Abstract
The valorisation of sewage sludge for sustainable agricultural use and biofuel production proposes an effective and beneficial management of sewage sludge in a closed-loop cycle. The management of sewage sludge biowaste is a rising problem due to increasing waste storage expenses. In this sense, the use of circular economy principles in sewage sludge management creates opportunities to develop new technologies for processing. The biorefinery model allows the application of wasteless technologies via sewage sludge valorisation in terms of agricultural use and biofuel production, especially with the hydrothermal carbonisation method. Applying hydrothermal carbonisation in the treatment of biosolid sewage sludge has numerous benefits due to processing highly hydrated organic waste into carbon hydro char, a high-quality solid biofuel. The direct use of sewage sludge in the soil does not allow for full use of its functional properties. However, the hydrothermal carbonisation of sewage sludge results in biocarbon pellets, making it a viable approach. This work also discusses the barriers (legal, chemical, biological, and technical) and possibilities related to sewage sludge biorefining processes.
1. Introduction
Biosolid sewage sludge (BS) is highly hydrated organic waste obtained in biological wastewater treatment plants (WWTPs). It contains fertiliser ingredients but also microbial and chemical pollutants. The global BS production ranges from 125 to 150 million t/y and is estimated to continuously grow to around 150–200 million t/y by 2025 [1,2,3,4], mainly as a result of the global population increase. The growing amount of municipal and industrial BS is one of the largest ecological issues. The amount of wastewater and its processing methods depend on the degree of national economic development. The processing and landfilling of sewage sludge from WWTPs are crucial issues in wastewater processing and management. Sewage sludge contains various toxic contaminants that threaten human and environmental health, calling for its adequate treatment and use [5,6,7].
In China, the total sewage sludge (80% water content) production was 39.04 million tons in 2019, with the potential to increase by 10% each year [8,9]. The estimated US sewage sludge production was 4.5 million dry metric tons in 2021 in public-owned treatment works (POTWs) [3]. The EU produced 9.0–9.5 million tons of sewage sludge (on a dry basis) in 2017 [8]. According to Eurostat, in 2020, the total amount of sewage sludge dry mass generated and disposed of in Europe was 8.7 million metric tons [10,11,12]. In the EU, the sewage sludge issue is being tackled with general directives and guides as well as state–national law-making prescriptions [13,14,15,16].
Typically, sewage sludge is processed in three main ways: direct use for farmland fertilisation, composting or reclamation of raw materials, and thermal processing [17,18,19]. Sewage sludge disposal for sustainable development (SD) [20,21,22] is a considerable issue because of the higher water content of the sludge (98–99%). Due to of the large volume, high wetness, low dewaterability, odour emissions, and content of pathogens, proper sludge disposal and processing methods are required not only to reduce cost and treatment complications but also to eliminate the risks to environmental and human health [23,24,25].
The main sewage sludge disposal methods are incineration, landfilling, or use for aboveground applications, including structural soil improvement, soil buffering, and soil improvement. Because of its specific characteristics, highlighting its considerable levels of nitrogen, phosphorus, and organic substances, sewage sludge is applied in agriculture to increase soil quality by enhancing the levels of plant-available nutrients. Apart from this, the use of sewage sludge stimulates soil microbial functions, such as respiration or soil enzyme activities, due to the reduction in the levels of organic substances in sewage sludge [26,27,28,29].
In EU countries, the agricultural use of sewage sludge in 2014–2015 amounted to 22.6% (2014) and 22.1% (2015) of the produced sludge and 23.3% (2014) and 23.1% (2015) of the disposed sludge. Among the different EU member states, these values ranged between 0% (Malta, Slovenia, and Slovakia) and 80% (Ireland). In Bulgaria, more than 50% of the sewage sludge was used in agriculture in 2015 [11,25].
On 2 May 2022, the 8th Environment Action Program was launched, which is the EU’s legally established joint program for environmental policy, containing a collection of priority goals for 2030 and the conditions necessary to fulfil them [30]. The action program reiterates the EU’s long-term vision for 2050, based on the European Green Deal. The strategic activities allow the conversion to a circular economy (CE), decreasing the impact on natural resources and recognising that human well-being and prosperity result from good environmental conditions. This is the EU’s base for the fulfilment of the United Nation’s 2030 Agenda and its Sustainable Development Goals [31].
The establishment of biorefineries is a promising solution for combining waste processing with the production of value-added compounds for the implementation of closed-loop production models with practically zero waste production [32,33,34]. Different biorefinery technologies, such as hydrothermal pretreatment (HTP) and anaerobic digestion (AD), allow the efficient processing of biowaste to produce valued products such as fertilisers or biofuels. The implementation of renewable energy production is a crucial activity to reach Sustainable Development Goals as it facilitates the substitution of the contaminating and harmful “grey economy” with an environmentally friendly “green economy” [30,31]. Wastewater Management is one of the most important issues in the conversion towards a CE. A special scope of interest refers to sewage sludge, the byproduct of municipal wastewater processing. The production of biofuels and the recovery and reuse of materials from sewage sludge can be economically and environmentally beneficial as the dry sludge contains 50–70% organic substances [4,35,36,37,38].
The CE is an economic model to improve environmental features, economic growth, and public justice, benefitting the current and following generations [39,40,41]. At the microeconomic level [42], CE implementation results in the improvement of the production models and increased cooperation with other firms in the delivery chain to obtain a more economically effective closed-loop cycle [28]. Based on the CE principles, reuse, treatment, and renovation of products results in a decreased need for resources and energy [40,43]. One of the assumptions of the CE is that combustion for energy recovery is a beneficial option, whereas waste landfilling is the last resort. By that means, the production value chain and life cycle keep the best achievable effectiveness and quality if possible and are as energy effective as possible [20,39,40].
At present, biofuel is a basic renewable energy source. The use of biofuels represents a possibility to decrease greenhouse gas emissions compared to the use of crude oil fuels and the storage of CO2 in the soil. This results from the processes used for the production of biofuels and their byproducts. Keeping soil organic carbon at an initial phase of developing a biofuel decreases climate transition. This should help farmers to manage soil carbon throughout the life cycle of biofuel manufacturing [21,44,45].
Biofuel is necessary for the decarbonised transport sector and the development of low-carbon proposals for present technologies, such as light-duty vehicles for the near future and heavy-duty lorries, ships, and airplanes, with a small number of optional beneficial solutions in the further future. The biofuel demand in 2022 achieved a record of 4300 PJ (170,000 m3), but a considerably higher biofuel production is required to achieve the net zero emission goal (NZE) by 2050. According to proposed NZE scenarios, biofuel manufacturing should obtain over 10,000 PJ by 2030, which requires a yearly average biofuel production increase of approximately 11%. Advanced raw material consumption should also grow; biofuels should be produced from waste and agricultural residues, and nonfood energy harvest must meet >40% of total biofuel demand in 2030, in comparison to 9% in 2021 [6,19,34].
However, biofermentation is the main way to produce drop-in fuel. It is still in the early stage of development and requires more studies and pilot-scope research to work out less expensive and sustainable processes to implement biofermentation technologies into the industry. It is estimated that in the future, ethanol and biodiesel will be fundamental biofuels [6,44]. For the manufacture of second-generation 2G biofuels, crops containing mostly cellulose are used. These 2G biofuels are produced from biomass obtained from fields intended for this purpose and not used as arable lands. Lignocellulosic biomass (LCB) is the most abundant accessible bioresource, with a worldwide yield reaching 1.3 billion t/y. It is one of the most important carbon raw materials to deliver fuels to transportation systems for light- and heavy-duty use. The use of LCB can improve energy safety by decreasing the demand for crude oils, promoting agricultural progression, and reducing greenhouse gas emissions. LCB hydrolysis allows for the release of various reducing sugars, which are effective raw materials in obtaining biofuels such as bioethanol or biogas [29,37,46].
This work presents a review of the current solutions for the processing of sewage sludge into biofuels and/or their use in agriculture, considering the use of diverse technological methods to offer cost-effective and sustainable management of sewage sludge in a closed-loop cycle. This is supplemented by an analysis of the barriers and opportunities of the hydrothermal carbonisation of sewage sludge.
In this paper, characteristics of sludge from municipal wastewater treatment are carried out in Section 2; here, the main features of treatments and their ability to meet the above-mentioned goals are discussed. Sewage sludge applications are then analysed in Section 3. Sewage sludge use in agriculture is discussed in Section 4 and thermal treatment of sewage sludge in Section 5. HTC hydrothermal carbonisation of sewage sludge is analysed in Section 6. An economic evaluation of biocarbon pellet production from sewage sludge is presented in Section 7 and conclusions in Section 8.
2. Characteristics of Sludge from Municipal Wastewater Treatment
Table 1 shows the amount of sewage sludge in European countries over the last few years. In 2020, Poland produced and used over 570,000 t (dry matter, DM) of sewage sludge. It is estimated that a maximum of 80% of the municipal wastewater generated in Poland will be processed in the following 10 years. The sewage sludge produced in Poland has characteristics similar to those of the sludge produced throughout the EU. The largest producer of sewage sludge in Europe, Germany, produces and uses approximately 1,800,000 t (dry matter) of sewage sludge per year [11].

Table 1.
Sewage sludge production in European countries [1000 t/y DM], 2016–2020 [10,11,12].
Sewage sludge disposal methods vary depending on the country, socio-economic development, and regulatory requirements. Most sewage sludge has rather considerable fertiliser properties regarding its organic substances and macro-elements, but its use in agriculture is strongly inhibited due to its high heavy metal content. The sanitary properties of sewage sludge are not adequate, impeding its use in the environment without specific pretreatment. Due to the global need for adequate sewage sludge disposal, other methods of sludge use are proposed. The three most important sludge applications are agricultural use, area reclamation, and disposal. Sludge processing for reuse has become another possibility and is linked to the recycling or reuse of organic materials [10,22,27]. The disposal processes of sewage sludge are presented in Table 2.

Table 2.
Production and use routes of sewage sludge in European countries [1000 t/y DM] [10,22,27].
The combustion heat ranges from 16 to 18 MJ/kg. Preliminary raw sludge has a combustion heat of approximately 25.5 MJ/kg, digested sludge has a heat of approximately 11.6 MJ/kg, excess sludge of approximately 20.9 MJ/kg, and sludge from chemical precipitation of approximately 16.3 MJ/kg. Sludge drying reduces the initial sludge weight by 25–30% [47]. The main crystalline phases of the dry sludge are composed of calcium carbonate and silicon oxide; in the ash phase, calcium sulphate, silicon oxide, and iron oxide(III) are common.
Treatment plants generate several waste types (Table 3). Screenings (containing 30–40% dry matter) contain mechanical contaminants of various sizes, depending on the type and density of the grids (typically 3, 6, 10, or 20 mm) used to capture them at the treatment plant; sand from sand traps—the “heavy” fraction of pollutants, which, in addition to sand, includes stones, glass, pieces of metal, and significant amounts of organic compounds (up to 20–30%), making it necessary to wash it to reduce its organic compound content to less than 3%; preliminary sludge, comprising residue after the sedimentation of suspended solids from wastewater in primary settling tanks, containing up to 80% of the amount of organic impurities contained in the treated wastewater; excess sludge formed during biological wastewater treatment, namely activated slurry from biological reactors, excessive in quantity. This sludge represents the largest amount of waste from wastewater treatment. The concentrations of mineral substances in this sludge are low (approximately 30%), and the rest is organic matter [7,20,23,28].

