Abstract
In this research, TiO2 nanotubes (NTs) were produced by electrochemical anodization of a Ti substrate where different NH4F wt.% in the electrolyte was added. NTs with diameter of 65–90 nm and 3.3–4.9 µm length were obtained and sensitized with binary cadmium chalcogenides nanoparticles, CdS and CdSe, by successive ionic layer adsorption and reaction method (SILAR). Additionally, both anions S and Se were deposited onto Cd, labeled as CdSSe and CdSeS, to evaluate the effect of the deposition order of the anion from the precursor solution to form cadmium chalcogenides. The structural, optical, and electrochemical performance were analyzed through the SEM, XRD, XPS, UV-VIS, lineal voltammetry and chronoamperometry characterizations. The increase of NH4F wt.% from 1.5% to 4.5% produced a decrement of the diameter and length attributed to the fluoride ions concentration causing solubility of the NTs. XRD confirmed the TiO2 anatase and hexagonal CdS structures. From the EDS and XPS results, the presence of small amount of Se in the sensitized samples demonstrated the doping effect of Se instead of forming ternary semiconductor. With the sensitization of the TiO2 NTs with the nanoparticles, an improved hydrogen generation was observed (reaching 1.068 mL h−1 cm−2) in the sample with CdSSe. The improvement was associated to a synergetic effect in the light absorption and higher cadmium chalcogenide amount deposited when sulfur ions were deposited before selenium.
1. Introduction
Solar water splitting for hydrogen generation has received much more attention in recent years because access to affordable, safe, sustainable, and modern energy is part of the sustainable development goals of the United Nations; as well as the development of new surface modification systems that make hydrogen production more efficient on the photocatalyst materials used for this purpose [1,2].
The development of stable, highly efficient, and low-cost photoelectrodes remains the main challenge to boost the photoelectrochemical devices. Carbon-based materials have been tested for photoelectrocatalytic application [3]; however, metal oxides are the most selected materials for photoelectrodes because they are chemically stable and resistant to corrosion. However, they present some disadvantages such as large bandgap, poor electrical conductivity and short charge carrier lifetime and diffusion length. Among the different alternatives, the most common semiconductor tested for photoelectrochemical water splitting is TiO2. It is a wide band gap semiconductor (3.2 eV), with preferential absorption in the UV range representing 4% of the solar spectrum, wasting most available solar energy [4].
To improve the photocatalytic activity of TiO2, it has been proposed to increase the active surface area, modify the band gap, and sensitize to increase light absorption [5,6,7]. The morphology of TiO2 varies from compact barrier layer, nanoparticles, nanorods, nanobelts, nanofibers to nanotubes (NTs) [8,9,10]. In this way, NTs can be obtained by template, hydrothermal, and anodization methods [4]. Electrochemical anodization consists of immersing titanium in an electrolyte and applying an electrical stimulus for a specific time. The final morphology and properties of the TiO2 nanotubes formed depend on parameters such as voltage/current, time, electrolyte temperature, nature of the electrolyte, concentration, and pH [11,12,13,14,15,16,17]. Additional advantages of the TiO2 NTs include high mechanical strength, improved electronic properties related to the quantum confinement effect, as high electron mobility, and the chance of embed specific ions into the NTs wall, as dopants or co-catalysts [18,19].
On the other hand, the sensitization of TiO2 electrodes with binary semiconductors such as CdS, CdSe, PbS, PbSe, and Cu2O has received interest to solve the problem of poor light absorption in the visible region [10,20,21,22]. Other strategies such as co-sensitizing and core-shell structures have been probed [23,24,25,26]. Nevertheless, there are few reports concerning ternary nanomaterials. The use of ternary semiconductor materials present advantages such as tunable band gap, and synergistic effect for light harvesting [26].
Li et al. obtained a CdSxSe1−x alloy by the hydrothermal method varying the S:Se ratio and deposited it onto TiO2 NTs to fabricate quantum dot-sensitized solar cells (QDSSCs) [27]. They found that an increment in Se content to reach the stoichiometry CdS0.5Se0.5 resulted in an improvement of the photovoltaic performance attributed to the increased light absorption and improvement of charge transport by the tuned S:Se ratio. It agrees with the study carried out by Ai et al., where the optimized composition of the photoelectrode was CdS0.52Se0.48 deposited by thermal vapor onto TiO2 nanowires that showed improved stability for long-time hydrogen generation [28]. Likewise, Sung et al. synthesized CdSxSe1−x nanowires and TiO2/CdSxSe1−x core-shell nanocables by thermal vapor deposition and tested in a photoelectrochemical cell (PEC) [29]. They reported a multishell structures with alloy phases obtained with the chosen deposition method. The highest hydrogen generation rate was 600 µmol h−1 cm−2 with the TiO2/CdS0.2Se0.8 sample, however, this deposition technique is expensive and not easily scalable. Tyagi et al. prepared CdS1−xSex quantum dots (QDs) by successive ionic layer absorption and reaction (SILAR) and tested in QDSSCs. In the SILAR process, for the anionic precursor solution they mixed Na2S and Se powder as sources to obtain Na2S1−xSex solution. In their study, an increment in the power conversion efficiency was observed when the sulfur concentration was increased [30]. It is noteworthy to mention that the SILAR method allows one to obtain QDs or bulk material by the control of cycles number, and it has the advantage of allowing a higher charge of light absorbing semiconductor layer than the pre-synthesized colloidal QDs [31].
Furthermore, doping has been used as strategy to improve catalyst performance for the hydrogen evolution reaction by creating a suitable electronic environment [32]. Yang et al., synthesized Se-doped CdS nanocrystals by the hot injection method by varying the reaction temperature and Cd:oleic acid ratio [33]. The optical and structural properties were reported; however, no further information was provided. Shi et al., obtained a Se-doped CdS QDs catalyst by solvothermal procedure modifying the Se content. They found that Se shifted the Fermi level position to higher energy causing effective capture of photogenerated electrons, inhibition of recombination charges and prolonged carriers lifetime [34]. Poornaprakash et al., effectively tested Er-doped CdS nanoparticles for H2 production and organic pollutants degradation [35]. Likewise, CdS nanoparticles with Mn, Cu, Ni and N as dopants have been evaluated [36,37,38,39]. In-doped CdS-ZnO and Sr-doped CdS-ZnS composites have demonstrated enhanced photocatalytic activity attributed to improved charge transport, band structure modification, and passivation of defect centers [40,41].
Although several works have been performed using doped Cd chalcogenides nanoparticles, they were tested in systems where the catalysts are dispersed in the electrolyte, but the main problem with this system is the removal of catalyst powder after reactions.
In this work, TiO2 NTs photoelectrodes were obtained by anodization where the effect of the electrolyte concentration in the morphology by changing the NH4F wt.% during the process was analyzed. Furthermore, the electrochemical performance for the solar hydrogen generation with anodized TiO2 NTs sensitized with binary cadmium chalcogenides nanoparticles, CdS and CdSe, were evaluated, as well as the combination of anions, S and Se labeled as CdSSe and CdSeS, to assess the effect of the deposition order of the anion from the precursor solution. Its morphological, structural, optical, and electrochemical properties were investigated. The increased NH4F wt.% resulted in a decrease in diameter and length of the TiO2 NTs. Additionally, the formation of cadmium chalcogenide on the TiO2 NTs was obtained, and it was demonstrated that when sulfur is deposited followed by selenium, it allowed for a more efficient deposition, and therefore an improved hydrogen evolution rate.
