An Experimental Study on the Effect of Nanofluids on the Thermal Conductivity and Rheological Properties of a Coolant for Liquids
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Preparation
2.2. Thermal Conductivity and Rheological Investigation
2.3. Experimental Uncertainty
3. Results and Discussion
3.1. The Basic Properties of the Investigated Nanofluids
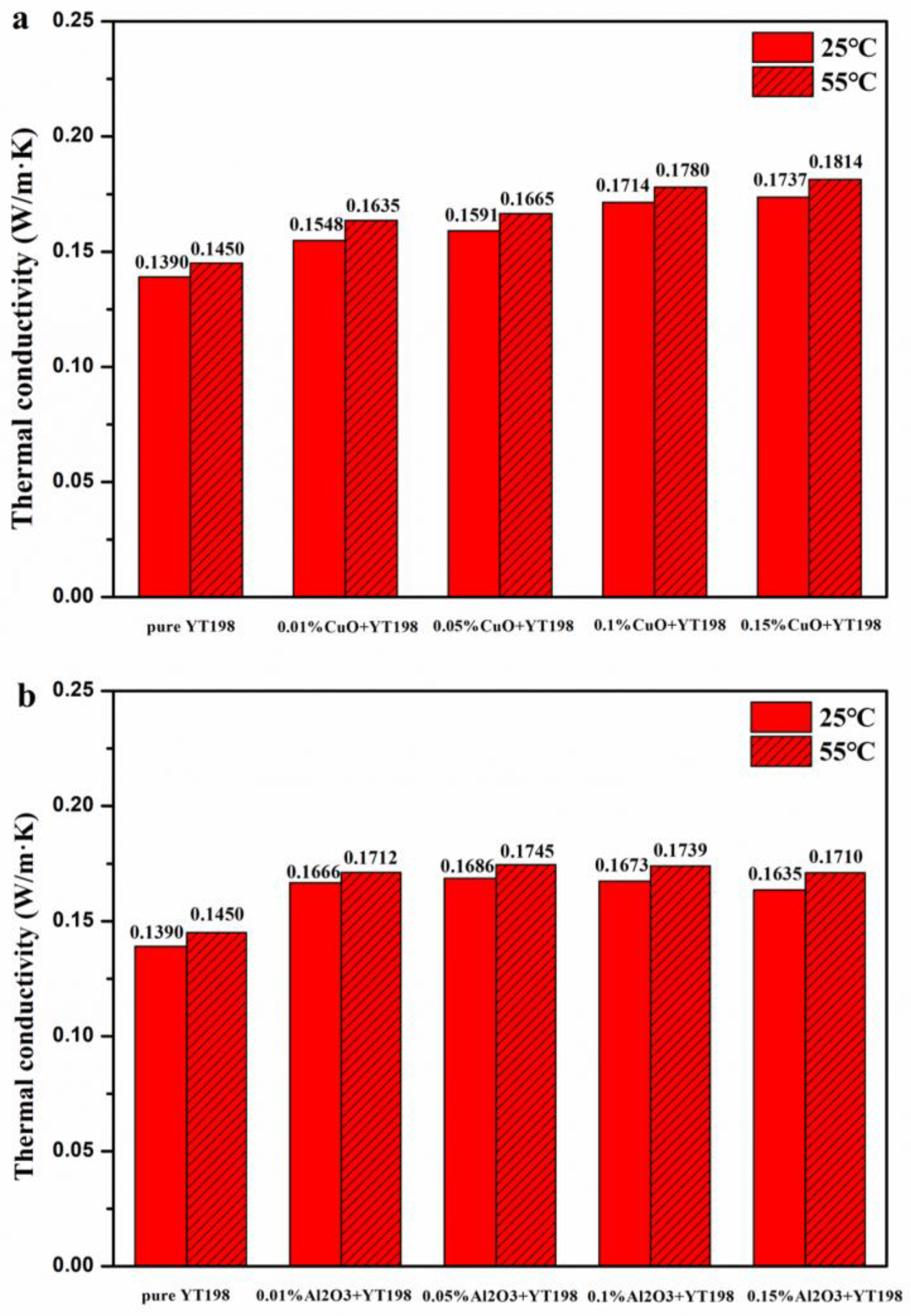
