Thermal Ageing of Dry Cellulose Paper Impregnated with Different Insulating Liquids—Comparative Studies of Materials Properties
Abstract
1. Introduction
2. Materials and Methods
3. Results
3.1. Results of the Paper Properties Tests
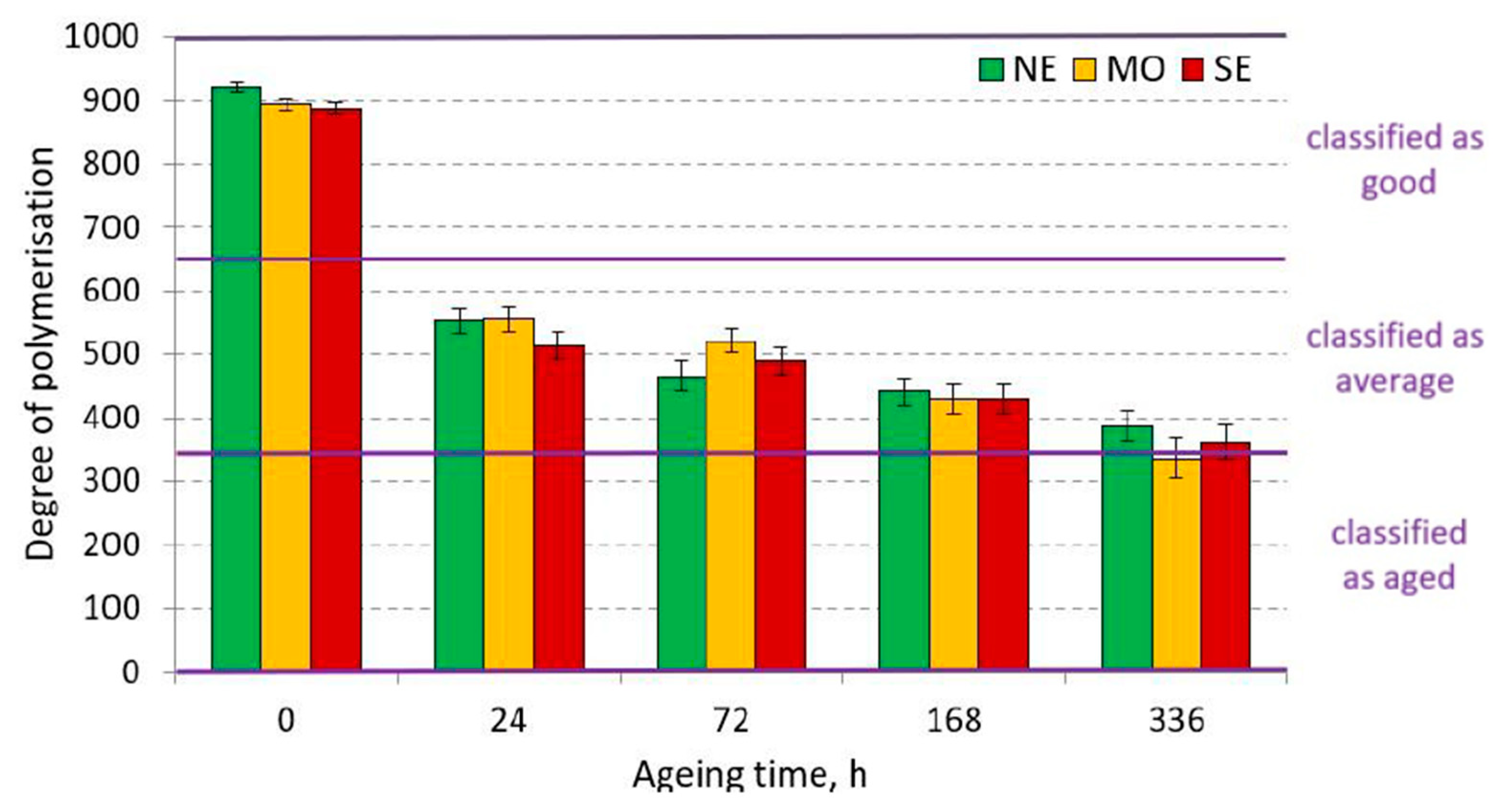
3.1.1. Degree of Polymerisation of Cellulose
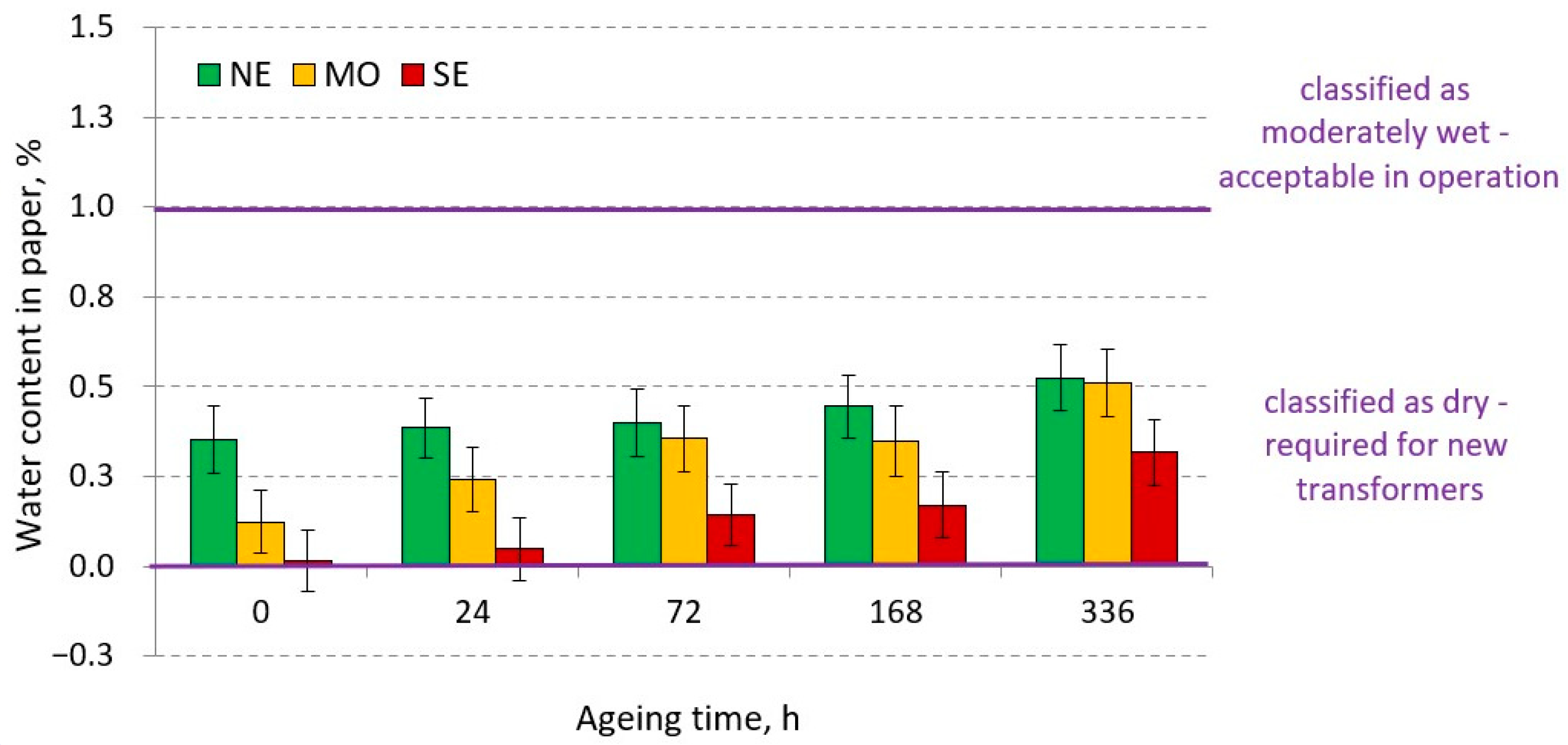
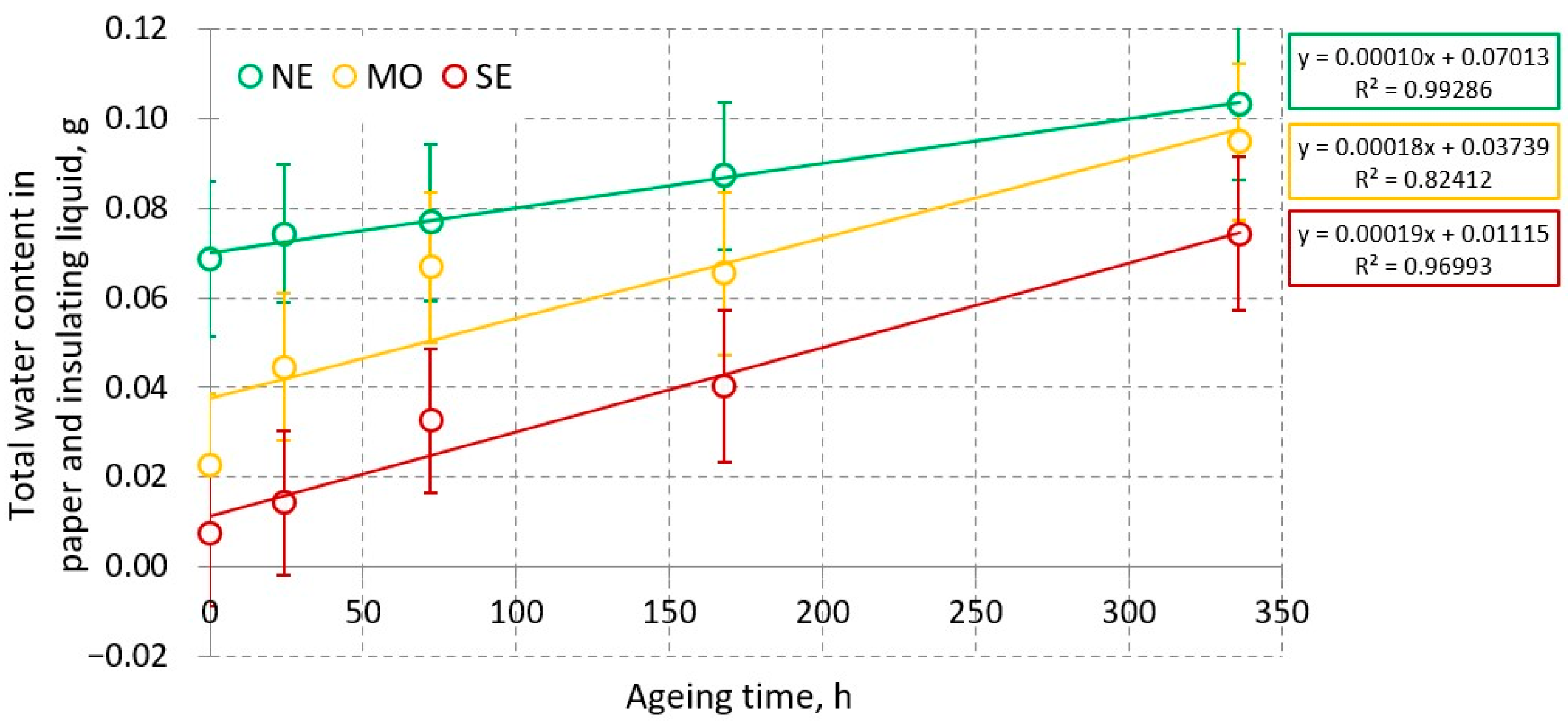
3.1.2. Water Content of Paper
3.2. Test Results on the Properties of Electro-Insulating Liquids
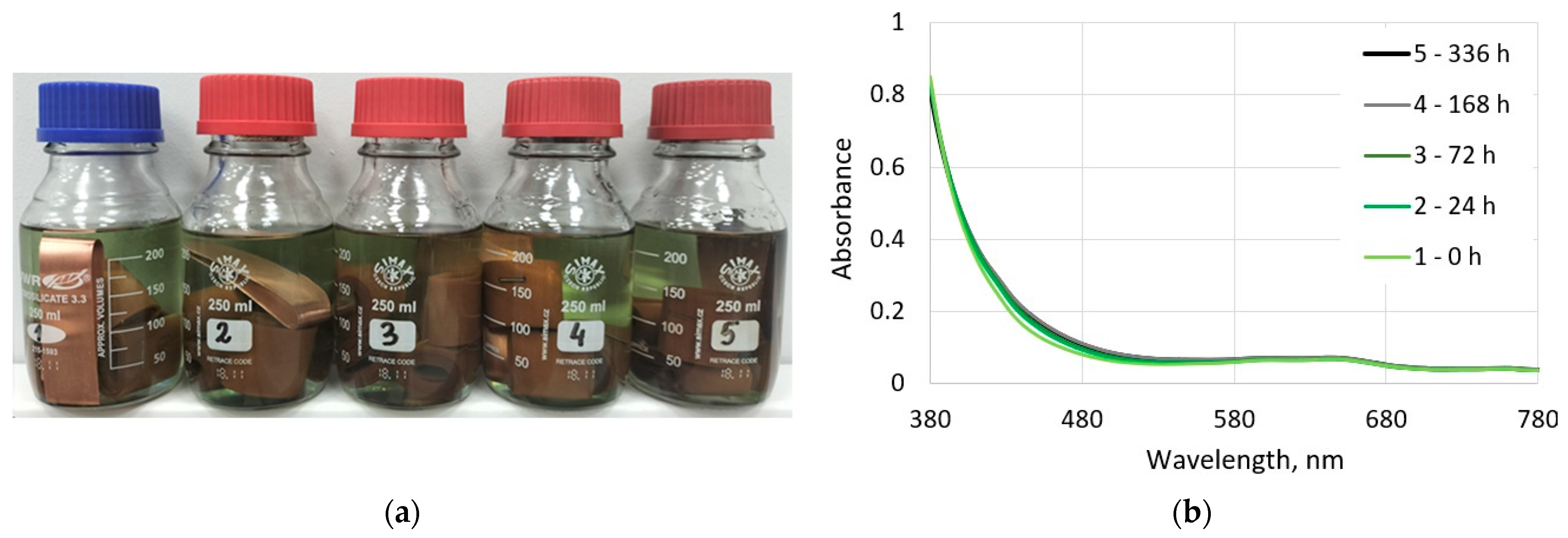
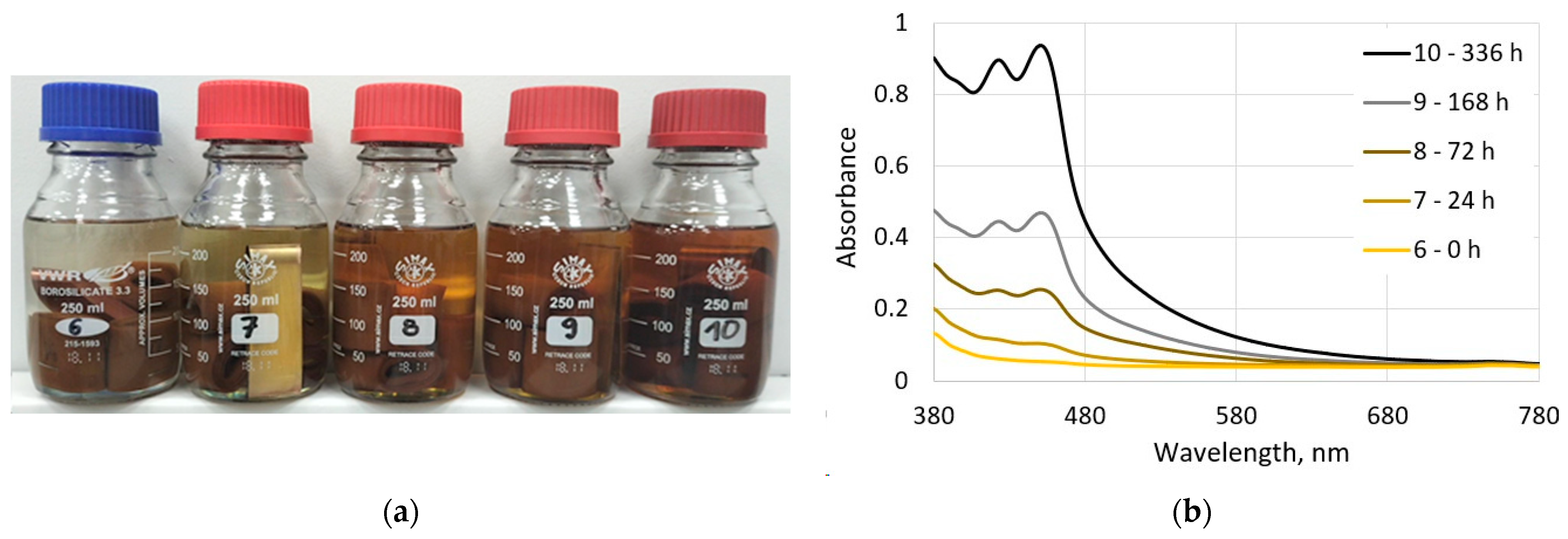
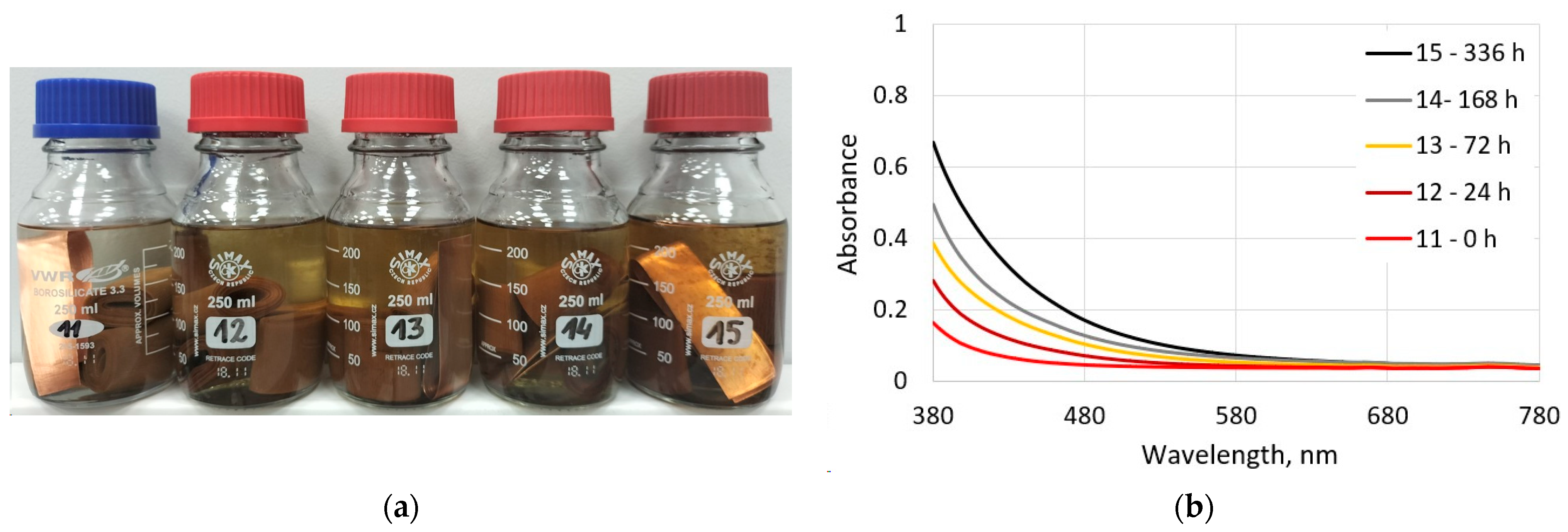
3.2.1. Visual and Spectrophotometric Assessments of the Ageing Degree of Electro-Insulating Liquids
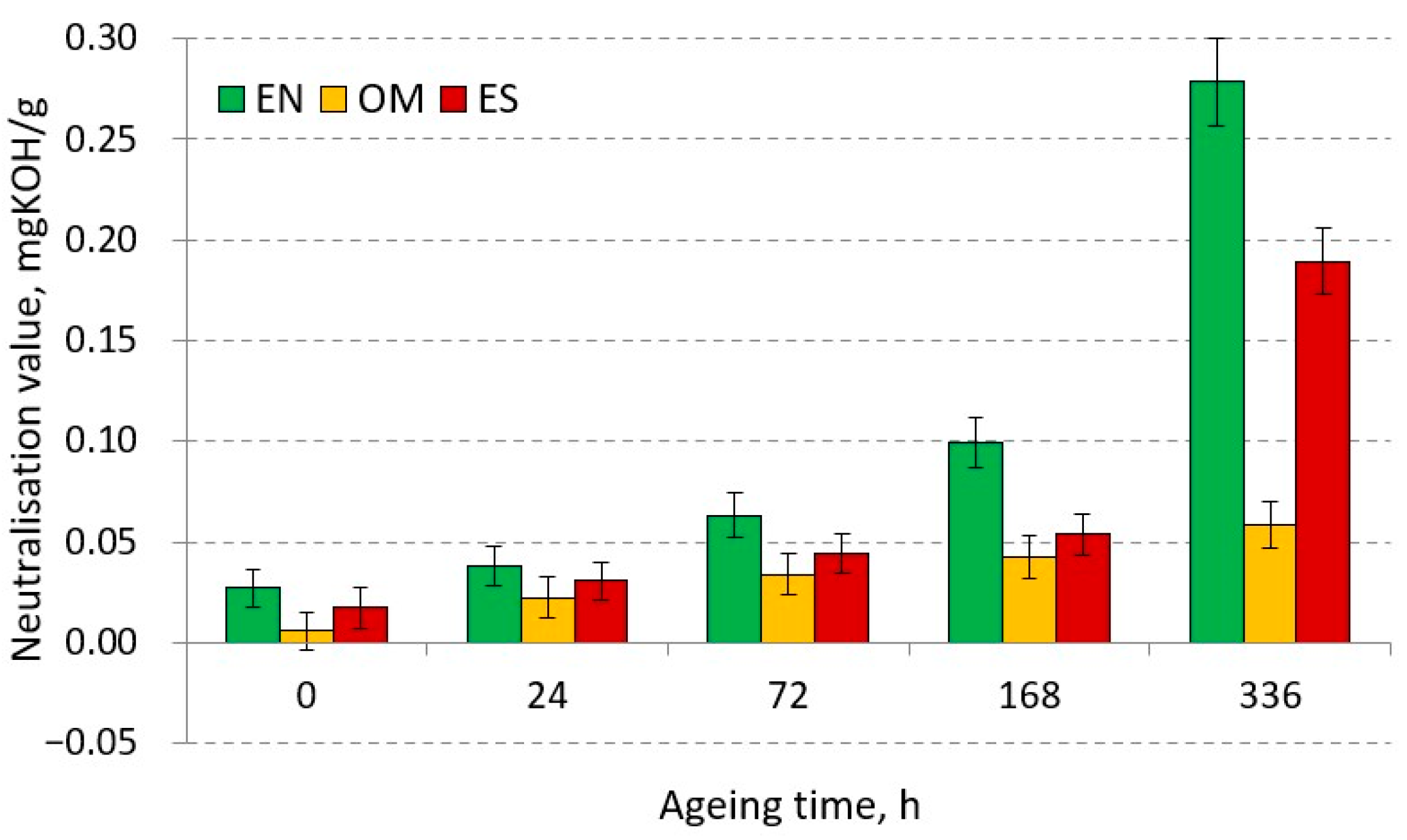
3.2.2. Neutralisation Value
3.2.3. Interfacial Tension
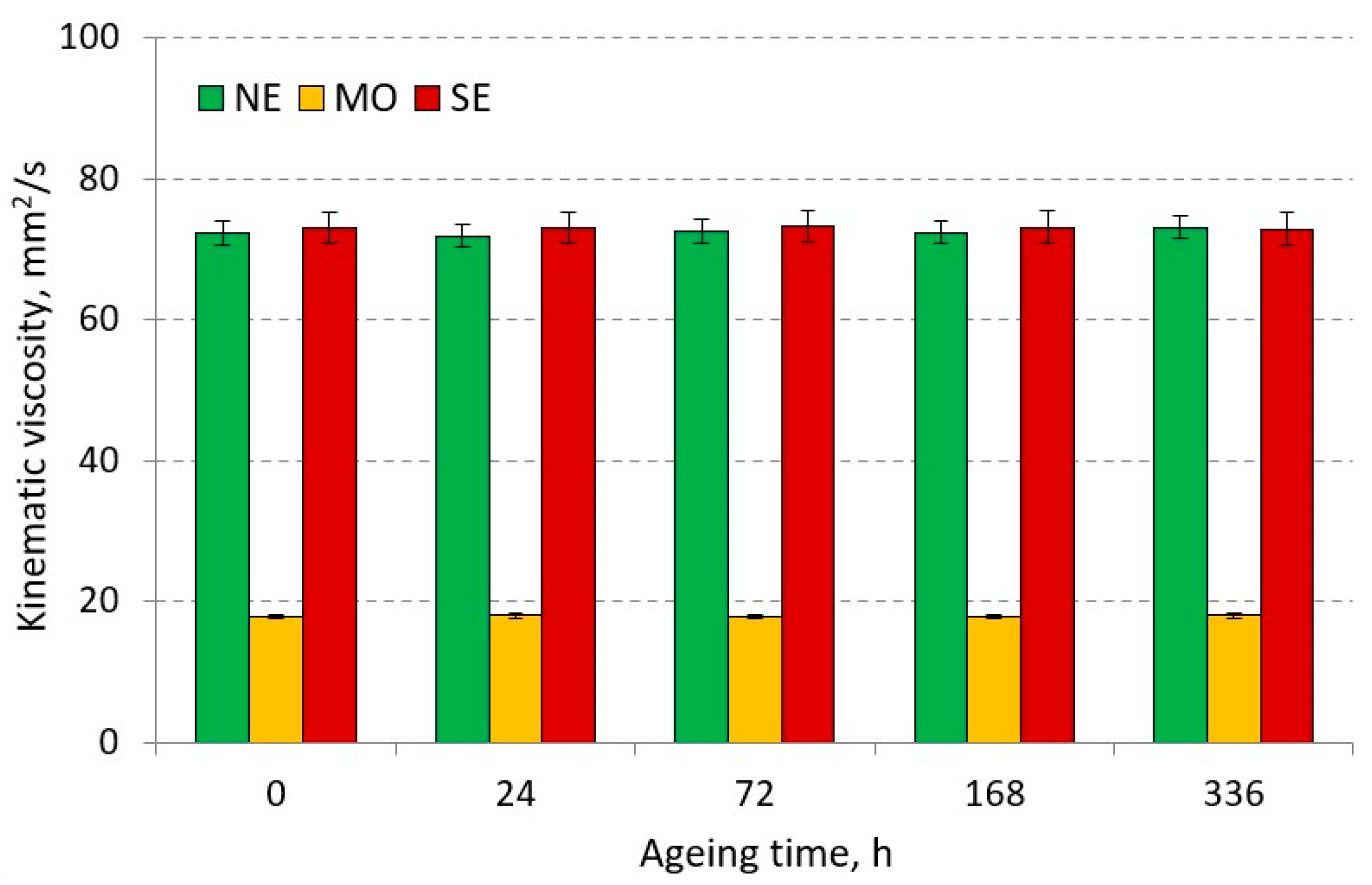
3.2.4. Kinematic Viscosity
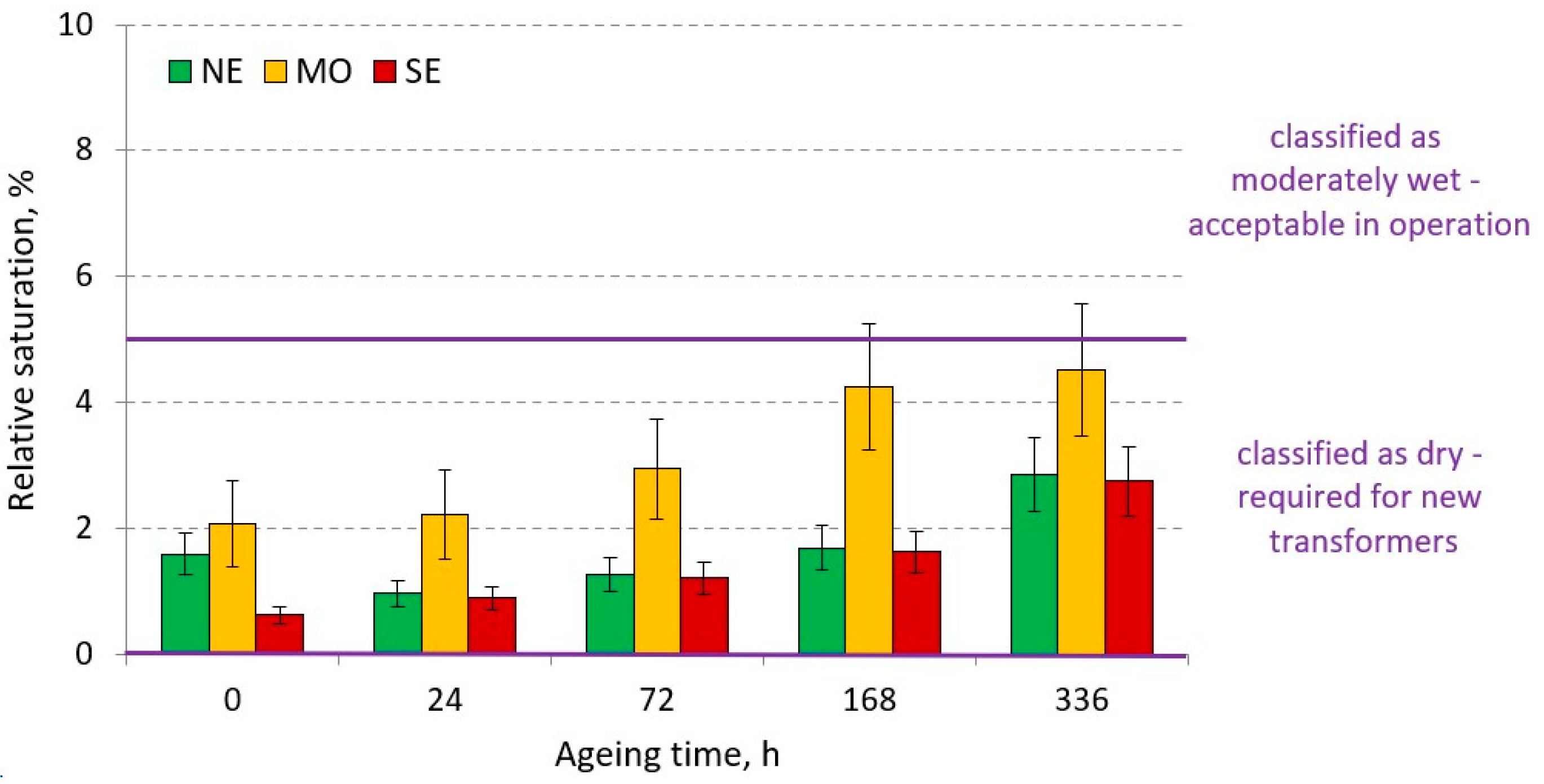
3.2.5. Water Content of Electro-Insulating Liquids
3.2.6. Dissipation Factor, Dielectric Permittivity, and Volume Resistivity
4. Discussion
5. Conclusions
Funding
Data Availability Statement
Conflicts of Interest
References
- El-Harbawi, M.; Al-Mubaddel, F. Risk of Fire and Explosion in Electrical Substations Due to the Formation of Flammable Mixtures. Sci. Rep. 2020, 10, 6295. [Google Scholar] [CrossRef]
