Assessing the Performance of Continuous-Flow Microbial Fuel Cells and Membrane Electrode Assembly with Electrodeposited Mn Oxide Catalyst
Abstract
1. Introduction
2. Materials and Methods
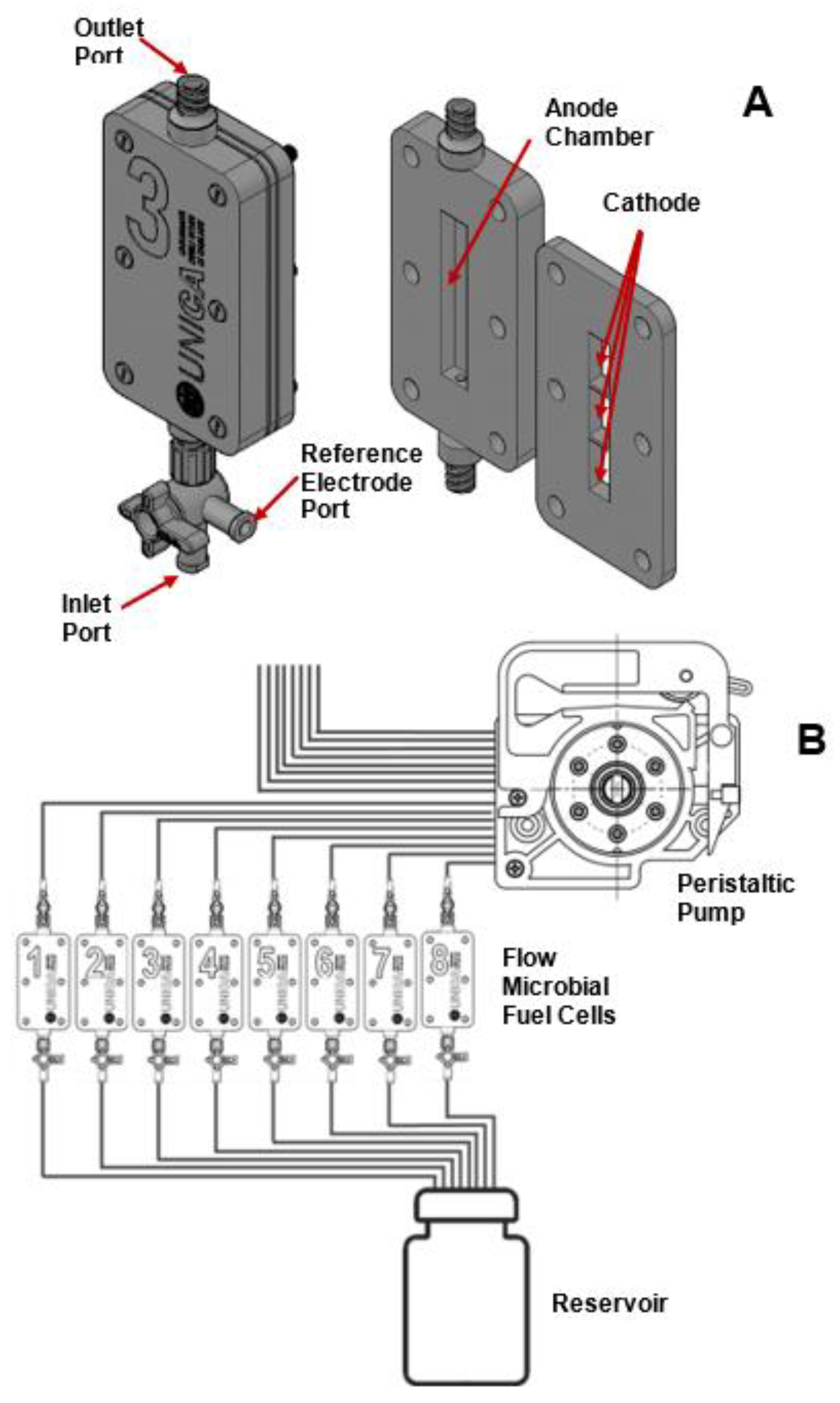
2.1. Preparation of the MEAs
2.2. MFC Set up and Operation
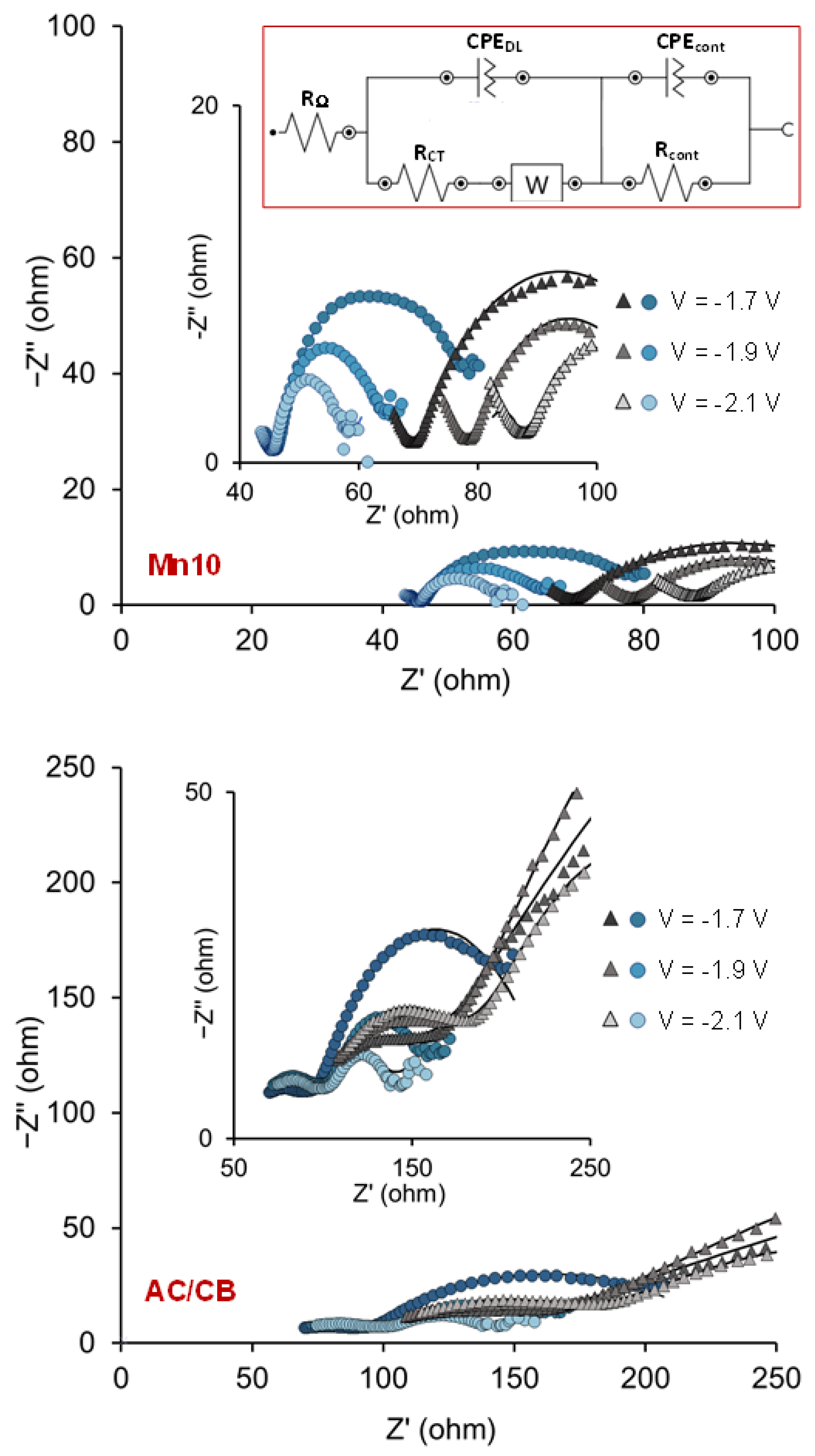
2.3. EIS Characterization
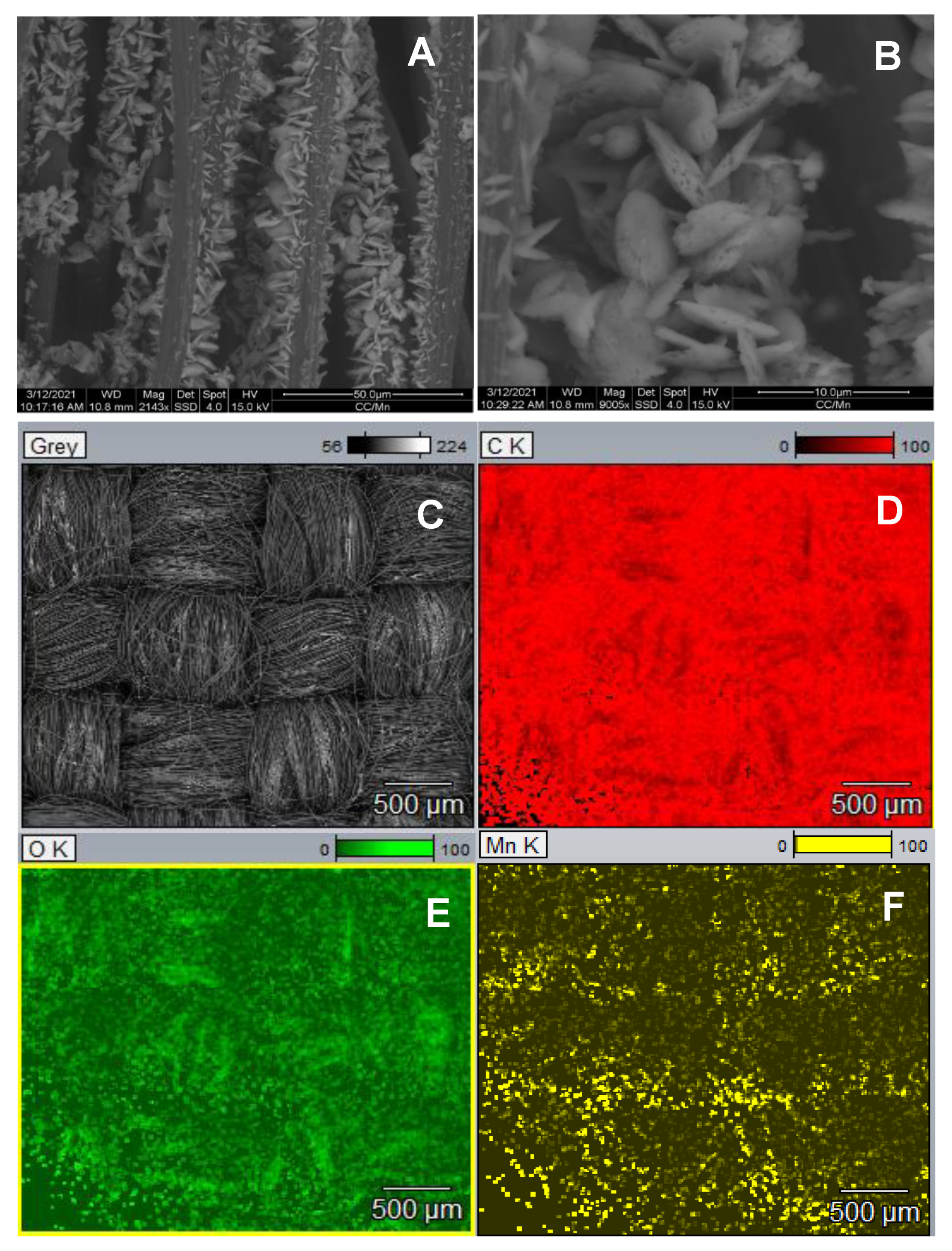
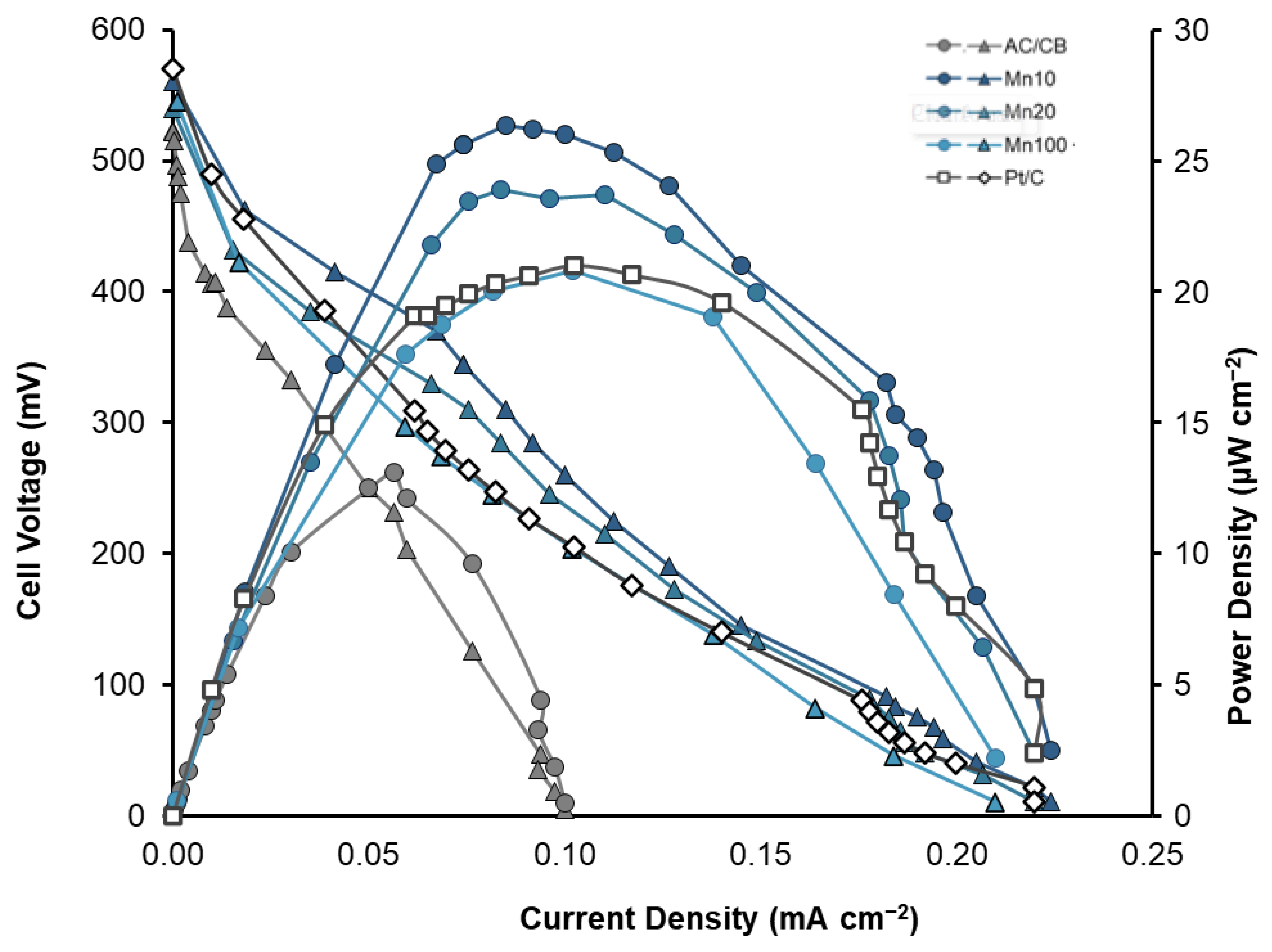
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Naha, A.; Debroy, R.; Sharma, D.; Shah, M.P.; Nath, S. Microbial Fuel Cell: A State-of-the-Art and Revolutionizing Technology for Efficient Energy Recovery. Clean. Circ. Bioecon. 2023, 5, 100050. [Google Scholar] [CrossRef]
- Chen, J.; Yang, J.; Wang, R.; Yang, Y.; Liu, Y. Design and Research Progress of Nano Materials in Cathode Catalysts of Microbial Fuel Cells: A Review. Int. J. Hydrogen Energy 2022, 47, 18098–18108. [Google Scholar] [CrossRef]
- Ucar, D.; Zhang, Y.; Angelidaki, I. An Overview of Electron Acceptors in Microbial Fuel Cells. Front. Microbiol. 2017, 8, 248548. [Google Scholar] [CrossRef] [PubMed]
- Ben Liew, K.; Daud, W.R.W.; Ghasemi, M.; Leong, J.X.; Su Lim, S.; Ismail, M. Non-Pt Catalyst as Oxygen Reduction Reaction in Microbial Fuel Cells: A Review. Int. J. Hydrogen Energy 2014, 39, 4870–4883. [Google Scholar] [CrossRef]
- Elangovan, K.; Saravanan, P.; Campos, C.H.; Sanhueza-Gómez, F.; Khan, M.M.R.; Chin, S.Y.; Krishnan, S.; Viswanathan Mangalaraja, R. Outline of Microbial Fuel Cells Technology and Their Significant Developments, Challenges, and Prospects of Oxygen Reduction Electrocatalysts. Front. Chem. Eng. 2023, 5, 1228510. [Google Scholar] [CrossRef]
- Ramya, M.; Senthil Kumar, P. A Review on Recent Advancements in Bioenergy Production Using Microbial Fuel Cells. Chemosphere 2022, 288, 132512. [Google Scholar] [CrossRef] [PubMed]
