1. Introduction
The increasing pressure on the aviation sector to reduce greenhouse gas emissions means that the chemical composition of fuels for turbine aircraft engines will change as a result of the introduction of various sustainable aviation fuels (SAFs) [
1,
2]. This may affect the operation of engines and their durability and reliability. As a result, adding SAFs to fuels should be assessed in terms of the effects on flight safety. Gan et al. [
3] emphasized that safety is the most important condition for using the new fuel. Fuel quality is known to have a direct impact on flight safety. The quality of aviation fuel is determined through production, storage, transport and distribution. Fuel quality affects ignition and re-ignition, flame extinguishment, combustion efficiency and the life of hot tip components, which under the worst conditions can result in dangerous accidents. All these accidents may occur as a result of changes in the fuel structure related to the chemical composition of Jet A-1 fuel and, consequently, as a result of changes in combustion chemistry. There have been differences between the SAF safety requirements and the ASTM standards due to differences in the goal of each of the standards. Airworthiness standards focused on aircraft engines. However, ASTM standards covered fuel properties, and the physicochemical properties requirements for SAF were limited to those previously specified for Jet-A or Jet-A1.
The testing algorithm for the approval of the new SAF fuel developed by ASTM assumed a number of testing stages, including engine testing [
4,
5,
6]. These tests are long-lasting and expensive and require the consumption of a relatively large amount of tested fuel [
7]. This situation generated interest in new tests and research methods that would allow for the selection of new SAFs obtained from new raw materials or using new technologies.
Typical Jet A-1 is a diverse mixture of normal paraffins, isoparaffins, aromatic compounds and cycloparaffins with carbon numbers ranging from C
7 to C
18. Most computational models for aviation fuels have relied on simpler surrogates and chemical mechanisms developed from experimental data or fit-for-purpose relationships for specific operating points [
7,
8,
9,
10,
11,
12]. Yu et al. [
12] used the thermophysical model of a real fluid, which was based on the equation of state, as a way to departure from the ideal gas state in order to obtain thermodynamic properties and the phase boundary and in general. The aim of their work was to establish a framework applicable to SAFs in order to assess their performances and discuss limitations and necessary improvements. Tyliszczak et al. [
13] proposed the use of a real fluid modeling framework built on thermodynamic principles and generalized correlations to characterize sustainable aviation fuels over a wide range of jet engine operating conditions. They took into account fuel atomization and its evaporation, but they did not take into account the impact of SAFs on the fuel oxidation reaction in a complex chain of chemical reactions.
Smooke et al. [
14] dealt with the combustion process in gas turbine simulations. The simulations were performed using a steady flamelet model with chemical mechanisms with 16 species and 25 elementary reactions [
15] and for the GRI-2.11 mechanisms with 49 species and 277 elementary reactions [
16]. In their conclusion, the authors noted that the kinetics of chemical reactions have a large impact on the combustion process and its proper consideration can bring the modeling results closer to experimental data.
Fuel consumption was the subject of the research of Białecki [
17]. Using statistical methods, the authors determined the relationship between various physicochemical properties of fuels and fuel consumption. Their model did not take into account the complexity of the chemical reaction chains and interactions between fuels/blends components, which influence fuel consumption. In this context, the technical readiness of aircraft refueling vehicles was also analyzed [
18].
Similar research has been conducted by Mehl et al. [
19]. A mathematical model of the combustion process was developed using regression analysis. The model described the relationship between the physicochemical properties of fuels and selected parameters characterizing the operation of a miniature jet engine. This model enabled predicting engine operating parameters and the emissions characteristics due to the correlation between the properties of chosen fuels and the operating parameters of a miniature turbine engine.
Gan et al. [
3] focused on methodological aspects related to defining general models aimed at capturing the basic aspects of fuel chemistry. The methodology adopted by the authors assumed that the research began with an experimental assessment of the oxidation of the tested fuels in well-characterized conditions. The combustion process was carried out in a high-temperature flow reactor coupled with a molecular beam mass spectrometer. This configuration allowed for an in-depth study of the relevant features of combustion chemistry by simultaneously identifying many intermediates and thus paths in the reaction chain that determine the course of the process. The proposed kinetic models require the identification of all important reaction paths controlling fuel combustion and, for each elementary step, the determination of the temperature- and pressure-dependent reaction rate. In this way, reaction rate constants, including reverse reactions, could be defined for reversible elementary steps. To achieve this, it was necessary to:
Identify the appropriate reaction intermediates (the number of which determines the number of equations required to calculate the composition of the system);
Determine the reaction rate for the thousands of parameters it contains, along with the thermodynamic properties of each species.
Gan et al. [
3] pointed to significant discrepancies in modeling and experimental results, which were caused by the high complexity of the combustion process, even when measured under controlled reactor conditions.
To summarize the short review of modeling methods presented above, it can be concluded that work on the development of tools for predicting the impact of SAF on the operation and reliability of aircraft turbine engines is indeed much needed. The review showed that these methods were based on the study of the combustion reactions of surrogate fuels (single hydrocarbons), the identification of several dozen/several hundred elementary reactions and an attempt to assemble them into a combustion model using numerical tools. These models were therefore created for relatively low-complex fuels, burned in controlled and stable conditions.
It should be emphasized that the subjects of research usually included mixtures of Jet A-1 fuel with SAFs, i.e., fuels containing over a thousand chemical compounds. The question that the models should help answer is as follows: To what extent does SAFs change the fuel combustion process under specific engine operating conditions? To make this possible, the model should take into account, on the one hand, data on the chemical composition and properties of the fuel, and on the other, critical engine operating parameters. The research methods used so far to assess the possibility of using fuel for use in aviation assume a gradual complexity of the research system range from laboratory tests to stand modeling the operation of individual engine components, ending with engine tests in a dynamometer.
