High-Capacity Energy Storage Devices Designed for Use in Railway Applications
Abstract
1. Introduction
1.1. Possibility of Energy Saving in Transport
1.2. Materials Used in Supercapacitors
- ➢
- Eco-friendliness and renewability—starch is a biodegradable, renewable material, making it an environmentally friendly choice. This is particularly valuable in reducing the ecological impact of energy storage devices, which is a growing concern in material science and engineering.
- ➢
- High power density—starch-derived materials can deliver high power density, which is advantageous in applications where rapid charging and discharging are more critical than overall energy storage capacity.
- ➢
- Cost-effectiveness—starch is an abundant and low-cost material. This can reduce the overall production cost of supercapacitors, making them more accessible for broader applications.
1.3. Challenges of Using Supercapacitors in Transport, Particularly Rail
- ➢
- Energy density limitations
- ➢
- Cost
- ➢
- Voltage balancing
- ➢
- Temperature sensitivity
- ➢
- Energy recovery efficiency
- ➢
- Space and weight considerations
- ➢
- Integration with existing infrastructure
- ➢
- Controlled energy discharge
2. Methods and Materials
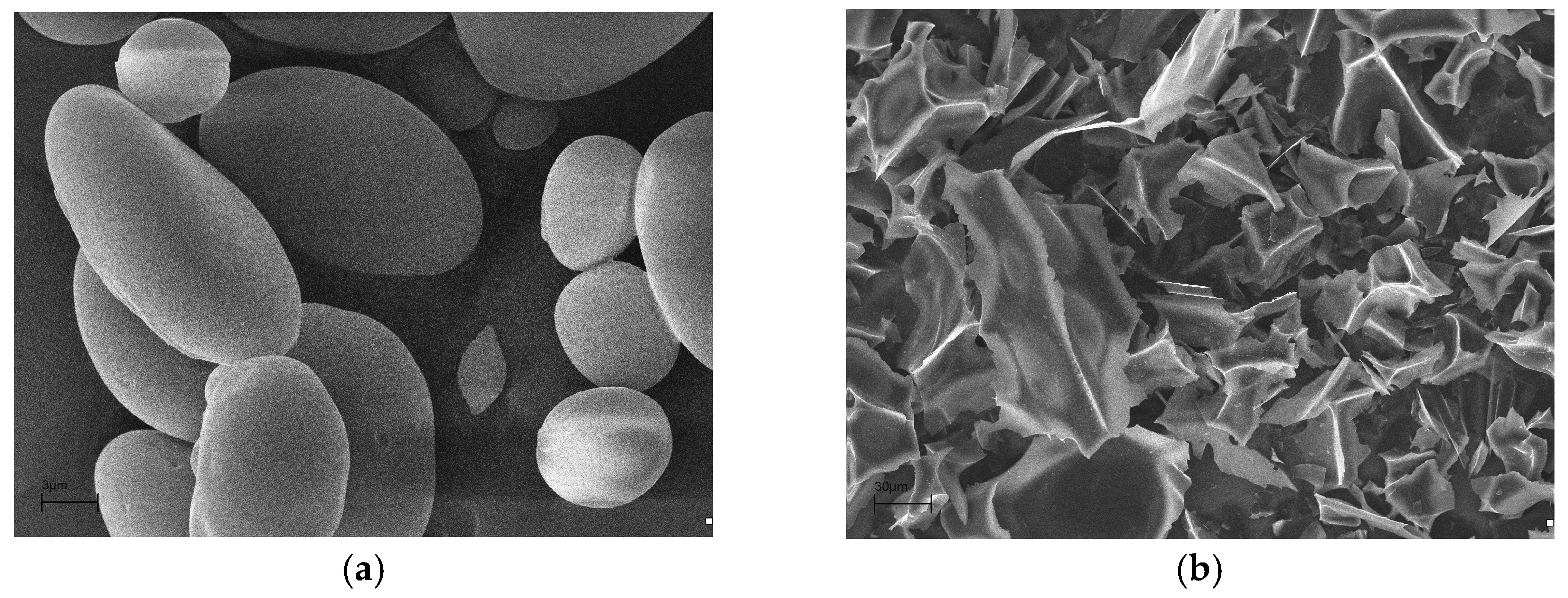
2.1. Synthesis of Carbon Material from Starch
2.2. Research Object
- Current and voltage at the pantograph before the main switch;
- Current and voltage at braking resistors;
- Current and voltage at the output of traction rectifier;
- Speed from the GPS device and from sensors installed on the axles.
