Numerical Analysis of Soot Dynamics in C3H8 Oxy-Combustion with CO2 and H2O
Abstract
1. Introduction
2. Experiment and Simulation
2.1. Experimental Instrument
2.2. Experiment Principle
2.3. Experimental Condition
2.4. Numerical Simulation
3. Results and Analysis
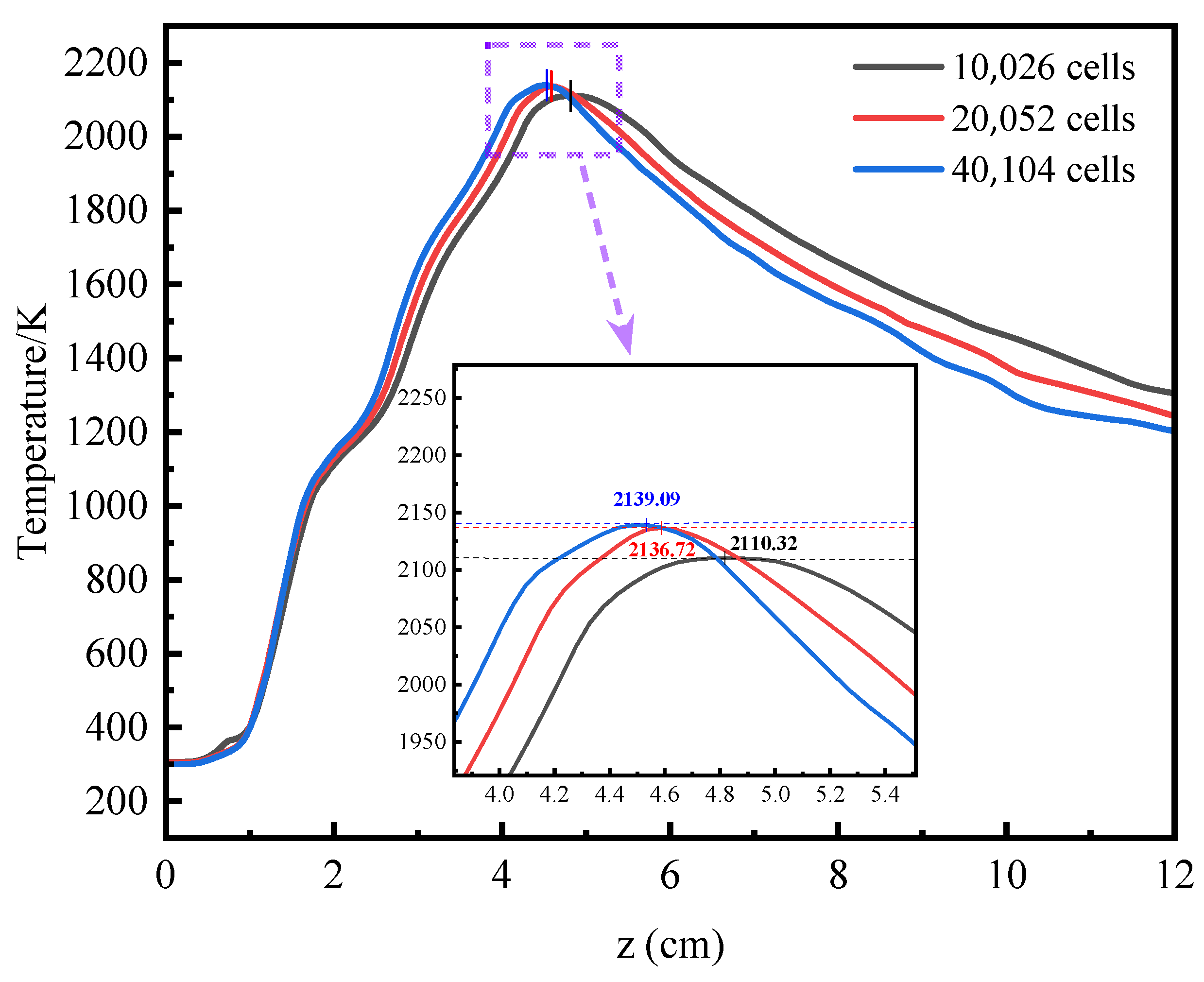
3.1. Verification
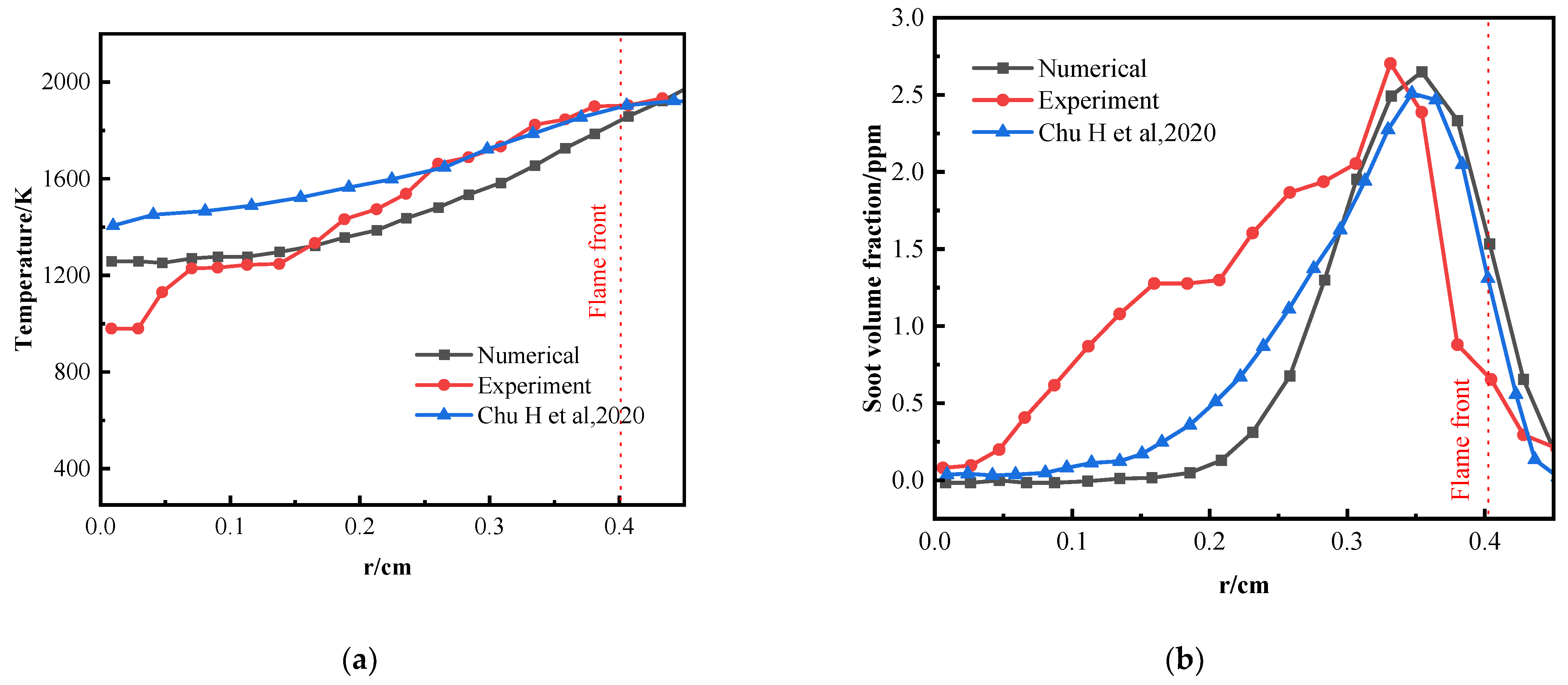
3.2. Numerical and Experimental Comparisons
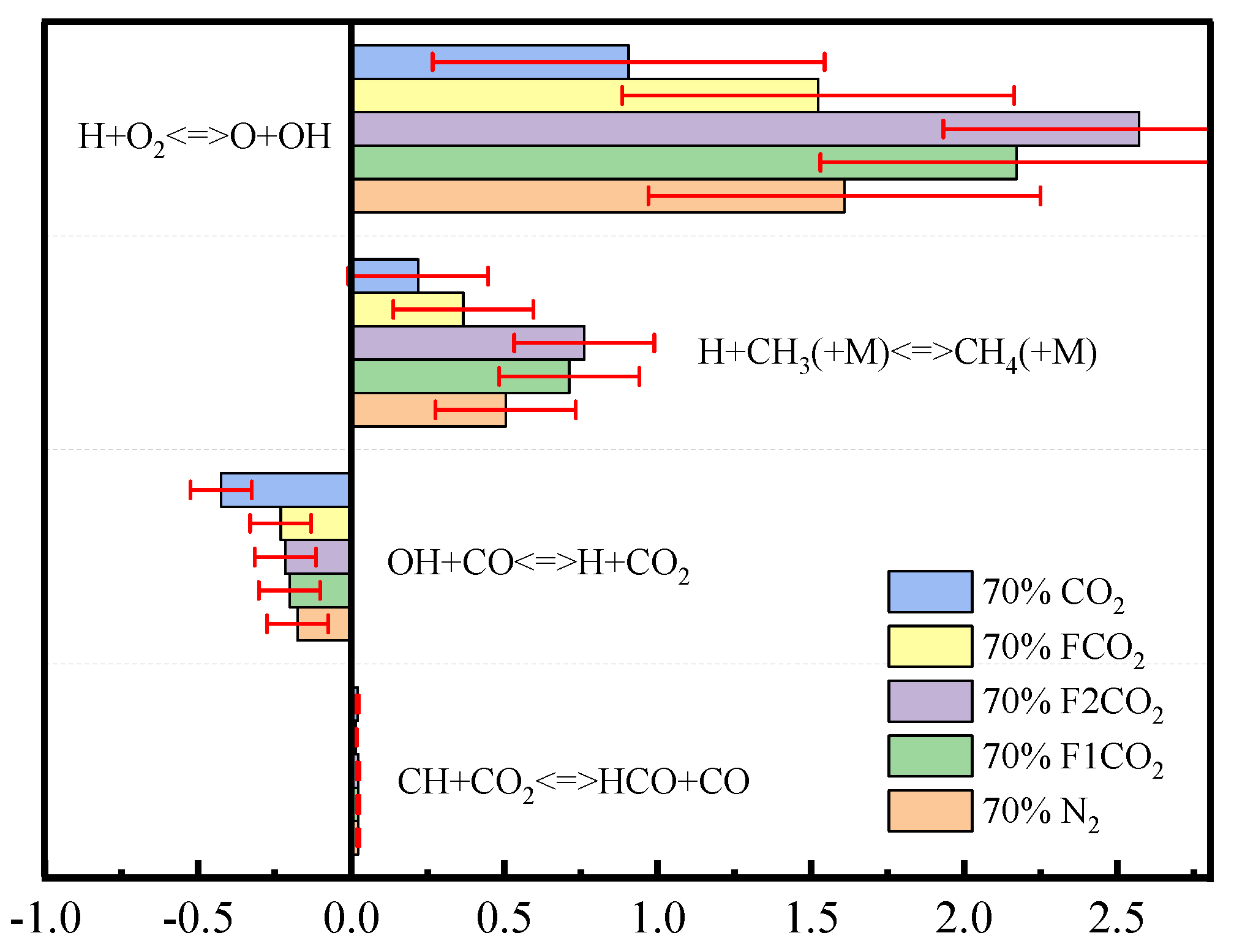
3.3. Impacts of CO2 Addition: Combustion Sensitivity Analysis
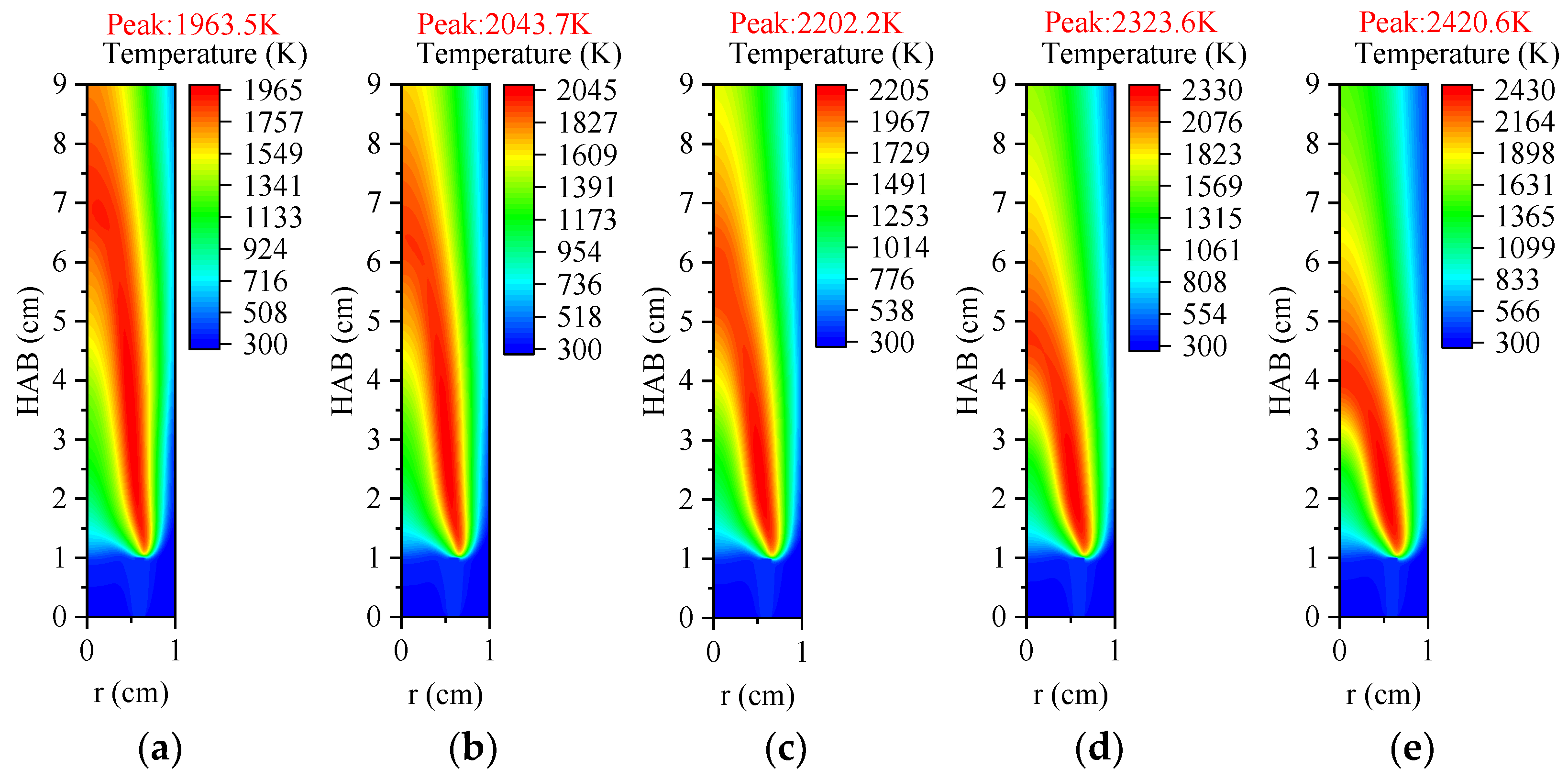
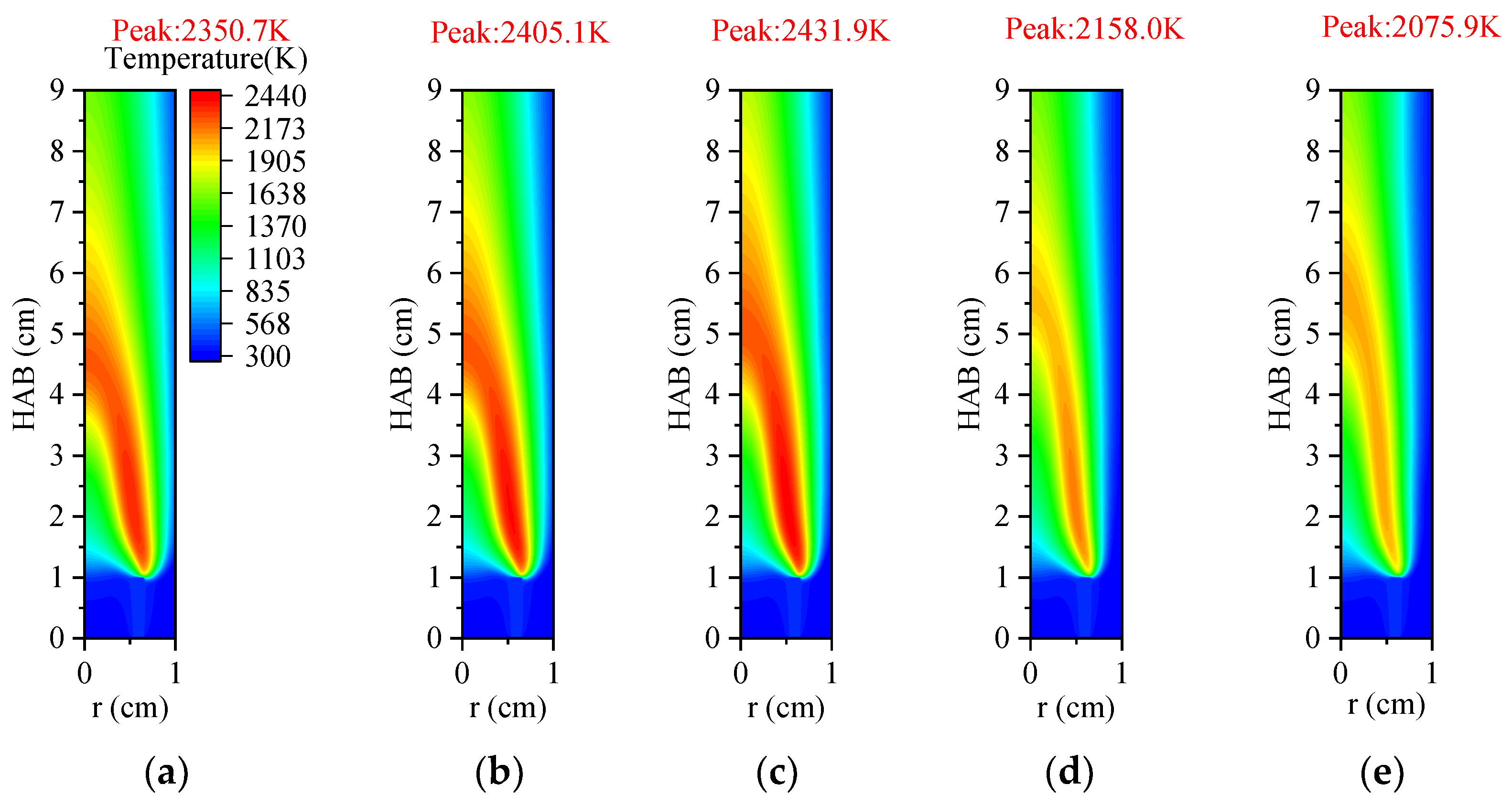
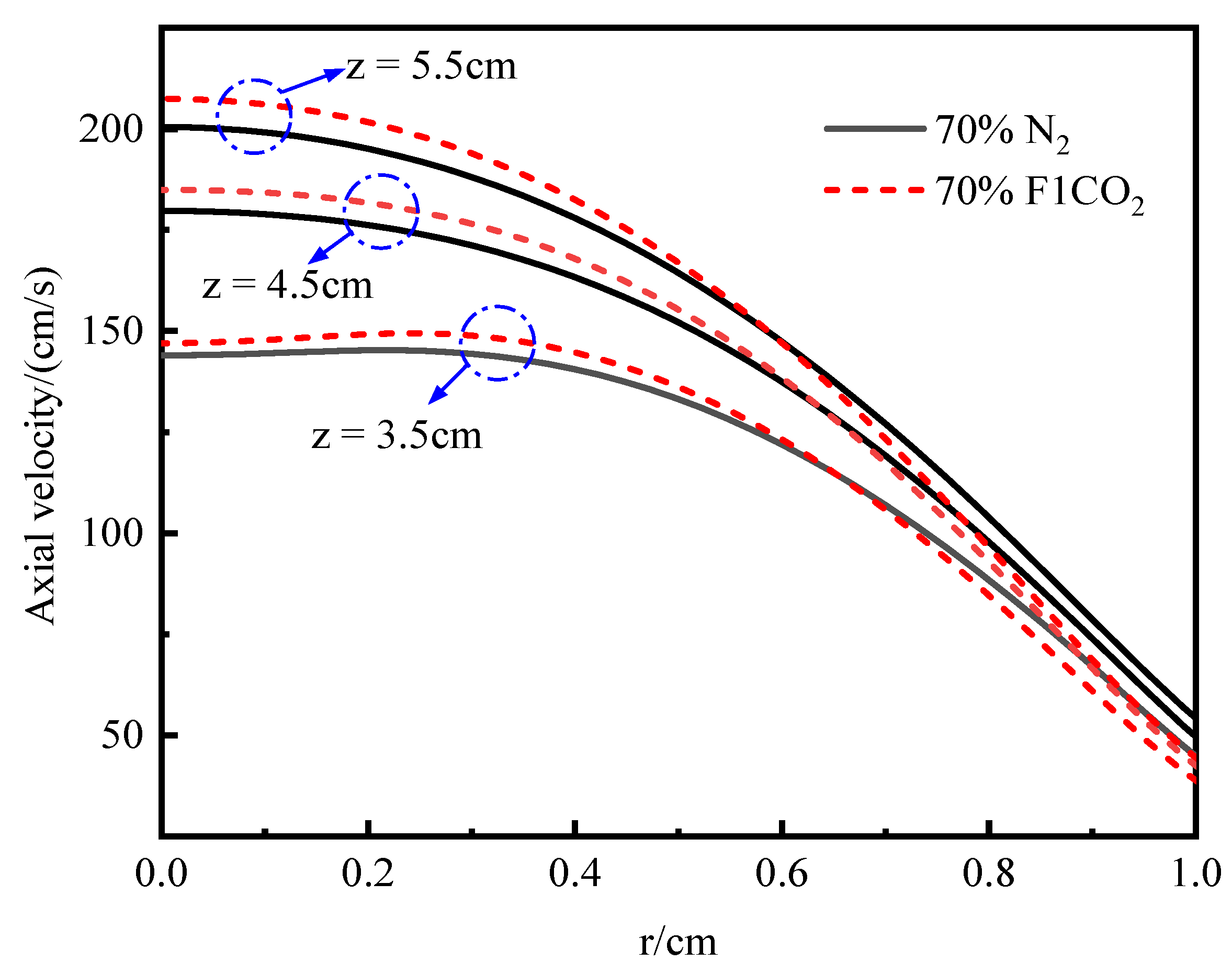
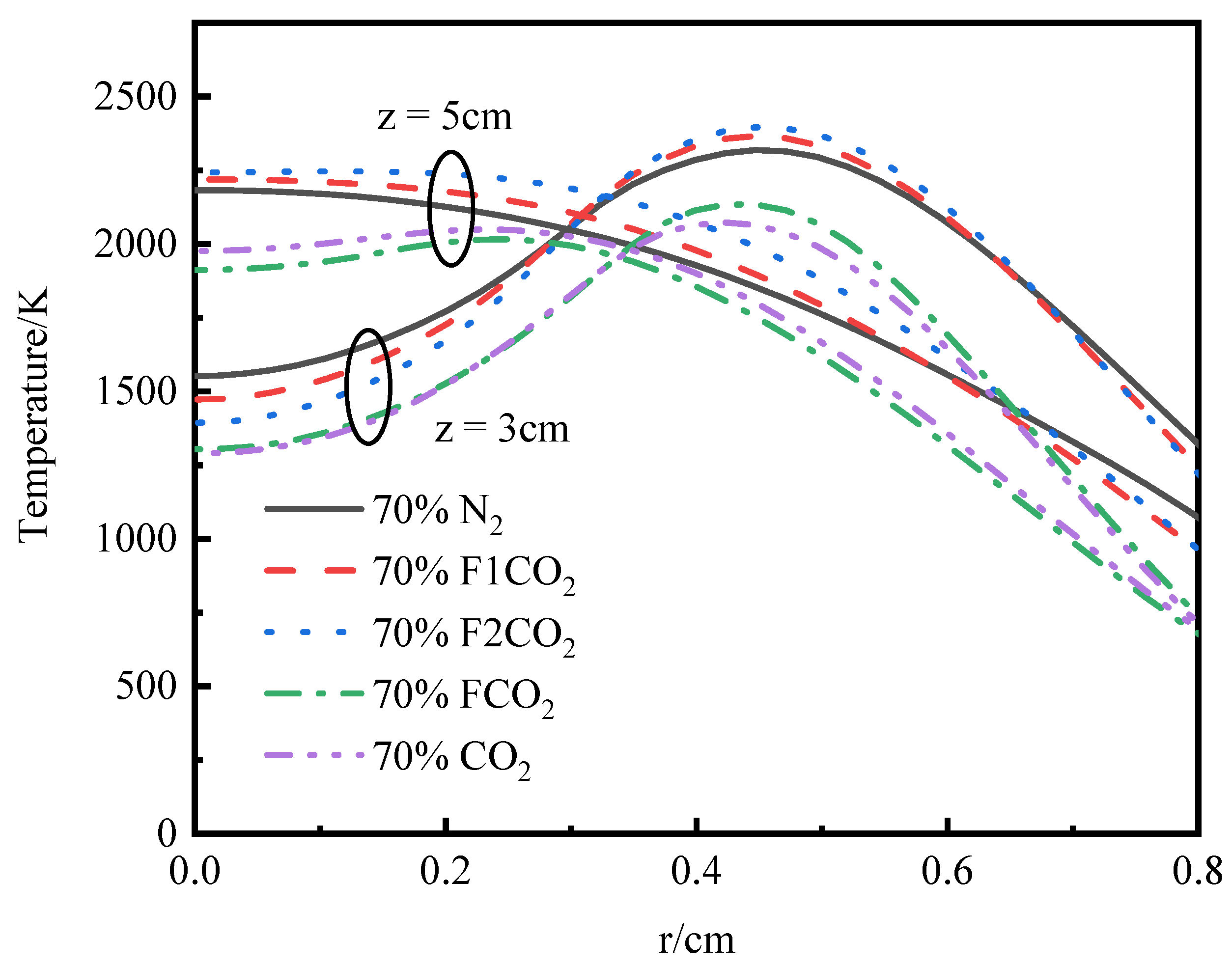
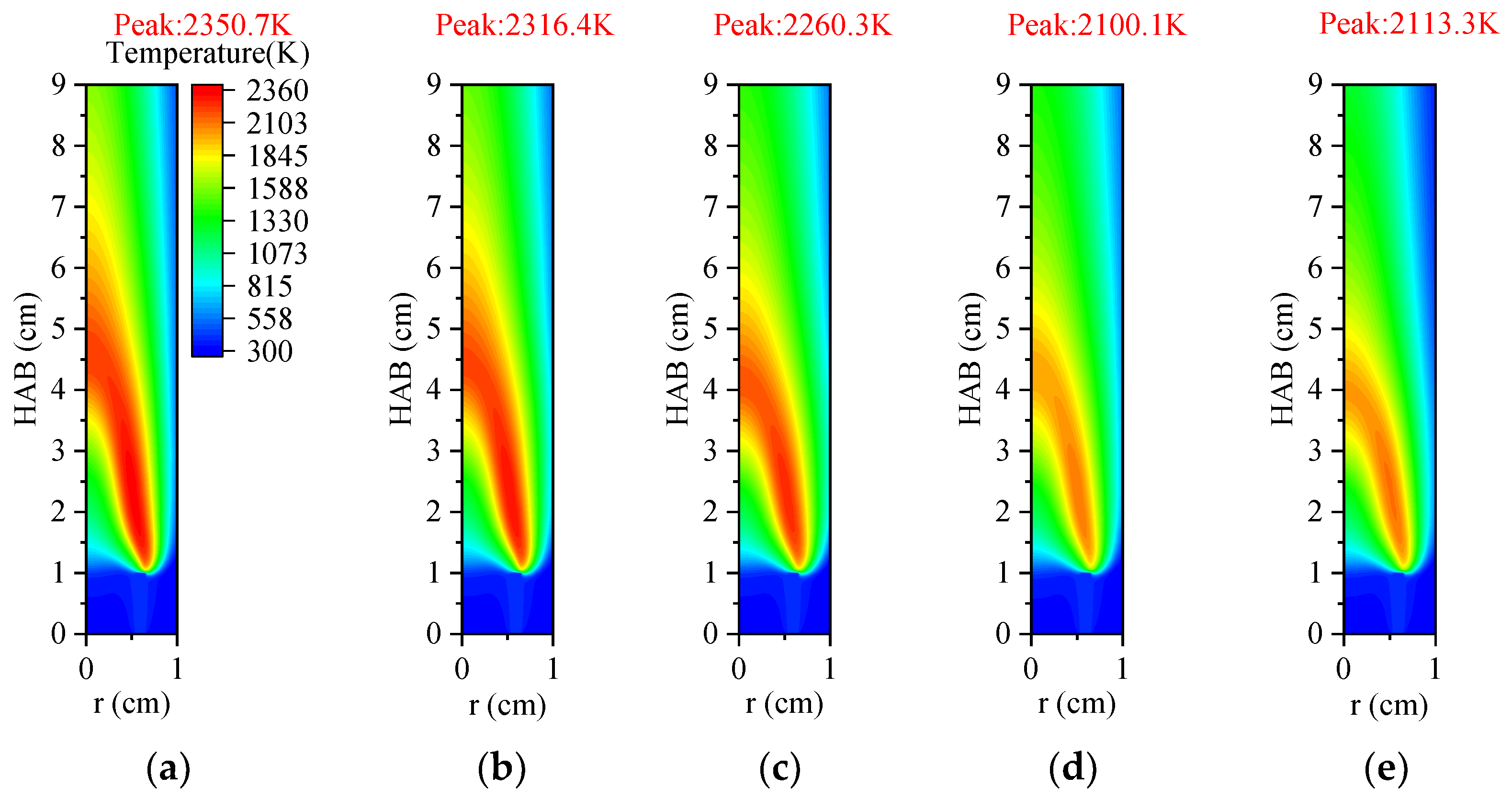
3.3.1. Temperature and Axial Flow Velocity
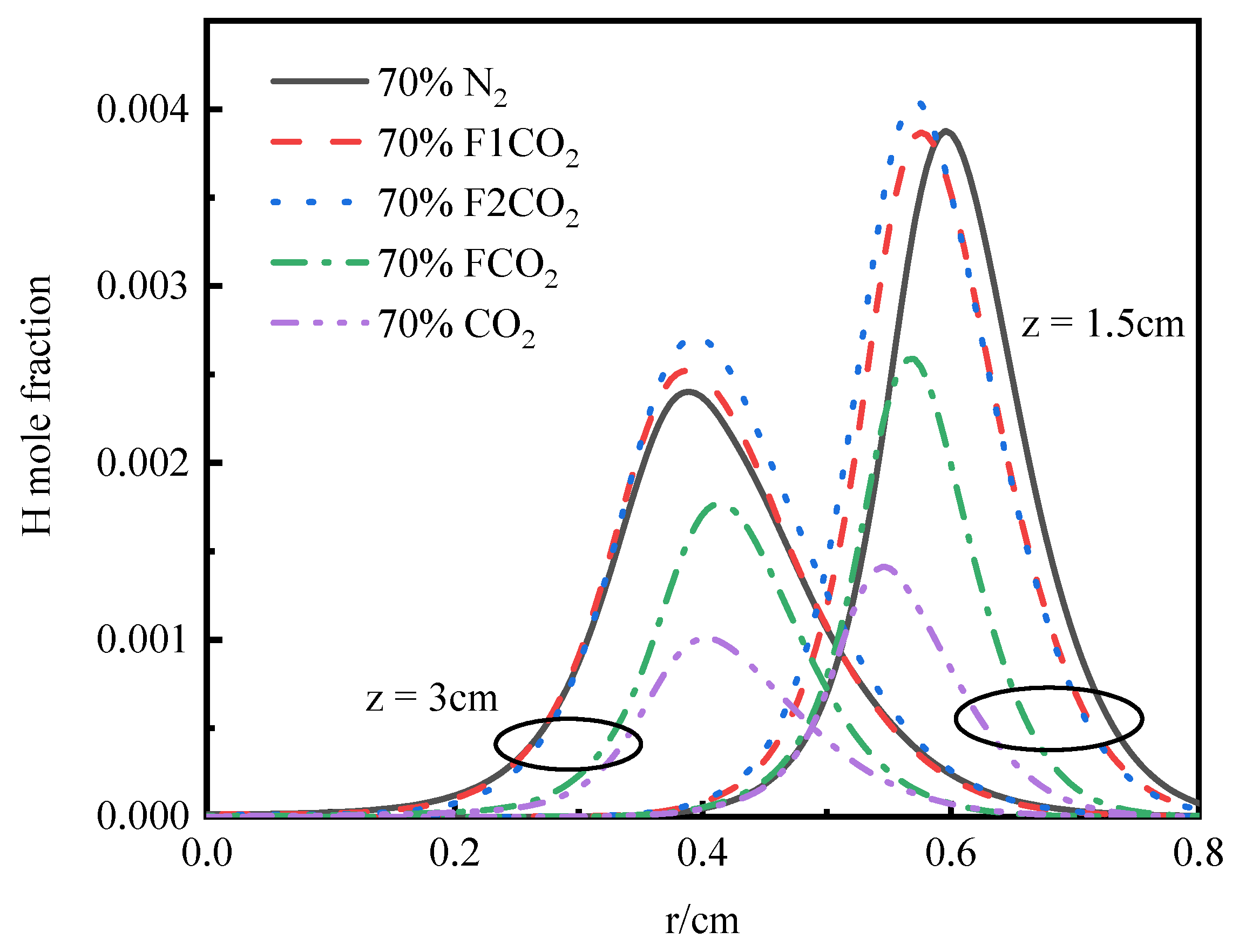
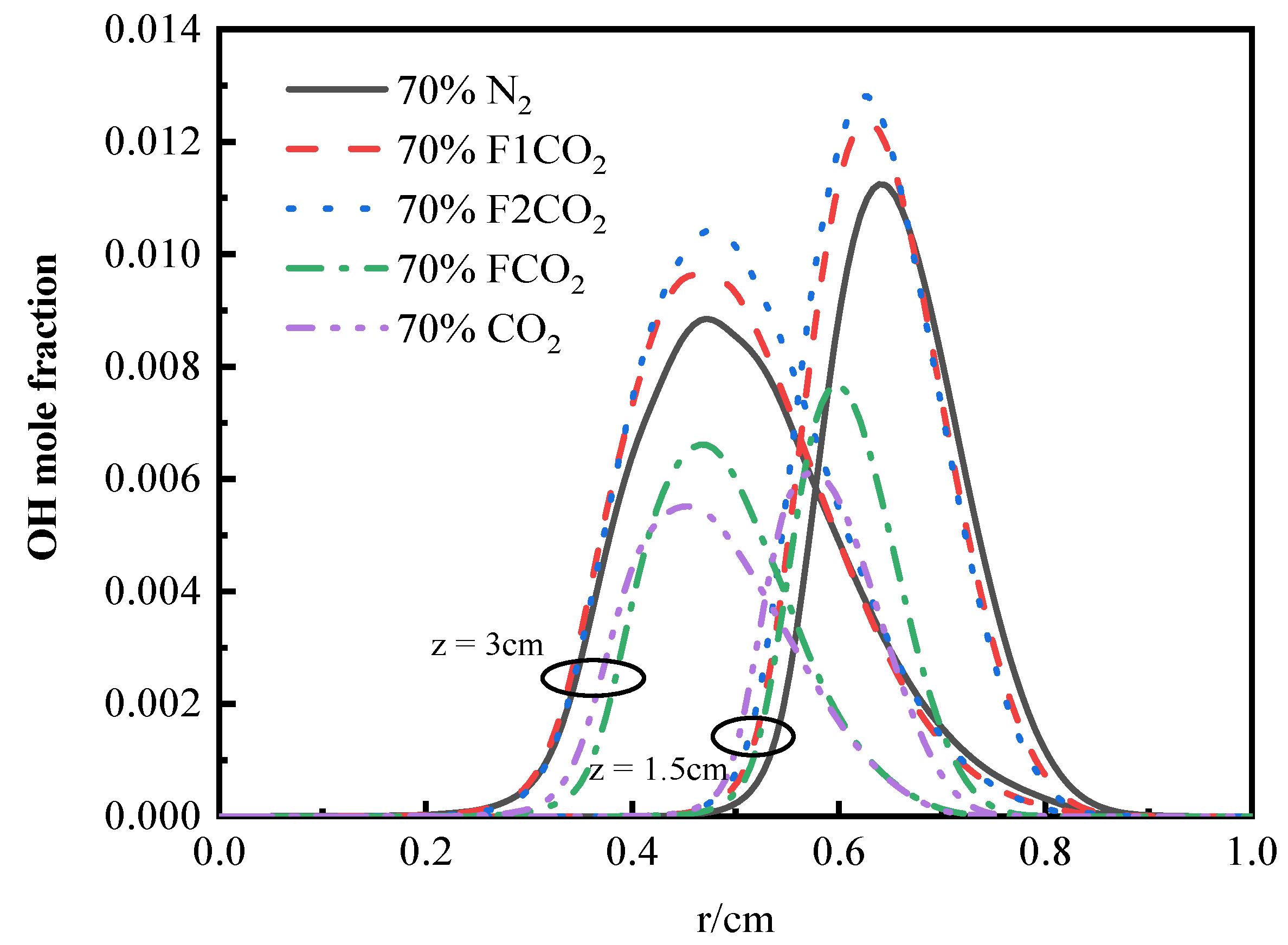
3.3.2. OH Radical Distribution and Flame Height
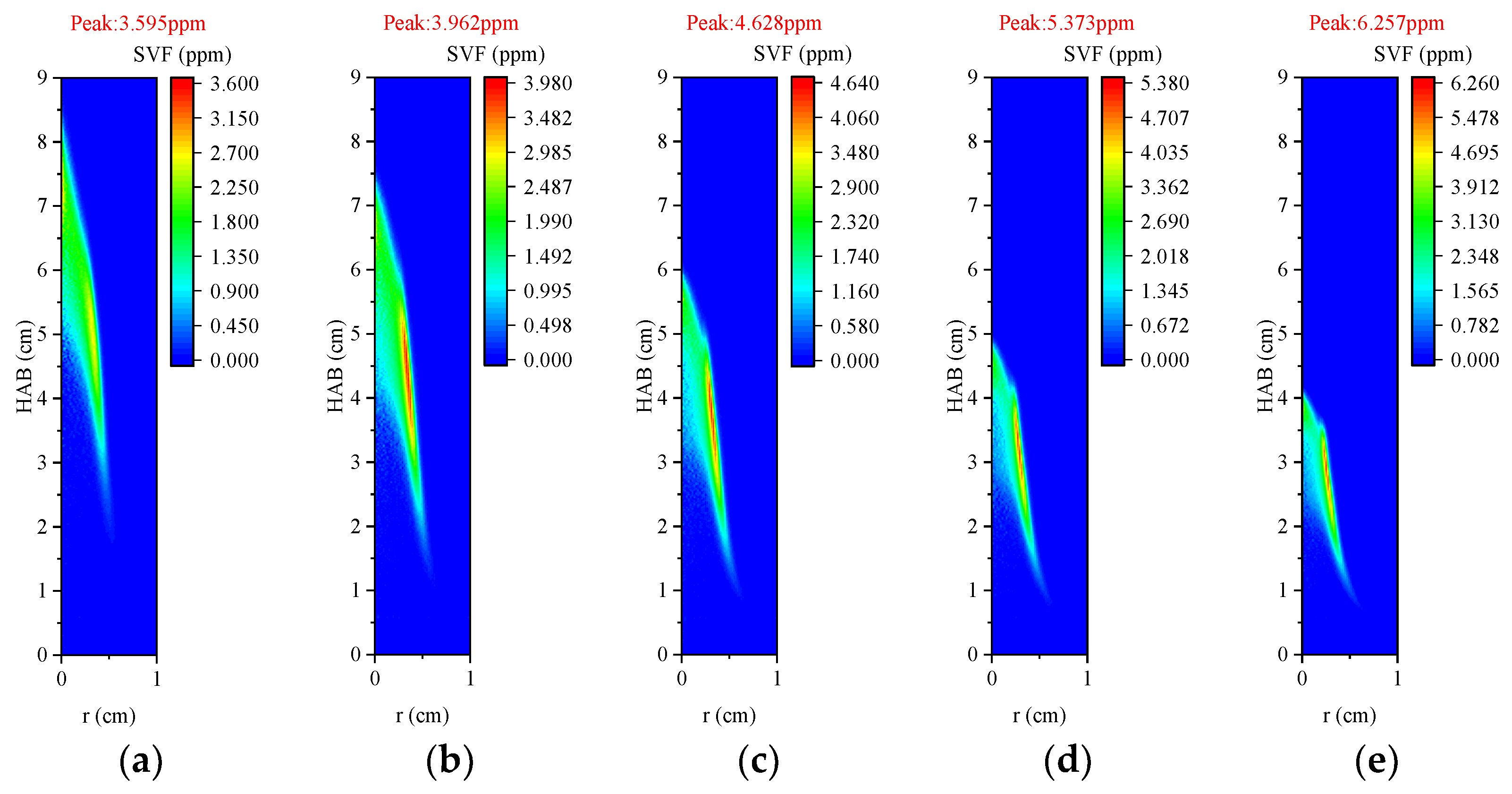
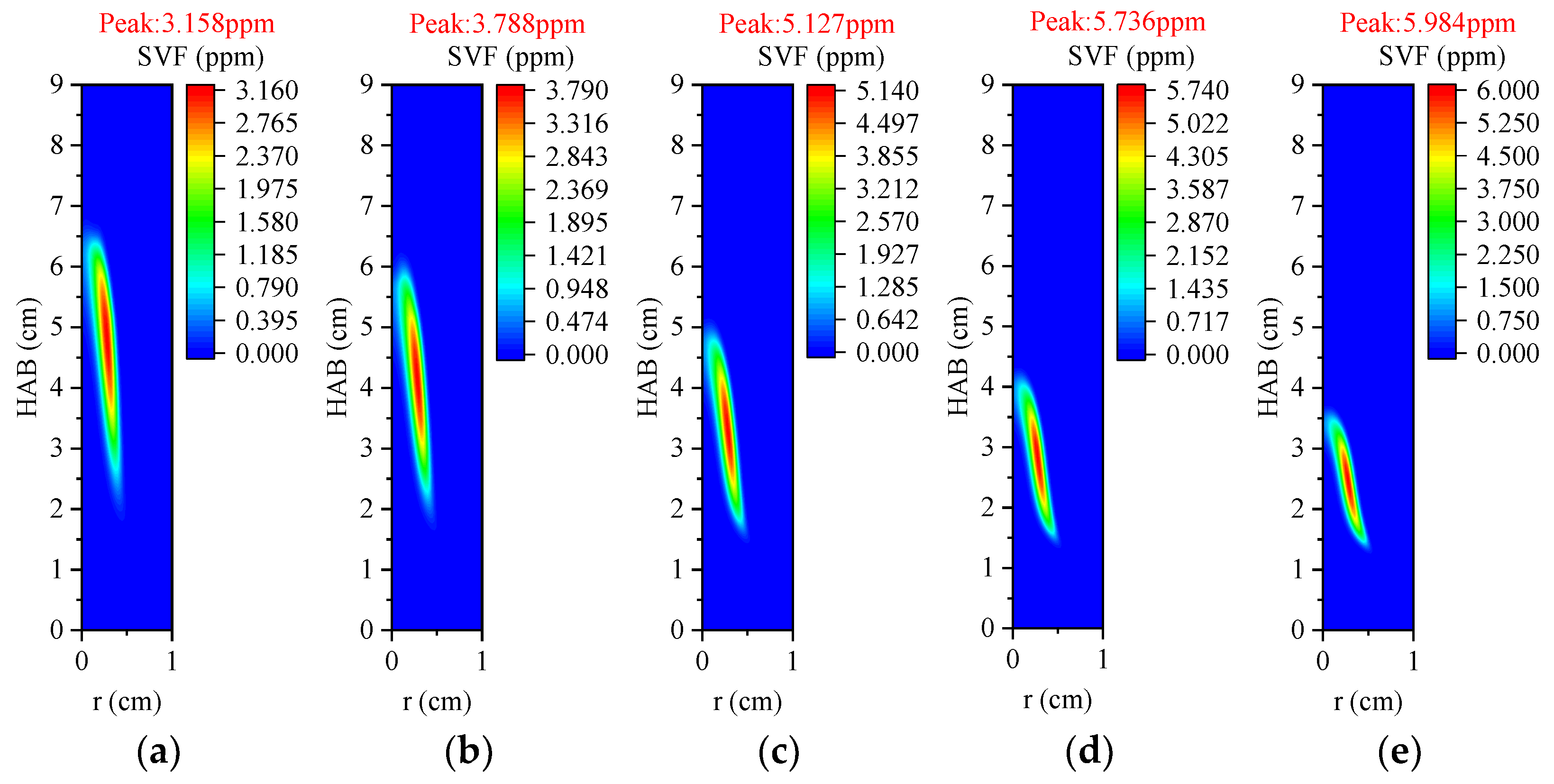
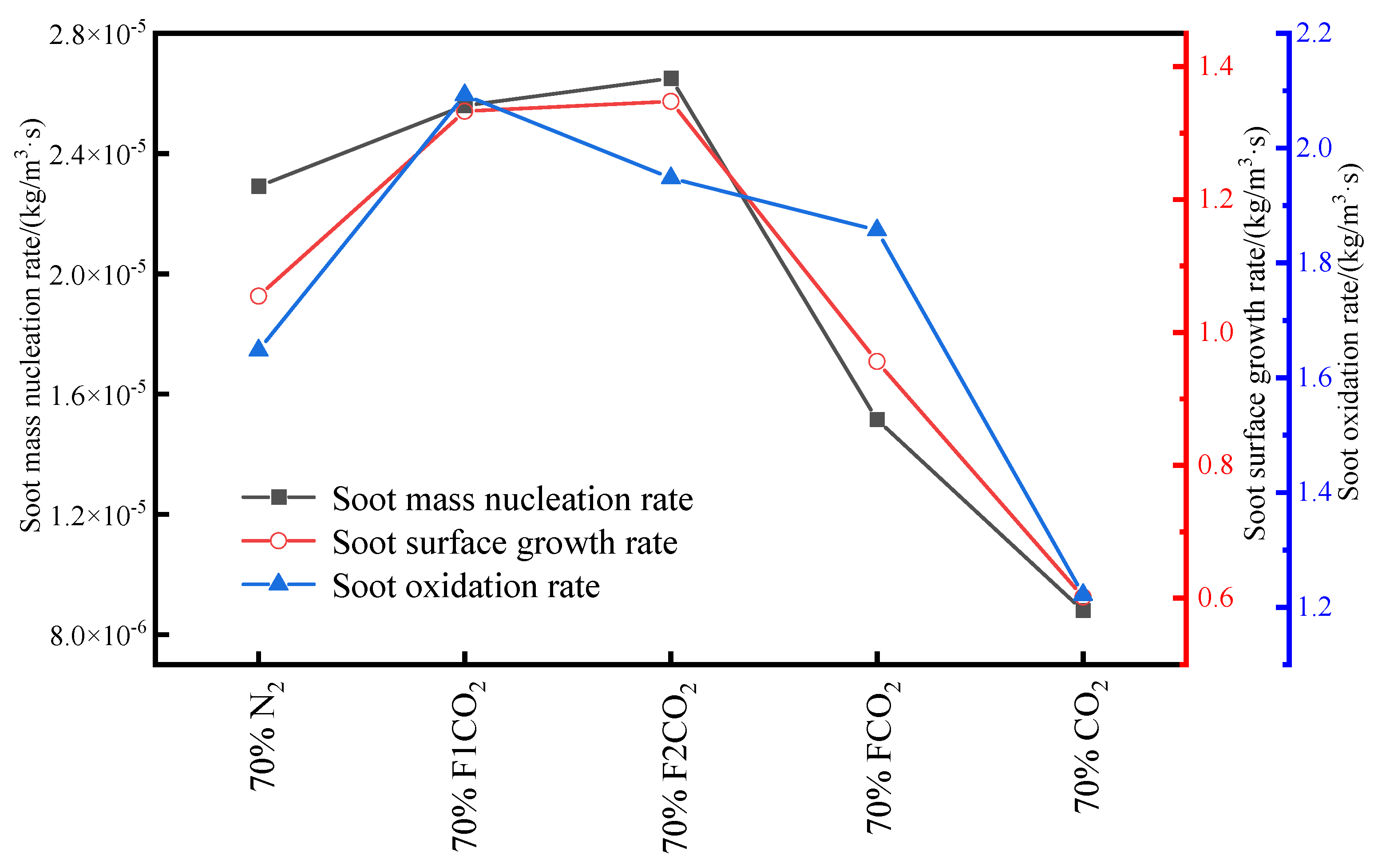
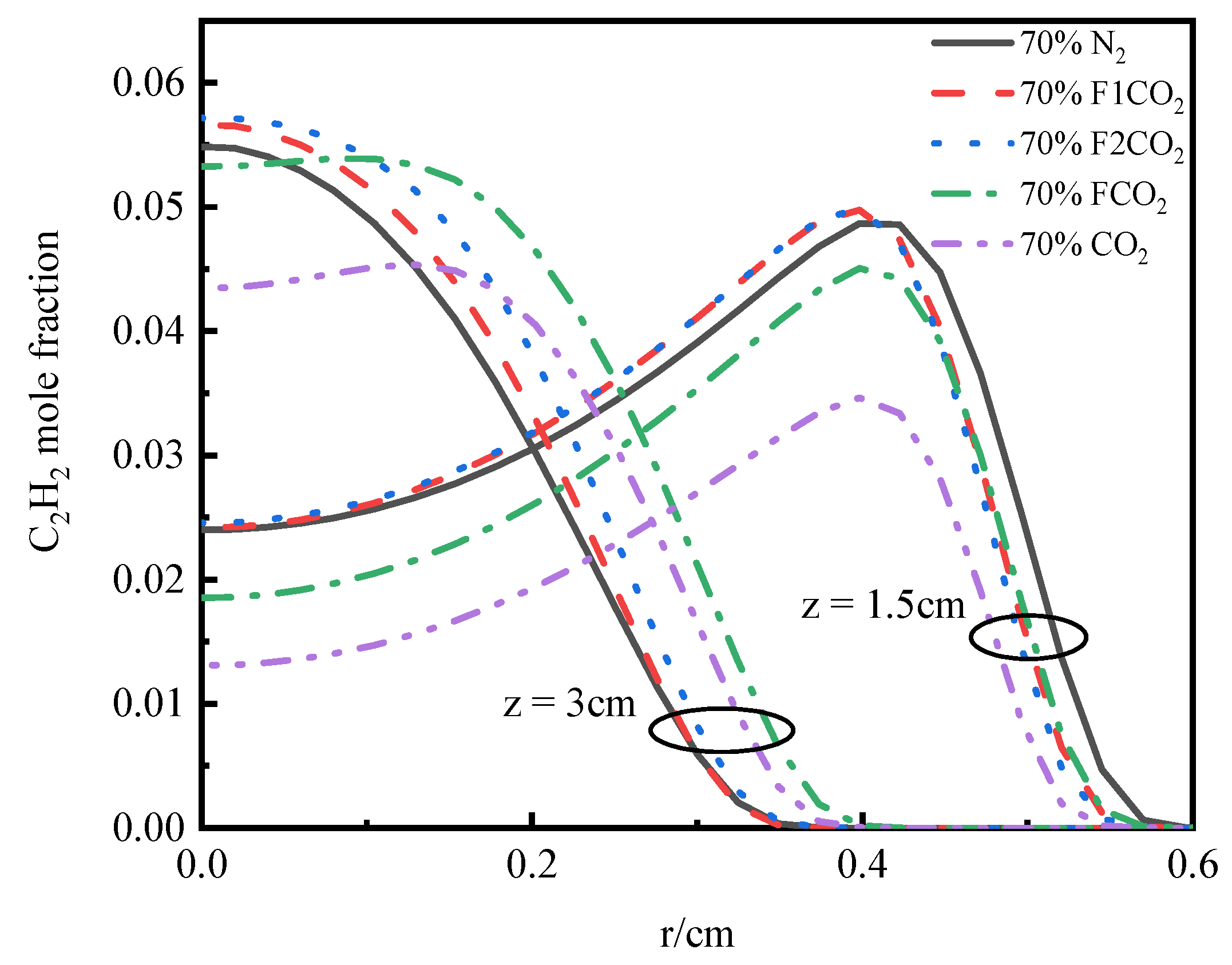
3.3.3. Volume Fraction of Soot
3.4. Impacts of H2O Addition: Combustion Sensitivity Analysis
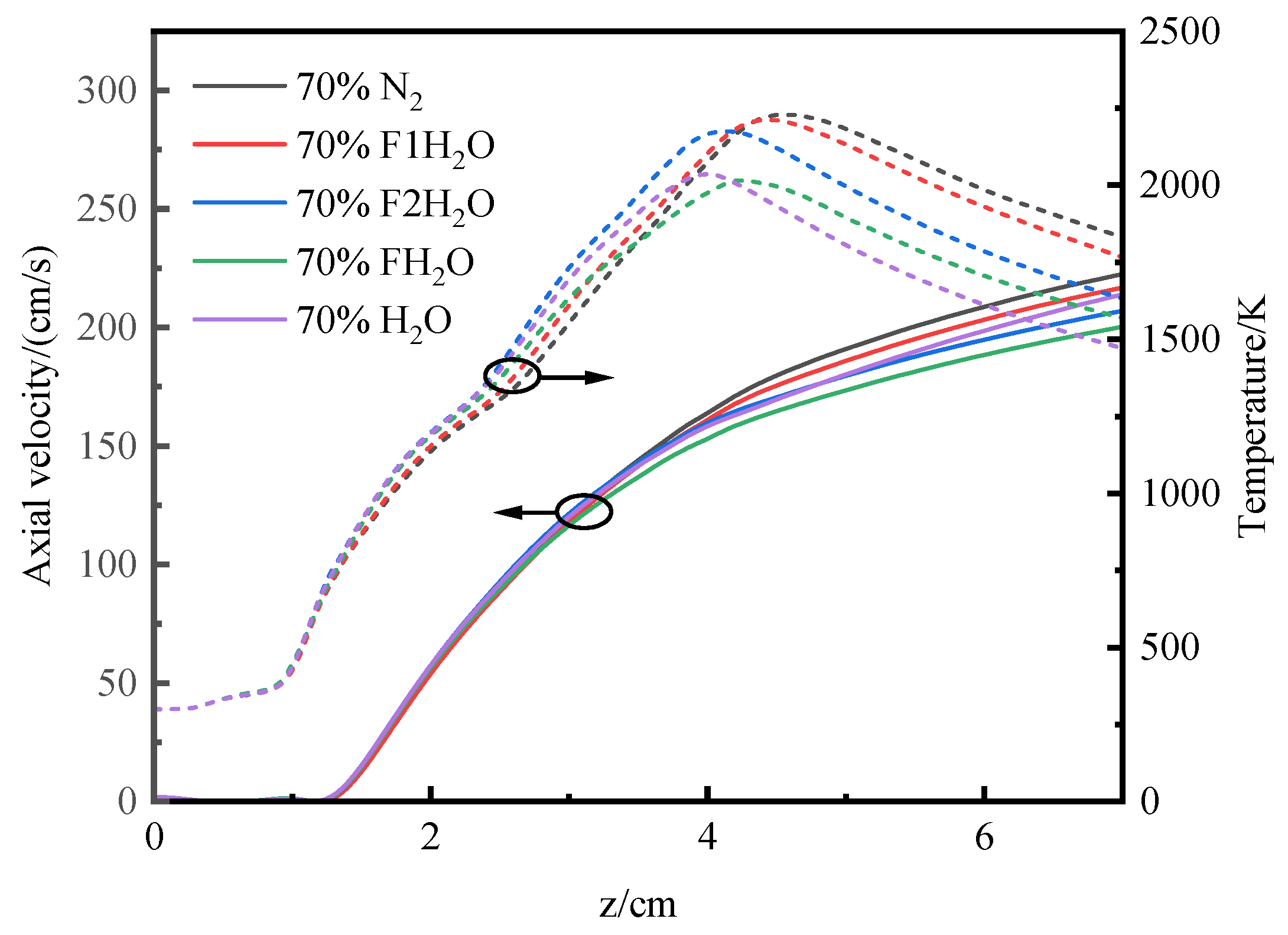
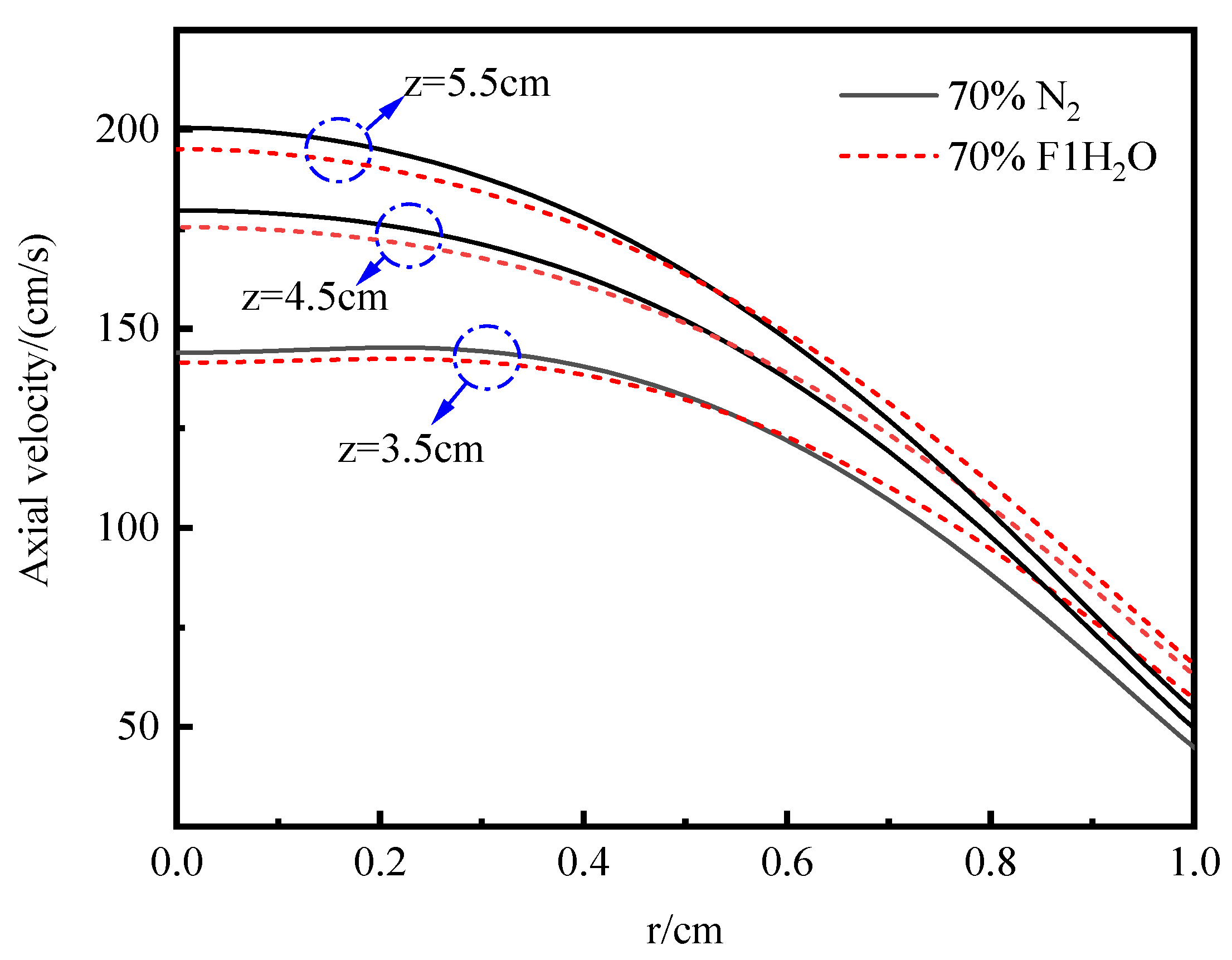
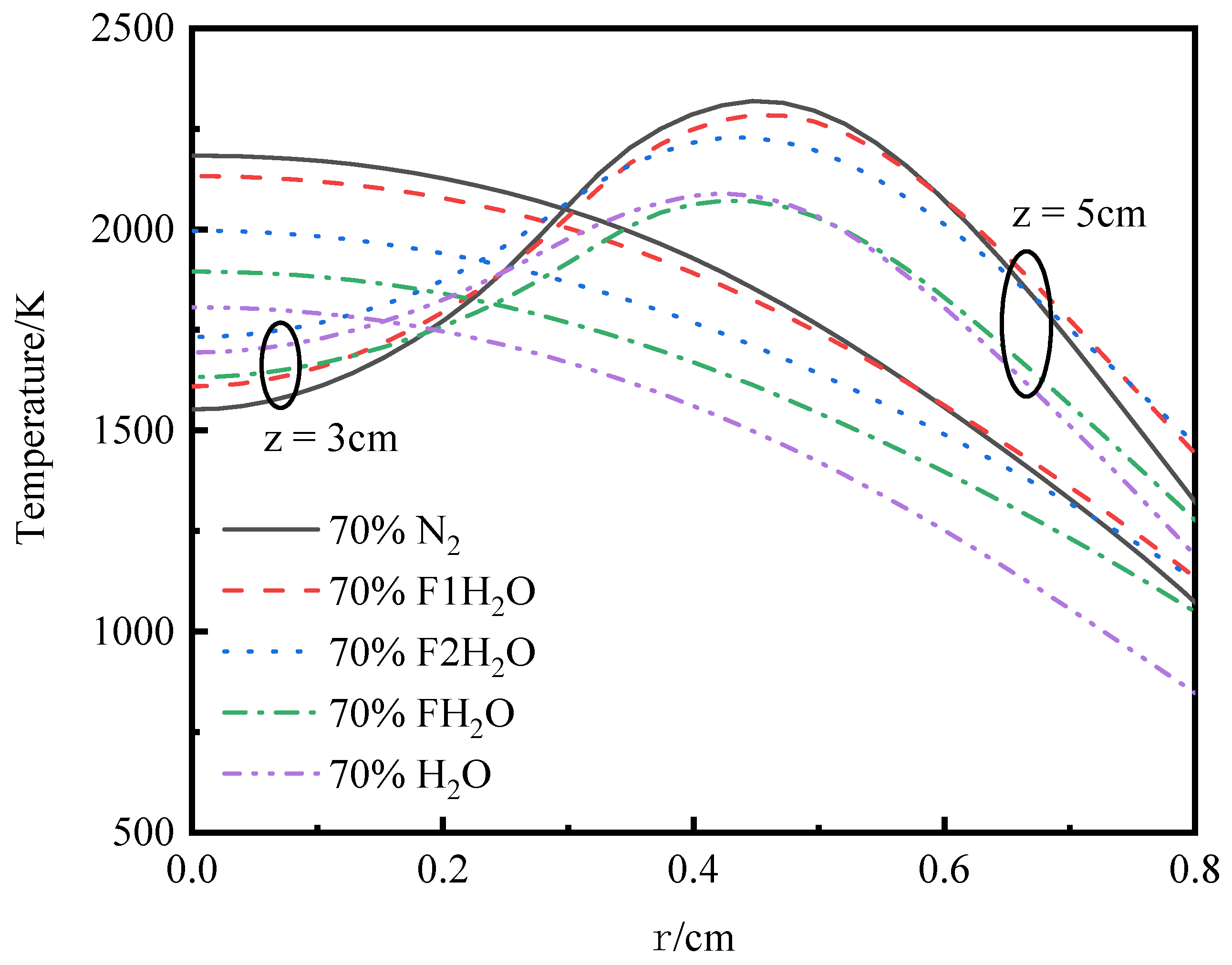
3.4.1. Temperature and Axial Flow Velocity
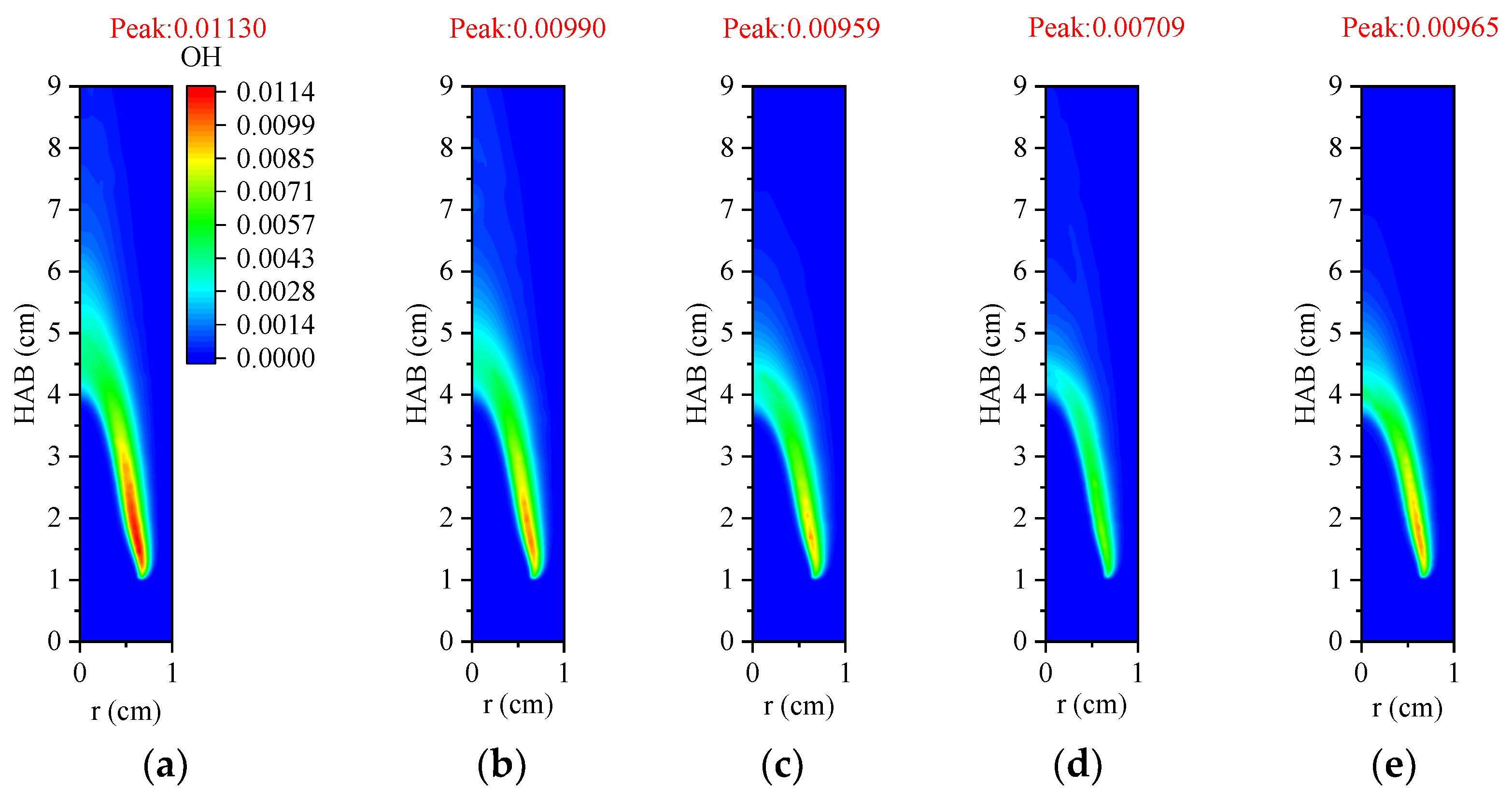
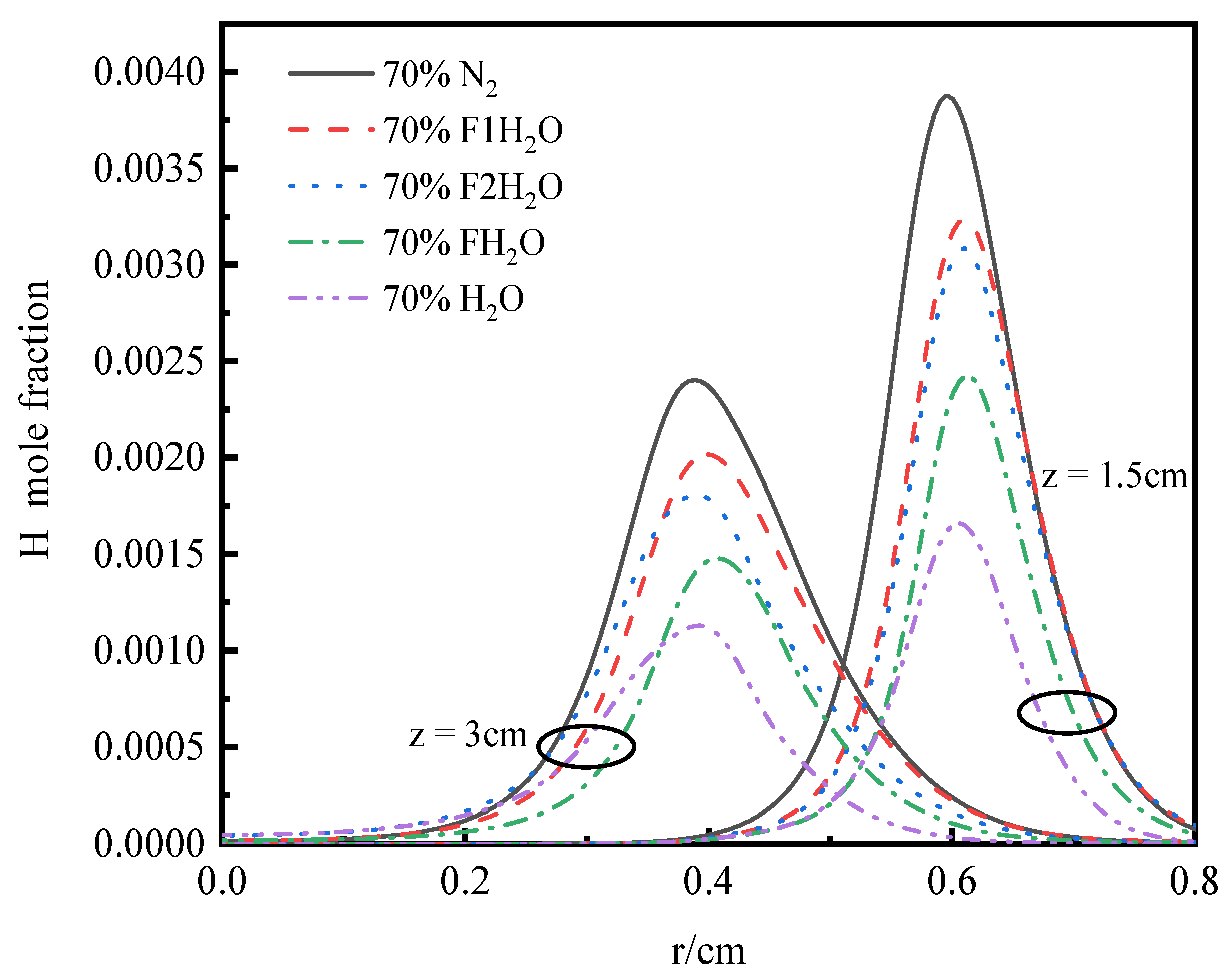
3.4.2. OH Radical Distribution and Flame Height
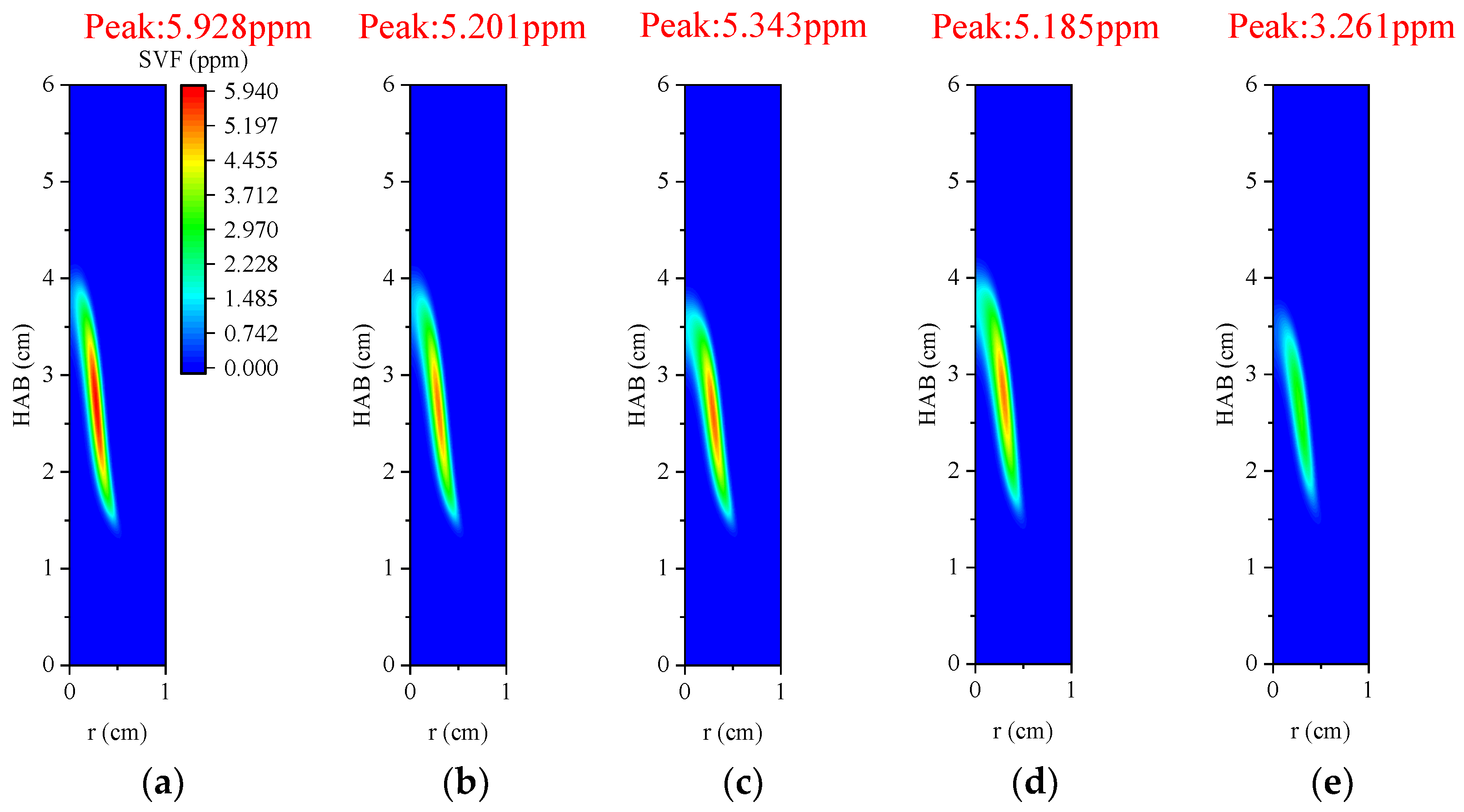
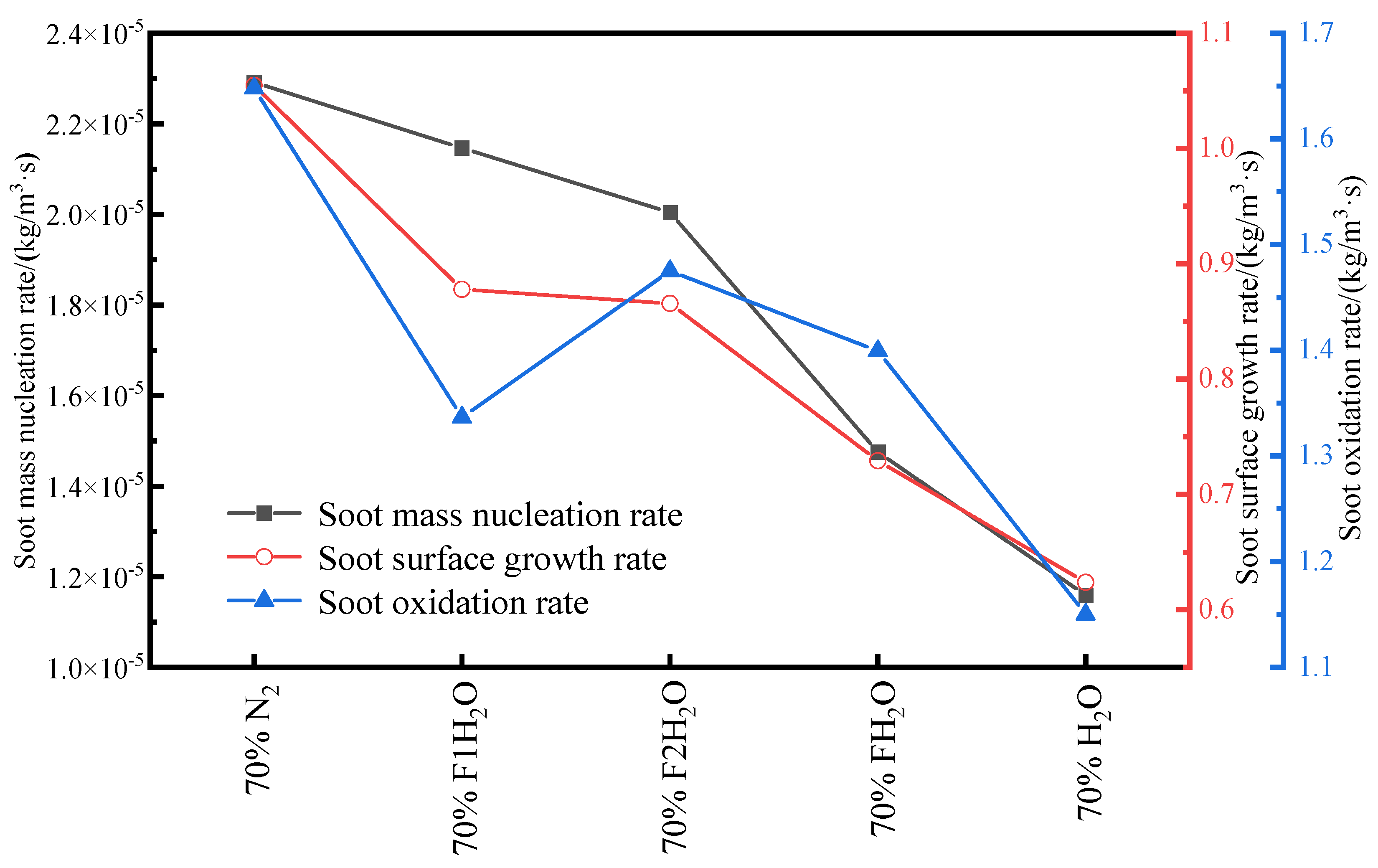
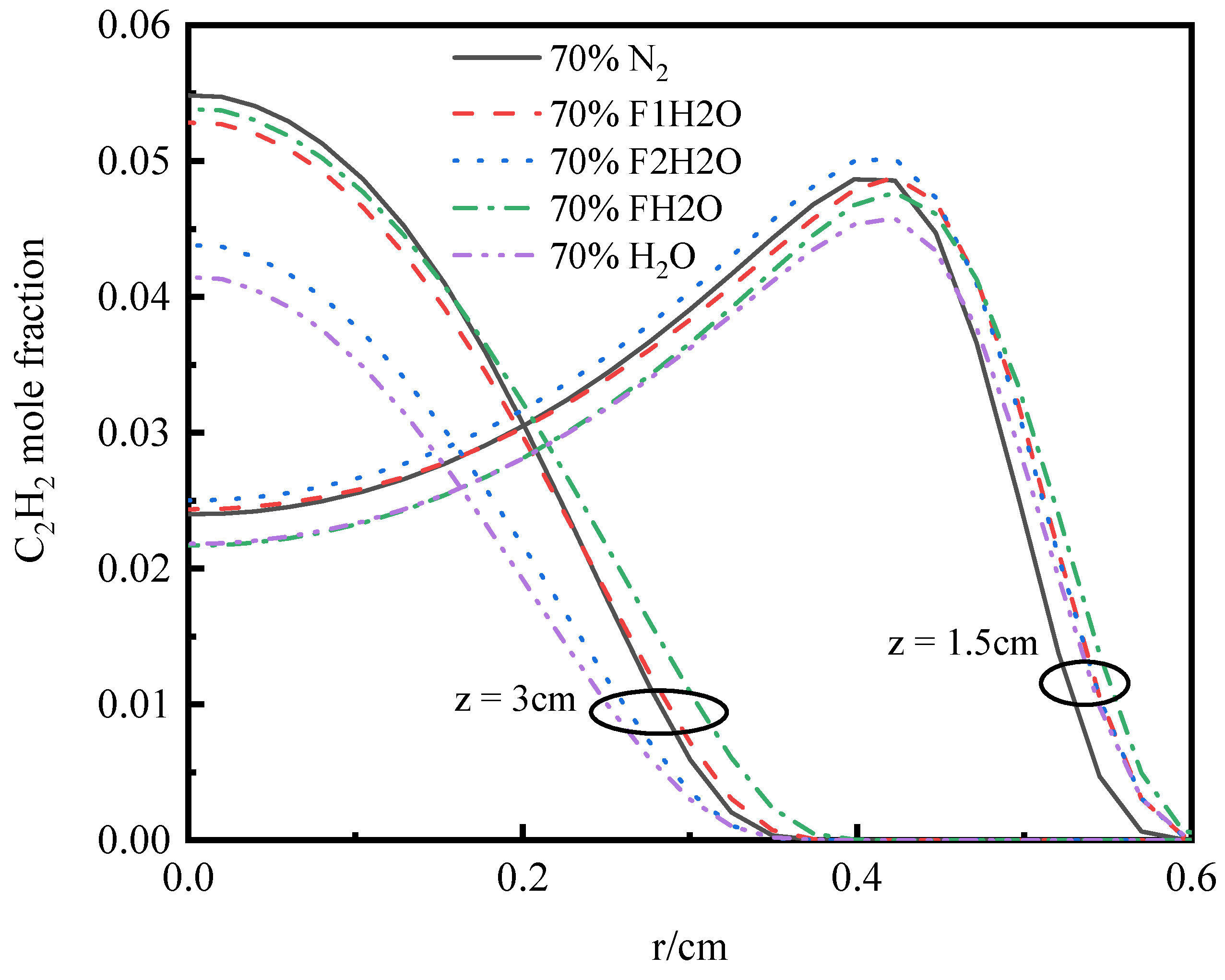
3.4.3. Volume Fraction of Soot
4. Conclusions
- (1)
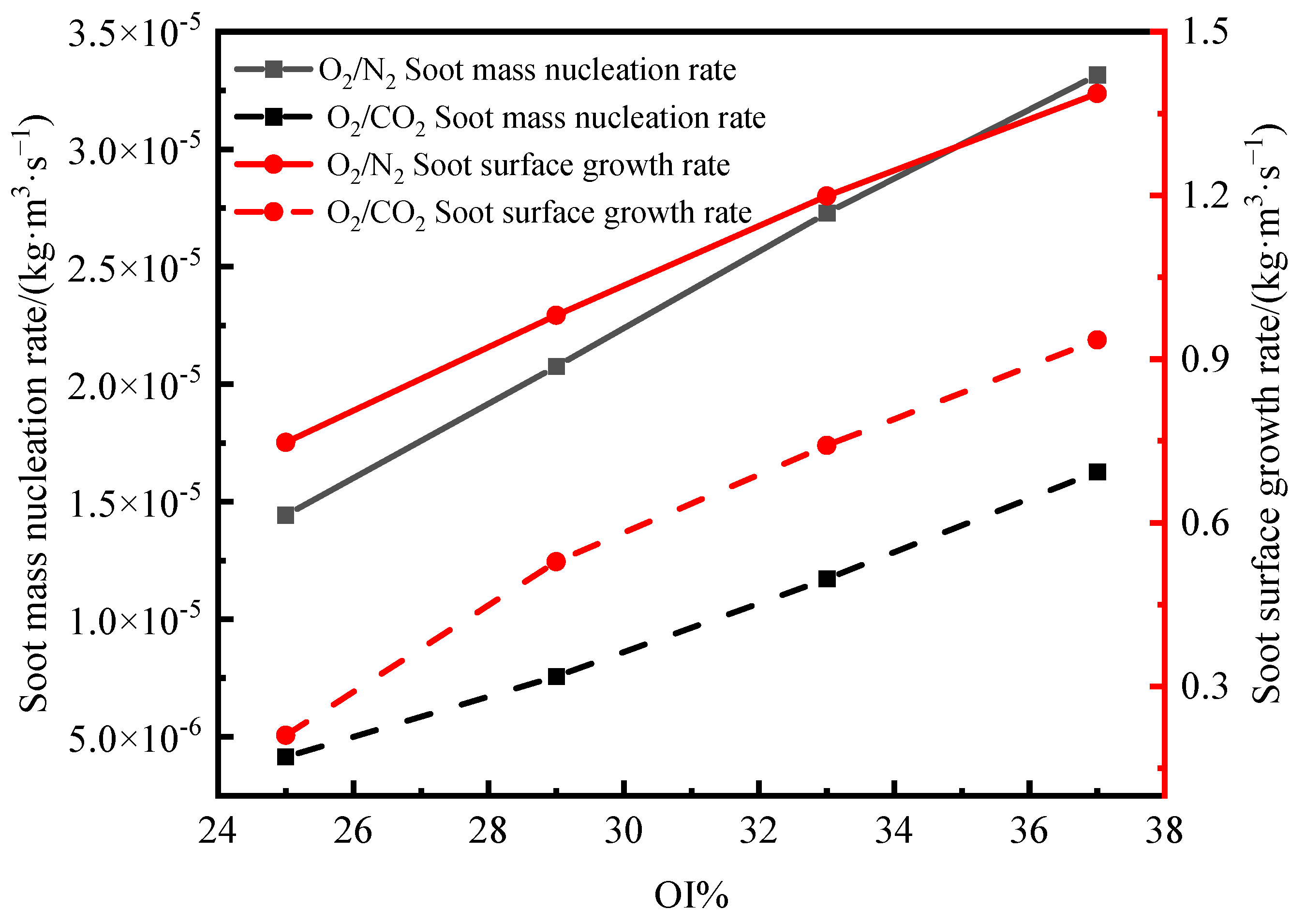
- Under an O2 index (OI) of 30%, substituting CO2 for N2 effectively suppresses soot formation. This replacement moves the high-temperature zone from the flame edges to the tip, thus, removing the splitting effect of the flame tip. The reduction in SVF is a result of decreased soot mass nucleation and surface growth rates, which occur when N2 is substituted with CO2.
