Study on Surfactants for the Removal of Water from Deliquification Natural Gas Wells to Enhance Production
Abstract
1. Introduction
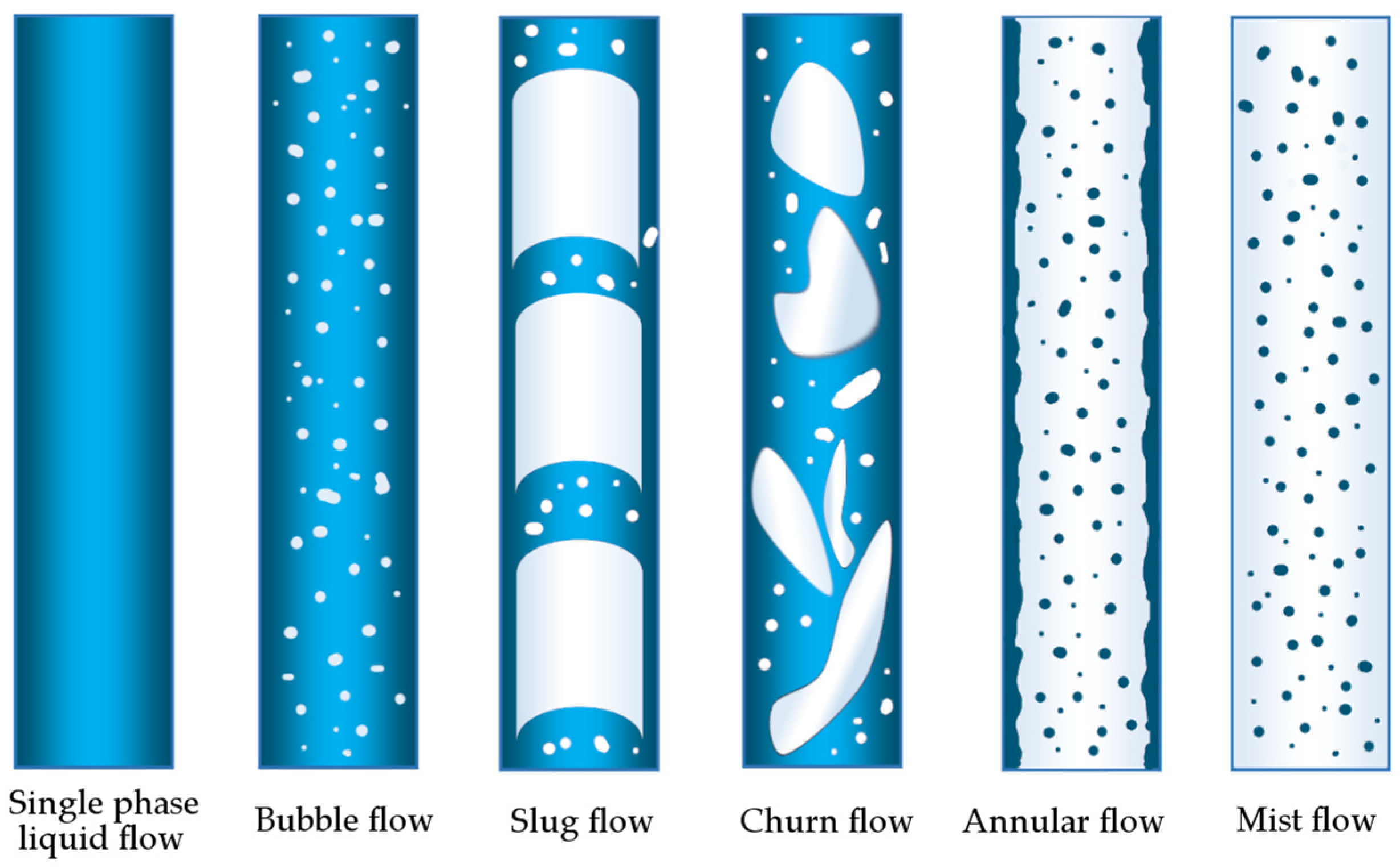
- annular flow occurring at high gas velocities, where the gas is in a continuous phase, and the liquid is present as dispersed droplets in the gas and a thin film on the pipe wall;
- mass flow, occurring at lower gas velocities, where the flow changes from a continuous gaseous phase to a continuous liquid phase, implying a transition to a return flow where the liquid film reaches a certain point and starts to flow downwards;
- slug flow, occurring as the velocity of the gas flow decreases further, during which gas bubbles intermingle with liquid films;
- bubble flow, occurring at even lower gas flow rates, where the gas is in the form of small bubbles floating in the liquid.
2. Materials and Methods
2.1. Materials
- BioLight 30/380—stick containing ethoxylated C16-18 alcohols as non-ionic surfactants, d (25 °C) = 0.800 g/cm3, held on the water surface;
- BioFoam 30—stick containing ethoxylated C16-18 alcohols as non-ionic surfactants, d (25 °C) = 1.100 g/cm3;
- BioCond 30—stick containing non-ionic and amphoteric surfactants, the main ingredient being (2-hydroxy-3-sulfopropyl)dimethyl [3-[(1-oxododecyl)amino]propyl]ammonium hydroxide, d (25 °C) = 1.100 g/cm3, recommended for foaming waters with high condensate content;
- BioAcid 30/380—stick whose main ingredient is amidosulphonic acid (60%) (non-ionic surfactant) with the addition of a corrosion inhibitor, d (25 °C) = 1.400 g/cm3;
- BioCond Plus 30/380—non-ionic and amphoteric surfactant-based stick, whose main ingredient is (2-hydroxy-3-sulfopropyl)dimethyl[3-[(1-oxododecyl)amino]propyl]ammonium hydroxide (15%), d (25 °C) = 1.120 g/cm3, recommended for foaming waters with a high hydrocarbon condensate content.