2. Materials and Methods
Materials: Titanium foil (99.7%, 2 mm thickness) ammonium fluoride (NH4F), ethylene glycol (HOCH2CH2OH), cadmium acetate dihydrate (Cd(CH3COO)2·2H2O), sodium sulfite nonahydrate (Na2S·9H2O), and selenium powder were obtained from Sigma-Aldrich. Sodium sulfite (Na2SO3), methanol, and ethanol were obtained from Fermont.
2.1. TiO2 Nanotubes Anodization
The Ti foil was cut in dimensions of 2 cm × 1 cm to ensure a homogeneous surface; before anodizing, it was mechanically roughened through successive grades of SiC paper up to 1200 grade. All samples were chemically polished in a mixture of HF (40 wt.%):HNO3 (70 wt.%):H2O with a volume ratio of 1:4:5 for 1 min, at room temperature under continuous stirring, finally rinsed in distilled water and dried in cold air. The anodizing process was performed using a two-electrode arrangement with a Pt mesh as counter electrode, using a power supply Keithley model 2410 (Ektronix, Inc., Beaverton, OR, USA) and applying 50 V for 30 min at room temperature with constant stirring. The electrolyte concentration was varied changing the NH4F wt.% from 1.5% to 4.5% in ethylene glycol solution and adding 4 vol% H2O. After the anodic oxidation, the substrates were rinsed with water and a thermal treatment was carried out at 450 °C for 1 h.
2.2. Cadmium Chalcogenides Nanoparticles Formation and Deposition by SILAR
The CdS, CdSe, CdSSe and CdSeS nanoparticles were deposited onto the TiO2 NTs by SILAR method. It consists in the successive immersion of the TiO2 NTs photoelectrode for 1 min in 0.05 M Cd(CH3COO)2 dissolved in ethanol and Na2S 0.05 M in methanol:water 1:1 v/v as Cd and S precursor solution [42]. Between each immersion, the electrodes were rinsed in ethanol and methanol:water, respectively, to remove excess unreacted or deposited ions. For the deposition of CdSe, the Cd source solution was the same, while the Se precursor solution used was 0.1 M Na2SeSO3, which was obtained from 0.3 M of Na2SO3 dissolved in 100 mL of water and adding 0.79 g of Se powder. It was maintained under vigorous stirring at 250 °C in a hotplate with a reflux system for 3 h [43]. Five SILAR cycles were performed to obtain CdS and CdSe nanoparticles. For the doped samples, CdSSe or CdSeS, the Cd, S, and Se precursor solutions were used in a cycle changing the sequence of S and Se.
2.3. Photoelectrochemical Cell
The electrochemical measurements were carried out in a three-electrode configuration cell, where the TiO2/Cd-nanoparticles was the photoelectrode, Pt wire was the counter electrode, and Ag/AgCl was the reference electrode. The electrolyte was distilled water with 0.25 M Na2SO3 and 0.35 M Na2S as sacrificial hole scavengers. For the hydrogen evolution, it was collected and quantified in a sealed syringe containing the Pt wire.
2.4. Characterizations
The scanning electron microscopy was performed using a FEI Nova NanoSem200 microscopy (FEI Company, Hillsboro, OR, USA), to obtain the surface morphology of the TiO2 NTs. The NTs size was determined using ImageJ software version 1.8.0. The micrographs of the TiO2 photoelectrodes with cadmium chalcogenide nanoparticles and EDS spectra were obtained using a Jeol JSM-6010Plus/LA microscope (JEOL, Inc., Peabody, MA, USA). The UV-VIS characterization was performed using a UV-Vis NIR spectrophotometer model Cary 5000 (Agilent, Santa Clara, CA, USA). The X-ray diffraction measurements were recorded with a Rigaku D-Max 2200 X-ray diffractometer (Rigaku, The Woodlands, TX, USA) with monochromatized Cu-K radiation (λ = 1.54 Å). The high-resolution C 1s X-ray photoelectron spectroscopy (XPS) was performed using a Thermo Scientific Escalab 250Xi instrument (Thermo Scientific Inc., Waltham, MA, USA). The lineal sweep voltammetry and chronoamperometry were obtained using a potentiostat/galvanostat EC Epsilon coupled with a 100 W full spectrum Led chip COB DIY lamp (380–840 nm). The chronoamperometry was performed at 0 V applied under intermittent light. In the PEC for hydrogen generation, the photoelectrodes were illuminated with 100 mW cm−2 illumination intensity using a 450 W oriel xenon lamp model 66021, equipped with an A. M. 1.5 G filter. The externa quantum efficiency (EQE) was performed with a monochromator adaptor, where light from the PEC was reflected.
3. Results and Discussion
3.1. Nanotubes Formation
To assess the effect of electrolytic composition on the morphology of TiO2 nanotubes (NTs), anodic layers were grown using three different concentrations of NH4F in the anodization bath. Figure 1 depicts the characteristic current density versus time curve generated during the anodization treatments performed at constant potential.
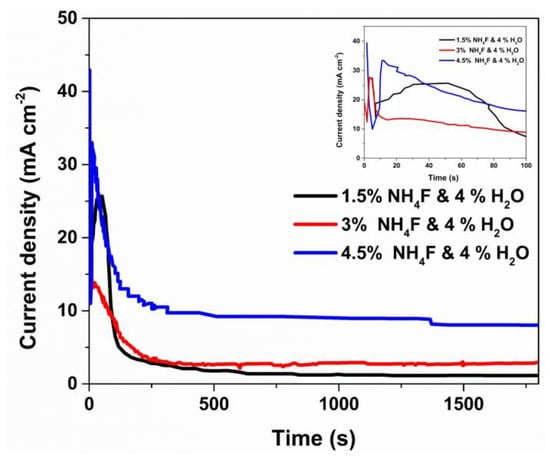
Figure 1.
Current density vs. time curve of the TiO2 NTs anodization obtained with different NH4F wt.% in the electrolyte.
In all cases, the typical behavior of these curves during titanium and its alloy’s anodization processes was observed. Initially, current densities sharply decreased, from maximum values of 43 mA cm−2 for the titanium sample anodized with 4.5 wt.% of NH4F to around 12 mA cm−2 for the three studied conditions. This abrupt decrease in the early stages of the anodization treatment is associated with the initial formation of a compact layer of titanium oxide that inhibits the electrolyte-substrate interaction [14,44]. Subsequently, the current density slightly increases in all studied conditions, a phenomenon associated with pore nucleation on the surface of the compact layer created initially. The extent of this stage depends on various factors such as the amount of fluorides present in the anodization bath, surface finish, substrate chemical composition, solution pH, and water content, among others [45]. Finally, a gradual decrease in current density is observed until stabilization is reached after 150 s for all three conditions studied. At this stage, the current density is associated with the competition between the growth and dissolution of the anodic layer, as well as the presence of secondary reactions such as medium evolution [12].