3.2. Thermal Conductivity Enhancement of Nanofluids
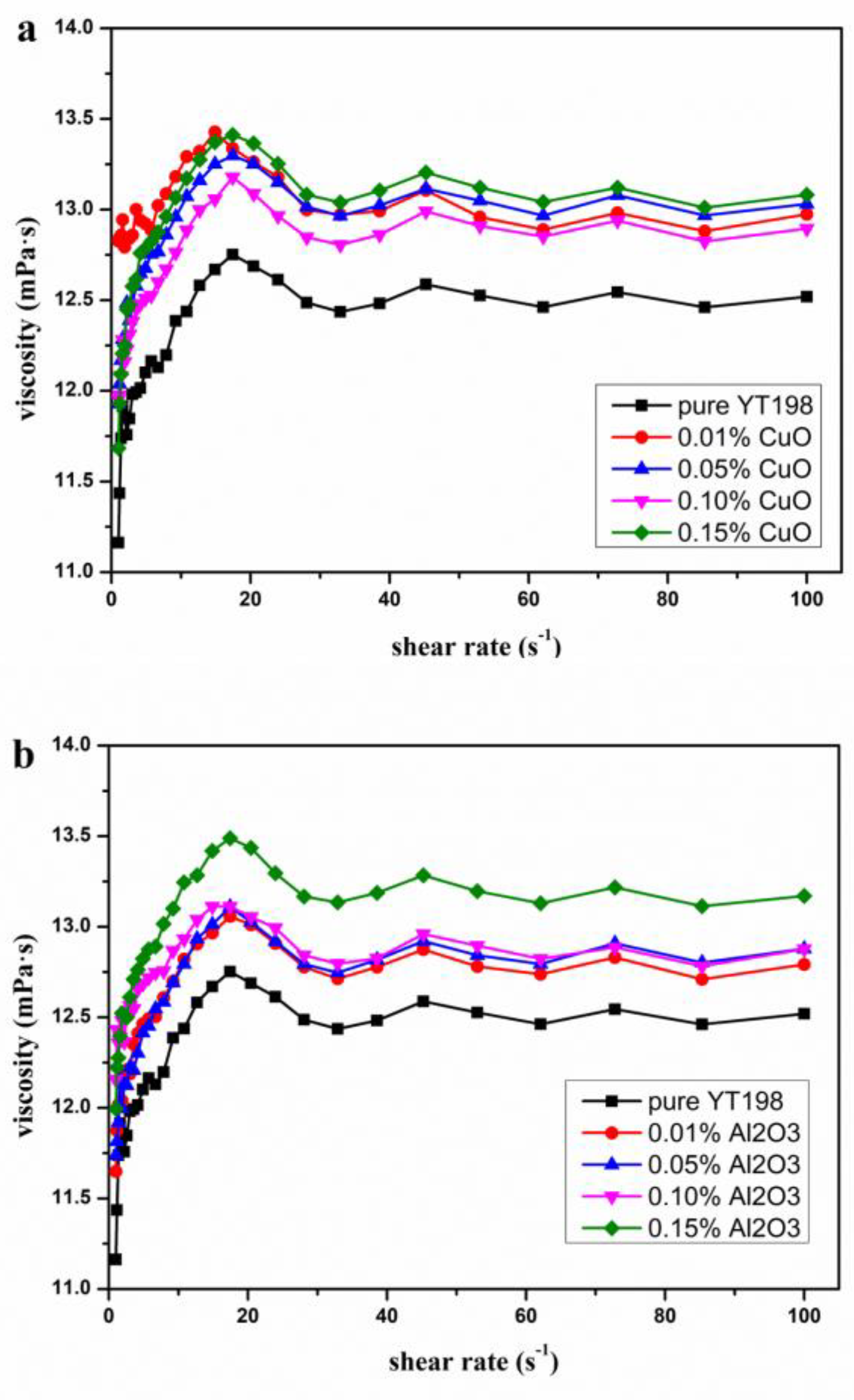
3.3. Rheological Properties of Various Nanofluids at Different Shear Rates
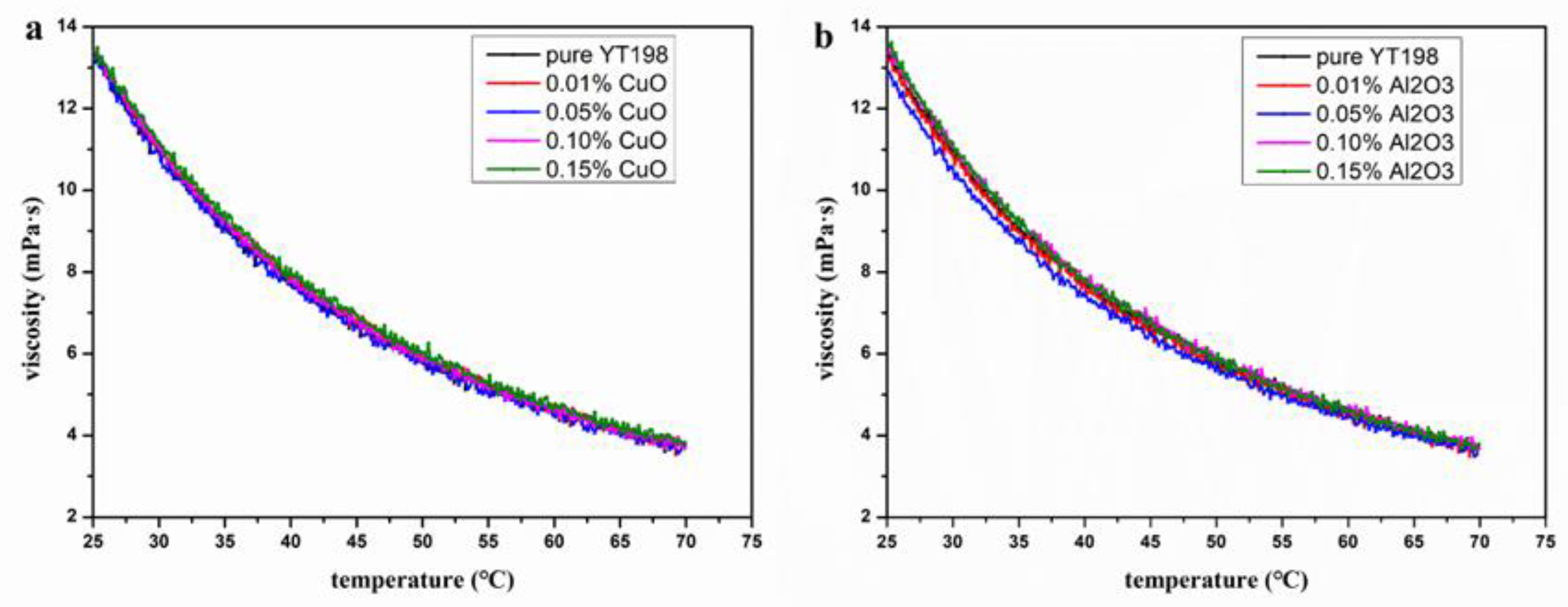
3.4. Effects of Temperature on Rheological Properties of Nanofluids
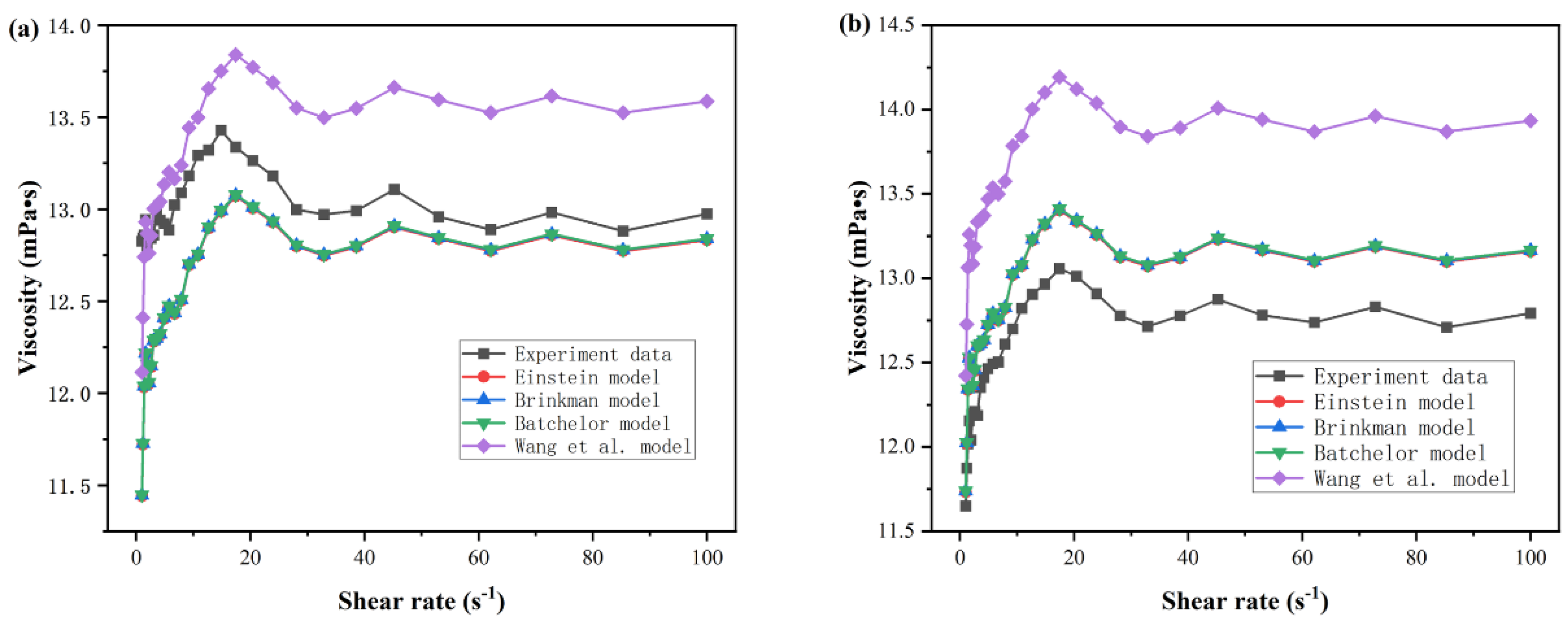
3.5. Derivation of Empirical Correlation
4. Conclusions
- There are several parameters that have influences on the effect of a nanofluid coolant, including but not limited to particle size and size distribution, particle shape and morphology, the concentration of nanoparticles and temperature. These factors must be comprehensively and adequately studied in future works.
- The stability of nanofluids is critical for their use in practical applications. Thus, more investigations about the surface modifications of nanoparticles and the use of surfactants should be conducted in order to determine an appropriate surfactant with remarkable stability, moderate viscosity and thermal conductivity.
- The long-term effects of using these nanofluids in cooling systems, such as corrosion or clogging, deserve more attention. A life cycle analysis (LCA) of cooling systems using nanofluids may be necessary to evaluate whether a nanofluid is suitable for long-term use.