2.2. Methods
2.2.1. Analysis of Formation Waters
2.2.2. Natural Gas Analysis
2.3. Experimental Procedures
2.3.1. Tests Under Laboratory Conditions
2.3.2. Tests Under Well Conditions
3. Results and Discussion
3.1. Laboratory Tests
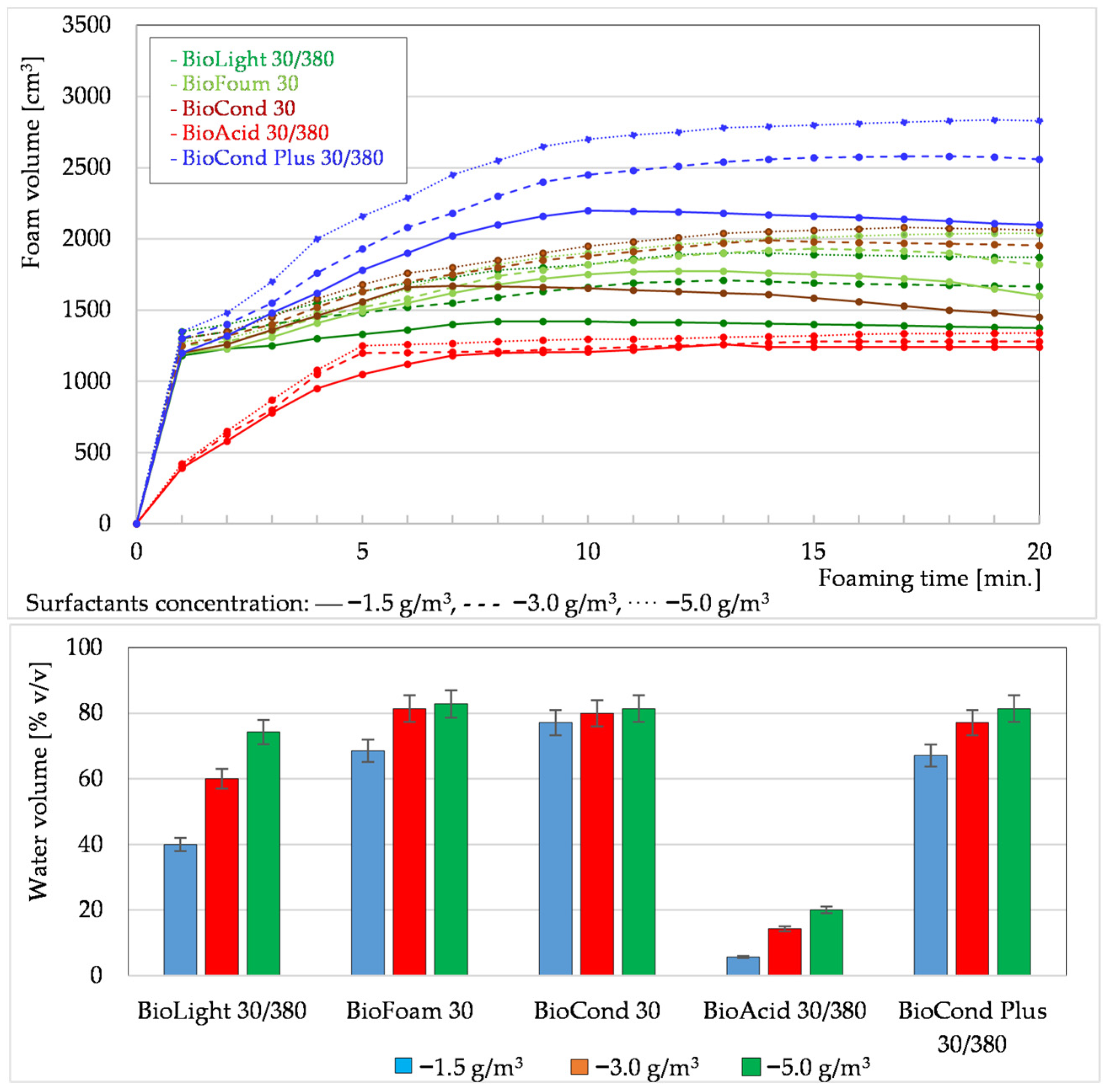
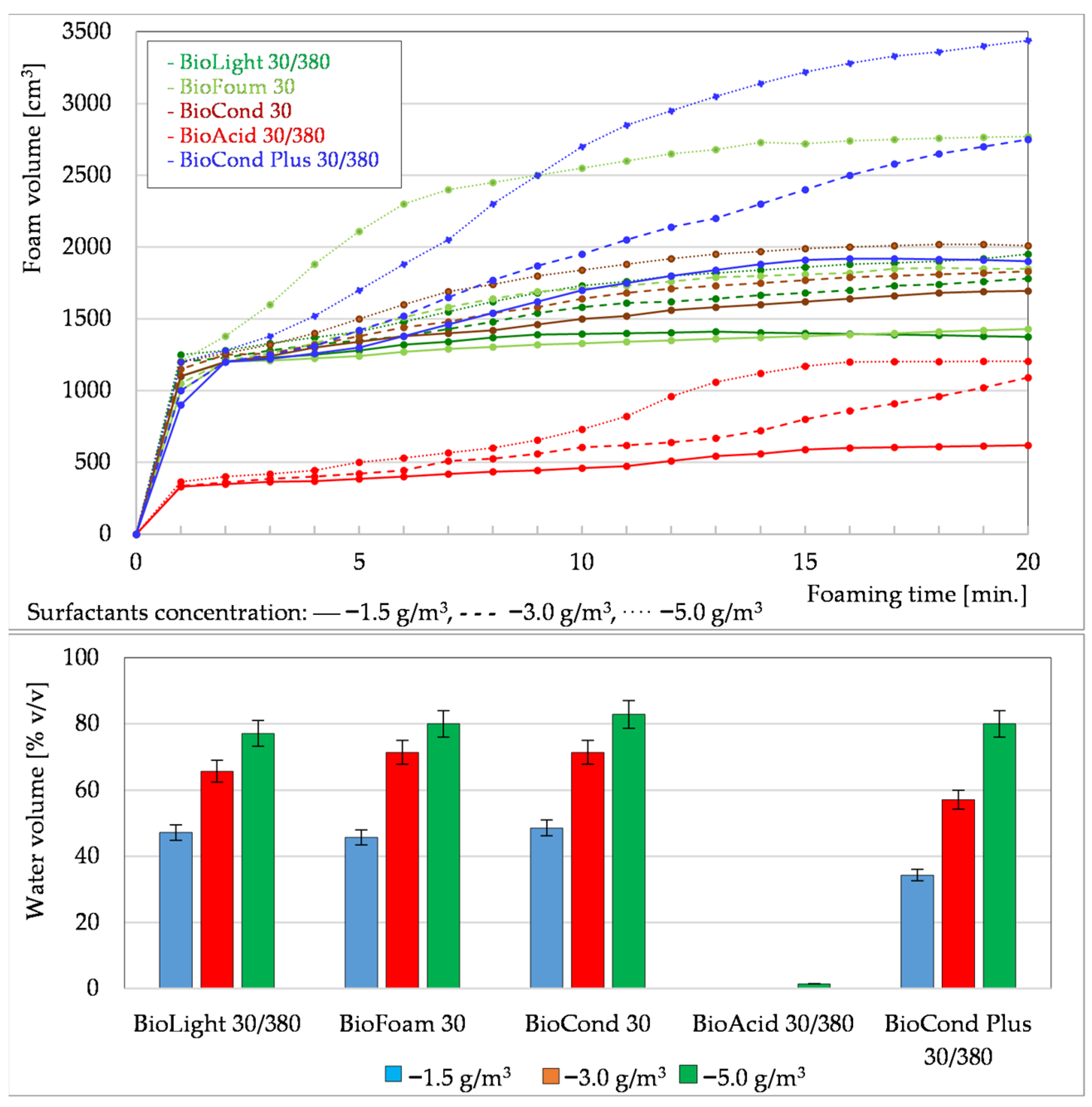
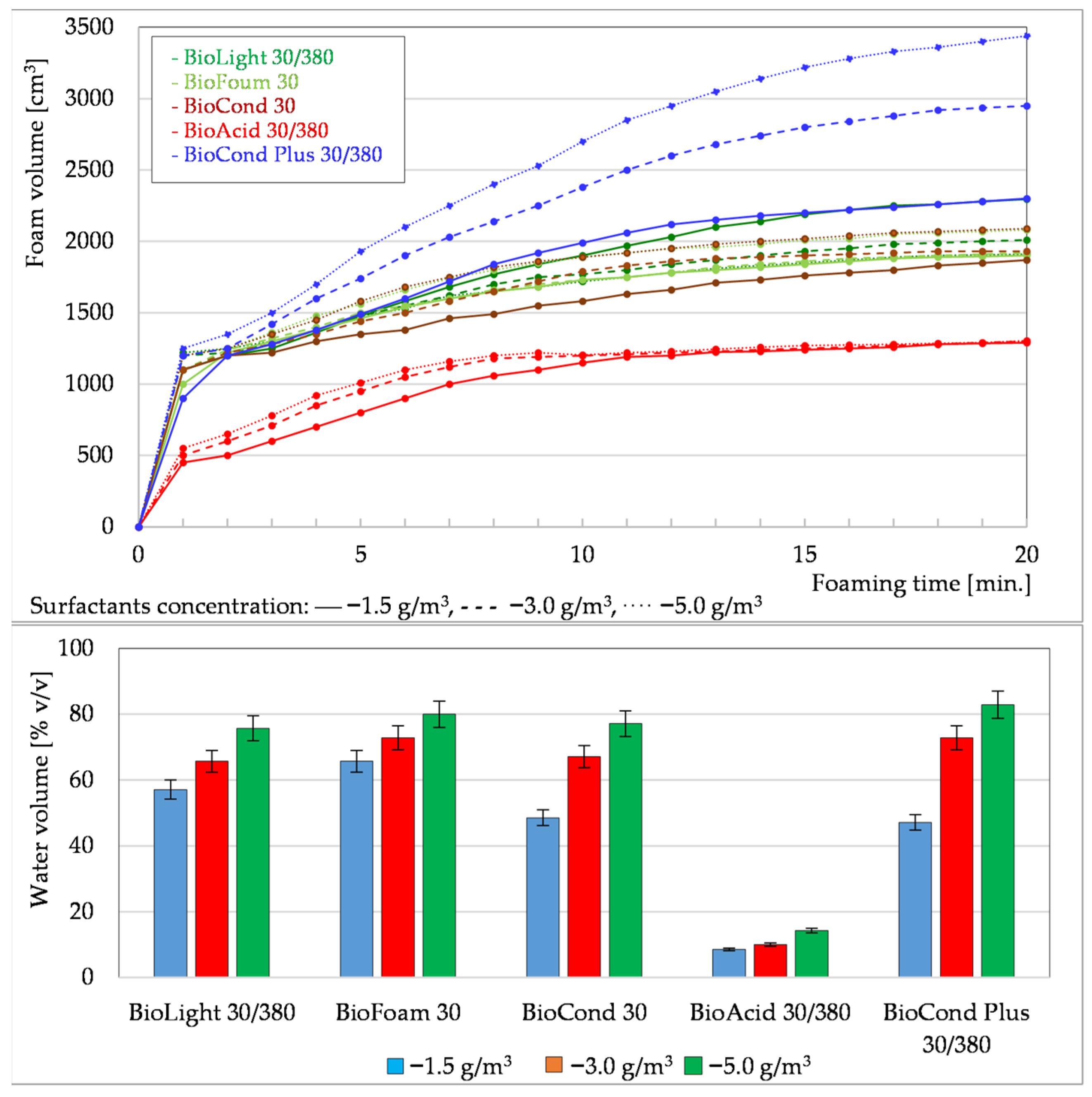
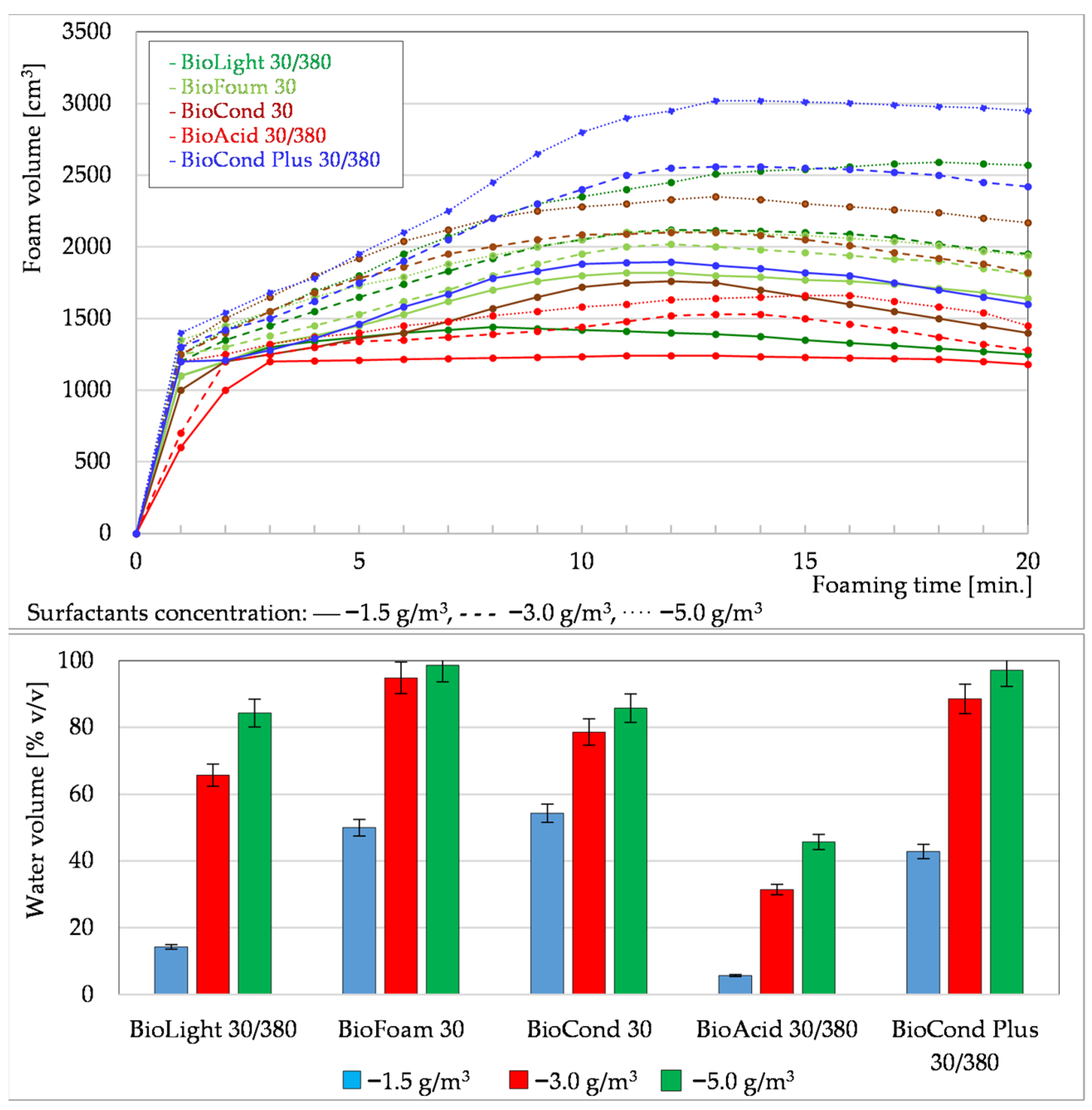
3.1.1. Foaming Tests of Formation Waters Using Surfactants
3.1.2. Measurement of Surface Tension of Solutions
3.1.3. Summary of Foaming Tests Carried Out Under Laboratory Conditions
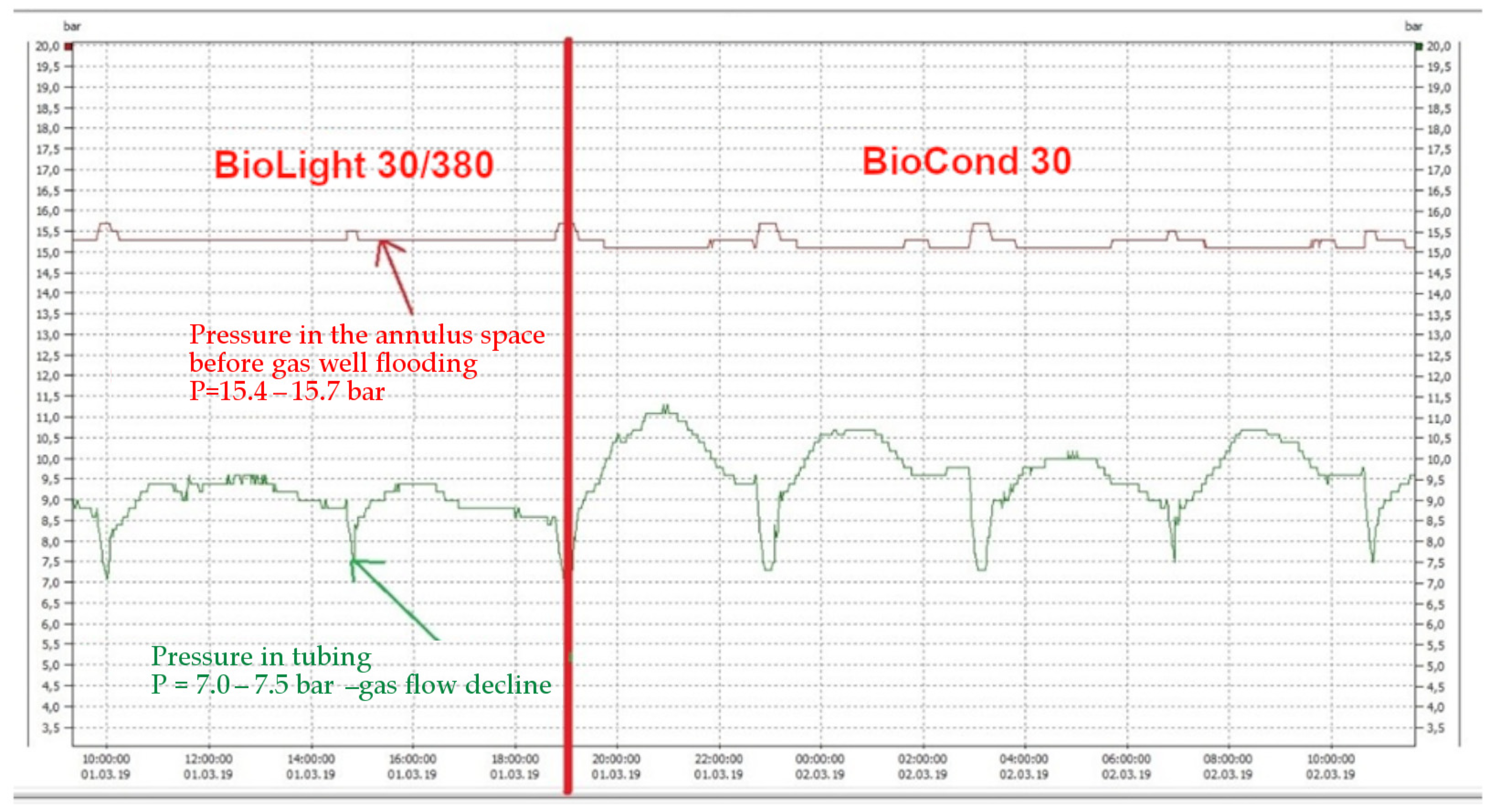
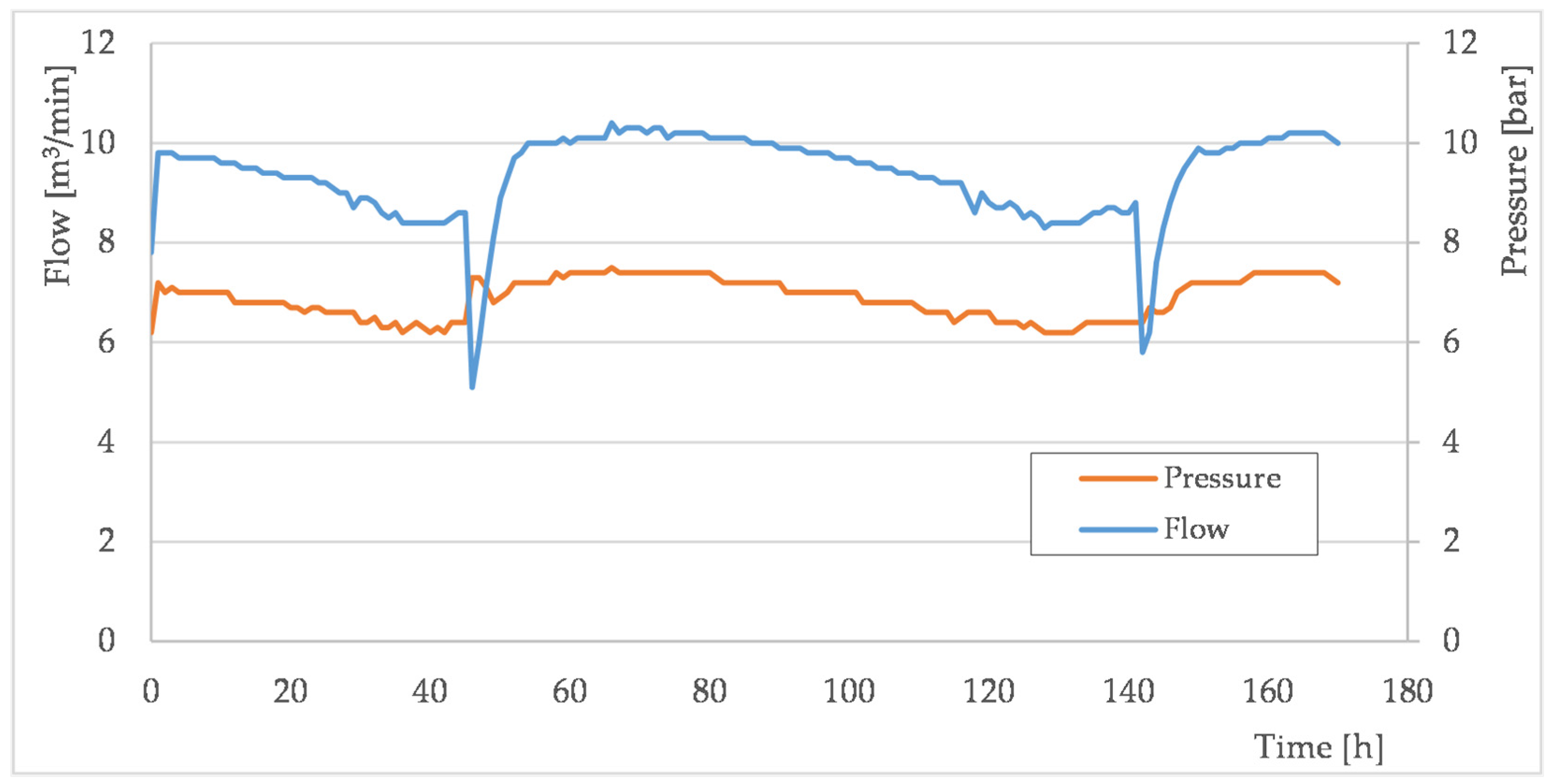
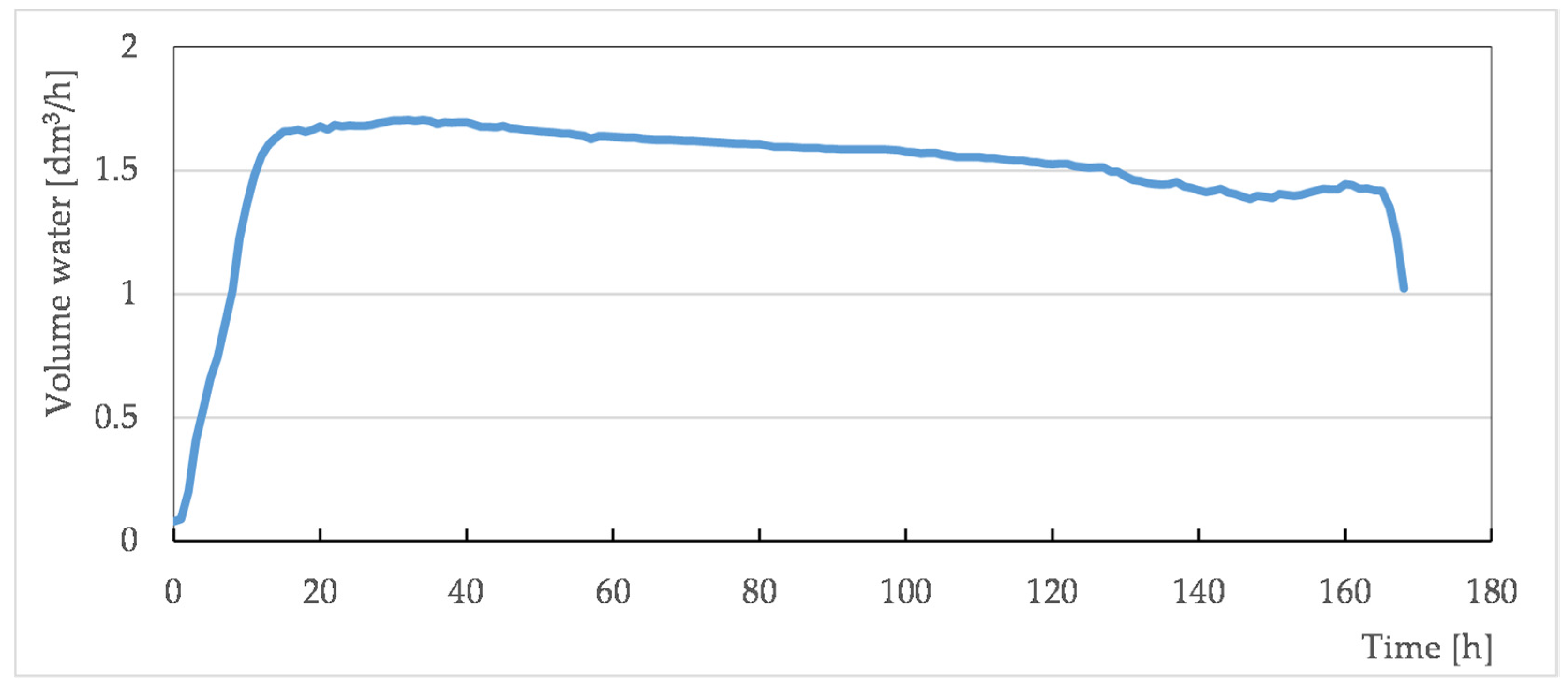
3.2. Well Tests of Formation Water Foaming Using Surfactants
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Beggs, H.D. Production Optimization Using NODALTM Analysis, 2nd ed; OGCI: London, UK, 2003. [Google Scholar]
- Adeyemi, G.A.; Ling, K.; Fadairo, A. Experimental investigation of the impact of soybean oil-derived surfactant as a surface-active agent during gas well deliquification. Pet. Res. 2024. [Google Scholar] [CrossRef]
- He, X.; Lei, W.; Xu, X.; Xu, J.; Qiu, J.; Dai, W.; Cui, L.; Ma, J.; Shen, Z.; Li, Y. Gas well deliquification in the presence of high content of condensate: From laboratory to field test. In Proceedings of the SPE Middle East Oil & Gas Show and Conference, Event Canceled. 1–28 December 2021; SPE: Denver, CO, USA, 2021; p. D041S050R001. [Google Scholar] [CrossRef]
- Szpunar, T.; Budak, P. Ilościowe ujęcie zagadnienia usuwania wody z odwiertów gazowych. Naft.-Gaz 2012, 1, 27–31. [Google Scholar]
- Falkowicz, S.; Dubiel, S.; Cicha-Szot, R. Case studies of water shut-off treatments in oil and gas production wells. Miner. Resour. Manag. 2012, 28, 125–136. [Google Scholar] [CrossRef]
- Chang, P.; Bai, B. An improved method of gas well deliquification using supersonic nozzle. Int. J. Heat Mass Transf. 2017, 108, 2262–2272. [Google Scholar] [CrossRef]
- Kluk, D.; Steliga, T.; Bęben, D. Badania wpływu środków pieniących na skuteczność wynoszenia wody złożowej z odwiertu. Naft.-Gaz 2023, 79, 420–427. [Google Scholar] [CrossRef]