The main idea of models described above was to take into account as many elementary reactions as possible and numerically integrate them into one combustion reaction chain. These elementary reactions were defined for individual reagents (hydrocarbons) and were selected based on similarity to the chemical composition of the tested fuel. This approach made it possible to create a model describing the combustion process that was highly consistent with the experimental data, but inconsistencies always appear. These inconsistencies were due to two reasons:
Arbitrary selection of elementary reactions;
The model constructed in this way does not take into account the interactions between fuel components, which in some cases may change the structure of the reaction chain and the rate relations of individual elementary reactions.
Another weakness of these models was the poor connection between the fuel combustion reaction and the design and operating conditions of the engine.
Kulczycki et al. [
20] previously described the reactivity model
when applied to combustion processes.
Chemical reactivity refers to the ability of a chemical substance to undergo various reactions. Chemical reactivity is either treated as a qualitative feature of a substance or element or as a quantitative feature whose description was derived from the density functional theory. In the latter case, reactivity was related to the electronic structure of a specific reagent. The application of this concept was recently observed by Elshakre et al. [
21].
The model described by Kulczycki et al. [
20] introduced the coefficient of reactivity
. This parameter is characteristic of any given fuel and is defined as the ratio of the engine operating parameters (e.g., thrust) to the parameters describing the fuel combustion kinetics. The basic difference between the models presented above is the assumption that the entire chain of fuel combustion reactions is treated as one elementary reaction. With this assumption, measurable quantities such as fuel consumption, temperature in the combustion chamber and the chemical composition of exhaust gases could be treated as quantities characterizing an elementary reaction with all its consequences. Thanks to this assumption, the activation energy can be determined using the Arrhenius equation. According to this equation, for a given elementary reaction, activation energy is a characteristic quantity with a constant value. It was assumed that if changes in engine operating parameters only caused changes in the reaction rates that made up the combustion process, then it is the same elementary reaction. In the α
i reactivity model, the assumed activation energy was treated as a criterion for the invariability of the fuel combustion reaction chain. This seemed to be the most important element of the
model—it was possible to change the engine’s operating parameters, e.g., fuel mass flow rate, and define a range of values for this parameter that would not cause changes in the combustion reaction chain, i.e., would not cause a change in the activation energy value.
Another important consequence was the ability to determine how changing the concentration of a selected fuel component would affect changes in the structure of the combustion reaction chain.
