Abstract
The current research on CO2 cosolvent primarily focuses on reducing the minimum miscibility pressure and improving oil recovery. However, investigations into the impact of additive agents on the phase behavior of crude oil during the CO2 injection process are relatively limited. In this study, we introduced tributyl citrate as a cosolvent to the CO2 injection process. By comparing the phase parameters of crude oil and changes in component composition in the residual oil before and after the addition of tributyl citrate, we explored the influence patterns of this cosolvent during CO2 injection. The experiments show that the optimum concentration of tributyl citrate is 0.3%. After the addition of tributyl citrate, the bubble point pressure of crude oil is reduced from 14.28 MPa to 13.36 MPa, and the density is decreased from 1.00 g/cm3 to 0.95 g/cm3. These alterations of bubble point pressure and density indicate an enhanced solubility of CO2 and improved miscibility with the oil, coinciding with an increased volume expansion coefficient rising from 1.12 to 1.18 under 20 MPa and a decrease in viscosity from 0.73 mPa·s to 0.64 mPa·s. Tributyl citrate primarily affects the properties of crude oil by reducing interfacial tension and the content of heavy components in the dissolution system. The addition of tributyl citrate stabilizes the deposition trend of heavy components in crude oil and promotes the transformation of heavy components into light components, thereby enhancing the efficiency of CO2 extraction. This study provides valuable insights into a novel and simple method to further increase oil recovery in the CO2 injection process.
1. Introduction
As global energy demand continues to grow, the increasing demand for energy in production and daily lives highlights the contradiction in the supply and demand of petroleum. The development and utilization of traditional petroleum resources face significant challenges. The gradual depletion of oil reserves, aging oil fields, and the increasingly severe environmental protection situation make the search for more efficient and sustainable oilfield production technologies an urgent task. Since Whorton obtained the first CO2 enhanced oil recovery (CO2-EOR) technology patent in 1952, CO2-EOR technology has gradually become a research focus in petroleum development [1]. After 70 years of development, this technology has gradually formed an industrial scale and matured.
CO2-EOR, as an important technology to improve crude oil recovery, has been widely applied in three phases of oil production, making it one of the highly effective measures for enhancing crude oil recovery [2,3,4,5]. The core idea of CO2 flooding is to alter the physical and chemical environment within the reservoir by injecting CO2, thereby improving the fluidity of crude oil and increasing the recovery [6,7,8]. However, in practical applications, a series of interactions and phase behavior issues exist between CO2 and crude oil [9,10,11,12]. These issues are closely related to the phase changes of crude oil. When conditions such as temperature, pressure, CO2 concentration, etc., change, the phase behavior of crude oil will also change (e.g., bubble point pressure, volume coefficient, viscosity, gas–liquid composition, and composition), and the minimum miscibility pressure will vary, thereby affecting the efficiency of the oil recovery process [13,14,15,16]. To overcome these challenges, researchers employ co-solvents to regulate the phase behavior between CO2 and crude oil, enhance the solubility of CO2, and promote the miscibility of CO2 with crude oil, ultimately achieving the goal of increased production [17,18,19,20].
Currently, scholars have injected different effective chemicals to decrease the minimum miscibility pressure, which are mainly classified into four categories: low molecular weight hydrocarbons, low carbon alcohols, toll oil, and surfactants [21]. Low-molecular-weight hydrocarbons, such as propane, butane, and liquefied petroleum gas (LPG), can directly mix with crude oil in any proportion and maintain a single phase, allowing for a one-time contact miscibility. Therefore, the extraction capacity of CO2 for intermediate hydrocarbons can be increased by increasing the content of light hydrocarbons [22,23,24]. Low-carbon alcohols can dissolve in both supercritical CO2 and crude oil, reducing the interfacial tension between the two while increasing the viscosity and density of CO2, thereby enhancing the dissolution of CO2 in crude oil [25,26]. Toll oil, composed mainly of fatty acids, resins, and sterols in a ratio of 5:4:1, can dissolve well in both crude oil and CO2, increasing the viscosity of supercritical CO2 and reducing the polarity of crude oil- CO2, thus facilitating the formation of a single phase [27]. Surfactants are organic compounds with amphiphilic groups, containing two groups with different polarities and hydrophilic properties. Their addition in small amounts can significantly reduce the interfacial tension in the solution, alter the interface state of the solution system, and result in a series of effects such as wetting, emulsification, and solubilization [28,29,30].
Research indicates that low-molecular-weight hydrocarbons and low-carbon alcohols can notably decrease miscibility pressure. Nevertheless, their extensive injection requirements and associated costs present significant challenges to the broad implementation of light hydrocarbons in oilfields [31]. In contrast, surfactants, with lower material needs and a cost-effective profile, exhibit a pronounced enhancement in reducing miscibility pressure. This positions them as a more promising strategy for improving the phase behavior between carbon dioxide and crude oil.
