An Experimental Study on the Performance and Emissions of an 8% Water-in-Diesel Emulsion Stabilized by a Hydrophilic Surfactant Blend
Abstract
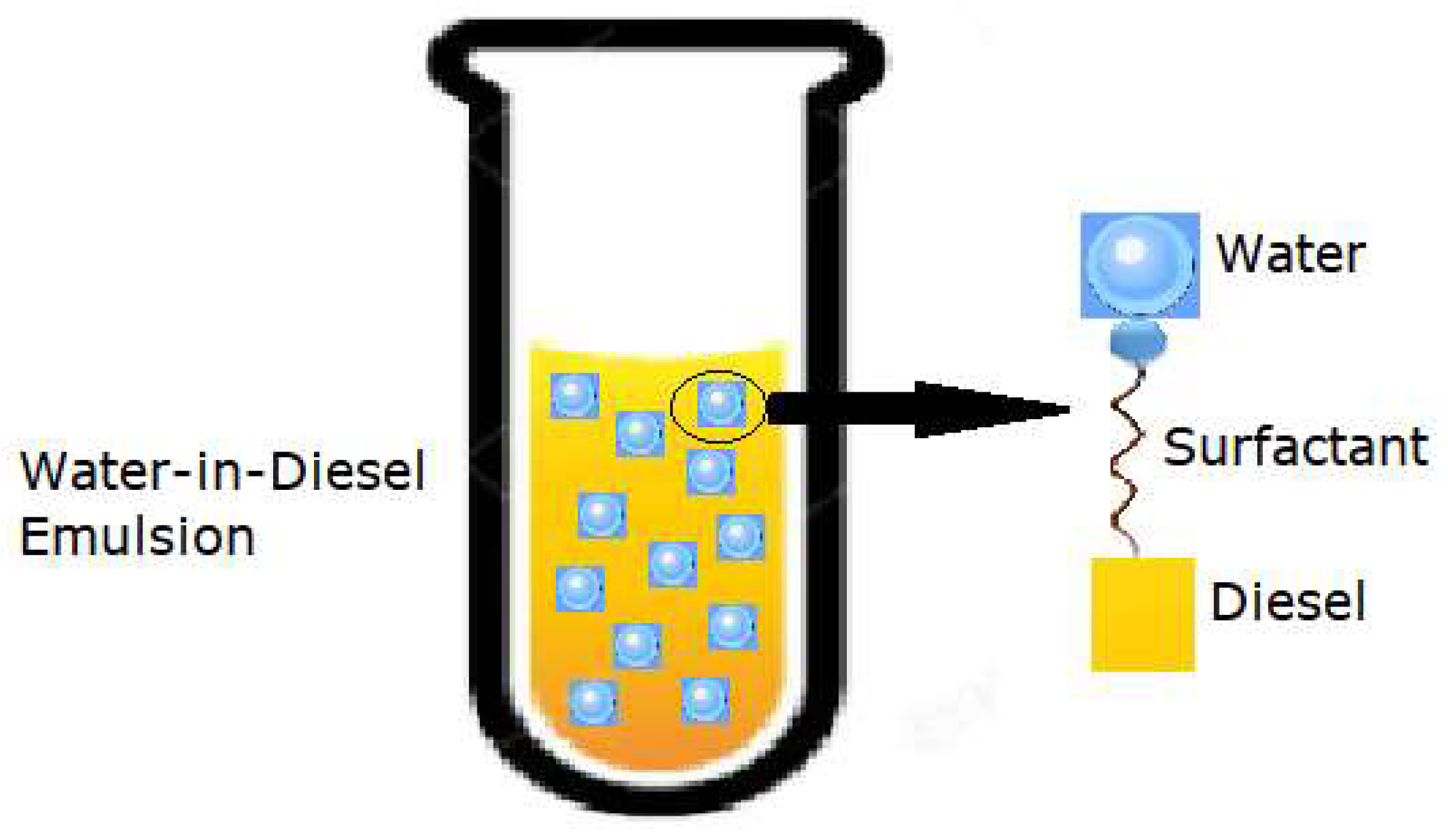
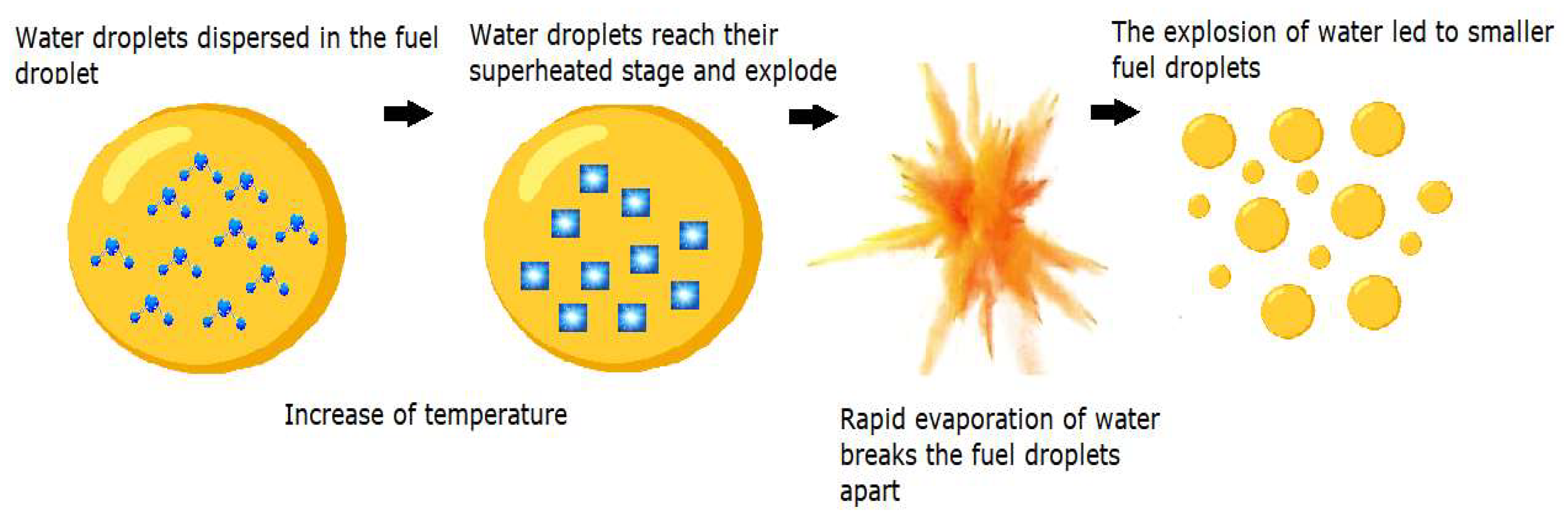
1. Introduction
2. Materials and Methods
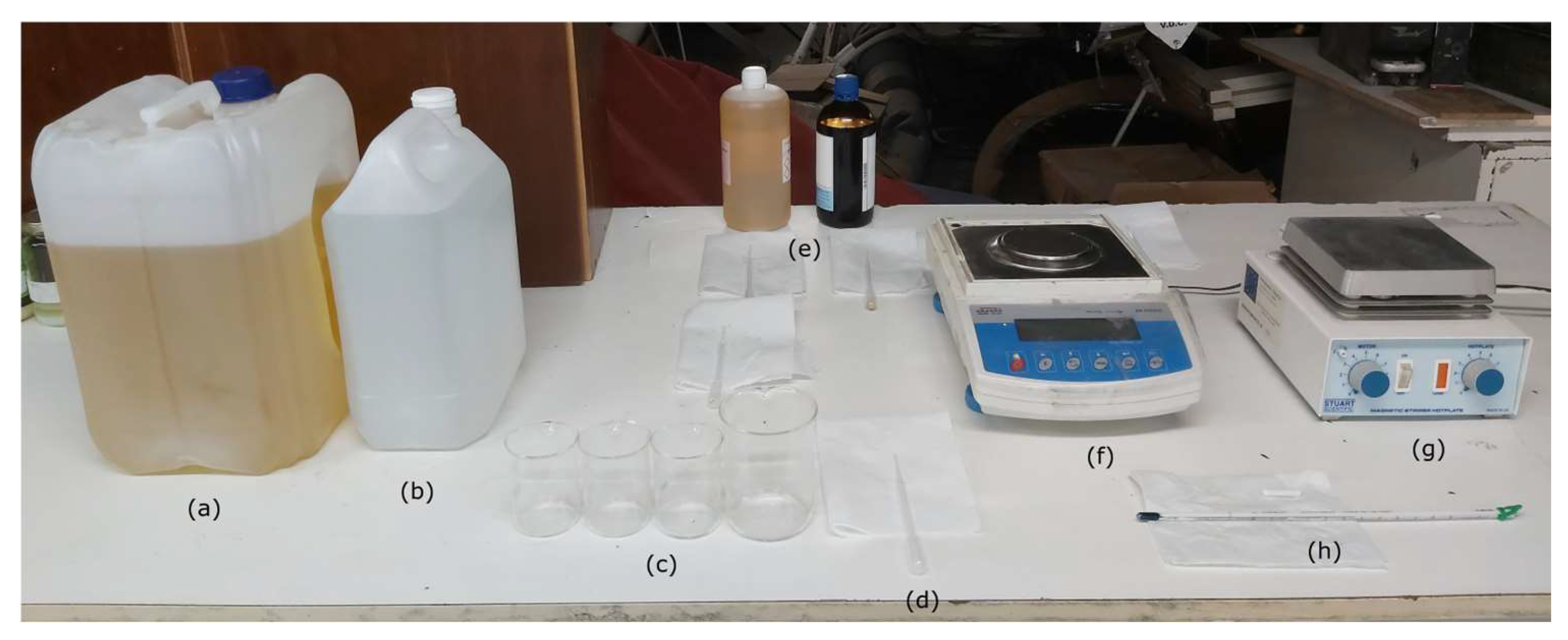
2.1. Emulsion Preparation
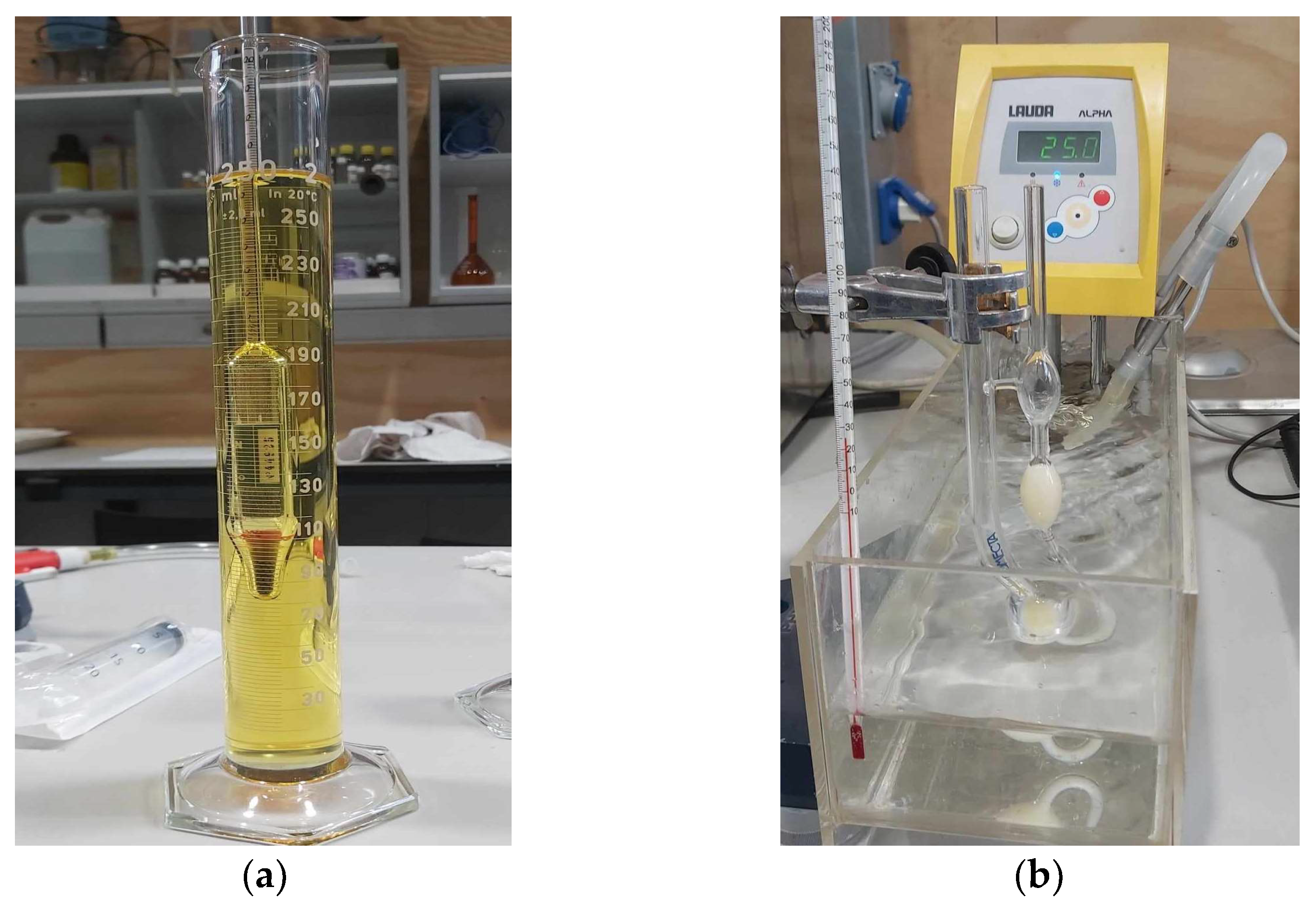
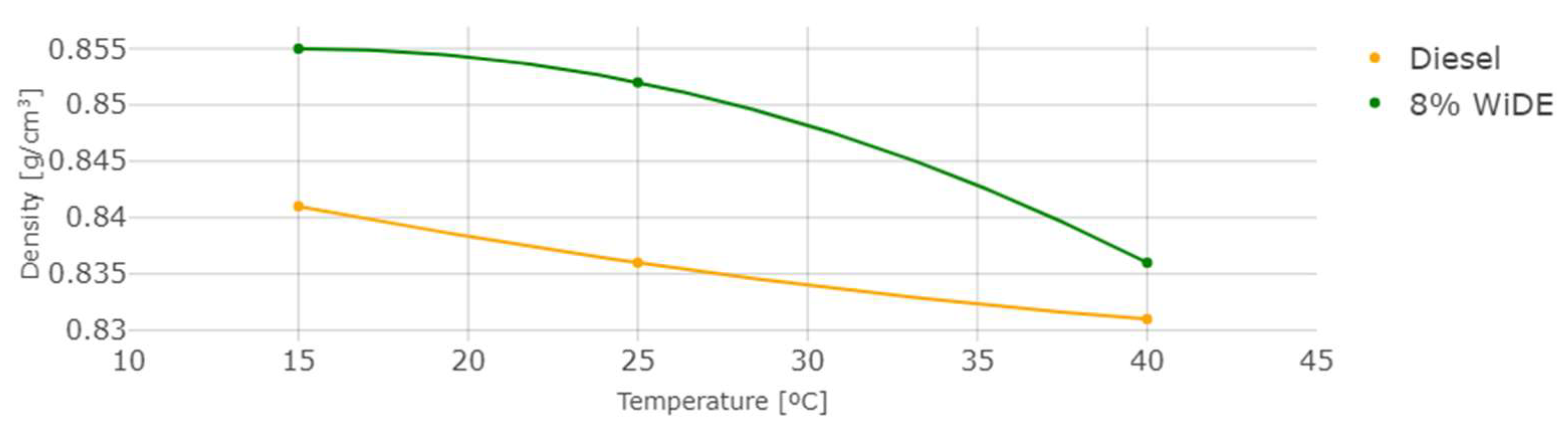
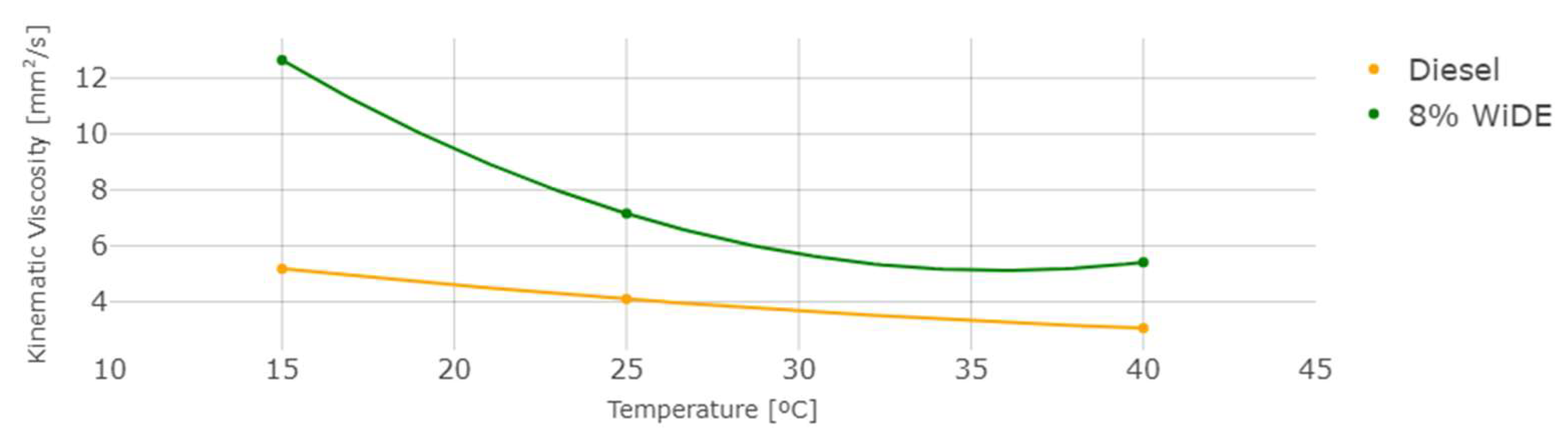
Density, Viscosity and Heating Value
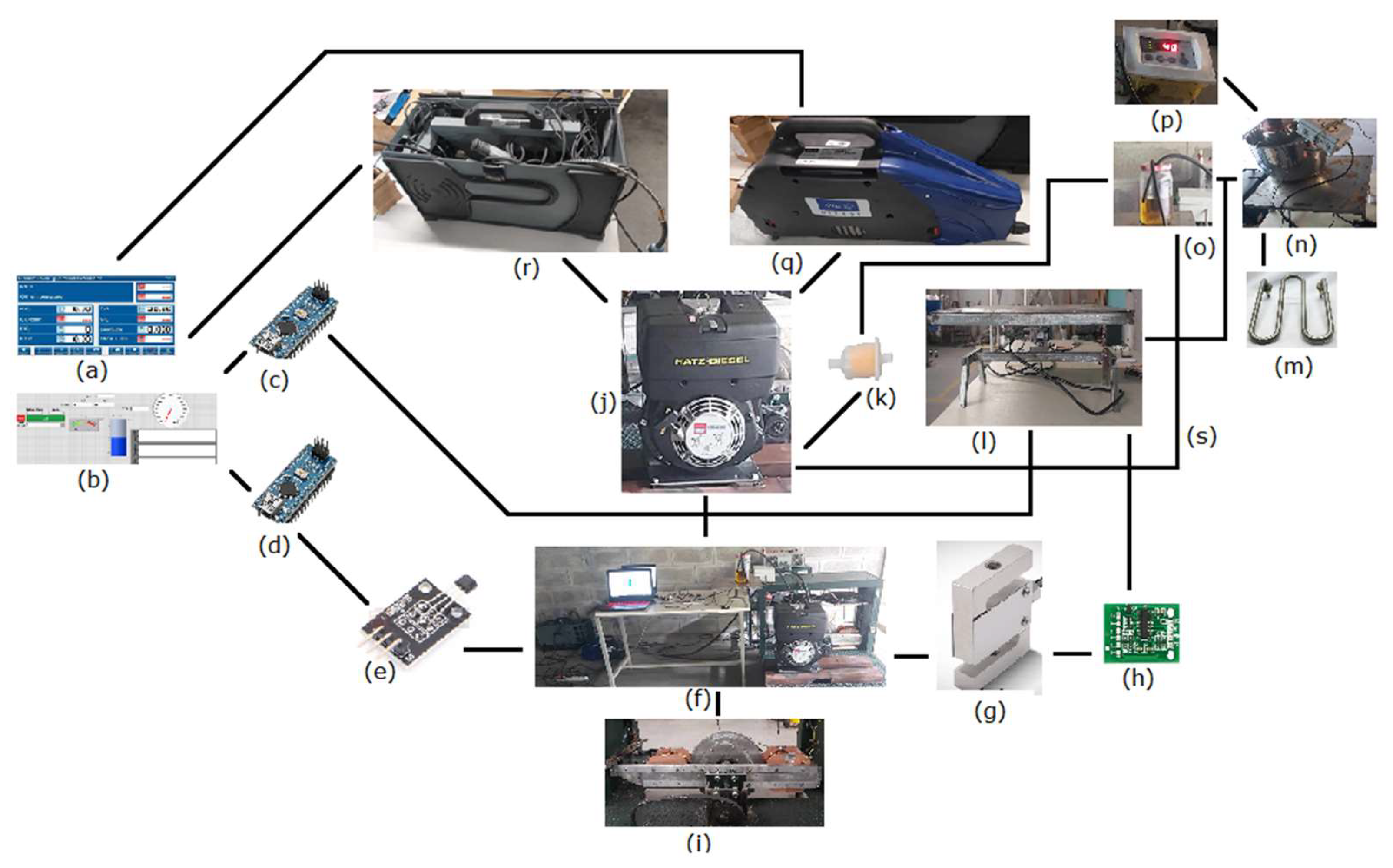
2.2. Engine Testing
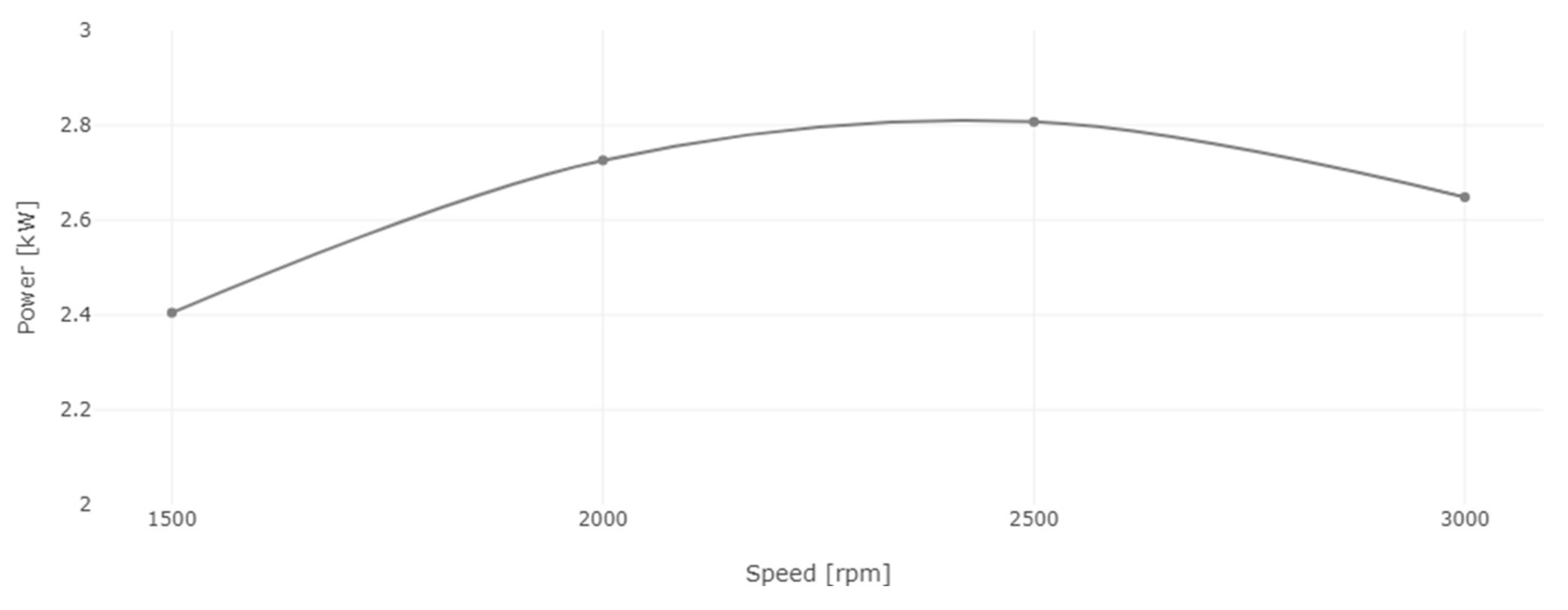
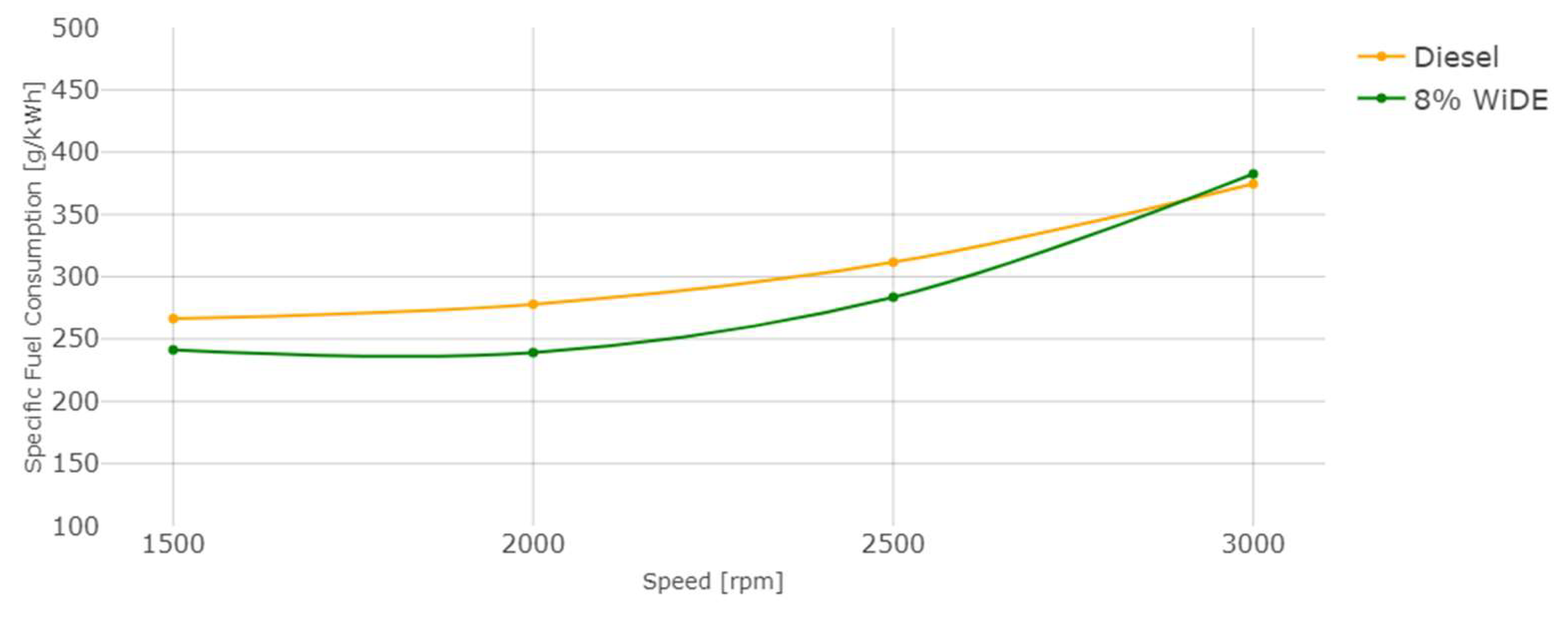
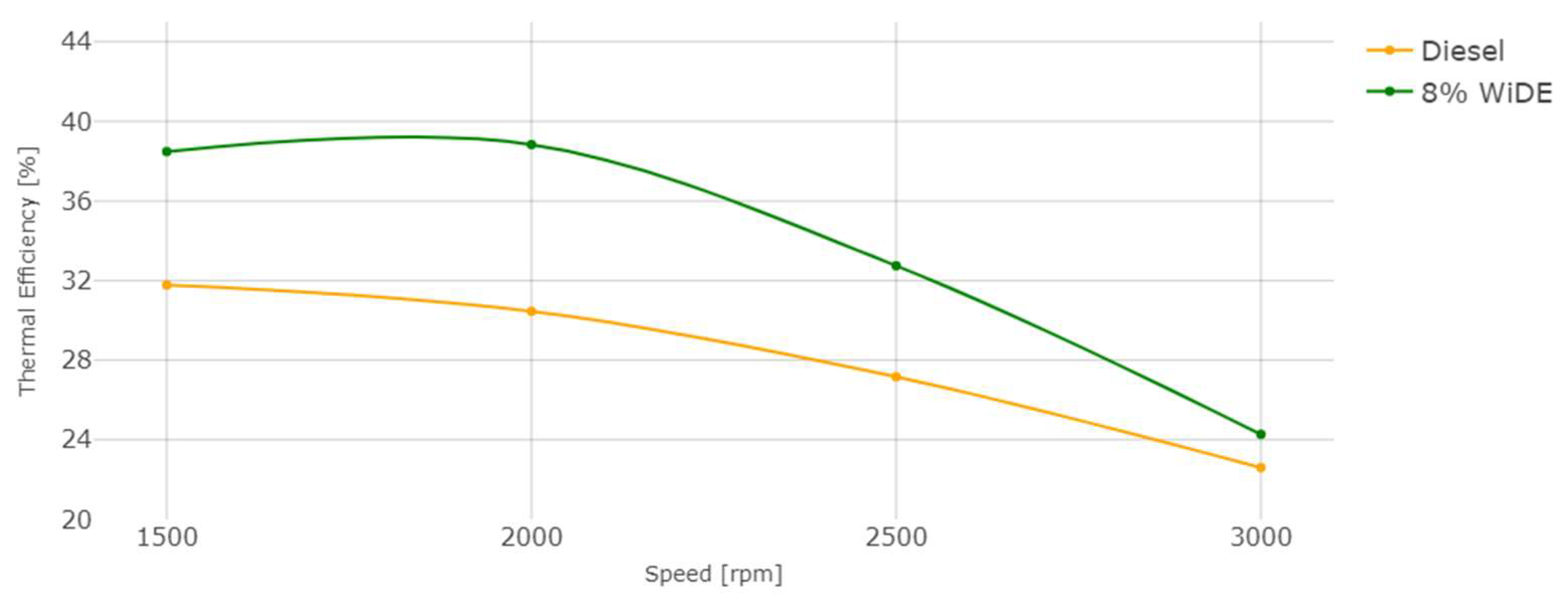
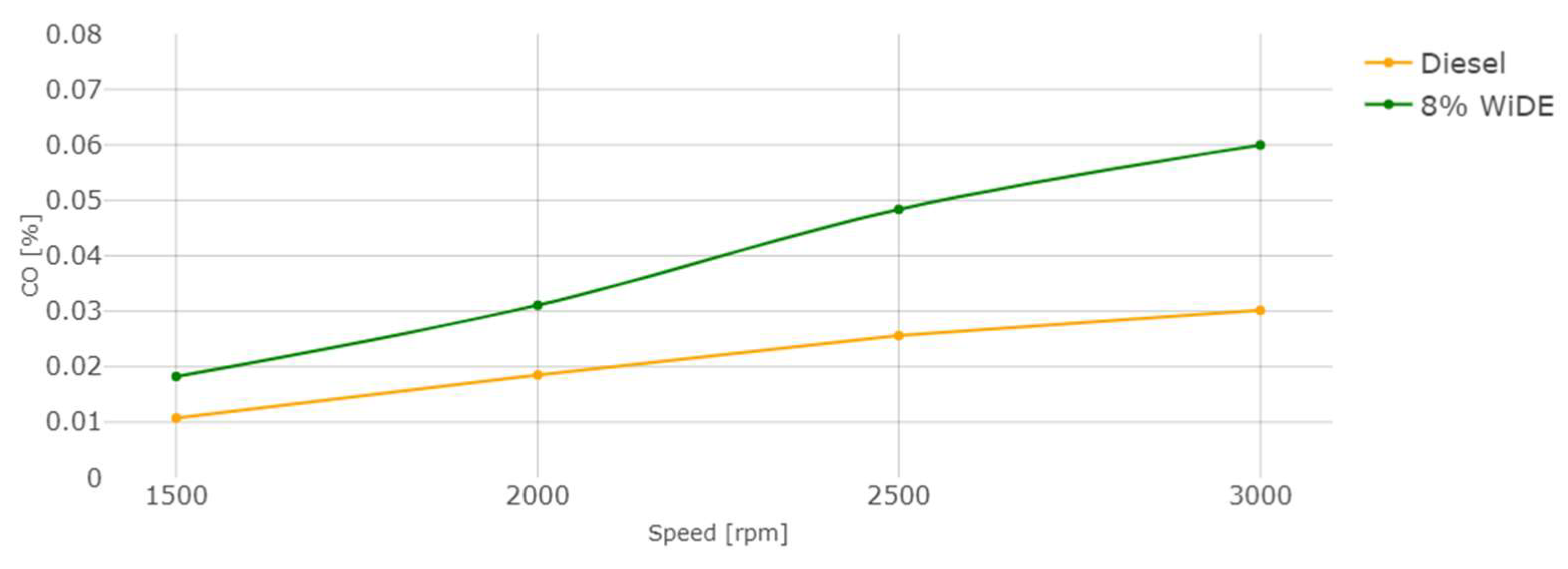
3. Results and Discussion
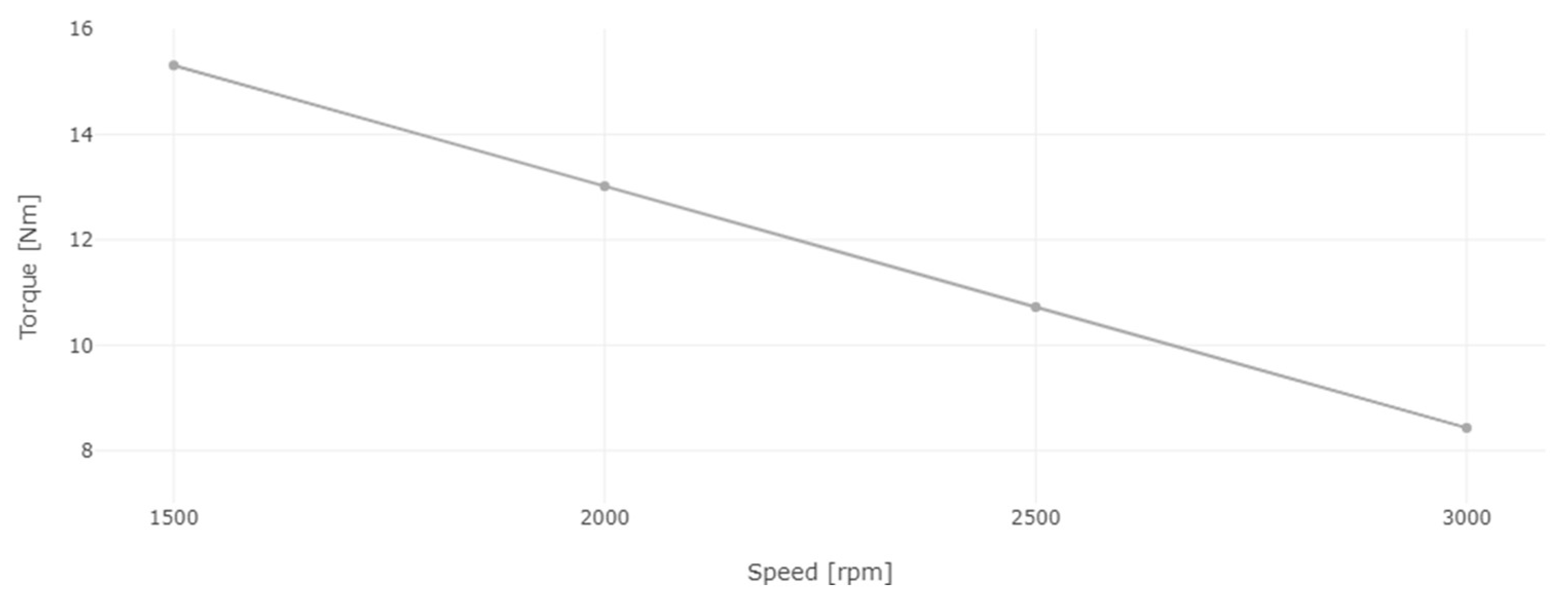
3.1. Performance
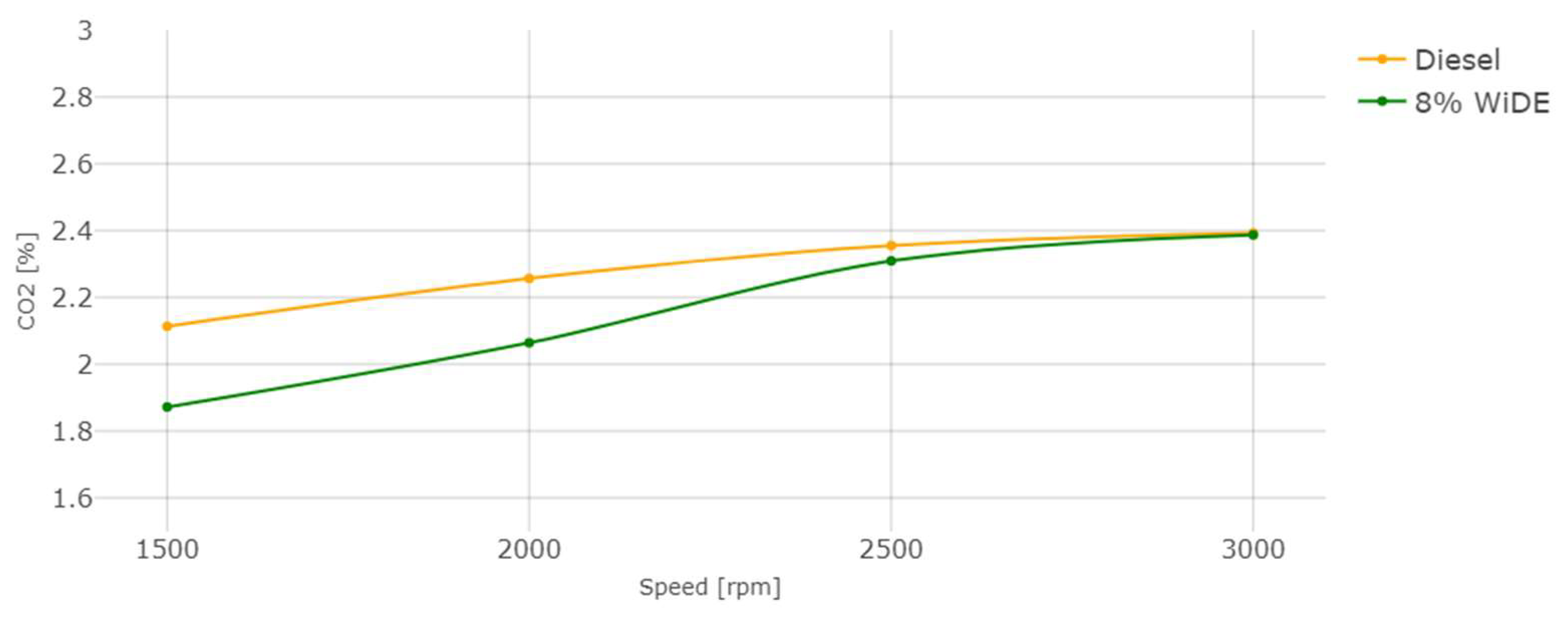
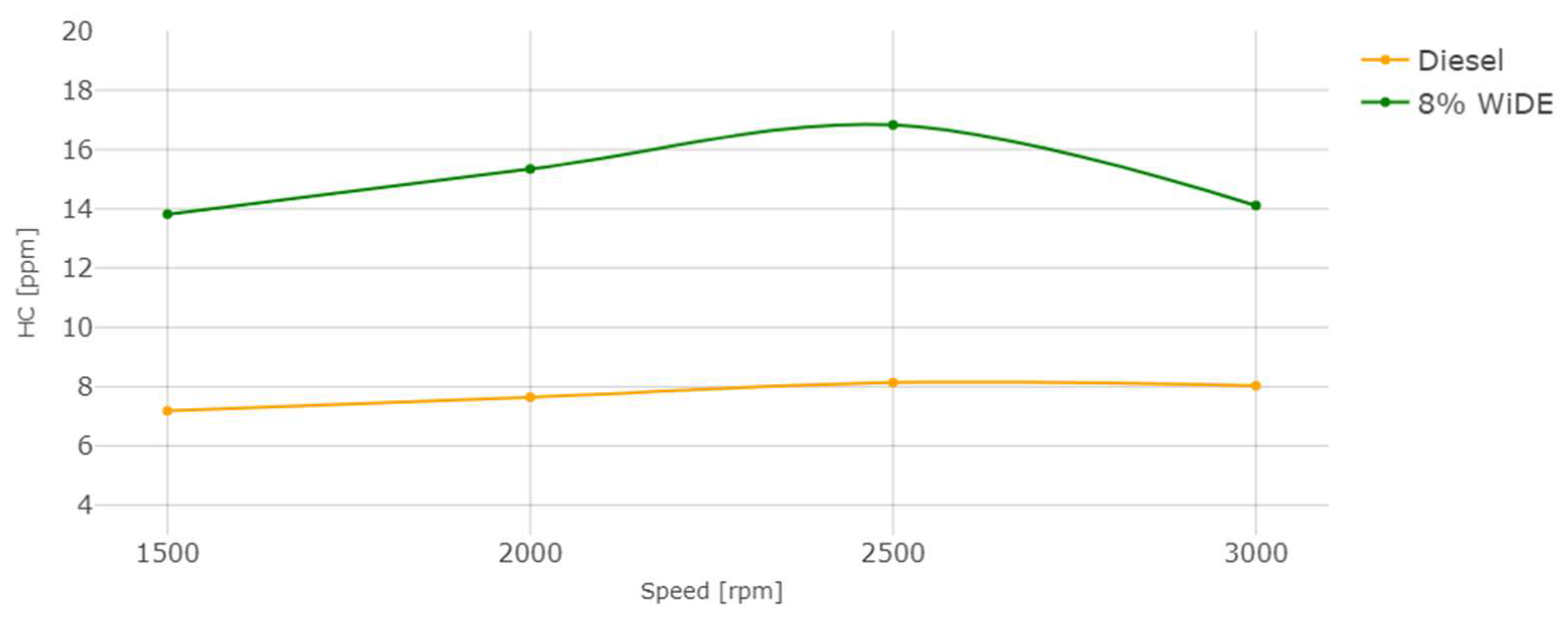
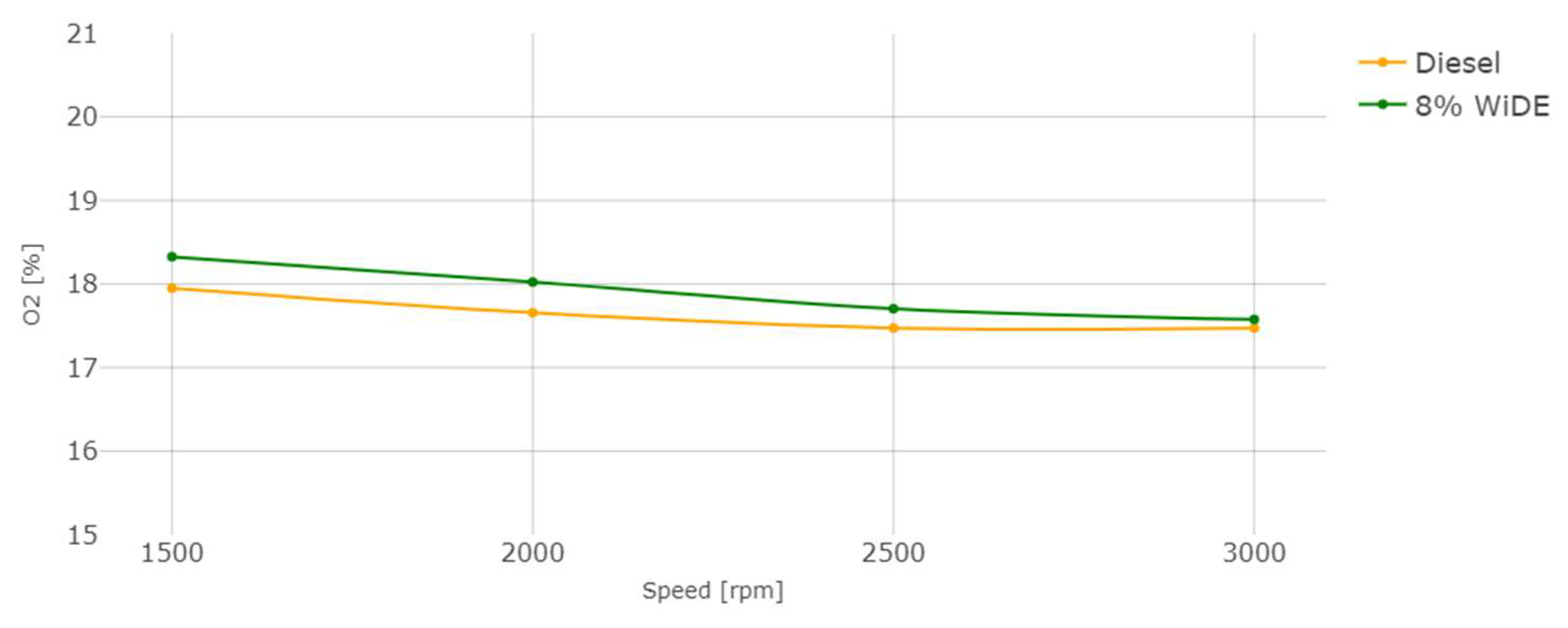
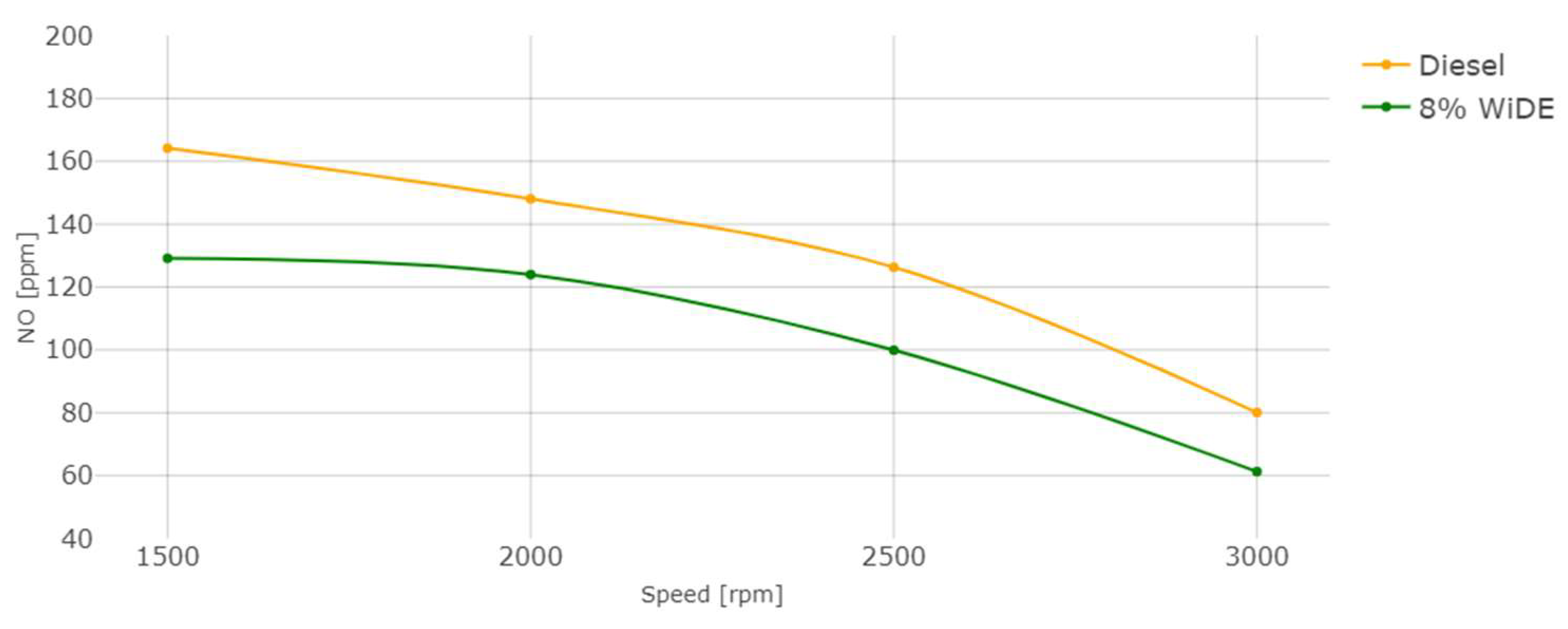
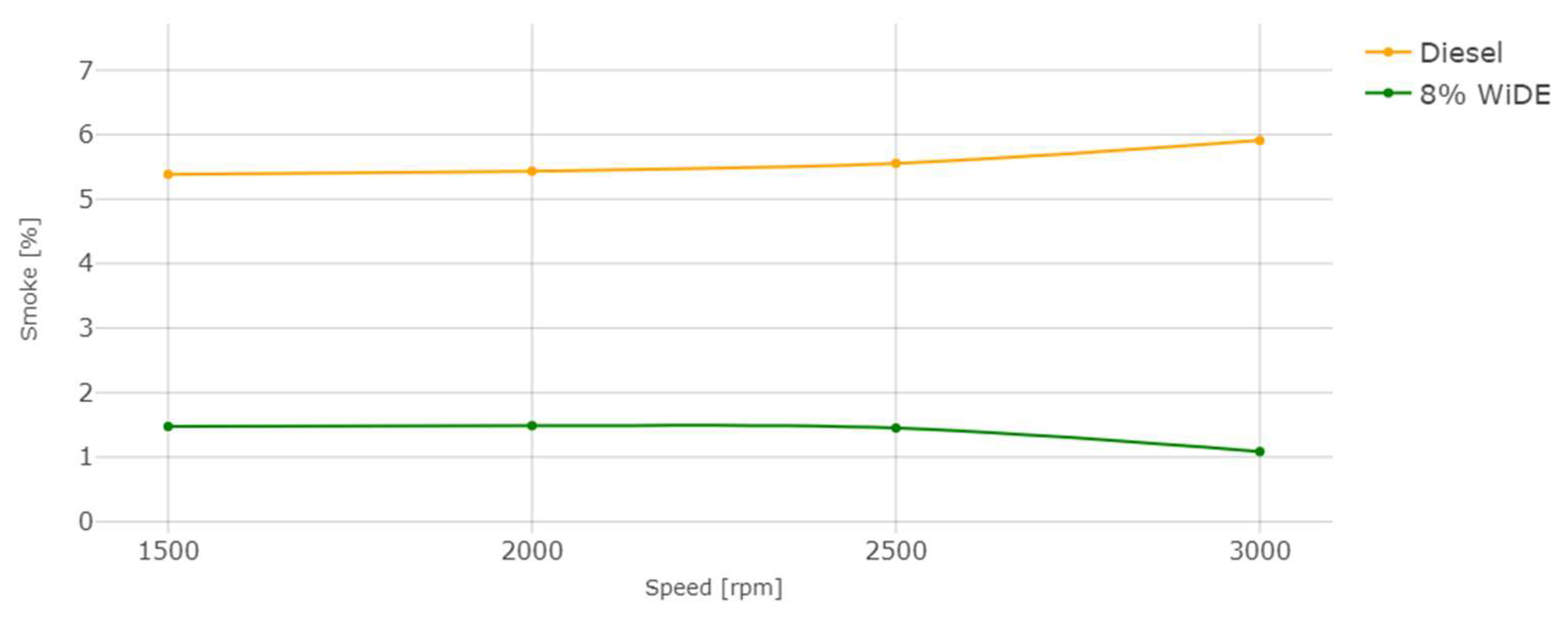
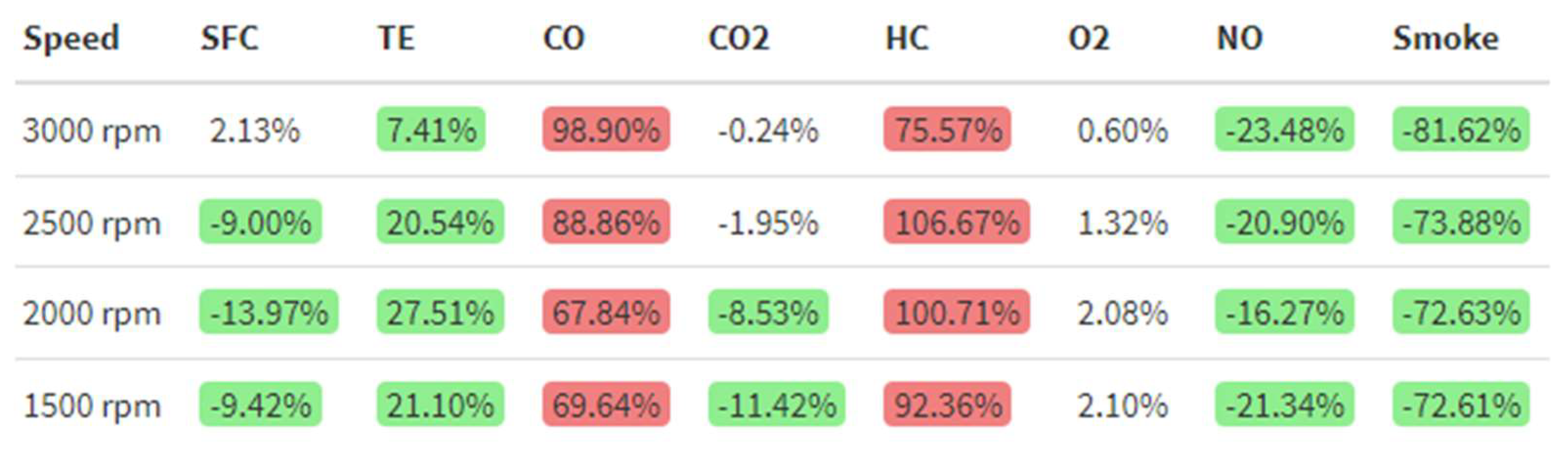
3.2. Emissions
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ağbulut, Ü.; Sarıdemir, S. A general view to converting fossil fuels to cleaner energy source by adding nanoparticles. Int. J. Ambient Energy 2021, 42, 1569–1574. [Google Scholar] [CrossRef]
