Enhancing the Electrochemical Performance of ZnO-Co3O4 and Zn-Co-O Supercapacitor Electrodes Due to the In Situ Electrochemical Etching Process and the Formation of Co3O4 Nanoparticles
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of the ZnO-Co3O4 and h-Zn-Co-O
2.3. Characterization Techniques
2.4. Electrode Preparation
2.5. Electrochemical Analysis of Electrode and Hybrid Supercapacitor
3. Results and Discussion
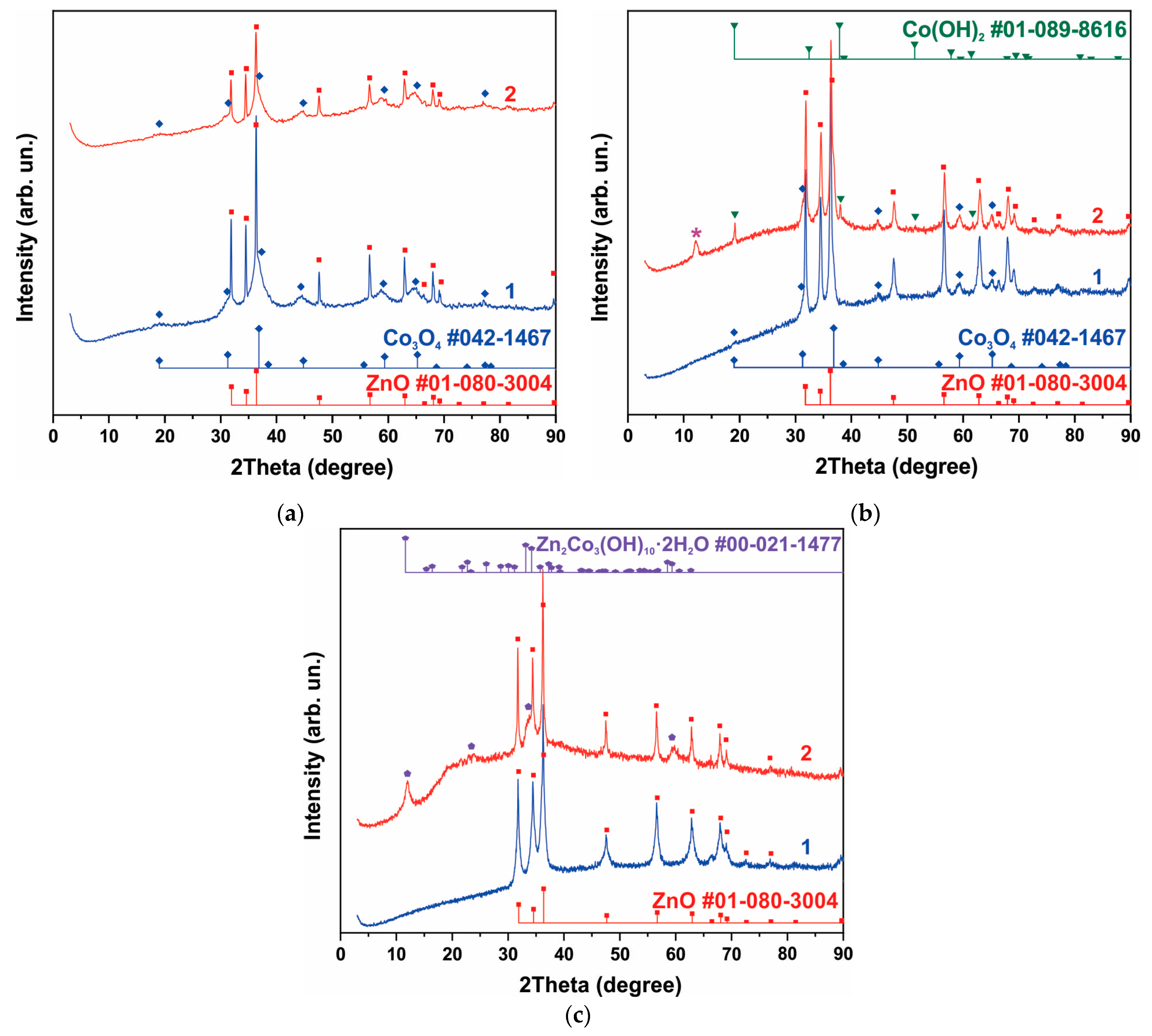
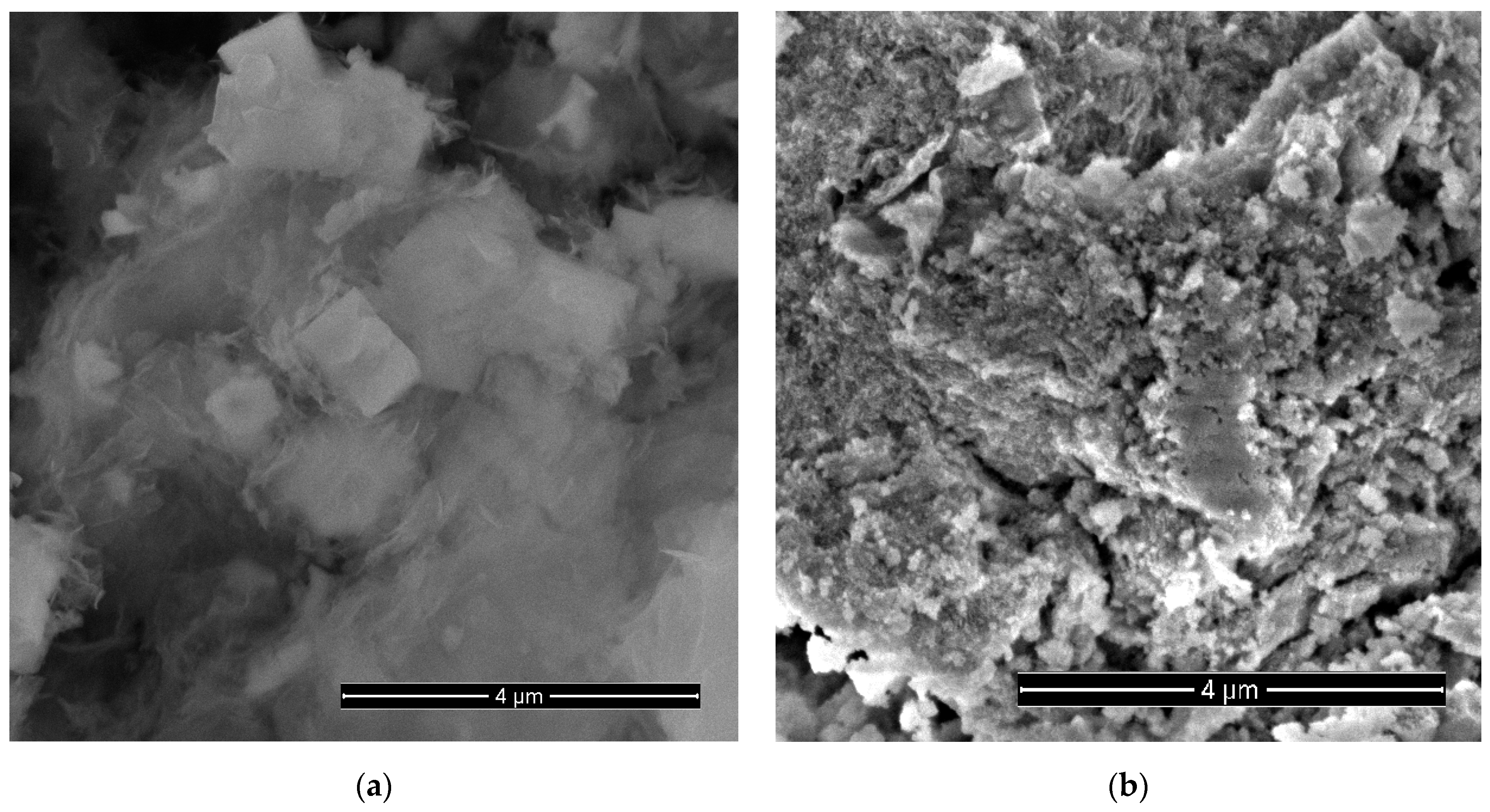
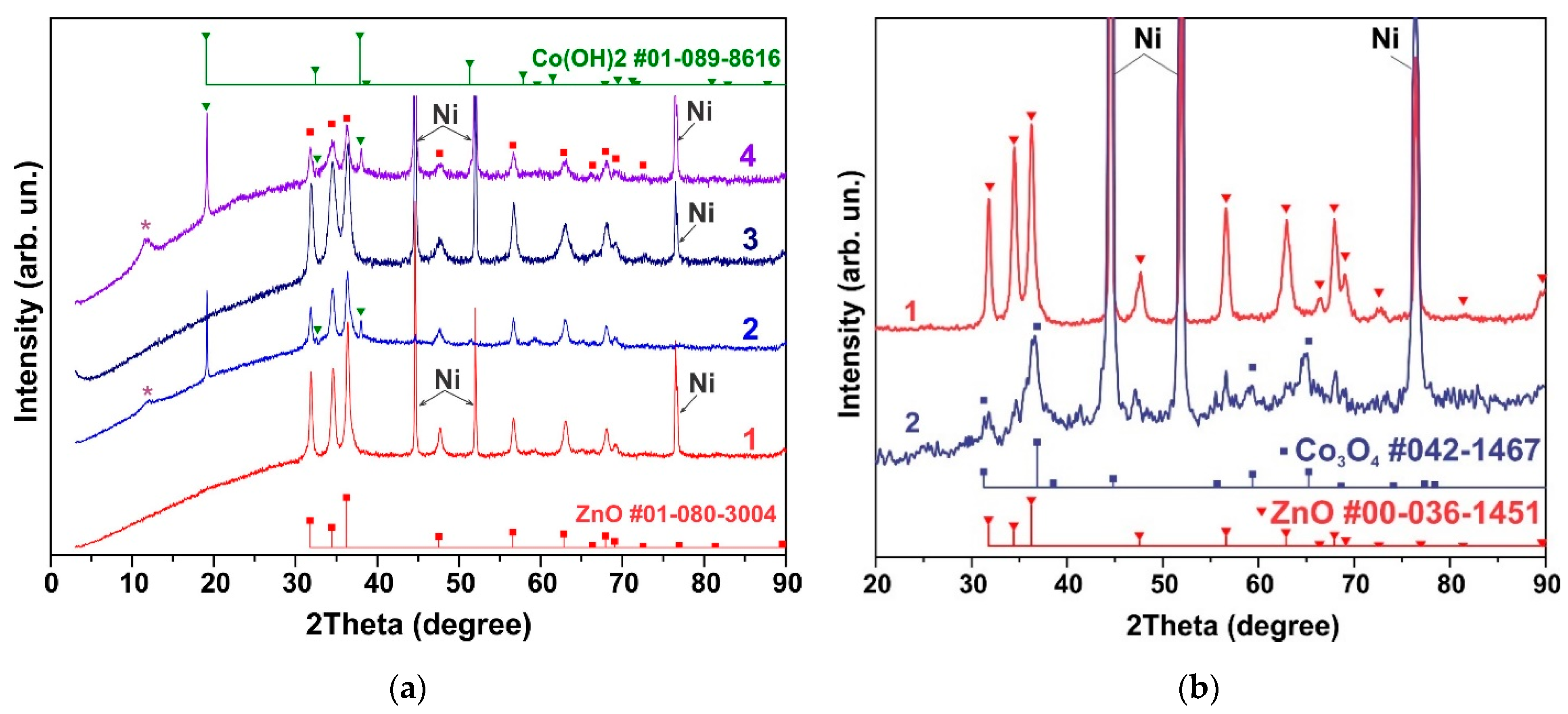
3.1. Morphology and Structure
3.2. Raman Spectra
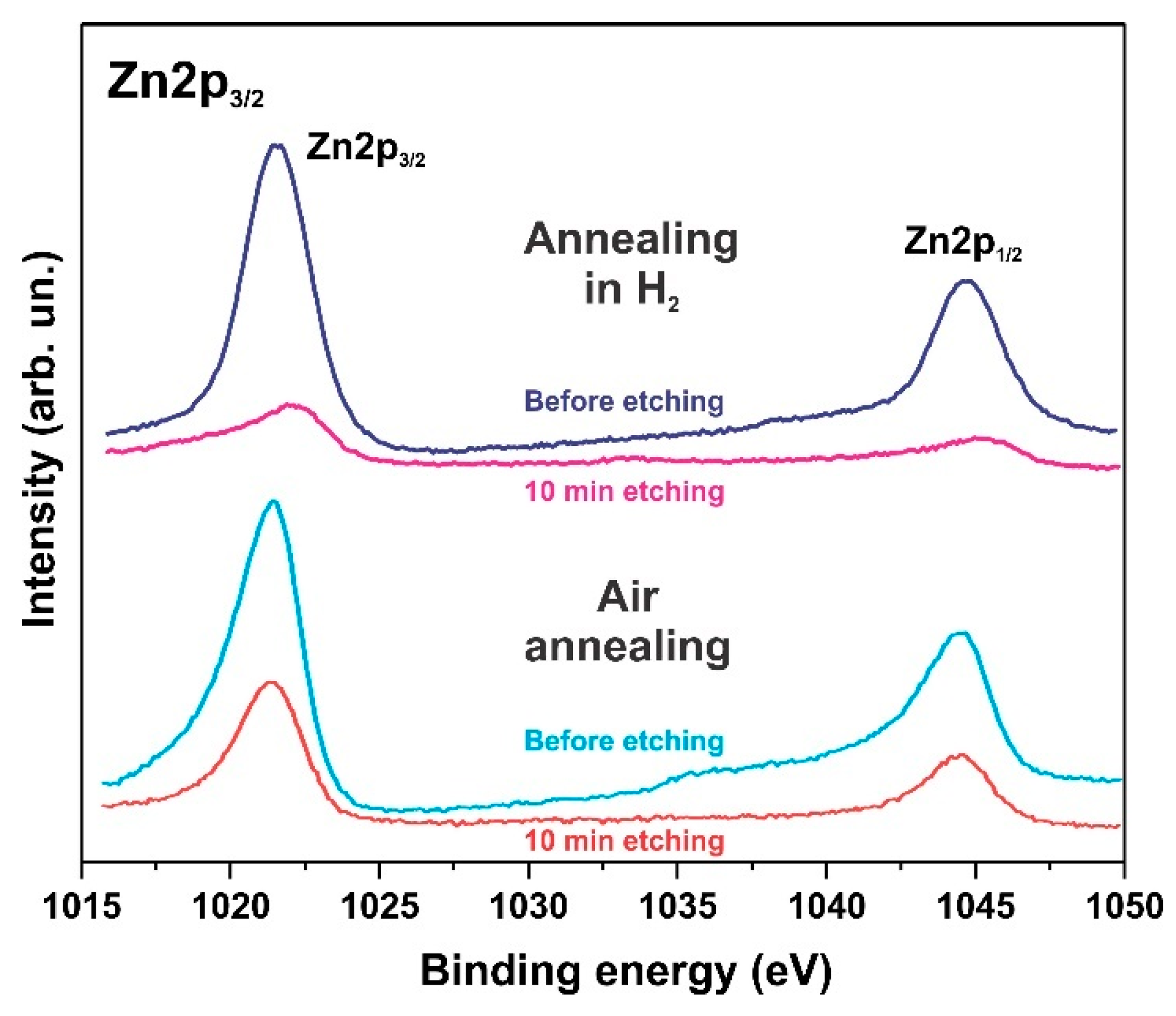
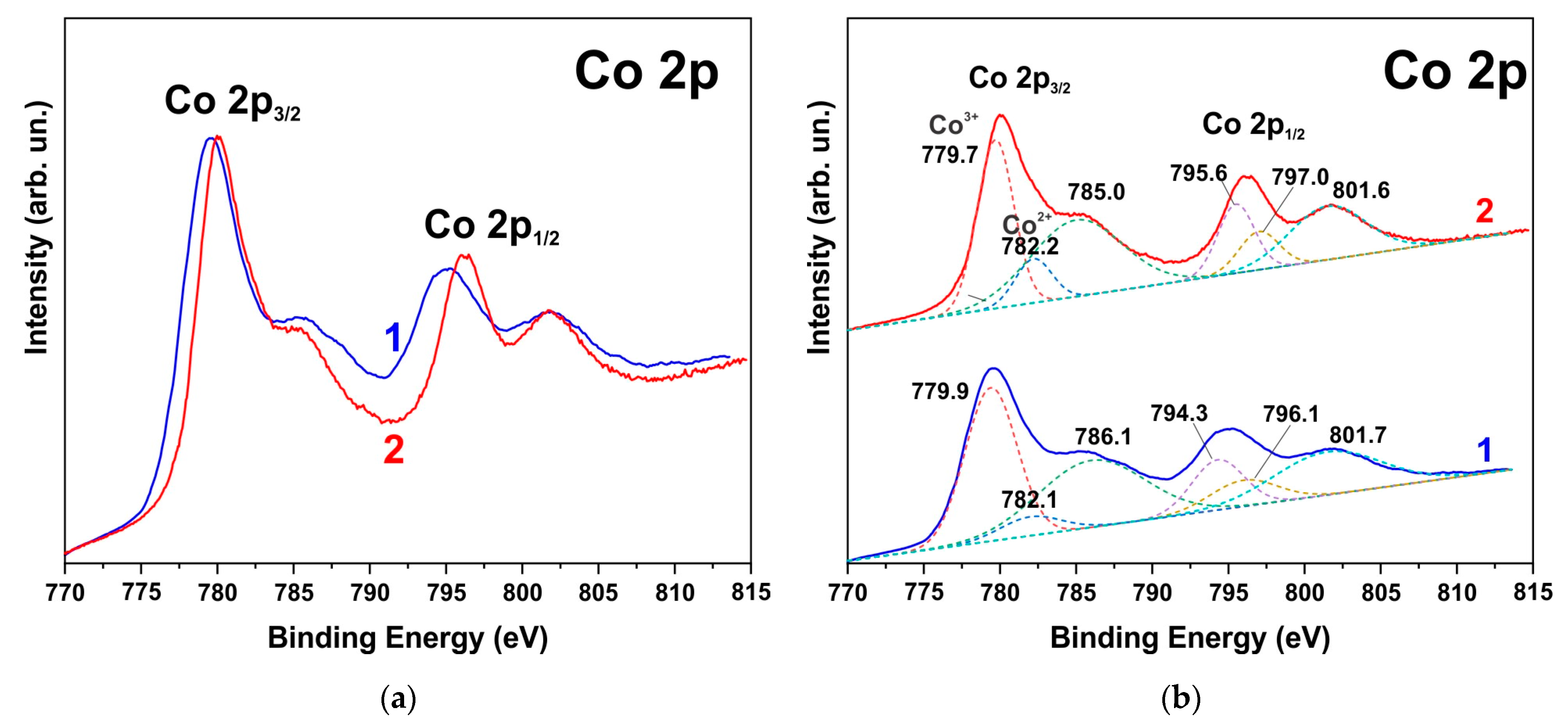
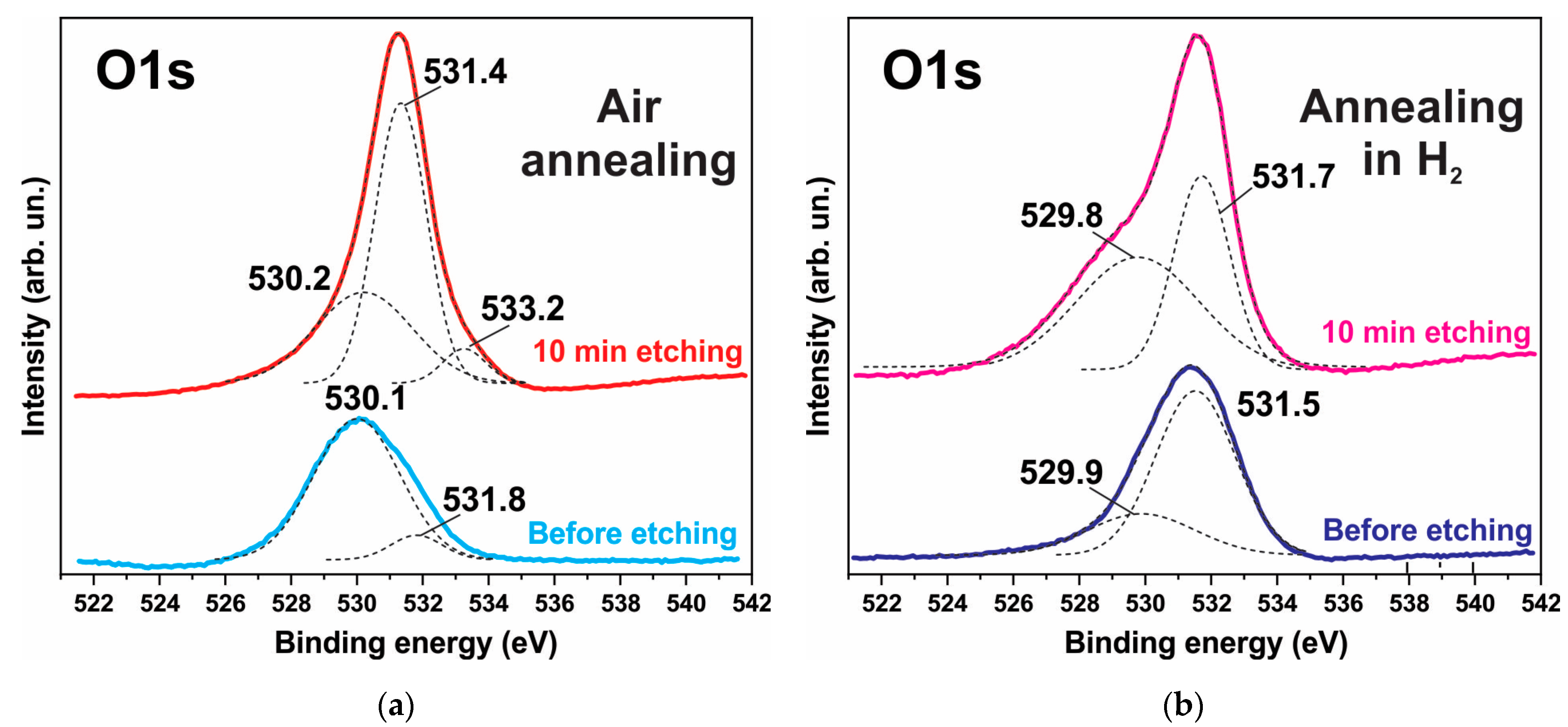
3.3. XPS Spectra
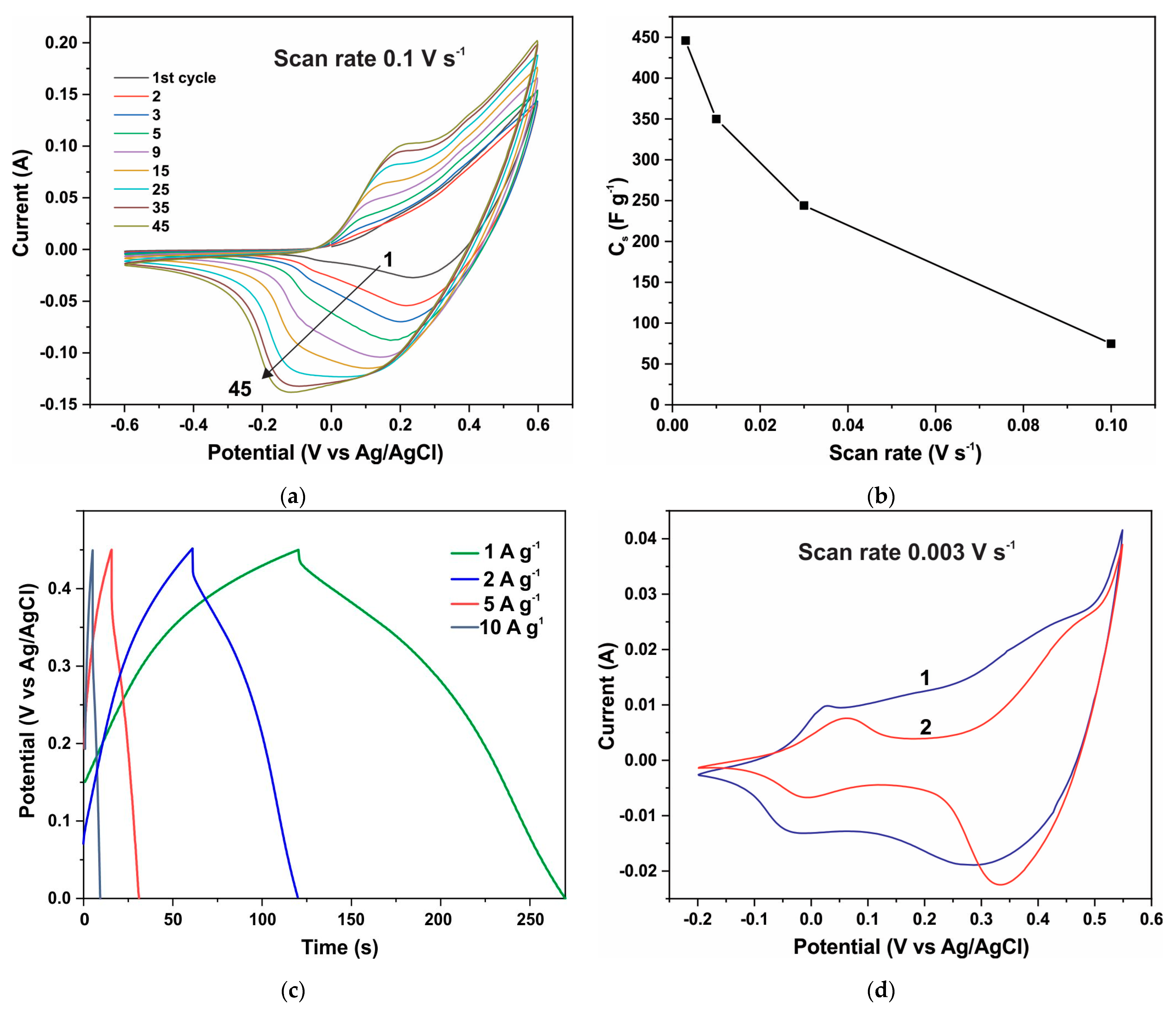
3.4. Electrochemical Measurements of Electrodes
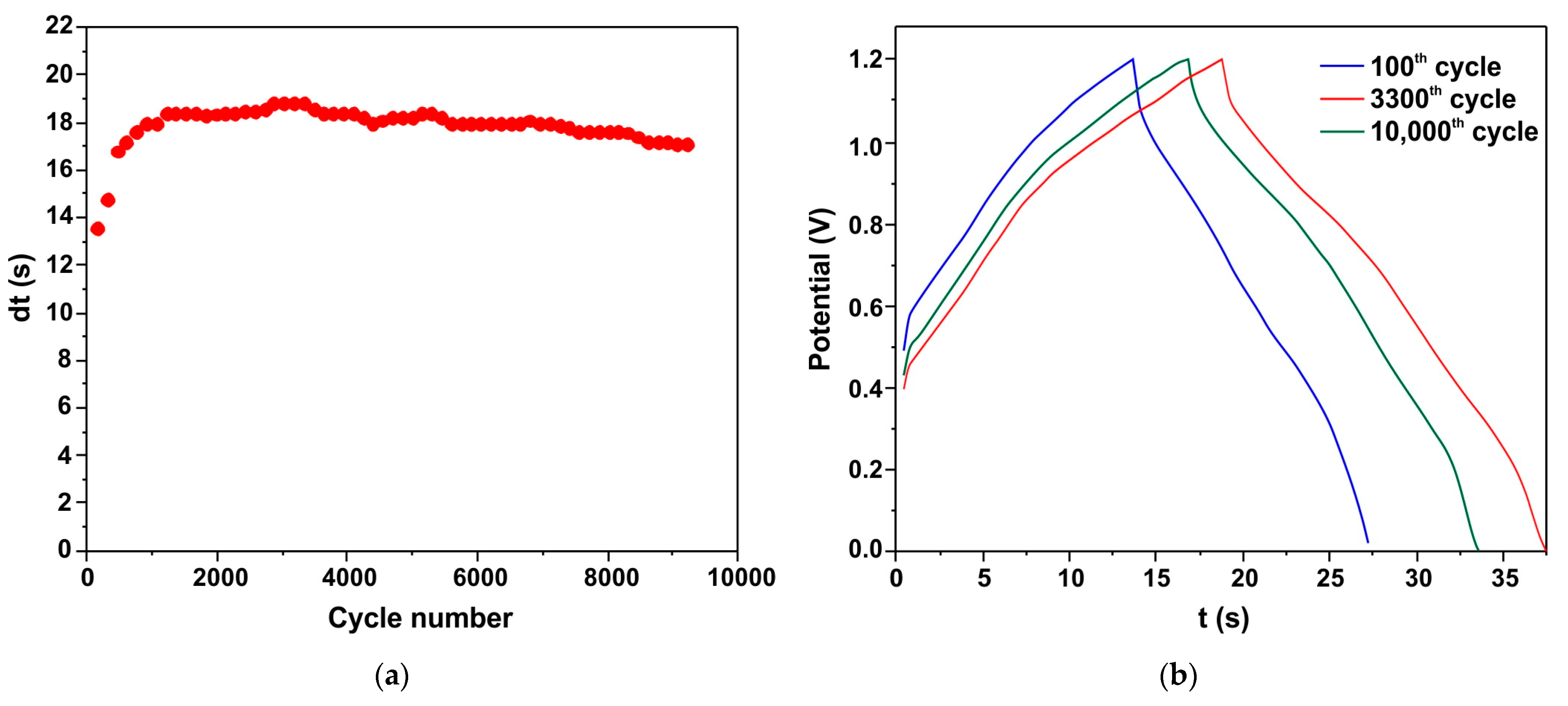
3.5. Electrochemical Measurements of a Hybrid Capacitor
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Wisz, G.; Virt, I.; Sagan, P.; Potera, P.; Yavorskyi, R. Structural, Optical and Electrical Properties of Zinc Oxide Layers Produced by Pulsed Laser Deposition Method. Nanoscale Res. Lett. 2017, 12, 253. [Google Scholar] [CrossRef] [PubMed]
- Vyas, S. A Short Review on Properties and Applications of Zinc Oxide Based Thin Films and Devices. Johns. Matthey Technol. Rev. 2020, 64, 202–218. [Google Scholar] [CrossRef]
- Vayssieres, L.; Keis, K.; Hagfeldt, A.; Lindquist, S.-E. Three-Dimensional Array of Highly Oriented Crystalline ZnO Microtubes. Chem. Mater. 2001, 13, 4395–4398. [Google Scholar] [CrossRef]
- Zhao, Y.; Tong, F.; Wang, M.H. Effect of cobalt doping on microstructures and dielectric properties of ZnO. Can. J. Chem. 2019, 97, 227–232. [Google Scholar] [CrossRef]
- Arshad, M.; Ansari, M.M.; Ahmed, A.S.; Tripathi, P.; Ashraf, S.; Naqvi, A.; Azam, A. Band gap engineering and enhanced photoluminescence of Mg doped ZnO nanoparticles synthesized by wet chemical route. J. Lumin. 2015, 161, 275–280. [Google Scholar] [CrossRef]
- Hassan, M.M.; Khan, W.; Azam, A.; Naqvi, A. Influence of Cr incorporation on structural, dielectric and optical properties of ZnO nanoparticles. J. Ind. Eng. Chem. 2015, 21, 283–291. [Google Scholar] [CrossRef]
- Gürbüz, O.; Okutan, M. Structural, electrical, and dielectric properties of Cr doped ZnO thin films: Role of Cr concentration. Appl. Surf. Sci. 2016, 387, 1211–1218. [Google Scholar] [CrossRef]
- Tabib, A.; Sdiri, N.; Elhouichet, H.; Férid, M. Investigations on electrical conductivity and dielectric properties of Na doped ZnO synthesized from sol gel method. J. Alloy. Compd. 2015, 622, 687–694. [Google Scholar] [CrossRef]
