Enhancing DMC Production from CO2: Tuning Oxygen Vacancies and In Situ Water Removal
Abstract
1. Introduction
2. Materials and Methods
2.1. Catalyst Synthesis
2.2. Catalyst Characterizations
2.3. Catalytic Performance Evaluation
3. Results and Discussion
3.1. Material Characterizations
3.2. Catalytic Performance in Liquid Phase
3.3. Catalytic Performance in Gas Phase
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Jin, B.; Wang, K.; Yu, H.; He, X.; Liang, X. Engineering oxygen vacancy-rich CeOx overcoating onto Ni/Al2O3 by atomic layer deposition for bi-reforming of methane. Chem. Eng. J. 2023, 459, 141611. [Google Scholar] [CrossRef]
- Tian, L.; Tan, Z.; Wang, Q.; Liao, Y.S.; Chou, J.P.; Wu, J.M.; Liu, G.; Peng, Y.K. Cerium coordination-dependent surface intermediates regulate activity in dimethyl carbonate synthesis from CO2 and methanol. Appl. Catal. B 2023, 336, 122914. [Google Scholar] [CrossRef]
- Stoian, D.; Medina, F.; Urakawa, A. Improving the Stability of CeO2 Catalyst by Rare Earth Metal Promotion and Molecular Insights in the Dimethyl Carbonate Synthesis from CO2 and Methanol with 2-Cyanopyridine. ACS Catal. 2018, 8, 3181–3193. [Google Scholar] [CrossRef]
- Liu, K.; Liu, C. Synthesis of dimethyl carbonate from methanol and CO(2) under low pressure. RSC Adv. 2021, 11, 35711–35717. [Google Scholar] [CrossRef] [PubMed]
- Nomanbhay, S.; Ong, M.Y.; Chew, K.W.; Show, P.L.; Lam, M.K.; Chen, W.-H. Organic Carbonate Production Utilizing Crude Glycerol Derived as By-Product of Biodiesel Production: A Review. Energies 2020, 13, 1483. [Google Scholar] [CrossRef]
- Ein-Eli, Y. Dimethyl carbonate (DMC) electrolytes—The effect of solvent purity on Li–ion intercalation into graphite anodes. Electrochem. Commun. 2002, 4, 644–648. [Google Scholar] [CrossRef]
- Han, S. Structure and dynamics in the lithium solvation shell of nonaqueous electrolytes. Sci. Rep. 2019, 9, 5555. [Google Scholar] [CrossRef]
- Tundo, P.; Selva, M. The chemistry of dimethyl carbonate. Acc. Chem. Res. 2002, 35, 706–716. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.; Tiwari, A.; Thompson, M.; Chen, Z.; Cleary, T.P.; Lee, T.B.K. A Practical Method for N-Methylation of Indoles Using Dimethyl Carbonate. Org. Proc. Res. Dev. 2001, 5, 604–608. [Google Scholar] [CrossRef]