- Mustayen, A.G.M.B.; Rasul, M.G.; Wang, X.; Negnevitsky, M.; Hamilton, J.M. Remote areas and islands power generation: A review on diesel engine performance and emission improvement techniques. Energy Convers. Manag. 2022, 260, 115614. [Google Scholar] [CrossRef]
- Mohd Tamam, M.Q.; Yahya, W.J.; Ithnin, A.M.; Abdullah, N.R.; Kadir, H.A.; Rahman, M.M.; Rahman, H.A.; Abu Mansor, M.R.; Noge, H. Performance and emission studies of a common rail turbocharged diesel electric generator fueled with emulsifier free water/diesel emulsion. Energy 2023, 268, 126704. [Google Scholar] [CrossRef]
- Vijay Kumar, M.; Babu, A.V.; Reddy, C.R.; Pandian, A.; Bajaj, M.; Zawbaa, H.M.; Kamel, S. Investigation of the combustion of exhaust gas recirculation in diesel engines with a particulate filter and selective catalytic reactor technologies for environmental gas reduction. Case Stud. Therm. Eng. 2022, 40, 102557. [Google Scholar] [CrossRef]
- Wijeyakulasuriya, S.; Kim, J.; Probst, D.; Srivastava, K.; Yang, P.; Scarcelli, R.; Senecal, P.K. Enabling Powertrain Technologies for Euro 7/VII Vehicles with Computational Fluid Dynamics. Transp. Eng. 2022, 9, 100127. [Google Scholar] [CrossRef]
- Woo, S.; Lee, K. Effect of injection strategy and water content on water emulsion fuel engine for low pollutant compression ignition engines. Fuel 2023, 343, 127809. [Google Scholar] [CrossRef]
- Marzuki, N.H.C.; Wahab, R.A.; Hamid, M.A. An overview of nanoemulsion: Concepts of development and cosmeceutical applications. Biotechnol. Biotechnol. Equip. 2019, 33, 779–797. [Google Scholar] [CrossRef]