Table 3.
Properties of sewage sludge (mixed preliminary and excess sludge) [20,23,28,48,49,50,51].
3. Sewage Sludge Applications
The sewage sludge processing methods depend on the sludge characteristics. For example, for sewage sludge neutralisation with lime, dewatering, thickening, desiccation, composting, and biofermentation processes are used. Sludge containing macronutrients could be used for fertilisation, but this requires purification when the sludge is obtained from sewage. The harmful substances resulting from industrial and economic treatment methods impede the use of such sludge in agriculture. However, certain sludge types need to be landfilled or combusted, depending on their characteristics [47,52]. Municipal sewage sludge is important in environmental processes and needs to be pretreated prior to disposal [53,54,55]. Sludge is processed to achieve dewatering (amount decrease), the elimination of organic substances, the killing of microorganisms, and the removal of hazardous substances. The basic technologies considered are dewatering, biofermentation, conditioning, composting, desiccation, and combustion (Figure 1).
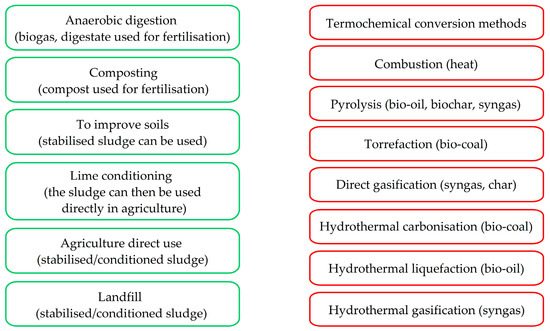
Figure 1.
Sewage sludge disposal methods (agricultural marked in green and thermochemical in red).
The conversion of sewage sludge into biofuels and their use in agriculture via various technical routes are effective and sustainable methods for the management of sewage sludge in a closed-loop cycle (Figure 2). The development of renovative closed-loop cycles, material and economic, and the connection of reuse, renovation, and recycling methods in CEs influences total sustainable development and consumption [56,57]. The CE, as an industrial system [40], shifts towards the exploitation of renewable energy, impairing reuse and allowing the removal of waste as a result of the eco-design of substances, goods, models, and industrial patterns [58,59] (Figure 2).
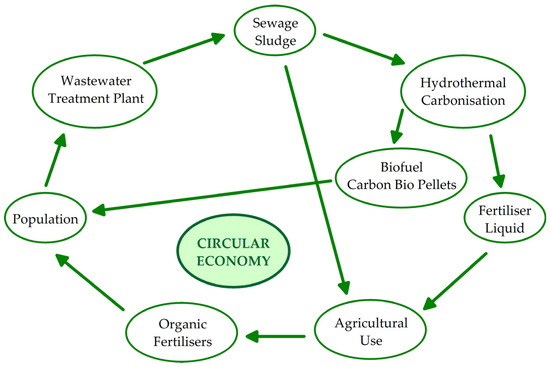
Figure 2.
Agricultural use and hydrothermal carbonisation of sewage sludge as an example of a CE industrial system.
The EU Directives [13,14,15,16] forbade the landfilling of untreated waste, introducing limitations in this area. Landfilling and water deposition of sewage sludge need to consider the following characteristics [15,16]: total organic carbon > 5.0% DM; loss at combustion > 8.0% DM; and upper calorific value ≤ 6.0 MJ/kg. Most EU countries established more stringent thresholds for heavy metal contents in sewage sludge compared to the sustainable development requirements (Table 4) [60,61,62].

Table 4.
Maximum permissible heavy metal contents in sewage sludge [60,62].
Sewage sludge fertiliser improves the structure of agricultural land for the production of industrial crops. Fertilization with sewage sludge causes an increase in annual growth and in the number of new shoots. It is generally assumed that sewage sludge is safe when it is obtained from treatment plants and has undergone various processes (i.e., biochemical stabilisation, inactivation of pathogenic organisms, thickening, and dewatering) [32,33,51].
The agricultural use of sewage sludge is stipulated in the Sludge Directive, which determines the permissible levels of heavy metals in sewage sludge for land use to prevent contamination of the environment and ensure public health safety [14,60,62]. This regulation also applies to fertilisers derived from organic or secondary raw materials as well as waste products converted into crop nutrients. Stabilised sewage sludge can be used for fertilisation, and the following stabilisation methods are applied: adding lime to achieve a pH of 12 for at least 2 h; storing raw liquid sludge for at least 3 months; stabilising raw sludge using lime or other coagulants; and dewatering and landfilling for at least 3 months [21,24,62].
Typical sewage sludge uses are composting for agricultural use, gasification, pyrolysis, and incineration [63]. The incineration of sewage sludge has several disadvantages, such as the emission of greenhouse gases, dioxins, and furans. In addition, the resulting ash also needs to be disposed of after extra processing. This method requires large amounts of energy and is therefore not commonly used [47,48]. The European Commission’s goal is to diminish such waste by 50% by 2050 [12,30].
Sewage Sludge Management in Poland
The amount of sewage sludge produced in the processing of municipal wastewater in Poland [64,65] is currently increasing due to the increase in the length of sewage networks and the volume of municipal wastewater treated, along with improvements in the efficiency of treatment processes at WWT plants (Table 5). This is the result of some actions connected with the adaptation of sewage sludge management to European Union directives [14,15,16]. In Poland, sewage sludge is obtained in 3276 (data from 2019) municipal sewage treatment plants [64,65]. Most of them (estimated > 80%) are small sewage treatment plants (for 10–30,000 inhabitants), producing up to 2500 t/y of hydrated sludge. They have the greatest problems regarding the disposal of sewage sludge.

Table 5.
Municipal sewage and municipal sludge in Poland per number of inhabitants.
Adequately treated sewage sludge, i.e., thickened, stabilised, dewatered, dried, and combusted, can be used in various ways. Table 6 shows how municipal sewage sludge was managed in 2009–2016. During the period under review, a change in trends in sewage sludge processing was noted, mainly resulting from the new Polish legislations in force, based on EU regulations [15,16]. These influenced the organisation of Polish sewage sludge management (Table 6).

Table 6.
Methods of sewage sludge [t/y of dry mass] disposal in Poland [65].
4. Sewage Sludge Use in Agriculture
The agricultural use of sewage sludge influences the physicochemical and biological characteristics of soils and replenishes them with basic crop nutrients [14,21,25,66]. However, sewage sludge is a complex biological waste that can contain toxic compounds, potentially resulting in chemical and biological pollution. Plastics and heavy metals are the basic soil impurities and can accumulate and be transported in the environment, adversely affecting the growth of microorganisms and plants. The threat of environmental pollution by fertilising the soil with sewage sludge is due to the contents of heavy metals, pharmaceuticals, and organic substances. Drugs can be released into the environment through several ways, but mainly with sewage entering the sewage treatment plant and, after treatment, sewage sludge. Soil quality and fertility are preserved because of microbial activities, and sewage sludge can enhance these activities due to its high level of organic substances [32,33,34]. The use of sewage sludge with low heavy metal contents in the soil can positively impact organic carbon, microbial action, and biomass. On the other side, if the sewage sludge contains a high quantity of heavy metals, losses in soil carbon contents and microbial activities can be expected.
The content of contaminants in EU organic-mineral fertiliser must not exceed established threshold values [32,33,60]. For example, for cadmium (Cd), this value is 3 mg/kg dry weight, whereas the total P2O5 content is <5% (m/m) in organic-mineral fertiliser. The threshold values for some cumulative micronutrients are as follows (mg/kg): arsenic—1000; cadmium—200; lead—600; and mercury—100.
The use of sludge in agriculture is banned in certain cases, such as on pastures or crops grown for forage, if the pasture is to be used for grazing animals, within 3 weeks before harvest, and for fruit and vegetables during the growth period. However, these prescriptions do not apply to fruit trees and to soils intended for growing fruits and vegetables that have direct contact with the soil and are consumed raw. The ban applies for 10 months preceding the harvest of these plants and during their harvest [32,33].
Benefits and Risks of Using Sewage Sludge
Sewage sludge slurry is generated during the biological treatment of municipal wastewater. It is mostly human waste but can also contain industrial wastewater, animal wastewater, and agri-food treated wastewater, in addition to rainwater. Some components of sewage sludge are potential toxic elements (PTEs) that are harmless if they are below established standards [14,49,61,66]. Sewage sludge comprises nitrogen and/or phosphorus, which are required for plant growth. Sludge is a good source of organic substances, improving soil properties. Treated sludge has lower numbers of pathogens and is less susceptible to fermentation. Processing methods can change the available nitrogen in sludge. To obtain benefits from sludge, it has to be used during the growing period and in appropriate amounts. The use of dewatered sludge improves the water-holding capacity and soil characteristics (Table 7 and Table 8). Liquid fermented sludge releases ammonium nitrogen, which has a higher plant availability.

Table 7.
Examples of sewage sludge treatment [15,62,65,66,67].

Table 8.
Crops for which processed or unprocessed sewage sludge can be used [14,25].
Sludge should be tested at least every 6 months or when the characteristics of the wastewater change in terms of dry and organic substances, pH, total N, ammonium, total P, and potential toxic elements (PTEs) (Table 9) [67].

Table 9.
Potential toxic element (PTE) limits for agricultural field soils [49,61,66].
5. Thermal Treatment of Sewage Sludge
Thermochemical processes allow the use of diverse biomass feedstocks for the production of heat energy and solid, liquid, and gaseous biofuels such as biochar, bio-oil, and biogas. Nevertheless, certain methods cannot be upscaled because of their low energy efficiency, higher costs, and the production of hazardous substances.
Ideally, the conversion technologies for energy efficiency improvement and the production of sustainable energy from sewage sludge should be improved [5,68,69]. Sewage sludge has different physical–chemical characteristics compared to typical solid fuels (biomass or coal), making its treatment more complex [70,71,72]. It generally contains N and P compounds, low-toxic organic substances, hazardous heavy metals (Pb, Ni, Cd, Hg, and As), organic contaminations (dioxins, pesticides, and aromatic hydrocarbons), pathogens, inorganic substances (Si, Ca, and Mg compounds), and a varying concentration of water. To decrease the amount of sludge that needs to be processed or disposed of, it is crucial to remove excess water. Water is generally removed in two phases, namely the dewatering and thickening stages. The water content should be significantly decreased so that the sludge can be used for energy recovery. Sewage sludge can be considered a fuel because of its high heating value (10–14 MJ) and volatile content [5,54,73].
Thermal treatment methods include incineration and coincineration [12,47,48]. The use of incineration for municipal sewage sludge typically requires a drying step. In Poland, 33% of thermally treated sewage sludge is coincinerated in cement plants and 67% is combusted in mono-incineration sewage sludge plants. Currently, the country operates 11 sewage sludge monocombustion installations, incinerating 160,300 t/year of sludge dry matter. The largest incinerators are listed in Table 10. So far, the cocombustion of sewage sludge under domestic conditions is only possible in cement plants because of legal regulations that allow the treatment of sewage sludge as biomass, which facilitates the elimination of additional fees for CO2 emissions.

Table 10.
Characteristics of sludge monocombustion plants.