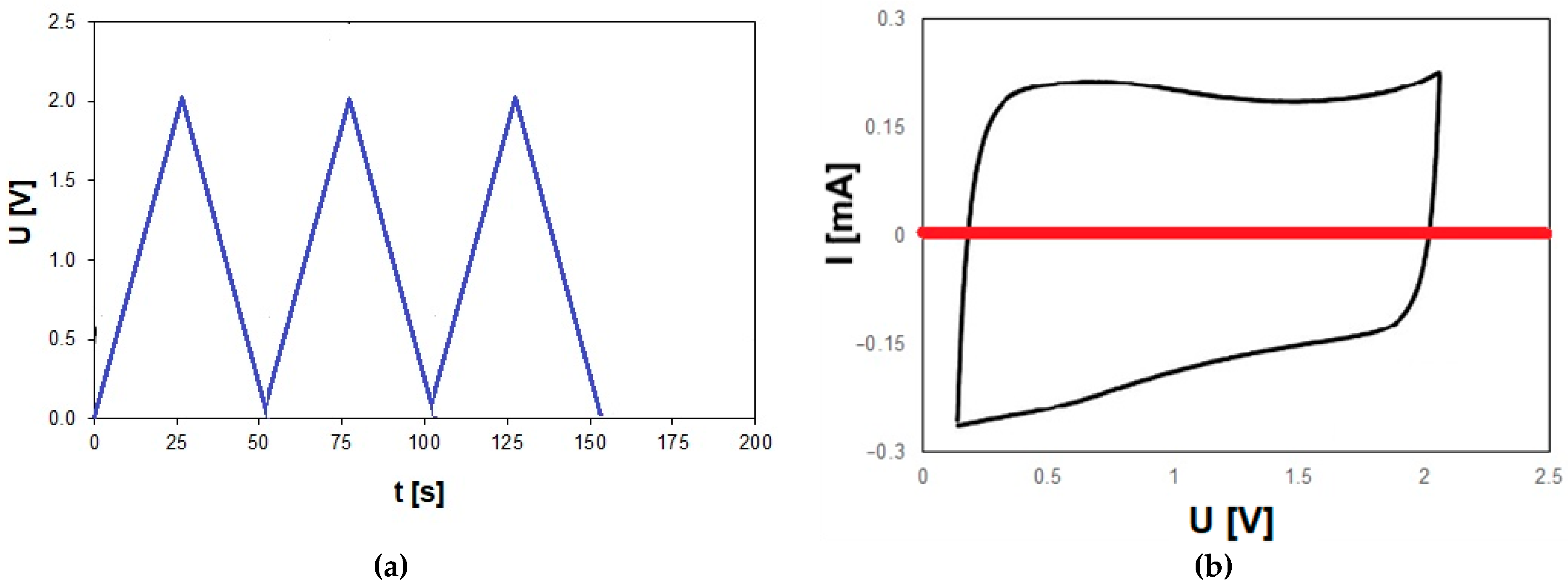
2.3. Electrochemical Properties
- Identifying surface contaminants.
- Estimating the relative specific surface area and surface roughness.
- Determining the potential at which oxidation–reduction reactions take place.
- Qualitatively analyzing specific substances.
- Assessing the capacitance of the electrode.
- Evaluating the kinetics of electron transfer.