- Prevost, T.A.; Oommen, T.V. Cellulose insulation in oil-filled power transformers: Part I—History and development. IEEE Electr. Insul. Mag. 2006, 22, 28–35. [Google Scholar] [CrossRef]
- Rozga, P.; Beroual, A.; Przybylek, P.; Jaroszewski, M.; Strzelecki, K. A Review on Synthetic Ester Liquids for Transformer Applications. Energies 2020, 13, 6429. [Google Scholar] [CrossRef]
- García, B.; Ortiz, A.; Renedo, C.; García, D.F.; Montero, A. Use Performance and Management of Biodegradable Fluids as Transformer Insulation. Energies 2021, 14, 6357. [Google Scholar] [CrossRef]
- Dombek, G.; Gielniak, J. Fire safety and electrical properties of mixtures of synthetic ester/mineral oil and synthetic ester/natural ester. IEEE Trans. Dielectr. Electr. Insul. 2018, 25, 1846–1852. [Google Scholar] [CrossRef]
- Przybylek, P. Determination of Mineral Oil Concentration in the Mixture with Synthetic Ester Using Near-Infrared Spectroscopy. Energies 2023, 16, 6381. [Google Scholar] [CrossRef]
- Martin, R.; Athanassatou, H.; Duart, J.C.; Perrier, C.; Sitar, I.; Walker, J.; Claiborne, C.; Boche, T.; Cherry, D.; Darwin, A.; et al. Experiences in Service with New Insulating Liquids; Cigré Technical Brochure 436; International Council on Large Electric Systems (CIGRE): Paris, France, 2010. [Google Scholar]
