Spinning of Carbon Nanofiber/Ni–Cu–S Composite Nanofibers for Supercapacitor Negative Electrodes
Abstract
1. Introduction
2. Experimental Section
2.1. Chemicals and Materials
2.2. Material Characterization Instruments and Equipment
2.3. Preparation of the CNFs/Ni-Cu and CNFs/Ni–Cu–S Electrodes
2.4. Electrochemical Measurements
3. Results and Discussion
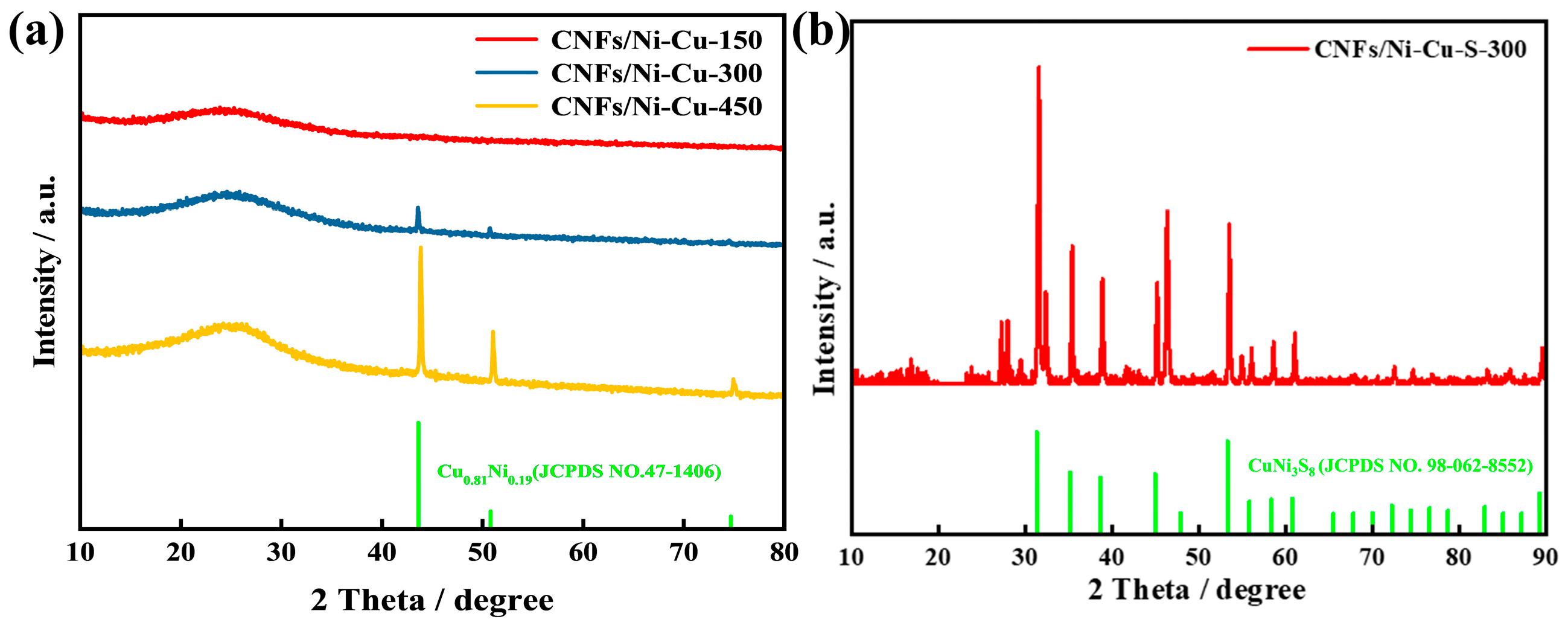
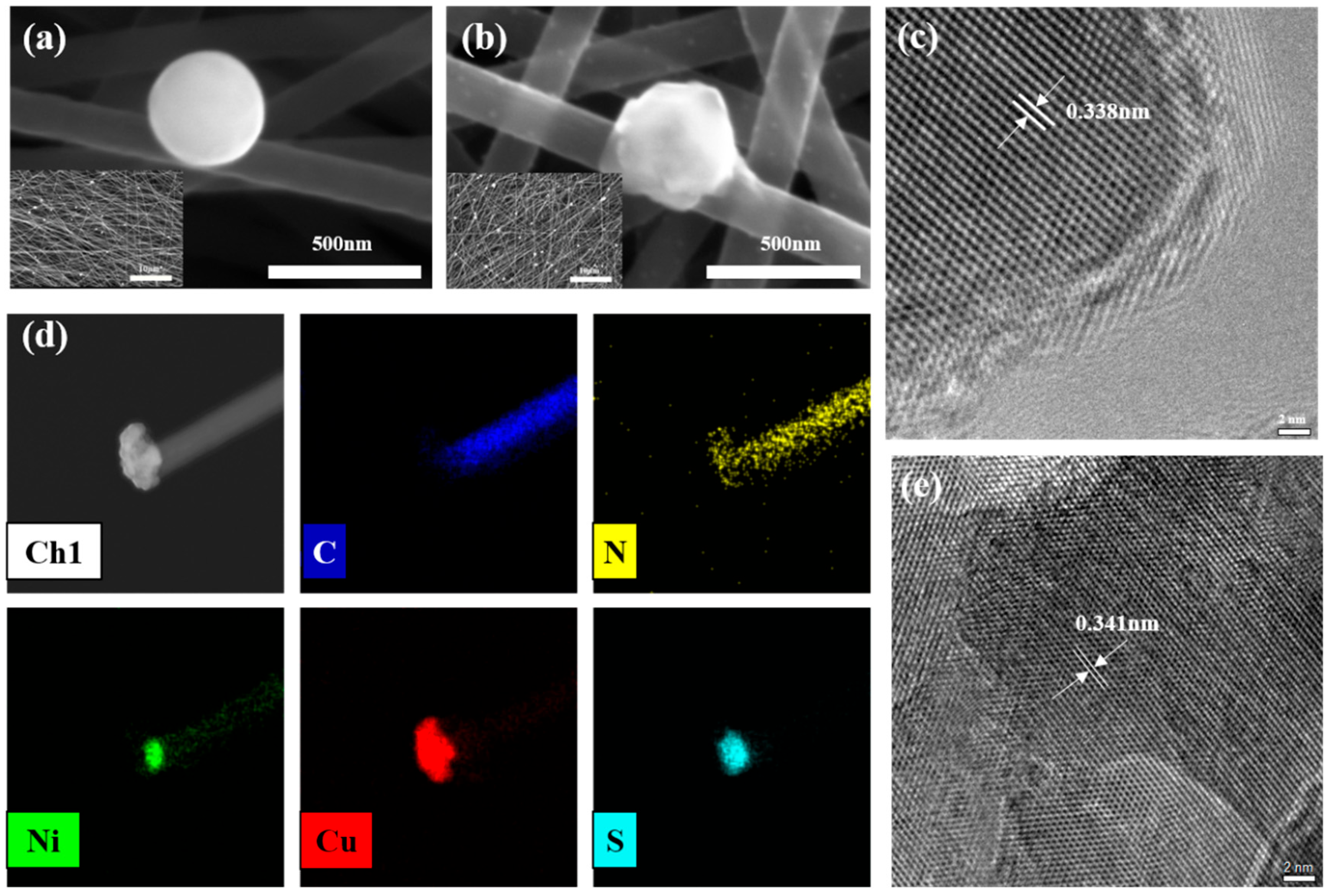
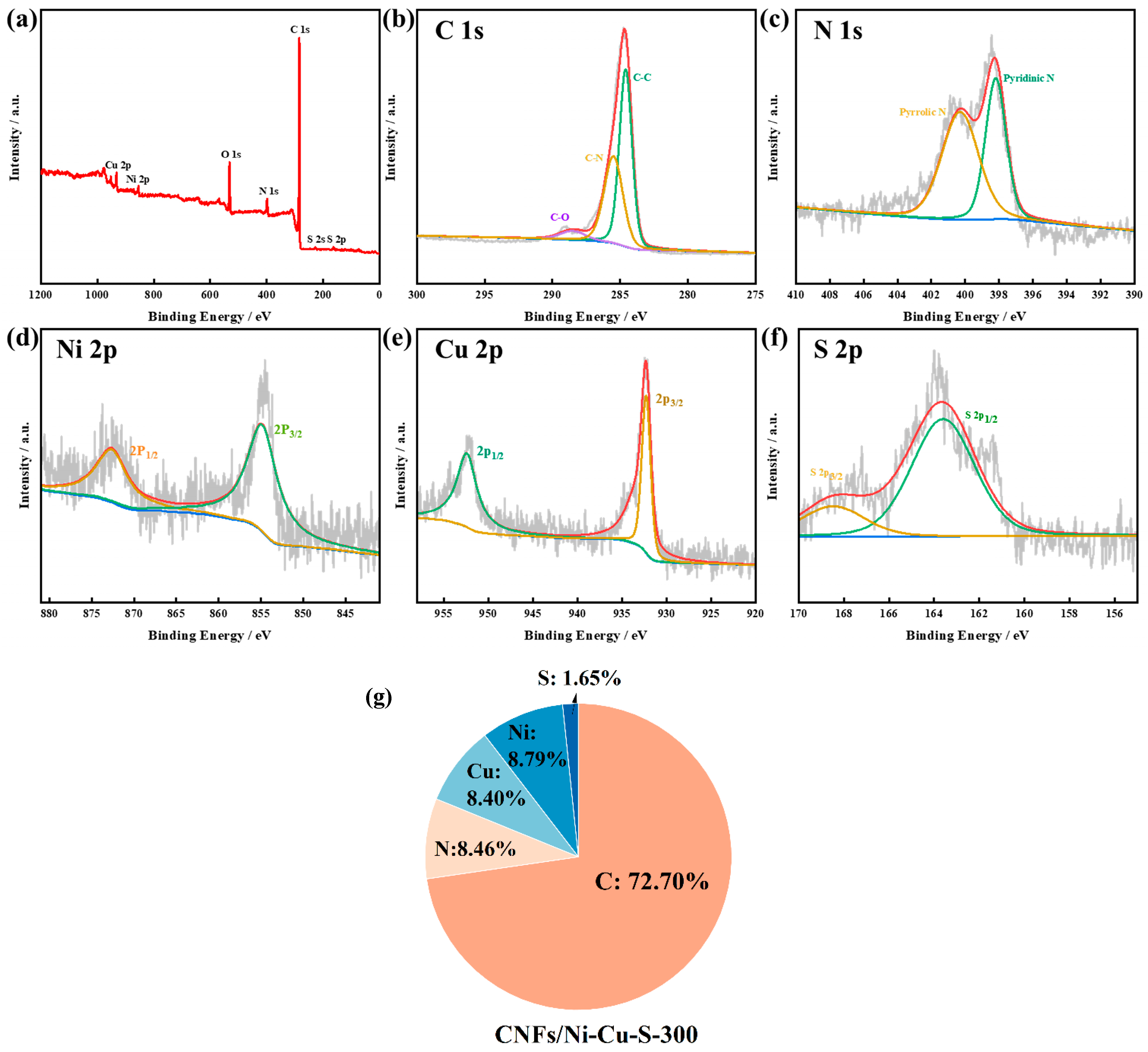
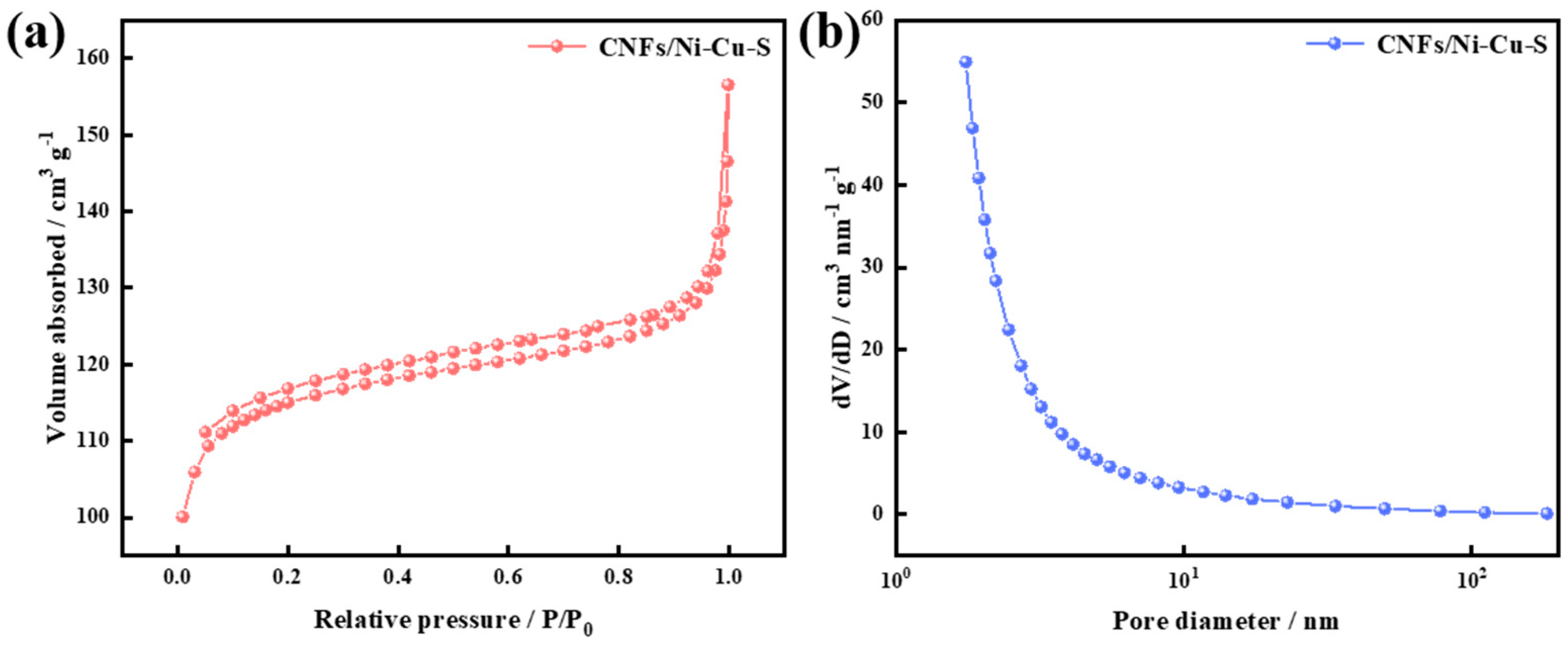
3.1. Material Characterization
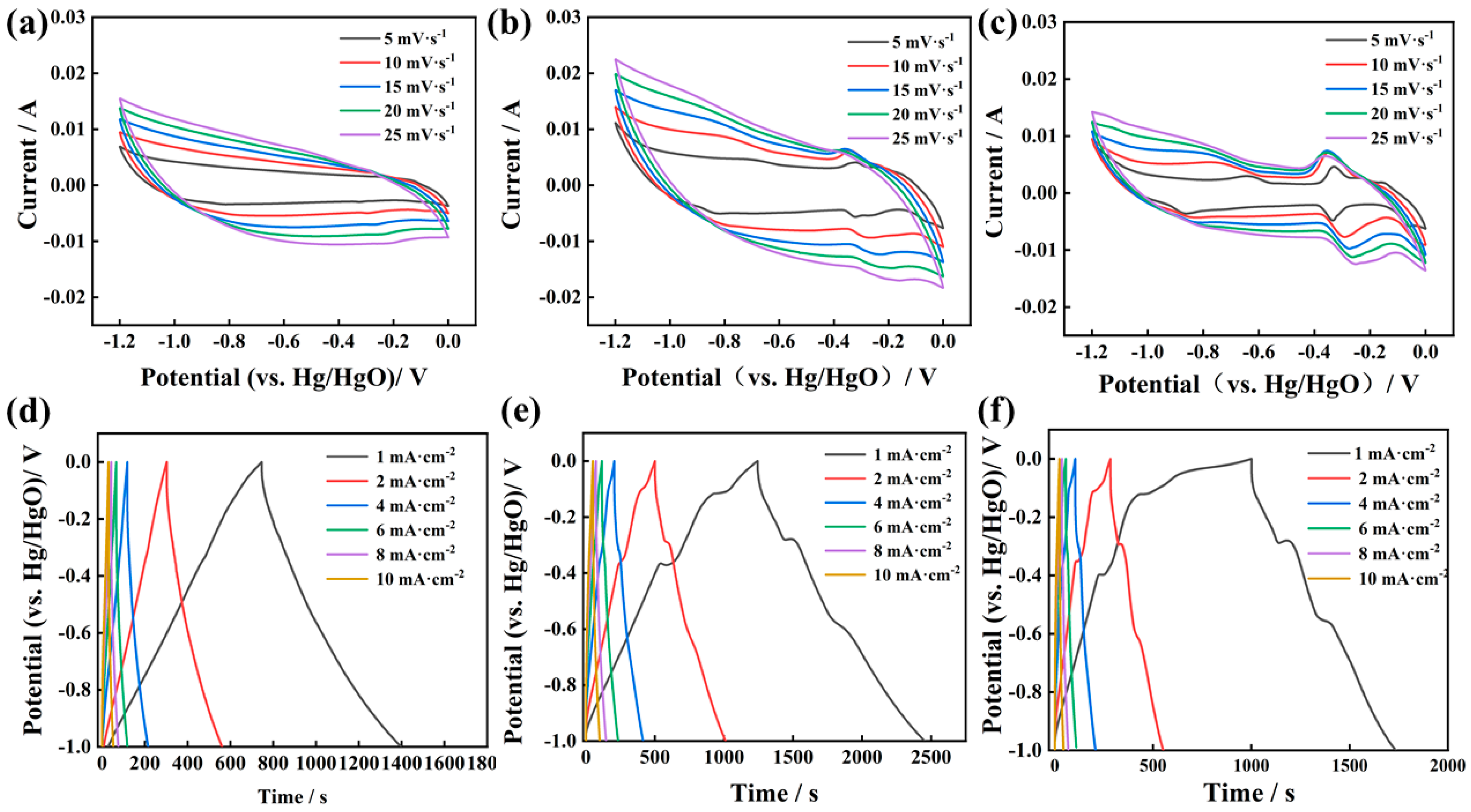
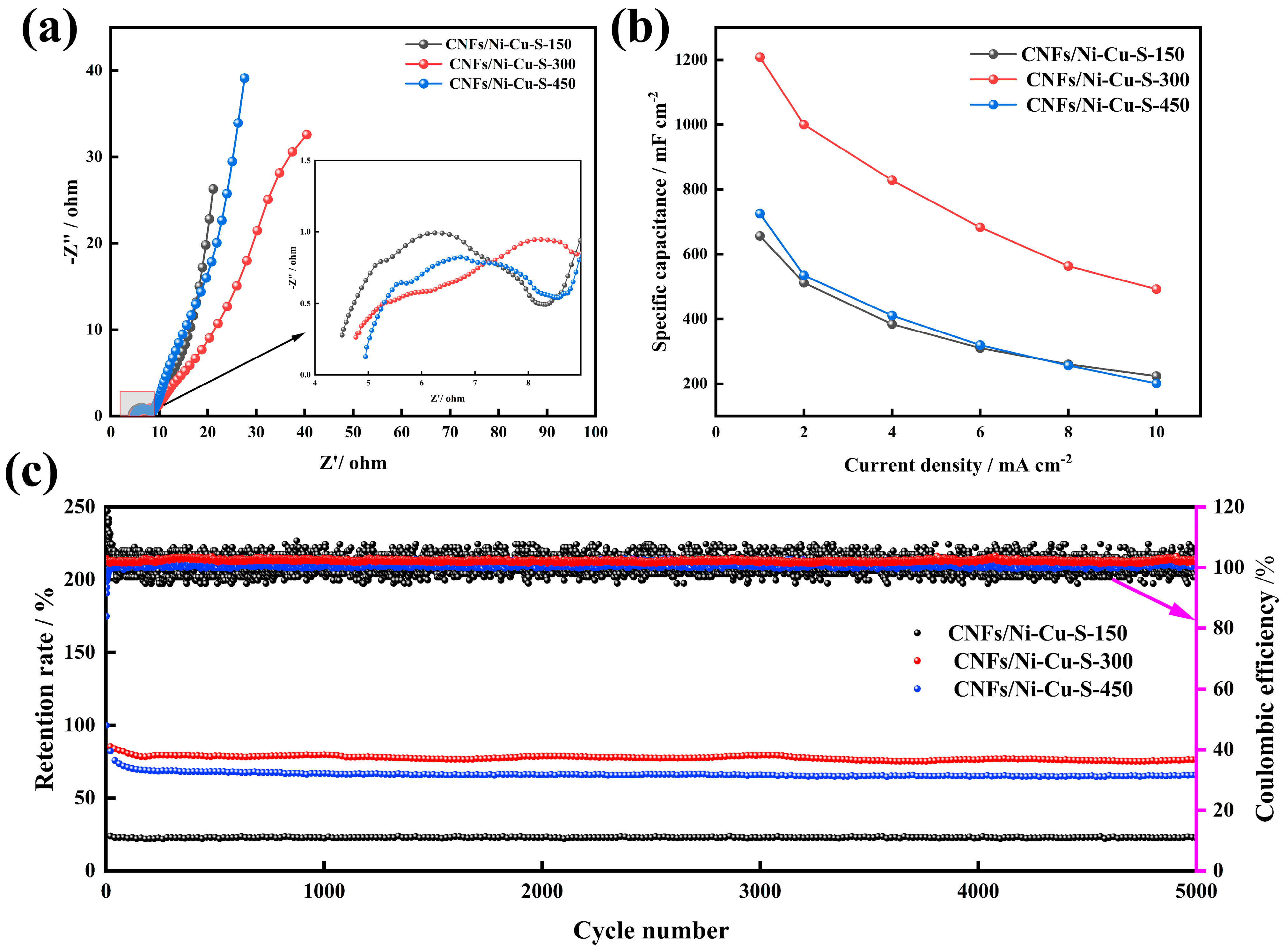
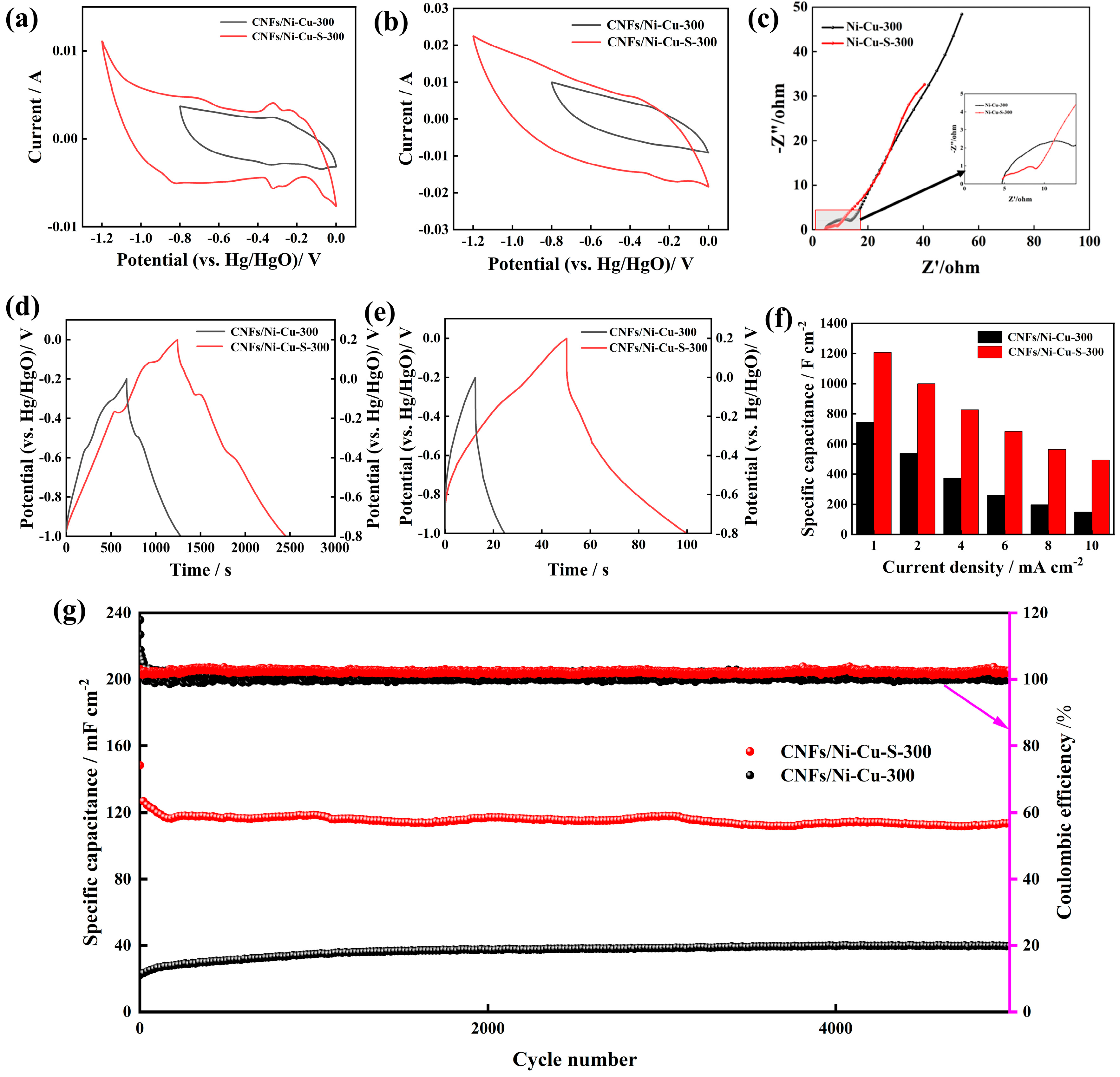
3.2. Electrochemical Performance
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Du, Y.Q.; Zhang, B.Y.; Kang, R.K.; Zhou, W.; Zhang, W.Y.; Jin, H.X.; Wan, J.Q.; Zhang, J.X.; Chen, G.W. Boron-doping-induced defect engineering enables high performance of a graphene cathode for aluminum batteries. Inorg. Chem. Front. 2022, 9, 925–934. [Google Scholar] [CrossRef]
- Su, Z.H.; Huang, J.H.; Wang, R.H.; Zhang, Y.; Zeng, L.X.; Zhang, Y.F.; Fan, H.S. Multilayer structure covalent organic frameworks (COFs) linking by double functional groups for advanced K plus batteries. J. Colloid. Interf. Sci. 2023, 639, 7–13. [Google Scholar] [CrossRef] [PubMed]
- Zhao, D.C.; Zhang, Z.; Ren, J.H.; Xu, Y.Y.; Xu, X.Y.; Zhou, J.; Gao, F.; Tang, H.; Liu, S.P.; Wang, Z.L.; et al. Fe2VO4 nanoparticles on rGO as anode material for high-rate and durable lithium and sodium ion batteries. Chem. Eng. J. 2023, 451, 138882. [Google Scholar] [CrossRef]
- Liang, G.; Li, X.; Wang, Y.; Yang, S.; Huang, Z.; Yang, Q.; Wang, D.; Dong, B.; Zhu, M.; Zhi, C. Building durable aqueous K-ion capacitors based on MXene family. Nano Res. Energy 2022, 1, e9120002. [Google Scholar] [CrossRef]