2.4. Supercapacitor Energy and Power
3. Results and Discussion
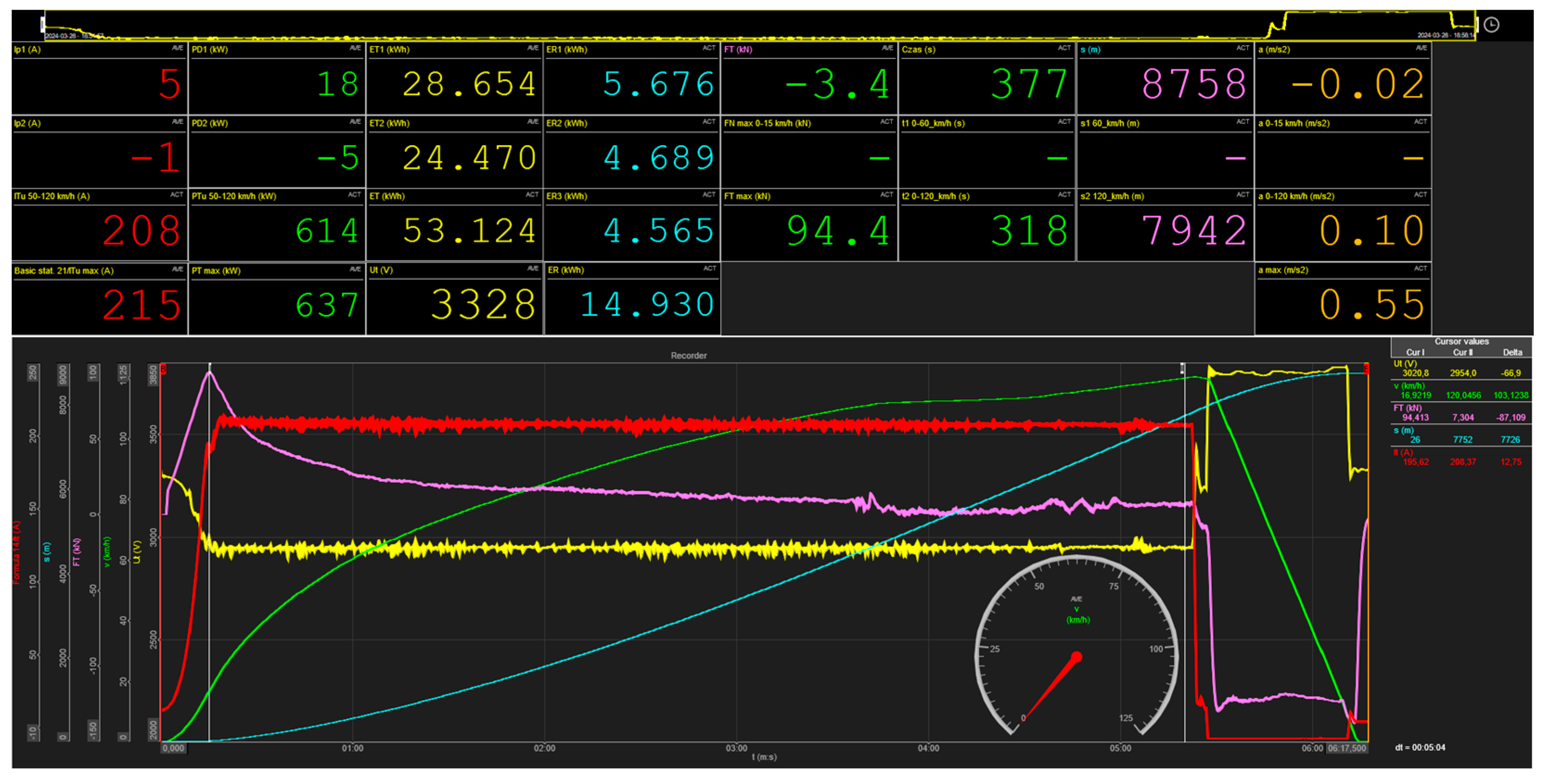
3.1. Measurement Results of Railway Vehicle Electricity Consumption Estimation
3.2. Development of Electrode Material from Starch
- DC supplies may incorporate multiple parallel supercapacitors to enhance signal filtration and eliminate AC ripple. This method enables the utilization of smaller supercapacitors with superior ripple characteristics while achieving higher capacitance values.