- Meira, M.; Álvarez, R.E.; Verucchi, C.J.; Catalano, L.J.; Ruschetti, C.R. Thermal Aging Analysis of Mineral Oil and Natural Ester Immersed Windings. In Proceedings of the IEEE Electrical Insulation Conference (EIC), Knoxville, TN, USA, 22 June–3 July 2020. [Google Scholar] [CrossRef]
- Przybylek, P.; Gielniak, J. The Use of Methanol Vapour for Effective Drying of Cellulose Insulation. Energies 2023, 16, 4465. [Google Scholar] [CrossRef]
- Lundgaard, L.E.; Hansen, W.; Linhjell, D.; Painter, T.J. Ageing of Oil-impregnated Paper in Power Transformers. IEEE Trans. Power Deliv. 2004, 19, 230–239. [Google Scholar] [CrossRef]
- Emsley, A.M.; Xiao, X.; Heywood, R.J.; Ali, M. Degradation of cellulosic insulation in power transformers. Part 3: Effects of oxygen and water on aging in oil. IEE Proc. Sci. Meas. Technol. 2000, 147, 115–119. [Google Scholar] [CrossRef]
- Lawson, W.G.; Simmons, M.A.; Gale, P.S. Thermal Ageing of Cellulose Paper Insulation. IEEE Trans. Electr. Insul. 1977, EI-12, 61–66. [Google Scholar] [CrossRef]
- Prosiński, S. Chemia Drewna, 2nd ed.; Państwowe Wydawnictwo Rolnicze i Leśne: Warsaw, Poland, 1984; pp. 154–196. [Google Scholar]
- Bródka, B.; Mościcka-Grzesiak, H. Budowa i struktura celulozy w aspekcie procesów degradacji izolacji papierowo-olejowej. Przegląd Elektrotech. 2006, 1k/2006, 53–56. [Google Scholar]
