 2794–2802. [Google Scholar] [CrossRef]
- Ganesapillai, M.; Mehta, R.; Tiwari, A.; Sinha, A.; Bakshi, H.S.; Chellappa, V.; Drewnowski, J. Waste to Energy: A Review of Biochar Production with Emphasis on Mathematical Modelling and Its Applications. Heliyon 2023, 9, e14873. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Heidari, M.; Regmi, B.; Salaudeen, S.; Arku, P.; Thimmannagari, M.; Dutta, A. Hydrothermal Carbonization of Fruit Wastes: A Promising Technique for Generating Hydrochar. Energies 2018, 11, 2022. [Google Scholar] [CrossRef]
- Braz, C.E.; Crnkovic, P. Physical Chemical Characterization of Biomass Samples for Application in Pyrolysis Process. Chem. Eng. Trans. 2014, 37, 523–528. [Google Scholar] [CrossRef]
- Xia, G.; You, W.; Manickam, S.; Yoon, J.Y.; Xuan, X.; Sun, X. Numerical Simulation of Cavitation-Vortex Interaction Mechanism in an Advanced Rotational Hydrodynamic Cavitation Reactor. Ultrason. Sonochem. 2024, 105, 106849. [Google Scholar] [CrossRef] [PubMed]
- Mamvura, T.A.; Danha, G. Biomass Torrefaction as an Emerging Technology to Aid in Energy Production. Heliyon 2020, 6, e03531. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.H.; Cheng, W.Y.; Lu, K.M.; Huang, Y.P. An Evalution on Improvement of Pulverized Biomass Property for Solid Fuel through Torrefaction. Appl. Energy 2011, 88, 3636–3644. [Google Scholar] [CrossRef]
- Nhuchhen, D.R.; Basu, P. Experimental Investigation of Mildly Pressurized Torrefaction in Air and Nitrogen. Energy Fuels 2014, 28, 3110–3121. [Google Scholar] [CrossRef]
- Ipiales, R.P.; de La Rubia, M.A.; Diaz, E.; Mohedano, A.F.; Rodriguez, J.J. Integration of Hydrothermal Carbonization and Anaerobic Digestion for Energy Recovery of Biomass Waste: An Overview. Energy Fuels 2021, 35, 17032–17050. [Google Scholar] [CrossRef]
- Chen, W.-H.; Kuo, P.-C. A Study on Torrefaction of Various Biomass Materials and Its Impact of Lignocellulosic Structure Simulated by a Thermogravimetry. Energy 2010, 35, 2580–2586. [Google Scholar] [CrossRef]

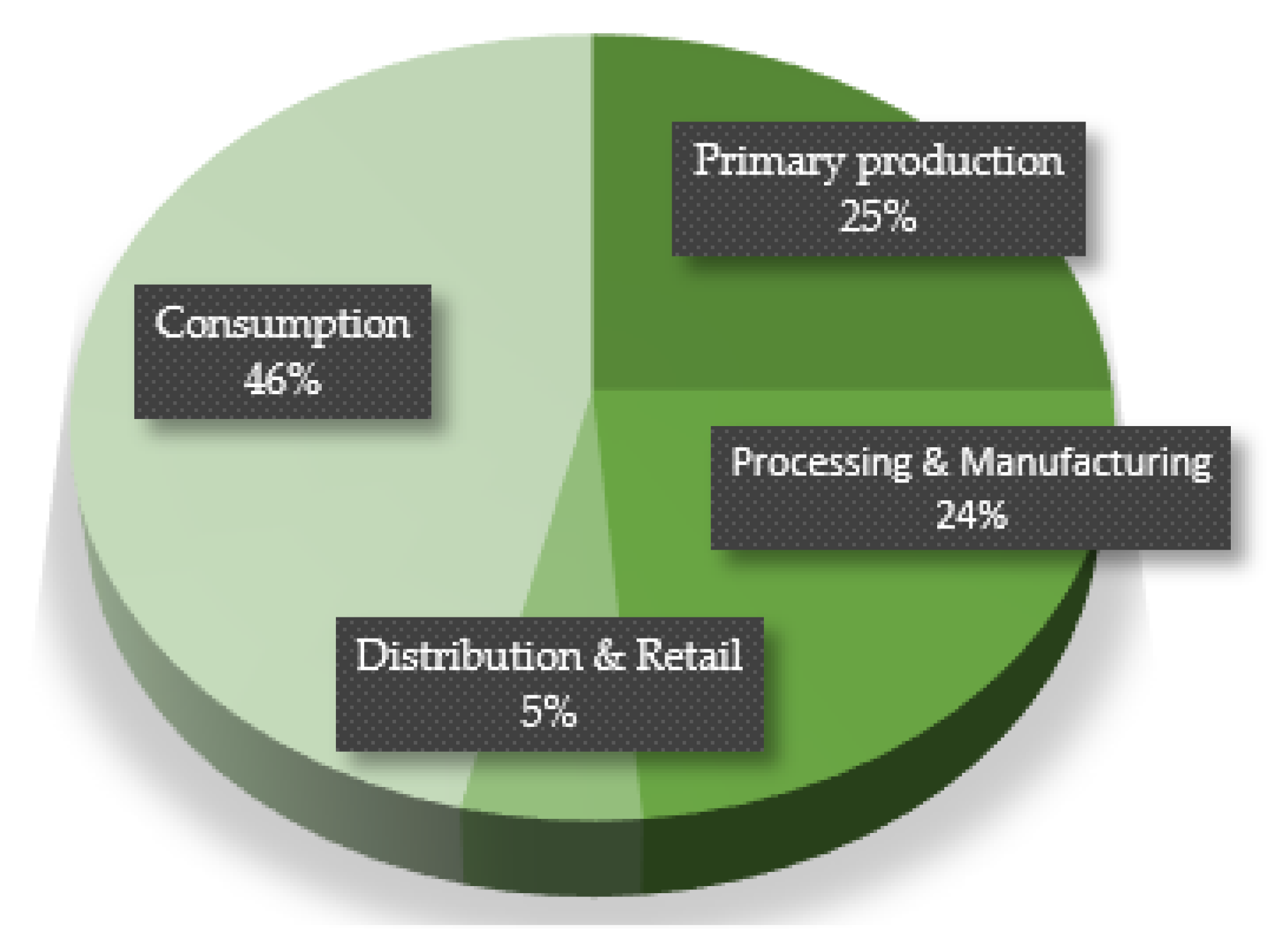
| Source | Plant Sources | Animal Resources |
|---|---|---|
| Primary production |
|
|
| Processing and manufacturing |
| |
| Retail and marketing |
| |
| Final preparation and consumption |
| |
| Technology | Characteristic | Processing Cycle | The Results |
|---|---|---|---|
| Incineration |
|
|
|
| Fast pyrolysis |
|
|
|
| Gasification |
|
|
|
| Hydrothermal Carbonization (HTC) |
|
|
|