- There are various models that can predict the thermal conductivity and viscosity of nanofluids separately. In future works, more mechanisms such as Brownian diffusion, particle aggregation, thermomigration and nanolayer formation can be taken into consideration. Therefore, models that will be developed in the future can be more accurate.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Patra, A.K.; Nayak, M.K.; Misra, A. Viscosity of nanofluids—A review. Int. J. Thermofluid Sci. Technol. 2020, 7, 070202. [Google Scholar] [CrossRef]
- Nabil, M.F.; Azmi, W.H.; Hamid, K.A.; Mamat, R.; Hagos, F.Y. An experimental study on the thermal conductivity and dynamic viscosity of TiO2-SiO2 nanofluids in water: Ethylene glycol mixture. Int. Commun. Heat Mass Transf. 2017, 86, 181–189. [Google Scholar] [CrossRef]
- Khodadadi, H.; Toghraie, D.; Karimipour, A. Effects of nanoparticles to present a statistical model for the viscosity of MgO-Water nanofluid. Powder Technol. 2019, 342, 166–180. [Google Scholar] [CrossRef]
- Saxena, G.; Raj, J. A critical review on applications of nano-fluid as coolant. Int. J. Eng. Manag. Res. 2017, 7, 304–311. [Google Scholar]
- Saravanakumar, P.T.; Surya, M.; Vijay, D.; Santhoshkumar, G. Improving performance in engine cooling system using nano fluids. Int. Res. J. Eng. Technol. 2017, 4, 1495–1499. [Google Scholar]
- Junior, L.C.C.; Nogueira, E. Influence of the coolant flow containing silver nanoparticles (Ag) from an aqueous solution based on ethylene glycol (EG50%) on the thermal-hydraulic performance of an automotive radiator. World J. Nano Sci. Eng. 2020, 10, 14. [Google Scholar] [CrossRef]
- Sandhya, M.; Ramasamy, D.; Sudhakar, K.; Kadirgama, K.; Harun, W.S.W. Hybrid nano-coolants in automotive heat transfer–an updated report. Maejo Int. J. Energy Environ. Commun. 2023, 2, 43–57. [Google Scholar] [CrossRef]
- Erkan, A.; Tüccar, G.; Tosun, E.; Özgür, T. Comparison of effects of nanofluid utilization (Al2O3, SiO2, TiO2) with reference water in automotive radiators on exergetic properties of diesel engines. SN Appl. Sci. 2021, 3, 365. [Google Scholar] [CrossRef]
- Sundari, K.G.; Asirvatham, L.G.; Ninolin, E.; Surekha, B. Feasibility of Glycerin/Al2O3 nanofluid for automotive cooling applications. J. Therm. Eng. 2020, 6, 619–632. [Google Scholar] [CrossRef]
- Halelfadl, S.; Mare, T.; Estelle, P. Efficiency of carbon nanotubes water based nanofluids as coolants. Exp. Therm. Fluid Sci. 2014, 53, 104–110. [Google Scholar] [CrossRef]
- Suganthi, K.S.; Rajan, K.S. Improved transient heat transfer performance of ZnO propylene glycol nanofluids for energy management. Energy Convers Manag. 2015, 96, 115–123. [Google Scholar] [CrossRef]
- Asadi, A.; Pourfattah, F. Heat transfer performance of two oil-based nanofluids containing ZnO and MgO nanoparticles: A comparative experimental investigation. Powder Technol. 2019, 343, 296–308. [Google Scholar] [CrossRef]
- Guo, W.; Li, G.; Zheng, Y.; Dong, C. Measurement of the thermal conductivity of SiO2 nanofluids with an optimized transient hot wire method. Thermochim. Acta 2018, 661, 84–97. [Google Scholar] [CrossRef]
