A Direct Chemical Approach to Mitigate Environment Lead Contamination in Perovskite Solar Cells
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
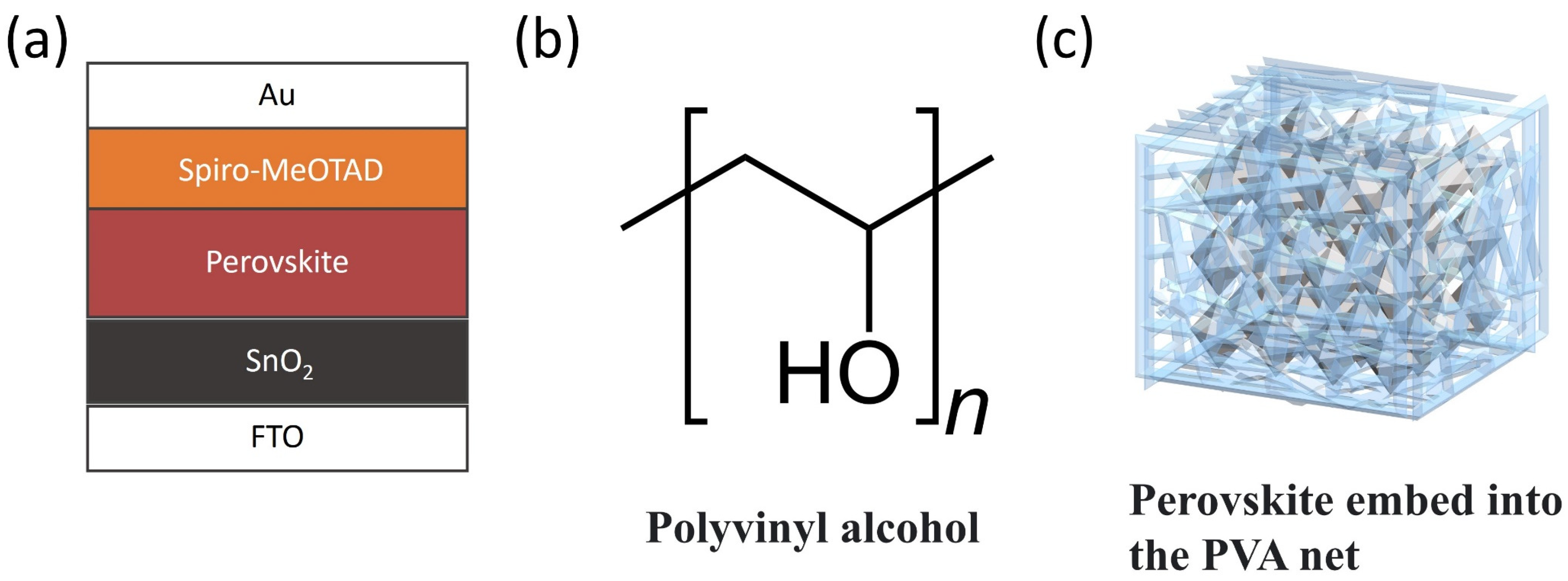
2.2. Device Fabrication
2.3. Lead Leakage Tests Procedure
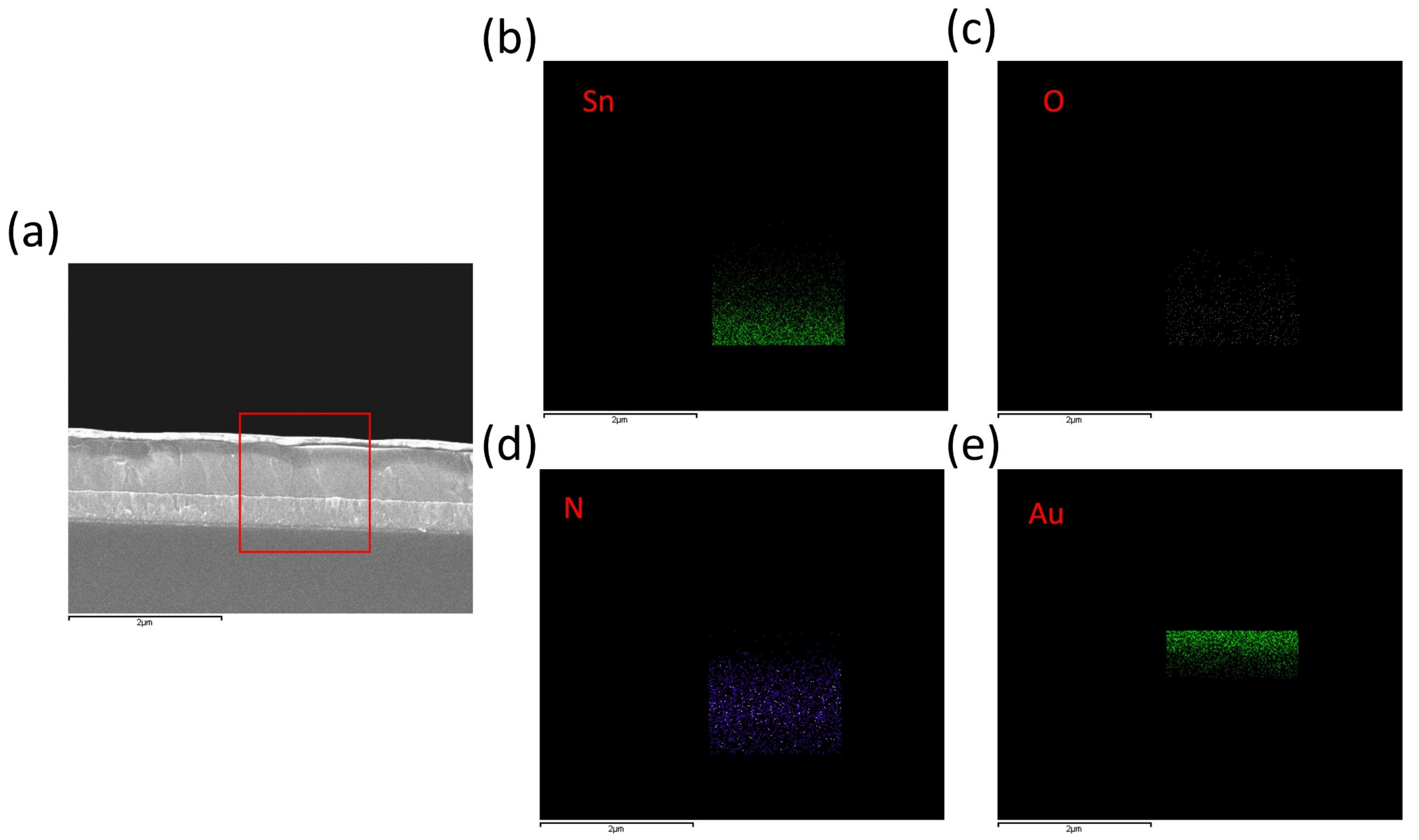
2.4. Characterizations
2.5. Stability Measurement
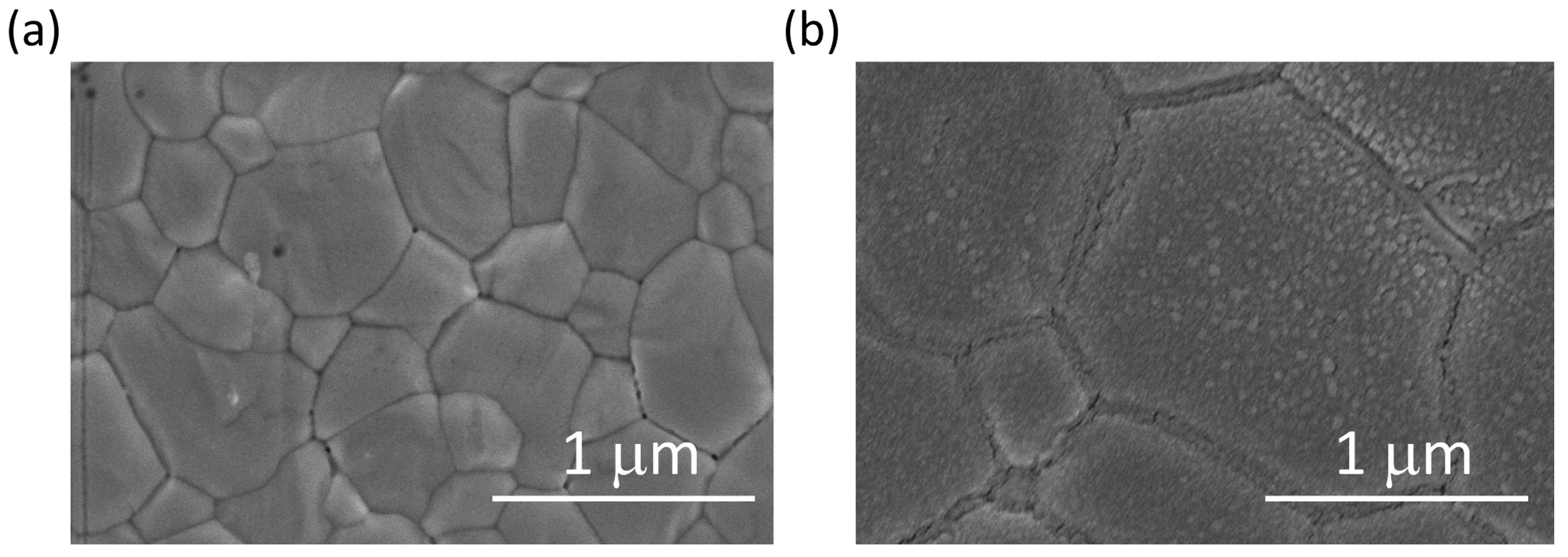
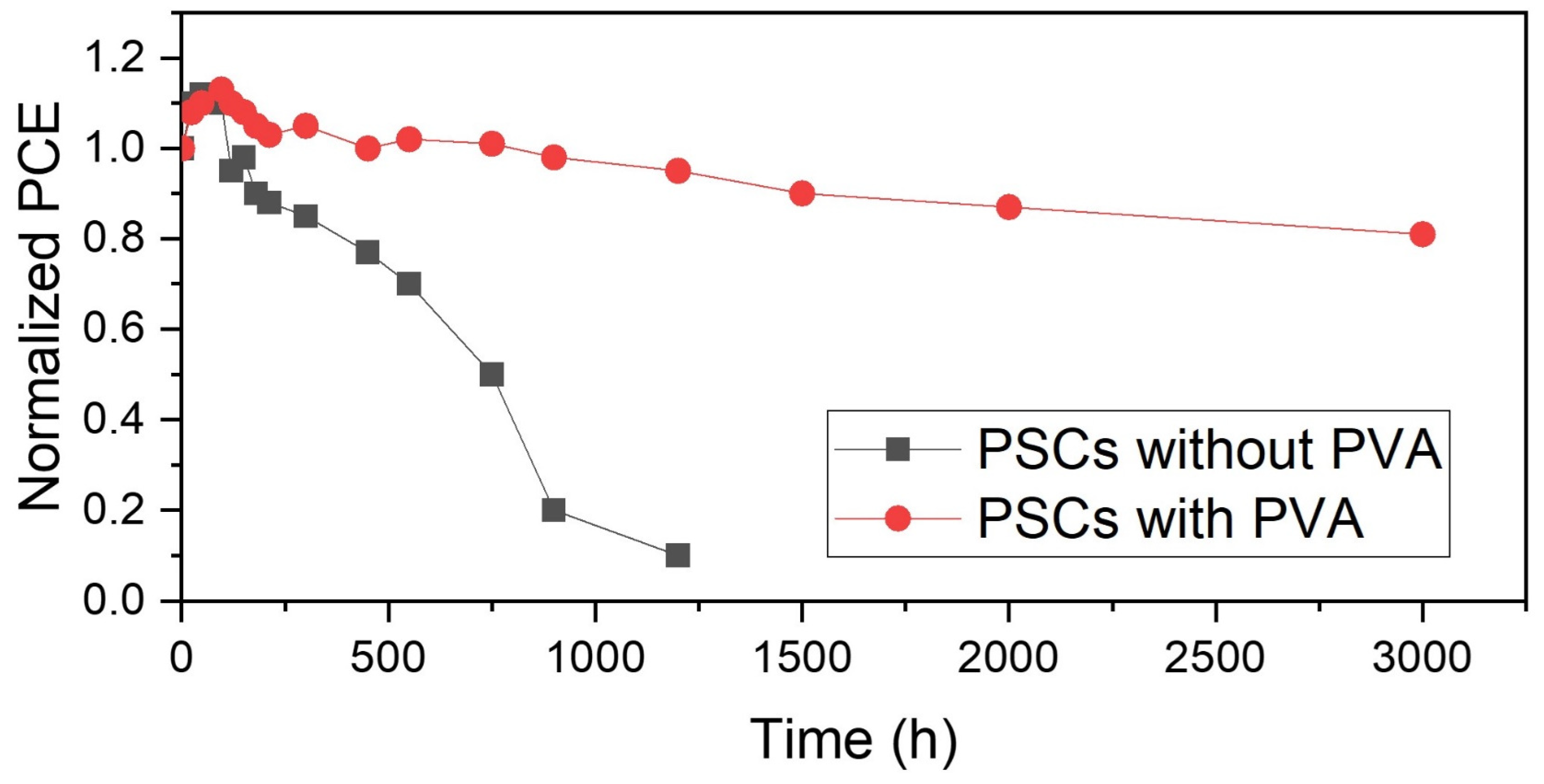
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Green, M.A.; Dunlop, E.D.; Hohl-Ebinger, J.; Yoshita, M.; Kopidakis, N.; Bothe, K.; Hinken, D.; Rauer, M.; Hao, X. Solar cell efficiency tables (Version 60). Prog. Photovolt. Res. Appl. 2022, 30, 687–701. [Google Scholar] [CrossRef]
- Huo, X.; Sun, W.; Wang, K.; Liu, W.; Yin, R.; Sun, Y.; Gao, Y.; You, T.; Yin, P. Preparation of High-Efficiency (>14%) HTL-Free Carbon-Based All-Inorganic Perovskite Solar Cells by Passivation with PABr Derivatives. ACS Appl. Mater. Interfaces 2023, 15, 9382–9391. [Google Scholar] [CrossRef]
- Zhang, H.; Yu, W.; Guo, J.; Xu, C.; Ren, Z.; Liu, K.; Yang, G.; Qin, M.; Huang, J.; Chen, Z. Excess PbI2 Management via Multimode Supramolecular Complex Engineering Enables High-Performance Perovskite Solar Cells. Adv. Energy Mater. 2022, 12, 2201663. [Google Scholar] [CrossRef]
- Yang, G.; Ren, Z.; Liu, K.; Qin, M.; Deng, W.; Zhang, H.; Wang, H.; Liang, J.; Ye, F.; Liang, Q.; et al. Stable and low-photovoltage-loss perovskite solar cells by multifunctional passivation. Nat. Photonics 2021, 15, 681–689. [Google Scholar] [CrossRef]
- Bhandari, S.; Roy, A.; Mallick, T.K.; Sundaram, S. Impact of different light induced effect on organic hole-transporting layer in perovskite solar cells. Mater. Lett. 2020, 268, 127568. [Google Scholar] [CrossRef]