Wang et al. [32] introduced the surfactants TXIB and NP-9 when injecting CO2 at pressures below the minimum miscibility pressure (MMP). They observed an increase in recovery from 72.61% to 86.52% and 93.04%, indicating that co-solvents can effectively enhance the miscibility of CO2 and crude oil. Almobarak et al. [33] utilized various surfactants to reduce the minimum miscibility pressure and found that some chemicals could reduce the MMP of the CO2-crude oil system by 22%, with surfactants being more effective than alcohol-based chemicals. Luo et al. [34] reduced the miscibility pressure by using a propoxy-based surfactant (CPO) and found that at a dosage of 0.5 wt%, the MMP of the CO2-crude oil system decreased by 22.3%. Additionally, Zhang et al. [35] discovered a synergistic effect between ethanol and surfactants. The combination of 0.5 wt% non-ionic polyether surfactant 2EH-PO5-EO9 and 7 wt% ethanol reduced the MMP and FCMP by 21.1% and 24.0%, respectively. However, most of the aforementioned studies mainly focus on the effects of reducing the minimum miscibility pressure and improving recovery, with limited research on the specific impact of different co-solvents on the phase behavior of crude oil. As co-solvents are inherently oil-soluble/gas-soluble surfactants or small-molecule low-carbon alcohols and hydrocarbons, their introduction alters the phase behavior of crude oil, affecting parameters such as oil solubility, density, and expansion coefficient. When phase parameters change, they can impact the effectiveness of co-solvents in reducing interfacial tension, thereby influencing recovery.
In recent years, tributyl citrate has gained widespread attention as a cosolvent in the process of carbon dioxide displacement [36]. Its molecular structure, being an esterification product of citric acid, contains multiple polar functional groups, thereby exhibiting high interfacial activity at the oil–water interface. As a lipophilic compound, it is miscible with most organic solvents, dissolves well in crude oil, and its solubility in supercritical carbon dioxide increases with pressure. Moreover, compared to commonly used cosolvents such as low molecular weight alkanes and low-carbon alcohols, tributyl citrate demonstrates significant advantages in improving the physical properties of crude oil (such as reducing viscosity and surface tension), environmental friendliness, and economic benefits [37]. These characteristics not only make tributyl citrate an ideal choice for optimizing the carbon dioxide displacement process but also highlight the importance and urgency of researching the interactions between tributyl citrate and crude oil.
Given the relatively limited research on the phase behavior of tributyl citrate in the CO2-crude oil system, this study employs an ultra-high-pressure full-visual PVT (Pressure-Volume-Temperature) system to acquire phase parameters of the crude oil system under various conditions. By examining the alterations in the phase behavior of the crude oil system at different pressures, the study aims to elucidate the influence patterns of tributyl citrate on the phase behavior of crude oil. This research contributes to a more profound understanding of the impact of tributyl citrate on the phase characteristics of the CO2-crude oil system.
2. Experimental Section
2.1. Experimental Materials
The experimental crude oil used is light oil from Changqing oilfield, and the relevant component parameters are shown in Table 1. Gas chromatography (GC) and mass spectrometry (MS) were employed to analyze the oil composition, as shown in Table 2. The tributyl citrate used in the experiment is produced by Shanghai Macklin Biochemical Technology Co., Ltd. (Shanghai, China), and is of analytical purity. The CO2 used is produced by Yantai Deyi Gas Co., Ltd. (Yantai, China), with a purity of 99.9%. The petroleum ether used for cleaning the experimental equipment is produced by China National Pharmaceutical Group Chemical Reagent Co., Ltd. (Shanghai, China), and is of analytical purity.

Table 1.
Deaerated crude oil four-component testing.

Table 2.
Compositional analysis of the oil samples.
2.2. Experimental Apparatus
The CO2 injection experiment utilized the high-temperature and high-pressure visualization PVT-240 testing system from the French company ST (It is located in Frépillon, France), as shown in Figure 1. The main volume of the equipment is 240 mL, with a testing accuracy of 0.01 mL. The temperature is controlled by a built-in constant temperature control system, with a testing temperature range of 0 to 200 °C and an accuracy of 0.1 °C. A conical piston is used in the PVT cell, forming an annular volume space between the PVT cell wall and the piston. An automatic pump controls the piston to achieve different test pressures, with a pressure range of 0.01 to 150 MPa and a testing accuracy of 0.01 MPa. The equipment is equipped with a high-definition camera that can record the interaction between oil and CO2-oil crude during the experimental process.
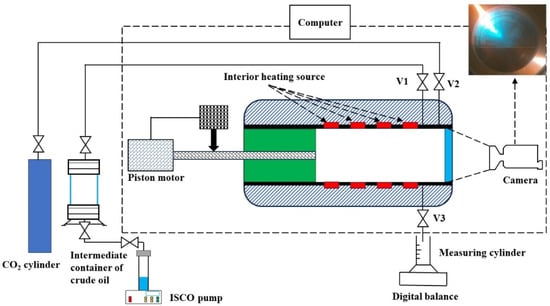
Figure 1.
Schematic diagrams of the high pressure full-visual pvt apparatus.
Mixing tributyl citrate with the CO2-crude oil system reduces interfacial tension. To find the best concentration, the pendant drop tensiometer, shown in Figure 2, is used. This device includes a 400 mL high-pressure container, a micro-injector, heating, and gas injection systems. The interfacial tension between CO2 and crude oil at different pressures is calculated by measuring the droplet shape in the device, using Andreas’ formula.
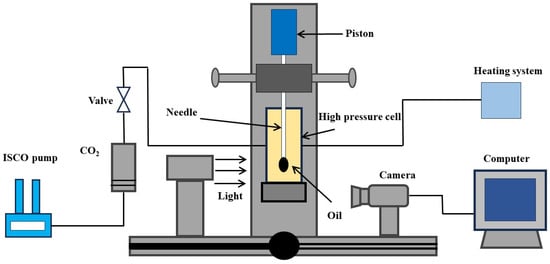
Figure 2.
