Muti-Filler Composites Reinforced with Multiwalled Carbon Nanotubes and Chopped Carbon Fibers for the Bipolar Plate of Fuel Cells
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Sample Preparation
2.3. Characterizations
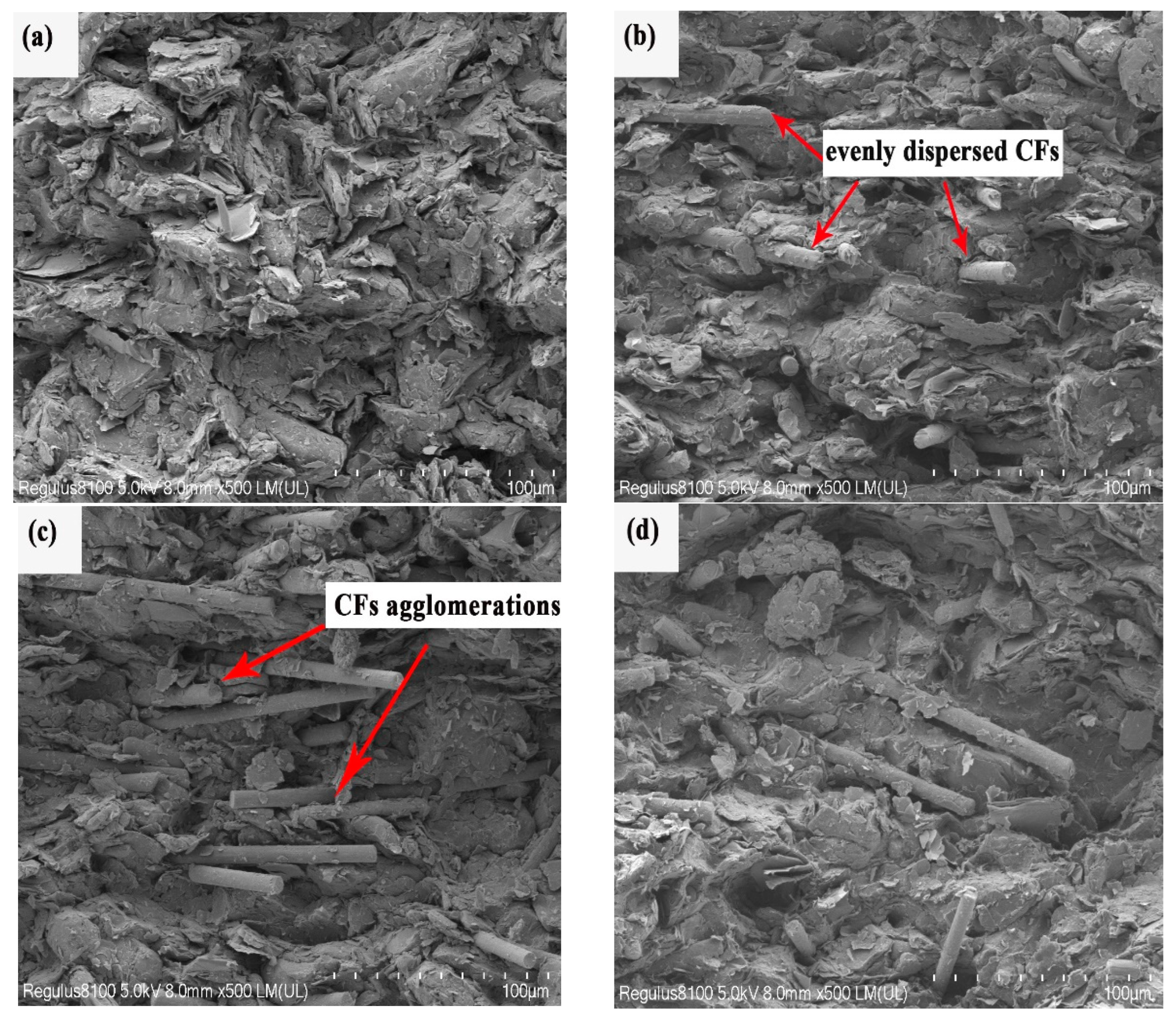
3. Results and Discussion
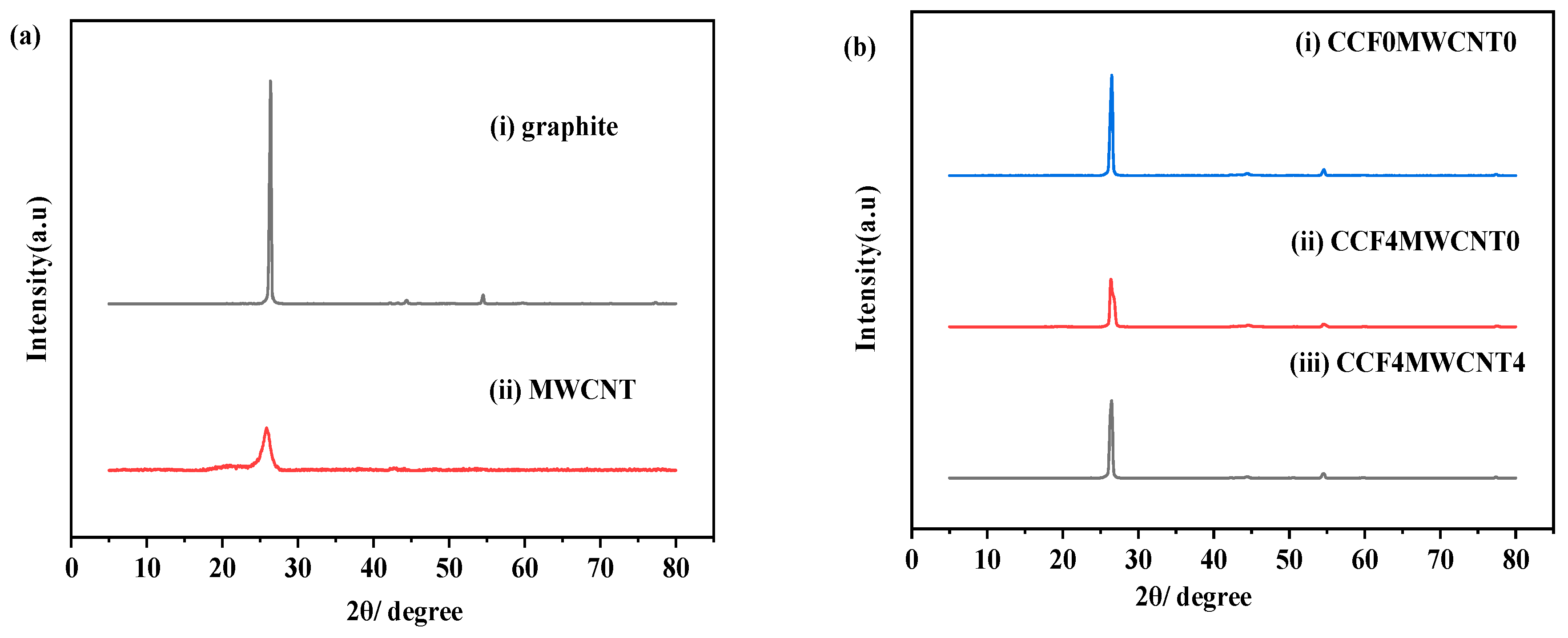
3.1. XRD Analysis
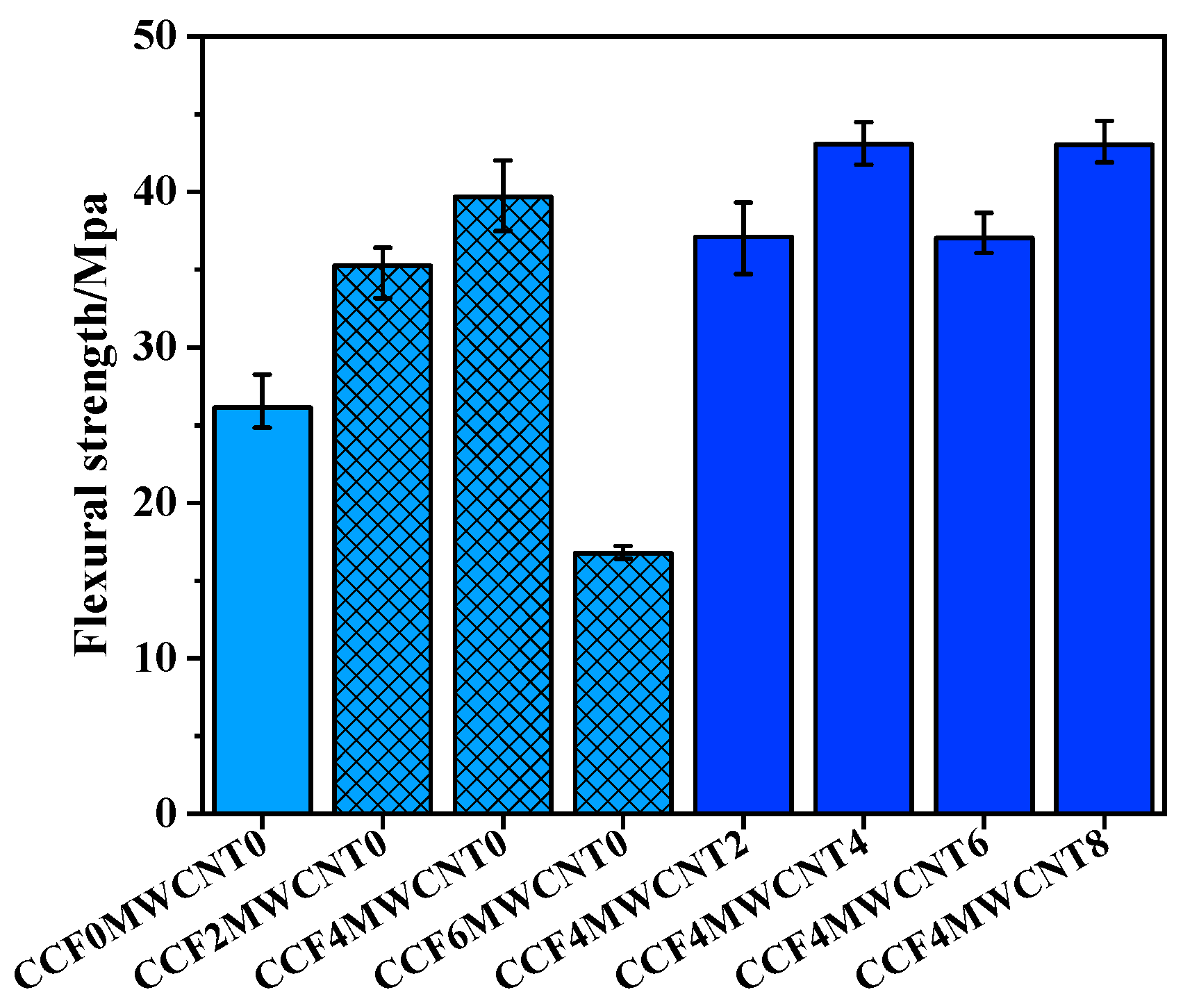
3.2. Flexural Strength of Composites
3.3. Electrical Conductivity of Composites
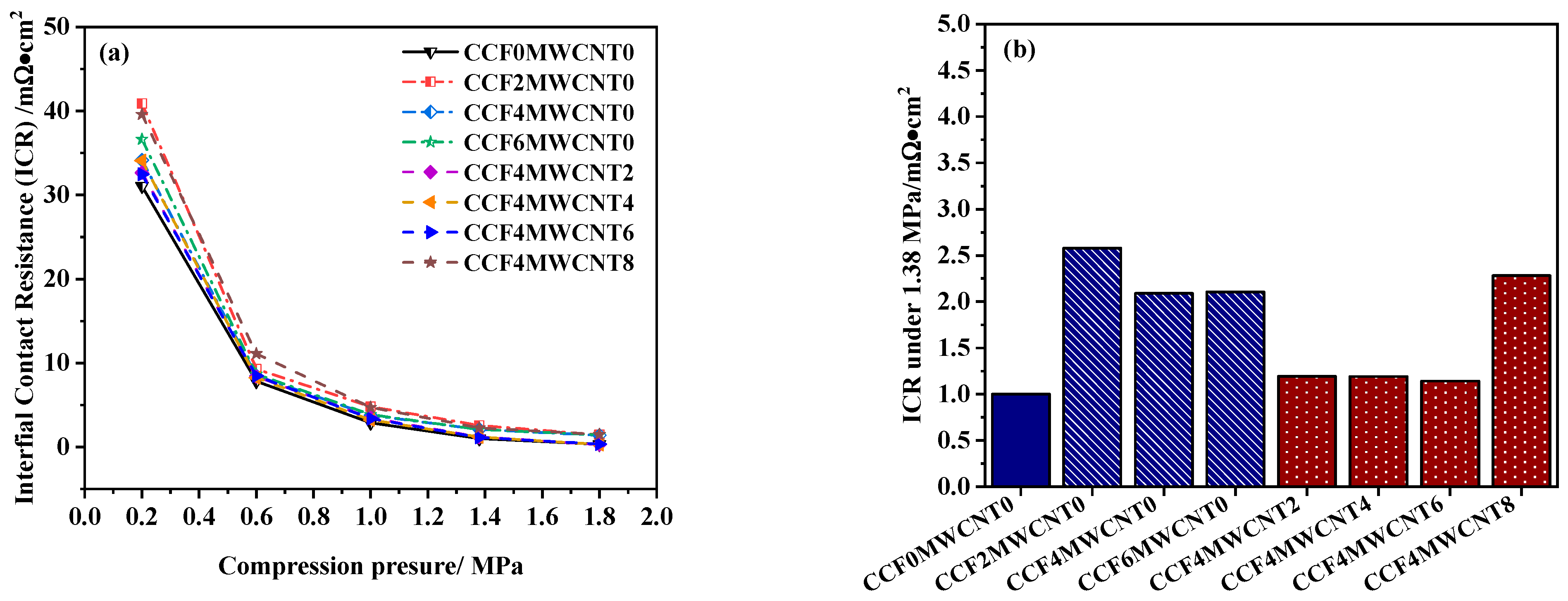
3.4. ICR of Composites
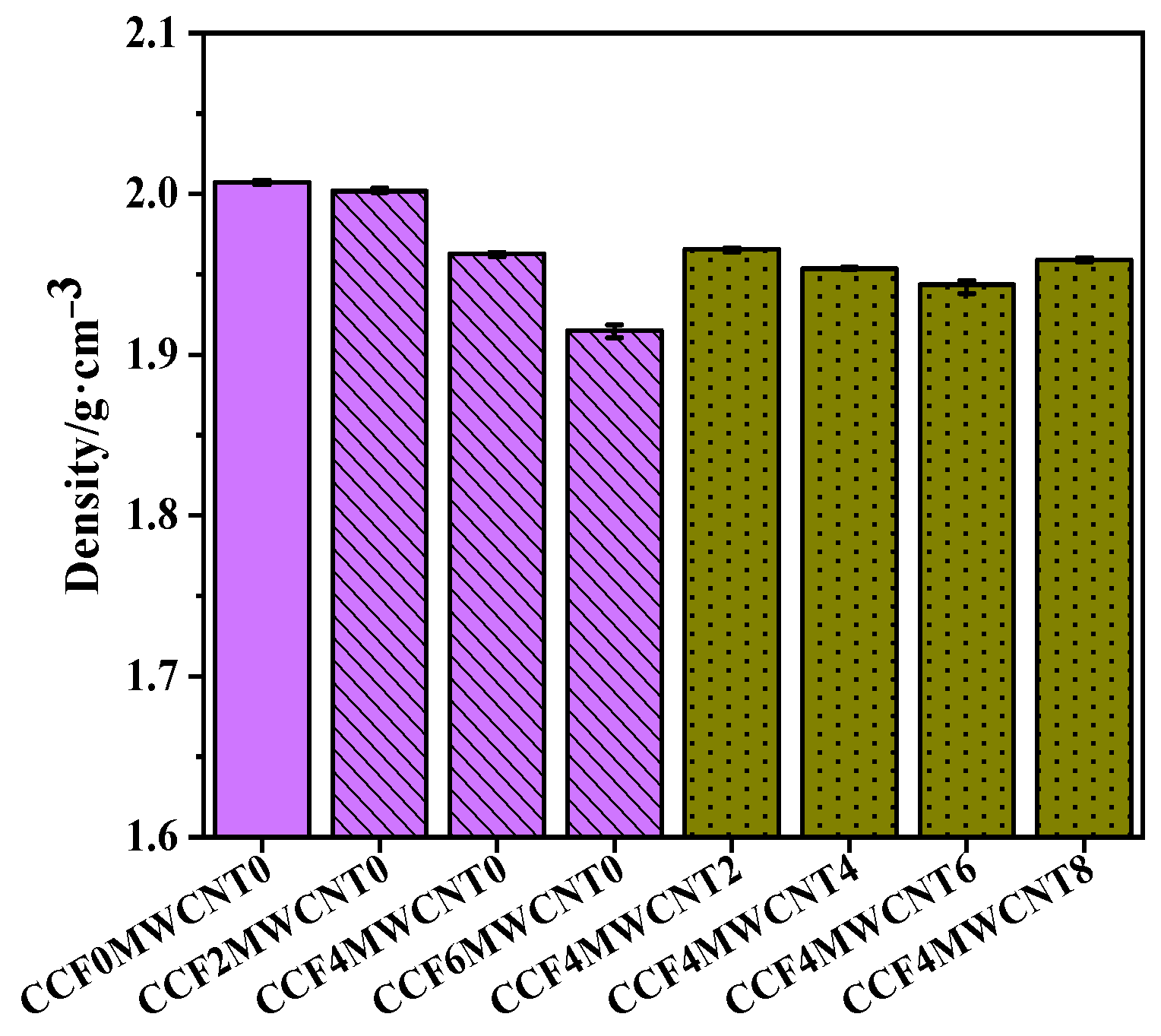
3.5. Density of Composites
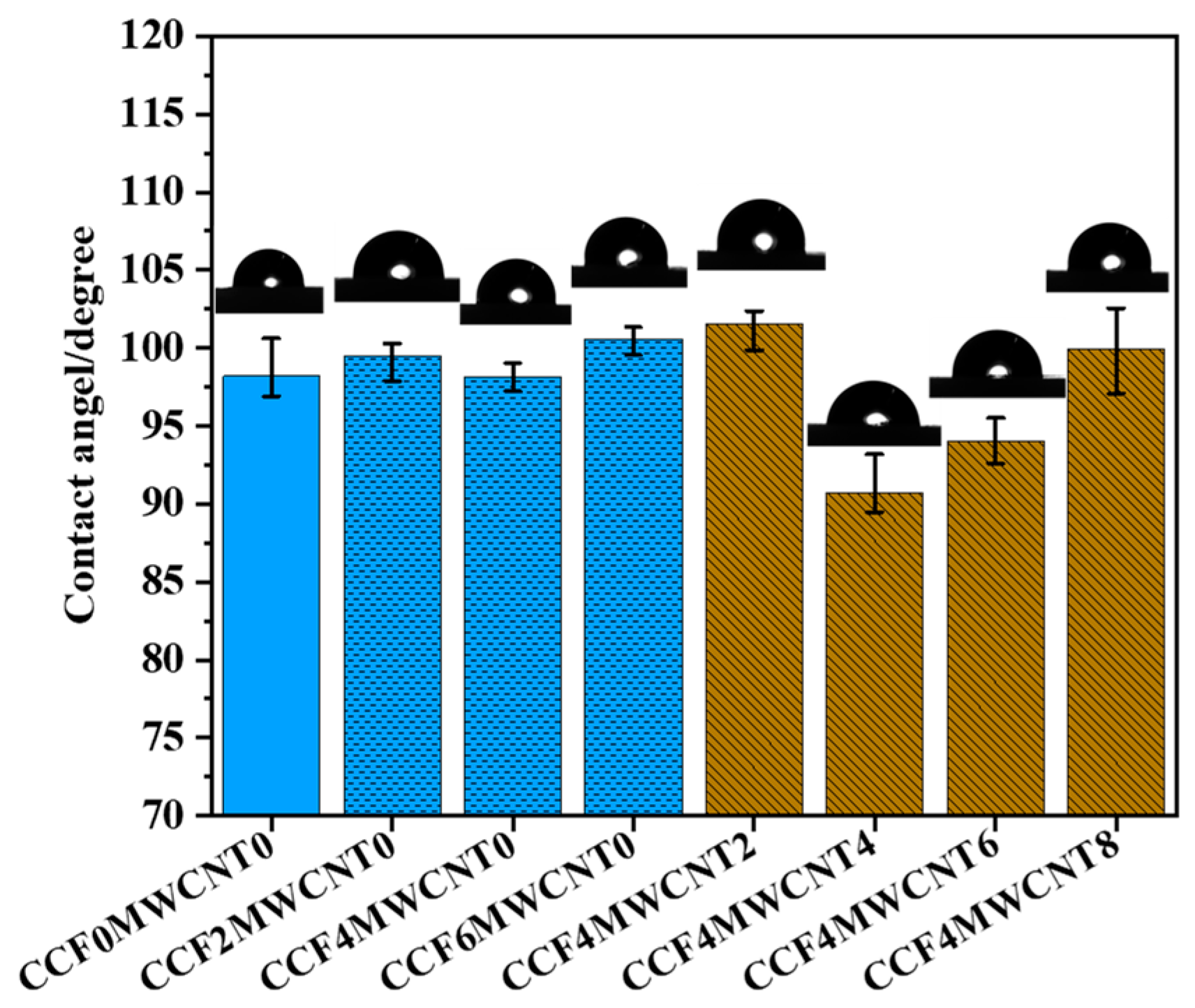
3.6. Hydrophobicity Behavior of Composites
3.7. Corrosion Behavior
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Lee, D.; Choe, J.; Nam, S.; Lim, J.W.; Choi, I.; Lee, D.G. Development of non-woven carbon felt composite bipolar plates using the soft layer method. Compos. Struct. 2017, 160, 976–982. [Google Scholar] [CrossRef]
- Sepehr, S.; Majid, K.; Mohammad, M. Fabrication of multi-filler thermoset-based composite bipolar plates for PEMFCs applications: Molding defects and properties characterizations. Int. J. Hydrogen Energy 2020, 45, 14119–14132. [Google Scholar]