- Vladut, C.M.; Mihaiu, S.; Mocioiu, O.C.; Atkinson, I.; Pandele-Cusu, J.; Anghel, E.M.; Calderon-Moreno, J.M.; Zaharescu, M. Thermal studies of Mn2+-doped ZnO powders formation by sol–gel method. J. Therm. Anal. Calorim. 2019, 135, 2943–2951. [Google Scholar] [CrossRef]
- Han, C.; Duan, L.; Zhao, X.; Hu, Z.; Niu, Y.; Geng, W. Effect of Fe doping on structural and optical properties of ZnO films and nanorods. J. Alloy. Compd. 2019, 770, 854–863. [Google Scholar] [CrossRef]
- Raha, S. Ahmaruzzaman ZnO nanostructured materials and their potential applications: Progress, challenges and perspectives. Nanoscale Adv. 2022, 4, 1868–1925. [Google Scholar] [CrossRef]
- Clarke, B.; Ghandi, K. The Interplay of Growth Mechanism and Properties of ZnO Nanostructures for Different Applications. Small 2023, 19, e2302864. [Google Scholar] [CrossRef]
- Wang, J.; Chen, R.; Xiang, L.; Komarneni, S. Synthesis, properties and applications of ZnO nanomaterials with oxygen vacancies: A review. Ceram. Int. 2018, 44, 7357–7377. [Google Scholar] [CrossRef]
- Theerthagiri, J.; Salla, S.; Senthil, R.A.; Nithyadharseni, P.; Madankumar, A.; Arunachalam, P.; Maiyalagan, T.; Kim, H.-S. A review on ZnO nanostructured materials: Energy, environmental and biological applications. Nanotechnology 2019, 30, 392001. [Google Scholar] [CrossRef] [PubMed]
- Yin, X.; Hua, Y.; Gao, Z. Two-Dimensional Materials for High-Performance Oxygen Evolution Reaction: Fundamentals, Recent Progress, and Improving Strategies. Renewables 2023, 1, 190–226. [Google Scholar] [CrossRef]
- Goktas, S.; Goktas, A. A comparative study on recent progress in efficient ZnO based nanocomposite and heterojunction photocatalysts: A review. J. Alloy. Compd. 2021, 863, 158734. [Google Scholar] [CrossRef]
- Mehr, M.E.; Maleki-Ghaleh, H.; Yarahmadi, M.; Kavanlouei, M.; Siadati, M.H. Synthesis and characterization of photocatalytic zinc oxide/titanium oxide (core/shell) nanocomposites. J. Alloy. Compd. 2021, 882, 160777. [Google Scholar] [CrossRef]
- Bakranova, D.; Seitov, B.; Bakranov, N. Preparation and Photocatalytic/Photoelectrochemical Investigation of 2D ZnO/CdS Nanocomposites. Chemengineering 2022, 6, 87. [Google Scholar] [CrossRef]
- Seitov, B.; Kurbanbekov, S.; Bakranova, D.; Abdyldayeva, N.; Bakranov, N. Study of the Photoelectrochemical Properties of 1D ZnO Based Nanocomposites. Catalysts 2021, 11, 1235. [Google Scholar] [CrossRef]
