Analysis and Optimization of a s-CO2 Cycle Coupled to Solar, Biomass, and Geothermal Energy Technologies
Abstract
1. Introduction
2. Materials and Methods
2.1. Location
2.2. System Configuration
2.3. Reciprocating Internal Combustion Engine
2.4. Concentrating Solar Tower
2.5. Supercritical CO2 Brayton Cycle
2.6. Double-Flash Geothermal Rankine Cycle
2.7. Optimization Methodology
3. Results and Discussion
3.1. Validation of the Model
3.2. Performance of the Concentrating Solar Tower
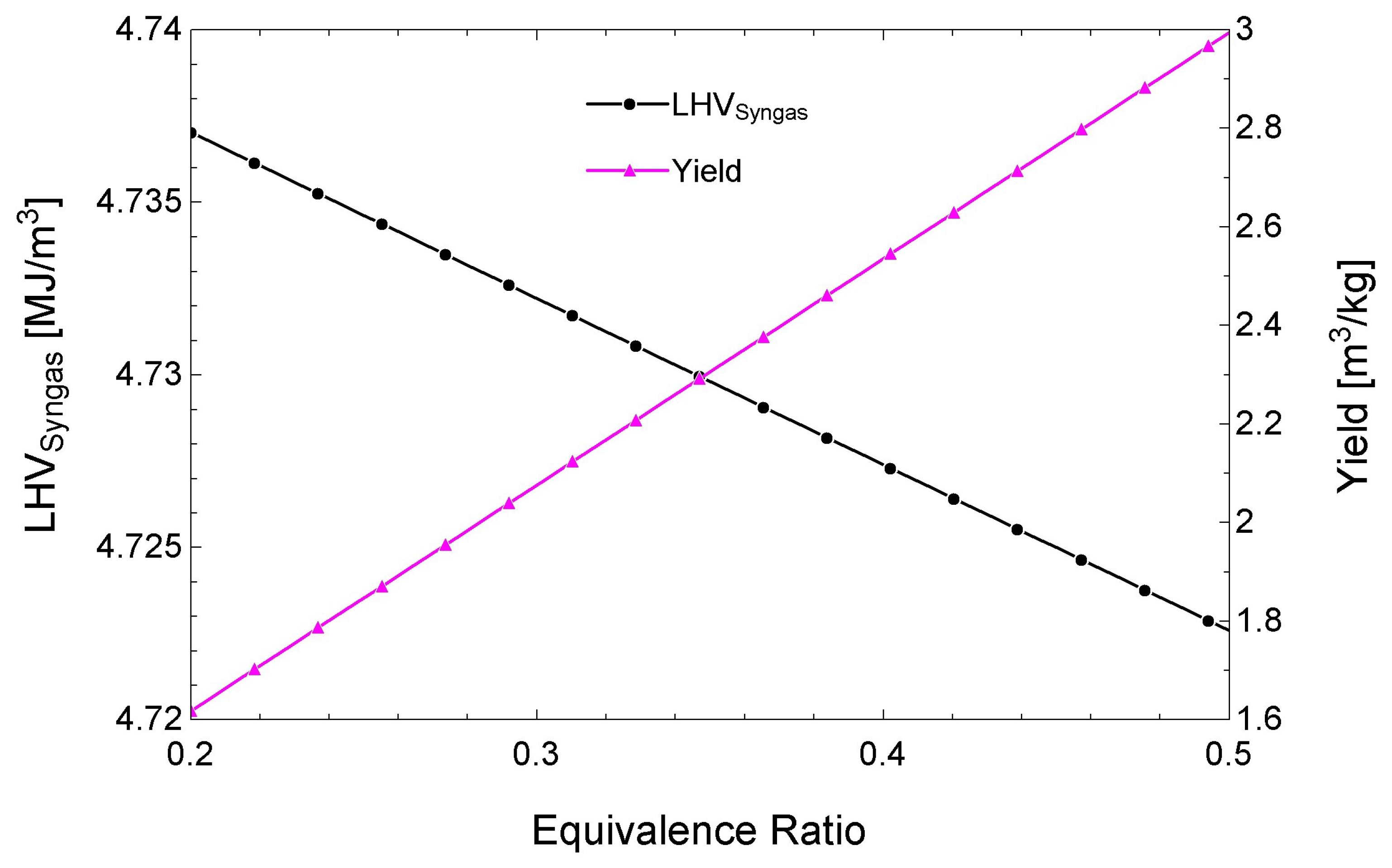
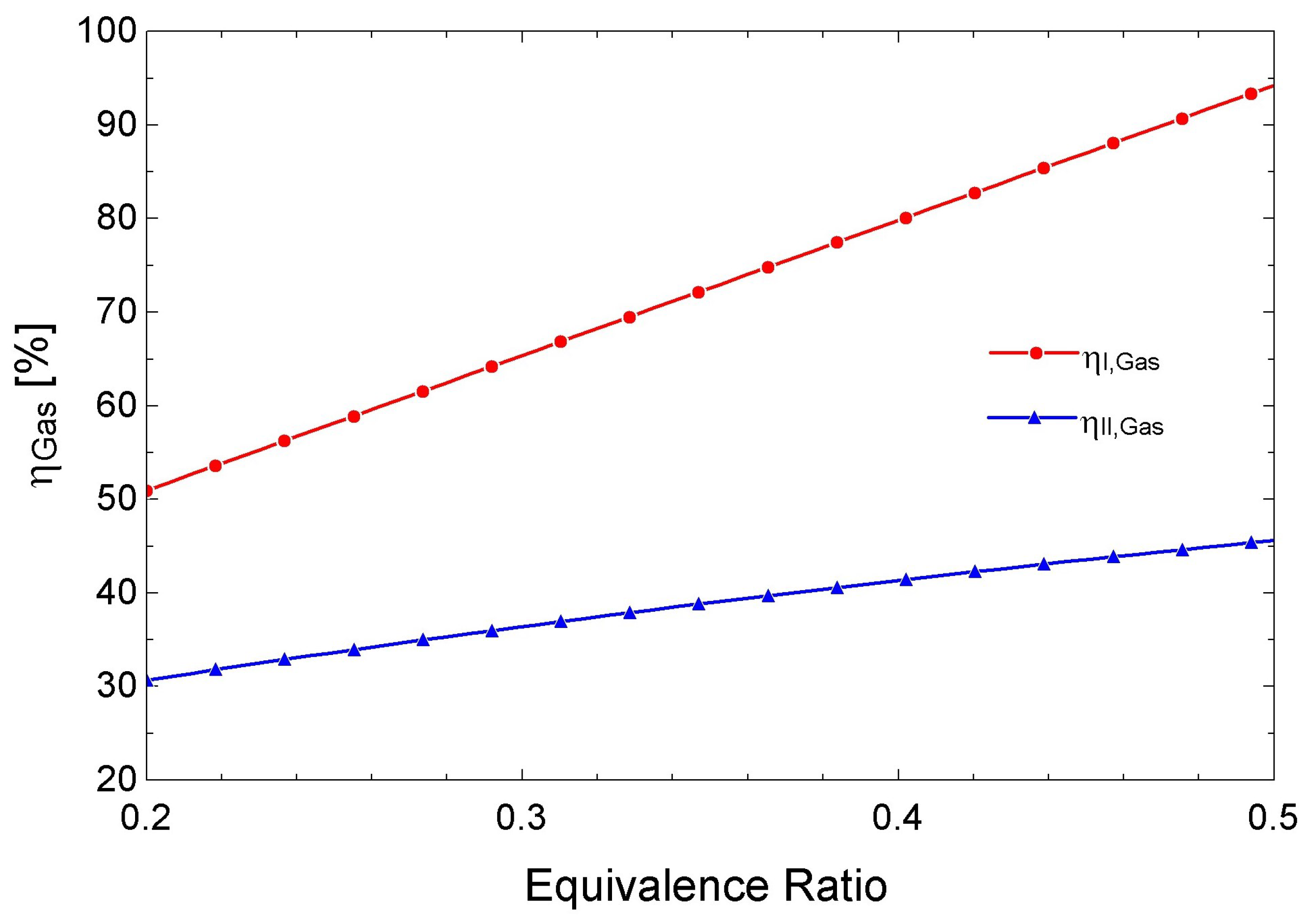
3.3. Performance of the Biomass Gasifier
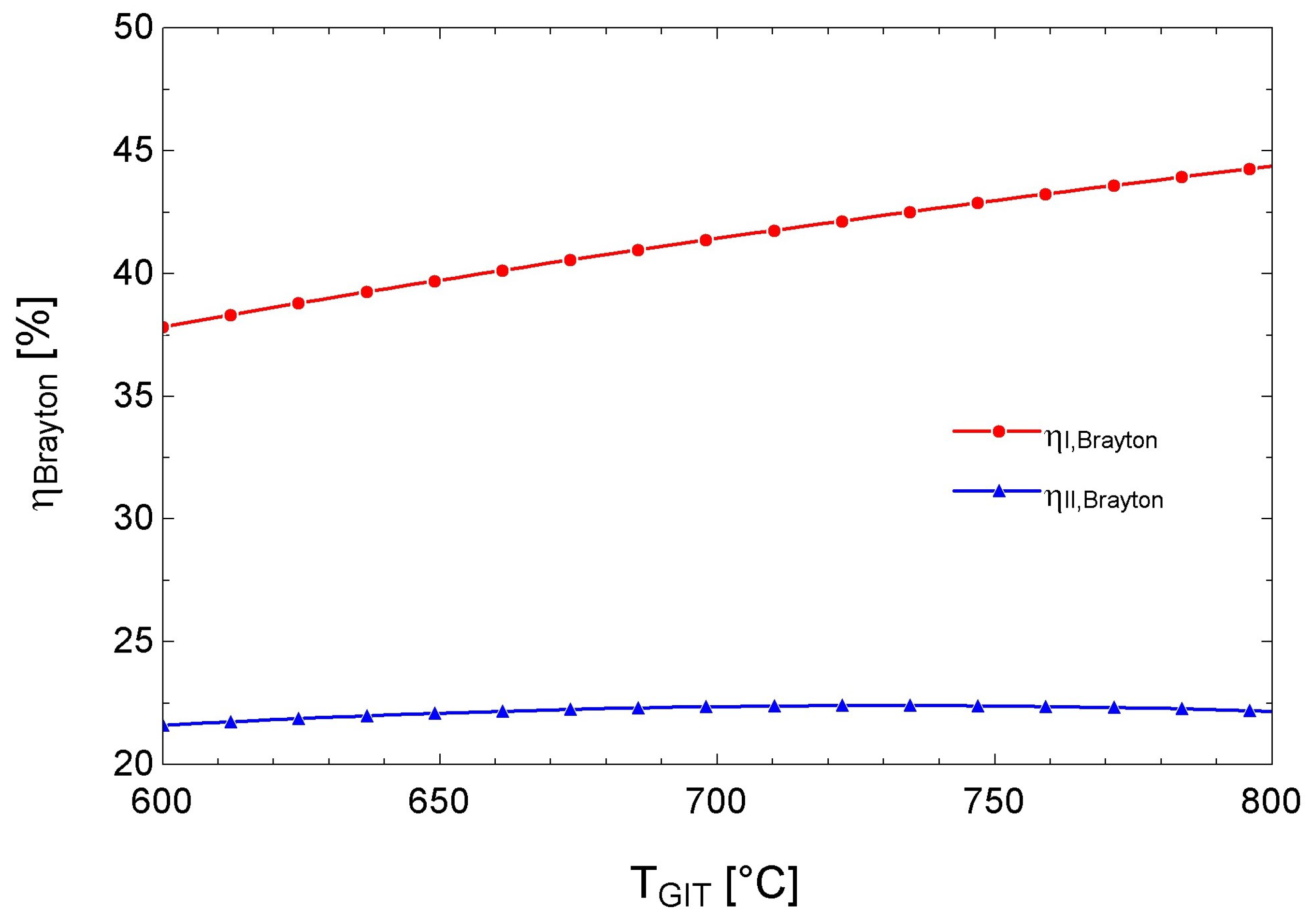
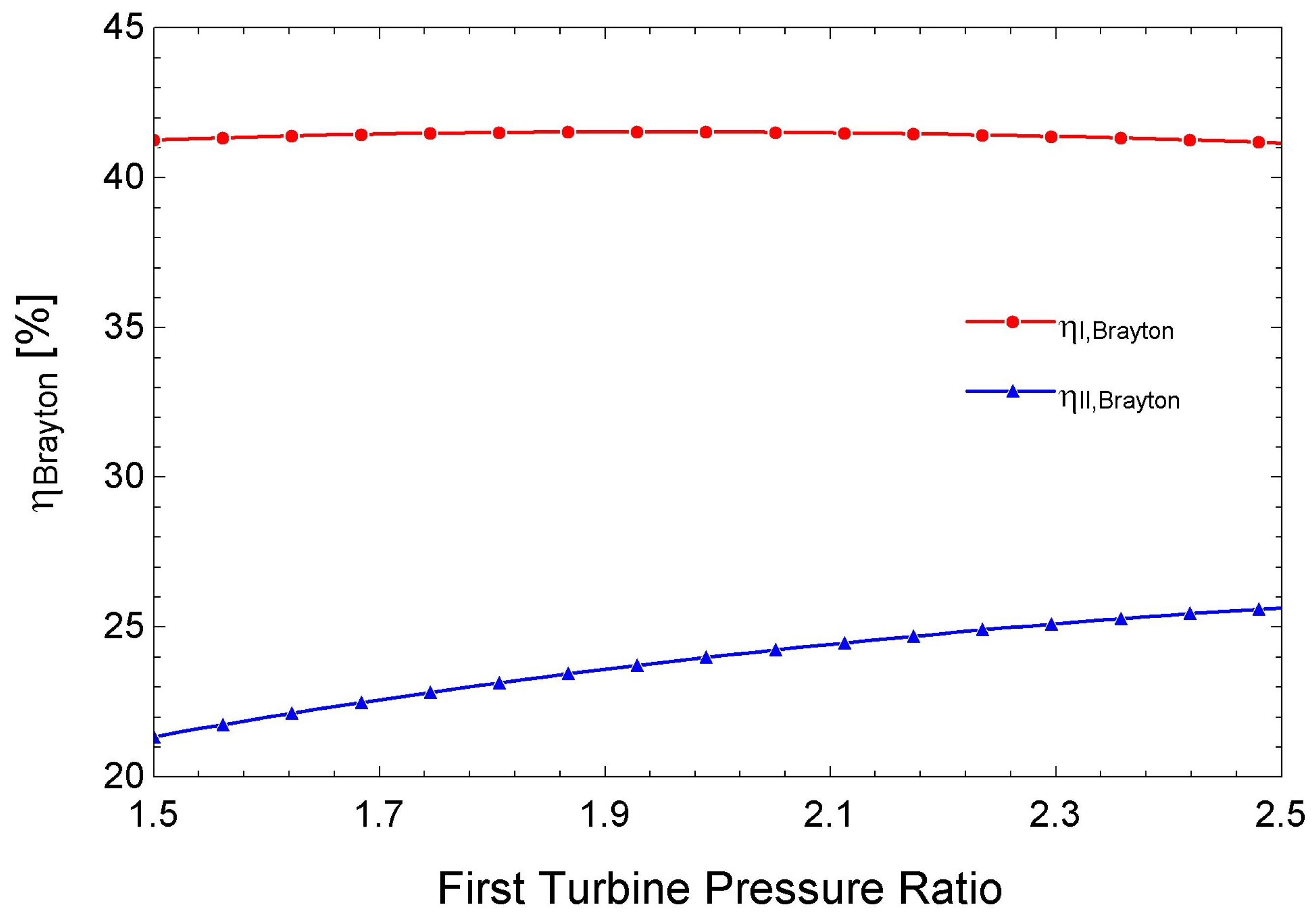
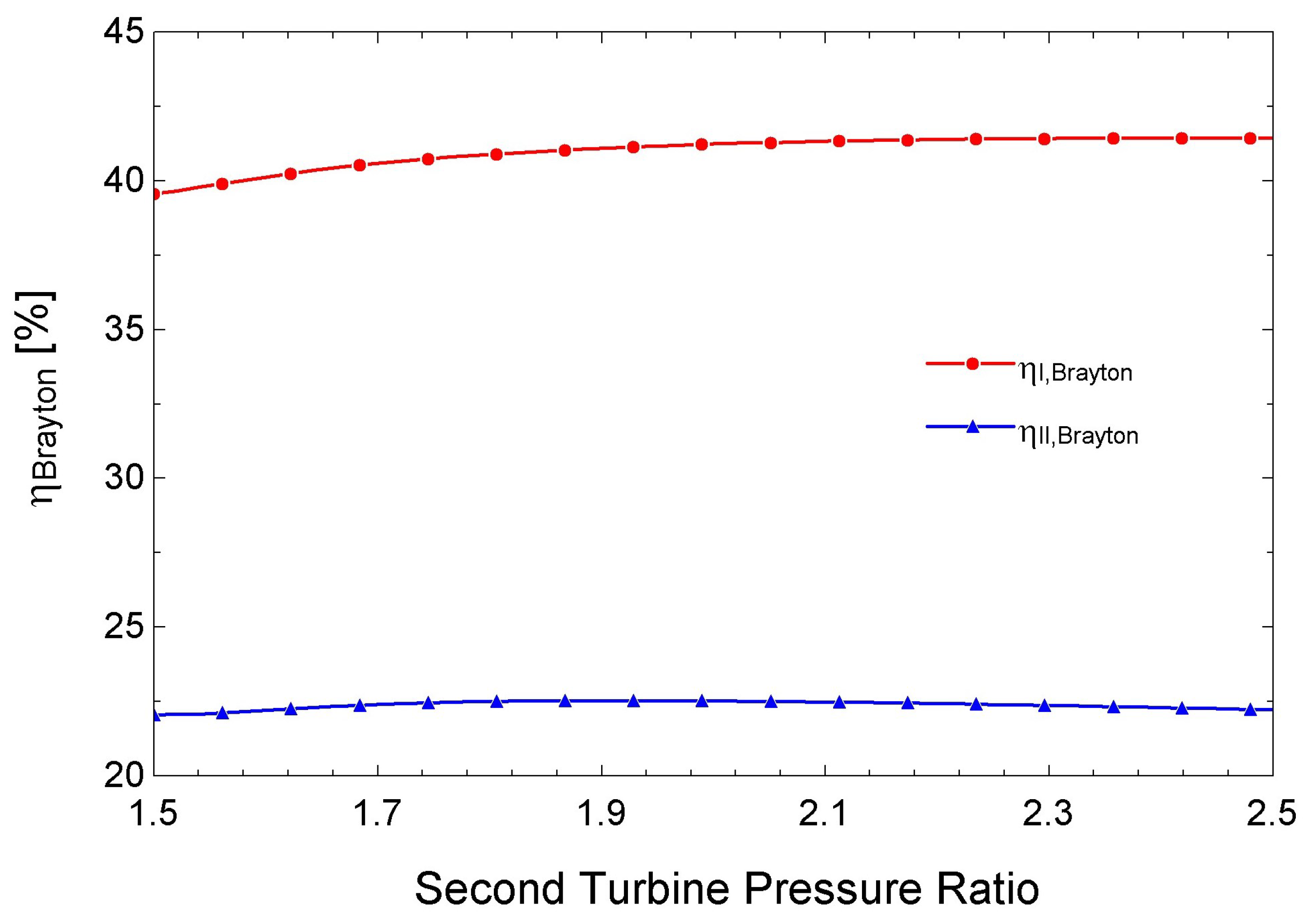
3.4. Performance of the s-CO2 Brayton Cycle
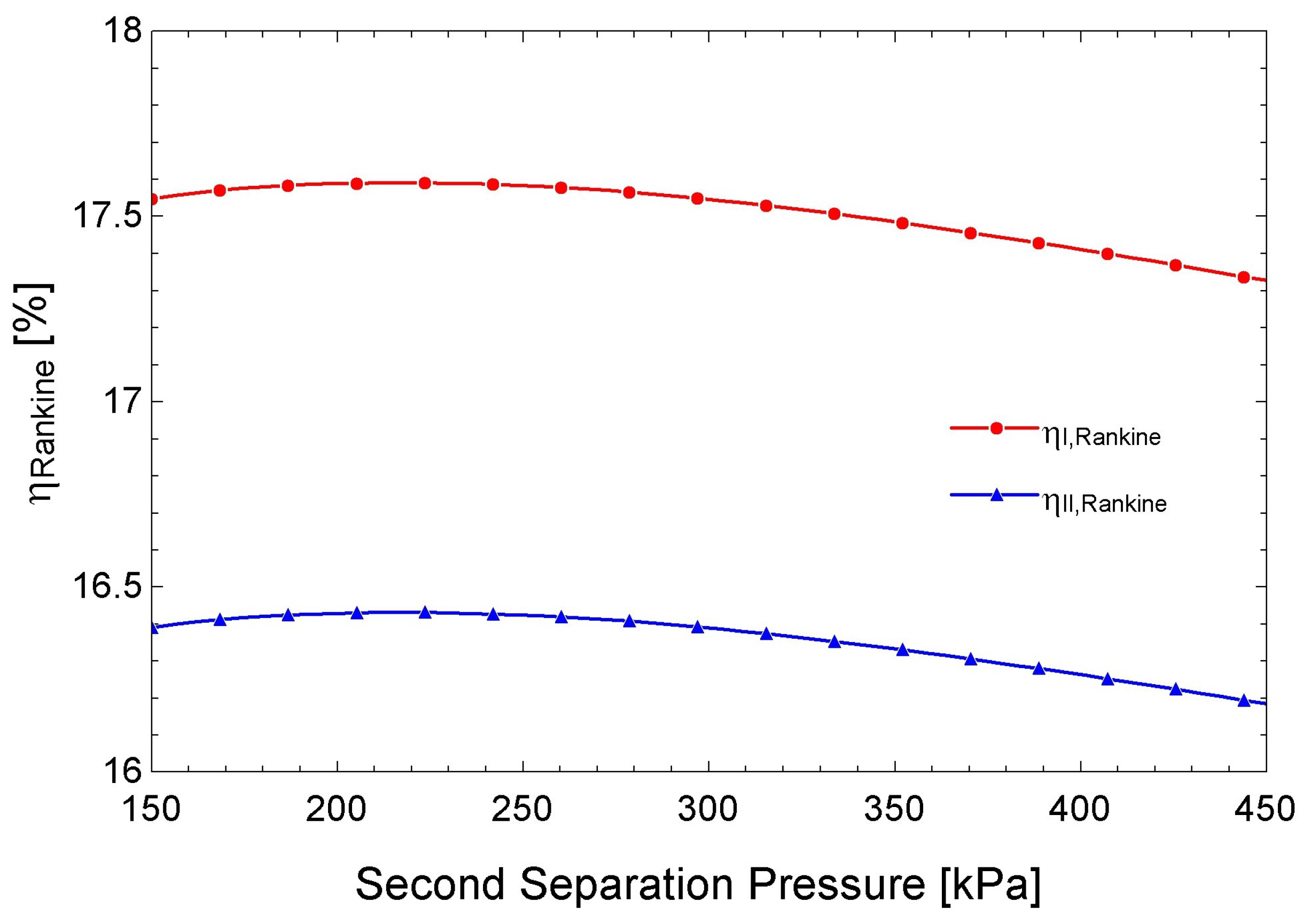
3.5. Performance of the Double-Flash Geothermal Rankine Cycle
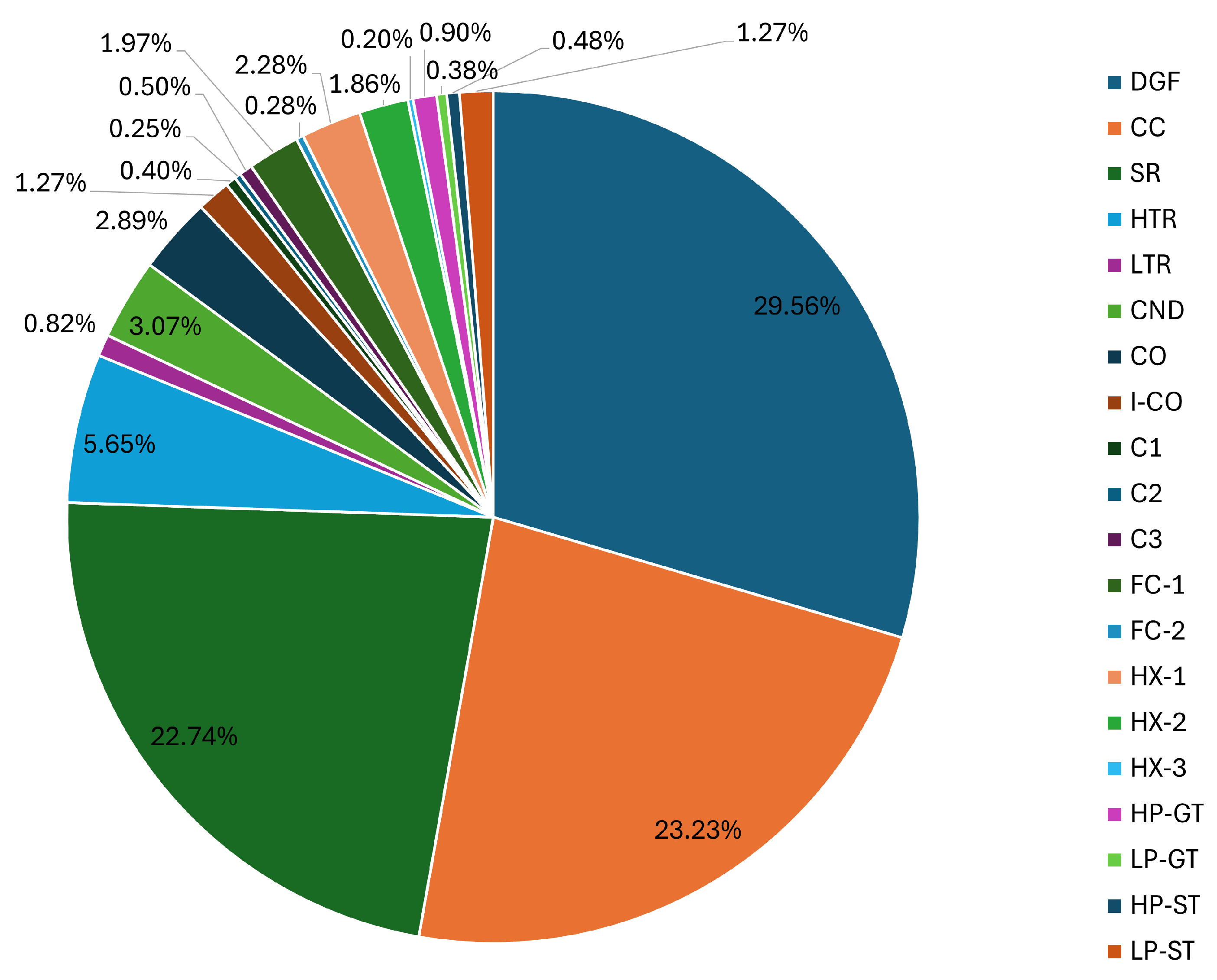
3.6. Optimum Performance Conditions of the Integrated Power System
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| Symbols: | |
| Efficiency (%) | |
| Absortivity (%) | |
| Thermal Emissivity (%) | |
| Pellets Correlation Factor (-) | |
| Solar Cone Angle (rad) | |
| Penalty Factor (-) | |
| Nominal Rotation Velocity (RPM) | |
| b | Stroke (m) |
| d | Diameter (m) |
| z | No. of Cylinders (-) |
| l | Displacement (m3) |
| h | Specific Enthalpy (kJ/kg) |
| e | Specific Exergy (kJ/kg) |
| Mass Flow (kg/s) | |
| Power (kW) | |
| T | Temperature (°C) |
| Exergy (kW) | |
| P | Pressure (kP) |
| Subscripts: | |
| 0 | Ambient Conditions |
| Syngas | |
| Biomass | |
| Mixture | |
| Inlet | |
| Outlet | |
| Acronyms: | |
| Direct Normal Irradiation | |
| Concentrating Solar Power | |
| International Energy Agency | |
| Levelized Cost Of Electricity | |
| Cumulative Density Function | |