- Shang, Z.; Wycisk, R.; Pintauro, P. Electrospun Composite Proton-Exchange and Anion-Exchange Membranes for Fuel Cells. Energies 2021, 14, 6709. [Google Scholar] [CrossRef]
- Banerjee, A.; Calay, R.K.; Eregno, F.E. Role and Important Properties of a Membrane with Its Recent Advancement in a Microbial Fuel Cell. Energies 2022, 15, 444. [Google Scholar] [CrossRef]
- Rodríguez, J.; Mais, L.; Campana, R.; Piroddi, L.; Mascia, M.; Gurauskis, J.; Vacca, A.; Palmas, S. Comprehensive Characterization of a Cost-Effective Microbial Fuel Cell with Pt-Free Catalyst Cathode and Slip-Casted Ceramic Membrane. Int. J. Hydrogen Energy 2021, 46, 26205–26223. [Google Scholar] [CrossRef]
- Wilberforce, T.; Abdelkareem, M.A.; Elsaid, K.; Olabi, A.G.; Sayed, E.T. Role of Carbon-Based Nanomaterials in Improving the Performance of Microbial Fuel Cells. Energy 2022, 240, 122478. [Google Scholar] [CrossRef]
- Quan, X.; Mei, Y.; Xu, H.; Sun, B.; Zhang, X. Optimization of Pt-Pd Alloy Catalyst and Supporting Materials for Oxygen Reduction in Air-Cathode Microbial Fuel Cells. Electrochim. Acta 2015, 165, 72–77. [Google Scholar] [CrossRef]
- Osgood, H.; Devaguptapu, S.V.; Xu, H.; Cho, J.; Wu, G. Transition Metal (Fe, Co, Ni, and Mn) Oxides for Oxygen Reduction and Evolution Bifunctional Catalysts in Alkaline Media. Nano Today 2016, 11, 601–625. [Google Scholar] [CrossRef]
- Aysla Costa De Oliveira, M.; D’Epifanio, A.; Ohnuki, H.; Mecheri, B. Platinum Group Metal-Free Catalysts for Oxygen Reduction Reaction: Applications in Microbial Fuel Cells. Catalysts 2020, 10, 475. [Google Scholar] [CrossRef]
- Neethu, B.; Khandelwal, A.; Ghangrekar, M.M.; Ihjas, K.; Swaminathan, J. Microbial Fuel Cells—Challenges for Commercialization and How They Can Be Addressed. In Scaling Up of Microbial Electrochemical Systems; Elsevier: Amsterdam, The Netherlands, 2022; pp. 393–418. [Google Scholar] [CrossRef]
