CO2–Water–Rock Interaction and Its Influence on the Physical Properties of Continental Shale Oil Reservoirs
Abstract
1. Introduction
2. Experimental
2.1. Experimental Material
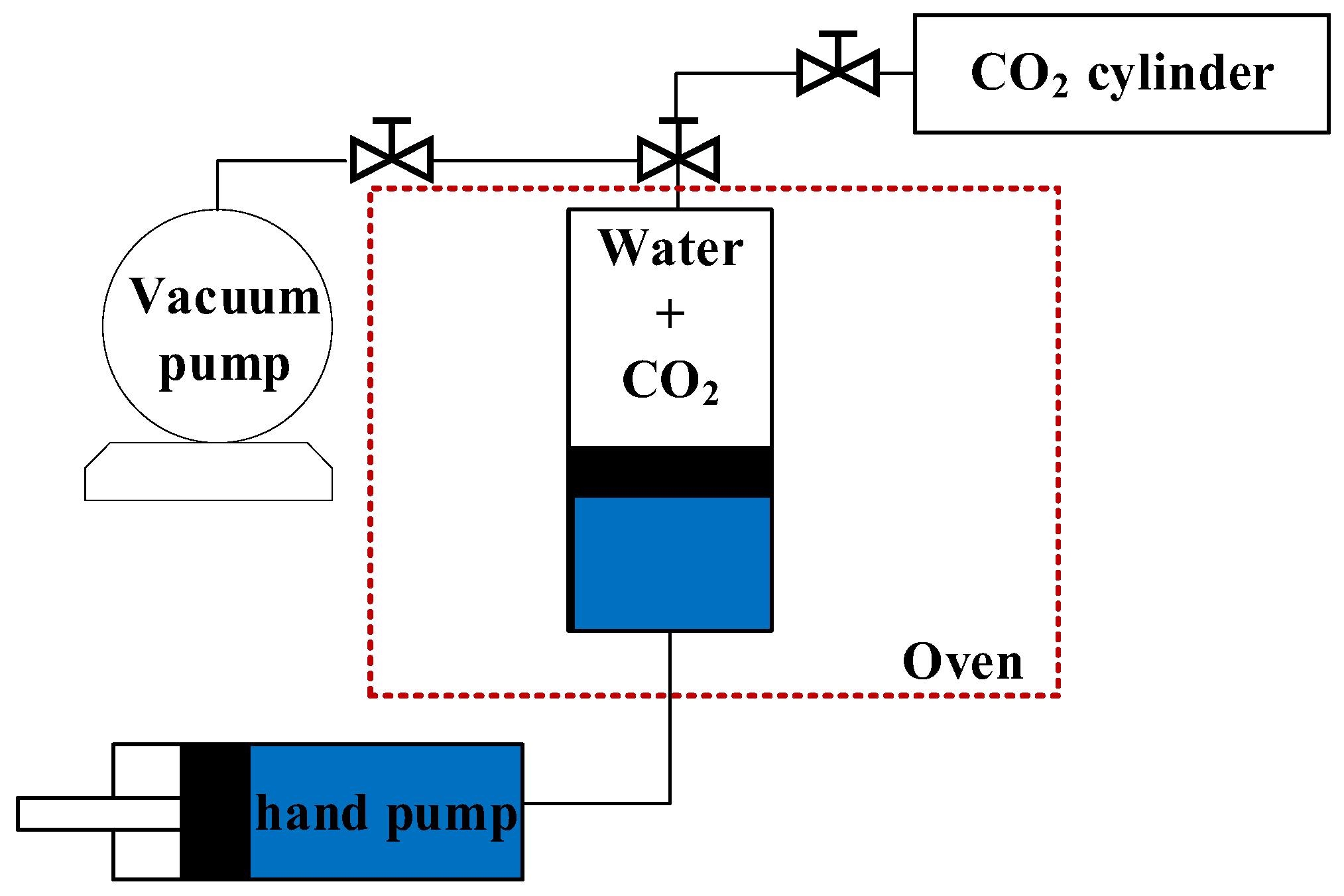
2.2. CO2–Water–Rock Static Reaction
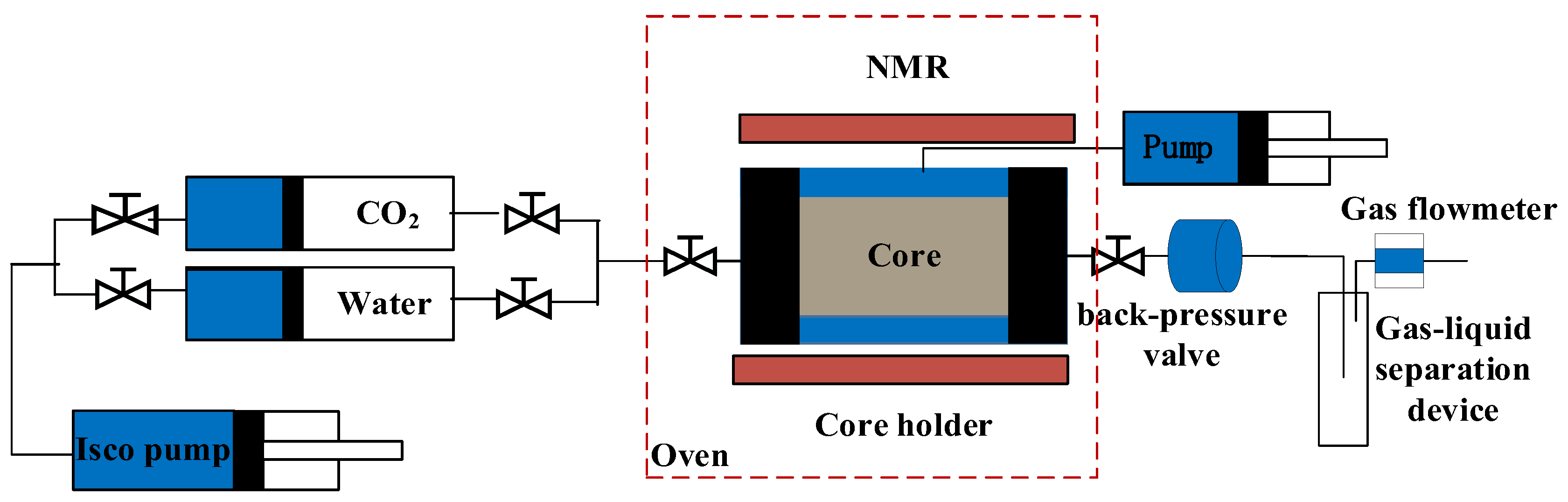
2.3. CO2–Water–Rock Dynamic Displacement Experiment
3. Results and Discussion
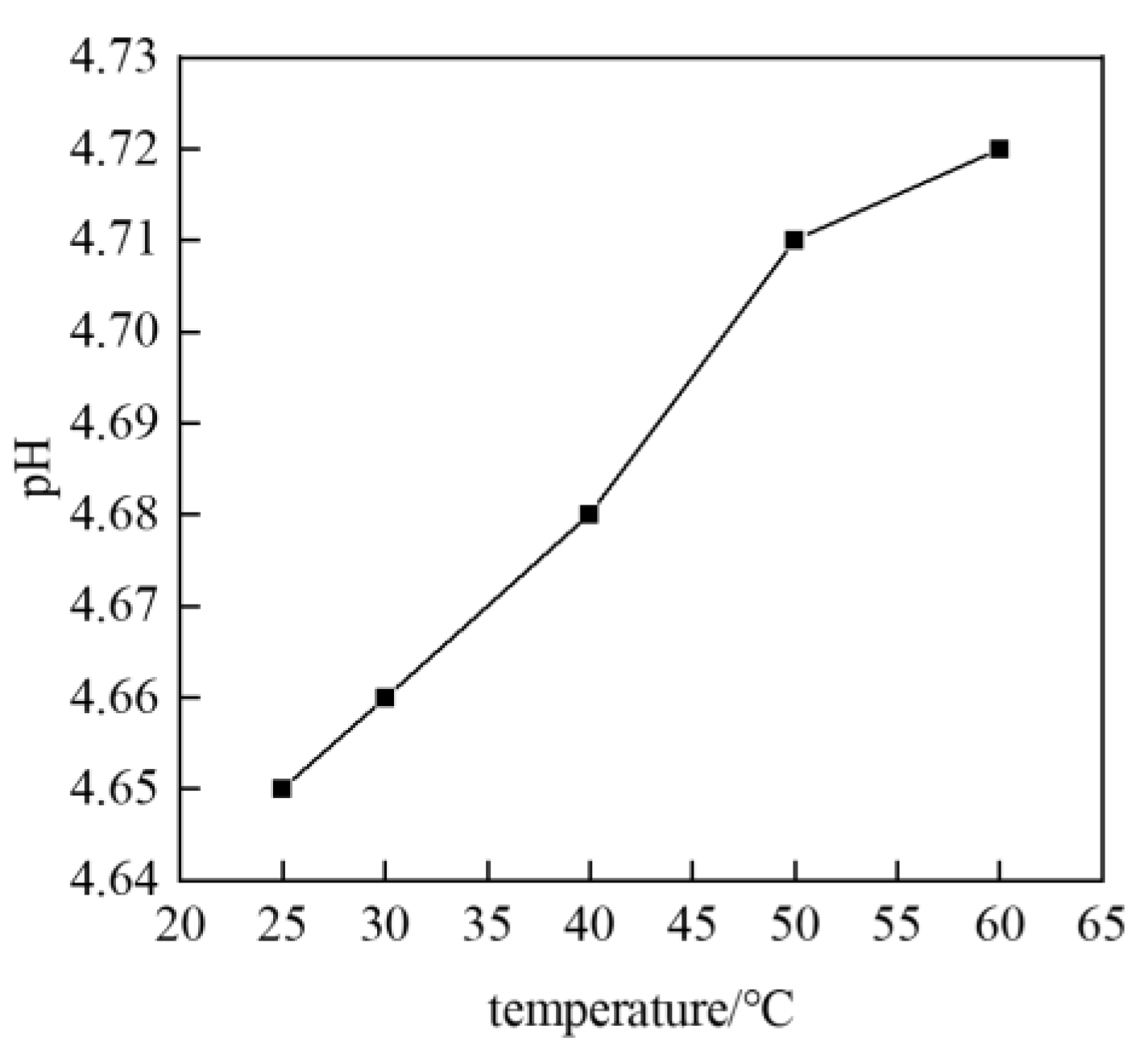
3.1. Influence of Pressure and Temperature Conditions on Formation Water pH Value
