Experimental Study of the Fluid Contents and Organic/Inorganic Hydrocarbon Saturations, Porosities, and Permeabilities of Clay-Rich Shale
Abstract
1. Introduction
2. Experimental Methods
2.1. Rock Samples
2.2. Pore-Structure Characterization
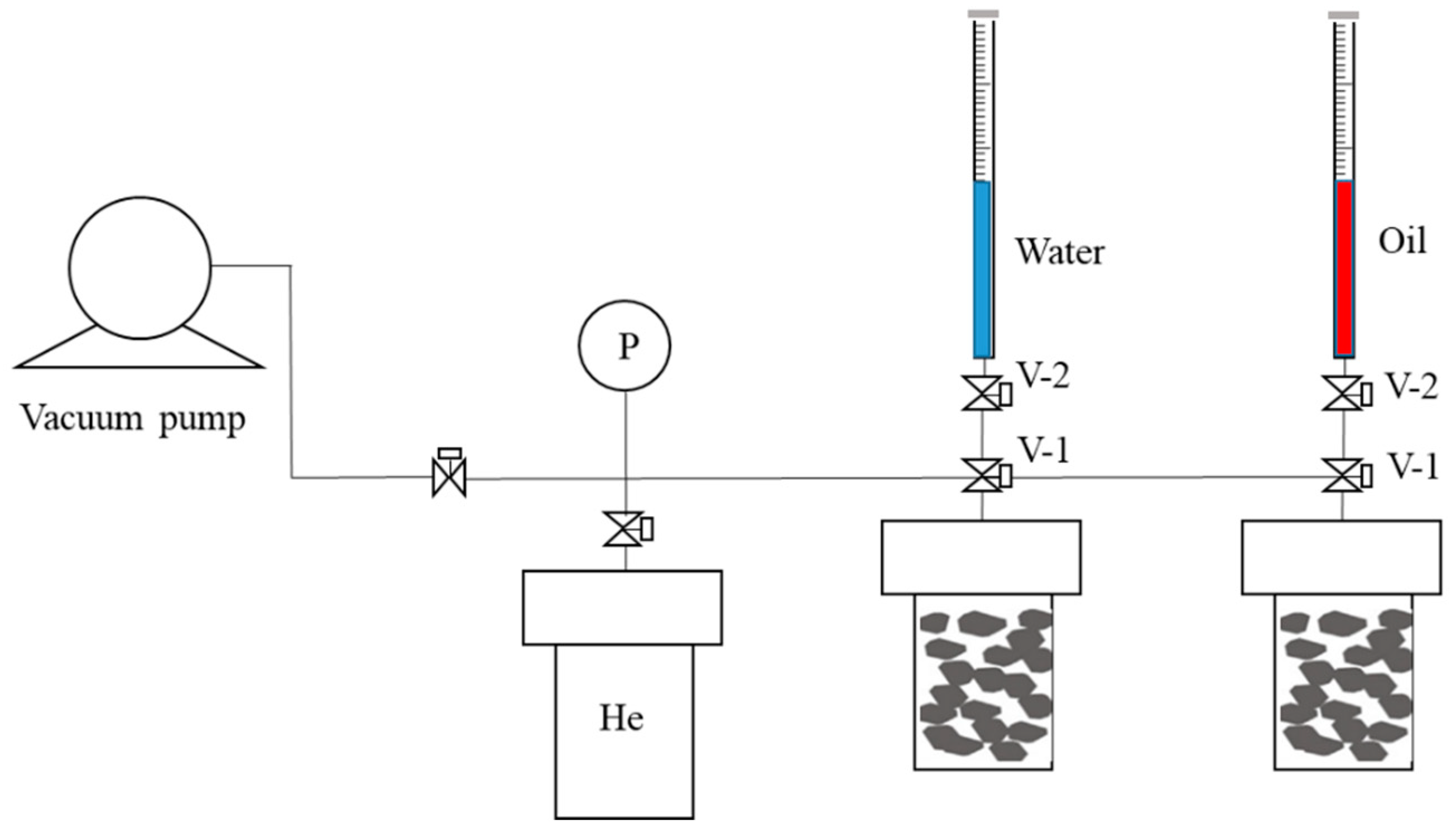
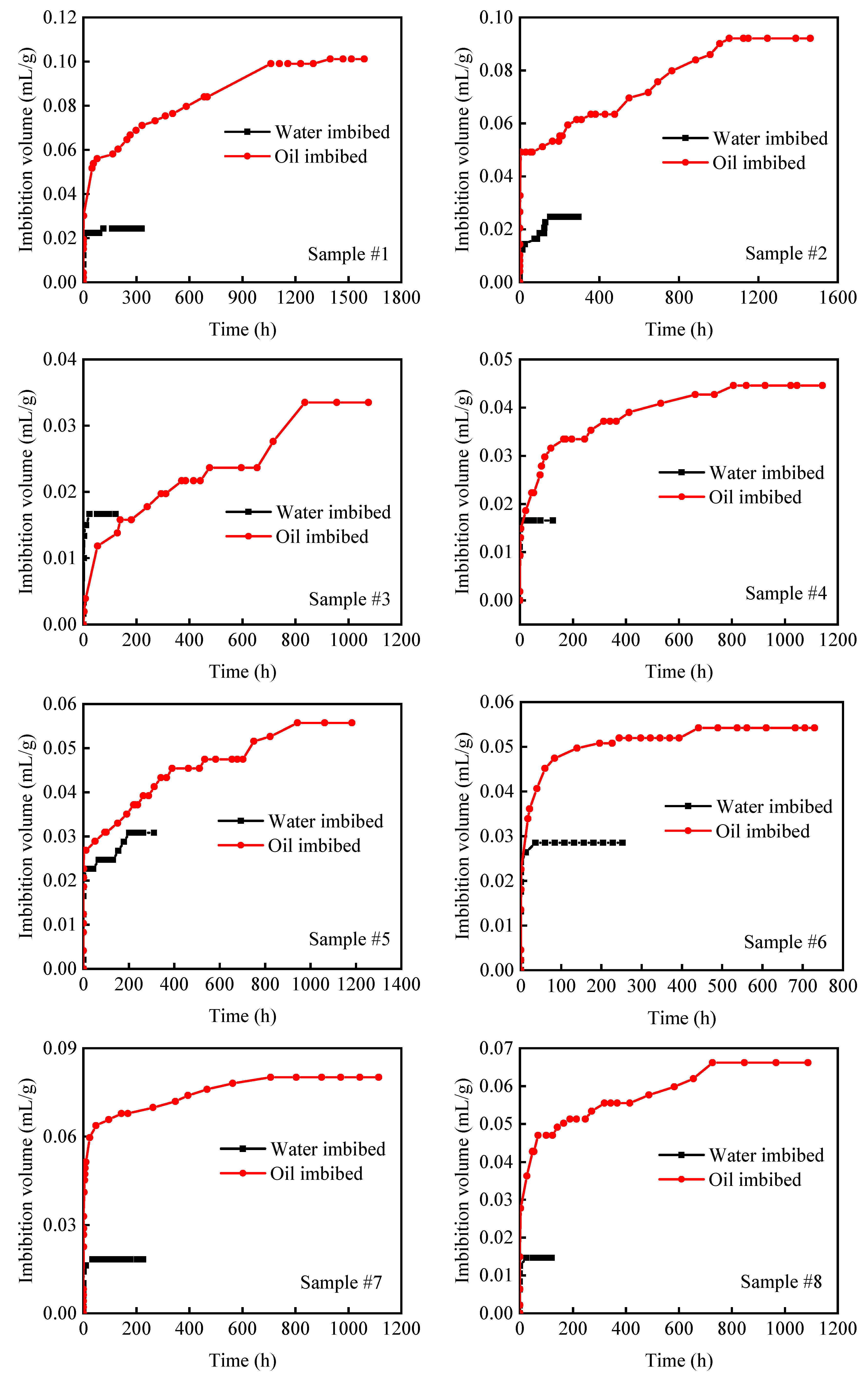
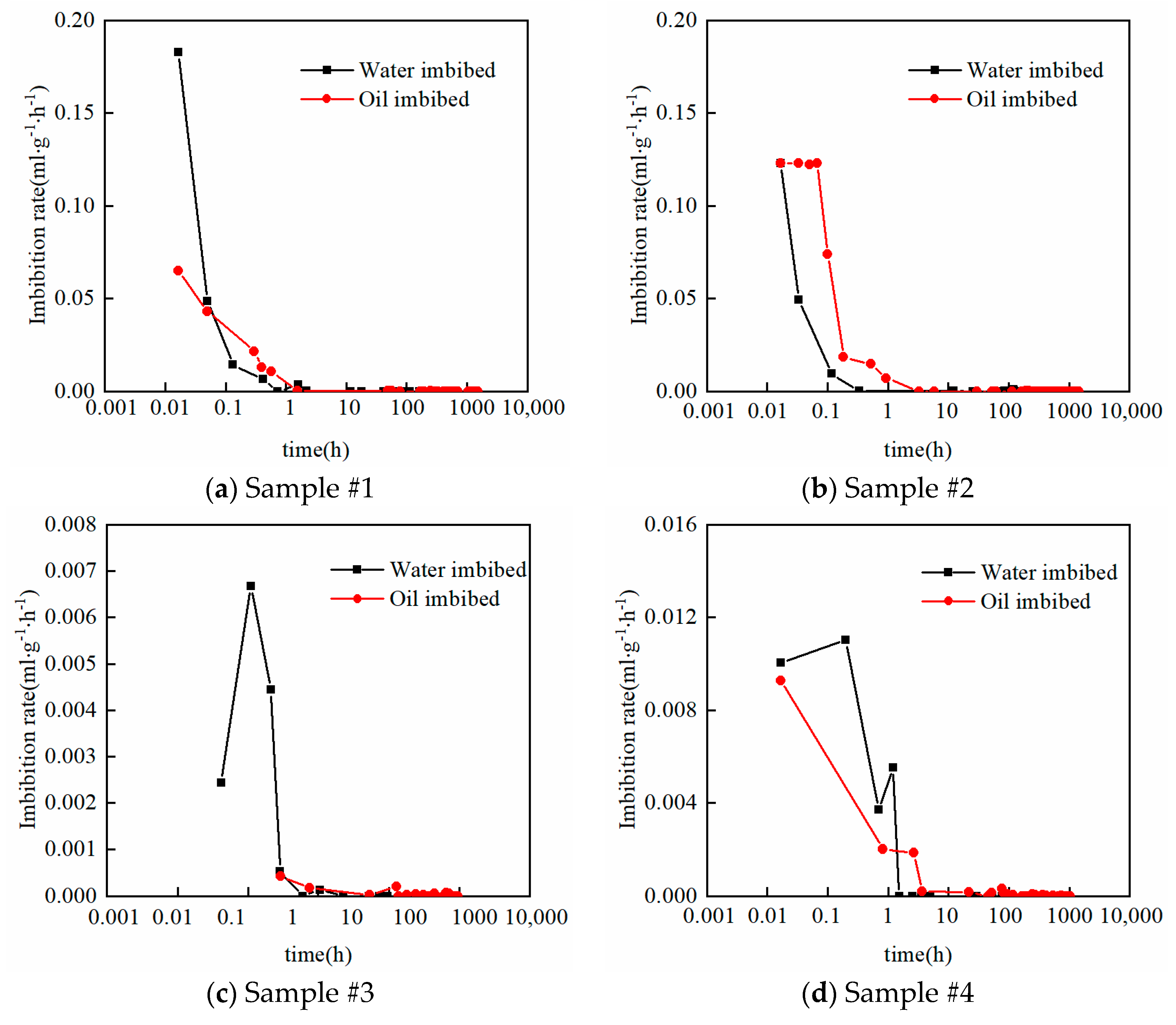
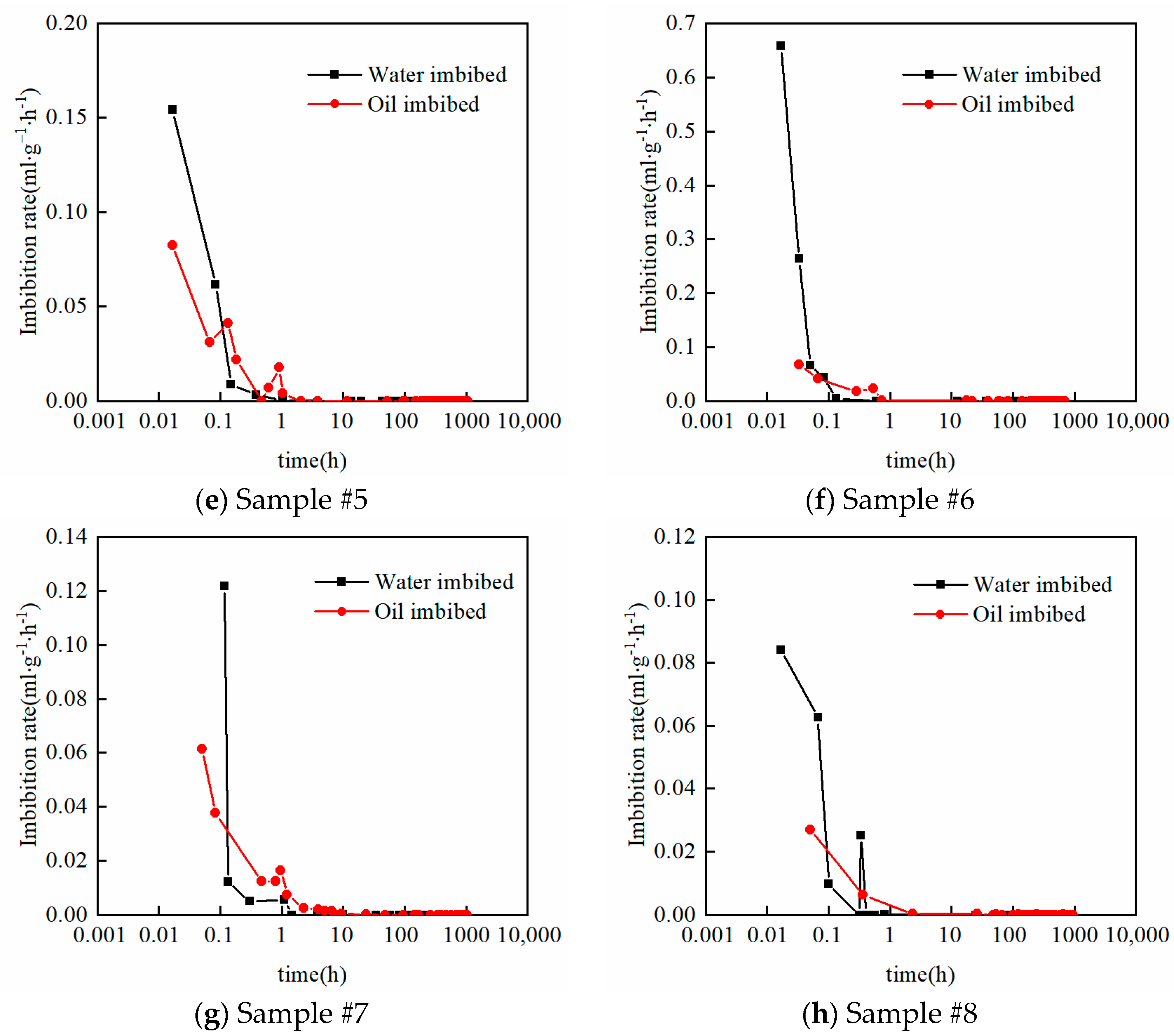
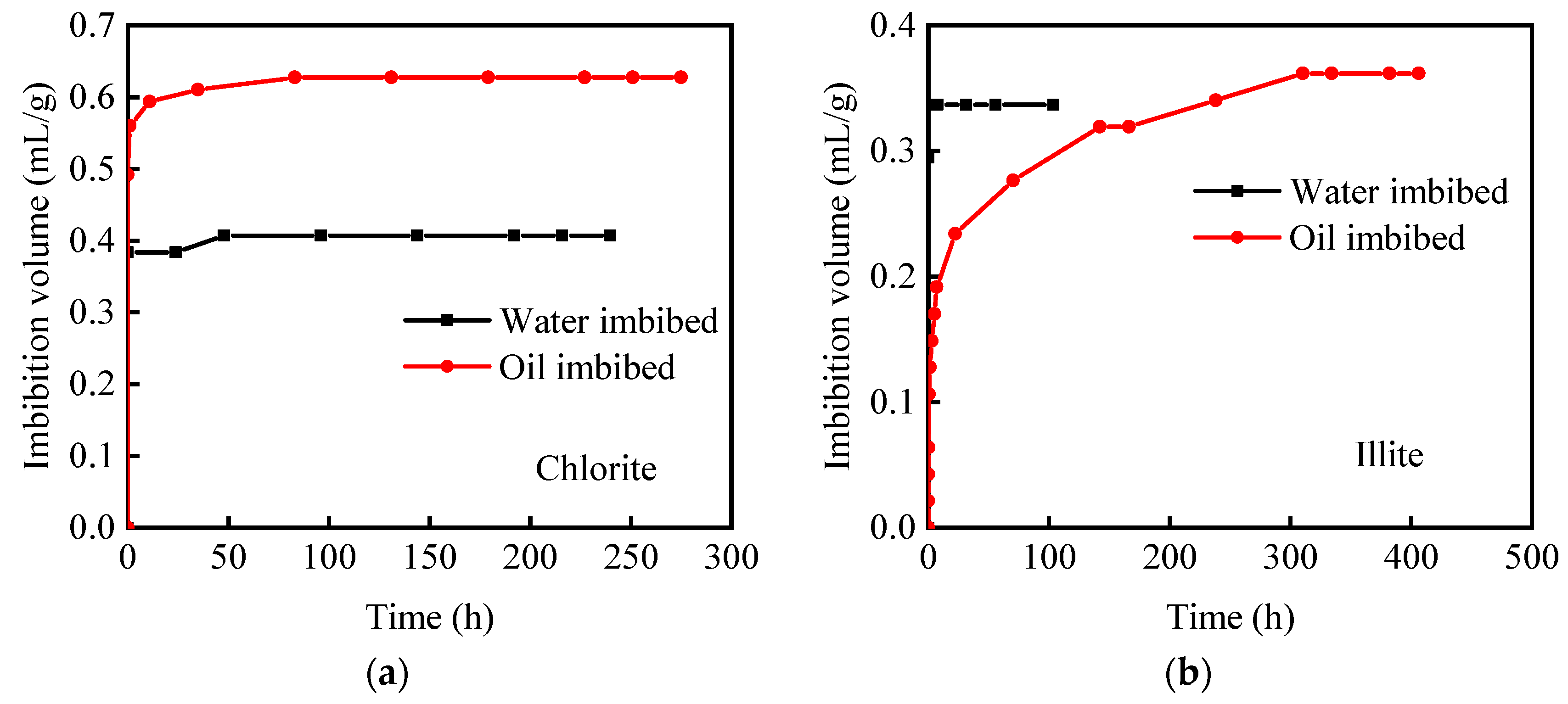
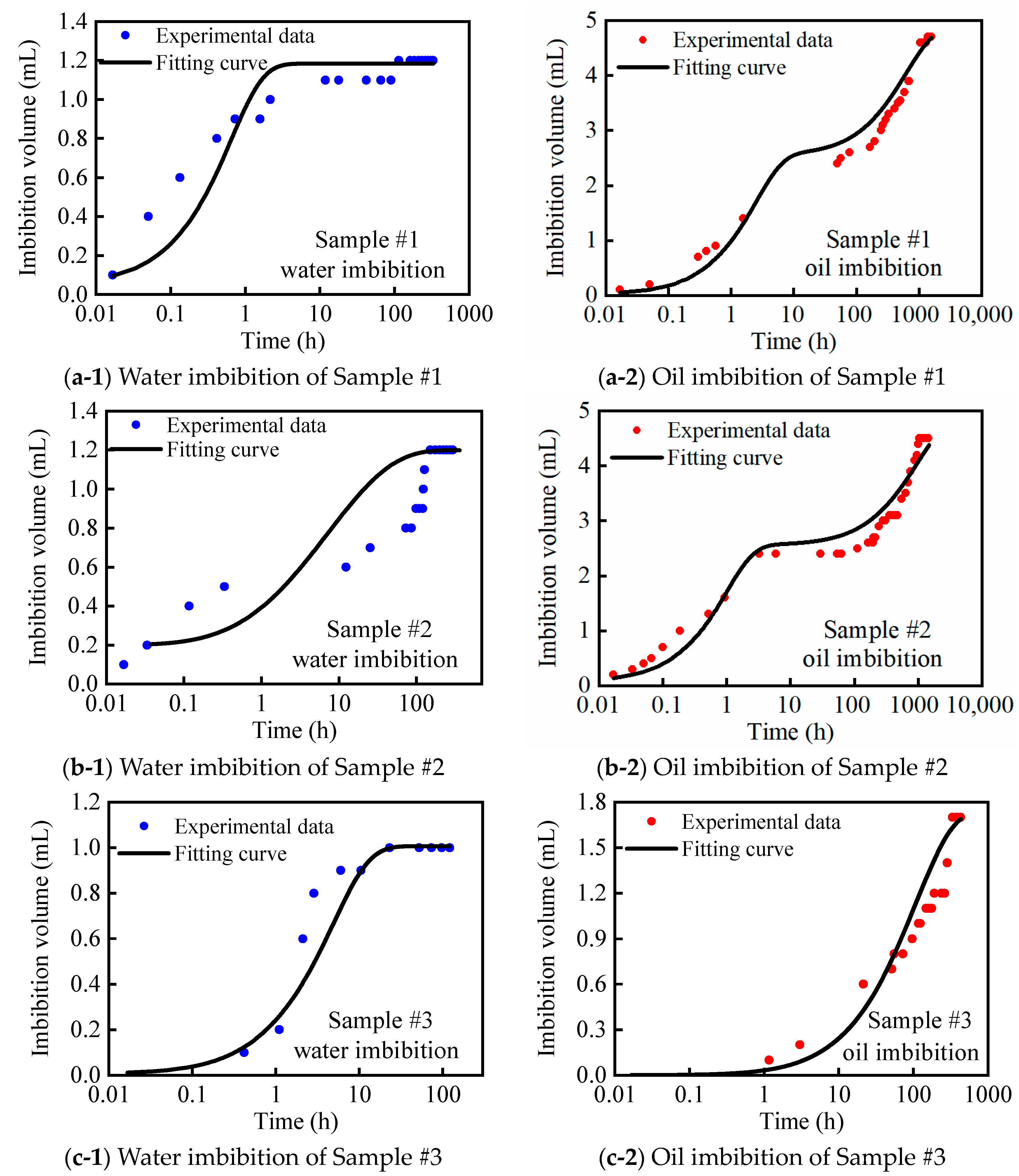
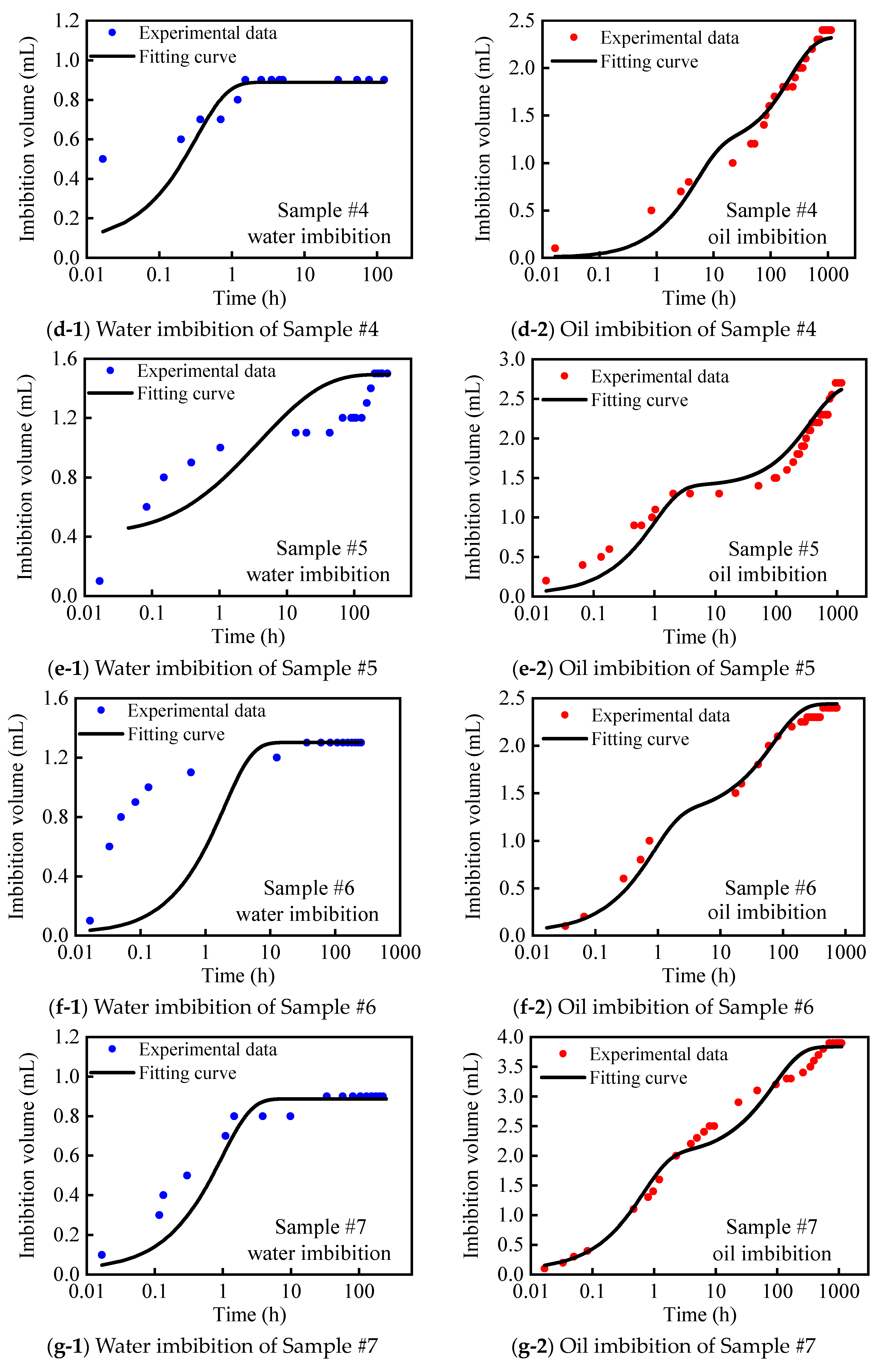
