Abstract
Global warming is presently one of the most pressing issues the planet faces, with the emission of greenhouse gasses being a primary concern. Among these gasses, CO2 is the most detrimental because, among all the greenhouse gasses resulting from anthropogenic sources, CO2 currently contributes the largest share to global warming. Therefore, to reduce the adverse effects of climate change, many countries have signed the Paris Agreement, according to which net zero emissions of CO2 will be achieved by 2050. In this respect, Carbon Capture and Sequestration (CCS) is a critical technology that will play a vital role in achieving the net zero goal. It allows CO2 from emission sources to be injected into suitable subsurface geological formations, aiming to confine CO2 underground for hundreds of years. Therefore, the confinement of CO2 is crucial, and the success of CCS projects depends on it. One of the main components on which the confinement of the CO2 relies is the integrity of the cement. As it acts as the barrier that restricts the movement of the sequestrated CO2 to the surface. However, in a CO2-rich environment, cement reacts with CO2, leading to the deterioration of its physical, chemical, transfer, morphological, and mechanical properties. This degradation can create flow paths that enable the leakage of sequestered CO2 to the surface, posing risks to humans, animals, and the environment. To address this issue, numerous studies have investigated the use of various additives in cement to reduce carbonation, thus enhancing the cement’s resistance to supercritical (sc) CO2 and maintaining its integrity. This paper provides a comprehensive review of current research on cement carbonation tests conducted by different authors. It includes detailed descriptions of the additives used, testing setups, curing conditions, methodologies employed, and experimental outcomes. This study will help to provide a better understanding of the carbonation process of the cement sample exposed to a CO2-rich environment, along with the pros and cons of the additives used in the cement. A significant challenge identified in this research is the lack of a standardized procedure for conducting carbonation tests, as each study reviewed employed a unique methodology, making direct comparisons difficult. Nonetheless, the paper provides an overview of the most commonly used temperatures, pressures, curing durations, and carbonation periods in the studies reviewed.
1. Introduction
Climate change is one of the world’s biggest problems because it has far-reaching impacts on the social, economic, and environmental spheres. In that respect, the release of greenhouse gasses in the atmosphere plays a crucial role in global warming as the accumulation of those gasses due to various human activities causes the Earth’s temperature to rise [1,2]. Among all greenhouse gasses, CO2 is regarded as the most dangerous because its absorption of infrared radiation leads to vibrational deformation and stretching of the CO2 molecules, which in turn contributes to the warming of the Earth’s surface [3,4]. It is reported that the Earth’s temperature has risen by approximately 1 °C since pre-industrial times [5]. If the current emission of CO2 into the atmosphere continues, it is predicted that the temperature rise by 2030 will be 1.5 °C higher than in the pre-industrial era, which will have an adverse effect on human life [5].
It is reported that about 76% of greenhouse gas emissions come from 20 countries (European Union, India, China, United States, Australia, Canada, Saudi Arabia, Turkey, UK, Japan, Indonesia, Italy, Australia, France, Argentina, Mexico, South Korea, South Africa, Germany, and Brazil). Whereas 3.8% of the contribution is from the least developed countries while small island countries emit less than 1% [6]. Therefore, to control global warming and reduce greenhouse gas emissions, the Paris Agreement was signed by different countries on 12 December 2015 [7]. According to this agreement, 45% of greenhouse gas emissions will be reduced by 2030, while the net zero target will be achieved by 2050. Net zero is a balance that dictates that the amount of CO2 emitted into the atmosphere should be equal to the CO2 removed from the atmosphere. Therefore, developed nations, which are significant contributors to greenhouse gas emissions, have initiated various measures to reduce their carbon footprint. These include adopting renewable energy sources for power generation and utilizing hydrogen as a substitute for fossil fuels. While these approaches can help lower emissions, Carbon Capture and Sequestration (CCS) remains the most effective option for removing CO2 from the atmosphere and can help achieve the ambitious net-zero target [8].
The CCS project could begin with the development of a new site dedicated explicitly to CO2 sequestration. Hence, the well will be designed and constructed according to the regulations and acidic environment and will be less prone to failure. However, this approach will be cost-intensive. On the other hand, the CCS project can be more cost-effective if it uses old oil and gas fields where abandoned wells are present. In such a case, a new injection well is drilled in that field, and the CO2 is injected from that well. Nevertheless, issues related to well integrity may arise as the CO2 plume migrates toward the abandoned wells. Therefore, a thorough risk assessment should be conducted in these fields to evaluate the feasibility of the project.
The concept of Carbon Capture and Storage (CCS) was first introduced in 1977 when CO2 emitted from a coal power plant was captured and injected into a suitable subsurface geological formation [9]. These suitable formations for CO2 injection include saline aquifers, coal beds, and depleted oil and gas reservoirs [10]. Currently, anthropogenic CO2 can be captured and geologically stored from activities such as electricity generation and industrial processes, including hydrogen production [11]. An example of this process is shown in Figure 1, where CO2 with a high content is extracted from natural gas and injected into an underground formation. The sequestration of CO2 in the subsurface can also take place through the Enhanced Oil Recovery (EOR) method, which makes this technique a multifaceted process because while CO2 is captured in underground geological formations, oil production will increase [12,13]. This approach can help in removing CO2 from the atmosphere while also enhancing energy production. In this technique, the CO2 is captured from the emission source and is injected into the mature oil field. When injected into the reservoir, the CO2 will act as a solvent and reduce the oil’s viscosity, increasing its mobility. At the same time, it helps to increase the pressure of the reservoir and improve production. Nonetheless, the impact of CCS-EOR is debatable when it comes to the long-term strategies of climate change. Critics argue that in one place, the CO2 in the atmosphere is removed by the sequestration process, while on the other hand, the increase in fossil fuel production will further delay the shift toward renewable energy, which can help to reduce greenhouse gas emissions in the environment.
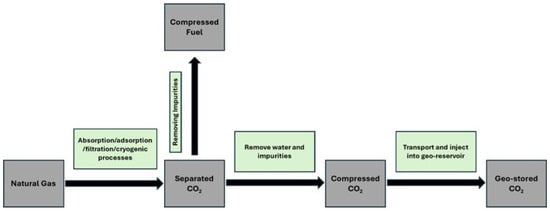
Figure 1.
CO2 being processed and injected in the suitable geological formation [12].
The effectiveness of any Carbon Capture and Storage (CCS) project relies on three main factors: injectivity, capacity, and confinement. Capacity is related to the amount of CO2 that can be stored in the subsurface without fracturing the caprock, and it depends on various factors that include thickness, effective porosity, TOC (total organic carbon), apparent gas saturation of reservoir, permeability, and absorption isotherm [14]. However, it must be noted that the reservoir is not used to its full theoretical capacity due to the technical limitations [15]. Injectivity defines the rate at which CO2 can be injected into the geological formation and depends on the completion technique used. Nonetheless, the reservoir has its own limitations, which make high injection rates impossible to achieve [16]. The most critical factor among the three parameters is confinement, as the success of a CCS project depends on it. For a CCS project to be deemed successful, the sequestered CO2 must remain underground for hundreds of years, or if leakage occurs, it should be at a very low rate of between 0.01 and 0.1% per year [17]. If the leakage rate increases and the sequestered CO2 escapes to the surface, it could have a detrimental impact on the ecosystem, human health, and the environment. Leakage pathways might include fractures in the formation or caprock, but the most significant risk comes from the micro annulus that can form in the cement sheath behind the casing or in the plugs of abandoned wells. One of the primary factors compromising the integrity of the cement is its reaction with supercritical (sc) CO2.
In general, cement is basic in nature, having a pH value that ranges from 12.0 to 13.8 [18]. In contrast, formation water mixed with CO2 becomes acidic, with a pH ranging from 3 to 5, depending on downhole pressure and temperature [19]. Due to the difference in the pH, the reaction between the CO2-saturated formation water with the cement initiates so that the equilibrium in the system is achieved. The rate of carbonation reaction in cement exposed to scCO2 environments is primarily controlled by the diffusion rate of scCO2 into the cement matrix [20]. Therefore, it is crucial to understand how this reaction occurs and how it affects the transfer and mechanical properties of the cement as the reaction progresses.
2. Chemical Compositions and Cement Reactions
2.1. Chemical Composition of Class G Cement
In the petroleum industry, Class G cement is the most commonly used cement for well applications, with a Blaine-specific fineness of 280–340 m2/kg [21]. Table 1 provides the elemental composition of Class G cement as determined by X-ray fluorescence (XRF) analysis [22]. Whereas Table 2 outlines the compound compositions of Class G cement and their specific functions.

Table 1.
Element composition of Class G cement from XRF testing [22].

Table 2.
Compound composition and their function in Class G cement [23].
2.2. Cement Hydration Reaction
The strength, stiffness, and solidification of the cement are achieved when the dry cement is mixed with the water. This process is called cement hydration, and for this reason, it is referred to as hydraulic cement, as its strength is gained through a chemical reaction with water. Moreover, it is important to note that cement hydration is an exothermic reaction, meaning that heat is released as the cement reacts with water.
2.3. Reaction of Silicate Phase
As shown in Table 2, the silicate phase is the most abundant in cement, making up nearly 80 wt% of its composition. Within this silicate phase, over 70% consists of C3S, while C2S accounts for 20%. The following equation gives the reaction of the silicate phase with the water. It must be noted that the formula of the compound used in the reactions will be according to the cement chemistry notation [24,25,26].
Tri/Dicalcium Silicate + Water → Calcium Silicate Hydrate + Calcium Hydroxide (Portlandite)
2C3S + 7H → C3S2H8 + 3CH ΔH = 120 Cal/g
2C2S + 7H → C3S2H8 + CH ΔH = 62 Cal/g
The reactions of C3S (tricalcium silicate) and C2S (dicalcium silicate) are similar and produce the same products. However, the initial strength in cement is primarily associated with C3S, as its reaction rate is faster than that of C2S. In contrast, the strength development during the later stages of cement curing is attributed to C2S.
The reaction of silicates produces calcium silicate hydrate (C-S-H), the primary binding material in cement, which makes up approximately 50 to 60% of the volume in hydrated cement. The strength of C-S-H is derived from Van der Waals forces (about 35%) and ionic/covalent bonding (about 65%) [27]. Calcium hydroxide, also referred to as portlandite, is crystalline in nature and constitutes approximately 20 to 25% of the volume.
2.4. Reaction of Aluminate Phase
Ettringite is produced when tricalcium aluminate and gypsum react with each other in the presence of water [28].
Tricalcium aluminate + gypsum + water → ettringite + heat
C3A + 3CSH2 + 26H → C6AS3H32 ΔH = 207 cal/g
Ettringite is composed of elongated crystals that remain stable only in gypsum solution. It does not improve cement strength. In hydrated cement, ettringite occupies approximately 15 to 20% of the total volume.
After gypsum is consumed completely, ettringite loses its stability and undergoes a reaction with any unconsumed C3A, resulting in the formation of mono-sulfate aluminate hydrate.
Tricalcium aluminate + water + ettringite → mono-sulfate aluminate hydrate
2C3A + C6AS3H32 + 22H → 3C4ASH18
It must be noted that mono-sulfate aluminate hydrate is only stable in the absence of a sulfate environment. Once it comes in contact with the sulfate, the mono-sulfate aluminate hydrate converts back to ettringite. At this stage, the length of the ettringite crystal is 2.5 times bigger than that of the mono-sulfate aluminate hydrate, which can lead to the formation of cracks and micro-annuli in the cement [24,25,26].
2.5. Reaction of Ferrite Phase
The reaction of the ferrite phase proceeds in two steps.
- Ferrite + gypsum + water → ettringite + ferric aluminum hydroxide + lime
C4AF + 3CSH2 + 3H → C6(A,F)S3H32 + (A,F)H3 + CH
- 2.
- While in the second step, ferrite will react with the produced ettringite and yield garnet
Ferrite + ettringite + Portlandite + water → garnet
C4AF + C6(A,F)S3H32 + 2CH + 23H → 3C4(A,F)SH18 + (A,F)H3
The garnet produced in the ferrite reaction occupies space in the hydrated cement and does not contribute to strength development.
From the above reactions, it is clear that neither the aluminate nor the ferrite phase contributes to the strength of the cement. The only phase that provides strength is the silicate phase and its products. When the cement is exposed to supercritical CO2 (scCO2), it targets and degrades the products of the silicate phase, thereby compromising the properties and integrity of the cement. The detailed reaction of the scCO2 with the cement will be discussed in the following section.
2.6. Supercritical CO2
When the pressure and temperature of the CO2 go above the critical point (7.37 MPa and 31 °C), the state of the CO2 changes to the supercritical phase [29]. At this point, CO2 has the viscosity of gas and density of liquid. Therefore, when the CO2 is pumped into the subsurface, the pressure and temperature of the bottom hole convert the injected CO2 into the supercritical phase, due to which its diffusivity and reactivity increase and become more severe than any other CO2 phase. The phase diagram of CO2 is illustrated in Figure 2.
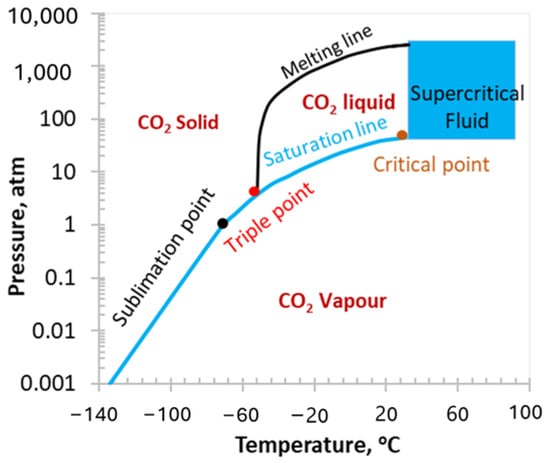
Figure 2.
Phase diagram of CO2 [20].
Due to the low viscosity of the scCO2, the mobility of the injected CO2 is increased, which will improve its efficient distribution in the reservoir. The higher density of scCO2 will compress more CO2 in the given sequestrated geological formation, improving the storage capacity. On the other hand, the injected scCO2 can increase the pressure in the reservoir, which can cause the activation of the existing fault or cause a fracture in the surrounding formation. The corrosive nature of scCO2 can affect the integrity of the wellbore material, such as that of cement and casing. Moreover, the monitoring of the scCO2 in the sequestrated reservoir requires specialized techniques to ascertain that it has not migrated beyond the planned storage area. This monitoring and verification of the scCO2 movement can cause an increase in the project cost.
Overall, it can be said that scCO2 is effective in maximizing the capacity of the injected CO2; however, careful consideration should be given to the material selection, monitoring, and designing of the CCS wells in order to mitigate the risk associated with scCO2 unique properties.
2.7. Reaction of Cement with Carbon Dioxide
The interaction between carbon dioxide and cement begins when CO2 dissolves in the formation water, resulting in the formation of carbonic acid. The reaction is presented in Equation (7) [17,30].
CO2 (aq) + H2O → H2CO3 (aq)
The carbonic acid reacts with the cement matrix, and the first component to be attacked is portlandite.
Ca(OH)2 (s) + H2CO3 (aq) → CaCO3(s) + H2O (aq)
As a result of this reaction, the polymorph of calcium carbonate is formed. It is commonly believed that the carbonation of cement is detrimental to the cement properties, which is incorrect. This step is also known as the self-healing process because, at this stage, the transfer properties of the cement decrease, and the mechanical properties increase. The reason is that the molar volume of calcium carbonate (54.13 cm3/mol) is bigger than the molar volume of the replace component, which is portlandite (31.66 cm3/mol).
If the reaction were to stop at this stage, it would be beneficial for the cement’s integrity. However, the diffusivity of carbonic acid in the cement continues, which converts carbonate to bicarbonate, which is soluble in the water and leaches out of the cement matrix. Hence, the formation of bicarbonate marks the beginning of the degradation of cement [31].
CaCO3 (s) + H2CO3 (aq) → Ca(HCO3)2 → Ca2+ (aq) + 2HCO3− (aq)
At the last stage of the bicarbonate, carbonic acid starts to attack the main binding material, C-S-H, yielding calcium carbonate and amorphous silica gel [20].
3H2CO3 + Ca3Si2O7 · 4H2O → 3 CaCO3 + 2 SiO2 · H2O + 5 H2O
At this stage, the cement will lose its integrity, leading to increased porosity and permeability, while its mechanical strength is compromised. This increase in transfer properties occurs because the molar volume of CaCO3 is smaller than that of C-S-H (calcium silicate hydrate). As CaCO3 replaces C-S-H, more voids are created within the cement matrix, contributing to the deterioration of its transfer properties [32]. While the primary cause of the reduction in the mechanical properties of the cement is the formation of amorphous silica gel [17,30]. Figure 3 illustrates various chemically altered zones identified after the carbonation test, as reported by [17,33].
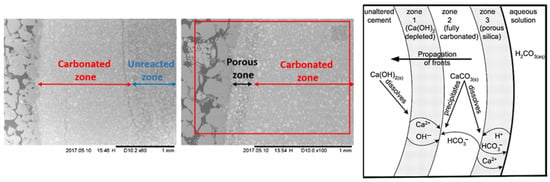
Figure 3.
Carbonation zones in the cement [17,33].
3. Review of Experimental Testing by Different Authors
As evident from the above reactions, the interaction between cement and CO2 leads to a degradation of the cement’s mechanical and transfer properties, which can pose significant well integrity challenges in CCS wells. As a result, numerous studies have been carried out to investigate the effects of carbonation and bicarbonation on various well-cement composites. In this respect, different authors used different setups for the carbonation test. Some of the authors used static reactors, while others used dynamic reactors. Hence, this paper will provide a comprehensive review of testing setups, additives used, methodologies employed, curing parameters, and the conclusions drawn from those studies.