- Bogachuk, D.; Tsuji, R.; Martineau, D.; Narbey, S.; Herterich, J.P.; Wagner, L.; Suginuma, K.; Ito, S.; Hinsch, A. Comparison of highly conductive natural and synthetic graphites for electrodes in perovskite solar cells. Carbon 2021, 178, 10–18. [Google Scholar] [CrossRef]
- Zhang, H.; Ren, Z.; Liu, K.; Qin, M.; Wu, Z.; Shen, D.; Zhang, Y.; Chandran, H.T.; Hao, J.; Lee, C.S. Controllable Heterogenous Seeding-Induced Crystallization for High-Efficiency FAPbI3-Based Perovskite Solar Cells Over 24%. Adv. Mater. 2022, 34, 2204366. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Qin, M.; Chen, Z.; Yu, W.; Ren, Z.; Liu, K.; Huang, J.; Zhang, Y.; Liang, Q.; Chandran, H.T. Bottom-Up Quasi-Epitaxial Growth of Hybrid Perovskite from Solution Process—Achieving High-Efficiency Solar Cells via Template-Guided Crystallization. Adv. Mater. 2021, 33, 2100009. [Google Scholar] [CrossRef]
- Qin, M.; Xue, H.; Zhang, H.; Hu, H.; Liu, K.; Li, Y.; Qin, Z.; Ma, J.; Zhu, H.; Yan, K. Precise Control of Perovskite Crystallization Kinetics via Sequential A-Site Doping. Adv. Mater. 2020, 32, 2004630. [Google Scholar] [CrossRef]
- Zhang, H.; Chen, Z.; Qin, M.; Ren, Z.; Liu, K.; Huang, J.; Shen, D.; Wu, Z.; Zhang, Y.; Hao, J. Multifunctional Crosslinking-Enabled Strain-Regulating Crystallization for Stable, Efficient α-FAPbI3-Based Perovskite Solar Cells. Adv. Mater. 2021, 33, 2008487. [Google Scholar] [CrossRef]
- Chen, B.; Rudd, P.N.; Yang, S.; Yuan, Y.; Huang, J. Imperfections and their passivation in halide perovskite solar cells. Chem. Soc. Rev. 2019, 48, 3842–3867. [Google Scholar] [CrossRef]
- Huang, J.; Yuan, Y.; Shao, Y.; Yan, Y. Understanding the physical properties of hybrid perovskites for photovoltaic applications. Nat. Rev. Mater. 2017, 2, 1–19. [Google Scholar] [CrossRef]