- Franco, M.A.; Conti, P.P.; Andre, R.S.; Correa, D.S. A review on chemiresistive ZnO gas sensors. Sensors Actuators Rep. 2022, 4, 100100. [Google Scholar] [CrossRef]
- Kang, Y.; Yu, F.; Zhang, L.; Wang, W.; Chen, L.; Li, Y. Review of ZnO-based nanomaterials in gas sensors. Solid State Ionics 2021, 360, 115544. [Google Scholar] [CrossRef]
- Krishna, K.G.; Umadevi, G.; Parne, S.; Pothukanuri, N. Zinc oxide based gas sensors and their derivatives: A critical review. J. Mater. Chem. C 2023, 11, 3906–3925. [Google Scholar] [CrossRef]
- Ivanishcheva, A.P.; Sysoev, V.V.; Abdullin, K.A.; Nesterenko, A.V.; Khubezhov, S.A.; Petrov, V.V. The Application of Combined Visible and Ultraviolet Irradiation to Improve the Functional Characteristics of Gas Sensors Based on ZnO/SnO2 and ZnO/Au Nanorods. Chemosensors 2023, 11, 200. [Google Scholar] [CrossRef]
- Shanmugam, N.R.; Muthukumar, S.; Prasad, S. A review on ZnO-based electrical biosensors for cardiac biomarker detection. Futur. Sci. OA 2017, 3, FSO196. [Google Scholar] [CrossRef] [PubMed]
- Napi, M.L.M.; Sultan, S.M.; Ismail, R.; How, K.W.; Ahmad, M.K. Electrochemical-Based Biosensors on Different Zinc Oxide Nanostructures: A Review. Materials 2019, 12, 2985. [Google Scholar] [CrossRef]
- Napi, M.L.M.; Noorden, A.F.A.; Tan, M.L.P.; Jamaluddin, H.; Hamid, F.A.; Hashim, U.; Ahmad, M.R.; Sultan, S.M. Review—Three Dimensional Zinc Oxide Nanostructures as an Active Site Platform for Biosensor: Recent Trend in Healthcare Diagnosis. J. Electrochem. Soc. 2020, 167, 137501. [Google Scholar] [CrossRef]
- Krishna, M.S.; Singh, S.; Batool, M.; Fahmy, H.M.; Seku, K.; Shalan, A.E.; Lanceros-Mendez, S.; Zafar, M.N. A review on 2D-ZnO nanostructure based biosensors: From materials to devices. Mater. Adv. 2023, 4, 320–354. [Google Scholar] [CrossRef]
- Rodrigues, J.; Pereira, S.O.; Zanoni, J.; Rodrigues, C.; Brás, M.; Costa, F.M.; Monteiro, T. ZnO Transducers for Photoluminescence-Based Biosensors: A Review. Chemosensors 2022, 10, 39. [Google Scholar] [CrossRef]
- Tripathy, N.; Kim, D.-H. Metal oxide modified ZnO nanomaterials for biosensor applications. Nano Converg. 2018, 5, 27. [Google Scholar] [CrossRef]
- Paltusheva, Z.U.; Ashikbayeva, Z.; Tosi, D.; Gritsenko, L.V. Highly Sensitive Zinc Oxide Fiber-Optic Biosensor for the Detection of CD44 Protein. Biosensors 2022, 12, 1015. [Google Scholar] [CrossRef]
- Bakranova, D.; Seitov, B.; Bakranov, N. Photocatalytic and Glucose Sensing Properties of ZnO-Based Nanocoating. Chemengineering 2023, 7, 22. [Google Scholar] [CrossRef]