- Zhang, Y.W.; Du, H.L.; Lu, J.; Li, Z.; Lu, T.; Hu, Y.X.; Ma, Y.T. Enhanced cyclic stability of Ga2O3@PDA-C nanospheres as pseudocapacitive anode materials for lithium-ion batteries. Fuel 2023, 334, 126683. [Google Scholar] [CrossRef]
- Wang, Z.P.; Cheng, J.L.; Zhou, J.W.; Zhang, J.X.; Huang, H.; Yang, J.; Li, Y.C.; Wang, B. All-climate aqueous fiber-shaped supercapacitors with record areal energy density and high safety. Nano Energy 2018, 50, 106–117. [Google Scholar] [CrossRef]
- Fang, B.; Wei, Y.-Z.; Suzuki, K.; Kumagai, M. Surface modification of carbonaceous materials for EDLCs application. Electrochim. Acta 2005, 50, 3616–3621. [Google Scholar] [CrossRef]
- Yang, S.; Liu, Y.; Hao, Y.; Yang, X.; Goddard, W.A., III; Zhang, X.L.; Cao, B. Oxygen-vacancy abundant ultrafine Co3O4/graphene composites for high-rate supercapacitor electrodes. Adv. Sci. 2018, 5, 1700659. [Google Scholar] [CrossRef] [PubMed]
- Levitt, A.S.; Alhabeb, M.; Hatter, C.B.; Sarycheva, A.; Dion, G.; Gogotsi, Y. Electrospun MXene/carbon nanofibers as supercapacitor electrodes. J. Mater. Chem. A 2019, 7, 269–277. [Google Scholar] [CrossRef]
- Elseman, A.; Fayed, M.; Mohamed, S.; Rayan, D.; Allam, N.; Rashad, M.; Song, Q. A novel composite CoFe2O4@CSs as electrode by easy one-step solvothermal for enhancing the electrochemical performance of hybrid supercapacitors. ChemElectroChem 2020, 7, 526–534. [Google Scholar] [CrossRef]