- Certain applications demand capacity rates surpassing those offered by commercially available supercapacitors. Supercapacitor banks address this need, such as those employed for power factor correction with inductive loads or energy storage in automotive applications like KERS (Kinetic Energy Recovery System) for regenerative braking.
- 1 kWh = 3600 kJ
- Therefore, 15 kWh = 15 × 360,015 × 3600 kJ = 54,000 kJ = 54,000,000 J.
- The energy stored in a supercapacitor is given by Equation (7), where E is the energy, C is the capacitance, and U is the voltage.
- Given that the capacitance C is 130 F/g, we need to know the voltage U at which the supercapacitors operate.
- The energy stored in one supercapacitor E = 1/2 × 130 F × U2; U = 1.8 V
- Simplifying, E = 65 × 1.82 = 220.32 J/g.
- To determine the number of supercapacitors needed, we carry out the following:
- Total energy required = 54,000,000 J
- Energy per supercapacitor = 65 × U2 J (assuming 1 g per capacitor)
- Number of supercapacitors N:
- To determine the total mass of supercapacitors, we consider the following:
- Total energy = 54,000,000 J
- Energy stored per gram = 220.32 J/g
- Total mass M:
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Correction Statement
References
- Andrych-Zalewska, M.; Chłopek, Z.; Merkisz, J.; Pielecha, J. Research on the Results of the WLTP Procedure for a Passenger Vehicle. Maint. Reliab./Eksploat. I Niezawodn. 2024, 26, 1–15. [Google Scholar] [CrossRef]
- Rymaniak, Ł.; Merkisz, J.; Szymlet, N.; Kamińska, M.; Weymann, S. Use of Emission Indicators Related to CO2 Emissions in the Ecological Assessment of an Agricultural Tractor. Eksploat. I Niezawodn. 2021, 23, 605–611. [Google Scholar] [CrossRef]
- Ziółkowski, A.; Fuć, P.; Jagielski, A.; Bednarek, M.; Konieczka, S. Comparison of the Energy Consumption and Exhaust Emissions between Hybrid and Conventional Vehicles, as Well as Electric Vehicles Fitted with a Range Extender. Energies 2023, 16, 4669. [Google Scholar] [CrossRef]
- Pielecha, I.; PIelecha, J. Simulation Analysis of Electric Vehicles Energy Consumption in Driving Tests. Eksploat. I Niezawodn. 2020, 22, 130–137. [Google Scholar] [CrossRef]
- Pielecha, I.; Szwajca, F. Cooperation of a PEM Fuel Cell and a NiMH Battery at Various States of Its Charge in a FCHEV Drive. Eksploat. I Niezawodn. 2021, 23, 468–475. [Google Scholar] [CrossRef]
- Kęska, A. The Actual Toxicity of Engine Exhaust Gases Emitted from Vehicles: The Development and Perspectives of Biological and Chemical Measurement Methods. ACS Omega 2023, 8, 24718–24726. [Google Scholar] [CrossRef]
- Merkisz, J.; Pielecha, J. Nanoparticle Emissions from Combustion Engines; Springer: Berlin/Heidelberg, Germany, 2015; ISBN 3-319-15928-3. [Google Scholar]