- Li, Q.; Wang, Y.; Wang, Y.; San, J.; Li, Q.; Foster, G. Synthetic Process on Hydroxyl-Containing Polydimethylsiloxane as a Thickener in CO2 Fracturing and Thickening Performance Test. Energy Sources Part A Recovery Util. Environ. Eff. 2018, 40, 1137–1143. [Google Scholar] [CrossRef]
- Li, Q.; Zhang, C.; Yang, Y.; Ansari, U.; Han, Y.; Li, X.; Cheng, Y. Preliminary Experimental Investigation on Long-Term Fracture Conductivity for Evaluating the Feasibility and Efficiency of Fracturing Operation in Offshore Hydrate-Bearing Sediments. Ocean. Eng. 2023, 281, 114949. [Google Scholar] [CrossRef]
- Duangprasert, T.; Sirivat, A.; Siemanond, K.; Wilkes, J.O. Vertical two-phase flow regimes and pressure gradients under the influence of SDS surfactant. Exp. Therm. Fluid Sci. 2008, 32, 808–817. [Google Scholar] [CrossRef]
- Van Nimwegen, A.T.; Portela, L.M.; Henkes, R.A.W.M. The effect of surfactants on upward air–water pipe flow at various inclinations. Int. J. Multiph. Flow 2016, 78, 132–147. [Google Scholar] [CrossRef]
- Amani, P.; Rudolph, V.; Hurter, S.; Firouzi, M. Sustainable dewatering of unconventional gas wells using engineered multiphase flow dynamics. Fuel 2022, 324, 124675. [Google Scholar] [CrossRef]
- Sun, Y.Q.; Zhang, Y.P.; Liu, Q.W.; Fan, Z.Z.; Li, N.; Wei, A.Q. Research progress on new highly efficient foam drainage agents for gas wells (A review). Pet. Chem. 2023, 63, 1119–1131. [Google Scholar] [CrossRef]
- Zhang, Z.; Wang, Z.; Gao, Y.; Li, H.; Wang, J.; Sun, B. Experimental study on the effect of surfactants on the characteristics of gas carrying liquid in vertical churn and annular flows. J. Pet. Sci. Eng. 2019, 180, 347–356. [Google Scholar] [CrossRef]
- Ajani, A.; Kelkar, M.; Sarica, C.; Pereyra, E. Effect of surfactants on liquid loading in vertical wells. Int. J. Multiph. Flow 2016, 83, 183–201. [Google Scholar] [CrossRef]
- Liang, Z.; Wang, F.; Deng, X. A novel technology of combining foam injection and compression to lift liquid in water flooded gas wells. J. Nat. Gas Sci. Eng. 2014, 19, 147–151. [Google Scholar] [CrossRef]
- Oyewole, P.O.; Lea, J.F. Artificial lift selection strategy for the life of a gas well with some liquid production. In All Days; SPE: Denver, CO, USA, 2008; p. SPE-115950-MS. [Google Scholar] [CrossRef]