- (2)
- Thermal and chemical interactions with CO2 drive the temperature differences in O2/CO2 combustion mode, while transport and density effects play a less important role. The chemical role of CO2 leads to a reduction in OH radical concentration and temperature, which in turn results in an elongated flame height.
- (3)
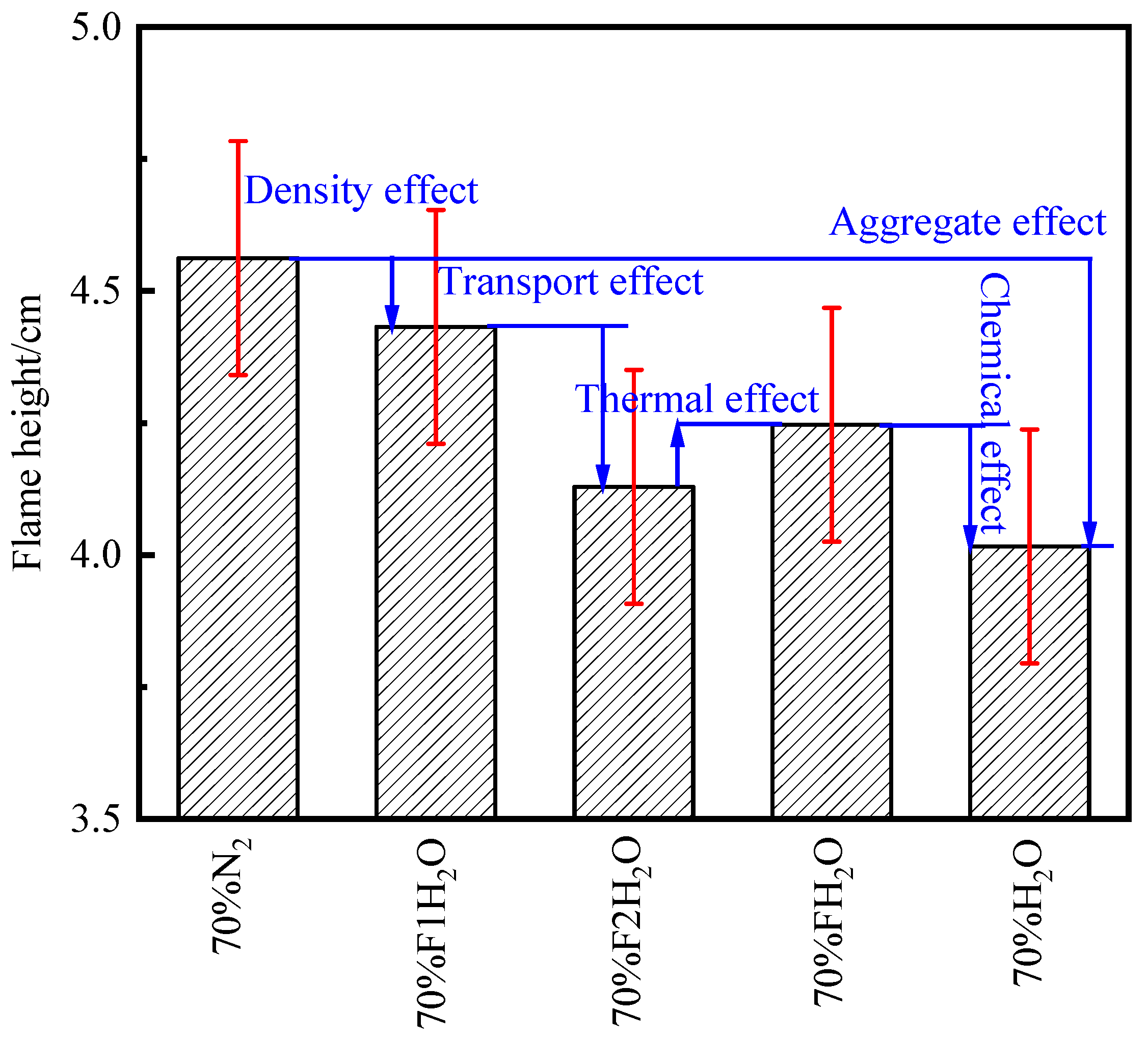
- The differences in temperature distribution during O2/H2O combustion are primarily attributed to the thermal effects and chemical interactions of water vapor. In addition to its chemical effects, H2O has significant thermal effects. The replacement of N2 with H2O leads to a reduction in flame height. The thermal effects of both CO2 and H2O lead to a reduction in OH concentration and a decrease in temperature. However, H2O’s density effect diverges from that of CO2, and its influence on transport processes is less pronounced, highlighting the distinct roles these species play in the system’s dynamics.
- (4)
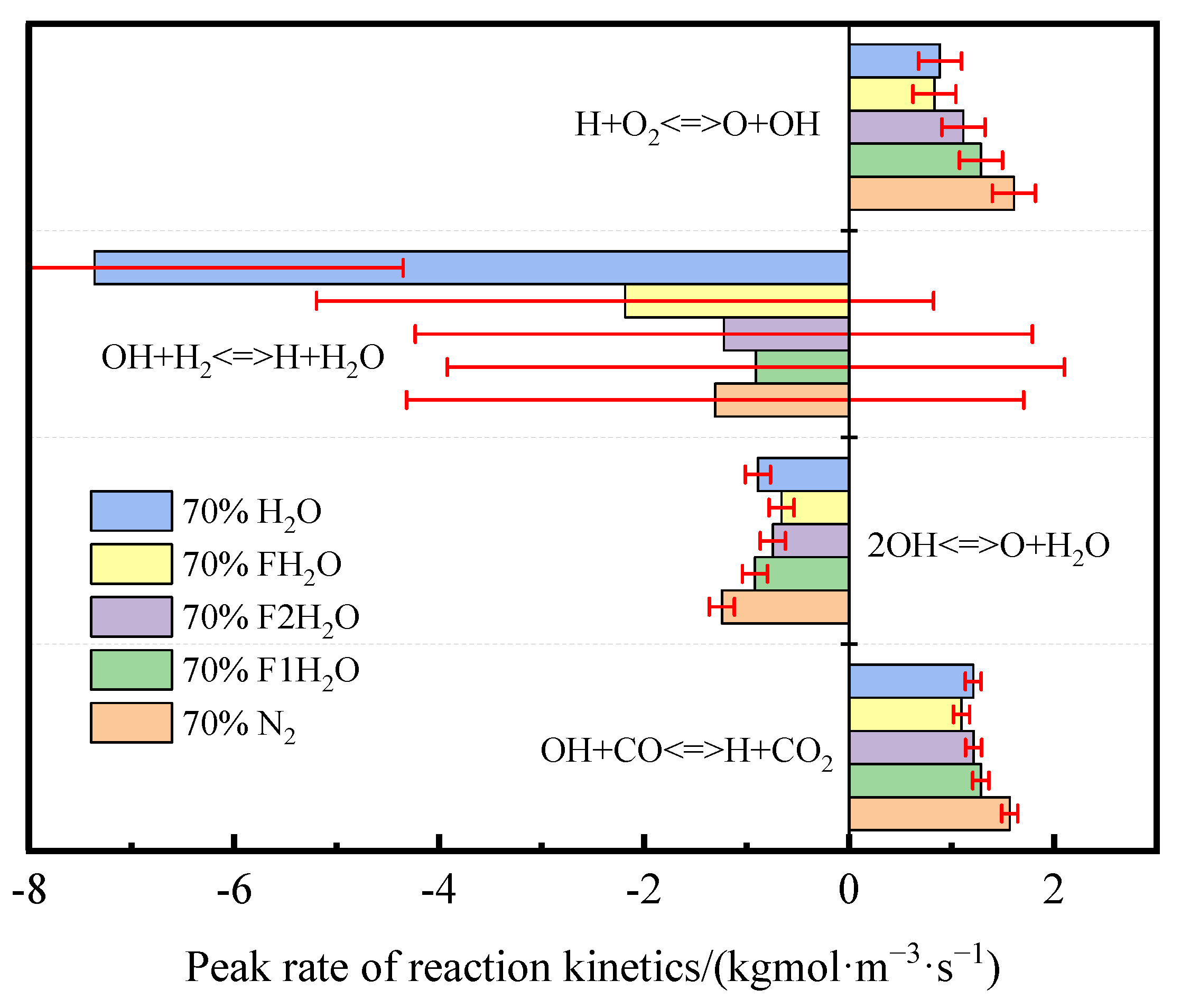
- In the oxidizer, CO2 primarily impacts soot nucleation and surface growth rates by lowering flame temperature and reducing the mole fraction of H radicals, rather than by enhancing soot oxidation. In contrast, in oxy-steam combustion, the reaction OH + H2 ⇋ H + H2O plays a crucial role in suppressing soot formation. Here, the chemical effects of water vapor significantly inhibit soot generation by increasing OH radical concentrations.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Nomenclature and Abbreviations
| r | Radial distance |
| z | Axial distance |
| PAHs | polycyclic aromatic hydrocarbons |
| HACA | Hydrogen extraction surface addition |
| OI | Oxygen-index |
| Molar mass | |
| SVF | Soot volume fraction |
| HAB | Height above burner |
| C2H2 | acetylene |
References
- Greiner, P.T.; York, R.; Mcgee, J.A. When are fossil fuels displaced? An exploratory inquiry into the role of nuclear electricity production in the displacement of fossil fuels. Heliyon 2022, 8, e08795. [Google Scholar] [CrossRef] [PubMed]