- Vellaiyan, S. Recent advancements in water emulsion fuel to explore efficient and cleaner production from various biodiesels: A retrospective review. Renew. Sustain. Energy Rev. 2023, 187, 113704. [Google Scholar] [CrossRef]
- Kapadia, H.; Brahmbhatt, H.; Dabhi, Y.; Chourasia, S. Investigation of emulsion and effect on emission in CI engine by using diesel and bio-diesel fuel: A review. Egypt. J. Pet. 2019, 28, 323–337. [Google Scholar] [CrossRef]
- Jhalani, A.; Sharma, D.; Soni, S.L.; Sharma, P.K. Effects of process parameters on performance and emissions of a water-emulsified diesel-fueled compression ignition engine. Energy Sources Part A Recover. Util. Environ. Eff. 2023, 45, 4242–4254. [Google Scholar] [CrossRef]
- Jin, C.; Wei, J. The combined effect of water and nanoparticles on diesel engine powered by biodiesel and its blends with diesel: A review. Fuel 2023, 343, 127940. [Google Scholar] [CrossRef]
- Anil, M.D.; Hemadri, V.B.; Swamy, M. Experimental investigation on impact of water in diesel emulsion in a single-cylinder research diesel engine. Int. J. Ambient Energy 2023, 44, 399–412. [Google Scholar] [CrossRef]
- Gowrishankar, S.; Krishnasamy, A. Emulsification—A promising approach to improve performance and reduce exhaust emissions of a biodiesel fuelled light-duty diesel engine. Energy 2023, 263, 125782. [Google Scholar] [CrossRef]