- Jordan, D.C.; Kurtz, S.R. Photovoltaic degradation rates—An analytical review. Prog. Photovolt. Res. Appl. 2013, 21, 12–29. [Google Scholar] [CrossRef]
- Gao, Y.; Hu, Y.; Yao, C.; Zhang, S. Recent Advances in Lead-Safe Perovskite Solar Cells. Adv. Funct. Mater. 2022, 32, 2208225. [Google Scholar] [CrossRef]
- Wang, X.; Dong, B.; Feng, M.; Xue, D.-J.; Wang, S.-M. Sustainable management of lead in perovskite solar cells. J. Mater. Chem. A 2022, 10, 15861–15864. [Google Scholar] [CrossRef]
- Li, X.; Zhang, F.; Wang, J.; Tong, J.; Xu, T.; Zhu, K. On-device lead-absorbing tapes for sustainable perovskite solar cells. Nat. Sustain. 2021, 4, 1038–1041. [Google Scholar] [CrossRef]
- Park, S.Y.; Park, J.-S.; Kim, B.J.; Lee, H.; Walsh, A.; Zhu, K.; Kim, D.H.; Jung, H.S. Sustainable lead management in halide perovskite solar cells. Nat. Sustain. 2020, 3, 1044–1051. [Google Scholar] [CrossRef]
- Piñón Reyes, A.C.; Ambrosio Lázaro, R.C.; Monfil Leyva, K.; Luna López, J.A.; Flores Méndez, J.; Heredia Jiménez, A.H.; Muñoz Zurita, A.L.; Severiano Carrillo, F.; Ojeda Durán, E. Study of a lead-free perovskite solar cell using CZTS as HTL to achieve a 20% PCE by SCAPS-1D simulation. Micromachines 2021, 12, 1508. [Google Scholar] [CrossRef]
- Rabhi, S.; Benzouid, H.; Slami, A.; Dadda, K. Modeling and Numerical Simulation of a CH3NH3SnI3 Perovskite Solar Cell Using the SCAPS1-D Simulator. Eng. Proc. 2023, 56, 97. [Google Scholar] [CrossRef]
- Ghosh, S.; Yasmin, S.; Ferdous, J.; Saha, B.B. Numerical Analysis of a CZTS Solar Cell with MoS2 as a Buffer Layer and Graphene as a Transparent Conducting Oxide Layer for Enhanced Cell Performance. Micromachines 2022, 13, 1249. [Google Scholar] [CrossRef]