- Shaw, R.; Naskar, S.; Das, T.; Chowdhury, A. A Review on the Advanced Techniques Used for the Capturing and Storage of CO2 from Fossil Fuel Power Plants. In Sustainability in Environmental Engineering and Science, Proceedings of the Select Proceedings of SEES 2019 Springer, Singapore; Springer: Berlin/Heidelberg, Germany, 2020; pp. 193–197. [Google Scholar] [CrossRef]
- Luo, Z.; Xiang, Q.; Cheng, Q.; Lou, C. Changing characteristics of flame images under different oxy-fuel atmospheres in a 3-MW pilot-scale furnace. IEEE Trans. Instrum. Meas. 2016, 65, 2265–2271. [Google Scholar] [CrossRef]
- Li, Y.; Fan, W.; Wang, Y.; Chen, C. Characteristics of char gasification in staged oxygen-enriched combustion in a down flame furnace. Energy Fuels 2016, 30, 1675–1684. [Google Scholar] [CrossRef]
- Lou, C.; Chen, X.; Yan, W.; Tian, Y.; Kumfer, B.M. Effect of stoichiometric mixture fraction on soot fraction and emission spectra with application to oxy-combustion. Proc. Combust. Inst. 2019, 37, 4571–4578. [Google Scholar] [CrossRef]
- Lou, C.; Li, Z.; Zhang, Y.; Kumfer, B.M. Soot formation characteristics in laminar co-flow flames with application to oxy-combustion. Combust. Flame 2021, 227, 371–383. [Google Scholar] [CrossRef]
- Park, J.; Kim, S.G.; Lee, K.M.; Kim, T.K. Chemical effect of diluents on flame structure and NO emission characteristic in methane-air counterflow diffusion flame. Int. J. Energy Res. 2002, 26, 1141–1160. [Google Scholar] [CrossRef]
- Wang, Y.; Chung, S.H. Formation of soot in counterflow diffusion flames with carbon dioxide dilution. Combust. Sci. Technol. 2016, 188, 805–817. [Google Scholar] [CrossRef]
- Chen, L.; Zheng, X.; Zhou, J.; Wu, J.; Wu, X.; Gao, X.; Gréhan, G.; Cen, K. Effects of carbon dioxide addition on the soot particle sizes in an ethylene/air flame. Aerosol Air Qual. Res. 2017, 17, 2522–2532. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, F.; Lou, C. Experimental and numerical investigations of soot formation in laminar coflow ethylene flames burning in O2/N2 and O2/CO2 atmospheres at different O2 mole fractions. Energy Fuels 2018, 32, 6252–6263. [Google Scholar] [CrossRef]