- Abdolbaqi, M.K.; Sidik, N.A.C.; Rahim, M.F.A.; Mamat, R.; Azmi, W.H. Experimental investigation and development of new correlation for thermal conductivity and viscosity of BioGlycol/water based SiO2 nanofluids. Int. Commun. Heat Mass Transf. 2016, 77, 54–63. [Google Scholar] [CrossRef]
- Krishnakumar, T.S.; Sheeba, A.; Mahesh, V.; Jose, P.M. Heat transfer studies on ethylene glycol/water nanofluid containing TiO2 nanoparticles. Int. J. Refrig. 2019, 102, 55–61. [Google Scholar] [CrossRef]
- Perez-Tavernier, J.; Vallejo, J.P.; Cabaleiro, D.; Fernandez-Seara, J.; Lugo, L. Heat transfer performance of a nano-enhanced propylene glycol: Water mixture. Int. J. Therm. Sci. 2019, 139, 413–423. [Google Scholar] [CrossRef]
- Yashawantha, K.M.; Vinod, A.V. ANN modelling and experimental investigation on effective thermal conductivity of ethylene glycol: Water nanofluids. J. Therm. Anal. Calorim. 2020, 145, 609–630. [Google Scholar] [CrossRef]
- Leong, K.Y.; Saidur, R.; Kazi, S.N.; Mamun, A.H. Performance investigation of an automotive car radiator operated with nanofluid-based coolants (nanofluid as a coolant in a radiator). Appl. Therm. Eng. 2010, 30, 2685–2692. [Google Scholar] [CrossRef]
- Subhedar, D.G.; Ramani, B.M.; Gupta, A. Experimental investigation of heat transfer potential of Al2O3/Water-Mono Ethylene Glycol nanofluids as a car radiator coolant. Case Stud. Therm. Eng. 2018, 11, 26–34. [Google Scholar] [CrossRef]
- Mhamed, B.; Sidik, N.A.; Akhbar, M.F.A.; Mamat, R.; Najafi, G. Experimental study on thermal performance of MWCNT nanocoolant in Perodua Kelisa 1000cc radiator system. Int. Commun. Heat Mass Transf. 2016, 76, 156–161. [Google Scholar] [CrossRef]
- Goudarzi, K.; Jamali, H. Heat transfer enhancement of Al2O3-EG nanofluid in a car radiator with wire coil inserts. Appl. Therm. Eng. 2017, 118, 510–517. [Google Scholar] [CrossRef]
- Kumar, A.; Hassan, M.A.; Chand, P. Heat transport in nanofluid coolant car radiator with louvered fins. Powder Technol. 2020, 376, 631–642. [Google Scholar] [CrossRef]
- Said, Z.; El Haj Assad, M.; Hachicha, A.A.; Bellos, E.; Abdelkareem, M.A.; Alazaizeh, D.Z. Enhancing the performance of automotive radiators using nanofluids. Renew Sustain. Energy Rev. 2019, 112, 183–194. [Google Scholar] [CrossRef]
- Tijani, A.S.; Sudirman, A.S. Thermos-physical properties and heat transfer characteristics of water/anti-freezing and Al2O3/CuO based nanofluid as a coolant for car radiator. Int. J. Heat Mass Transf. 2018, 118, 48–57. [Google Scholar] [CrossRef]
- Muruganandam, M.; Mukesh Kumar, P.C. Experimental analysis on internal combustion engine using MWCNT/water nanofluid as a coolant. Mater. Today Proc. 2020, 21, 248–252. [Google Scholar] [CrossRef]
- Behrangzade, A.; Heyhat, M.M. The effect of using nano-silver dispersed water based nanofluid as a passive method for energy efficiency enhancement in a plate heat exchanger. Appl. Therm. Eng. 2016, 102, 311–317. [Google Scholar] [CrossRef]
- Hassani, S.M.; Khoshvaght, A.M.; Mazloumi, S.H. Influence of chevron fin interruption on thermo-fluidic transport character-istics of nanofluid-cooled electronic heat sink. Chem. Eng. Sci. 2018, 191, 436–447. [Google Scholar] [CrossRef]