- Rekha, S.M.; Neelamana, H.V.; Bhat, S.V. Recent Advances in Solution-Processed Zinc Oxide Thin Films for Ultraviolet Photodetectors. ACS Appl. Electron. Mater. 2023, 5, 4051–4066. [Google Scholar] [CrossRef]
- Boruah, B.D. Zinc oxide ultraviolet photodetectors: Rapid progress from conventional to self-powered photodetectors. Nanoscale Adv. 2019, 1, 2059–2085. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.; Sakurai, M.; Aono, M. ZnO-Based Ultraviolet Photodetectors. Sensors 2010, 10, 8604–8634. [Google Scholar] [CrossRef] [PubMed]
- Nandi, S.; Kumar, S.; Misra, A. Zinc oxide heterostructures: Advances in devices from self-powered photodetectors to self-charging supercapacitors. Mater. Adv. 2021, 2, 6768–6799. [Google Scholar] [CrossRef]
- Shang, S.; Dong, Y.; Zhang, W.; Ren, W. Fabrication and Performance of UV Photodetector of ZnO Nanorods Decorated with Al Nanoparticles. Nanomaterials 2022, 12, 3768. [Google Scholar] [CrossRef] [PubMed]
- Young, S.-J.; Liu, Y.-H.; Shiblee, M.D.N.I.; Ahmed, K.; Lai, L.-T.; Nagahara, L.; Thundat, T.; Yoshida, T.; Arya, S.; Furukawa, H.; et al. Flexible Ultraviolet Photodetectors Based on One-Dimensional Gallium-Doped Zinc Oxide Nanostructures. ACS Appl. Electron. Mater. 2020, 2, 3522–3529. [Google Scholar] [CrossRef]
- Wibowo, A.; Marsudi, M.A.; Amal, M.I.; Ananda, M.B.; Stephanie, R.; Ardy, H.; Diguna, L.J. ZnO nanostructured materials for emerging solar cell applications. RSC Adv. 2020, 10, 42838–42859. [Google Scholar] [CrossRef] [PubMed]
- Consonni, V.; Briscoe, J.; Kärber, E.; Li, X.; Cossuet, T. ZnO nanowires for solar cells: A comprehensive review. Nanotechnology 2019, 30, 362001. [Google Scholar] [CrossRef]
- Peksu, E.; Coskun, A.; Karaagac, H. Recent progress in solar cells based on one dimensional ZnO nanostructures. Nanotechnology 2023, 34, 352003. [Google Scholar] [CrossRef]
- Wang, L.-H.; Ren, L.-L.; Qin, Y.-F. The Review of Hybridization of Transition Metal-Based Chalcogenides for Lithium-Ion Battery Anodes. Materials 2023, 16, 4448. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves, J.M.; da Silva, M.I.; Silva, M.N.T.; Martins, P.R.; Nossol, E.; Toma, H.E.; Angnes, L. Recent progress in ZnCo2O4 and its composites for energy storage and conversion: A review. Energy Adv. 2022, 1, 793–841. [Google Scholar] [CrossRef]
- Najib, S.; Erdem, E. Current progress achieved in novel materials for supercapacitor electrodes: Mini review. Nanoscale Adv. 2019, 1, 2817–2827. [Google Scholar] [CrossRef] [PubMed]