- Ziółkowski, A.; Fuć, P.; Lijewski, P.; Jagielski, A.; Bednarek, M.; Kusiak, W. Analysis of Exhaust Emissions from Heavy-Duty Vehicles on Different Applications. Energies 2022, 15, 7886. [Google Scholar] [CrossRef]
- Conzen, J.; Lakshmipathy, S.; Kapahi, A.; Kraft, S.; DiDomizio, M. Lithium Ion Battery Energy Storage Systems (BESS) Hazards. J. Loss Prev. Process Ind. 2023, 81, 104932. [Google Scholar] [CrossRef]
- Gao, Z.; Zhang, Y.; Song, N.; Li, X. Biomass-Derived Renewable Carbon Materials for Electrochemical Energy Storage. Mater. Res. Lett. 2017, 5, 69–88. [Google Scholar] [CrossRef]
- Kim, B.K.; Sy, S.; Yu, A.; Zhang, J. Electrochemical Supercapacitors for Energy Storage and Conversion. Handb. Clean Energy Syst. 2015, 1–25. [Google Scholar] [CrossRef]
- Ranaweera, C.; Kahol, P.; Ghimire, M.; Mishra, S.; Gupta, R.K. Orange-Peel-Derived Carbon: Designing Sustainable and High-Performance Supercapacitor Electrodes. C 2017, 3, 25. [Google Scholar] [CrossRef]
- Qi, M.; Liu, Y.; Landon, R.S.; Liu, Y.; Moon, I. Assessing and Mitigating Potential Hazards of Emerging Grid-Scale Electrical Energy Storage Systems. Process Saf. Environ. Prot. 2021, 149, 994–1016. [Google Scholar] [CrossRef]
- Rosewater, D.; Williams, A. Analyzing System Safety in Lithium-Ion Grid Energy Storage. J. Power Sources 2015, 300, 460–471. [Google Scholar] [CrossRef]
- Wu, Y.; Zhang, T. Risk Assessment of Offshore Wave-Wind-Solar-Compressed Air Energy Storage Power Plant through Fuzzy Comprehensive Evaluation Model. Energy 2021, 223, 120057. [Google Scholar] [CrossRef]
- Sawczuk, W.; Jüngst, M.; Ulbrich, D.; Kowalczyk, J. Modeling the Depth of Surface Cracks in Brake Disc. Materials 2021, 14, 3890. [Google Scholar] [CrossRef]
- Sawczuk, W.; Merkisz-Guranowska, A.; Ulbrich, D.; Kowalczyk, J.; Cañás, A.-M.R. Investigation and Modelling of the Weight Wear of Friction Pads of a Railway Disc Brake. Materials 2022, 15, 6312. [Google Scholar] [CrossRef]
- Dai, H. Carbon Nanotubes: Opportunities and Challenges. Surf. Sci. 2002, 500, 218–241. [Google Scholar] [CrossRef]
- Frackowiak, E.; Gautier, S.; Gaucher, H.; Bonnamy, S.; Beguin, F. Electrochemical Storage of Lithium in Multiwalled Carbon Nanotubes. Carbon 1999, 37, 61–69. [Google Scholar] [CrossRef]
- Wang, X.; Sun, L.; Susantyoko, R.A.; Fan, Y.; Zhang, Q. Ultrahigh Volumetric Capacity Lithium Ion Battery Anodes with CNT–Si Film. Nano Energy 2014, 8, 71–77. [Google Scholar] [CrossRef]
- Wong, S.I.; Sunarso, J.; Wong, B.T.; Lin, H.; Yu, A.; Jia, B. Towards Enhanced Energy Density of Graphene-Based Supercapacitors: Current Status, Approaches, and Future Directions. J. Power Sources 2018, 396, 182–206. [Google Scholar] [CrossRef]
- Ke, Q.; Wang, J. Graphene-Based Materials for Supercapacitor Electrodes—A Review. J. Mater. 2016, 2, 37–54. [Google Scholar] [CrossRef]