Due to the high costs of thermal treatment, this method is especially used in large urban zones. The most popular systems for the thermal treatment of sewage sludge are fluidised bed combustion technologies. The sewage sludge has to be dried before combustion, causing additional costs. The ash obtained during incineration is disposed of in regional, secure landfills. The cost of incinerating sewage sludge is significantly higher than that of agricultural use. However, it is largely used for sludge recovery and waste reduction. Japan, for example, developed a sewage sludge energy transformation method for high-scale urban sewage-processing installations [74].
The combustion method is essential in the management of waste such as sewage sludge. The treatment allows for the decomposition of toxic organic materials and a waste reduction of 70–90%. The renewable energy obtained from sewage sludge incineration could be used as electricity or heat. The ash produced after combustion is treated as a hazardous material because of its high heavy metal content and is used, for example, in the production of cement and concrete, as a material for road building, and for glass and ceramics [29,43,47,48].
Thermochemical Treatment of Sewage Sludge for Energy Conversion
Thermochemical technologies [75] include direct incineration, torrefaction, gasification (direct), and pyrolysis, which usually require the preliminary drying of feedstock with high water contents (>20%) and hydrothermal technologies (i.e., liquefaction, gasification, and carbonization) [75,76,77,78,79].
Direct combustion technology is the incineration of sewage sludge to produce heat energy, which can be used, for example, for the production of electricity in steam turbines or connected heat and power (CHP) cogeneration systems [29,47,48,74]. The dried sludge is incinerated in the combustion chamber, where it is converted into inert ash at temperatures above 850 °C. The combustion system includes the following operations: sludge feed, mechanical dewatering, drying, combustion, ash disposal, and air pollution control. Energy recovery through sludge incineration has several technological, environmental, social, and economic benefits.
With the dry torrefaction technology, the biomass is heated in the absence of oxygen at 200–300 °C and converted into a coal-like product [80,81,82,83]. The preliminary heating of sewage sludge allows the removal of unbound water. The torrefied biomass has a lower water content and a higher heating value than the raw biomass and could be used as a substitute for coal. However, the commercial development of torrefaction requires a deeper knowledge of the method, the characteristics of the torrefied substances, and the characteristics of the volatiles. The torrefied biomass contains all ash components of the raw biomass as the corrosive sediments on the boiler tube are not eliminated.
Pyrolysis technology requires temperatures of 300–800 °C or higher under oxygen-free conditions. Initially, pyrolysis produces vapours from the volatile substances of the sludge, the nonvolatile compounds are decomposed, and the inert compounds, tars, and gases are obtained. Pyrolysis results in three different product types: gas, oil, and pyrolysis residues [45,46,50,84] and needs exceptional energy consumption with extremal treatment temperature requirements resulting in the emission of potentially toxic GHG and CO. Therefore, industrial pyrolysis units require a purification system to treat the produced flue gases. Pyrolysis oil is dark brown and can be used instead of fossil fuels after further purification and dewatering. Pyrolysis gases are noncondensable gases obtained from the pyrolysis of sewage sludge, containing hydrogen, carbon monoxide, dioxide, methane, and ethane. These gases can be used in different applications after separation and purification. Pyrolysis is scalable and can be performed in batches, allowing for a 90% reduction in the sewage sludge volume. The low-pressure technology requires minimal preliminary feedstock treatment [84,85,86,87].
There are six types of pyrolysis technologies, depending on the used process parameters, namely vacuum, slow, fast, flash, microwave, and catalytic pyrolysis [84,88,89,90,91]. The differences among slow, fast, and flash pyrolysis are based on the temperature range or heating yield. In vacuum pyrolysis, the biomass is decomposed at temperatures from 350 to 600 °C and pressure in the range of 8–20 kPa [91,92]. Slow pyrolysis allows for the decomposition of biomass at temperatures up to 400–950 °C, with a lower heating rate and a longer reaction period to obtain higher biochar yields as the vapours can react longer to obtain more solids, with low production of bio-oil (tar) and gases [92,93]. In fast pyrolysis, the biomass is heated at a set temperature of 1250 °C, using a short reaction period; higher heating rates result in high yields of bio-oil as the reaction period is too short for the production of solids. The bio-oil obtained via fast pyrolysis has a low pH, making it more corrosive [90].
Some limitations of fast pyrolysis are that it requires raw materials with low water content as well as higher heating rates and temperatures [90,92]. Flash or ultra-fast pyrolysis is a more developed process of fast pyrolysis. The reaction temperature is in the range of 700–1000 °C, sometimes even reaching 1200 °C, with a higher heating rate than that used in fast pyrolysis (>1000 °C/s) and a short reaction period of 0.1–1 s [88,89]. The high heating rate, combined with the high temperature and short reaction period of the vapours, results in bio-oil with a low moisture and char content [88,93]. Microwave-assisted pyrolysis (MAP) requires high heating rates and has transformation yields higher than those of typical pyrolysis; decomposing the biomass at lower temperatures of 100–150 °C [78,93] results in larger amounts of bio-oil [94,95]. The MAP method is divided into three types, moderate, fast, and catalytic fast MAP. Moderate MAP uses microwave heating of the biomass to higher temperatures (300 °C or more). Fast microwave-assisted pyrolysis (FMAP) uses a fixed pyrolysis temperature and allows for a higher bio-oil efficiency than moderate MAP. Catalytic fast microwave-assisted pyrolysis is an improved MAP as it uses catalysts, increasing the heating rates and pyrolysis temperatures and resulting in the microwave absorbance of the reaction catalysts [78,94,95].
Gasification technology involves heating the biomass at 500–1400 °C in a gasifier, using an atmospheric pressure of 33 bar, with the injection of oxygen in the presence of a gasification element and a catalyst, allowing for partial oxidation to obtain synthetic gas. Optionally, the biomass is directed through steam, resulting in high amounts of hydrogen and methane [77,96,97,98,99,100]. Syngas has a low heating value and can be used as biofuel for heating, diesel engines, and electricity production in gaseous turbines. It can also be processed to produce liquid fuels with the Fischer–Tropsch method and can be treated to separate the hydrogen, which is an alternative fuel [98,100].
Four types of gasifiers are typically applied in biomass gasification, i.e., fixed and fluidised bed, entrained flow, and plasma gasifiers [99]. However, gasification causes corrosion, with negative impacts on human and environmental health. Gasification with air results in a mixture of carbon monoxide and dioxide, hydrogen, methane, nitrogen, and tar. This mix is difficult to incinerate, particularly in a turbine, as the heating value of the syngas is 5 MJ/m3. In the gasification process using oxygen, the heating value of the obtained gas is 10–12 MJ/m3, and the gaseous product lacks nitrogen. Using oxygen instead of air is costlier, but the quality of the obtained gas is higher. When steam is applied, the contents of methane and hydrocarbons increase and the resulting gas has a heating value of 15–20 MJ/m3. Direct gasification allows for the maximum conversion of syngas, biochar formation, a higher hydrogen efficiency, decreased emissions, and higher yield. It is elastic in its capacity, comprehensive, and allows the treatment of raw biomass to biofuel gas with higher yields, using biomass with lower water content. Materials such as sewage sludge, emitting sulphur in the form of hydrogen sulphide, require gas purification.
Hydrothermal liquefaction (HTL), also named hydrous pyrolysis, is a thermochemical method using subcritical water (SCW), wherein the biomass is treated at temperatures of 250–400 °C, a pressure of 4–25 MPa [101,102], and with catalysts (usually alkali) for an adequate period to break the biopolymeric structure, producing a liquid fuel named ‘crude’ or bio-oil. To eliminate reactor clogging, a high-pressure pump is needed to pump highly concentrated biomass materials. Higher temperatures and pressures can result in fracture, rupture, or burst if the pipeline or reactor is not properly protected. Hot compressed water can cause electrochemical corrosion due to the influence of alkali salts on reactor walls and pipelines.
Hydrothermal gasification, also called ‘hydrogasification’, is a thermochemical method in which the biomass is heated in water at a supercritical state at 375 °C and a pressure of 22 MPa to produce syngas [103,104,105,106]. In contrast to typical or dry gasification, the specific characteristic of water at a supercritical state results in an exceptionally rapid reaction course without the generation of char and tar, generating gas of high quality [103]. The high solubility of the semifinished products in water under supercritical parameters inhibits tar and coke production; thus, the reactive compounds from the biomass are diluted in water, which results in a reduction in the reaction index of polymerisation to the undesirable product such as tar and coke and allows a higher gas efficiency at lower temperatures [105].
Hydrothermal carbonisation (HTC) is a thermal conversion method for the sustainable development of biocoal production from biomass and waste [107,108,109], requiring heating biomass mixed with water at 160–260 °C at a pressure of 20 bar for a few hours [110,111,112,113]. This process does not result in the emission of hazardous gases, minimising greenhouse gas emissions. The obtained hydrocarbons can be widely used, i.e., as biofuels, catalysts, absorbents, in environmental remediation, and as soil improvers. The HTC technology involves the thermochemical treatment of waste at lower temperatures than pyrolysis or gasification. Hydrothermal carbonisation allows for the management of a wide range of sewage sludge and the yield of value-added products [114,115,116]. The main product is the solid carbonised product biocoal, which has numerous applications and is a substitute for fossil coal. The advantages and disadvantages of thermochemical methods of sewage sludge treatment are summarised in Table 11.

Table 11.
Thermochemical methods of sewage sludge conversion.
6. HTC Hydrothermal Carbonisation of Sewage Sludge
The hydrothermal carbonisation process was first studied by Bergius in 1913 [117] and imitates the natural creation of carbon from biomass, which takes from 50,000 to 50 million years. The HTC process is an example of industrial ecology [118,119] based on analogies with natural ecological systems that, in nature, operate in appropriate networks of connections, creating interactive systems. The industrial HTC process is the same as that occurring in nature, but the parameters (temperature 180–220 °C and pressure 20–25 bar) are intensified to shorten the reaction time, which can range from one to a few hours, depending on the type of biomass used. It is carried out in a closed reactor at temperatures of 180–280 °C under pressure (2–6 MPa) for 5–240 min (Figure 3). The basic product of HTC is a coal-like material termed hydrocarbon, and the byproduct is an aqueous phase AHL (rich in nutrients) [120,121,122,123,124]. During HTC, the subcritical effects of water change the physical and chemical structure of the raw material mainly through hydrolysis reactions, but aromatisation, dehydration, decarboxylation, and recondensation reactions also occur. Removing the carboxyl and hydroxyl compounds significantly decreases the O/C ratio, and the final product has a higher energy density [121,122,123].
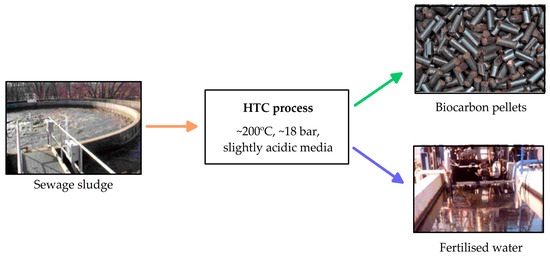
Figure 3.
Hydrothermal carbonisation (HTC) process.
