Underground Gas Storage in Saline Aquifers: Geological Aspects
Abstract
1. Introduction
2. General Characteristics of Aquifer Storage
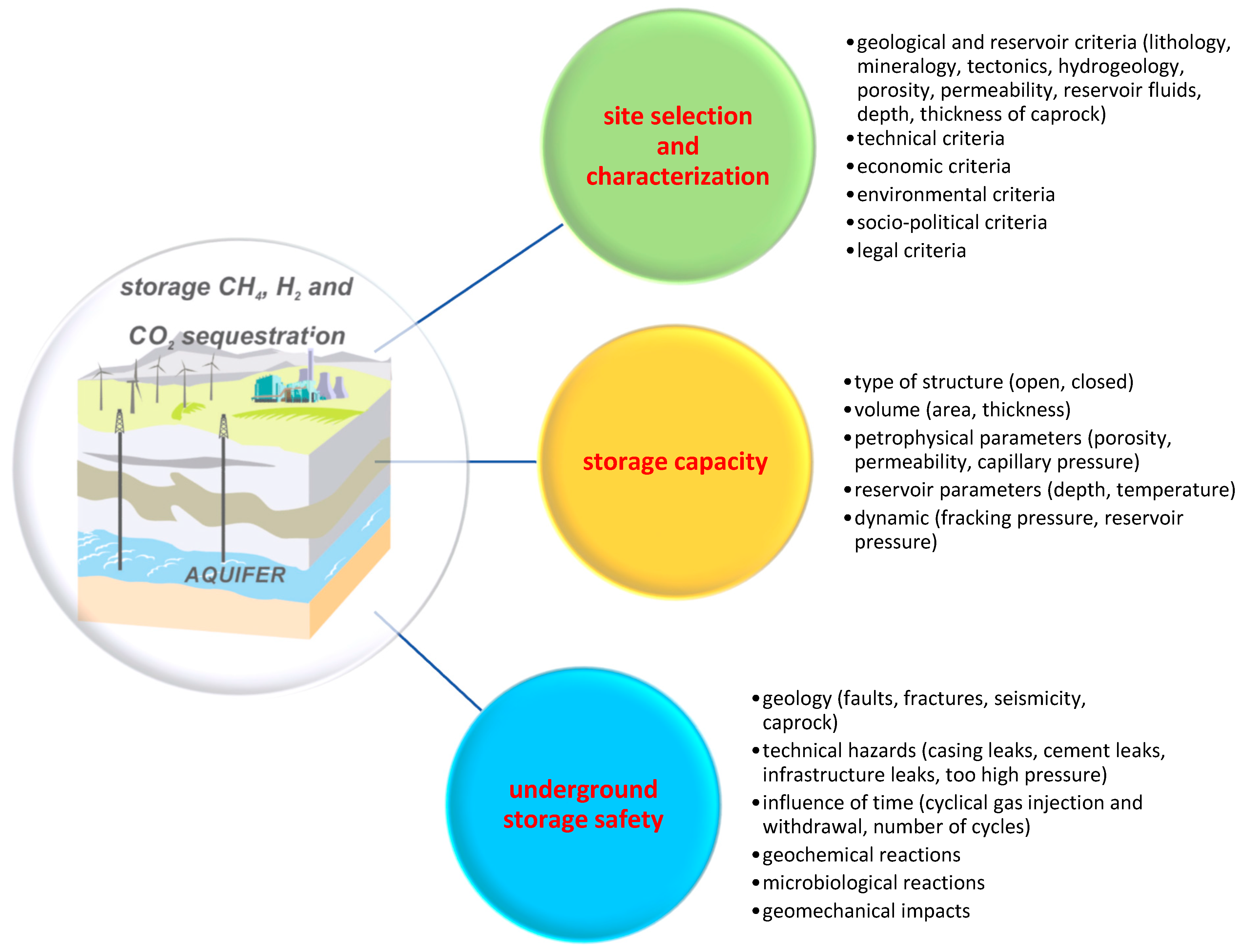
3. Selected Geological Aspects of Gas Storage in Aquifers
3.1. Selection and Characterization of Potential Gas Storage Sites
3.2. Storage Capacity
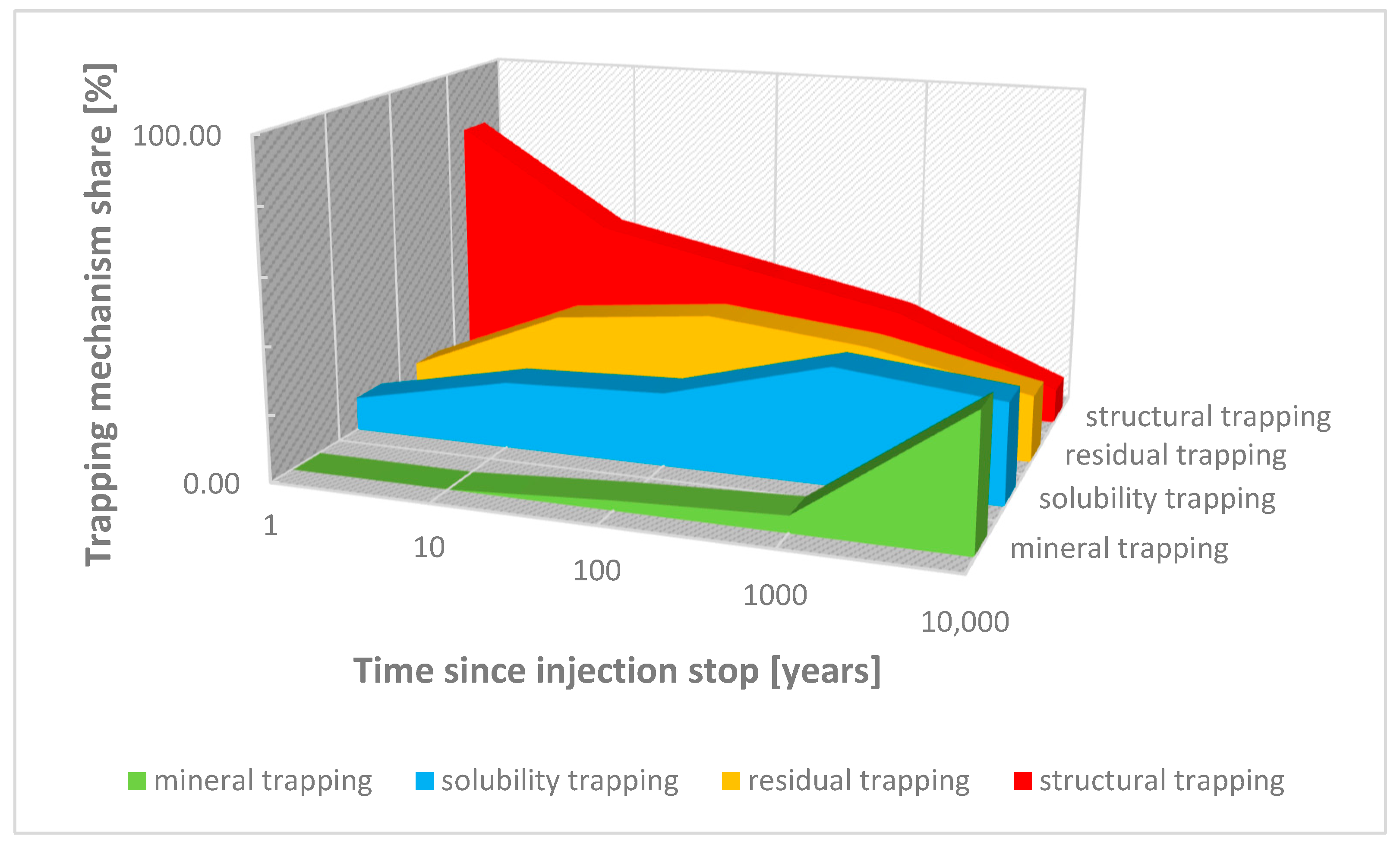
3.3. Safety of Gas Storage in Aquifers
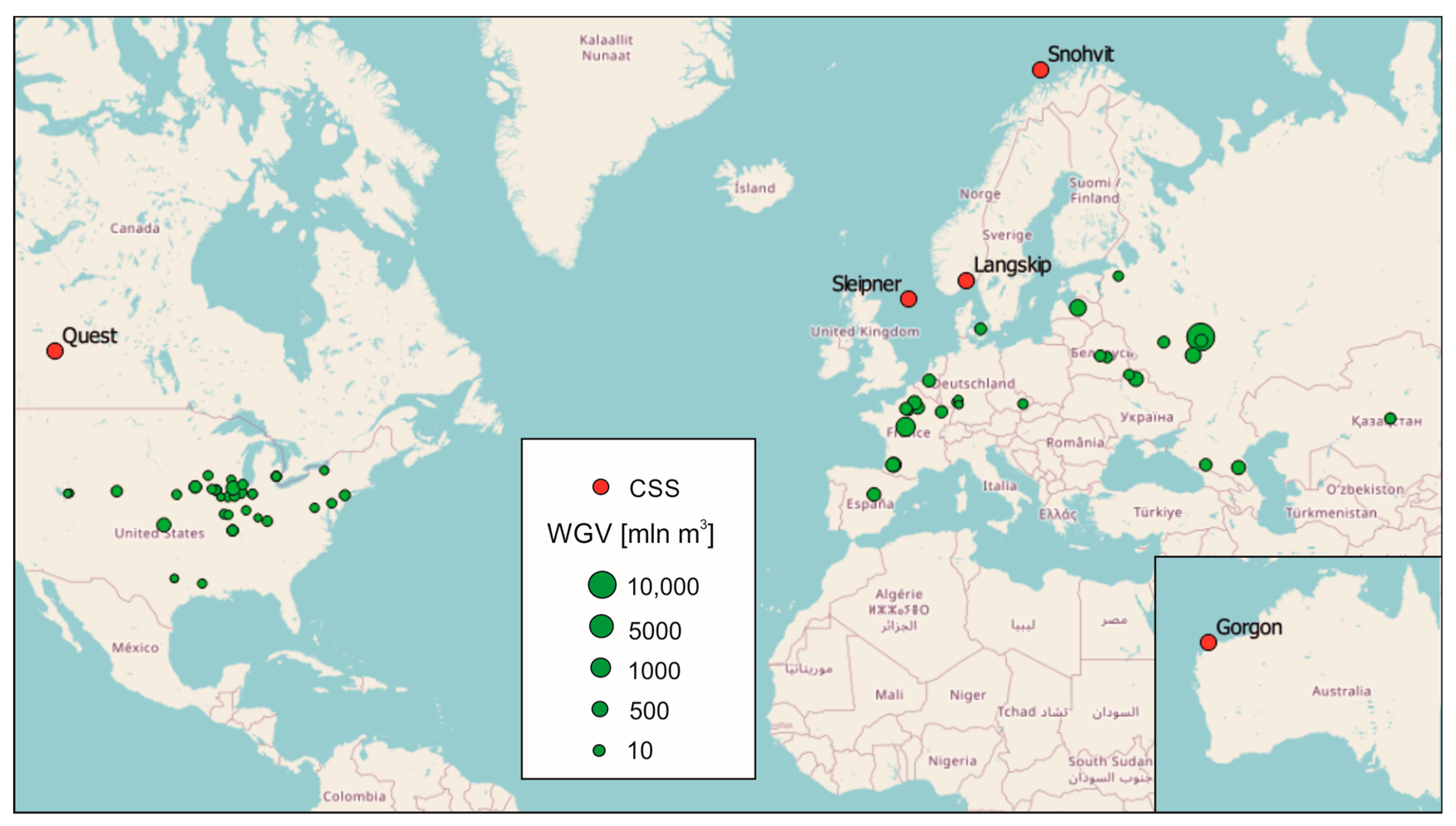
4. Natural Gas, CO2, and Hydrogen Underground Storage Facilities
5. Conclusions
- The choice of a suitable structure and its detailed characterization determine the success of the whole process of storing gases in aquifers. When selecting the best site for an underground storage facility, various factors must be considered, including geological, technical, economic, environmental, social, political, administrative, and legal aspects. Currently, there is no standardized approach for selecting locations for underground gas storage facilities. Research indicates a wide array of methods utilized globally, demonstrating diverse approaches for evaluating CO2 and hydrogen storage facility locations.
- The second very important aspect is a reliable estimate of the storage capacity, which determines the feasibility of constructing storage in a given aquifer formation. The assessment of the storage capacity should be based on the geological parameters of the rock formation intended for underground storage; however, it should also take into account the processes occurring during injection, particularly that of pressure build-up.
- With regard to the safety of the storage and its purpose, the tightness of the underground storage has been identified as the most important feature, determining the success of the entire gas storage process. The degree of tightness of an underground storage facility depends on the type of geological structure and the gas stored.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Uliasz-Misiak, B.; Przybycin, A. Present and future status of the underground space use in Poland. Environ. Earth Sci. 2016, 75, 1430. [Google Scholar] [CrossRef]
- Evans, D.; Stephenson, M.; Shaw, R. The present and future use of ‘land’ below ground. Land Use Policy 2009, 26, S302–S316. [Google Scholar] [CrossRef]
- UNFCCC. Adoption of the Paris Agreement: Draft Decision; UNFCCC: Bonn, Germany, 2015; pp. 1–32. [Google Scholar]
- EU Commission. A Strategy for Competitive, Sustainable and Secure Energy; COM/2010/639; EU Monitor: Brussels, Belgium, 2010; pp. 1–21.
- Abe, J.O.; Popoola, A.P.I.; Ajenifuja, E.; Popoola, O.M. Hydrogen energy, economy and storage: Review and recommendation. Int. J. Hydrogen Energy 2019, 44, 15072–15086. [Google Scholar] [CrossRef]
- Heinemann, N.; Alcalde, J.; Miocic, J.M.; Hangx, S.J.T.; Kallmeyer, J.; Ostertag-Henning, C.; Hassanpouryouzband, A.; Thaysen, E.M.; Strobel, G.J.; Schmidt-Hattenberger, C.; et al. Enabling large-scale hydrogen storage in porous media—The scientific challenges. Energy Environ. Sci. 2021, 14, 853–864. [Google Scholar] [CrossRef]
- Uliasz-Misiak, B.; Misiak, J.; Lewandowska-Śmierzchalska, J. Hydrogen Storage in Porous Rocks: A Bibliometric Analysis of Research Trends. Energies 2024, 17, 805. [Google Scholar] [CrossRef]
- Wan, C.; Liu, X.; Wang, J.; Chen, F.; Cheng, D.G. Heterostructuring 2D CO2P nanosheets with 0D CoP via a salt-assisted strategy for boosting hydrogen evolution from ammonia borane hydrolysis. Nano Res. 2023, 16, 6260–6269. [Google Scholar] [CrossRef]
- Wan, C.; Li, G.; Wang, J.; Xu, L.; Cheng, D.G.; Chen, F.; Asakura, Y.; Kang, Y.; Yamauchi, Y. Modulating Electronic Metal-Support Interactions to Boost Visible-Light-Driven Hydrolysis of Ammonia Borane: Nickel-Platinum Nanoparticles Supported on Phosphorus-Doped Titania. Angew. Chem. Int. Ed. 2023, 62, e202305371. [Google Scholar] [CrossRef] [PubMed]
- Wan, C.; Liang, Y.; Zhou, L.; Huang, J.; Wang, J.; Chen, F.; Zhan, X.; Cheng, D. guo Integration of morphology and electronic structure modulation on cobalt phosphide nanosheets to boost photocatalytic hydrogen evolution from ammonia borane hydrolysis. Green Energy Environ. 2024, 9, 333–343. [Google Scholar] [CrossRef]
- Evans, J.P. An Appraisal of Underground Gas Storage Technologies and Incidents, for the Development of Risk Assessment Methodology; Sustainable and Renewable Energy Programme; British Geological Survey: Nottingham, UK, 2007. [Google Scholar]
- Kunstman, A.; Poborska-Młynarska, K.; Urbańczyk, K. Construction of storage caverns in salt deposits-geological and mining aspects. Przegląd Geol. 2009, 57, 819–928. (In Polish) [Google Scholar]