The highest current density, observed in the equilibrium zone, was present at the maximum fluoride condition of 4.5 wt.%, while it decreased with decreasing NH4F concentration. This behavior is associated with the increase in F− ion concentration in the anodization bath, which accelerates the dissolution processes of the oxide layer formed by an increase in the formation of soluble [TiF6]2− species, along with medium evolution processes, as reported by several authors in both acidic and organic media [46,47].
3.2. Morphology
Figure 2 shows the SEM micrographs of TiO2 NTs obtained with different NH4F wt.% in the electrolyte. A homogeneous pore formation in all samples was observed, however, when the NH4F concentration was low (1.5%), Figure 2a, the formation of wires over the TiO2 NTs which can provide a higher surface for the cadmium chalcogenide nanoparticles deposition was noted. The NTs diameter distribution of the samples was calculated using ImageJ software and the corresponding histograms are presented in Figure 2b,e,h. A decrement of the pore diameter from 90 nm to 68 nm with the increment of NH4F concentration was observed. Similar effect was obtained with the TiO2 NTs length, that decreased from 4.95 µm to 3.86 µm. The increased TiO2 NTs size with the lower NH4F wt.% agrees with the decreased current density observed in Figure 1.
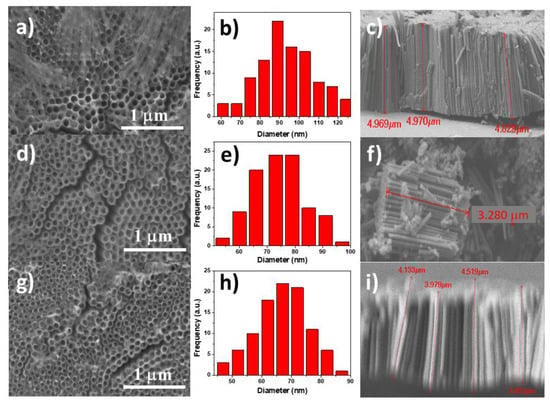
Figure 2.
SEM images of the top view, histograms and cross section of the anodized TiO2 NTs obtained with (a–c) 1.5% NH4F, (d–f) 3% NH4F, and (g–i) 4.5% NH4F.
Figure 3 present SEM images of the TiO2 NTs after the cadmium chalcogenides nanoparticles deposition. It is interesting to note that in all cases the cadmium chalcogenides nanoparticles were deposited on the top surface instead of penetrating the TiO2 NTs structure; this could be attributed to the number of SILAR cycles and to the compactness of TiO2 NTs. In the samples with CdS and CdSe, there are zones where the diameter of the NTs can be observed (marked with a red circle), while the cadmium nanoparticles form agglomerations. In the case of CdSeS the covered surface increased due to the increase in the immersion steps in the SILAR cycle, and in the sample with CdSSe, the surface was almost completely covered.
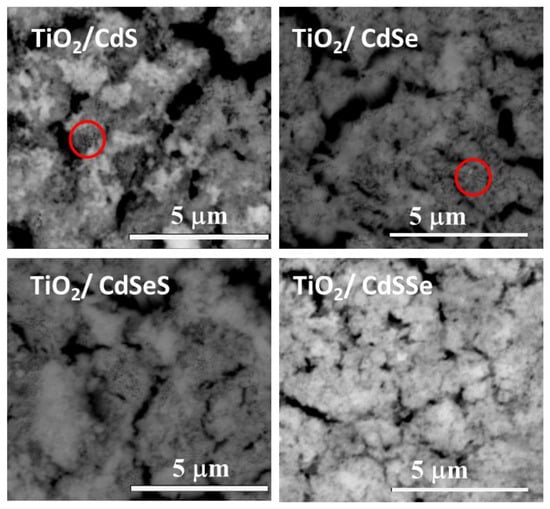
Figure 3.
Top view of the SEM images of TiO2 NTs deposited with CdS, CdSe, CdSeS and CdSSe nanoparticles. Red circles show uncoated TiO2 NTs.
Table 1 presents the estimated atomic weight percentage of the components detected from EDS analysis performed on the photoelectrodes surface. The low amount of cadmium chalcogenide nanoparticles deposited on the TiO2 NTs is evident. Moreover, the %Se was lower than %S, which might indicate selenium is doping the CdS instead of forming a ternary semiconductor, as it has been established that for the ternary CdSSe formation is necessary an annealing over 230 °C [48], meanwhile in this study the SILAR deposition was carried out at room temperature. In the samples with both S and Se, it is observed that when S was deposited before Se, the covering of the nanoparticles on the TiO2 NTs surface was enhanced according to the CdSSe sample. This suggests that the Se ions did not react with the deposited Cd ions but rinsed them or only doped the formed CdS.

Table 1.
Chemical composition (atomic %) of the TiO2 NTs photoelectrodes sensitized with cadmium chalcogenides nanoparticles obtained from EDS analysis.
3.3. Structural Characteristics
The X-ray diffraction patterns of the TiO2 NTs obtained with 1.5% NH4F electrolyte after thermal annealing and sensitizing with cadmium chalcogenide nanoparticles samples are demonstrated in Figure 4. In all samples, the main peaks associated with the tetragonal TiO2 anatase phase (PDF#21-1272) corresponding to (101), (004), (105), (211), (204) and (116) planes were observed, marked with *. Additionally, in the electrode with CdS deposited, two peaks at 28.4° and 47.8° were identified which correspond to (101) and (103) planes attributed to the hexagonal CdS crystalline structure (PDF#41-1049). The intensity of the 28.4° peak increased in the photoelectrode sensitized with CdSSe and CdSeS. The sample with CdSe showed the characteristic peaks of TiO2 and a small peak at 28.4° which can be associated to the sulfur reacting with cadmium from the Na2SeSO3 precursor solution. The absence of the CdSe peaks in the XRD diffractogram can be related to the small content of Se in the samples, as was discussed from the EDS results. Furthermore, a small peak at 40° was observed in the samples corresponding to Ti foil substrate. The crystallite size was calculated from the broadening of the XRD peaks using Scherrer equation [49]:
where D is the crystallite size, 0.9 is the Scherrer constant, λ is the wavelength of the XRD radiation (0.1541 nm for Cu Kα), β is the full width at half-maximum (FWHM), and θ is the diffraction angle. The estimated crystallite size of TiO2 NTs was 13.7 nm, while for CdS was 14.1 nm. The higher crystallite size of the CdS explain its deposition over the TiO2 NTs.