- Xie, Y.; Wang, J.; Xu, N.; Yu, S.; Huang, Z. Comparative study on the effect of CO2 and H2O dilution on laminar burning characteristics of CO/H2/air mixtures. Int. J. Hydrogen Energy 2014, 39, 3450–3458. [Google Scholar] [CrossRef]
- Xu, H.; Liu, F.; Sun, S.; Zhao, Y.; Meng, S.; Tang, W. Effects of H2O and CO2 diluted oxidizer on the structure and shape of laminar coflow syngas diffusion flames. Combust. Flame 2017, 177, 67–78. [Google Scholar] [CrossRef]
- Liu, F.; Consalvi, J.L.; Fuentes, A. Effects of water vapor addition to the air stream on soot formation and flame properties in a laminar co flow ethylene/air diffusion flame. Combust. Flame 2014, 161, 1724–1734. [Google Scholar] [CrossRef]
- Ying, Y.; Liu, D. Effects of water addition on soot properties in ethylene inverse diffusion flames. Fuel 2019, 247, 187–197. [Google Scholar] [CrossRef]
- Cepeda, F.; Jerez, A.; Demarco, R.; Liu, F.; Fuentes, A. Influence of water-vapor in oxidizer stream on the sooting behavior for laminar co-flow ethylene diffusion flames. Combust. Flame 2019, 210, 114–125. [Google Scholar] [CrossRef]
- Qiu, L.; Zheng, Y.; Hua, Y.; Zhuang, Y.; Qian, Y.; Cheng, X. Effects of water vapor addition on the flame structure and soot formation in a laminar ethanol/air co flow flame. Combust. Sci. Technol. 2021, 193, 626–642. [Google Scholar] [CrossRef]
- Hu, X.; Wei, H. Experimental investigation of laminar flame speeds of propane in O2/CO2 atmosphere and kinetic simulation. Fuel 2020, 268, 117347. [Google Scholar] [CrossRef]
- Zhang, Y.; Xin, Y.; Si, M.; Liu, F.; Lou, C. Experimental Study on Soot Formation and Primary Particle Size in Oxy-Combustion Ethylene Diffusion Flames under CO2 Substitution for N2. Case Stud. Therm. Eng. 2023, 48, 103060. [Google Scholar] [CrossRef]