- Pan, Z.D.; Yu, S.; Wang, L.F.; Li, C.Y.; Meng, F.; Wang, N.; Zhou, S.X.; Xiong, Y.; Wang, Z.L.; Wu, Y.T.; et al. Recent Advances in Porous Carbon Materials as Electrodes for Supercapacitors. Nanomaterials 2023, 13, 1744. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Deng, C.; Huang, Z.; Zhang, Q.; Chai, X.; Yi, D.; Fang, Y.; Wu, M.; Wang, X.; Tang, Y. Dual-Silica Template-Mediated Synthesis of Nitrogen-Doped Mesoporous Carbon Nanotubes for Supercapacitor Applications. Small 2023, 19, 2205725. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.K.; Lv, Y.Y.; Chi, H.Z.; Xi, J.H.; Xiong, Q.W.; Qin, H.Y. All-Solid-State Supercapacitor with High Volumetric Energy for Flexible Application. J. Electrochem. Soc. 2019, 166, A2797–A2804. [Google Scholar] [CrossRef]
- Orlando, J.D.; Lima, R.M.A.P.; Li, L.; Sydlik, S.A.; de Oliveira, H.P. Electrochemical Performance of N-Doped Carbon-Based Electrodes for Supercapacitors. ACS Appl. Electron. Mater. 2022, 4, 5040–5054. [Google Scholar] [CrossRef]
- Wang, H.W.; Yi, H.; Chen, X.; Wang, X.F. Asymmetric supercapacitors based on nano-architectured nickel oxide/graphene foam and hierarchical porous nitrogen-doped carbon nanotubes with ultrahigh-rate performance. J. Mater. Chem. A 2014, 2, 3223–3230. [Google Scholar] [CrossRef]
- Cheng, Y.; Zhai, M.M.; Hu, J.B. The fabrication of NiCuS from NiCu film on nickel foam for methanol electrooxidation and supercapacitors. Appl. Surf. Sci. 2019, 480, 505–513. [Google Scholar] [CrossRef]
- Dai, T.T.; Cai, B.; Yang, X.J.; Jiang, Y.; Wang, L.Y.; Wang, J.S.; Li, X.S.; Lü, W. Asymmetric supercapacitors based on SnNiCoS ternary metal sulfide electrodes. Nanotechnology 2023, 34, 225401. [Google Scholar] [CrossRef]
- Beknalkar, S.A.; Teli, A.M.; Shin, J.C. Current innovations and future prospects of metal oxide electrospun materials for supercapacitor technology: A review. J. Mater. Sci. Technol. 2023, 166, 208–233. [Google Scholar] [CrossRef]