- Roßberg, K.; Trapp, V. Graphite-Based Bipolar Plates; SGL Technologies GmbH: Meitingen, Germany, 2010; p. 2. [Google Scholar]
- Li, Y.; Jia, X.; Zhang, W.; Fang, C.; Wang, X.; Qin, F.; Yamaura, S.; Yokoyama, Y. Effects of Alloying Elements on the Thermal Stability and Corrosion Resistance of an Fe-based Metallic Glass with Low Glass Transition Temperature. Metall. Mater. Trans. A 2013, 45, 2393. [Google Scholar] [CrossRef]
- Jeong, K.I.; Oh, J.; Song, S.A.; Lee, D.; Kim, S.S. A review of composite bipolar plates in proton exchange membrane fuel cells: Electrical properties and gas permeability. Compos. Struct. 2021, 262, 113617. [Google Scholar] [CrossRef]
- Planes, E.; Flandin, L.; Alberola, N. Polymer composites bipolar plates for PEMFCs. Energy Procedia 2012, 20, 311–323. [Google Scholar] [CrossRef]
- Shu, H.L.; Chuan, Y.Y.; Cheng, C.W.; Yu, F.L.; Chen, C.M.M.; Ching, H.Y.; Ming, C.T.; Ming, Y.Y.; Min, C.H.; Shuo, J.L.; et al. Preparation and properties of carbon nanotube/polypropylene nanocomposite bipolar plates for polymer electrolyte membrane fuel cells. J. Power Sources 2008, 185, 1225–1232. [Google Scholar]