- Wu, J.; Chen, L.; Bengtsson, P.E.; Zhou, J.; Zhang, J.; Wu, X.; Cen, K. Effects of carbon dioxide addition to fuel on soot evolution in ethylene and propane diffusion flames. Combust. Flame 2019, 199, 85–95. [Google Scholar] [CrossRef]
- Liu, H.; Zheng, S.; Zhou, H. Measurement of soot temperature and volume fraction of axisymmetric ethylene laminar flames using hyperspectral tomography. IEEE Trans. Instrum. Meas. 2016, 66, 315–324. [Google Scholar] [CrossRef]
- Wu, Y.H.; Hu, Y.H.; Jiang, F.; Zhang, L.H. Design of temperature measurement system based on two-color imaging in adaptive optics of CCD. In Proceedings of the 5th International Symposium on Advanced Optical Manufacturing and Testing Technologies, Dalian, China, 26–29 April 2010; Volume 7656. [Google Scholar] [CrossRef]
- Bowen, L.; Chengjing, W.; Yindi, Z.; Bing, L.; Fanjin, Z.; Takyi, S.A.; Tontiwachwuthikuld, P. Effect of CO2/H2O addition on laminar diffusion flame structure and soot formation of oxygen-enriched ethylene. J. Energy Inst. 2022, 102, 160–175. [Google Scholar] [CrossRef]
- Legros, G.; Wang, Q.; Bonnety, J.; Kashif, M.; Morin, C.; Consalvi, J.L.; Liu, F. Simultaneous soot temperature and volume fraction measurements in axis-symmetric flames by a two-dimensional modulated absorption/emission technique. Combust. Flame 2015, 162, 2705–2719. [Google Scholar] [CrossRef]
- Smyth, K.C.; Shaddix, C.R. The elusive history of m∼= 1.57 − 0.56 I for the refractive index of soot. Combust. Flame 1996, 107, 314–320. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, F.; Clavel, D.; Smallwood, G.; Lou, C. Measurement of Soot Volume Fraction and Primary Particle Diameter in Oxygen Enriched Ethylene Diffusion Flames Using the Laser-induced Incandescence Technique. Energy 2019, 177, 421–432. [Google Scholar] [CrossRef]
- Charest MR, J.; Groth CP, T.; Gülder Ö, L. Effects of gravity and pressure on laminar coflow methane-air diffusion flames at pressures from 1 to 60 atmospheres. Combust. Flame 2011, 158, 860–875. [Google Scholar] [CrossRef]
