Lithium-Ion Batteries on Board: A Review on Their Integration for Enabling the Energy Transition in Shipping Industry
Abstract
1. Introduction
2. Use of Batteries on Ships
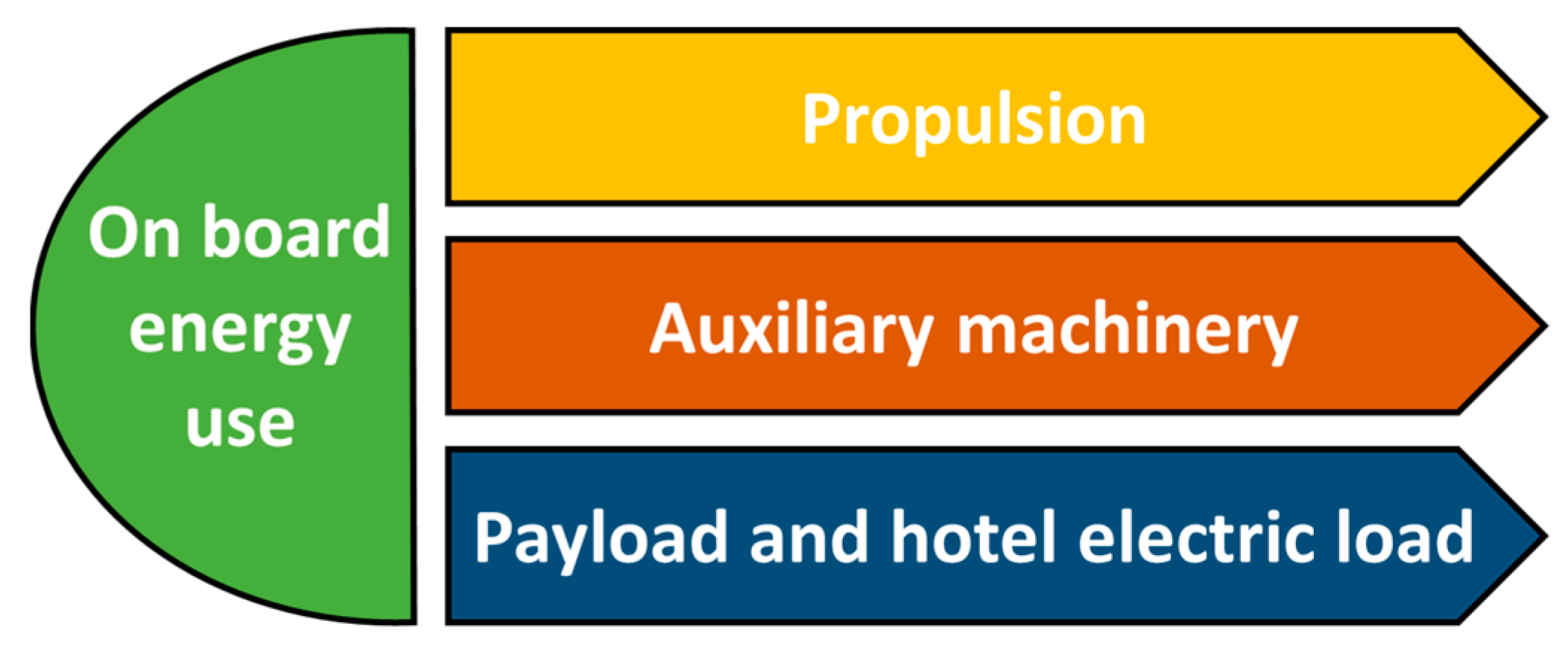
2.1. Possible Battery Services for Ships
2.2. Battery Use by Ship Type and Reference Projects
3. Lithium-Ion Batteries for the Marine Industry and Future Perspectives
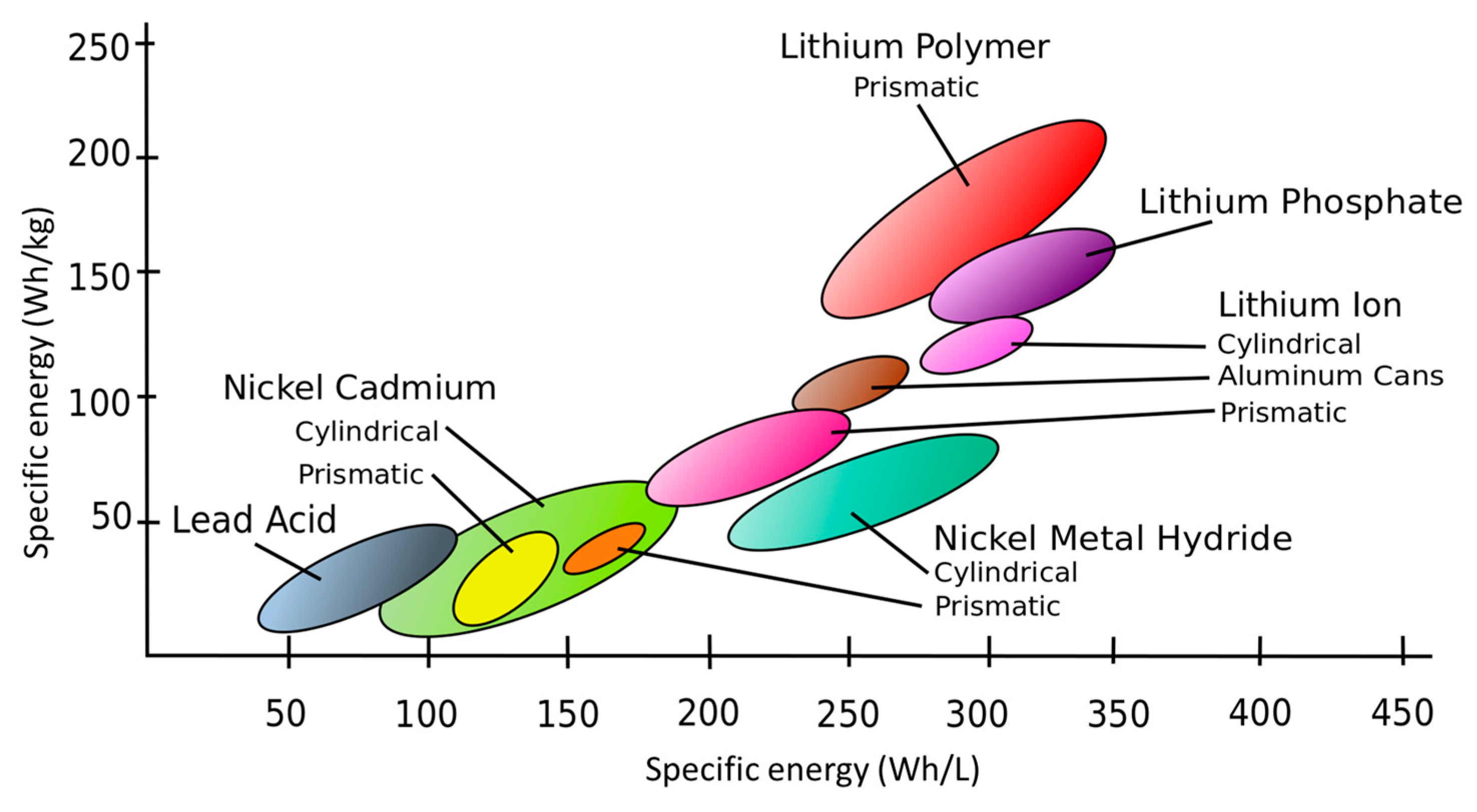
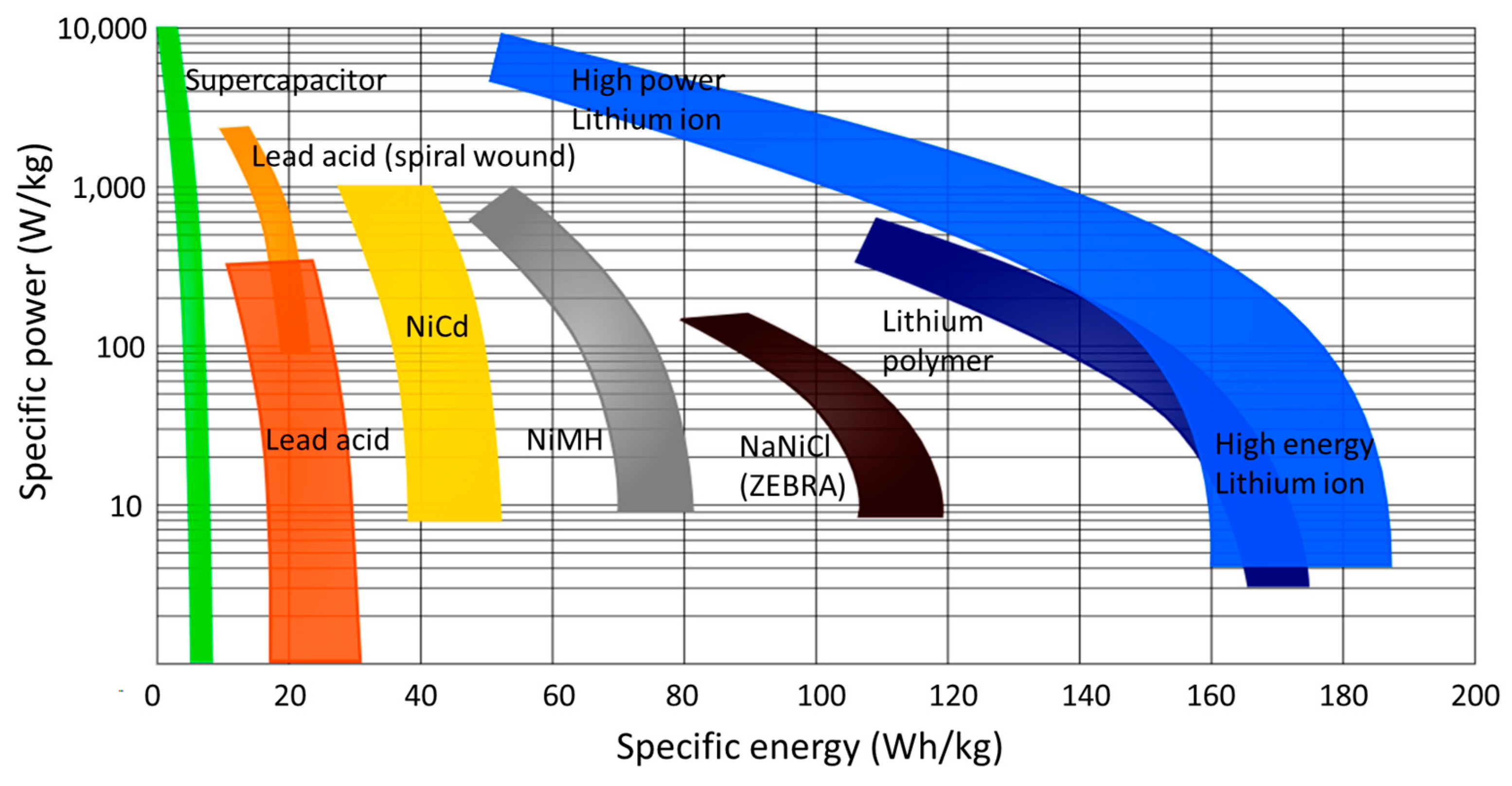
3.1. Lithium-Ion Battery Types
| Chemistry | Cycles Number | Cell Voltage (V) | Specific Capacity (Ah/kg) | Volumetric Capacity (Ah/L) | Specific Energy (Wh/kg) | Main Features | Uses IN Marine Applications |
|---|---|---|---|---|---|---|---|
| LCO | 500–1000 [53] | 3.8 [54] 3.9 [55] | 145 [54] 130–155 [55] | 550 [54] | 150–190 [53] | High specific energy Low thermal stability Limited safety and power Low cycle life Toxicity and High cost due to Cobalt | - |
| LMO | 1000–1500 [53] | 4.1 [54] 4 [55] 3.7 [56] | 120 [54] 100–120 [55] | 596 [54] | 100–140 [53] | Average number of cycles High C-rates Low specific energy Low toxicity Low cost (Cobalt free) | LMO used in combination with LTO anodes |
| NMC | 1000–2000 [53] | 3.7 [54] | 170 [54] 160–170 [57] | 600 [54] | 140–200 [53] | High voltage High specific energy Rapid aging in terms of specific energy fade Good thermal stability Tailored composition possible | Largely adopted, thanks to flexible features in terms of energy and power capabilities |
| NCA | 1000–1500 [53] | 3.7 [54] | 200 [54] | 700 [54] | 200–250 [53] | High specific energy Good calendar life Low cycle life Low safety Moderate cost due to limited Co | Used for application with high energy request |
| LFP | up to 2000 [53] 1000–2500 [55] | 3.4 [54] 3.3 [55] | 165 [54] 150–170 [55] | 589 [54] | 90–140 [53] | High safety High number of cycles Low specific energy High self-discharge Wide SoC window (15–100%) “Eco-friendly” materials Low cost for Co absence | Used for applications due to the best safety and flat voltage curve |
