Effect of Composting Ashes from Biomass Combustion on Polycyclic Aromatic Hydrocarbon Content
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Incubation Process
2.3. Determination of PAH Content
2.4. PAH Toxicity Analysis
3. Results and Discussion
3.1. PAH Content in Biomass Ash
3.2. PAH Content of the Tested Mixtures after the Incubation Process
3.3. Risk Indicators in the Tested Samples (CEQ, MEQ TCDD-TEQ, ΣPAHcarc/ΣPAH)
4. Conclusions
- An analysis of the content of a group of hydrocarbons consisting of a smaller number of aromatic rings (2–4) showed a huge impact of the temperature of the composting process on the reduction in PAHs. The highest degree of degradation was obtained for indeno(1,2,3-c,d)pyrene (InP), where the content of this congener decreased by approximately 46% during incubation at 20 °C, and by 76% at 40 °C.
- In both composting methods, acenaphthene turned out to be the most resistant to degradation, the content of which decreased significantly after mixing with compost, but increased during incubation. After incubation at 20 °C, its content increased by 123.07%, and after incubation at 40 °C, it increased by 159.07%.
- The composting process has a beneficial effect on reducing the total number of polycyclic aromatic hydrocarbons in ash from biomass combustion. As a result of the co-composting of ashes, a decrease in PAH16 content was found by 37.2%.
- Analyzing the calculated TEQ = CEQ, MEQ, and TCDD-TEQ indicators, a significant decrease was found in the composting process. The TCDD-TEQ carcinogenicity index decreased the most (45.45%).
- The harmfulness of the tested ΣPAHcarc/ΣPAH samples was minimally reduced from 0.22 to 0.21 when composted at 20 °C, but increased to 0.27 when composted at 40 °C.
- The ΣPAHcarc/ΣPAH coefficient for the test samples was well below 1, which means that the resulting composts do not pose a threat to humans.
- To sum up, it was concluded that the research results showed a positive effect of co-composting ashes on the content of PAHs. Reducing the content of ΣPAH16 and reducing the overall harmfulness of ΣPAHcarc/ΣPAH means that ash from biomass combustion has great potential for use as a fertiliser. Therefore, the management of waste from biomass combustion in the form of compost in agriculture would enable an effective and very simple reduction of PAHs entering the environment.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Redukcja Emisji Gazów Cieplarnianych: Cele i Przepisy Unii Europejskiej. Available online: https://www.europarl.europa.eu/news/pl/headlines/society/20180305STO99003/redukcja-emisji-gazow-cieplarnianych-cele-i-przepisy-unii-europejskiej (accessed on 1 November 2023).
