A Complete Assessment of the Emission Performance of an SI Engine Fueled with Methanol, Methane and Hydrogen
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Apparatus
| Species | Principle Measurement | Measurement Range | Resolution |
|---|---|---|---|
| CO | Non-dispersive infrared (NDIR) | 0–10% vol. | 0.01%vol. |
| CO2 | Non-dispersive infrared (NDIR) | 0–20% vol. | 0.1%vol. |
| THC | Non-dispersive infrared (NDIR) | 0–20,000 ppm vol. | 1 ppm |
| NOx | Chemiluminescent detector (CLD) | 0–5000 ppm vol. | 1 ppm |
| Properties | Range |
|---|---|
| Particle Size Range | 5.6–560 nm |
| Particle Size Resolution | 16 channels per decade (32 total) |
| Electrometer Channels | 22 |
| Maximum Data Rate | 10 size distribution per second |
| Inlet Aerosol Temperature | 10–52 °C |
| Storage Temperature | −20 to 50 °C |
2.2. Procedure
3. Results
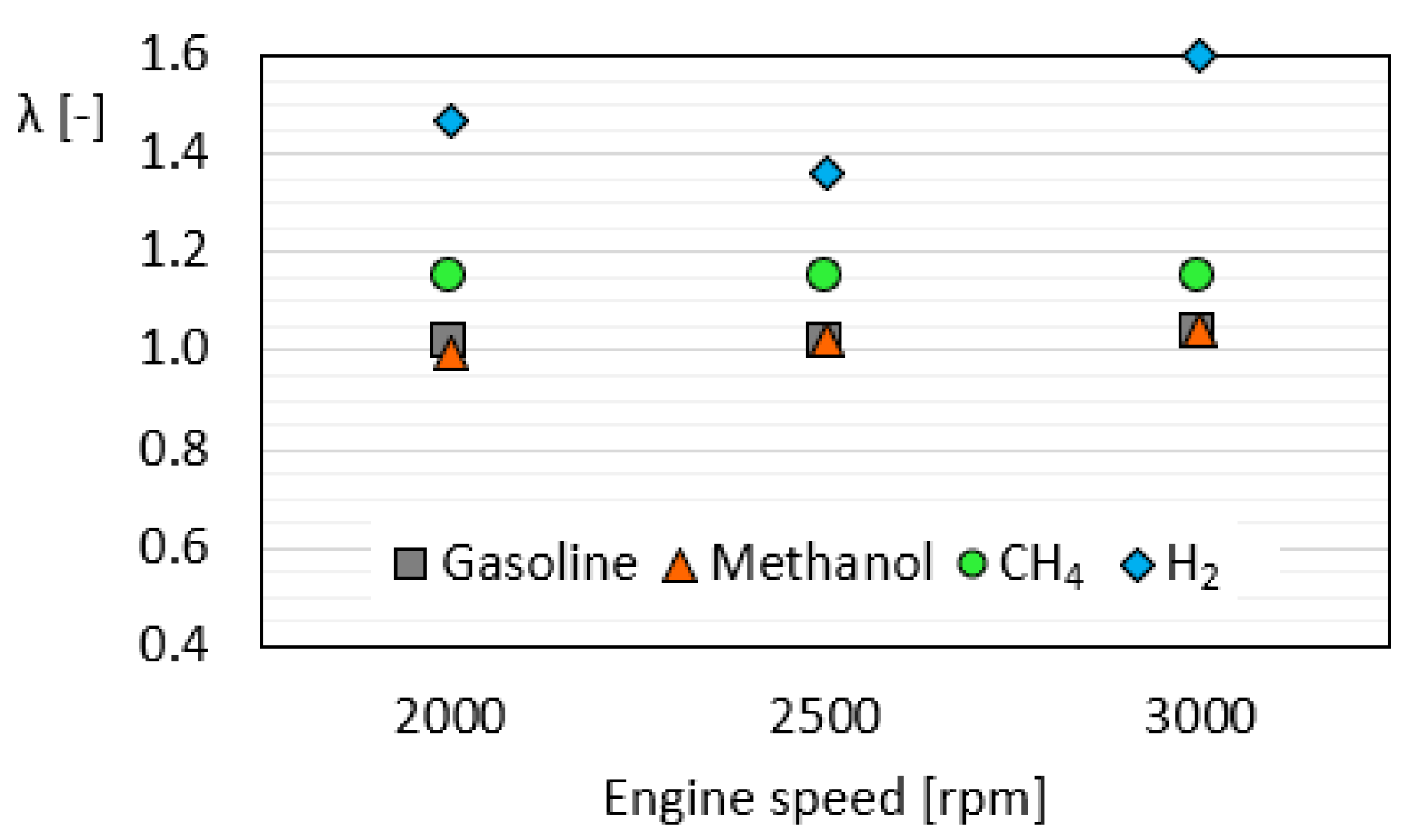
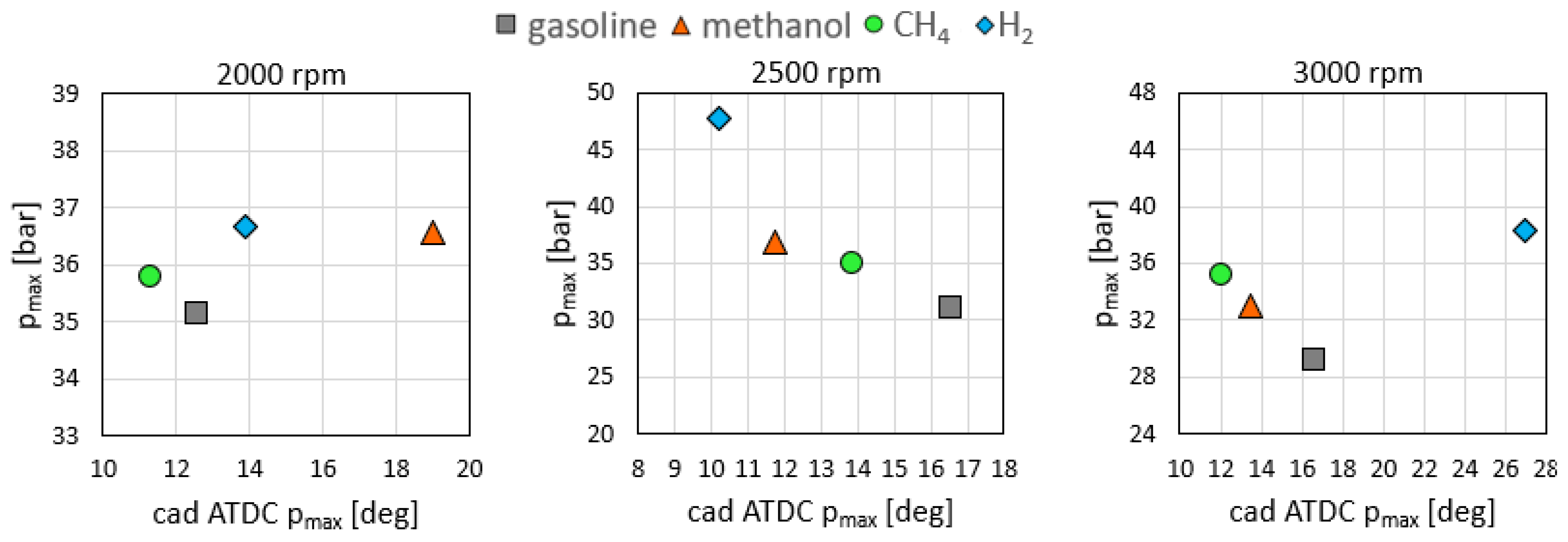
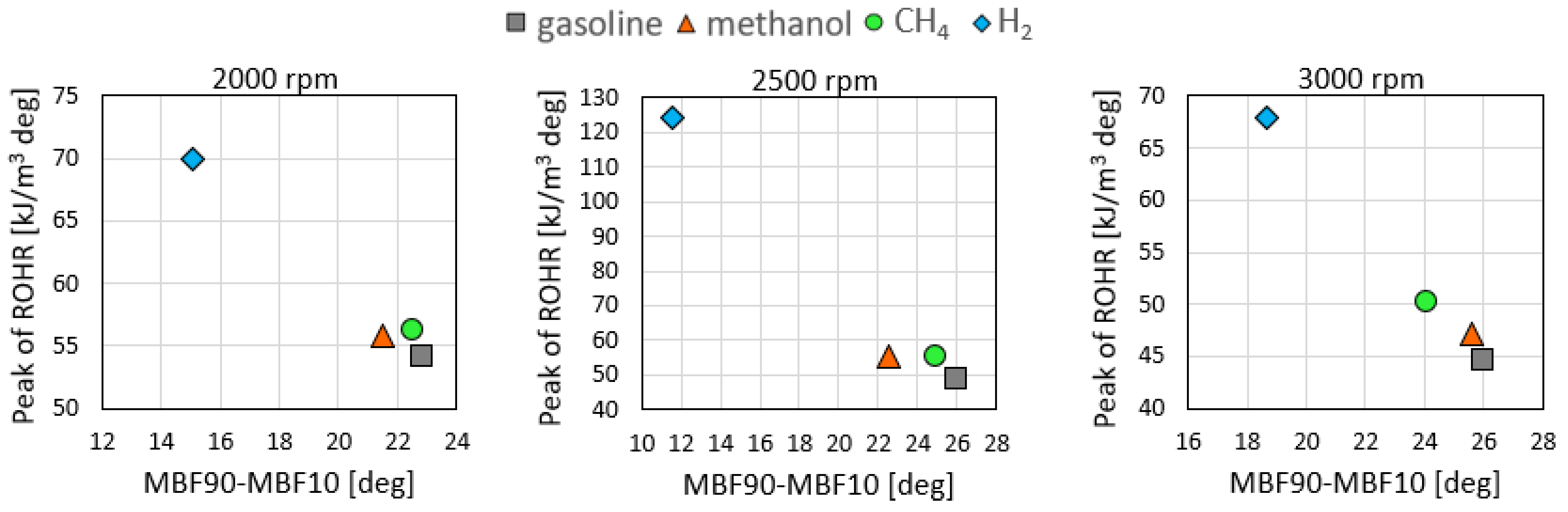
3.1. Combustion Analysis
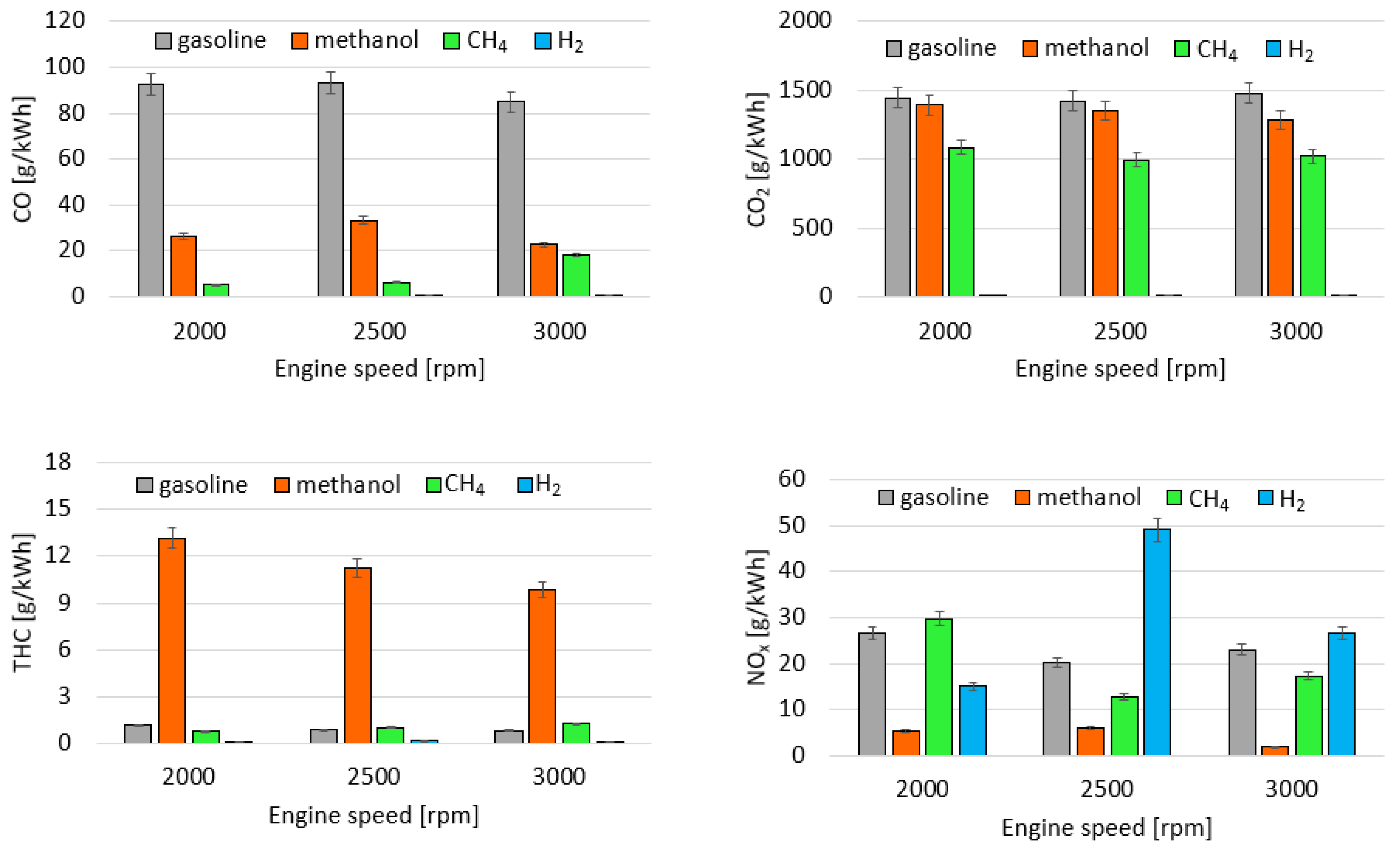
3.2. Exhaust Emission Analysis
4. Conclusions
- The indicative data revealed that methanol is characterized by advanced—up to 5 cad—and faster combustion with a higher pressure peak—up to 18%—and heat release compared to the reference fuel.