| LTO | above 3000 [58] | 2.6 with LMO [59] | 175 [54] | 77 with LFP cathode [59] | 50–80 [60] | High cycle life High safety High C-rates Low specific energy | Used for applications thanks to fast charging capability, high power and very high cycling |
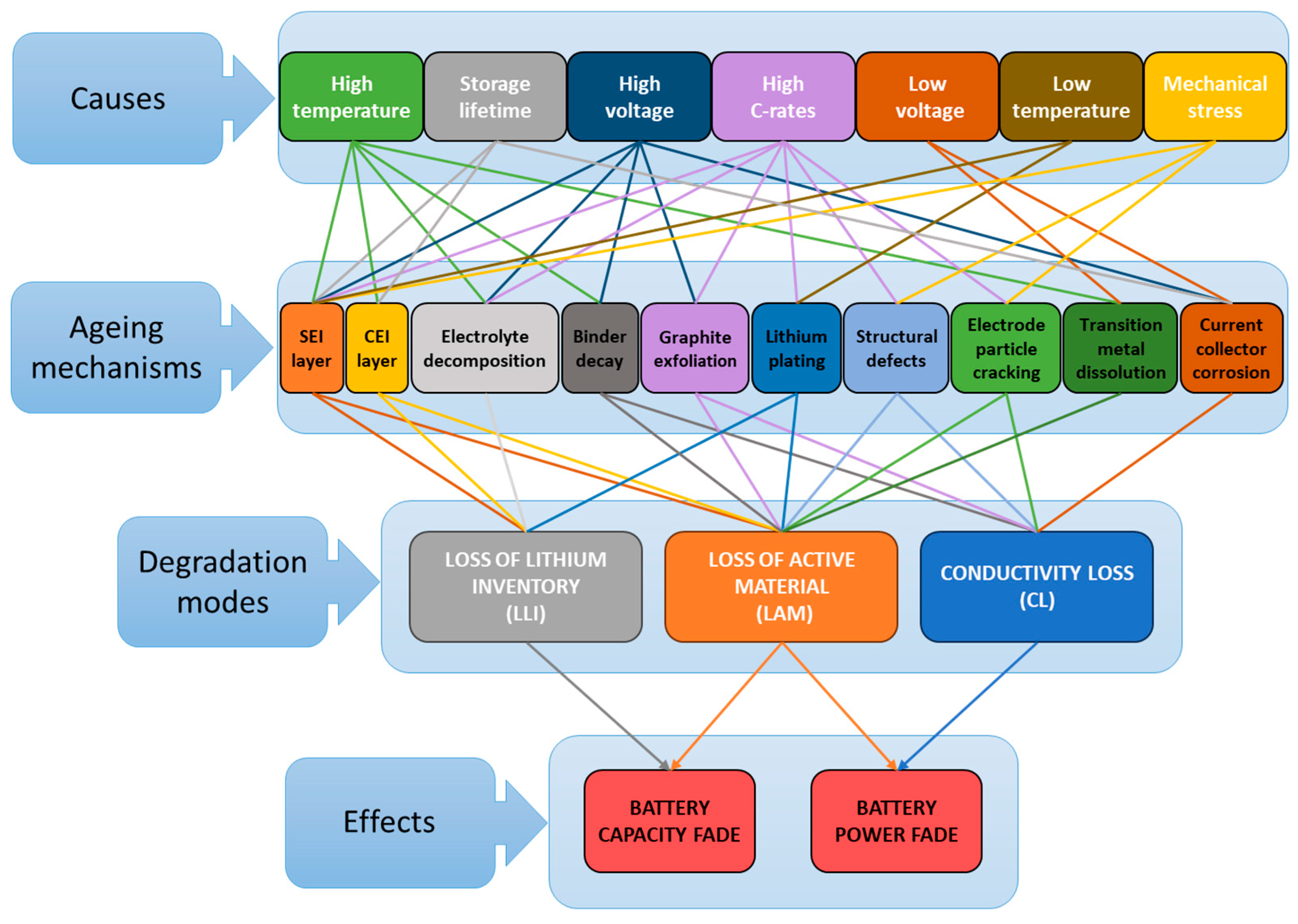
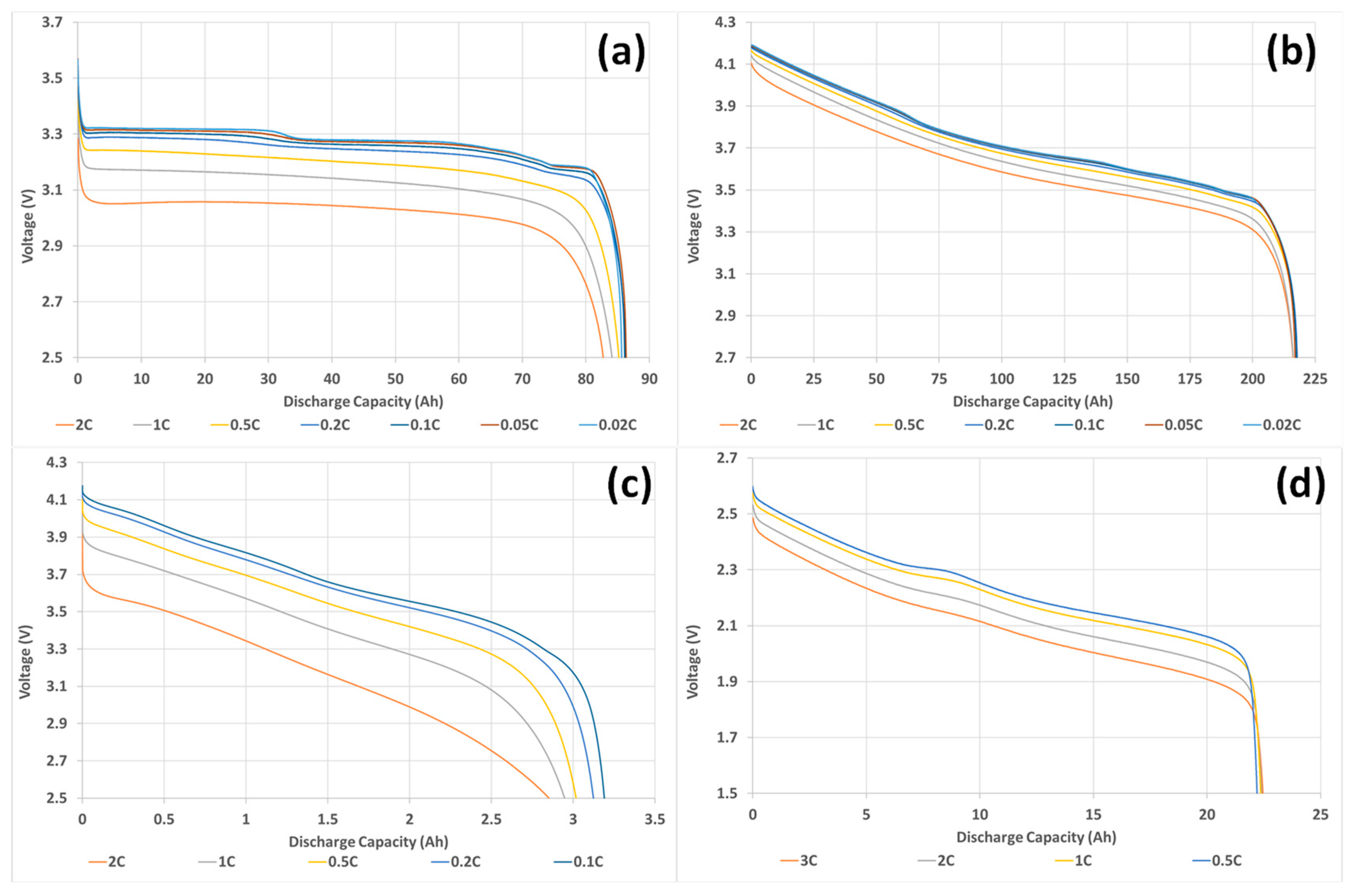
3.2. Lithium-Ion Battery Ageing
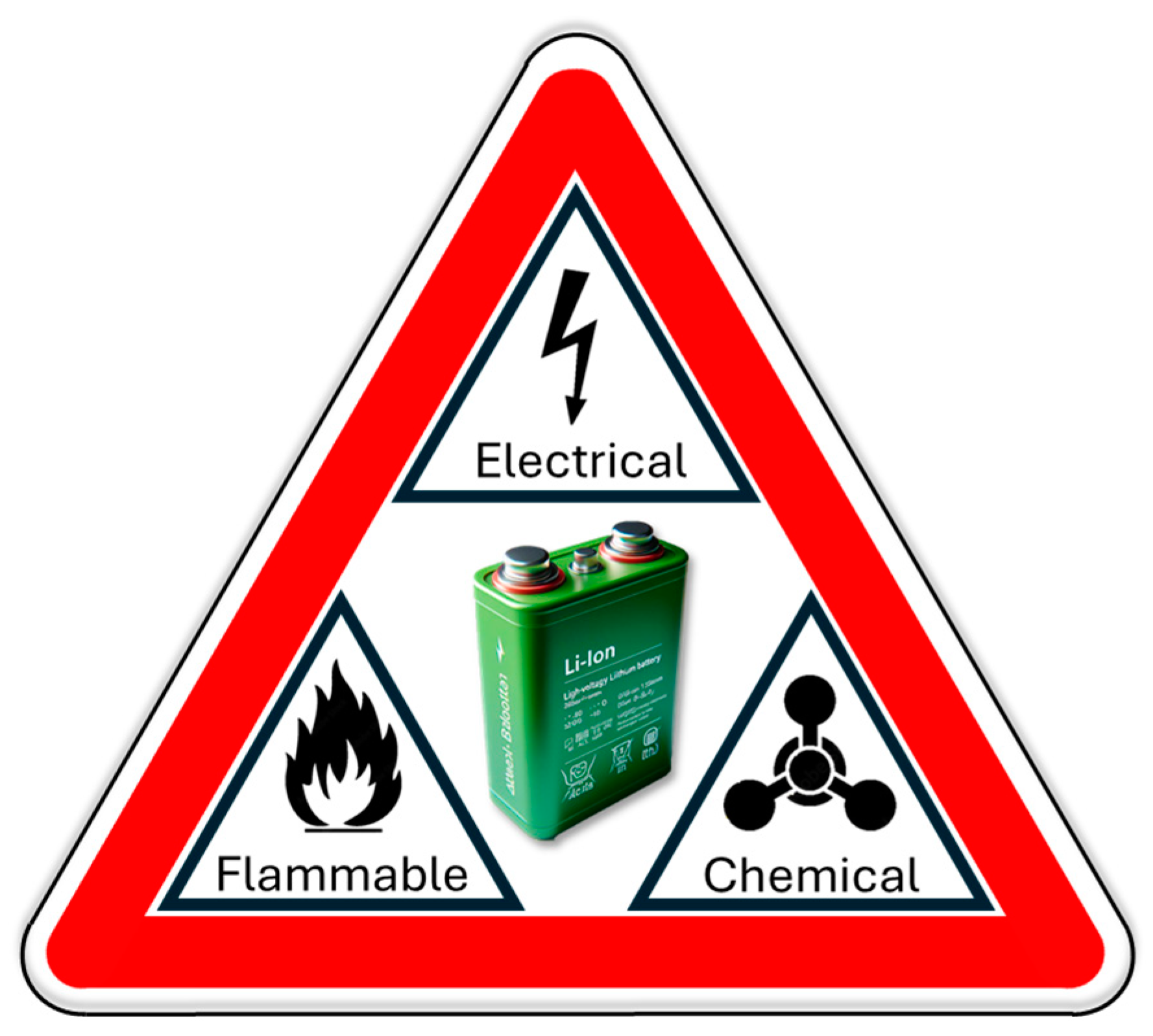
3.3. Lithium-Ion Battery Hazards
3.4. Battery Perspectives in the Marine Industry
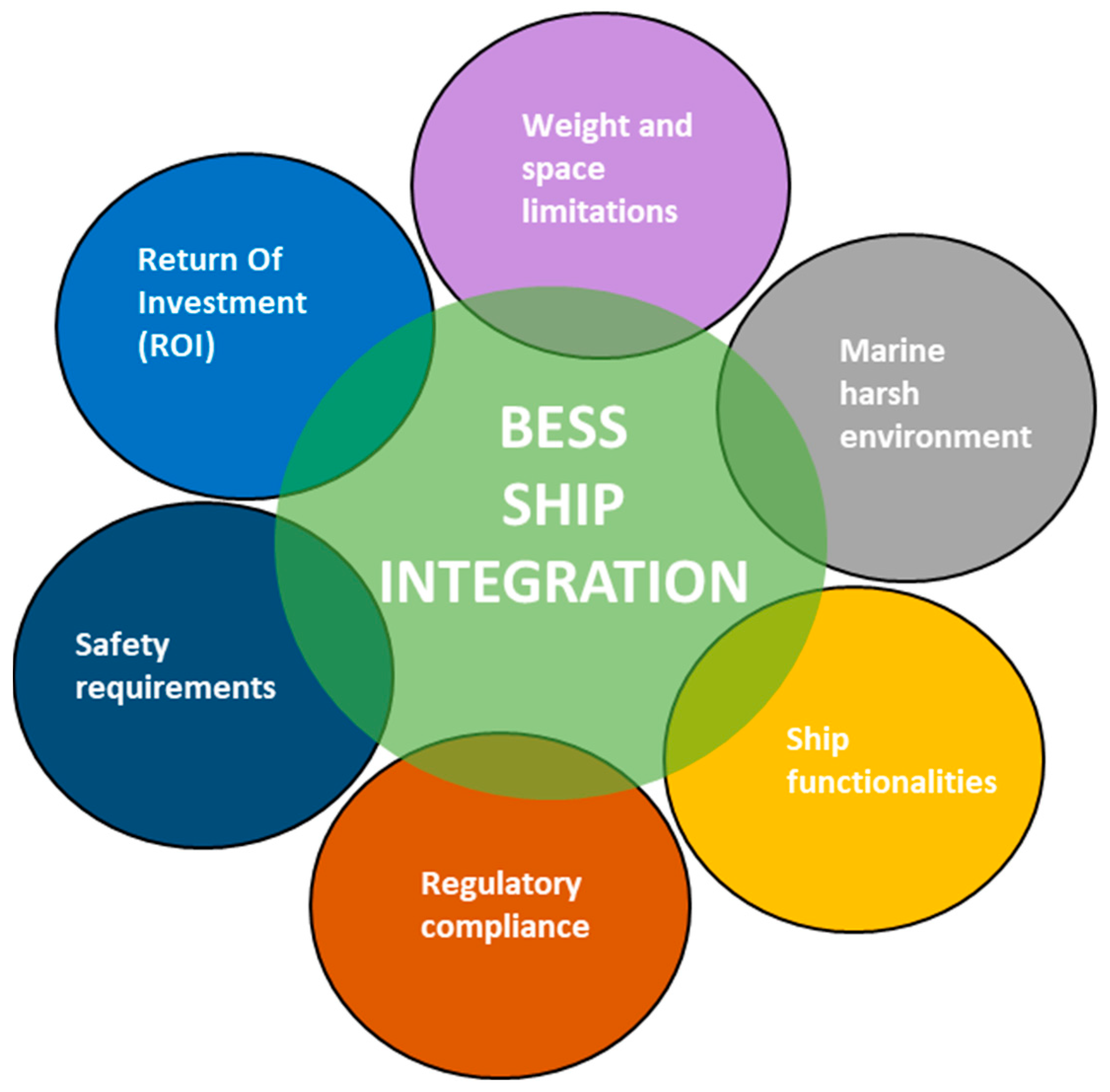
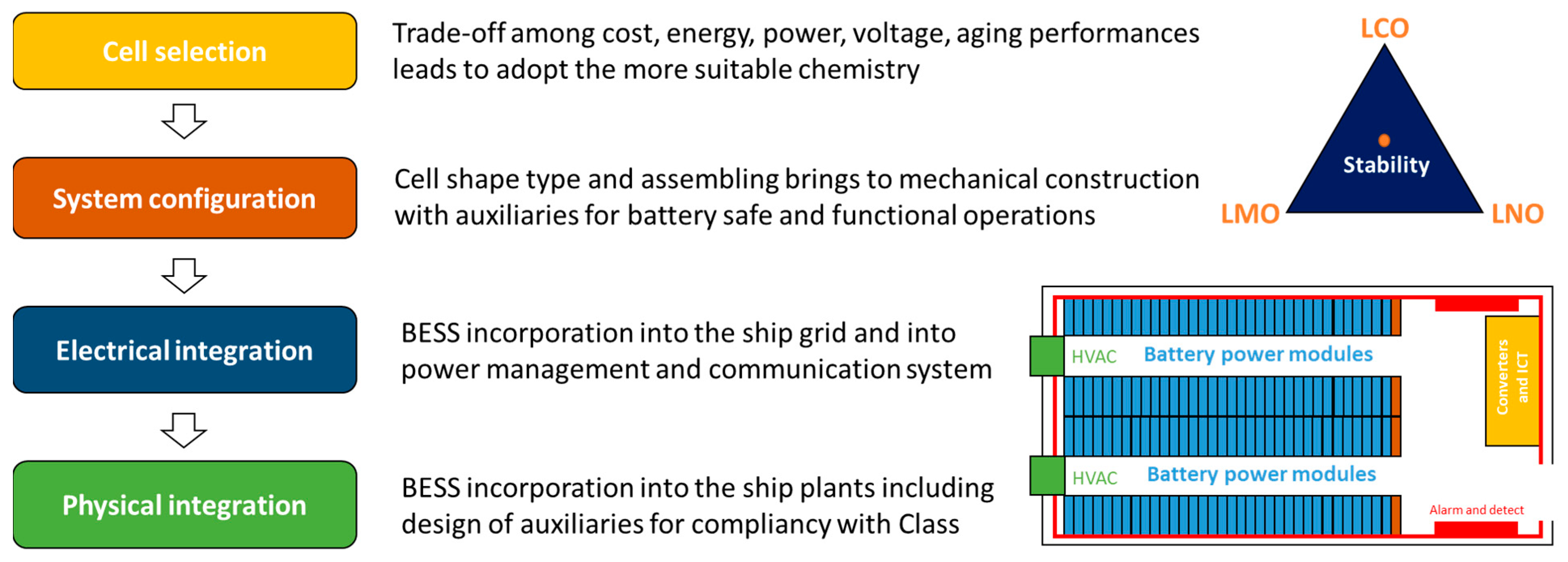
4. Battery System Integration for Ships
4.1. Battery Selection Criteria for Ship Applications
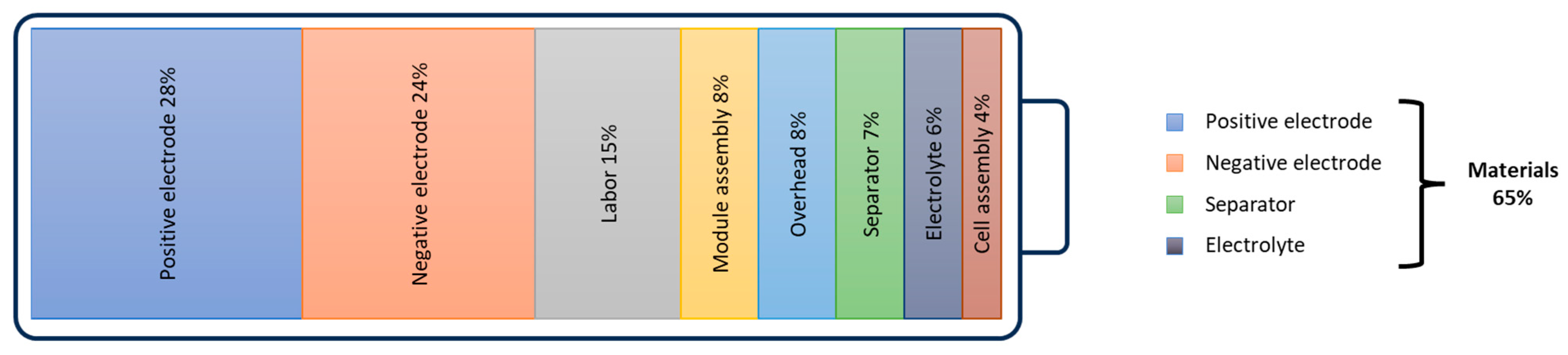
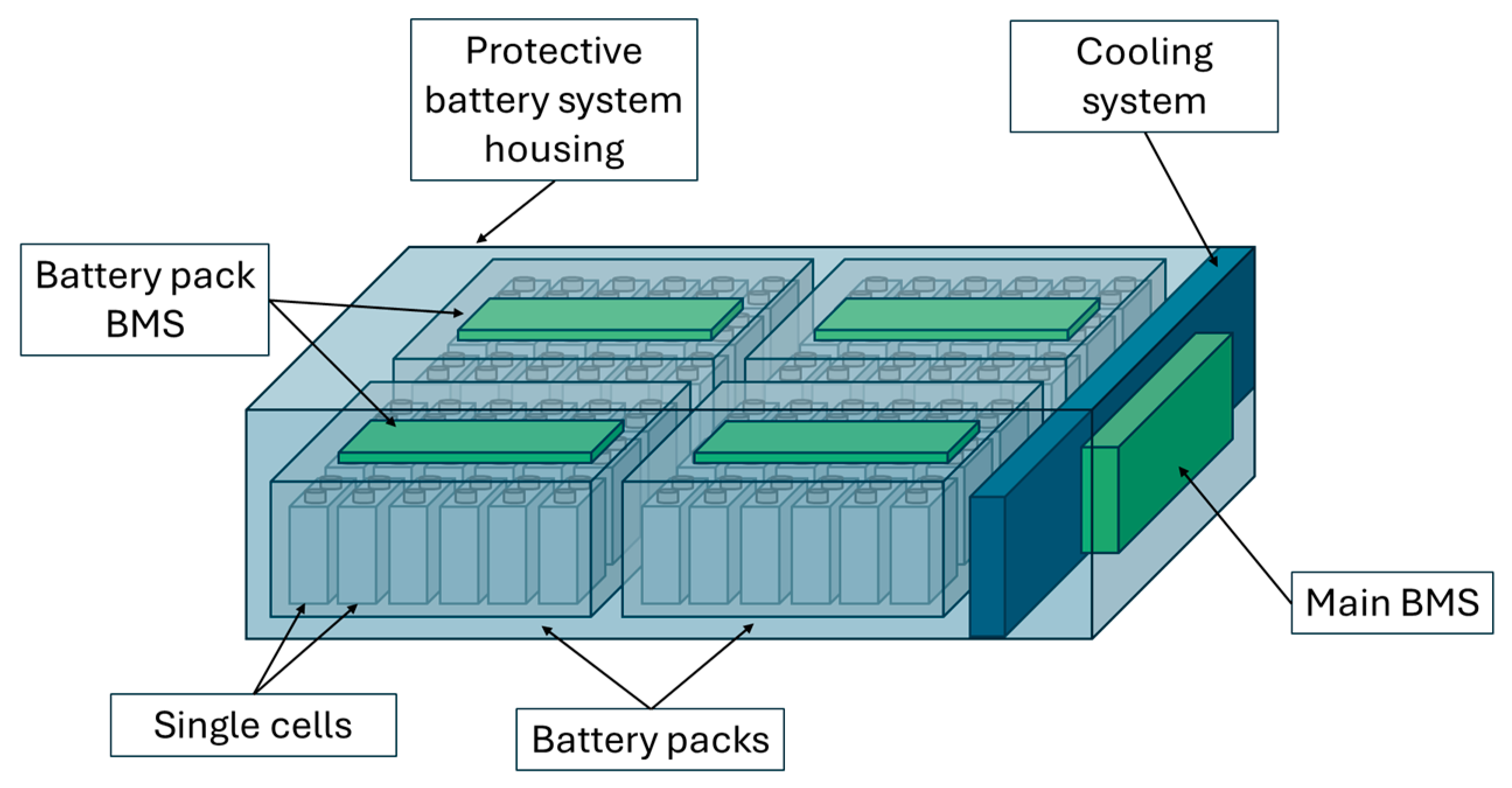
4.2. From Cell to Battery System Configuration
4.2.1. Mechanical Construction and Casing
4.2.2. Considerations at System Level
4.3. Battery Electrical and ITC Integration
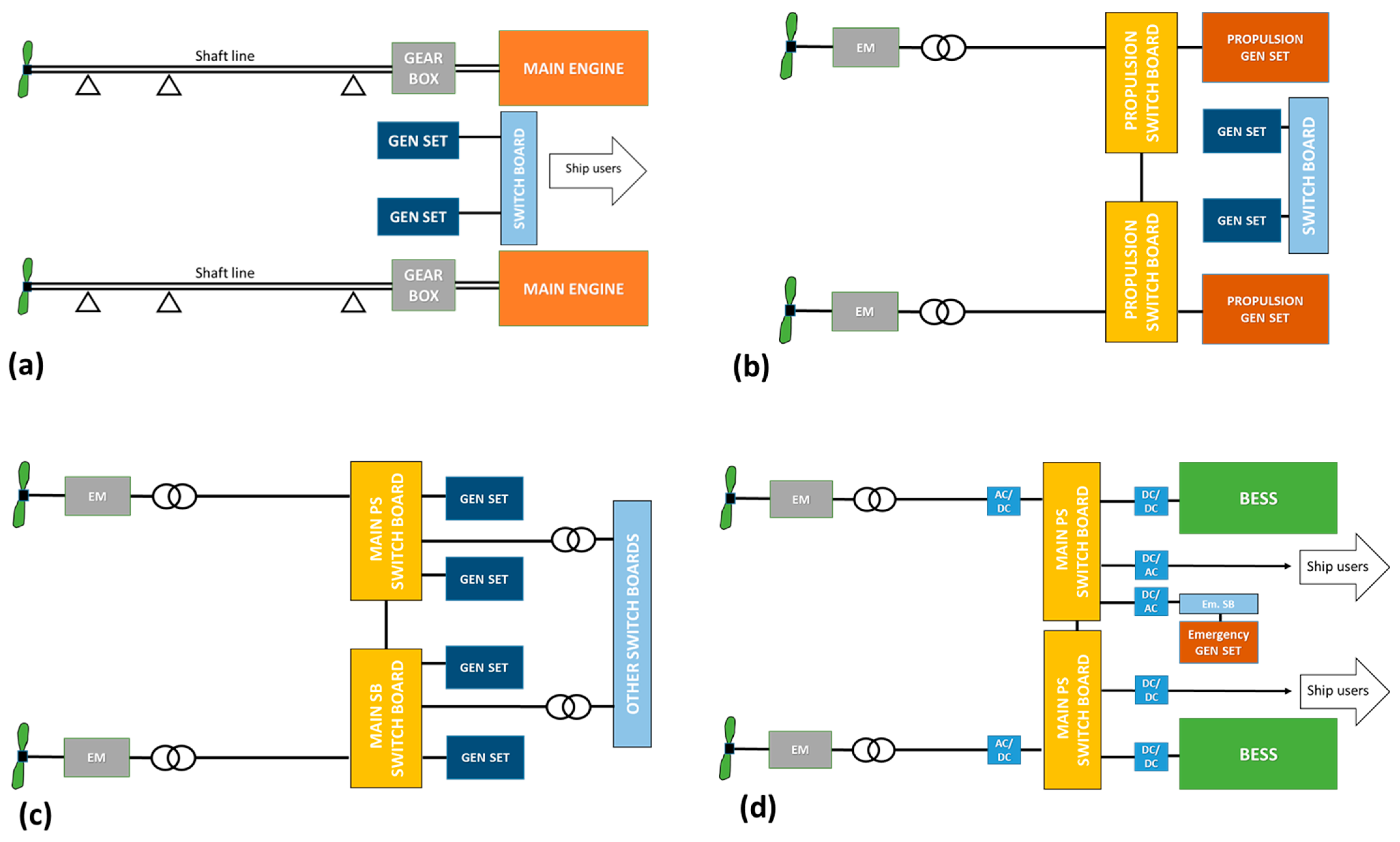
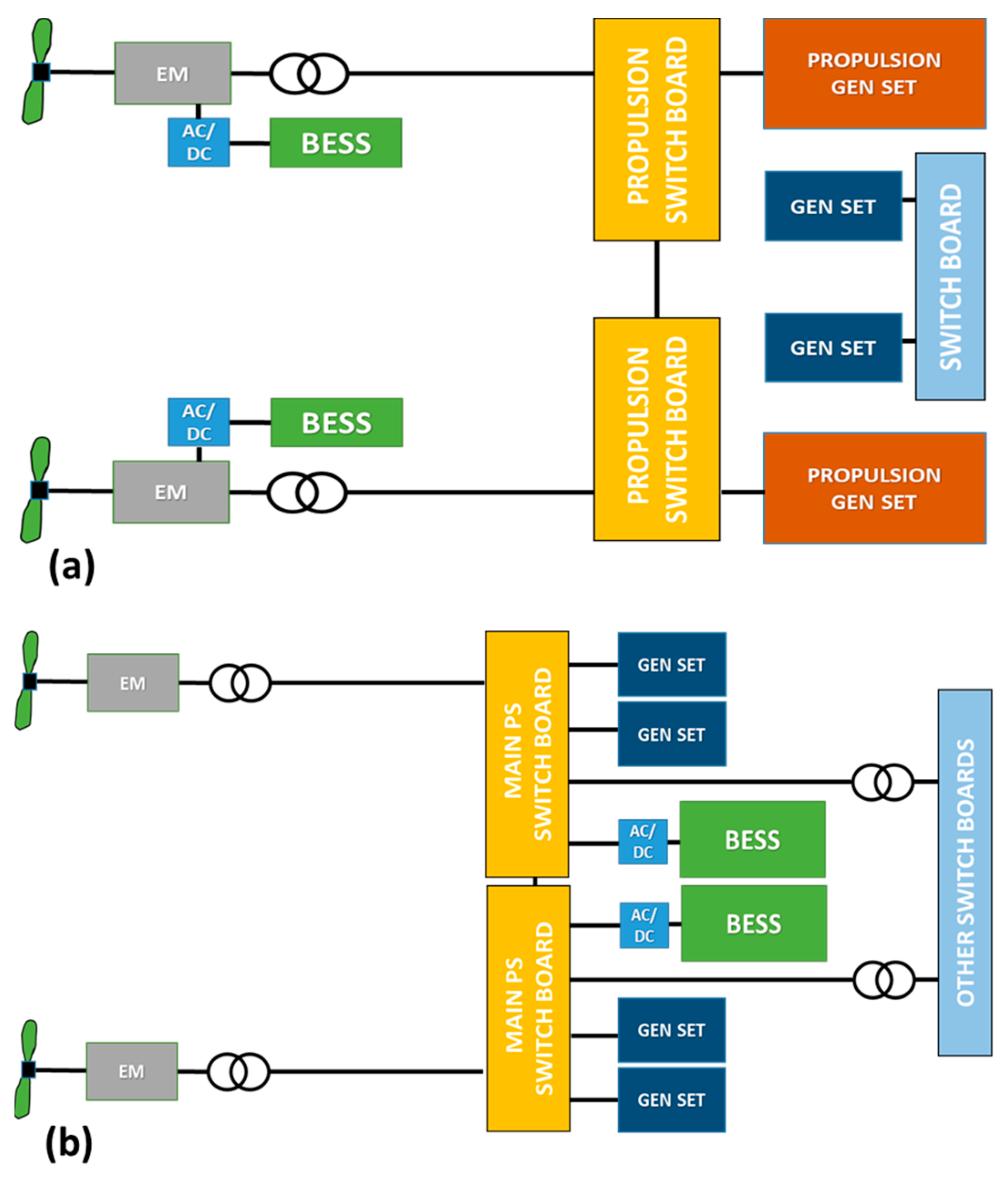
4.3.1. Ship Electrical Grid Topology and Distribution
4.3.2. BESSs for Power Quality
4.3.3. Integrated Power and Energy System
4.4. Physical Battery Integration on Board
5. Regulations for BESSs: Class Rules and International Codes
5.1. Approval Process for BESSs on Board Ships and Technical Standards
5.2. General Technical Content of BESS Rules for Ship and Comparison among Main Class Societies
6. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Reusser, C.A.; Pérez Osses, J.R. Challenges for Zero-Emissions Ship. J. Mar. Sci. Eng. 2021, 9, 1042. [Google Scholar] [CrossRef]
- IMO. Fourth IMO Greenhouse Gas Study 2020—Executive Summary; International Maritime Organization: London, UK, 2021. [Google Scholar]
- Psaraftis, H.N. Green Maritime Transportation: Market Based Measures. In Green Transportation Logistics: The Quest for Win-Win Solutions; Springer: Cham, Germany, 2016; ISBN 9783319171753. [Google Scholar]