- Epelle, E.I.; Obande, W.; Udourioh, G.A.; Afolabi, I.C.; Desongu, K.S.; Orivri, U.; Gunes, B.; Okolie, J.A. Perspectives and prospects of underground hydrogen storage and natural hydrogen. Sustain. Energy Fuels 2022, 6, 3324–3343. [Google Scholar] [CrossRef]
- Tarkowski, R. Some aspects of underground hydrogen storage. Przegląd Geol. 2017, 65, 282–291. [Google Scholar]
- Alms, K.; Ahrens, B.; Graf, M.; Nehler, M. Linking geological and infrastructural requirements for large-scale underground hydrogen storage in Germany. Front. Energy Res. 2023, 11, 1172003. [Google Scholar] [CrossRef]
- Matos, C.R.; Carneiro, J.F.; Silva, P.P. Overview of Large-Scale Underground Energy Storage Technologies for Integration of Renewable Energies and Criteria for Reservoir Identification. J. Energy Storage 2019, 21, 241–258. [Google Scholar] [CrossRef]
- Caglayan, D.G.; Weber, N.; Heinrichs, H.U.; Linßen, J.; Robinius, M.; Kukla, P.A.; Stolten, D. Technical potential of salt caverns for hydrogen storage in Europe. Int. J. Hydrogen Energy 2020, 45, 6793–6805. [Google Scholar] [CrossRef]
- Panfilov, M. Underground and pipeline hydrogen storage. In Compendium of Hydrogen Energy; Elsevier: Amsterdam, The Netherlands, 2016; Volume 2, pp. 91–115. [Google Scholar]
- Zivar, D.; Kumar, S.; Foroozesh, J. Underground hydrogen storage: A comprehensive review. Int. J. Hydrogen Energy 2021, 46, 23436–23462. [Google Scholar] [CrossRef]
- Aftab, A.; Hassanpouryouzband, A.; Xie, Q.; Machuca, L.L.; Sarmadivaleh, M. Toward a Fundamental Understanding of Geological Hydrogen Storage. Ind. Eng. Chem. Res. 2022, 61, 3233–3253. [Google Scholar] [CrossRef]
- Acar, C.; Dincer, I. Review and evaluation of hydrogen production options for better environment. J. Clean. Prod. 2019, 218, 835–849. [Google Scholar] [CrossRef]
- Tarkowski, R.; Uliasz-Misiak, B.; Tarkowski, P. Storage of hydrogen, natural gas, and carbon dioxide—Geological and legal conditions. Int. J. Hydrogen Energy 2021, 46, 20010–20022. [Google Scholar] [CrossRef]
- Hendriks, C.A.; Blok, K. Underground storage of carbon dioxide. Energy Convers. Manag. 1995, 36, 539–542. [Google Scholar] [CrossRef]
- Holt, T.; Jensen, J.I.; Lindeberg, E. Underground storage of CO2 in aquifers and oil reservoirs. Energy Convers. Manag. 1995, 36, 535–538. [Google Scholar] [CrossRef]
- Holloway, S. Underground sequestration of carbon dioxide—A viable greenhouse gas mitigation option. Energy 2005, 30, 2318–2333. [Google Scholar] [CrossRef]
- Ajayi, T.; Gomes, J.S.; Bera, A. A review of CO2 storage in geological formations emphasizing modeling, monitoring and capacity estimation approaches. Pet. Sci. 2019, 16, 1028–1063. [Google Scholar] [CrossRef]
- Bachu, S. CO2 storage in geological media: Role, means, status and barriers to deployment. Prog. Energy Combust. Sci. 2008, 34, 254–273. [Google Scholar] [CrossRef]
- Alcalde, J.; Flude, S.; Wilkinson, M.; Johnson, G.; Edlmann, K.; Bond, C.E.; Scott, V.; Gilfillan, S.M.V.; Ogaya, X.; Stuart Haszeldine, R. Estimating geological CO2 storage security to deliver on climate mitigation. Nat. Commun. 2018, 9, 2201. [Google Scholar] [CrossRef] [PubMed]
- Aminu, M.D.; Nabavi, S.A.; Rochelle, C.A.; Manovic, V. A review of developments in carbon dioxide storage. Appl. Energy 2017, 208, 1389–1419. [Google Scholar] [CrossRef]
- Schultz, R.A.; Heinemann, N.; Horváth, B.; Wickens, J.; Miocic, J.M.; Babarinde, O.O.; Cao, W.; Capuano, P.; Dewers, T.A.; Dusseault, M.; et al. An overview of underground energy-related product storage and sequestration. Geol. Soc. Lond. Spec. Publ. 2023, 528, 15–35. [Google Scholar] [CrossRef]
- Metz, B.; Davidson, O.; de Coninck, H.C.; Loos, M.; Meyer, L. IPCC (Intergovernmental Panel on Climate Change). In IPCC Special Report on Carbon Dioxide Capture and Storage; Prepared by Working Group III of the Intergovernmental Panel on Climate Change; Cambridge University Press: New York, NY, USA, 2005. [Google Scholar]
- GWPC-IOGCC. Underground Gas Storage Regulatory Considerations: A Guide for State and Federal Regulatory Agencies; Interstate Oil and Gas Compact Commission: Oklahoma City, OK, USA, 2017.
- Evans, D.J. A review of underground fuel storage events and putting risk into perspective with other areas of the energy supply chain. Geol. Soc. Spec. Publ. 2009, 313, 173–216. [Google Scholar] [CrossRef]
- Al-Shafi, M.; Massarweh, O.; Abushaikha, A.S.; Bicer, Y. A review on underground gas storage systems: Natural gas, hydrogen and carbon sequestration. Energy Rep. 2023, 9, 6251–6266. [Google Scholar] [CrossRef]
- Tarkowski, R.; Uliasz-Misiak, B. Use of underground space for the storage of selected gases (CH4, H2, and CO2)—Possible conflicts of interest. Gospod. Surowcami Miner. Resour. Manag. 2021, 37, 141–160. [Google Scholar] [CrossRef]