- Zhao, D.H.; Wang, H.; Bai, Y.; Yang, H.; Song, H.F.; Li, B.H. Preparation of Advanced Multi-Porous Carbon Nanofibers for High-Performance Capacitive Electrodes in Supercapacitors. Polymers 2023, 15, 213. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Sajjad, M.; Zheng, Y.Q.; Zhao, M.M.; Li, Z.J.; Wu, Z.Y.; Kang, K.; Qiu, L. Covalent organic framework templated ordered nanoporous C as stable energy efficient supercapacitor electrode material. Carbon 2021, 182, 144–154. [Google Scholar] [CrossRef]
- Yadav, D.; Amini, F.; Ehrmann, A. Recent advances in carbon nanofibers and their applications—A review. Eur. Polym. J. 2020, 138, 109963. [Google Scholar] [CrossRef]
- Azwar, E.; Wan Mahari, W.A.; Chuah, J.H.; Vo, D.V.N.; Ma, N.L.; Lam, W.H.; Lam, S.S. Transformation of biomass into carbon nanofiber for supercapacitor application—A review. Int. J. Hydrogen Energy 2018, 43, 20811–20821. [Google Scholar] [CrossRef]
- Kshetri, T.; Tran, D.T.; Nguyen, D.C.; Kim, N.H.; Lau, K.-T.; Lee, J.H. Ternary graphene-carbon nanofibers-carbon nanotubes structure for hybrid supercapacitor. Chem. Eng. J. 2020, 380, 122543. [Google Scholar]
- Luo, W.; Xue, H. The synthesis and electrochemical performance of NiCo2O4 embedded carbon nanofibers for high-performance supercapacitors. Fuller. Nanotub. Carbon Nanostruct. 2019, 27, 189–197. [Google Scholar] [CrossRef]
- Fan, P.Z.; Ye, C.W.; Xu, L. One-dimensional nanostructured electrode materials based on electrospinning technology for supercapacitors. Diam. Relat. Mater. 2023, 134, 109803. [Google Scholar] [CrossRef]
- Thejas Prasannakumar, A.; Mohan, R.R.; V, M.; Varma, S.J. Progress in Conducting Polymer-Based Electrospun fibers for Supercapacitor Applications: A Review. ChemistrySelect 2023, 8, e202203564. [Google Scholar] [CrossRef]