- Kumar, P.; With, P.; Srivastava, V.C.; Shukla, K.; Gläser, R.; Mishra, I.M. Dimethyl carbonate synthesis from carbon dioxide using ceria–zirconia catalysts prepared using a templating method: Characterization, parametric optimization and chemical equilibrium modeling. RSC Adv. 2016, 6, 110235–110246. [Google Scholar] [CrossRef]
- Eta, V.; Mäki-Arvela, P.i.; Leino, A.-R.; Kordás, K.n.; Salmi, T.; Murzin, D.Y.; Mikkola, J.-P. Synthesis of Dimethyl Carbonate from Methanol and Carbon Dioxide: Circumventing Thermodynamic Limitations. Ind. Eng. Chem. Res. 2010, 49, 9609–9617. [Google Scholar] [CrossRef]
- Bansode, A.; Urakawa, A. Continuous DMC Synthesis from CO2 and Methanol over a CeO2 Catalyst in a Fixed Bed Reactor in the Presence of a Dehydrating Agent. ACS Catal. 2014, 4, 3877–3880. [Google Scholar] [CrossRef]
- Kulthananat, T.; Kim-Lohsoontorn, P.; Seeharaj, P. Ultrasonically assisted surface modified CeO(2) nanospindle catalysts for conversion of CO(2) and methanol to DMC. Ultrason. Sonochem. 2022, 90, 106164. [Google Scholar] [CrossRef] [PubMed]
- Kumar, P.; With, P.; Srivastava, V.C.; Gläser, R.; Mishra, I.M. Efficient ceria-zirconium oxide catalyst for carbon dioxide conversions: Characterization, catalytic activity and thermodynamic study. J. Alloys Compd. 2017, 696, 718–726. [Google Scholar] [CrossRef]
- Chen, L.; Wang, S.; Zhou, J.; Shen, Y.; Zhao, Y.; Ma, X. Dimethyl carbonate synthesis from carbon dioxide and methanol over CeO2 versus over ZrO2: Comparison of mechanisms. RSC Adv. 2014, 4, 30968–30975. [Google Scholar] [CrossRef]
- Bian, J.; Xiao, M.; Wang, S.-J.; Lu, Y.-X.; Meng, Y.Z. Carbon nanotubes supported Cu–Ni bimetallic catalysts and their properties for the direct synthesis of dimethyl carbonate from methanol and carbon dioxide. Appl. Surf. Sci. 2009, 255, 7188–7196. [Google Scholar] [CrossRef]
- Han, D.; Chen, Y.; Wang, S.; Xiao, M.; Lu, Y.; Meng, Y. Effect of In-Situ Dehydration on Activity and Stability of Cu–Ni–K2O/Diatomite as Catalyst for Direct Synthesis of Dimethyl Carbonate. Catalysts 2018, 8, 343. [Google Scholar] [CrossRef]
- Pimprom, S.; Sriboonkham, K.; Dittanet, P.; Föttinger, K.; Rupprechter, G.; Kongkachuichay, P. Synthesis of copper–nickel/SBA-15 from rice husk ash catalyst for dimethyl carbonate production from methanol and carbon dioxide. J. Ind. Eng. Chem. 2015, 31, 156–166. [Google Scholar] [CrossRef]