3.1. Case Study 1
Dugid and Scherer [34] performed a set of experiments to understand the behavior of cement when exposed to the flowing carbonated brine solution. The cement used in this study consisted of neat Class H and Class H + 6% bentonite (BWOC, By Weight of Cement). Two sets of experiments were conducted at 20 and 50 °C, and 10 MPa. In each test, two cylindrical molds were placed in a plastic reactor filled with CO2-saturated brine, which was pumped and circulated upwards and around the specimens. The brine solution contained 0.5 M NaCl. These two experiments simulated the condition of the sandstone and limestone reservoirs. The dynamic experimental setup is presented in Figure 4. For the sandstone reservoir simulation, the pH of the effluent was controlled at 3.4 and 2.7, while for the limestone reservoir, the pH was kept at 5. The neat cement sample was prepared according to API standards 10A and 10B [35,36] with a water-to-cement (W/C) ratio of 0.38, while the cement mixed with bentonite had a water-to-solid (W/S) ratio of 0.70. The CO2-rich brine was circulated at 8.3–8.4 mL/min for the 20 °C test and 8.6–8.7 mL/min for the 50 °C experiment. Both samples, mixed per API standards, were vacuum-treated for two minutes to remove air pockets from the slurry. The cement was then transferred into a pastry bag and poured into greased 10 mL disposable plastic pipette molds for curing. The samples were initially cured for three days in a 0.5 M NaCl solution at either 20 °C or 50 °C, followed by a year immersion in NaCl solution before undergoing carbonation testing. The dimensions of the cylindrical samples were about 7.5 mm × 200 mm (Diameter × Height). The samples were subjected to corrosion testing for 31 days. XRD and Electron Probe Microanalysis (EPMA) analyses revealed that the outer rim of the samples exposed to 20 and 50 °C with pH levels of 2.4 and 3.7 were fully degraded. The degradation rate of these samples was dependent on the dissolution rate of the CaCO3 layer. It was also observed that the sample exposed to the same pH but at different temperatures had a higher degradation than those placed at the same temperature with a different pH. As for the comparison between the neat Class H and Class H + 6% bentonite. It was found that neat cement performed better than the sample containing bentonite in its composition. This was due to the higher W/S ratio used in the bentonite cement composite, which resulted in higher porosity and, consequently, greater degradation. The most corrosive condition was found to be pH 2.4 at 50 °C, representing a 1 km depth sandstone reservoir condition. However, cement degradation was also recorded in other sandstone environments, albeit at a slower rate. The least severe environment was the limestone condition simulated at 50 °C and pH 5 for a 1 km depth reservoir, where the cement samples showed no signs of degradation. Thus, it was determined that cement degradation is affected by factors such as the type of reservoir, temperature, and pH.
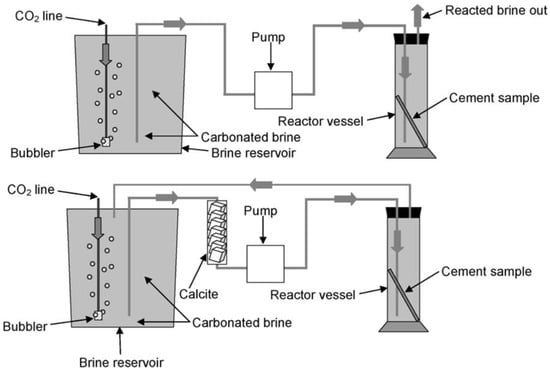
Figure 4.
Carbonation setup used by the studies carried out by Duguid and Scherer [34]. Upper section represents the sandstone reservoir condition, while lower part corresponds to the limestone reservoir condition.
3.2. Case Study 2
Laudet, et al. [37] conducted the carbonation test on the Class G and Class G + 35% Silica Flour (SF). They utilized two methods of testing: one static and the second one dynamic. In the static test, the samples were placed in the static reactor without any confining pressure. In the dynamic testing, they used coupled chemomechanical techniques, where the specimen was subjected to the CO2 environment while simultaneously being exposed to triaxial stresses. Although the second test is referred to as a dynamic test, but both tests were conducted in static reactors. The curing conditions for the samples varied depending on the experiment. For both the static and dynamic tests involving Class G cement, the samples were cured for more than 28 days at 90 °C. The SF cement composite was cured by exposing the samples to 140 °C and 20.7 MPa for the first ten days. Afterward, the samples were placed at atmospheric pressure and temperature of 90 °C (for the carbonation test at 140 °C). They wanted to replicate the condition for Class G + 35% SF in which the cement sheath is rapidly exposed to the CO2 environment. Cylindrical samples were used for the static (20 mm × 40 mm) and dynamic (25 mm × 50 mm) tests. The experimental setup in which the carbonation test was conducted is shown in Figure 5. The samples were immersed completely in the water present in the static reactor and were subjected to the pressure of 8 MPa and temperature of 140 or 90 °C (depending on the testing condition). The dynamic test lasted for 12 days, while the static test extended up to 88 days. After the testing period, it was reported that the neat sample and Class G incorporating 35% SF went through partial or full carbonation. However, the reaction rate for the neat Class G cement was controlled by diffusion, which evolved linearly with the square root of time. In contrast, for the SF cement composite, the progression of the carbonation front was constrained by reaction kinetics, with the carbonation front advancing linearly over time. Moreover, the dynamic test, designed to study chemomechanical effects, revealed that the cement was able to maintain its integrity and preserve its transfer properties. They concluded that in a CO2-rich environment, the cement’s integrity is unlikely to be compromised by the reaction with CO2. Instead, integrity loss is more likely to result from factors such as brine lixiviation and mechanical-induced damage (micro annulus, hydro frac, etc.).
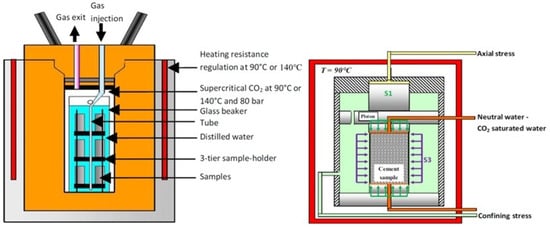
Figure 5.
Static reactor used for the testing of dynamic and static carbonation tests by Laudet, et al. [37].
3.3. Case Study 3
Santra and Sweatman [38] study showed that the reaction of cement with CO2 is inevitable due to the thermodynamically favorable conditions in the CCS environment. However, the effects of carbonation can be mitigated by incorporating various additives into the cement. In this respect, they conducted the carbonation test on Class G and Classified cement samples. The samples were first cured for 28 days in a water-filled pressure vessel at 13.8 MPa (2000 psi) and 60 °C (140°F) before being exposed to the carbonation test. The dimensions of the cylindrical samples were 63.5 × 25.4 mm (Dia × Height). After curing, the samples were placed in a static test cell (Figure 6) filled with fresh water and supercritical CO2, maintaining the same temperature and pressure as during the curing conditions (140 °F and 2000 psi). The first set of samples was subjected to exposure for 15 days, while the second set was exposed for 90 days. It was concluded from this study that the percentage of carbonation and its depth are dependent upon the portlandite produced during the cement hydration and W/C ratio. The modified cement performed better in the simulated CCS environment. The authors suggested that cement designed specifically for CCS wells must be capable of withstanding geomechanical stresses. They concluded that the ionic content of the formation fluid tends to increase the pH of carbonic acid, thereby reducing its corrosive effect on the cement sheath surrounding the well. Furthermore, they found that incorporating pozzolanic material into the cement could be advantageous, as it reduces the cement’s transfer properties. Alternatively, using non-Portland cement, such as resin-based cement or high alumina phosphate, could completely prevent cement carbonation since no Portlandite would be present in their composition.
Figure 6.
Static testing cell used by Santra and Sweatman [38] for their studies.
3.4. Case Study 4
Syed et al. [39] conducted carbonation testing to evaluate the micro annulus sealing behavior at the cement casing interface under simulated downhole temperature and pressure conditions. They used the findings of this research to develop a methodology that will assist in assessing the integrity of the CCS wells. In that respect, three experiments were conducted that represented different brine salinities, reservoir depths, and pressure. In these tests, a constant flow of CO2 through the micro-annulus was maintained. The decrease in the micro annulus permeability over time, observed in experiments, was used in the real reservoir model. This model was then applied to all three scenarios to assess how leakage at the cement casing interface affects the overall integrity of CO2 storage wells. The experiment was conducted in the well-bore test cell presented in Figure 7, while Figure 8 represents the entire testing setup with ancillary equipment and wellbore cell. For this study, Class G cement was utilized, which was initially allowed to cure for a period of 28 days within the wellbore cell under ambient temperature and pressure conditions. After which, the wellbore cell was attached to the CO2 flow setup, and different temperatures and pressures were applied depending on the scenarios under consideration. The temperature and pressure in scenarios one, two and three consisted of 40 °C and 10 MPa, 34 °C and 7.3 MPa, and 92 °C and 12.5 MPa, respectively. The test was conducted for 80 days. The result of the testing was utilized in the reservoir simulation. It was found that under shallow reservoir conditions, the flow of CO2 through the micro-annulus gradually reduced its permeability, demonstrating self-healing capability due to the carbonation of the cement sample. Consequently, this phenomenon suggests that over an extended period, micro annulus is unlikely to present a significant threat of CO2 leakage. On the contrary, if the leakage occurs in a deep well where temperature and pressure are high, the self-healing process does not initiate due to the complete hydration of the cement. Therefore, in such a scenario, a contingency plan for remediation is essential to guarantee the safe storage of CO2 underground.
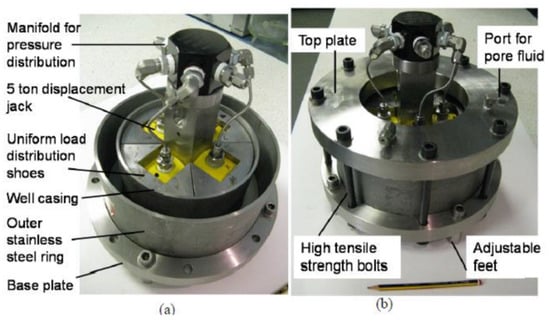
Figure 7.
Well-bore test cell used by Syed et al. [39]. (a) Central mechanism of the testing chamber in which annulus is shown where the cement was poured in (b) Complete assembly of the wellbore cell.
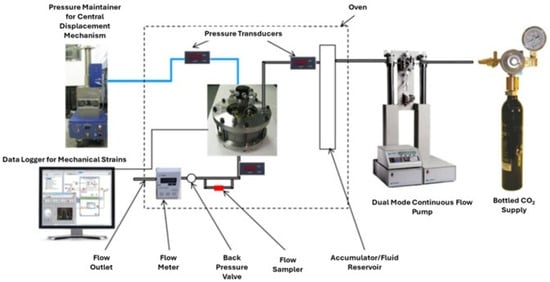
Figure 8.
Setup to which the wellbore test cell was connected by Syed, et al. [39].
3.5. Case Study 5
Connell et al. [40] conducted an experimental investigation into the effects of cement degradation at the cement-formation interface and conducted simulations to evaluate the subsequent impact of this degradation on the overall well integrity. A core flooding test approach was taken for the experimental studies in which the cement sandstone core plug was exposed to the temperature, pressure, and water chemistry according to the CO2 storage site. The sandstone cement composite simulates the formation-cement interface and permits water flow through the core plug due to the sandstone’s high permeability, facilitating regular water sampling. Moreover, it also helps to increase the reaction rate as fresh fluid comes in contact with the cement sample. XRD analysis was performed after each core flooding test to identify the cement mineralogy. Class G cement was used in this research and was cured in brine solution. Figure 9 shows the sample in which the discoloration of the cement surface can be noticed, which is caused by the perception of salt during the curing period. For the core flooding experiment, a triaxial cell was used, whose schematic is presented in Figure 9, in which the inflow and outflow path of the water can be seen. The analysis was also made on the water flowing across the cement sandstone composite plug in order to calculate the erosion of the cement. This was achieved by calculating the cumulative dissolution of CaCO3 through water chemistry and flow rate measurements. Therefore, each set of experiments involves two sets of observations. One analyzes the water before it is flown through the sample, and one after it has gone through it. From their studies, they concluded that if the formation water is saturated with calcium and carbonate ions, then the dissolution of CaCO3 from the cement that is produced due to carbonation will become difficult, and hence, the integrity of the cement can be maintained. However, if the formation water is undersaturated with the Ca2+ and CO32− ions and there is a continuous flow of water that can carry the solute away, then in such a scenario, cement degradation can occur. Therefore, it can be said that the formation like carbonate will possess less risk toward cement degradation as the formation water in that rock will be saturated with the carbonate and calcium ion and hence can maintain the equilibrium with the cement. In contrast, the cement exposed to the sandstone will be at greater risk of losing its integrity. Hence, in the sandstone case, it must be ensured that the seal between the casing cement interface is good enough so that the CO2-saturated water does not flow through it and the dissolution of the CaCO3 from the cement can be avoided. The same observation was made by [34,41], who concluded that the cement sample exposed to the sandstone environment will be degraded more as compared to the samples present in the limestone environment because of the equilibrium created between the cement and limestone formation water.
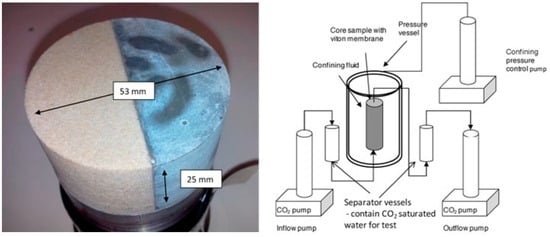
Figure 9.
Cement sandstone plug composite and the testing setup of core flooding used by Connell, et al. [40].
3.6. Case Study 6
Yang et al. [42] studies showed that the novel additive ACA (consisting of latex, nanocrystalline SiO2, latex, and superfine pitch mixed in a specific ratio) might enhance the durability of cement in CO2-rich environments. The quantity of ACA added in the Class G cement consisted of 5 and 8 wt%. Before being exposed to the corrosion, the specimens underwent curing in a HPHT vessel at 20 MPa and 85 °C for a duration of seven days. The dimensions of the sample used in this study were cubical and cylindrical. The cubical sample had a dimension of 50 mm on each side and was used for the compressive strength test. Whereas for the measurement of the permeability, the cylindrical samples were utilized, which had the dimensions of dia = 25 mm and h = 40 mm. After the initial curing, the samples were placed in the carbonation vessel (Figure 10), which had 0.4 M NaCl at temperatures and pressures of 85 °C and 20 MPa (CO2 partial pressure was about 7.5 MPa). The carbonation test lasted for 30 days. Compressive strength testing revealed that strength retrogression was approximately 58.08% for the control sample (ACA 0%) and 16.14% and 7.38% for Class G cement with 5% and 8% ACA, respectively. Permeability measurements showed that the ACA 0% sample’s permeability increased from 0.4 to 2.5 mD after 30 days of carbonation test. In contrast, the ACA 8% sample’s permeability remained nearly unchanged between 10 and 30 days of CO2 exposure, staying below 0.5 mD. The carbonation depth indicated that the carbon front advanced approximately 6.11 mm in the control sample after 30 days, whereas in the ACA 8% sample, it was limited to just 0.89 mm. Therefore, it was concluded that the incorporation of ACA reduced the cement’s permeability and improved its compressive strength after the carbonation period. The incorporation of ACA led to a significant reduction in Portlandite, which was transformed into a carbonation-resistant compound, primarily C-S-H. Moreover, ACA mitigates the dissolution and leaching effects by consuming high Ca/S hydration products and CH. Although both ACA quantities in the cement performed better than the control sample, the 8% ACA showed better resistance against CO2 attack than the 5% ACA.
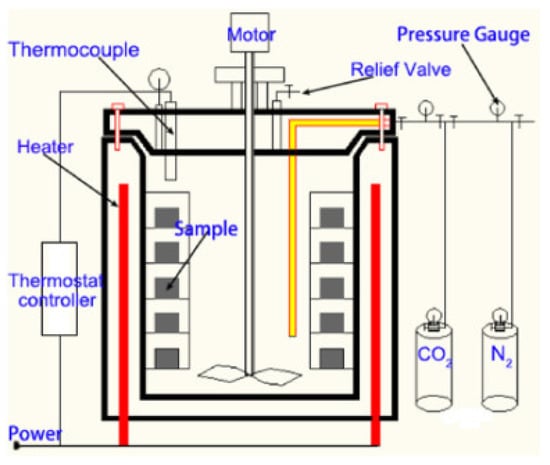
Figure 10.
Static carbonation vessel used in studies conducted by Yang, et al. [42].
3.7. Case Study 7
Costa et al. [43] did studies on the carbonation of Class G cement, which was based on sedimentation. In that respect, two slurries of Class G with two different additives were prepared. The first additive could maintain the element in suspension, resulting in a stable system, while the second additive caused particle suspension, leading to an unstable system. After the preparation of the slurry, it was placed in the cylindrical metal mold having the dimension of 203 mm height and 25 mm internal dia (ID) and was immersed in a water bath maintained at 70 °C and atmospheric pressure for a duration of 24 h. After curing for a day, the sample was cut into four equal parts with a 50 mm length. The specimens were then placed in CO2-saturated water in an autoclave (Figure 11) for 30 days at a temperature of 70 °C and a pressure of 13.8 MPa (2000 psi). The carbonated samples were then analyzed through the static sedimentation test, XRD, and acid-base indicator. It was noted that the carbonation of the cement behaves in a certain pattern when sedimentation of the particle occurs. The author named that pattern the hourglass effect. Therefore, a model based on fluid mechanics was proposed to describe the sedimentation and carbonation behavior of the unstable slurry. It was suggested that ensuring sedimentation stability is crucial for accurate carbonate cement studies; otherwise, sedimentation issues in the cement could lead to false negative or false positive results.
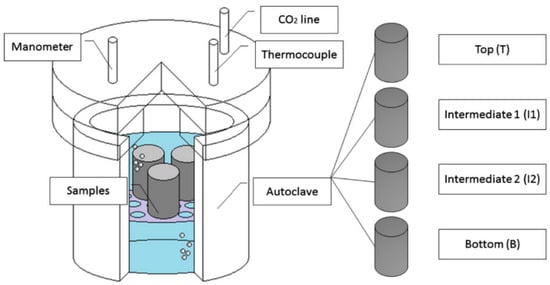
Figure 11.
Autoclave used for the studies of Costa et al. [43,44].
3.8. Case Study 8
Costa et al. [44] conducted their second experimental study on cement carbonation in 2018. In this study, three Class G cement slurries were tested, each cured for varying durations, to evaluate the impact of curing time on cement carbonation. The specimens were cured for various durations consisting of 8 h, 7 days, and 28 days at a temperature of 70 °C and atmospheric pressure before being subjected to the carbonation test. The dimensions of the samples and the autoclave used were the same as those of [43]. Different curing durations were considered to simulate early and late time scenarios, reflecting the conditions under which cement might be exposed to a CO2 environment in real field conditions. Prior to being placed in the autoclave, the samples underwent XRD analysis. Following this, the specimens were exposed to scCO2-saturated water for 30 days at 70 °C and 13.8 MPa (2000 psi). After this exposure, XRD analysis was performed once again, along with density and mass measurements. For the determination of the carbonated area, the samples were cut in the longitudinal direction and sprayed with a pH indicator, which consisted of ethanol and phenolphthalein diluted in deionized water. Phenolphthalein is a colorless liquid that acts as an indicator and has a pH of 9; when put in a solution or on a surface that has a pH of more than 9, it changes its color to pink [45]. They found that the carbonation depth was greater in the samples cured for 7 days compared to those cured for 8 h and 28 days. The reason for 8 h and 28 days showing the same results is that in the 8 h cured sample, not many hydrated products were developed that could react with CO2. In contrast, the 28-day samples have already formed many products, resulting in a closed and dense matrix with low transfer properties. On the other hand, the sample cured for 7 days showed a significant formation of hydrated reactive products when exposed to a CO2-rich environment. Since the 7-day period allows for the formation of fewer hydrated products compared to the 28-day period, it is reasonable to conclude that the 7-day samples had more space between the compounds in the cement matrix, indicating higher porosity and permeability, which favors the penetration of CO2 into the sample.