- Altin, Y.; Bedeloglu, A.C. Polyacrylonitrile/polyvinyl alcohol-based porous carbon nanofiber electrodes for supercapacitor applications. Int. J. Energ. Res. 2021, 45, 16497–16510. [Google Scholar] [CrossRef]
- Zhu, H.; Yu, D.; Zhang, S.; Chen, J.; Wu, W.; Wan, M.; Wang, L.; Zhang, M.; Du, M. Morphology and structure engineering in nanofiber reactor: Tubular hierarchical integrated networks composed of dual phase octahedral CoMn2O4/carbon nanofibers for water oxidation. Small 2017, 13, 1700468. [Google Scholar] [CrossRef] [PubMed]
- Ismar, E.; Karazehir, T.; Ates, M.; Sarac, A.S. Electrospun carbon nanofiber web electrode: Supercapacitor behavior in various electrolytes. J. Appl. Polym. Sci. 2018, 135, 45723. [Google Scholar] [CrossRef]
- Sun, J.; Chen, Y.-R.; Huang, K.; Li, K.; Wang, Q. Interfacial electronic structure and electrocatalytic performance modulation in Cu0.81Ni0.19 nanoflowers by heteroatom doping engineering using ionic liquid dopant. Appl. Surf. Sci. 2020, 500, 144052. [Google Scholar]
- Huang, Q.; Li, C.; Tu, Y.; Jiang, Y.; Mei, P.; Yan, X. Spinel CoFe2O4/carbon nanotube composites as efficient bifunctional electrocatalysts for oxygen reduction and oxygen evolution reaction. Ceram. Int. 2021, 47, 1602–1608. [Google Scholar]
- Li, T.F.; Lv, Y.J.; Su, J.H.; Wang, Y.; Yang, Q.; Zhang, Y.W.; Zhou, J.C.; Xu, L.; Sun, D.M.; Tang, Y.W. Anchoring CoFe2O4 Nanoparticles on N-Doped Carbon Nanofibers for High-Performance Oxygen Evolution Reaction. Adv. Sci. 2017, 4, 1700226. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.G.; Jin, D.D.; Zhou, R.; Shen, C.; Xie, K.Y.; Wei, B.Q. One-step synthesis of NiCoS ultrathin nanosheets on conductive substrates as advanced electrodes for high-efficient energy storage. J. Power Sources 2016, 306, 100–106. [Google Scholar] [CrossRef]