Activation energy is a quantity strongly assigned to the conditions in which the combustion process takes place. Therefore, it cannot be assigned to any given fuel and treated as a feature characterizing the fuel itself in various combustion conditions. The reactivity coefficient that was introduced into the reactivity model is a quantity that determines the relationship between engine operating parameters, the value of which results from fuel combustion (e.g., thrust) and the kinetic characteristics of the combustion reaction chain. The mathematical assumptions of the reactivity model introduced a limitation: both the engine operating parameters and the quantities describing the kinetics of the combustion reaction must be functions of one and the same independent variable (e.g., fuel mass flow rate). Thanks to this, the reactivity coefficient αi could be assigned to a specific fuel burned in different conditions (different engines and different engine operating conditions) as long as the structure of the combustion reaction chain remained the same in these different conditions.
Significance needs to be given to the phrase “the same combustion reactions chain”. In this application, it was assumed that the same chain meant that the changes in the combustion process conditions or/and fuel chemical structure did not introduce new elementary reactions but only changed the relations between the rate of elemental reactions. In this situation, the coefficient of reactivity αi described the property of the fuel, determining how the change in the rate (kinetic parameters) of combustion reactions caused changes in the operating parameters of the turbine engine.
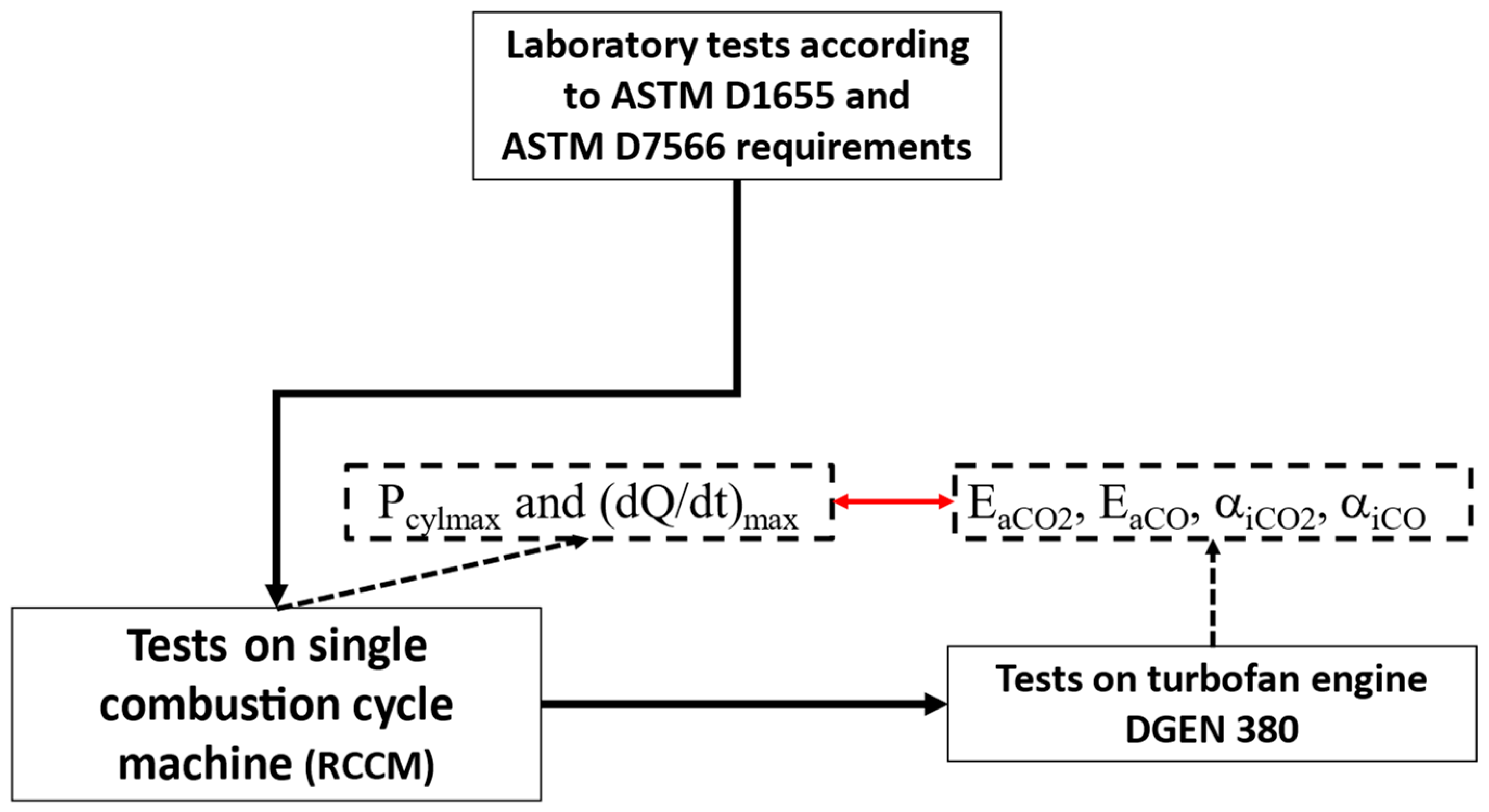
The aim of this article was to verify the thesis that the reactivity coefficient
and the activation energy (resultant for the entire combustion reactions chain), as described by Kulczycki et al. [
20], can be criteria for assessing the impact of SAFs (of different types and concentrations) on the combustion process in a RCCM (rapid compression combustion machine) [