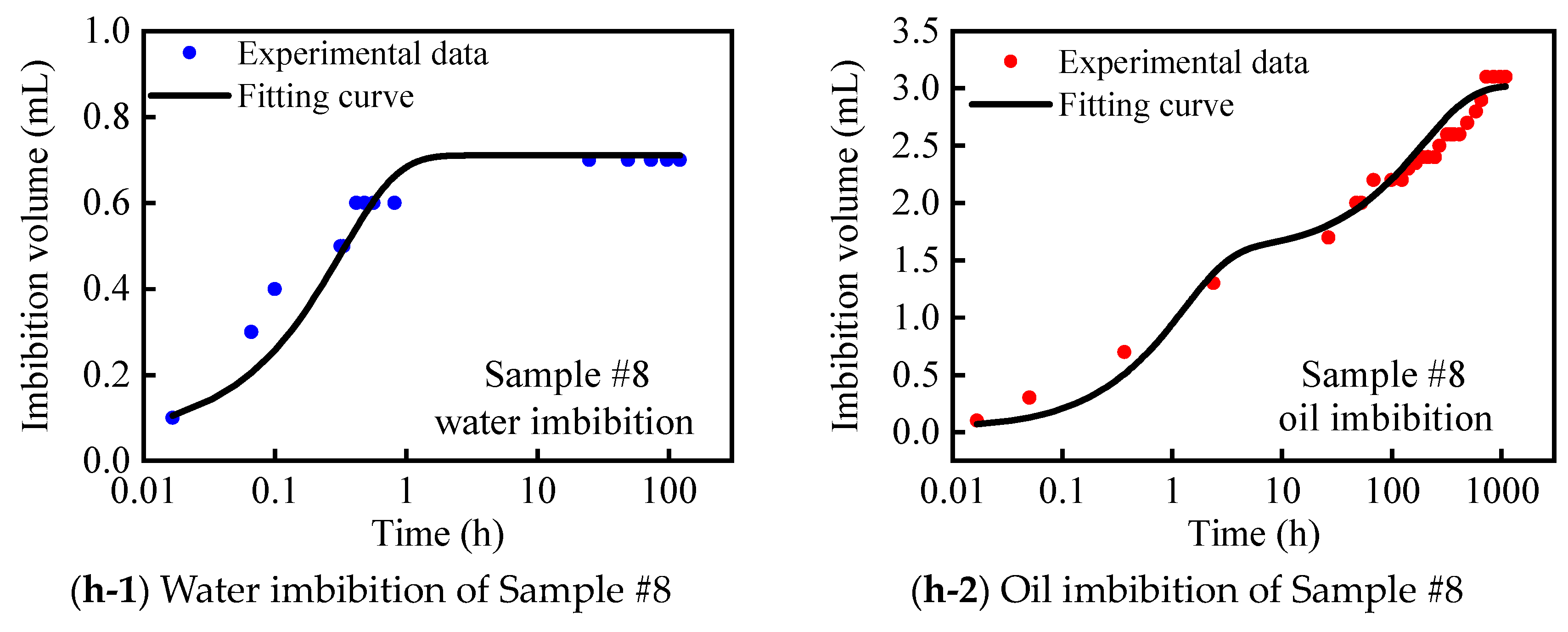
2.3. Vacuum-Imbibition Tests
- (1)
- The shale samples were broken into blocks of approximately 1 cm × 1 cm × 0.5 cm and extracted with CH2Cl2 for 15 days, and then dried for 48 h at 100 °C.
- (2)
- The processed rock-samples were divided into two parts of equal weight and placed in two sealed cells for water and oil imbibition tests. An air tightness test was conducted.
- (3)
- The samples were vacuumed, and then saturated with helium gas. The saturated gas content was obtained according to Boyle’s law to calculate the total pore volumes and porosities of the shale rock samples.