Hot pressured water has some advantages over chemicals as a reaction component as it is environmentally safe, nonexpensive, and readily available. The hydrogen bonds of the water weaken upon pressure, resulting in a change of the dielectric constant, which allows the water to catalyse the reaction, acting as both a base and an acid at temperatures of 200–280 °C, maximising its degree of ionisation. At these temperatures, the dielectric constant of water is diminished, and it therefore reacts as a nonpolar solvent. During HTC, hemicellulose and cellulose are hydrolysed into oligomers and monomers, whereas lignin remains mostly unchanged. Hydrothermal pre-treatment allows for improving enzymatic cellulose saccharification [123,124]. The HTC method does not use an additional catalyst, and the pressure is rather moderate, which keeps the production costs low.
The HTC of biomass is aimed at developing cleaner technologies for hydrated organic waste use. The major value of HTC is that, as the reaction occurs in water, the biomass does not need an initial drying stage, thus reducing energy consumption and process period, facilitating the direct processing of high-moisture waste and sewage sludge [125,126,127]. Lignocellulosic biomass is a raw material in the production of biofuels and biomaterials for the sustainable development of biorefineries to facilitate the generation of highly valuable materials and second-generation biofuels. In high-moisture biomass, at subcritical temperatures and pressures, lignocellulosic feedstock reacts in set types of reactions such as hydrolysis, dehydration, bond breaking, the formation of new links, and condensation. The final products of these processes could be divided into liquid products (or water-soluble components), solid products (first- and second-rate chars and carbon dots), and gases (water vapor, carbon monoxide, dicarbon oxide, and methane). The produced hydrothermal carbon, regardless of the raw material, has a hydrogen content in the range of 4–6%. However, the hydrothermal carbon produced when carbonising food waste has an average hydrogen content of 7.2%. A low content of oxygen observed in the preliminary raw materials is integrated within the hydrothermal carbon following carbonisation, which is advantageous in terms of the different uses of hydrothermal carbon as an energy source sludge [52,128,129,130].
Hydrothermal carbons are applicable and sustainable types of substances for use in energy storage. The product obtained from the carbonisation of biomass determines the bioenergy potential of using hydrothermal carbon as a substitute for natural fuel [42,52,128]. Hydrothermal carbons generated from biomass have high energy densities (up to 35 MJ/kg dry mass) and structures such as coal, allowing for changing and/or supplementing fossil-fuel-derived energy [130,131]. Hydrocarbon obtained using the HTC process differs significantly from biocarbon (obtained via slow dry pyrolysis) and appears superior in several aspects. In addition to its carbon neutrality, it contains slightly less alkali and alkaline earth metals and heavy metals and has a slightly higher calorific value under the same conditions [130,131,132]. The HTC advantage is that without energy-consuming drying, biomass can be converted into carbon-containing solids. The energy density of the hydrocarbon is considerably higher than that of the input material. Harmful organic particles and rested micro-pollutants are also broken down during HTC [132,133].
Although there is no explicit definition in terms of the used temperature, HTC can be divided into two basic types. The high-temperature method operates in a temperature range of 300–800 °C, allowing the synthesis of carbon nanotubes, graphical carbon substances, and activated carbon. Functional carbon-containing substances can be obtained with dehydration and polymerisation reactions in a lower-temperature HTC at 300 °C. The carbon content increases after carbonisation, the oxygen and mineral contents are reduced, and there is negligible gas production. Process temperature, pressure, and time are essential parameters influencing HTC, and the type of biomass used affects HTC products [134,135].
HTC is mainly used to manufacture a solid biofuel such as coal, with a higher energy density. It can be burned to obtain energy or used as soil fertiliser (also carbon sequestration). This is one of the most promising waste treatment processes because it can use large amounts of water. The process mainly results in the removal of carboxyl and OH groups and widely reduces the O/C ratio to obtain an energy-dense final product. Solid yields from this reaction are in the range of 35–65% of the preliminary dry raw material, with a high heating value of HHV (13–30 MJ/kg), depending on the basic energy content of the biomass. Since HTC involves an aqueous environment, the water content of lignin has an advantageous effect on the total process. Generally, lignin carbonization takes place in a set of stages, with the first phase being the reaction of water and lignin under increased pressure. In addition to having excellent physicochemical properties conducive to hydrothermal treatment, water produces H+ and OH−, which are catalysts of the process. Finally, several reactions such as hydrolysis, alkylation, condensation, demethoxylation, and bond breaking (particularly C-C and C-O-C) occur, resulting in the formation of valuable carbon products from lignin. Generally, HTC takes place at the temperature range of 553–573 K and a pressure of 20–25 MPa, i.e., under almost supercritical conditions [134].
HTC has some benefits in the transformation of both dry and wet biomass into hydrochar, resulting in a clean solid biofuel. The energy properties of the hydrochar improve as the reaction intensity increases (higher temperature and longer reaction time). There are two methods to decrease the water amount and maximise energy recovery: anaerobic digestion of the liquid byproducts after HTC [135,136] and in-process recycling after hydrothermal liquid AHL [137]. The optimisation of the HTC process parameters is a main goal of AHL in-process recycling HTC, and further studies in this area are needed, particularly testing the effect of process water valorisation.
There are two categories of HTC methods, depending on the process temperature: high temperatures above 400 °C and low temperatures below 250 °C. High-temperature HTC yields a variety of carbon products, namely multiwalled carbon nanotubes, fullerenes, and carbon spheres with various nanotextures [134,138]. Low-temperature HTC has been applied to different sugars, where biocarbon was manufactured as the main product.
HTC proceeds to create opportunities to use raw materials with a high moisture content (>80%), such as sewage sludge, which is a global problem. Compared to the feedstock, the synthetic hydrocarbons contain higher carbon levels. This results from dehydration and decarboxylation reactions, removing hydrogen and oxygen from the raw material in the water and carbon dioxide. Hydrocarbon also has lower nitrogen and sulphur contents in comparison to the feedstock as these are transferred as oxides to the solution during processing. Hydrocarbon has a low ash content (nonburnable substances), similar to that of other coals (including biocarbon), due to the inorganic substances that accumulate after incineration and are extracted into the after-hydrothermal solution during HTC [135,138].
Hydrothermal carbonisation has several advantages as a method of biomass carbonisation. The main advantage of this technology is that it takes place in an aqueous solution, and the water content of the biomass is not a limiting factor. There are no toxic waste products and the excess process water contains soluble components that facilitate plant growth, such as N, K, and Fe. The HTC reaction is exothermic, with a low thermal energy consumption, and there is no need to evaporate moisture from the raw biomass. Hydrocarbon concentrates the majority of the carbon contained in the original biomass. It has a low content of water-soluble chemicals (which are leached with water), such as sulphur, chloride, and potassium. Impurity separation and hydrocarbon upgrading equipment are being installed alongside HTC reactors for a variety of market applications [133,139,140,141].
Hydrothermal Carbonisation of Sewage Sludge According to the Ingelia Technology
Ingelia developed a method of the hydrothermal carbonisation of biomass to recover the carbon comprised in the organic waste, irrespective of humidity and heterogeneity, to produce a solid carbon biofuel (hydrochar). Hydrochar can be a substitute for industrial fossil fuels as biofuel with a higher heating value, low humidity, high yield of incineration, and without CO2 emissions. Ingelia developed and implemented hydrothermal industrial processes (Figure 4) based on studies at the Max Planck Institute [132,142], which started a cooperation with Ingelia before 2007 [133,142,143,144]. The HTC technology has been developed by Ingelia since 2007, and the world’s first industrial HTC plant has been operating since 2010. The HTC method dehydrates the carbohydrate under controlled pressure and temperature and in an acidic medium in a rather short time (4 to 16 h) [135]. The concept of an industrial HTC facility for sewage sludge has been designed, and the economic and technical data have been described [140,143].
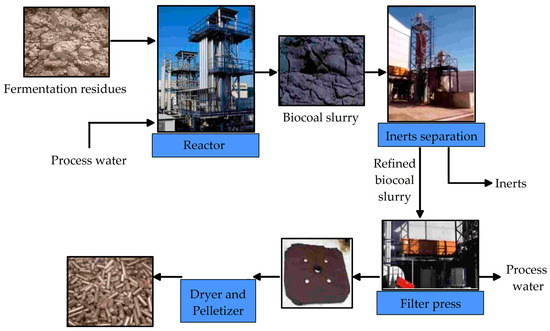
Figure 4.
Flow sheet of hydrothermal carbonisation [37].
The major advantage of HTC is that the reaction takes place in an aqueous medium, and the water content of the biomass does not play a role. The biomass is converted into two products, namely hydrocarbon and nitrogen-laden water. The process is versatile because the chemical reaction occurs with any type of hemicellulose biomass. There are no emissions or toxic waste products, and the excess process water could be used as soluble fertiliser containing nutrients such as N, K, and Fe. The process is highly efficient because the reaction captures most of the carbon in the biomass in hydrocarbons. The exothermic reaction occurring in the process results in a low heat-energy consumption. The process concentrates the energy content from biomass into a solid biofuel and produces liquid byproducts which can be used for fertilisation. The main product of HTC is a solid carbon product (biocarbon) that has a much higher energy recovery value compared to those obtained via other organic waste valorisation techniques, both from an environmental and an economic viewpoint. The byproduct is a concentrated liquid with fertilizing properties. In HTC, the biomass is immersed in water and heated at 180–260 °C in a closed process at a pressure of 20–100 bar, i.e., under subcritical conditions within a few hours. The HTC reaction times indicate that the major reaction takes place in the first 30 min [132,143,144].
Ingelia’s innovation of the HTC process is an example of development based on system innovations introduced with the launch of the first commercial plants (Figure 5). The Ingelia technology is easily scalable, based on a customised pumping system for the wastewater/water mixture, which improves process efficiency. It uses vertical reverse-flow reactors without moving parts or heat exchangers inside. The temperature and pressure control system ensures stable conditions inside the HTC reaction area throughout the retention time. Biochemical extraction avoids the presence of hydrocarbon molecules in the process water. The post-treatment equipment has been developed based on equipment used in the coal industry (coal washing) to separate inert and impurities and reduce the ash content [145,146].
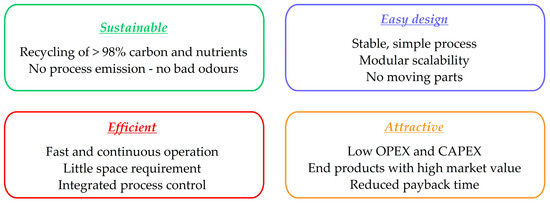
Figure 5.
Advantages of the Ingelia hydrothermal carbonisation process.
Ingelia’s HTC process is in line with the CE concept of recycling, especially that of the carbon contained in biowaste and sludge in the form of biocarbon, which can be used as a solid fuel, according to PN-EN ISO 17225-8:2023-10 [147] or, in some cases, even as a material (carbon source) for the metallurgical or chemical sector (Table 12). The adaptation of HTC products to specific sectors (such as the steel and the energy sectors) is still being investigated [143,146].

Table 12.
Characteristic of carbon biopellets.
The byproduct, fertiliser solution, is the water obtained from the moisture content of the biomass. It contains soluble elements which act as biofertilisers and reduce the need for chemical fertilisers, thereby protecting groundwater quality. The concentration of nutrients through microfiltration and osmosis allows for NPK recovery. The goal is to use this solution as a commercial biofertiliser and for soil reclamation (organic matter and nutrient content).
