The Relevance of Lithium Salt Solvate Crystals in Superconcentrated Electrolytes in Lithium Batteries
Abstract
1. Introduction
2. Theoretical Methods
3. Results and Discussion
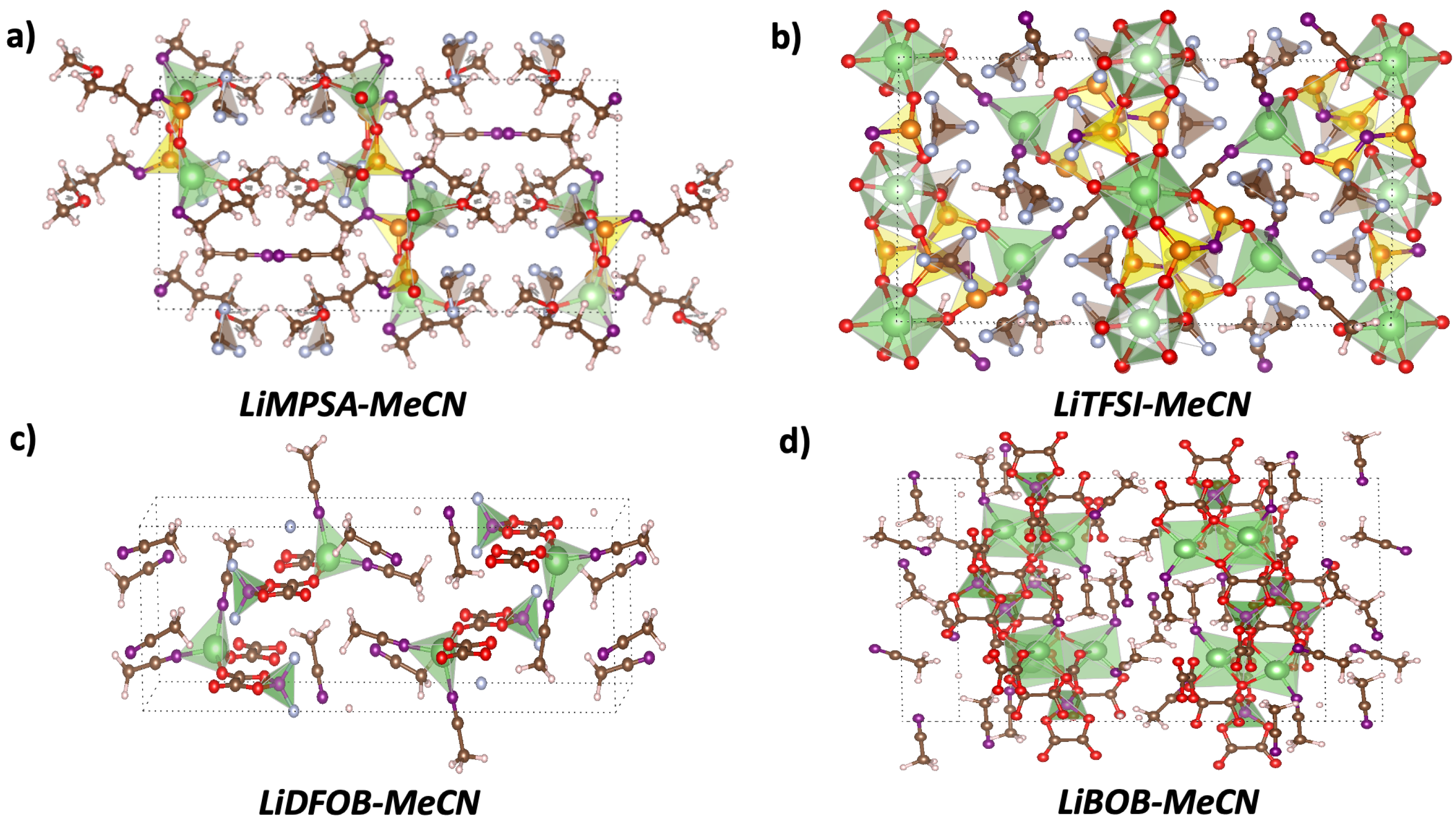
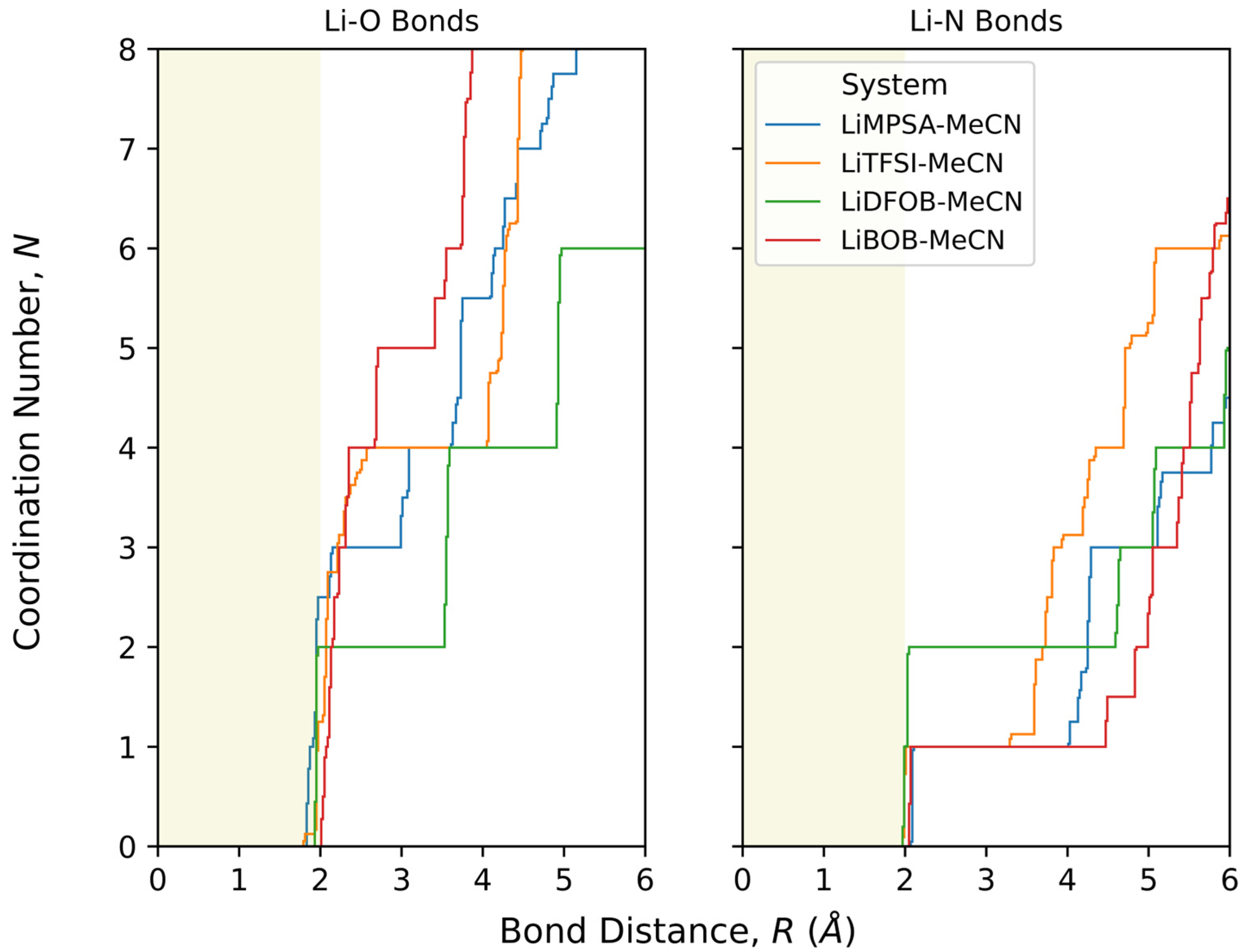
3.1. Structure Analysis
3.1.1. LiMPSA-MeCN Solvate Crystal
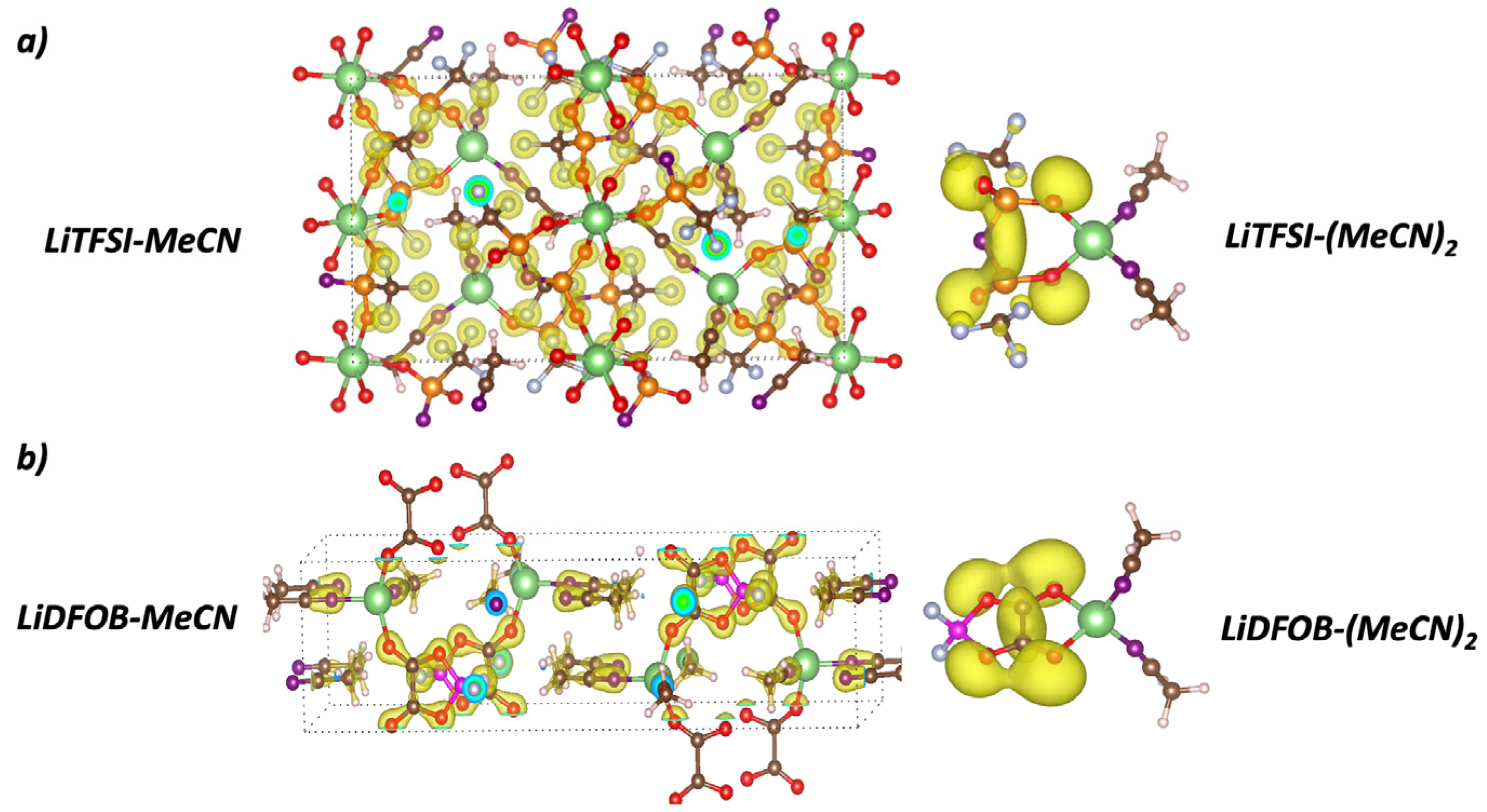
3.1.2. LiTFSI-MeCN Solvate Crystal
3.1.3. LiDFOB-MeCN Solvate Crystal
3.1.4. LiBOB-MeCN Solvate Crystal
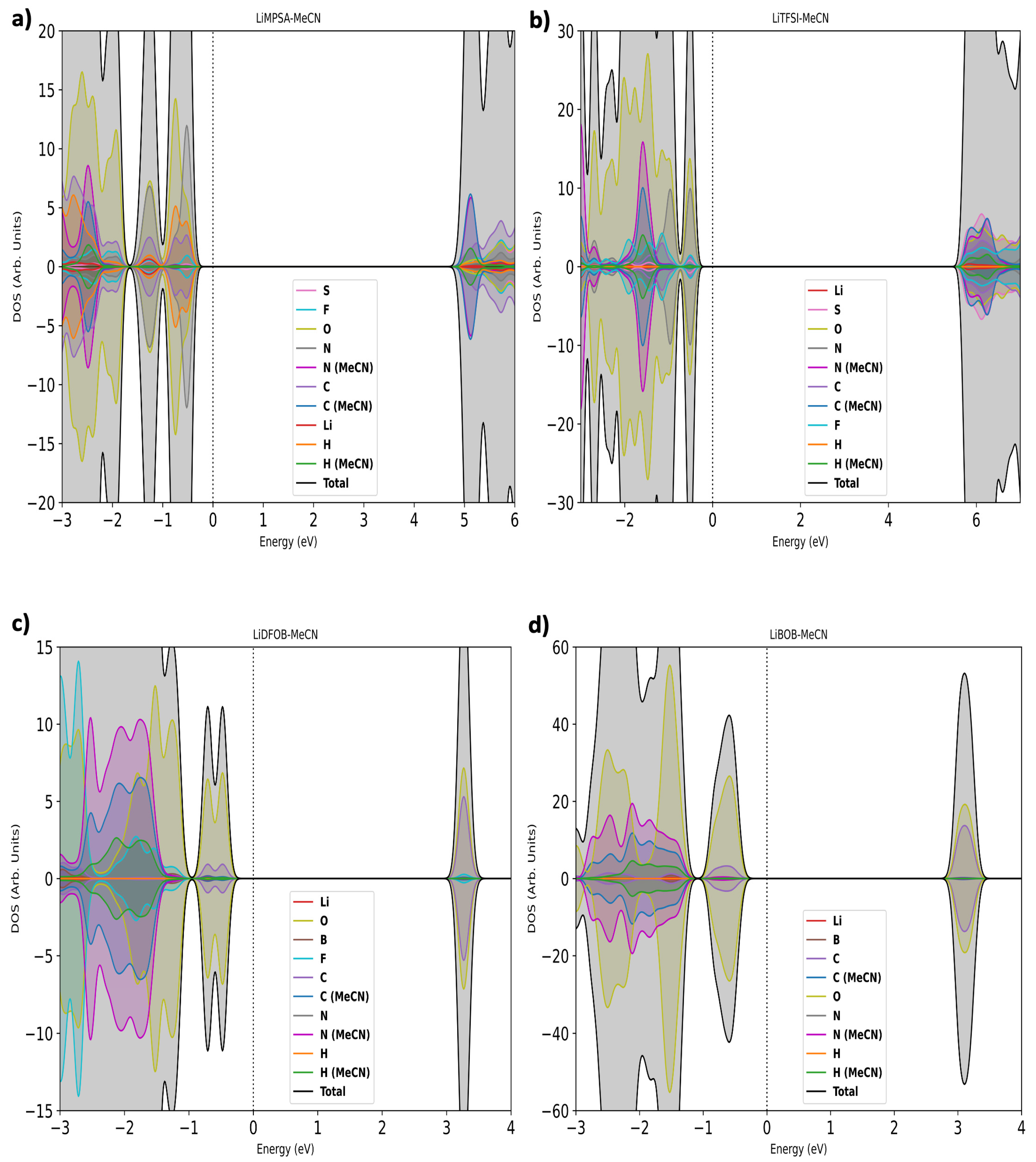
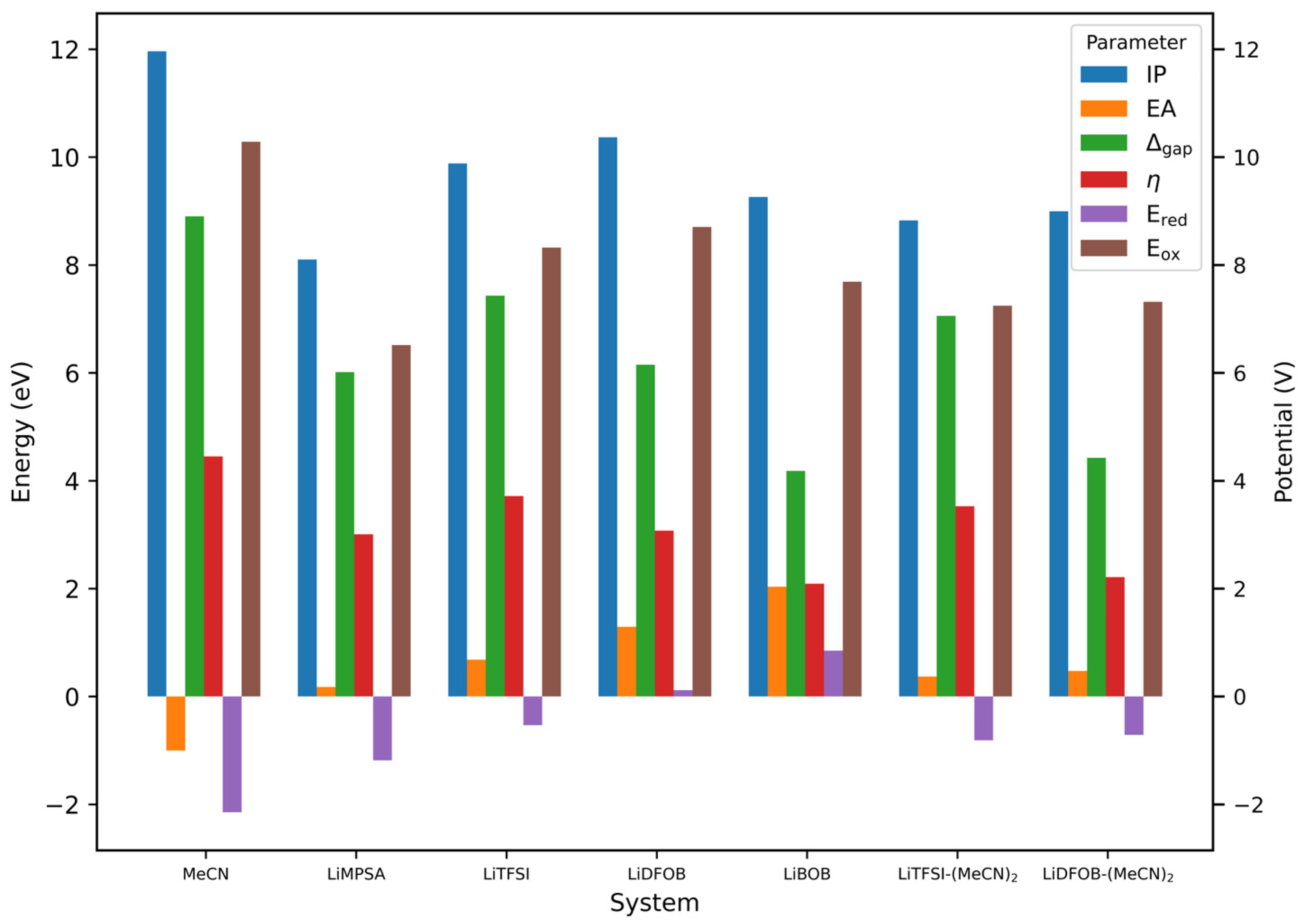
3.2. Electronic Properties of Solvate Crystals
3.3. Electrochemical Stability of MeCN and Lithium Salt Molecule
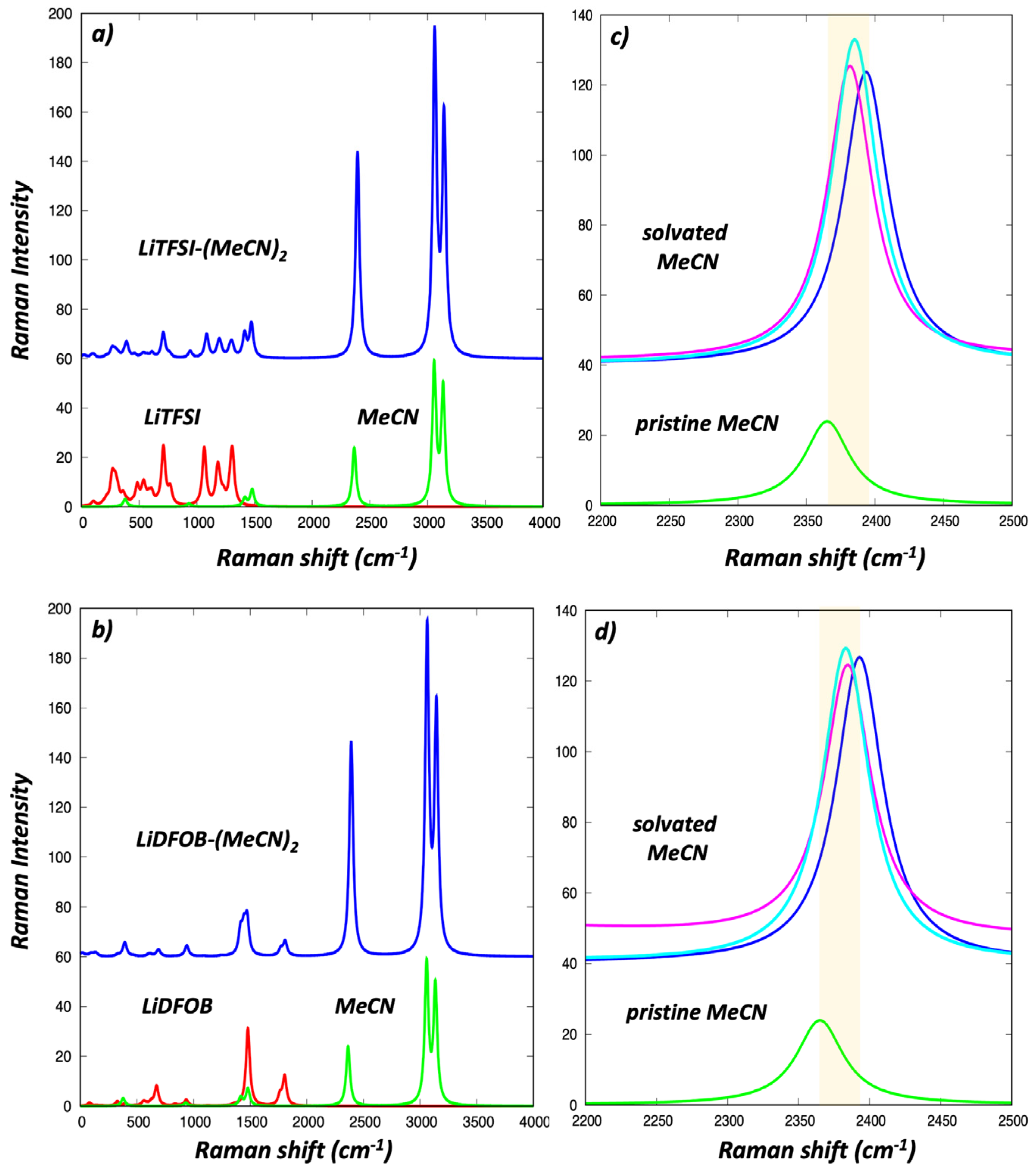
3.4. Electrochemical Stability and Electronic and Raman Signature of Salt-MeCN Molecular Solvates
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Grey, C.P.; Hall, D.S. Prospects for lithium-ion batteries and beyond—A 2030 vision. Nat. Commun. 2020, 11, 6279. [Google Scholar] [CrossRef] [PubMed]
- Goodenough, J.B.; Park, K.S. The Li-Ion Rechargeable Battery: A Perspective. J. Am. Chem. Soc. 2013, 135, 1167–1176. [Google Scholar] [CrossRef] [PubMed]
- Kang, X. Electrolytes and Interphases in Li-Ion Batteries and Beyond. Chem. Rev. 2014, 114, 11503–11618. [Google Scholar]
- Riddick, J.A.; Bungh, W.B.; Sakano, T.K. Organic Solvents: Physical Properties and Methods of Purification, 4th ed.; Willey: New York, NY, USA, 1986. [Google Scholar]