- Łabętowicz, J.; Rutkowska, B.; Sosulski, T.; Stępień, W.; Szara, E.; Szulc, W.; Szymańska, M. Fertilizers from waste as a source of nutrients for fertilizing crops. In Agricultural Use of Waste and By-Products as a Link in the Circular Economy, 1st ed.; SGGW Publishing House: Warsaw, Poland, 2020; pp. 182–189. [Google Scholar]
- Meinhard, D. The Paris Agreement: Historic breakthrough or high stakes experiment? Clim. Law 2016, 6, 1–20. [Google Scholar]
- Stępień, M.; Białecka, B. Inventory of innovative energy waste recovery technologies. SWwIP 2017, 6, 108–123. [Google Scholar]
- Chrzanowski, Z.; Baran, B.; Dudziak, M.; Katzor, R. Poland as a potential source of combustion by-products (UPS) for European markets. In Proceedings of the XXVI International Conference Ashes from the Energy Industry, Sopot, Poland, 8–10 October 2019. [Google Scholar]
- Markowski, G. Economic use of ashes after burning hard coal and biomass. Eunomia–Rozw. Zrównoważony–Sustain. Dev. 2018, 2, 71–80. [Google Scholar]
- Bies, J.; Bochenek, D.; Dzik, M.; Hejne, J.; Karpińska, K.; Kiełczykowska, A.; Kruszewska, D.; Nowakowska, B.; Pawłowska, T.; Sulik, J.; et al. Environmental Protection 2019; Główny Urząd Statystyczny Statistics Poland: Warsaw, Poland, 2019. [Google Scholar]
- Vassilev, S.V.; Baxter, D.; Andersen, L.K.; Vassileva, C.G. An overview of the chemical composition of biomass. Fuel 2010, 89, 913–933. [Google Scholar] [CrossRef]
- Wheatley, A.D.; Sadhra, S. Polycyclic aromatic hydrocarbons in solid residues from waste incineration. Chemosphere 2004, 55, 743–749. [Google Scholar] [CrossRef]
- Bignal, K.L.; Langridge, S.; Zhou, J.L. Release of polycyclic aromatic hydrocarbons, carbon monoxide and particulate matter from biomass combustion in a wood-fired boiler under varying boiler conditions. Atmos. Environ. 2008, 42, 8863–8871. [Google Scholar] [CrossRef]
- Li, Z.; Chen, L.; Liu, S.; Ma, H.; Wang, L.; An, C.; Zhang, R. Characterization of PAHs and PCBs in fly ashes of eighteen coal-fired power plants. Aerosol Air Qual. Res. 2016, 16, 3175–3186. [Google Scholar] [CrossRef]