- Przybyłek, P. Rozpuszczalność wody w estrze syntetycznym oraz mieszaninie estru z olejem mineralnym w aspekcie suszenia izolacji celulozowej. Przegląd Elektrotech. 2016, 10, 92–95. [Google Scholar] [CrossRef]
- Przybylek, P. Water saturation limit of insulating liquids and hygroscopicity of cellulose in aspect of moisture determination in oil-paper insulation. IEEE Trans. Dielectr. Electr. Insul. 2016, 23, 1886–1893. [Google Scholar] [CrossRef]
- Zhao, Y.; Qian, Y.; Wei, B.; Wang, R.; Rapp, K.J.; Xu, Y. In-Service Ageing Comparison Study of Natural Ester and Mineral Oil Filled Distribution Transformers. In Proceedings of the IEEE International Conference on Dielectric Liquids (ICDL), Rome, Italy, 23–27 June 2019. [Google Scholar] [CrossRef]
- Suwarno; Ritonga, A. Effects of Thermal Aging on the Characteristics of Kraft Paper in Various Liquid Insulating Materials. In Proceedings of the International Symposium on Electrical Insulating Materials (ISEIM), Tokyo, Japan, 13–17 September 2020. [Google Scholar]
- Yang, L.; Liao, R.; Caixin, S.; Zhu, M. Influence of vegetable oil on the thermal aging of transformer paper and its mechanism. IEEE Trans. Dielectr. Electr. Insul. 2021, 18, 692–700. [Google Scholar] [CrossRef]