- Du, Y.; Qin, C.; Xu, Y.; Xu, D.; Bai, J.; Ma, G.; Ding, M. Ni nanoparticles dispersed on oxygen vacancies-rich CeO2 nanoplates for enhanced low-temperature CO2 methanation performance. Chem. Eng. J. 2021, 418, 129402. [Google Scholar] [CrossRef]
- Ren, J.; Mebrahtu, C.; van Koppen, L.; Martinovic, F.; Hofmann, J.P.; Hensen, E.J.M.; Palkovits, R. Enhanced CO2 methanation activity over La2-xCexNiO4 perovskite-derived catalysts: Understanding the structure-performance relationships. Chem. Eng. J. 2021, 426, 131760. [Google Scholar] [CrossRef]
- Ye, R.P.; Li, Q.; Gong, W.; Wang, T.; Razink, J.J.; Lin, L.; Qin, Y.-Y.; Zhou, Z.; Adidharma, H.; Tang, J.; et al. High-performance of nanostructured Ni/CeO2 catalyst on CO2 methanation. Appl. Catal. B 2020, 268, 118474. [Google Scholar] [CrossRef]
- Zhang, T.; Wang, W.; Gu, F.; Xu, W.; Zhang, J.; Li, Z.; Zhu, T.; Xu, G.; Zhong, Z.; Su, F. Enhancing the low-temperature CO2 methanation over Ni/La-CeO2 catalyst: The effects of surface oxygen vacancy and basic site on the catalytic performance. Appl. Catal. B 2022, 312, 121385. [Google Scholar] [CrossRef]
- Liu, B.; Li, C.; Zhang, G.; Yao, X.; Chuang, S.S.C.; Li, Z. Oxygen Vacancy Promoting Dimethyl Carbonate Synthesis from CO2 and Methanol over Zr-Doped CeO2 Nanorods. ACS Catal. 2018, 8, 10446–10456. [Google Scholar] [CrossRef]
- Jing, R.; Lu, X.; Wang, J.; Xiong, J.; Qiao, Y.; Zhang, R.; Yu, Z. CeO(2)-Based Frustrated Lewis Pairs via Defective Engineering: Formation Theory, Site Characterization, and Small Molecule Activation. Small 2024, e2310926. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Geng, S.; Liu, F.; Yao, M.; Ma, J.; Cao, J.; Li, Z. Uncovering the role of yttrium in a cerium-based binary oxide in the catalytic conversion of carbon dioxide and methanol to dimethyl carbonate. J. Colloid Interface Sci. 2023, 652, 1984–1993. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Li, C.; Zhang, G.; Yan, L.; Li, Z. Direct synthesis of dimethyl carbonate from CO2 and methanol over CaO–CeO2 catalysts: The role of acid–base properties and surface oxygen vacancies. New J. Chem. 2017, 41, 12231–12240. [Google Scholar] [CrossRef]