- Jayapriya, J.; Gummadi, S.N. Scaling up and Applications of Microbial Fuel Cells. In Scaling Up of Microbial Electrochemical Systems: From Reality to Scalability; Elsevier: Amsterdam, The Netherlands, 2022; pp. 309–338. [Google Scholar] [CrossRef]
- Wang, H.; Long, X.; Sun, Y.; Wang, D.; Wang, Z.; Meng, H.; Jiang, C.; Dong, W.; Lu, N. Electrochemical Impedance Spectroscopy Applied to Microbial Fuel Cells: A Review. Front. Microbiol. 2022, 13, 973501. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.; Chang, I.S.; Dinsdale, R.M.; Guwy, A.J. Accurate Measurement of Internal Resistance in Microbial Fuel Cells by Improved Scanning Electrochemical Impedance Spectroscopy. Electrochim. Acta 2021, 366, 137388. [Google Scholar] [CrossRef]
- Antonopoulou, G.; Bampos, G.; Ntaikou, I.; Alexandropoulou, M.; Dailianis, S.; Bebelis, S.; Lyberatos, G. The Biochemical and Electrochemical Characteristics of a Microbial Fuel Cell Used to Produce Electricity from Olive Mill Wastewater. Energy 2023, 282, 128804. [Google Scholar] [CrossRef]
- Manohar, A.K.; Mansfeld, F. The Internal Resistance of a Microbial Fuel Cell and Its Dependence on Cell Design and Operating Conditions. Electrochim. Acta 2009, 54, 1664–1670. [Google Scholar] [CrossRef]
- Bazina, N.; Ahmed, T.G.; Almdaaf, M.; Jibia, S.; Sarker, M. Power Generation from Wastewater Using Microbial Fuel Cells: A Review. J. Biotechnol. 2023, 374, 17–30. [Google Scholar] [CrossRef] [PubMed]
- Aelterman, P.; Rabaey, K.; Pham, H.T.; Boon, N.; Verstraete, W. Continuous Electricity Generation at High Voltages and Currents Using Stacked Microbial Fuel Cells. Environ. Sci. Technol. 2006, 40, 3388–3394. [Google Scholar] [CrossRef] [PubMed]