- Buhaug, Ø.; Corbett, J.; Endresen, Ø.; Eyring, V.; Faber, J.; Hanayama, S.; Lee, D.; Lee, D.; Lindstad, H.; Markowska, A.; et al. Second IMO GHG Study 2009; International Maritime Organization: London, UK, 2009. [Google Scholar]
- Hansen, J.F.; Wendt, F. History and State of the Art in Commercial Electric Ship Propulsion, Integrated Power Systems, and Future Trends. Proc. IEEE 2015, 103, 2229–2242. [Google Scholar] [CrossRef]
- Sulligoi, G.; Vicenzutti, A.; Menis, R. All-Electric Ship Design: From Electrical Propulsion to Integrated Electrical and Electronic Power Systems. IEEE Trans. Transp. Electrif. 2016, 2, 507–521. [Google Scholar] [CrossRef]
- Zhou, Z.; Benbouzid, M.; Frédéric Charpentier, J.; Scuiller, F.; Tang, T. A Review of Energy Storage Technologies for Marine Current Energy Systems. Renew. Sustain. Energy Rev. 2013, 18, 390–400. [Google Scholar] [CrossRef]
- Wang, Z.; Carriveau, R.; Ting, D.S.K.; Xiong, W.; Wang, Z. A Review of Marine Renewable Energy Storage. Int. J. Energy Res. 2019, 43, 6108–6150. [Google Scholar] [CrossRef]
- Adeyemo, A.A.; Alves, E.; Marra, F.; Brandao, D.; Tedeschi, E. Suitability Assessment of High-Power Energy Storage Technologies for Offshore Oil and Gas Platforms: A Life Cycle Cost Perspective. J. Energy Storage 2023, 61, 106643. [Google Scholar] [CrossRef]
- Stampatori, D.; Raimondi, P.P.; Noussan, M. Li-Ion Batteries: A Review of a Key Technology for Transport Decarbonization. Energies 2020, 13, 2638. [Google Scholar] [CrossRef]
- Verma, J.; Kumar, D. Recent Developments in Energy Storage Systems for Marine Environment. Mater. Adv. 2021, 2, 6800–6815. [Google Scholar] [CrossRef]
- Papanikolaou, A.D. Review of the Design and Technology Challenges of Zero-Emission, Battery-Driven Fast Marine Vehicles. J. Mar. Sci. Eng. 2020, 8, 941. [Google Scholar] [CrossRef]