- Wang, S.; Zhao, L.; Wang, W.; Zhao, Y.; Zhang, G.; Ma, X.; Gong, J. Morphology control of ceria nanocrystals for catalytic conversion of CO2 with methanol. Nanoscale 2013, 5, 5582–5588. [Google Scholar] [CrossRef]
- Fu, Z.; Zhong, Y.; Yu, Y.; Long, L.; Xiao, M.; Han, D.; Wang, S.; Meng, Y. TiO2-Doped CeO2 Nanorod Catalyst for Direct Conversion of CO2 and CH3OH to Dimethyl Carbonate: Catalytic Performance and Kinetic Study. ACS Omega 2018, 3, 198–207. [Google Scholar] [CrossRef]
- Luo, M.; Qin, T.; Liu, Q.; Yang, Z.; Wang, F.; Li, H. Novel Fe-modified CeO2 Nanorod Catalyst for the Dimethyl Carbonate Formation from CO2 and Methanol. ChemCatChem 2022, 14, e202200253. [Google Scholar] [CrossRef]
- Wang, Z.Q.; Zhang, M.J.; Hu, X.B.; Dravid, V.P.; Xu, Z.N.; Guo, G.C. CeO2−x quantum dots with massive oxygen vacancies as efficient catalysts for the synthesis of dimethyl carbonate. Chem. Commun. 2020, 56, 403–406. [Google Scholar] [CrossRef]
- Honda, M.; Suzuki, A.; Noorjahan, B.; Fujimoto, K.; Suzuki, K.; Tomishige, K. Low pressure CO(2) to dimethyl carbonate by the reaction with methanol promoted by acetonitrile hydration. Chem. Commun. 2009, 30, 4596–4598. [Google Scholar] [CrossRef]
- Fang, W.; Wang, C.; Liu, Z.; Wang, L.; Liu, L.; Li, H.; Xu, S.; Zheng, A.; Qin, X.; Liu, L.; et al. Physical mixing of a catalyst and a hydrophobic polymer promotes CO hydrogenation through dehydration. Science 2022, 377, 406–410. [Google Scholar] [CrossRef] [PubMed]
- Honda, M.; Tamura, M.; Nakagawa, Y.; Sonehara, S.; Suzuki, K.; Fujimoto, K.; Tomishige, K. Ceria-catalyzed conversion of carbon dioxide into dimethyl carbonate with 2-cyanopyridine. ChemSusChem 2013, 6, 1341–1344. [Google Scholar] [CrossRef] [PubMed]
- Honda, M.; Tamura, M.; Nakagawa, Y.; Nakao, K.; Suzuki, K.; Tomishige, K. Organic carbonate synthesis from CO2 and alcohol over CeO2 with 2-cyanopyridine: Scope and mechanistic studies. J. Catal. 2014, 318, 95–107. [Google Scholar] [CrossRef]
- Aouissi, A.; Al-Deyab, S.S. Comparative study between gas phase and liquid phase for the production of DMC from methanol and CO2. J. Nat. Gas Chem. 2012, 21, 189–193. [Google Scholar] [CrossRef]