- Chen, C.-M.; Zhang, Q.; Yang, M.-G.; Huang, C.-H.; Yang, Y.-G.; Wang, M.-Z. Structural Evolution during Annealing of Thermally Reduced Graphene Nanosheets for Application in Supercapacitors. Carbon 2012, 50, 3572–3584. [Google Scholar] [CrossRef]
- Fan, Z.; Zhao, Q.; Li, T.; Yan, J.; Ren, Y.; Feng, J.; Wei, T. Easy Synthesis of Porous Graphene Nanosheets and Their Use in Supercapacitors. Carbon 2012, 50, 1699–1703. [Google Scholar] [CrossRef]
- Li, C.; Zhang, X.; Wang, K.; Zhang, H.; Sun, X.; Ma, Y. Three Dimensional Graphene Networks for Supercapacitor Electrode Materials. New Carbon Mater. 2015, 30, 193–206. [Google Scholar] [CrossRef]
- Rudge, A.; Davey, J.; Raistrick, I.; Gottesfeld, S.; Ferraris, J.P. Conducting Polymers as Active Materials in Electrochemical Capacitors. J. Power Sources 1994, 47, 89–107. [Google Scholar] [CrossRef]
- Pigłowska, M.; Kurc, B.; Galiński, M.; Fuć, P.; Kamińska, M.; Szymlet, N.; Daszkiewicz, P. Challenges for Safe Electrolytes Applied in Lithium-Ion Cells—A Review. Materials 2021, 14, 6783. [Google Scholar] [CrossRef]
- Kurc, B.; Pigłowska, M.; Rymaniak, Ł.; Fuć, P. Modern Nanocomposites and Hybrids as Electrode Materials Used in Energy Carriers. Nanomaterials 2021, 11, 538. [Google Scholar] [CrossRef]
- Zhang, J.; Gu, M.; Chen, X. Supercapacitors for Renewable Energy Applications: A Review. Micro Nano Eng. 2023, 21, 100229. [Google Scholar] [CrossRef]
- Gifford, P.; Adams, J.; Corrigan, D.; Venkatesan, S. Development of Advanced Nickel/Metal Hydride Batteries for Electric and Hybrid Vehicles. J. Power Sources 1999, 80, 157–163. [Google Scholar] [CrossRef]
- Kouchachvili, L.; Yaïci, W.; Entchev, E. Hybrid Battery/Supercapacitor Energy Storage System for the Electric Vehicles. J. Power Sources 2018, 374, 237–248. [Google Scholar] [CrossRef]
- Muhiuddin, M.; Khan, A.Z.; Devi, N.A.; Bharadishettar, N.; Meti, S.; Siddique, A.B.; Bhat, K.U.; Akhtar, W.; Rahman, M.R. Cost Effective Synthesis of Sulfur and Nitrogen Co-Doped Graphene Aerogel and Application in Binder Free Supercapacitor. J. Appl. Phys. 2024, 136, 034901. [Google Scholar] [CrossRef]
- Barić, T.; Glavaš, H.; Barukčić, M. Impact of Balance Resistor Uncertainty on Voltages across Supercapacitors. J. Energy Storage 2019, 22, 131–136. [Google Scholar] [CrossRef]
- Xiong, G.; Kundu, A.; Fisher, T.S. Influence of Temperature on Supercapacitor Performance. In Thermal Effects in Supercapacitors; Xiong, G., Kundu, A., Fisher, T.S., Eds.; Springer International Publishing: Cham, Switzerland, 2015; pp. 71–114. ISBN 978-3-319-20242-6. [Google Scholar]
- Zou, Z.; Cao, J.; Cao, B.; Chen, W. Evaluation Strategy of Regenerative Braking Energy for Supercapacitor Vehicle. ISA Trans. 2015, 55, 234–240. [Google Scholar] [CrossRef] [PubMed]
