Municipal Plastic Waste Recycling through Pyrogasification
Abstract
1. Introduction
2. Fundamentals on Pyrolysis
2.1. Properties of Feedstock: HDPE, LDPE, PP, PS, PET, PVC
| Plastic Waste | Proximate Analysis (wt% 1) | Ultimate Analysis (wt% 2) | HHV (MJ/kg) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| M | VM | FC | A | C | H | O 3 | N | S | Cl | Ref | Ref. | ||
| HDPE | 0.2 | 99.1 | 0 | 0.6 | 86.0 | 13.8 | 0.1 | 0 | 0.1 | 0 | [29,30] | 44.6 | [29,31,32,33] |
| LDPE | 0 | 99.5 | 0 | 0.5 | 86.1 | 13.9 | 0 | 0 | 0 | 0 | [34,35] | 45.5 | |
| PP | 0.1 | 95.3 | 0.8 | 2.7 | 83.8 | 14.4 | 0.1 | 0.3 | 0.9 | 0.5 | [29,36] | 45.6 | |
| PS | 0 | 99.7 | 0.3 | 0 | 91.1 | 8.2 | 0.4 | 0.1 | 0.2 | 0 | [37,38] | 41.5 | |
| PET | 0 | 89.5 | 8.5 | 0 | 63.6 | 4.1 | 32.3 | 0 | 0 | 0 | [29,39] | 22.8 | |
| PVC | 0 | 95.5 | 4.6 | 0 | 38.5 | 4.6 | 0 | 0.1 | 0.3 | 56.5 | [40,41] | 20.1 | |
| Domestic | - | 93.4 | 5.3 | 1.2 | 84.4 | 12.4 | 0.1 | 2.7 | 0.4 | 0 | [27] | 40.4 | [27] |
2.2. Reactors
2.3. Operational Conditions
3. Case Scenario
3.1. Input Data
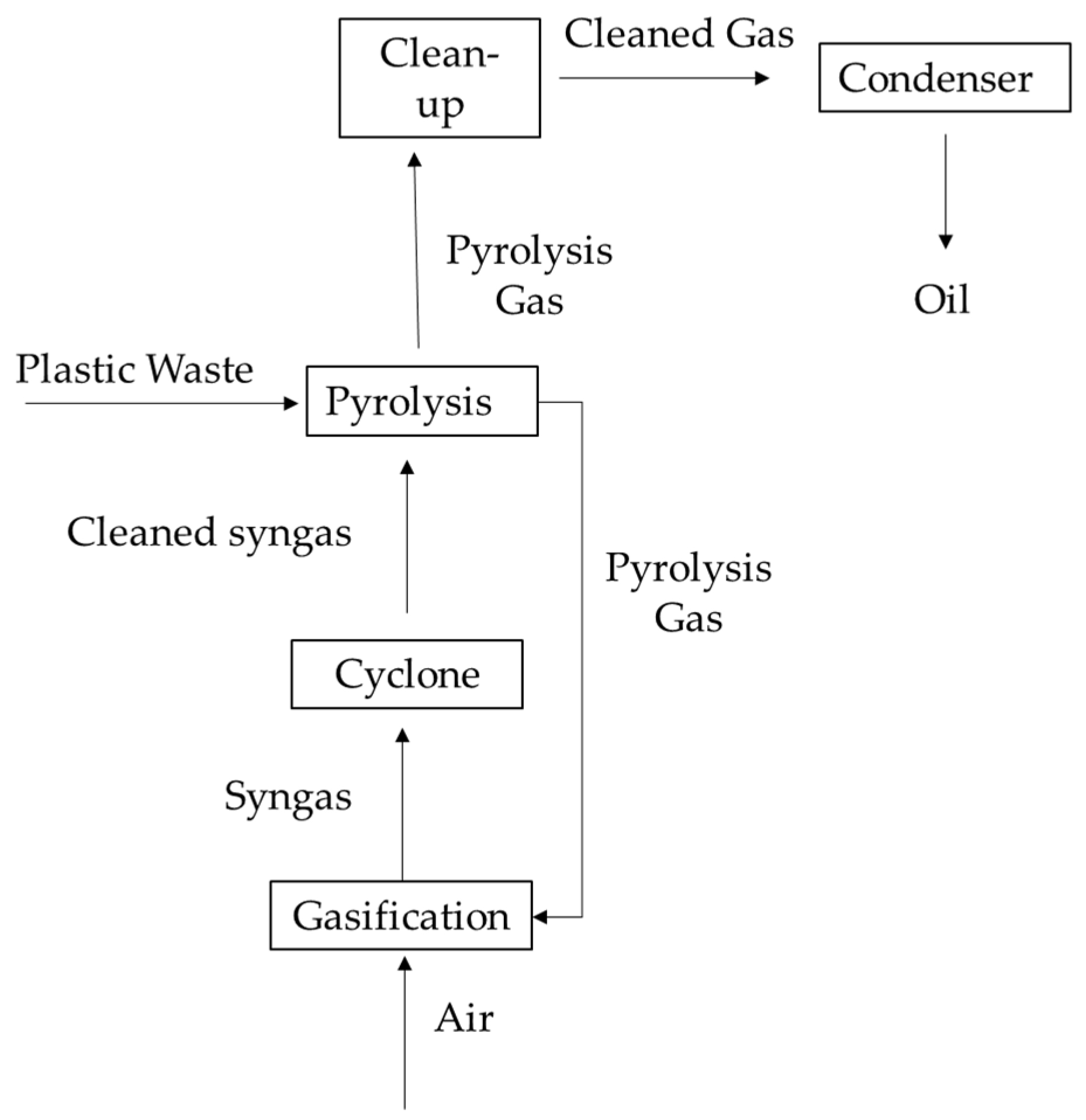
3.2. Description of the Technology
3.3. Definition and Quantification of Products
- -
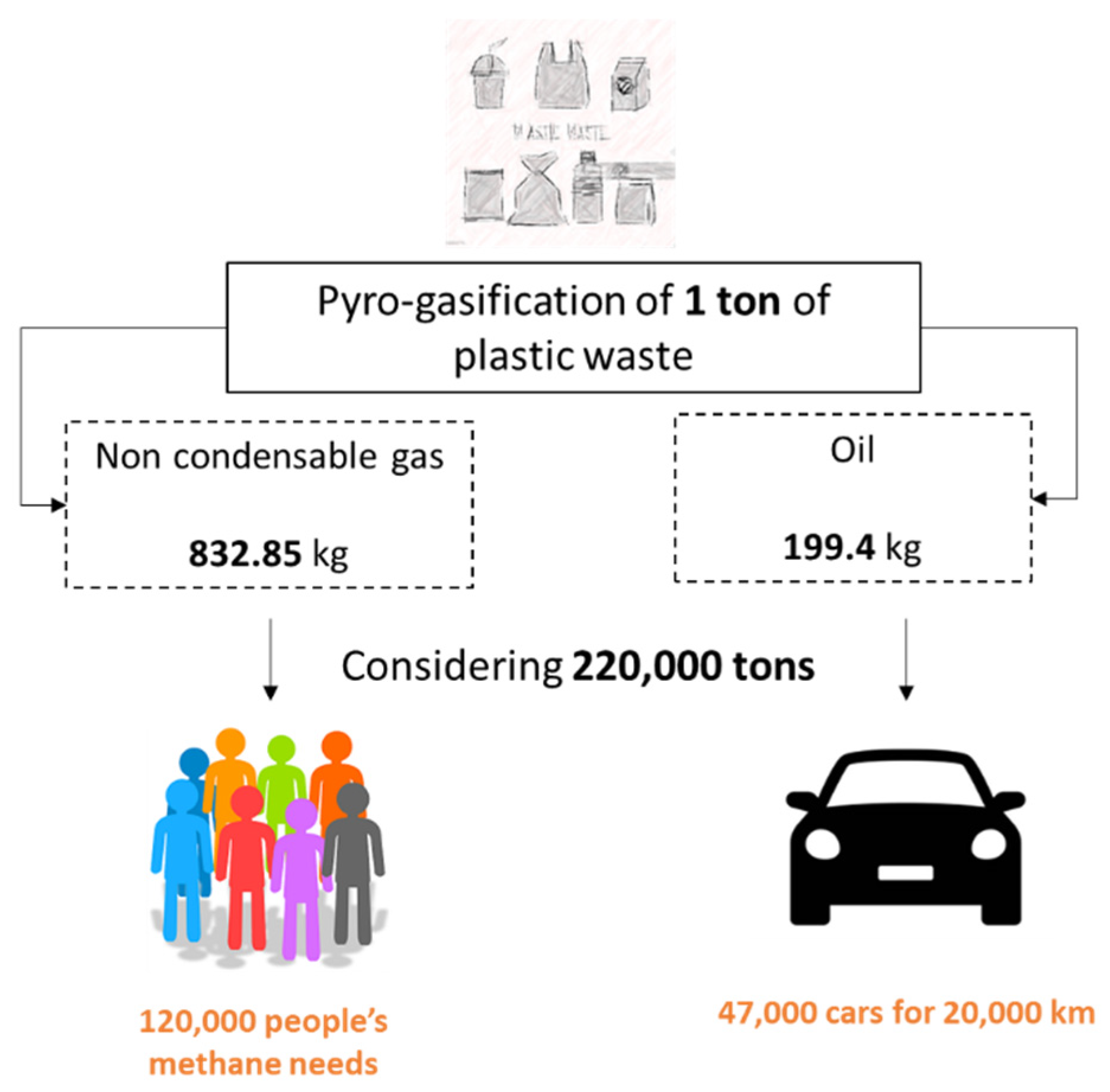
- A total of 183.227 tons of green gas (calorific value of 16 MJ/kg (corresponding to 0.0044 MWh/kg)), which can generate 0.81 million MWh, providing 44% of the initial thermal energy contained in the feedstock. Methane’s heating value is 40 MJ/m3 [84], equivalent to 0.0111 MWh/m3, which correspond to 73 million m3 of methane. Considering that a family composed of 2.3 people (https://www.istat.it/en, accessed on 10 January 2024) consumes 1400 m3 of methane in one year (https://www.arera.it/en, accessed on 10 January 2024), the production of green gas would satisfy the annual methane need of 120,000 inhabitants.
- -
- Approximately 43.9 thousand tons of liquid green fuel are generated, which can provide 0.57 million MWh, representing 30% of the initial thermal energy in the feedstock. If the produced liquid has a density equal to that of diesel, 0.85 kg/L [85], a total of 52.6 million liters can be produced, corresponding to the consumption of over 47,000 cars (for approximately 70,000 inhabitants) traveling 20,000 km per year at 18 km/L (estimated from https://www.quattroruote.it/magazine/, accessed on 10 January 2024)
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- COREPLA Relazione Sulla Gestione 2022. Available online: www.corepla.it (accessed on 12 December 2023).