- Shu, H.L.; Cheng, C.W.; Chuan, Y.Y.; Min, C.H.; Chen, C.M.M.; Ming, C.T.; Ay, S.; Ming, Y.Y.; Yu, F.L.; Po, L.L. Preparation and properties of functionalized multiwalled carbon nanotubes/polypropylene nanocomposite bipolar plates for polymer electrolyte membrane fuel cells. J. Power Sources 2010, 195, 263–270. [Google Scholar]
- Min, C.H.; Shu, H.L.; Yu, F.L.; Cheng, C.W.; Han, M.T.; Chen, C.M.M.; Shie, H.L.; Ming, Y.Y.; Po, L.L. Polypropylene-grafted multiwalled carbon nanotube reinforced polypropylene composite bipolar plates in polymer electrolyte membrane fuel cells. Energy Environ. Sci. 2011, 4, 543. [Google Scholar]
- Ha, E.L.; Song, H.H.; Seung, A.S.; Seong, S.K. Novel fabrication process for carbon fiber composite bipolar plates using sol gel and the double percolation effect for PEMFC. Compos. Struct. 2015, 134, 44–51. [Google Scholar]
- Ha, E.L.; Yong, S.C.; Seong, S.K. Feasibility study on carbon-felt-reinforced thermoplastic composite materials for PEMFC bipolar plates. Compos. Struct. 2017, 180, 378–385. [Google Scholar]