- Kolodziejski, M.; Michalska-Pozoga, I. Battery Energy Storage Systems in Ships’ Hybrid/Electric Propulsion Systems. Energies 2023, 16, 1122. [Google Scholar] [CrossRef]
- Szymborski, J. Lead-Acid Batteries for Use in Submarine Applications. In Proceedings of the IEEE Symposium on Autonomous Underwater Vehicle Technology, San Antonio, TX, USA, 20–21 June 2002. [Google Scholar] [CrossRef]
- Mutarraf, M.U.; Terriche, Y.; Niazi, K.A.K.; Vasquez, J.C.; Guerrero, J.M. Energy Storage Systems for Shipboard Microgrids—A Review. Energies 2018, 11, 3492. [Google Scholar] [CrossRef]
- Alnes, O.; Eriksen, S.; Vartdal, B.-J. Battery-Powered Ships: A Class Society Perspective. IEEE Electrif. Mag. 2017, 5, 10–21. [Google Scholar] [CrossRef]
- Kaur, D.; Singh, M.; Singh, S. Lithium-Sulfur Batteries for Marine Applications. In Lithium-Sulfur Batteries: Materials, Challenges and Applications; Elsevier: Amsterdam, The Netherlands, 2022; ISBN 9780323919340. [Google Scholar] [CrossRef]
- Thongam, J.S.; Tarbouchi, M.; Okou, A.F.; Bouchard, D.; Beguenane, R. All-Electric Ships—A Review of the Present State of the Art. In Proceedings of the 2013 8th International Conference and Exhibition on Ecological Vehicles and Renewable Energies, EVER 2013, Monte Carlo, Monaco, 27–30 March 2013; pp. 1–8. [Google Scholar] [CrossRef]
- Vieira, G.T.T.; Peralta, C.O.; Salles, M.B.C.; Carmo, B.S. Reduction of CO2 Emissions in Ships with Advanced Energy Storage Systems. In Proceedings of the 2017 6th International Conference on Clean Electrical Power: Renewable Energy Resources Impact, ICCEP 2017, Santa Margherita Ligure, Italy, 27–29 June 2017; pp. 564–571. [Google Scholar] [CrossRef]
- Wu, P.; Bucknall, R. Marine Propulsion Using Battery Power. In Proceedings of the Shipping in Changing Climates Conference, Newcastle, UK, 10–11 November 2016. Available online: https://discovery.ucl.ac.uk/id/eprint/1528988/ (accessed on 28 January 2024).