- Mondal, P.K.; Mandal, B.K. A comparative study on the performance and emissions from a CI engine fuelled with water emulsified diesel prepared by mechanical homogenization and ultrasonic dispersion method. Energy Rep. 2019, 5, 639–648. [Google Scholar] [CrossRef]
- Woo, S.; Kim, W.; Lee, J.; Lee, K. Fuel properties of water emulsion fuel prepared using porous membrane method for low pollutant engine at various temperatures. Energy Rep. 2021, 7, 6638–6650. [Google Scholar] [CrossRef]
- Noor El-Din, M.R.; El-Hamouly, S.H.; Mohamed, H.M.; Mishrif, M.R.; Ragab, A.M. Water-in-diesel fuel nanoemulsions: Preparation, stability and physical properties. Egypt. J. Pet. 2013, 22, 517–530. [Google Scholar] [CrossRef]
- Deore, S.L.; Kale, S.N. Emulsion Micro Emulsion and Nano Emulsion: A Review. Syst. Rev. Pharm. 2017, 8, 39–47. [Google Scholar] [CrossRef]
- Patel, K.R.; Dhiman, V.D. A review on emission and performance of water diesel micro-emulsified mixture-diesel engine. Int. J. Environ. Sci. Technol. 2022, 19, 8027–8042. [Google Scholar] [CrossRef]
- Sazhin, S.S.; Rybdylova, O.; Crua, C.; Heikal, M.; Ismael, M.A.; Nissar, Z.; Aziz, A.R.B.A. A simple model for puffing/micro-explosions in water-fuel emulsion droplets. Int. J. Heat Mass Transf. 2019, 131, 815–821. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, Q.; Wang, X.; Xu, J. Micro-explosion characteristics and mechanism of multi-component fuel droplet with high volatility differential. Fuel 2023, 333, 126370. [Google Scholar] [CrossRef]