- Pattarakamon, C.; Jantrawan, P. Wet vs. dry dispersion methods for multiwall carbon nanotubes in the high graphite content phenolic resin composites for use as bipolar plate application. Electrochim. Acta 2015, 158, 1–6. [Google Scholar]
- Hu, B.; Chang, F.L.; Xiang, L.Y.; He, G.J.; Cao, X.W.; Yin, X.C. High performance polyvinylidene fluoride/graphite/multiwalled carbon nanotubes composite bipolar plate for PEMFC with segregated conductive networks. Int. J. Hydrogen Energy 2021, 46, 25666–25676. [Google Scholar] [CrossRef]
- Yang, B.L.; Choong, H.L.; Kyung, M.K.; Dae, S.L. Preparation and properties on the graphite/polypropylene composite bipolar plates with a 304 stainless steel by compression molding for PEM fuel cell. Int. J. Hydrogen Energy 2011, 36, 7621–7627. [Google Scholar]
- Kang, Y.; Daniel, A.; Ayou, H.; Jim, P.Z.; Zhiyong, L.; Nam, N. Highly Conductive and Strong Graphite-Phenolic Resin Composite for Bipolar Plate Applications. Energy Fuels 2017, 31, 14320–14331. [Google Scholar]
- Kyungmun, K.; Sunghyun, P.; Ahrae, J.; Kise, L.; Hyunchul, J. Development of ultralight and thin bipolar plates using epoxy-carbon fiber prepregs and graphite composites. Int. J. Hydrogen Energy 2017, 42, 1691–1697. [Google Scholar]