- Nguyen, C.T.; Roy, G.; Gauthier, C.; Galanis, N. Heat transfer enhancement using Al2O3–water nanofluid for an electronic liquid cooling system. Appl. Therm. Eng. 2007, 27, 1501–1506. [Google Scholar] [CrossRef]
- Chein, R.; Chuang, J. Experimental microchannel heat sink performance studies using nanofluids. Int. J. Therm. Sci. 2007, 46, 157–166. [Google Scholar] [CrossRef]
- Sarafraz, M.M.; Arya, A.; Hormozi, F.; Nikkhah, V. On the convective thermal performance of a CPU cooler working with liquid gallium and CuO/water nanofluid: A comparative study. Appl. Therm. Eng. 2017, 112, 1373–1381. [Google Scholar] [CrossRef]
- Sun, S.; Jin, X.; Liu, C.; Meng, Q.; Zhang, Y. Study on in-situ measurement method of THF hydrate thermal conductivity. Int. J. Heat Mass Transf. 2018, 127, 88–96. [Google Scholar] [CrossRef]
- Gu, C.; Lv, Y.; Fan, X.; Zhao, C.; Dai, C.; Zhao, G. Study on rheology and microstructure of phenolic resin cross-linked nonionic polyacrylamide (NPAM) gel for profile control and water shutoff treatments. J. Pet. Sci. Eng. 2018, 169, 546–552. [Google Scholar] [CrossRef]
- Amaral, C.; Pinto, S.; Silva, T. Development of polyurethane foam incorporating phase change material for thermal energy storage. J. Energy Storage 2020, 28, 101177. [Google Scholar] [CrossRef]
- Alex, R.; Alexander, M.T.; Gabriel, F.; Gaurav, S.; Laurent, P. Thermal conductivity of cementitious composites containing microencapsulated phase change materials. Int. J. Heat Mass Transf. 2017, 104, 71–82. [Google Scholar]
- Maxim, P.; Daniil, R.; Pavel, S. Stability and rheology of carbon-containing composite liquid fuels under subambient temperatures. Energy 2023, 278, 127912. [Google Scholar]
- Theodorsson, E. Uncertainty in measurement and total error: Tools for coping with diagnostic uncertainty. Clin. Lab. Med. 2017, 37, 15–34. [Google Scholar] [CrossRef] [PubMed]
- Tehmina, A.; Man-Hoe, K. Influence of particle size on the effective thermal conductivity of nanofluids: A critical review. Appl. Energy 2020, 264, 114684. [Google Scholar]
- Nishant, K.; Shiriram, S. Experimental study of thermal conductivity and convective heat transfer enhancement using CuO and TiO2 nanoparticles. Int. Commun. Heat Mass 2016, 76, 98–107. [Google Scholar]
- Durga, B.J.; Tami, S.G.; Subashini, G.; Saravanan, P.; Muthumareeswaran, M.; Abdullah, N.A.; Nirmala, G.A. Ultrasonic Interferometry and Physiothermal properties of Al2O3/CuO nanofluids. Case Stud. Therm. Eng. 2024, 55, 104120. [Google Scholar]
- Dan, L.; Zhang, K.; Wang, Q.; Liu, N. Surface modification boosts dispersion stability of nanoparticles in dielectric fluids. J. Ind. Eng. Chem. 2024, 132, 518–528. [Google Scholar] [CrossRef]
- Younes, H.; Mao, M.; Murshed, S.S.; Lou, D.; Hong, H.; Peterson, G.P. Peterson. Nanofluids: Key parameters to enhance thermal conductivity and its applications. Appl. Therm. Eng. 2022, 10, 118202. [Google Scholar] [CrossRef]
- Yılmaz, D.A.; Gürü, M. Nanofluids: Preparation, stability, properties, and thermal performance in terms of thermo-hydraulic, thermodynamics and thermo-economic analysis. J. Therm. Anal. Calorim. 2022, 147, 7631–7664. [Google Scholar] [CrossRef]