- Islamova, A.; Tkachenko, P.; Shlegel, N.; Kuznetsov, G. Secondary Atomization of Fuel Oil and Fuel Oil/Water Emulsion through Droplet-Droplet Collisions and Impingement on a Solid Wall. Energies 2023, 16, 1008. [Google Scholar] [CrossRef]
- Shen, S.; Liu, H.; Liu, Y.; Liu, X.; Hu, H.; Hu, Z.; Wang, T. Dynamic details inside water-in-oil (W/O) emulsion droplet and its impact on droplet evaporation and micro-explosion. Fuel 2023, 338, 127254. [Google Scholar] [CrossRef]
- Rosli, M.A.F.; Aziz, A.R.A.; Ismael, M.A.; Elbashir, N.O.; Zainal, A.E.Z.; Baharom, M.; Mohammed, S.E. Experimental study of micro-explosion and puffing of gas-to-liquid (GTL) fuel blends by suspended droplet method. Energy 2021, 218, 119462. [Google Scholar] [CrossRef]
- Zhang, H.; Lu, Z.; Wang, T.; Che, Z. Mist formation during micro-explosion of emulsion droplets. Fuel 2022, 339, 127350. [Google Scholar] [CrossRef]
- Moussa, O.; Tarlet, D.; Massoli, P.; Bellettre, J. Investigation on the conditions leading to the micro-explosion of emulsified fuel droplet using two colors LIF method. Exp. Therm. Fluid Sci. 2020, 116, 110106. [Google Scholar] [CrossRef]