| Hydrothermal liquefaction (HTL) Torrefaction |
|
|
|
| Biomass | C | N | O | H | S | Ash Content | VM % | FC % | HHV (MJ/kg) | O/C | H/C |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Animal-based [33] | [30] | ||||||||||
| Egg | 23.49 | 3.03 | 7.65 | 23.11 | 1.02 | 62.50 | 18.90 | 18.60 | 10.09 | 1.24 | 1.17 |
| Fish | 44.42 | 7.41 | 28.67 | 7.90 | 1.25 | 10.35 | 75.56 | 14.09 | 15.20 | 0.49 | 2.12 |
| Meat (Beef fat—processing) | 70.95 | 0.70 | 16.90 | 11.01 | - | 0.44 | 99.50 | 0.06 | 36.64 | 0.11 | 1.16 |
| Manure and whey | 28.2 | 1.7 | 20.3 | 3.6 | 0.5 | 45.7 | - | - | 15.9 | 0.54 | 1.53 |
| Plant-based [33] | [30,34] | ||||||||||
| Pumpkin | 48.85 | 3.50 | 39.33 | 6.67 | - | 1.65 | 92.32 | 6.03 | 19.12 | 0.60 | 1.63 |
| Potatoes | 42.20 | 2.15 | 48.05 | 5.84 | - | 1.76 | 93.39 | 4.85 | 14.12 | 0.86 | 1.65 |
| Carrot | 42.45 | 2.17 | 47.63 | 5.55 | - | 2.19 | 81.86 | 15.95 | 13.88 | 0.84 | 1.56 |
| Grape pomace (GP) | 44.14 | 1.27 | 41.91 | 6.18 | - | 6.50 | 76.22 | 17.28 | - | 0.79 | 1.68 |
| Garlic | 42.96 | 1.08 | 37.40 | 5.49 | 0.88 | 12.20 | 67.64 | 20.17 | 15.83 | 0.65 | 1.52 |
| Peanut | 59.27 | 3.30 | 22.39 | 8.18 | - | 6.86 | 93.95 | 0.81 | 27.77 | 0.29 | 1.64 |
| Apple chip pomace (ACP) | 47.94 | 1.96 | 40.90 | 6.66 | 0.07 | 2.47 | 81.65 | 15.88 | - | 0.64 | 1.66 |
| Rice husk | 46.26 | 1.36 | 45.92 | 6.46 | - | 11.80 | 73.50 | 14.70 | 16.20 | 0.74 | 1.67 |
| Corn stalk | 45.67 | 0.31 | 47.60 | 6.42 | - | 2.59 | 87.19 | 2.59 | 17.65 | 0.78 | 1.68 |
| Wheat straw | 51.25 | 0.63 | 42.81 | 5.18 | 0.13 | 3.70 | 80.00 | 7.80 | 17.10 | 0.63 | 1.21 |
| Empty fruit bunces | 48.30 45.53 | 1.00 | 43.70 | 6.66 | 0.34 | 3.00 | 82.21 | 10.41 | 19.45 | 0.68 | 1.65 |
| Olive pulp- including kernels | 51.91 | 1.65 | 40.45 | 5.99 | - | 3.10 | 75.20 | 21.80 | 21.70 | 0.58 | 1.38 |
| Coconut husk | 50.05 | 0.41 | 43.63 | 5.80 | 0.10 | 3.40 | 61.80 | 34.80 | 19.10 | 0.66 | 1.39 |
| Banana peel | 47.50 | 1.00 | 45.50 | 7.03 | - | 8.30 | - | - | 9.19 | 0.71 | 1.77 |
| Energy crops | 40.30 | 2.1 | 24.0 | 4.6 | 0.3 | 28.7 | - | - | 16.4 | 0.44 | 1.37 |
| Vegetable, garden and fruit waste | 29.50 | 2.0 | 21.4 | 3.0 | 0.3 | 43.8 | - | - | 14.9 | 0.54 | 1.22 |
| Torrefaction [24,34] | Hydrothermal Carbonization [12,33,34,35] | |||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Biomass (Animal- and Plant-Based) | C | N | O | H | S | Ash | HHV (MJ/kg) | O/C | H/C | C | N | O | H | S | Ash | HHV (MJ/kg) | O/C | H/C |