- Chen, Z.; Zhou, F.; Rahman, S.S. Effect of Cap Rock Thickness and Permeability on Geological Storage of CO2: Laboratory Test and Numerical Simulation. Energy Explor. Exploit. 2014, 32, 943–964. [Google Scholar] [CrossRef]
- England, W.A.; Mackenzie, A.S.; Mann, D.M.; Quigley, T.M. The movement and entrapment of petroleum fluids in the subsurface. J. Geol. Soc. London. 1987, 144, 327–347. [Google Scholar] [CrossRef]
- Shukla, R.; Ranjith, P.; Haque, A.; Choi, X. A review of studies on CO2 sequestration and caprock integrity. Fuel 2010, 89, 2651–2664. [Google Scholar] [CrossRef]
- Verga, F. What’s Conventional and What’s Special in a Reservoir Study for Underground Gas Storage. Energies 2018, 11, 1245. [Google Scholar] [CrossRef]
- Chadwick, A.; Arts, R.; Bernstone, C.; May, F.; Thibeau, S.; Zweigel, P. Best Practice for the Storage of CO2 in Saline Aquifers. Observations and Guidelines from the SACS and CO2STORE Projects; British Geological Survey Occasional Publication 14; British Geological Survey: Nottingham, UK, 2008. [Google Scholar]
- Ismail, I.; Gaganis, V. Carbon Capture, Utilization, and Storage in Saline Aquifers: Subsurface Policies, Development Plans, Well Control Strategies and Optimization Approaches—A Review. Clean Technol. 2023, 5, 609–637. [Google Scholar] [CrossRef]
- Nicot, J.P. Evaluation of large-scale CO2 storage on fresh-water sections of aquifers: An example from the Texas Gulf Coast Basin. Int. J. Greenh. Gas Control 2008, 2, 582–593. [Google Scholar] [CrossRef]
- Bachu, S.; Adams, J.J. Sequestration of CO2 in geological media in response to climate change: Capacity of deep saline aquifers to sequester CO2 in solution. Energy Convers. Manag. 2003, 44, 3151–3175. [Google Scholar] [CrossRef]
- Potdar, R.S.; Vishal, V. Trapping mechanism of CO2 storage in deep saline aquifers: Brief review. In Geologic Carbon Sequestration: Understanding Reservoir Behavior; Springer: Cham, Switzerland, 2016; pp. 47–58. [Google Scholar] [CrossRef]
- Zhang, D.; Song, J. Mechanisms for geological carbon sequestration. Procedia IUTAM 2015, 10, 319–327. [Google Scholar] [CrossRef]
- Han, W.S.; McPherson, B.J.; Lichtner, P.C.; Wang, F.P. Evaluation of trapping mechanisms in geologic CO2 sequestration: Case study of SACROC northern platform, a 35-year CO2 injection site. Am. J. Sci. 2010, 310, 282–324. [Google Scholar] [CrossRef]
- Raza, A.; Arif, M.; Glatz, G.; Mahmoud, M.; Al Kobaisi, M.; Alafnan, S.; Iglauer, S. A holistic overview of underground hydrogen storage: Influencing factors, current understanding, and outlook. Fuel 2022, 330, 125636. [Google Scholar] [CrossRef]
- Katz, D.L.; Tek, M.R. Storage of Natural Gas in Saline Aquifers. Water Resour. Res. 1970, 6, 1515–1521. [Google Scholar] [CrossRef]
- Ang, L.; Yongming, L.; Xi, C.; Zhongyi, Z.; Yu, P. Review of CO2 sequestration mechanism in saline aquifers. Nat. Gas Ind. B 2022, 9, 383–393. [Google Scholar] [CrossRef]
- Iglauer, S. Dissolution Trapping of Carbon Dioxide in Reservoir Formation Brine—A Carbon Storage Mechanism; Nakajima, H., Ed.; IntechOpen: Rijeka, Croatia, 2011; ISBN 978-953-307-636-2. [Google Scholar]
- Szulczewski, M.L.; Hesse, M.A.; Juanes, R. Carbon dioxide dissolution in structural and stratigraphic traps. J. Fluid Mech. 2013, 736, 287–315. [Google Scholar] [CrossRef]
- Chabab, S.; Théveneau, P.; Coquelet, C.; Corvisier, J.; Paricaud, P. Measurements and predictive models of high-pressure H2 solubility in brine (H2O+NaCl) for underground hydrogen storage application. Int. J. Hydrogen Energy 2020, 45, 32206–32220. [Google Scholar] [CrossRef]
- Kimbrel, E.H.; Herring, A.L.; Armstrong, R.T.; Lunati, I.; Bay, B.K.; Wildenschild, D. Experimental characterization of nonwetting phase trapping and implications for geologic CO2 sequestration. Int. J. Greenh. Gas Control 2015, 42, 1–15. [Google Scholar] [CrossRef]
- Jafari Raad, S.M.; Leonenko, Y.; Hassanzadeh, H. Hydrogen storage in saline aquifers: Opportunities and challenges. Renew. Sustain. Energy Rev. 2022, 168, 112846. [Google Scholar] [CrossRef]
- Hurter, S.; Labregere, D.; Berge, J. Simulations for CO2 Injection Projects With Compositional Simulator. In Proceedings of the SPE Offshore Europe Conference, OnePetro, Aberdeen, UK, 4–7 September 2007; pp. 214–220. [Google Scholar]
- Fang, Y.; Baojun, B.; Dazhen, T.; Dunn-Norman, S.; Wronkiewicz, D. Characteristics of CO2 sequestration in saline aquifers. Pet. Sci. 2010, 7, 83–92. [Google Scholar] [CrossRef]