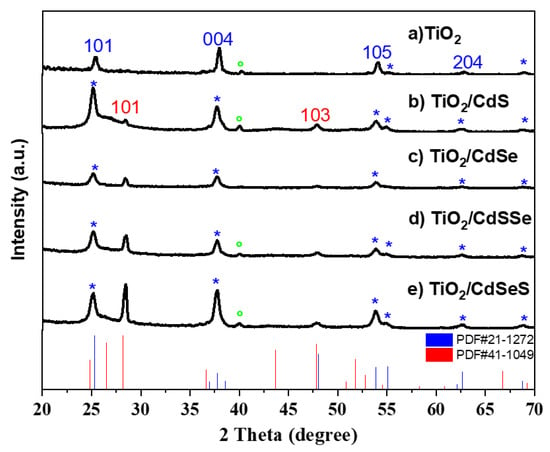
Figure 4.
XRD diffractogram of the anodized TiO2 NTs sensitized with binary and selenium-doped cadmium chalcogenide nanoparticles. PDF #21-1272 corresponding to TiO2 and #41-1049 corresponding to CdS are included. The peak indicated with ° corresponds to the Ti foil substrate. The peaks with * represent all TiO2 anatase observed planes.
To verify the influence of the order precursor solutions in the cadmium nanoparticles formation by SILAR method, the XPS spectra of the TiO2 NTs photoelectrodes sensitized with cadmium sulfur and selenide changing the order of S and Se deposited are shown in Figure 5. The survey spectrum shows the presence of Ti, O, Cd and S, as well as the peak of C from the air (Figure 5a). The characteristic peaks of Ti 2p at 458 and 464 eV observed in Figure 5b correspond to the Ti 2p3/2 and Ti 2p1/2 associated to Ti4+ [50]. The peak observed at 529.7 eV is assigned to the O2− in the TiO2 lattice, while the peak at 531.6 eV can be attributed to the surface-adsorbed hydroxyl (Figure 5c) [22,51]. It is interesting to note that the area ratio of the two O 1s peaks changed with the different order of the S and Se deposition. When S was deposited before Se (CdSSe sample), the OH area peak was higher than the TiO2 area peak, this might indicate the formation of oxygen vacancies in the lattice [52], whereas in the sample where Se was deposited before S (CdSeS), the higher area observed was in the peak related to TiO2.
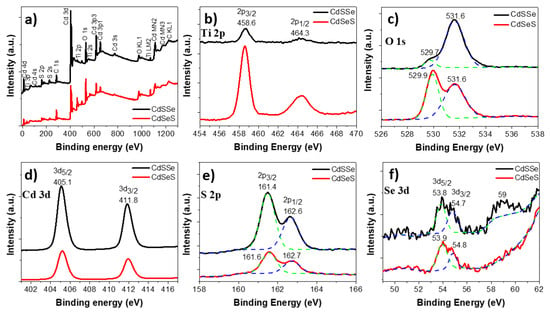
Figure 5.
(a) Survey spectrum and (b–f) XPS spectra of the TiO2 NTs sensitized with cadmium sulfide and selenide deposited by SILAR with different order of S and Se deposition. Dashed lines show the deconvoluted spectra.
Additionally, Figure 5d shows two peaks located at 405.1 and 411.8 eV corresponding to Cd 3d5/2 and Cd 3d3/2, which are consistent to Cd2+ [53,54]. The two peaks observed at 161 and 162 eV are related to S 2p3/2 and 2p1/2 orbits in agreement with S2− (Figure 5e) [55]. Comparing the two samples with different order of deposition, a slight shift from 161.6 to 161.4 eV and from 161.7 to 161.6 eV was observed when S was deposited first (CdSSe). It could be attributed to sulfur vacancies generated, and therefore, the possibility of selenium doping the lattice [55]. Figure 5f shows in both samples the presence of Se 3d5/2 and 3d3/2 doublets at 53.8 and 54.7 eV associated to CdSe [56,57]. However, in the CdSSe sample, an additional peak at 59 eV was observed, which can be attributed to SeO32− [58] from the Se precursor solution suggesting that not all the Se reacted with the cadmium sulfur nanoparticles.
3.4. Optical Properties
The absorbance of the sensitized TiO2 NTs samples is shown in Figure 6. In the photoelectrode with TiO2/CdS, two absorption bands were observed at 350 nm and 500 nm, corresponding to the light absorption of the TiO2 and CdS, respectively [23]. The sample with CdSe deposited showed an abrupt decrease in absorption at 400 nm which corresponds to the TiO2 absorption edge and a peak at 600 nm attributed to CdSe absorption, indicating that the main light absorption contribution was made by the TiO2 NTs [59]. For the samples with sulfur and selenium, a wider absorption spectrum was obtained, where the three absorption peaks corresponding to TiO2 (350 nm), CdS (500 nm) and CdSe (600 nm) were observed.
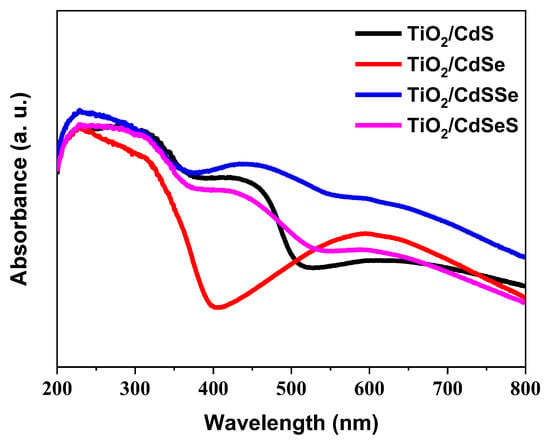
Figure 6.
Absorbance spectra of the anodized TiO2 NTs sensitized with binary and selenium-doped cadmium chalcogenide nanoparticles.
3.5. Electrochemical Measurement
The electrochemical characterization of the anodized TiO2 NTs sensitized with binary and selenium-doped cadmium chalcogenide nanoparticles by the SILAR method is presented in Figure 7. The lineal sweep voltammetry of the TiO2 TNs photoelectrodes obtained with 1.5% NH4F and 4% H2O electrolyte sensitized with CdS, CdSe, CdSSe and CdSeS is shown in Figure 7a. At 0 V vs. Ag/AgCl, the pristine TiO2 NTs electrode showed 0.15 mA cm−2. The current density of the electrode sensitized with CdS was very close (1.46 mA cm−2) comparing to the current obtained with CdSe (1.35 mA cm−2). For the Se-doped samples, the electrode deposited with CdSSe showed a higher current density (2.41 mA cm−2) while the photoelectrode with CdSeS demonstrated 1.78 mA cm−2. This agrees with the trend observed in Table 1 concerning the order of anions deposited.
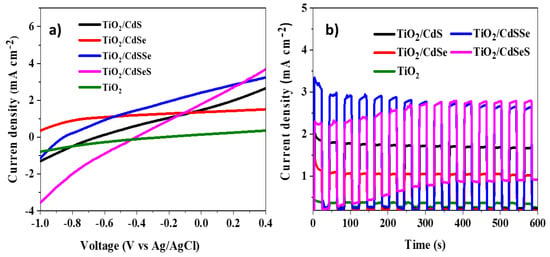
Figure 7.
(a) Lineal sweep voltammetry and (b) chronoamperometry response at transient illumination of the anodized TiO2 NTs photoelectrodes sensitized with binary and selenium-doped cadmium chalcogenide nanoparticles.