| Probability Density Function | |
| High Heating Value | |
| Low Heating Value | |
| Heliostat Field | |
| Solar Receiver | |
| High Pressure Gas Turbine | |
| Low Pressure Gas Turbine | |
| High Pressure Steam Turbine | |
| Low Pressure Steam Turbine | |
| High Temperature Recuperator | |
| Low Temperature Recuperator | |
| Compressor 1 | |
| Compressor 2 | |
| Compressor 3 | |
| Combustion Chamber | |
| Downdraft Gasifier | |
| Cyclone | |
| Filter | |
| Internal Combustion Engine | |
| Heat Exchanger 1 | |
| Heat Exchanger 2 | |
| Heat Exchanger 3 | |
| Condenser | |
| Flash Chamber 1 | |
| Flash Chamber 2 | |
| Expansion Valve 1 | |
| Expansion Valve 2 | |
| Production Well | |
| Injection Well | |
| Cooler | |
| Intercooler | |
| Cold Gas Efficiency |
Appendix A
| State | Fluid | T (°C) | P (kPa) | h (kJ/kg) | s (kJ/kg-K) | e (kJ/kg) | m (kg/s) | W (MW) |
|---|---|---|---|---|---|---|---|---|
| 0 | Air | 23.4 | 100.8 | −5.1 | 0.242 | - | - | - |
| 0′ | Carbon dioxide | 23.4 | 100.8 | −2.3 | −0.005 | - | - | - |
| 0″ | Water | 23.4 | 100.8 | 98.2 | 0.345 | - | - | - |
| 1 | Biomass | 23.4 | - | - | - | - | 0.626 | - |
| 2 | Air | 23.4 | 100.8 | 0 | 0 | 55.1 | 1.360 | - |
| 3 | Air | 23.4 | 100.8 | 0 | 0 | 55.1 | 2.395 | - |
| 4 | Air | 23.4 | 100.8 | 0 | 0 | 55.1 | 8.112 | - |
| 5 | Air | 300 | 98.8 | 10.0 | 0.024 | 58.01 | 1.360 | - |
| 6 | Syngas | 794.1 | 98.8 | 45.8 | 0.075 | 4144 | 1.986 | - |
| 7 | Syngas | 230 | 94.8 | 11.5 | 0.030 | 4110 | 1.986 | - |
| 8 | Syngas | 216 | 92.9 | - | - | 9.6 | 1.883 | - |
| 9 | Syngas | 216 | 5 | 10.7 | 0.072 | 4094 | 1.817 | - |
| 10 | Syngas | 216 | 5 | 10.7 | 0.072 | 4171 | 1.298 | - |
| 11 | Syngas | 216 | 5 | 10.7 | 0.072 | 4094 | 0.479 | - |
| 12 | Syngas | 40 | 5 | 0.905 | 0.048 | 4094 | 2.874 | - |
| 13 | Exhaust gas | 600 | 5 | 903.2 | 7.678 | - | 2.874 | - |