- Tong, D.; Trusler, J.P.M.; Vega-Maza, D. Solubility of CO2 in aqueous solutions of CaCl2 or MgCl2 and in a synthetic formation brine at temperatures up to 423 K and pressures up to 40 MPa. J. Chem. Eng. Data 2013, 58, 2116–2124. [Google Scholar] [CrossRef]
- Gunter, W.D.; Wiwehar, B.; Perkins, E.H. Aquifer disposal of CO2-rich greenhouse gases: Extension of the time scale of experiment for CO2-sequestering reactions by geochemical modelling. Mineral. Petrol. 1997, 59, 121–140. [Google Scholar] [CrossRef]
- De Silva, G.P.D.; Ranjith, P.G.; Perera, M.S.A. Geochemical aspects of CO2 sequestration in deep saline aquifers: A review. Fuel 2015, 155, 128–143. [Google Scholar] [CrossRef]
- Rimmelé, G.; Barlet-Gouédard, V.; Renard, F. Evolution of the Petrophysical and Mineralogical Properties of Two Reservoir Rocks Under Thermodynamic Conditions Relevant for CO2 Geological Storage at 3 km Depth. Oil Gas Sci. Technol. Rev. L’institut Français Pétrole 2010, 65, 565–580. [Google Scholar] [CrossRef]
- Shiraki, R.; Dunn, T.L. Experimental study on water–rock interactions during CO2 flooding in the Tensleep Formation, Wyoming, USA. Appl. Geochem. 2000, 15, 265–279. [Google Scholar] [CrossRef]
- Bachu, S.; Gunter, W.D.; Perkins, E.H. Aquifer disposal of CO2: Hydrodynamic and mineral trapping. Energy Convers. Manag. 1994, 35, 269–279. [Google Scholar] [CrossRef]
- Thiyagarajan, S.R.; Emadi, H.; Hussain, A.; Patange, P.; Watson, M. A comprehensive review of the mechanisms and efficiency of underground hydrogen storage. J. Energy Storage 2022, 51, 104490. [Google Scholar] [CrossRef]
- Saeed, M.; Jadhawar, P.; Bagala, S. Geochemical Effects on Storage Gases and Reservoir Rock during Underground Hydrogen Storage: A Depleted North Sea Oil Reservoir Case Study. Hydrogen 2023, 4, 323–337. [Google Scholar] [CrossRef]
- Yekta, A.E.; Pichavant, M.; Audigane, P. Evaluation of geochemical reactivity of hydrogen in sandstone: Application to geological storage. Appl. Geochem. 2018, 95, 182–194. [Google Scholar] [CrossRef]
- Hemme, C.; van Berk, W. Potential risk of H2S generation and release in salt cavern gas storage. J. Nat. Gas Sci. Eng. 2017, 47, 114–123. [Google Scholar] [CrossRef]
- Pijnenburg, R.P.J.; Verberne, B.A.; Hangx, S.J.T.; Spiers, C.J. Inelastic Deformation of the Slochteren Sandstone: Stress-Strain Relations and Implications for Induced Seismicity in the Groningen Gas Field. J. Geophys. Res. Solid Earth 2019, 124, 5254–5282. [Google Scholar] [CrossRef]
- Peng, D.D.; Fowler, M.; Elkamel, A.; Almansoori, A.; Walker, S.B. Enabling utility-scale electrical energy storage by a power-to-gas energy hub and underground storage of hydrogen and natural gas. J. Nat. Gas Sci. Eng. 2016, 35, 1180–1199. [Google Scholar] [CrossRef]
- Hagemann, B.; Rasoulzadeh, M.; Panfilov, M.; Ganzer, L.; Reitenbach, V. Hydrogenization of underground storage of natural gas: Impact of hydrogen on the hydrodynamic and biochemical behavior. Comput. Geosci. 2016, 20, 595–606. [Google Scholar] [CrossRef]
- Gregory, S.P.; Barnett, M.J.; Field, L.P.; Milodowski, A.E. Subsurface Microbial Hydrogen Cycling: Natural Occurrence and Implications for Industry. Microorganisms 2019, 7, 53. [Google Scholar] [CrossRef]
- Bernardez, L.A.; De Lima, L.R.P.A.; De Jesus, E.B.; Ramos, C.L.S.; Almeida, P.F. A kinetic study on bacterial sulfate reduction. Bioprocess Biosyst. Eng. 2013, 36, 1861–1869. [Google Scholar] [CrossRef]
- Jørgensen, B.B.; Isaksen, M.F.; Jannasch, H.W. Bacterial Sulfate Reduction Above 100C in Deep-Sea Hydrothermal Vent Sediments. Science 1992, 258, 1756–1757. [Google Scholar] [CrossRef]
- Machel, H.G. Bacterial and thermochemical sulfate reduction in diagenetic settings—Old and new insights. Sediment. Geol. 2001, 140, 143–175. [Google Scholar] [CrossRef]
- Thaysen, E.M.; McMahon, S.; Strobel, G.J.; Butler, I.B.; Ngwenya, B.T.; Heinemann, N.; Wilkinson, M.; Hassanpouryouzband, A.; McDermott, C.I.; Edlmann, K. Estimating microbial growth and hydrogen consumption in hydrogen storage in porous media. Renew. Sustain. Energy Rev. 2021, 151, 111481. [Google Scholar] [CrossRef]
- Ramesh Kumar, K.; Honorio, H.; Chandra, D.; Lesueur, M.; Hajibeygi, H. Comprehensive review of geomechanics of underground hydrogen storage in depleted reservoirs and salt caverns. J. Energy Storage 2023, 73, 108912. [Google Scholar] [CrossRef]
- Miocic, J.M.; Heinemann, N.; Alcalde, J.; Edlmann, K.; Schultz, R.A. Enabling Secure Subsurface Storage in Future Energy Systems; Geological Society of London Special Publication; Geological Society of London: London, UK, 2023; Volume 528, pp. 1–7. [Google Scholar] [CrossRef]
- DOE/NETL. BEST PRACTICES: Monitoring, Verification, and Accounting (MVA) for Geologic Storage Projects DOE/NETL; NETL: Albany, NY, USA, 2017.