The HTC plants using sewage sludge as feedstock should be located next to a wastewater treatment plant with anaerobic digestion to optimise sludge and water logistics and to take advantage of the energy value of the organic matter of the process water in a plant using anaerobic digestion. The modular design of HTC plants enables the decentralised transformation of large and seasonal biomass into high-caloric biocarbon, thereby reducing biomass logistics and road transport. The metallurgical industry is facing the need to switch to greenhouse-gas-free carbon sources. Ingelia has supplied HTC biocarbon to a Swedish research project on the use of biocarbon in a cupola, blast furnace, and electric arc furnace, with promising results [140].
7. Economic Evaluation of Biocarbon Pellet Production from Sewage Sludge
The minimum efficiency of the installation of a system is determined by the capacity of one reactor, which is approximately 700 kg/h (biomass with 50% humidity) or 1.2 t/h (biomass with 80% humidity), i.e., 5600–10,600 t/y (8000 h/r working time).
Poland has the largest number (estimated at >80%) of small wastewater treatment plants (for 10–30,000 residents), producing up to 2500 t/y of hydrated sludge. The country also has huge sludge disposal problems. Below, we present calculations for processing sewage sludge with capacities in the range of 10,000 to 1,000,000 t/y (Table 13).

Table 13.
Economic effects of sewage sludge treatment with the hydrothermal carbonisation method.
The prices of hydrated sewage sludge (70% H2O) vary, according to our data from the sewage treatment plant used for this study. The prices of carbon bio pellets were estimated on the basis of data from the Polish market, at a level that can currently be considered below the average prices of bio pellets of various types. The prices of the fertiliser solution were estimated based on the prices of N and K in manufactured fertilisers. Operating costs and revenue at the sludge collection price were estimated using our data. All evaluations should be considered moderately conservative.
A payback period of 1.7 or 2.4 years indicates high profitability of investment (less than 5 years is favourable for this type of investment). With gas prices continuing to rise, the possibility of replacing gas with a low-cost biofuel appears to be profitable. The actual 2023 natural gas price (with a calorific value of 35.14 MJ/m3) is 3.23 [EUR/m3] [148].
8. Conclusions
Biorefinery technologies enable the efficient use of biosolid sewage sludge in agriculture, offering a sustainable solution, using regionally available resources, and promoting food safety and security. This, in turn, prevents the landfilling of sewage sludge and influences nutrient recovery regulations.
Different thermochemical methods, especially HTC, facilitate the efficient processing of sewage sludge to recover valuable materials for the production of bioenergy. In this context, HTC is a superior method to handle sewage sludge as it takes place in a water solution, is universal as the chemical reaction works with any type of hemicelluloses biomass, does not produce any emissions and toxic waste, and results in excess water with high levels of N, K, and Fe used as fertiliser solution.
It is also highly efficient as it captures most of the carbon of the biomass in the hydrochar. The reaction as such is exothermic, with a low thermal energy consumption. However, adequate standardising and marketing approaches are necessary to support the use of such products.
The implementation of innovative biological waste management processes promotes a circular economy. Nevertheless, there are challenges in the commercial use of these methods that require solutions, such as the scaling up of biofuel manufacturing, commercialisation, and the seasonal use of sewage sludge in agriculture.
Author Contributions
Conceptualization, Z.K.; methodology, Z.K., A.M., J.K. and A.G.; software, A.M. and P.K.; validation, A.M., P.K., J.C. and A.G.-C.; formal analysis, Z.K., J.K., A.G. and J.C.; investigation, Z.K., A.M., A.G. and J.C.; resources, A.M., P.K. and A.G.-C.; data curation, Z.K.; writing—original draft preparation, Z.K. and A.M.; writing—review and editing, Z.K. and A.M.; visualization, A.M. and A.G.-C.; supervision, Z.K. and J.K.; project administration, Z.K. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
Data are contained within the article.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- U.S. Energy Information Administration. Biomass Explained. Available online: https://www.eia.gov/energyexplained/biomass/ (accessed on 10 November 2023).
- Biosolids Program Strategy: Fiscal Year 2020–2025; U.S. Environmental Protection Agency Office of Water Office of Science and Technology: Washington, DC, USA, 2021. Available online: https://static1.squarespace.com/static/54806478e4b0dc44e1698e88/t/6192943a23b235491251859c/1636996157317/epa+biosolids+strategy+2020-2025_october2021 (accessed on 12 November 2023).
- United States Environmental Protection Agency. Basic Information about Biosolids. Available online: https://www.epa.gov/biosolids/basic-information-about-biosolids#Biosolids%20Uses5 (accessed on 10 November 2023).
- Vaithyanathan, V.K.; Cabana, H. Integrated Biotechnology Management of Biosolids: Sustainable Ways to Produce Value—Added Products. Front. Water. 2021, 3, 729679. [Google Scholar] [CrossRef]
- Prajapat, N.; Raval, A.D.; Pitroda, J.R.; Kulkarni, V.V. A Review on Sewage Sludge Applications and Utilization. Int. J. Eng. Res. 2019, 8, 109–112. [Google Scholar]
- Speight, J.G.; Singh, K. Environmental Management of Energy from Biofuels and Biofeedstocks; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2014. [Google Scholar] [CrossRef]
- Aziz, S.Q.; Mustafa, J.S. Wastewater sludge characteristics, treatment techniques, and energy production. Recycl. Sustain. Develop. 2022, 15, 9–27. [Google Scholar] [CrossRef]
- Wei, L.; Zhu, F.; Li, Q.; Xue, C.; Xia, X.; Yu, H.; Zhao, Q.; Jiang, J.; Bai, S. Development, current state and future trends of sludge management in China: Based on exploratory data and CO2-equivalent emissions analysis. Environ. Int. 2020, 144, 106093. [Google Scholar] [CrossRef]
- Pelkmans, L.; Kejun, D.; Zhongying, W.; Dongming, R. Implementation of Bioenergy in China; Country Reports; IEA Bioenergy: Paris, France, 2021; p. 10. Available online: https://www.ieabioenergy.com/wp-content/uploads/2021/11/CountryReport2021_China_final.pdf (accessed on 16 November 2023).
- Eurostat. Sewage Sludge Production and Disposal. Available online: https://data.europa.eu/data/datasets/g1a4auwbnkfrmzm3dg6zg?locale=en (accessed on 10 November 2023).
- Sewage Sludge Generation in Europe 2020, by Country. Published by Statista Research Department. Available online: https://www.statista.com/statistics/1393771/sewage-sludge-generation-europe/ (accessed on 4 November 2023).
- Bianchini, A.; Bonfiglioli, L.; Pellegrini, M.; Saccani, C. Sewage sludge management in Europe: A critical analysis of data quality. Int. J. Environ. Waste Manag. 2016, 18, 226–238. [Google Scholar] [CrossRef]
- Council Directive of 18 March 1991 Amending Directive 75/442/EEC on Waste (91/156/EEC). Official Journal of the European Communities. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:31999L0156 (accessed on 2 November 2023).
- Council Directive of 12 June 1986 on the Protection of the Environment, and in Particular of the Soil, When Sewage Sludge Is Used in Agriculture (86/278/EEC) Consolidated Text Access the Current Version (01/01/2023). Available online: http://data.europa.eu/eli/dir/1986/278/2009-04-20 (accessed on 2 November 2023).
- Council Directive 1999/31/EC of 26 April 1999 on the Landfill of Waste. Official Journal of the European Communities. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:31999L0031 (accessed on 2 November 2023).
- European Commission, Working Document Sludge and Biowaste, 21 September 2010, Brussels. Available online: https://www.compostnetwork.info/download/no-012010-eu-working-document-sludge-biowaste/ (accessed on 19 December 2023).
- Vávrová, K.; Králík, T.; Janota, L.; Šolcováb, O.; Čárský, M.; Soukup, K.; Vítek, M. Process Economy of Alternative Fuel Production from Sewage Sludge and Waste Celluloses Biomass. Energies 2023, 16, 518. [Google Scholar] [CrossRef]
- Canziani, R.; Spinosa, L. 1—Sludge from wastewater treatment plants. In Industrial and Municipal Sludge. Emerging Concerns and Scope for Resource Recovery; Prasad, M.N.V., Favas, P., Vithanage, M., Mohan, S.V., Eds.; Elsevier: Amsterdam, The Netherlands; Butterworth-Heinemann: Oxford, UK, 2019; pp. 3–30. [Google Scholar]
- Facchini, F.; Mummolo, G.; Vitti, M. Scenario Analysis for Selecting Sewage Sludge-to-Energy/Matter Recovery Processes. Energies 2021, 14, 276. [Google Scholar] [CrossRef]
- Bonfiglioli, L.; Bianchini, A.; Pellegrini, M.; Saccani, C. Sewage sludge: Characteristics and recovery options. J. Alma Mater Stud.—Univ. Di Bologna 2014, 1–21. [Google Scholar] [CrossRef]
- Kumar, V.; Chopra, A.K.; Kumar, A. A review on sewage sludge (Biosolids) a resource for sustainable agriculture. Arch. Agric. Environ. Sci. 2017, 2, 340–347. [Google Scholar] [CrossRef]
- Grobelak, A.; Czerwińska, K.; Murtaś, A. 7—General considerations on sludge disposal, industrial and municipal sludge. In Industrial and Municipal Sludge. Emerging Concerns and Scope for Resource Recovery; Prasad, M.N.V., de Campos Favas, P.J., Vithanage, M., Mohan, S.V., Eds.; Elsevier Inc.: Amsterdam, The Netherlands, 2019; pp. 135–153. [Google Scholar] [CrossRef]
- Iticescu, C.; Georgescu, L.P.; Murariu, G.; Circiumaru, A.; Timofti, M. The Characteristics of Sewage Sludge Used on Agricultural Lands. AIP Conf. Proc. 2018, 2022, 020001. [Google Scholar] [CrossRef]
- Delibacak, S.; Voronina, L.; Morachevskaya, E.; Ongun, A.R. Use of sewage sludge in agricultural soils: Useful or harmful. Eur. J. Soil Sci. 2020, 9, 126–139. [Google Scholar] [CrossRef]
- Hudcová, H.; Vymazal, J.; Rozkošný, M. Present restrictions of sewage sludge application in agriculture within the European Union. Soil Water Res. 2019, 14, 104–120. [Google Scholar] [CrossRef]
- El Hammoudani, Y.; Dimane, F.; El Ouarghi, H. Characterization of Sewage Sludge Generated from Wastewater Treatment Plant in Relation to Agricultural Use. Environ. Water Sci. Public Health Territ. Intell. J. 2019, 3, 47–52. Available online: http://revues.imist.ma/?journal=ewash-ti (accessed on 12 December 2023).
- Kacprzak, M.; Neczaj, E.; Fijałkowski, K.; Grobelak, A.; Grosser, A.; Worwag, M.; Rorat, A.; Brattebo, H.; Almås, Å.; Singh, B.R. Sewage sludge disposal strategies for sustainable development. Environ. Res. 2017, 156, 39–46. [Google Scholar] [CrossRef] [PubMed]
- Gherghel, A.; Teodosiu, C.; De Gisi, S. A review on wastewater sludge valorisation and its challenges in the context of a circular economy. J. Clean. Prod. 2019, 228, 244–263. [Google Scholar] [CrossRef]
- Kowalski, Z.; Kulczycka, J.; Makara, A.; Verhé, R.; De Clercq, G. Assessment of Energy Recovery from Municipal Waste Management Systems Using Circular Economy Quality Indicators. Energies 2022, 15, 8625. [Google Scholar] [CrossRef]
- European Commission, Environment Action Programme to 2030. Available online: https://environment.ec.europa.eu/strategy/environment-action-programme-2030_en (accessed on 16 November 2023).