- Saha, D.; Roy, B. Influence of areca nut husk nano-additive on combustion, performance, and emission characteristics of compression ignition engine fuelled with plastic-grocery-bag derived oil-water-diesel emulsion. Energy 2023, 268, 126682. [Google Scholar] [CrossRef]
- Alves, L.; Holz, L.I.V.; Fernandes, C.; Ribeirinha, P.; Mendes, D.; Fagg, D.P.; Mendes, A. A comprehensive review of NOx and N2O mitigation from industrial streams. Renew. Sustain. Energy Rev. 2022, 155, 111916. [Google Scholar] [CrossRef]
- Rajpoot, A.S.; Saini, G.; Chelladurai, H.M.; Shukla, A.K.; Choudhary, T. Comparative combustion, emission, and performance analysis of a diesel engine using carbon nanotube (CNT) blended with three different generations of biodiesel. Environ. Sci. Pollut. Res. 2023, 30, 125328–125346. [Google Scholar] [CrossRef]
- Gautam, P.S.; Vishnoi, P.K.; Gupta, V.K. The effect of water emulsified diesel on combustion, performance and emission characteristics of diesel engine. Mater. Today Proc. 2022, 52, 1041–1047. [Google Scholar] [CrossRef]
- Deepak, B.; Mohamed Ibrahim, M. A critical review on emulsion fuel formulation and its applicability in compression ignition engine. Biofuels 2023, 1, 1–19. [Google Scholar] [CrossRef]
- Sartomo, A.; Santoso, B.; Ubaidillah; Muraza, O. Recent progress on mixing technology for water-emulsion fuel: A review. Energy Convers. Manag. 2020, 213, 112817. [Google Scholar] [CrossRef]