| Manure and whey | - | - | - | - | - | - | - | - | - | 35.5 | - | 13.6 | 3.8 | - | - | 14.5 | 0.28 | 1.28 |
| Chicken | - | - | - | - | - | - | - | - | - | 66.1 | 6.46 | 16.22 | 9.9 | 0.3 | 1.02 | 32.97 | 0.18 | 1.8 |
| Grape pomace [35] | - | - | - | - | - | - | - | - | - | 61.46 | 1.72 | 29.97 | 5.16 | - | 1.69 | - | 0.36 | 1.01 |
| Apple chip pomace | - | - | - | - | - | - | - | - | - | 62.10 | 2.26 | 27.86 | 6.94 | 0.07 | 0.77 | - | 0.34 | 1.34 |
| Rice husk | 55.82 | 0.91 | - | - | 0.02 | 21.24 | - | - | - | 49.26 | 0.68 | 43.57 | 6.48 | - | 12.10 | 16.50 | 0.66 | 1.57 |
| Corn fiber | - | - | - | - | - | - | - | - | - | 49.25 | 0.25 | 44.28 | 6.21 | - | 0.53 | 19.47 | 0.67 | 1.15 |
| Wheat straw | - | - | - | - | - | - | - | - | - | 53.02 | 0.63 | 40.88 | 5.36 | 0.11 | 1.30 | 19.30 | 0.57 | 1.21 |
| Empty fruit bunces | 47.07 | 1.35 | 42.24 | 4.95 | 0.11 | - | - | 0.67 | 1.26 | 54.30 | 1.02 | 38.29 | 4.14 | 0.24 | 4.16 | 22.07 | 0.53 | 0.91 |
| Olive pulp- including kernels | 51.8 | 0.1 | 41.5 | 6.1 | 0.02 | 0.5 | 19.6 | 0.60 | 1.41 | 61.16 | 1.68 | 30.63 | 6.53 | - | 4.80 | 24.30 | 0.37 | 1.28 |
| Coconut husk | - | - | - | - | - | - | - | - | - | 59.52 | 0.50 | 34.17 | 5.71 | 0.10 | 0.30 | 23.90 | 0.43 | 1.15 |
| Energy crops | - | - | - | - | - | - | - | - | - | 41.2 | - | 21.9 | 3.9 | - | - | 23.1 | ||
| Raw vegetables, fruits and peels [12] | 55.86 | 3.15 | 10.93 | 5.15 | - | 24.91 | 23.83 | 0.15 | 1.10 | - | - | - | - | - | - | - | 0.52 | 1.41 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Škorjanc, A.; Goričanec, D.; Urbancl, D. Assessing Energy Potential and Chemical Composition of Food Waste Thermodynamic Conversion Products: A Literature Review. Energies 2024, 17, 1897. https://doi.org/10.3390/en17081897
Škorjanc A, Goričanec D, Urbancl D. Assessing Energy Potential and Chemical Composition of Food Waste Thermodynamic Conversion Products: A Literature Review. Energies. 2024; 17(8):1897. https://doi.org/10.3390/en17081897
Chicago/Turabian StyleŠkorjanc, Andreja, Darko Goričanec, and Danijela Urbancl. 2024. "Assessing Energy Potential and Chemical Composition of Food Waste Thermodynamic Conversion Products: A Literature Review" Energies 17, no. 8: 1897. https://doi.org/10.3390/en17081897
APA StyleŠkorjanc, A., Goričanec, D., & Urbancl, D. (2024). Assessing Energy Potential and Chemical Composition of Food Waste Thermodynamic Conversion Products: A Literature Review. Energies, 17(8), 1897. https://doi.org/10.3390/en17081897