- Sardon, H.; Dove, A.P. Plastics Recycling with a Difference. Science 2018, 360, 380–381. [Google Scholar] [CrossRef]
- Gwada, B.; Ogendi, G.; Makindi, S.M.; Trott, S. Composition of Plastic Waste Discarded by Households and Its Management Approaches. Glob. J. Environ. Sci. Manag. 2019, 5, 83–94. [Google Scholar]
- Kosior, E.; Mitchell, J. Current Industry Position on Plastic Production and Recycling. In Plastic Waste and Recycling; Elsevier: Amsterdam, The Netherlands, 2020; pp. 133–162. [Google Scholar]
- Lanorte, A.; De Santis, F.; Nolè, G.; Blanco, I.; Loisi, R.V.; Schettini, E.; Vox, G. Agricultural Plastic Waste Spatial Estimation by Landsat 8 Satellite Images. Comput. Electron. Agric. 2017, 141, 35–45. [Google Scholar] [CrossRef]
- Benson, N.U.; Fred-Ahmadu, O.H.; Bassey, D.E.; Atayero, A.A. COVID-19 Pandemic and Emerging Plastic-Based Personal Protective Equipment Waste Pollution and Management in Africa. J. Environ. Chem. Eng. 2021, 9, 105222. [Google Scholar] [CrossRef]
- Ellen MacArthur More Plastic than Fish in the Sea by 2050. Available online: https://www.ellenmacarthurfoundation.org/ (accessed on 10 January 2024).
- Huang, J.; Chen, H.; Zheng, Y.; Yang, Y.; Zhang, Y.; Gao, B. Microplastic Pollution in Soils and Groundwater: Characteristics, Analytical Methods and Impacts. Chem. Eng. J. 2021, 425, 131870. [Google Scholar] [CrossRef]
- Guo, J.-J.; Huang, X.-P.; Xiang, L.; Wang, Y.-Z.; Li, Y.-W.; Li, H.; Cai, Q.-Y.; Mo, C.-H.; Wong, M.-H. Source, Migration and Toxicology of Microplastics in Soil. Environ. Int. 2020, 137, 105263. [Google Scholar] [CrossRef]
- Mazhandu, Z.S.; Muzenda, E.; Mamvura, T.A.; Belaid, M.; Nhubu, T. Integrated and Consolidated Review of Plastic Waste Management and Bio-Based Biodegradable Plastics: Challenges and Opportunities. Sustainability 2020, 12, 8360. [Google Scholar] [CrossRef]
- Directive 2008/98/EC; European Parliament and of the Council of 19 November 2008 on Waste and Repealing Certain Directives. European Union: Brussels, Belgium, 2018.
- Faheem, S.M.; Khan, M.A. Waste Management Methods and Sustainablity. In Advances in Bioprocess Technology; Springer International Publishing: Cham, Germany, 2015; pp. 57–78. [Google Scholar]
- Kibria, M.d.G.; Masuk, N.I.; Safayet, R.; Nguyen, H.Q.; Mourshed, M. Plastic Waste: Challenges and Opportunities to Mitigate Pollution and Effective Management. Int. J. Environ. Res. 2023, 17, 20. [Google Scholar] [CrossRef]
- Directive (EU) 2018; European Parliament and of the Council of 30 May 2018 Amending Directive 1999/31/EC on the Landfill of Waste. European Union: Brussels, Belgium, 2018.
- Li, X.; Wang, J.; Zhang, T.; Yang, S.; Sun, M.; Qian, X.; Wang, T.; Zhao, Y. Sustainable Catalytic Strategies for the Transformation of Plastic Wastes into Valued Products. Chem. Eng. Sci. 2023, 276, 118729. [Google Scholar] [CrossRef]
- Chang, S.H. Plastic Waste as Pyrolysis Feedstock for Plastic Oil Production: A Review. Sci. Total Environ. 2023, 877, 162719. [Google Scholar] [CrossRef]
- Armenise, S.; SyieLuing, W.; Ramírez-Velásquez, J.M.; Launay, F.; Wuebben, D.; Ngadi, N.; Rams, J.; Muñoz, M. Plastic Waste Recycling via Pyrolysis: A Bibliometric Survey and Literature Review. J. Anal. Appl. Pyrolysis 2021, 158, 105265. [Google Scholar] [CrossRef]
- Huang, J.; Veksha, A.; Chan, W.P.; Giannis, A.; Lisak, G. Chemical Recycling of Plastic Waste for Sustainable Material Management: A Prospective Review on Catalysts and Processes. Renew. Sustain. Energy Rev. 2022, 154, 111866. [Google Scholar] [CrossRef]