Figure 7b presents the photocurrent response of the sensitized photoelectrodes at transient illumination applying 0 V bias. A clear increment of the photocurrent response was observed with the cadmium chalcogenide sensitizing the TiO2 NTs and a very stable current response. The photocurrent transient increased with the doped samples, showing an increasing trend with the TiO2/CdSeS electrode and a slightly decreasing trend with the TiO2/CdSSe electrode. The higher current density was obtained with the CdSSe sensitized photoelectrode. This enhanced photocurrent agrees with the improved light absorption obtained caused by the presence of both S and Se, and as has been reported, the CdS doping produce the improvement of charge transport by the alignment of energy bands, especially the addition of Se brings the fermi level closer to the conduction band, thereby allowing for an effective capture of electrons and eliminating charge recombination [28,29,34,36].
The electrochemical characterization of the TiO2 NTs photoelectrodes obtained with different NH4F concentration and sensitized with CdSSe nanoparticles is shown in Figure 8. From the lineal sweep voltammetry, a slightly higher current density in the sample obtained with 1.5% NH4F at 0 V vs. Ag/AgCl was observed (Figure 8a), associated to the larger TiO2 NTs. The transient photocurrent response showed high current density with the lower NH4F concentration, nevertheless, this decreased with time. With the increased NH4F concentration, the transient photocurrent response was very stable, see Figure 8b.
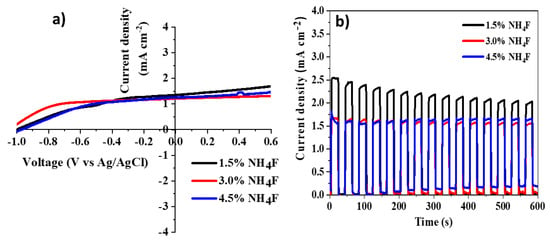
Figure 8.
(a) Lineal sweep voltammetry and (b) chronoamperometry response at transient illumination of the anodized TiO2 NTs photoelectrodes with different NH4F concentration in the electrolyte and sensitized with CdSSe nanoparticles.
The hydrogen evolution was performed in a three-cell configuration where the photoelectrodes were illuminated, while the hydrogen was generated in the Pt wire and collected in a locked syringe. Na2SO3 and Na2S were added as sacrificial agents to prevent corrosion of the photoelectrode. The possible reaction mechanism in the PEC assembly has been reported as follows [60]:
photoanode
cathode
electrolyte
The hydrogen rate obtained with the photoelectrodes in the photoelectrochemical cell at 0 V bias under 1 sun illumination is presented in Figure 9a. The higher hydrogen generation with the TiO2/CdSSe sample (1.068 mL h−1 cm−2) compared with the TiO2/CdSeS sample (0.695 mL h−1 cm−2) is evident, which is in agreement with the lineal sweep voltammetry response. The external quantum efficiency (EQE) (Figure 9b), which is defined as the number of available electrons produced by the incident photons and collected from the cell to the external circuit [61], demonstrated the response of the photoelectrodes tested mainly in the visible range until 550 nm attributed to the CdS and an additional small response until 650 nm related to the Se doping. The increase in EQE suggests that the amount of photogenerating semiconductor is higher in the sample with CdSSe, which is consistent with Table 1. Therefore, it can be concluded that the enhanced hydrogen generation of this sample is caused by an increase in deposited photoactive material due to the order of deposition. The integrated current density curve resulting from the EQE confirmed the improved performance of the TiO2/CdSSe photoelectrode regarding the TiO2/CdSeS.
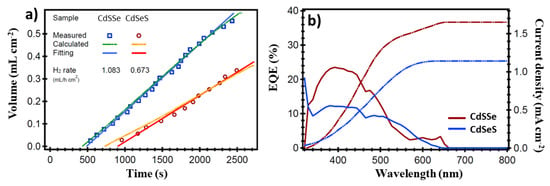
Figure 9.
(a) Volume of hydrogen generated, and (b) external quantum efficiency and integrated current density curve of the TiO2/CdSSe and TiO2/CdSeS photoelectrodes in the photoelectrochemical cell under 1 sun illumination.
Although the amount of hydrogen generated with these photoelectrodes was low compared with similar PECs reported (see Table 2), this system can be improved by obtaining larger diameter NTs or enhancing the cadmium chalcogenide nanoparticles deposition technique, for instance, with QDs that can be deposited inside the NTs and have advantage such as quantum confinement and multiple carrier generation.

Table 2.
Performance parameters of the PECs based on TiO2, and cadmium sulfide selenide nanoparticles reported in literature and this work.
4. Conclusions
TiO2 nanotubes were obtained by electrochemical anodization testing different NH4F wt.% in the electrolyte, causing a decrement of the diameter and length. The TiO2 NTs were sensitized with binary CdS and CdSe nanoparticles, as well as Se doping CdS by SILAR. In the doped samples, the order of selenium and sulfur deposition demonstrated an improvement of nanoparticles covering the TiO2 NTs when the sulfur precursor was first deposited instead of selenium (CdSSe sample). This enhancement was attributed to the increased surface of the TiO2 NTs when 1.5% NH4F was added, and a more efficient deposition of the nanoparticles. Therefore, the higher hydrogen generation rate was obtained with the TiO2/CdSSe photoelectrode tested in the PEC. This demonstrated the potential suitability of these nanomaterials for photoelectrochemical devices.
Author Contributions
Conceptualization, A.C.-P. and J.M.H.-L.; Data curation, J.A.C.; Formal analysis, I.Z. and L.R.-G.; Investigation, J.A.C.; Methodology, J.A.C.; Supervision, A.C.-P.; Writing—review & editing, A.C.-P., I.Z., L.R.-G., J.A.H.-M., K.C.S., S.L.L. and J.M.H.-L. All authors have read and agreed to the published version of the manuscript.
Funding
We appreciate the financial support of the Programa de Apoyo a la Investigación Científica y Tecnológica (PAICYT) projects IT1267-20 and IT1674-21 from UANL.
Data Availability Statement
Data are contained within the article.
Acknowledgments
The authors gratefully acknowledge the help of Nayeli Pineda Aguilar for SEM, Alonso Concha Balderrama for XRD and Luis Gerardo Silva Vidaurri for XPS measurements from CIMAV-Monterrey. J. Alfaro Chacón acknowledge the scholarship graded by CONAHCYT for the master program.
Conflicts of Interest
The authors declare no conflicts of interest or personal relationships that could have appeared to influence the work reported in this paper.
References
- United Nations. Sustainable Development Goals. Available online: https://www.un.org/sustainabledevelopment/ (accessed on 1 February 2024).