- Lu, Y.; Zhang, Z.W.; Liu, X.M.; Wang, W.X.; Peng, T.; Guo, P.F.; Sun, H.B.; Yan, H.L.; Luo, Y.S. NiCoS/carbon nanotube nanocomposites with a chain-like architecture for enhanced supercapacitor performance. Crystengcomm 2016, 18, 7696–7706. [Google Scholar] [CrossRef]
- Wu, D.; Zhang, W.; Cheng, D. Facile synthesis of Cu/NiCu electrocatalysts integrating alloy, core–shell, and one-dimensional structures for efficient methanol oxidation reaction. ACS Appl. Mater. Interfaces 2017, 9, 19843–19851. [Google Scholar] [CrossRef] [PubMed]
- Fang, X.R.; Cheng, P.; Sun, K.; Fu, Y.J.; Liu, D.Q.; He, D.Y. Zn-N Codoped Carbon Nanofiber Interlayer for Anchor and Catalysis of Polysulfides in Lithium-Sulfur Batteries. ACS Appl. Energ. Mater. 2022, 5, 8189–8197. [Google Scholar] [CrossRef]
- William, J.J.; Babu, I.M.; Muralidharan, G. Nickel bismuth oxide as negative electrode for battery-type asymmetric supercapacitor. Chem. Eng. J. 2021, 422, 130058. [Google Scholar] [CrossRef]
- Shen, B.S.; Guo, R.S.; Lang, J.W.; Liu, L.; Liu, L.Y.; Yan, X.B. A high-temperature flexible supercapacitor based on pseudocapacitive behavior of FeOOH in an ionic liquid electrolyte. J. Mater. Chem. A 2016, 4, 8316–8327. [Google Scholar] [CrossRef]
- Qi, J.Q.; Chang, Y.; Sui, Y.W.; He, Y.Z.; Meng, Q.K.; Wei, F.X.; Zhao, Y.L.; Jin, Y.X. Facile Construction of 3D Reduced Graphene Oxide Wrapped NiS Nanoparticles on Ni Foam for High-Performance Asymmetric Supercapacitor Electrodes. Part. Part. Syst. Charact. 2017, 34, 1700196. [Google Scholar] [CrossRef]