- Dale, S.J.; Hebner, R.E.; Sulligoi, G. Electric Ship Technologies. Proc. IEEE 2015, 103, 2225–2228. [Google Scholar] [CrossRef]
- Kim, K.; Park, K.; Ahn, J.; Roh, G.; Chun, K. A Study on Applicability of Battery Energy Storage System (BESS) for Electric Propulsion Ships. In Proceedings of the 2016 IEEE Transportation Electrification Conference and Expo, Asia-Pacific, ITEC Asia-Pacific 2016, Busan, Republic of Korea, 1–4 June 2016; pp. 203–207. [Google Scholar]
- Kim, S.Y.; Choe, S.; Ko, S.; Sul, S.K. A Naval Integrated Power System with a Battery Energy Storage System: Fuel Efficiency, Reliability, and Quality of Power. IEEE Electrif. Mag. 2015, 3, 22–33. [Google Scholar] [CrossRef]
- Tang, R.; Wu, Z.; Li, X. Optimal Operation of Photovoltaic/Battery/Diesel/Cold-Ironing Hybrid Energy System for Maritime Application. Energy 2018, 162, 697–714. [Google Scholar] [CrossRef]
- Lee, K.J.; Shin, D.; Yoo, D.W.; Choi, H.K.; Kim, H.J. Hybrid Photovoltaic/Diesel Green Ship Operating in Standalone and Grid-Connected Mode—Experimental Investigation. Energy 2013, 49, 475–483. [Google Scholar] [CrossRef]
- Nyanya, M.N.; Vu, H.B.; Schönborn, A.; Ölçer, A.I. Wind and Solar Assisted Ship Propulsion Optimisation and Its Application to a Bulk Carrier. Sustain. Energy Technol. Assess. 2021, 47, 101397. [Google Scholar] [CrossRef]
- Pan, P.; Sun, Y.; Yuan, C.; Yan, X.; Tang, X. Research Progress on Ship Power Systems Integrated with New Energy Sources: A Review. Renew. Sustain. Energy Rev. 2021, 144, 111048. [Google Scholar] [CrossRef]
- Spagnolo, G.S.; Papalillo, D.; Martocchia, A.; Makary, G. Solar-Electric Boat. J. Transp. Technol. 2012, 02, 144–149. [Google Scholar] [CrossRef]
- Leem, J.; Park, J.W.; Lee, H.J. A Study of the Method for Calculating the Optimal Generator Capacity of a Ship Based on Lng Carrier Operation Data. Electronics 2021, 10, 258. [Google Scholar] [CrossRef]
- He, W.; Mo, O.; Remøy, A.; Valøen, L.O.; Såtendal, H.; Howie, A.; Vie, P.J.S. Accelerating Efficient Installation and Optimization of Battery Energy Storage System Operations Onboard Vessels. Energies 2022, 15, 4908. [Google Scholar] [CrossRef]
- Godjevac, M.; Visser, K.; Boonen, E.J.; Malikouti, C.; Mestemaker, B.T.W.; Lyu, Z.; Van Der Veen, F. Electrical Energy Storage for Dynamic Positiong Operations: Investigation of Three Application Case. In Proceedings of the 2017 IEEE Electric Ship Technologies Symposium (ESTS 2017), Arlington, VA, USA, 14–17 August 2017; pp. 182–186. [Google Scholar] [CrossRef]
- Castellan, S.; Menis, R.; Pigani, M.; Sulligoi, G.; Tessarolo, A. Modeling and Simulation of Electric Propulsion Systems for All-Electric Cruise Liners. In Proceedings of the IEEE Electric Ship Technologies Symposium (ESTS 2007), Arlington, VA, USA, 21–23 May 2007; 2014, pp. 1–8. [CrossRef]
- Ko, S.; Yun, J.; Sul, S.K.; Kang, S.W.; Park, W.; Kim, S.H. Control Strategy of Electric Propulsion System to Improve Ship Dynamics. In Proceedings of the 2021 IEEE Transportation Electrification Conference and Expo (ITEC 2021), Chicago, IL, USA, 21–25 June 2021. pp. 528–532. [CrossRef]
- Luckose, L.; Hess, H.L.; Johnson, B.K. Fuel Cell Propulsion System for Marine Applications. In Proceedings of the IEEE Electric Ship Technologies Symposium (ESTS 2009), Baltimore, MD, USA, 20–22 April 2009; pp. 574–580. [Google Scholar] [CrossRef]
- Park, D.; Zadeh, M.K.; Skjetne, R. DC-DC Converter Control for Peak-Shaving in Shipboard DC Power System via Hybrid Control. In Proceedings of the Proceedings of the 15th IEEE Conference on Industrial Electronics and Applications (ICIEA 2020), Kristiansand, Norway, 9–13 November 2020; pp. 528–532. [Google Scholar] [CrossRef]
- Acanfora, M.; Balsamo, F.; Fantauzzi, M.; Lauria, D.; Proto, D. Design of an Electrical Energy Storage System for Hybrid Diesel Electric Ship Propulsion Aimed at Load Levelling in Irregular Wave Conditions. Appl. Energy 2023, 350, 121728. [Google Scholar] [CrossRef]
- Bordin, C.; Mo, O. Including Power Management Strategies and Load Profiles in the Mathematical Optimization of Energy Storage Sizing for Fuel Consumption Reduction in Maritime Vessels. J. Energy Storage 2019, 23, 425–441. [Google Scholar] [CrossRef]
- Kim, S.Y.; Choe, S.; Ko, S.; Kim, S.; Sul, S.K. Electric Propulsion Naval Ships with Energy Storage Modules through AFE Converters. J. Power Electron. 2014, 14, 402–412. [Google Scholar] [CrossRef][Green Version]
- IDTechEx. Electric Leisure & Sea-Going Boats and Ships 2021–2040. 2021. Available online: https://www.idtechex.com/en/research-report/electric-leisure-and-sea-going-boats-and-ships-2021-2040/739 (accessed on 18 February 2024).
- Akbarzadeh, M.; De Smet, J.; Stuyts, J. Battery Hybrid Energy Storage Systems for Full-Electric Marine Applications. Processes 2022, 10, 2418. [Google Scholar] [CrossRef]
- Lan, H.; Wen, S.; Hong, Y.Y.; Yu, D.C.; Zhang, L. Optimal Sizing of Hybrid PV/Diesel/Battery in Ship Power System. Appl. Energy 2015, 158, 26–34. [Google Scholar] [CrossRef]
- Reddy, M.V.; Mauger, A.; Julien, C.M.; Paolella, A.; Zaghib, K. Brief History of Early Lithium-Battery Development. Materials 2020, 13, 1884. [Google Scholar] [CrossRef]
- Inal, O.B.; Charpentier, J.F.; Deniz, C. Hybrid Power and Propulsion Systems for Ships: Current Status and Future Challenges. Renew. Sustain. Energy Rev. 2022, 156, 111965. [Google Scholar] [CrossRef]
- Sabihuddin, S.; Kiprakis, A.E.; Mueller, M. A Numerical and Graphical Review of Energy Storage Technologies. Energies 2015, 8, 172–216. [Google Scholar] [CrossRef]
- Tsiropoulos, I.; Tarvydas, D.; Lebedeva, N. Li-Ion Batteries for Mobility and Stationary Storage Applications; Publications Office of the European Union: Luxembourg, 2018; ISBN 978-92-79-97254-6. [Google Scholar] [CrossRef]
- Fleischmann, J.; Hanicke, M.; Horetsky, E.; Ibrahim, D.; Jautelat, S.; Linder, M.; Schaufuss, P.; Torscht, L.; van de Rijt, A. Battery 2030: Resilient, Sustainable, and Circular; McKinsey & Company: Chicago, IL, USA, 2023; pp. 2–18. [Google Scholar]
- Goldie-Scot, L. A Behind the Scenes Take on Lithium-Ion Battery Prices; BloombergNEF: 2019. Available online: https://about.bnef.com/blog/behind-scenes-take-lithium-ion-battery-prices/ (accessed on 18 February 2024).
- Berckmans, G.; Messagie, M.; Smekens, J.; Omar, N.; Vanhaverbeke, L.; Mierlo, J. Van Cost Projection of State of the Art Lithium-Ion Batteries for Electric Vehicles up to 2030. Energies 2017, 10, 1314. [Google Scholar] [CrossRef]
- Wang, L.; Chen, B.; Ma, J.; Cui, G.; Chen, L. Reviving Lithium Cobalt Oxide-Based Lithium Secondary Batteries-toward a Higher Energy Density. Chem. Soc. Rev. 2018, 47, 6505–6602. [Google Scholar] [CrossRef]
- Schwenzel, J.; Thangadurai, V.; Weppner, W. New Trends in Intercalation Compounds for Energy Storage and Conversion. In Proceedings of the International Symposium; ECS: Paris, France, 2003; pp. 575–583. ISBN 1566774039/9781566774031. [Google Scholar]
- Du Pasquier, A.; Plitz, I.; Menocal, S.; Amatucci, G. A Comparative Study of Li-Ion Battery, Supercapacitor and Nonaqueous Asymmetric Hybrid Devices for Automotive Applications. J. Power Sources 2003, 115, 171–178. [Google Scholar] [CrossRef]
- Dahn, J.R.; Fuller, E.W.; Obrovac, M.; von Sacken, U. Thermal Stability of LixCoO2, LixNiO2 and λ-MnO2 and Consequences for the Safety of Li-Ion Cells. Solid State Ion. 1994, 69, 265–270. [Google Scholar] [CrossRef]
- Zubi, G.; Dufo-López, R.; Carvalho, M.; Pasaoglu, G. The Lithium-Ion Battery: State of the Art and Future Perspectives. Renew. Sustain. Energy Rev. 2018, 89, 292–308. [Google Scholar] [CrossRef]
- Nitta, N.; Wu, F.; Lee, J.T.; Yushin, G. Li-Ion Battery Materials: Present and Future. Mater. Today 2015, 18, 252–264. [Google Scholar] [CrossRef]
- Sarasketa-Zabala, E. A Novel Approach for Lithium-Ion Battery Selection and Lifetime Prediction; Mondragon University: Guipúzcoa, Spain, 2014. [Google Scholar]
- Shi, L.; Xie, W.; Ge, Q.; Wang, S.; Chen, D.; Qin, L.; Fan, M.; Bai, L.; Chen, Z.; Shen, H.; et al. Preparation and Electrochemical Performances of LiNi0.5Mn0.5O2 Cathode Materials for Lithium-Ion Batteries. Int. J. Electrochem. Sci. 2015, 10, 4696–4705. [Google Scholar] [CrossRef]
- Hassoun, J.; Reale, P.; Scrosati, B. Recent Advances in Liquid and Polymer Lithium-Ion Batteries. J. Mater. Chem. 2007, 17, 3668. [Google Scholar] [CrossRef]
- Takami, N.; Inagaki, H.; Tatebayashi, Y.; Saruwatari, H.; Honda, K.; Egusa, S. High-Power and Long-Life Lithium-Ion Batteries Using Lithium Titanium Oxide Anode for Automotive and Stationary Power Applications. J. Power Sources 2013, 244, 469–475. [Google Scholar] [CrossRef]
- Sandhya, C.P.; John, B.; Gouri, C. Lithium Titanate as Anode Material for Lithium-Ion Cells: A Review. Ionics 2014, 20, 601–620. [Google Scholar] [CrossRef]
- EMSA Electrical Energy Storage for Ships. Battery Systems for Maritime Application—Technology Sustainability and Safety; EMSA: Lisbon, Portugal, 2020; Available online: https://www.emsa.europa.eu/publications/item/3895-study-on-electrical-energy-storage-for-ships.html (accessed on 18 February 2024).