22,
23] and turbine engine. The thesis was formulated as the
reactivity model application enabled the use of the RCCM test results to predict the behavior of the tested fuel during combustion in turbine engine DGEN 380.
4. Discussion
4.1. The Relationships Between Parameters Determined During DGEN Engine Tests (, , and ) and the and Determined During RCCM Tests
Defining the influence of SAF on the combustion process in a turbine engine required a reliance on simpler preliminary tests. This made it possible to select various new fuel candidates for future, more complex research.
The results presented above were used to tentatively answer the following questions:
Are the activation energy and coefficient of reactivity parameters quantitatively describing the influence of SAF on the combustion process in various engines, including RCCM tests?
Is the quantitative relationship between and determined in fuels containing SAFs burned in different engines?
Can the RCCM be used to predict and for given fuels containing SAFs in various turbine engines?
To solve the problem of how the SAFs of a given chemical structure change the combustion process in relation to conventional Jet A-1 fuel, the ratios of
to
determined for both blends containing SAFs and for fossil Jet A-1 fuel were analyzed. Below (in
Figure 9,
Figure 10,
Figure 11 and
Figure 12) the influence of SAFs of different chemical structures on the value of
(activation energy determined for the chemical chain of complete combustion to
) and reactivity coefficients in a chain of reactions ending in the production of
and
was shown.
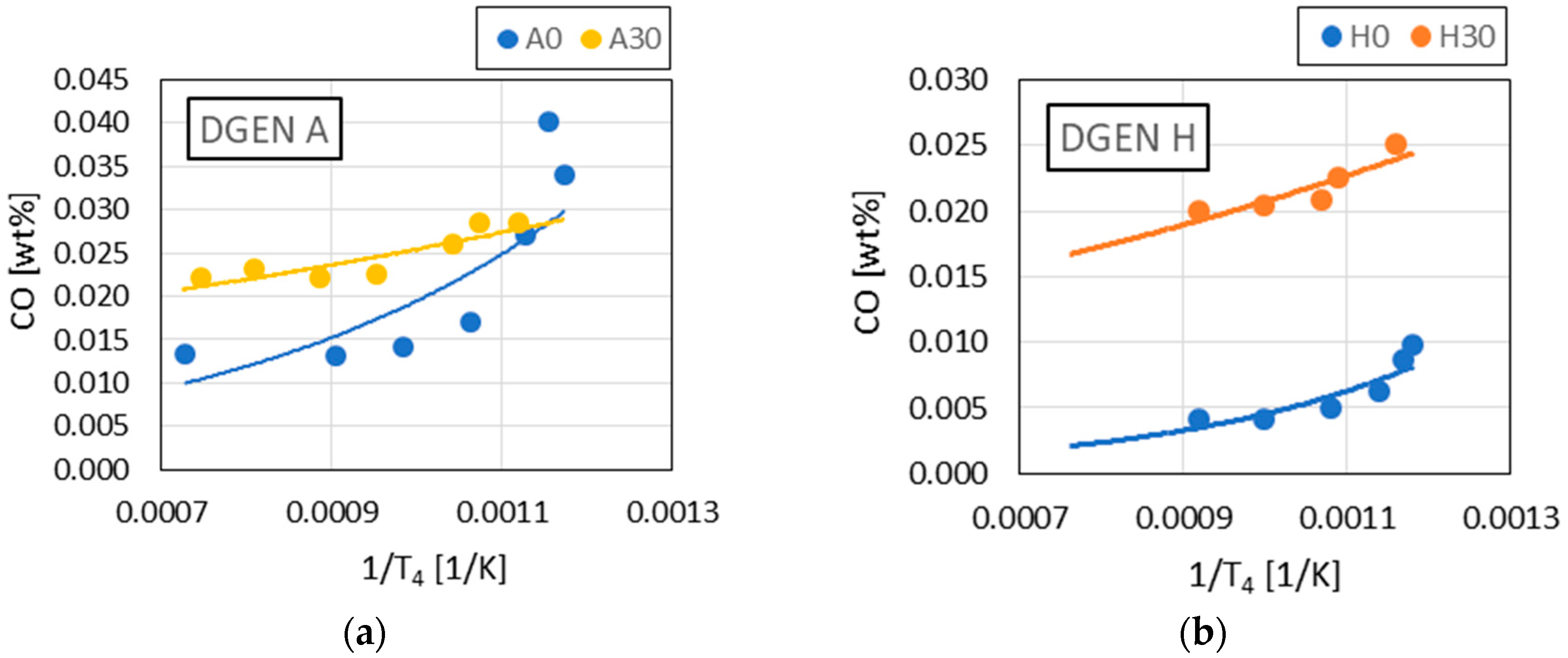
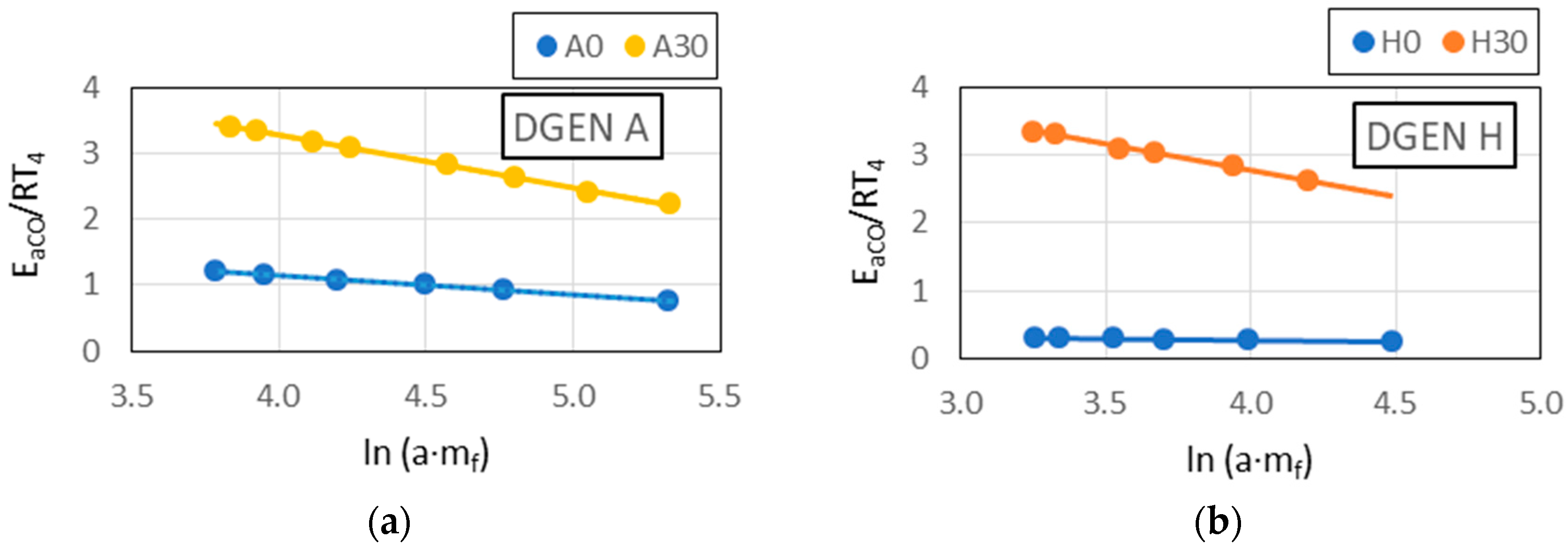