- Rupich, M.W.; Pitts, L.; Abraham, K.M.J. Characterization of Reactions and Products of the Discharge and Forced Overdischarge of Li /SO2 Cells. Electrochem. Soc. 1982, 129, 1857–1861. [Google Scholar] [CrossRef]
- Jeong, S.-K.; Seo, H.-Y.; Kim, D.-H.; Han, H.-K.; Kim, J.-G.; Lee, Y.B.; Iriyama, Y.; Abe, T.; Ogumi, Z. Suppression of dendritic lithium formation by using concentrated electrolyte solutions. Electrochem. Commun. 2008, 10, 635–638. [Google Scholar] [CrossRef]
- Yamada, Y.; Furukawa, K.; Sodeyama, K.; Kikuchi, K.; Yaegashi, M.; Tateyama, Y.; Yamada, A. Unusual Stability of Acetonitrile-Based Superconcentrated Electrolytes for Fast-Charging Lithium-Ion Batteries. J. Am. Chem. Soc. 2014, 136, 5039–5046. [Google Scholar] [CrossRef] [PubMed]
- Azov, V.A.; Egorova, K.S.; Seitkalieva, M.M.; Kashin, A.S.; Ananikov, V.P. “Solvent-in-Salt” Systems for Design of New Materials in Chemistry, Biology and Energy Research. Chem. Soc. Rev. 2018, 47, 1250–1284. [Google Scholar] [CrossRef] [PubMed]
- Borodin, O.; Self, J.; Persson, K.A.; Wang, C.; Xu, K. Uncharted Waters: Super-Concentrated Electrolytes. Joule 2020, 4, 69–100. [Google Scholar] [CrossRef]
- Yamada, Y.; Yamada, A. Superconcentrated Electrolytes for Lithium Batteries. J. Electrochem. Soc. 2015, 162, A2406–A2423. [Google Scholar] [CrossRef]
- Seo, D.M.; Borodin, O.; Han, S.-D.; Boyle, P.D.; Henderson, W.A. Electrolyte Solvation and Ionic Association II. Acetonitrile-Lithium Salt Mixtures: Highly Dissociated Salts. J. Electrochem. Soc. 2012, 159, A1489–A1500. [Google Scholar] [CrossRef]