Schematic diagram of the pendant drop tensiometer.
2.3. Experimental Procedure
2.3.1. Experiment Preparation
The experiment adhered to the stringent guidelines set forth by the SY/T 5542-2009 standard test method [38] for reservoir fluid physical properties. Recognizing the susceptibility of crude oil and CO2 volumes to fluctuations in temperature and pressure, rigorous measures were taken to ensure volumetric precision upon injection. This entailed a meticulous cleansing protocol, whereby the equipment underwent 2–3 cycles of purification with petroleum ether, followed by a thorough nitrogen drying process. Subsequently, the system was finely tuned through a CO2 calibration process, the PVT cell underwent a comprehensive evaluation, and the ambient temperature within the cell was adjusted to the predetermined set point.
2.3.2. Constant Mass Expansion Experiment
This experiment aims to explore how pressure and volume interact in a constant mass of crude oil at reservoir temperature, which is crucial for understanding the CO2-crude oil system’s behavior. The test begins by opening valves V1 and V2 on the PVT cell, and setting the ISCO pump to 1 mL/min to add the oil and CO2 into the cell. After injecting the samples and closing the valves, the cell’s pressure is adjusted to 20 MPa and heated to 85 °C. A stirrer helps dissolve CO2 in the oil until a stable volume is reached. The experiment starts by adjusting the PVT cell to control pressure and heating the cell to 85 °C. Pressure is increased to 20 MPa to keep conditions stable. We use a stirrer to mix CO2 into the crude oil until the cell’s volume stabilizes. The test involves gradually lowering the pressure by 1 to 2 MPa per step. After reaching the bubble point pressure, we shift to expanding the volume step by step, increasing it by 0.5 to 20 mL each time until it is three times the size of the original sample. Concluding the experiment, a 0.3% volumetric ratio of tributyl citrate is amalgamated into the crude oil, after which the aforementioned protocol is diligently replicated.
2.3.3. Differential Liberation Experiment
The experiment setup is similar to the constant mass expansion experiment. After preparation, the PVT cell pressure is raised to the bubble point. Pressure intervals are then set based on this bubble point pressure, and we conduct five degassing stages. After each stage, the volume of oil and gas is measured, and important properties, such as density and gas-to-oil ratio, are calculated. Controlled degassing is then initiated by opening valve V3, allowing for the release of all gas and subsequent pumping out of the residual oil, the volumes and masses of which are meticulously recorded. In the final analysis, the impact of tributyl citrate on the oil is discerned through an examination of the residual oil’s components and carbon number distribution.
2.3.4. Tributyl Citrate Concentration Optimization Experiment
Before the experiment, tributyl citrate is mixed into crude oil at specific ratios to prepare the samples. The high-pressure chamber’s visual windows and syringes are cleaned with petroleum ether. After loading the syringe with the oil sample, the chamber is sealed, and then heated to the target temperature. CO2 is introduced to achieve the desired pressure, setting the appropriate CO2 and oil density. The oil droplet’s volume is carefully adjusted to maintain equilibrium, ensuring optimal shape and dispersion for accurate interfacial tension measurements.
3. Results and Discussion
3.1. Tributyl Citrate Concentration Optimization
Figure 3 demonstrates the impact of varying concentrations of tributyl citrate on the interfacial tension of crude oil. The data indicate a substantial reduction in interfacial tension, plummeting from 26.52 mN/m to 3.04 mN/m—an 88.54% decrease—as the concentration of tributyl citrate ester is augmented up to 0.3%. Notably, past the 0.3% concentration threshold, the interfacial tension plateaus, suggesting that further increments in tributyl citrate ester concentration yield no significant additional reduction in tension. This plateau points to a saturation point, implying that beyond this concentration, tributyl citrate ester’s ability to lower interfacial tension is maximized. The pronounced efficiency of tributyl citrate ester at minimal concentrations likely arises from its molecular alignment at the CO2-oil interface, effectively diminishing the forces maintaining the separation of the two phases.
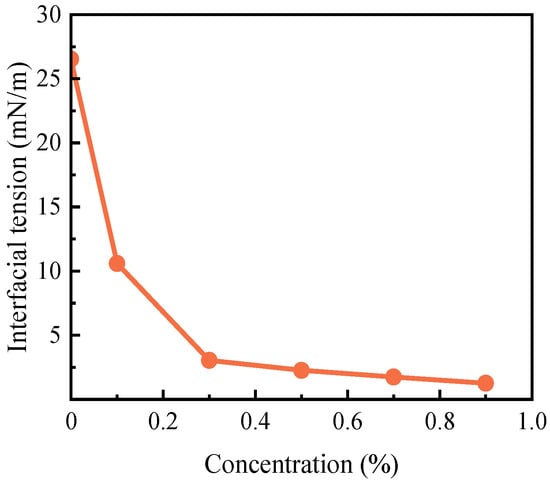
Figure 3.
Changes in crude oil bubble point pressure before and after adding 0.3% tributyl citrate. Changes in the surface tension of butyl citrate at different concentrations.
3.2. Bubble Point Pressure Variation
The bubble point pressure is the pressure at which the first batch of gas bubbles emerges as the system pressure decreases (Figure 4). Typically, the pressure corresponding to the intersection of the single-phase and two-phase regions on the pressure–volume curve is considered as the bubble point pressure.