- Adeyemo, A.A.; Alves, E.; Marra, F.; Brandao, D.; Tedeschi, E. Suitability Assessment of High-Power Energy Storage Technologies for Offshore Oil and Gas Platforms: A Life Cycle Cost Perspective. J. Energy Storage 2023, 61, 106643. [Google Scholar] [CrossRef]
- Kurc, B.; Lijewski, P.; Rymaniak, Ł.; Fuć, P.; Pigłowska, M.; Urbaniak, R.; Ciupek, B. High-Energy Solid Fuel Obtained from Carbonized Rice Starch. Energies 2020, 13, 4096. [Google Scholar] [CrossRef]
- Liu, Y.; Chen, J.; Cui, B.; Yin, P.; Zhang, C. Design and Preparation of Biomass-Derived Carbon Materials for Supercapacitors: A Review. C 2018, 4, 53. [Google Scholar] [CrossRef]
- Wang, X.; Xie, X.; Xie, K.; Liu, Q.; Li, Y.; Tan, X.; Dong, H.; Li, X.; Dong, Z.; Xia, Q. Chitin and Cuticle Proteins Form the Cuticular Layer in the Spinning Duct of Silkworm. Acta Biomater. 2022, 145, 260–271. [Google Scholar] [CrossRef]
- Yang, X.; Fei, B.; Ma, J.; Liu, X.; Yang, S.; Tian, G.; Jiang, Z. Porous Nanoplatelets Wrapped Carbon Aerogels by Pyrolysis of Regenerated Bamboo Cellulose Aerogels as Supercapacitor Electrodes. Carbohydr. Polym. 2018, 180, 385–392. [Google Scholar] [CrossRef]
- Zhuo, H.; Hu, Y.; Chen, Z.; Zhong, L. Cellulose Carbon Aerogel/PPy Composites for High-Performance Supercapacitor. Carbohydr. Polym. 2019, 215, 322–329. [Google Scholar] [CrossRef]
- Cáceres, G.; Fullenkamp, K.; Montané, M.; Naplocha, K.; Dmitruk, A. Encapsulated Nitrates Phase Change Material Selection for Use as Thermal Storage and Heat Transfer Materials at High Temperature in Concentrated Solar Power Plants. Energies 2017, 10, 1318. [Google Scholar] [CrossRef]
- Nazir, H.; Batool, M.; Osorio, F.J.B.; Isaza-Ruiz, M.; Xu, X.; Vignarooban, K.; Phelan, P.; Kannan, A.M. Recent Developments in Phase Change Materials for Energy Storage Applications: A Review. Int. J. Heat Mass Transf. 2019, 129, 491–523. [Google Scholar] [CrossRef]
- Wang, Z.; Sun, S.; Lin, X.; Liu, C.; Tong, N.; Sui, Q.; Li, Z. A Remote Integrated Energy System Based on Cogeneration of a Concentrating Solar Power Plant and Buildings with Phase Change Materials. Energy Convers. Manag. 2019, 187, 472–485. [Google Scholar] [CrossRef]
- Zhao, B.; Cheng, M.; Liu, C.; Dai, Z. Thermal Performance and Cost Analysis of a Multi-Layered Solid-PCM Thermocline Thermal Energy Storage for CSP Tower Plants. Appl. Energy 2016, 178, 784–799. [Google Scholar] [CrossRef]


| Technical Data | Value |
|---|---|
| Track width [mm] | 1435 |
| Overall length with bumpers [mm] | 78,600 |
| Top speed—electric drive [km/h] | 160 |
| Top speed—diesel drive [km/h] | 120 |
| Axle arrangement | 2’Bo’ + Bo’2’+ Bo’2’ |
| Traction motor power [kW] | 6 × 300 |
| Total power of combustion engine power [kW] | 900 |