- Verma, S.K.; Masto, R.E.; Gautam, S.; Choudhury, D.P.; Ram, L.C.; Maiti, S.K.; Maity, S. Investigations on PAHs and trace elements in coal and its combustion residues from a power plant. Fuel 2015, 162, 138–147. [Google Scholar] [CrossRef]
- White, P.A. The genotoxicity of priority polycyclic aromatic hydrocarbons in complex mixtures. Mutat. Res. 2002, 515, 85–98. [Google Scholar] [CrossRef]
- Poluszyńska, J. Assessment of the possibility of contamination of the soil and ground environment with polycyclic aromatic hydrocarbons (PAHs) contained in fly ash from power boilers. Pr. Inst. Ceram. I Mater. Bud. 2013, 6, 60–71. [Google Scholar]
- Regulation of the Minister of the Environment of 28 January 2009 Amending the Regulation on the Conditions to Be Met When Introducing Sewage into Water or Land and on Substances Particularly Harmful to the Aquatic Environment. Available online: https://isap.sejm.gov.pl/isap.nsf/DocDetails.xsp?id=WDU20090270169 (accessed on 19 February 2009).
- Dwivedi, A.; Jain, M.K. Fly ash–waste management and overview: A Review. Recent Res. Sci. Technol. 2014, 6, 30–35. [Google Scholar]
- Diaz-Loya, E.I.; Allouche, E.N.; Eklund, S.; Joshi, A.R.; Kupwade-Patil, K. Toxicity mitigation and solidification of municipal solid waste incinerator fly ash using alkaline activated coal ash. Waste Manag. 2012, 32, 1521–1527. [Google Scholar] [CrossRef]
- Jura, J.; Ulewicz, M. The influence of ashes from biomass combustion on selected properties of cement mortars. Przegląd Bud. 2017, 88, 54–55. [Google Scholar]
- Thomas, B.S.; Yang, J.; Mo, K.H.; Abdalla, J.A.; Hawileh, R.A.; Ariyachandra, E. Biomass ashes from agricultural wastes as supplementary cementitious materials or aggregate replacement in cement/geopolymer concrete: A comprehensive review. J. Build. Eng. 2021, 40, 102332. [Google Scholar] [CrossRef]
- Ogundiran, M.B.; Nugteren, H.W.; Witkamp, G.J. Geopolymerisation of fly ashes with waste aluminium anodising etching solutions. J. Environ. Manag. 2016, 181, 118–123. [Google Scholar] [CrossRef]
- Davidovits, J. Geopolymer cement. A review. Geopolymer Institute. Tech. Pap. 2013, 21, 1–11. [Google Scholar]
- Mikuła, J.; Łach, M.; Mierzwiński, D. Methods of managing ashes and slags from waste incineration plants. EEET 2017, 18, 37–46. [Google Scholar]