3.2. Change in Ion Concentration in Formation Water during Static Reaction
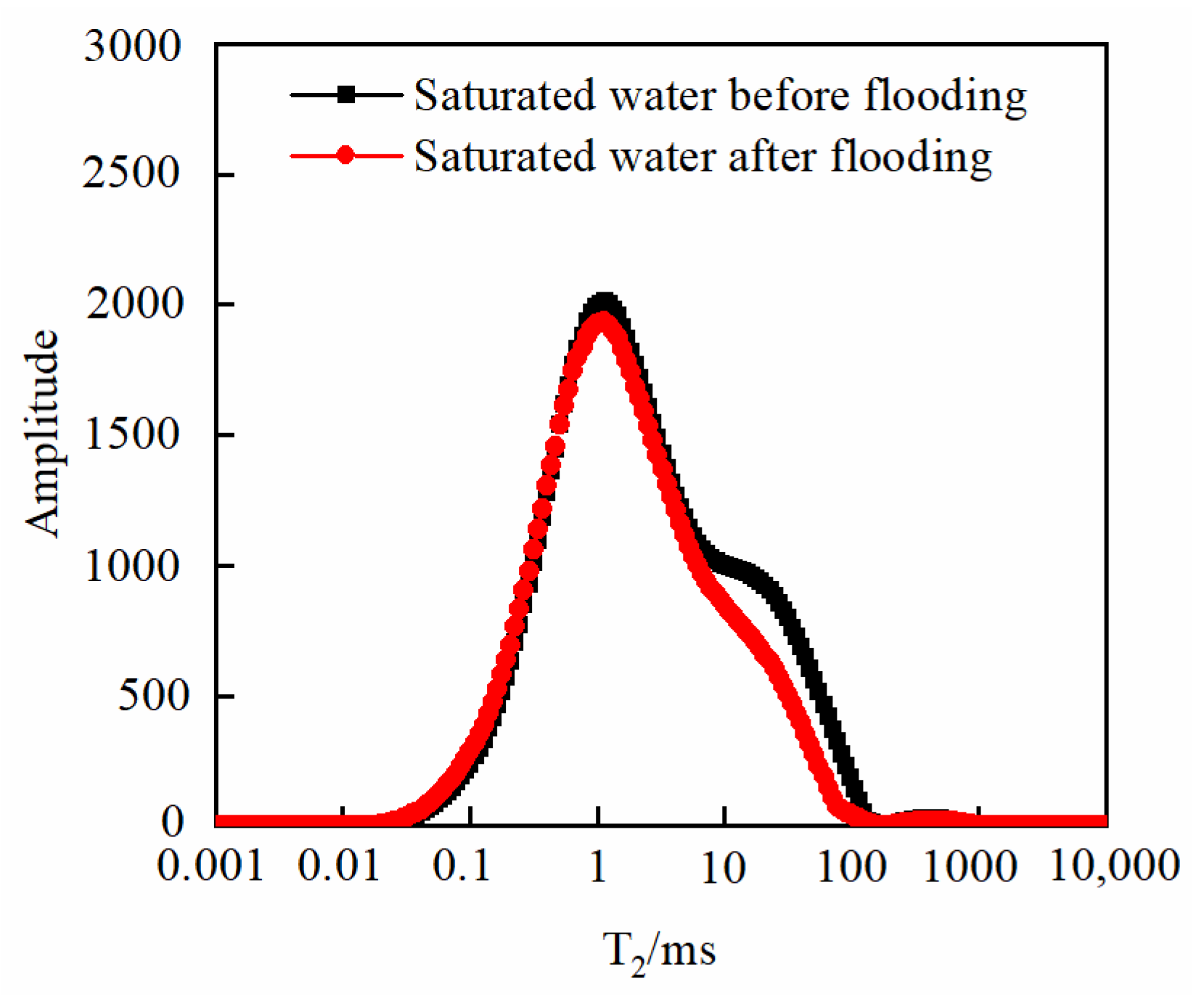
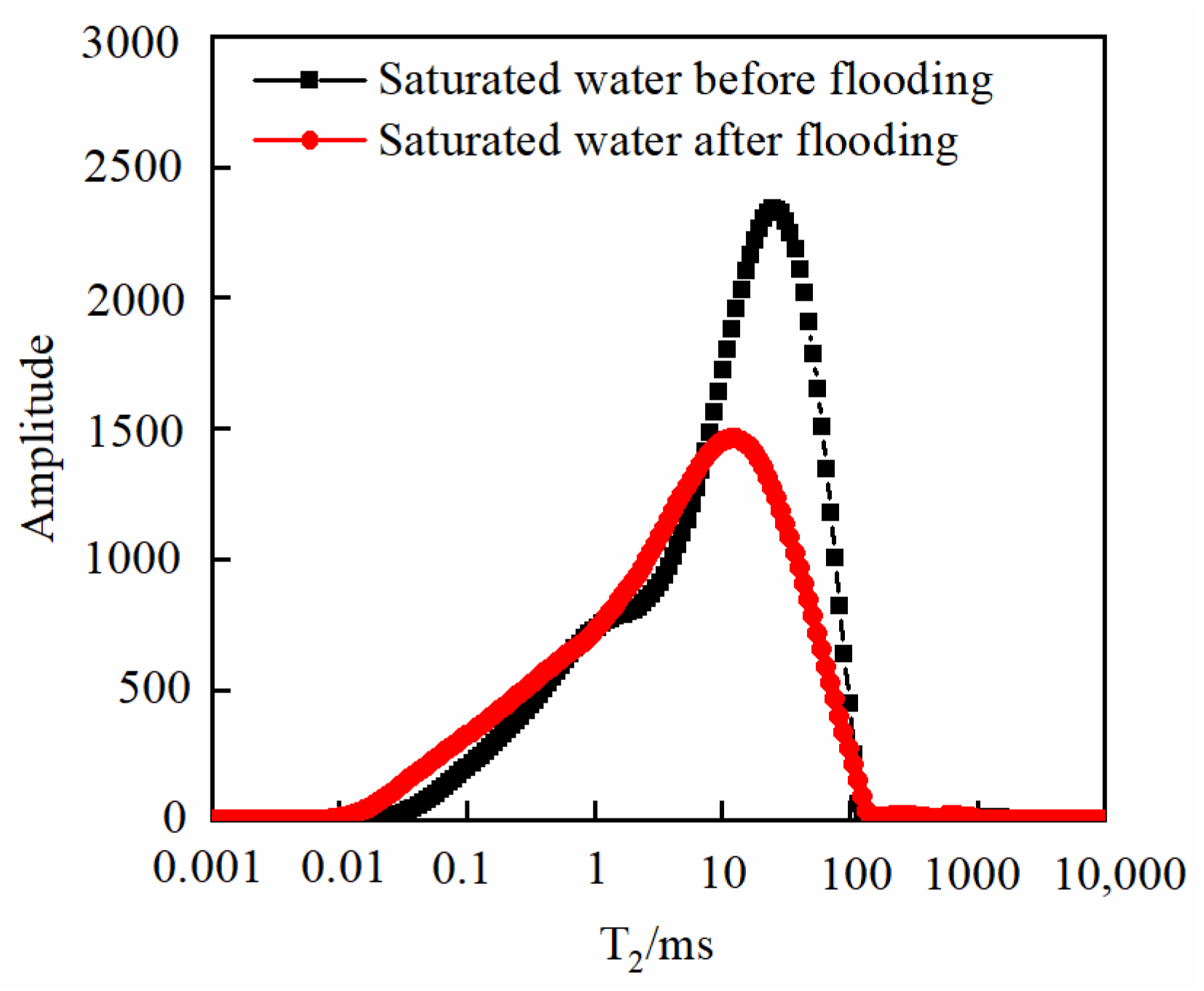
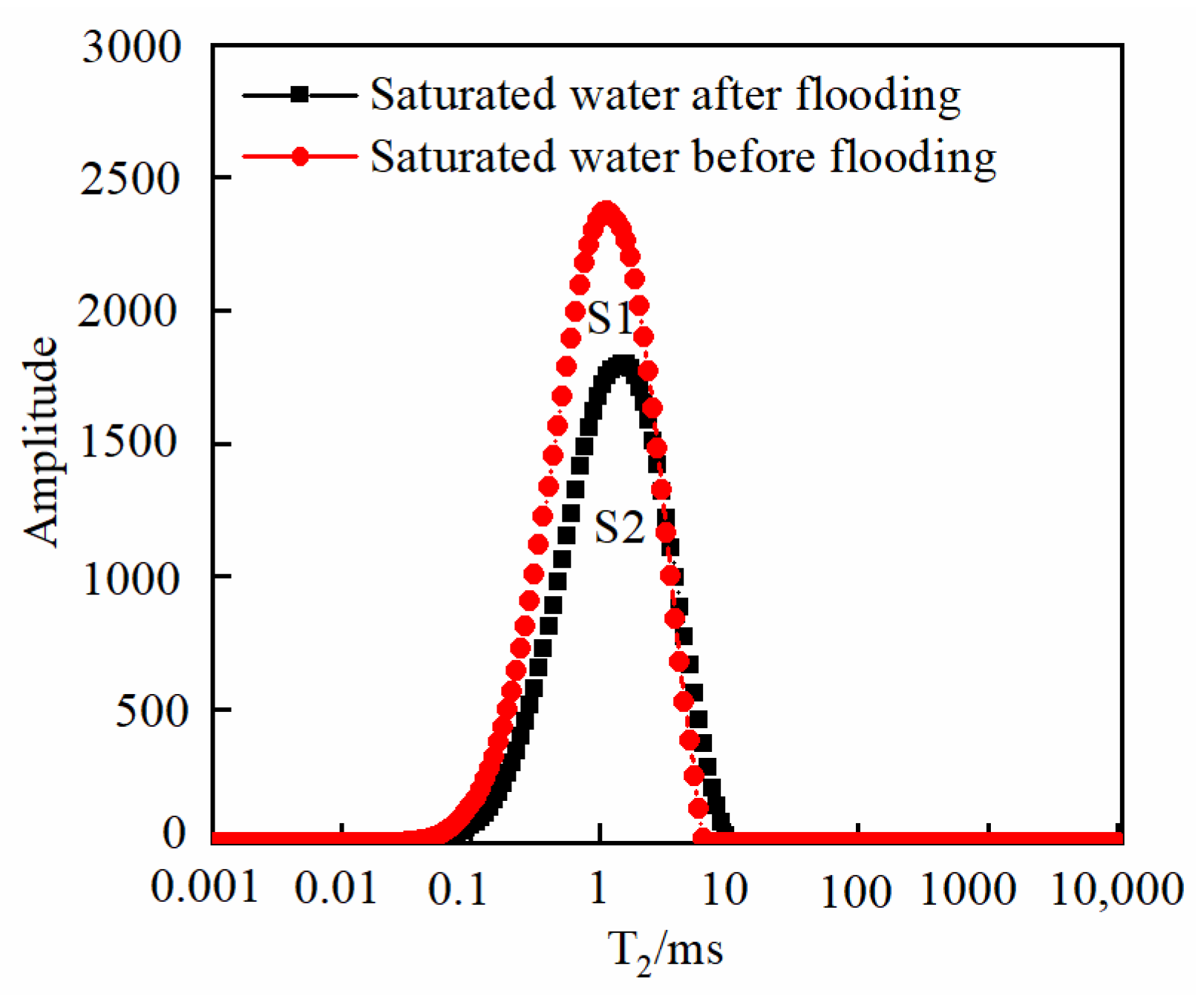
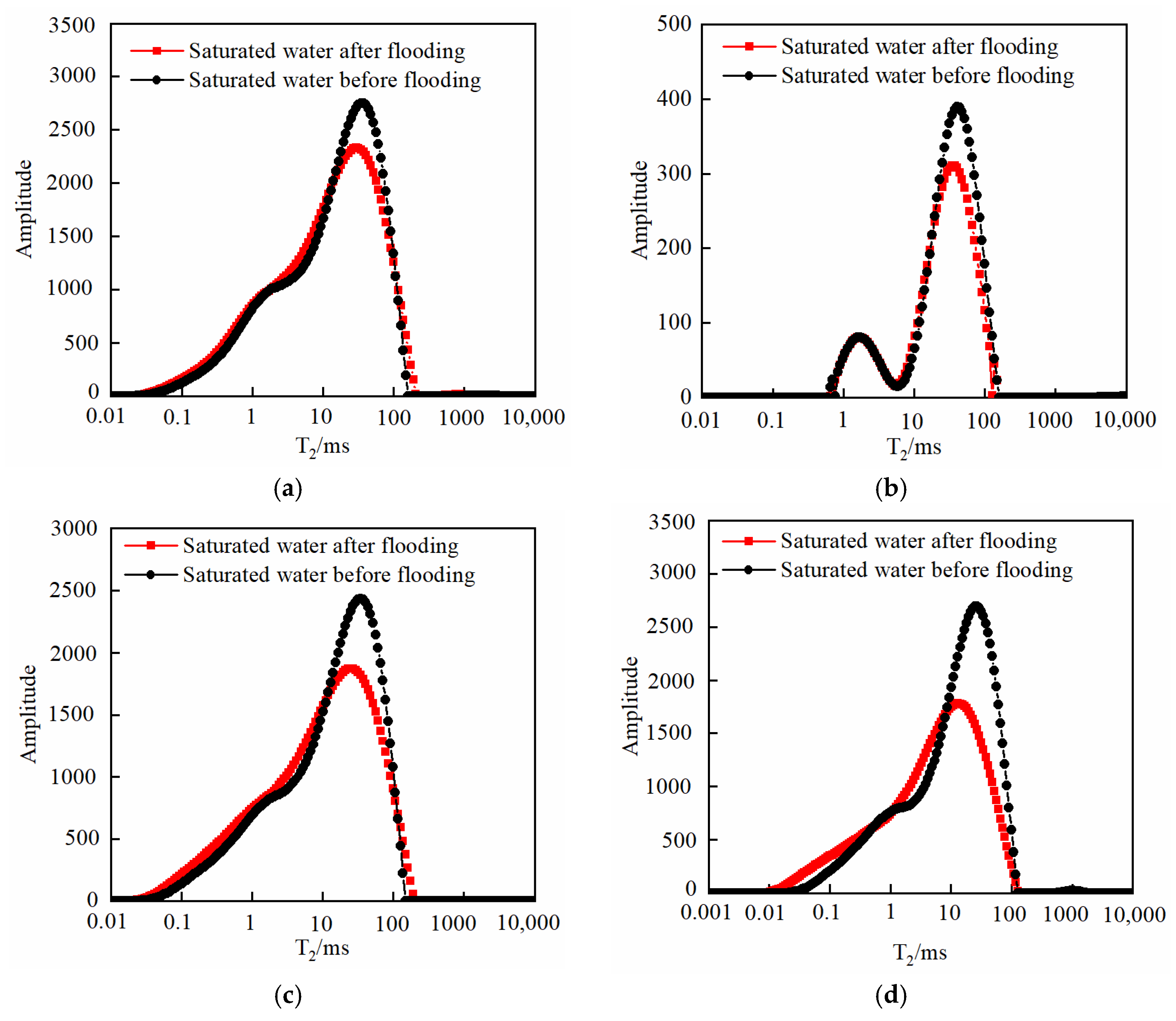
3.3. Changes in Core Physical Properties after CO2 Displacement
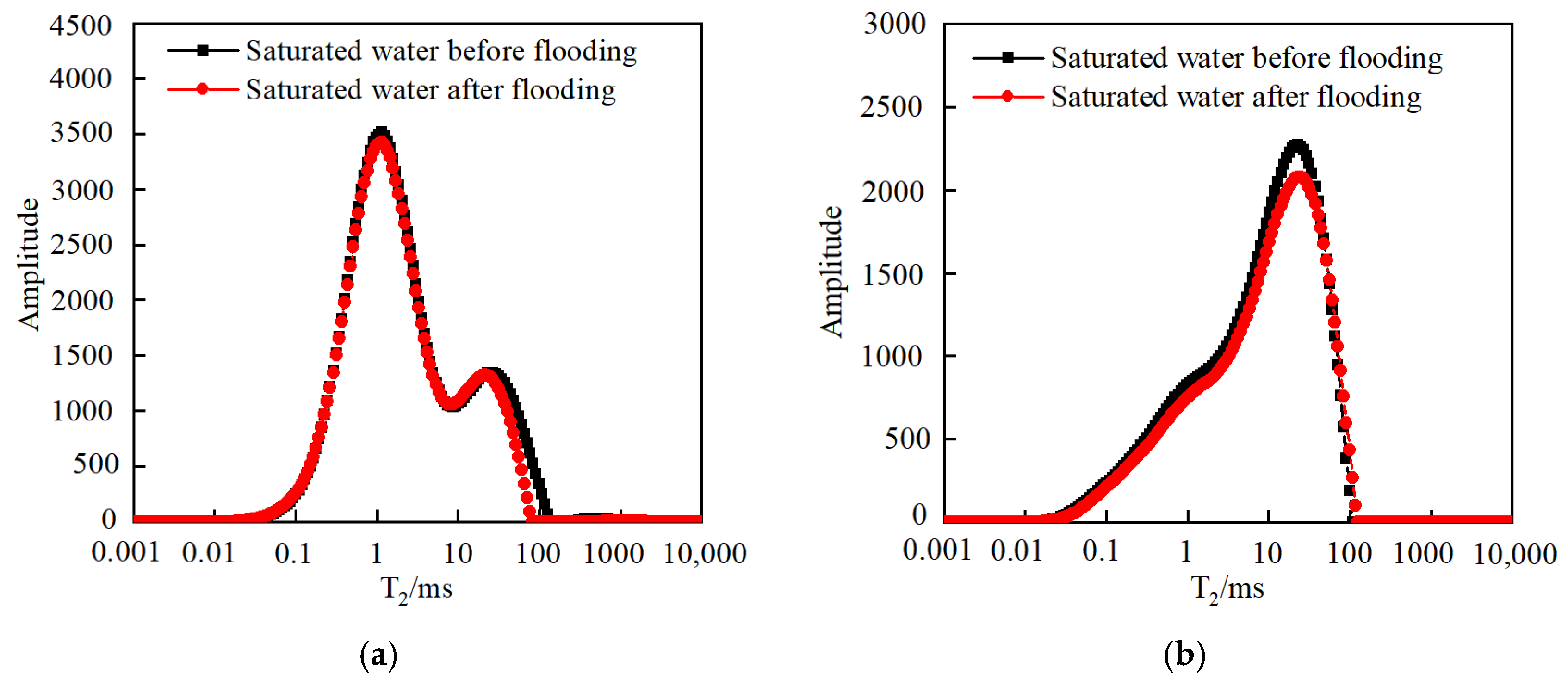
3.3.1. Effect of Injection PV Number
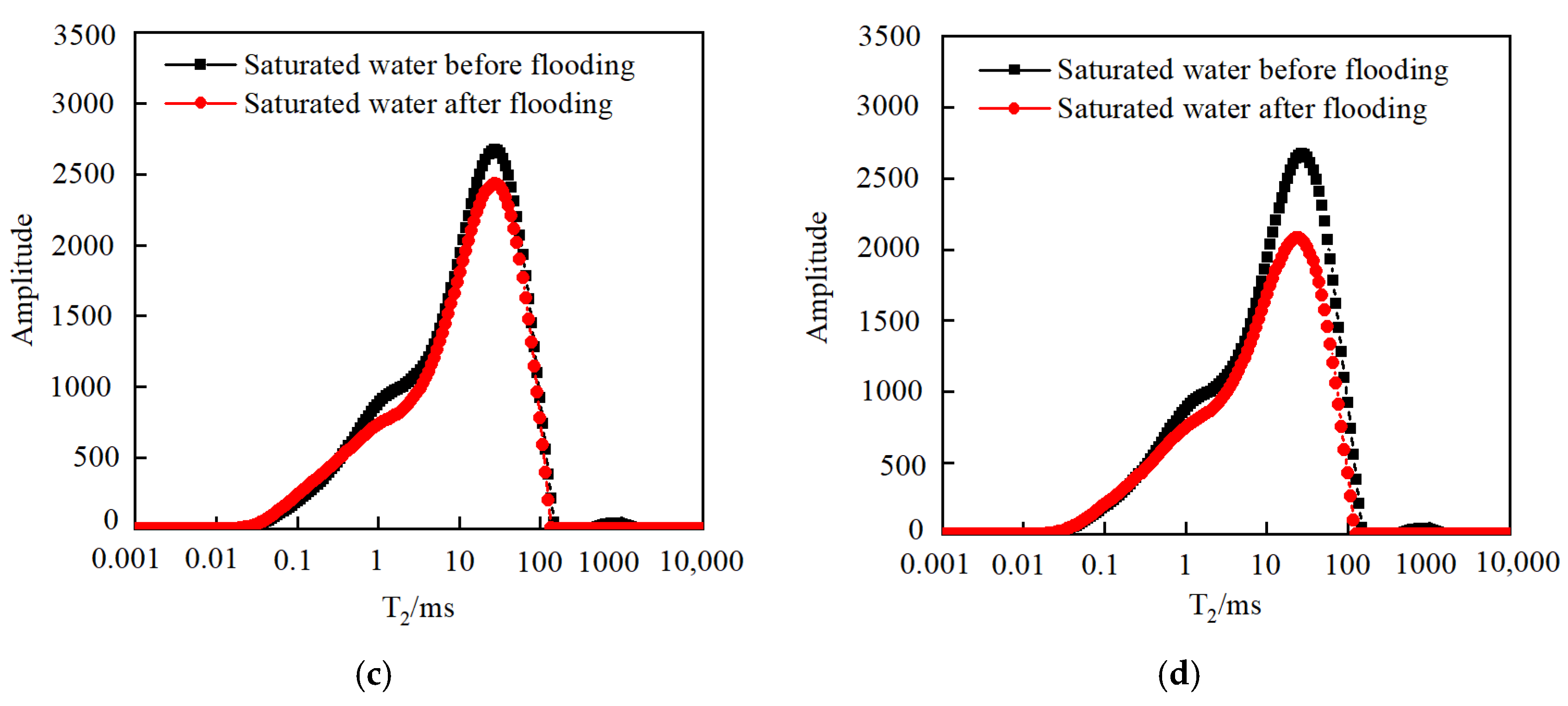
3.3.2. Effect of Pressure
3.3.3. Effect of Temperature