| 14 | Exhaust gas | 100 | 5 | 374.1 | 6.785 | - | 2.874 | - |
| 15 | Syngas | 216 | 5 | 10.7 | 0.072 | 4171 | 9.410 | - |
| 16 | Syngas | 2082 | 5 | 27.0 | 8.9 | 70.05 | 9.410 | - |
| 17 | Ashes | - | - | - | - | 13,834 | 0.143 | - |
| 18 | Tar | - | - | - | - | 12,817 | 0.066 | - |
| 19 | ICE power | - | - | - | - | - | - | 1 |
| 20 | GT power | - | - | - | - | - | - | 14.6 |
| 21 | ST power | - | - | - | - | - | - | 4.6 |
| 22 | Solar power | - | - | - | - | - | - | 21.0 |
| 23 | Carbon dioxide | 700.2 | 25,000 | 715 | 0.166 | 666.3 | 91.8 | - |
| 24 | Carbon dioxide | 573.5 | 10,000 | 564.7 | 0.179 | 512 | 91.8 | - |
| 25 | Carbon dioxide | 700.2 | 10,000 | 720.9 | 0.351 | 617.3 | 91.8 | - |
| 26 | Carbon dioxide | 643 | 6640 | 652 | 0.357 | 546.7 | 91.8 | - |
| 27 | Carbon dioxide | 184.4 | 6640 | 120.4 | −0.442 | 252.3 | 91.8 | - |
| 28 | Carbon dioxide | 107.3 | 6640 | 32.1 | −0.654 | 226.7 | 91.8 | - |
| 29 | Carbon dioxide | 43.4 | 6640 | −59.7 | −0.920 | 213.8 | 91.8 | - |
| 30 | Carbon dioxide | 77.5 | 10,000 | −40.8 | −0.914 | 231 | 91.8 | - |
| 31 | Carbon dioxide | 77.5 | 10,000 | −40.8 | −0.914 | 231 | 40.7 | - |
| 32 | Carbon dioxide | 43.4 | 10,000 | −171.1 | −1.311 | 218.2 | 40.7 | - |
| 33 | Carbon dioxide | 81.4 | 25,000 | −144.2 | −1.302 | 242.7 | 40.7 | - |
| 34 | Carbon dioxide | 159.4 | 25,000 | 24.4 | −0.900 | 292 | 40.7 | - |
| 35 | Carbon dioxide | 77.5 | 10,000 | −40.8 | −0.914 | 231 | 51.1 | - |
| 36 | Carbon dioxide | 159.4 | 25,000 | 12.6 | −0.900 | 280.3 | 51.1 | - |
| 37 | Carbon dioxide | 159.4 | 25,000 | 17.8 | −0.900 | 285.5 | 91.8 | - |
| 38 | Carbon dioxide | 569.4 | 25,000 | 549.4 | −0.016 | 554.9 | 91.8 | - |
| 39 | Carbon dioxide | 549.4 | 25,000 | 524.3 | −0.046 | 538.7 | 91.8 | - |
| 40 | Carbon dioxide | 529.4 | 25,000 | 499.2 | −0.077 | 522.8 | 91.8 | - |