- Chen, Y.; Li, Y.; Chen, W.; Xu, W.W.; Han, Z.k.; Waheed, A.; Ye, Z.; Li, G.; Baiker, A. Continuous dimethyl carbonate synthesis from CO2 and methanol over BixCe1−xOδ monoliths: Effect of bismuth doping on population of oxygen vacancies, activity, and reaction pathway. Nano Res. 2021, 15, 1366–1374. [Google Scholar] [CrossRef]
- Choudhury, B.; Choudhury, A. Ce3+ and oxygen vacancy mediated tuning of structural and optical properties of CeO2 nanoparticles. Mater. Chem. Phys. 2012, 131, 666–671. [Google Scholar] [CrossRef]
- Deshpande, S.; Patil, S.; Kuchibhatla, S.V.N.T.; Seal, S. Size dependency variation in lattice parameter and valency states in nanocrystalline cerium oxide. Appl. Phys. Lett. 2005, 87, 133113. [Google Scholar] [CrossRef]
- Liu, J.; Zhao, Z.; Xu, C.; Liu, J. Structure, synthesis, and catalytic properties of nanosize cerium-zirconium-based solid solutions in environmental catalysis. Chin. J. Catal. 2019, 40, 1438–1487. [Google Scholar] [CrossRef]
- Christou, S.Y.; Efstathiou, A.M. The Effects of P-Poisoning of CexZr1−xO2 on the Transient Oxygen Storage and Release Kinetics. Top Catal. 2013, 56, 232–238. [Google Scholar] [CrossRef]
- Yashima, M.; Arashi, H.; Kakihana, M.; Yoshimura, M. Raman Scattering Study of Cubic–Tetragonal Phase Transition in Zr1−xCexO2 Solid Solution. J. Am. Ceram. Soc. 2005, 77, 1067–1071. [Google Scholar] [CrossRef]
- Yashima, M.; Ohtake, K.; Kakihana, M.; Yoshimura, M. Synthesis of Metastable Tetragonal (t′) Zirconia–Ceria Solid Solutions by the Polymerized Complex Method. J. Am.Ceram. Soc. 2005, 77, 2773–2776. [Google Scholar] [CrossRef]
- Zhou, Y.; Deng, J.; Lan, L.; Wang, J.; Yuan, S.; Gong, M.; Chen, Y. Remarkably promoted low-temperature reducibility and thermal stability of CeO2–ZrO2–La2O3–Nd2O3 by a urea-assisted low-temperature (90 °C) hydrothermal procedure. J. Mater. Sci. 2017, 52, 5894–5907. [Google Scholar] [CrossRef]
- Gao, R.; Zhang, D.; Maitarad, P.; Shi, L.; Rungrotmongkol, T.; Li, H.; Zhang, J.; Cao, W. Morphology-Dependent Properties of MnOx/ZrO2–CeO2 Nanostructures for the Selective Catalytic Reduction of NO with NH3. J. Phys. Chem. C 2013, 117, 10502–10511. [Google Scholar] [CrossRef]
- Wang, N.; Qian, W.; Chu, W.; Wei, F. Crystal-plane effect of nanoscale CeO2 on the catalytic performance of Ni/CeO2 catalysts for methane dry reforming. Catal. Sci. Technol. 2016, 6, 3594–3605. [Google Scholar] [CrossRef]