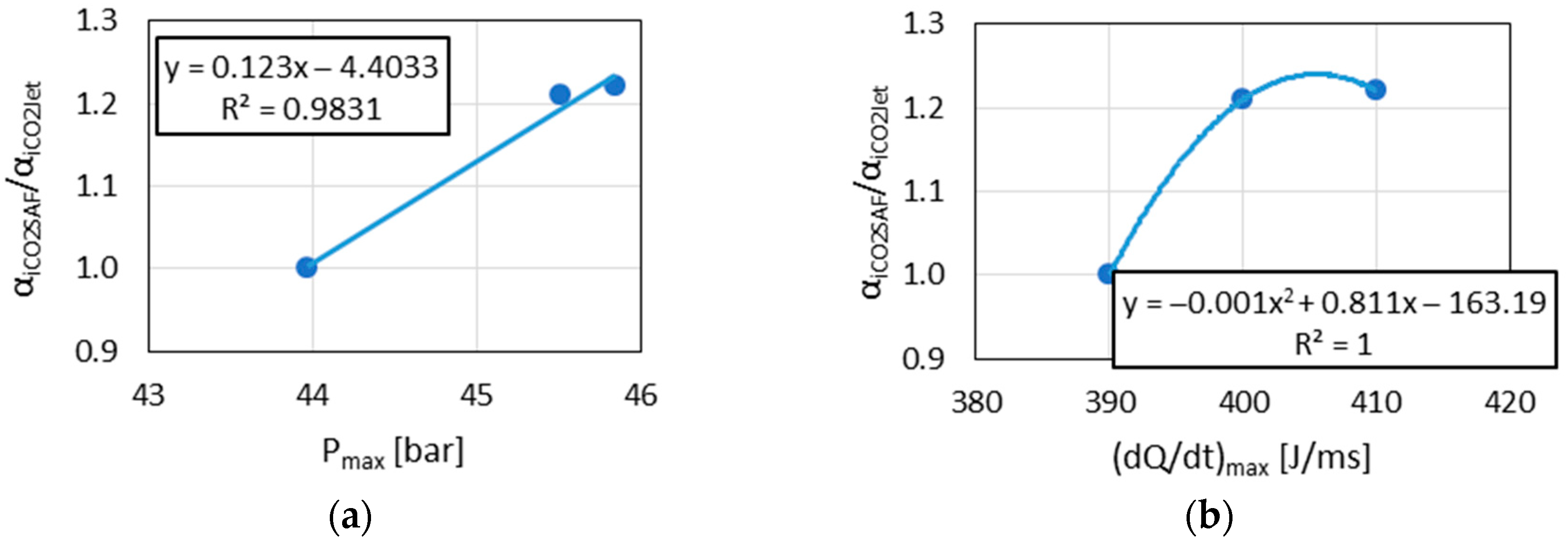
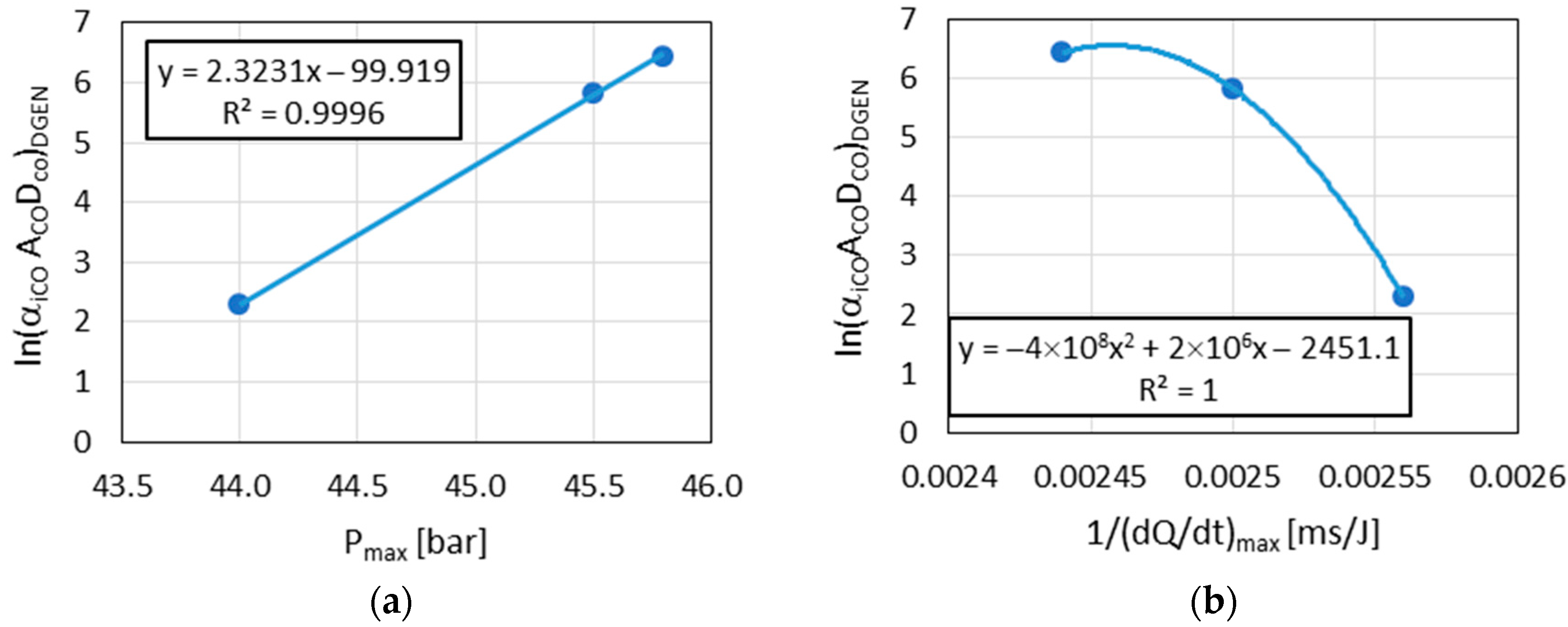
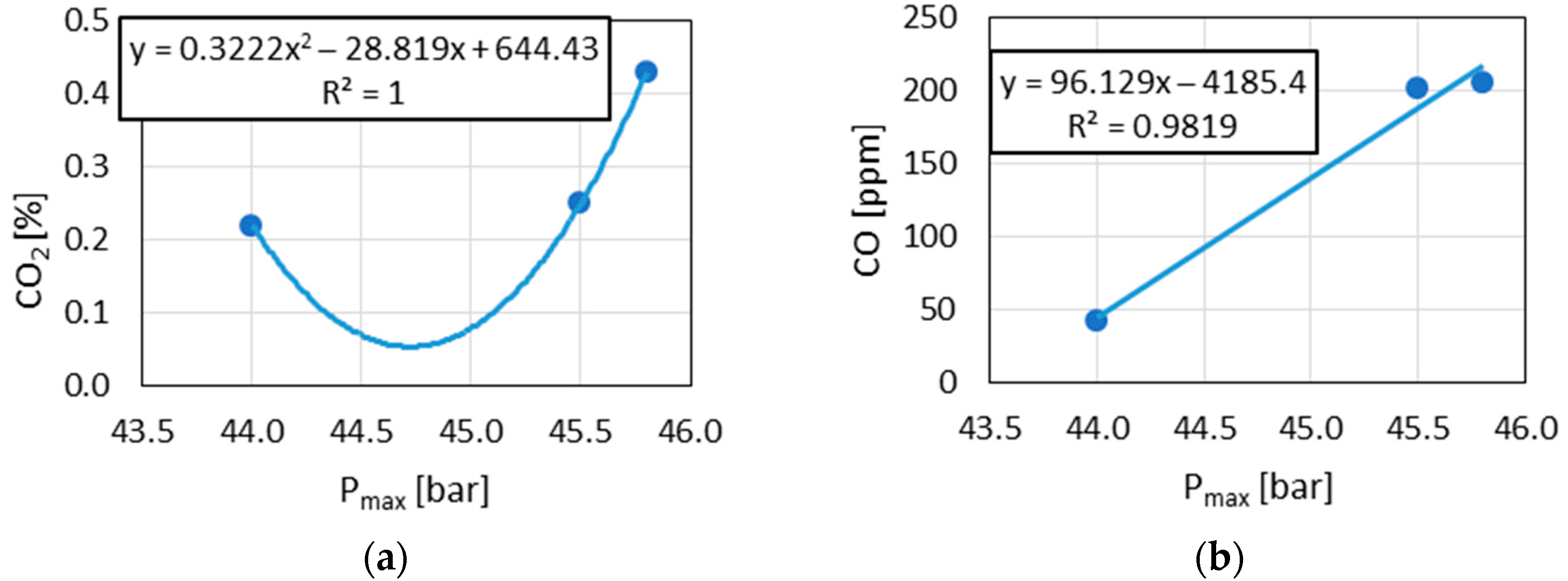
The observed linear relationships between and with Pmax in the results indicated the similarity of the combustion reaction chains in the DGEN 380 engine and in the RCCM stand.
4.2. The Possibility of Predicting the and Concentration in Exhaust Gases Using RCCM Test Results
The above results (
Figure 9,
Figure 10 and
Figure 12) indicated the linear relationships in the cases where
was used as the result of RCCM tests. These were the reasons that
was chosen to predict
and
concentration in exhaust gases emitted during DGEN engine tests.
Equations (9), (10), (14) and (15) use the fuel flow rate
as the independent variable. This variable, like reactivity coefficient
and activation energy
, was not dependent on
. On the other hand, the concentrations of
and
in exhaust gases did depend on
. The prediction of
and
concentrations in exhaust gases based on RCCM test results would be possible for assumed values of
. To verify this claim, the
, 90 and 150 dm
3/h values were chosen. The obtained results are shown in
Figure 12,
Figure 13,
Figure 14 and
Figure 15.
The demonstrated correlations between and in relation to combustion pressure did not indicate typical trends in the changes in these components. However, this article only made an attempt to create analytical procedures in this aspect. Increasing the number of data points would make it possible to obtain a more typical trend of these two exhaust components (i.e., inversely proportional concentration to concentration in exhaust gases).
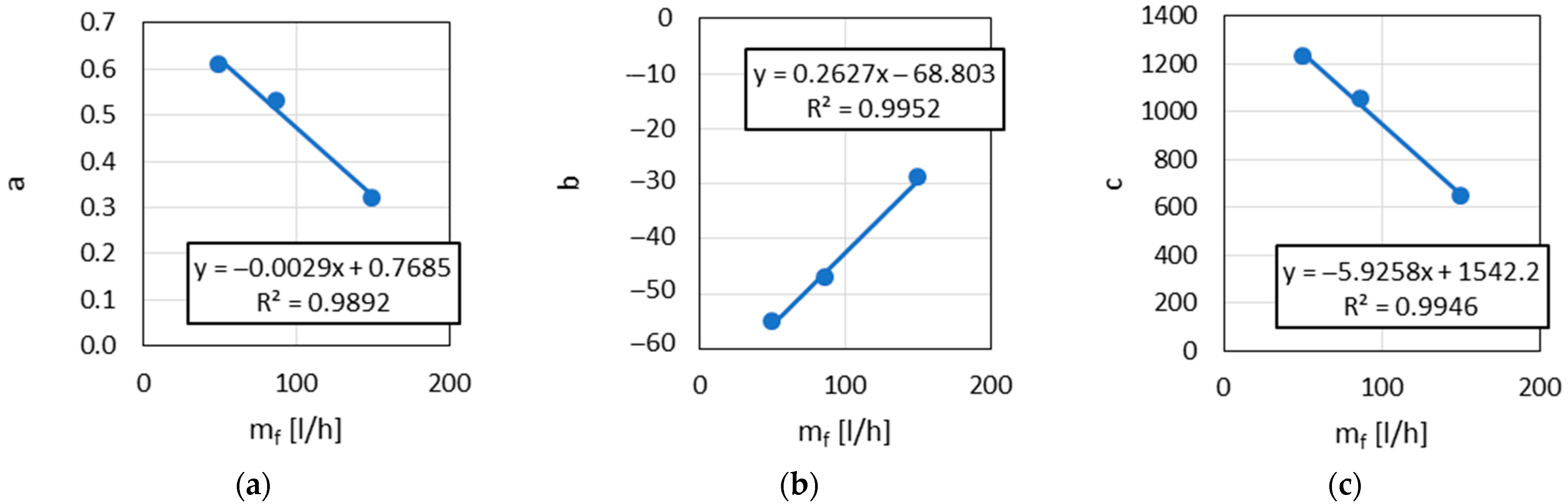
The following empirical relationships were found:
The parameters a, b, c,
and
(
Figure 16) were found to be functions of
.