- Sunderland, P.B.; Faeth, G.M. Soot formation in hydrocarbon/air laminar jet diffusion flames. Combust. Flame 1996, 105, 132–146. [Google Scholar] [CrossRef]
- Smith, T.F.; Shen, Z.F.; Friedman, J.N. Evaluation of coefficients for the weighted sum of gray gases model. J. Heat Transfer. 1982, 104, 602–608. [Google Scholar] [CrossRef]
- Chu, H.; Yan, Y.; Xiang, L.; Han, W.; Ren, F.; Peng, L. Effect of oxygen-rich combustion on soot formation in laminar co-flow propane diffusion flames. J. Energy Inst. 2020, 93, 822–832. [Google Scholar] [CrossRef]
- Appel, J.; Bockhorn, H.; Frenklach, M. Kinetic modeling of soot formation with detailed chemistry and physics: Laminar premixed flames of C2 hydrocarbons. Combust. Flame 2000, 121, 122–136. [Google Scholar] [CrossRef]
- Kalvakala, K.C.; Katta, V.R.; Aggarwal, S.K. Effects of oxygen-enrichment and fuel unsaturation on soot and NOx emissions in ethylene, propane, and propene flames. Combust. Flame 2018, 187, 217–229. [Google Scholar] [CrossRef]
- Mahmoud, N.M.; Zhong, W.; Ibrahim, J.N.; Wang, Q. Flame Structure and Soot-Precursor Formation of Coflow n-Heptane Diffusion Flame Burning in O2/N2 and O2/CO2 Atmosphere. J. Energy Eng. 2021, 147, 04021027. [Google Scholar] [CrossRef]
- Wang, Y.; Gu, M.; Gao, Y.; Liu, X.; Lin, Y. An experimental and numerical study of soot formation of laminar coflow H2/C2H4 diffusion flames in O2-CO2 atmosphere. Combust. Flame 2020, 221, 50–63. [Google Scholar] [CrossRef]
- Lee, K.B.; Thring, M.W.; Beer, J.M. On the rate of combustion of soot in a laminar soot flame. Combust. Flame 1962, 6, 137–145. [Google Scholar] [CrossRef]
- Zhang, Q.; Thomson, M.J.; Guo, H.; Liu, F.; Smallwood, G.J. A numerical study of soot aggregate formation in a laminar coflow diffusion flame. Combust. Flame 2009, 156, 697–705. [Google Scholar] [CrossRef]