- Sharma, D.K.; Bapat, S.; Brandes, W.F.; Rice, E.; Castaldi, M.J. Technical Feasibility of Zero Waste for Paper and Plastic Wastes. Waste Biomass Valorization 2019, 10, 1355–1363. [Google Scholar] [CrossRef]
- Uthpalani, P.G.I.; Premachandra, J.K.; De Silva, D.S.M.; Weerasinghe, V.P.A. Pyrolysis as a Value Added Method for Plastic Waste Management: A Review on Converting LDPE and HDPE Waste into Fuel. Ceylon J. Sci. 2023, 52, 277–296. [Google Scholar] [CrossRef]
- Moorthy Rajendran, K.; Chintala, V.; Sharma, A.; Pal, S.; Pandey, J.K.; Ghodke, P. Review of Catalyst Materials in Achieving the Liquid Hydrocarbon Fuels from Municipal Mixed Plastic Waste (MMPW). Mater. Today Commun. 2020, 24, 100982. [Google Scholar] [CrossRef]
- Achilias, D.S.; Roupakias, C.; Megalokonomos, P.; Lappas, A.A.; Antonakou, Ε.V. Chemical Recycling of Plastic Wastes Made from Polyethylene (LDPE and HDPE) and Polypropylene (PP). J. Hazard. Mater. 2007, 149, 536–542. [Google Scholar] [CrossRef] [PubMed]
- Sakhiya, A.K.; Anand, A.; Kaushal, P. Production, Activation, and Applications of Biochar in Recent Times. Biochar 2020, 2, 253–285. [Google Scholar] [CrossRef]
- Nanda, S.; Berruti, F. Thermochemical Conversion of Plastic Waste to Fuels: A Review. Environ. Chem. Lett. 2021, 19, 123–148. [Google Scholar] [CrossRef]
- Matuszewska, A.; Owczuk, M.; Biernat, K. Current Trends in Waste Plastics’ Liquefaction into Fuel Fraction: A Review. Energies 2022, 15, 2719. [Google Scholar] [CrossRef]
- Kumar, S.; Panda, A.K.; Singh, R.K. A Review on Tertiary Recycling of High-Density Polyethylene to Fuel. Resour. Conserv. Recycl. 2011, 55, 893–910. [Google Scholar] [CrossRef]
- Ghodke, P.K.; Sharma, A.K.; Moorthy, K.; Chen, W.H.; Patel, A.; Matsakas, L. Experimental Investigation on Pyrolysis of Domestic Plastic Wastes for Fuel Grade Hydrocarbons. Processes 2023, 11, 71. [Google Scholar] [CrossRef]
- Jouhara, H.; Ahmad, D.; van den Boogaert, I.; Katsou, E.; Simons, S.; Spencer, N. Pyrolysis of Domestic Based Feedstock at Temperatures up to 300 °C. Therm. Sci. Eng. Prog. 2018, 5, 117–143. [Google Scholar] [CrossRef]
- Chattopadhyay, J.; Pathak, T.S.; Srivastava, R.; Singh, A.C. Catalytic Co-Pyrolysis of Paper Biomass and Plastic Mixtures (HDPE (High Density Polyethylene), PP (Polypropylene) and PET (Polyethylene Terephthalate)) and Product Analysis. Energy 2016, 103, 513–521. [Google Scholar] [CrossRef]
- Baloch, H.A.; Siddiqui, M.T.H.; Nizamuddin, S.; Mubarak, N.M.; Khalid, M.; Srinivasan, M.P.; Griffin, G.J. Solvothermal Co-Liquefaction of Sugarcane Bagasse and Polyethylene under Sub-Supercritical Conditions: Optimization of Process Parameters. Process Saf. Environ. Prot. 2020, 137, 300–311. [Google Scholar] [CrossRef]
- Özsin, G.; Pütün, A.E. A Comparative Study on Co-Pyrolysis of Lignocellulosic Biomass with Polyethylene Terephthalate, Polystyrene, and Polyvinyl Chloride: Synergistic Effects and Product Characteristics. J. Clean. Prod. 2018, 205, 1127–1138. [Google Scholar] [CrossRef]
- Suthar, M. Plastic Waste as an Alternate Fuel. Int. J. Eng. Res. 2020, V9, 1254–1261. [Google Scholar] [CrossRef]
- Das, P.; Tiwari, P. The Effect of Slow Pyrolysis on the Conversion of Packaging Waste Plastics (PE and PP) into Fuel. Waste Manag. 2018, 79, 615–624. [Google Scholar] [CrossRef] [PubMed]