3.9. Case Study 9
Costa et al. [46] conducted an additional set of tests to investigate the impact of carbonation on Class G cement when exposed to scCO2-saturated water and to scCO2 medium alone (wet scCO2). The dimensions of the sample were the same as those used in their previous studies [43,44]. The specimens were cured for 24 h at 70 °C and atmospheric conditions before being placed in the autoclave. The setup used in their studies is shown in Figure 12. The samples were subjected to the CO2-rich environment for 30 days at a temperature of 70 °C and a pressure of 13.78 MPa (2000 psi). After the carbonation period, the samples underwent phenolphthalein testing, as well as XRD and TGA analysis. It was found that the sample exposed to scCO2-saturated water experienced 35% more attack by CO2 as compared to the samples present in the scCO2 medium alone. Whereas XRD analysis revealed that the amount of CaCO3 in the samples exposed to the saturated medium was about 48.07%, while the samples in the scCO2 had only 14.08% of CaCO3. The differences in observed behaviors can be explained by the presence and accessibility of water and CO2 molecules. These components are essential for generating the acidic conditions required for the chemical reactions to occur. The upper section of the autoclave was rich in scCO2 but had a limited availability of H2O molecules. In contrast, the lower section had a high availability of H2O and a greater concentration of dissolved CO2 molecules, resulting in a more aggressive saturated environment.
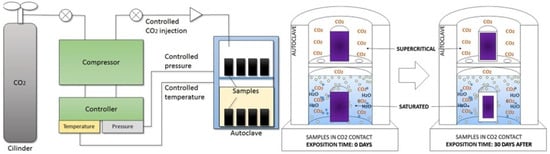
Figure 12.
Testing schematic used for the carbonation testing by Costa et al. [46].
3.10. Case Study 10
Xu et al. [47] performed an experimental study in which the Class G cement was modified by adding amorphous nano silica latex (ANL) to improve its resistance against CO2. ANL consisted of 1:3 mass ratio of amorphous nano silica to latex. The addition of ANL made in the cement consisted of 2, 4, 6, and 8 wt%. However, it was reported that adding 8% ANL to the cement increases its viscosity, so the amount of ANL added should be kept below 8%. The W/S ratio used for the mixing of the cement was 0.44. After the preparation, the cement slurry was transferred into cylindrical molds with dimensions of 25.44 mm in diameter and 30 mm in height. The samples were initially cured in water within an HPHT vessel for 15 days at 90 °C and 20 MPa. The specimens were then placed in a 4 M NaCl solution in the carbonation vessel for 90 days at the same temperature (90 °C) and pressure (20 MPa) conditions. The carbonation vessel used in this study is presented in Figure 13. After the carbonation period, the samples were subjected to several tests: Energy Dispersive Spectroscopy (EDS) to measure carbonation depth, a phenolphthalein indicator test, compressive strength testing, gas permeability testing to determine permeability, and Scanning Electron Microscopy (SEM) for microstructure analysis. It was found that the cement composite containing 6% ANL had an initial permeability of approximately one-seventh of that of the control sample. Following the carbonation period, it was recorded that the permeability and carbonation depth of Class G cement were 14.7 and 15.7 times more than that of the cement incorporating 6% ANL in its composition. Because ANL had the capacity to initiate a pozzolanic reaction, converting portlandite into a long chain of C-S-H with a low Ca/S ratio. This reaction leads to decreased permeability, enhanced compressive strength, a reduced CO2 diffusion rate, and a lower portlandite content, which in turn decreases the pH of the cement. However, this study did not provide specific details on the mixing process of ANL with cement. In contrast, the detailed procedures for mixing cement with nano silica (NS) for CCS wells are thoroughly explained in the studies conducted by Abid et al. [48].
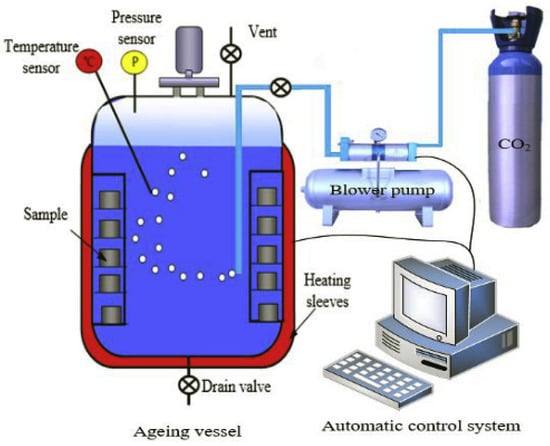
Figure 13.
HPHT carbonation vessel used by Xu et al. [47].
3.11. Case Study 11
The study carried out by Mahmoud and Elkatatny, [49] investigated the mechanical strength retrogression of the cement sample incorporating different quantities of synthetic polypropylene fiber (PPF) after being exposed to the scCO2 environment. The mechanical strength assessed in this study included both compressive and tensile strength. The base/control sample consisted of Saudi Class G cement mixed with silica flour, an expandable agent, a friction reducer, and deionized water. For the PPF cement composite, 0.125% (PPF 1), 0.25% (PPF 2), and 0.375 wt% (PPF 3) of PPF were added to the control sample. The incorporation of silica flour into the cement slurries was a critical aspect of this study, particularly given the high-temperature environments that the cement samples would encounter during testing. This inclusion was based on a study by Mahmoud and Elkatatny [50], which demonstrated that adding silica flour to cement significantly improves its resistance to strength degradation in high-temperature environments. Strength retrogression is a phenomenon where cement loses strength and durability when exposed to elevated temperatures over time, typically due to the breakdown of certain hydration products. Silica flour counteracts this effect by reacting with Portlandite and forming additional C-S-H phases. These C-S-H phases exhibit greater stability at high temperatures than the original cement hydration products, thereby maintaining or even improving the cement’s strength and durability under these challenging conditions. The cement slurry was prepared according to API standards, after which it was poured into different mold sizes depending on the targeted test. Table 3 presents the dimensions of the samples utilized in this investigation. The initial curing of the samples was carried out in 0.5 M NaCl solution at 130 °C for 24 h. XRD analysis was performed after the initial curing to analyze the quartz and CH content in the samples. The results revealed a lower concentration of CH and quartz in PF 1 compared to PPF 0. This indicated that in the 0.125% PPF cement composite, CH and quartz were converted into more stable C-S-H. Therefore, it was expected that the PPF 1 would perform better when subjected to the CO2-rich environment. As in this sample, the amount of CH is lower, making it less susceptible to CO2 attack. Additionally, it will have a more stable C-S-H in its cement matrix. On the other hand, it was observed that incorporating more than 0.125% of PPF into the cement led to an increase in the amount of quartz and CH. Therefore, it was anticipated that the PPF 2 and 3 samples would not perform well under carbonation conditions. For the carbonation test, the specimens were placed in an HPHT vessel (Figure 14) containing a 0.5 M NaCl solution for ten and twenty days while maintaining the temperature and pressure of 130 °C and 10 MPa, respectively. The carbonation depth testing showed that the ingression of CO2 increased with the increase in the carbonation days. The carbonation depths in the base sample were about 2586 μm after 10 days and 3425 μm after 20 days of reaction. In the case of PPF 1, the carbonation depths were reduced to 1588 μm after 10 days of reaction and 2355 μm after 20 days of exposure. PPF 1 showed the lowest carbonation front among all the samples. The carbonation rate for the PPF 1 sample was calculated to be 118 μm/Day after 20 days of exposure, whereas the control sample showed a rate of about 171 μm/Day. The samples containing PPF showed a 34% higher compressive strength than the base cement after a 10-day period. This improvement became even more significant after 20 days, with the PPF samples showing a 50.6% higher compressive strength than the control cement. A similar pattern was noted in tensile strength, where the neat sample displayed tensile strengths of approximately 5.75 MPa and 5.03 MPa after 10 and 20 days of exposure, respectively. In contrast, PPF1 had tensile strengths of about 6.86 MPa and 6.41 MPa over the same time periods. It must be noted that the degradation of mechanical properties of PPF 2 and 3 was more than PPF 1. The lowest permeability was also observed from the PPF 1, and when compared with the control sample, it was about 25 and 35.1% less after 10 and 20 days of reaction with the scCO2-saturated brine solution. These results indicate that adding 0.125 wt% PPF to the cement enhances its resistance to carbonation-induced degradation and retains its mechanical and transfer properties.

Table 3.
Method and dimension of the sample used in the post-carbonation phase Mahmoud and Elkatatny [49].
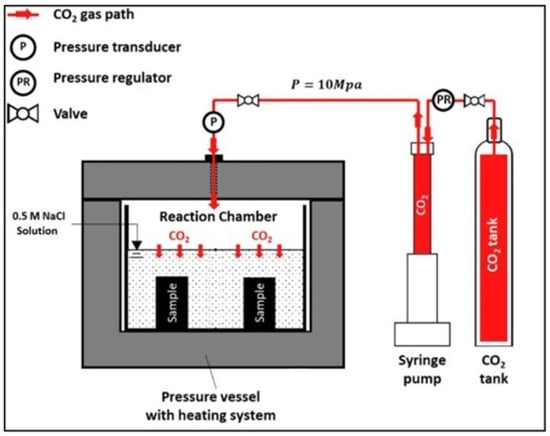
Figure 14.
HPHT vessel used for the studies carried out by Mahmoud and Elkatatny [49].
3.12. Case Study 12
In their second set of studies, Mahmoud and Elkatatny, [51] examined the impact of olive waste on Class G cement in scCO2-saturated brine environment. Olive waste, a natural organic byproduct generated during the processing of olives for olive oil production, was used in this study without any preprocessing. Its mineralogical composition comprised 12.3% quartz, 18.5% tridymite, and 69.2% sylvite. The base cement consisted of Saudi Class G with 35 wt% of silica flour, and different quantities of defoamer, expandable, dispersion, and fluid loss control agent. The control sample was named OW 0. Meanwhile, 0.1, 0.2, and 0.3 wt% of olive waste were added to the base sample and were titled OW 1, OW 2, and OW 3, respectively. The preparation of the samples followed API standards, with a W/C ratio of 0.44 being employed in the mixing process. They were prepared in various sizes depending on the testing target, as detailed in Table 1. For the initial curing, the molds were placed in the deionized water at 75 °C and ambient pressure for 24 h. After this, an XRD analysis was performed, and it was found that no sufficient decrease in CH was noticed in the olive waste cement composite as compared to the base cement. This minimal change could be attributed to the relatively small quantity of olive waste incorporated into the cement mixture. Hence, it was believed that these olive waste cement composites might not perform well when subjected to a CO2-enriched environment. Following the initial curing, the specimens were placed in deionized water within an HPHT vessel and carbonated for 10 and 20 days while maintaining a pressure of 10 MPa and a temperature of 130 °C. Figure 15 illustrates the experimental setup used for the carbonation testing. The micro-CT scan technique was employed to measure the progression of the carbonation front. The cylindrical sample used for this testing had dimensions of 38.1 × 101.6 mm. For sample OW 0, the carbonation depths after 10 and 20 days were 1469 μm and 2362 μm, respectively. These depths were reduced to 1269 μm and 1913 μm for the sample containing 0.1 wt.% OW in its composition. The carbonation depth of OW 1 was the lowest among all the samples tested. The compressive strength of the control sample was approximately 105.8 MPa after 10 days of carbonation and 100.8 MPa after 20 days. In contrast, the OW cement composite had a slightly lower compressive strength, with OW 1 showing 104.9 MPa after 10 days and 100.2 MPa after 20 days of carbonation. A similar pattern was noted in the tensile strength measurements. After 20 days of immersion in CO2-saturated brine, the tensile strengths for samples OW 1, OW 2, and OW 3 were 4.47 MPa, 4.46 MPa, and 4.20 MPa, respectively. When compared to the base cement (OW 0), these values indicate reductions of 2.6%, 2.8%, and 8.5% in tensile strength. The lower performance of the OW samples can be attributed to two main factors. First, as highlighted by Alkheder, et al. [52] impurities present in olive waste can interfere with the cement’s ability to develop mechanical strength. Second, the quantity of OW added to the cement may not have been sufficient to make a significant impact. In terms of permeability, measurements showed that after 10 and 20 days of exposure, sample OW1 had 14.3% and 33.9% lower permeability, respectively, compared to the control sample. This reduced permeability contributed to the lower carbonation depth in OW 1, as it slowed the leaching of CaCO3 from the cement matrix.
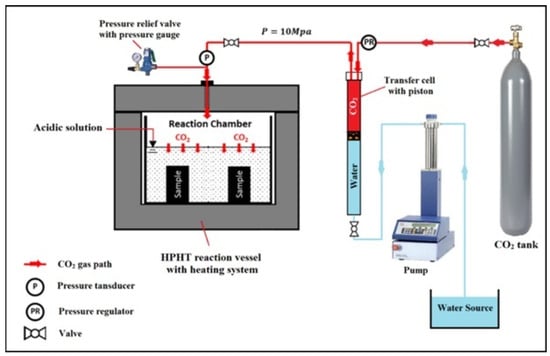
Figure 15.
Carbonation setup used for the studies of olive waste cement composite conducted by [51].
3.13. Case Study 13
Ledesma, et al. [53] analyzed the impact of pozzolanic material on Class G cement in the simulated CCS environment. The cement was replaced with 5% and 10% (by weight of cement) pozzolanic materials, which included zeolite and fly ash (FA). The zeolite was commercially synthetic in nature and was of two types (4A-1 and 4A-2), while the FA was sourced from a coal-fired plant. The cement slurries were prepared according to the API standard 10A. It must be noted that pozzolanic material and cement were mixed in the dry state for 1 h before mixing with the cement. After the slurry was prepared, it was poured into the cylindrical mold, with dimensions of height = 46 mm and diameter = 23 mm. The initial curing of the cement was performed by placing the molds in a water-filled high-pressure vessel at a temperature of 60 °C and a pressure of 6 MPa for 8 h. Following the initial curing, the cement samples were submerged in deionized water inside a 1 L stainless steel high-pressure vessel, as shown in Figure 16. The CO2 degradation experiments were performed under 15 MPa pressure and 90 °C temperature conditions. These parameters were chosen to replicate the environment typically found at a depth of roughly 1.5 km within a wellbore. In such settings, the pressure increases following a gradient of approximately 10 MPa for every kilometer of depth. The samples were subjected to a CO2-rich environment for 7 and 14 days. The test was conducted in the static condition (no flow) to simulate an environment where the well is at a distance from the injection well or after the CO2 injection period had ended. Upon completion of the degradation period, the pressure was gradually released, after which different experiments were conducted on the specimen. The post-carbonation test consisted of XRD, compressive strength, and SEM. The XRD and SEM analyses revealed that the pozzolanic cement reacted similarly to conventional oil well cement. The reaction mechanism comprised several distinct regions: an area depleted of portlandite, a zone of increased porosity, and regions of carbonation and bi-carbonation. Nevertheless, standard cement appears to have a denser carbonated area compared to pozzolanic cement (FA or zeolite). Moreover, the properties of pozzolans, including particle size, morphology, chemical composition, morphology, and specific surface area, affect the characteristics of cement paste and its resistance to CO2 attack. Overall, the 4A-1 zeolite demonstrated better resistance to CO2 attack than the 4A-2 zeolite. Samples with 5 and 10% FA exposed for seven days of carbonation showed reduced thickness of the chemically altered layer. However, with an exposure time of 14 days, 10% FA cement composite showed a significant increase in the carbonated layer thickness. It was concluded that 14 days is insufficient for the carbonation test, as the reaction between the CO2 and cement is still ongoing, and a more extended exposure period is needed to fully observe the extent of cement degradation.
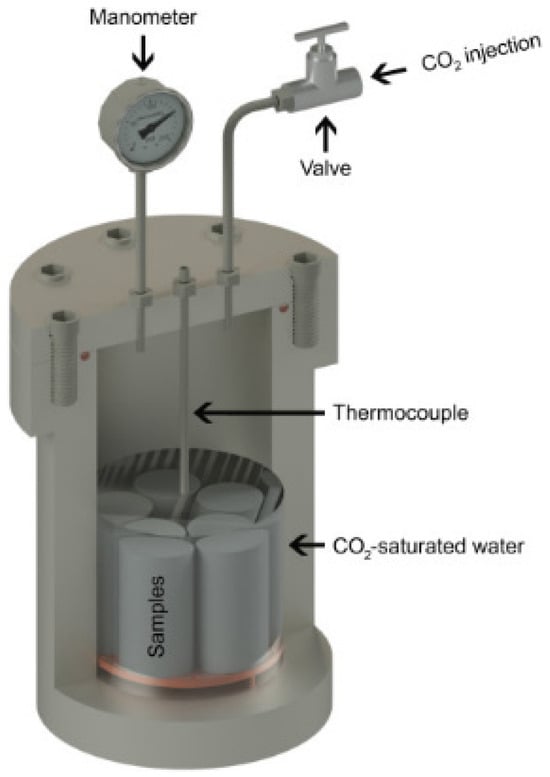
Figure 16.
High-pressure vessel used for the studies by Ledesma, et al. [53].