- Yang, S.; Fu, W.; Zhang, Z.; Chen, H.; Li, C.-Z. Recent advances in perovskite solar cells: Efficiency, stability and lead-free perovskite. J. Mater. Chem. A 2017, 5, 11462–11482. [Google Scholar] [CrossRef]
- Giustino, F.; Snaith, H.J. Toward lead-free perovskite solar cells. ACS Energy Lett. 2016, 1, 1233–1240. [Google Scholar] [CrossRef]
- Zheng, W.; Sun, R.; Liu, Y.; Wang, X.; Liu, N.; Ji, Y.; Wang, L.; Liu, H.; Zhang, Y. Excitation management of lead-free perovskite nanocrystals through doping. ACS Appl. Mater. Interfaces 2021, 13, 6404–6410. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.; Song, P.; Tian, H.; Tian, C.; Tian, W.; Nan, Z.; Cai, Y.; Yang, P.; Sun, C.; Chen, J. Lead leakage preventable fullerene-porphyrin dyad for efficient and stable perovskite solar cells. Adv. Funct. Mater. 2022, 32, 2110139. [Google Scholar] [CrossRef]
- Luo, H.; Li, P.; Ma, J.; Han, L.; Zhang, Y.; Song, Y. Sustainable Pb management in perovskite solar cells toward eco-friendly development. Adv. Energy Mater. 2022, 12, 2201242. [Google Scholar] [CrossRef]
- Niu, T.; Lu, J.; Tang, M.-C.; Barrit, D.; Smilgies, D.-M.; Yang, Z.; Li, J.; Fan, Y.; Luo, T.; McCulloch, I. High performance ambient-air-stable FAPbI 3 perovskite solar cells with molecule-passivated Ruddlesden–Popper/3D heterostructured film. Energy Environ. Sci. 2018, 11, 3358–3366. [Google Scholar] [CrossRef]
- Xiao, X.; Wang, M.; Chen, S.; Zhang, Y.; Gu, H.; Deng, Y.; Yang, G.; Fei, C.; Chen, B.; Lin, Y. Lead-adsorbing ionogel-based encapsulation for impact-resistant, stable, and lead-safe perovskite modules. Sci. Adv. 2021, 7, eabi8249. [Google Scholar] [CrossRef] [PubMed]