Figure 4.
Constant composition expansion experiment process.
Figure 5 and Table 3 depict the pressure–volume relationship curve of the crude oil system both before and after the introduction of tributyl citrate at 85 °C. Both systems exhibit a linear trend until reaching the bubble point pressure. Once the bubble point pressure is reached, CO2 rapidly separates within the system, leading to a swift expansion of the crude oil. The PV relationship curves of the two systems were fitted, revealing an initial bubble point pressure of 14.28 MPa for the crude oil. Following the addition of tributyl citrate, the bubble point pressure decreased to 13.36 MPa. The addition of tributyl citrate manifests a dual effect: firstly, it diminishes the interfacial tension between CO2 and crude oil, enhancing the miscibility of CO2 with crude oil and consequently reducing the bubble point pressure. Secondly, tributyl citrate enables crude oil to dissolve more CO2 at the same pressure, further contributing to the decrease in the bubble point pressure. The combined impact of these two aspects results in a notable decrease in the bubble point pressure after the addition of tributyl citrate.
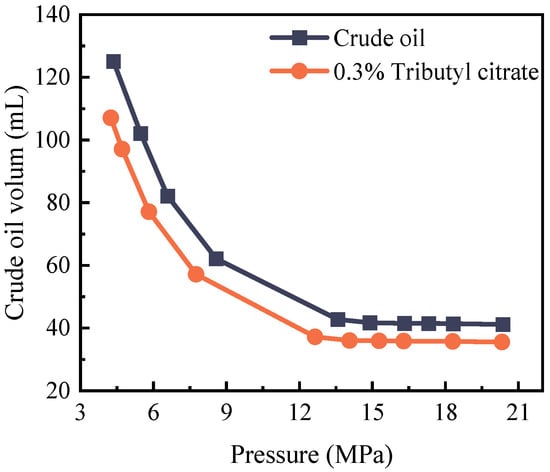
Figure 5.
Changes in crude oil bubble point pressure before and after adding 0.3% tributyl citrate.

Table 3.
Changes in crude oil bubble point pressure before and after adding 0.3% tributyl citrate.
3.3. Density Variation
The variation in crude oil density under the influence of tributyl citrate is depicted in Figure 6. Prior to the addition of tributyl citrate, as pressure increases, the solubility of CO2 in the crude oil also increases. The light components in the crude oil are extracted, leading to an increase in heavy component content, manifested as an increase in density. At the bubble point pressure (14.86 MPa), the density is recorded as 1.00 g/cm3. Following the addition of tributyl citrate, the increased solubility of CO2 in the crude oil contributes to an overall decrease in crude oil density (the density at the bubble point pressure is 0.95 g/cm3). This observed trend signifies an improvement in the miscibility of CO2 with crude oil.
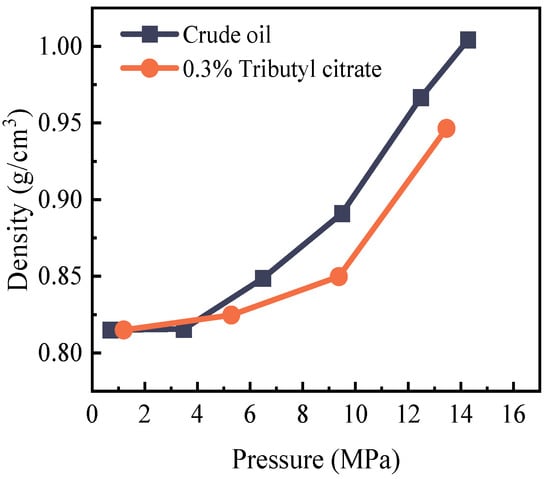
Figure 6.
Changes in crude oil density before and after adding 0.3% tributyl citrate (below bubble point pressure).
3.4. Changes in Solubility
CO2 gradually dissolves into the crude oil through the diffusion process, exerting a profound influence on the properties of the crude oil. The experimental findings are illustrated in Figure 7. As evident from the graph, with the rise in system pressure, there is an augmentation in the influx of CO2 molecules into the crude oil, leading to elevated solubility. Consequently, under high pressure, the CO2–crude oil interaction becomes more comprehensive, and the extraction impact on light components becomes more pronounced. Following the addition of tributyl citrate, the light component content of the system increases, thereby diminishing the interfacial tension between CO2 and crude oil. This augmentation in the CO2 extraction effect culminates in enhanced solubility of crude oil.
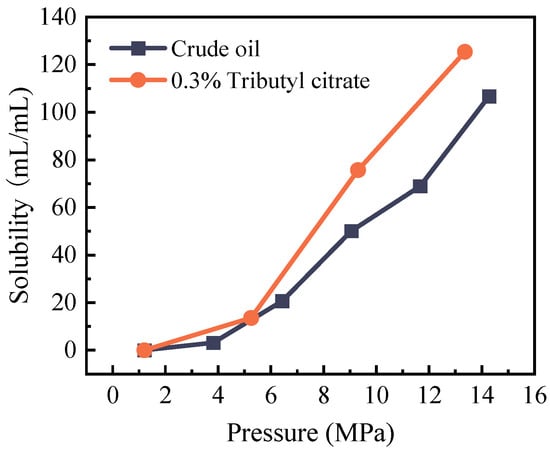
Figure 7.