- Sathish, S.; Aravind Kumar, J.; Prabu, D.; Annam Renita, A.; Murugesan, K.; Rajasimman, M.; Joo, S.-W.; Vasseghian, Y.; Wang, C. Latest Avenues on Solar Light-Driven Photocatalytic Hydrogen Generation Using Surface Modified Nanomaterials towards Sustainable Environment and Circular Bioeconomy. Fuel 2023, 340, 127398. [Google Scholar] [CrossRef]
- Sangiorgi, N.; Tuci, G.; Sanson, A.; Peruzzini, M.; Giambastiani, G. Metal-Free Carbon-Based Materials for Electrocatalytic and Photo-Electrocatalytic CO2 Reduction. Rend. Lincei. Sci. Fis. Nat. 2019, 30, 497–513. [Google Scholar] [CrossRef]
- Chu, S.; Li, W.; Yan, Y.; Hamann, T.; Shih, I.; Wang, D.; Mi, Z. Roadmap on Solar Water Splitting: Current Status and Future Prospects. Nano Futures 2017, 1, 022001. [Google Scholar] [CrossRef]
- Arifin, K.; Yunus, R.M.; Minggu, L.J.; Kassim, M.B. Improvement of TiO2 Nanotubes for Photoelectrochemical Water Splitting: Review. Int. J. Hydrogen Energy 2021, 46, 4998–5024. [Google Scholar] [CrossRef]
- Ismael, M. A Review and Recent Advances in Solar-to-Hydrogen Energy Conversion Based on Photocatalytic Water Splitting over Doped-TiO2 Nanoparticles. Sol. Energy 2020, 211, 522–546. [Google Scholar] [CrossRef]
- Mátravölgyi, B.; Hergert, T.; Thurner, A.; Varga, B.; Sangiorgi, N.; Bendoni, R.; Zani, L.; Reginato, G.; Calamante, M.; Sinicropi, A.; et al. Synthesis and Investigation of Solar-Cell Photosensitizers Having a Fluorazone Backbone. Eur. J. Org. Chem. 2017, 2017, 1843–1854. [Google Scholar] [CrossRef]
- Ge, M.; Cao, C.; Huang, J.; Li, S.; Chen, Z.; Zhang, K.-Q.; Al-Deyab, S.S.; Lai, Y. A Review of One-Dimensional TiO2 Nanostructured Materials for Environmental and Energy Applications. J. Mater. Chem. A 2016, 4, 6772–6801. [Google Scholar] [CrossRef]
- Domínguez-Jaimes, L.P.; Cedillo-González, E.I.; Luévano-Hipólito, E.; Acuña-Bedoya, J.D.; Hernández-López, J.M. Degradation of Primary Nanoplastics by Photocatalysis Using Different Anodized TiO2 Structures. J. Hazard. Mater. 2021, 413, 125452. [Google Scholar] [CrossRef] [PubMed]
- Dubey, P.K.; Kumar, R.; Tiwari, R.S.; Srivastava, O.N.; Pandey, A.C.; Singh, P. Surface Modification of Aligned TiO2 Nanotubes by Cu2O Nanoparticles and Their Enhanced Photo Electrochemical Properties and Hydrogen Generation Application. Int. J. Hydrogen Energy 2018, 43, 6867–6878. [Google Scholar] [CrossRef]
- Indira, K.; Mudali, U.K.; Nishimura, T.; Rajendran, N. A Review on TiO2 Nanotubes: Influence of Anodization Parameters, Formation Mechanism, Properties, Corrosion Behavior, and Biomedical Applications. J. Bio-Tribo-Corros. 2015, 1, 28. [Google Scholar] [CrossRef]
- Sun, Y.; Yan, K.P. Effect of Anodization Voltage on Performance of TiO2 Nanotube Arrays for Hydrogen Generation in a Two-Compartment Photoelectrochemical Cell. Int. J. Hydrogen Energy 2014, 39, 11368–11375. [Google Scholar] [CrossRef]
- Qin, L.; Chen, Q.; Lan, R.; Jiang, R.; Quan, X.; Xu, B.; Zhang, F.; Jia, Y. Effect of Anodization Parameters on Morphology and Photocatalysis Properties of TiO2 Nanotube Arrays. J. Mater. Sci. Technol. 2015, 31, 1059–1064. [Google Scholar] [CrossRef]
- Ghani, T.; Mujahid, M.; Mehmood, M.; Ubaidullah, M.; Shah, A.; Mahmood, A. Effect of Processing Temperature on the Morphology and Crystal Structure of Anodic TiO2 Nanotubes. J. Electron. Mater. 2020, 49, 1881–1888. [Google Scholar] [CrossRef]
- Nischk, M.; Mazierski, P.; Gazda, M.; Zaleska, A. Ordered TiO2 Nanotubes: The Effect of Preparation Parameters on the Photocatalytic Activity in Air Purification Process. Appl. Catal. B Environ. 2014, 144, 674–685. [Google Scholar] [CrossRef]
- Jiang, L.; Zhang, J.; Chen, B.; Zhang, S.; Zhang, Z.; Wan, W.; Song, Y. Morphological Comparison and Growth Mechanism of TiO2 Nanotubes in HBF4 and NH4F Electrolytes. Electrochem. Commun. 2022, 135, 107200. [Google Scholar] [CrossRef]
- David, T.M.; Dev, P.R.; Wilson, P.; Sagayaraj, P.; Mathews, T. A Critical Review on the Variations in Anodization Parameters toward Microstructural Formation of TiO2 Nanotubes. Electrochem. Sci. Adv. 2022, 2, e202100083. [Google Scholar] [CrossRef]
- Roy, P.; Berger, S.; Schmuki, P. TiO2 Nanotubes: Synthesis and Applications. Angew. Chem. Int. Ed. 2011, 50, 2904–2939. [Google Scholar] [CrossRef]
- Zhou, X.; Liu, N.; Schmuki, P. Photocatalysis with TiO2 Nanotubes: “Colorful” Reactivity and Designing Site-Specific Photocatalytic Centers into TiO2 Nanotubes. ACS Catal. 2017, 7, 3210–3235. [Google Scholar] [CrossRef]
- Jin-Nouchi, Y.; Hattori, T.; Sumida, Y.; Fujishima, M.; Tada, H. PbS Quantum Dot-Sensitized Photoelectrochemical Cell for Hydrogen Production from Water under Illumination of Simulated Sunlight. ChemPhysChem 2010, 11, 3592–3595. [Google Scholar] [CrossRef]
- Liao, W.; Wang, B.; Liu, Z. Photoelectrochemical Hydrogen Production at Peak Efficiency over 10% via PbSe QDs/TiO2 Nanotube Array Photoanodes. Int. J. Hydrogen Energy 2017, 42, 10962–10970. [Google Scholar] [CrossRef]
- Yu, J.; Gong, C.; Wu, Z.; Wu, Y.; Xiao, W.; Su, Y.; Sun, L.; Lin, C. Efficient Visible Light-Induced Photoelectrocatalytic Hydrogen Production Using CdS Sensitized TiO2 Nanorods on TiO2 Nanotube Arrays. J. Mater. Chem. A 2015, 3, 22218–22226. [Google Scholar] [CrossRef]