- Liang, J.; Wang, C.; Wang, Y.; Xu, Z.; Lu, Z.; Ma, Y.; Zhu, H.; Hu, Y.; Xiao, C.; Yi, X. All-inorganic perovskite solar cells. J. Am. Chem. Soc. 2016, 138, 15829–15832. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Wu, X.; Wu, S.; Gao, D.; Dong, H.; Huang, F.; Hu, X.; Jen, A.K.-Y.; Zhu, Z. An effective and economical encapsulation method for trapping lead leakage in rigid and flexible perovskite photovoltaics. Nano Energy 2022, 93, 106853. [Google Scholar] [CrossRef]
- Chao, L.; Niu, T.; Xia, Y.; Chen, Y.; Huang, W. Ionic Liquid for Perovskite Solar Cells: An Emerging Solvent Engineering Technology. Acc. Mater. Res. 2021, 2, 1059–1070. [Google Scholar] [CrossRef]
- Wu, P.; Wang, S.; Li, X.; Zhang, F. Beyond efficiency fever: Preventing lead leakage for perovskite solar cells. Matter 2022, 5, 1137–1161. [Google Scholar] [CrossRef]
- Rong, Y.; Hu, Y.; Mei, A.; Tan, H.; Saidaminov, M.I.; Seok, S.I.; McGehee, M.D.; Sargent, E.H.; Han, H. Challenges for commercializing perovskite solar cells. Science 2018, 361, eaat8235. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Liang, C.; Sun, M.; Sun, F.; Ji, C.; Wan, X.; Li, D.; You, F.; He, Z. Controlled Crystallization of CsRb-Based Multi-Cation Perovskite Using a Blended Sequential Process for High-Performance Solar Cells. Solar RRL 2021, 5, 2100050. [Google Scholar] [CrossRef]
- Schmidt, F.; Amrein, M.; Hedwig, S.; Kober-Czerny, M.; Paracchino, A.; Holappa, V.; Suhonen, R.; Schäffer, A.; Constable, E.C.; Snaith, H.J. Organic solvent free PbI2 recycling from perovskite solar cells using hot water. J. Hazard. Mater. 2023, 447, 130829. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.; Fei, C.; Chen, S.; Gu, H.; Xiao, X.; Huang, J. Recycling lead and transparent conductors from perovskite solar modules. Nat. Commun. 2021, 12, 5859. [Google Scholar] [CrossRef] [PubMed]