- Rossi, R.; Hall, D.M.; Wang, X.; Regan, J.M.; Logan, B.E. Quantifying the Factors Limiting Performance and Rates in Microbial Fuel Cells Using the Electrode Potential Slope Analysis Combined with Electrical Impedance Spectroscopy. Electrochim. Acta 2020, 348, 136330. [Google Scholar] [CrossRef]
- Sonawane, J.M.; Mahadevan, R.; Pandey, A.; Greener, J. Recent Progress in Microbial Fuel Cells Using Substrates from Diverse Sources. Heliyon 2022, 8, e12353. [Google Scholar] [CrossRef] [PubMed]
- Ortiz-Medina, J.F.; Call, D.F. Electrochemical and Microbiological Characterization of Bioanode Communities Exhibiting Variable Levels of Startup Activity. Front. Energy Res. 2019, 7, 478969. [Google Scholar] [CrossRef]
- Rossi, R.; Cario, B.P.; Santoro, C.; Yang, W.; Saikaly, P.E.; Logan, B.E. Evaluation of Electrode and Solution Area-Based Resistances Enables Quantitative Comparisons of Factors Impacting Microbial Fuel Cell Performance. Environ. Sci. Technol. 2019, 53, 3977–3986. [Google Scholar] [CrossRef]
- Lepage, G.; Albernaz, F.O.; Perrier, G.; Merlin, G. Characterization of a Microbial Fuel Cell with Reticulated Carbon Foam Electrodes. Bioresour. Technol. 2012, 124, 199–207. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Guo, S.; Wang, J.; Li, C. Fundamental Development and Research of Cathodic Compartment in Microbial Fuel Cells: A Review. J. Environ. Chem. Eng. 2022, 10, 107918. [Google Scholar] [CrossRef]
- Sekar, N.; Ramasamy, R.P. Electrochemical Impedance Spectroscopy for Microbial Fuel Cell Characterization. J. Microb. Biochem. Technol. 2013, 5, 1948–5948. [Google Scholar] [CrossRef]
- Lee, M.; Kakarla, R.; Min, B. Performance of an Air-Cathode Microbial Fuel Cell under Varied Relative Humidity Conditions in the Cathode Chamber. Bioprocess Biosyst. Eng. 2019, 42, 1247–1254. [Google Scholar] [CrossRef]