- (4)
- The samples were then vacuumed again, and oil and water were imbibed into the 2 cells. The imbibed volumes of oil and water were recorded, along with the time. The entire experimental process was conducted at a constant temperature of 25 °C. The n-dodecane (n-C12) was used as the oil phase, and the KCl solution with 8% mass concentration was used as the water phase.
2.4. Two-Dimensional Nuclear Magnetic Resonance Characterization
3. Results and Discussion
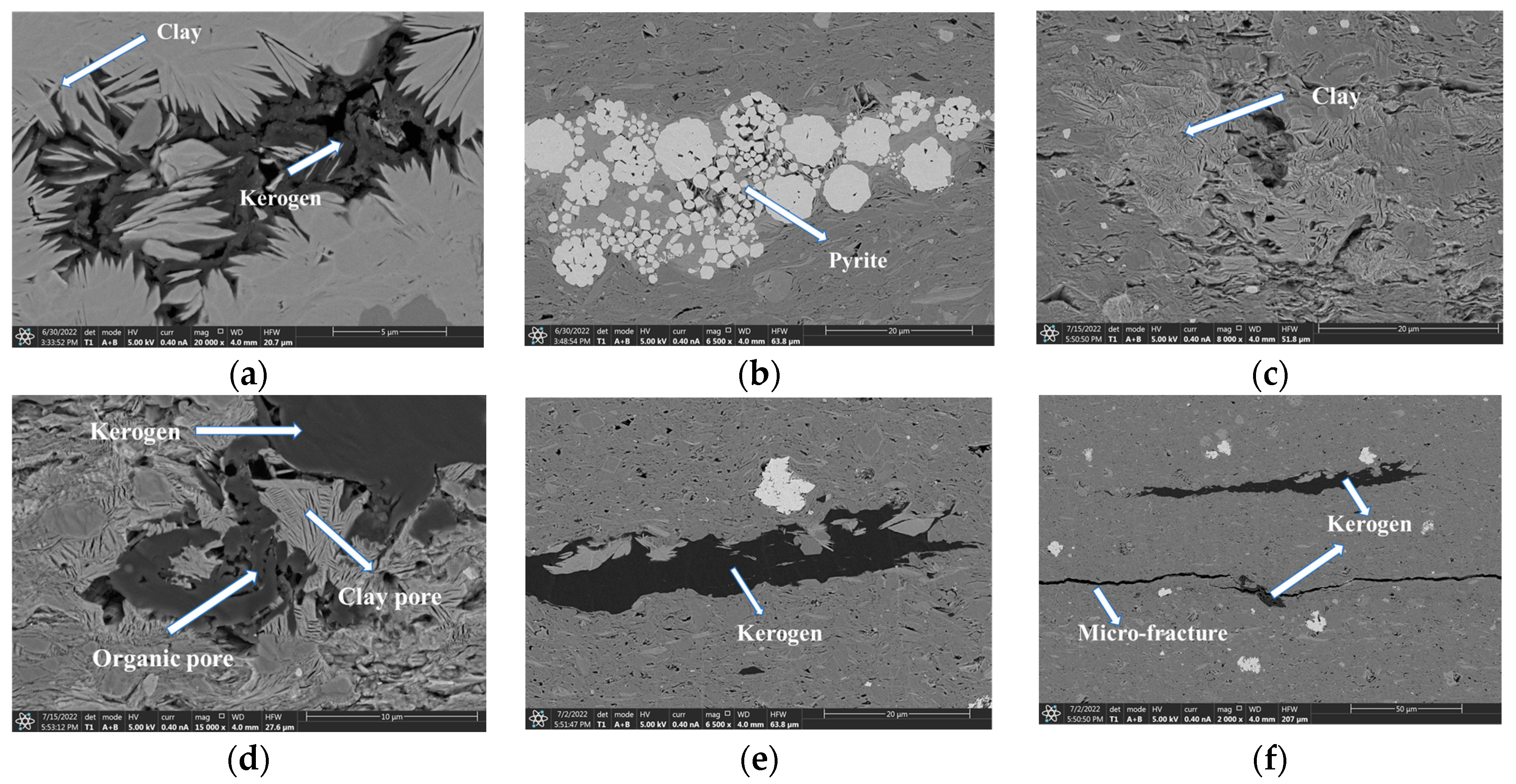
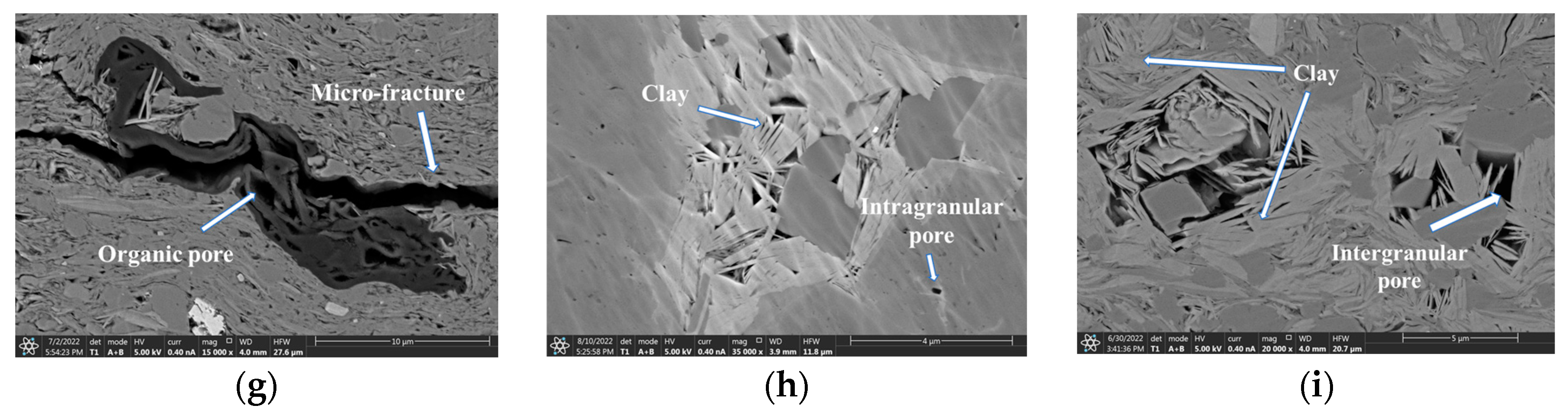
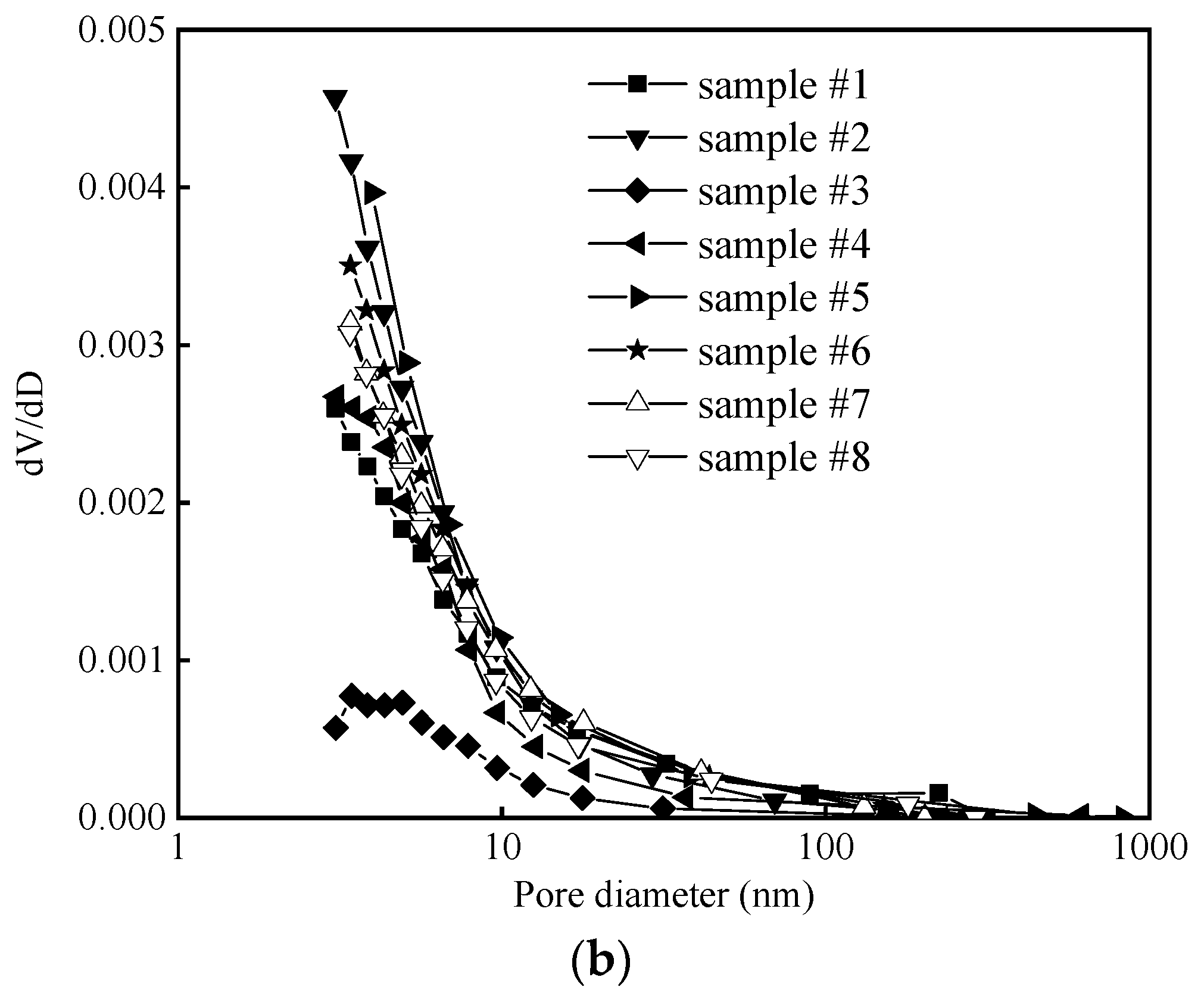
3.1. Microstructure of Pore-Space and Pore Size Distribution
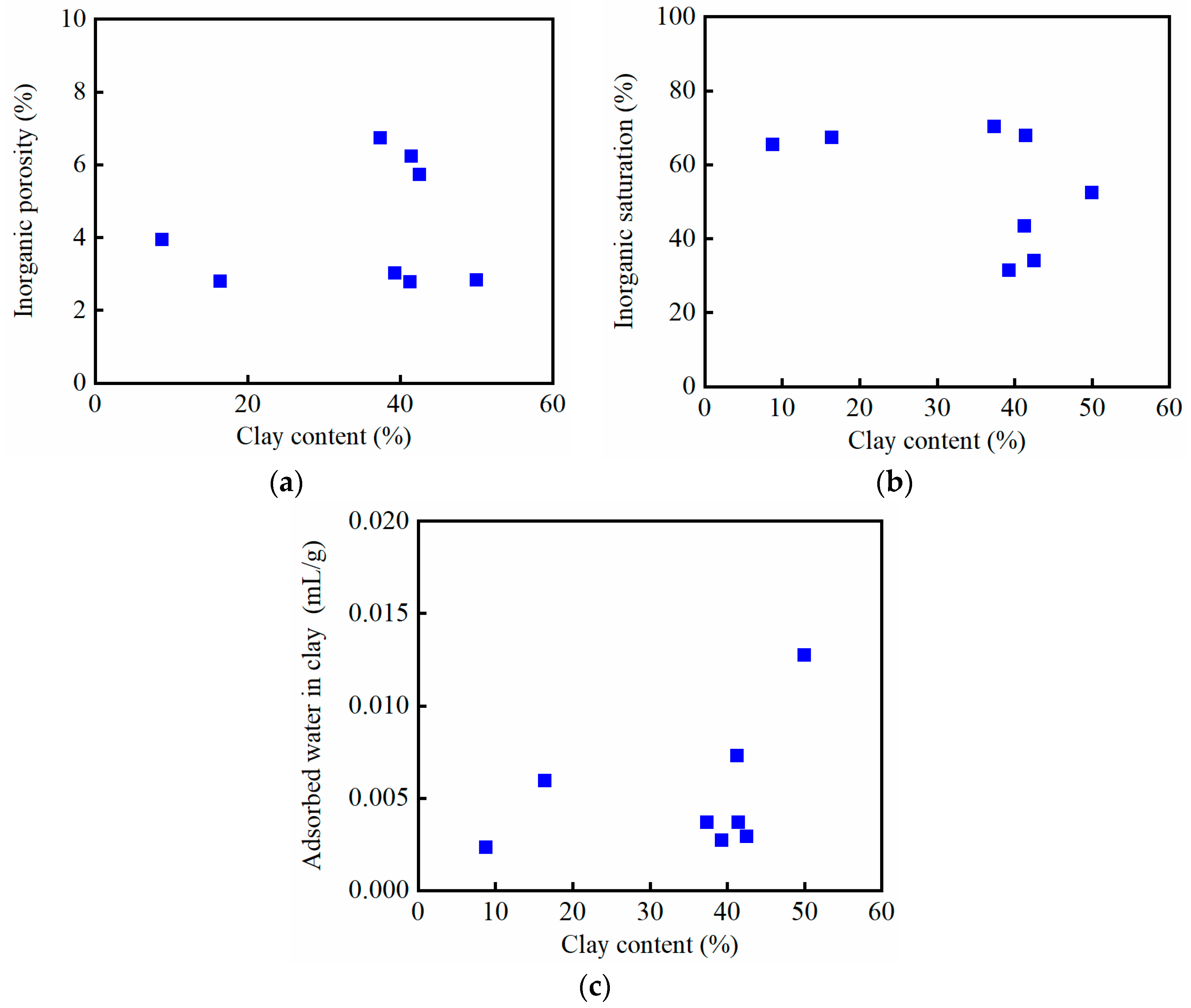
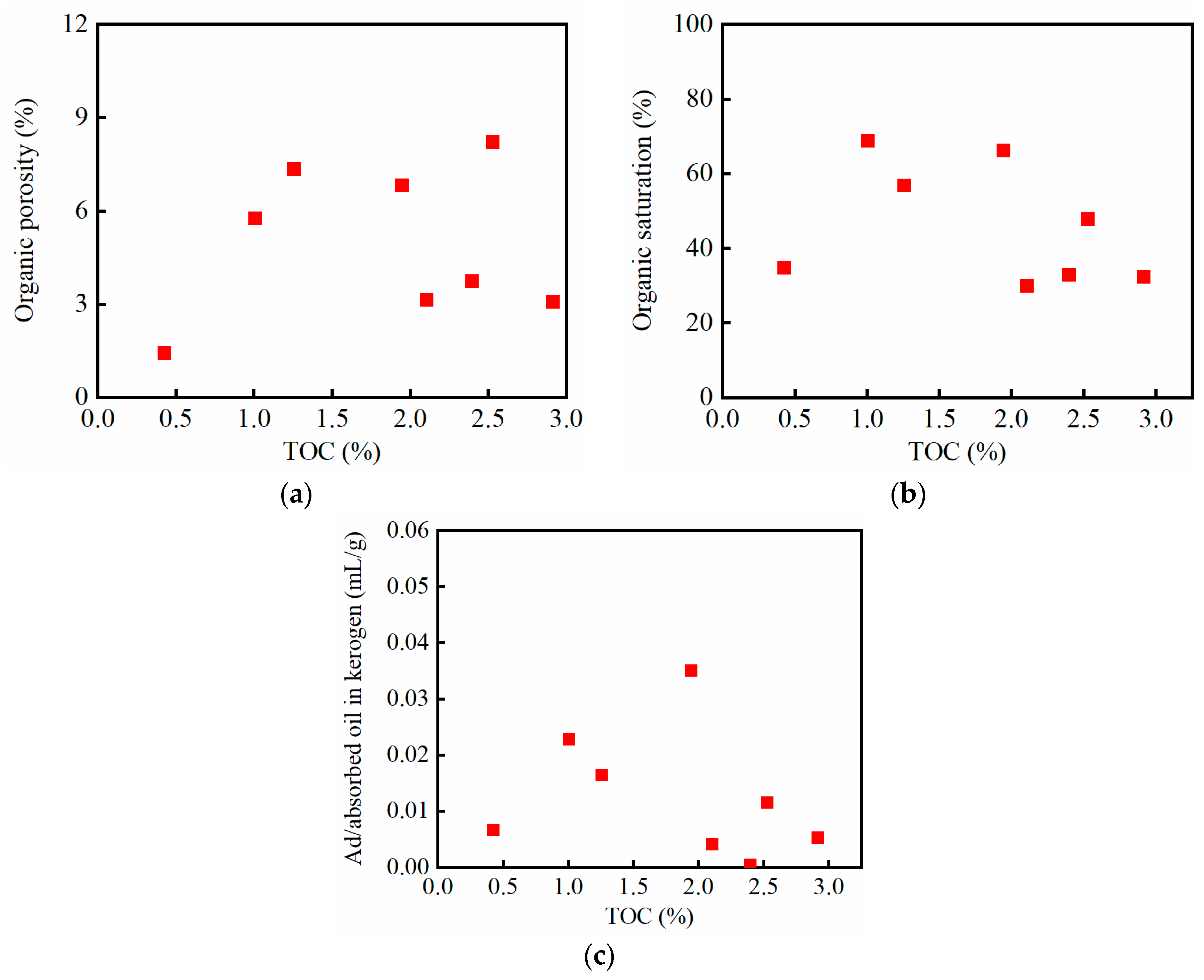
3.2. Inorganic and Organic Saturations and Porosities
- (1)
- Inorganic porosity: The ratio of the inorganic pore volume to the apparent volume of the crushed sample. The inorganic pore volume is calculated by subtracting the adsorbed water volume in clay from the gas-measured pore volume.
- (2)
- Organic porosity: The ratio of the organic pore volume to the apparent volume of the crushed sample. The organic pore volume is the gas-measured pore volume minus the inorganic pore volume.
- (3)
- Inorganic saturation: The ratio of the volume of oil imbibed into the inorganic material to the total volume of imbibed oil. The oil imbibed into the inorganic material is the sum of the free oil in the inorganic pores (inorganic pore volume) and the clay-adsorbed oil.
- (4)
- Organic saturation: The ratio of the volume of oil imbibed into the organic matter to the total volume of imbibed oil. The oil imbibed into the organic matter is the sum of the free oil in organic pores and the ad/absorbed oil in the organic matter.
- (5)
- The percentage of adsorbed water in clay: The ratio of the clay-adsorbed water volume to the water volume within the inorganic matter.
- (6)
- The percentages of ad/absorbed oil in organic matter: The ratio of the volume of ad/absorbed oil in the organic matter to the volume of oil within the organic matter.