- Provis, J.L.; Duxson, P.; Harrex, R.M.; Yong, C.Z.; Van Deventer, J.S.J. Valorisation of fly ashes by geopolymerisation. Glob. Nest J. 2009, 11, 147–154. [Google Scholar]
- Komnitsas, K.; Zaharaki, D.; Bartzas, G. Effect of sulphate and nitrate anions on heavy metal immobilisation in ferronickel slag geopolymers. Appl. Clay Sci. 2013, 73, 103–109. [Google Scholar] [CrossRef]
- Ogundiran, M.B.; Nugteren, H.W.; Witkamp, G.J. Immobilisation of lead smelting slag within spent aluminate—Fly ash based geopolymers. J. Hazard. Mater. 2013, 248, 29–36. [Google Scholar] [CrossRef]
- Ahmari, S.; Zhang, L. Durability and leaching behavior of mine tailings-based geopolymer bricks. Constr. Build. Mater. 2013, 44, 743–750. [Google Scholar] [CrossRef]
- Kowalczyk-Juśko, A. Źródła biomasy na cele energetyczne. In Bioenergetyka Podkarpacka; Editor Kościk, B., Ed.; PWSZ: Jarosław, Poland, 2007; pp. 105–185. [Google Scholar]
- Kalembasa, D. Quantity and chemical composition of ash from biomass of energy crops. Acta Agrophys. 2006, 7, 909–914. [Google Scholar]
- Niedziółka, I.; Zuchniarz, A. Energy analysis of selected types of biomass of agricultural origin. Motrol. Motoryz. I Energetyka Rol. 2006, 8, 232–237. [Google Scholar]
- Wójcik, M.; Stachowicz, F.; Masłoń, A. Possibility of using fly ash to improve sewage sludge dewatering. JCEEA 2017, 64, 377–393. [Google Scholar]
- Uliasz-Bocheńczuk, A.; Pawluk, A.; Pyzalski, M. Characteristics of ashes from biomass combustion in fluidized bed boilers. Miner. Resour. Manag. 2016, 32, 149–162. [Google Scholar]
- Act of 10 July 2007 on Fertilizers and Fertilization (Journal of Laws of 2007, No. 147, Item 1033). Available online: https://isap.sejm.gov.pl/isap.nsf/DocDetails.xsp?id=wdu20071471033 (accessed on 7 October 2007).
- Regulation of the Minister of Agriculture and Rural Development of 18 June 2008 on the Implementation of Certain Provisions of the Act on Fertilizers and Fertilization (Table 1) (Journal of Laws of 2008, No. 119, Item 765). Available online: https://isap.sejm.gov.pl/isap.nsf/DocDetails.xsp?id=wdu20081190765 (accessed on 18 June 2008).
- Piekarczyk, M. Content of available forms of some macro- and microelements in light soil fertilized with ash from winter wheat straw. Fragm. Agron. 2013, 30, 91–99. [Google Scholar]
- Niezgoda, D. Profitability of replacing mineral fertilizers with plant protection products in the plant production process. Zagadnienia Ekon. Rolnej 2015, 344, 3. [Google Scholar] [CrossRef]