- Liu, F.; Ai, Y.; Kong, W. Effect of hydrogen and helium addition to fuel on soot formation in an axisymmetric coflow laminar methane/air diffusion flame. Int. J. Hydrogen Energy 2014, 39, 3936–3946. [Google Scholar] [CrossRef]
- Wang, Y.; Gu, M.; Zhu, Y.; Cao, L.; Zhu, B.; Wu, J.; Lin, Y.; Huang, X. A review of the effects of hydrogen, carbon dioxide, and water vapor addition on soot formation in hydrocarbon flames. Int. J. Hydrogen Energy 2021, 46, 31400–31427. [Google Scholar] [CrossRef]
- Guo, H.; Smallwood, G.J. Numerical study on the influence of CO2 addition on soot formation in an ethylene/air diffusion flame. Combust. Sci. Technol. 2008, 180, 1695–1708. [Google Scholar] [CrossRef]
- Bedick, C.R.; Kolczynski, L.; Woodside, C.R. Combustion plasma electrical conductivity model development for oxy-fuel MHD applications. Combust. Flame 2017, 181, 225–238. [Google Scholar] [CrossRef]
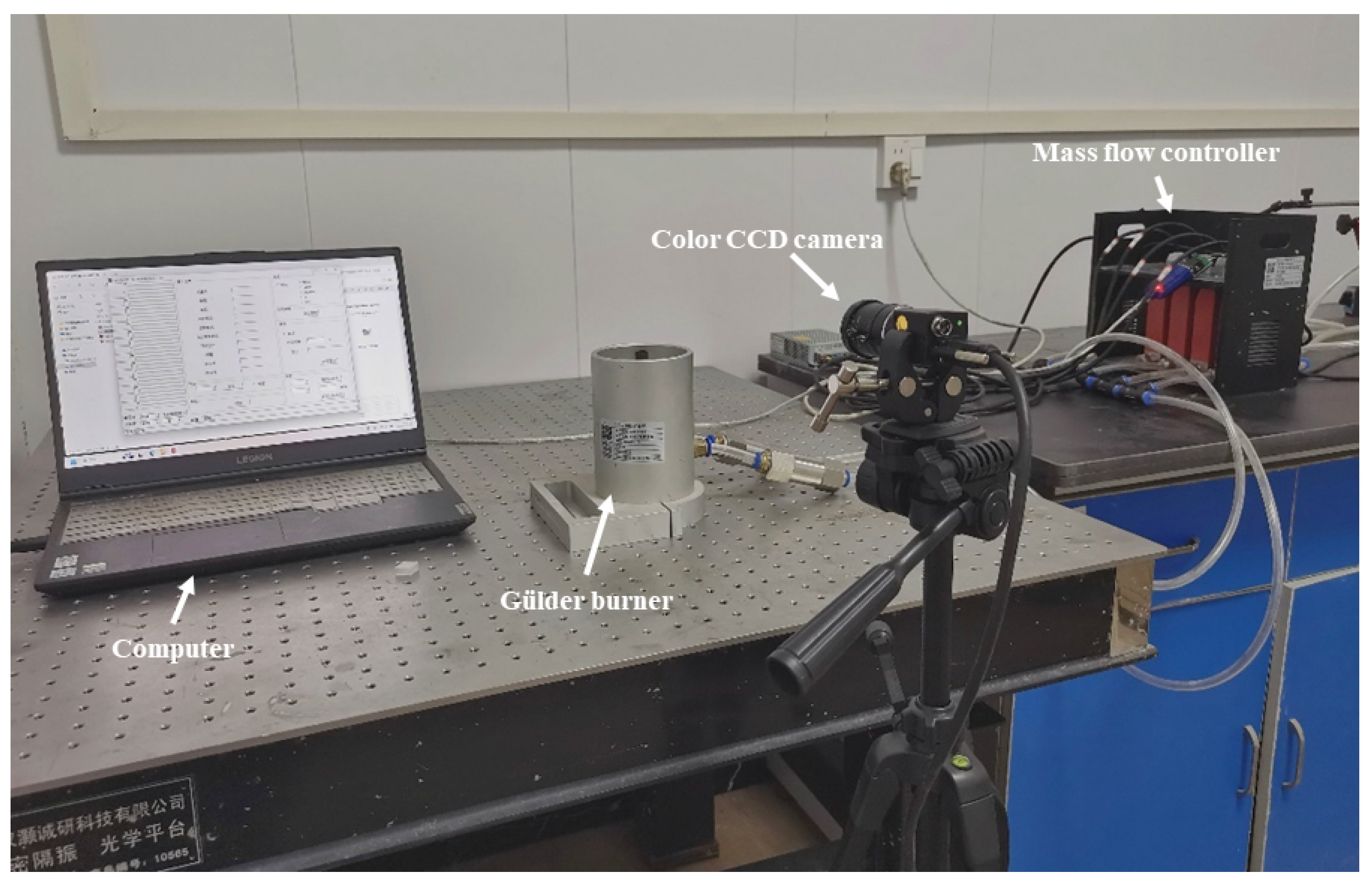
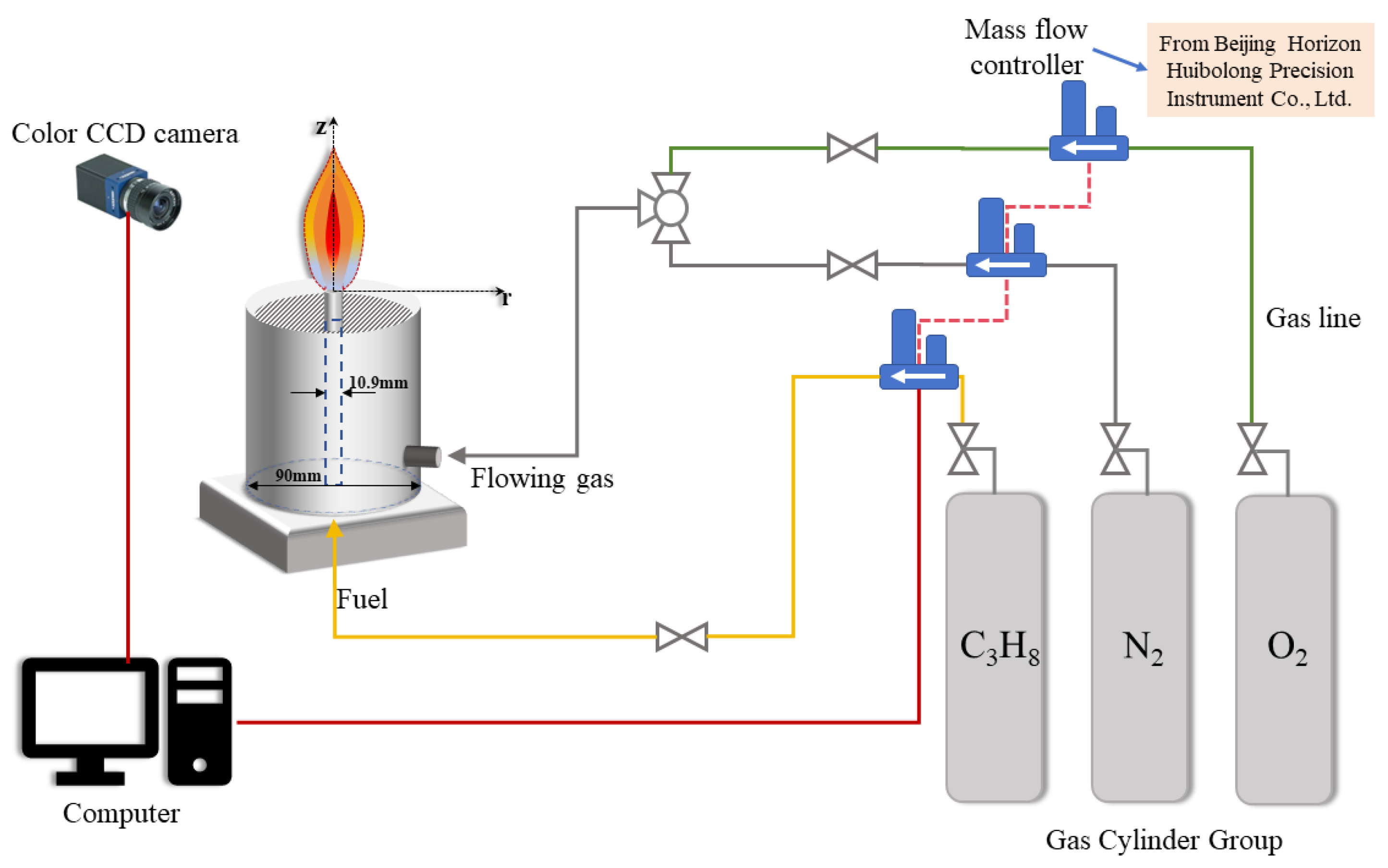

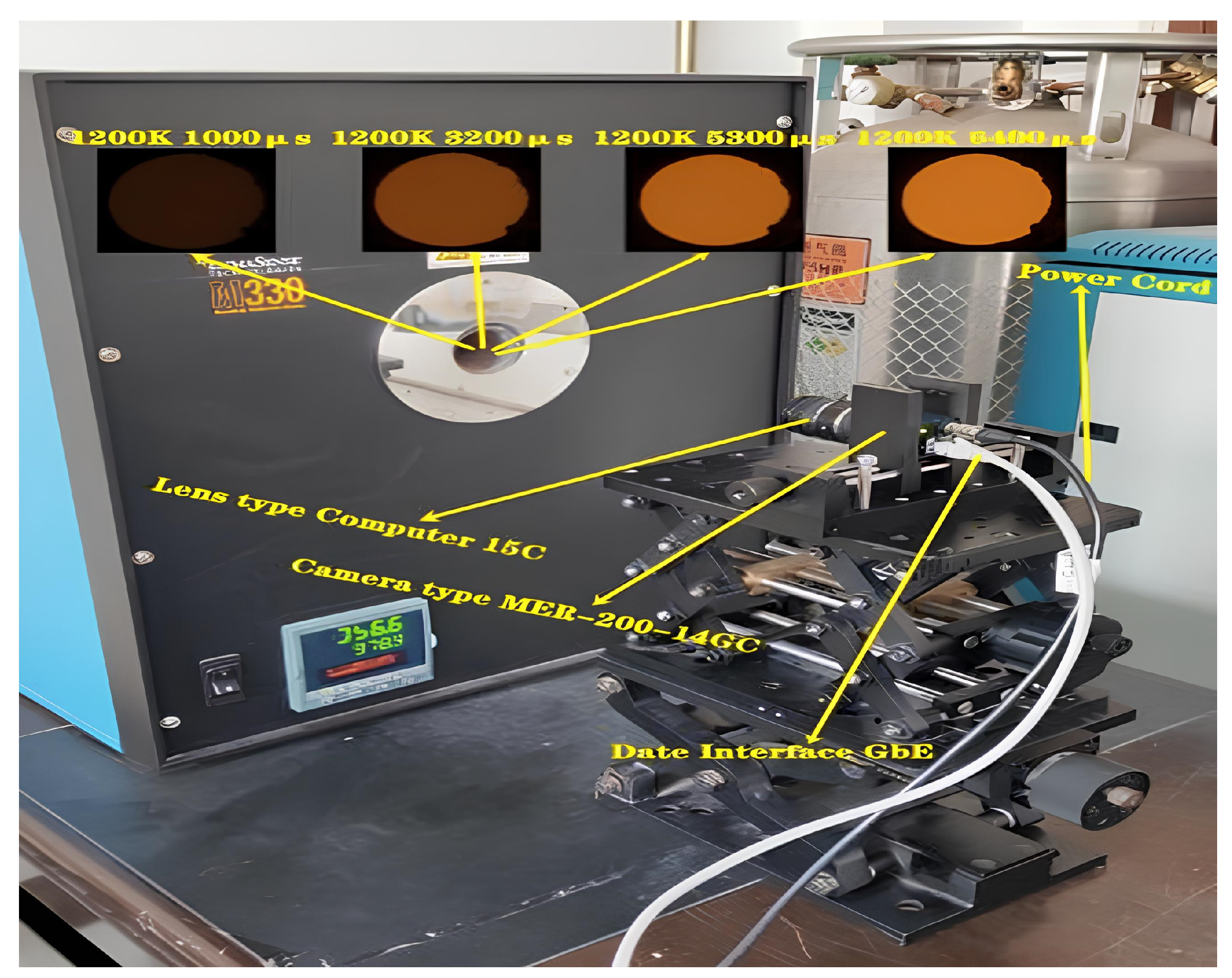
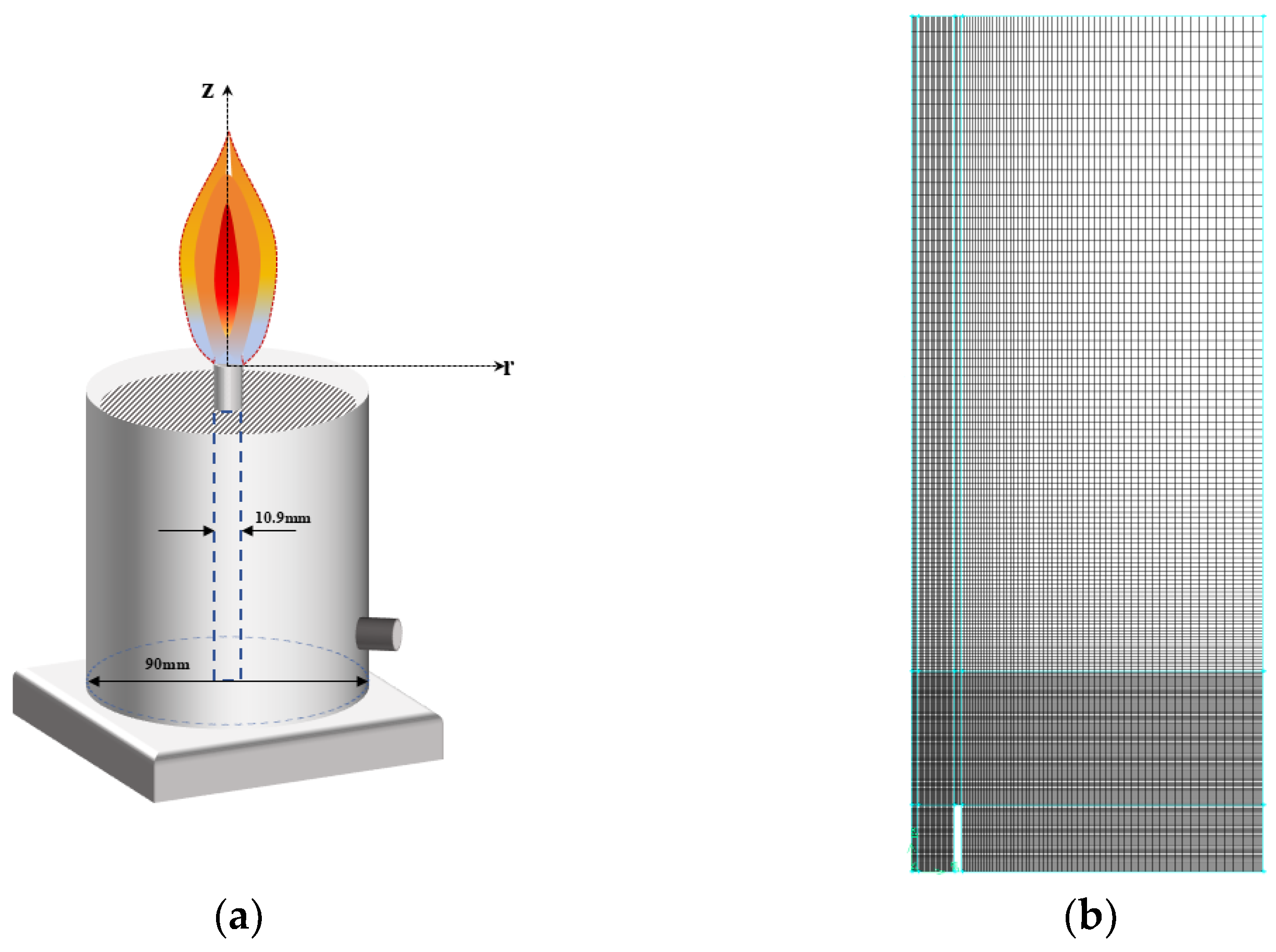

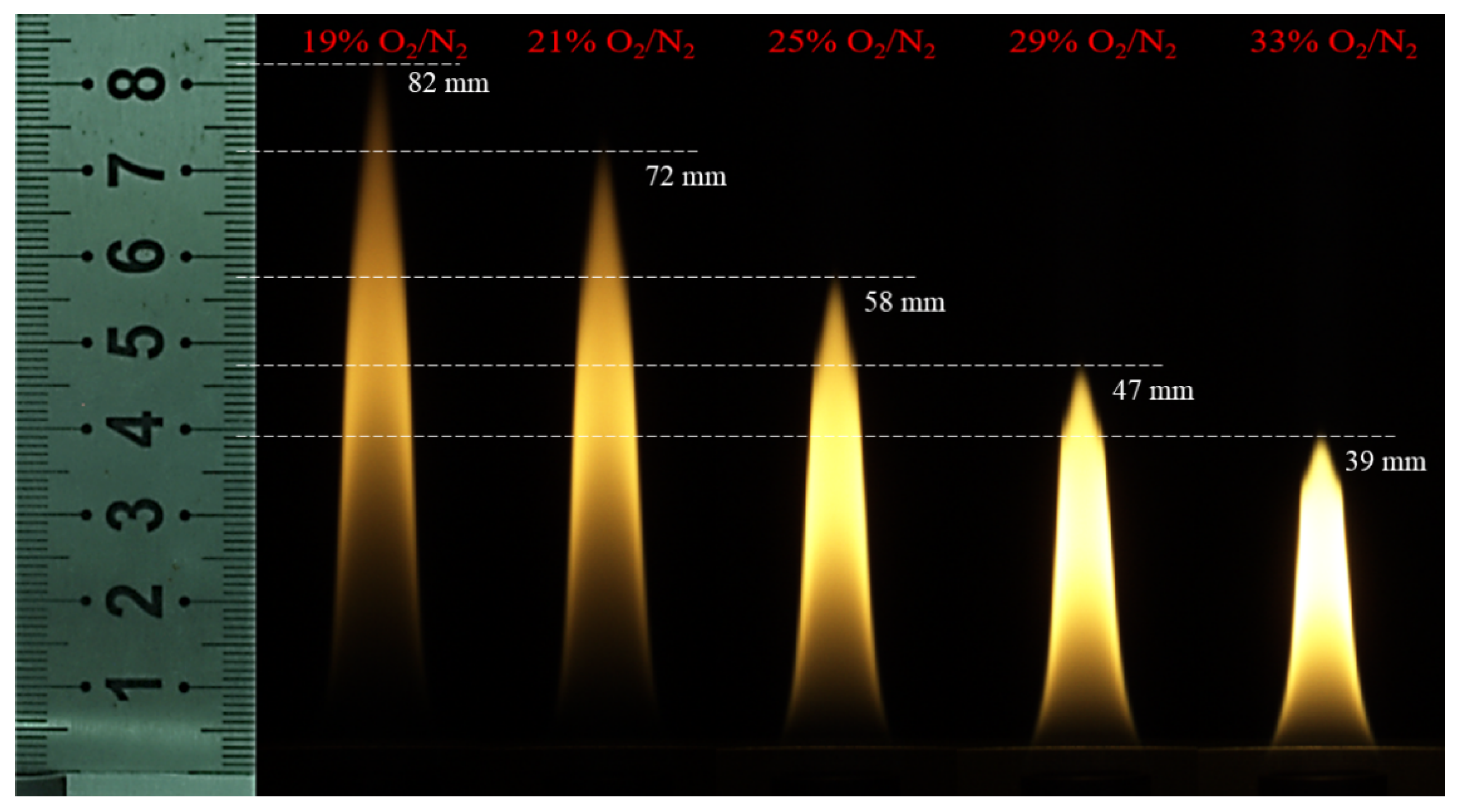






| Test Conditions | Fuel Flow/(mL·min−1) | OI | Co-Flow Air Flow Rate/(L·min−1) | |
|---|---|---|---|---|
| C3H8 | Total | N2 | ||
| Case 1 | 109.8 | 19% | 40 | 32.4 |
| Case 2 | 109.8 | 21% | 40 | 31.6 |
| Case 3 | 109.8 | 25% | 40 | 30 |
| Case 4 | 109.8 | 29% | 40 | 28.4 |
| Case 5 | 109.8 | 33% | 40 | 26.8 |
| Case | Oxidant Composition (Mole Fraction) | Remark | ||||||
|---|---|---|---|---|---|---|---|---|
| XO2 ** | XN2 | XF1CO2 | XF2CO2 | XFCO2 | XCO2 | The Velocity of Co-Current Gas Flow/(m·s−1) | ||
| 3-1 | 0.3 | 0.7 | 0.1783 | Bench | ||||
| 3-2 | 0.7 | 0.1783 | 1 and 2, Density effect | |||||
| 3-3 | 0.7 | 0.1783 | 2 and 3, Transport effect | |||||
| 3-4 | 0.7 | 0.1783 | 3 and 4, Thermal effect | |||||
| 3-5 | 0.7 | 0.1783 | 4 and 5, Chemical effect | |||||
| Case | Oxidant Composition (Mole Fraction) | Remark | ||||||
|---|---|---|---|---|---|---|---|---|
| XO2 | XN2 | XF1H2O | XF2H2O | XFH2O | XH2O | The Velocity of Co-Current Gas Flow/(m·s−1) | ||