- Feng, X.; Guo, Q.; Xiu, J.; Ying, Z.; Ng, K.W.; Huang, L.; Wang, S.; Pan, H.; Tang, Z.; He, Z. Close-loop recycling of perovskite solar cells through dissolution-recrystallization of perovskite by butylamine. Cell Rep. Phys. Sci. 2021, 2, 2. [Google Scholar] [CrossRef]
- Dewi, H.A.; Li, J.; Wang, H.; Chaudhary, B.; Mathews, N.; Mhaisalkar, S.; Bruno, A. Excellent Intrinsic Long-Term Thermal Stability of Co-Evaporated MAPbI3 Solar Cells at 85 °C. Adv. Funct. Mater. 2021, 31, 2100557. [Google Scholar] [CrossRef]
- Deng, Y.; Xu, S.; Chen, S.; Xiao, X.; Zhao, J.; Huang, J. Defect compensation in formamidinium–caesium perovskites for highly efficient solar mini-modules with improved photostability. Nat. Energy 2021, 6, 633–641. [Google Scholar] [CrossRef]
- Zong, Y.; Zhou, Y.; Zhang, Y.; Li, Z.; Zhang, L.; Ju, M.-G.; Chen, M.; Pang, S.; Zeng, X.C.; Padture, N.P. Continuous grain-boundary functionalization for high-efficiency perovskite solar cells with exceptional stability. Chem 2018, 4, 1404–1415. [Google Scholar] [CrossRef]
- Yang, G.; Chen, C.; Yao, F.; Chen, Z.; Zhang, Q.; Zheng, X.; Ma, J.; Lei, H.; Qin, P.; Xiong, L. Effective carrier-concentration tuning of SnO2 quantum dot electron-selective layers for high-performance planar perovskite solar cells. Adv. Mater. 2018, 30, 1706023. [Google Scholar] [CrossRef]
- Hu, Y.; He, Z.; Jia, X.; Zhang, S.; Tang, Y.; Wang, J.; Wang, M.; Sun, G.; Yuan, G.; Han, L. Dual functions of performance improvement and lead leakage mitigation of perovskite solar cells enabled by phenylbenzimidazole sulfonic acid. Small Methods 2022, 6, 2101257. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Zhang, H.; Zhang, Y.; Hu, R.; Jiang, M.; Zhang, R.; Lv, H.; Tian, J.; Chu, L.; Zhang, J. Enhancing the performance of inverted perovskite solar cells via grain boundary passivation with carbon quantum dots. ACS Appl. Mater. Interfaces 2018, 11, 3044–3052. [Google Scholar] [CrossRef] [PubMed]