- Gaseous fuels are characterized by a lower combustion duration and higher heat release than gasoline. This result could be in part due to the injection configuration, highlighting the positive effect of the DI technology for gaseous fuels.
- For hydrogen fueling, the effect of the higher flame speed was more evident, resulting in the fastest combustion—up to 11 cad of combustion duration—at 2500 rpm.
- All the alternative fuels guarantee a reduction of CO and CO2 emissions.
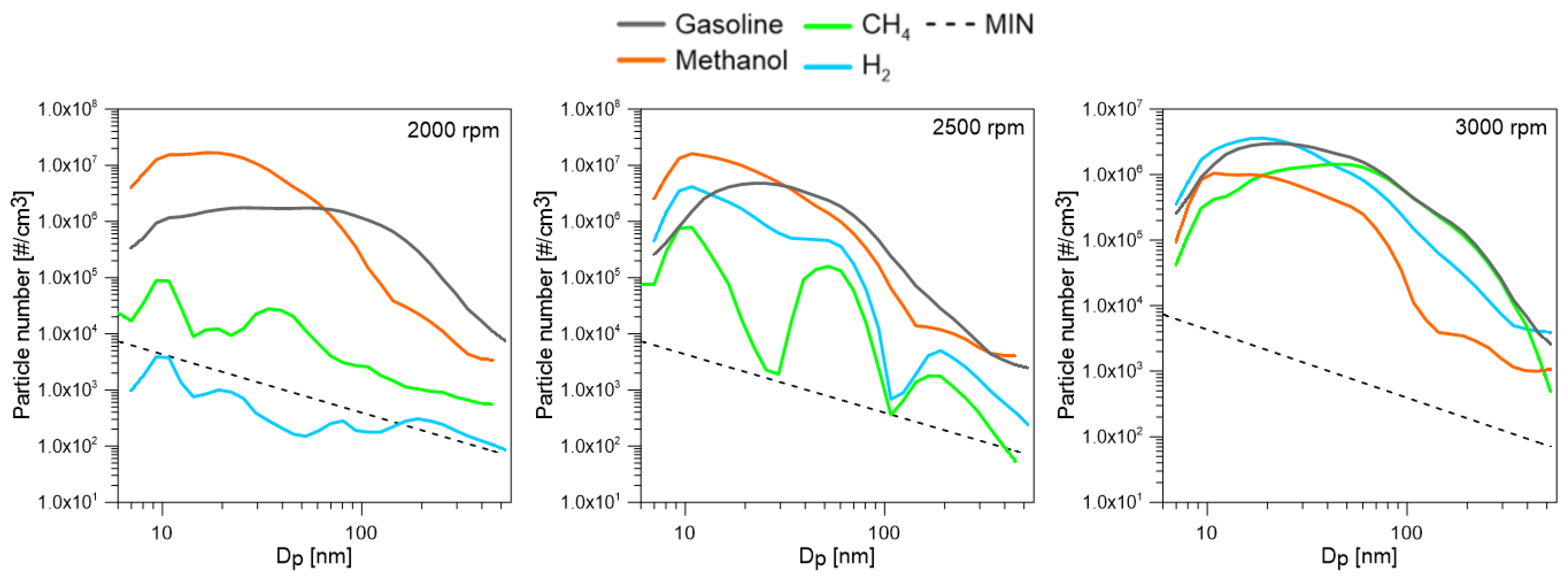
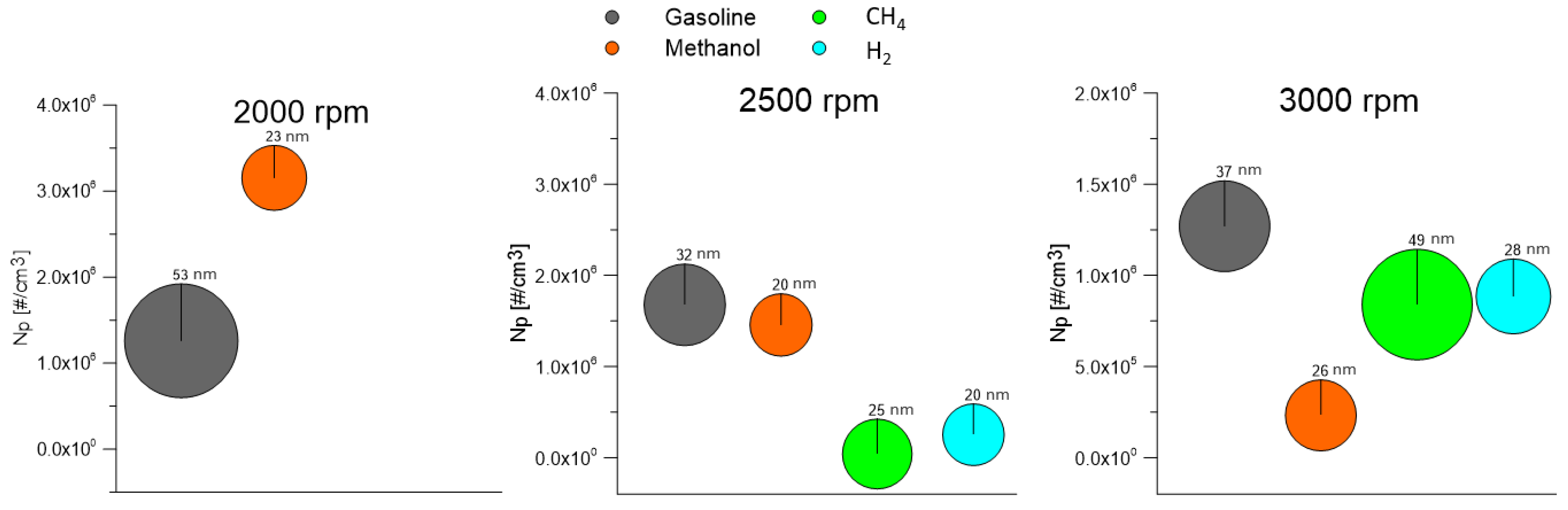
- Methanol shows high THC emissions, with a maximum value of 13 g/kWh at 2000 rpm, due to the less efficient fuel vaporization. NOx emissions are, instead, decreased. Regarding the particles, methanol shows not negligible emissions. In general, smaller size particles were measured, while their number depended on the engine test point. The nature of these particles is not clear. An important factor affecting their formation is, in fact, the lubricating oil.
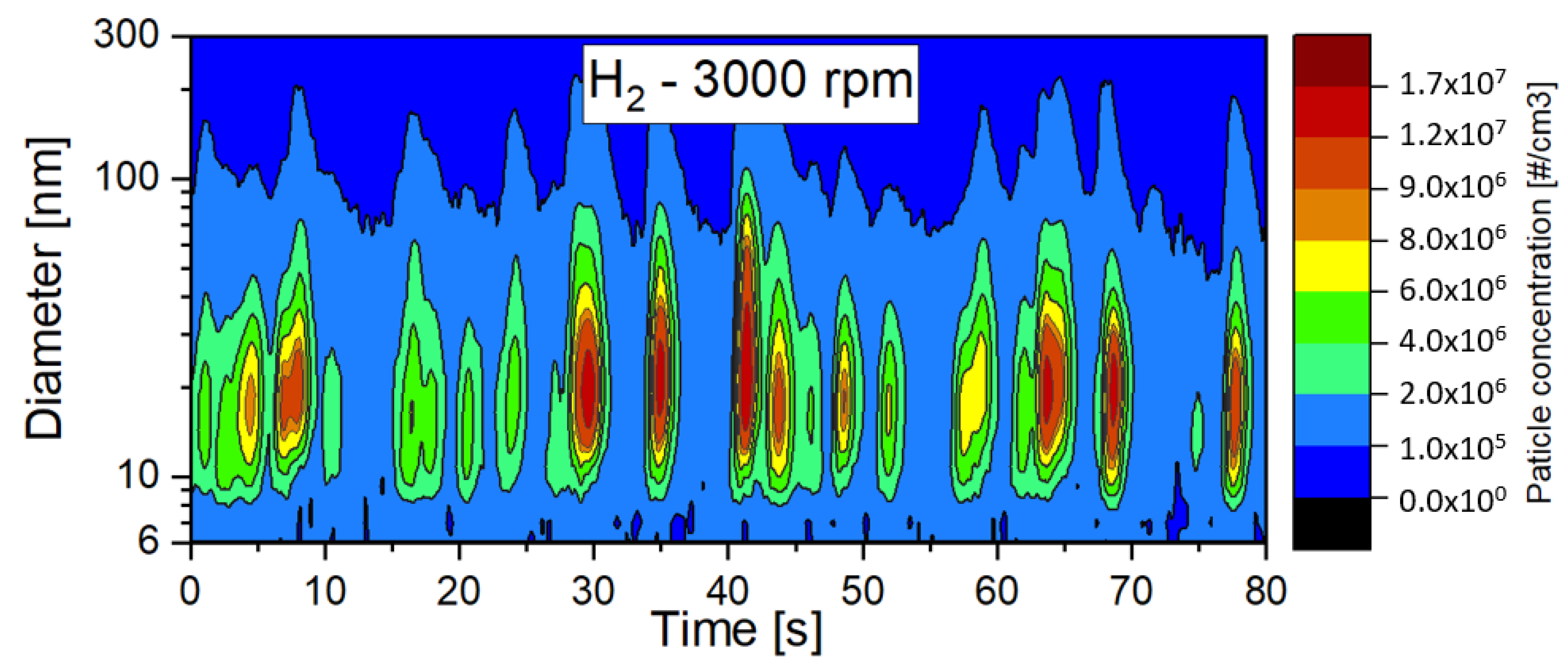
- For gaseous fuels, the emission levels also depended on the operating conditions that affect the in-cylinder temperature. When gaseous fuels were used, the effect of oil on the particle emissions was clear, especially for hydrogen, which should not emit particles on its own. Lower particle emissions with a larger diameter were measured for methane fueling. For hydrogen, an increase in particle emissions was observed with increases in engine speed.