- Yu, D.; Hui, H.; Ding, G.; Dong, N.; Li, S. Enhancement of Aromatics Production from Catalytic Co-Pyrolysis of Walnut Shell and LDPE via a Two-Step Approach. J. Anal. Appl. Pyrolysis 2021, 157, 105216. [Google Scholar] [CrossRef]
- Zheng, Y.; Li, D.; Pei, T.; Wang, J.; Liu, C.; Lu, Y.; Lin, X.; Li, J.; Zheng, Z. Mechanism of Synergistic Effects and Kinetic Analysis in Bamboo-LDPE Waste Ex-Situ Catalytic Co-Pyrolysis for Enhanced Aromatic Hydrocarbon Production via CeZrAl and HZSM-5 Dual Catalyst. J. Environ. Chem. Eng. 2022, 10, 107479. [Google Scholar] [CrossRef]
- Hakeem, I.G.; Aberuagba, F.; Musa, U. Catalytic Pyrolysis of Waste Polypropylene Using Ahoko Kaolin from Nigeria. Appl. Petrochem. Res. 2018, 8, 203–210. [Google Scholar] [CrossRef]
- Ahmad, N.; Ahmad, N.; Maafa, I.M.; Ahmed, U.; Akhter, P.; Shehzad, N.; Amjad, U.; Hussain, M. Thermal Conversion of Polystyrene Plastic Waste to Liquid Fuel via Ethanolysis. Fuel 2020, 279, 118498. [Google Scholar] [CrossRef]
- van der Westhuizen, S.; Collard, F.; Görgens, J. Pyrolysis of Waste Polystyrene into Transportation Fuel: Effect of Contamination on Oil Yield and Production at Pilot Scale. J. Anal. Appl. Pyrolysis 2022, 161, 105407. [Google Scholar] [CrossRef]
- Wang, Z.; Burra, K.G.; Li, X.; Zhang, M.; He, X.; Lei, T.; Gupta, A.K. CO2-Assisted Gasification of Polyethylene Terephthalate with Focus on Syngas Evolution and Solid Yield. Appl. Energy 2020, 276, 115508. [Google Scholar] [CrossRef]
- Ephraim, A.; Pham Minh, D.; Lebonnois, D.; Peregrina, C.; Sharrock, P.; Nzihou, A. Co-Pyrolysis of Wood and Plastics: Influence of Plastic Type and Content on Product Yield, Gas Composition and Quality. Fuel 2018, 231, 110–117. [Google Scholar] [CrossRef]
- Zhou, H.; Wu, C.; Onwudili, J.A.; Meng, A.; Zhang, Y.; Williams, P.T. Effect of Interactions of PVC and Biomass Components on the Formation of Polycyclic Aromatic Hydrocarbons (PAH) during Fast Co-Pyrolysis. RSC Adv. 2015, 5, 11371–11377. [Google Scholar] [CrossRef]
- Parku, G.K.; Collard, F.-X.; Görgens, J.F. Pyrolysis of Waste Polypropylene Plastics for Energy Recovery: Influence of Heating Rate and Vacuum Conditions on Composition of Fuel Product. Fuel Process. Technol. 2020, 209, 106522. [Google Scholar] [CrossRef]
- Gopalakrishnan, B.; Khanna, N.; Das, D. Dark-Fermentative Biohydrogen Production; Elsevier: Amsterdam, The Netherlands, 2019; ISBN 9780444642035. [Google Scholar]
- Han, J.; Yao, X.; Zhan, Y.; Oh, S.-Y.; Kim, L.-H.; Kim, H.-J. A Method for Estimating Higher Heating Value of Biomass-Plastic Fuel. J. Energy Inst. 2017, 90, 331–335. [Google Scholar] [CrossRef]
- Li, S.; Cañete Vela, I.; Järvinen, M.; Seemann, M. Polyethylene Terephthalate (PET) Recycling via Steam Gasification—The Effect of Operating Conditions on Gas and Tar Composition. Waste Manag. 2021, 130, 117–126. [Google Scholar] [CrossRef]
- Yu, J.; Sun, L.; Ma, C.; Qiao, Y.; Yao, H. Thermal Degradation of PVC: A Review. Waste Manag. 2016, 48, 300–314. [Google Scholar] [CrossRef]
- Starnes, W.H. Structural Defects in Poly(Vinyl Chloride). J. Polym. Sci. A Polym. Chem. 2005, 43, 2451–2467. [Google Scholar] [CrossRef]
- Esmizadeh, E.; Tzoganakis, C.; Mekonnen, T.H. Degradation Behavior of Polypropylene during Reprocessing and Its Biocomposites: Thermal and Oxidative Degradation Kinetics. Polymers 2020, 12, 1627. [Google Scholar] [CrossRef]
- Li, H.; Mašek, O.; Harper, A.; Ocone, R. Kinetic Study of Pyrolysis of High-density Polyethylene (HDPE) Waste at Different Bed Thickness in a Fixed Bed Reactor. Can. J. Chem. Eng. 2021, 99, 1733–1744. [Google Scholar] [CrossRef]