- Seo, D.M.; Borodin, O.; Balogh, D.; O’Connell, M.; Ly, Q.; Han, S.-D.; Passerini, S.; Henderson, W.A. Electrolyte Solvation and Ionic Association III. Acetonitrile-Lithium Salt Mixtures: Transport Properties. J. Electrochem. Soc. 2013, 160, A1061–A1070. [Google Scholar] [CrossRef]
- Dillon, R.E.A.; Stern, C.L.; Shriver, D.F. X-ray Structure Determinations of Li[CF3SO2N(CH2)3OCH3] and the Solid Electrolyte [Li⊂12-C-4] [CF3SO2N(CH2)3OCH3]. Chem. Mater. 2000, 12, 1122–1126. [Google Scholar] [CrossRef]
- Seo, D.M.; Boyle, P.D.; Henderson, W.A. Poly[bis(acetonitrile-κN)bis[μ_3-bis(trifluoromethanesulfonyl)imido-κ^4O,O’:O”:O”’]dilithium]. Acta Cryst. 2011, E67, m534. [Google Scholar]
- Han, S.-D.; Allen, J.L.; Jonsson, E.; Johansson, P.; McOwen, D.W.; Boyle, P.D.; Henderson, W.A. Solvate Structures and Computational/Spectroscopic Characterization of Lithium Difluoro(oxalate)borate (LiDFOB) Electrolytes. J. Phys. Chem. C 2013, 117, 5521–5531. [Google Scholar] [CrossRef]
- Zavalij, P.Y.; Yang, S.; Whittingham, M.S. Structural Chemistry of new lithium bis(oxalate)-borate solvates. Acta Cryst. 2004, B60, 716–724. [Google Scholar] [CrossRef] [PubMed]
- Kresse, G.; Furthmüller, J. Efficient Iterative Schemes for Ab Initio Total-Energy Calculations Using a Plane-Wave Basis Set. Phys. Rev. B 1996, 54, 11169–11186. [Google Scholar] [CrossRef]