- Veeken, K.; Hinai, K.; Shanfari, A.A.; Yamadi, A.; Zadjali, A.; Saidi, A.; Hadhrami, H.; Daraai, T.; Mans, J.; Yousfi, I.; et al. Evaluating performance of foam-assisted lift in sultanate of foman by dedicated field testing. In Proceedings of the Abu Dhabi International Petroleum Exhibition & Conference, Abu Dhabi, United Arab Emirates, 13–16 November 2017; SPE: Abu Dhabi, United Arab Emirates, 2017; p. D021S033R006. [Google Scholar] [CrossRef]
- Lea, J.F.; Rowlan, L. Gas Well Deliquification, 3rd ed.; Gulf Drilling Guides; Gulf Professional Publishing: Cambridge, MA, USA, 2019. [Google Scholar]
- Martins, A.; Marino, M.; Kerem, M.; Guzman, M. Foam assisted gas lift: The impact of different surfactant delivery methods on oil well performance. In SPE Europec Featured at 81st EAGE Conference and Exhibition; SPE: London, UK, 2019; p. D041S012R002. [Google Scholar] [CrossRef]
- Zhang, P.; Guo, D.; Cao, X.; Li, X.; Xia, W.; Peng, W.; Bian, J. Foam stability: The key to inhibiting slug generation in gas–liquid flow. J. Pet. Sci. Eng. 2022, 218, 110969. [Google Scholar] [CrossRef]
- Hearn, W. Gas well deliquification. In All Days; SPE: Abu Dhabi, United Arab Emirates, 2010; p. SPE-138672-MS. [Google Scholar] [CrossRef]
- Zhou, H.; Qu, C.; Lu, G.; Li, Z.; Wang, X.; Yin, H.; Feng, Y. Deliquification of low-productivity natural gas wells with in situ generated foams and heat. Energy Fuels 2021, 35, 9873–9882. [Google Scholar] [CrossRef]
- Seifert, U. Hydrodynamic Lift on bound vesicles. Phys. Rev. Lett. 1999, 83, 876–879. [Google Scholar] [CrossRef]
- Gcali, C.; Karami, H.; Pereyra, E.; Sarica, C. Surfactant batch treatment efficiency as an artificial lift method for horizontal gas wells. In Proceedings of the SPE Artificial Lift Conference and Exhibition–Americas, The Woodlands, TX, USA, 28–30 August 2018; SPE: The Woodlands, TX, USA, 2018; p. D012S001R004. [Google Scholar] [CrossRef]
- Kawale, D.; Van Nimwegen, A.T.; Portela, L.M.; Van Dijk, M.A.; Henkes, R.A.W.M. The relation between the dynamic surface tension and the foaming behaviour in a sparger setup. Colloids Surf. A Physicochem. Eng. Asp. 2015, 481, 328–336. [Google Scholar] [CrossRef]