- Al-Salem, S.M.; Dutta, A. Wax Recovery from the Pyrolysis of Virgin and Waste Plastics. Ind. Eng. Chem. Res. 2021, 60, 8301–8309. [Google Scholar] [CrossRef]
- Kodera, Y.; Ishihara, Y.; Kuroki, T. Novel Process for Recycling Waste Plastics To Fuel Gas Using a Moving-Bed Reactor. Energy Fuels 2006, 20, 155–158. [Google Scholar] [CrossRef]
- Kaminsky, W. Chemical Recycling of Plastics by Fluidized Bed Pyrolysis. Fuel Commun. 2021, 8, 100023. [Google Scholar] [CrossRef]
- Almohamadi, H.; Alamoudi, M.; Ahmed, U.; Shamsuddin, R.; Smith, K. Producing Hydrocarbon Fuel from the Plastic Waste: Techno-Economic Analysis. Korean J. Chem. Eng. 2021, 38, 2208–2216. [Google Scholar] [CrossRef]
- Hasan, M.M.; Rasul, M.G.; Khan, M.M.K.; Ashwath, N.; Jahirul, M.I. Energy Recovery from Municipal Solid Waste Using Pyrolysis Technology: A Review on Current Status and Developments. Renew. Sustain. Energy Rev. 2021, 145, 111073. [Google Scholar] [CrossRef]
- Arabiourrutia, M.; Elordi, G.; Lopez, G.; Borsella, E.; Bilbao, J.; Olazar, M. Characterization of the Waxes Obtained by the Pyrolysis of Polyolefin Plastics in a Conical Spouted Bed Reactor. J. Anal. Appl. Pyrolysis 2012, 94, 230–237. [Google Scholar] [CrossRef]
- Aguado, J.; Serrano, D.P.; Escola, J.M.; Garagorri, E. Catalytic Conversion of Low-Density Polyethylene Using a Continuous Screw Kiln Reactor. Catal. Today 2002, 75, 257–262. [Google Scholar] [CrossRef]
- Miandad, R.; Rehan, M.; Nizami, A.S.; El-Fetouh Barakat, M.A.; Ismail, I.M. Recycling of Solid Waste for Biofuels and Bio-Chemicals; Karthikeyan, O.P., Heimann, K., Muthu, S.S., Eds.; Springer: Singapore, 2016; ISBN 978-981-10-0148-2. [Google Scholar]
- Czajczyńska, D.; Anguilano, L.; Ghazal, H.; Krzyżyńska, R.; Reynolds, A.J.; Spencer, N.; Jouhara, H. Potential of Pyrolysis Processes in the Waste Management Sector. Therm. Sci. Eng. Prog. 2017, 3, 171–197. [Google Scholar] [CrossRef]
- Kumar, S.; Singh, R.K. Recovery of Hydrocarbon Liquid from Waste High Density Polyethylene by Thermal Pyrolysis. Braz. J. Chem. Eng. 2011, 28, 659–667. [Google Scholar] [CrossRef]
- Fivga, A.; Dimitriou, I. Pyrolysis of Plastic Waste for Production of Heavy Fuel Substitute: A Techno-Economic Assessment. Energy 2018, 149, 865–874. [Google Scholar] [CrossRef]
- Garcia-Nunez, J.A.; Pelaez-Samaniego, M.R.; Garcia-Perez, M.E.; Fonts, I.; Abrego, J.; Westerhof, R.J.M.; Garcia-Perez, M. Historical Developments of Pyrolysis Reactors: A Review. Energy Fuels 2017, 31, 5751–5775. [Google Scholar] [CrossRef]
- Wong, S.L.; Ngadi, N.; Abdullah, T.A.T.; Inuwa, I.M. Current State and Future Prospects of Plastic Waste as Source of Fuel: A Review. Renew. Sustain. Energy Rev. 2015, 50, 1167–1180. [Google Scholar] [CrossRef]
- Adrados, A.; de Marco, I.; Caballero, B.M.; López, A.; Laresgoiti, M.F.; Torres, A. Pyrolysis of Plastic Packaging Waste: A Comparison of Plastic Residuals from Material Recovery Facilities with Simulated Plastic Waste. Waste Manag. 2012, 32, 826–832. [Google Scholar] [CrossRef]
- Yaqoob, L.; Noor, T.; Iqbal, N. Conversion of Plastic Waste to Carbon-Based Compounds and Application in Energy Storage Devices. ACS Omega 2022, 7, 13403–13435. [Google Scholar] [CrossRef]
- Yek, P.N.Y.; Chan, Y.H.; Foong, S.Y.; Mahari, W.A.W.; Chen, X.; Liew, R.K.; Ma, N.L.; Tsang, Y.F.; Sonne, C.; Cheng, Y.W.; et al. Co-Processing Plastics Waste and Biomass by Pyrolysis–Gasification: A Review. Environ. Chem. Lett. 2023, 22, 171–188. [Google Scholar] [CrossRef]