- Kakati, B.K.; Sathiyamoorthy, D.; Verma, A. Electrochemical and mechanical behavior of carbon composite bipolar plate for fuel cell. Int. J. Hydrogen Energy 2010, 35, 4185–4194. [Google Scholar] [CrossRef]
- Biraj, K.K.; Avijit, G.; Anil, V. Efficient composite bipolar plate reinforced with carbon fiber and graphene for proton exchange membrane fuel cell. Int. J. Hydrogen Energy 2013, 38, 9362–9369. [Google Scholar]
- Joong, H.L.; Yun, K.J.; Chang, E.H.; Nam, H.K.; Peng, L.; Hong, K.L. Effect of carbon fillers on properties of polymer composite bipolar plates of fuel cells. J. Power Sources 2009, 193, 523–529. [Google Scholar]
- Bo, L.; Zhigang, S.; Liang, H.; Yong, G.; Shucheng, S. A novel graphite/phenolic resin bipolar plate modified by doping carbon fibers for the application of proton exchange membrane fuel cells. Prog. Nat. Sci. Mater. Int. 2020, 30, 876. [Google Scholar]
- Ali, A.; Morteza, S.; Mahmood, M.; Hadi, N.P. High performance polymeric bipolar plate based on polypropylene/graphite/graphene/nano-carbon black composites for PEM fuel cells. Renew. Energy 2016, 99, 867–874. [Google Scholar]