- Kakarla, R.; Kim, J.R.; Jeon, B.H.; Min, B. Enhanced Performance of an Air–Cathode Microbial Fuel Cell with Oxygen Supply from an Externally Connected Algal Bioreactor. Bioresour. Technol. 2015, 195, 210–216. [Google Scholar] [CrossRef]
- Lee, M.; Kondaveeti, S.; Jeon, T.; Kim, I.; Min, B. Influence of Humidity on Performance of Single Chamber Air-Cathode Microbial Fuel Cells with Different Separators. Processes 2020, 8, 861. [Google Scholar] [CrossRef]
- Vacca, A.; Palmas, S.; Mascia, M.; Piras, L.; Mais, L. Continuous-flow microbial fuel cells with electrodeposited MnOx as catalyst for the oxygen reduction reaction. In Proceedings of the 4th Edition of the E3 Mediterranean Symposium: Electrochemistry for Environment and Energy (E3MS), Orvieto, Italy, 15–17 September 2022. [Google Scholar]
- Qiu, S.; Guo, Z.; Naz, F.; Yang, Z.; Yu, C. An Overview in the Development of Cathode Materials for the Improvement in Power Generation of Microbial Fuel Cells. Bioelectrochemistry 2021, 141, 107834. [Google Scholar] [CrossRef]
- Liu, X.W.; Sun, X.F.; Huang, Y.X.; Sheng, G.P.; Zhou, K.; Zeng, R.J.; Dong, F.; Wang, S.G.; Xu, A.W.; Tong, Z.H. Nano-Structured Manganese Oxide as a Cathodic Catalyst for Enhanced Oxygen Reduction in a Microbial Fuel Cell Fed with a Synthetic Wastewater. Water Res. 2010, 44, 5298–5305. [Google Scholar] [CrossRef]
- Cheng, S.; Liu, H.; Logan, B.E. Power Densities Using Different Cathode Catalysts (Pt and CoTMPP) and Polymer Binders (Nafion and PTFE) in Single Chamber Microbial Fuel Cells. Environ. Sci. Technol. 2005, 40, 364–369. [Google Scholar] [CrossRef]
- Liu, H.; Logan, B.E. Electricity Generation Using an Air-Cathode Single Chamber Microbial Fuel Cell in the Presence and Absence of a Proton Exchange Membrane. Environ. Sci. Technol. 2004, 38, 4040–4046. [Google Scholar] [CrossRef]