3.14. Case Study 14
Another study on the effect of pozzolanic materials such as nano-silica (NS), Rice Husk Ash (RHA), and Palm Oil Fuel Ash (POFA) in cement was conducted by Abid, et al. [54]. In this study, the cement was replaced by different wt% of POFA (5, 10, 15, and 20%), RHA (1, 2, 3, 4, and 5%), and NS (0.25, 0.5, 1, and 1.5%). The sample preparation process adhered to the guidelines outlined in API Standard 10A. However, the API standard does not specify the mixing process for additives such as POFA and RHA. Therefore, the additives and cement were thoroughly hand-mixed for 5 min before starting the cement mixing process. The sample preparation for the NS cement composite followed the steps presented in [48]. The slurry was then transferred in a cylindrical mold with a length-to-height ratio of 1 (25.4 × 25.4 mm). The initial curing of cement was carried out for 28 days in the water bath at 50 °C and atmospheric pressure. Afterward, pre-carbonation tests were conducted to determine which concentration of cement composite of NS, POFA, and RHA performed better with respect to physical, rheological, and mechanical properties. These tests aimed to evaluate and select the cement samples with the best performance in each additive category. From this experiment, it was found that 5% POFA, 3% RHA, and 0.5 and 0.75% NS performed better and were exposed to the carbonation test along with the neat Class G sample in the static reactor, whose schematic and image are presented in Figure 17. This percentage of the pozzolanic material also corresponds to the saturation point, which means that above this quantity, the properties of cement, instead of becoming better, will start to deteriorate. The carbonation test was carried out with fresh water to accelerate the reaction of CO2 with the cement. The pressure and temperature were maintained at 20.68 MPa (3000 psi) and 65 °C for 40 days. Subsequently, post-carbonation tests were conducted on the carbonated sample, consisting of TGA, compressive strength, mass evaluation, and phenolphthalein tests. The post and pre-carbonation test results were compared to analyze the extent of cement degradation. It was noted that all the specimens went through carbonation; however, their performance was better than that of the neat cement. It was concluded that using POFA and RHA as partial cement replacements initially slows down compressive strength development (act as a retarded) but can ultimately improve strength over time. Nonetheless, agricultural waste cement composite did not perform well when exposed to a scCO2 environment as they had lower compressive strength after the CO2 exposure, more carbonated area, lowest amount of portlandite left in its composition due to the carbonation process, and also showed the highest percentage increase in mass distribution (comparison based on the pre and post carbonation test) that points towards the initiation of the bi-carbonation process. Conversely, nano silica (particularly in 0.5% and 0.75% cement composites) improved the strength of the cement upon replacement, showed a smaller carbonated area, had a lower percentage increase in mass, and enhanced overall cement performance under the simulated CCS site conditions.
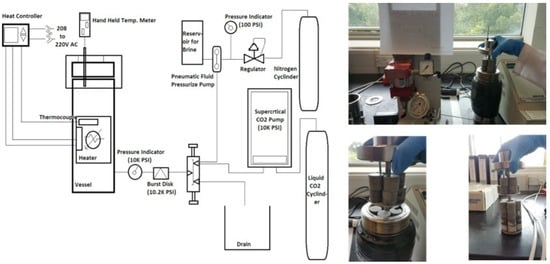
Figure 17.
Schematic and picture of the static reactor used by the studies carried out by Abid, et al. [54] and Tiong, et al. [55].
3.15. Case Study 15
Tiong et al. [55] investigated the use of Nano Glass Flakes (NGF) and Multi-Wall Carbon Nanotubes (MWCNT) as partial replacements for cement in order to improve its durability in scCO2 environments, which are common in CCS wells. It is important to note that the dispersion of nanoparticles is a crucial step in the mixing of the cement-containing nanoparticles. To achieve proper dispersion of the nanomaterials (NGF and MWNCT), the author utilized an ultrasonication device operating at 20 kHz and 500 watts. The sonication process was conducted at 50% amplitude for 15 min, ensuring uniform dispersion of the nanomaterials in the mixed water. This step is crucial for improving the distribution of the nanomaterials throughout the cement matrix. The cement was then mixed with the water-containing nanoparticles per API standards 10A and 10B. The initial curing of the sample was carried out for one day in a water bath at a temperature of 50 °C and atmospheric pressure. Different concentrations of nanoparticles were evaluated to identify the optimal level for improving cement composites in CCS well applications. Following a pre-carbonation test like that conducted by Abid et al. [54], the optimal concentrations were identified as 0.1 wt% and 0.005 wt% for MWCNTs, and 1 wt% and 0.5 wt% for NGF. The chosen cement samples were subsequently subjected to carbonation testing. These tests were conducted for 28 and 56 days under conditions of 70 °C and a pressure of 24.13 MPa (3500 psi), simulating the scCO2 environment. The carbonation static chamber used for this study was the same as that used by Abid et al. [54]. The phenolphthalein test conducted after 56 days of exposure revealed that the cement composite with 0.5 wt% NGF and 0.05 wt% MWCNTs showed the lowest carbonation levels. It was also noted that the cement samples with properly dispersed nanoparticles, achieved through ultrasonication, performed significantly better in the scCO2 environment than those mixed using conventional methods. Proper dispersion of nanoparticles ensured a more uniform distribution within the cement matrix, leading to enhanced resistance against carbonation and improved durability. In contrast, the samples mixed in the conventional way exhibited inferior performance, likely due to uneven nanoparticle distribution, which negatively affected the cement’s ability to withstand the harsh scCO2 conditions. These findings were corroborated by porosity and XRD test results, which showed that the 0.5 wt% NGF and 0.05 wt% MWCNTs samples had the lowest porosity and contained the least amount of aragonite, a polymorph of calcium carbonate (CaCO3), indicating less carbonation. The compressive strength of the samples at the post-carbonation stage was lower than in the pre-carbonation phase, suggesting that total carbonation had not occurred. If the samples had fully carbonated, their post-carbonation compressive strength would have been higher than in the pre-carbonation phase. Additionally, the authors concluded that NGFs are a more viable option than MWCNTs due to their significantly lower cost—50 USD/kg for NGFs compared to 700 USD/kg for MWCNTs—making NGFs a more cost-effective solution for enhancing cement composites in CCS well environments.
3.16. Case Study 16
Another study on the inclusion of nanoparticles in cement was investigated by Batista, et al. [56] for enhancing its performance against CO2. The nanoparticles used in the study consisted of nano-silica (NS), added in different quantities (0.5%, 1%, 1.5%, and 3 wt%). Class G cement was used as the control sample. The cement mixing was conducted according to API 10A and 10B standards. Due to the large surface area of NS and its influence on the cement’s rheological characteristics, a W/S ratio of 0.44 was utilized instead of the conventional water-to-binder ratio. Polymeric cylindrical molds were used with dimensions of 21.6 × 43.2 mm. The initial curing of the sample was conducted in a pressure vessel for 8 h at a temperature of 60 °C and a nitrogen pressure of 6 MPa (60 bars). The specimens were then subjected to a carbonation test. They were fully immersed in Milli-Q water within a high-capacity, 1 L pressure chamber constructed from AISI 316 stainless steel (Figure 18). The carbonation test lasted for 7 and 56 days, with the pressure and temperature in the pressure vessel maintained at 15 MPa (150 bars) and 90 °C, respectively. It is important to note that 99.99% pure CO2 was used to pressurize the HPHT vessel. To analyze the affected layer of the cement after exposure to scCO2-saturated water, techniques such as SEM, X-ray microtomography, EDS by line scan, and atomic force microscopy (AFM) were utilized. Additionally, compressive strength and Vickers microhardness tests were performed to evaluate the mechanical properties of the carbonated samples. It was found that the inclusion of NS in the cement significantly affected its rheological properties while having minimal impact on the density of the hardened cement. This observation aligns with the findings of Abid et al. [48]. The study found that all samples underwent the carbonation process, forming distinct carbonation zones. After seven days of carbonation, the Class G cement with 1.5% NS showed the best performance, with a chemically altered layer of 2.63 mm, compared to 3.06 mm for neat Class G cement. For longer exposure periods, the 0.5% NS cement composite performed better than other samples. This concentration of NS in cement has also been reported by Abid, et al. [54] to have shown good resistance in scCO2-rich environments. After 56 days of curing, the thickness of the altered layer for the 0.5% NS cement composite was 7.68 mm, while other samples had higher values, with the highest thickness observed in the sample containing 1% NS. The compressive strength of all NS cement composites after 56 days of exposure was greater than that of the control sample. Moreover, from the AFM image, microtomography, and SEM, it was found that changes in microstructure, density, and chemical composition from the edge to the core of the sample were less accentuated in the NS cement composite as compared to the control sample. The better performance of NS cement composite in the CO2-rich environment was likely due to the pozzolanic effect, which is enhanced by the large surface area of the NS particles. This effect decreases the quantity and size of portlandite crystals, resulting in a dense microstructure and improved properties of the cementitious matrix.
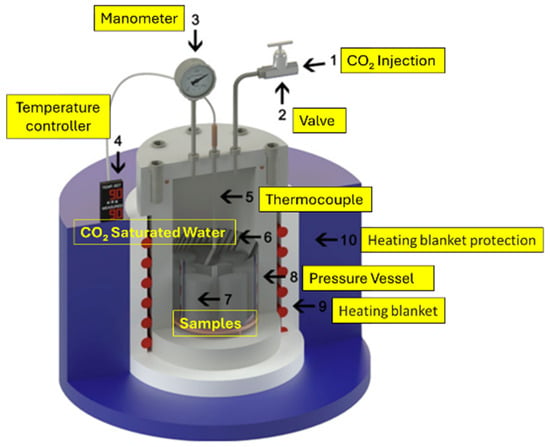
Figure 18.
Pressure vessel used by Batista, et al. [56] for their carbonation tests.
3.17. Case Study 17
Ponzi, et al. [57] explored the addition of supplementary cementing material (SCM) (Basalt powder (BP)) to class G cement for the CCS wells. BP provides limited pozzolanic reaction, has large inert friction, and has smaller particle sizes than Class G cement. The quantity of BP added to the cement consisted of 0.25, 0.50, 1, 2.5, and 5 wt%. API standards 10A and 10B were utilized for the mixing of the cement. The W/S ratio for the neat cement was maintained at 0.44, while the ratio changed with the increase in the solid content of Basalt in the cement, going to 0.419 for Class G + 5% BP. Once the cement slurry was mixed, it was transferred into cylindrical molds made of PVC. These molds were then submerged in a water bath for a curing period of 14 days. The curing conditions were maintained at a constant temperature of 65 °C and atmospheric pressure. Subsequent to the initial curing, the samples were cut into the dimensions of 22 × 44 mm to have a ratio of 1:2. Some of the samples, which were not intended for CO2 exposure, were immersed in a Ca(OH)2 solution. The remaining specimens were subjected to an HPHT environment using a static vessel (as shown in Figure 19). These samples were exposed to deionized water saturated with scCO2 for a period of 7 days. The carbonation experimental conditions were maintained at a temperature of 65 °C and a pressure of 15 MPa. The experiments conducted in the post-carbonation stage to analyze the carbonated and non-carbonated cement consisted of XRD, SEM-EDS, compressive strength test, phenolphthalein test, carbonated layer analysis through image analysis, and Micro CT. From the XRD analysis of Basalt, it was observed that only 9.9 wt% of SiO2 is present in its composition, due to which the pozzolanic reaction with the cement is not as intensive as it was in the case of [47,53,54]. It was found from the testing of the carbonated samples that the cement composite that incorporated ≤ 0.5% of Basalt and had a higher water-to-solid ratio performed well when exposed to the scCO2 environment. On the contrary, the sample that incorporated more than 0.5 wt% of BP suffered more degradation as the transfer properties were high, due to which the resistance against the CO2 was compromised, leading to the degradation of the compressive strength. The lower content of BP acts as the filler within the cement matrix, which reduces the transfer properties, restricts the ingression of CO2, and delays the progress of the carbonation front. Additionally, there is minimal reduction in the alkaline reserve of portlandite, which further strengthens the cement’s ability to resist scCO2 attack. However, a comprehensive set of tests must be conducted to ensure the viability of BP for large-scale field applications. These evaluations should focus on measuring the cement mixture’s free fluid content, thickening time, rheological behavior, and fluid loss characteristics. Moreover, the necessity for supplementary additives such as retardants, anti-foam agents, and dispersants must be assessed to ensure that the new formulations comply with the diverse specifications required for CCS projects.
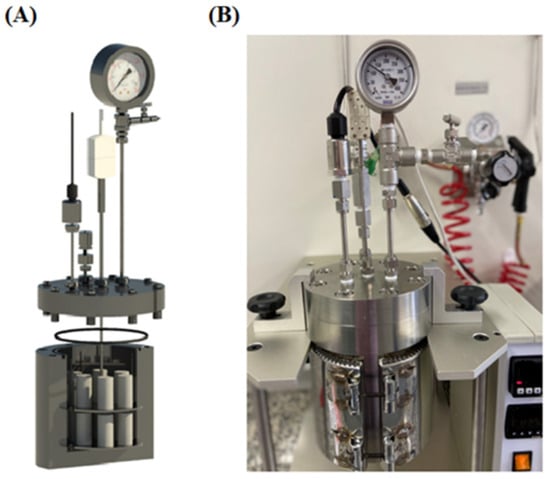
Figure 19.
HPHT static reactor used for the carbonation testing for the studies carried out by Ponzi, et al. [57]. (A) Cross-sectional veiw (B) Full setup of the carbonation vessel.
3.18. Case Study 18
As seen from the above studies, cement is subject to degradation when exposed to a CO2-rich environment. Therefore, Jani and Imqam [58] performed experimental studies comparing the carbonation resistance of two cement types. They examined a geopolymer cement made from Class C FA and alkali activation, and compared its performance under carbonation conditions with that of conventional Class H cement. The preparation of Class H cement followed API guidelines, while the geopolymer preparation adhered to the methods presented by Ahdaya and Imqam [59]. Following the preparation of the cement mixture, the resulting slurry was transferred into cylindrical molds measuring 50.8 mm in both diameter and height. These samples were then subjected to an initial curing process in a water bath for a period of 3 days. The curing conditions were maintained at a temperature of 43.3 °C under atmospheric pressure. Post-initial curing, the specimens were transferred to a custom-built apparatus designed specifically for carbonation testing that consisted of a syringe pump, two accumulators, and a CO2 cylinder. Figure 20 illustrates the schematic of this setup. The syringe pump was used to raise the CO2 pressure to the desired level while a pressure gauge monitored changes in pressure. Additionally, a pressure relief valve was installed to release CO2 pressure either after the experiment or in an emergency. The study conducted 12 experiments in total, with 6 at 3.45 MPa (500 psi) and the remaining at 10.34 MPa (1500 psi), all maintained at 43.3 °C for periods of 3, 7, and 14 days. The specimens were placed inside accumulator 2, which was filled with fresh water to accelerate the carbonation reaction, as CO2 solubility is higher in freshwater than in saline water [60]. These conditions mimicked two scenarios: 3.45 MPa and 43.3 °C to replicate the gas behavior of CO2, and 10.34 MPa and 43.3 °C to put CO2 into the supercritical state. In both scenarios, the CO2 dissolved in the water and reacted with the geopolymer and neat Class H cement. The water’s pH was measured before and after the carbonation test to record any change. Moreover, visual inspection, density change, and compressive strength were also made on the samples. It was found that the Class H compressive strength continued to decrease with an increased number of exposure days and reduced to about 27.77% and 41.54% for gaseous and supercritical states, respectively, at the 14-day mark. In contrast, geopolymer samples did not show any retrogression in compressive strength for 3, 7, or 14 days in the gaseous phase. In the supercritical state, a small reduction was noted which was about 8.8% at 7 days and 12.06% at 14 days. Visual inspection revealed millimeter-sized CaCO3 crystals on the surface of Class H cement, while no changes were observed on the geopolymer surface. Density remained unchanged for cement or geopolymer after 14 days of CO2 exposure. However, the pH of the water decreased after CO2 injection in the accumulator 2. From their studies, it was concluded that the carbonation problem faced by cement could be mitigated using geopolymer cement as it does not contain any hydration components that are susceptible to CO2 attack.
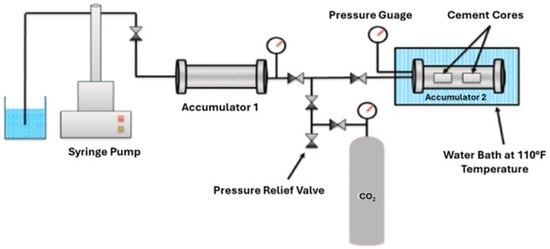
Figure 20.
Carbonation testing setup utilized by Jani and Imqam [58].
3.19. Case Study 19
Costa et al. [61] indicated that the physicochemical transformations of cement compromise its integrity when placed in a CO2 environment. In that regard, the cement must be properly designed to resist the attack of CO2. Therefore, they conducted a carbonation test on the cement with the different formulations and densities, i.e., 15.8, 16.5, and 17 ppg cement. The formulation starts with simple additives, and the composition becomes more complex at the end. Class G was utilized as the base cement in this formulation, and the mixing was conducted according to the API standard. The formulations were named Form A, B, and C. Additives used for the creation of different formulations are presented in Table 4. After the cement preparation, the slurry was transferred into the 203 mm long and 25 mm ID cylindrical mold and was placed in the water bath for 24 h at atmospheric pressure and 70 °C. After initial curing, the cement was removed from the mold and cut into four equal parts, each length of 50 mm. The specimens were then submerged in CO2-saturated water in the autoclave for 30, 60, and 90 days at a temperature of 70 °C and a pressure of 13.78 MPa. The autoclave used in the research had the same configuration as in their previous studies studies [43,44]. The testing conducted at the post-carbonation stage consisted of a sedimentation test, carbonation depth, and area calculation through image analysis. The flow chart of the methodology used in this study is shown in Figure 21. It was found that Form A, which was made with the simplest formulation, performed well in the CO2-rich environment but had low stability toward the sedimentation. Form B showed high sedimentation due to the addition of a dispersant, while Form C, which included both a dispersant and a fluid loss additive, demonstrated improved stability. All the samples went through carbonation, which increased over time. Nonetheless, the sample with complex additive addition, i.e., Form C, showed a stable carbonation depth for its high densities, i.e., 16.5 and 17 ppg, as these samples showed small variations in the carbonated area over different time periods. The study concluded that optimizing cement slurry with various additives and high density is beneficial for the cement subjected to CO2 exposure.

Table 4.
Cement formulation with different densities used for the carbonation test by Costa, et, al. [61].
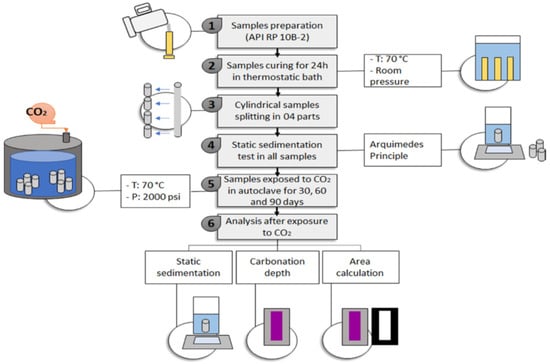
Figure 21.
Methodology used for the testing of different formulations of cement by Costa, et al. [61].