- Steliga, T.; Jakubowicz, P.; Kapusta, P. Changes in toxicity during treatment of wastewater from oil plant contaminated with petroleum hydrocarbons: Changes in toxicity during treatment of wastewater from oil plant. J. Chem. Technol. Biotechnol. 2015, 90, 1408–1418. [Google Scholar] [CrossRef]
- Miller, R.; Liggieri, L. (Eds.) Bubble and Drop Interfaces; CRC Press: Boca Raton, FL, USA, 2011. [Google Scholar] [CrossRef]
- Janocha, A. Zmiany napięcia powierzchniowego zasiarczonej wody złożowej na granicy z powietrzem. Naft.-Gaz 2014, 6, 365–369. [Google Scholar]
- Han, W.; Fan, J.; Lv, H.; Yan, Y.; Liu, C.; Dong, S. Excellent foaming properties of anionic-zwitterionic-Gemini cationic compound surfactants for gas well deliquification: Experimental and computational investigations. Colloids Surf. A Physicochem. Eng. Asp. 2022, 653, 129944. [Google Scholar] [CrossRef]
- Wang, Z.; Song, H. The synthesis of quaternary n-alkyl tropinium cationic surfactants and study on their properties: Effect of temperature, hydrophobic chain length and anions. J. Mol. Struct. 2022, 1268, 133732. [Google Scholar] [CrossRef]
- Dong, S.; Fan, J.; Lv, H.; Qiang, T.; Ji, Y.; Han, W. Excellent condensate and salt-resistant foam by anionic-zwitterionic-cationic Gemini surfactants compounds for gas well deliquification. J. Surfact. Deterg. 2024, 27, 135–145. [Google Scholar] [CrossRef]
- Xu, Q.; Nakajima, M.; Ichikawa, S.; Nakamura, N.; Roy, P.; Okadome, H.; Shiina, T. Effects of surfactant and electrolyte concentrations on bubble formation and stabilization. J. Colloid Interface Sci. 2009, 332, 208–214. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.; Panthi, K.; Weerasooriya, U.; Mohanty, K.K. Multistimuli-responsive foams using an anionic surfactant. Langmuir 2018, 34, 11010–11020. [Google Scholar] [CrossRef]
- Emami, H.; Ayatizadeh Tanha, A.; Khaksar Manshad, A.; Mohammadi, A.H. Experimental investigation of foam flooding using anionic and nonionic surfactants: A screening scenario to assess the effects of salinity and pH on foam stability and foam height. ACS Omega 2022, 7, 14832–14847. [Google Scholar] [CrossRef]