- Kresse, G.; Furthmüller, J. Efficiency of Ab-Initio Total Energy Calculations for Metals and Semiconductors Using a Plane-Wave Basis Set. Comput. Mater. Sci. 1996, 6, 15–50. [Google Scholar] [CrossRef]
- Blöchl, P.E. Projector Augmented-Wave Method. Phys. Rev. B 1994, 50, 17953. [Google Scholar] [CrossRef]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized Gradient Approximation Made Simple. Phys. Rev. Lett. 1996, 77, 3865. [Google Scholar] [CrossRef]
- Grimme, S.; Antony, J.; Ehrlich, S.; Krieg, H. A Consistent and Accurate Ab Initio Parametrization of Density Functional Dispersion Correction (Dft-D) for the 94 Elements H-Pu. J. Chem. Phys. 2010, 132, 154104. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; et al. Gaussian 16, Revision C.01; Gaussian, Inc.: Wallingford, CT, USA, 2016. [Google Scholar]
- Becke, A.D. Density-functional thermochemistry. III. The role of exact exchange. J. Chem. Phys. 1993, 98, 5648. [Google Scholar] [CrossRef]
- Assary, R.S.; Brushett, F.R.; Curtiss, L.A. Reduction potential predictions of some aromatic nitrogen-containing molecules. RSC Adv. 2014, 4, 57442–57451. [Google Scholar] [CrossRef]
- Marenich, A.V.; Ho, J.; Coote, M.L.; Cramer, C.J.; Truhlar, D.G. Computational electrochemistry: Prediction of liquid-phase reduction potentials. Phys. Chem. Chem. Phys. 2014, 16, 15068–15106. [Google Scholar] [PubMed]
- Lau, K.C.; Kandalam, A.K.; Costales, A.; Pandey, R. Equilibrium geometry and electron detachment energies of anionic Cr2O4, Cr2O5, and Cr2O6 clusters. Chem. Phys. Lett. 2004, 393, 112–117. [Google Scholar] [CrossRef]