- Cerdán-Pasarán, A.; López-Luke, T.; Zarazúa, I.; De la Rosa, E.; Fuentes-Ramírez, R.; Sanal, K.C.; Alatorre-Ordaz, A. Co-Sensitized TiO2 Electrodes with Different Quantum Dots for Enhanced Hydrogen Evolution in Photoelectrochemical Cells. J. Appl. Electrochem. 2019, 49, 475–484. [Google Scholar] [CrossRef]
- Carrera-Crespo, J.E.; Ramos-Sánchez, G.; De la Luz, V.; González, F.; Barrera, E.; González, I. Photoelectrochemical Hydrogen Generation on TiO2 Nanotube Arrays Sensitized with CdS@Sb2S3 Core Shell Particles. Int. J. Hydrogen Energy 2017, 42, 30249–30256. [Google Scholar] [CrossRef]
- Gao, X.-F.; Sun, W.-T.; Ai, G.; Peng, L.-M. Photoelectric Performance of TiO2 Nanotube Array Photoelectrodes Cosensitized with CdS/CdSe Quantum Dots. Appl. Phys. Lett. 2010, 96, 153104. [Google Scholar] [CrossRef]
- Ai, G.; Mo, R.; Xu, H.; Chen, Q.; Yang, S.; Li, H.; Zhong, J. Vertically Aligned TiO2/(CdS, CdTe, CdSTe) Core/Shell Nanowire Array for Photoelectrochemical Hydrogen Generation. J. Power Sources 2015, 280, 5–11. [Google Scholar] [CrossRef]
- Li, Z.; Yu, L.; Liu, Y.; Sun, S. CdSxSe1−x Alloyed Quantum Dots-Sensitized Solar Cells Based on Different Architectures of Anodic Oxidation TiO2 Film. J. Nanoparticle Res. 2014, 16, 2779. [Google Scholar] [CrossRef]
- Ai, G.; Mo, R.; Xu, H.; Chen, Q.; Yang, S.; Li, H.; Zhong, J. Composition-Optimized TiO2/CdSxSe1−x Core/Shell Nanowire Arrays for Photoelectrochemical Hydrogen Generation. J. Appl. Phys. 2014, 116, 174306. [Google Scholar] [CrossRef]
- Sung, T.K.; Kang, J.H.; Jang, D.M.; Myung, Y.; Jung, G.B.; Kim, H.S.; Jung, C.S.; Cho, Y.J.; Park, J.; Lee, C.L. CdSSe Layer-Sensitized TiO2 Nanowire Arrays as Efficient Photoelectrodes. J. Mater. Chem. 2011, 21, 4553–4561. [Google Scholar] [CrossRef]
- Tyagi, J.; Gupta, H.; Purohit, L.P. Ternary Alloyed CdS1−xSex Quantum Dots on TiO2/ZnS Electrodes for Quantum Dots-Sensitized Solar Cells. J. Alloys Compd. 2021, 880, 160480. [Google Scholar] [CrossRef]
- Becker, M.A.; Radich, J.G.; Bunker, B.A.; Kamat, P.V. How Does a SILAR CdSe Film Grow? Tuning the Deposition Steps to Suppress Interfacial Charge Recombination in Solar Cells. J. Phys. Chem. Lett. 2014, 5, 1575–1582. [Google Scholar] [CrossRef]
- Liu, T.; Diao, P.; Lin, Z.; Wang, H. Sulfur and Selenium Doped Nickel Chalcogenides as Efficient and Stable Electrocatalysts for Hydrogen Evolution Reaction: The Importance of the Dopant Atoms in and beneath the Surface. Nano Energy 2020, 74, 104787. [Google Scholar] [CrossRef]
- Yang, D.; Xu, S.; Chen, Q.; Wang, W. A Simple Organic Synthesis for CdS and Se-Doped CdS Nanocrystals. Colloids Surf. A Physicochem. Eng. Asp. 2007, 299, 153–159. [Google Scholar] [CrossRef]
- Shi, J.-W.; Sun, D.; Zou, Y.; Ma, D.; He, C.; Ji, X.; Niu, C. Trap-Level-Tunable Se Doped CdS Quantum Dots with Excellent Hydrogen Evolution Performance without Co-Catalyst. Chem. Eng. J. 2019, 364, 11–19. [Google Scholar] [CrossRef]
- Poornaprakash, B.; Chalapathi, U.; Kumar, M.; Subramanyam, K.; Vattikuti, S.V.P.; Pratap Reddy, M.S.; Park, S. Enhanced Photocatalytic Activity and Hydrogen Evolution of CdS Nanoparticles through Er Doping. Ceram. Int. 2020, 46, 21728–21735. [Google Scholar] [CrossRef]
- Liu, M.; Du, Y.; Ma, L.; Jing, D.; Guo, L. Manganese Doped Cadmium Sulfide Nanocrystal for Hydrogen Production from Water under Visible Light. Int. J. Hydrogen Energy 2012, 37, 730–736. [Google Scholar] [CrossRef]
- Su, J.; Zhang, T.; Li, Y.; Chen, Y.; Liu, M. Photocatalytic Activities of Copper Doped Cadmium Sulfide Microspheres Prepared by a Facile Ultrasonic Spray-Pyrolysis Method. Molecules 2016, 21, 735. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Chen, W.; Zhang, J.; Huang, C.; Mao, L. Nickel Nanoparticles Modified CdS–A Potential Photocatalyst for Hydrogen Production through Water Splitting under Visible Light Irradiation. Int. J. Hydrogen Energy 2015, 40, 340–345. [Google Scholar] [CrossRef]
- Li, W.; Wang, F.; Liu, X.; Dang, Y.; Li, J.; Ma, T.; Wang, C. Promoting Body Carriers Migration of CdS Nanocatalyst by N-Doping for Improved Hydrogen Production under Simulated Sunlight Irradiation. Appl. Catal. B Environ. 2022, 313, 121470. [Google Scholar] [CrossRef]
- Sasikala, R.; Gaikwad, A.P.; Sudarsan, V.; Gupta, N.; Bharadwaj, S.R. Cubic Phase Indium Doped Cadmium Sulfide Dispersed on Zinc Oxide: Enhanced Photocatalytic Activity for Hydrogen Generation from Water. Appl. Catal. A Gen. 2013, 464–465, 149–155. [Google Scholar] [CrossRef]
- Zhang, K.; Jing, D.; Chen, Q.; Guo, L. Influence of Sr-Doping on the Photocatalytic Activities of CdS–ZnS Solid Solution Photocatalysts. Int. J. Hydrogen Energy 2010, 35, 2048–2057. [Google Scholar] [CrossRef]
- Cerdán-Pasarán, A.; Esparza, D.; Zarazúa, I.; Reséndiz, M.; López-Luke, T.; De la Rosa, E.; Fuentes-Ramírez, R.; Alatorre-Ordaz, A.; Martínez-Benítez, A. Photovoltaic Study of Quantum Dot-Sensitized TiO2/CdS/ZnS Solar Cell with P3HT or P3OT Added. J. Appl. Electrochem. 2016, 46, 975–985. [Google Scholar] [CrossRef]