| 3-6 | 0.3 | 0.7 | 0.1783 | Bench | ||||
| 3-7 | 0.7 | 0.1783 | 1 and 2, Density effect | |||||
| 3-8 | 0.7 | 0.1783 | 2 and 3, Transport effect | |||||
| 3-9 | 0.7 | 0.1783 | 3 and 4, Thermal effect | |||||
| 3-10 | 0.7 | 0.1783 | 4 and 5, Chemical effect | |||||
| Cas | Density (kg/m3) | Heat Capacity (J/mol·K) | Isobaric Specific Heat Capacity (J/mol·K) | Coefficient of Thermal Conductivity (W/m·K) | Thermal Diffusivity (m2/s) |
|---|---|---|---|---|---|
| N2 | 0.34 | 32.963 | 41.293 | 0.097085 | 1.95 × 10−4 |
| CO2 | 0.54 | 46 | 54.322 | 0.070571 | 1.08 × 10−4 |
| H2O | 0.22 | 24.386 | 32.703 | 0.065991 | 1.68 × 10−4 |
| OI | Parameter | Current Numerical Results | Experimental Results | Chu et al. [29] Results | Relative Error (%): Current vs. Experimental | Relative Error (%): Current vs. Chu et al. [29] |
|---|---|---|---|---|---|---|
| 21% | Peak Temperature (K) | 1986.58 | 1933.31 | 1922.31 | 2.76 | 3.34 |
| Peak SVF (ppm) | 2.65 | 2.70 | 2.51 | 1.85 | 5.58 | |
| 25% | Peak Temperature (K) | 2161.71 | 2106.57 | 2172.41 | 2.62 | 0.49 |
| Peak SVF (ppm) | 4.71 | 4.86 | 4.52 | 3.09 | 4.20 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xin, Y.; Liang, B.; Zhang, Y.; Si, M.; Cunatt, J.J. Numerical Analysis of Soot Dynamics in C3H8 Oxy-Combustion with CO2 and H2O. Energies 2024, 17, 6232. https://doi.org/10.3390/en17246232
Xin Y, Liang B, Zhang Y, Si M, Cunatt JJ. Numerical Analysis of Soot Dynamics in C3H8 Oxy-Combustion with CO2 and H2O. Energies. 2024; 17(24):6232. https://doi.org/10.3390/en17246232
Chicago/Turabian StyleXin, Yue, Bowen Liang, Yindi Zhang, Mengting Si, and Jinisper Joseph Cunatt. 2024. "Numerical Analysis of Soot Dynamics in C3H8 Oxy-Combustion with CO2 and H2O" Energies 17, no. 24: 6232. https://doi.org/10.3390/en17246232
APA StyleXin, Y., Liang, B., Zhang, Y., Si, M., & Cunatt, J. J. (2024). Numerical Analysis of Soot Dynamics in C3H8 Oxy-Combustion with CO2 and H2O. Energies, 17(24), 6232. https://doi.org/10.3390/en17246232