- Fu, Y.; Liu, L.; Yu, H.-Z.; Wang, Y.-M.; Guo, Q.-X. Quantum chemical prediction of absolute standard redox potentials of diverse organic molecules and free radicals in acetonitrile. J. Am. Chem. Soc. 2005, 127, 7227–7234. [Google Scholar] [CrossRef]
- Boothroyd, S.; Kerridge, A.; Broo, A.; Buttar, D.; Anwar, J. Why Do Some Molecules Form Hydrates or Solvates? Cryst. Growth. Des. 2018, 18, 1903–1908. [Google Scholar] [CrossRef]
- Grothe, E.; Meekes, H.; Vlieg, E.; ter Horst, J.H.; de Gelder, R. Solvates, Salts, and Cocrystals: A Proposal for a Feasible Classification System. Cryst. Growth. Des. 2016, 16, 3237–3243. [Google Scholar] [CrossRef]
- Schkeryantz, L.; Nguyen, P.; McCulloch, W.D.; Moore, C.E.; Lau, K.C.; Wu, Y. Unusual Melting Trend in an Alkali Asymmetric Sulfonamide Salt Series: Single-Crystal Analysis and Modeling. Inorg. Chem. 2021, 60, 14679–14686. [Google Scholar] [CrossRef]
- Schkeryantz, L.; Nguyen, P.; McCulloch, W.D.; Moore, C.E.; Lau, K.C.; Wu, Y. K+ Single Cation Ionic Liquids Electrolytes with Low Melting Asymmetric Salt. J. Phys. Chem. C 2022, 126, 11407–11413. [Google Scholar] [CrossRef]
- van de Streek, J. All Series of Multiple Solvates (Including Hydrates) from the Cambridge Structural Database. CrystEngComm 2007, 9, 350–352. [Google Scholar] [CrossRef]