- Forouzandeh, P.; Kumaravel, V.; Pillai, S.C. Electrode Materials for Supercapacitors: A Review of Recent Advances. Catalysts 2020, 10, 969. [Google Scholar] [CrossRef]
- Nandi, D.; Mohan, V.B.; Bhowmick, A.K.; Bhattacharyya, D. Metal/metal oxide decorated graphene synthesis and application as supercapacitor: A review. J. Mater. Sci. 2020, 55, 6375–6400. [Google Scholar] [CrossRef]
- Yan, Z.; Sun, Z.; Yue, K.; Li, A.; Qian, L. CoO/ZnO nanoclusters immobilized on N-doped 3 D reduced graphene oxide for enhancing lithium storage capacity. J. Alloy. Compd. 2020, 836, 155443. [Google Scholar] [CrossRef]
- Abebe, E.M.; Ujihara, M. Influence of Temperature on ZnO/Co3O4 Nanocomposites for High Energy Storage Supercapacitors. ACS Omega 2021, 6, 23750–23763. [Google Scholar] [CrossRef] [PubMed]
- Angelin, M.D.; Rajkumar, S.; Ravichandran, A.; Merlin, J.P. Systematic investigation on the electrochemical performance of Cd-doped ZnO as electrode material for energy storage devices. J. Phys. Chem. Solids 2021, 161, 110486. [Google Scholar] [CrossRef]
- Zhou, H.; Fu, W.; Muhammad, M.; Xie, M.; Xie, E.; Han, W.-H. Self-assembled microspheres composed of porous ZnO/CoO nanosheets for aqueous hybrid supercapacitors. J. Phys. D Appl. Phys. 2019, 52, 505501. [Google Scholar] [CrossRef]
- Alver, U.; Tanrıverdi, A. Boron doped ZnO embedded into reduced graphene oxide for electrochemical supercapacitors. Appl. Surf. Sci. 2016, 378, 368–374. [Google Scholar] [CrossRef]
- Shaheen, I.; Ahmad, K.S.; Zequine, C.; Gupta, R.K.; Thomas, A.G.; Malik, M.A. Sustainable synthesis of organic framework-derived ZnO nanoparticles for fabrication of supercapacitor electrode. Environ. Technol. 2022, 43, 605–616. [Google Scholar] [CrossRef]
- Najib, S.; Bakan, F.; Abdullayeva, N.; Bahariqushchi, R.; Kasap, S.; Franzò, G.; Sankir, M.; Sankir, N.D.; Mirabella, S.; Erdem, E. Tailoring morphology to control defect structures in ZnO electrodes for high-performance supercapacitor devices. Nanoscale 2020, 12, 16162–16172. [Google Scholar] [CrossRef]
- Pradeeswari, K.; Venkatesan, A.; Pandi, P.; Karthik, K.; Krishna, K.V.H.; Kumar, R.M. Study on the electrochemical performance of ZnO nanoparticles synthesized via non-aqueous sol-gel route for supercapacitor applications. Mater. Res. Express 2019, 6, 105525. [Google Scholar] [CrossRef]
- Reddy, I.N.; Reddy, C.V.; Sreedhar, A.; Shim, J.; Cho, M.; Yoo, K.; Kim, D. Structural, optical, and bifunctional applications: Supercapacitor and photoelectrochemical water splitting of Ni-doped ZnO nanostructures. J. Electroanal. Chem. 2018, 828, 124–136. [Google Scholar] [CrossRef]