- Feng, Y.; Yang, Q.; Wang, X.; Logan, B.E. Treatment of Carbon Fiber Brush Anodes for Improving Power Generation in Air–Cathode Microbial Fuel Cells. J. Power Sources 2010, 195, 1841–1844. [Google Scholar] [CrossRef]
- Zang, L.; Liu, C.; Zhuang, L.; Li, W.; Zhou, S.; Zang, J. Manganese Dioxide as an Alternative Cathodic Catalyst to Platinum in Microbial Fuel Cells. Biosens. Bioelectron. 2009, 24, 2825–2829. [Google Scholar] [CrossRef]
- Córdoba-Torres, P. Relationship between Constant-Phase Element (CPE) Parameters and Physical Properties of Films with a Distributed Resistivity. Electrochim. Acta 2017, 225, 592–604. [Google Scholar] [CrossRef]
- Landesfeind, J.; Pritzl, D.; Gasteirger, H.A. An Analysis Protocol for Three-Electrode Li-Ion Battery Impedance Spectra: Part I. Analysis of a High-Voltage Positive Electrode. J. Electrochem. Soc. 2017, 164, A1773. [Google Scholar] [CrossRef]
- Teufl, T.; Pritzl, D.; Solchenbach, S.; Gasteiger, H.A.; Mendez, M.A. State of Charge Dependent Resistance Build-Up in Li-and Mn-Rich Layered Oxides during Lithium Extraction and Insertion. J. Electrochem. Soc. 2019, 166, A1275. [Google Scholar] [CrossRef]
- Noori, M.T.; Ghangrekar, M.M.; Mukherjee, C.K.; Min, B. Biofouling Effects on the Performance of Microbial Fuel Cells and Recent Advances in Biotechnological and Chemical Strategies for Mitigation. Biotechnol. Adv. 2019, 37, 107420. [Google Scholar] [CrossRef] [PubMed]
- Yesilbag, Y.O.; Tuzluca, F.N.; Ertugrul, M. The Use of α-MnOOH Nanosheets as Battery-Type Electrode for Supercapacitor Applications. J. Mater. Sci. Mater. Electron. 2019, 30, 8201–8209. [Google Scholar] [CrossRef]
- Majidi, M.R.; Shahbazi Farahani, F.; Hosseini, M.; Ahadzadeh, I. Low-Cost Nanowired α-MnO2/C as an ORR Catalyst in Air-Cathode Microbial Fuel Cell. Bioelectrochemistry 2019, 125, 38–45. [Google Scholar] [CrossRef]