| 41 | Water | 320 | 11,357 | 1461 | 3.447 | 442.8 | 15 | - |
| 42 | Water | 195.9 | 1426 | 1461 | 3.629 | 388.9 | 15 | - |
| 43 | Water | 195.9 | 1426 | 834.1 | 2.292 | 158.3 | 10.2 | - |
| 44 | Water | 195.9 | 1426 | 1037 | 2.726 | 233.1 | 10.2 | - |
| 45 | Water | 195.9 | 1426 | 834.1 | 2.292 | 158.3 | 9.1 | - |
| 46 | Water | 138.3 | 344.6 | 834.1 | 2.335 | 145.8 | 9.1 | - |
| 47 | Water | 138.3 | 344.6 | 582.1 | 1.722 | 75.4 | 8.1 | - |
| 48 | Water | 97.7 | 344.6 | 409.7 | 1.281 | 33.8 | 15 | - |
| 49 | Water | 138.3 | 344.6 | 2732 | 6.946 | 675.9 | 1.1 | - |
| 50 | Water | 239.8 | 344.6 | 2945 | 7.409 | 751.4 | 1.1 | - |
| 51 | Water | 195.9 | 1426 | 2790 | 6.462 | 877.6 | 4.8 | - |
| 52 | Water | 195.9 | 1426 | 2790 | 6.462 | 877.6 | 1.1 | - |
| 53 | Water | 195.9 | 1426 | 2790 | 6.452 | 877.6 | 5.9 | - |
| 54 | Water | 347.8 | 1426 | 3144 | 7.118 | 1037 | 5.9 | - |
| 55 | Water | 196.8 | 344.6 | 2856 | 7.229 | 716.3 | 5.9 | - |
| 56 | Water | 203.4 | 344.6 | 2870 | 7.258 | 721.4 | 6.9 | - |
| 57 | Water | 50 | 12.3 | 2409 | 7.510 | 185.6 | 6.9 | - |
| 58 | Water | 50 | 12.3 | 209.3 | 0.704 | 4.6 | 6.9 | - |
Appendix B
| Component | Cost Function | Value (USD) |
|---|---|---|
| C1 | 217,283.00 | |
| C2 | 334,768.00 | |
| C3 | 477,973.00 | |
| HP-ST | 107,756.00 | |
| LP-ST | 170,620.00 | |
| HP-GT | 18,600,000.00 | |
| LP-GT | 8,470,000.00 | |
| DGF | 4,990,000.00 | |
| CND | 122,799.00 | |
| CC | 6,500,000.00 | |
| HX-1 | 19,493.00 | |
| HX-2 | 17,964.00 | |
| HX-3 | 18,072.00 | |
| CO | 15,766.00 | |
| I-CO | 17,413.00 | |
| FC-1 | 4205.00 | |
| FC-2 | 2568.00 | |
| HTR | 16,840.00 | |
| LTR | 18,558.00 |
References
- Nations, U. World Population Prospects: The 2017 Revision, Key Findings and Advance Tables; Working Paper No. ESA/P/WP/248; United Nations: New York, NY, USA, 2017.