| Braking resistors maximum power (RH1, RH2, RH3) [kW] | 3 × 1000 |
| Number of seats for passengers | 156 |
| Mass of vehicle [kg] | 171,332 |
| No. | Specific Parameters | Time (t) and Distance (s) | Total Distance | Energy Consumed by the Vehicle ET and Returned to the Braking Resistors ER1 + ER3 + ER3 = ER Brake Setting “P”—Passanger | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 60 km/h | 120 km/h | ||||||||||||||||||
| ITu(1) | PTmax(2) | PTu(3) | FNmax(4) | amax(5) | av(6) | av(7) | t1 | s1 | t2 | s2 | s | ET | ER1 | ER2 | ER3 | ER | ET – ER | ER/ET | |
| A | kW | kW | kN | m/s2 | m/s2 | m/s2 | s | m | s | m | m | kWh | kWh | kWh | kWh | kWh | kWh | % | |
| 1 | 208 | 636 | 612 | 96 | 0.56 | 0.30 | 0.11 | 54 | 513 | 306 | 7 518 | 8 522 | 50.72 | 5.83 | 4.89 | 4.63 | 15.35 | 35.37 | 30.27 |
| 2 | 207 | 636 | 611 | 95 | 0.55 | 0.28 | 0.10 | 55 | 513 | 318 | 7 839 | 8 776 | 52.29 | 5.74 | 4.83 | 4.64 | 15.21 | 37.07 | 29.09 |
| 3 | 208 | 646 | 616 | 96 | 0.56 | 0.30 | 0.10 | 56 | 528 | 324 | 8 120 | 8 938 | 53.95 | 5.70 | 4.80 | 4.53 | 15.02 | 38.93 | 27.84 |
| 4 | 208 | 640 | 615 | 94 | 0.55 | 0.29 | 0.11 | 56 | 521 | 313 | 7 869 | 8 669 | 51.91 | 5.81 | 4.87 | 4.59 | 15.27 | 36.64 | 29.41 |
| 5 | 208 | 642 | 613 | 92 | 0.53 | 0.30 | 0.10 | 57 | 563 | 319 | 7 790 | 8 731 | 53.66 | 5.66 | 4.90 | 4.64 | 15.20 | 38.47 | 28.32 |
| 6 | 208 | 637 | 614 | 94 | 0.55 | 0.30 | 0.10 | 57 | 550 | 318 | 7 742 | 8 758 | 53.12 | 4.69 | 4.57 | 4.57 | 13.82 | 39.31 | 26.01 |
| 7 | 208 | 640 | 613 | 95 | 0.55 | 0.30 | 0.11 | 56 | 533 | 317 | 7 743 | 8 747 | 52.79 | 5.65 | 4.82 | 4.57 | 15.03 | 37.75 | 28.48 |
| Av. values | 208 | 640 | 613 | 95 | 0.55 | 0.30 | 0.10 | 56 | 532 | 316 | 7 803 | 8 734 | 52.63 | 5.58 | 4.81 | 4.60 | 14.98 | 37.65 | 28.49 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Woźniak, K.; Kurc, B.; Rymaniak, Ł.; Szymlet, N.; Pielecha, P.; Sobczak, J. High-Capacity Energy Storage Devices Designed for Use in Railway Applications. Energies 2024, 17, 5904. https://doi.org/10.3390/en17235904
Woźniak K, Kurc B, Rymaniak Ł, Szymlet N, Pielecha P, Sobczak J. High-Capacity Energy Storage Devices Designed for Use in Railway Applications. Energies. 2024; 17(23):5904. https://doi.org/10.3390/en17235904
Chicago/Turabian StyleWoźniak, Krystian, Beata Kurc, Łukasz Rymaniak, Natalia Szymlet, Piotr Pielecha, and Jakub Sobczak. 2024. "High-Capacity Energy Storage Devices Designed for Use in Railway Applications" Energies 17, no. 23: 5904. https://doi.org/10.3390/en17235904
APA StyleWoźniak, K., Kurc, B., Rymaniak, Ł., Szymlet, N., Pielecha, P., & Sobczak, J. (2024). High-Capacity Energy Storage Devices Designed for Use in Railway Applications. Energies, 17(23), 5904. https://doi.org/10.3390/en17235904