3.20. Case Study 20
Gu et al. [62] examined the impact of carbonation on the pore structure of cement. For the analysis of such a system, SEM-BSE was utilized along with the nontoxic low melting point metal intrusion. The sample used in this research consisted of Class G cement, in which 10 wt% of silica fume was added. The W/C ratio was maintained at 0.44, and the cement mixing was conducted according to the API guidelines. After the preparation of the sample, the slurry was poured into the 25 × 25 mm cylindrical mold and was transferred in an autoclave for initial curing at 20 MPa and 60 °C for 28 days. The selected curing conditions were designed to replicate the subsurface conditions found at the Shenhua CCS site in China. Subsequently, the specimens were subjected to humid scCO2 in the HPHT static kettle (Figure 22), where the temperature and pressure were maintained at 20 MPa and 60 °C. The duration of the exposure was 10 and 20 days. After exposure, the specimens were cut into 5 × 5 × 5 mm dimensions using a diamond blade saw and then placed in a vacuum at 60 °C for one week to remove liquid from the pore spaces. BSE-SEM analysis with metal intrusion was then performed. It was found that for cement exposed for 10 days, the primary altered layers observed from the surface to the core consisted of: (a). carbonated layer, (b). portlandite-dissolved layer, and (c). partially leached layer. After 20 days of exposure, the key altered layers included a partially leached zone, a porous leached area, a carbonated transition zone, and a fully carbonated region. The novelty of the study was that the partially leached and carbonation transition layers were not presented in the previous studies. It was found that in the partially leached, carbonate, and carbonated transition layers, a reduction in the porous area fraction (PAF) was observed, along with a decrease in large pores. However, in the CH dissolved and porous leached layers, an increase in the large pore, along with the increment of PAF, was noted. Therefore, it was concluded that the corrosion direction parallel to the flow can be effectively blocked. On the other hand, the porous leached layer can facilitate fluid flow in a direction perpendicular to the main corrosion pathway.
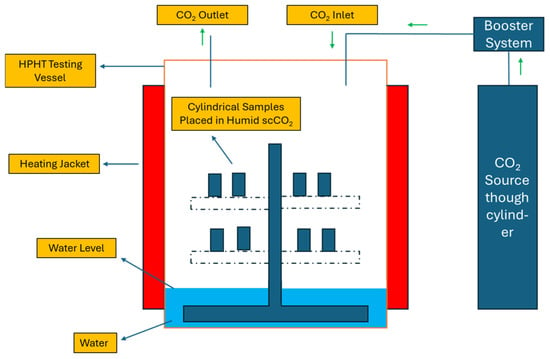
Figure 22.
HPHT testing cattle used for the pore characterization studies carried out by Gu, et al. [62]. The schematic picture has been modified.
3.21. Case Study 21
The study conducted by Moraes and Costa [63] demonstrated the impact of organo-modified montmorillonite (OMMT) nanoclay on cement’s resistance to carbonation when added to the mix. The OMMT quantity used to replace cement consisted of 0.5, 1, and 2 wt%. Class G was used as the control cement in this study. The preparation of the cement sample followed the procedure established by the API. However, as OMMT is hydrophobic in nature, the mixing of it with the cement was conducted in such a way that it was vigorously stirred for 3 h at 1000 RPM in the mixed water so that it would be properly dispersed, after which the cement was added. The cement slurry was then transferred into a polymeric mold having the dimensions of h = 46 mm and dia = 23 mm. The cement sample was then placed for curing in the pressure vessel for 8 h with the pressure and temperature of 6 MPa and 60 °C. Nitrogen gas was used to pressurize the vessel in the initial curing stage. After that, the specimens were quickly transferred to a static reactor for carbonation testing, conducted under conditions of 15 MPa pressure and 90 °C temperature. The samples were subjected to these conditions for varying durations of 7, 21, and 56 days. The static reactor contained two portions. The samples placed in the lower section were subjected to scCO2-saturated water (HSC), while the samples placed in the upper section were solely exposed to supercritical CO2, which was referred to as wet scCO2 (WSC). The image of the static reactor employed in this research is presented in Figure 23. The reactor was pressurized with the help of 99.9% of CO2. The analysis of the cement degradation with or without OMMT was conducted by determining the depth of the carbonation front, employing pycnometry, conducting SEM, performing XRD, and utilizing X-ray tomography. Moreover, the mechanical properties were determined through uniaxial compressive strength (UCS) and Vicker microhardness. Testing revealed that the inclusion of OMMT in the cement reduced its workability while increasing its specific mass. Cement with 1% and 2% OMMT showed a greater depth of carbonation front, even exceeding that of the control sample. However, the 0.5% OMMT inclusion resulted in the least carbonation depth among all the samples. It was also noted that the OMMT cement composite maintained its compressive strength after the carbonation test, which was attributed to the pozzolanic reaction that the nano clay facilitated in the cement. It was also reported that the degradation of the samples placed in HSC was more than those exposed to WSC. Therefore, this study indicates that incorporating OMMT into the cement composition can reduce the excessive precipitation of CaCO3, thereby influencing the diffusion rate of CO2 within the cement matrix.
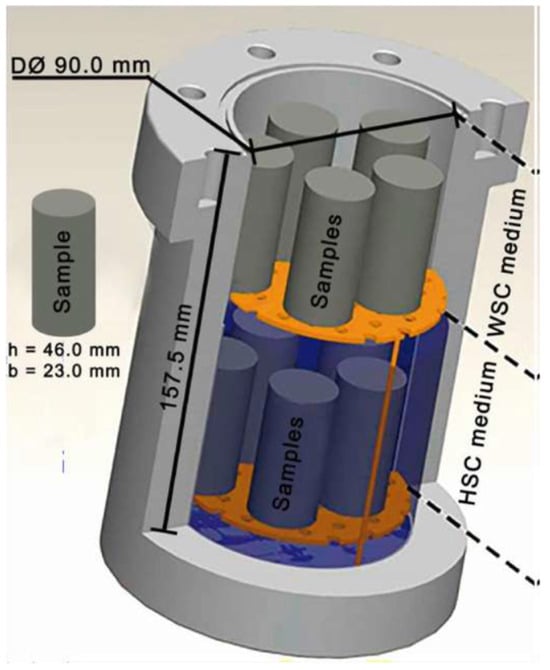
Figure 23.
Static reactor with two different compartments used for the studies of Moraes and Costa [63].
3.22. Case Study 22
Mei, et al. [64] studied the effect of treated and non-treated Ca-montmorillonite (CA MMT) in cement and observed its performance in a CO2-rich environment. Ca-montmorillonite is a natural and popular material and has been used for different applications where its modification is conducted through an inorganic and organic agent because of its multilayer structure and accessibility. If the unmodified Ca MMT is used in cement, it will capture the free water and impact the rheology of the cement. On the other hand, if the Ca MMT has been treated, it can reduce the tendency of Ca MMT to capture free fluid and avoid the degradation of the fluidity of the cement. For this study, the Ca MMT powder used for the cement was modified by immersing it in the scCO2 at pressure and temperature of 8 MPa and 60 °C for 24 h. Subsequently, the powder was dried in the oven to stabilize its structure and composition. They named modified Ca MMT as MC Ca-MMT while the non-treated was given the tag of Ca-MMT. The ratio of cement to the added Ca-MMT/MC Ca-MMT was 2:1. For the comparison purposes of these composite samples, a control sample was also prepared. The cement was mixed in accordance with the API standard. The initial cement curing was conducted at 1 wt% NaCl solution, with temperature and pressure of 60 °C and 17 MPa, respectively, for 14 days. Afterward, the specimens were subjected to carbonation testing by being submerged in a CO2-saturated brine solution at a partial CO2 pressure of 8 MPa and a temperature of 62 °C for 7, 14, and 28 days. Multiple dimensions of the specimens were used depending on the characterization test (morphological, compositional, and mechanical) to be conducted. Morphological testing was conducted using a transmission electron microscope (TEM) to examine the sample, while crystal structure analysis was performed using the selected area electron diffraction (SAED-TEM) technique. As the treatment of the Ca-MMT was carried out through scCO2, the absorption capacity was determined through the TGA. The composition of the sample was found through the XRD analysis. The mechanical properties were assessed according to API standard 10B, with compressive strength measurements conducted using a hydraulic press. The image analysis was performed using X-ray CT, where the scanning of the sample was conducted before and after the carbonation test. The results showed that scCO2 modifies Ca-MMT through two mechanisms: a physical process where scCO2 penetrates the layers, causing expansion and separation of agglomerated particles, and a chemical process where the interaction with calcium ions forms micro-calcite, reducing the chemical stability of Ca-MMT. When the CO2 ingresses in the MC-Ca-MMT cement composite, the already present micro calcite causes additional deposition of calcite, which will densify the cement structure and reduce the diffusion rate of CO2 in the cement matrix. This was backed by the CT image analysis of the chemically altered layer through a Probability Distribution Map (PDM). It was noted that after twenty-eight days of carbonation, the MC Ca-MMT had a smaller area of dissolution (22.94%) compared to the control (34.14%) and Ca-MMT cement composite (28.19%). The compressive strength analysis showed that both the control samples (CS) and the unmodified Ca-MMT (MT) cement composites continued to decrease over the 28-day CO2 exposure period, ultimately reaching compressive strengths of 16.12 MPa and 24.59 MPa, respectively. In contrast, the samples with modified Ca-MMT showed a different trend. Although the MC samples initially had lower compressive strength compared to the MT samples during the first week, by the end of the 28-day period, the MC28 samples achieved a compressive strength of 28.04 MPa. This indicates that the MC28 sample retained 72.43% of its initial compressive strength after twenty-eight days of carbon dioxide exposure, compared to 58.75% for the MT28 sample and 53.59% for the control sample. Based on the above finding, it was concluded that the modified Ca-MMT cement composite performed well in the CO2-rich environment as compared to MT and neat samples.
3.23. Case Study 23
Barría et al. [65] conducted a pyrochemical analysis to investigate the macroscale behavior of cement in a CCS environment. In that respect, the depth of chemically altered zones and the mechanical properties of the cement were assessed before and after the carbonation test. The pore size distribution (PSD) was determined with the help of a Mercury Intrusion Porositimeter (MIP), and compressive strength was measured by using a UCS machine. Whereas XRD was only performed on the carbonated sample. Beyond the experimental measurements, the authors conducted numerical modeling to explore how the mechanical and geochemical properties of the cement are altered under CO2 exposure. The cement used in the experimental phase was Class G cement, mixed with a W/C ratio of 0.44 in accordance with API standards. The cement slurry was then poured into the cylindrical molds with dimensions of 38 mm × 77 mm. The samples were initially cured for 24 h at 20 °C. Following this, they were extracted from the molds and immersed in lime-saturated water, where they remained for 28 days under ambient conditions. After the completion of the initial curing, the cement samples were put in wet scCO2 in the cylindrical reactor that had a dimension of 160 × 200 mm. The CO2 pressure was maintained at 20 MPa while the temperature was kept at 90 °C for a period of 30 days. It was found that the compressive strength and Young’s Modulus of the cement were reduced after exposure to CO2, and the precipitation of CaCO3 was also present. However, this trend was opposite to the common belief that the presence of calcium carbonate in the cement matrix reduces permeability and porosity while improving compressive strength due to the higher molar volume of CaCO3 compared to the portlandite it replaces. In this study, although the reduction in porosity was noted, the mechanical properties were compromised. The authors proposed that this could be attributed to the intrinsic amorphous nature of CaCO3, weak bonding between the carbonates and the pore walls, or significant degradation of C-S-H. This experimental result was backed by numerical modeling in which four zones were shown: (1). Degraded zone composed of silica gel, (2). Precipitation zone of CaCO3, (3). Ca(OH)2 depleted zone, and (4). Intact zone. The simulation results indicated that the cement’s mechanical properties would deteriorate due to the development of various bicarbonate layers.
3.24. Case Study 24
Peng, et al. [66] investigated the impact of an acidic environment, comprising H2S and CO2, on cement samples. To improve the resistance in the corrosive environment, the authors suggested the addition of modified water-based epoxy resin in the cement. The water-borne epoxy resin (WER) synthesis begins by dissolving epoxy resin (E-54) in a mixed solvent of n-butanol-acetone. This mixture was poured into a three-necked flask, where nitrogen gas was constantly injected. The flask was then heated to a specific temperature while being continuously stirred. An acetone solution containing benzoyl peroxide (BPO) as an initiator is then slowly added dropwise to the reaction mixture. Following this, an aqueous solution of 2-acrylamido-2-methylpropanesulfonic acid (AMPS) is also added gradually. The reaction is maintained at 100 °C for 5 h, leading to the formation of the desired water-borne epoxy resin product. Class G was used as a base cement in this study. The additive (WER) to cement ratio was 0.04, while the W/C ratio employed for the mixing consisted of 0.44, in accordance with API standards. After the preparation of the slurry, it was transferred into two cylindrical molds with dimensions of 25 × 50 mm and 25 × 25 mm. The molds were then placed in the pressurized kettle, where the samples were allowed to be cured for two days at a temperature and pressure condition of 90 °C and 20.7 MPa. Subsequently, the specimens were placed in the high-pressure corrosion kettle for 30 days. This kettle can be utilized for two distinct types of corrosion evaluations. The first method was gas-phase corrosion testing, where a small volume of water was placed in the lower part of the kettle. Gasses such as N2, H2S, and CO2 are then introduced into the kettle, with testing conducted at a temperature of 90 °C and a pressure of 10 MPa. The second method was liquid-phase corrosion testing. For this procedure, water was first added to the kettle in sufficient quantity to submerge the cement sample being evaluated. H2S and CO2 gases were then injected into the water. Finally, N2 gas was introduced to maintain the overall pressure in the kettle at 10 MPa. As with the gas phase test, the liquid phase corrosion evaluation was also conducted at 90 °C. In this setup, the system maintains a total pressure of 10 MPa, with H2S contributing a partial pressure of 1.5 MPa and CO2 accounting for 1 MPa of the total pressure. The schematic of the corrosion kettle is shown in Figure 24. To identify the structure of the epoxy resin before and after the modification FTIR (Fourier transform infrared spectrometer) was conducted. The alteration zone in the cement was identified through the phenolphthalein test, while the compressive strength of samples was determined with the help of the nYL300 pressure testing machine. A core displacement device was utilized for the measurement of the permeability of the specimens before and after the carbonation test. For the characterization of the hydration product, XRD was used. The morphological structure was analyzed through SEM analysis. After 30 days of exposure to the gaseous and liquid acidic environment, it was found that the strength degradation of the WER cement composite was about 1.2% and 9.8%, respectively, with corrosion depths of 10.6 and 3.1 mm. Whereas the mechanical strength retrogression of the control sample in the gaseous and liquid phases was 17.6 and 32.1%, respectively, with the carbonation depth of 36.6 and 16.1 mm. For permeability, it was found that in the control sample, it increased by 28.8% in the gas phase and 16.7% in the liquid phase. For the WER cement composite, the increase in the gaseous phase was 8.4%, while for the liquid phase, it was 21.7%. Therefore, incorporating WER into the cement improved strength retention, reduced corrosion depth, and enhanced permeability in both liquid and gas phases. These properties were notably better than those of the control samples. The WER performed better because the resin particles fill the pore spaces within the cement, making the matrix denser. Moreover, the three-dimensional network structure that develops after curing acts as a barrier, separating the cement-hydrated products from the acidic medium, thus improving the cement’s ability to withstand acidic environments.
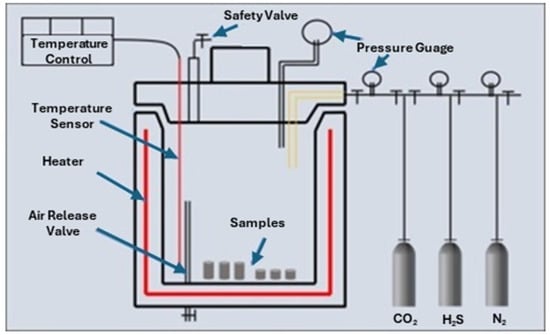
Figure 24.
HPHT corrosion kettle used by Peng, et al. [66] for their studies.
3.25. Case Study 25
Mahmoud, et al. [67] investigated the performance of a graphite cement composite when subjected to scCO2 conditions. The study used Class G cement as the control sample, with 35 wt% silica added, along with varying amounts of dispersant, expandable agent, fluid loss agent, and defoamer. The graphite in the control cement was added by 0.1 (Gr 1), 0.2 (Gr 2), and 0.3 (Gr 3) wt%. The cement was prepared in accordance with API 10A and 10B standards, with a W/C ratio of 0.44. The slurry was then transferred into different molds based on the testing requirements for each sample. Initial curing was conducted in a water bath at ambient pressure and 70 °C for 24 h. Afterward, XRD analysis was conducted on all the samples, and it was learned that the graphite cement composite contained a lower concentration of portlandite than the control specimen. It was recorded that the control sample had a concentration of 24.5% of CH, while for Gr 1, Gr 2, and Gr 3, it was 19.9, 18.7, and 21.7%, respectively. This indicates that the small amount of SiO2 present in the graphite has converted CH into secondary C-S-H. SEM analysis further supported this finding, showing large portlandite particles throughout the control sample matrix, while Gr 2 primarily displayed C-S-H products with minimal CH. This microstructural variation is critical, as portlandite is less stable in CO2-rich environments compared to C-S-H. Thus, the graphite cement composite was expected to perform better than the control samples. The compressive strength of the cement specimens was measured following the initial curing phase and subsequently compared to the strength of carbonated samples at a later stage. The samples were then submerged in deionized water within a reaction vessel (Figure 15) for ten days at 130 °C and 10 MPa, creating a scCO2-saturated water environment. The ingression of the carbonation front was measured through Micro CT scan with the sample having the dimension of 38.1 × 76.2 mm. The carbonation depth analysis showed the following measurements: 2081 μm for the control sample, 1600 μm for Gr1, 1460 μm for Gr2, and 1788 μm for Gr3. Notably, the Gr2 sample showed around 29.8% less CO2 ingression than the control sample. The sample’s permeability in the post-carbonation stage was measured through Hagen–Poiseuille law [68,69]. The specimen dimensions used for this test consisted of 38.1 × 15.2 mm (dia × height). In the pre-carbonation phase, the control sample showed the highest permeability at 0.0075 millidarcys, exceeding all graphite cement composite samples. Sample Gr2 had the lowest permeability at 0.0055 millidarcy, a 26.7% reduction compared to the control. This suggests that graphite incorporation effectively improves the cement’s pore structure and barrier properties, with the decrease in permeability attributed to the pozzolanic reaction. After carbonation, the permeability of all the samples was further decreased because of the formation of CaCO3 in the cement matrix. However, the permeability of the Gr2 sample remained 31.4% lower compared to the control sample, even in the post-carbonation phase. Cubic samples measuring 50.8 mm on each side were used for compressive strength testing. It was observed that the compressive strength of all the specimens incorporating graphite was higher than the control sample. Even after carbonation, the same trend was observed. Gr2 had a compressive strength of 97.4 MPa after ten days of carbonation testing, while the control sample had 83.7 MPa. This increase in strength was attributed to the formation of CaCO3, which helps in densifying the cement structure. The tensile strength was evaluated using the Brazilian tensile strength method on sample dimensions measuring 38.1 × 22.9 mm. In the pre- and post-carbonation stages, the tensile strength of the graphite cement composite was higher than that of the control sample. However, among all the graphite compositions, Gr2 performed best and showed an increase in tensile strength of 13.4 and 23.8% at pre- and post-carbonation tests, respectively, when compared to the control sample. In conclusion, the study found that adding graphite to cement improves its resistance to CO2-saturated water, with the Gr2 cement sample (containing 0.2% graphite) demonstrating the best overall performance.