- Frimpong, G.K.; Oommen, T.V.; Asano, R. A Survey of Aging Characteristics of Cellulose Insulation in Natural Ester and Mineral Oil. IEEE Electr. Insul. Mag. 2011, 27, 36–48. [Google Scholar] [CrossRef]
- Garcia, B.; Garcia, T.; Primo, V.; Burgos, J.C.; Urquiza, D. Studying the Loss of Life of Natural-Ester-Filled Transformer Insulation: Impact of Moisture on the Aging Rate of Paper. IEEE Electr. Insul. Mag. 2017, 33, 15–23. [Google Scholar] [CrossRef]
- Chauvelon, G.; Saulnier, L.; Buleon, A.; Thibault, J.F.; Gourson, C.; Benhaddou, R.; Granet, R.; Krausz, P. Acidic Activation of Cellulose and Its Esterification by Long-Chain Fatty Acid. J. Appl. Polym. Sci. 1999, 72, 1933–1940. [Google Scholar] [CrossRef]
- Rapp, K.J.; McShane, C.P.; Luksich, J. Interaction Mechanisms of Natural Ester Dielectric Fluid and Kraft Paper. In Proceedings of the IEEE International Conference on Dielectric Liquids (ICDL), Coimbra, Portugal, 26 June–1 July 2005. [Google Scholar] [CrossRef]
- NYTRO® 10XN Product Data Sheet by NYNAS. Available online: https://www.nynas.com/en/products/transformer-oils/products/nytro-10xn/ (accessed on 13 December 2023).
- MIDEL 7131 Product Data Sheet by M&I MATERIALS. Available online: https://www.midel.com/app/uploads/2023/11/MIDEL-7131-Product-Brochure.pdf (accessed on 13 December 2023).