- Ge, S.; Chen, D.; Yin, L.; Hong, L.; Zhou, H.; Huang, Z. Municipal Solid Wastes Pyro-Gasification Using High-Temperature Flue Gas as Heating Resource and Gasifying Agent. Waste Manag. 2022, 149, 114–123. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Fu, Z.; Wang, W.; Ji, G.; Zhao, M.; Li, A. Kinetics, Product Evolution, and Mechanism for the Pyrolysis of Typical Plastic Waste. ACS Sustain. Chem. Eng. 2022, 10, 91–103. [Google Scholar] [CrossRef]
- Akhtar, J.; Saidina Amin, N. A Review on Operating Parameters for Optimum Liquid Oil Yield in Biomass Pyrolysis. Renew. Sustain. Energy Rev. 2012, 16, 5101–5109. [Google Scholar] [CrossRef]
- Al-Salem, S.M.; Antelava, A.; Constantinou, A.; Manos, G.; Dutta, A. A Review on Thermal and Catalytic Pyrolysis of Plastic Solid Waste (PSW). J. Environ. Manag. 2017, 197, 177–198. [Google Scholar] [CrossRef] [PubMed]
- López, A.; de Marco, I.; Caballero, B.M.; Adrados, A.; Laresgoiti, M.F. Deactivation and Regeneration of ZSM-5 Zeolite in Catalytic Pyrolysis of Plastic Wastes. Waste Manag. 2011, 31, 1852–1858. [Google Scholar] [CrossRef]
- Wong, S.L.; Armenise, S.; Nyakuma, B.B.; Bogush, A.; Towers, S.; Lee, C.H.; Wong, K.Y.; Lee, T.H.; Rebrov, E.; Muñoz, M. Plastic Pyrolysis over HZSM-5 Zeolite and Fluid Catalytic Cracking Catalyst under Ultra-Fast Heating. J. Anal. Appl. Pyrolysis 2023, 169, 105793. [Google Scholar] [CrossRef]
- Marino, A.; Aloise, A.; Hernando, H.; Fermoso, J.; Cozza, D.; Giglio, E.; Migliori, M.; Pizarro, P.; Giordano, G.; Serrano, D.P. ZSM-5 Zeolites Performance Assessment in Catalytic Pyrolysis of PVC-Containing Real WEEE Plastic Wastes. Catal. Today 2022, 390–391, 210–220. [Google Scholar] [CrossRef]
- Vargas, M.; Tupayachy-Quispe, D.; Roudet, F.; Duquesne, S.; Almirón, J. Catalytic Pyrolysis of Plastic Materials Using Natural Zeolite Catalysts Synthesized from Volcanic Ash. IOP Conf. Ser. Mater. Sci. Eng. 2021, 1150, 012018. [Google Scholar] [CrossRef]
- Yuan, R.; Shen, Y. Catalytic Pyrolysis of Biomass-Plastic Wastes in the Presence of MgO and MgCO3 for Hydrocarbon-Rich Oils Production. Bioresour. Technol. 2019, 293, 122076. [Google Scholar] [CrossRef]
- Singh, M.V.; Kumar, S.; Sarker, M. Waste HD-PE Plastic, Deformation into Liquid Hydrocarbon Fuel Using Pyrolysis-Catalytic Cracking with a CuCO3 Catalyst. Sustain. Energy Fuels 2018, 2, 1057–1068. [Google Scholar] [CrossRef]
- Gebre, S.H.; Sendeku, M.G.; Bahri, M. Recent Trends in the Pyrolysis of Non-Degradable Waste Plastics. ChemistryOpen 2021, 10, 1202–1226. [Google Scholar] [CrossRef]
- Sun, K.; Huang, Q.; Chi, Y.; Yan, J. Effect of ZnCl2-Activated Biochar on Catalytic Pyrolysis of Mixed Waste Plastics for Producing Aromatic-Enriched Oil. Waste Manag. 2018, 81, 128–137. [Google Scholar] [CrossRef] [PubMed]
- Panda, A.K.; Alotaibi, A.; Kozhevnikov, I.V.; Shiju, N.R. Pyrolysis of Plastics to Liquid Fuel Using Sulphated Zirconium Hydroxide Catalyst. Waste Biomass Valorization 2020, 11, 6337–6345. [Google Scholar] [CrossRef]
- Luo, W.; Fan, Z.; Wan, J.; Hu, Q.; Dong, H.; Zhang, X.; Zhou, Z. Study on the Reusability of Kaolin as Catalysts for Catalytic Pyrolysis of Low-Density Polyethylene. Fuel 2021, 302, 121164. [Google Scholar] [CrossRef]
- Li, K.; Wang, Y.; Zhou, W.; Cui, T.; Yang, J.; Sun, Z.; Min, Y.; Lee, J.-M. Catalytic Pyrolysis of Film Waste over Co/Ni Pillared Montmorillonites towards H2 Production. Chemosphere 2022, 299, 134440. [Google Scholar] [CrossRef] [PubMed]