3.3.4. Effect of Core Permeability
4. Conclusions
- (1)
- CO2 has a notable effect on the pH value of the formation water. To mitigate this influence during CO2 injection for reservoir development purposes, it is necessary to increase the injection pressure to reduce the pH value of the reservoir. By creating an acidic environment, some minerals in the reservoir can be dissolved, and the reservoir’s permeability can be enhanced to some extent.
- (2)
- As the temperature increased, the solubility of CO2 in the formation water decreased. Consequently, the concentration of CO32− ions decreased, and the amount of carbonate precipitation gradually decreased. Conversely, increasing the pressure promoted the ionization of carbonic acid and bicarbonate, leading to an increase in CO2 solubility. Upon pressure release, the CO2 concentration decreased, resulting in an increase in formation water precipitation.
- (3)
- The average particle size of the produced liquid increased by 1579 nm, and the PDI increased by 0.147. It is important to note that the core is susceptible to corrosion under high-temperature and -pressure conditions. As the temperature and pressure increased, the degree of dissolution gradually intensified. The concentration of Na+ and K+ ions primarily increased due to the dissolution of plagioclase and potassium feldspar minerals. Furthermore, the concentration of Ca2+ ions primarily increased as a result of the dissolution of dolomite and calcite carbonate minerals.