- FR3® Fluid Product Data Sheet by CARGILL. Available online: https://www.cargill.com/bioindustrial/doc/1432243645220/fr3-most-proven-natural-ester.pdf (accessed on 13 December 2023).
- Cybulski, M.; Przybylek, P. The application of a molecular sieve for drying the insulation system of a power transformer in terms of improving its operational reliability. Eksploat. Niezawodn. Maint. Reliab. 2022, 24, 502–509. [Google Scholar] [CrossRef]
- Oommen, T.V.; Prevost, T.A. Cellulose Insulation in Oil-Filled Power Transformers: Part II Maintaining Insulation Integrity and Life. IEEE Electr. Insul. Mag. 2006, 22, 5–14. [Google Scholar] [CrossRef]
- IEC 60422; Mineral Insulating Oils in Electrical Equipment, Supervision and Maintenance Guidance. International Electrotechnical Commission (IEC): Geneva, Switzerland, 2013.
- IEC 60450; Measurement of the Average Viscometric Degree of Polymerization of New and Aged Cellulosic Electrically Insulating Materials. International Electrotechnical Commission (IEC): Geneva, Switzerland, 2004.
- IEC 60814; Insulating Liquids—Oil-Impregnated Paper and Pressboard—Determination of Water by Automatic Coulometric Karl Fischer Titration. International Electrotechnical Commission (IEC): Geneva, Switzerland, 1997.