- Steen, M. Greenhouse Gas Emissions from Fossil Fuel Fired Power Generation Systems; Institute for Advanced Materials, Joint Research Centre, European Commission: Ispra, Italy, 2001. [Google Scholar]
- Colgan, J.D. The International Energy Agency; Challenges for the 21st Century. GPPi Energy Policy Paper; Global Public Policy Institute (GPPI): Berlin, Germany, 2009; Volume 6. [Google Scholar]
- Sengupta, M.; Xie, Y.; Lopez, A.; Habte, A.; Maclaurin, G.; Shelby, J. The national solar radiation data base (NSRDB). Renew. Sustain. Energy Rev. 2018, 89, 51–60. [Google Scholar] [CrossRef]
- Le Bert, G.H.; Gutiérrez-Negrín, L.C.; Quijano León, H.L.; Ornelas Celis, A.; Espíndola, S.; Hernandez Carrillo, I. Evaluación de la energía geotérmica en México. In Informe Para el Banco Interamericano de Desarrollo y la Comisión Reguladora de Energía; Comisión Reguladora de Energía: Mexico City, Mexico, 2011. [Google Scholar]
- Valdez-Vazquez, I.; Acevedo-Benítez, J.A.; Hernández-Santiago, C. Distribution and potential of bioenergy resources from agricultural activities in Mexico. Renew. Sustain. Energy Rev. 2010, 14, 2147–2153. [Google Scholar] [CrossRef]
- Binotti, M.; Astolfi, M.; Campanari, S.; Manzolini, G.; Silva, P. Preliminary assessment of sCO2 power cycles for application to CSP Solar Tower plants. Energy Procedia 2017, 105, 1116–1122. [Google Scholar] [CrossRef]
- Zhou, C. Hybridisation of solar and geothermal energy in both subcritical and supercritical Organic Rankine Cycles. Energy Convers. Manag. 2014, 81, 72–82. [Google Scholar] [CrossRef]
- Astolfi, M.; Xodo, L.; Romano, M.C.; Macchi, E. Technical and economical analysis of a solar–geothermal hybrid plant based on an Organic Rankine Cycle. Geothermics 2011, 40, 58–68. [Google Scholar] [CrossRef]
- Lentz, Á.; Almanza, R. Solar–geothermal hybrid system. Appl. Therm. Eng. 2006, 26, 1537–1544. [Google Scholar] [CrossRef]
- Cardemil, J.M.; Cortés, F.; Díaz, A.; Escobar, R. Thermodynamic evaluation of solar-geothermal hybrid power plants in northern Chile. Energy Convers. Manag. 2016, 123, 348–361. [Google Scholar] [CrossRef]
- Boukelia, T.E.; Arslan, O.; Bouraoui, A. Thermodynamic performance assessment of a new solar tower-geothermal combined power plant compared to the conventional solar tower power plant. Energy 2021, 232, 121109. [Google Scholar] [CrossRef]
- Javadi, M.A.; Abhari, M.K.; Ghasemiasl, R.; Ghomashi, H. Energy, exergy and exergy-economic analysis of a new multigeneration system based on double-flash geothermal power plant and solar power tower. Sustain. Energy Technol. Assess. 2021, 47, 101536. [Google Scholar] [CrossRef]
- Kulhánek, M.A.D.V.; Dostal, V. Thermodynamic analysis and comparison of supercritical carbon dioxide cycles. In Proceedings of the Supercritical CO2 Power Cycle Symposium, Boulder, CO, USA, 24–25 May 2011; pp. 1–7. [Google Scholar]
- Feher, E.G. The supercritical thermodynamic power cycle. Energy Convers. 1968, 8, 85–90. [Google Scholar] [CrossRef]
- Angelino, G. Carbon dioxide condensation cycles for power production. J. Eng. Power 1968, 90, 287–295. [Google Scholar] [CrossRef]
- Dostal, V. A Supercritical Carbon Dioxide Cycle for Next Generation Nuclear Reactors. Ph.D. Thesis, Massachusetts Institute of Technology, Department of Nuclear Engineering, Cambridge, MA, USA, 2004. [Google Scholar]
- Moisseytsev, A.; Sienicki, J.J. Investigation of alternative layouts for the supercritical carbon dioxide Brayton cycle for a sodium-cooled fast reactor. Nucl. Eng. Des. 2009, 239, 1362–1371. [Google Scholar] [CrossRef]
- Padilla, R.V.; Too, Y.C.S.; Benito, R.; Stein, W. Exergetic analysis of supercritical CO2 Brayton cycles integrated with solar central receivers. Appl. Energy 2015, 148, 348–365. [Google Scholar] [CrossRef]
- Conboy, T.; Wright, S.; Pasch, J.; Fleming, D.; Rochau, G.; Fuller, R. Performance characteristics of an operating supercritical CO2 Brayton cycle. J. Eng. Gas Turbines Power. 2012, 134, 111703. [Google Scholar] [CrossRef]
- Iverson, B.D.; Conboy, T.M.; Pasch, J.J.; Kruizenga, A.M. Supercritical CO2 Brayton cycles for solar-thermal energy. Appl. Energy 2013, 111, 957–970. [Google Scholar] [CrossRef]
- Wang, X.; Liu, Q.; Bai, Z.; Lei, J.; Jin, H. Thermodynamic analysis of the cascaded supercritical CO2 cycle integrated with solar and biomass energy. Energy Procedia 2017, 105, 445–452. [Google Scholar] [CrossRef]
- Yu, W.; Gong, Q.; Gao, D.; Wang, G.; Su, H.; Li, X. Thermodynamic analysis of supercritical carbon dioxide cycle for internal combustion engine waste heat recovery. Processes 2020, 8, 216. [Google Scholar] [CrossRef]
- Nkhonjera, L.; Ansari, S.; Liu, X. Development of hybrid CSP biomass gasification process with supercritical carbon dioxide cycle for power generation. In AIP Conference Proceedings; AIP Publishing: New York, NY, USA, 2020; Volume 2303, p. 080005. [Google Scholar]
- Cao, Y.; Habibi, H.; Zoghi, M.; Raise, A. Waste heat recovery of a combined regenerative gas turbine-recompression supercritical CO2 Brayton cycle driven by a hybrid solar-biomass heat source for multi-generation purpose: 4E analysis and parametric study. Energy 2021, 236, 121432. [Google Scholar] [CrossRef]
- Altinkaynak, M.; Ozturk, M. Thermodynamic analysis of a novel integrated system operating with gas turbine, s-CO2 and t-CO2 power systems for hydrogen production and storage. Int. J. Hydrogen Energy 2022, 47, 3484–3503. [Google Scholar] [CrossRef]
- Manesh, M.K.; Mehrabian, M.; Nourpour, M.; Onishi, V. Risk and 4E analyses and optimization of a novel solar-natural gas-driven polygeneration system based on Integration of Gas Turbine–SCO2–ORC-solar PV-PEM electrolyzer. Energy 2023, 263, 125777. [Google Scholar] [CrossRef]
- Chitgar, N.; Moghimi, M. Design and evaluation of a novel multi-generation system based on SOFC-GT for electricity, fresh water and hydrogen production. Energy 2020, 197, 117162. [Google Scholar] [CrossRef]
- Molina Pfennig, P.D.; González-Baheza, A. Evaluación de los recursos de radiación solar en los municipios de Baja California Sur, México, mediante un modelo de aptitud socioambiental. Región Soc. 2020, 32, e1296. [Google Scholar] [CrossRef] [PubMed]
- Barragán, M.A.M.; Ochoa, E.d.J.R.; García, A.Z.; Buriticá, N.G.M. Energía geotérmica en México, una alternativa con el medio ambiente: Cerro Prieto. p. 71. Available online: https://revistas.udem.edu.co/index.php/fragua/issue/download/238/51#page=71 (accessed on 11 April 2023).