- Xiong, Q.Q.; Xia, X.H.; Tu, J.P.; Chen, J.; Zhang, Y.Q.; Zhou, D.; Gu, C.D.; Wang, X.L. Hierarchical Fe2O3@Co3O4 Nanowire Array Anode for High-Performance Lithium-Ion Batteries. J. Power Sources 2013, 240, 344–350. [Google Scholar] [CrossRef]
- Sevda, S.; Chayambuka, K.; Sreekrishnan, T.R.; Pant, D.; Dominguez-Benetton, X. A Comprehensive Impedance Journey to Continuous Microbial Fuel Cells. Bioelectrochemistry 2015, 106, 159–166. [Google Scholar] [CrossRef] [PubMed]
- Xochitl, D.B.; Sevda, S.; Vanbroekhoven, K.; Pant, D. The Accurate Use of Impedance Analysis for the Study of Microbial Electrochemical Systems. Chem. Soc. Rev. 2012, 41, 7228–7246. [Google Scholar] [CrossRef]
- Wen, Q.; Wu, Y.; Zhao, L.X.; Sun, Q.; Kong, F.Y. Electricity Generation and Brewery Wastewater Treatment from Sequential Anode-Cathode Microbial Fuel Cell. J. Zhejiang Univ. Sci. B 2010, 11, 87–93. [Google Scholar] [CrossRef] [PubMed]
- Mateo, S.; Mascia, M.; Fernandez-Morales, F.J.; Rodrigo, M.A.; Di Lorenzo, M. Assessing the Impact of Design Factors on the Performance of Two Miniature Microbial Fuel Cells. Electrochim. Acta 2019, 297, 297–306. [Google Scholar] [CrossRef]
- Tremouli, A.; Antonopoulou, G.; Bebelis, S.; Lyberatos, G. Operation and Characterization of a Microbial Fuel Cell Fed with Pretreated Cheese Whey at Different Organic Loads. Bioresour. Technol. 2013, 131, 380–389. [Google Scholar] [CrossRef]
- Yuan, H.; Hou, Y.; Abu-Reesh, I.M.; Chen, J.; He, Z. Oxygen Reduction Reaction Catalysts Used in Microbial Fuel Cells for Energy-Efficient Wastewater Treatment: A Review. Mater. Horiz. 2016, 3, 382–401. [Google Scholar] [CrossRef]
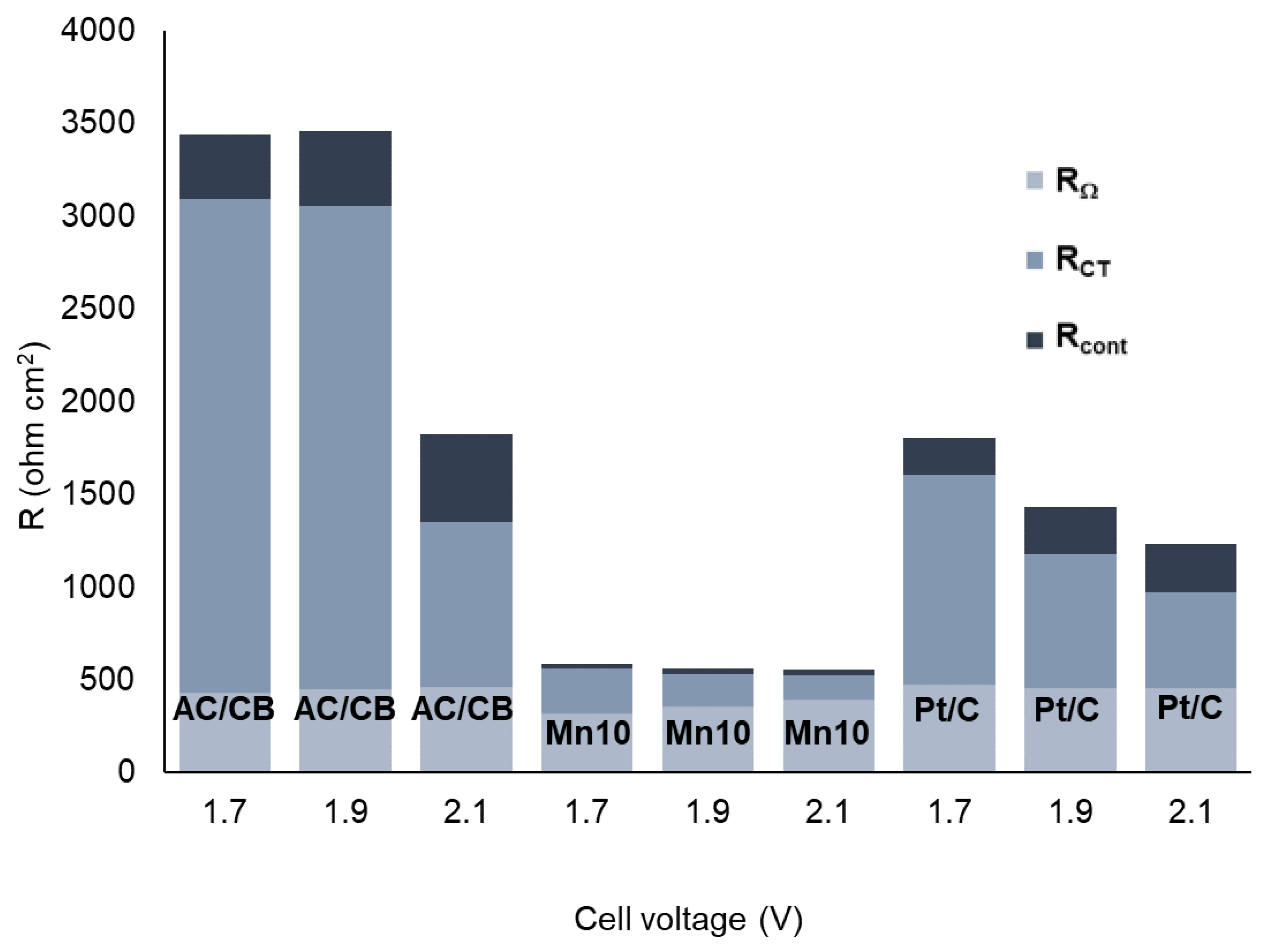
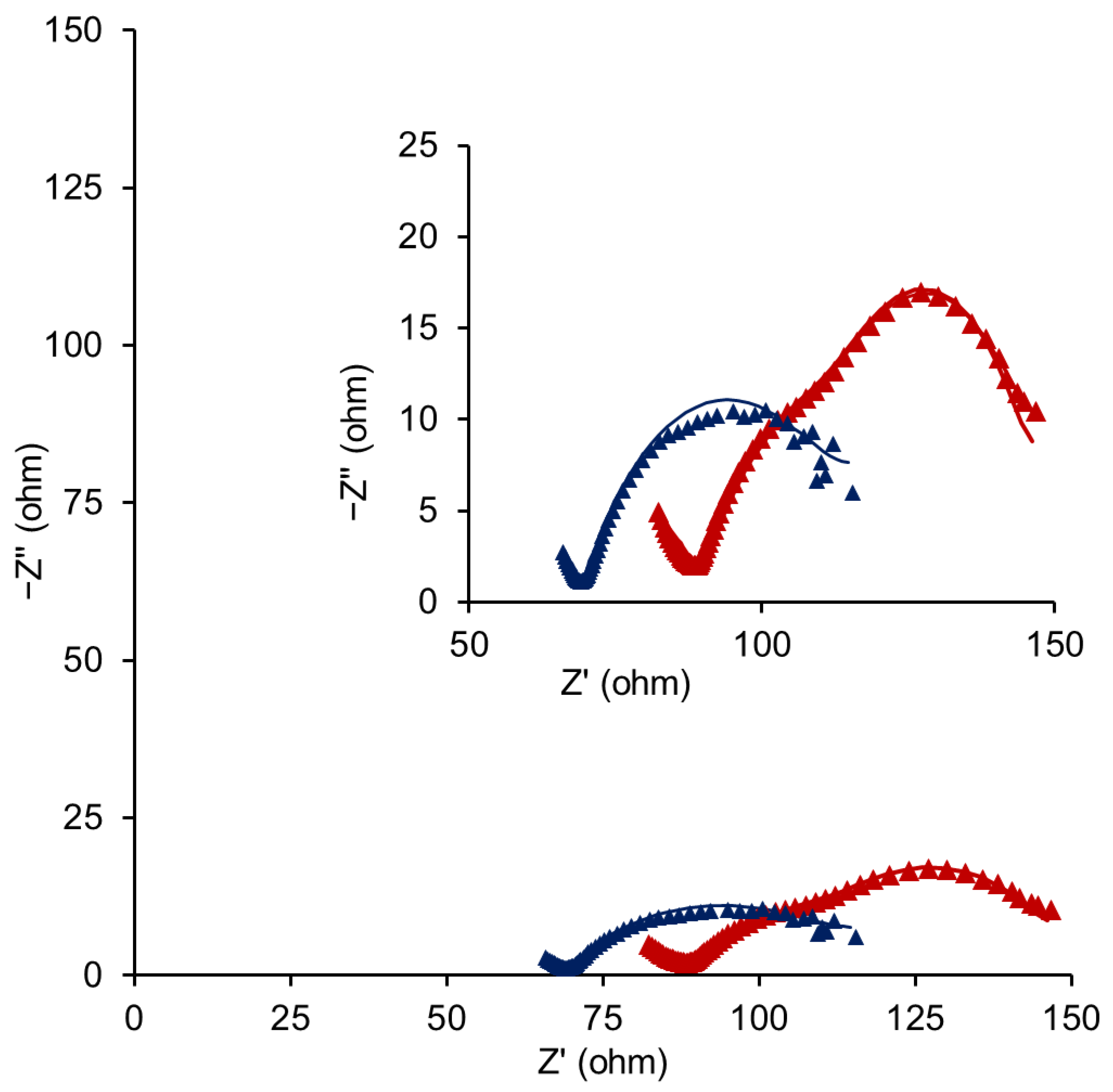
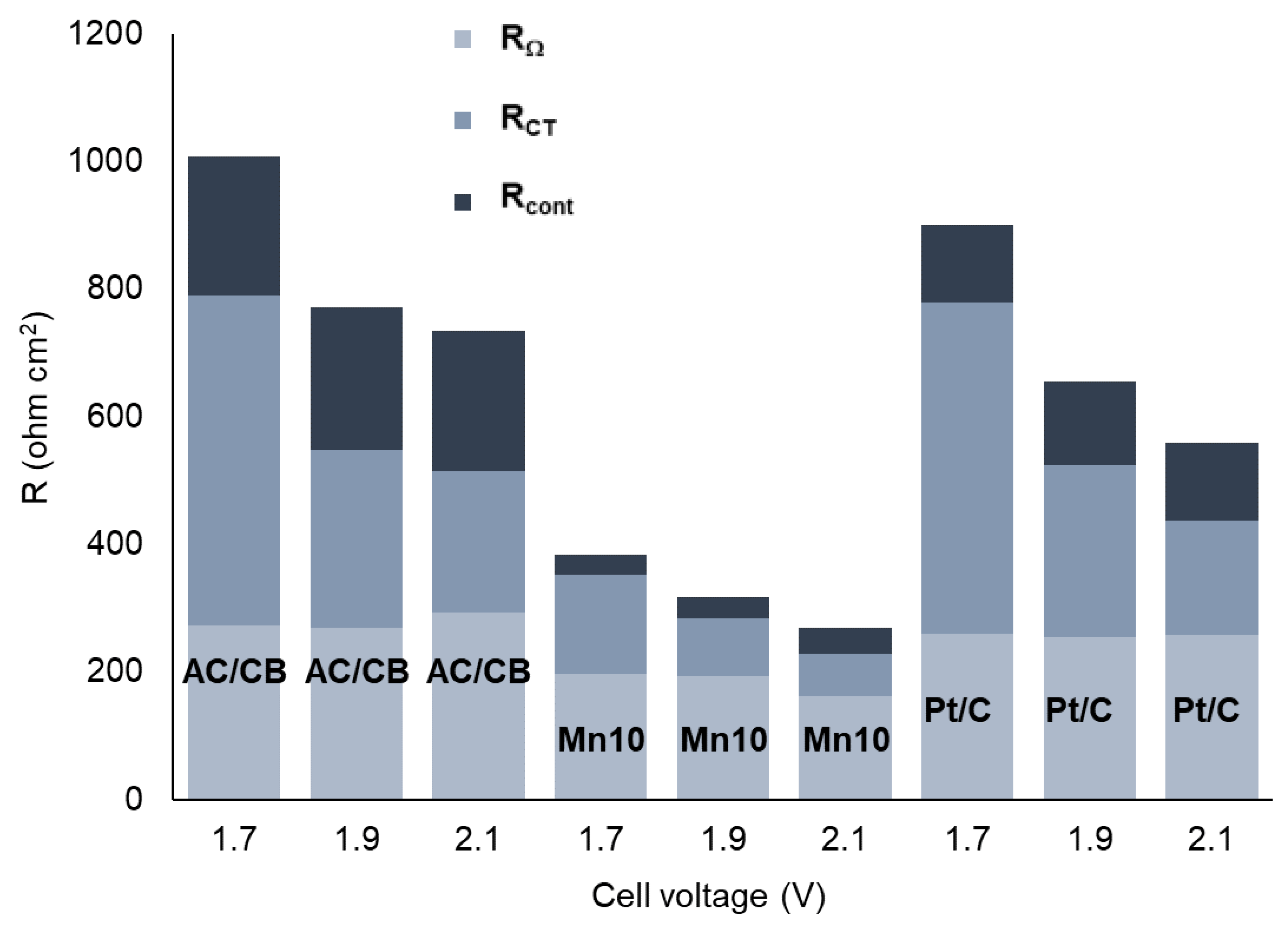
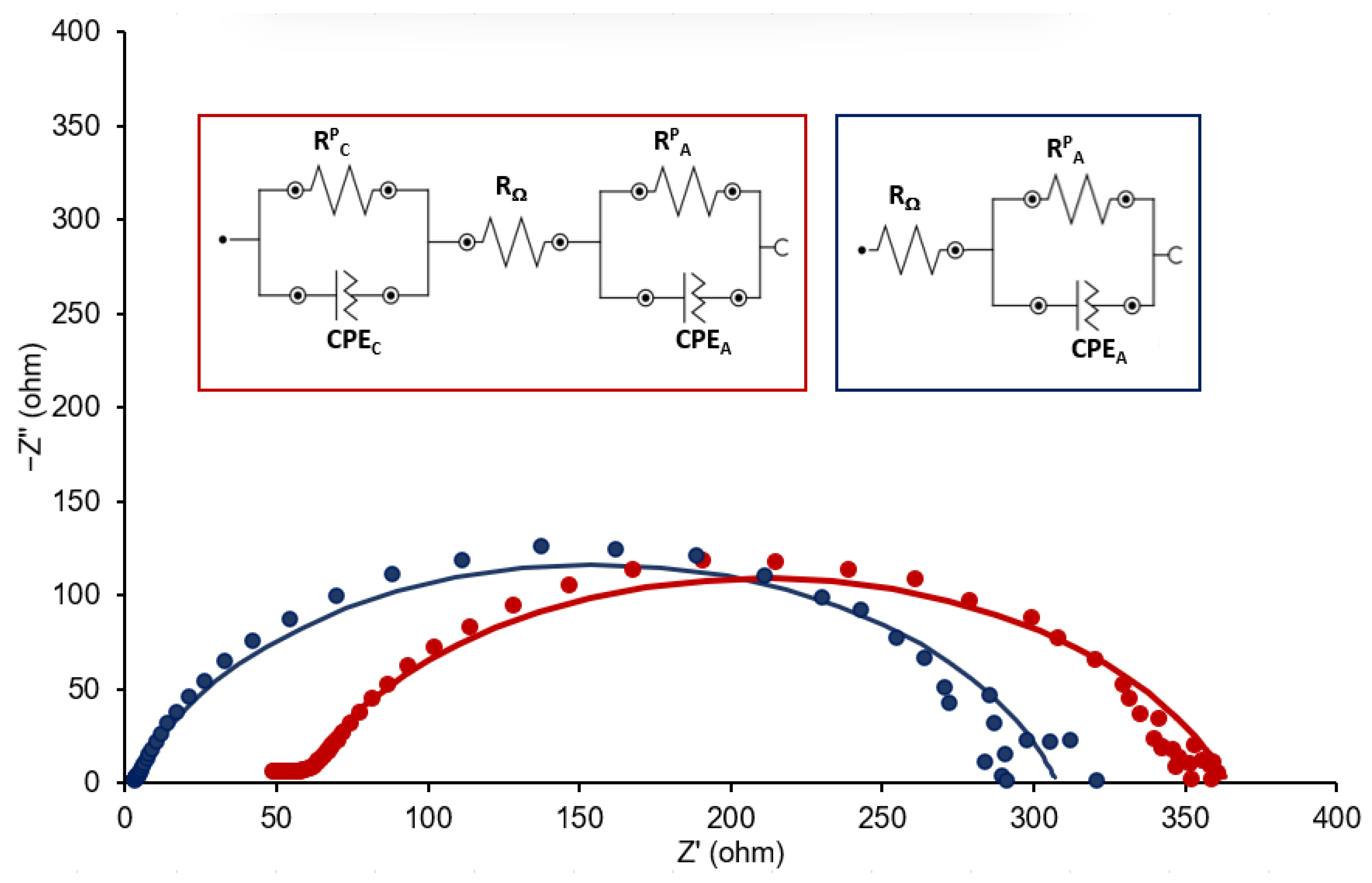
| CDL (F/cm2) | Ccont (F/cm2) | |||||
|---|---|---|---|---|---|---|