Changes in the solubility of crude oil at 85 °C before and after adding 0.3% tributyl citrate.
3.5. Changes in Expansion Coefficient
The volume expansion coefficient represents the volume ratio of the oil sample before and after CO2 injection under identical conditions. This parameter serves as an indicator of CO2′s expansion impact on crude oil post-injection. The changes in the crude oil volume expansion coefficient with system pressure, both before and after the introduction of tributyl citrate, are illustrated in Figure 8.
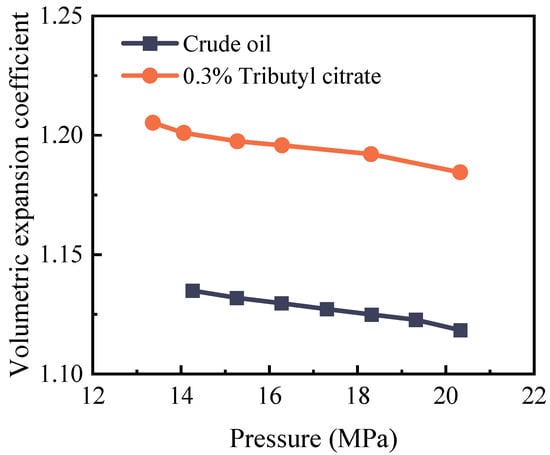
Figure 8.
Changes in the coefficient of volume expansion of crude oil at 85 °C before and after adding 0.3% tributyl citrate.
From the graph, it can be observed that with increasing pressure, the volume expansion coefficient of crude oil gradually decreases. When the pressure reaches 20 MPa, the volume expansion coefficient of crude oil can reach 1.12. After the addition of tributyl citrate, the polarity difference between CO2 and crude oil decreases, leading to an increase in the solubility of CO2 in crude oil. The expansion coefficient of tributyl citrate–crude oil increases to 1.18.
3.6. Changes in Viscosity
The substantial decrease in crude oil viscosity resulting from CO2 dissolution is another major factor contributing to increased oil recovery. The direct impact of viscosity reduction is improved fluidity of the reservoir. A 10% reduction in crude oil viscosity corresponds to a tenfold increase in reservoir fluidity. Viscosity tests were conducted on the fluid system post-CO2 injection to assess viscosity changes in the CO2-crude oil system at various pressures before and after the addition of tributyl citrate. The experimental results are depicted in Figure 9.
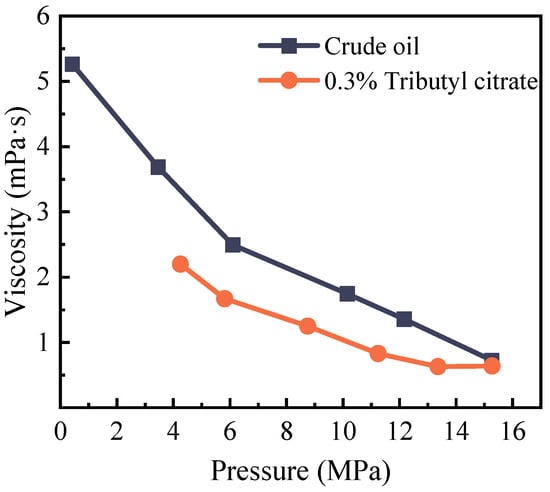
Figure 9.
Changes in the viscosity of crude oil at 85 °C before and after adding 0.3% tributyl citrate.
From the graph, it is evident that, before reaching saturation pressure, the viscosity of the reservoir crude oil gradually increases as the system pressure decreases. Near the saturation pressure, the viscosity of the reservoir crude oil reaches its minimum at only 0.63 mPa·s (before the addition of tributyl citrate, the viscosity of the crude oil was 0.73 mPa·s). The reason for this phenomenon is that, before reaching the saturation pressure, as the system pressure decreases, the dissolved gas in the crude oil continuously separates, causing a gradual reduction in the quantity of light components in the crude oil and an increase in the relative amount of heavy components. This leads to an increase in crude oil viscosity as the system pressure decreases. When the system pressure exceeds the saturation pressure, the viscosity of the reservoir crude oil decreases as the system pressure decreases. This is mainly because, as the system pressure decreases, the internal friction between crude oil liquid molecules gradually decreases, resulting in a gradual reduction of shear stress between liquid layers, leading to a gradual decrease in crude oil viscosity.
After the addition of tributyl citrate to the crude oil, its molecules can disperse and penetrate the heavy components through hydrogen bonding. It partially disassembles the aggregate structure and reduces the content of resin and asphaltene molecules in the aggregate, reducing the cohesive force within the crude oil and preventing re-aggregation. Therefore, in addition to reducing the surface tension of crude oil, tributyl citrate also has a certain viscosity reduction function.
3.7. Changes in Four Components and Carbon Number Distribution
The residual oil resulting from the reaction between crude oil and CO2 in the PVT cylinder was collected. The changes in crude oil components before and after the addition of tributyl citrate were studied using methods of crude oil four-component and carbon number distribution analysis. The results are presented in Figure 10 and Figure 11.