- United Nations 2030 Agenda for Sustainable Development. Available online: https://www.un.org/sustainabledevelopment/development-agenda/ (accessed on 16 November 2023).
- Chojnacka, K. Valorization of biorefinery residues for sustainable fertilizer production: A comprehensive review. Biomass Convers. Biorefin. 2023, 13, 14359–14388. [Google Scholar] [CrossRef]
- Regulation (EU) 2019/1009 of the European Parliament and of the Council of June 5, 2019, Laying Down Rules for Making EU Fertilizer Products Available on the Market of EU Fertilising Products and Amending Regulations (EC) No 1069/2009 and (EC) No 1107/2009 and Repealing Regulation (EC) No 2003/2003. Official Journal of the European Union. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:32019R1009 (accessed on 10 December 2023).
- Goyal, H.B.; Seal, D.; Saxena, R.C. Bio-fuels from thermochemical conversion of renewable resources: A review. Renew. Sustain. Energy Rev. 2008, 12, 504–517. [Google Scholar] [CrossRef]
- Danso-Boateng, E.; Achaw, O.W. Bioenergy and biofuel production from biomass using thermochemical conversions technologies—A review. AIMS Energy 2022, 10, 585–647. [Google Scholar] [CrossRef]
- Child, M. Industrial-Scale Hydrothermal Carbonization of Waste Sludge Materials for Fuel Production. Master’s Thesis, Lappeenranta University of Technology, Lappeenranta, Finland, 2014. [Google Scholar]
- Kowalski, Z.; Kulczycka, J.; Verhé, R.; Desender, L.; De Clercq, G.; Makara, A.; Generowicz, N.; Harazin, P. Second-generation biofuel production from the organic fraction of municipal solid waste. Front. Energy Res. 2022, 10, 919415. [Google Scholar] [CrossRef]
- Peterson, A.A.; Vogel, F.; Lachance, R.P.; Fröling, M.; Antal, M.J.; Tester, J.W. Thermochemical biofuel production in hydrothermal media: A review of sub- and supercritical water technologies. Energy Environ. Sci. 2008, 1, 32–65. [Google Scholar] [CrossRef]
- Ellen MacArthur Foundation. Towards the Circular Economy, Vol. 3: Accelerating the Scale-up across Global Supply Chains. 2014. Available online: https://www.ellenmacarthurfoundation.org/towards-the-circular-economy-vol-3-accelerating-the-scale-up-across-global (accessed on 12 November 2023).
- Ghisellini, P.; Cialani, C.; Ulgiati, S. A review on circular economy: The expected transition to a balanced interplay of environmental and economic systems. J. Clean. Prod. 2016, 114, 11–32. [Google Scholar] [CrossRef]
- Korhonen, J.; Honkasalo, A.; Seppälä, J. Circular economy: The concept and its limitations. Ecol. Econ. 2018, 143, 37–46. [Google Scholar] [CrossRef]
- Kowalski, Z.; Makara, A. The circular economy model used in the Polish agro-food consortium: A case study. J. Clean. Prod. 2021, 284, 124751. [Google Scholar] [CrossRef]
- Kowalski, Z.; Makara, A. Sustainable Systems for the Production of District Heating Using Meat-Bone Meal as Biofuel: A Polish Case Study. Energies 2022, 15, 3615. [Google Scholar] [CrossRef]
- Osman, A.I.; Mehta, N.; Elgarahy, A.M.; Al-Hinai, A.; Al-Muhtaseb, A.H.; Rooney, D.W. Conversion of biomass to biofuels and life cycle assessment: A review. Environ. Chem. Lett. 2021, 19, 4075–4118. [Google Scholar] [CrossRef]
- Huang, C.; Mohamed, B.A.; Li, L.Y. Comparative life-cycle energy and environmental analysis of sewage sludge and biomass co-pyrolysis for biofuel and biochar production. Chem. Eng. J. 2023, 457, 141284. [Google Scholar] [CrossRef]
- Carrier, M.; Hugo, T.; Gorgens, J.; Knoetze, H. Comparison of slow and vacuum pyrolysis of sugar cane bagasse. J. Anal. Appl. Pyrol. 2011, 90, 18–26. [Google Scholar] [CrossRef]
- Kowalski, Z.; Kulczycka, J.; Makara, A.; Harazin, P. Quantification of material recovery from meat waste incineration—An approach to an updated food waste hierarchy. J. Hazard Mater. 2021, 416, 126021. [Google Scholar] [CrossRef] [PubMed]
- Kowalski, Z.; Banach, M.; Makara, A. Optimisation of the co-combustion of meat–bone meal and sewage sludge in terms of the quality produced ashes used as substitute of phosphorites. Environ. Sci. Pollut. Res. 2021, 28, 8205–8214. [Google Scholar] [CrossRef]
- Fijalkowski, K.; Rorat, A.; Grobelak, A.; Kacprzak, M.J. The presence of contaminations in sewage sludge—The current situation. J Environ. Manag. 2017, 203, 1126–1136. [Google Scholar] [CrossRef]
- Moško, J.; Pohořelý, M.; Cajthaml, T.; Jeremiáš, M.; Robles-Aguilar, A.A.; Skoblia, S.; Beňo, Z.; Innemanová, P.; Linhartová, L.; Michalíková, K.; et al. Effect of pyrolysis temperature on removal of organic pollutants present in anaerobically stabilized sewage sludge. Chemosphere 2021, 265, 129082. [Google Scholar] [CrossRef]
- Abbasi, T.; Patnaik, P.; Rahi, R.; Abbasi, S.A. A circular biorefinery-integrating wastewater treatment with the generation of an energy precursor and an organic fertilizer. Sustainability 2022, 14, 5714. [Google Scholar] [CrossRef]
- Escala, M.; Zumbühl, T.; Koller, C.; Junge, R.; Krebs, R. Hydrothermal carbonization as an energy-efficient alternative to established drying technologies for sewage sludge: A feasibility study on a laboratory scale. Energy Fuels 2013, 27, 454–460. [Google Scholar] [CrossRef]
- Ruiz, H.A.; Conrad, M.; Sun, S.-N.; Sanchez, A.; Rocha, G.J.M.; Romaní, A.; Castro, E.; Torres, A.; Rodríguez-Jasso, R.M.; Andrade, L.P.; et al. Engineering aspects of hydrothermal pretreatment: From batch to continuous operation, scale-up and pilot reactor under biorefinery concept. Bioresour. Technol. 2020, 299, 122685. [Google Scholar] [CrossRef] [PubMed]
- Singh, D.K.; Garg, A. 18-A review on hydrothermal pretreatment of sewage sludge: Energy recovery options and major challenges. In Advanced Organic Waste Management, Sustainable Practices and Approaches; Hussain, C., Hait, S., Eds.; Elsevier Inc.: Amsterdam, The Netherlands, 2022; pp. 297–314. [Google Scholar] [CrossRef]
- Baruah, J.; Nath, B.K.; Sharma, R.; Kumar, S.; Deka, R.C.; Baruah, D.C.; Kalita, E. Recent Trends in the Pretreatment of Lignocellulosic Biomass for Value-Added Products. Front. Energy Res. 2018, 6, 141. [Google Scholar] [CrossRef]
- Kwapinski, W.; Kolinovic, I.; Leahy, J.J. Sewage Sludge Thermal Treatment Technologies with a Focus on Phosphorus Recovery: A Review. Waste Biomass Valorization 2021, 12, 5837–5852. [Google Scholar] [CrossRef]
- Ellen MacArthur Foundation. SUN, McKinsey Centre for Business and Environment. Growth Within: A Circular Economy Vision for a Competitive Europe. 2015. Available online: https://www.ellenmacarthurfoundation.org/growth-within-a-circular-economy-vision-for-a-competitive-europe (accessed on 12 November 2023).
- Lieder, M.; Rashid, A. Towards circular economy implementation: A comprehensive review in the context of manufacturing industry. J. Clean. Prod. 2016, 115, 36–51. [Google Scholar] [CrossRef]
- Rizos, V.; Behrens, A.; Van der Gaast, W.; Hofman, E.; Ioannou, A.; Kafyeke, T.; Flamos, A.; Rinaldi, R.; Papadelis, S.; Hirschnitz-Garbers, M.; et al. Implementation of circular economy business models by small and medium-sized enterprises (SMEs): Barriers and enablers. Sustainability 2016, 8, 1212. [Google Scholar] [CrossRef]
- Kowalski, Z.; Wzorek, Z.; Gorazda, K.; Jodko, M.; Przewrocki, P.; Kulczycka, J. Thermal utilization of sewage sludge in Poland. Miner. Energy 2003, 18, 34–41. [Google Scholar] [CrossRef]
- Wiśniowska, E.; Grobelak, A.; Kokot, P.; Kacprzak, M. 10—Sludge legislation-comparison between different countries. In Industrial and Municipal Sludge. Emerging Concerns and Scope for Resource Recovery; Prasad, M.N.V., de Campos Favas, P.J., Vithanage, M., Mohan, S.V., Eds.; Elsevier Inc.: Amsterdam, The Netherlands, 2019; pp. 201–224. [Google Scholar] [CrossRef]
- Lamastra, L.; Suciu, N.A.; Trevisan, M. Sewage sludge for sustainable agriculture: Contaminants’ contents and potential use as fertilizer. Chem. Biol. Technol. Agric. 2018, 5, 10. [Google Scholar] [CrossRef]
- Syed-Hassan, S.S.A.; Wang, Y.; Hu, S.; Su, S.; Xiang, J. Thermochemical processing of sewage sludge to energy and fuel: Fundamentals, challenges, and considerations. Renew. Sust. Energy Rev. 2017, 80, 888–913. [Google Scholar] [CrossRef]
- Statistics Poland. 2022. Available online: https://stat.gov.pl (accessed on 2 November 2023).
- Przydatek, G.; Wota, A.K. Analysis of the comprehensive management of sewage sludge in Poland. J. Mater. Cycles Waste Manag. 2020, 22, 80–88. [Google Scholar] [CrossRef]
- Singh, R.P.; Agrawal, M. Potential benefits and risks of land application of sewage sludge. Waste Manag. 2008, 28, 347–358. [Google Scholar] [CrossRef]
- Sewage Sludge in Agriculture: Code of Practice for England, Wales and Northern Ireland. Department for Environment, Food & Rural Affairs and Environment Agency. Available online: https://www.gov.uk/government/publications/sewage-sludge-in-agriculture-code-of-practice (accessed on 14 November 2023).
- Energy Technology Perspectives: Scenarios and Strategies to 2050. International Energy Agency. Available online: https://iea.blob.core.windows.net/assets/0e190efb-daec-4116-9ff7-ea097f649a77/etp2008.pdf (accessed on 8 November 2023).