- Desai, N.D.; Ghanwat, V.B.; Khot, K.V.; Mali, S.S.; Hong, C.K.; Bhosale, P.N. Effect of Substrate on the Nanostructured Bi2Se3 Thin Films for Solar Cell Applications. J. Mater. Sci. Mater. Electron. 2016, 27, 2385–2393. [Google Scholar] [CrossRef]
- Hernández-López, J.M.; Conde, A.; de Damborenea, J.J.; Arenas, M.A. TiO2 Nanotubes with Tunable Morphologies. RSC Adv. 2014, 4, 62576–62585. [Google Scholar] [CrossRef]
- Liu, G.; Wang, K.; Hoivik, N.; Jakobsen, H. Progress on Free-Standing and Flow-through TiO2 Nanotube Membranes. Sol. Energy Mater. Sol. Cells 2012, 98, 24–38. [Google Scholar] [CrossRef]
- Acevedo-Peña, P.; Lartundo-Rojas, L.; González, I. Effect of Water and Fluoride Content on Morphology and Barrier Layer Properties of TiO2 Nanotubes Grown in Ethylene Glycol-Based Electrolytes. J. Solid State Electrochem. 2013, 17, 2939–2947. [Google Scholar] [CrossRef]
- Omidvar, H.; Goodarzi, S.; Seif, A.; Azadmehr, A.R. Influence of Anodization Parameters on the Morphology of TiO2 Nanotube Arrays. Superlattices Microstruct. 2011, 50, 26–39. [Google Scholar] [CrossRef]
- Chen, Z.; Peng, W.; Zhang, K.; Zhang, J.; Yanagida, M.; Han, L. Surface Ion Transfer Growth of Ternary CdS1−XSex Quantum Dots and Their Electron Transport Modulation. Nanoscale 2012, 4, 7690–7697. [Google Scholar] [CrossRef]
- Kim, J.-Y.; Sekino, T.; Park, D.J.; Tanaka, S.-I. Morphology Modification of TiO2 Nanotubes by Controlling the Starting Material Crystallite Size for Chemical Synthesis. J. Nanoparticle Res. 2011, 13, 2319–2327. [Google Scholar] [CrossRef]
- Shaddad, M.N.; Cardenas-Morcoso, D.; García-Tecedor, M.; Fabregat-Santiago, F.; Bisquert, J.; Al-Mayouf, A.M.; Gimenez, S. TiO2 Nanotubes for Solar Water Splitting: Vacuum Annealing and Zr Doping Enhance Water Oxidation Kinetics. ACS Omega 2019, 4, 16095–16102. [Google Scholar] [CrossRef]
- Wiatrowski, A.; Mazur, M.; Obstarczyk, A.; Wojcieszak, D.; Kaczmarek, D.; Morgiel, J.; Gibson, D. Comparison of the Physicochemical Properties of TiO2 Thin Films Obtained by Magnetron Sputtering with Continuous and Pulsed Gas Flow. Coatings 2018, 8, 412. [Google Scholar] [CrossRef]
- Bharti, B.; Kumar, S.; Lee, H.-N.; Kumar, R. Formation of Oxygen Vacancies and Ti3+ State in TiO2 Thin Film and Enhanced Optical Properties by Air Plasma Treatment. Sci. Rep. 2016, 6, 32355. [Google Scholar] [CrossRef] [PubMed]
- Yue, D.; Qian, X.; Zhang, Z.; Kan, M.; Ren, M.; Zhao, Y. CdTe/CdS Core/Shell Quantum Dots Cocatalyzed by Sulfur Tolerant [Mo3S13]2− Nanoclusters for Efficient Visible-Light-Driven Hydrogen Evolution. ACS Sustain. Chem. Eng. 2016, 4, 6653–6658. [Google Scholar] [CrossRef]
- Li, W.; Li, M.; Xie, S.; Zhai, T.; Yu, M.; Liang, C.; Ouyang, X.; Lu, X.; Li, H.; Tong, Y. Improving the Photoelectrochemical and Photocatalytic Performance of CdO Nanorods with CdS Decoration. CrystEngComm 2013, 15, 4212–4216. [Google Scholar] [CrossRef]
- Li, G.; Sun, Y.; Hong, X.; Lu, W.; Chen, W.; Deng, Y.; Sun, Z.; Sun, W. Enhanced Photocatalytic Hydrogen Production on Tin Disulfide Self-Assembled from Ultrathin Sheets with Sulfur Vacancies Generated by Doping Indium Ions. J. Mater. Sci. 2021, 56, 10847–10858. [Google Scholar] [CrossRef]
- Han, J.; Liu, Y.; Wang, Y.; Zhao, R.; Wang, L. Construction of Ternary CdxMo1−xSe Quantum Dots for Enhanced Photocatalytic Hydrogen Production. J. Mater. Sci. 2020, 55, 1117–1125. [Google Scholar] [CrossRef]
- Gualdrón-Reyes, A.F.; Meléndez, A.M.; Mejía-Escobar, M.A.; Jaramillo, F.; Niño-Gómez, M.E. The Role of Boron in the Carrier Transport Improvement of CdSe-Sensitized B,N,F-TiO2 Nanotube Solar Cells: A Synergistic Strategy. N. J. Chem. 2018, 42, 14481–14492. [Google Scholar] [CrossRef]
- Zaiats, G.; Yanover, D.; Vaxenburg, R.; Shapiro, A.; Safran, A.; Hesseg, I.; Sashchiuk, A.; Lifshitz, E. PbSe/CdSe Thin-Shell Colloidal Quantum Dots. Z. Phys. Chem. 2015, 229, 3–21. [Google Scholar] [CrossRef]
- Cerdán-Pasarán, A.; López-Luke, T.; Esparza, D.; Zarazúa, I.; De la Rosa, E.; Fuentes-Ramírez, R.; Alatorre-Ordaz, A.; Sánchez-Solís, A.; Torres-Castro, A.; Zhang, J.Z. Photovoltaic Properties of Multilayered Quantum Dot/Quantum Rod-Sensitized TiO2 Solar Cells Fabricated by SILAR and Electrophoresis. Phys. Chem. Chem. Phys. 2015, 17, 18590–18599. [Google Scholar] [CrossRef]
- Liu, G.; Ling, Z.; Wang, Y.; Zhao, H. Near-Infrared CdSexTe1−X@CdS “Giant” Quantum Dots for Efficient Photoelectrochemical Hydrogen Generation. Int. J. Hydrogen Energy 2018, 43, 22064–22074. [Google Scholar] [CrossRef]
- Farah Khaleda, M.Z.; Vengadaesvaran, B.; Rahim, N.A. Spectral Response and Quantum Efficiency Evaluation of Solar Cells: A Review. In Energy Materials; Elsevier: Hoboken, NJ, USA, 2021; pp. 525–566. ISBN 9780128237106. [Google Scholar]
- Chi, C.; Liau, S.; Lee, Y. The Heat Annealing Effect on the Performance of CdS/CdSe-Sensitized TiO2 Photoelectrodes in Photochemical Hydrogen Generation. Nanotechnology 2010, 21, 025202. [Google Scholar] [CrossRef]
- Lee, Y.; Chi, C.; Liau, S. CdS/CdSe Co-Sensitized TiO2 Photoelectrode for Efficient Hydrogen Generation in a Photoelectrochemical Cell. Chem. Mater. 2010, 22, 922–927. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).