- Jin, J.C.L.; Tsang, C.W.; Xu, B.; Liang, C. Catalytic combustion of methane over Pd/Ce–Zr oxides washcoated monolithic catalysts under oxygen lean conditions. RSC Adv. 2015, 5, 102147–102156. [Google Scholar] [CrossRef]
- Liu, Y.; Hu, C.; Bian, L. Highly Dispersed Pd Species Supported on CeO2 Catalyst for Lean Methane Combustion: The Effect of the Occurrence State of Surface Pd Species on the Catalytic Activity. Catalysts 2021, 11, 772. [Google Scholar] [CrossRef]
- Rao, R.; Jin, P.; Huang, Y.; Hu, C.; Dong, X.; Tang, Y.; Wang, F.; Luo, F.; Fang, S. A surface control strategy of CeO2 nanocrystals for enhancing adsorption removal of Congo red. Colloids Interface Sci. Commun. 2022, 49, 100631. [Google Scholar] [CrossRef]
- Chen, Z.; Chen, L.; Jiang, M.; Gao, X.; Huang, M.; Li, Y.; Ren, L.; Yang, Y.; Yang, Z. Controlled synthesis of CeO2 nanorods and their promotional effect on catalytic activity and aging resistibility for diesel soot oxidation. Appl. Surf. Sci. 2020, 510, 145401. [Google Scholar] [CrossRef]
- Tana; Zhang, M.; Li, J.; Li, H.; Li, Y.; Shen, W. Morphology-dependent redox and catalytic properties of CeO2 nanostructures: Nanowires, nanorods and nanoparticles. Catal. Today 2009, 148, 179–183. [Google Scholar] [CrossRef]
- Hojo, H.; Hirota, K.; Ito, S.; Einaga, H. Reduction Mechanism for CeO2 Revealed by Direct Observation of the Oxygen Vacancy Distribution in Shape-Controlled CeO2. Adv. Mater. Interfaces 2022, 10, 2201954. [Google Scholar]
- Shah, P.M.; Day, A.N.; Davies, T.E.; Morgan, D.J.; Taylor, S.H. Mechanochemical preparation of ceria-zirconia catalysts for the total oxidation of propane and naphthalene Volatile Organic Compounds. Appl. Catal. B 2019, 253, 331–340. [Google Scholar] [CrossRef]
- Bao, H.; Qian, K.; Chen, X.; Fang, J.; Huang, W. Spectroscopic study of microstructure-reducibility relation of CexZr1−xO2 solid solutions. Appl. Surf. Sci. 2019, 467, 361–369. [Google Scholar] [CrossRef]
- Désaunay, T.; Bonura, G.; Chiodo, V.; Freni, S.; Couzinié, J.P.; Bourgon, J.; Ringuedé, A.; Labat, F.; Adamo, C.; Cassir, M. Surface-dependent oxidation of H2 on CeO2 surfaces. J. Catal. 2013, 297, 193–201. [Google Scholar] [CrossRef]