- Erdemir, F.; Tuzcu, E.; Bilgin, S.; Alver, Ü.; Çanakçı, A. Influence of fluorine doping of zinc oxide on its electrochemical performance in supercapacitors. Mater. Chem. Phys. 2021, 259, 124033. [Google Scholar] [CrossRef]
- Ali, A.; Ammar, M.; Ali, M.; Yahya, Z.; Javaid, M.Y.; Hassan, S.U.; Ahmed, T. Mo-doped ZnO nanoflakes on Ni-foam for asymmetric supercapacitor applications. RSC Adv. 2019, 9, 27432–27438. [Google Scholar] [CrossRef]
- Pallavolu, M.R.; Nallapureddy, J.; Nallapureddy, R.R.; Neelima, G.; Yedluri, A.K.; Mandal, T.K.; Pejjai, B.; Joo, S.W. Self-assembled and highly faceted growth of Mo and V doped ZnO nanoflowers for high-performance supercapacitors. J. Alloy. Compd. 2021, 886, 161234. [Google Scholar] [CrossRef]
- Huang, Y.-Y.; Lin, L.-Y.; Li, X. Efficient battery supercapacitor hybrid devices with quaternary metal oxide electrodes based on nickel and cobalt. J. Energy Storage 2019, 25, 100826. [Google Scholar] [CrossRef]
- Radhamani, A.V.; Shareef, K.M.; Rao, M.S.R. ZnO@MnO2 Core–Shell Nanofiber Cathodes for High Performance Asymmetric Supercapacitors. ACS Appl. Mater. Interfaces 2016, 8, 30531–30542. [Google Scholar] [CrossRef]
- Huang, M.; Li, F.; Zhao, X.L.; Luo, D.; You, X.Q.; Zhang, Y.X.; Li, G. Hierarchical ZnO@MnO2 Core-Shell Pillar Arrays on Ni Foam for Binder-Free Supercapacitor Electrodes. Electrochimica Acta 2015, 152, 172–177. [Google Scholar] [CrossRef]
- Tajik, S.; Dubal, D.P.; Gomez-Romero, P.; Yadegari, A.; Rashidi, A.; Nasernejad, B.; Inamuddin; Asiri, A.M. Nanostructured mixed transition metal oxides for high performance asymmetric supercapacitors: Facile synthetic strategy. Int. J. Hydrogen Energy 2017, 42, 12384–12395. [Google Scholar] [CrossRef]
- Dutta, A.; Chatterjee, K.; Mishra, S.; Saha, S.K.; Akhtar, A.J. An insight into the electrochemical performance of cobalt-doped ZnO quantum dot for supercapacitor applications. J. Mater. Res. 2022, 37, 3955–3964. [Google Scholar] [CrossRef]
- He, Y.; Xie, L.; Ding, S.; Long, Y.; Zhou, X.; Hu, Q.; Lin, D. Core–shell nanostructured Zn–Co–O@CoS arrays for high-performance hybrid supercapacitors. Dalton Trans. 2021, 50, 4923–4931. [Google Scholar] [CrossRef]
- He, Y.; Zhou, X.; Ding, S.; Hu, Q.; Lin, D.; Wei, X. ZnO/CoO@NiCoS nanohybrids with double heterogeneous interface for high-performance hybrid supercapacitors. J. Alloy. Compd. 2021, 875, 160046. [Google Scholar] [CrossRef]
- Kumar, S.; Ahmed, F.; Shaalan, N.M.; Arshi, N.; Dalela, S.; Chae, K.H. Influence of Fe Doping on the Electrochemical Performance of a ZnO-Nanostructure-Based Electrode for Supercapacitors. Nanomaterials 2023, 13, 2222. [Google Scholar] [CrossRef] [PubMed]