- Katz, D.L.; Coats, K.H. Underground Storage of Fluids; Ulrich’s Books Inc.: Ann Arbor, MI, USA, 1968. [Google Scholar]
- Katz, D.L.; Tek, M.R. Overview on Underground Storage of Natural Gas. J. Pet. Technol. 1981, 33, 943–951. [Google Scholar] [CrossRef]
- Foster Associates Inc. Profile of Underground Natural Gas Storage Facilities and Market Hubs; Foster Associates Inc.: Washington, DC, USA, 1995. [Google Scholar]
- Behrouz, T.; Askari, A.; Forghaani, S.; Basirat, R.M.; Teymouri, A.; Bonyad, H. Fast Screening Method to Prioritize Underground Gas Storage Structures for Site Selection. In Proceedings of the International Gas Union Research Conference (IGRC 2014), Copenhagen, Denmark, 17–19 September 2014; Volume 3, pp. 2378–2386. [Google Scholar]
- Çetin Demirel, N.; Demirel, T.; Deveci, M.; Vardar, G. Location selection for underground natural gas storage using Choquet integral. J. Nat. Gas Sci. Eng. 2017, 45, 368–379. [Google Scholar] [CrossRef]
- Yousefi-Sahzabi, A.; Sasaki, K.; Djamaluddin, I.; Yousefi, H.; Sugai, Y. GIS modeling of CO2 emission sources and storage possibilities. Energy Procedia 2011, 4, 2831–2838. [Google Scholar] [CrossRef]
- Le Gallo, Y.; Lecomte, A. Global Industrial CCS Technology Roadmap—Sectoral Assessment: Source-Tosink Matching Final Report; UNIDO: Vienna, Austria, 2011. [Google Scholar]
- Bradshaw, J.; Dance, T. Mapping geological storage prospectivity of CO2 for the world’s sedimentary basins and regional source to sink matching. In Proceedings of the Greenhouse Gas Control Technologies; Elsevier Science Ltd.: Vancouver, BC, Canada, 2005; pp. 583–591. [Google Scholar]
- Delprat-Jannaud, F.; Korre, A.; Shi, J.Q.; McConnell, B.; Arvanitis, A.; Boavida, D.; Car, M.; Gastine, M.; Grunnaleite, I.; Bateman, K.; et al. State-of-the-Art of Review CO2 Storage Site Selection and Characterisation Methods; IFP Energies nouvelles: Solaize, France, 2013. [Google Scholar]
- DNV. CO2QUALSTORE Guideline for Selection and Qualification of Sites and Projects for Geological Storage of CO2; DNV: Hong Kong, China, 2010. [Google Scholar]
- Raza, A.; Rezaee, R.; Gholami, R.; Bing, C.H.; Nagarajan, R.; Hamid, M.A. A screening criterion for selection of suitable CO2 storage sites. J. Nat. Gas Sci. Eng. 2016, 28, 317–327. [Google Scholar] [CrossRef]
- Uliasz-Misiak, B. CO2 storage capacity classification and site selection criteria. Gospod. Surowcami Miner. Resour. Manag. 2009, 25, 97–108. [Google Scholar]
- Vangkilde-Pedersen, T.; Anthonsen, K.L.; Smith, N.; Kirk, K.; Neele, F.; van der Meer, B.; Le Gallo, Y.; Bossie-Codreanu, D.; Wojcicki, A.; Le Nindre, Y.M.; et al. Assessing European capacity for geological storage of carbon dioxide-the EU GeoCapacity project. Energy Procedia 2009, 1, 2663–2670. [Google Scholar] [CrossRef]
- Tarkowski, R.; Marek, S.; Uliasz-Misiak, B. Preliminary geological analysis of structures to store CO2 within the Belchatow area. Gospod. Surowcami Miner. 2009, 25, 37–45. (In Polish) [Google Scholar]
- Kaldi, J.G.; Gibson-Poole, C.M. Storage Capacity Estimation, Site Selection and Characterisation for CO2 Storage Projects; Cooperative Research Centre for Greenhouse Gas Technologies: Canberra, Australia, 2008. [Google Scholar]
- IEA GHG. CCS Site Characterisation Criteria; IEA Greenhouse Gas R&D Programme: Cheltenham, UK, 2009. [Google Scholar]
- Alcalde, J.; Heinemann, N.; James, A.; Bond, C.E.; Ghanbari, S.; Mackay, E.J.; Haszeldine, R.S.; Faulkner, D.R.; Worden, R.H.; Allen, M.J. A criteria-driven approach to the CO2 storage site selection of East Mey for the acorn project in the North Sea. Mar. Pet. Geol. 2021, 133, 105309. [Google Scholar] [CrossRef]
- CSA Z741-2012 (R2017); Geological Storage of Carbon Dioxide. Geological Society: London, UK, 2017; pp. 1–80.
- Llamas, B.; Cámara, A. Application of multicriteria algorithm to select suitable areas for storing CO2: CO2SiteAssess software. Energy Procedia 2014, 63, 4977–4986. [Google Scholar] [CrossRef]
- Llamas, B.; Cienfuegos, P. Multicriteria Decision Methodology to Select Suitable Areas for Storing CO2. Energy Environ. 2012, 23, 249–264. [Google Scholar] [CrossRef]
- Hsu, C.-W.; Chen, L.T.; Hu, A.H.; Chang, Y.M. Site selection for carbon dioxide geological storage using analytic network process. Sep. Purif. Technol. 2012, 94, 146–153. [Google Scholar] [CrossRef]
- Deveci, M.; Demirel, N.Ç.; John, R.; Özcan, E. Fuzzy multi-criteria decision making for carbon dioxide geological storage in Turkey. J. Nat. Gas Sci. Eng. 2015, 27, 692–705. [Google Scholar] [CrossRef]
- Firtina Ertiş, İ. Appliaction of multi-criteria decision making for geological carbon dioxide storage area in Turkey. Anadolu Univ. J. Sci. Technol. A Appl. Sci. Eng. Year 2018, 19, 536–545. [Google Scholar] [CrossRef]
- Nemati, B.; Mapar, M.; Davarazar, P.; Zandi, S.; Davarazar, M.; Jahanianfard, D.; Mohammadi, M. A sustainable approach for site selection of underground hydrogen storage facilities using fuzzy-delphi methodology. J. Settl. Spat. Plan. 2020, 2020, 5–16. [Google Scholar] [CrossRef]
- Lewandowska-Śmierzchalska, J.; Tarkowski, R.; Uliasz-Misiak, B. Screening and ranking framework for underground hydrogen storage site selection in Poland. Int. J. Hydrogen Energy 2018, 43, 4401–4414. [Google Scholar] [CrossRef]
- Bouteldja, M.; Acosta, T.; Carlier, B.; Reveillere, A.; Jannel, H.; Fournier, C. Definition of Selection Criteria for a Hydrogen Storage Site in Depleted Fields or Aquifers, Hystories Project: Rueil-Malmaison, France, 2021.
- Kruck, O.; Crotogino, F. Benchmarking of Selected Storage Options—“HyUnder” Project; European Commission: Brussels, Belgium, 2013.