- Almusaiteer, K.A.; Al-Mayman, S.I.; Mamedov, A.; Al-Zeghayer, Y.S. In Situ IR Studies on the Mechanism of Dimethyl Carbonate Synthesis from Methanol and Carbon Dioxide. Catalysts 2021, 11, 517. [Google Scholar] [CrossRef]
- Huo, L.; Wang, T.; Xuan, K.; Li, L.; Pu, Y.; Li, C.; Qiao, C.; Yang, H.; Bai, Y. Synthesis of Dimethyl Carbonate from CO2 and Methanol over Zr-Based Catalysts with Different Chemical Environments. Catalysts 2021, 11, 710. [Google Scholar] [CrossRef]
- Tomishige, K.; Ikeda, Y.; Sakaihori, T.; Fujimoto, K. Catalytic properties and structure of zirconia catalysts for direct synthesis of dimethyl carbonate from methanol and carbon dioxide. J. Catal. 2000, 192, 355–362. [Google Scholar] [CrossRef]
- Huang, P.; Chu, J.; Fu, J.; Yu, J.; Li, S.; Guo, Y.; Zhao, C.; Liu, J. Influence of reduction conditions on the structure-activity relationships of NaNO3-promoted Ni/MgO dual function materials for integrated CO2 capture and methanation. Chem. Eng. J. 2023, 467, 143431. [Google Scholar] [CrossRef]
- Everett Espino, O.E.; Zonetti, P.C.; Celin, R.R.; Costa, L.T.; Alves, O.C.; Spadotto, J.C.; Appel, L.G.; de Avillez, R.R. The tendency of supports to generate oxygen vacancies and the catalytic performance of Ni/ZrO2 and Ni/Mg(Al)O in CO2 methanation. Catal. Sci. Technol. 2022, 12, 1324–1338. [Google Scholar] [CrossRef]
- Yang, G.; Jia, A.; Li, J.; Li, F.; Wang, Y. Investigation of synthesis parameters to fabricate CeO2 with a large surface and high oxygen vacancies for dramatically enhanced performance of direct DMC synthesis from CO2 and methanol. Mol. Catal. 2022, 528, 112471. [Google Scholar] [CrossRef]
- Lan, Y.-P.; Sohn, H.Y. Effect of oxygen vacancies and phases on catalytic properties of hydrogen-treated nanoceria particles. Mater. Res. Express 2018, 5, 035501. [Google Scholar] [CrossRef]
- Porqueras, I.; Person, C.; Bertran, E. Influence of the film structure on the properties of electrochromic CeO2 thin films deposited by e-beam PVD. Thin Solid Films 2004, 447, 119–124. [Google Scholar] [CrossRef]
- Mogensen, M. Physical, chemical and electrochemical properties of pure and doped ceria. Solid State Ion. 2000, 129, 63–94. [Google Scholar] [CrossRef]
- Chen, Z.; Ding, Y.; Fang, N.; Liu, C. Fabrication and photocatalytic activities of dark brown CeO2 with a crystalline-core/disordered-shell heterostructure. Mater. Res. Express 2018, 5, 065905. [Google Scholar] [CrossRef]