- Johri, N.; Kadam, S.; Veeken, K.; Prakash, A.; Suvarna, H.; Singh, P.; Daukia, S.; Kadirus, S.; Goswami, A.; Aakash, S. Conquer liquid loading in raageshwari gas field by automated intermittent production and deliquification. In Proceedings of the ADIPEC, Abu Dhabi, United Arab Emirates, 2–5 October 2023; SPE: Abu Dhabi, United Arab Emirates, 2023; p. D021S075R003. [Google Scholar] [CrossRef]
- Gao, F.; Dong, S.; Wang, D.; Lv, H.; Han, W. A novel foaming formulation with excellent salt-resistant for foam assisted deliquification of gas wells. J. Dispers. Sci. Technol. 2024, 1–11. [Google Scholar] [CrossRef]
- Yakhshi-Tafti, E.; Kumar, R.; Cho, H.J. Measurement of surface interfacial tension as a function of temperature using pendant drop images. Int. J. Optomechatronics 2011, 5, 393–403. [Google Scholar] [CrossRef]
- Paswan, B.K.; Kumar, S.; Mahto, V. Evaluation of a soybean oil derived surfactant in the development of oil-in-water (O/W) emulsion drilling mud for shale formation. J. Pet. Sci. Eng. 2022, 217, 110926. [Google Scholar] [CrossRef]
- Fadairo, A.; Adeyemi, G.; Ogunkunle, T.; Lawal, O.; Oredeko, O. Modelling minimum flow rate required for unloading liquid in gas wells. In Proceedings of the SPE Nigeria Annual International Conference and Exhibition, Virtual, 11 August 2020; p. D013S004R009. [Google Scholar] [CrossRef]
- Zhang, Z.; Xiong, Y.; Pu, H.; Sun, Z. Effect of the variations of thermophysical properties of drilling fluids with temperature on wellbore temperature calculation during drilling. Energy 2021, 214, 119055. [Google Scholar] [CrossRef]
- Tavakkoli, M.; Panuganti, S.R.; Khemka, Y.; Valdes, H.; Vargas, F.M. Foam-assisted gas lift: A novel experimental setup to investigate the feasibility of using a commercial surfactant for increasing oil well productivity. J. Pet. Sci. Eng. 2021, 201, 108496. [Google Scholar] [CrossRef]
- Cao, Q.; Cao, Q. Effects of Temperature on the properties of the new gel foam. In Advances in Transdisciplinary Engineering; Chen, M., Giorgetti, M., Li, Z., Chen, Z., Jin, B., Agarwal, R.K., Eds.; IOS Press: Amsterdam, The Netherlands, 2022. [Google Scholar] [CrossRef]
- Yang, L.; Estrada, L.P. Surfactant adsorbed at the oil-water interface and its elimination. GEP 2015, 3, 40–46. [Google Scholar] [CrossRef]