- See, K.A.; Wu, H.-L.; Lau, K.C.; Shin, M.; Cheng, L.; Balasubramanian, M.; Gallagher, K.G.; Curtiss, L.A.; Gewirth, A.A. Effect of Hydrofluoroether Cosolvent Addition on Li Solvation in Acetonitrile-Based Solvate Electrolytes and Its Influence on S Reduction in a Li–S Battery. ACS Appl. Mater. Interfaces 2016, 8, 34360–34371. [Google Scholar] [CrossRef] [PubMed]
- Lau, K.C.; Dunlap, B.I. Lattice dielectric and thermodynamic properties of yttria stabilized zirconia solids. J. Phys. Condens. Matter 2009, 21, 145402. [Google Scholar] [CrossRef] [PubMed]
- Ugata, Y.; Thomas, M.L.; Mandai, T.; Ueno, K.; Dokko, K.; Watanabe, M. Li-Ion Hopping Conduction in Highly Concentrated Lithium Bis (Fluorosulfonyl) Amide/Dinitrile Liquid Electrolytes. Phys. Chem. Chem. Phys. 2019, 21, 9759–9768. [Google Scholar] [CrossRef] [PubMed]
- Camacho-Forero, L.E.; Smith, T.W.; Balbuena, P.B. Effects of High and Low Salt Concentration in Electrolytes at Lithium–Metal Anode Surfaces. J. Phys. Chem. C 2016, 121, 182–194. [Google Scholar] [CrossRef]
- Dokko, K.; Watanabe, D.; Ugata, Y.; Thomas, M.L.; Tsuzuki, S.; Shinoda, W.; Hashimoto, K.; Ueno, K.; Umebayashi, Y.; Watanabe, M. Direct Evidence for Li Ion Hopping Conduction in Highly Concentrated Sulfolane-Based Liquid Electrolytes. J. Phys. Chem. B 2018, 122, 10736–10745. [Google Scholar] [CrossRef]
- Chen, S.; Zheng, J.; Yu, L.; Ren, X.; Engelhard, M.H.; Niu, C.; Lee, H.; Xu, W.; Xiao, J.; Liu, J.; et al. High-Efficiency Lithium Metal Batteries with Fire-Retardant Electrolytes. Joule 2018, 2, 1548. [Google Scholar] [CrossRef]