- Zenasni, M.; Belhadj, H.; Kiari, M.; Alelyani, M.; Alhailiy, A.B.; Benyoucef, A.; Bakkour, Y. Synthesis, characterization, and enhanced electrochemical behavior of polypyrrole doped ZrO2–ZnO electrode materials for supercapacitor applications. Front. Energy Res. 2023, 11, 1244699. [Google Scholar] [CrossRef]
- Ammar, A.U.; Bakan-Misirlioglu, F.; Aleinawi, M.H.; Franzo, G.; Condorelli, G.G.; Yesilbag, F.N.T.; Yesilbag, Y.O.; Mirabella, S.; Erdem, E. All-in-one supercapacitors with high performance enabled by Mn/Cu doped ZnO and MXene. Mater. Res. Bull. 2023, 165, 112334. [Google Scholar] [CrossRef]
- Altaf, C.T.; Colak, T.O.; Rostas, A.M.; Mihet, M.; Lazar, M.D.; Iatsunskyi, I.; Coy, E.; Yildirim, I.D.; Misirlioglu, F.B.; Erdem, E.; et al. GO/ZnO-based all-solid-state photo-supercapacitors: Effect of GO:ZnO ratio on composite properties and device performance. J. Energy Storage 2023, 68, 107694. [Google Scholar] [CrossRef]
- Chauhan, P.S.; Kumar, S.; Mondal, A.; Sharma, P.; Parekh, M.N.; Panwar, V.; Rao, A.M.; Misra, A. Stacked vanadium pentoxide–zinc oxide interface for optically-chargeable supercapacitors. J. Mater. Chem. A 2023, 11, 95–107. [Google Scholar] [CrossRef]
- Mubeen, K.; Shah, M.Z.U.; Sajjad, M.; Irshad, A.; Ali, Z.; Zafar, Z.; Shah, A. Boosting the electrochemical performance of ZnO nanomaterials through a conductive CuS matrix for aqueous supercapacitors. New J. Chem. 2023, 47, 7819–7829. [Google Scholar] [CrossRef]
- Shaheen, I.; Hussain, I.; Zahra, T.; Memon, R.; Alothman, A.A.; Ouladsmane, M.; Qureshi, A.; Niazi, J.H. Electrophoretic Fabrication of ZnO/CuO and ZnO/CuO/rGO Heterostructures-based Thin Films as Environmental Benign Flexible Electrode for Supercapacitor. Chemosphere 2023, 322, 138149. [Google Scholar] [CrossRef] [PubMed]
- Ali, A.; Hameed, I.; Hussain, I.; Mujahid, R.; Ahmad, R.T.M.; Yahya, Z.; Waqas, M.; Ammar, M. High performance asymmetric supercapacitor based on hydrothermally synthesized ZnO nanosheets embedded on Ni foam. J. Mater. Sci. Mater. Electron. 2023, 34, 744. [Google Scholar] [CrossRef]
- Pern, F.J.; To, B.; Glick, S.H.; Sundaramoorthy, R.; DeHart, C.; Glynn, S.; Perkins, C.; Mansfield, L.; Gessert, T. Variations in damp heat-induced degradation behavior of sputtered ZnO window layer for CIGS solar cells. In Proceedings of the Reliability of Photovoltaic Cells, Modules, Components, and Systems III, San Diego, CA, USA, 1–5 August 2010; Available online: https://www.spiedigitallibrary.org/conference-proceedings-of-spie/7773/1/Variations-in-damp-heat-induced-degradation-behavior-of-sputtered-ZnO/10.1117/12.863078.short (accessed on 15 November 2023).