- Kinate, B.B.; Nmegbu, C.G.J.; Amadichuku, N. Investigation of the Impact of Surfactant Concentration on Gas Well Deliquification. In Proceedings of the SPE Nigeria Annual International Conference and Exhibition, Lagos, Nigeria, 31 July–2 August 2023; SPE: Lagos, Nigeria, 2023; p. D031S021R002. [Google Scholar] [CrossRef]
- Wang, H.; Liu, J.; Yang, Q.; Wang, Y.; Li, S.; Sun, S.; Hu, S. Study on the influence of the external conditions and internal components on foam performance in gas recovery. Chem. Eng. Sci. 2021, 231, 116279. [Google Scholar] [CrossRef]
- Wang, S.; Chen, C.; Yuan, N.; Ma, Y.; Ogbonnaya, O.I.; Shiau, B.; Harwell, J.H. Design of extended surfactant-only EOR formulations for an ultrahigh salinity oil field by using hydrophilic lipophilic deviation (HLD) approach: From laboratory screening to simulation. Fuel 2019, 254, 115698. [Google Scholar] [CrossRef]
- Yang, J.; Jovancicevic, V.; Ramachandran, S. Foam for gas well deliquification. Colloids Surf. A Physicochem. Eng. Asp. 2007, 309, 177–181. [Google Scholar] [CrossRef]



| Type of Foaming Stick | Surfactant Concentration | |||
|---|---|---|---|---|
| 0 [g/m3] | 1.5 [g/m3] | 3.0 [g/m3] | 5.0 [g/m3] | |
| Surface Tension [mN/m] | ||||
| Brine from A well | ||||
| BioLight 30/380 | 65.8 ± 5.5 | 63.5 ± 5.4 | 62.5 ± 5.4 | 61.8 ± 5.3 |
| BioFoam 30 | 65.8 ± 5.5 | 62.0 ± 5.4 | 61.3 ± 5.3 | 60.5 ± 5.2 |
| BioCond 30 | 65.8 ± 5.5 | 61.5 ± 5.3 | 61.0 ± 5.3 | 60.8 ± 5.2 |
| BioAcid 30/380 | 65.8 ± 5.5 | 63.1 ± 5.4 | 63.0 ± 5.4 | 61.0 ± 5.3 |
| BioCond Plus 30/380 | 65.8 ± 5.5 | 62.0 ± 5.4 | 61.6 ± 5.3 | 60.5 ± 5.2 |
| Brine from B well | ||||
| BioLight 30/380 | 71.8 ± 5.8 | 63.5 ± 5.4 | 61.8 ± 5.3 | 61.6 ± 5.3 |
| BioFoam 30 | 71.8 ± 5.8 | 63.6 ± 5.4 | 63.0 ± 5.4 | 61.0 ± 5.3 |
| BioCond 30 | 71.8 ± 5.8 | 63.8 ± 5.4 | 63.7 ± 5.4 | 61.8 ± 5.4 |
| BioAcid 30/380 | 71.8 ± 5.8 | 67.0 ± 5.6 | 64.2 ± 5.5 | 63.0 ± 5.5 |
| BioCond Plus 30/380 | 71.8 ± 5.8 | 62.4 ± 5.3 | 60.7 ± 5.2 | 59.5 ± 5.1 |
| Brine from C well | ||||
| BioLight 30/380 | 70.0 ± 5.7 | 64.2 ± 5.5 | 63.9 ± 5.5 | 64.0 ± 5.5 |
| BioFoam 30 | 70.0 ± 5.7 | 64.8 ± 5.5 | 63.4 ± 5.4 | 63.0 ± 5.4 |
| BioCond 30 | 70.0 ± 5.7 | 65.2 ± 5.5 | 63.6 ± 5.4 | 63.0 ± 5.4 |
| BioAcid 30/380 | 70.0 ± 5.7 | 65.6 ± 5.6 | 63.8 ± 5.4 | 63.0 ± 5.4 |