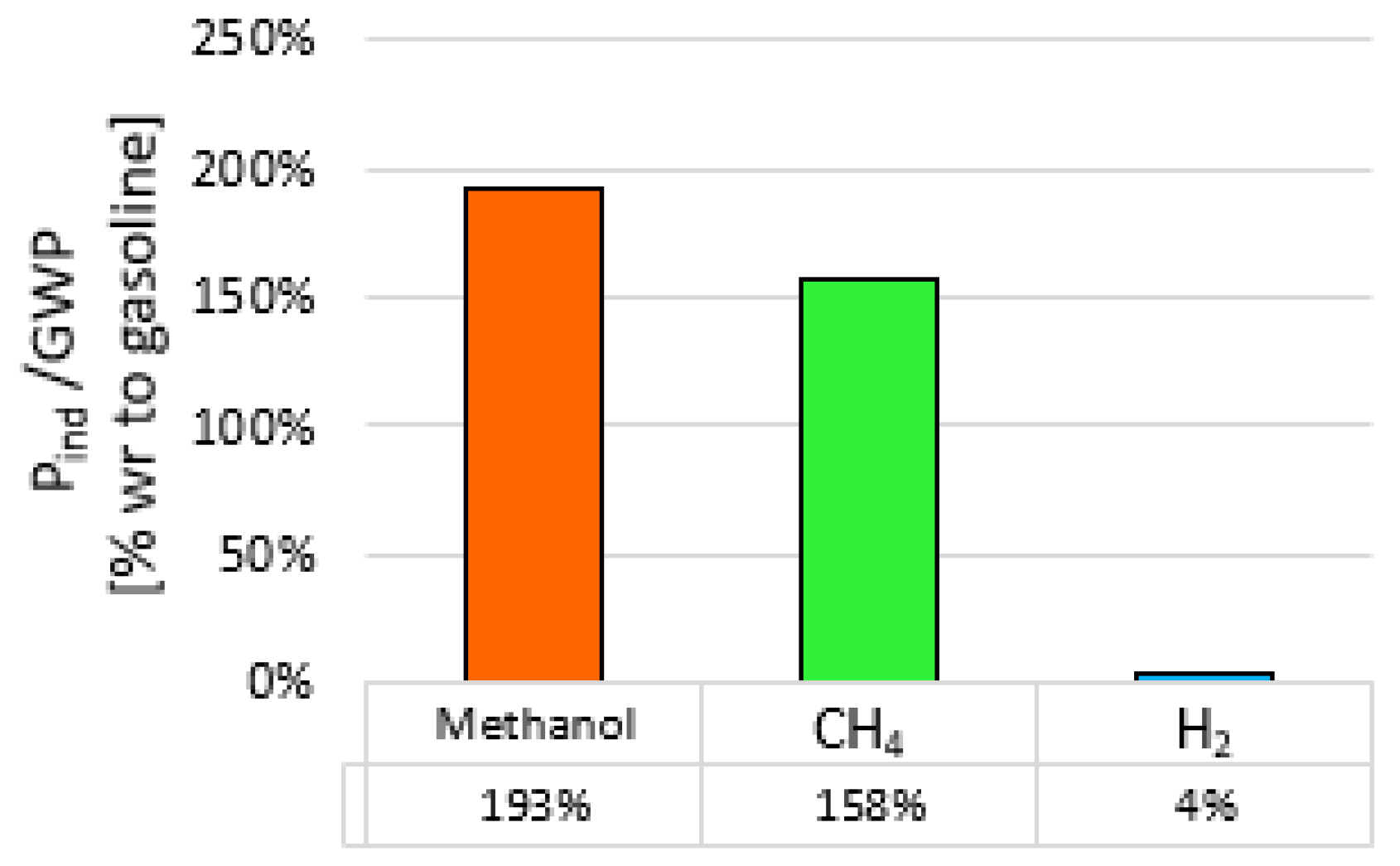
- All the tested fuels contributed to sustainable mobility thanks to the benefit in terms of total CO2 reductions with respect to gasoline, with the best performance achieved by methanol.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AFRst | Stoichiometric Air/Fuel Ratio |
| AM | Accumulation Mode |
| ATDC | After Top Dead Center |
| cad | Crank Angle Degree |
| CLD | Chemiluminescent Detector |
| CO | Carbon Monoxide |
| CO2 | Carbon Dioxide |
| DI | Direct Injection |
| Dm | Mean Diameter |
| DOI | Duration of Injection |
| EEPS | Engine Exhaust Particle Spectrometer |
| ETU | Engine Timing Unit |
| EV | Electric Vehicle |
| GWP | Global Warming Potential |
| HOV | Heat of Vaporization |
| ICE | Internal Combustion Engine |
| Imep | Indicated Mean Effective Pressure |
| λ | Excess Air Ratio |
| LHV | Low Heating Value |
| MBF10 | cad for 10% of Mass Fraction Burned |
| MBF90 | cad for 90% of Mass Fraction Burned |
| NDIR | Non-Dispersive Infrared |
| NM | Nucleation Mode |
| NOx | Nitrogen Oxides |
| Np | Particle Number |
| ON | Octane Number |
| PFI | Port Fuel Injection |
| Pind | Indicated Power |
| PM | Particulate Matter |
| PSD | Particle Size Distribution |
| ROHR | Rate of Heat Release |
| SD | Single Diluter |
| SI | Spark Ignition |
| SOI | Start of Injection |