Consequently Equation (17) could be expressed as a function of
and
.
Equation (18) can also be expressed as:
The empirically obtained relationships indicate that parameters of Equations (17) and (18) are functions of the fuel flow rate . These functions were obtained for the mineral Jet A-1 fuel and its blends with the SAF components A and H.
4.3. The Accuracy of the Obtained Model
Modeling the fuel combustion process in turbine engines is a tool for predicting the changes in this process under various engine operating conditions, as well as when powered by different fuels. The recent introduction of SAFs into aviation has generated particular interest in modeling the effect of the chemical composition and properties of fuels on the combustion process. Two directions of such investigations were observed:
The first group—statistical models—require a large database, and their accuracy can be increased by using, for example, neural networks. However, the parameters of the relationships obtained in this way cannot be physically interpreted, and their values will change with the growth of the database. An example is also the relationships presented in
Section 4.2. The accuracy of statistical models applied to the composition of exhaust gases emitted by turbine engines was typically not sufficient. The main reason for this was because of the relatively low repeatability, and even less reproducibility, of exhaust gas composition measurements. The causes were not precisely determined, but many studies show that the causes could due to the inhomogeneity of the exhaust gas stream and the variability of the properties of the air drawn in by the engine. It should be noted, however, that regardless of the indicated disadvantages of statistical models, they are very useful for predicting the fuel combustion process in aircraft turbine engines. They allow for the prediction of the effects of physicochemical properties of fuels during the combustion process in turbine engines [
17] as well as for the prediction of the effects of fuel quality on the emissions of exhaust components.
The second group consists of models based on the fundamental principles of the structure of matter. An example of this is neural network-based molecular dynamics simulation [
41]. These models use several databases and describe a chain of reactions consisting of several hundred elementary reactions. For a chemically simple fuel, such as methane, 798 elementary reactions were identified. This clearly indicates significant limitations in the use of this group of models in relation to the combustion of chemically complex fuels, such as the mineral fuel Jet A-1 (consisting of about 1000 chemical compounds) and its mixtures with SAF components.
As was shown in the introduction, the reactivity model αi assumed that the combustion process consisted of one elemental reaction: fuel → exhaust gases. Due to this assumption, the operating parameters of the engine, such as fuel flow mf, temperature in combustion chamber and the concentration of the combustion products in exhaust gases, can be applied to a kinetic equation, and the activation energy Ea, related to this assumed elemental reaction can thus be determined. When another operating parameter—thrust F—is used, the reactivity coefficient αi can be determined. Both the activation energy Ea and the coefficient of reactivity αi characterize the behavior of fuel during combustion. When approaching the problem of how SAF components influence the combustion process, the fuels containing various SAFs are treated as various substrates—i.e., the “fuel” in the assumed elemental reaction. For each fuel, the Ea and αi can be determined and assigned to the given fuel. The comparison of the values of these parameters obtained for Jet A-1 fuel and mixtures containing BSCs makes it possible to assess the impact of BSCs on the combustion process and minimize the effect of low measurement repeatability.
If the structure of a fuel combustion chemical reaction chain is similar in different devices (as assumed for the αi reactivity model), then the Ea and αi values determined for one device (e.g., DGEN 380 engine) can be correlated with the values which characterize the combustion process in the second device (e.g., RCCM).
Based on the assumption of the reactivity model, the reactivity coefficient αi and the activation energy assigned to the entire combustion reaction chain are treated as features of the fuel. These features characterize the fuel undergoing combustion reactions. If the combustion reaction chains in different devices are similar or the same, then αi and Ea determined for one device characterize the fuel’s behavior during combustion in the second device. Consequently, the fuel combustion model built on the basis of test results in one device can be used to describe the combustion process in another device. Model verification, consisting of checking the consistency of the measured values with the predicted ones, allows conclusions to be drawn regarding the similarity of the combustion chemical reaction chains. The procedure does not allow for determining of individual chain reactions, but it does allow for the assessment of the behavior of the new fuel (SAF) in turbine engines, and the similarity of the reaction chains, e.g., in relation to Jet A-1 fuel, allows for the assessment of the extent to which the addition of the SAF component changes/disturbs the combustion reaction chain.
Hence, based on
Figure 10,
Figure 11 and
Figure 12, through P
max determined on RCCM, it becomes possible to predict the values of E
a and α
i for DGEN. This in turn allows for the prediction of the SAF influence on the combustion process, including on
and
concentrations in exhaust gases. Of course, the relationships presented in this article require verification using a much larger database.
5. Conclusions
The results shown in this article indicate that SAFs added to the jet fuel change the combustion process, i.e., they change the relationships between the rates of elemental reactions in the chemical reaction chain. These changes depend on the chemical structure of the SAF component and its concentration in the mixture with Jet A-1 fuel.