| BioCond Plus 30/380 | 70.0 ± 5.7 | 65.0 ± 5.5 | 62.2 ± 5.3 | 60.0 ± 5.2 |
| Brine from D well | ||||
| BioLight 30/380 | 71.8 ± 5.8 | 65.1 ± 5.5 | 64.0 ± 5.4 | 63.0 ± 5.4 |
| BioFoam 30 | 71.8 ± 5.8 | 63.0 ± 5.4 | 62.0 ± 5.3 | 61.0 ± 5.3 |
| BioCond 30 | 71.8 ± 5.8 | 64.3 ± 5.4 | 64.0 ± 5.4 | 63.0 ± 5.4 |
| BioAcid 30/380 | 71.8 ± 5.8 | 65.2 ± 5.5 | 64.0 ± 5.4 | 63.5 ± 5.4 |
| BioCond Plus 30/380 | 71.8 ± 5.8 | 62.6 ± 5.3 | 62.0 ± 5.3 | 61.5 ± 5.3 |
| Tap water | ||||
| BioLight 30/380 | 72.6 ± 5.9 | 70.3 ± 5.8 | 67.3 ± 5.7 | 61.2 ± 5.3 |
| BioFoam 30 | 72.6 ± 5.9 | 69.9 ± 5.8 | 66.8 ± 5.6 | 59.8 ± 5.1 |
| BioCond 30 | 72.6 ± 5.9 | 70.0 ± 5.8 | 67.0 ± 5.6 | 60.3 ± 5.2 |
| BioAcid 30/380 | 72.6 ± 5.9 | 70.1 ± 5.8 | 67.2 ± 5.7 | 60.6 ± 5.2 |
| BioCond Plus 30/380 | 72.6 ± 5.9 | 69.5 ± 5.7 | 66.2 ± 5.6 | 59.2 ± 5.0 |
| Parameter | Well A | Well B | Well C | Well A + BioFoum 30 | Well B + BioFoum 30 | Well C + BioCond Plus 30/380 |
|---|---|---|---|---|---|---|
| Pressure [MPa] | 0.61 | 0.62 | 0.62 | 0.75 | 0.81 | 0.86 |
| Gas production [m3/min] | 1.29 | 2.99 | 8.2 | 2.29 | 4.15 | 10.3 |
| Water production [dm3/day] | 1.0 | 0.45 | 0.08 | 16 | 17 | 36 |
| Frequency of dosing surfactant sticks [day] | - | - | - | 7 | 18 | 7 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kluk, D.; Steliga, T.; Bęben, D.; Jakubowicz, P. Study on Surfactants for the Removal of Water from Deliquification Natural Gas Wells to Enhance Production. Energies 2024, 17, 5924. https://doi.org/10.3390/en17235924
Kluk D, Steliga T, Bęben D, Jakubowicz P. Study on Surfactants for the Removal of Water from Deliquification Natural Gas Wells to Enhance Production. Energies. 2024; 17(23):5924. https://doi.org/10.3390/en17235924
Chicago/Turabian StyleKluk, Dorota, Teresa Steliga, Dariusz Bęben, and Piotr Jakubowicz. 2024. "Study on Surfactants for the Removal of Water from Deliquification Natural Gas Wells to Enhance Production" Energies 17, no. 23: 5924. https://doi.org/10.3390/en17235924
APA StyleKluk, D., Steliga, T., Bęben, D., & Jakubowicz, P. (2024). Study on Surfactants for the Removal of Water from Deliquification Natural Gas Wells to Enhance Production. Energies, 17(23), 5924. https://doi.org/10.3390/en17235924