| SOS | Start of Spark |
| THC | Total Hydrocarbons |
| TP | Throttle Position |
| WOT | Wide Open Throttle |
| WtT | Well-to-Tank |
References
- 2050 Long-Term Strategy. n.d. Available online: https://climate.ec.europa.eu/eu-action/climate-strategies-targets/2050-long-term-strategy_en (accessed on 27 March 2023).
- Adhikari, M.; Ghimire, L.P.; Kim, Y.; Aryal, P.; Khadka, S.B. Identification and analysis of barriers against electric vehicle use. Sustainability 2020, 12, 4850. [Google Scholar] [CrossRef]
- Kaunda, R.B. Potential environmental impacts of lithium mining. J. Energy Nat. Resour. Law 2020, 38, 237–244. [Google Scholar] [CrossRef]
- Serrano, J.R.; Novella, R.; Piqueras, P. Why the development of internal combustion engines is still necessary to fight against global climate change from the perspective of transportation. Appl. Sci. 2019, 9, 4597. [Google Scholar] [CrossRef]
- Karczewski, M.; Chojnowski, J.; Szamrej, G. A review of low-CO2 emission fuels for a dual-fuel RCCI engine. Energies 2021, 14, 5067. [Google Scholar] [CrossRef]
- Breuer, J.L.; Scholten, J.; Koj, J.C.; Schorn, F.; Fiebrandt, M.; Samsun, R.C.; Albus, R.; Görner, K.; Stolten, D.; Peters, R. An Overview of Promising Alternative Fuels for Road, Rail, Air, and Inland Waterway Transport in Germany. Energies 2022, 15, 1443. [Google Scholar] [CrossRef]