- Serôdio, J.C.F. Method, System, Apparatus and Formulations for Producing Oil-based Blends and Microemulsions and Nanoemulsions. WO2021090010A1, 14 May 2021. [Google Scholar]
- Preetika, R.; Mehta, P.S.; Kaisare, N.S.; Basavaraj, M.G. Kinetic stability of surfactant stabilized water-in-diesel emulsion fuels. Fuel 2019, 236, 1415–1422. [Google Scholar] [CrossRef]
- Pulkrabek, W.W. Engineering Fundamentals of the Internal Combustion Engine, 2nd ed.; Pearson: Platteville, WI, USA, 2003; p. 251. [Google Scholar]
- Arévalo-Ramírez, R.; Aros-Taglioni, J. Parametric analysis based on energy and exergy balances of a condensing boiler. J. Mech. Sci. Technol. 2023, 37, 1463–1471. [Google Scholar] [CrossRef]





| Surfactants | Chemical Formula | HLB Value | % (m/m) |
|---|---|---|---|
| Cocamide DEA | CH3(CH2)10C(=O)N(CH2CH2OH)2 | 13.5 | 91 |
| Span 80 | C24H44O6 | 4 | 9 |
| Fuel | HHv (MJ/Kg) | HHVv (MJ/L) | LHVp (MJ/Kg) | LHVp (MJ/L) |
|---|---|---|---|---|
| Diesel | 45.49 | 38.26 | 42.53 | 35.77 |
| 8% WiDE | 41.68 | 35.64 | 38.77 | 33.15 |
| Engine Specifications | Parameters Values |
|---|---|
| Operation cycle | 4-stroke |
| Cylinders | 1 |
| Valves per cylinder | 2 |
| Bore [mm] | 88 |
| Stroke [mm] | 76 |
| Displacement [cm3] | 462 |
| Injection system | Direct injection |
| Injection pressure [bar] | 200 |
| Compression ratio | 20.5:1 |
| Empty weight [kg] | 48 |
| Cooling system | Air cooling |