- Runlin, F.; Junsheng, Z.; Yuhang, P.; Jing, C.; Zize, Z.; Dongmei, Y.; Cunman, Z.; Pingwen, M. The conductive network optimization of composite graphite plates and its orphological analysis. Chem. Eng. J. 2022, 446, 136652. [Google Scholar]
- Shu, H.L.; Chih, H.H.; Chen, C.M.M.; Chuan, Y.Y.; Yu, F.L.; Cheng, C.W. Preparation and properties of carbon nanotube-reinforced vinyl ester/nanocomposite bipolar plates for polymer electrolyte membrane fuel cells. J. Power Sources 2008, 176, 175–182. [Google Scholar]
- Radzuan, N.M.; Sulong, A.B.; Somalu, M.R.; Abdullah, A.T.; Husaini, T.; Rosli, R.E.; Majlan, E.H.; Rosli, M.I. Fibre orientation effect on polypropylene/milled carbon fiber composites in the presence of carbon nanotubes or graphene as a secondary filler: Application on PEM fuel cell bipolar plate. Int. J. Hydrogen Energy 2019, 44, 30618–30626. [Google Scholar] [CrossRef]
- Dongjie, Z.; Haibin, Z.; Zhiyong, G.; Yu, G.; Qing, L.; Guilong, W.; Guoqun, Z. Surface treatment of multiwalled carbon nanotubes and the formation of the multiscale conductivity network in long carbon fiber reinforced polypropylene. Polym. Compos. 2022, 43, 4645–4659. [Google Scholar]