- IEC 62961; Insulating Liquids—Test Methods for the Determination of Interfacial Tension of Insulating Liquids—Determination with the Ring Method. International Electrotechnical Commission (IEC): Geneva, Switzerland, 2018.
- ISO 3104; Petroleum Products—Transparent and Opaque Liquids—Determination of Kinematic Viscosity and Calculation of Dynamic Viscosity. ISO: Geneva, Switzerland, 2020.
- IEC 60247; Insulating Liquids—Measurement of Relative Permittivity, Dielectric Dissipation Factor (tan d) and d.c. Resistivity. International Electrotechnical Commission (IEC): Geneva, Switzerland, 2008.
- Lundgaard, L.E.; Allan, D.; Atanasowa-Höhlein, I.; Clavreul, R.; Dahlund, M.O.; Gasser, H.P.; Heywood, R.; Krause, C.; Lessard, M.C.; Saha, T.K.; et al. Ageing of Cellulose in Mineral-Oil Insulated Transformers; Cigré Technical Brochure 323; International Council on Large Electric Systems (CIGRE): Paris, France, 2007. [Google Scholar]
- IEC 62975; Natural Esters—Guidelines for Maintenance and Use in Electrical Equipment. International Electrotechnical Commission (IEC): Geneva, Switzerland, 2021.
- IEC 61203; Synthetic Organic Esters for Electrical Purposes—Guide for Maintenance of Transformer Esters in Equipment. International Electrotechnical Commission (IEC): Geneva, Switzerland, 1992.