- Rodríguez-Alejandro, D.A. Optimización de un Gasificador de Biomasa Basado en la Exergo-Caracterización. Master’s Thesis, Universidad de Guanajuato, Salamanca, Mexico, 2012. [Google Scholar]
- Alejandro, D.A.R. Gasificación de Biomasa: Modelado, Simulación y Análisis Experimental. Ph.D. Thesis, Universidad de Guanajuato, Guanajuato, Mexico, 2017. [Google Scholar]
- Ebrahimi, A.; Ghorbani, B.; Ziabasharhagh, M.; Rahimi, M.J. Biomass gasification process integration with Stirling engine, solid oxide fuel cell, and multi-effect distillation. J. Therm. Anal. Calorim. 2021, 145, 1283–1302. [Google Scholar] [CrossRef]
- Atoche Sánchez, E.A. Estudio de Factibilidad de un Sistema Moto-Generador Empleando Gasificación de Biomasa. Bachelor Thesis, Universidad de Piura, Piura, Peru, 2018. [Google Scholar]
- Alsagri, A.S.H. Thermoeconomic and Optimization Analysis of Advanced Supercritical Carbon Dioxide Power Cycles in Concentrated Solar Power Application; University of Dayton: Dayton, OH, USA, 2018. [Google Scholar]
- U.S. National Renewable Energy Laboratory. Available online: https://www.nrel.gov/ (accessed on 3 November 2023).
- Scheffler, O.C. Optimization of an Optical Field for a Central Receiver Solar Thermal Power Plant; Stellenbosch University: Stellenbosch, South Africa, 2015. [Google Scholar]
- DiPippo, R. Geothermal Power Plants: Principles, Applications, Case Studies and Environmental Impact; Butterworth-Heinemann: Woburn, MA, USA, 2012. [Google Scholar]
- Powell, M.J.D. A direct search optimization method that models the objective and constraint functions by linear interpolation. In Advances in Optimization and Numerical Analysis; Mathematics and Its Applications; Gomez, S., Hennart, J.P., Eds.; Springer: Berlin/Heidelberg, Germany, 1994; Volume 275, pp. 51–67. [Google Scholar] [CrossRef]
- Rodriguez-Alejandro, D.A.; Nam, H.; Maglinao, A.L., Jr.; Capareda, S.C.; Aguilera-Alvarado, A.F. Development of a modified equilibrium model for biomass pilot-scale fluidized bed gasifier performance predictions. Energy 2016, 115, 1092–1108. [Google Scholar] [CrossRef]
- Karagoz, M.; Uysal, C.; Agbulut, U.; Saridemir, S. Energy, exergy, economic and sustainability assessments of a compression ignition diesel engine fueled with tire pyrolytic oil-diesel blends. J. Clean. Prod. 2020, 264, 121724. [Google Scholar] [CrossRef]
- Comision Federal de Electricidad. Available online: https://www.cfe.mx/hogar/tarifas/Pages/Acuerdosdetarifasant.aspx (accessed on 15 September 2024).
- Mamaghani, A.H.; Najafi, B.; Shirazi, A.; Rinaldi, F. 4E analysis and multi-objective optimization of an integrated MCFC (molten carbonate fuel cell) and ORC (organic Rankine cycle) system. Energy 2015, 82, 650–663. [Google Scholar] [CrossRef]