- Ramírez-Herrera, C.A.; Tellez-Cruz, M.M.; Pérez-González, J.; Solorza-Feria, O.; Flores-Vela, A.; Cabañas-Moreno, J.G. Enhanced mechanical properties and corrosion behavior of polypropylene/multiwalled carbon nanotubes/carbon nanofibers nanocomposites for application in bipolar plates of proton exchange membrane fuel cells. Int. J. Hydrogen Energy 2021, 46, 26110–26125. [Google Scholar] [CrossRef]
- Fatih, D.; Alparslan, T.; Kadir, A.; Selahattin, C. Carbon nanotube (CNT) modified carbon fiber/epoxy composite plates for the PEM fuel cell bipolar plate application. Int. J. Hydrogen Energy 2023, 48, 1090–1106. [Google Scholar]
- Sirawit, W.; Manunya, O.; Chanchira, J.; Panagiotis, K.; Sarawut, R. Highly filled graphite/graphene/carbon nanotube in polybenzoxazine composites for bipolar plate in PEMFC. Int. J. Hydrogen Energy 2020, 45, 30898–30910. [Google Scholar]
- Avijit, G.; Pranab, G.; Pinakeshwar, M.; Anil, V. Effect of carbon fiber length and graphene on carbon-polymer composite bipolar plate for PEMFC. J. Solid State Electrochem. 2014, 18, 3427–3436. [Google Scholar]
- GB-T 20042.6-2011; Proton Exchange Membrane Fuel Cell—Part 6: Test Method of Bipolar Plate Properties. Standards Press of China: Beijing, China, 2011.