| Rated torque [Nm] | 23.4 |
| Rater power [kW] | 7.3 |
| Measuring Ranges | Accuracy |
|---|---|
| CO: 0–15% vol. | ±0.03% vol. |
| CO2: 0–20% vol. | ±0.5% vol. |
| HC: 0–30,000 ppm vol. | ±10 ppm vol. |
| O2: 0–25% vol. | ±5% of Maximum |
| NO: 0–5000 ppm vol. | ±5 ppm vol. |
| Measuring Ranges | Accuracy |
|---|---|
| Opacity: 0–100% | ±0.1% |
| Absorption (k-value): 0–99.99 m−1 | ±0.01 m−1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oliveira, P.; Brójo, F. An Experimental Study on the Performance and Emissions of an 8% Water-in-Diesel Emulsion Stabilized by a Hydrophilic Surfactant Blend. Energies 2024, 17, 1328. https://doi.org/10.3390/en17061328
Oliveira P, Brójo F. An Experimental Study on the Performance and Emissions of an 8% Water-in-Diesel Emulsion Stabilized by a Hydrophilic Surfactant Blend. Energies. 2024; 17(6):1328. https://doi.org/10.3390/en17061328
Chicago/Turabian StyleOliveira, Pedro, and Francisco Brójo. 2024. "An Experimental Study on the Performance and Emissions of an 8% Water-in-Diesel Emulsion Stabilized by a Hydrophilic Surfactant Blend" Energies 17, no. 6: 1328. https://doi.org/10.3390/en17061328
APA StyleOliveira, P., & Brójo, F. (2024). An Experimental Study on the Performance and Emissions of an 8% Water-in-Diesel Emulsion Stabilized by a Hydrophilic Surfactant Blend. Energies, 17(6), 1328. https://doi.org/10.3390/en17061328