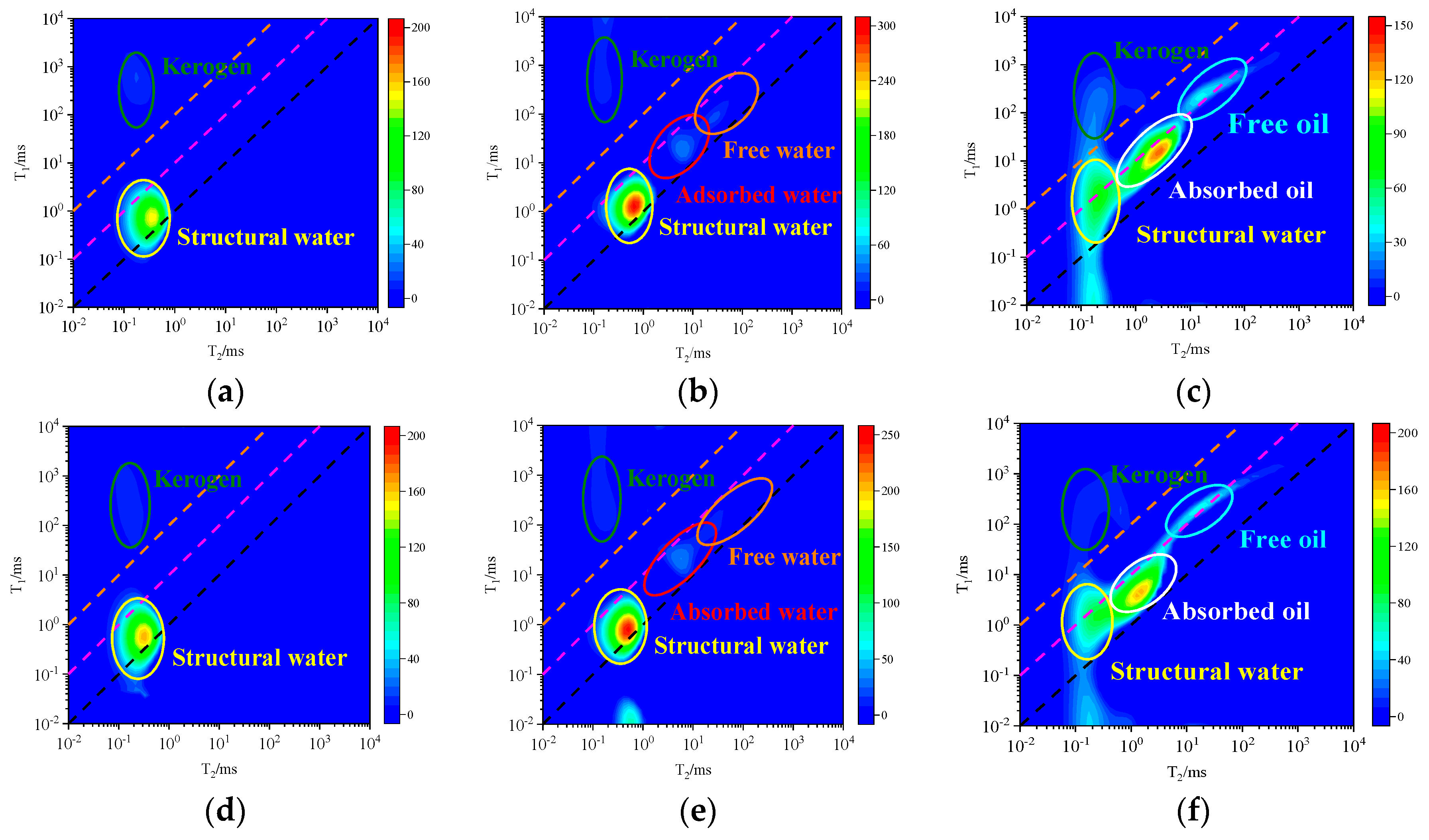
3.3. NMR Spectrum of Fluid in Different States
3.4. Mathematical Model Coupling Organic and Inorganic Pores Considering Ad/Absorbed Fluid in Organic Matter and Clay
- (1)
- Oil exists in the form of a free state in inorganic pores, in the form of a free state and an adsorbed state in clay pores, and in the forms of an adsorbed state and a free state in organic pores.
- (2)
- Water only flows into inorganic pores. Oil can enter both organic and inorganic pores.
- (3)
- The matrix and the fluid are slightly compressible, and the influence of gravity is neglected.
- (4)
- The shale sample is spherical and isotropic.
- (5)
- During the experiment, the temperature and boundary pressure remained constant.
- (6)
- The adsorption of clay and kerogen on oil is considered. The adsorption amount is not a function of time, which means that the adsorption process is instantaneous.
4. Conclusions
- (1)
- Based on the different interactions between oil, water, and gas and the organic and inorganic pores in shale, a fluid saturation method was established to distinguish the contents of each fluid phase. For the shale samples tested, the organic porosities are 1–9%, the inorganic porosities are 2–7%, the organic saturations are 29–69%, and the inorganic saturations are 31–71%. The oil in shale can be divided into inorganic free-oil, organic free-oil, and oil ad/absorbed in the kerogen. A total of 2% to 58% of the organic oil content is ad/absorbed in the kerogen.
- (2)
- A two-dimensional nuclear magnetic resonance method was used to characterize the occurrence-states of the oil and water in the shale samples. Water was mainly in the forms of free water and structural water, while oil was mainly in the forms of free oil and adsorbed oil. The signal intensity of oil is significantly higher than that of water, indicating that the imbibed oil volume is higher than the imbibed water volume.
- (3)
- Based on the inorganic–organic coupling mathematical model, by considering ad/absorbed fluid, the organic and inorganic permeabilities of the shale samples were determined. The organic permeability is one to three orders of magnitude lower than that of the inorganic permeability.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Nomenclature
| Abbreviations | |
| TOC | Total organic matter content |
| GY | Guye well |
| BJH | Barret–Joyner–Halenda method |
| NMR | Nuclear magnetic resonance |
| T2 | The transverse relaxation |
| T1 | The longitudinal relaxation |
| SEM | Scanning electron microscopy |
| XRD | X-ray diffraction |
| PDI | Polydispersity Index |
| Symbols | |
| q | The cross-flow between inorganic and organic pores, kg/(m3∙s) |
| Cs | Adsorption mass per unit volume of clay, kg/m3 |
| Rm | The percentage of clay mass, Fraction |
| rl | The density of liquid, kg/m3 |
| kim | The permeability of inorganic pores, m2 |
| μl | The viscosity of liquid, Pa·s |
| Pim | The pressure of inorganic pores, Pa |
| ct_im | The compression coefficient of inorganic pores, 1/Pa |
| t | The time, s |
| kom | The permeability of organic pores, m2 |
| Pom | The pressure of organic pores, Pa |
| ct_om | The compression coefficient of organic pores, 1/Pa |
| qm | The flow of ad/absorbed oil per unit volume, kg/(m3∙s) |
| β | Proportion of organic matter, Fraction |
| α | Cross-flow coefficient, 1/m2 |
References
- Liu, X.; Jian, X.; Liang, L. Investigation of pore structure and fractal characteristics of organic-rich Yanchang formation shale in central China by nitrogen adsorption/desorption analysis. Plateau Meteorol. 2015, 22, 62–72. [Google Scholar] [CrossRef]
- Jian, X.; Liu, X.; Liang, L. Experimental study on the pore structure characteristics of the Upper Ordovician Wufeng Formation shale in the southwest portion of the Sichuan Basin, China. J. Nat. Gas Sci. Eng. 2015, 22, 530–539. [Google Scholar]
- Hensen, E.; Smit, B. Why clays swell. J. Phys. Chem. B 2002, 106, 12664–12667. [Google Scholar] [CrossRef]
- Tunega, D.; Gerzabek, M.H.; Lischka, H. Ab Initio Molecular Dynamics Study of a Monomolecular Water Layer on Octahedral and Tetrahedral Kaolinite Surfaces. J. Phys. Chem. B 2004, 108, 5930–5936. [Google Scholar] [CrossRef]
- Norrish, K.; Quirk, J. Crystalline Swelling of Montmorillonite: Manner of Swelling of Montmorillonite. Nature 1954, 173, 256–257. [Google Scholar] [CrossRef]
- Wang, Z.; Yue, X.; Han, D. Effect of clay mineral and fluid on flow characteristics of the low-permeability cores. Pet. Geol. Recovery Effic. 2007, 14, 89–92. [Google Scholar]
- Liang, J.; Fang, Y. Experimenal study of seepage characteristics of tiny-particle clay. Chin. J. Rock Mech. Eng. 2010, 29, 1222–1230. [Google Scholar]
- Skipper, N.; Lock, P.; Titiloye, J.; Swenson, Z.; Howells, W.; Fernandez-Alonso, F. The structure and dynamics of 2-dimensional fluids in swelling clays. Chem. Geol. 2006, 230, 182–196. [Google Scholar] [CrossRef]
- Wei, J.; Yang, H.; Zhu, J.; Shen, W.; Yuan, P.; He, H.; Chen, M. Molecular dynamic simulation of interlayer micro-structure in orgnaic montmorilionite. Mineral. Petrol. 2009, 29, 33–37. [Google Scholar]
- Jiang, J.; Yu, C.; Zhou, Y. The study and aplication of clay swelling model considering wettability. Pet. Geol. Eng. 2016, 30, 87–89, 144. [Google Scholar]
- Zou, C.; Yang, Z.; Cui, J.; Zhu, R.; Hou, L.; Tao, S.; Yuan, X.; Wu, S.; Lin, S.; Wang, L.; et al. Formation mechanism, geological characteristics and development strategy of nonmarine shale oil in China. Pet. Explor. Dev. 2013, 40, 14–26. [Google Scholar] [CrossRef]
- Zhang, J.; Lin, L.; Li, Y.; Tang, X.; Zhu, L.; Xing, Y.; Jiang, S.; Jing, T.; Yang, S. Classification and evaluation of shale oil. Earth Sci. Front. 2012, 19, 322–331. [Google Scholar]