- Yoo, J.J.; Seo, G.; Chua, M.R.; Park, T.G.; Lu, Y.; Rotermund, F.; Kim, Y.-K.; Moon, C.S.; Jeon, N.J.; Correa-Baena, J.-P. Efficient perovskite solar cells via improved carrier management. Nature 2021, 590, 587–593. [Google Scholar] [CrossRef]
- Li, X.; Zhang, F.; He, H.; Berry, J.J.; Zhu, K.; Xu, T. On-device lead sequestration for perovskite solar cells. Nature 2020, 578, 555–558. [Google Scholar] [CrossRef]
- Jung, M.; Ji, S.-G.; Kim, G.; Seok, S.I. Perovskite precursor solution chemistry: From fundamentals to photovoltaic applications. Chem. Soc. Rev. 2019, 48, 2011–2038. [Google Scholar] [CrossRef]
- Snaith, H.J. Perovskites: The emergence of a new era for low-cost, high-efficiency solar cells. J. Phys. Chem. Lett. 2013, 4, 3623–3630. [Google Scholar] [CrossRef]
- Bai, S.; Da, P.; Li, C.; Wang, Z.; Yuan, Z.; Fu, F.; Kawecki, M.; Liu, X.; Sakai, N.; Wang, J.T.-W. Planar perovskite solar cells with long-term stability using ionic liquid additives. Nature 2019, 571, 245–250. [Google Scholar] [CrossRef]
- Bi, D.; Yi, C.; Luo, J.; Décoppet, J.-D.; Zhang, F.; Zakeeruddin, S.M.; Li, X.; Hagfeldt, A.; Grätzel, M. Polymer-templated nucleation and crystal growth of perovskite films for solar cells with efficiency greater than 21%. Nat. Energy 2016, 1, 1–5. [Google Scholar] [CrossRef]
- Chen, S.; Deng, Y.; Xiao, X.; Xu, S.; Rudd, P.N.; Huang, J. Preventing lead leakage with built-in resin layers for sustainable perovskite solar cells. Nat. Sustain. 2021, 4, 636–643. [Google Scholar] [CrossRef]