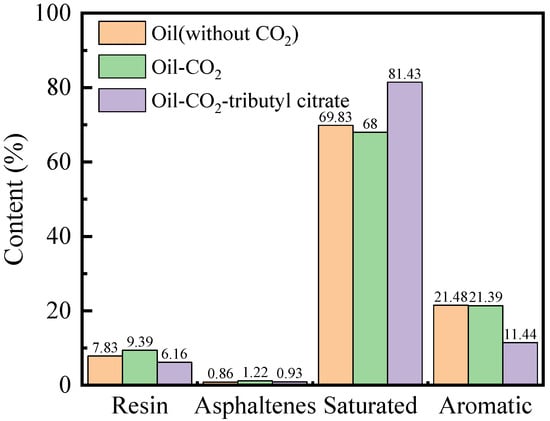
Figure 10.
Changes in crude oil four components under the action of surfactants.
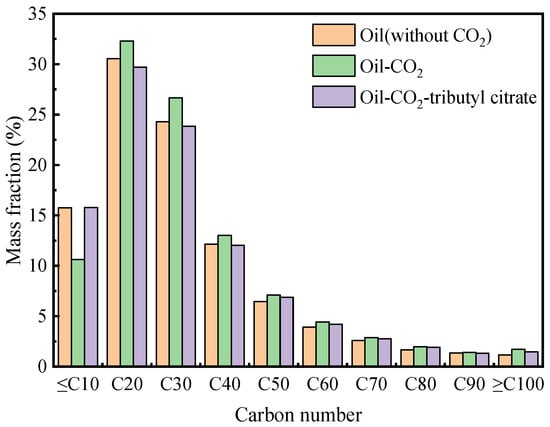
Figure 11.
Changes in crude oil carbon number distribution under the action of surfactants.
From the graph, it is evident that after the addition of CO2, the saturated fraction content in the crude oil decreased by 1.83%, while the aromatic fraction content remained largely unchanged. The resin and asphaltene content increased by 1.56% and 0.36%, respectively. Components ≤ C10 were depleted, and the content of components ≥ C20 increased. This indicates that CO2 extraction removed light components from the crude oil, leading to the deposition of heavy components in the remaining oil. After the addition of tributyl citrate, the saturated fraction content in the remaining oil increased to 81.43%, while the asphaltene and resin content decreased to 0.93% and 6.16%, respectively. This suggests that the addition of tributyl citrate transformed heavy components into light components, increasing the content of light components in the crude oil system. The addition of tributyl citrate also led to an increase in the content of C20 components and a decrease in the content of C30 components in the remaining oil, indicating that the tributyl citrate mainly transformed the C30 components.
4. Conclusions
The role of tributyl citrate is primarily manifested in two aspects: reducing interfacial tension and decreasing the content of heavy components in the dissolution system. Following the introduction of tributyl citrate, bubble point pressure, CO2 solubility, and expansion coefficient exhibit an increasing trend, while density and viscosity show a decrease. A detailed analysis of the changes in the residual oil components reveals that the addition of tributyl citrate stabilizes the tendency of heavy components to deposit in the original oil. Simultaneously, it facilitates the transformation of soluble heavy components into lighter ones, thereby further enhancing the efficiency of CO2 extraction.
While this study provides significant insights, it acknowledges certain limitations. The precise mechanisms by which tributyl citrate interacts with the CO2-crude oil system at the core scale remain to be fully elucidated, particularly in the microscale transformation of heavy components. Future research should, therefore, broaden the scope to examine the effects of tributyl citrate on the crude oil system under varied conditions, enhancing our overall understanding.
This research contributes valuable theoretical insights into heavy component deposition during CO2 flooding and the strategic selection of cosolvents. It also reveals the potential of tributyl citrate in boosting oil recovery, highlighting the need for further exploration of its wider impacts and applications in CO2 production enhancement.
Author Contributions
Methodology, J.L.; Formal analysis, F.X. and L.Y.; Data curation, R.Y.; Writing—original draft, F.X.; Writing—review & editing, M.Z.; Supervision, M.Z. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
Data is contained within the article.
Conflicts of Interest
Authors F.X., L.Y., J.L., R.Y. were employed by the company Petrochina Changqing Oilfield Company. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- Whorton, L.P.; Brownscombe, E.R.; Dyes, A.B. Method for Producing Oil by Means of Carbon Dioxide. U.S. Patent US2623596A, 30 December 1952. Available online: https://patents.google.com/patent/US2623596A/en (accessed on 8 December 2023).
- Warrlich, G.; Al-Waili, I.; Said, D.; Diri, M.; Al-Bulushi, N.; Strauss, J.; Al-Kindi, M.; Al-Hadhrami, F.; Heel, T.V.; Wunnik, J.V.; et al. PDOS EOR Screening Methodology for Heavy-Oil Fractured Carbonate Fields—A Case Study. In Proceedings of the SPE EOR Conference at Oil and Gas West Asia, Muscat, Oman, 16–18 April 2012. [Google Scholar] [CrossRef]
- Mohammadian, E.; Jan, B.M.; Azdarpour, A.; Hamidi, H.; Othman, N.H.B.; Dollah, A. CO2-EOR/Sequestration: Current Trends and Future Horizons. In Enhanced Oil Recovery Processes—New Technologies; IntechOpen: London, UK, 2019; Available online: https://www.intechopen.com/chapters/69763 (accessed on 8 December 2023).