- Cao, X.; Ren, X.; Zou, L.; Engelhard, M.H.; Huang, W.; Wang, H.; Matthews, B.E.; Lee, H.; Niu, C.; Arey, B.W.; et al. Monolithic solid–electrolyte interphases formed in fluorinated orthoformate-based electrolytes minimize Li depletion and pulverization. Nat. Energy 2019, 4, 796. [Google Scholar] [CrossRef]
- Goodenough, J.B.; Kim, Y. Challenges for Rechargeable Li Batteries. Chem. Mater. 2010, 22, 587–603. [Google Scholar] [CrossRef]
- Manthiram, A. Materials Challenges and Opportunities of Lithium Ion Batteries. J. Phys. Chem. Lett. 2011, 2, 176–184. [Google Scholar] [CrossRef]
- Assary, R.S.; Curtiss, L.A.; Redfern, P.C.; Zhang, Z.; Amine, K. Computational Studies of Polysiloxanes: Oxidation Potentials and Decomposition Reactions. J. Phys. Chem. C 2011, 115, 12216–12223. [Google Scholar] [CrossRef]
- Reed, J.L. Electronegativity: Chemical Hardness I. J. Phys. Chem. A 1997, 101, 7396–7400. [Google Scholar] [CrossRef]
- Parr, R.G.; Pearson, R.G. Absolute hardness: Companion parameter to absolute electronegativity. J. Am. Chem. Soc. 1983, 105, 7512–7516. [Google Scholar] [CrossRef]
- Parr, R.G.; Yang, W. Density Functional Theory of Atoms and Molecules, 1st ed.; Oxford University Press: New York, NY, USA, 1989; pp. 70–98. [Google Scholar]
- Jiménez-Hoyos, C.A.; Janesko, B.G.; Scuseria, G.E. Evaluation of range-separated hybrid density functionals for the prediction of vibrational frequencies, infrared intensities, and Raman activities. Phys. Chem. Chem. Phys. 2008, 10, 6621–6629. [Google Scholar] [CrossRef] [PubMed]