- Szewczyk, P. Kompostowanie/stabilizacja tlenowa. Przegląd Komunal. 2016, 4, 45–48. [Google Scholar]
- Piotrowska-Cyplik, A.; Cyplik, P.; Czarnecki, Z. Measurement of dehydrogenase activity and traditional method of microorganisms count estimation as indicators of microorganisms activity in compost from municipal sewage sludge. J. Res. Appl. Agric. Eng. 2007, 52, 22–26. [Google Scholar]
- Smolewska, M.E.; Krasowska, M.; Piekut, J.; Smolewski, M.; Bagińska, E. Assessment of PAH content in soil and aboveground parts of Lolium perenne L. next to the communication arteries of the urban agglomeration. Stud. Quat. 2022, 39. [Google Scholar]
- Nisbet, I.C.T.; LaGoy, P.K. Toxic equivalency factors (TEFs) for polycyclic aromatic hydrocarbons (PAHs). Regul. Toxicol. Pharmacol. 1992, 16, 290–300. [Google Scholar] [CrossRef]
- Rogula-Kozłowska, W.; Kozielska, B.; Klejnowski, K. Concentration, origin and health hazard from fine particle-bound PAH at three characteristic sites in Southern Poland. Bull. Environ. Contam. Toxicol. 2013, 91, 349–355. [Google Scholar] [CrossRef]
- Willett, K.L.; Gardinali, P.R.; Sericano, J.L.; Wade, T.L.; Safe, S.H. Characterization of the H4IIE rat hepatoma cell bioassay for evaluation of environmental samples containing polynuclear aromatic hydrocarbons (PAHs). Arch. Environ. Contam. Toxicol. 1996, 32, 442–448. [Google Scholar] [CrossRef]
- Bourotte, C.; Forti, M.C.; Taniguchi, S.; Bícego, M.C.; Lotufo, P.A. A wintertime study of PAHs in fine and coarse aerosols in São Paulo city, Brazil. Atmos. Environ. 2005, 39, 3799–3811. [Google Scholar] [CrossRef]
- Masto, R.E.; Sarkar, E.; George, J.; Kumari, J.; Dutta, P.; Ram, L.C. PAHs and potentially toxic elements in the fly ash and bed ash of biomass fired power plants. Fuel Process. Technol. 2015, 132, 139–152. [Google Scholar] [CrossRef]
- Zhai, J.; Burke, I.T.; Stewart, D.I. Potential reuse options for biomass combustion ash as affected by the persistent organic pollutants (POPs) content. J. Hazard. Mater. 2022, 5, 100038. [Google Scholar] [CrossRef]
- Mackiewicz-Walec, E.; Krzebietke, S.J. Content of polycyclic aromatic hydrocarbons in soil in a multi-annual fertilisation regime. Environ. Monit. Assess. 2020, 192, 314. [Google Scholar] [CrossRef]