3.26. Case Study 26
Wu et al. [70] investigated the impact of weighing material on the cement’s resistance to carbonation. In that respect, three weighing materials were utilized that consisted of hematite (Fe2O3), barite (BaSO4), and hausmannite (Mn3O4). Class G was used as a base cement in this research. The quantity of weighing material added to the cement was 50 wt%, while 35 wt% of silica flour was included in all cement compositions to prevent strength degradation in high-temperature environments. Other additives like defoamer, fluid loss additive, and dispersant were also included in the cement design. The mixing of the cement was conducted in such a way that liquid admixture was added in the mix water first and was stirred at ~3800 to 4200 rpm; afterward, the solid materials were added slowly within the next 30 s. Then, the mixture was allowed to be mixed for 11,000 to 12,000 rpm for 35 s. Subsequently, the slurry was poured into cylindrical molds with dimensions of 25 mm × 25 mm. The samples were then subjected to initial curing conditions in a testing kettle at 150 °C and 21 MPa for a duration of 3 days. After initial curing, the cylindrical samples were submerged in fresh water in the testing vessel (Figure 25), maintained at 150 °C and 30 MPa with a gas mix of 30% N2 and 70% CO2. The carbonation testing period consisted of 21 days. The tests conducted during the post-carbonation stage included compressive strength measurements, carbonation depth assessment using the phenolphthalein test, XRD analysis, pore size distribution analysis, and SEM for morphological determination. Different tests were conducted on various days throughout the 30-day period. The results indicated that the carbonation depth in all the samples increased with the increase in CO2 exposure time. Different zones of chemically altered layers were noticed in the samples. Among the weighing cement composite, hausmannite cement demonstrates the best resistance to carbonation, followed by hematite cement, while barite cement showed the least resistance. Barite cement composite showed a carbonation depth 50.2% greater than hausmannite cement and 31.3% higher than hematite cement. The rate of carbonation in these samples was found to be 0.31 mm/day for barite, 0.24 mm/day for hematite, and 0.21 mm/day for hausmannite. The compressive strength tests were conducted in two batches: the first batch consisted of samples that were not exposed to CO2, while the other batch was subjected to carbonation testing. After 14 days of curing, samples subjected to CO2-rich conditions displayed lower compressive strength compared to those not exposed to the CO2 environment. However, the hausmannite cement composite had no significant changes in compressive strength following CO2 exposure, while the barite cement composite was the most adversely affected one. XRD analysis was performed on the specimens after 30 days of exposure, showing the presence of CaCO3 polymorphs, including calcite, aragonite, and vaterite. The analysis showed that calcite was the predominant polymorph in all the carbonated specimens. Vaterite was mainly found in the carbonation zone of the cement containing barite in its composition, while aragonite was present in smaller quantities but was more abundant in the carbonation zones of hematite and hausmannite cement composites. The significant presence of vaterite in barite cement indicates its high carbonation rate. Analysis of the carbonated zone’s pore size distribution revealed that the hausmannite cement composite showed the highest increase in gel pores (diameters under 10 nm). Larger pores (over 100 nm in diameter) were most prevalent in the barite cement composite at 4.8%, followed by hematite cement, while hausmannite cement had the lowest percentage at 1.9%. It was presented by [71,72] that a pore size greater than 50 nm is considered harmful and causes the degradation of the cement matrix. Hence, their study concluded that hausmannite was the most effective in improving the resistance of oil well cement to CO2 attack.
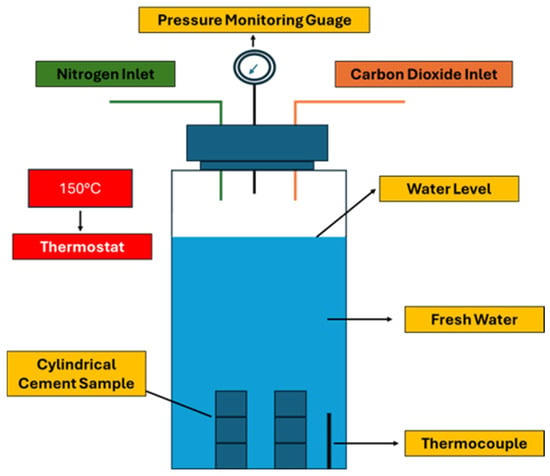
Figure 25.
HPHT static testing vessel used for the studies of Wu, et al. [70]. The picture has been modified.
3.27. Case Study 27
Kravanja and Knez [73] compared the carbonation results of neat cement with a metakaolin (MK) cement composite. The study used Class G cement, with 15 wt% MK replacing the cement in the composite. The cement was mixed in accordance with API standards and had a W/C ratio of 0.44. After the preparation of the sample, the slurry was transferred into the triple mold having the dimension of 40 × 40 × 160 mm and was placed in the water bath for 24 h at 50 °C and ambient pressure. Subsequently, some of the specimens were taken out of the mold for the carbonation test, while the remaining samples were placed back in the water bath and were treated as reference samples. The reactor used in this test had a volume of 5 L. The carbonation test was conducted for twenty-eight days at a temperature and pressure of 50 °C and 15 MPa, respectively. The reactor was divided into two parts. In the top section, two samples were placed that were exposed to scCO2 conditions and were given the names SUP G and SUP G + MK, while in the lower section, two samples were placed in scCO2 saturated 0.5 wt% NaCl solution. The samples were tagged as SAT G and SAT G + MK. Figure 26 shows the static reactor utilized in this study. The post-carbonation test consisted of mechanical testing (flexural and compressive strength), phenolphthalein test for determining the carbonation depth, XRD, TGA, measurement of the transfer properties through nitrogen desorption/absorption method, and surface morphology through SEM. It was noted that the flexural and compressive strength of the sample placed in the SUP and SAT environment increased as compared to the specimens cured in ambient conditions. For compressive strength, the increase was 17.10% and 28.44% for SUP and SAT conditions for Class G + 15%MK, respectively. After the carbonation test, the flexural strength of both Class G and MK cement composite showed improvements of 9.98% and 10.87%, respectively, compared to pre-carbonation stage. The improvement in the mechanical properties was due to the pozzolanic reaction of the cement with the MK that yields secondary C-S-H and from the carbonation reaction that replaces CH with CaCO3. The average carbonation depth of the neat sample placed in the SAT and SUP conditions was about 3.20 and 2.15 mm, respectively. While for the metakolin cement composite, it was about 5.76 mm for SAT condition and 4.50 mm for SUP condition. It was also interesting to find that the carbonation rate increased for the sample placed in the SAT condition than that of the SUP. For the neat sample, the rate increased from 4 to 12%, while for Class G + 15% MK, the rise was from 36 to 39%. This result aligned with Moraes and Costa [63], which demonstrated that scCO2-saturated water presents a more aggressive environment compared to the pure scCO2 condition as more CO2 can be dissolved in the liquid medium and react with the cement aggressively. They concluded that the below-par performance of MK in the cement could be due to the fact that it creates a lightweight cement with low density, and such samples are more prone to vigorous carbonation. The polymorph of CaCO3 was found in all specimens exposed to the CO2-rich conditions. Class G samples in the SUP and SAT conditions had CaCO3 of about 17.3 and 25%, respectively, while G + 15% MK was about 31.3 and 33.2%. The presence of CaCO3 is likely a critical factor, as its precipitation can potentially result in the development of pores and cracks, creating pathways that facilitate more intense CO2 diffusion and upward movement. This result was backed by the absorption/desorption test of SUP G + MK and SAT G + MK, which revealed that the largest specific area was present in these samples, which resulted in a higher absorption of CO2 in its matrix. The authors suggested that the addition of the pozzolanic material in the cement should be carried out with care, particularly when the cement will be subjected to a CO2-rich environment. There is a further need to conduct experiments on the pozzolanic-based material, especially for MK, to ensure its performance in CCS well conditions.
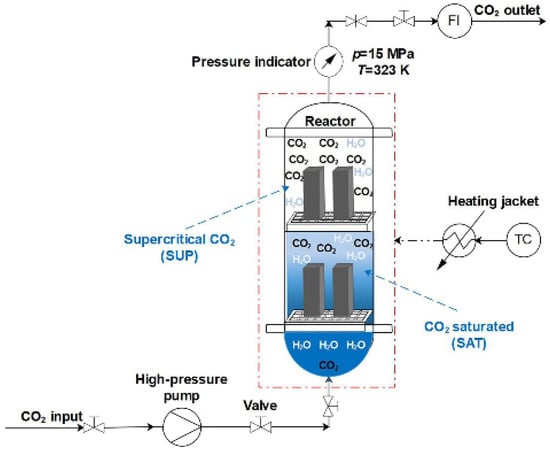
Figure 26.
Reactor used for the carbonation test studies carried out by Kravanja and Knez [73].
3.28. Case Study 28
Yan, et al. [74] studied the chemo-mechanical behavior of cement incorporating silica fume, latex, and liquid silica at high temperatures and high CO2 partial pressure. The base material for the study was Class G cement, to which 40 wt% silica fume (Mix 15), 10 wt% liquid silica (Mix 16), and 16 wt% latex (Mix 20) were added. The cement mixture was formulated according to API standards, using a W/C ratio of 0.44. Subsequently, the resulting slurry was poured into cubic molds measuring 50 × 50 × 50 mm, which were then cored to obtain the cylindrical samples with the dimensions of 25 × 50 mm. Each representative sample was placed in an HPHT autoclave containing 2 L of synthetic brine. For the deoxidization, nitrogen was introduced into the vessel for a period of 2 h. The vessel’s temperature was raised to 150 °C, while the pressure was increased to 4 MPa, creating a CO2-saturated brine environment within the testing chamber. These parameters were taken to simulate the reservoir condition of the southwest oil field in China. The testing was conducted for 14 and 28-day time periods. The experimental design of this study consisted of measuring the porosity with the helium porosity meter, permeability through the transient pulse method (PDP-200), compressive strength, and microstructural analysis through indentation test, SEM-EDS imaging, and XRD analysis. The carbonation depth in the sample was observed with the help of the phenolphthalein test. The results indicate varying carbonation resistance among different cement mixes. The silica fume blend (Mix 15) showed a carbonation depth of 3.24 mm at 14 days, which raised to 4.79 mm by day 28, representing a 47% increase. Similarly, the liquid silica blend (Mix 16) showed a 1.67 mm carbonation depth at 14 days, followed by a more than 48% increase by day 28. This ongoing carbonation in both silica-based mixes suggests that they may be more prone to corrosion. In contrast, the latex-blended cement performed better, showing the smallest radial carbonation depth among all tested mixtures. This enhanced performance is likely attributable to the latex’s ability to form a denser matrix within the cement. This denser structure provides improved resistance to CO2 penetration, effectively slowing down the carbonation process. Compressive strength tests showed a decrease in strength for all samples after 28 days of curing, with the highest retrogression observed from Mix 16 (26%), then Mix 20 (18%), and the least was from Mix 15 (13%). The experimental results from the transfer property test showed diverse responses among the cement mixes when subjected to CO2-saturated brine. Mix 16 demonstrated the most favorable outcome, with a relative decrease in permeability, indicating enhanced resistance to fluid penetration. In contrast, Mix 15 exhibited a 600% rise in permeability despite a 44% decline in porosity, suggesting significant structural alterations. Mix 20 showed a more moderate response with a 70% permeability increase and 86% porosity decrease. The general trend of decreased porosity across all mixes might be caused by carbonate precipitation in pore spaces, but the varying permeability changes indicate complex structural modifications in each mix. Different changes in transfer properties observed after the carbonation test can be attributed to a complex interplay of factors, including the type and amount of reactants, the conditions under which reactions occurred, and the subsequent mineralogical composition and microstructural characteristics of the formed products. For Mix 20 and Mix 15, the observed rise in permeability despite reduced porosity likely stems from alterations in the existing mineralogy, leading to the formation of a more interconnected and open pore network. The decrease in permeability observed in Mix 16 may be attributed to two primary reasons: either a reduction in the size of pores or the development of more complex mineral formations within the cement matrix. These changes likely resulted in a more tortuous path for fluids to flow through the cement matrix. Micro-indentation of the specimen revealed a decrease in mechanical properties, including hardness and average elastic modulus, especially at the outer rim of the sample exposed to CO2. This finding was supported by XRD analysis, which indicated a higher concentration of CaCO3 in the outer layer of the sample, with no detectable C-S-H present. Additionally, the absence of CH in these zones suggests that the cement samples have undergone carbonation. The addition of silica base additive in the cement initiates the pozzolanic reaction that yields secondary C-S-H and lowers the Ca/S ratio in the cement. However, the formation of secondary C-S-H is thermodynamically unstable and eventually converts into C-S-H phases with lower strength. They concluded that micromechanical properties of various cement phases differ significantly, with the elastic modulus and hardness of the hydration products (CH and C-S-H) being notably distinct from those of the carbonation product (CaCO3). Therefore, this research underscores the importance of using admixtures to reduce the impact of CO2 corrosion in HPHT environments.
3.29. Case Study 29
Zhang, et al. [75] investigated the impact that polymer had on the anti-corrosion properties of cement in a carbonation environment. The research focused on the influence of different corrosion factors on the corrosion depth of polymer-modified cement composites. The study utilized the Box–Behnken experimental design, a statistical method, to investigate the effect of various corrosion-related variables on the extent of corrosion penetration. Using the response surface methodology, the authors analyzed the interplay between various influencing factors and their combined effects on multiple corrosion depths. Class G cement was used as a control sample in this study. In addition to the base cement, several additives were incorporated to enhance specific properties. These additives included 2-Acrylamido-2-methylpropane sulfonic acid polymer material, which acted as a water loss agent; a polyacid-modified material used as a retarder; aldehyde ketone condensation polymer serving as a dispersant; and micro silicon to improve the cement strength. To impart the anti-corrosion property to the cement, a polymer preservative was utilized, whose primary component was water-borne resin emulsion. The wt% of the polymer preservative used was 12%. The mixing of the cement was conducted at 4000 RPM, in which all the components were thoroughly mixed with each other. The slurry was poured into the 25.4 × 25.4 mm cylindrical molds and placed in the water bath for 24 h at 60 °C and atmospheric pressure. The carbonation of the cement was conducted using the HPHT corrosion tester. The experiment was to be conducted at five different levels of temperatures, pressures, and time periods in order to note their impact on cement carbonation. However, from the Box–Behnken experimental design, 3 levels were selected that consisted of: days 30, 40, and 50; pressure (MPa), 15, 20, and 25; and temperature (°C) 65, 70, and 75. Testing conducted at 20 MPa and 70 °C revealed that as the curing period increased, the carbonation depth in both the controlled and cement polymer composite also increased. However, at the 30-day mark, the sample containing polymer in its composition showed a 57.9% reduction in carbonation depth compared to the control sample. The impact of pressure on corrosion rate was observed by maintaining a constant carbonation period of 7 days and a temperature of 70 °C. The results showed that as pressure increased, the depth of the carbonation front also increased, with the other variables (carbonation period and temperature) held constant. The carbonation depth for the polymer cement composite increased to 0.11 mm when the pressure was raised from 5 to 25 MPa. The effect of temperature was found by keeping the time period (7 days) and pressure (20 MPa) constant. It was recorded that the corrosion depth increased by 0.14 mm as the temperature rose from 50 °C to 90 °C. The relation of temperature with the corrosion depth is also presented in the study conducted by [76]. This study [75] presented that while the inclusion of polymer enhanced the cement’s ability against CO2 attack, carbonation of the sample still occurred. The degradation of polymer-modified cement composite was influenced by three key factors: the duration of corrosion exposure, CO2 pressure, and temperature of the exposed environment. Analysis of the fitted data reveals specific relationships between these factors and the extent of corrosion: (a) corrosion depth has a logarithmic relationship with temperature, (b) a square root relationship exists between corrosion depth and CO2 pressure, and (c) there is a linear correlation between corrosion depth and exposure time.
3.30. Case Study 30
To improve the ability of the cement matrix against CO2 attack, Sedić et al. [77] introduced a calcium aluminate cement (CAC) system. In this system, they further incorporated hollow glass microfibers (soda-lime-borosilicate, 4.5 wt%) and rubber latex (butadiene copolymer latex, 27 wt%) to improve its durability and performance in CO2-rich environments. The performance of the CAC composite was compared against a control sample, which consisted of Class G cement having 7.5 wt% of glass microsphere (PC-μ). The CAC microsphere composite was represented as CAC-μ, while the one with the latex was given the acronym CAC-L. The preparation of these CAC composites is presented in Sedic [78]. The density of all the samples was kept at 1650 g/cm3, while the compressive strength, API filtration, free water, thickening time, and rheology were all in the acceptable range. After the cement slurry preparation, it was poured into the plastic cylindrical molds, which had dimensions of 38.1 mm ID and 68.3 mm length. The initial curing was conducted in the water bath at ambient pressure and temperature of 80 °C for a duration of 30 days. Subsequently, the sample size was reduced in height to 40 mm by cutting the portion from the top and bottom. Then, the specimens were immersed in water within an HPHT chamber for the carbonation test, which lasted 80 days. Pressure and temperature were maintained at 7 MPa and 100 °C, respectively. The post-carbonation tests involved evaluating compressive strength, assessing transport properties, and conducting morphological characterization. The results showed that in the pre-carbonation stage, the CAC composite showed higher compressive strength compared to the control sample. However, in the post-carbonation phase, the compressive strength of all the samples was reduced. In which the highest retrogression was noticed from the CAC-L while CAC-μ showed the lowest. Analysis of porosity reveals that CAC samples demonstrate significantly lower porosity levels when compared to the reference samples. However, after exposure to CO2, all specimen types experienced an increase in porosity due to degradation. Among these, PC-µ samples showed a particularly pronounced increase in porosity, indicating a greater susceptibility to CO2-induced degradation compared to the other cement types tested. As for the permeability, it was noticed that before the exposure to the CO2, the control sample showed the lowest permeability. However, after the exposure, CAC-µ and control sample presented the lowest permeability value. In terms of carbonation depth, using phenolphthalein as an indicator, it was observed that the control sample had undergone complete carbonation, with no visible purple color, indicating that the entire cement was carbonated. Meanwhile, for the CAC composite, the carbonation depth was 1 to 3 mm. It was found that the resistance of CAC-µ > CAC-L > PC-µ. This result was backed by the morphological analysis carried out through SEM, TGA, and XRD analysis.