- (4)
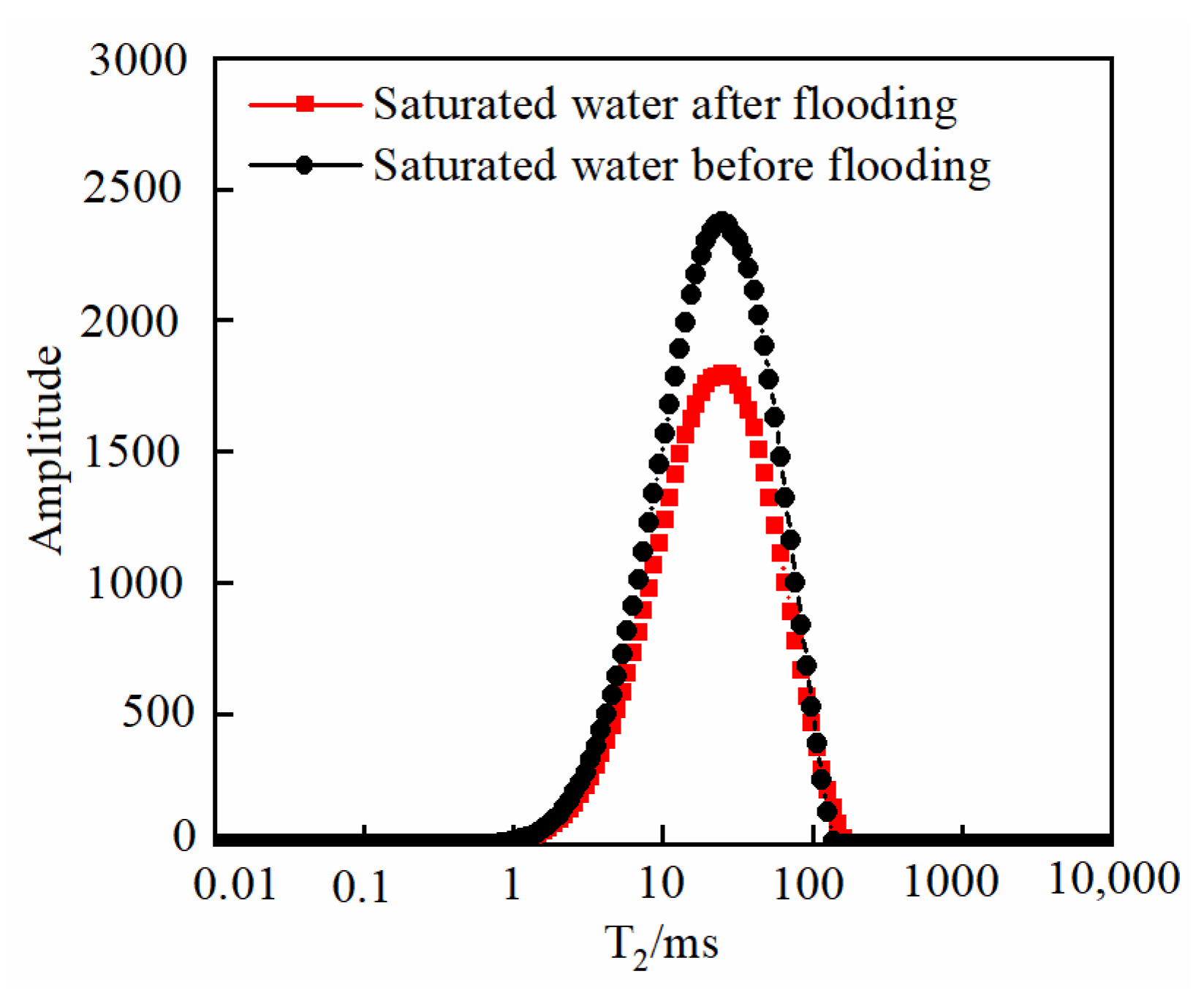
- As the injected PV increased, CO2 first entered the macropores, resulting in a decrease in the amount of secondary saturated water within the macropores and an increasing degree of pore throat blockage. As the injected PV continued to increase, the dissolution process led to the formation of small cracks, resulting in an increased amount of secondary saturated water compared to the initial stage, and the volume of small pores increased. Pressure plays a significant role in the CO2–water–rock reaction. When the pressure reaches the supercritical state, the dissolution process intensifies at the pore throat. The resulting debris from dissolution can then block the flow path, leading to a significant reduction in signal within larger pores and causing severe blockage. Temperature also has an effect on the water–rock reaction. As the temperature increases to the critical temperature, the macropores are the first to become blocked, and the degree of blockage in the macropores gradually increases. The small pores are also blocked, leading to a decrease in the porosity of the core.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Nomenclature
| Abbreviations | |
| SEM | scanning electron microscopy |
| ICP-MS | inductively coupled plasma–mass spectrometry |
| T2 | transverse relaxation time |
| NMR | nuclear magnetic resonance |
| XRD | X-ray diffraction |
| PDI | polydispersity index |
| Symbols | |
| PV | the pore volume, dimensionless |
| B | the pore plugging rate, fraction |
| S1 | the peak area value of saturated water before flooding |
| S2 | the peak area value of saturated water after flooding |
References
- Jarboe, P.; Candela, P.; Zhu, W.; Kaufman, A. Extraction of hydrocarbons from high-maturity Marcellus shale using supercritical carbon dioxide. Energy Fuels 2015, 29, 7897–7909. [Google Scholar] [CrossRef]
- Jin, L.; Hawthorne, S.; Sorensen, J.; Pekot, L.; Kurz, B.; Smith, S.; Heebink, L.; Herdegen, V.; Bosshart, N.; Dalkhaa, C.; et al. Advancing CO2 enhanced oil recovery and storage in unconventional oil play—Experimental studies on Bakken shales. Appl. Energy 2017, 208, 171–183. [Google Scholar] [CrossRef]
- Gamadi, T.; Sheng, J.; Soliman, M.; Menouar, H.; Watson, M.; Emadibaladehi, H. An Experimental Study of Cyclic CO2 Injection to Improve Shale Oil Recovery, SPE Improved Oil Recovery Symposium; Society of Petroleum Engineers: Tulsa, OK, USA, 2014; pp. 1–9. [Google Scholar]
- Jiang, Y.; Luo, Y.; Lu, Y.; Qin, C.; Liu, H. Effects of supercritical CO2 treatment time, pressure, and temperature on microstructure of shale. Energy 2016, 97, 173–181. [Google Scholar] [CrossRef]
- Yin, H.; Zhou, J.; Jiang, Y.; Xian, X.; Liu, Q. Physical and structural changes in shale associated with supercritical CO2 exposure. Fuel 2016, 184, 289–303. [Google Scholar] [CrossRef]
- Psarras, P.; Holmes, R.; Vishal, V.; Wilcox, J. Methane and CO2 adsorption capacities of kerogen in the Eagle Ford shale from molecular simulation. Acc. Chem. Res. 2017, 50, 1818–1828. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Z.; Li, M.; Lin, M.; Peng, B.; Sun, L. Review of the CO2-Water-Rock Interaction in Reservoir. Bulletin of Mineralogy. Petrol. Geochem. 2011, 30, 104–112. [Google Scholar]
- Dove, P.M.; Crerar, D.A. Kinetics of quartz dissolution in electrolyte solutions using a hydrothermal mixed flow reactor. Geochim. Cosmochim. Acta 1990, 54, 955–969. [Google Scholar] [CrossRef]
- Ross, G.D.; Todd, A.C.; Tweedie, J.A. The Effect of Simulated CO2 Flooding on the Permeability of Reservoir Rocks; Elsevier: Amsterdam, The Netherlands, 1981; Volume 37, pp. 169–174. [Google Scholar]
- Bowker, K.A.; Shuler, P.J. Carbon dioxide injection and resultant alteration of the Weber Sandstone, Rangely Field, Colorado. AAPG Bull. 1991, 39, 129–136. [Google Scholar]
- Sayegh, S.G.; Krause, F.F.; Girard, M.; DeBree, C. Rock/fluid interactions of carbonated brines in a sandstone reservoir: Pembina Cardium, Alberta, Canada. SPE Form. Eval. 1990, 5, 399–405. [Google Scholar] [CrossRef]