- Abam, F.; Diemuodeke, O.; Ekwe, E.; Samuel, O.; Farooq, M. Exergoeconomic and environmental modeling of integrated polygeneration power plant with biomass-based syngas supplemental firing. Energies 2020, 13, 6018. [Google Scholar] [CrossRef]
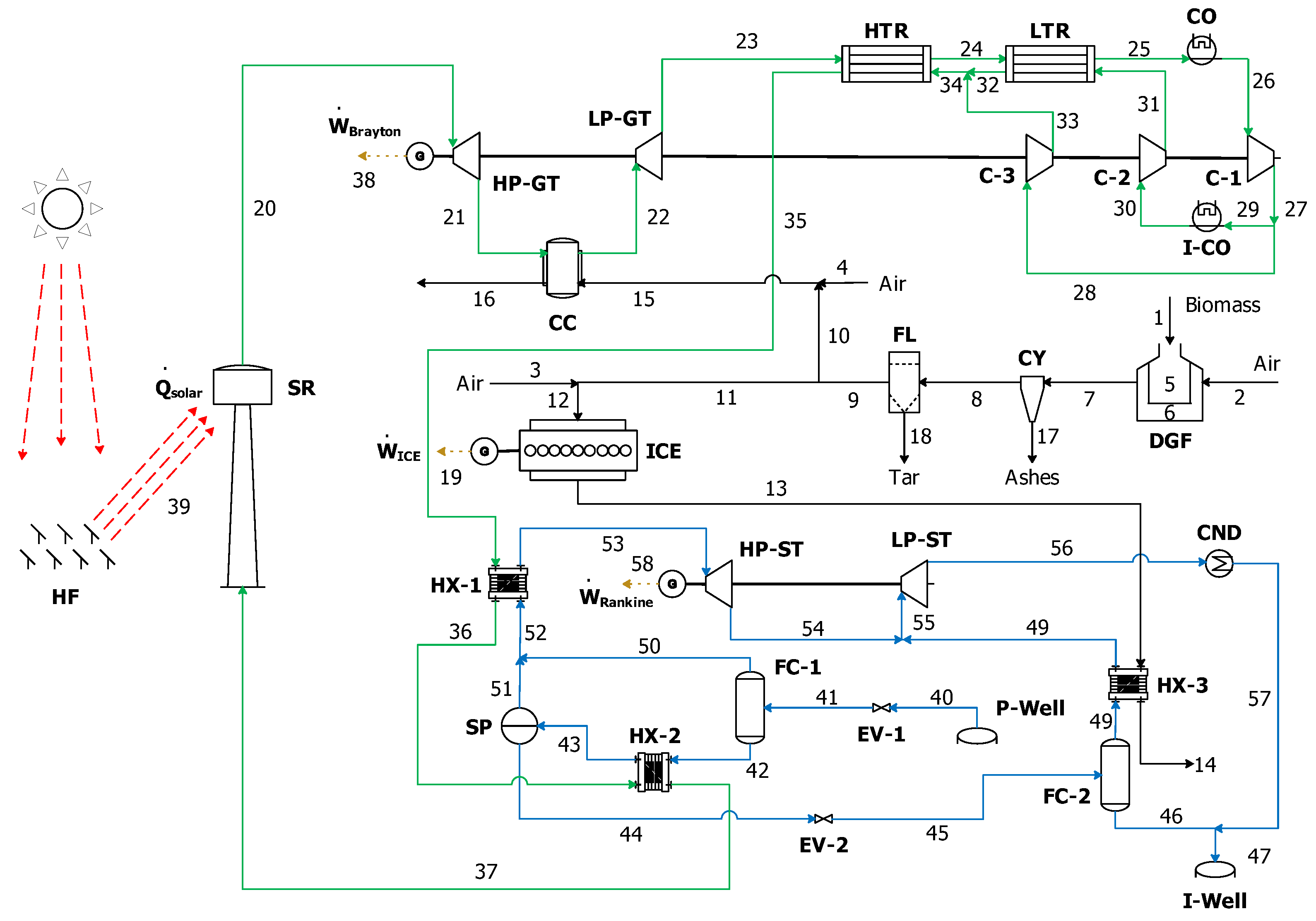
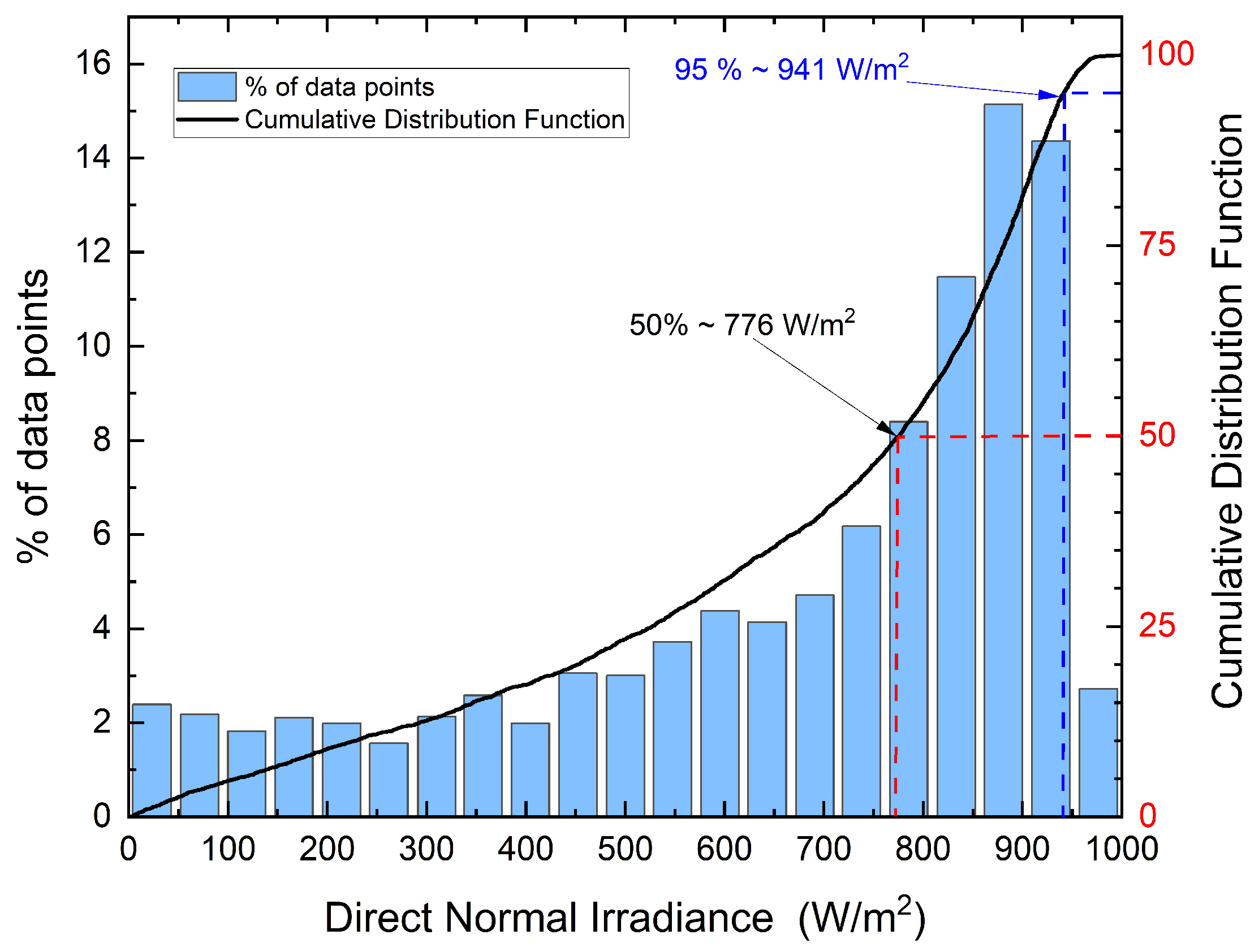

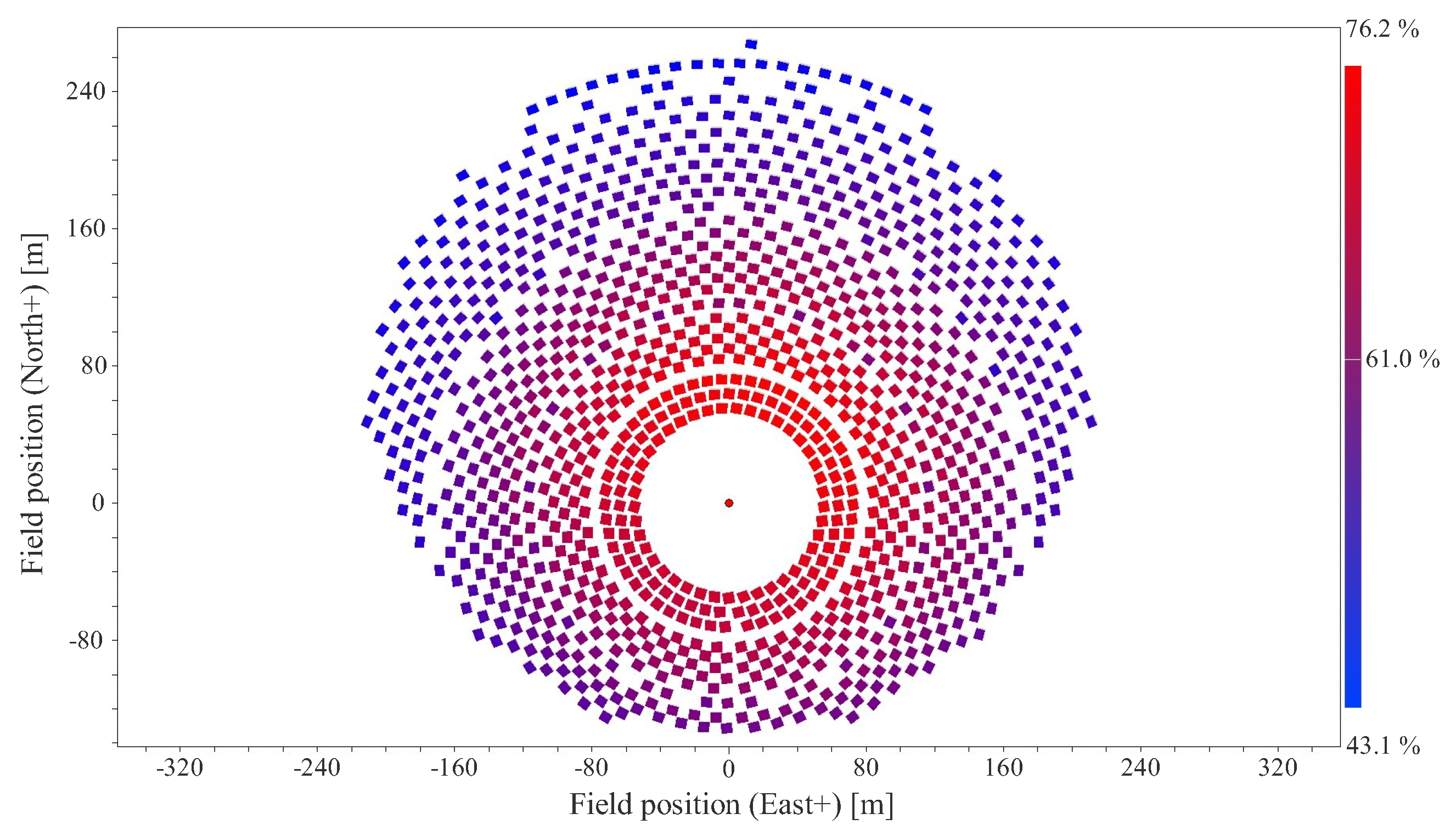


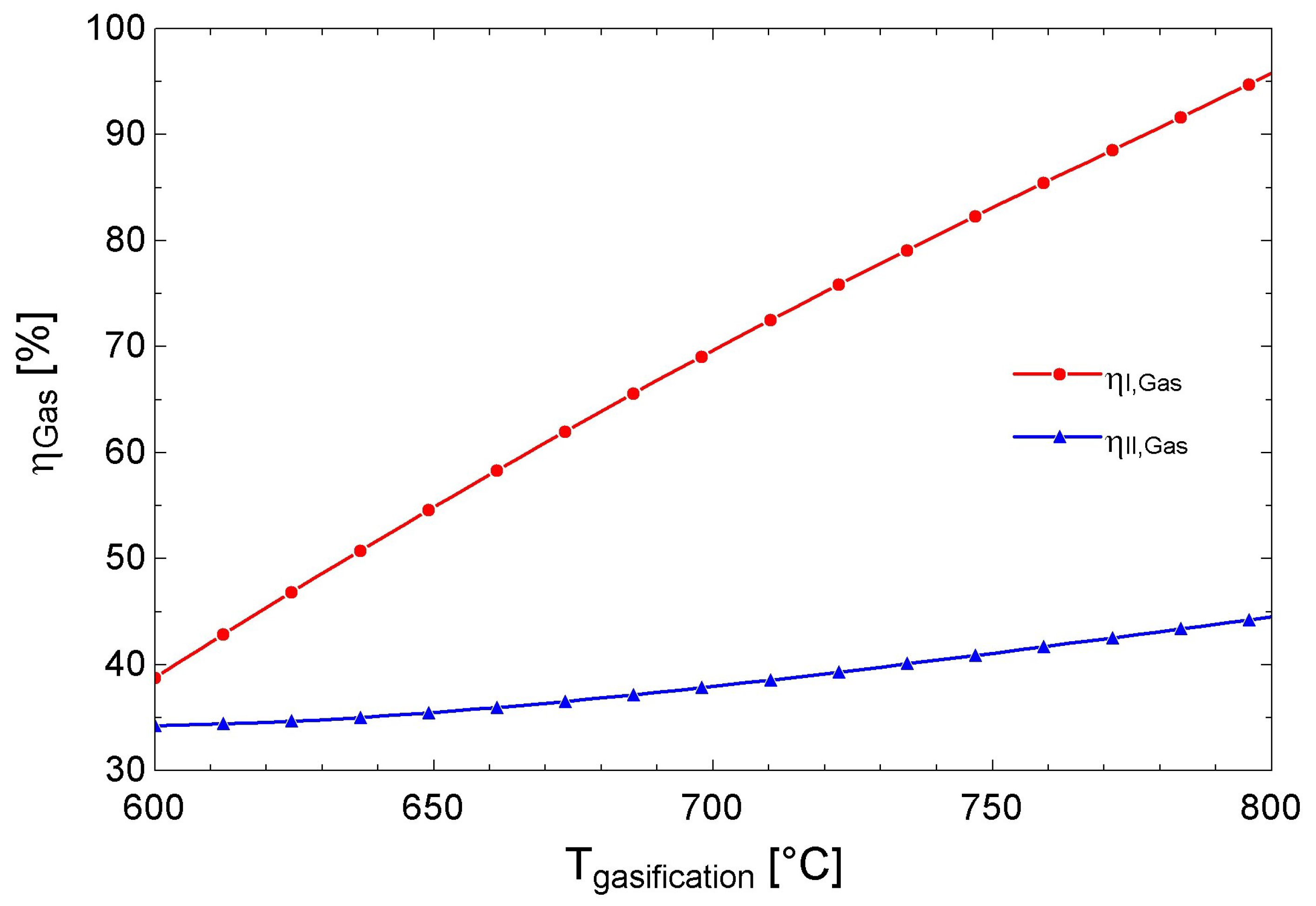


| Well No. | Temperature [°C] | Production [Ton/h] | Enthalpy (kJ/kg) |
|---|---|---|---|
| 1 | 305 | 20 | 1300 |
| 2 | 322 | 53 | 1520 |
| 3 | 333 | 49 | 1620 |
| 4 | 320 | 57 | 1461 |
| Parameter | Value | Units |
|---|---|---|
| Nominal power, | 1000 | kW |
| Syngas pressure, | 3–5 | kPa |
| Exhaust gas temperature, | 600 | °C |
| Nominal rotational velocity, | 720 | RPM |
| Compression ratio, | 9:1 | - |
| No. of cylinders, z | 9 | - |
| Diameter, d | 0.300 | m |
| Stroke, b | 0.380 | m |
| Displacement, l | 0.242 | m3 |
| Variable | Value |
|---|---|
| Location | 115°14′ W; 32°25′ N |
| Direct normal irradiation, DNI | 941 W/m2 |
| Unitary heliostat size | m |
| Solar cone angle, | 0.005 rad |
| Coefficient of convection, | 10 W/m2 K |
| Absorptivity, | 0.95 |
| Thermal emissivity, | 0.85 |
| Fouling factor, | 0.95 |
| Reflectivity factor, | 0.95 |
| View factor, | 1 |
| Convective loss factor, | 1 |
| Variable | Value | Units |
|---|---|---|
| Rankine Cycle | ||
| HP-ST efficiency, | 85 | % |
| LP-ST efficiency, | 85 | % |
| First flash pressure, | 0.8668 | MPa |
| Second flash pressure, | 0.2768 | MPa |
| Brayton Cycle | ||
| HP-GT efficiency, | 93 | % |
| LP-GT efficiency, | 93 | % |
| C-1 efficiency, | 89 | % |
| C-2 efficiency, | 89 | % |
| C-3 efficiency, | 89 | % |
| Heat exchanger effectiveness, | 95 | % |
| CO2 max pressure, | 25 | MPa |
| CO2 split fraction, | 0.8606 | - |
| Gas turbine inlet temperature, | 700 | °C |
| Initial temperture difference, | 20 | °C |
| Decision Variable | Lower Bound | Guess Value | Upper Bound |
|---|---|---|---|
| Solar tower system | |||
| 1 | 50 | 200 | |
| 1 | 10 | 50 | |
| 1 | 10 | 50 | |
| s-CO2 Brayton cycle | |||
| 600 °C | 700 °C | 800 °C | |
| 1.5 | 1.662 | 2.5 | |
| 1.5 | 2.317 | 2.5 | |
| 0.5 | 0.8606 | 0.9 | |
| Rankine cycle | |||
| 500 kPa | 1200 kPa | 1500 kPa | |
| 150 kPa | 395 kPa | 450 kPa | |
| ICE cycle | |||
| 600 °C | 700 °C | 800 °C | |
| 0.2 | 0.35 | 0.5 | |
| Variable | Value | Units |
|---|---|---|
| Tower height | 73.71 | m |
| Receiver height | 1.86 | m |
| Diameter of the receiver | 6.91 | m |
| Number of heliostats | 1116 | - |