- Bae, C.; Kim, J. Alternative fuels for internal combustion engines. Proc. Combust. Inst. 2017, 36, 3389–3413. [Google Scholar] [CrossRef]
- Verhelst, S.; Turner, J.W.; Sileghem, L.; Vancoillie, J. Methanol as a fuel for internal combustion engines. Prog. Energy Combust. Sci. 2019, 70, 43–88. [Google Scholar] [CrossRef]
- Boretti, A. Renewable hydrogen to recycle CO2 to methanol. Int. J. Hydrogen Energy 2013, 38, 1806–1812. [Google Scholar] [CrossRef]
- Di Iorio, S.; Catapano, F.; Magno, A.; Sementa, P.; Vaglieco, B.M. The Potential of Ethanol/Methanol Blends as Renewable Fuels for DI SI Engines. Energies 2023, 16, 2791. [Google Scholar] [CrossRef]
- Turner, J.W.; Lewis, A.G.; Akehurst, S.; Brace, C.J.; Verhelst, S.; Vancoillie, J.; Sileghem, L.; Leach, F.; Edwards, P.P. Alcohol fuels for spark-ignition engines: Performance, efficiency and emission effects at mid to high blend rates for binary mixtures and pure components. Proc. Inst. Mech. Eng. Part D J. Automob. Eng. 2018, 232, 36–56. [Google Scholar] [CrossRef]
- Turner, J.W.; Lewis, A.G.; Akehurst, S.; Brace, C.J.; Verhelst, S.; Vancoillie, J.; Sileghem, L.; Leach, F.; Edwards, P.P. Alcohol Fuels for Spark-Ignition Engines: Performance, Efficiency, and Emission Effects at Mid to High Blend Rates for Ternary Mixtures. Energies 2020, 13, 6390. [Google Scholar] [CrossRef]