- Xu, G.; Liu, Z.; Zhang, C.; Cui, G.; Chen, L. Strategies for Improving the Cyclability and Thermo-Stability of LiMn2O4-Based Batteries at Elevated Temperatures. J. Mater. Chem. A 2015, 3, 4092–4123. [Google Scholar] [CrossRef]
- Selinis, P.; Farmakis, F. Review—A Review on the Anode and Cathode Materials for Lithium-Ion Batteries with Improved Subzero Temperature Performance. J. Electrochem. Soc. 2022, 169, 010526. [Google Scholar] [CrossRef]
- Nayak, P.K.; Erickson, E.M.; Schipper, F.; Penki, T.R.; Munichandraiah, N.; Adelhelm, P.; Sclar, H.; Amalraj, F.; Markovsky, B.; Aurbach, D. Review on Challenges and Recent Advances in the Electrochemical Performance of High Capacity Li- and Mn-Rich Cathode Materials for Li-Ion Batteries. Adv. Energy Mater. 2018, 8, 1702397. [Google Scholar] [CrossRef]
- Tu, J.; Zhao, X.B.; Cao, G.S.; Zhuang, D.G.; Zhu, T.J.; Tu, J.P. Enhanced Cycling Stability of LiMn2O4 by Surface Modification with Melting Impregnation Method. Electrochim. Acta 2006, 51, 6456–6462. [Google Scholar] [CrossRef]
- Wohlfahrt-Mehrens, M.; Vogler, C.; Garche, J. Aging Mechanisms of Lithium Cathode Materials. J. Power Sources 2004, 127, 58–64. [Google Scholar] [CrossRef]
- Zheng, H.; Sun, Q.; Liu, G.; Song, X.; Battaglia, V.S. Correlation between Dissolution Behavior and Electrochemical Cycling Performance for LiNi1/3Co1/3Mn1/3O2-Based Cells. J. Power Sources 2012, 207, 134–140. [Google Scholar] [CrossRef]
- Zhao, H.; Lam, W.Y.A.; Sheng, L.; Wang, L.; Bai, P.; Yang, Y.; Ren, D.; Xu, H.; He, X. Cobalt-Free Cathode Materials: Families and Their Prospects. Adv. Energy Mater. 2022, 12, 2103894. [Google Scholar] [CrossRef]
- Bi, K.; Zhao, S.X.; Huang, C.; Nan, C.W. Improving Low-Temperature Performance of Spinel LiNi0.5Mn1.5O4 Electrode and LiNi0.5Mn1.5O4/Li4Ti5O12 Full-Cell by Coating Solid-State Electrolyte Li-Al-Ti-P-O. J. Power Sources 2018, 389, 240–248. [Google Scholar] [CrossRef]
- Wang, F.; Borodin, O.; Ding, M.S.; Gobet, M.; Vatamanu, J.; Fan, X.; Gao, T.; Eidson, N.; Liang, Y.; Sun, W.; et al. Hybrid Aqueous/Non-Aqueous Electrolyte for Safe and High-Energy Li-Ion Batteries. Joule 2018, 2, 2178. [Google Scholar] [CrossRef]
- Sun, Y.K.; Yoon, C.S.; Lee, Y.S. Electrochemical Properties and Structural Characterization of Layered Li[Ni0.5Mn0.5]O2 Cathode Materials. Electrochim. Acta 2003, 48, 2589–2592. [Google Scholar] [CrossRef]
- Wang, X.; Ding, Y.L.; Deng, Y.P.; Chen, Z. Ni-Rich/Co-Poor Layered Cathode for Automotive Li-Ion Batteries: Promises and Challenges. Adv. Energy Mater. 2020, 10, 1903864. [Google Scholar] [CrossRef]
- Choi, J.U.; Voronina, N.; Sun, Y.K.; Myung, S.T. Recent Progress and Perspective of Advanced High-Energy Co-Less Ni-Rich Cathodes for Li-Ion Batteries: Yesterday, Today, and Tomorrow. Adv. Energy Mater. 2020, 10, 2002027. [Google Scholar] [CrossRef]
- Wang, B.; Zhang, F.L.; Zhou, X.A.; Wang, P.; Wang, J.; Ding, H.; Dong, H.; Liang, W.B.; Zhang, N.S.; Li, S.Y. Which of the Nickel-Rich NCM and NCA Is Structurally Superior as a Cathode Material for Lithium-Ion Batteries? J. Mater. Chem. A 2021, 9, 13540–13551. [Google Scholar] [CrossRef]
- Brand, M.; Gläser, S.; Geder, J.; Menacher, S.; Obpacher, S.; Jossen, A.; Quinger, D. Electrical Safety of Commercial Li-Ion Cells Based on NMC and NCA Technology Compared to LFP Technology. World Electr. Veh. J. 2013, 6, 572–580. [Google Scholar] [CrossRef]
- Yao, K.P.C.; Kalaga, K.; Gilbert, J.A.; Okasinski, J.; Almer, J.; Abraham, D.P. (Invited) Lithium-Ion Transport in Ncm Oxides: Effect of Crystallographic Evolution during Delithiation. ECS Meet. Abstr. 2017, MA2017-02, 223. [Google Scholar] [CrossRef]
- Sahore, R.; O’Hanlon, D.C.; Tornheim, A.; Lee, C.-W.; Garcia, J.C.; Iddir, H.; Balasubramanian, M.; Bloom, I. Revisiting the Mechanism Behind Transition-Metal Dissolution from Delithiated LiNixMnyCozO2 (NMC) Cathodes. J. Electrochem. Soc. 2020, 167, 020513. [Google Scholar] [CrossRef]
- Das, D.; Manna, S.; Puravankara, S. Electrolytes, Additives and Binders for NMC Cathodes in Li-Ion Batteries—A Review. Batteries 2023, 9, 193. [Google Scholar] [CrossRef]
- Sergi, F.; Arista, A.; Agnello, G.; Ferraro, M.; Andaloro, L.; Antonucci, V. Characterization and Comparison between Lithium Iron p Hosphate and Lithium-Polymers Batteries. J. Energy Storage 2016, 8, 235–243. [Google Scholar] [CrossRef]
- Hu, J.; Huang, W.; Yang, L.; Pan, F. Structure and Performance of the LiFePO4 cathode Material: From the Bulk to the Surface. Nanoscale 2020, 12, 15036–15044. [Google Scholar] [CrossRef] [PubMed]
- Camargos, P.H.; dos Santos, P.H.J.; dos Santos, I.R.; Ribeiro, G.S.; Caetano, R.E. Perspectives on Li-Ion Battery Categories for Electric Vehicle Applications: A Review of State of the Art. Int. J. Energy Res. 2022, 46, 19258–19268. [Google Scholar] [CrossRef]
- Stenina, I.; Minakova, P.; Kulova, T.; Yaroslavtsev, A. Electrochemical Properties of LiFePO4 Cathodes: The Effect of Carbon Additives. Batteries 2022, 8, 111. [Google Scholar] [CrossRef]
- Orendorff, C.J.; Doughty, D.H. Lithium Ion Battery Safety. Interface Mag. 2012, 21, 35. [Google Scholar] [CrossRef]
- Fu, R.; Zhou, X.; Fan, H.; Blaisdell, D.; Jagadale, A.; Zhang, X.; Xiong, R. Comparison of Lithium-Ion Anode Materials Using an Experimentally Verified Physics-Based Electrochemical Model. Energies 2017, 10, 2174. [Google Scholar] [CrossRef]
- Ghosh, A.; Ghamouss, F. Role of Electrolytes in the Stability and Safety of Lithium Titanate-Based Batteries. Front. Mater. 2020, 7, 186. [Google Scholar] [CrossRef]
- Zhao, B.; Ran, R.; Liu, M.; Shao, Z. A Comprehensive Review of Li4Ti5O12-Based Electrodes for Lithium-Ion Batteries: The Latest Advancements and Future Perspectives. Mater. Sci. Eng. R Rep. 2015, 98, 1–71. [Google Scholar] [CrossRef]
- Soltani, M.; Ibrahim, T.; Stroe, A.I.; Stroe, D.I. Comparison of High-Power Energy Storage Devices for Frequency Regulation Application (Performance, Cost, Size, and Lifetime). In Proceedings of the IECON Proceedings (Industrial Electronics Conference), Brussels, Belgium, 17–20 October 2022; pp. 1–6. [Google Scholar] [CrossRef]
- Vermeer, W.; Chandra Mouli, G.R.; Bauer, P. A Comprehensive Review on the Characteristics and Modeling of Lithium-Ion Battery Aging. IEEE Trans. Transp. Electrif. 2022, 8, 2205–2232. [Google Scholar] [CrossRef]
- Gauthier, R.; Luscombe, A.; Bond, T.; Bauer, M.; Johnson, M.; Harlow, J.; Louli, A.; Dahn, J.R. How Do Depth of Discharge, C-Rate and Calendar Age Affect Capacity Retention, Impedance Growth, the Electrodes, and the Electrolyte in Li-Ion Cells? J. Electrochem. Soc. 2022, 169, 020518. [Google Scholar] [CrossRef]
- Spitthoff, L.; Shearing, P.R.; Burheim, O.S. Temperature, Ageing and Thermal Management of Lithium-Ion Batteries. Energies 2021, 14, 1248. [Google Scholar] [CrossRef]
- Wu, W.; Wang, S.; Wu, W.; Chen, K.; Hong, S.; Lai, Y. A Critical Review of Battery Thermal Performance and Liquid Based Battery Thermal Management. Energy Convers. Manag. 2019, 182, 262–281. [Google Scholar] [CrossRef]
- Li, R.; Li, W.; Singh, A.; Ren, D.; Hou, Z.; Ouyang, M. Effect of External Pressure and Internal Stress on Battery Performance and Lifespan. Energy Storage Mater. 2022, 52, 395–429. [Google Scholar] [CrossRef]
- Birkl, C.R.; Roberts, M.R.; McTurk, E.; Bruce, P.G.; Howey, D.A. Degradation Diagnostics for Lithium Ion Cells. J. Power Sources 2017, 341, 373–386. [Google Scholar] [CrossRef]
- Christensen, P.A.; Anderson, P.A.; Harper, G.D.J.; Lambert, S.M.; Mrozik, W.; Rajaeifar, M.A.; Wise, M.S.; Heidrich, O. Risk Management over the Life Cycle of Lithium-Ion Batteries in Electric Vehicles. Renew. Sustain. Energy Rev. 2021, 148, 111240. [Google Scholar] [CrossRef]
- Lebedeva, N.P.; Boon-Brett, L. Considerations on the Chemical Toxicity of Contemporary Li-Ion Battery Electrolytes and Their Components. J. Electrochem. Soc. 2016, 163, A821–A830. [Google Scholar] [CrossRef]
- Diekmann, J.; Grützke, M.; Loellhoeffel, T.; Petermann, M.; Rothermel, S.; Winter, M.; Nowak, S.; Kwade, A. Potential Dangers During the Handling of Lithium-Ion Batteries. In Sustainable Production, Life Cycle Engineering and Management; Springer: Cham, Germany, 2018; pp. 39–51. [Google Scholar]
- Larsson, F.; Andersson, P.; Blomqvist, P.; Mellander, B.E. Toxic Fluoride Gas Emissions from Lithium-Ion Battery Fires. Sci. Rep. 2017, 7, 10018. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Li, J.; Zhou, T.; Yang, K.; Wei, S.; Tang, N.; Dang, N.; Li, H.; Qiu, X.; Chen, L. Toxicity, a Serious Concern of Thermal Runaway from Commercial Li-Ion Battery. Nano Energy 2016, 27, 313–319. [Google Scholar] [CrossRef]
- Buston, J.E.H.; Gill, J.; Lisseman, R.; Morton, J.; Musgrove, D.; Williams, R.C.E. Experimental Determination of Metals Generated during the Thermal Failure of Lithium Ion Batteries. Energy Adv. 2023, 2, 170–179. [Google Scholar] [CrossRef]
- Baird, A.R.; Archibald, E.J.; Marr, K.C.; Ezekoye, O.A. Explosion Hazards from Lithium-Ion Battery Vent Gas. J. Power Sources 2020, 446, 227257. [Google Scholar] [CrossRef]
- Liu, K.; Liu, Y.; Lin, D.; Pei, A.; Cui, Y. Materials for Lithium-Ion Battery Safety. Sci. Adv. 2018, 4, eaas9820. [Google Scholar] [CrossRef]