- Qu, X. The Experiment Research of CO2-Sandstone Interaction and Application CO2 Gas Reservoir; Jilin University: Changchun, China, 2007. [Google Scholar]
- Shi, M. Experiment research of compatibility between CO2 and formation water, reservoir respectively. J. Chongqing Univ. Sci. Technol. 2011, 13, 55–57. [Google Scholar]
- Yu, Z.; Yang, S.; Liu, L.; Li, S.; Yang, Y. An experimental study on water-rock interaction during water flooding in formations saturated with CO2. Acta Pet. Sin. 2012, 33, 1032–1042. [Google Scholar]
- Wang, G.; Zhao, J.; Zhang, F.; Tao, Y.; Yang, X.; Wang, H. Interactions of CO2-brine-rock in sandstone reservoir. J. Cent. South Univ. 2013, 44, 1167–1173. [Google Scholar]
- Cui, G.; Zhang, L.; Tan, C.; Ren, S.; Zhuang, Y.; Enechukwu, C. Injection of supercritical CO2 for geothermal exploitation from sandstone and carbonate reservoirs: CO2–water–rock interactions and their effects. J. CO2 Util. 2017, 20, 113–128. [Google Scholar] [CrossRef]
- Siqueira, A.T.; Iglesias, S.R.; Ketzer, M.J. Carbon dioxide injection in carbonate reservoirs—A review of CO2-water-rock interaction studies. Greenh. Gases 2017, 7, 802–816. [Google Scholar] [CrossRef]
- Xiao, N.; Li, S.; Lin, M. Experimental Investigation of CO2-Water-Rock Interactions during CO2 Flooding in Carbonate Reservoir. Open J. Yangtze Oil Gas 2017, 2, 108–124. [Google Scholar] [CrossRef]
- Liu, N.; Cheng, J. Geochemical effects of cement mineral variations on water–rock–CO2 interactions in a sandstone reservoir as an experiment and modeling study. Greenh. Gases 2019, 9, 789–810. [Google Scholar] [CrossRef]
- Ma, B.; Cao, Y.; Zhang, Y.; Eriksson, A.K. Role of CO2-water-rock interactions and implications for CO2 sequestration in Eocene deeply buried sandstones in the Bonan Sag, eastern Bohai Bay Basin, China. Chem. Geol. 2020, 541, 119585. [Google Scholar] [CrossRef]
- Ahmat, K.; Cheng, J.; Yu, Y.; Zhao, R.; Li, J. CO2-Water-Rock Interactions in Carbonate Formations at the Tazhong Uplift, Tarim Basin, China. Minerals 2022, 12, 635. [Google Scholar] [CrossRef]





| Number | Plagioclase | Calcite | Quartz | Clay | Feldspar | Dolomite |
|---|---|---|---|---|---|---|
| #1 | 24.0 | 2.1 | 44.7 | 10.7 | 11.9 | 6.6 |
| #2 | 15.0 | 6.0 | 48.0 | 9.7 | 12.3 | 9.0 |
| #3 | 5.0 | 13.0 | 33.0 | 34.0 | 9.0 | 6.0 |
| #4 | 24.0 | 6.0 | 24.0 | 18.0 | 16.0 | 12.0 |
| #5 | 26.7 | 2.3 | 44.0 | 9.0 | 10.0 | 8.0 |
| #6 | 19.0 | 6.8 | 48.1 | 12.0 | 9.1 | 5.0 |
| #7 | 23.2 | 3.0 | 32.0 | 15.0 | 6.8 | 20.0 |
| #8 | 18.6 | 9.2 | 42.7 | 14.0 | 8.7 | 7.0 |
| #9 | 10.4 | 7.4 | 44.2 | 18.0 | 4.0 | 16.0 |
| #10 | 21.5 | 7.7 | 41.8 | 16.0 | 4.0 | 9.0 |
| #11 | 21.9 | 8.7 | 37.4 | 15.0 | 9.0 | 8.0 |
| #12 | 28.0 | 6.1 | 45.6 | 8.2 | 6.1 | 6.0 |
| #13 | 16.9 | 13.0 | 46.1 | 5.0 | 12.0 | 7.0 |
| #14 | 23.3 | 5.4 | 38.4 | 10.0 | 13.9 | 9.0 |
| #15 | 22.5 | 5.9 | 43.4 | 9.0 | 11.2 | 8.0 |
| Number | Length/cm | Diameter/cm | Porosity/% | Permeability/mD |
|---|---|---|---|---|
| #1 | 4.78 | 2.48 | 13.55 | 5.40 × 10−2 |
| #2 | 4.74 | 2.48 | 7.73 | 1.60 × 10−2 |
| #3 | 4.77 | 2.48 | 10.25 | 2.30 × 10−2 |
| #4 | 4.79 | 2.46 | 9.81 | 3.30 × 10−2 |
| #5 | 4.86 | 2.49 | 15.34 | 1.17 × 10−1 |
| #6 | 4.76 | 2.48 | 12.34 | 1.12 × 10−1 |
| #7 | 4.75 | 2.47 | 12.66 | 5.40 × 10−2 |
| #8 | 5.75 | 2.47 | 8.14 | 3.30 × 10−2 |
| #9 | 5.29 | 2.48 | 5.53 | 3.00 × 10−3 |
| #10 | 5.14 | 2.48 | 11.34 | 1.17 × 10−1 |
| #11 | 3.81 | 2.48 | 11.67 | 1.12 × 10−1 |
| #12 | 4.77 | 2.47 | 12.89 | 8.82 × 10−1 |
| #13 | 3.56 | 2.48 | 5.53 | 3.00 × 10−3 |
| #14 | 5.04 | 2.47 | 11.34 | 1.70 × 10−2 |
| #15 | 4.32 | 2.47 | 12.89 | 8.82 × 10−1 |
| Ion Species | Ion Content/(mg/L) |
|---|---|
| Ca2+ | 5683.46 |
| Mg2+ | 421.803 |
| Ba2+ | 169.06 |
| Na+ | 15,030.60 |
| Sr2+ | 443.25 |
| K+ | 292.26 |
| CO32− | - |
| HCO3− | 252 |
| Experiment Number | Pressure/MPa | Temperature/°C | ρ(K+)/(mg·L−1) | ρ(Na+)/(mg·L−1) | ρ(Ca2+)/(mg·L−1) |
|---|---|---|---|---|---|
| 1 | 5 | 60 | 2.34 | 17.64 | 80.32 |
| 2 | 10 | 60 | 4.67 | 21.56 | 115.68 |
| 3 | 15 | 60 | 6.23 | 27.83 | 174.36 |
| 4 | 20 | 60 | 7.68 | 37.97 | 220.71 |
| 5 | 20 | 30 | 3.31 | 16.51 | 100.03 |
| 6 | 20 | 40 | 4.37 | 24.88 | 167.28 |