- Kim, Y.; Parker, W. A technical and economic evaluation of the pyrolysis of sewage sludge for the production of bio-oil. Bioresour. Technol. 2008, 99, 1409–1416. [Google Scholar] [CrossRef]
- Özbay, N.; Pütün, A.E.; Uzun, B.B.; Pütün, E. Biocrude from biomass: Pyrolysis of cottonseed cake. Renew. Energy 2001, 24, 615–625. [Google Scholar] [CrossRef]
- Tan, Y.Y.; Huong, Y.Z.; Tang, F.E.; Saptoro, A. A review of sewage sludge dewatering and stabilization in reed bed system: Towards the process-based modeling. Int. J. Environ. Sci. Technol. 2024, 21, 997–1020. [Google Scholar] [CrossRef]
- Qiu, C.; Xu, W.; Wang, Y.; Yang, J.; Su, X.; Lin, Z. Hydrothermal alkaline conversion of sewage sludge: Optimization of process parameters and characterization of humic acid. Environ Sci. Pollut. Res. 2021, 28, 57695–57705. [Google Scholar] [CrossRef] [PubMed]
- Rathika, K.; Kumar, S.; Yadav, B.R. Enhanced energy and nutrient recovery via hydrothermal carbonisation of sewage sludge: Effect of process parameters. Sci. Total Environ. 2024, 906, 167828. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Nakakubo, T. Design of a sewage sludge energy conversion technology introduction scenario for large city sewage treatment plants in Japan: Focusing on zero fuel consumption. J. Clean. Prod. 2022, 379, 134794. [Google Scholar] [CrossRef]
- Gao, N.; Kamran, K.; Quan, C.; Williams, P.T. Thermochemical conversion of sewage sludge: A critical review. Prog. Energ. Combust. 2020, 79, 100843. [Google Scholar] [CrossRef]
- Sharma, R.; Jasrotia, K.; Singh, N.; Ghosh, P.; Srivastava, S.; Sharma, N.R.; Singh, J.; Kanwar, R.; Kumar, A. A Comprehensive Review on Hydrothermal Carbonization of Biomass and its Applications. Chem. Afr. 2020, 3, 1–19. [Google Scholar] [CrossRef]
- Stirling, R.J.; Snape, C.E.; Meredith, W. The impact of hydrothermal carbonisation on the char reactivity of biomass. Fuel Process. Technol. 2018, 177, 152–158. [Google Scholar] [CrossRef]
- Suresh, A.; Alagusundaram, A.; Kumar, P.S.; Nguyen Vo, D.-V.; Christopher, F.C.; Balaji, B.; Viswanathan, V.; Sankar, S. Microwave pyrolysis of coal, biomass and plastic waste: A review. Environ Chem. Lett. 2021, 19, 3609–3629. [Google Scholar] [CrossRef]
- Watson, J.; Zhang, Y.; Si, B.; Chen, W.T.; de Souza, R. Gasification of biowaste: A critical review and outlooks. Renew. Sustain. Energy Rev. 2018, 83, 1–17. [Google Scholar] [CrossRef]
- Arias, B.; Pevida, C.; Fermoso, J.; Plaza, M.G.; Rubiera, J.; Pis, J.J. Influence of torrefaction on the grindability and reactivity of woody biomass. Fuel Process. Technol. 2008, 89, 169–175. [Google Scholar] [CrossRef]
- Bergman, P.C.A.; Kiel, J.H.A. Torrefaction for biomass upgrading. In Proceedings of the 14th European Biomass Conference & Exhibition, Paris, France, 17–21 October 2005. [Google Scholar]
- Niu, Y.; Lv, Y.; Lei, Y.; Liu, S.; Liang, Y.; Wang, D.; Hui, S. Biomass torrefaction: Properties, applications, challenges, and economy. Renew. Sust. Energ. Rev. 2019, 115, 109395. [Google Scholar] [CrossRef]
- Sadaka, S.; Negi, S. Improvements of biomass physical and thermochemical characteristics via torrefaction process. Environ. Prog. Sustain. Energy 2009, 28, 427–434. [Google Scholar] [CrossRef]
- Li, L.; Rowbotham, J.S.; Greenwell, H.C.; Dyer, P.W. Chapter 8—An introduction to pyrolysis and catalytic pyrolysis: Versatile techniques for biomass conversion. In New and Future Developments in Catalysis; Suib, S.L., Ed.; Elsevier B.V.: Amsterdam, The Netherlands, 2013; pp. 173–208. [Google Scholar] [CrossRef]
- Demirbas, A.; Arin, G. An overview of biomass pyrolysis. Energy Sources 2002, 24, 471–482. [Google Scholar] [CrossRef]
- Mohan, D.; Pittman, C.U.; Steele, P.H. Pyrolysis of wood/biomass for bio-oil: A critical review. Energy Fuels 2006, 20, 848–889. [Google Scholar] [CrossRef]
- Iliopoulou, E.F.; Triantafyllidis, K.S.; Lappas, A.A. Overview of catalytic upgrading of biomass pyrolysis vapors toward the production of fuels and high-value chemicals. Wiley Interdiscip. Rev. Energy Environ. 2019, 8, e322. [Google Scholar] [CrossRef]
- Ratnasari, D.K.; Yang, W.; Jönsson, P.G. Catalytic pyrolysis of lignocellulosic biomass: The influence of the catalyst regeneration sequence on the composition of upgraded pyrolysis oils over a H ZSM-5/Al-MCM-41 catalyst mixture. ACS Omega 2020, 5, 28992–29001. [Google Scholar] [CrossRef]
- Czernik, S.; Bridgwater, A.V. Overview of applications of biomass fast pyrolysis oil. Energy Fuels 2004, 18, 590–598. [Google Scholar] [CrossRef]
- Luna-Murillo, B.; Pala, M.; Paioni, A.L.; Baldus, M.; Ronsse, F.; Prins, W.; Bruijnincx, P.C.A.; Weckhuysen, B.M. Catalytic fast pyrolysis of biomass: Catalyst characterization reveals the feed-dependent deactivation of a technical ZSM-5-based catalyst. ACS Sustain. Chem. Eng. 2021, 9, 291–304. [Google Scholar] [CrossRef]
- Bridgwater, A.V. Review of fast pyrolysis of biomass and product upgrading. Biomass Bioenergy 2012, 38, 68–94. [Google Scholar] [CrossRef]
- Tripathi, M.; Sahu, J.N.; Ganesan, P. Effect of process parameters on production of biochar from biomass waste through pyrolysis: A review. Renew. Sustain. Energy Rev. 2016, 55, 467–481. [Google Scholar] [CrossRef]
- Zhang, Q.; Chang, J.; Wang, T.; Xu, Y. Review of biomass pyrolysis oil properties and upgrading research. Energy Convers Manag. 2007, 48, 87–92. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, P.; Liu, S.; Fan, L.; Zhou, N.; Min, M.; Cheng, Y.; Peng, P.; Anderson, E.; Wang, Y.; et al. Microwave-assisted pyrolysis of biomass for bio-oil production. In Pyrolysis; Samer, M., Ed.; IntechOpen: London, UK, 2017; pp. 129–166. [Google Scholar] [CrossRef]
- Budarin, V.L.; Shuttleworth, P.S.; De Bruyn, M.; Farmer, T.J.; Gronnow, M.J.; Pfaltzgraff, L.; Macquarrie, D.J.; Clark, J.H. The potential of microwave technology for the recovery, synthesis and manufacturing of chemicals from bio-wastes. Catal. Today 2015, 239, 80–89. [Google Scholar] [CrossRef]
- Werle, S.; Dudziak, M. 25-Gasification of sewage sludge. In Industrial and Municipal Sludge, Emerging Concerns and Scope for Resource Recovery; Prasad, M.N.V., de Campos Favas, P.J., Vithanage, M., Mohan, S.V., Eds.; Elsevier Inc.: Amsterdam, The Netherlands, 2019; pp. 575–593. [Google Scholar] [CrossRef]
- Ahmad, A.A.; Zawawi, N.A.; Kasim, F.H.; Inayat, A.; Khasri, A. Assessing the gasification performance of biomass: A review on biomass gasification process conditions, optimization, and economic evaluation. Renew. Sustain. Energy Rev. 2016, 53, 1333–1347. [Google Scholar] [CrossRef]
- Choi, Y.K.; Cho, M.H.; Kim, J.S. Steam/oxygen gasification of dried sewage sludge in a two-stage gasifier: Effects of the steam to fuel ratio and ash of the activated carbon on the production of hydrogen and tar removal. Energy 2015, 91, 160–167. [Google Scholar] [CrossRef]
- Matsumura, Y.; Minowa, T. Fundamental design of a continuous biomass gasification process using a supercritical water fluidized bed. Int. J. Hydrog. Energy 2004, 29, 701–707. [Google Scholar] [CrossRef]
- Sansaniwal, S.K.; Pal, K.; Rosen, M.A.; Tyagi, S.K. Recent advances in the development of biomass gasification technology: A comprehensive review. Renew. Sustain. Energy Rev. 2017, 72, 363–384. [Google Scholar] [CrossRef]
- Sandquist, J.; Tschentscher, R.; del Alamo Serrano, G. Hydrothermal liquefaction of organic resources in biotechnology: How does it work and what can be achieved? Appl. Microbiol. Biotechnol. 2019, 103, 673–684. [Google Scholar] [CrossRef]
- Chuntanapum, A.; Matsumura, Y. Formation of tarry material from 5-HMF in subcritical and supercritical water. Ind. Eng. Chem. Res. 2009, 48, 9837–9846. [Google Scholar] [CrossRef]
- Matsumura, Y. Chapter 9—Hydrothermal Gasification of Biomass. In Recent Advances in Thermo-Chemical Conversion of Biomass; Pandey, A., Bhaskar, T., Stöcker, M., Sukumaran, R.K., Eds.; Elsevier B.V.: Amsterdam, The Netherlands, 2015; pp. 251–267. [Google Scholar] [CrossRef]
- Kruse, A.; Bernolle, P.; Dahmen, N.; Dinjus, E.; Maniam, P. Hydrothermal gasification of biomass: Consecutive reactions to long-living intermediates. Energy Environ. Sci. 2010, 3, 136–143. [Google Scholar] [CrossRef]
- Kruse, A. Hydrothermal biomass gasification. J. Supercrit. Fluids 2009, 47, 391–399. [Google Scholar] [CrossRef]
- Alptekin, F.M.; Celiktas, M.S. Review on Catalytic Biomass Gasification for Hydrogen Production as a Sustainable Energy Form and Social, Technological, Economic, Environmental, and Political Analysis of Catalysts. ACS Omega 2022, 7, 24918–24941. [Google Scholar] [CrossRef]
- IEA International Energy Agency. Tracking Clean Energy Progress 2023. Available online: https://www.iea.org/reports/tracking-clean-energy-progress-2023 (accessed on 17 November 2023).
- Working Party on Agricultural Policies and Markets. Available online: https://one.oecd.org/document/TAD/CA/APM/WP(2009)11/FINAL/en/pdf (accessed on 15 November 2023).