- Han, S.-D.; Borodin, O.; Allen, J.L.; Seo, D.M.; McOwen, D.W.; Yun, S.-H.; Henderson, W.A. Electrolyte Solvation and Ionic Association IV. Acetonitrile- Difluoro(oxalato)borate (LiDFOB) Mixtures. J. Electrochem. Soc. 2013, 160, A2100–A2110. [Google Scholar] [CrossRef]
| System | Binding Energy, Eb (eV/Li) | Electronic Band Gap, Eg (eV) |
|---|---|---|
| LiMPSA-MeCN | 3.68 | 5.65 |
| LiTFSI-MeCN | 2.52 | 6.72 |
| LiDFOB-MeCN | 3.90 | 3.75 |
| LiBOB-MeCN | 3.90 | 3.70 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Klorman, J.A.; Lau, K.C. The Relevance of Lithium Salt Solvate Crystals in Superconcentrated Electrolytes in Lithium Batteries. Energies 2023, 16, 3700. https://doi.org/10.3390/en16093700
Klorman JA, Lau KC. The Relevance of Lithium Salt Solvate Crystals in Superconcentrated Electrolytes in Lithium Batteries. Energies. 2023; 16(9):3700. https://doi.org/10.3390/en16093700
Chicago/Turabian StyleKlorman, Jake A., and Kah Chun Lau. 2023. "The Relevance of Lithium Salt Solvate Crystals in Superconcentrated Electrolytes in Lithium Batteries" Energies 16, no. 9: 3700. https://doi.org/10.3390/en16093700
APA StyleKlorman, J. A., & Lau, K. C. (2023). The Relevance of Lithium Salt Solvate Crystals in Superconcentrated Electrolytes in Lithium Batteries. Energies, 16(9), 3700. https://doi.org/10.3390/en16093700