- Wu, X.; Gao, B.; Ye, X.; Bian, R.; Nie, H.; Lu, F. Shale oil accumulation conditions and exploration potential of faulted basins in the east of China. Oil Gas Geol. 2013, 34, 455–462. [Google Scholar]
- Fangxing, N.; Xuejun, W.; Xuefeng, H. An analysis on occurrence state and mobility of shale oil in Jiyang depression. Xinjiang Oil Gas 2015, 11, 1–5. [Google Scholar]
- Yang, C.; Sheng, G.; Dang, Z. Sorption mechanism of polycyclic aromatic hydrocarbons (PAHs) on kerogen. Environ. Chem. 2007, 26, 472–475. [Google Scholar]
- Cai, J. Organo-Clay Complexes in Muddy Sediments and Mudstones; Science Press: Beijing, China, 2004; pp. 53–144. [Google Scholar]
- Juanhong, G.; Yanrong, Z.; Yonghe, Y. Evolutional characteristics of the kerogen molecular structure during the low-mature stage: An infrared spectra analysis. Geochimica 2014, 43, 529–537. [Google Scholar]
- Qian, M.; Jiang, Q.; Li, M.; Li, Z.; Liu, P.; Ma, Y.; Cao, T. Quantitative characterization of extractable organic matter in lacustrine shale with different occurrences. Pet. Geol. Exp. 2017, 39, 278–286. [Google Scholar]
- Jiang, Q.; Li, M.; Qian, M.; Li, Z.; Li, Z.; Huang, Z.; Zhang, C.; Ma, Y. Quantitative characterization of shale oil in different occurrence states and its application. Pet. Geol. Exp. 2016, 38, 842–849. [Google Scholar]
- Wang, S.; Feng, Q.; Zha, M.; Lu, S.; Qin, Y.; Xia, T.; Zhang, C. Molecular dynamics simulation of liquid alkane occurrence state in pores and fractures of shale organic matter. Pet. Explor. Dev. 2015, 42, 772–778. [Google Scholar] [CrossRef]
- Sang, Q.; Zhang, S.; Li, Y.; Dong, M.; Bryant, S. Determination of organic and inorganic hydrocarbon saturations and effective porosities in shale using vacuum-imbibition method. Int. J. Coal Geol. 2018, 200, 123–134. [Google Scholar] [CrossRef]
- Li, S.; Sang, Q.; Dong, M.; Luo, P. Determination of inorganic and organic permeabilities of shale. Int. J. Coal Geol. 2019, 215, 103296. [Google Scholar] [CrossRef]
- Barrett, E.P.; Joyner, L.G.; Halenda, P.P. The determination of pore volume and area distributions in porous substances. I. Computations from nitrogen isotherms. J. Am. Chem. Soc. 1951, 73, 373–380. [Google Scholar] [CrossRef]
- Li, J.; Huang, W.; Lu, S.; Wang, M.; Chen, G.; Tian, W.; Guo, Z. Nuclear Magnetic Resonance T1-T2 Map Division Method for Hydrogen-Bearing Components in Continental Shale. Energy Fuels 2018, 32, 9043–9054. [Google Scholar] [CrossRef]


| Number | Samples | Clay | Quartz | Feldspar | Calcite | Dolomite | Pyrite | Ankerite | Barite | TOC |
|---|---|---|---|---|---|---|---|---|---|---|
| #1 | GY 7 | 50.1 | 32.7 | 9.0 | 7.4 | 6.2 | 1.9 | 5.4 | - | 2.5 |
| #2 | GY 10HC-1 | 42.6 | 37.0 | 9.4 | - | 8.5 | 2.5 | - | - | 2.0 |
| #3 | GY 10HC-2 | 8.8 | 6.1 | - | - | 81.3 | - | - | 3.8 | 0.4 |
| #4 | GY 4HC-189 | 16.4 | 19.6 | 2.7 | 1.7 | 58.2 | 1.4 | - | - | 2.4 |
| #5 | GY 9HC-208 | 37.4 | 35.4 | 16.5 | 0.7 | 6.4 | 3.6 | - | - | 2.1 |
| #6 | GY 9HC-210 | 41.5 | 37.8 | 16.7 | - | - | 4.0 | - | - | 2.9 |
| #7 | GY 16-24 | 41.3 | 31.7 | 14.8 | - | 6.1 | 6.1 | - | - | 1.3 |
| #8 | GY 16-32 | 39.3 | 33.6 | 18.9 | 4.3 | - | 3.9 | - | - | 1.0 |
| Sample Number | Illite | Kaolinite | Chlorite | Illite/Smectite |
|---|---|---|---|---|
| #1 | 41 | - | 20 | 39 |
| #2 | 66 | - | 7 | 27 |
| #3 | 42 | 31 | 27 | - |
| #4 | 39 | - | 37 | 24 |
| #5 | 64 | - | 10 | 26 |
| #6 | 68 | - | 9 | 23 |
| #7 | 44 | - | 18 | 38 |
| #8 | 60 | - | 7 | 33 |
| #1 | 41 | - | 20 | 39 |
| Sample Number | Bulk Volume (cm3) | Oil Volume in Organic Pores (mL/g) | Oil Volume in Inorganic Pores (mL/g) | Helium Saturation Volume (mL/g) | Organic Saturation (%) | Inorganic Saturation (%) | Organic Porosity (%) | Inorganic Porosity (%) | Percentage of Organic Pores (%) |
|---|---|---|---|---|---|---|---|---|---|
| #1 | 20.434 | 0.101 | 0.024 | 0.048 | 47.59 | 52.41 | 8.86 | 2.82 | 75.87 |
| #2 | 18.502 | 0.092 | 0.025 | 0.048 | 66.09 | 33.91 | 6.81 | 5.72 | 54.36 |
| #3 | 21.818 | 0.034 | 0.017 | 0.019 | 34.62 | 65.38 | 1.41 | 3.94 | 26.27 |
| #4 | 20.708 | 0.045 | 0.017 | 0.025 | 32.80 | 67.20 | 3.72 | 2.79 | 57.13 |
| #5 | 19.689 | 0.056 | 0.031 | 0.040 | 29.85 | 70.15 | 3.11 | 6.72 | 31.63 |
| #6 | 18.220 | 0.054 | 0.029 | 0.037 | 32.16 | 67.84 | 3.06 | 6.22 | 33.00 |
| #7 | 19.591 | 0.080 | 0.018 | 0.040 | 56.75 | 43.25 | 7.33 | 2.77 | 72.59 |
| #8 | 18.984 | 0.066 | 0.015 | 0.035 | 68.65 | 31.35 | 5.71 | 3.01 | 65.46 |
| Sample Number | Inorganic Permeability (mD) | Organic Permeability (mD) |
|---|---|---|
| #1 | 4.00 × 10−2 | 6.00 × 10−5 |
| #2 | 2.38 × 10−2 | 1.19 × 10−5 |
| #3 | 1.33 × 10−3 | 1.33 × 10−4 |
| #4 | 4.00 × 10−2 | 1.00 × 10−5 |
| #5 | 4.35 × 10−2 | 1.74 × 10−5 |
| #6 | 5.71 × 10−2 | 4.76 × 10−4 |
| #7 | 4.55 × 10−2 | 2.12 × 10−4 |
| #8 | 3.08 × 10−2 | 1.15 × 10−4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, F.; Li, B.; Cao, S.; Zhang, J.; Xu, Q.; Sang, Q. Experimental Study of the Fluid Contents and Organic/Inorganic Hydrocarbon Saturations, Porosities, and Permeabilities of Clay-Rich Shale. Energies 2024, 17, 524. https://doi.org/10.3390/en17020524
Wang F, Li B, Cao S, Zhang J, Xu Q, Sang Q. Experimental Study of the Fluid Contents and Organic/Inorganic Hydrocarbon Saturations, Porosities, and Permeabilities of Clay-Rich Shale. Energies. 2024; 17(2):524. https://doi.org/10.3390/en17020524
Chicago/Turabian StyleWang, Fenglan, Binhui Li, Sheng Cao, Jiang Zhang, Quan Xu, and Qian Sang. 2024. "Experimental Study of the Fluid Contents and Organic/Inorganic Hydrocarbon Saturations, Porosities, and Permeabilities of Clay-Rich Shale" Energies 17, no. 2: 524. https://doi.org/10.3390/en17020524
APA StyleWang, F., Li, B., Cao, S., Zhang, J., Xu, Q., & Sang, Q. (2024). Experimental Study of the Fluid Contents and Organic/Inorganic Hydrocarbon Saturations, Porosities, and Permeabilities of Clay-Rich Shale. Energies, 17(2), 524. https://doi.org/10.3390/en17020524