- Guo, W.; Zhang, B.; Liang, Y.; Qiu, R.; Wei, X.; Niu, P.; Zhang, H.; Li, Z. Improved method and practice for site selection of underground gas storage under complex geological conditions. J. Nat. Gas Sci. Eng. 2022, 108, 104813. [Google Scholar] [CrossRef]
- Bachu, S.; Bonijoly, D.; Bradshaw, J.; Burruss, R.; Holloway, S.; Christensen, N.P.; Mathiassen, O.M. CO2 storage capacity estimation: Methodology and gaps. Int. J. Greenh. Gas Control 2007, 1, 430–443. [Google Scholar] [CrossRef]
- Gorecki, C.D.; Ayash, S.C.; Liu, G.; Braunberger, J.R.; Dotzenrod, N.W. A comparison of volumetric and dynamic CO2 storage resource and efficiency in deep saline formations. Int. J. Greenh. Gas Control 2015, 42, 213–225. [Google Scholar] [CrossRef]
- Sopher, D.; Juhlin, C.; Erlström, M. A probabilistic assessment of the effective CO2 storage capacity within the Swedish sector of the Baltic Basin. Int. J. Greenh. Gas Control 2014, 30, 148–170. [Google Scholar] [CrossRef]
- Uliasz-Misiak, B. Regional-scale CO2 storage capacity estimation in Mesozoic aquifers of Poland. Oil Gas Sci. Technol. 2011, 66, 37–45. [Google Scholar] [CrossRef]
- Bradshaw, J.; Allinson, G.; Bradshaw, B.E.; Nguyen, V.; Rigg, A.J.; Spencer, L.; Wilson, P. Australia’s CO2 geological storage potential and matching of emission sources to potential sinks. Energy 2004, 29, 1623–1631. [Google Scholar] [CrossRef]
- Brennan, S.T.; Burruss, R.C.; Merrill, M.D.; Freeman, P.A.; Ruppert, L.F.; Mcnutt, M.K. A Probabilistic Assessment Methodology for the Evaluation of Geologic Carbon Dioxide Storage; US Geological Survey: Reston, VA, USA, 2010. [Google Scholar]
- Popova, O.H.; Small, M.J.; McCoy, S.T.; Thomas, A.C.; Karimi, B.; Goodman, A.; Carter, K.M. Comparative analysis of carbon dioxide storage resource assessment methodologies. Environ. Geosci. 2012, 19, 105–124. [Google Scholar] [CrossRef]
- Kopp, A.; Class, H.; Helmig, R. Investigations on CO2 storage capacity in saline aquifers—Part 2: Estimation of storage capacity coefficients. Int. J. Greenh. Gas Control 2009, 3, 277–287. [Google Scholar] [CrossRef]
- Calvo, R.; Gvirtzman, Z. CO2 Storage Capacity Assessment in the Deep Saline Aquifers of Southern Israel. Energy Procedia 2013, 37, 5118–5123. [Google Scholar] [CrossRef][Green Version]
- Cavanagh, A.; Wildgust, N. Pressurization and brine displacement issues for deep saline formation CO2 storage. Energy Procedia 2011, 4, 4814–4821. [Google Scholar] [CrossRef]
- Van der Meer, L.G.H.B.; Yavuz, F. CO2 storage capacity calculations for the Dutch subsurface. Energy Procedia 2009, 1, 2615–2622. [Google Scholar] [CrossRef]
- Bachu, S. Review of CO2 storage efficiency in deep saline aquifers. Int. J. Greenh. Gas Control 2015, 40, 188–202. [Google Scholar] [CrossRef]
- Luboń, K. CO2 storage capacity of a deep aquifer depending on the injection well location and cap rock capillary pressure. Gospod. Surowcami Miner. 2020, 36, 173–196. [Google Scholar]
- Zhang, Z.; Agarwal, R.K.; Zhang, Z.; Agarwal, R.K. Numerical simulation and optimization of CO2 sequestration in saline aquifers for vertical and horizontal well injection. Comput. Geosci. 2012, 16, 891–899. [Google Scholar] [CrossRef]
- Akai, T.; Kuriyama, T.; Kato, S.; Okabe, H. Numerical modelling of long-term CO2 storage mechanisms in saline aquifers using the Sleipner benchmark dataset. Int. J. Greenh. Gas Control 2021, 110, 103405. [Google Scholar] [CrossRef]
- Wang, Y.; Xu, Y.; Zhang, K. Investigation of CO2 storage capacity in open saline aquifers with numerical models. Procedia Eng. 2012, 31, 886–892. [Google Scholar] [CrossRef][Green Version]
- Zhou, Q.; Birkholzer, J.T.; Tsang, C.F.; Rutqvist, J. A method for quick assessment of CO2 storage capacity in closed and semi-closed saline formations. Int. J. Greenh. Gas Control 2008, 2, 626–639. [Google Scholar] [CrossRef]
- Ehlig-Economides, C.; Economides, M.J. Sequestering carbon dioxide in a closed underground volume. J. Pet. Sci. Eng. 2010, 70, 123–130. [Google Scholar] [CrossRef]
- Cavanagh, A.J.; Yousefi, S.H.; Wilkinson, M.; Groenenberg, R.M. Hydrogen Storage Potential of Existing European Gas Storage Sites in Depleted Gas Fields and Aquifers; European Commission: Brussels, Belgium, 2022.
- Luboń, K.T.; Tarkowski, R. Numerical simulation of hydrogen storage in the Konary deep saline aquifer trap. Gospod. Surowcami Miner. Miner. Resour. Manag. 2023, 39, 103–124. [Google Scholar] [CrossRef]
- Bouteldja, M.; Gallo, Y. Le From hydrogen storage potential to hydrogen capacities in underground hydrogen storages. In Proceedings of the 84th EAGE Annual Conference & Exhibition, Vienna, Austria, 5–8 June 2023; Volume 2023, pp. 1–5. [Google Scholar] [CrossRef]
- Wallace, R.L.; Cai, Z.; Zhang, H.; Zhang, K.; Guo, C. Utility-scale subsurface hydrogen storage: UK perspectives and technology. Int. J. Hydrogen Energy 2021, 46, 25137–25159. [Google Scholar] [CrossRef]
- Scafidi, J.; Wilkinson, M.; Gilfillan, S.M.V.; Heinemann, N.; Haszeldine, R.S. A quantitative assessment of the hydrogen storage capacity of the UK continental shelf. Int. J. Hydrogen Energy 2021, 46, 8629–8639. [Google Scholar] [CrossRef]
- Heinemann, N.; Scafidi, J.; Pickup, G.; Thaysen, E.M.; Hassanpouryouzband, A.; Wilkinson, M.; Satterley, A.K.; Booth, M.G.; Edlmann, K.; Haszeldine, R.S. Hydrogen storage in saline aquifers: The role of cushion gas for injection and production. Int. J. Hydrogen Energy 2021, 46, 39284–39296. [Google Scholar] [CrossRef]
- Zhao, Q.; Wang, Y.; Chen, C. Numerical simulation of the impact of different cushion gases on underground hydrogen storage in aquifers based on an experimentally-benchmarked equation-of-state. Int. J. Hydrogen Energy 2024, 50, 495–511. [Google Scholar] [CrossRef]
- Lord, A.S.; Kobos, P.H.; Borns, D.J. Geologic storage of hydrogen: Scaling up to meet city transportation demands. Int. J. Hydrogen Energy 2014, 39, 15570–15582. [Google Scholar] [CrossRef]
- Luboń, K.; Tarkowski, R. Numerical simulation of hydrogen injection and withdrawal to and from a deep aquifer in NW Poland. Int. J. Hydrogen Energy 2020, 45, 2068–2083. [Google Scholar] [CrossRef]
- Chai, M.; Chen, Z.; Nourozieh, H.; Yang, M. Numerical simulation of large-scale seasonal hydrogen storage in an anticline aquifer: A case study capturing hydrogen interactions and cushion gas injection. Appl. Energy 2023, 334, 120655. [Google Scholar] [CrossRef]
- Rütters, H.; Möller, I.; May, F.; Flornes, K.; Hladik, V.; Arvanitis, A.; Gülec, N.; Bakiler, C.; Dudu, A.; Kucharic, L.; et al. State-of-the-Art of Monitoring Methods to Evaluate CO2 Storage Site Performance. CGS Europe Key Report 2013, 1, 109. [Google Scholar] [CrossRef]