- Kumar, A.; Kumar, N.; Baredar, P.; Shukla, A. A review of biomass energy resources, potential, conversion, and policy in India. Renew. Sustain. Energy Rev. 2015, 45, 530–539. [Google Scholar] [CrossRef]
- Arauzo, P.J.; Atienza-Martínez, M.; Ábrego, J.; Olszewski, M.P.; Cao, Z.; Kruse, A. Combustion characteristics of hydrochar and pyrochar derived from digested sewage sludge. Energies 2020, 13, 4164. [Google Scholar] [CrossRef]
- Marinovic, A.; Pileidis, F.D.; Titirici, M.M. Chapter—5: Hydrothermal Carbonisation (HTC): History, State-of-the-Art and Chemistry. In Porous Carbon Materials from Sustainable Precursors; White, R.J., Ed.; Royal Society of Chemistry: London, UK, 2015; pp. 129–155. [Google Scholar] [CrossRef]
- Kruse, A.; Funke, A.; Titirici, M.-M. Hydrothermal conversion of biomass to fuels and energetic materials. Curr. Opin. Chem. Biol. 2013, 17, 515–521. [Google Scholar] [CrossRef]
- Danso-Boateng, E.; Holdich, R.G.; Shama, G.; Wheatley, A.D.; Sohail, M.; Martin, S.J. Kinetics of faecal biomass hydrothermal carbonisation for hydrochar production. Appl. Energy 2013, 111, 351–357. [Google Scholar] [CrossRef]
- Libra, J.A.; Ro, K.S.; Kammann, C.; Funke, A.; Berge, N.D.; Neubauer, Y.; Titirici, M.-M.; Fühenr, C.; Bens, O.; Kern, J.; et al. Hydrothermal carbonization of biomass residuals: A comparative review of the chemistry, processes and applications of wet and dry pyrolysis. Biofuels 2011, 2, 71–106. [Google Scholar] [CrossRef]
- Funke, A.; Ziegler, F. Hydrothermal carbonization of biomass: A summary and discussion of chemical mechanisms for process engineering. Biofuels Bioprod. Biorefining 2010, 4, 160–177. [Google Scholar] [CrossRef]
- Cebi, D.; Celiktas, M.S.; Sarptas, H. A Review on Sewage Sludge Valorization via Hydrothermal Carbonization and Applications for Circular Economy. Circ. Econ. Sustain. 2022, 2, 1345–1367. [Google Scholar] [CrossRef]
- Bergius, F. Die Anwendung Hoher Drucke bei Chemischen Vorgängen und Eine Nachbildung des Entstehungsprozesses der Steinkohle; Publisher Knapp: Halle, Germany, 1913. [Google Scholar]
- Kowalski, Z.; Kulczycka, J.; Makara, A.; Mondello, G.; Salomone, R. Industrial Symbiosis for Sustainable Management of Meat Waste: The Case of Smiłowo Eco-Industrial Park, Poland. Int. J. Environ. Res. Public Health 2023, 20, 5162. [Google Scholar] [CrossRef]
- Choudhary, C. Industrial Ecology: Concepts, System View and Approaches. Int. J. Eng. Res. Technol. 2012, 1, 1–6. [Google Scholar] [CrossRef]
- Djandja, O.S.; Yin, L.-X.; Wang, Z.-C.; Duan, P.-G. From wastewater treatment to resources recovery through hydrothermal treatments of municipal sewage sludge: A critical review. Process Saf. Environ. Prot. 2021, 151, 101–127. [Google Scholar] [CrossRef]
- Dos Santos Dalbelo, T.; Rutkowski, E.W. Industrial Ecology: Ultimate of the Industrial Revolution Toward Sustainability. In Industry, Innovation and Infrastructure; Leal Filho, W., Azul, A.M., Brandli, L., Lange Salvia, A., Wall, T., Eds.; Springer: Cham, Switzerland, 2021; pp. 553–563. [Google Scholar] [CrossRef]
- Li, A.; Antizar-Ladislao, B.; Khraisheh, M. Bioconversion of municipal solid waste to glucose for bio-ethanol production. Bioprocess Biosyst. Eng. 2007, 30, 189–196. [Google Scholar] [CrossRef]
- Lu, X.; Jordan, B.; Berge, N.D. Thermal conversion of municipal solid waste via hydrothermal carbonization: Comparison of carbonization products to products from current waste management techniques. Waste Manag. 2012, 32, 1353–1365. [Google Scholar] [CrossRef]
- Nicolae, S.A.; Au, H.; Modugno, P.; Luo, H.; Szego, A.E.; Qiao, M.; Li, L.; Yin, W.; Heeres, H.J.; Berge, N.; et al. Recent advances in hydrothermal carbonisation: From tailored carbon materials and biochemicals to applications and bioenergy. Green Chem. 2020, 22, 4747–4800. [Google Scholar] [CrossRef]
- Owsianiak, M.; Ryberg, M.; Renz, M.; Hitzl, M.; Hauschild, M. Environmental performance of hydrothermal carbonization of four wet biomass waste streams at industry-relevant scales. ACS Sustain. Chem. Eng. 2016, 4, 6783–6791. [Google Scholar] [CrossRef]
- González-Arias, J.; Sánchez, M.E.; Cara-Jiménez, J.; Baena-Moreno, F.M.; Zhang, Z. Hydrothermal carbonization of biomass and waste: A review. Environ. Chem. Lett. 2022, 20, 211–221. [Google Scholar] [CrossRef]
- Fuertes, A.B.; Camps Arbestain, M.; Sevilla, M.; Maciá-Agulló, J.A.; Fiol, S.; López, R.; Smernik, R.J.; Aitkenhead, W.P.; Arce, F.; Macias, F. Chemical and structural properties of carbonaceous products obtained by pyrolysis and hydrothermal carbonization of corn stover. Aust. J. Soil Res. 2010, 48, 618–626. [Google Scholar] [CrossRef]
- Liu, X.; Zhai, Y.; Li, S.; Wang, B.; Wang, T.; Liu, Y.; Qiu, Z.; Li, C. Hydrothermal carbonization of sewage sludge: Effect of feed-water pH on hydrochar’s physicochemical properties, organic component and thermal behavior. J. Hazard. Mater. 2020, 388, 122084. [Google Scholar] [CrossRef]
- Zhai, Y.; Peng, C.; Xu, B.; Wang, T.; Li, C.; Zeng, G.; Zhu, Y. Hydrothermal carbonization of sewage sludge for char production with different waste biomass: Effects of reaction temperature and energy recycling. Energy 2017, 127, 167–174. [Google Scholar] [CrossRef]
- Wang, W.; Chen, W.-H.; Jang, M.-F. Characterization of Hydrochar Produced by Hydrothermal Carbonization of Organic Sludge. Future Cities Environ. 2020, 6, 13. [Google Scholar] [CrossRef]
- Titirici, M.M. Chapter 12—Hydrothermal Carbons: Synthesis, Characterization, and Applications. In Novel Carbon Adsorbents; Tascón, J.M.D., Ed.; Elsevier Ltd.: Amsterdam, The Netherlands, 2012; pp. 351–399. [Google Scholar] [CrossRef]
- Titirici, M.M.; Thomas, A.; Antonietti, M. Back in the black: Hydrothermal carbonization of plant material as an efficient chemical process to treat the CO2 problem? New J. Chem. 2007, 31, 787–789. [Google Scholar] [CrossRef]
- Burguete, P.; Corma, A.; Hitzl, M.; Modrego, R.; Ponce, E.; Renz, M. Fuel and chemicals from wet lignocellulosic biomass waste streams by hydrothermal carbonization. Green Chem. 2016, 18, 1051–1060. [Google Scholar] [CrossRef]
- Davies, G.; El Sheikh, A.; Collett, C.; Yakub, I.; McGregor, J. Chapter 5—Catalytic carbon materials from biomass. In Emerging Carbon Materials for Catalysis; Sadjadi, S., Ed.; Elsevier Inc.: Amsterdam, The Netherlands, 2021; pp. 161–195. [Google Scholar] [CrossRef]
- Danso-Boateng, E.; Shama, G.; Wheatley, A.D.; Martin, S.J.; Holdich, R.G. Hydrothermal carbonization of sewage sludge: Effect of process conditions on product characteristics and methane production. Bioresour Technol. 2015, 177, 318–327. [Google Scholar] [CrossRef]
- Parmar, K.R.; Ross, A.B. Integration of Hydrothermal Carbonisation with Anaerobic Digestion; Opportunities for Valorisation of Digestate. Energies 2019, 12, 1586. [Google Scholar] [CrossRef]
- Picone, A.; Volpe, M.; Giustra, M.G.; Di Bella, G.; Messineo, A. Hydrothermal carbonization of lemon peel waste: Preliminary results on the effects of temperature during process water recirculation. Appl. Syst. Innov. 2021, 4, 19. [Google Scholar] [CrossRef]
- Inagaki, M.; Kang, F.; Toyoda, M.; Konno, H. Advanced materials science and engineering of carbon. MRS Bull. 2014, 39, 1018. [Google Scholar] [CrossRef][Green Version]
- Hernandez, M.; Salimbeni, A.; Hitzl, M.; Zhang, J.; Wang, G.; Wang, K.; Wang, C. Evaluation of Utilising Ingelia Hydrochar Produced from Organic Residues for Blast Furnaces Injection—Comparison with Anthracite and Bituminous Coal. In Proceedings of the 26th European Biomass Conference & Exhibition (EUBCE 2018) in Copenhagen, Copenhagen, Denmark, 14–17 May 2018; pp. 1560–1568. [Google Scholar] [CrossRef]
- Hernandez, M.L.; Hitzl, M.; Corma, A.; Renz, M. Hydrothermal Carbonisation of Organic Fraction Municipal Solid Waste. In Proceedings of the HTC 2017—The 1st International Symposium on Hydrothermal Carbonisation: Possibilities and Limits for Feedstocks, Processes and Applications, London, UK, 3–4 April 2017. [Google Scholar]
- Hitzl, M.; Corma, A.; Pomares, F.; Renz, M. The hydrothermal carbonization (HTC) plant as a decentral biorefinery for wet biomass. Catal. Today 2015, 257, 154–159. [Google Scholar] [CrossRef]
- Titirici, M.M.; Antonietti, M.; Baccile, N. Hydrothermal carbon from biomass: A comparison of the local structure from poly- to monosaccharides and pentoses/hexoses. Green Chem. 2008, 10, 1204–1212. [Google Scholar] [CrossRef]
- Hernández, M.L.; Latorre, M.B.A.; Hitzl, M. Industrial HTC Technology applied to Sewage Sludge Treatment. In Proceedings of the International VDI Conference 2017 Sewage Sludge Treatment, Copenhagen, Denmark, 17–18 May 2017. [Google Scholar]
- Renz, M.; Corma, A.; Hitzl, M. Biocoal Water Fuel (BWF) obtained by Hydro Thermal Carbonization (HTC). In Proceedings of the 21st European Biomass Conference and Exhibition, Copenhagen, Denmark, 3–7 June 2013. [Google Scholar]
- Tasca, A.L.; Puccini, M.; Gori, R.; Corsi, I.; Raspolli Galletti, A.M.; Vitolo, S. Hydrothermal carbonization of sewage sludge: A critical analysis of process severity, hydrochar properties and environmental implications. Waste Manag. 2019, 93, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Ingelia. Ingelia Biorefinery—from Organic Waste to High Value Bioproducts. Available online: https://ingelia.com/index.php/quienes-somos/english-ingelias-hystory/?lang=en (accessed on 10 November 2023).
- PN-EN ISO 17225-8:2023-10; Solid Biofuels—Fuel Specifications and Classes—Part 8: Fuel Classes from Thermally Processed and Compressed Biomass for Non-Industrial and Industrial Use. Polish Committee for Standardization: Warsaw, Poland, 2023.
- Natural Gas (Henry Hub). Markets Insider. Available online: https://markets.businessinsider.com/news/commodities/natural-gas-price?miRedirects=1 (accessed on 15 December 2023).
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).