- Zhang, Z.; Wen, M.; Cui, Y.; Ming, Z.; Wang, T.; Zhang, C.; Ampah, J.D.; Jin, C.; Huang, H.; Liu, H. Effects of methanol application on carbon emissions and pollutant emissions using a passenger vehicle. Processes 2022, 10, 525. [Google Scholar] [CrossRef]
- Geng, P.; Zhang, H.; Yang, S. Experimental investigation on the combustion and particulate matter (PM) emissions from a port-fuel injection (PFI) gasoline engine fueled with methanol–ultralow sulfur gasoline blends. Fuel 2015, 145, 221–227. [Google Scholar] [CrossRef]
- Ho, C.S.; Peng, J.; Yun, U.; Zhang, Q.; Mao, H. Impacts of methanol fuel on vehicular emissions: A review. Front. Environ. Sci. Eng. 2022, 16, 121. [Google Scholar] [CrossRef]
- Wei, Y.; Zhu, Z.; Liu, S.; Liu, H.; Shi, Z.; Zeng, Z. Investigation on injection strategy affecting the mixture formation and combustion of a heavy-duty spark-ignition methanol engine. Fuel 2023, 334, 126680. [Google Scholar] [CrossRef]
- Larsson, T.; Mahendar, S.K.; Christiansen-Erlandsson, A.; Olofsson, U. The effect of pure oxygenated biofuels on efficiency and emissions in a gasoline optimised disi engine. Energies 2021, 14, 3908. [Google Scholar] [CrossRef]
- Svensson, E.; Li, C.; Shamun, S.; Johansson, B.; Tuner, M.; Perlman, C.; Lehtiniemi, H.; Mauss, F. Potential Levels of Soot, NOx, HC and CO for Methanol Combustion. SAE Tec Pap 2016-01-0887. 2016. Available online: https://saemobilus.sae.org/content/2016-01-0887/ (accessed on 18 January 2024).