- Chen, Y.; Kang, Y.; Zhao, Y.; Wang, L.; Liu, J.; Li, Y.; Liang, Z.; He, X.; Li, X.; Tavajohi, N.; et al. A Review of Lithium-Ion Battery Safety Concerns: The Issues, Strategies, and Testing Standards. J. Energy Chem. 2021, 59, 83–99. [Google Scholar] [CrossRef]
- Röder, P.; Baba, N.; Wiemhöfer, H.D. A Detailed Thermal Study of a Li[Ni0.33Co0.33Mn0.33]O2/LiMn2O4-Based Lithium Ion Cell by Accelerating Rate and Differential Scanning Calorimetry. J. Power Sources 2014, 248, 978–987. [Google Scholar] [CrossRef]
- Golubkov, A.W.; Fuchs, D.; Wagner, J.; Wiltsche, H.; Stangl, C.; Fauler, G.; Voitic, G.; Thaler, A.; Hacker, V. Thermal-Runaway Experiments on Consumer Li-Ion Batteries with Metal-Oxide and Olivin-Type Cathodes. RSC Adv. 2014, 4, 3633–3642. [Google Scholar] [CrossRef]
- Park, J.S.; Oh, S.M.; Sun, Y.K.; Myung, S.T. Thermal Properties of Fully Delithiated Olivines. J. Power Sources 2014, 256, 479–484. [Google Scholar] [CrossRef]
- Yim, C.H.; Houache, M.S.E.; Baranova, E.A.; Abu-Lebdeh, Y. Understanding Key Limiting Factors for the Development of All-Solid-State-Batteries. Chem. Eng. J. Adv. 2023, 13, 100436. [Google Scholar] [CrossRef]
- Miao, Y.; Hynan, P.; Von Jouanne, A.; Yokochi, A. Current Li-Ion Battery Technologies in Electric Vehicles and Opportunities for Advancements. Energies 2019, 12, 1074. [Google Scholar] [CrossRef]
- Abraham, K.M.; Jiang, Z. A Polymer Electrolyte-Based Rechargeable Lithium/Oxygen Battery. J. Electrochem. Soc. 1996, 143, 1–5. [Google Scholar] [CrossRef]
- Imanishi, N.; Yamamoto, O. Perspectives and Challenges of Rechargeable Lithium–Air Batteries. Mater. Today Adv. 2019, 4, 100031. [Google Scholar] [CrossRef]
- Rudola, A.; Rennie, A.J.R.; Heap, R.; Meysami, S.S.; Lowbridge, A.; Mazzali, F.; Sayers, R.; Wright, C.J.; Barker, J. Commercialisation of High Energy Density Sodium-Ion Batteries: Faradion’s Journey and Outlook. J. Mater. Chem. A 2021, 9, 8279–8302. [Google Scholar] [CrossRef]
- Usiskin, R.; Lu, Y.; Popovic, J.; Law, M.; Balaya, P.; Hu, Y.S.; Maier, J. Fundamentals, Status and Promise of Sodium-Based Batteries. Nat. Rev. Mater. 2021, 6, 1020–1035. [Google Scholar] [CrossRef]
- Nurohmah, A.R.; Nisa, S.S.; Stulasti, K.N.R.; Yudha, C.S.; Suci, W.G.; Aliwarga, K.; Widiyandari, H.; Purwanto, A. Sodium-Ion Battery from Sea Salt: A Review. Mater. Renew. Sustain. Energy 2022, 11, 71–89. [Google Scholar] [CrossRef]
- Chen, J.; Xu, W.; Wang, X.; Yang, S.; Xiong, C. Progress and Applications of Seawater-Activated Batteries. Sustainability 2023, 15, 1635. [Google Scholar] [CrossRef]
- Arnold, S.; Wang, L.; Presser, V. Dual-Use of Seawater Batteries for Energy Storage and Water Desalination. Small 2022, 18, e2107913. [Google Scholar] [CrossRef]
- Shoman, W.; Yeh, S.; Sprei, F.; Plötz, P.; Speth, D. Battery Electric Long-Haul Trucks in Europe: Public Charging, Energy, and Power Requirements. Transp. Res. Part D Transp. Environ. 2023, 121, 103825. [Google Scholar] [CrossRef]
- Kortsari, A.; Mitropoulos, L.; Heinemann, T.; Hagbarth Mikkelsen, H. Prototype and Full-Scale Demonstration of Next-Generation 100% Electrically Powered Ferry for Passengers and Vehicles: Evaluation report of the E-Ferry; E-Ferry Project: Ærø, Denmark, Tech. Rep; 2020. [Google Scholar] [CrossRef]
- Chin, C.S.; Xiao, J.; Ghias, A.M.Y.M.; Venkateshkumar, M.; Sauer, D.U. Customizable Battery Power System for Marine and Offshore Applications: Trends, Configurations, and Challenges. IEEE Electrif. Mag. 2019, 7, 46–55. [Google Scholar] [CrossRef]
- Trzesniowski, M. Electric Drives. In Powertrain; Springer: Wiesbaden, Germany, 2023; pp. 179–241. [Google Scholar]
- Van den Bossche, P.; Vergels, F.; Van Mierlo, J.; Matheys, J.; Van Autenboer, W. SUBAT: An Assessment of Sustainable Battery Technology. J. Power Sources 2006, 162, 913–919. [Google Scholar] [CrossRef]
- Zhou, W.; Liu, Z.; Chen, W.; Sun, X.; Luo, M.; Zhang, X.; Li, C.; An, Y.; Song, S.; Wang, K.; et al. A Review on Thermal Behaviors and Thermal Management Systems for Supercapacitors. Batteries 2023, 9, 128. [Google Scholar] [CrossRef]
- Grohol, M.; Veeh, C. Study on the Critical Raw Materials for the EU 2023—Final Report; Publications Office of the European Union: Luxembourg, 2023; Available online: https://data.europa.eu/doi/10.2873/725585 (accessed on 28 January 2024).
- Lehmusto, M.; Santasalo-Aarnio, A. Mathematical Framework for Total Cost of Ownership Analysis of Marine Electrical Energy Storage Inspired by Circular Economy. J. Power Sources 2022, 528, 231164. [Google Scholar] [CrossRef]
- Tarascon, J.M.; Armand, M. Issues and Challenges Facing Rechargeable Lithium Batteries. Nature 2001, 414, 359–367. [Google Scholar] [CrossRef] [PubMed]
- Bielewski, M.; Blagoeva, D.; Cordella, M.; Di Persio, F.; Gaudillat, P.; Hildebrand, S.; Mancini, L.; Mathieux, F.; Moretto, P.; Paffumi, E.; et al. Analysis of Sustainability Criteria for Lithium-Ion Batteries Including Related Standards and Regulations; European Commission: Brussels, Belgium, 2020. [Google Scholar]
- Bravo Diaz, L.; He, X.; Hu, Z.; Restuccia, F.; Marinescu, M.; Barreras, J.V.; Patel, Y.; Offer, G.; Rein, G. Review—Meta-Review of Fire Safety of Lithium-Ion Batteries: Industry Challenges and Research Contributions. J. Electrochem. Soc. 2020, 167, 090559. [Google Scholar] [CrossRef]
- Rahimi-Eichi, H.; Ojha, U.; Baronti, F.; Chow, M.Y. Battery Management System: An Overview of Its Application in the Smart Grid and Electric Vehicles. IEEE Ind. Electron. Mag. 2013, 7, 4–16. [Google Scholar] [CrossRef]
- DNV GL. Handbook for Maritime and Offshore Battery Systems; DNV: Oslo, Norway, 2016. [Google Scholar]
- McCoy, T.J. Trends in Ship Electric Propulsion. In Proceedings of the IEEE Power Engineering Society Summer Meeting, Chicago, IL, USA, 21–25 July 2002; Volume 1, pp. 343–346. [Google Scholar] [CrossRef]
- Chalfant, J.S.; Chryssostomidis, C. Analysis of Various All-Electric-Ship Electrical Distribution System Topologies. In Proceedings of the 2011 IEEE Electric Ship Technologies Symposium (ESTS 2011), Alexandria, VA, USA, 10–13 April 2011; pp. 72–77. [Google Scholar] [CrossRef]
- Bosich, D.; Vicenzutti, A.; Pelaschiar, R.; Menis, R.; Sulligoi, G. Toward the Future: The MVDC Large Ship Research Program. In Proceedings of the 2015 AEIT International Annual Conference (AEIT 2015), Naples, Italy, 14–16 October 2015; pp. 1–6. [Google Scholar] [CrossRef]
- Apsley, J.M.; González-Villaseñor, A.; Barnes, M.; Smith, A.C.; Williamson, S.; Schuddebeurs, J.D.; Norman, P.J.; Booth, C.D.; Burt, G.M.; McDonald, J.R. Propulsion Drive Models for Full Electric Marine Propulsion Systems. IEEE Trans. Ind. Appl. 2009, 45, 676–684. [Google Scholar] [CrossRef]
- Jayasinghe, S.G.; Meegahapola, L.; Fernando, N.; Jin, Z.; Guerrero, J.M. Review of Ship Microgrids: System Architectures, Storage Technologies and Power Quality Aspects. Inventions 2017, 2, 4. [Google Scholar] [CrossRef]
- Arcidiacono, V.; Menis, R.; Sulligoi, G. Improving Power Quality in All Electric Ships Using a Voltage and VAR Integrated Regulator. In Proceedings of the IEEE Electric Ship Technologies Symposium (ESTS 2007), Arlington, VA, USA, 21–23 May 2007; pp. 322–327. [Google Scholar] [CrossRef]
- Vu, L.; Nguyen, T.-T.; Nguyen, B.L.-H.; Anam, M.I.; Vu, T. Real-Time Hybrid Controls of Energy Storage and Load Shedding for Integrated Power and Energy Systems of Ships. Electr. Power Syst. Res. 2024, 229, 110191. [Google Scholar] [CrossRef]
- Planakis, N.; Papalambrou, G.; Kyrtatos, N. Ship Energy Management System Development and Experimental Evaluation Utilizing Marine Loading Cycles Based on Machine Learning Techniques. Appl. Energy 2022, 307, 118085. [Google Scholar] [CrossRef]
- Pan, X.; Zhu, X.; Zhao, F. More Environmental Sustainability Routing and Energy Management for All Electric Ships. Front. Energy Res. 2022, 9, 821236. [Google Scholar] [CrossRef]
- Rose, M.W.; Cuzner, R.M. Fault Isolation and Reconfiguration in a Three-Zone System. In Proceedings of the 2015 IEEE Electric Ship Technologies Symposium (ESTS 2015), Old Town Alexandria, VA, USA, 21–24 June 2015; pp. 409–414. [Google Scholar] [CrossRef]
- Wang, S.; Zhen, L.; Psaraftis, H.N.; Yan, R. Implications of the EU’s Inclusion of Maritime Transport in the Emissions Trading System for Shipping Companies. Engineering 2021. [Google Scholar] [CrossRef]
- Hamann, R.; Peschmann, J. Goal-Based Standards and Risk-Based Design. Sh. Technol. Res. 2013, 60, 46–56. [Google Scholar] [CrossRef]
- IEC 62619 CMV; Secondary Cells and Batteries Containing Alkaline or Other Non-Acid Electrolytes—Safety Requirements for Secondary Lithium Cells and Batteries, for Use in Industrial Applications. International Electrotechnical Commission: Geneva, Switzerland, 2022.
- IEC 62620:2014/AMD1:2023; Amendment 1—Secondary Cells and Batteries Containing Alkaline or Other Non-Acid Electrolytes—Secondary Lithium Cells and Batteries for Use in Industrial Applications. International Electrotechnical Commission: Geneva, Switzerland, 2023.
- UL 1973; ANSI/CAN/UL Batteries for Use in Stationary and Motive Auxiliary Power Applications. Underwriter Laboratories Inc.: Northbrook, IL, USA, 2022.
- UL 1642; Lithium Batteries. Underwriters Laboratories Inc.: Northbrook, IL, USA, 2020.
- 143. UN 38.3; Manual of Tests and Criteria on Transport of Dangerous Goods. United Nations: New York, NY, USA, 2019.