- Zhang, Y.; Jackson, C.; Zahasky, C.; Nadhira, A.; Krevor, S. European carbon storage resource requirements of climate change mitigation targets. Int. J. Greenh. Gas Control 2022, 114, 103568. [Google Scholar] [CrossRef]
- Celia, M.A.; Bachu, S.; Nordbotten, J.M.; Bandilla, K.W. Status of CO2 storage in deep saline aquifers with emphasis on modeling approaches and practical simulations. Water Resour. Res. 2015, 51, 6846–6892. [Google Scholar] [CrossRef]
- Qin, J.; Zhong, Q.; Tang, Y.; Rui, Z.; Qiu, S.; Chen, H. CO2 storage potential assessment of offshore saline aquifers in China. Fuel 2023, 341, 127681. [Google Scholar] [CrossRef]
- Uliasz-Misiak, B.; Lewandowska-Smierzchalska, J.; Matuła, R. Hydrogen Storage Potential in Natural Gas Deposits in the Polish Lowlands. Energies 2024, 17, 374. [Google Scholar] [CrossRef]
- Le Gallo, Y.; Bouteldja, M. 3D Multi-Realization Simulations for Fluid Flow and Mixing Issues at European Scale; Geological Society: London, UK, 2023. [Google Scholar]
- Vavra, C.L.; Kaldi, J.G.; Sneider, R.M. Geological applications of capillary pressure: A review. Am. Assoc. Pet. Geol. Bull. 1992, 76, 840–850. [Google Scholar] [CrossRef]
- Veil, J.A.; Puder, M.G.; Elcock, D.; Redweik, J.R.J. A White Paper Describing Produced Water from Production of Crude Oil, Natural Gas, and Coal Bed Methane; Argonne National Laboratory: Lemont, IL, USA, 2004. [Google Scholar]
- Wei, L.; Jie, C.; Deyi, J.; Xilin, S.; Yinping, L.; Daemen, J.J.K.; Chunhe, Y. Tightness and suitability evaluation of abandoned salt caverns served as hydrocarbon energies storage under adverse geological conditions (AGC). Appl. Energy 2016, 178, 703–720. [Google Scholar] [CrossRef]
- Vilarrasa, V.; Makhnenko, R.; Gheibi, S. Geomechanical analysis of the influence of CO 2 injection location on fault stability. J. Rock Mech. Geotech. Eng. 2016, 8, 805–818. [Google Scholar] [CrossRef]
- Song, Y.; Jun, S.; Na, Y.; Kim, K.; Jang, Y.; Wang, J. Geomechanical challenges during geological CO2 storage: A review. Chem. Eng. J. 2023, 456, 140968. [Google Scholar] [CrossRef]
- Rutqvist, J. The Geomechanics of CO2 Storage in Deep Sedimentary Formations. Geotech. Geol. Eng. 2012, 30, 525–551. [Google Scholar] [CrossRef]
- Dautriat, J.; Gland, N.; Guelard, J.; Dimanov, A.; Raphanel, J.L. Axial and Radial Permeability Evolutions of Compressed Sandstones: End Effects and Shear-band Induced Permeability Anisotropy. Pure Appl. Geophys. 2009, 166, 1037–1061. [Google Scholar] [CrossRef]
- Ostermeier, R.M. Deepwater Gulf of Mexico Turbidites—Compaction Effects on Porosity and Permeability. SPE Form. Eval. 1995, 10, 79–85. [Google Scholar] [CrossRef]
- Suckale, J. Moderate-to-large seismicity induced by hydrocarbon production. Lead. Edge 2010, 29, 310–319. [Google Scholar] [CrossRef]
- Hangx, S.; Bakker, E.; Bertier, P.; Nover, G.; Busch, A. Chemical–mechanical coupling observed for depleted oil reservoirs subjected to long-term CO2-exposure—A case study of the Werkendam natural CO2 analogue field. Earth Planet. Sci. Lett. 2015, 428, 230–242. [Google Scholar] [CrossRef]
- Song, J.; Zhang, D. Comprehensive Review of Caprock-Sealing Mechanisms for Geologic Carbon Sequestration. Environ. Sci. Technol. 2012, 47, 9–22. [Google Scholar] [CrossRef] [PubMed]
- Dewhurst, D.N.; Piane, C.D.; Esteban, L.; Sarout, J.; Josh, M.; Pervukhina, M.; Clennell, M. Ben Microstructural, Geomechanical, and Petrophysical Characterization of Shale Caprocks. Geophys. Monogr. Ser. 2018, 238, 1–30. [Google Scholar] [CrossRef]
- Van Noort, R. Effects of clay swelling or shrinkage on shale caprock integrity. In Proceedings of the 80th EAGE Conference and Exhibition, Copenhagen, Denmark, 11–14 June 2018; Volume 2018, pp. 1–5. [Google Scholar] [CrossRef]
- Zeng, L.; Vialle, S.; Ennis-King, J.; Esteban, L.; Sarmadivaleh, M.; Sarout, J.; Dautriat, J.; Giwelli, A.; Xie, Q. Role of geochemical reactions on caprock integrity during underground hydrogen storage. J. Energy Storage 2023, 65, 107414. [Google Scholar] [CrossRef]
- Spiers, C.J.; Hangx, S.J.T.; Niemeijer, A.R. New approaches in experimental research on rock and fault behaviour in the Groningen gas field. Geol. Mijnb./Neth. J. Geosci. 2017, 96, s55–s69. [Google Scholar] [CrossRef]
- Ugarte, E.R.; Salehi, S. A Review on Well Integrity Issues for Underground Hydrogen Storage. J. Energy Resour. Technol. Trans. ASME 2022, 144, 042001. [Google Scholar] [CrossRef]
- IEA GHG. Long Term Integrity of CO2 Storage-Well Abandonment; Technical Study; IEA GHG: Cheltenham, UK, 2009. [Google Scholar]
- Wu, L.; Yi, L.; Taoutaou, S.; Yao, K.; Fan, Y.; Liu, W.; Fan, X.; Liu, H.; Liu, L. Maintaining Well Integrity in Underground Gas Storage Wells in China Using a Novel Cementing Technology. In Proceedings of the Abu Dhabi International Petroleum Exhibition and Conference, Abu Dhabi, United Arab Emirates, 10–13 November 2014; Volume 4, pp. 2794–2803. [Google Scholar] [CrossRef]
- Li, B.; Zhou, F.; Li, H.; Duguid, A.; Que, L.; Xue, Y.; Tan, Y. Prediction of CO2 leakage risk for wells in carbon sequestration fields with an optimal artificial neural network. Int. J. Greenh. Gas Control 2018, 68, 276–286. [Google Scholar] [CrossRef]
- Aftab, A.; Hassanpouryouzband, A.; Martin, A.; Kendrick, J.E.; Thaysen, E.M.; Heinemann, N.; Utley, J.; Wilkinson, M.; Haszeldine, R.S.; Edlmann, K. Geochemical Integrity of Wellbore Cements during Geological Hydrogen Storage. Environ. Sci. Technol. Lett. 2023, 10, 551–556. [Google Scholar] [CrossRef]
- Iorio, V.S.; Cracolici, F.; Parrozza, F.; Sabatino, L.M.F.; Massara, E.P.; Consonni, A.; Tritto, C.; De Simoni, M. Cement to Safeguard the Wells Integrity in Underground Hydrogen Storage: An Experimental Investigation. Chem. Eng. Trans. 2022, 96, 307–312. [Google Scholar] [CrossRef]
- Bois, A.P.; Vu, M.H.; Ghabezloo, S.; Sulem, J.; Garnier, A.; Laudet, J.B.Î. Cement Sheath Integrity for CO2 Storage—An Integrated Perspective. Energy Procedia 2013, 37, 5628–5641. [Google Scholar] [CrossRef]
- Bai, M.; Zhang, Z.; Fu, X. A review on well integrity issues for CO2 geological storage and enhanced gas recovery. Renew. Sustain. Energy Rev. 2016, 59, 920–926. [Google Scholar] [CrossRef]
- Kanezaki, T.; Narazaki, C.; Mine, Y.; Matsuoka, S.; Murakami, Y. Effects of hydrogen on fatigue crack growth behavior of austenitic stainless steels. Int. J. Hydrogen Energy 2008, 33, 2604–2619. [Google Scholar] [CrossRef]
- Reitenbach, V.; Ganzer, L.; Albrecht, D.; Hagemann, B. Influence of added hydrogen on underground gas storage: A review of key issues. Environ. Earth Sci. 2015, 73, 6927–6937. [Google Scholar] [CrossRef]
- UGS Storage Committee—Underground Gas Storage Database. Available online: http://ugs.igu.org/index.php/ugs_list/get_list (accessed on 18 December 2023).
- OpenStreetMap. Available online: https://www.openstreetmap.org/about (accessed on 19 December 2023).