- Rao, U.M.; Fofana, I.; N’cho, J.S. On Some Imperative IEEE Standards for Usage of Natural Ester Liquids in Transformers. IEEE Access 2020, 8, 45446–145456. [Google Scholar] [CrossRef]
- Atanasova-Höhlein, I.; Končan-Gradnik, M.; Gradnik, T.; Čuček, B.; Przybylek, P.; Siodla, K.; Brede Liland, K.; Senja, L.; Qiang, L. Experience with Capacitive On-Line Sensors for Moisture Evaluation in Transformer Insulation. IEEE Electr. Insul. Mag. 2019, 35, 18–26. [Google Scholar] [CrossRef]
- Rao, U.M.; Sood, Y.R.; Jarial, R.K. Oxidation Stability Enhancement of a Blend of Mineral and Synthetic Ester Oils. IEEE Electr. Insul. Mag. 2016, 32, 43–47. [Google Scholar] [CrossRef]
- Kouassi, K.; Fofana, I.; Cissé, L.; Hadjadj, Y.; Yapi, K.; Diby, K. Impact of Low Molecular Weight Acids on Oil Impregnated Paper Insulation Degradation. Energies 2018, 11, 1465. [Google Scholar] [CrossRef]
- Jeanneton, A.; Perrier, C.; Beroual, A. Thermal Aging Study of Alternative Liquids for Oil-Paper High-Voltage Insulation in Current Transformers. In Proceedings of the IEEE International Conference on Dielectric Liquids (ICDL), Sevilla, Spain, 29 May–2 June 2022. [Google Scholar] [CrossRef]
- Münster, T.; Werle, P.; Hämel, K.; Preusel, J. Thermally Accelerated Aging of Insulation Paper for Transformers with Different Insulating Liquids. Energies 2021, 14, 3036. [Google Scholar] [CrossRef]



| Property | Mineral Oil | Synthetic Ester | Natural Ester |
|---|---|---|---|
| Water content, ppm | <20 | 50 | 4–50 |
| Neutralisation value, mgKOH/goil | <0.01 | 0.02 | 0.01–0.05 |
| Interfacial tension at 25 °C, mN/m | 49 | - | - |
| Dissipation factor at 90 °C - | <0.001 | <0.008 | 0.01–0.03 |
| Resistivity at 90 °C, GΩ∙m | - | >20 | - |
| Kinematic viscosity at 40 °C, mm2/s (cSt) | 7.6 | 29 | 32–34 |
| Sample Number | Kind of Liquid | Ageing Time, h |
|---|---|---|
| 1 | Natural ester | 0 |
| 2 | Natural ester | 24 |
| 3 | Natural ester | 72 |
| 4 | Natural ester | 168 |
| 5 | Natural ester | 336 |
| 6 | Mineral oil | 0 |
| 7 | Mineral oil | 24 |
| 8 | Mineral oil | 72 |
| 9 | Mineral oil | 168 |
| 10 | Mineral oil | 336 |
| 11 | Synthetic ester | 0 |
| 12 | Synthetic ester | 24 |
| 13 | Synthetic ester | 72 |
| 14 | Synthetic ester | 168 |
| 15 | Synthetic ester | 336 |
| Sample Number | Kind of Liquid | Ageing Time, h | tanδ, % | C, pF | εr, - | R, GΩ | ρ, GΩ m |
|---|---|---|---|---|---|---|---|
| 1 | NE | 0 | 0.49 | 183.1 | 3.1 | 7.0 | 47.59 |
| 2 | NE | 24 | 0.57 | 183.5 | 3.1 | 6.1 | 41.49 |
| 3 | NE | 72 | 0.37 | 184.4 | 3.1 | 7.4 | 50.10 |
| 4 | NE | 168 | 0.71 | 184.7 | 3.1 | 5.2 | 34.98 |
| 5 | NE | 336 | 0.46 | 184.2 | 3.1 | 5.9 | 39.73 |
| 6 | MO | 0 | 0.07 | 129.8 | 2.2 | 362.0 | 2454 |
| 7 | MO | 24 | 0.01 | 129.9 | 2.2 | 485.0 | 3288 |
| 8 | MO | 72 | 0.01 | 129.9 | 2.2 | 364.0 | 2467 |
| 9 | MO | 168 | 0.02 | 129.9 | 2.2 | 370.0 | 2508 |
| 10 | MO | 336 | 0.08 | 129.9 | 2.2 | 194.6 | 1319 |
| 11 | SE | 0 | 0.24 | 189.9 | 3.2 | 12.0 | 81.63 |
| 12 | SE | 24 | 1.41 | 189.4 | 3.2 | 4.2 | 28.34 |
| 13 | SE | 72 | 1.30 | 189.4 | 3.2 | 4.7 | 32.07 |
| 14 | SE | 168 | 1.89 | 189.2 | 3.2 | 5.5 | 37.08 |
| 15 | SE | 336 | 2.53 | 189.1 | 3.2 | 3.7 | 25.02 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Przybylek, P. Thermal Ageing of Dry Cellulose Paper Impregnated with Different Insulating Liquids—Comparative Studies of Materials Properties. Energies 2024, 17, 784. https://doi.org/10.3390/en17040784
Przybylek P. Thermal Ageing of Dry Cellulose Paper Impregnated with Different Insulating Liquids—Comparative Studies of Materials Properties. Energies. 2024; 17(4):784. https://doi.org/10.3390/en17040784
Chicago/Turabian StylePrzybylek, Piotr. 2024. "Thermal Ageing of Dry Cellulose Paper Impregnated with Different Insulating Liquids—Comparative Studies of Materials Properties" Energies 17, no. 4: 784. https://doi.org/10.3390/en17040784
APA StylePrzybylek, P. (2024). Thermal Ageing of Dry Cellulose Paper Impregnated with Different Insulating Liquids—Comparative Studies of Materials Properties. Energies, 17(4), 784. https://doi.org/10.3390/en17040784