- Friengfung, P.; Jamkrajang, E.; Sunphorka, S.; Kuchonthara, P.; Mekasut, L. NiO/Dolomite Catalyzed Steam/O2 Gasification of Different Plastics and Their Mixtures. Ind. Eng. Chem. Res. 2014, 53, 1909–1915. [Google Scholar] [CrossRef]
- Jiang, G.; Sanchez Monsalve, D.A.; Clough, P.; Jiang, Y.; Leeke, G.A. Understanding the Dechlorination of Chlorinated Hydrocarbons in the Pyrolysis of Mixed Plastics. ACS Sustain. Chem. Eng. 2021, 9, 1576–1589. [Google Scholar] [CrossRef]
- Yuan, X. Converting Waste Plastics into Liquid Fuel by Pyrolysis: Developments in China. In Feedstock Recycling and Pyrolysis of Waste Plastics; Wiley: Hoboken, NJ, USA, 2006; pp. 729–755. [Google Scholar]
- Eswara, A.K.; Misra, S.C.; Ramesh, U.S. Introduction to Natural Gas: A Comparative Study of Its Storage, Fuel Costs and Emissions for a Harbor Tug. In Proceedings of the Annual Meeting of Society of Naval Architects & Marine Engineers (SNAME), Bellevue, DC, USA, 8 November 2013. [Google Scholar]
- Speight, J.G. Production, Properties and Environmental Impact of Hydrocarbon Fuel Conversion. In Advances in Clean Hydrocarbon Fuel Processing; Elsevier: Amsterdam, The Netherlands, 2011; pp. 54–82. [Google Scholar]
- Neuwahl, F.; Cusano, G.; Benavides, J.G.; Holbrook, S.; Roudier, S. European IPPC Bureau Best Available Techniques (BAT) Reference Document for Waste Incineration; Publications Office of the European Union: Luxembourg, 2019. [Google Scholar]
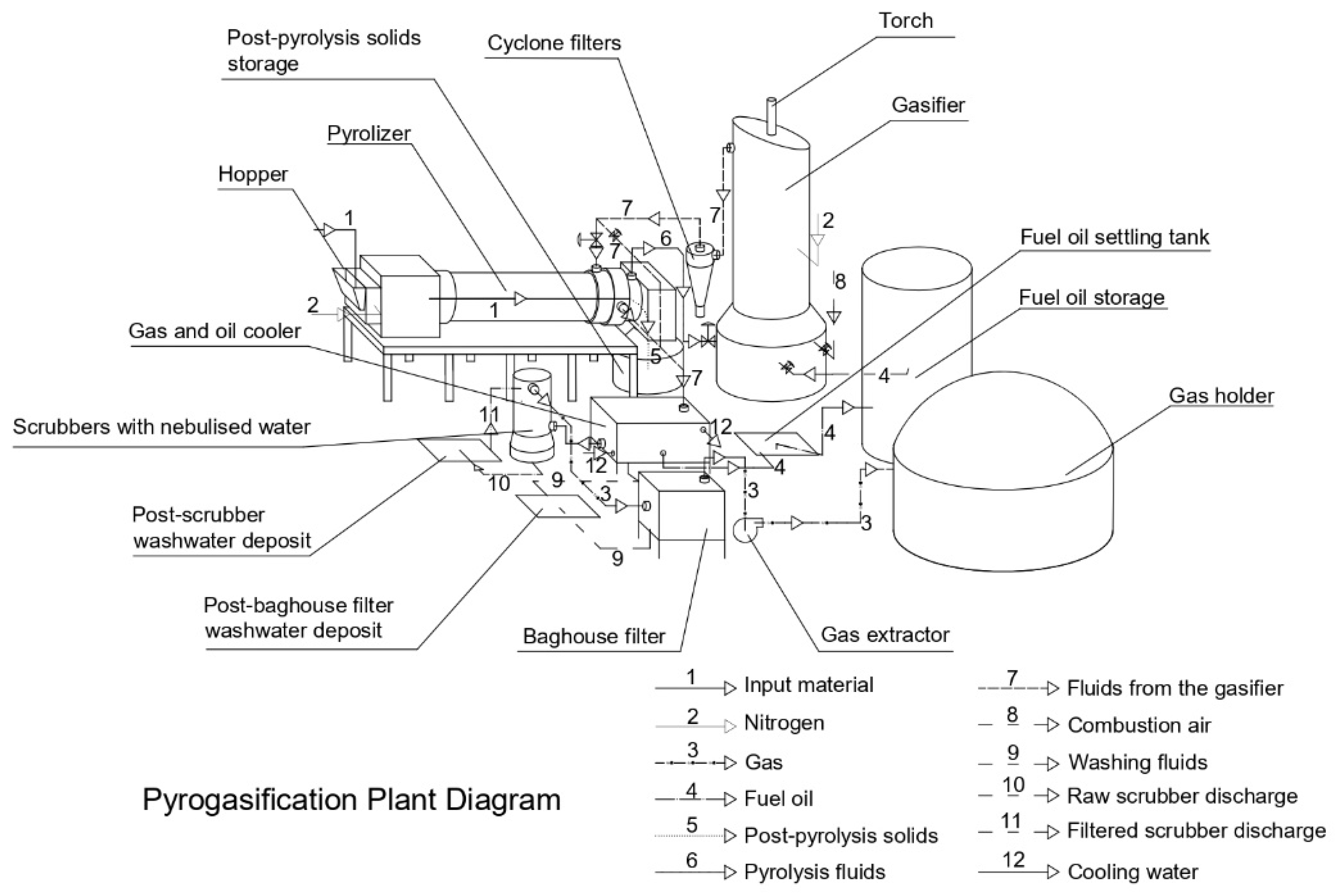

| Feedstock | Fixed Bed—Batch | Fluidized Bed | Spouted Bed | Moving Bed |
|---|---|---|---|---|
| Plastics from MSW | G 25, L 34, W 6 | G 28.5, L 36.5 | G 19.6, L 37.6, W 7.8 | G 26.6, L 35.6, W 2.8 |
| Inerts 28, Char 5, Ash 2 | ||||
| Plasmix | G 32, L 43, W 12 | G 36, L 46, W 5 | G 30, L 49, W 8 | G 34, L 45, W 8 |
| Inerts 6; Char 5; Ash 2 | ||||
| Component | Weight (kg) | HHV (MJ/kg) | Energy (MJ) |
|---|---|---|---|
Mixed plastic waste:
| 600 | 42 | 25.200 |
| Plastic associated with paper | 167 | 35 | 5.845 |
| Glass and inerts | 180 | - | - |
| Metals (Al, …) | 53 | - | - |
| Total | 1000 | 31.045 | 31.045 |
| Component | Volume (Nm3) | % vol | Weight (kg) | HHV (MJ/kg) | Total Energy (MJ) |
|---|---|---|---|---|---|
| H2 | 152 | 19 | 13.6 | 120 | 1.632 |
| CO | 138 | 18 | 172 | 10.05 | 1.729 |
| CO2 | 44 | 6 | 86.4 | 0 | 0 |
| CH4 | 19 | 2 | 13.6 | 50 | 680 |
| C2–C6 | 99 | 13 | 188.5 | 50 | 9.425 |
| C5–C9 | 41.5 | 5 | 160 | 47.8 | 7.648 |
| C10–C13 | 5.5 | 1 | 39.4 | 49 | 1.931 |
| N2 | 287 | 37 | 359 | 0 | 0 |
| Total | 786 | 100 | 1032.25 | 23.045 |
| Weight (kg) | Energy (MJ) | |
|---|---|---|
| Non-condensable gas | 832.85 | 13.466 |
| Condensed product | 199.4 | 9.579 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moliner, C.; Pasquale, G.; Arato, E. Municipal Plastic Waste Recycling through Pyrogasification. Energies 2024, 17, 1206. https://doi.org/10.3390/en17051206
Moliner C, Pasquale G, Arato E. Municipal Plastic Waste Recycling through Pyrogasification. Energies. 2024; 17(5):1206. https://doi.org/10.3390/en17051206
Chicago/Turabian StyleMoliner, Cristina, Giovanni Pasquale, and Elisabetta Arato. 2024. "Municipal Plastic Waste Recycling through Pyrogasification" Energies 17, no. 5: 1206. https://doi.org/10.3390/en17051206
APA StyleMoliner, C., Pasquale, G., & Arato, E. (2024). Municipal Plastic Waste Recycling through Pyrogasification. Energies, 17(5), 1206. https://doi.org/10.3390/en17051206