- Zhang, N.; Wei, M.; Bai, B.J. Comprehensive Review of Worldwide CO2 Immiscible Flooding. In Proceedings of the SPE Improved Oil Recovery Conference, Tulsa, OK, USA, 14 April 2018. [Google Scholar] [CrossRef]
- Bruce, H.L.; Li, X.C.; Wei, N. CO2-EOR in China: A comparative review. Int. J. Greenh. Gas. Con. 2020, 103, 103173. [Google Scholar] [CrossRef]
- Cai, M.Y.; Su, Y.L.; Hao, Y.M.; Guo, Y.C.; Elsworth, D.; Li, L.; Li, D.S.; Li, X.Y. Monitoring oil displacement and CO2 trapping in low-permeability media using NMR: A comparison of miscible and immiscible flooding. Fuel 2021, 305, 121606. [Google Scholar] [CrossRef]
- Kumar, N.; Sampaio, M.A.; Ojha, K.; Hoteit, H.; Mandal, A. Fundamental aspects, mechanisms and emerging possibilities of CO2 miscible flooding in enhanced oil recovery: A review. Fuel 2022, 330, 125633. [Google Scholar] [CrossRef]
- Zhao, M.W.; Yan, X.W.; Wang, X.Y.; Yan, R.Q.; Dai, C.L. The development of a smart gel for CO2 mobility control in heterogeneity reservoir. Fuel 2023, 342, 127844. [Google Scholar] [CrossRef]
- Han, H.S.; Yuan, S.Y.; Li, S.; Liu, X.L.; Chen, X.L. Dissolving capacity and volume expansion of carbon dioxide in chain n-alkanes. Petrol. Explor. Dev. 2015, 42, 97–103. [Google Scholar] [CrossRef]
- Li, S.; Zhang, K.; Ma, D.S.; Qin, J.S.; Chen, X.L. Correlation of miscible ability between key components of formation crude and CO2. Reserv. Eval. Dev. 2013, 3, 30–33. Available online: https://10.3969/j.issn.2095-1426.2013.05.006 (accessed on 9 December 2023).
- Han, H.S.; Li, S.; Chen, X.L.; Qin, J.S.; Zeng, B.Q. Main control factors of carbon dioxide on swelling effect of crude hydrocarbon components. Acta Pet. Sin. 2016, 37, 392–398. Available online: http://www.syxb-cps.com.cn/EN/Y2016/V37/I3/392 (accessed on 9 December 2023).
- Nascimento, F.P.; Paredes, M.L.L.; Bernardes, A.P.D.; Pessoa, F.L.P. Phase behavior of CO2/toluene, CO2/n-decane and CO2/toluene/n-decane: Experimental measurements and thermodynamic modeling with SAFT-VR Mie equation of state. J. Supercrit. Fluids 2019, 154, 104634. [Google Scholar] [CrossRef]
- Cao, M.; Gu, Y.A. Temperature effects on the phase behaviour, mutual interactions and oil recovery of a light crude oil–CO2 system. Fluid Phase Equilibria 2013, 356, 78–89. [Google Scholar] [CrossRef]
- Zuo, M.S.; Chen, H.; Qi, X.Y.; Liu, X.Y.; Xu, C.H.; Yu, H.Z.; Brahim, M.S.; Wu, Y.; Liu, H.P. Effects of CO2 injection volume and formation of in-situ new phase on oil phase behavior during CO2 injection for enhanced oil recovery(EOR)in tight oil reservoirs. Chem. Eng. J. 2023, 452, 139454. [Google Scholar] [CrossRef]
- Al-Marzouqi, A.H.; Zekri, A.Z.; Jobe, B.; Dowaidar, A. Supercritical fluid extraction for the determination of optimum oil recovery conditions. J. Petrol. Sci. Eng. 2007, 55, 37–47. [Google Scholar] [CrossRef]
- Hawthorne, S.B.; Miller, D.J. Comparison of CO2 and Produced Gas Hydrocarbons to Dissolve and Mobilize Bakken Crude Oil at 10.3, 20.7, and 34.5 MPa and 110 °C. Energy Fuels 2020, 34, 10882–10893. [Google Scholar] [CrossRef]
- Yang, Y.; Li, X.L.; Guo, P.; Zhuo, Y.Y.; Sha, Y. Improving oil recovery in the CO2 flooding process by utilizing nonpolar chemical modifiers. Chin. J. Chem. Eng. 2016, 24, 646–650. [Google Scholar] [CrossRef]
- Wu, Y.N.; Liu, Q.X.; Liu, D.Y.; Cao, X.P.; Yuan, B.; Zhao, M.W. CO2 responsive expansion hydrogels with programmable swelling for in-depth CO2 conformance control in porous media. Fuel 2023, 332, 126047. [Google Scholar] [CrossRef]
- Zhao, M.W.; Li, Y.; Wang, T.; Gao, M.W.; Zhang, B.H.; Song, X.G.; Wang, X.; Guan, B.S.; Liu, P.; Dai, C.L. The dissolution characteristic of nonionic surfactants in supercritical CO2. J. Mol. Liq. 2020, 305, 112846. [Google Scholar] [CrossRef]
- Yan, X.W.; Zhao, M.W.; Yan, R.Q.; Wang, X.Y.; Dai, C.L. Statistical analysis of gelation mechanism of high-temperature CO2-responsive smart gel system. J. Mol. Liq. 2023, 377, 121521. [Google Scholar] [CrossRef]