3.31. Case Study 31
Barría, et al. [79] studies the effect of bacterial nanocellulose (BNC) on cement against the wet scCO2. BNC is a unique biopolymer with several notable characteristics. It is insoluble in water-based solutions, possesses exceptional mechanical strength, and demonstrates high thermal resistance [80]. The reference sample used in this study consisted of Class G oil well cement. The ultrasound technique was utilized to disperse BNC in the mixed water. Multiple steps were taken to use BNC as an additive in the cement, which is described in detail by the studies presented by Barría, et al. [81] BNC was added to the cement by 0.05 (BNC 05) and 0.15 (BNC 15) wt%. The cement mixing followed API standards with a W/C ratio of 0.44. The slurry was poured into cylindrical molds with dimensions of 76 mm in height and 38 mm in diameter. The initial curing was conducted in ambient conditions for 24 h and was then de-molded and placed in lime-saturated water for 28 days. The carbonation test was performed in a static titanium vessel. The samples were exposed to the wet scCO2 (a thin layer of water at the bottom of the vessel was placed to maintain humidity) for 30 and 120 days at temperatures and pressures of 90 °C and 20 MPa, respectively. The samples were placed on the grid and lowered in the vessel to avoid contact between the samples and the water. The schematic of the setup is presented in Figure 27. Compressive strength, density, porosity measurement, carbonation depth, and XRD analysis were conducted in the post-carbonation test. It was found that the density of all the samples was increased. The control sample showed a bulk density increase of about 1.4% after 30 days and 2.5% after 120 days. In contrast, the sample with 0.05 wt% BNC showed a minor increase in bulk density, with increments of 1.5 and 2% after 30 and 120 days, respectively. The carbonation depth for the 0.05 wt% BNC sample was approximately 5.6 mm, compared to 8.7 mm for the control sample. The mercury intrusion porosimeter (MIP) found that for the uncarbonated sample, the presence of capillary pore for the neat cement, 0.05 and 0.15 BNC cement composite was about 19, 12, and 16%, respectively. After 30 days of the carbonation period, small pores (less than 0.010 μm) started to appear. However, as the carbonation period continued, at 120 days, the quantity of smaller pores was reduced due to the dissolution of CH and C-S-H. It was observed that the pore population above 30 days of exposure was more toward 0.100 μm. At the core of the samples, capillary porosity decreased for neat cement and 0.15% BNC, but remained unchanged for 0.05% BNC additive cement. XRD analysis revealed an increase in hydrated products in the BNC cement composites during the carbonation stage. After the exposure to the CO2 environment, the amount of aragonite was more at the outer rim of the sample, indicating the dissolution of CaCO3. Mechanical strength measurements showed a continuous decline for neat cement, while BNC cement composites maintained their strength from 30 to 120 days. The compressive strengths at 120 days were 42 MPa for BNC15, 45 MPa for BNC05, and 36 MPa for neat cement. The young modulus values were 16.5 GPa for the control sample and 22 and 21 GPa for BNC05 and BNC15, respectively. Therefore, it was concluded that the cement incorporating 0.05% BNC in its composition performed better under wet supercritical CO2 conditions compared to neat Class G cement.
Figure 27.
Carbonation testing setup used by the studies carried out by Barría, et al. [79]. This schematic image has been modified.
3.32. Case Study 32
Shine, et al. [82] conducted carbonation tests on a cement mixture composed of 50% Class G cement and 50% Class F fly ash, referred to as fit-for-purpose cement (FFPC), and compared it to standard Class G cement. In the FFPC, cross-linked polyvinyl alcohol was added as the primary supporting additive to form the matrix. The samples were subjected to wet and dry scCO2 for 60 and 180 days at 74 °C and 6.89 MPa. Post-carbonation testing involved XRF to determine the CaO content in the carbonated sample and a CT scan to detect the carbonation front. Mechanical and transfer properties were also measured, including Poisson’s ratio, Young’s modulus, compressive strength, porosity, and permeability. It was recorded that the compressive strength of the FFPC increased after exposure to both CO2-rich environments, while it was reduced for the control sample. Whereas an increase in the Young Modulus was noticed in both samples. While the FFPC showed an increase in Poisson’s ratio in both CO2-rich environments, the control sample exhibited a decrease in wet conditions but an increase in dry conditions. XRF testing did not reveal significant observations, but the Ca/S ratio of the FFPC was 1:1, compared to 1:3 for Class G cement after 180 days of exposure. XRD analysis indicated that less calcite was present in the Class G cement than in the FFPC, suggesting that the carbonation process within FFPC systems is likely to reach a steady-state condition. After 180 days of CO2 exposure, the FFPC showed a 50% lower permeability and reduced porosity than the control sample. The carbonation front in the FFPC extended in a homogeneous radial form, helping the cement maintain its integrity in both wet and dry conditions, whereas the control sample showed less stable carbonation front progression. Overall, the results indicate that the FFPC design approach can lead to more resilient and effective cementing solutions for CCS applications. Hence, the authors suggested that the cement design for CCS wells should be customized according to the well conditions rather than depending on a generic or overly conservative approach.
3.33. Case Study 33
Lende, et al. [83] presented the mapping and quantification of the CO2 progression front in the cement. This study examined how cement reacts to the flow behavior of wet supercritical CO2 and CO2-saturated water as they were forced through a cylindrical cement sample. The authors used a high-pressure gradient to artificially enhance the flow rate through the sample. This experimental setup allowed them to study the effects of the highly pressurized, corrosive CO2-rich fluids flowing through the cement under accelerated conditions. Therefore, the carbonation of the cement was carried under dynamic conditions. The study was carried out in four steps: (a) molding and curing of the sample, (b) introduction of water-saturated or wet scCO2 axially through the cement core with high differential pressure and the simultaneous measurement of the flow rate, (c) using the indentation method to measure the carbonated area in the radial and axial direction, and (d) comparing the pre- and post-carbonation results. The sample used in the testing consisted of Ordinary Portland cement with a silica blend in its composition. The initial curing of the sample was conducted in fresh water for 28 days at pressure and temperature of 31 MPa and 80 °C. The dimensions of the cylindrical sample consisted of diameter = 38 mm and height = 76 to 80 mm. The carbonation testing was performed in the dynamic state and was named Forced Flow Test. The design detail of the testing setup is presented in Lende, et al. [84]. The post-carbonation test consisted of: (a) permeability, (b) uniaxial load test, (c) Brazilian tensile strength, and (d) indentation tests. The permeability test revealed that the control sample’s permeability decreased from 0.12 to 0.04 mD under wet scCO2 exposure, while it changed from 0.12 to 0.10 mD in CO2-saturated water. The tensile strength measurements showed significant variability due to sample inhomogeneity caused by sedimentation during early curing. The Rockwell hardness method was used for the indentation test on the control sample. While the phenolphthalein method was also utilized in parallel to find the cement’s carbonation depth. The carbonation depth was approximately 10 mm for samples exposed to CO2-saturated water and 20 mm for those exposed to wet scCO2, with the latter showing greater degradation due to the higher molarity and lower viscosity of the CO2 medium. High indentation depths were observed in control samples exposed for 90 days. Their studies concluded that the curing of the sample can be accelerated by increasing the temperature, while the forced axial method can enhance the carbonation rate in the cement samples. The exposure of the cement to CO2 in the static tests can be increased by increasing the surface area of the samples. The indentation test method is a simple yet effective way to map the cement sample’s response to CO2 exposure, offering a more comprehensive understanding of performance than conventional testing methods, particularly for samples with inhomogeneous characteristics.
Table 5 summarizes the critical parameters and key findings from the studies examined in this paper.

Table 5.
Critical parameters and finding of the studies reviewed in this paper.
4. Discussions
From the studies reviewed, it was observed that most carbonation tests were performed under quasi-static conditions, which are often considered representative of CCS well downhole environments due to the absence of continuous flow paths in the geological setting setting [85,86,87]. However, this assumption holds true only as long as the cement remains intact and no flow paths develop. Once a flow path forms within the cement matrix or at the cement-casing or cement-formation interface, the system transitions to a dynamic state. This shift accelerates the rate of cement carbonation as the cement sheath is continually exposed to fresh effluent, altering the equilibrium that would have been maintained under static conditions. Therefore, assessing the cement under these dynamic conditions is crucial, and more testing in dynamic states should be conducted.
It was also noted that most samples were exposed to water-saturated conditions. This approach is taken because the solubility of CO2 decreases with increasing salinity, allowing more CO2 to dissolve in fresh water and thereby accelerating the carbonation reaction [88]. While fresh water does not accurately replicate downhole brine conditions, it was used in testing because it enables more pronounced and rapid reactions between scCO2-saturated water and cement, allowing for effective assessment of carbonation within shorter timeframes.
Some authors exposed cement samples to scCO2-saturated water and wet scCO2. These studies consistently reported that cement degradation was more severe in water-saturated conditions. This is because the presence of water creates a harsher and more reactive environment for the cement matrix, whereas CO2, in its pure supercritical state, is less damaging.
To enhance the resistance of cement against CO2 attack, many researchers have incorporated pozzolanic materials into the cement mix. These materials initiate the pozzolanic reaction, which can be summarized as follows [89]:
Portlandite (a product of cement hydration) + Silicon dioxide (from pozzolanic material) → Secondary Calcium Silicate Hydrate
3Ca(OH)2 + 2SiO2 → 3CaO·2SiO2·3H2O
This reaction reduces the amount of calcium hydroxide in the cement. Portlandite is the first component to be attacked by CO2, so its reduction leads to the formation of more stable C-S-H (Calcium Silicate Hydrate), which strengthens the cement and improves its stability in CO2-rich environments. Furthermore, in terms of thermodynamic reactions, Yang, et al. [42] reported that the Gibbs Free Energy (ΔGT) governs the carbonation rate in cement samples. Their studies revealed that cement hydration products with lower ΔGT—or, in other words, a higher Ca/S ratio—are more susceptible to carbonation, with portlandite being a prime example. Therefore, if portlandite (CH) can be converted into products with a lower Ca/S ratio and higher ΔGT, the cement will be better able to resist scCO2 attack. This can be achieved through the pozzolanic reaction, which reduces the amount of CH (portlandite) in the hydrated cement. It was also observed that nearly all the studies used cylindrical samples for their testing instead of the standard API 2-inch cubes. This is because the carbonation reaction is less noticeable on cubes within a short timeframe than on cylindrical samples [45].
Table 6 summarizes the frequently reported days, temperatures, and pressures used by the authors in this study for both initial curing and carbonation periods, as depicted in the histograms of Figure 28 and Figure 29. Only one study involved curing the samples for a year in the initial phase, but this point of data was omitted from the histogram to prevent interference with the x-axis scale.

Table 6.
Commonly used temperatures, pressures, and curing durations employed by the authors reviewed in this study.
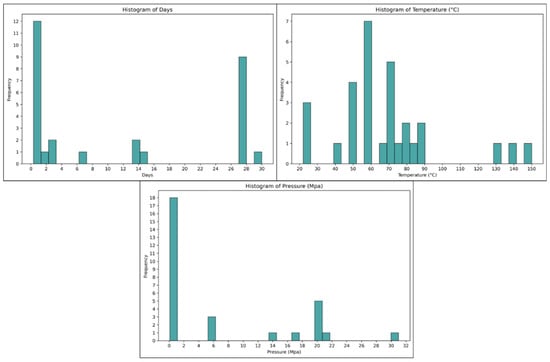
Figure 28.
Histogram of initial curing.
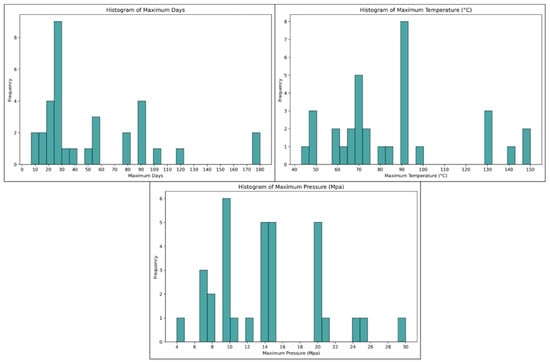
Figure 29.
Histogram of carbonation period.
5. Conclusions
This review highlights the inevitability of cement degradation in a CCS environment. However, incorporating additives, particularly pozzolanic materials, through their pozzolanic reaction can help control and slow the rate of carbonation and degradation of cement samples. Notably, there is no standardized procedure for conducting carbonation tests, as each author employs a distinct methodology, making it challenging to directly compare the results of different studies. Nevertheless, this paper outlines the most frequently used temperatures, pressures, and durations for initial curing or carbonation in the studies reviewed. It is worth noting that the majority of tests were conducted under static conditions, which may not accurately reflect scenarios where CO2 leaks through the cement sheath or at the interface. In almost all the tests, freshwater was used instead of brine to accelerate the carbonation reaction within the short duration of the testing. While most studies adhered to the API standards for cement mixing, cylindrical samples were used instead of the cubical samples recommended by API.
6. Recommendation for Further Research
- A standardized protocol of carbonation testing should be developed as it will help to compare the results of one test with the other, which will assist in developing better well cement for the CCS wells.
- More tests in the dynamic conditions should be conducted to simulate the actual downhole condition of the CCS wells.
- For the integrity of the cement in the CCS well, numerical models should be used and can be verified through the long-term carbonation testing of the cement.
- Different studies should be conducted on various cement additives and formulations so that the cement recipe can be optimized for the carbon storage wells.
- For a better assessment of the morphological properties of the cement, techniques such as SEM and X-ray tomography should be extensively used.
Author Contributions
Conceptualization, K.A., A.F.B.V. and C.T.; methodology, K.A., A.F.B.V. and C.T.; formal analysis, K.A., A.F.B.V. and C.T.; investigation, K.A., A.F.B.V. and C.T.; resources, C.T.; data curation, K.A. and C.T.; writing—original draft preparation, K.A., A.F.B.V. and C.T.; writing—review and editing, K.A., A.F.B.V. and C.T.; visualization, K.A; supervision, C.T. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
The original contributions presented in the study are included in the article, further inquiries can be directed to the corresponding author.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Smith, J.L.; Bryz-Gornia, C.J.; Bhatia, S.K.; Walowsky, D. A comparative study of RECPs: Index properties and field performance. Geotech. Spec. Publ. 2005, 1607–1616. [Google Scholar] [CrossRef]
- Parry, M.; Canziani, O.; Palutikof, J.; van der Linden, P.; Hanson, C. Climate Change 2007: Impacts, Adaptation and Vulnerability; Cambridge University Press: Cambridge, UK, 2007. [Google Scholar]
- Cox, P.M.; Betts, R.A.; Jones, C.D.; Spall, S.A.; Totterdell, I.J. Acceleration of global warming due to carbon-cycle feedbacks in a coupled climate model. Nature 2000, 408, 184–187. [Google Scholar] [CrossRef] [PubMed]
- Abdullah, M.; Baharak, S. Sustainable conversion of natural gas to hydrogen using transition metal carbides. Int. J. Hydrogen Energy 2024, 90, 61–103. [Google Scholar] [CrossRef]
- Fawzy, S.; Osman, A.I.; Doran, J.; Rooney, D.W. Strategies for mitigation of climate change: A review. Environ. Chem. Lett. 2020, 18, 2069–2094. [Google Scholar] [CrossRef]
- UN. 2023. Emissions Gap Report 2023. UN. Available online: https://www.unep.org/resources/emissions-gap-report-2023 (accessed on 10 August 2024).
- Change, UN Climate. 2024. The Paris Agreement. What Is the Paris Agreement? UN. Available online: https://unfccc.int/process-and-meetings/the-paris-agreement (accessed on 10 August 2024).
- Nationalgrid. What Is Carbon Capture and Storage? 2024. Available online: https://www.nationalgrid.com/stories/energy-explained/what-is-ccs-how-does-it-work (accessed on 23 July 2024).