- Poluszyńska, J.; Jarosz-Krzemińska, E.; Helios-Rybicka, E. Studying the effects of two various methods of composting on the degradation levels of polycyclic aromatic hydrocarbons (PAHs) in sewage sludge. Water Air Soil Pollut. 2017, 228, 1–10. [Google Scholar] [CrossRef]
- Antizar-Ladislao, B.; Lopez-Real, J.; Beck, A.J. Bioremediation of polycyclic aromatic hydrocarbons (PAH) in an aged coal-tar-contaminated soil using different in-vessel composting approaches. J. Hazard. Mater. 2006, 137, 1583–1588. [Google Scholar] [CrossRef]
- Košnář, Z.; Wiesnerová, L.; Částková, T.; Kroulíková, S.; Bouček, J.; Mercl, F.; Tlustoš, P. Bioremediation of polycyclic aromatic hydrocarbons (PAHs) present in biomass fly ash by co-composting and co-vermicomposting. J. Hazard. Mater. 2019, 369, 79–86. [Google Scholar] [CrossRef]
- Huygens, D.; Saveyn, H.; Tonini, D.; Eder, P.; Delgado Sancho, L. Technical proposals for selected new fertilising materials under the Fertilising Products Regulation (Regulation (EU) 2019/1009). FeHPO CaHPO. 2019, 4. [Google Scholar]
- Swedish National Board of Forestry. Recommendations for the Extraction of Forest Fuel and Compensation Fertilising; National Board of Forestry, Publishing Company: Jönköping, Sweden, 2002; pp. 15–17. [Google Scholar]
- Szatyłowicz, E.; Skoczko, I. Evaluation of the PAH content in soot from solid fuels combustion in low power boilers. Energies 2019, 12, 4254. [Google Scholar] [CrossRef]
- Košnář, Z.; Mercl, F.; Tlustoš, P. Long-term willows phytoremediation treatment of soil contaminated by fly ash polycyclic aromatic hydrocarbons from straw combustion. Environ. Pollut. 2020, 264, 114787. [Google Scholar] [CrossRef]
- Kozielska, B.; Żeliński, J.; Cieślar, M. Occurrence of polycylic aromatic hydrocarbons in bottom ash from individual heating devices. Sci. Pap. Main Sch. Fire Serv. 2022, 83, 7–18. [Google Scholar]
- Jelonek, Z.; Fabiańska, M.; Jelonek, I. Quantitative assessment of organic and inorganic contaminants in charcoal. Resources 2021, 10, 69. [Google Scholar] [CrossRef]
- Szatyłowicz, E.; Hawrylik, E. Assessment of migration of PAHs contained in soot of solid fuel combustion into the aquatic environment. Water 2022, 14, 3079. [Google Scholar] [CrossRef]

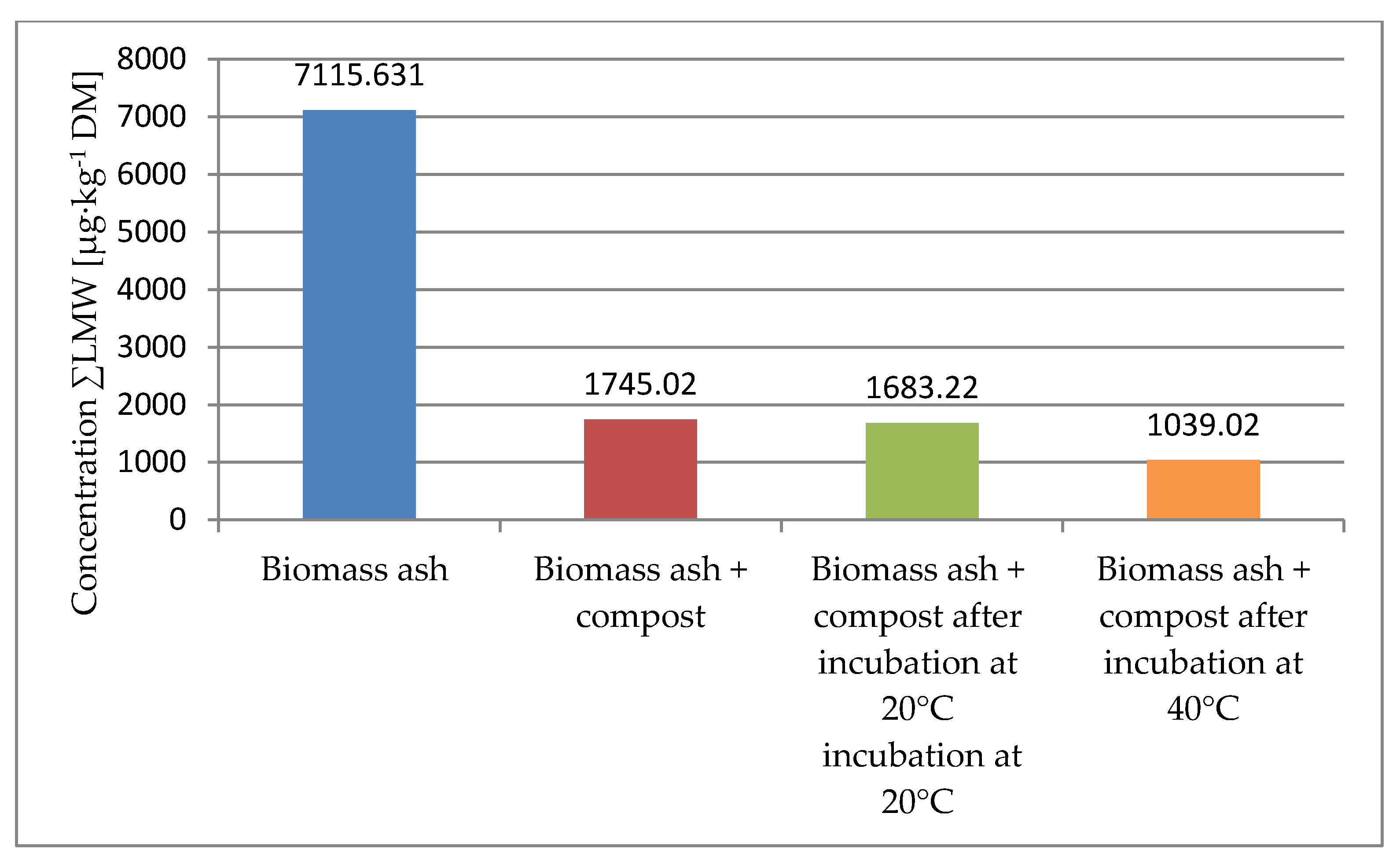
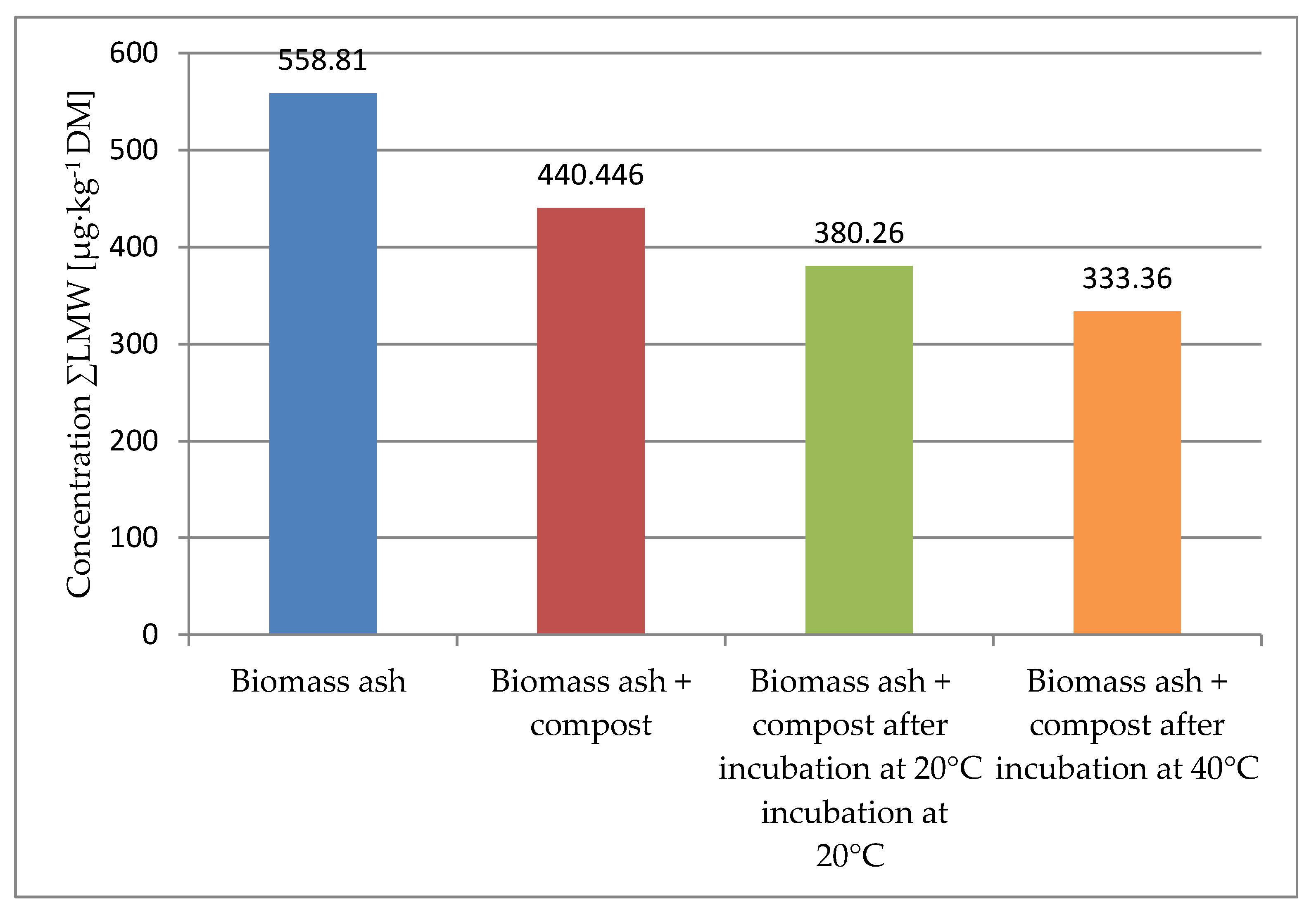
| Compound Name | Biomass Ash | Biomass Ash + Compost | Biomass Ash + Compost After Incubation at 20 °C | Biomass Ash + Compost After Incubation at 40 °C |
|---|---|---|---|---|
| [µg∙kg−1 DM] | ||||