- Hu, W.; Chen, R.Q.; Xie, W.; Zou, L.L.; Qin, N.; Bao, D.H. CoNiS Nanosheet Arrays Supported on Nickel Foams with Ultrahigh Capacitance for Aqueous Asymmetric Supercapacitor Applications. ACS Appl. Mater. Interfaces 2014, 6, 19318–19326. [Google Scholar] [CrossRef]
- Fan, P.; Xu, L. Core-Shell Carbon Nanofibers@Ni(OH)2/NiO Composites for High-Performance Asymmetric Supercapacitors. Materials 2022, 15, 8377. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Jiang, G.; Sun, S.; Xu, B.; Zhou, J.; Zhang, Y.; Yao, J. Decoration of carbon nanofibers with NiCo2S4 nanoparticles for flexible asymmetric supercapacitors. J. Alloys Compd. 2018, 731, 560–568. [Google Scholar] [CrossRef]
- Li, B.; Tian, Z.; Li, H.; Yang, Z.; Wang, Y.; Wang, X. Self-supporting graphene aerogel electrode intensified by NiCo2S4 nanoparticles for asymmetric supercapacitor. Electrochim. Acta 2019, 314, 32–39. [Google Scholar]
- Xu, L.; Wang, W.; Liu, Y.; Liang, D. Nanocellulose-Linked MXene/Polyaniline Aerogel Films for Flexible Supercapacitors. Gels 2022, 8, 798. [Google Scholar] [PubMed]


| Sample | Pre-Oxidation Temperature [°C] | Carbonization Temperature [°C] | Vulcanization Temperature [°C] | Ni(NO3)2·6H2O Addition Amount [mg] | Cu(NO3)2·3H2O Addition Amount [mg] |
|---|---|---|---|---|---|
| NCS/CNFs-150 | 230 | 800 | 550 | 150 | 150 |
| NCS/CNFs-300 | 230 | 800 | 550 | 300 | 300 |
| NCS/CNFs-450 | 230 | 800 | 550 | 450 | 450 |
| Sample | BET Surface Area (m2 g−1) | BJH Desorption Cumulative Surface Area (m2 g−1) | Total Pore Volume (cm3 g−1) | BJH Desorption Cumulative Volume (cm3 g−1) | Average Pore Diameter (nm) | BJH Desorption Median Pore Diameter (nm) |
|---|---|---|---|---|---|---|
| CNFs/Ni–Cu–300 | 42.609 | 24.359 | 0.008 | 0.059 | 6.065 | 9.762 |