- Marchetti, C. On geoengineering and the CO2 problem. Clim. Chang. 1977, 1, 59–68. [Google Scholar] [CrossRef]
- Abid, K. Designing a New Cement Composition Using Agricultural Wastes for Underground Gas Storage. Ph.D. Thesis, Curtin University, Perth, Australia, 2018. [Google Scholar]
- Kaldi, J.G.; Gibson-Poole, C.M.; Payenberg, T.H.D. Geological Input to Selection and Evaluation of CO2 Geosequestration Sites. In Carbon Dioxide Sequestration in Geological Media; The American Association of Petroleum Geologists: Tulsa, OK, USA, 2009. [Google Scholar]
- Sathsarani, H.B.S.; Sampath, K.H.S.M.; Ranathunga, A.S. Utilization of fly ash-based geopolymer for well cement during CO2 sequestration: A comprehensive review and a meta-analysis. Gas Sci. Eng. 2023, 113, 204974. [Google Scholar] [CrossRef]
- Ghedan, S. Global Laboratory Experience of CO2-EOR Flooding. In SPE/EAGE Reservoir Characterization and Simulation; SPE: Abu Dhabi, UAE, 2009. [Google Scholar]
- Godec, M.; Koperna, G.; Petrusak, R.; Oudinot, A. Assessment of Factors Influencing CO2 Storage Capacity and Injectivity in Eastern U.S. Gas Shales. Energy Procedia 2013, 37, 6644–6655. [Google Scholar] [CrossRef]
- Bachu, S.; Didier, B.; John, B.; Robert, B.; Sam, H.; Niels, P.C.; Odd, M.M. CO2 storage capacity estimation: Methodology and gaps. Int. J. Greenh. Gas Control. 2007, 1, 430–443. [Google Scholar] [CrossRef]
- Raza, A.; Rezaee, R.; Gholami, R.; Rasouli, V.; Bing, C.H.; Nagarajan, R.; Hamid, M.A. Injectivity and quantification of capillary trapping for CO2 storage: A review of influencing parameters. J. Nat. Gas Sci. Eng. 2015, 26, 510–517. [Google Scholar] [CrossRef]
- Kutchko, B.G.; Strazisar, B.R.; Huerta, N.; Lowry, G.V.; Dzombak, D.A.; Thaulow, N. CO2 Reaction with Hydrated Class H Well Cement under Geologic Sequestration Conditions: Effects of Flyash Admixtures. Environ. Sci. Technol. 2009, 43, 3947–3952. [Google Scholar] [CrossRef]
- Sumra, Y.; Payam, S.; Zainah, I. The pH of Cement-based Materials: A Review. Cem. Mater. 2020, 35, 908–924. [Google Scholar] [CrossRef]
- Haghi, R.K.; Chapoy, A.; Pereira, L.M.C.; Yang, J.; Tohidi, B. pH of CO2 saturated water and CO2 saturated brines: Experimental measurements and modelling. Int. J. Greenh. Gas Control. 2017, 66, 190–203. [Google Scholar] [CrossRef]
- Abid, K.; Raoof, G.; Paul, C.; Brabha, H.N. A review on cement degradation under CO2-rich environment of sequestration projects. J. Nat. Gas Sci. Eng. 2015, 27, 1149–1157. [Google Scholar] [CrossRef]
- Kurdowski, W. Cement and Concrete Chemistry; Springer: Berlin/Heidelberg, Germany, 2014. [Google Scholar]
- de Souza, O.A.; Dweck, J.; de Moraes Rego Fairbairno, E.; da Fonseca Martins Gomes, O.V. Crystalline admixture effects on crystal formation phenomenaduring cement pastes’ hydration. J. Therm. Anal. Calorim. 2019, 139, 3361–3375. [Google Scholar] [CrossRef]
- Adams, N.; Tommie, C. Drilling Engineering: A Complete Well Planning Approach; Penn Well Pub. Co.: Red Lion, PA, USA, 1985. [Google Scholar]
- Mamlouk, M.S.; Zaniewski, J.P. Materials for Civil and Construction Engineers; Addison Wesley Longman, Inc.: Saddle River, NJ, USA, 1999. [Google Scholar]
- Kosmatka, S.H.; Wilson, M.L. Design and Control of Concrete Mixtures; Portland Cement Association: Skokie, IL, USA, 1988. [Google Scholar]
- Mindess, S.; Young, J.F.; Darwin, D. Concrete; Prentice-Hall, Inc.: Hoboken, NJ, USA, 1981. [Google Scholar]
- Ge, J.; Xu, F.; Wei, H.; Wang, Q.; Peng, H.; Zhou, J.; Li, H. The Influence Mechanism of Interfacial Characteristics between CSH and Montmorillonite on the Strength Properties of Cement-Stabilized Montmorillonite Soil. Materials 2023, 16, 7141. [Google Scholar] [CrossRef]
- Nelson, E.B. Well Cementing; Schlumberger Educational Service: Adelaide, Australia, 1990. [Google Scholar]
- Peach, J.; Eastoe, J. Supercritical carbon dioxide: A solvent like no other. Beilstein J. Org. Chem. 2014, 10, 1878–1895. [Google Scholar] [CrossRef]
- Kutchko, B.G.; Brian, R.S.; Dzombak, D.A.; Lowry, G.V.; Thaulow, N. Degradation of Well Cement by CO2 under Geologic Sequestration Conditions. Environ. Sci. Technol. 2007, 41, 4787–4792. [Google Scholar] [CrossRef]
- Omosebi, O.; Maheshwari, H.; Ahmed, R.; Shah, S.; Osisanya, S. Experimental study of the effects of CO2 concentration and pressure at elevated temperature on the mechanical integrity of oil and gas well cement. J. Nat. Gas Sci. Eng. 2017, 44, 299–313. [Google Scholar] [CrossRef]
- Nygaard, R. Well Design and Well Integrity; Energy and Environmental System Group: Newburgh, IN, USA, 2010. [Google Scholar]
- Berntsen, A.; Todorovic, J.; Raphaug, M.; Torsæter, M. Salt clogging during supercritical CO2 injection into a downscaled borehole. Int. J. Greenh. Gas Control. 2019, 86, 201–210. [Google Scholar] [CrossRef]
- Duguid, A.; Scherer, G.W. Degradation of oilwell cement due to exposure to carbonated brine. Int. J. Greenh. Gas Control. 2010, 4, 546–560. [Google Scholar] [CrossRef]
- API Recommended Practice 10B-2; Recommended Practice for Testing Well Cements. API: Washington, DC, USA, 2013.
- API Specication 10-A; Specification for Cements and Materials for Well Cementing. API: Washington, DC, USA, 2005.
- Laudet, J.-B.; Garnier, A.; Neuville, N.; Le Guen, Y.; Fourmaintraux, D.; Rafai, N.; Burlion, N.; Shao, J.-F. The behavior of oil well cement at downhole CO2 storage conditions: Static and dynamic laboratory experiments. Energy Procedia 2011, 4, 5251–5258. [Google Scholar] [CrossRef]
- Santra, A.; Sweatman, R. Understanding the long-term chemical and mechanical integrity of cement in a CCS environment. Energy Procedia 2011, 4, 5243–5250. [Google Scholar] [CrossRef]
- Syed, A.; Shi, J.-Q.; Durucan, S.; Korre, A.; Nash, G. Experimental and numerical investigations into CO2 interactions. Energy Procedia 2014, 63, 5707–5714. [Google Scholar] [CrossRef]
- Connell, L.; Down, D.; Lu, M.; Hay, D.; Heryanto, D. An investigation into the integrity of wellbore cement in CO2 storage wells: Core flooding experiments and simulations. Int. J. Greenh. Gas Control. 2015, 37, 424–440. [Google Scholar] [CrossRef]
- Duguid, A.; Radonjic, M.; Scherer, G.W. Degradation of cement at the reservoir/cement interface from exposure to carbonated brine. Int. J. Greenh. Gas Control. 2011, 5, 1413–1428. [Google Scholar] [CrossRef]
- Yang, Y.; Yuan, B.; Wang, Y.; Zhang, S.; Zhu, L. Carbonation resistance cement for CO2 storage and injection wells. J. Pet. Sci. Eng. 2016, 146, 883–889. [Google Scholar] [CrossRef]
- de Sena, C.B.L.; de Oliveira Freitas, J.C.; Santos, P.H.S.; de Araújo Melo, D.M.; de Oliveira, Y.H. Effects of carbon dioxide in Portland cement: A relation between static sedimentation and carbonation. Constr. Build. Mater. 2017, 150, 450–458. [Google Scholar]
- Sena, C.B.L.; de Oliveira Freitas, J.C.; Santos, P.H.S.; de Araújo Melo, D.M.; da Silva Araujo, R.G.; de Oliveira, Y.H. Carbonation in oil well Portland cement: Influence of hydration time prior to contact with CO2. Constr. Build. Mater. 2018, 159, 252–260. [Google Scholar]
- Bruckdorfer, R.A. Carbon Dioxide Corrosion in Oilwell Cements. In Proceedings of the SPE Rocky Mountain Petroleum Technology Conference/Low-Permeability Reservoirs Symposium, Billings, Montana, 19–21 May 1986. [Google Scholar]
- de Sena, C.B.L.; de Oliveira Freitas, J.C.; Santos, P.H.S.; da Silva Araújo, R.G.; Oliveira, J.F.D.S.; de Araújo Melo, D.M. Study of carbonation in a class G Portland cement matrix at supercritical and saturated environments. Constr. Build. Mater. 2018, 180, 308–3019. [Google Scholar] [CrossRef]
- Xu, B.; Yuan, B.; Wang, Y.; Zeng, S.; Yang, Y. Nanosilica-latex reduction carbonation-induced degradation in cement of CO2 geological storage wells. J. Nat. Gas Sci. Eng. 2019, 65, 237–247. [Google Scholar] [CrossRef]
- Abid, K.; Raoof, G.; Henry, E.; Masood, M.; Chua, H.B.; Golam, M. A methodology to improve nanosilica based cements used in CO2 sequestration sites. Petroleum 2018, 4, 198–208. [Google Scholar] [CrossRef]
- Mahmoud, A.A.; Salaheldin, E. Improving class G cement carbonation resistance for applications of geologic carbon sequestration using synthetic polypropylene fiber. J. Nat. Gas Sci. Eng. 2020, 76, 103184. [Google Scholar] [CrossRef]
- Mahmoud, A.A.; Elkatatny, S. The Effect of Silica Content on the Changes in the Mechanical Properties of Class G Cement at High Temperature from Slurry to Set. In Proceedings of the 53rd U.S. Rock Mechanics/Geomechanics Symposium, New York, NY, USA, 23–26 June 2019. [Google Scholar]
- Mahmoud, A.A.; Salaheldin, E. Improved durability of Saudi Class G oil-well cement sheath in CO2 rich environments using olive waste. Constr. Build. Mater. 2020, 262, 120623. [Google Scholar] [CrossRef]
- Alkheder, S.; Yasmeen, T.O.; Madhar, T. Effect of olive waste (Husk) on behavior of cement paste. Case Stud. Constr. Mater. 2016, 5, 19–25. [Google Scholar] [CrossRef]
- Ledesma, R.B.; Lopes, N.F.; Bacca, K.G.; de Moraes, M.K.; Batista, G.D.S.; Pires, M.R.; da Costa, E.M. Zeolite and fly ash in the composition of oil well cement: Evaluation of degradation by CO2 under geological storage condition. J. Pet. Sci. Eng. 2020, 185, 106656. [Google Scholar] [CrossRef]
- Abid, K.; Raoof, G.; Golam, M. A pozzolanic based methodology to reinforce Portland cement used for CO2 storage sites. J. Nat. Gas Sci. Eng. 2020, 73, 103062. [Google Scholar] [CrossRef]
- Tiong, M.; Gholami, R.; Abid, K.; Rahman, M.E. Nanomodification: An efficient method to improve cement integrity in CO2 storage sites. J. Nat. Gas Sci. Eng. 2020, 84, 103612. [Google Scholar] [CrossRef]
- Santos, B.G.D.; Schemmer, L.B.; de Abreu Siqueira, T.; da Costa, E.M. Chemical resistance and mechanical properties of nanosilica addition in oil well cement. J. Pet. Sci. Eng. 2021, 196, 107742. [Google Scholar]
- Ponzi, G.G.D.; Santos, V.H.J.M.D.; Martel, R.B.; Pontin, D.; de Guimarães e Stepanha, A.S.; Schütz, M.K.; Menezes, S.C.; Einloft, S.M.O.; Vecchia, F.D. Basalt powder as a supplementary cementitious material in cement paste for CCS wells: Chemical and mechanical resistance of cement formulations for CO2 geological storage sites. Int. J. Greenh. Gas Control. 2021, 109, 103337. [Google Scholar] [CrossRef]
- Jani, P.; Imqam, A. Class C fly ash-based alkali activated cement as a potential alternative cement for CO2 storage applications. J. Pet. Sci. Eng. 2021, 201, 108408. [Google Scholar] [CrossRef]
- Ahdaya, M.; Abdulmohsin, I. Fly ash Class C based geopolymer for oil well cementing. J. Pet. Sci. Eng. 2019, 179, 750–757. [Google Scholar] [CrossRef]
- Spycher, N.; Pruess, K. CO2-H2O mixtures in the geological sequestration of CO2. II. Partitioning in chloride brines at 12–100 °C and up to 600 bar. Geochim. Cosmochim. Acta 2015, 69, 3309–3320. [Google Scholar] [CrossRef]
- Costa, B.L.S.; Freitas, J.C.O.; Araujo, R.G.S.; Oliveira, Y.H.; Santiago, R.C.; Oliveira, F.S. Analysis of different oil well cement slurry formulations exposed to a CO2-rich environment. J. CO2 Util. 2021, 51, 101636. [Google Scholar] [CrossRef]
- Gu, T.; Zheng, Y.; Yue, H.; Zheng, Y. Characterization of the Pore Structure of Well Cement under Carbon Capture and Storage Conditions by an Image-Based Method with a Combination of Metal Intrusion. ACS Omega 2021, 6, 2110–2120. [Google Scholar] [CrossRef]
- Moraes, M.K.; da Costa, E.M. Effect of adding organo-modified montmorillonite nanoclay on the performance of oil-well cement paste in CO2-rich environments. Cem. Concr. Compos. 2022, 127, 104400. [Google Scholar] [CrossRef]
- Mei, K.; Zhang, L.; Wang, Y.; Cheng, X.; Xue, Q.; Gan, M.; Fu, X.; Zhang, C.; Li, X. Structural evolution in micro-calcite bearing Ca-montmorillonite reinforced oilwell cement during CO2 invasion. Constr. Build. Mater. 2022, 315, 125744. [Google Scholar] [CrossRef]
- Barría, J.C.; Bagheri, M.; Manzanal, D.; Shariatipour, S.M.; Pereira, J.-M. Poromechanical analysis of oil well cements in CO2-rich environments. Int. J. Greenh. Gas Control. 2022, 119, 103734. [Google Scholar] [CrossRef]
- Peng, Z.; Lv, F.; Feng, Q.; Zheng, Y. Enhancing the CO2-H2S corrosion resistance of oil well cement with a modified epoxy resin. Constr. Build. Mater. 2022, 326, 126854. [Google Scholar] [CrossRef]
- Mahmoud, A.A.; Elkatatny, S.; Al-Majed, A.; Al Ramadan, M. The Use of Graphite to Improve the Stability of Saudi Class G Oil-Well Cement against the Carbonation Process. ACS Omega 2022, 7, 5764–5773. [Google Scholar] [CrossRef]
- Mahmoud, A.A.; Elkatatny, S.; Ahmed, A.; Gajbhiye, R. Influence of Nanoclay Content on Cement Matrix for Oil Wells Subjected to Cyclic Steam Injection. Materials 2019, 12, 1452. [Google Scholar] [CrossRef]
- Sanjuán, M.A.; Martialay, R.M. Influence of the age on air permeability of concrete. J. Mater. Sci. 1995, 303, 5657–5662. [Google Scholar] [CrossRef]
- Wu, Z.; Song, J.; Xu, M.; Liu, W.; Chen, R.; Pu, L.; Zhou, S. Effect of weighting materials on carbonation of oil well cement-based composites under high temperature and CO2-rich environment. Arab. J. Chem. 2023, 16, 104670. [Google Scholar] [CrossRef]
- Wang, S.Y.; McCaslin, E.; White, C.E. Effects of magnesium content and carbonation on the multiscale pore structure of alkali-activated slags. Cem. Concr. Res. 2020, 130, 105979. [Google Scholar] [CrossRef]
- Zhang, C.; Song, Y.; Wang, W.; Guo, X.; Li, H. The influence of sulfonated asphalt on the mechanical properties and microstructure of oil well cement paste. Constr. Build. Mater. 2017, 132, 438–445. [Google Scholar] [CrossRef]
- Kravanja, G.; Knez, Ž. Carbonization of Class G well cement containing metakaolin under supercritical and saturated environments. Constr. Build. Mater. 2023, 376, 131050. [Google Scholar] [CrossRef]
- Yan, W.; Wei, H.-G.; Muchiri, N.D.; Li, F.-L.; Zhang, J.-R.; Xu, Z.-X. Degradation of chemical and mechanical properties of cements with different formulations in CO2-containing HTHP downhole environment. Pet. Sci. 2023, 20, 1119–1128. [Google Scholar] [CrossRef]
- Zhang, Y.; Xie, J.; Zhao, W.; Dai, J.; Gao, F. Corrosion Characteristics of Polymer-Modified Oil Well Cement-Based Composite Materials in Geological Environment Containing Carbon Dioxide. Polymers 2024, 16, 2187. [Google Scholar] [CrossRef]
- Xu, B.; Yuan, B.; Wang, Y.; Zhu, L. H2S-CO2 mixture corrosion-resistant Fe2O3-amended wellbore cement for sour gas storage and production wells. Constr. Build. Mater. 2018, 188, 161–169. [Google Scholar] [CrossRef]
- Sedić, K.; Ukrainczyk, N.; Mandić, V.; Međimurec, N.G. Carbonation study of new calcium aluminate cement-based CO2 injection well sealants. Constr. Build. Mater. 2024, 419, 135517. [Google Scholar] [CrossRef]
- Sedic, K. Optimization of Cement Slurry Compositions for Cementing Protective; University of Zagreb: Zagreb, Croatia, 2017. [Google Scholar]
- Barría, J.C.; Manzanal, D.; Ghabezloo, S.; Pereira, J.M. Effect of supercritical carbonation on porous structure and mechanical strength of cementitious materials modified with bacterial nanocellulose. Mater. Struct. 2024, 56, 180. [Google Scholar] [CrossRef]
- Cerrutti, P.; Roldán, P.; García, R.M.; Galvagno, M.A.; Vázquez, A.; Foresti, M.L. Production of bacterial nanocellulose from wine industry residues: Importance of fermentation time on pellicle characteristics. J. Appl. Polym. Sci. 2015, 133, 43109. [Google Scholar] [CrossRef]
- Barría, J.C.; Analía, V.; Jean-Michel, P.; Diego, M. Effect of bacterial nanocellulose on the fresh and hardened states of oil well cement. J. Pet. Sci. Eng. 2021, 199, 108259. [Google Scholar] [CrossRef]
- Shine, J.M.; Qasmi, U.; Elhancha, A. Exploring CCUS Well Construction Using Fit-For-Purpose Cement Designs. In Proceedings of the International Petroleum Technology Conference, Dhahran, Saudi Arabia, 14 February 2024. [Google Scholar]
- Lende, G.; Sørensen, E.; Jandhyala, S.R.K.; van Noort, R. State of the art Test Method to Quantify Progression Rate of Carbonation of Wellbore Sealing Materials. In Proceedings of the SPE Europe Energy Conference and Exhibition, Turin, Italy, 26–28 June 2024. [Google Scholar]
- Lende, G.; Clausen, J.A.; Kvassnes, A.J. Evaluation of New Innovative Cement Blend for Enhanced CO2 and H2S Resistance. In Proceedings of the SPE/IADC International Drilling Conference and Exhibition, Virtual, 8–12 March 2021. [Google Scholar]
- Teodoriu, C.; Bello, O. A review of cement testing apparatus and methods under CO2 environment and their impact on well integrity prediction—Where do we stand? J. Pet. Sci. Eng. 2020, 187, 106736. [Google Scholar] [CrossRef]
- Bagheri, M.; Seyed, M.S.; Eshmaiel, G. A review of oil well cement alteration in CO2-rich environments. Constr. Build. Mater. 2018, 186, 946–968. [Google Scholar] [CrossRef]
- Lavrov, A. Stiff cement, soft cement: Nonlinearity, arching effect, hysteresis, and irreversibility in CO2-well integrity and near-well geomechanics. Int. J. Greenh. Gas Control. 2018, 70, 236–242. [Google Scholar] [CrossRef]
- Zou, X.; Zhu, Y.; Lv, J.; Zhou, Y.; Ding, B.; Liu, W.; Xiao, K.; Zhang, Q. Toward Estimating CO2 Solubility in Pure Water and Brine Using Cascade Forward Neural Network and Generalized Regression Neural Network: Application to CO2 Dissolution Trapping in Saline Aquifers. ACS Omega 2024, 9, 4705–4720. [Google Scholar] [CrossRef]
- Lin, Y.; Alengaram, U.J.; Ibrahim, Z. Effect of treated and untreated rice husk ash, palm oil fuel ash, and sugarcane bagasse ash on the mechanical, durability, and microstructure characteristics of blended concrete—A comprehensive review. J. Build. Eng. 2023, 78, 107500. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).