- Cho, H.M.; He, B.-Q. Spark ignition natural gas engines—A review. Energy Convers. Manag. 2007, 48, 608–618. [Google Scholar] [CrossRef]
- Akal, D.; Öztuna, S.; Büyükakın, M.K. A review of hydrogen usage in internal combustion engines (gasoline-Lpg-diesel) from combustion performance aspect. Int. J. Hydrogen Energy 2020, 45, 35257–35268. [Google Scholar] [CrossRef]
- Duc, K.N.; Duy, V.N.; Hoang-Dinh, L.; Viet, T.N.; Le-Anh, T. Performance and emission characteristics of a port fuel injected, spark ignition engine fueled by compressed natural gas. Sustain. Energy Technol. Assess. 2019, 31, 383–389. [Google Scholar] [CrossRef]
- Ma, F.; Liu, H.; Wang, Y.; Li, Y.; Wang, J.; Zhao, S. Combustion and emission characteristics of a port-injection HCNG engine under various ignition timings. Int. J. Hydrogen Energy 2008, 33, 816–822. [Google Scholar] [CrossRef]
- Ortiz-Imedio, R.; Ortiz, A.; Ortiz, I. Comprehensive analysis of the combustion of low carbon fuels (hydrogen, methane and coke oven gas) in a spark ignition engine through CFD modeling. Energy Convers. Manag. 2021, 251, 114918. [Google Scholar] [CrossRef]
- Yosri, M.; Palulli, R.; Talei, M.; Mortimer, J.; Poursadegh, F.; Yang, Y.; Brear, M. Numerical investigation of a large bore, direct injection, spark ignition, hydrogen-fuelled engine. Int. J. Hydrogen Energy 2023, 48, 17689–17702. [Google Scholar] [CrossRef]
- Thawko, A.; Eyal, A.; Tartakovsky, L. Experimental comparison of performance and emissions of a direct-injection engine fed with alternative gaseous fuels. Energy Convers. Manag. 2021, 251, 114988. [Google Scholar] [CrossRef]
- Thawko, A.; Tartakovsky, L. The Mechanism of Particle Formation in Non-Premixed Hydrogen Combustion in a Direct-Injection Internal Combustion Engine. Fuel 2022, 327, 125187. [Google Scholar] [CrossRef]
- Di Iorio, S.; Sementa, P.; Vaglieco, B.; Catapano, F. An Experimental Investigation on Combustion and Engine Performance and Emissions of a Methane-Gasoline Dual-Fuel Optical Engine. SAE Technical Paper 2014-01-1329. 2014. Available online: https://saemobilus.sae.org/content/2014-01-1329/ (accessed on 18 January 2024).
- Holman, J.P. Experimental Methods for Engineers; McGraw-Hill: New York, NY, USA, 2012. [Google Scholar]
- TSI Incorporated. Engine Exhaust Particle Sizer TM Spectrometer Model 3090. Available online: https://tsi.com/products/particle-sizers/fast-particle-sizer-spectrometers/engine-exhaust-particle-sizer-(eeps)-3090/ (accessed on 30 March 2015).
- TSI. Updated Inversion Matrices for Engine Exhaust Particle Sizer TM (Eeps TM) Spectrometer Model 3090—Application Note EEPS-005 (A4). 2015. Available online: https://tsi.com/getmedia/22bf0106-13d9-4503-b179-bc76cb55e100/Updated_Inversion_Matrices_EEPS-005-A4-web?ext=.pdf (accessed on 18 January 2024).
- AVL Emission Tester Series 4000. 2007. Available online: https://www.yumpu.com/en/document/view/7031754/avl-emission-tester-series-4000-product-brochure-avl-ditest (accessed on 18 January 2024).