- Xue, D.-J.; Hou, Y.; Liu, S.-C.; Wei, M.; Chen, B.; Huang, Z.; Li, Z.; Sun, B.; Proppe, A.H.; Dong, Y. Regulating strain in perovskite thin films through charge-transport layers. Nat. Commun. 2020, 11, 1514. [Google Scholar] [CrossRef]
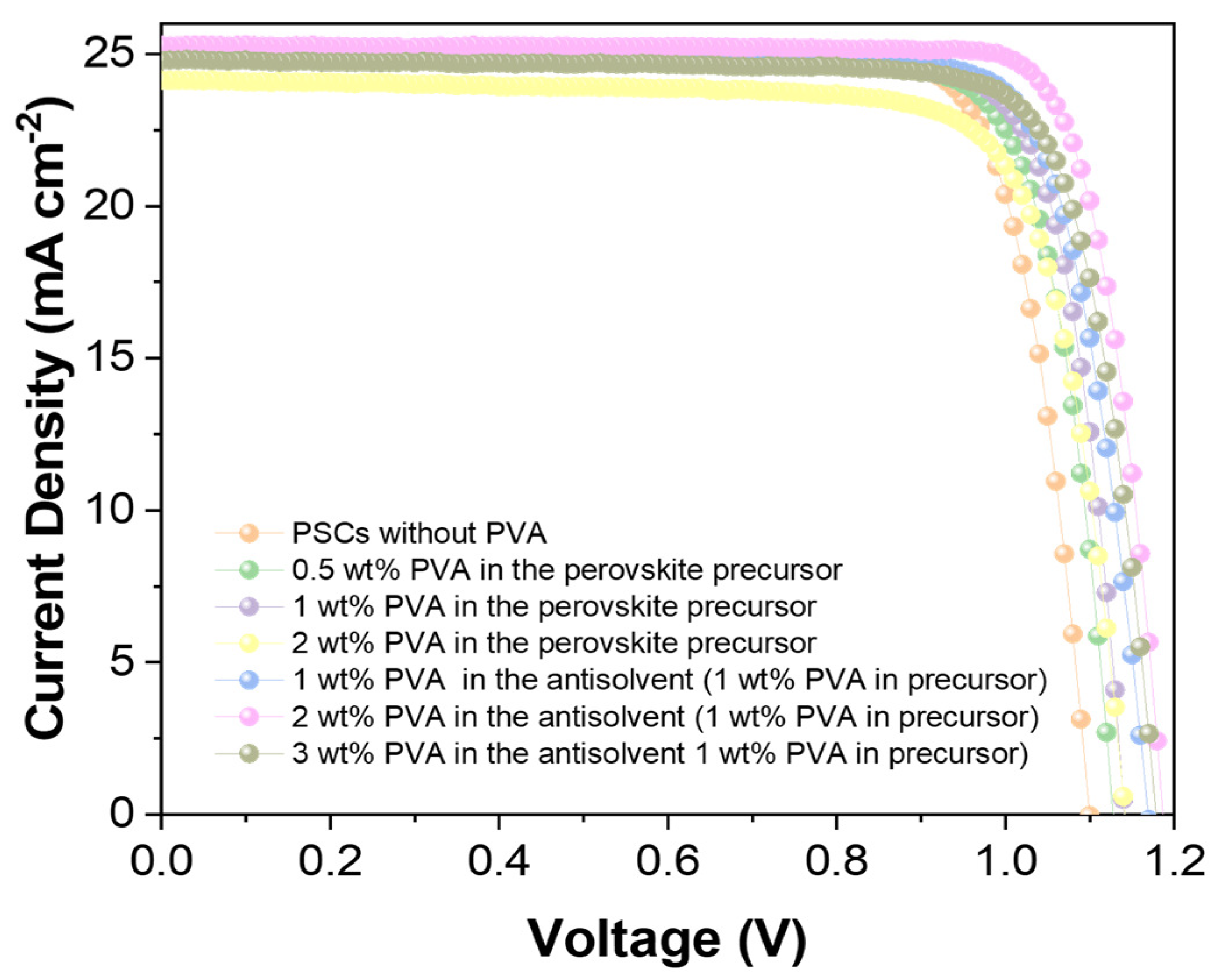
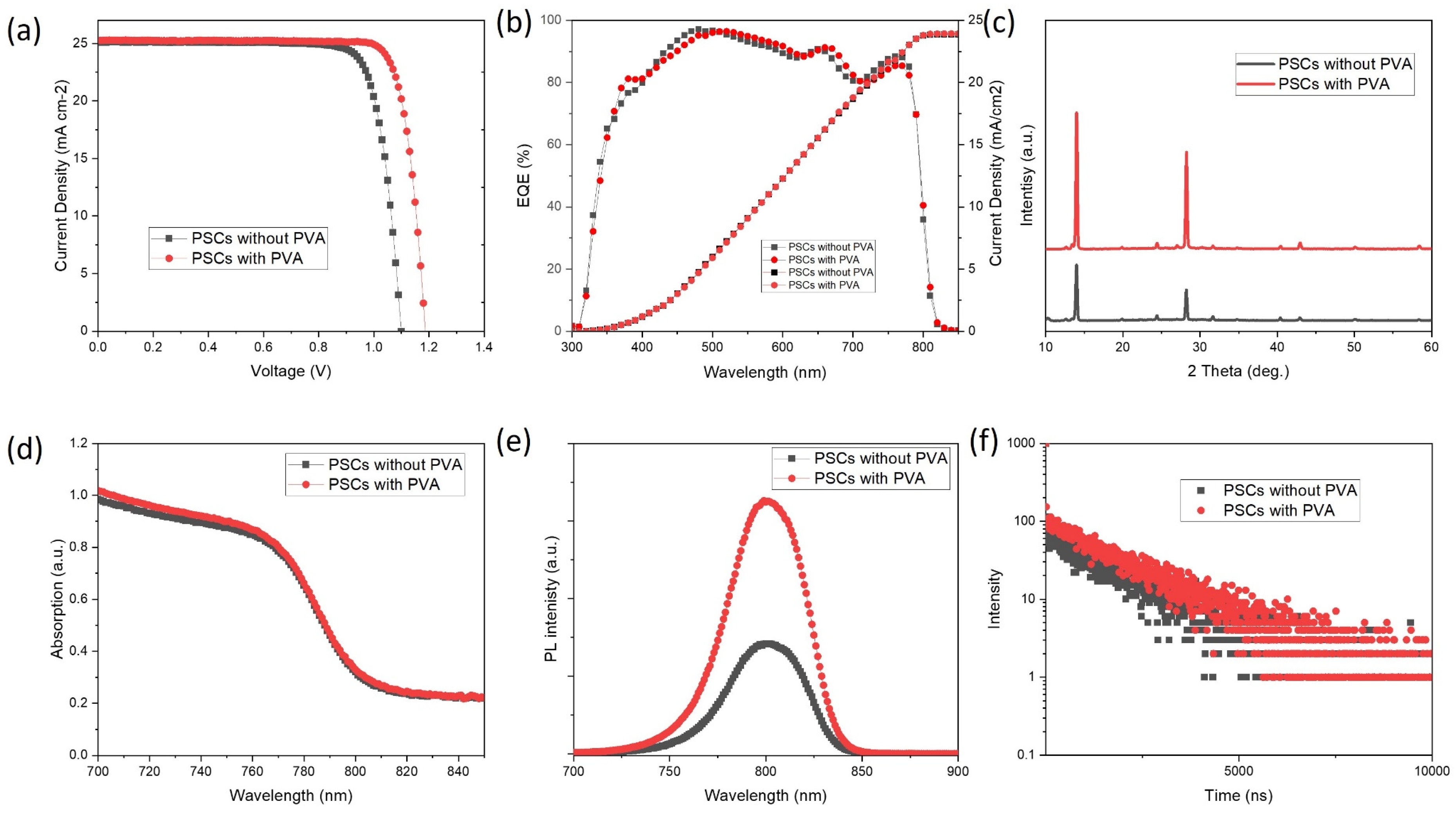
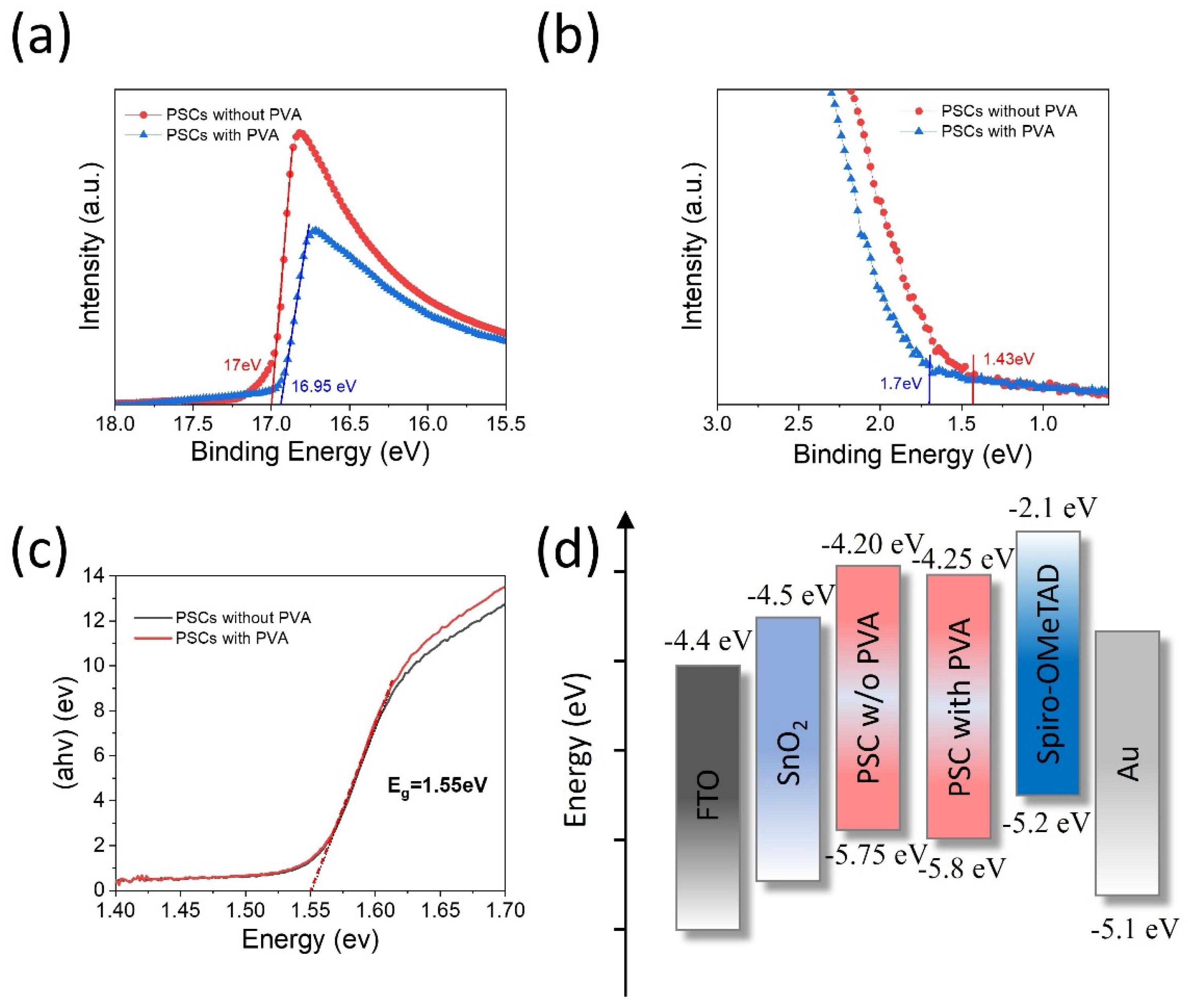

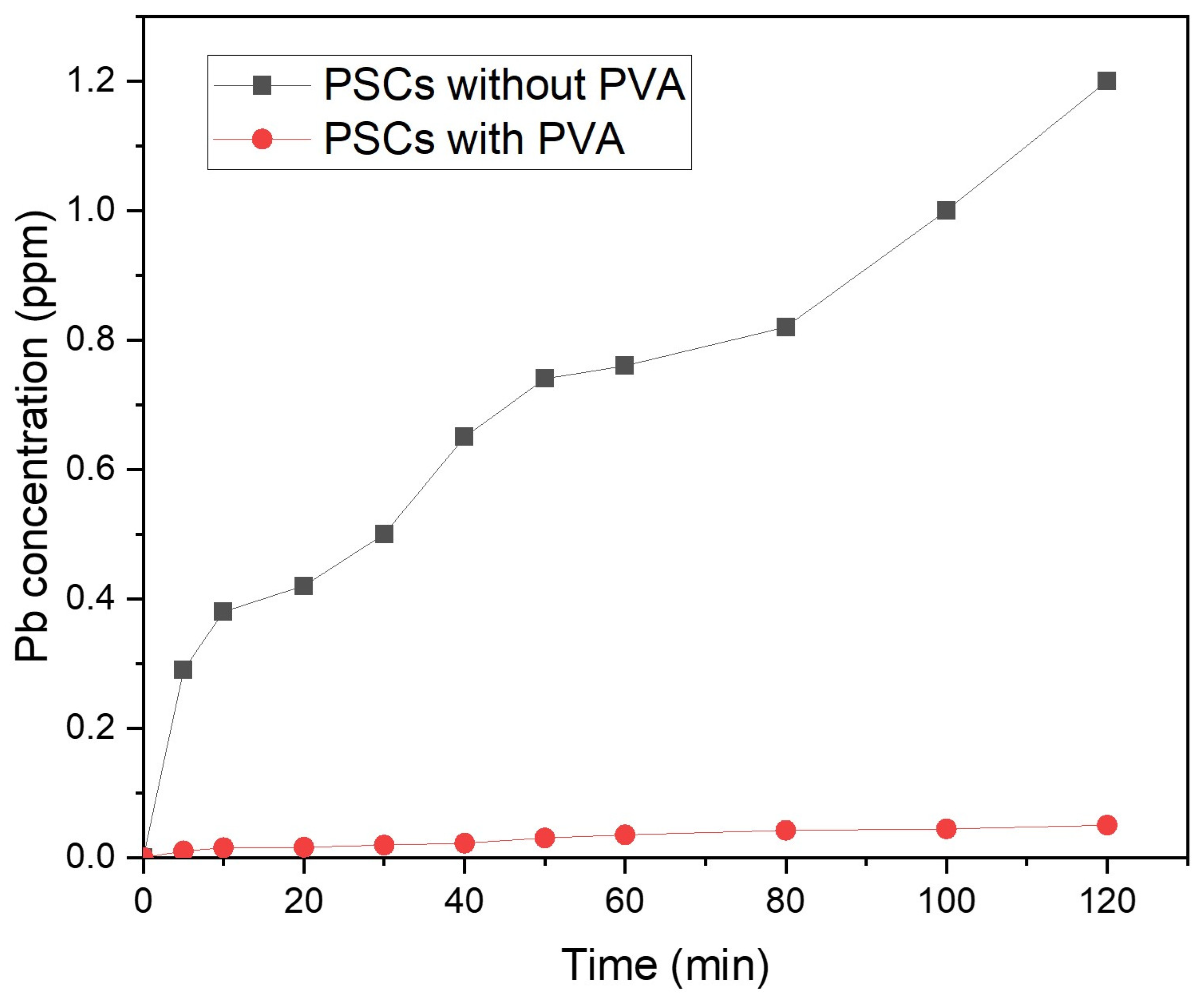
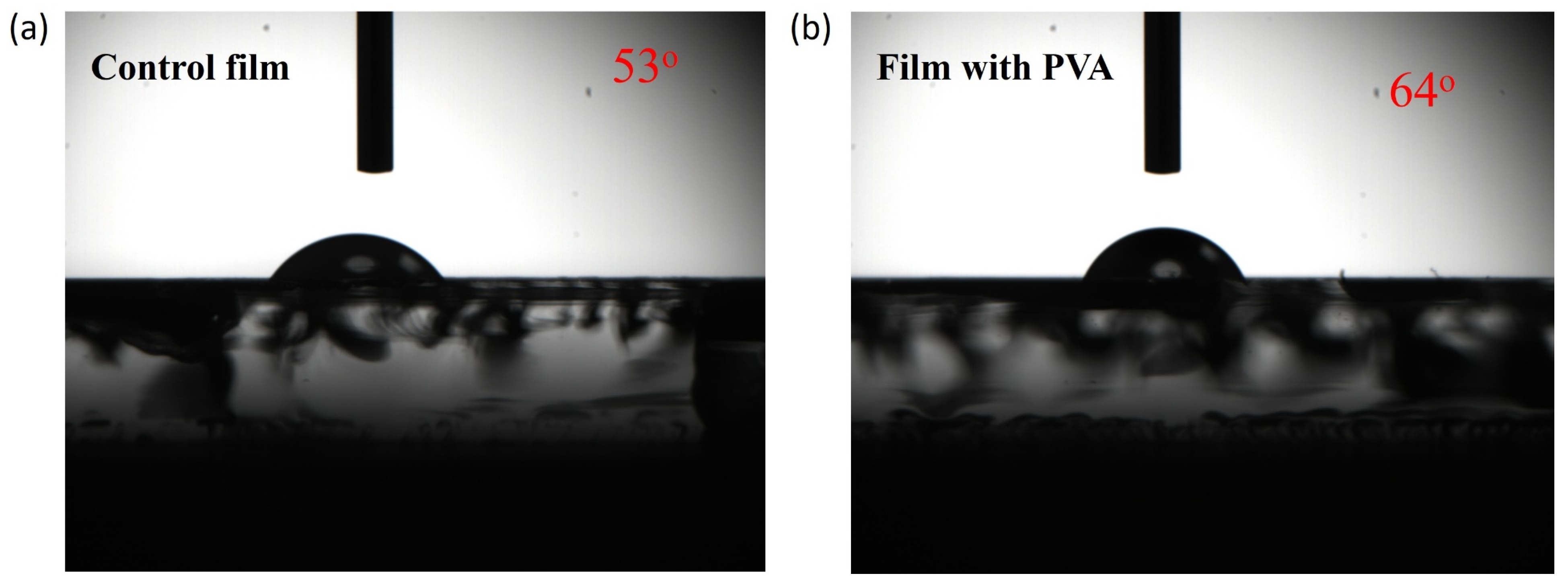
| Jsc | Voc | FF | PCE | |
|---|---|---|---|---|
| PSCs without PVA | 25.0 | 1.1 | 0.81 | 22.3 |
| PSCs with PVA | 25.3 | 1.19 | 0.82 | 24.7 |
| 1 wt% PVA in the Antisolvent | 2 wt% PVA in the Antisolvent | 3 wt% PVA in the Antisolvent | |
|---|---|---|---|
| Jsc | 25.19462 | 25.29255 | 24.78955 |
| Voc | 1.16953 | 1.18982 | 1.17976 |
| FF | 0.80265 | 0.82372 | 0.79856 |
| Efficiency | 23.65078 | 24.78869 | 23.35446 |
| A1 | τ1 | A2 | τ2 | |
|---|---|---|---|---|
| PSCs without PVA | 104 | 13.4 | 60 | 1600 |
| PSCs with PVA | 506 | 4.38 | 98 | 1757 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, B.; Jia, Z.; Chen, Z. A Direct Chemical Approach to Mitigate Environment Lead Contamination in Perovskite Solar Cells. Energies 2024, 17, 1629. https://doi.org/10.3390/en17071629
Liu B, Jia Z, Chen Z. A Direct Chemical Approach to Mitigate Environment Lead Contamination in Perovskite Solar Cells. Energies. 2024; 17(7):1629. https://doi.org/10.3390/en17071629
Chicago/Turabian StyleLiu, Benjamin, Zihan Jia, and Zhiliang Chen. 2024. "A Direct Chemical Approach to Mitigate Environment Lead Contamination in Perovskite Solar Cells" Energies 17, no. 7: 1629. https://doi.org/10.3390/en17071629
APA StyleLiu, B., Jia, Z., & Chen, Z. (2024). A Direct Chemical Approach to Mitigate Environment Lead Contamination in Perovskite Solar Cells. Energies, 17(7), 1629. https://doi.org/10.3390/en17071629