| Total area of heliostats | 38,971 | m2 |
| Cosine efficiency | 88.06 | % |
| Attenuation efficiency | 97.70 | % |
| Intersection efficiency | 78.34 | % |
| Shadow efficiency | 100.00 | % |
| Locking efficiency | 99.35 | % |
| Reflectivity efficiency | 90.25 | % |
| Optical efficiency | 57.41 | % |
| Incident heat rate on the heliostats | 36,632 | kW |
| Heat rate absorbed by the receiver | 21,031 | kW |
| Heat rate transmitted to the working flow | 19,822 | kW |
| Incident flux on the receiver | 549.23 | kW/m2 |
| Element | Dry Base | Humid Base |
|---|---|---|
| (%) | (%) | |
| C | 45.7 | 39.9 |
| H | 6.9 | 4.9 |
| O | 42.0 | 36.5 |
| N | 0 | 0 |
| S | 0 | 0 |
| Humidity | 0 | 13.2 |
| Ashes | 5.4 | 5.5 |
| Total | 100 | 100 |
| Constituent | Symbol | Content |
|---|---|---|
| Hydrogen | 21.53% | |
| Nitrogen | 40.65% | |
| Carbon monoxide | 17.22% | |
| Carbon dioxide | 11.20% | |
| Methane | 0.65% | |
| Water | 8.76% |
| System | (%) | (%) | (MW) | (%) | (%) | (MW) |
|---|---|---|---|---|---|---|
| Individual Performance | Optimized System | |||||
| Solar Tower | 78.7 | - | - | 76.4 | - | - |
| Gasifier | 69.7 | 48.2 | - | 83.9 | 54.7 | - |
| Brayton | 41.4 | 22.4 | 10.9 | 43.8 | 28.6 | 15.0 |
| Rankine | 17.4 | 16.3 | 4.2 | 18.7 | 17.2 | 4.6 |
| ICE | 9.6 | 19.2 | 1.0 | 10.1 | 20.5 | 1.0 |
| Overall | 31.5 | 25.7 | 16.1 | 38.8 | 30.9 | 20.6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Anaya-Reyes, O.; Salgado-Transito, I.; Rodríguez-Alejandro, D.A.; Zaleta-Aguilar, A.; Martínez-Pérez, C.B.; Cano-Andrade, S. Analysis and Optimization of a s-CO2 Cycle Coupled to Solar, Biomass, and Geothermal Energy Technologies. Energies 2024, 17, 5077. https://doi.org/10.3390/en17205077
Anaya-Reyes O, Salgado-Transito I, Rodríguez-Alejandro DA, Zaleta-Aguilar A, Martínez-Pérez CB, Cano-Andrade S. Analysis and Optimization of a s-CO2 Cycle Coupled to Solar, Biomass, and Geothermal Energy Technologies. Energies. 2024; 17(20):5077. https://doi.org/10.3390/en17205077
Chicago/Turabian StyleAnaya-Reyes, Orlando, Iván Salgado-Transito, David Aarón Rodríguez-Alejandro, Alejandro Zaleta-Aguilar, Carlos Benito Martínez-Pérez, and Sergio Cano-Andrade. 2024. "Analysis and Optimization of a s-CO2 Cycle Coupled to Solar, Biomass, and Geothermal Energy Technologies" Energies 17, no. 20: 5077. https://doi.org/10.3390/en17205077
APA StyleAnaya-Reyes, O., Salgado-Transito, I., Rodríguez-Alejandro, D. A., Zaleta-Aguilar, A., Martínez-Pérez, C. B., & Cano-Andrade, S. (2024). Analysis and Optimization of a s-CO2 Cycle Coupled to Solar, Biomass, and Geothermal Energy Technologies. Energies, 17(20), 5077. https://doi.org/10.3390/en17205077