- Equinor The Sleipner Area—Equinor. Available online: https://www.equinor.com/energy/sleipner (accessed on 16 December 2023).
- Chadwick, R.A.; Zweigel, P.; Gregersen, U.; Kirby, G.A.; Holloway, S.; Johannessen, P.N. Geological reservoir characterization of a CO2 storage site: The Utsira Sand, Sleipner, northern North Sea. Energy 2004, 29, 1371–1381. [Google Scholar] [CrossRef]
- Eiken, O.; Ringrose, P.; Hermanrud, C.; Nazarian, B.; Torp, T.A.; Høier, L. Lessons learned from 14 years of CCS operations: Sleipner, In Salah and Snøhvit. Energy Procedia 2011, 4, 5541–5548. [Google Scholar] [CrossRef]
- Chadwick, R.A.; Eiken, O. Offshore CO2 storage: Sleipner natural gas field beneath the North Sea. In Geological Storage of Carbon Dioxide (CO2): Geoscience, Technologies, Environmental Aspects and Legal Frameworks; Gluyas, J., Mathias, S., Eds.; Woodhead Publishing: Cambridge, UK, 2013; pp. 227–250. ISBN 9780857094278. [Google Scholar]
- Harvey, S.; Hopkins, J.; Kuehl, H.; O’Brien, S.; Mateeva, A. Quest CCS Facility: Microseismic System Monitoring. In Proceedings of the 15th International Conference on Greenhouse Gas Control Technologies, Abu Dhabi, United Arab Emirates, 15–18 March 2021; pp. 1–16. [Google Scholar]
- Michael, K.; Golab, A.; Shulakova, V.; Ennis-King, J.; Allinson, G.; Sharma, S.; Aiken, T. Geological storage of CO2 in saline aquifers—A review of the experience from existing storage operations. Int. J. Greenh. Gas Control 2010, 4, 659–667. [Google Scholar] [CrossRef]
- Government of Alberta. Quest Carbon Capture and Storage Project 2014; Government of Alberta: Edmonton, AB, Canada, 2014.
- Government of Alberta. Quest Carbon Capture and Storage Project: Annual Report, 2021—Open Government; Government of Alberta: Edmonton, AB, Canada, 2022.
- Tremosa, J.; Castillo, C.; Quang Vong, C.; Kervévan, C.; Lassin, A.; Audigane, P.; Trémosa, J. Long-term assessment of geochemical reactivity of CO2 storage in highly saline aquifers: Application to Ketzin, In Salah and Snøhvit storage sites. Int. J. Greenh. Gas Control 2014, 20, 2–26. [Google Scholar] [CrossRef]
- Equinor The Snøhvit Future Project—Equinor. Available online: https://www.equinor.com/energy/snohvit-future (accessed on 16 December 2023).
- Estublier, A.; Lackner, A.S. Long-term simulation of the Snøhvit CO2 storage. Energy Procedia 2009, 1, 3221–3228. [Google Scholar] [CrossRef]
- Hansen, O.; Gilding, D.; Nazarian, B.; Osdal, B.; Ringrose, P.; Kristoffersen, J.B.; Eiken, O.; Hansen, H. Snøhvit: The History of Injecting and Storing 1 Mt CO2 in the Fluvial Tubåen Fm. Energy Procedia 2013, 37, 3565–3573. [Google Scholar] [CrossRef]
- Equinor. Northen Ligths Project Concept Report; FEED Report; Equinor: Stavanger, Norway, 2020. [Google Scholar]
- Equinor The Northern Lights Project—Equinor. Available online: https://www.equinor.com/energy/northern-lights (accessed on 16 December 2023).
- Trupp, M.; Ryan, S.; Barranco Mendoza, I.; Leon, D.; Scoby-Smith, L. Developing the world’s largest CO2 Injection System—A history of the Gorgon Carbon Dioxide Injection System. In Proceedings of the 15th International Conference on Greenhouse Gas Control Technologies, GHGT-15, Abu Dhabi, United Arab Emirates, 15–18 March 2021; Elsevier BV: Amsterdam, The Netherlands, 2021; pp. 1–13. [Google Scholar]
- Duong, C.; Bower, C.; Hume, K.; Rock, L.; Tessarolo, S. Quest carbon capture and storage offset project: Findings and learnings from 1st reporting period. Int. J. Greenh. Gas Control 2019, 89, 65–75. [Google Scholar] [CrossRef]
- Rock, L.; O’Brien, S.; Tessarolo, S.; Duer, J.; Bacci, V.O.; Hirst, B.; Randell, D.; Helmy, M.; Blackmore, J.; Duong, C.; et al. The Quest CCS Project: 1st Year Review Post Start of Injection. Energy Procedia 2017, 114, 5320–5328. [Google Scholar] [CrossRef]
- Hematpur, H.; Abdollahi, R.; Rostami, S.; Haghighi, M.; Blunt, M.J. Review of underground hydrogen storage: Concepts and challenges. Adv. Geo-Energy Res. 2023, 7, 111–131. [Google Scholar] [CrossRef]
- Miocic, J.; Heinemann, N.; Edlmann, K.; Scafidi, J.; Molaei, F.; Alcalde, J. Underground hydrogen storage: A review. Geol. Soc. Lond. Spec. Publ. 2022, 528, 2022. [Google Scholar] [CrossRef]
- Kruck, O.; Crotogino, F.; Prelicz, R.; Rudolph, T. A Overview on All Known Underground Storage Technologies for Hydrogen; Elsevier: Amsterdam, The Netherlands, 2013. [Google Scholar]
| Parameter | UGS | CCS | UHS | |||
|---|---|---|---|---|---|---|
| Positive Indicator | Negative Indicator | Positive Indicator | Negative Indicator | Positive Indicator | Cautionary Indicator | |
| Depth (m below surface level) | 200–3000 | <1000 >3000 | 800–2500 | <800 >3500 | 500–2500 | <500 |
| Thickness of low-permeability caprock (m) | >20 | <20 | >50 | <20 | >50 | <20 |
| Porosity (%) | >10–15 | <10 | >20 | <10 | >10 | <10 |
| Permeability (mD) | >300 | <100 | >300 | <100 | >50 | <50 |
| Water mineralization (g/L) | - | - | >100 | <30 | >100 | <100 |
| Lithology | Porosity [%] | Permeability [mD] | Water Hydrochemical Type | Total Dissolved Solids [g/L] | CCS Project |
|---|---|---|---|---|---|
| sandstones | 27–42 | 1000–8000 | Na-Cl | 35 | Sleipner |
| sandstones with interbedded shales | 10–15 | 185–883 | Na-Cl | 159.4 | Snowhvit |
| sandstones and siltstones | 0–25.3 | 0–272 | Na-Cl | 37 | Gorgon |
| sandstones | 16 | 20–2000 | Ca-Mg-HCO3 to NaCl and Na-SO4 | 58–18,500 | Quest |
| Country | Project Name | Storage Volume (Million m3) | Depth [m] | Operational Years as Town Gas Storage | Current Status |
|---|---|---|---|---|---|
| France | Beynes | 330 | 430 | 1956–1972 | Natural gas storage |
| Germany | Hähnlein | 160 | 500 | - | Natural gas storage |
| Eschenfelden | 168 | 600 | - | Natural gas storage | |
| Engelborstel | - | - | 1955–1998 | Decommissioned | |
| Ketzin | 130 | 200–400 | 1964–2000 | Decommissioned | |
| Czech Republic | Lobodice | 100 | 400–500 | 1965–1995 | Natural gas storage |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Uliasz-Misiak, B.; Misiak, J. Underground Gas Storage in Saline Aquifers: Geological Aspects. Energies 2024, 17, 1666. https://doi.org/10.3390/en17071666
Uliasz-Misiak B, Misiak J. Underground Gas Storage in Saline Aquifers: Geological Aspects. Energies. 2024; 17(7):1666. https://doi.org/10.3390/en17071666
Chicago/Turabian StyleUliasz-Misiak, Barbara, and Jacek Misiak. 2024. "Underground Gas Storage in Saline Aquifers: Geological Aspects" Energies 17, no. 7: 1666. https://doi.org/10.3390/en17071666
APA StyleUliasz-Misiak, B., & Misiak, J. (2024). Underground Gas Storage in Saline Aquifers: Geological Aspects. Energies, 17(7), 1666. https://doi.org/10.3390/en17071666