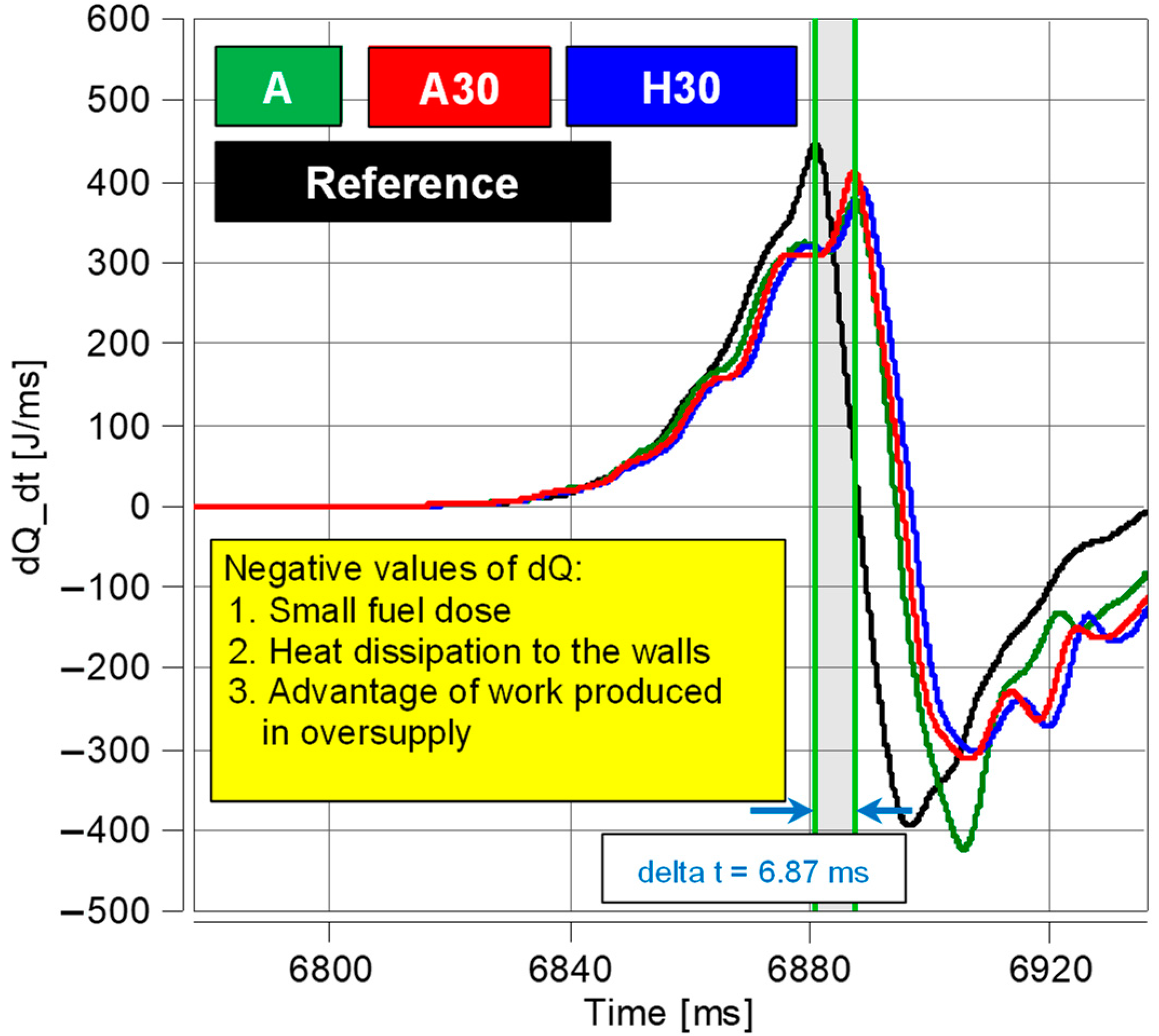
The tests using RCCM indicated differences in the combustion of the analyzed fuels and their additives. The results of the analyses from both stand tests confirm that the highest efficiency was observed for the A30 fuel.
The obtained results of the DGEN 380 tests made it possible to determine the activation energy
assigned to the combustion reaction chain of
and
. The values of activation energy
determined for Jet A-1 and blends containing A and H components in concentrations of 5, 20 and 30 wt. % are shown in
Table 4. The activation energy values assigned to the reactions chain leading to
formation
were determined for Jet A-1, A30 and H30 blends. The following values were obtained:
Jet A-1: ; ;
A30: ; ;
H30: ; .
The significantly different values obtained for each blend indicate differences in the structure of combustion reaction chains due to SAF addition to Jet A-1 fuel.
The same conclusions could be formulated by comparing values of the fuel reactivity coefficient determined in the tested blends.
Jet A-1: ; ;
A30: ; ;
H30: ; .
The values of obtained during indicator RCCM tests for Jet A-1, A30 and H30 blends correlated with both the activation energy and coefficients of reactivity. This suggests that the influence of SAF addition to Jet A-1 fuel on the structures of chemical reaction chains during RCCM tests was similar to that seen in DGEN 380 tests.
Based on these results, it was possible to determine the relationships between and concentrations in exhaust gases emitted by the DGEN 380 engine and the determined during RCCM tests. This indicated the possibility of the preliminary prediction of and emissions from the DGEN 380 engine based on the test performed at the RCCM stand. The parameters of these relationships were dependent on fuel flow rate . As the result, the Equations (19) and (20) were formulated, which can be used to predict the and concentrations in exhaust gases emitted during DGEN tests using the determined from the RCCM tests.
The thesis that the application of the reactivity model to the combustion process description enables the RCCM tests results to be used to predict the behavior of the tested fuel during combustion in the turbine engine DGEN 380 was thus confirmed. The relationships described in this paper should be confirmed for other components approved for aviation and for other turbine engines. In addition, the test results presented here should be confirmed by testing a larger number of SAFs and testing should be extended to turbine engines other than the DEGN 380.