| CNFs/Ni–Cu–S–300 | 371.063 | 54.281 | 0.149 | 0.086 | 2.562 | 6.361 |
| Electrode Materials | Electrolyte | C/Current Density | Capacity Retention Rate/Cycle Number |
|---|---|---|---|
| NiCo2O4-CNFs [24] | 6 M KOH | 836 F g−1/5 A g−1 | 80.9%/2000 cycles |
| CNFs@Ni(OH)2/NiO [41] | 3 M KOH | 695 F g−1/1 A g−1 | 77%/2000 cycles |
| NiCo2S4@CNFs [42] | 3 M KOH | 527.8 F g−1/0.2 A g−1 | 90%/3000 cycles |
| NiCo2S4/GA [43] | 3 M KOH | 704.34 F g−1/1 A g−1 | 80.3%/1500 cycles |
| MXene/CNF–PANI [44] | 3 M H2SO4 | 327 F g−1/3 mA cm−2 | 84.1%/3000 cycles |
| CNFs/Ni–Cu–S–300 (This work) | 1 M KOH | 1208 mF cm−2/1 mA cm−2 | 76.5%/5000 cycles |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Q.; Wang, Y.; Wei, G.; Fang, X.; Lan, N.; Zhao, Y.; Liu, Q.; Lin, S.; He, D. Spinning of Carbon Nanofiber/Ni–Cu–S Composite Nanofibers for Supercapacitor Negative Electrodes. Energies 2024, 17, 1449. https://doi.org/10.3390/en17061449
Li Q, Wang Y, Wei G, Fang X, Lan N, Zhao Y, Liu Q, Lin S, He D. Spinning of Carbon Nanofiber/Ni–Cu–S Composite Nanofibers for Supercapacitor Negative Electrodes. Energies. 2024; 17(6):1449. https://doi.org/10.3390/en17061449
Chicago/Turabian StyleLi, Qiong, Yu Wang, Ganghui Wei, Xiaorong Fang, Ni Lan, Yonggang Zhao, Qiming Liu, Shumei Lin, and Deyan He. 2024. "Spinning of Carbon Nanofiber/Ni–Cu–S Composite Nanofibers for Supercapacitor Negative Electrodes" Energies 17, no. 6: 1449. https://doi.org/10.3390/en17061449
APA StyleLi, Q., Wang, Y., Wei, G., Fang, X., Lan, N., Zhao, Y., Liu, Q., Lin, S., & He, D. (2024). Spinning of Carbon Nanofiber/Ni–Cu–S Composite Nanofibers for Supercapacitor Negative Electrodes. Energies, 17(6), 1449. https://doi.org/10.3390/en17061449