- Chen, A.-L.; Xu, D.; Chen, X.-Y.; Zhang, W.-Y.; Liu, X.-H. Measurements of zinc oxide solubility in sodium hydroxide solution from 25 to 100 °C. Trans. Nonferrous Met. Soc. China 2012, 22, 1513–1516. [Google Scholar] [CrossRef]
- Wang, X.; Xu, J.; Yu, X.; Xue, K.; Yu, J.; Zhao, X. Structural evidence of secondary phase segregation from the Raman vibrational modes in Zn1 xCoxO (0 < x < 0.6). Appl. Phys. Lett. 2007, 91, 031908. [Google Scholar] [CrossRef]
- Pourbaix, M. Atlas of Electrochemical Equilibria in Aqueous Solutions, 2nd ed.; NACE International: Brussels, Belgium, 1974. [Google Scholar]
- Peek, E.; Åkre, T.; Asselin, E. Technical and business considerations of cobalt hydrometallurgy. JOM 2009, 61, 43–53. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abdullin, K.; Gabdullin, M.; Kalkozova, Z.; Kudryashov, V.; Mirzaeian, M.; Yelemessov, K.; Baskanbayeva, D.; Serikkanov, A. Enhancing the Electrochemical Performance of ZnO-Co3O4 and Zn-Co-O Supercapacitor Electrodes Due to the In Situ Electrochemical Etching Process and the Formation of Co3O4 Nanoparticles. Energies 2024, 17, 1888. https://doi.org/10.3390/en17081888
Abdullin K, Gabdullin M, Kalkozova Z, Kudryashov V, Mirzaeian M, Yelemessov K, Baskanbayeva D, Serikkanov A. Enhancing the Electrochemical Performance of ZnO-Co3O4 and Zn-Co-O Supercapacitor Electrodes Due to the In Situ Electrochemical Etching Process and the Formation of Co3O4 Nanoparticles. Energies. 2024; 17(8):1888. https://doi.org/10.3390/en17081888
Chicago/Turabian StyleAbdullin, Khabibulla, Maratbek Gabdullin, Zhanar Kalkozova, Vladislav Kudryashov, Mojtaba Mirzaeian, Kassym Yelemessov, Dinara Baskanbayeva, and Abay Serikkanov. 2024. "Enhancing the Electrochemical Performance of ZnO-Co3O4 and Zn-Co-O Supercapacitor Electrodes Due to the In Situ Electrochemical Etching Process and the Formation of Co3O4 Nanoparticles" Energies 17, no. 8: 1888. https://doi.org/10.3390/en17081888
APA StyleAbdullin, K., Gabdullin, M., Kalkozova, Z., Kudryashov, V., Mirzaeian, M., Yelemessov, K., Baskanbayeva, D., & Serikkanov, A. (2024). Enhancing the Electrochemical Performance of ZnO-Co3O4 and Zn-Co-O Supercapacitor Electrodes Due to the In Situ Electrochemical Etching Process and the Formation of Co3O4 Nanoparticles. Energies, 17(8), 1888. https://doi.org/10.3390/en17081888