- Gabruś, E.; Nastaj, J.; Tabero, P.; Aleksandrzak, T. Experimental studies on 3A and 4A zeolite molecular sieves regeneration in TSA process: Aliphatic alcohols dewatering–water desorption. Chem. Eng. J. 2015, 259, 232–242. [Google Scholar] [CrossRef]
- Liu, P.; Lin, T.; Guo, L.; Liu, X.; Gong, K.; Yao, T.; An, Y.; Zhong, L. Tuning cobalt carbide wettability environment for Fischer-Tropsch to olefins with high carbon efficiency. Chin. J. Catal. 2023, 48, 150–163. [Google Scholar] [CrossRef]



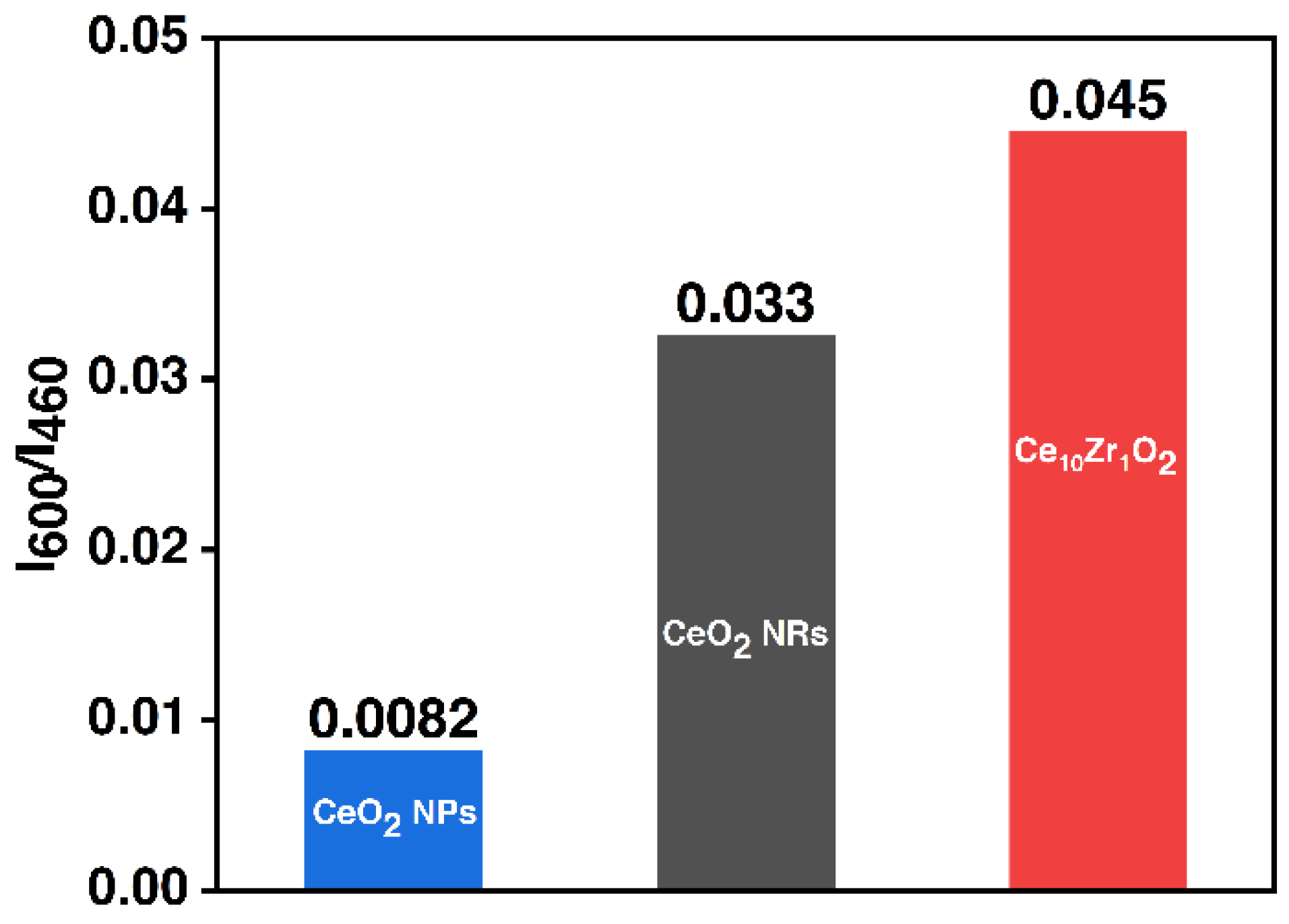

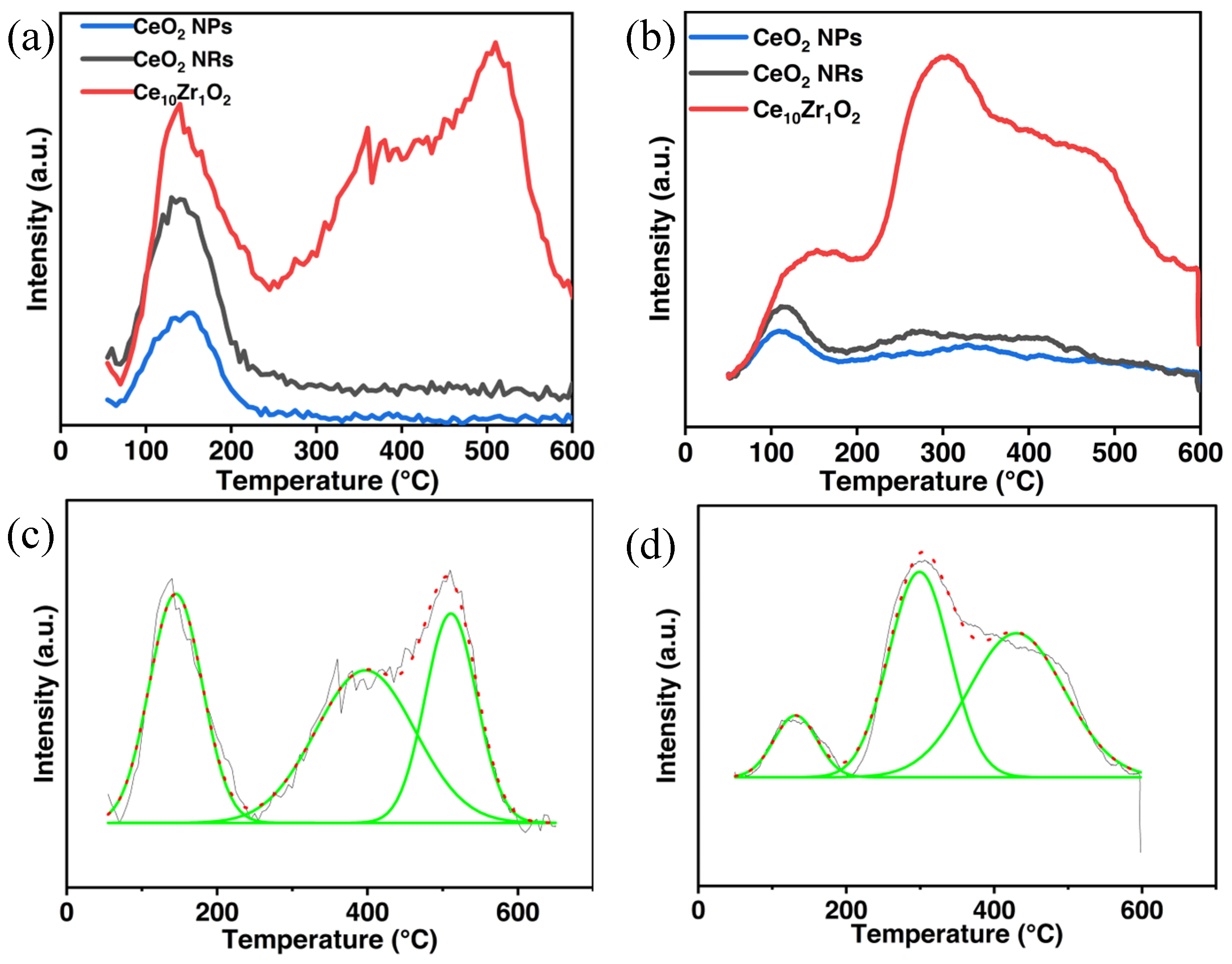

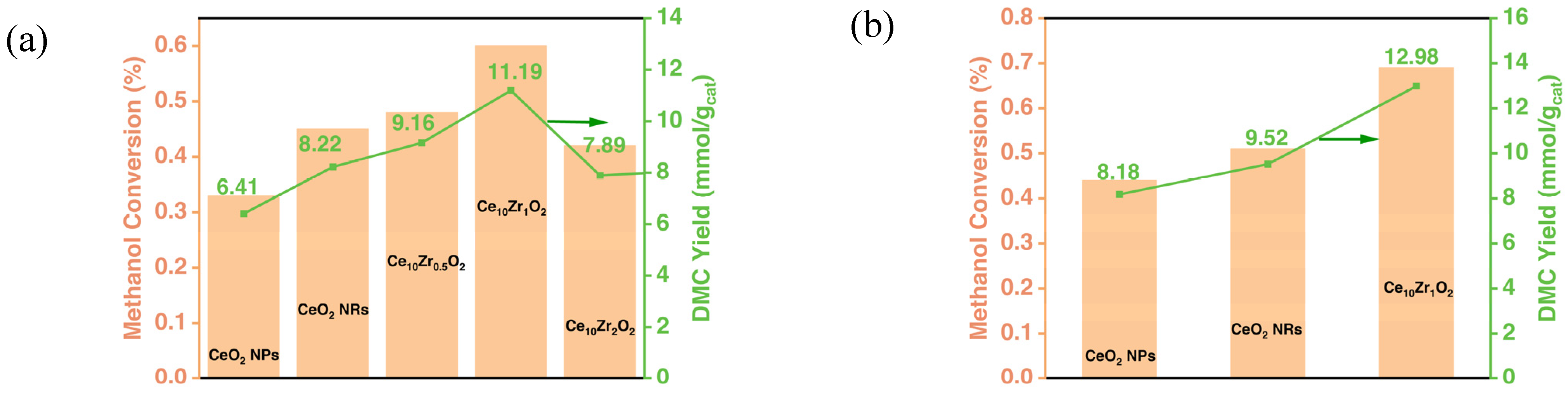
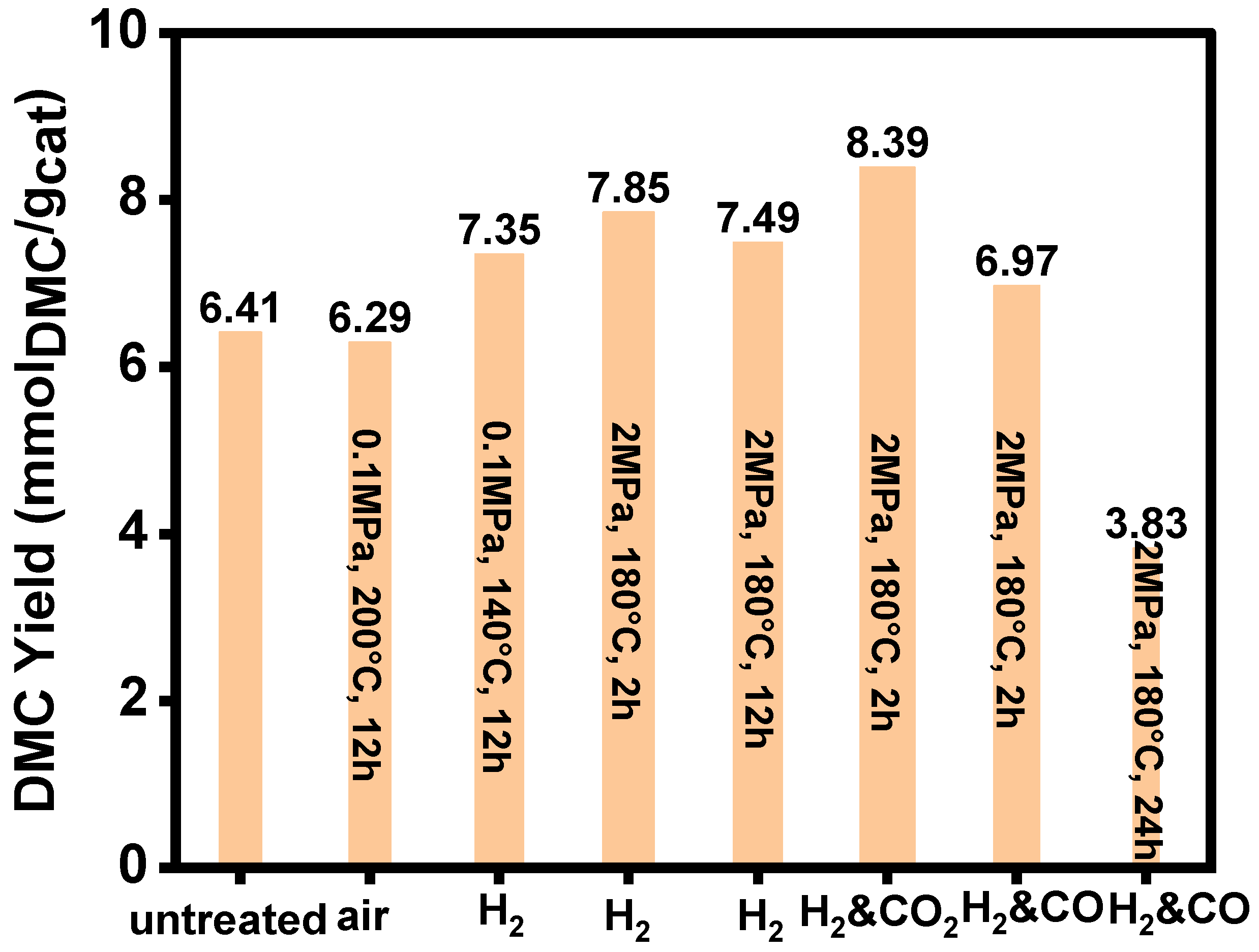

| Sample IDs | 2θ | Interplanar Spacing (Å) | Lattice Parameter | SBET (m2/g) |
|---|---|---|---|---|
| CeO2 NRs | 28.73 | 3.105 | 5.405 | 71 |
| Ce10Zr1O2 NRs | 28.61 | 3.117 | 5.362 | 102 |
| 2-CP (g) | CeO2 NPs (g) | Pressure (MPa) | DMC Yield (mmolDMC/gcat) | Methanol Conversion (%) |
|---|---|---|---|---|
| 0 | 0.1 | 3 | 6.41 | 0.33 |
| 1.04 | 0.1 | 3 | 27.67 | 5.54 |
| 10.4 | 0.3 | 3 | 239.00 | 72.52 |
| 10.4 | 0.3 | 5 | 289.67 | 87.00 |
| Catalysts | Mass (g) | Pressure (MPa) | DMC Yield (mmolDMC/gcat) | Methanol Conversion (%) |
|---|---|---|---|---|
| CeO2 NPs | 1 | 1.2 | 8.63 | 0.46 |
| 20%CeO2/3A | 1 | 1.2 | 7.12 | 0.38 |
| 20%CeO2/SiO2 | 1 | 1.2 | 17.8 | 0.95 |
| CeO2 NPs * | 0.1 | 3 | 6.41 | 0.33 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, K.; Li, S.; Yu, M.; Liang, X. Enhancing DMC Production from CO2: Tuning Oxygen Vacancies and In Situ Water Removal. Energies 2024, 17, 839. https://doi.org/10.3390/en17040839
Wang K, Li S, Yu M, Liang X. Enhancing DMC Production from CO2: Tuning Oxygen Vacancies and In Situ Water Removal. Energies. 2024; 17(4):839. https://doi.org/10.3390/en17040839
Chicago/Turabian StyleWang, Kaiying, Shiguang Li, Miao Yu, and Xinhua Liang. 2024. "Enhancing DMC Production from CO2: Tuning Oxygen Vacancies and In Situ Water Removal" Energies 17, no. 4: 839. https://doi.org/10.3390/en17040839
APA StyleWang, K., Li, S., Yu, M., & Liang, X. (2024). Enhancing DMC Production from CO2: Tuning Oxygen Vacancies and In Situ Water Removal. Energies, 17(4), 839. https://doi.org/10.3390/en17040839