- Sun, L.; Zhang, Y.; Gao, W.; Li, X.; Jing, D. Rheology of methanol-based metal oxide nanofluids: The effect of temperature and particle type and mass fraction. Interfacial Phenom. Heat Transf. 2020, 8, 165–181. [Google Scholar] [CrossRef]
- Sun, L.; Zhao, Q.; Zhang, Y.; Gao, W.; Jing, D. Insights into the rheological behavior of ethanol-based metal oxide nanofluids. J. Mol. Liq. 2021, 323, 115006. [Google Scholar] [CrossRef]
- Amir, Y.B.; Adnan, Q. Viscosity of CuO nanofluids: Experimental investigation and modelling with FFBP-ANN. Thermochim. Acta 2022, 714, 179267. [Google Scholar]
- Kole, M.; Dey, T.K. Viscosity of alumina nanoparticles dispersed in car engine coolant. Exp. Therm. Fluid Sci. 2010, 34, 677–683. [Google Scholar] [CrossRef]
- Chen, Y.; Huang, Y.; Li, K. Temperature effect on the aggregation kinetics of CeO2 nanoparticles in monovalent and divalent electrolytes. J. Environ. Anal. Toxicol. 2012, 2, 158–162. [Google Scholar]
- Maxwell, J. A physical treatise on electricity and magnetism. J. Frankl. Inst. 1881, 111, 386–389. [Google Scholar]
- Einstein, A. Eine neue bestimmung der moleküldimensionen. Ann. Phys. 1906, 324, 289–306. [Google Scholar] [CrossRef]
- Brinkman, H. The viscosity of concentrated suspensions and solutions. J. Chem. Phys. 1952, 20, 571. [Google Scholar] [CrossRef]
- Batchelor, G.K. The effect of Brownian motion on the bulk stress in a suspension of spherical particles. J. Fluid Mech. 1977, 83, 97. [Google Scholar] [CrossRef]
- Wang, X.; Xu, X.; Choi, S.U. Thermal conductivity of nanoparticle-fluid mixture. J. Thermophys. Heat Transf. 1999, 13, 474–480. [Google Scholar] [CrossRef]







| Density (20 °C) | Pour Point | Flash Point | Acidity | Specific Heat (40 °C) |
|---|---|---|---|---|
| 804.3 kg/m3 | −38 °C | 198 °C | 0.03 mgKOH/g | 2.089 KJ/kg·K |
| Breakdown Voltage | Relative Permittivity (90 °C) | Volume Resistivity (20 °C) | Surface Tension | Global Warming Potential |
| 62 KV | 2.039 | 1.9 × 1010 Ω·cm | 16 mN/m | 0 |
| Nanoparticle | Particle Size | Density (20 °C) | Melting Point | Boiling Point |
|---|---|---|---|---|
| CuO | 40 nm | 6.315 g/cm3 | 1326 °C | 1026 °C |
| Al2O3 | 80 nm | 1.06 g/cm3 | 2000 °C | 2977 °C |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, L.; Geng, J.; Dong, K.; Sun, Q. An Experimental Study on the Effect of Nanofluids on the Thermal Conductivity and Rheological Properties of a Coolant for Liquids. Energies 2024, 17, 1313. https://doi.org/10.3390/en17061313
Sun L, Geng J, Dong K, Sun Q. An Experimental Study on the Effect of Nanofluids on the Thermal Conductivity and Rheological Properties of a Coolant for Liquids. Energies. 2024; 17(6):1313. https://doi.org/10.3390/en17061313
Chicago/Turabian StyleSun, Le, Jiafeng Geng, Kaijun Dong, and Qin Sun. 2024. "An Experimental Study on the Effect of Nanofluids on the Thermal Conductivity and Rheological Properties of a Coolant for Liquids" Energies 17, no. 6: 1313. https://doi.org/10.3390/en17061313
APA StyleSun, L., Geng, J., Dong, K., & Sun, Q. (2024). An Experimental Study on the Effect of Nanofluids on the Thermal Conductivity and Rheological Properties of a Coolant for Liquids. Energies, 17(6), 1313. https://doi.org/10.3390/en17061313