| 7 | 20 | 50 | 5.96 | 30.45 | 190.33 |
| 8 | 20 | 60 | 7.68 | 37.97 | 220.71 |
| Particle Size/nm | PDI | |
|---|---|---|
| 1377 | 0.296 | |
| Pre-reaction | 1081 | 0.284 |
| 1130 | 0.296 | |
| 2500 | 0.362 | |
| Post-reaction | 2854 | 0.423 |
| 2971 | 0.534 |
| Experiment Number | Displacement Pressure/MPa | PV Number | Displacement Pressure Difference/MPa | Experimental Temperature/°C |
|---|---|---|---|---|
| 1 | 10 | 25 | 1 | 60 |
| 2 | 10 | 50 | 1 | 60 |
| 3 | 10 | 75 | 1 | 60 |
| 4 | 10 | 100 | 1 | 60 |
| Core Number | Permeability (before) /mD | Permeability (after) /mD | Rate of Change /% | Porosity (before) /% | Porosity (after) /% | Rate of Change /% | Pore Throat Blockage Rate/% |
|---|---|---|---|---|---|---|---|
| #1 | 3.30 × 10−2 | 4.90 × 10−2 | 9.26 | 13.55 | 12.66 | 6.56 | 7.21 |
| #2 | 3.30 × 10−2 | 1.40 × 10−2 | 12.50 | 7.73 | 7.12 | 7.89 | 8.42 |
| #3 | 2.30 × 10−2 | 2.00 × 10−2 | 13.04 | 10.25 | 8.84 | 13.75 | 11.66 |
| #4 | 3.30 × 10−2 | 2.70 × 10−2 | 18.18 | 9.81 | 8.14 | 17.06 | 17.94 |
| Experiment Number | Displacement Pressure/MPa | PV Number | Displacement Pressure Difference/MPa | Experimental Temperature/°C |
|---|---|---|---|---|
| 1 | 5 | 50 | 1 | 60 |
| 2 | 10 | 50 | 1 | 60 |
| 3 | 15 | 50 | 1 | 60 |
| 4 | 20 | 50 | 1 | 60 |
| Core Number | Displacement Pressure /MPa | Permeability (before) /mD | Permeability (after) /mD | Rate of Change /% | Porosity (before) /% | Porosity (after) /% | Rate of Change /% | Pore Throat Blockage Rate/% |
|---|---|---|---|---|---|---|---|---|
| #5 | 5 | 1.17 × 10−1 | 1.05 × 10−1 | 10.26 | 15.34 | 13.51 | 11.93 | 8.21 |
| #6 | 10 | 1.12 × 10−1 | 0.95 × 10−1 | 15.20 | 12.34 | 10.16 | 17.67 | 8.74 |
| #7 | 15 | 5.40 × 10−2 | 4.60 × 10−2 | 14.81 | 12.66 | 10.46 | 17.37 | 13.41 |
| #8 | 20 | 3.30 × 10−2 | 2.90 × 10−2 | 12.12 | 8.14 | 6.78 | 16.71 | 13.27 |
| Experiment Number | Displacement Pressure/Mpa | PV Number | Displacement Pressure Difference/Mpa | Experimental Temperature/°C |
|---|---|---|---|---|
| 1 | 10 | 50 | 1 | 30 |
| 2 | 10 | 50 | 1 | 40 |
| 3 | 10 | 50 | 1 | 50 |
| 4 | 10 | 50 | 1 | 60 |
| Core Number | Experimental Temperature /°C | Permeability (before) /mD | Permeability (after) /mD | Rate of Change/% | Porosity (before) /% | Porosity (after) /% | Rate of Change /% | Pore Throat Blockage Rate/% |
|---|---|---|---|---|---|---|---|---|
| #9 | 30 | 3.00 × 10−3 | 2.90 × 10−3 | 5.22 | 5.53 | 5.28 | 4.58 | 2.78 |
| #10 | 40 | 1.17 × 10−1 | 1.05 × 10−1 | 10.21 | 11.34 | 10.28 | 9.34 | 7.84 |
| #11 | 50 | 1.12 × 10−1 | 1.00 × 10−1 | 10.48 | 11.67 | 9.94 | 12.86 | 10.22 |
| #12 | 60 | 8.82 × 10−1 | 7.42 × 10−1 | 15.88 | 12.89 | 12.12 | 15.20 | 14.78 |
| Core Number | Permeability (before) /mD | Permeability (after) /mD | Rate of Change /% | Porosity (before) /% | Porosity (after) /% | Rate of Change /% | Pore Throat Blockage Rate /% |
|---|---|---|---|---|---|---|---|
| #13 | 3.00 × 10−3 | 2.60 × 10−3 | 10.78 | 5.53 | 4.79 | 8.84 | 9.02 |
| #14 | 1.70 × 10−2 | 1.60 × 10−2 | 5.66 | 11.34 | 10.77 | 5.02 | 6.84 |
| #15 | 8.82 × 10−1 | 8.64 × 10−1 | 2.11 | 2.89 | 12.56 | 2.74 | 2.07 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cao, S.; Sang, Q.; Zhao, G.; Lan, Y.; Dong, D.; Wang, Q. CO2–Water–Rock Interaction and Its Influence on the Physical Properties of Continental Shale Oil Reservoirs. Energies 2024, 17, 477. https://doi.org/10.3390/en17020477
Cao S, Sang Q, Zhao G, Lan Y, Dong D, Wang Q. CO2–Water–Rock Interaction and Its Influence on the Physical Properties of Continental Shale Oil Reservoirs. Energies. 2024; 17(2):477. https://doi.org/10.3390/en17020477
Chicago/Turabian StyleCao, Sheng, Qian Sang, Guozhong Zhao, Yubo Lan, Dapeng Dong, and Qingzhen Wang. 2024. "CO2–Water–Rock Interaction and Its Influence on the Physical Properties of Continental Shale Oil Reservoirs" Energies 17, no. 2: 477. https://doi.org/10.3390/en17020477
APA StyleCao, S., Sang, Q., Zhao, G., Lan, Y., Dong, D., & Wang, Q. (2024). CO2–Water–Rock Interaction and Its Influence on the Physical Properties of Continental Shale Oil Reservoirs. Energies, 17(2), 477. https://doi.org/10.3390/en17020477