| Applied Cell Voltage (V) | Applied Cell Voltage (V) | |||||
| 1.7 | 1.9 | 2.1 | 1.7 | 1.9 | 2.1 | |
| AC/CB | 2.50 × 10−3 | 6.02 × 10−4 | 1.03 × 10−4 | 2.59 × 10−8 | 4.33 × 10−8 | 4.08 × 10−8 |
| Mn10 | 1.60 × 10−4 | 9.37 × 10−5 | 9.43 × 10−5 | 5.43 × 10−8 | 2.92 × 10−8 | 8.83 × 10−8 |
| Pt/C | 7.20 × 10−5 | 5.43 × 10−5 | 4.87 × 10−5 | 1.91 × 10−8 | 1.19 × 10−8 | 5.28 × 10−9 |
| CDL (F/cm2) | CCONT (F/cm2) | |||||
|---|---|---|---|---|---|---|
| Applied Cell Voltage (V) | Applied Cell Voltage (V) | |||||
| 1.7 | 1.9 | 2.1 | 1.7 | 1.9 | 2.1 | |
| AC/CB | 1.45 × 10−4 | 7.26 × 10−5 | 4.42 × 10−5 | 2.95 × 10−8 | 2.89 × 10−8 | 1.86 × 10−8 |
| Mn10 | 1.87 × 10−4 | 3.82 × 10−5 | 4.16 × 10−5 | 1.16 × 10−8 | 3.23 × 10−7 | 1.89 × 10−7 |
| Pt/C | 1.58 × 10−4 | 8.70 × 10−5 | 8.56 × 10−5 | 2.38 × 10−8 | 2.57 × 10−8 | 2.57 × 10−8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mais, L.; Mascia, M.; Vacca, A. Assessing the Performance of Continuous-Flow Microbial Fuel Cells and Membrane Electrode Assembly with Electrodeposited Mn Oxide Catalyst. Energies 2024, 17, 943. https://doi.org/10.3390/en17040943
Mais L, Mascia M, Vacca A. Assessing the Performance of Continuous-Flow Microbial Fuel Cells and Membrane Electrode Assembly with Electrodeposited Mn Oxide Catalyst. Energies. 2024; 17(4):943. https://doi.org/10.3390/en17040943
Chicago/Turabian StyleMais, Laura, Michele Mascia, and Annalisa Vacca. 2024. "Assessing the Performance of Continuous-Flow Microbial Fuel Cells and Membrane Electrode Assembly with Electrodeposited Mn Oxide Catalyst" Energies 17, no. 4: 943. https://doi.org/10.3390/en17040943
APA StyleMais, L., Mascia, M., & Vacca, A. (2024). Assessing the Performance of Continuous-Flow Microbial Fuel Cells and Membrane Electrode Assembly with Electrodeposited Mn Oxide Catalyst. Energies, 17(4), 943. https://doi.org/10.3390/en17040943