- Li, L.; Zhou, X.M.; Wang, R.J.; Zhang, X.; Ma, S.T.; Su, Y.L.; Wang, C.L.; Luo, W.T.; Sun, H.T. Microscopic experiment study on mechanisms of oil-gas interaction and CO2-surfactant flooding with different temperatures and pressures. J. CO2 Util. 2023, 69, 102389. [Google Scholar] [CrossRef]
- Bon, J.; Sarma, H.K.; Theophilos, A.M. An Investigation of Minimum Miscibility Pressure for CO2-Rich Injection Gases with Pentanes-Plus Fraction. In Proceedings of the SPE International Improved Oil Recovery Conference, Kuala Lumpur, Malaysia, 5–6 December 2005. [Google Scholar] [CrossRef]
- Almobarak, M.; Wu, Z.Y.; Myers, M.B.; Wood, C.D.; Al-Maskari, N.S.; Liu, Y.B.; Rommerskirchen, R.; Saeedi, A.; Xie, Q. Chemical-assisted minimum miscibility pressure reduction between oil and methane. J. Petrol. Sci. Eng. 2021, 196, 108094. [Google Scholar] [CrossRef]
- Li, H.Z.; Zheng, S.X.; Yang, D.Y. Enhanced swelling effect and viscosity reducti-on of solvent(s)/CO2/heavy-oil systems. SPE. J. 2013, 18, 695–707. [Google Scholar] [CrossRef]
- Saira, H.Y.; Furqan, L.H. Effect of alcohol-treated CO2 on interfacial tension between CO2 and oil, and oil swelling. Adv. Geo-Energy Res. 2021, 5, 407–421. [Google Scholar] [CrossRef]
- Shang, Q.Y.; Xia, S.Q.; Cui, G.W.; Tang, B.; Ma, P.S. Experiment and correlation of the equilibrium interfacial tension for paraffin+CO2 modified with ethanol. J. Chem. Thermodyn. 2018, 116, 206–212. [Google Scholar] [CrossRef]
- Djabbarah, N.F. Tall Oil as Additive in Gas Drive Hydrocarbon Oil Recovery. U.S. Patent US4736793, 12 January 1988. Available online: https://patents.google.com/patent/US4736793/en (accessed on 9 December 2023).
- Lv, W.; Gong, H.J.; Li, Y.J.; Li, Z.J.; Dong, M.Z. The potential and mechanism of nonionic polyether surfactants dissolved in CO2 to improve the miscibility of CO2–hydrocarbon systems. Fuel 2022, 326, 125012. [Google Scholar] [CrossRef]
- Lv, W.; Dong, M.Z.; Sarma, H.; Li, Y.J.; Li, Z.J.; Sun, J.T.; Gong, H.J. Effects of CO2-philic nonionic polyether surfactants on miscibility behaviors of CO2–hydrocarbon systems: Experimental and simulation approach. Chem. Eng. J. 2023, 464, 142701. [Google Scholar] [CrossRef]
- Fan, G.G.; Zhao, Y.J.; Li, Y.L.; Zhang, X.D.; Chen, H. Research for reducing the Minimum Miscible Pressure of crude oil and carbon dioxide by injecting citric acid isobutyl ester. Oil Gas Sci. Technol.–Rev. D’ifp Energ. Nouv. 2021, 76, 30. [Google Scholar] [CrossRef]
- Kong, S.Q.; Feng, G.; Liu, Y.L.; Li, K.J.; Li, K.J. Potential of dimethyl ether as an additive in CO2 for shale oil recovery. Fuel 2021, 296, 120643. [Google Scholar] [CrossRef]
- Wang, T.F.; Wang, L.L.; Meng, X.B.; Chen, Y.; Song, W.; Yuan, C.D. Key parameters and dominant EOR mechanism of CO2 miscible flooding applied in low-permeability oil reservoirs. Geoenergy Sci. Eng. 2023, 225, 211724. [Google Scholar] [CrossRef]
- Almobarak, M.; Wu, Z.Y.; Zhou, D.Y.; Fan, K.; Liu, Y.B.; Xie, Q. A review of chemical-assisted minimum miscibility pressure reduction in CO2 injection for enhanced oil recovery. Petroleum 2021, 7, 245–253. [Google Scholar] [CrossRef]
- Luo, H.; Zhang, Y.C.; Fan, W.Y.; Nan, G.Z.; Li, Z.M. Effects of the Non-ionic Surfactant (CiPOj) on the Interfacial Tension Behavior between CO2 and Crude Oil. Energy Fuels 2018, 32, 6708–6712. [Google Scholar] [CrossRef]
- Zhang, C.; Xi, L.H.; Wu, P.K.; Li, Z.M. A novel system for reducing CO2-crude oil minimum miscibility pressure with CO2-soluble surfactants. Fuel 2020, 281, 118690. [Google Scholar] [CrossRef]
- Al-Azani, K.H.; Abu-Khamsin, S.A.; Sultan, A.S. Solubilities of Carbon Dioxide in Ethyl Benzoate and Triethyl Citrate at High Temperatures and Pressures. J. Chem. Eng. Data 2020, 65, 1857–1868. [Google Scholar] [CrossRef]
- Al-Azani, K.H.; Abu-Khamsin, S.A.; Sultan, A.S. Experimental Study of Blending CO2 with Triethyl Citrate for Mitigating Gravity Override During Reservoir Flooding. Arab. J. Sci. Eng. 2021, 46, 6787–6796. [Google Scholar] [CrossRef]
- SY/T 5542-2009; Test Method for Reservoir Fluid Physical Properties. Standardization Administration of China: Beijing, China, 2009.
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).