- IEC 62281:2019+AMD1:2021+AMD2:2023 CSV; Safety of Primary and Secondary Lithium Cells and Batteries during Transport. International Electrotechnical Commission: Geneva, Switzerland, 2023.
- UL 9540; Energy Storage System and Equipment. Underwriters Laboratories Inc.: Northbrook, IL, USA, 2021.
- IEC 60529: 1989+AMD1:1999+AMD2:2013 CSV; Degrees of Protection Provided by Enclosures (IP Code). International Electrotechnical Commission: Geneva, Switzerland, 2013.
- IEC 61508:2010 CMV; Functional Safety of Electrical/Electronic/Programmable Electronic Safety-Related Systems. International Electrotechnical Commission: Geneva, Switzerland, 2010.
- IEC 60092-504; Electrical Installations in Ships—Part 504: Automation, Control and Instrumentation. International Electrotechnical Commission: Geneva, Switzerland, 2016.
- IEC 62061:2021; Safety of Machinery—Functional Safety of Safety-Related Control Systems. International Electrotechnical Commission: Geneva, Switzerland, 2021.
- EN 50110; Operation of Electrical Installations—Part 1: General Requirements. CENELEC: Brussel, Belgium, 2023.
- IEEE 45-2002; Recommended Practice for Electrical Installations on Shipboard. Institute of Electrical and Electronics Engineers: New York, NY, USA, 2002.
- IEEE 80005-1; Utility Connections in Port—Part 1: High Voltage Shore Connection (HVSC) Systems—General Requirements. Institute of Electrical and Electronics Engineers: New York, NY, USA, 2012.
- IEEE 80005-2; Utility Connections in Port—Part 2: High and Low Voltage Shore Connection Systems—Data Communication for Monitoring and Control. Institute of Electrical and Electronics Engineers: New York, NY, USA, 2016.
- IEC PAS 80005-3:2014; Utility Connections in Port—Part 3: Low Voltage Shore Connection (LVSC) Systems—General Requirements. International Electrotechnical Commission: Geneva, Switzerland, 2014.
- IEC 62613-1:2019; Plugs, Socket-Outlets and Ship Couplers for High-Voltage Shore Connection (HVSC) Systems—Part 1: General Requirements. International Electrotechnical Commission: Geneva, Switzerland, 2019.
- IEC 62613-2:2016; Plugs, Socket-Outlets and Ship Couplers for High-Voltage Shore Connection Systems (HVSC-Systems)—Part 2: Dimensional Compatibility and Interchangeability Requirements for Accessories to be Used by Various Types of Ships. International Electrotechnical Commission: Geneva, Switzerland, 2016.
- IEC 60309-5:2017; Plugs, Socket-Outlets and Couplers for Industrial Purposes—Part 5: Dimensional Compatibility and Interchangeability Requirements for Plugs, Socket-Outlets, Ship Connectors and Ship Inlets for Low-Voltage Shore Connection Systems (LVSC). International Electrotechnical Commission: Geneva, Switzerland, 2017.
- DNV GL. Rules for Classification of Ships. DNV: Oslo, Norway, 2018. [Google Scholar]
- DNV GL. Class Programme—Type Approval DNVGL-CP-0418, Lithium Batteries; DNV: Oslo, Norway, 2015. [Google Scholar]
- Bureau Veritas. Rules for the Classification of Steel Ships; Bureau Veritas: Paris, France, 2018. [Google Scholar]
- Registro Italiano Navale. Rules for the Certification, Installation and Testing of Lithium Based Storage Batteries; RINA: Genova, Italy, 2016. [Google Scholar]
- DNV GL. Environmental Test Specification for Electrical; Electronic and Programmable Equipment and Systems; DNV: Oslo, Norway, 2015. [Google Scholar]
- American Bureau of Shipping. Rules for Building and Classing Steel Vessels; DNV: Oslo, Norway, 2019. [Google Scholar]



| Ship Type | Power (MW) | Electrical Service | Battery Technology |
|---|---|---|---|
RoRo ferries | 2–10 | Load levelling Spinning reserve Full electric | ENERGY: High POWER: Service dependent CYCLES: Very high TYPE: NMC, LFP, LTO |
HSC ferries | 1–15 | Full electric Load levelling | ENERGY: High POWER: High CYCLES: Very high TYPE: NMC, LFP, LTO |
Cruises | 20–70 | Spinning reserve, immediate power Load levelling, grid stabilization Zero-emission short mission | ENERGY: Service dependant POWER: Medium high CYCLES: Medium high TYPE: NMC, LFP |
DP Class offshore vessels | 30–50 | Regenerative power Grid stabilization Spinning reserve | ENERGY: Service dependant POWER: Very high CYCLES: Service dependant TYPE: NMC, LFP, LTO, Supercapacitor |
Offshore Supply Vessels | 10–20 | Regenerative power Spinning reserve, peak levelling Grid stabilization | ENERGY: Medium low POWER: Very high CYCLES: Low TYPE: NMC, LFP, LTO |
Harbour tugs | 2–4 | Regenerative power Full electric Hybrid propulsion | ENERGY: High POWER: High CYCLES: Service dependant TYPE: NMC, LFP, LTO, Supercapacitor |
Fishing vessels | 0.5–5 | Load levelling, peak shaving Regenerative power Hybrid propulsion | ENERGY: Low POWER: Medium low CYCLES: Service dependant TYPE: NMC, LFP |
Shuttle tankers | 10–20 | Spinning reserve Peak shaving Load levelling | ENERGY: Medium POWER: Very high CYCLES: Low TYPE: NMC, LTO |
Various with cranes | 5–15 | Regenerative power Load levelling, peak shaving Zero-emission short mission | ENERGY: Low POWER: High CYCLES: High TYPE: NMC, LFP, LTO |
Yachts | 1–30 | Spinning reserve Peak shaving Load levelling Zero-emission short mission, port stay | ENERGY: High POWER: Low CYCLES: Low TYPE: NMC, LFP; LTO |
| Date | Standard/Code | Title | Note | Ref |
|---|---|---|---|---|
| 2022 | IEC 62619:2022 CMV | Secondary cells and batteries containing alkaline or other non-acid electrolytes—Safety requirements for secondary lithium cells and batteries, for use in industrial applications | [139] | |
| 2023 | IEC 62620:2014/AMD1:2023 | Amendment 1—Secondary cells and batteries containing alkaline or other non-acid electrolytes—Secondary lithium cells and batteries for use in industrial applications | [140] | |
| 2022 | UL 1973 | Batteries for Use in Stationary and Motive Auxiliary Power Applications | [141] | |
| 2020 | UL 1642 | Standard for Safety of Lithium Batteries | [142] | |
| 2019 | UN 38.3 | Manual of Tests and Criteria on Transport of Dangerous Goods | [143] | |
| 2019 | IEC 62281:2019+AMD1:2021+AMD2:2023 CSV | Safety of primary and secondary lithium cells and batteries during transport | [144] | |
| 2021 | UL 9540 | Energy Storage Systems and Equipment | [145] | |
| 2013 | IEC 60529: 1989+AMD1:1999+AMD2:2013 CSV | Degrees of protection provided by enclosures (IP Code) | [146] | |
| 2010 | IEC 61508:2010 CMV | Functional safety of electrical/electronic/programmable electronic safety-related systems | Relevant for BMS | [147] |
| 2016 | IEC 60092-504 | Electrical installations in ships—Part 504: Automation, control and instrumentation | [148] | |
| 2021 | IEC 62061:2021 | Safety of machinery—Functional safety of safety-related control systems | [149] | |
| 2023 | EN 50110 | Operation of electrical installations—Part 1: General requirements | Supporting electrical testing of batteries for ships | [150] |
| 2002 | IEEE 45-2002 | Recommended Practice for Electrical Installations on Shipboard | [151] | |
| 2012 | IEEE 80005-1 | Utility connections in port—Part 1: High Voltage Shore Connection (HVSC) Systems—General requirements | Relevant standards for land to ship connections relating to the use of shore power | [152] |
| 2016 | IEEE 80005-2 | Utility connections in port—Part 2: High and low voltage shore connection systems—Data communication for monitoring and control | [153] | |
| 2014 | IEC PAS 80005-3:2014 | Utility connections in port—Part 3: Low Voltage Shore Connection (LVSC) Systems—General requirements | [154] | |
| 2019 | IEC 62613-1:2019 | Plugs, socket-outlets and ship couplers for high-voltage shore connection (HVSC) systems—Part 1: General requirements | [155] | |
| 2016 | IEC 62613-2:2016 | Plugs, socket-outlets and ship couplers for high-voltage shore connection systems (HVSC-systems)—Part 2: Dimensional compatibility and interchangeability requirements for accessories to be used by various types of ships | [156] | |
| 2017 | IEC 60309-5:2017 | Plugs, socket-outlets and couplers for industrial purposes—Part 5: Dimensional compatibility and interchangeability requirements for plugs, socket-outlets, ship connectors and ship inlets for low-voltage shore connection systems (LVSC) | [157] |
| Class | Type of Document | Title |
|---|---|---|
| DNV | Class rule | DNV-GL General Rules Part 6 Additional Class Notation, Ch 2, Section 1 |
| DNV | Guideline | DNV-GL Handbook for Maritime and Offshore Battery Systems |
| DNV | Technical code | DNV-GL CP 0418 Electrical Energy Storage |
| DNV | Class rule | DNV GL rules for classification—ShipsRU SHIP Pt.4 Ch.8—Electrical Installations |
| DNV | Class rule | DNV GL rules for classification—ShipsRU SHIP Pt.4 Ch.9—Control and monitoring systems |
| DNV | Class rule | DNV GL rules for classification—ShipsRU SHIP Pt.6 Ch.2 Sec.1–Battery power |
| DNV | Technical Standard | DNV GL offshore standard OS D201 Electrical installations |
| DNV | Technical Standard | DNV GL offshore standard OS D202 Automation, Safety and Telecommunication Systems |
| DNV | Guideline | Class guideline DNV GL CG 0339—Environmental test specification for electrical, electronic and programmable equipment and systems |
| DNV | Guideline | DNV GL Guideline for large Maritime Battery Systems |
| ABS | Guideline | Use of lithium-ion Batteries in the Marine and Offshore Industries |
| ABS | Class rule | ABS Rules for Building and Classing Marine Vessels PART 4 |
| ABS | Class rule | ABS Advisory on Hybrid Electric Power Systems |
| ABS | Class rule | ABS Rules for Building and Classing Marine Vessels PART 1 Ch 1 Sec 4/Alternatives |
| ABS | Class rule | ABS Rules for Building and Classing Marine Vessels PART 1 Ch 1 Appendix 3/ABS Type Approval program |
| ABS | Class rule | ABS Rules for Building and Classing Marine Vessels PART 1 Ch 1 Appendix 4/ABS Type Approval program |
| LR | Guideline | Large Battery Installations: Key hazards to consider and Lloyd’s Register approach to approval |
| RINA | Class rule | Rules for the Certification, Installation and Testing of Lithium-Based Storage Batteries |
| ABS | Guideline | Guide for use of lithium batteries in the marine and offshore industries |
| BV | Class rule | Rules for Classification of Ships—Electric Hybrid (Pt F, Ch 11, Sec 22) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lucà Trombetta, G.; Leonardi, S.G.; Aloisio, D.; Andaloro, L.; Sergi, F. Lithium-Ion Batteries on Board: A Review on Their Integration for Enabling the Energy Transition in Shipping Industry. Energies 2024, 17, 1019. https://doi.org/10.3390/en17051019
Lucà Trombetta G, Leonardi SG, Aloisio D, Andaloro L, Sergi F. Lithium-Ion Batteries on Board: A Review on Their Integration for Enabling the Energy Transition in Shipping Industry. Energies. 2024; 17(5):1019. https://doi.org/10.3390/en17051019
Chicago/Turabian StyleLucà Trombetta, Giovanni, Salvatore Gianluca Leonardi, Davide Aloisio, Laura Andaloro, and Francesco Sergi. 2024. "Lithium-Ion Batteries on Board: A Review on Their Integration for Enabling the Energy Transition in Shipping Industry" Energies 17, no. 5: 1019. https://doi.org/10.3390/en17051019
APA StyleLucà Trombetta, G., Leonardi, S. G., Aloisio, D., Andaloro, L., & Sergi, F. (2024). Lithium-Ion Batteries on Board: A Review on Their Integration for Enabling the Energy Transition in Shipping Industry. Energies, 17(5), 1019. https://doi.org/10.3390/en17051019