- GB-T13465.1-2014; Test Method of Impermeable Graphite Materials—Part 1: General of Test Method for Mechanical Properties. Standards Press of China: Beijing, China, 2014.
- Ha, N.Y.; Jun, W.L.; Jung, D.S.; Dai, G.L. A graphite-coated carbon fiber epoxy composite bipolar plate for polymer electrolyte membrane fuel cell. J. Power Sources 2011, 196, 9868–9875. [Google Scholar]
- Mathur, R.B.; Dhakate, S.R.; Gupta, D.K.; Dhami, T.L.; Aggarwal, R.K. Effect of different carbon fillers on the properties of graphite composite bipolar plate. Mater. Process. Technol. 2008, 203, 184–192. [Google Scholar] [CrossRef]
- Amit, C.B.; Raghunathan, R. Interfacial contact resistance in polymer electrolyte membrane fuel cells: Recent developments and challenges. Renew. Sustain. Energy Rev. 2019, 115, 109351. [Google Scholar]






| Sample | Materials | |||
|---|---|---|---|---|
| Graphite Content /wt.% | PF Content /wt.% | CCF Content /wt.% | MWCNT Content /wt.% | |
| CCF0MWCNT0 | 85 | 15 | 0 | 0 |
| CCF2MWCNT0 | 83 | 15 | 2 | 0 |
| CCF4MWCNT0 | 81 | 15 | 4 | 0 |
| CCF6MWCNT0 | 79 | 15 | 6 | 0 |
| CCF4MWCNT2 | 79 | 15 | 4 | 2 |
| CCF4MWCNT4 | 77 | 15 | 4 | 4 |
| CCF4MWCNT6 | 75 | 15 | 4 | 6 |
| CCF0MWCNT0 | 85 | 15 | 0 | 0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wei, H.; Chang, G.; Xu, S.; Liu, J. Muti-Filler Composites Reinforced with Multiwalled Carbon Nanotubes and Chopped Carbon Fibers for the Bipolar Plate of Fuel Cells. Energies 2024, 17, 1603. https://doi.org/10.3390/en17071603
Wei H, Chang G, Xu S, Liu J. Muti-Filler Composites Reinforced with Multiwalled Carbon Nanotubes and Chopped Carbon Fibers for the Bipolar Plate of Fuel Cells. Energies. 2024; 17(7):1603. https://doi.org/10.3390/en17071603
Chicago/Turabian StyleWei, Huili, Guofeng Chang, Sichuan Xu, and Jinling Liu. 2024. "Muti-Filler Composites Reinforced with Multiwalled Carbon Nanotubes and Chopped Carbon Fibers for the Bipolar Plate of Fuel Cells" Energies 17, no. 7: 1603. https://doi.org/10.3390/en17071603
APA StyleWei, H., Chang, G., Xu, S., & Liu, J. (2024). Muti-Filler Composites Reinforced with Multiwalled Carbon Nanotubes and Chopped Carbon Fibers for the Bipolar Plate of Fuel Cells. Energies, 17(7), 1603. https://doi.org/10.3390/en17071603