- Catapano, F.; Di Iorio, S.; Luise, L.; Sementa, P.; Vaglieco, B.M. Influence of ethanol blended and dual fueled with gasoline on soot formation and particulate matter emissions in a small displacement spark ignition engine. Fuel 2019, 245, 253–262. [Google Scholar] [CrossRef]
- Apicella, B.; Catapano, F.; Di Iorio, S.; Magno, A.; Russo, C.; Sementa, P.; Tregrossi, A.; Vaglieco, B.M. Comprehensive analysis on the effect of lube oil on particle emissions through gas exhaust measurement and chemical characterization of condensed exhaust from a DI SI engine fueled with hydrogen. Int. J. Hydrogen Energy 2023, 48, 22277–22287. [Google Scholar] [CrossRef]
- Apicella, B.; Catapano, F.; Di Iorio, S.; Magno, A.; Russo, C.; Sementa, P.; Tregrossi, A.; Vaglieco, B.M. Comprehensive analysis on the effect of lube oil on particle emissions through gas exhaust measurement and chemical characterization of condensed exhaust from a DI SI engine fueled with hydrogen. Part 2: Effect of operating conditions. Int. J. Hydrogen Energy 2024, 49, 968–979. [Google Scholar] [CrossRef]
- Thawko, A.; Yadav, H.; Eyal, A.; Shapiro, M.; Tartakovsky, L. Particle emissions of direct injection internal combustion engine fed with a hydrogen-rich reformate. Int. J. Hydrogen Energy 2019, 44, 28342–28356. [Google Scholar] [CrossRef]
- Raza, M.; Chen, L.; Leach, F.; Ding, S. A Review of particulate number (PN) emissions from gasoline direct injection (gdi) engines and their control techniques. Energies 2018, 11, 1417. [Google Scholar] [CrossRef]
- Larsson, T.; Olofsson, U.; Erlandsson, A.C. Undiluted measurement of the particle size distribution of different oxygenated biofuels in a gasoline-optimised disi engine. Atmosphere 2021, 12, 1493. [Google Scholar] [CrossRef]
- Lou, D.; Wang, T.; Fang, L.; Tan, P.; Hu, Z.; Zhang, Y.; Xu, Z.; Cheng, C.; Wang, S.; Zhang, Y. Investigation of the combustion and particle emission characteristics of a GDI engine with a 50 MPa injection system. Fuel 2022, 315, 123079. [Google Scholar] [CrossRef]
- Hora, T.S.; Shukla, P.C.; Agarwal, A.K. Particulate emissions from hydrogen enriched compressed natural gas engine. Fuel 2016, 166, 574–580. [Google Scholar] [CrossRef]

| Engine | Spark Ignition | |
|---|---|---|
| Number of Cylinders | 1 | |
| Bore [mm] | 72 | |
| Stroke [mm] | 60 | |
| Displacement [cm³] | 244.3 | |
| Compression Ratio | 11.5:1 | |
| Max. Power [kW] | 16 @ 8000 rpm | |
| Max. Torque [Nm] | 20 @ 5500 | |
| Intake | Turbo-charged | |
| Injection System | Gaseous fuels—DI | Liquid fuels—PFI |
| Number of Nozzle Holes | 6 | 3 |
| pinj [bar] | 5 | 2.5 |
| Properties | Gasoline | Methanol | Methane | Hydrogen |
|---|---|---|---|---|
| Chemical Formula | C4–C12 | CH3OH | CH4 | H2 |
| Density at 15 °C [kg/L] | 746 | 796 | 0.67 | 0.08 |
| Boiling Point [°C] | 27–225 | 64 | −161.4 | −252.9 |
| LHV [MJ/kg] | 42.94 | 20.1 | 50 | 120 |
| AFRst | 14.5 | 6.4 | 17.24 | 34.20 |
| RON | 95.0 | 108.6 | >120 | >130 |
| Flammability Limits [vol %] | 1.4/7.6 | 6.7/36 | 5.3/14 | 4/75 |
| C [% mass] | 85.9 | 38.0 | 75 | - |
| H [% mass] | 13.4 | 12.1 | 25 | 100 |
| O [% mass] | 0.6 | 50.0 | - | - |
| Engine Speed [rpm]/Imep [bar] | Fuel | Injection Mode | SOI [cad BTDC] | SOS [cad ATDC] | TP [%] | DOI [cad] |
|---|---|---|---|---|---|---|
| 2000/6 | gasoline | PFI | 315 | 37.0 | 4 | 64 |
| methanol | PFI | 360 | 40.0 | 4 | 155 | |
| CH4 | DI | 305 | 22.5 | 8 | 175 | |
| H2 | DI | 310 | 9.6 | 95 | 200 | |
| 2500/6 | gasoline | PFI | 315 | 39.0 | 4 | 80 |
| methanol | PFI | 360 | 40.0 | 4 | 170 | |
| CH4 | DI | 315 | 24.3 | 14 | 238 | |
| H2 | DI | 330 | 6.5 | 95 | 320 | |
| 3000/6 | gasoline | PFI | 315 | 44.0 | 4 | 89 |
| methanol | PFI | 360 | 45.0 | 4 | 175 | |
| CH4 | DI | 315 | 28.5 | 10 | 270 | |
| H2 | DI | 352 | 10.0 | 95 | 245 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Catapano, F.; Di Iorio, S.; Magno, A.; Sementa, P.; Vaglieco, B.M. A Complete Assessment of the Emission Performance of an SI Engine Fueled with Methanol, Methane and Hydrogen. Energies 2024, 17, 1026. https://doi.org/10.3390/en17051026
Catapano F, Di Iorio S, Magno A, Sementa P, Vaglieco BM. A Complete Assessment of the Emission Performance of an SI Engine Fueled with Methanol, Methane and Hydrogen. Energies. 2024; 17(5):1026. https://doi.org/10.3390/en17051026
Chicago/Turabian StyleCatapano, Francesco, Silvana Di Iorio, Agnese Magno, Paolo Sementa, and Bianca Maria Vaglieco. 2024. "A Complete Assessment of the Emission Performance of an SI Engine Fueled with Methanol, Methane and Hydrogen" Energies 17, no. 5: 1026. https://doi.org/10.3390/en17051026
APA StyleCatapano, F., Di Iorio, S., Magno, A., Sementa, P., & Vaglieco, B. M. (2024). A Complete Assessment of the Emission Performance of an SI Engine Fueled with Methanol, Methane and Hydrogen. Energies, 17(5), 1026. https://doi.org/10.3390/en17051026