| Nafthalen (NAP) | 6795.77 | 1492.99 | 995.34 | 604.94 |
| Acenaftylen (ACY) | 9.922 | 8.898 | 3.1 | 3.54 |
| Acenaften (ACE) | 67.299 | 18.725 | 41.77 | 48.51 |
| Fluoren (FLU) | 5.666 | 4.936 | 4.28 | 4.14 |
| Fenantren (PHE) | 45.949 | 43.473 | 119.41 | 120.15 |
| Antracen (ANT) | 7.565 | 4.764 | 7.94 | 6.81 |
| Fluoranten (FLA) | 51.489 | 51.098 | 192.88 | 90.97 |
| Pirene (PYR) | 55.63 | 50.937 | 242.3 | 99.43 |
| Benzo(a)antracen (BaA) | 23.791 | 20.087 | 28.44 | 19.23 |
| Chrysen (CHR) | 52.55 | 49.11 | 47.76 | 41.3 |
| Benzo(b)fluoranten (BbF) | 101.331 | 94.586 | 57.45 | 51.46 |
| Benzo(k)fluoranten (BkF) | 69.817 | 63.471 | 61.19 | 28.52 |
| Benzo(a)piren (BaP) | 343.295 | 249.609 | 234.7 | 232 |
| Indeno(1,2,3-c,d)piren (InP) | 6.58 | 2.34 | 1,27 | 0.57 |
| Dibenzo(a,h)antracen (DBA) | 7.88 | 4.72 | 2.47 | 2.18 |
| Benzo(g,h,i)perylen (BgP) | 29.907 | 25.72 | 23.18 | 18.63 |
| Total PAHs | 7674.44 | 2185.46 | 2063.48 | 1372.38 |
| ΣLMW | 7115.631 | 1745.02 | 1683.22 | 1039.02 |
| ΣHMW | 558.81 | 440.446 | 380.26 | 333.36 |
| Indicator | Biomass Ash | Biomass Ash + Compost | Biomass Ash + Compost After Incubation at 20 °C | Biomass Ash + Compost After Incubation at 40 °C |
|---|---|---|---|---|
| [µg∙kg−1 DM] | ||||
| TEQ = CEQ | 413.47 | 296.04 | 266.36 | 256.19 |
| MEQ | 389.16 | 289.71 | 264.45 | 254.63 |
| TCDD-TEQ | 0.75 | 0.66 | 0.54 | 0.36 |
| ΣPAHcarc/ΣPAH | 0.08 | 0.22 | 0.21 | 0.27 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cwalina, P.; Krasowska, M.; Smolewska, M.E.; Koziak, K. Effect of Composting Ashes from Biomass Combustion on Polycyclic Aromatic Hydrocarbon Content. Energies 2024, 17, 840. https://doi.org/10.3390/en17040840
Cwalina P, Krasowska M, Smolewska ME, Koziak K. Effect of Composting Ashes from Biomass Combustion on Polycyclic Aromatic Hydrocarbon Content. Energies. 2024; 17(4):840. https://doi.org/10.3390/en17040840
Chicago/Turabian StyleCwalina, Paweł, Małgorzata Krasowska, Marzena Ewa Smolewska, and Kinga Koziak. 2024. "Effect of Composting Ashes from Biomass Combustion on Polycyclic Aromatic Hydrocarbon Content" Energies 17, no. 4: 840. https://doi.org/10.3390/en17040840
APA StyleCwalina, P., Krasowska, M., Smolewska, M. E., & Koziak, K. (2024). Effect of Composting Ashes from Biomass Combustion on Polycyclic Aromatic Hydrocarbon Content. Energies, 17(4), 840. https://doi.org/10.3390/en17040840






