Abstract
As a result of the depletion of fossil resources, ongoing population growth, and the industrialized economy, energy demand has been rising quickly throughout the world. India is now the world’s third-largest oil consumer, surpassing Japan and Russia. Today, biofuel research is conducted worldwide because surrounding two essential characteristics: sustainability and renewability. Biofuels have gained considerable significance as a result of dwindling oil sources, worries about energy security, and the escalating environmental issues associated with climate change and greenhouse gas emissions. In most cases, biofuels are produced by subjecting materials that have been densified to the process of heat conversion. In the disciplines of research and development, alternative energy development is a top focus. Due to the depletion of fossil fuel resources, it has become important to find innovative replacements for fossil fuels, such as biofuels, to generate heat and power. Biofuels may be generated using several methodologies, encompassing biological, chemical, and physical approaches. The three steps of densification systems’ pre-, during-, and post-pelletization procedures convert biomass into pellets. Several agricultural wastes, such as grain dust, crop leftovers, and fruit tree residues, are available as sources of agricultural energy. Bioenergy from biomass, such as leftovers and energy crops, can be used to produce contemporary energy carriers. This article focuses on an overview of sustainable and renewable biofuel resources and their commercialization.
1. Introduction
Among the most crucial resources in the world are fossil fuels. Fossil fuels comprise 80% of the fuels used for energy, with the transport sector consuming approximately 58% [1,2,3]. Due to the excessive use of fossil fuels, it has been discovered that the sources of oil reserves and fossil fuels play a significant role in the harmful gas emissions and air pollution leading to climate change, biodiversity loss caused by globalization, glacier melting, and rising sea levels, among other factors.
The demand for fossil fuel is too high, causing fast depletion of resources and affecting the economy, ultimately increasing the price of oil [4]. Biofuels could play an essential role in reducing carbon emissions. Biofuels have various advantages over petroleum, as follows:
- They can be extracted from biomass, producing biofuels from organic materials such as plants, agricultural residues, or other biological sources.
- They are biodegradable: Biodegradable fuels have the ability to spontaneously decompose in the environment over a period of time. They undergo decomposition and reintegrate into the natural cycle without producing persistent damage or contamination, rendering them a more ecologically sound substitute for some traditional fossil fuels that do not readily undergo biodegradation.
- Biofuels, generated from renewable biological sources such as plants and algae, can be manufactured and utilized in an ecologically and socially sustainable manner. They combust based on the carbon dioxide cycle.
- Environmentally friendly: The environmental friendliness of biofuels can vary depending on factors such as feedstock choice, land-use practices, and production methods. Some biofuels, particularly those produced from food crops, have been associated with negative environmental impacts, such as deforestation, habitat loss, and competition with food production.
The agricultural industry, which accounts for 12% of all global GHG emissions, is one of the top three CO2 emitters [5,6]. Studies have shown that both the energy business and the agricultural sector offer prospects for lowering GHG emissions [7]. Finding ways to reduce N2O emissions is essential for balancing the global warming potential (GWP) of agricultural production systems because 61% of the world’s N2O emissions originate from agriculture [8]. The only way to keep climate change within manageable bounds is with the help of the agricultural sector [9,10]. The industrial production of bioethanol will produce several byproducts, some of which may still be a good source of organic C and other nutritive elements. Bioethanol byproducts can be added to soil to increase the soil’s N mineralization and N delivery to crops to partially replace N fertilizer [11,12]. The requirement of minimizing nitrogen fertilizer use in crop production is highlighted by the role of nitrogen fertilizer in lowering overall energy use and carbon emissions. Therefore, it is projected that using maize straw to make bioethanol will lower GHG emissions in both the agriculture and energy sectors. Bioethanol, derived from several crop grains and non-crop plants, is renowned for its generally carbon-neutral nature. Consequently, it has gained global recognition as a viable substitute for gasoline. China is presently ranked as the world’s third largest producer of bioethanol, following the United States and Brazil. Its annual consumption of bioethanol amounts to 2.6 million tons, which represents around 4.0% of the global consumption [13]. The production of bioethanol has challenges in terms of food security and limited availability of land suitable for cultivating non-food plants [14]. The main obstacle for bioethanol production is in ensuring a sufficient supply of raw materials. Corn stover (CS), a highly productive yet underused material, is a promising source for bioethanol production. At the federal and provincial levels, there has not been much analysis of the potential for bioethanol derived from maize straw and its effects on GHG emissions in the energy and agriculture sectors.
In the recent past, corn straw has been burned directly in the field or has been inefficiently used for heating and cooking in rural areas. To reduce air pollution caused by burning, the Chinese government has pushed the recycling of maize straw, including its usage as fertilizer, feed, industrial material, biofuel, and base material. More than half of them were able to improve crop production [15,16] and soil organic carbon (SOC) sequestration [17] by returning maize straw to the field directly as fertilizer. This was the case for more than half of them. Due to the high C/N ratio that it possesses, corn straw has the potential to be immediately returned to the soil, where it can then boost greenhouse gas emissions and the soil’s capacity to mineralize carbon [18,19]. Therefore, it is best to refrain from using corn straw directly on fields and instead put it to use in the manufacture of biofuel. In 2019, the amount of CS feedstock that may possibly be used in the manufacturing of bioethanol reached 20.7 Mt, which represented 48.6% of the total national CS yield. In light of this, the output of bioethanol derived from CS might reach 40.3 million metric tons in 2019, which would be sufficient to meet 4.4% of China’s overall demand for petroleum. Therefore, both the use of corn straw and the amount of direct return must be considered in order to reduce GHG emissions in agricultural systems. By expanding production and product, this will aid in the expansion of the agricultural sector [20,21]. Biofuels may be produced in solid, liquid, or gaseous forms. Due to its manufacture from a variety of crop grains and non-crop plants and its reputation for being comparatively carbon neutral, bioethanol has gained popularity as a gasoline substitute all over the world [22].
After the United States and Brazil, China is currently the third largest producer of bioethanol worldwide; its 2.6 million tons of annual bioethanol consumption represents roughly 4.0% of global consumption [13]. By properly exploiting renewable resources, we can lower GHG emissions while also boosting the rural economy [23,24]. Biomass refineries, can create the necessary energy, fuels, and chemicals while having a lower environmental impact [25,26]. Biomass, or stored energy and CO2 from the atmosphere, is used by plants. Cyanobacteria, which are photosynthetic nonpathogenic, can convert CO2 directly into ethylene, a fuel molecule. GM cyanobacteria have also been produced for mass ethylene production [27].
2. Biomass
Biomass pertains to organic matter obtained from live or deceased organisms, which serves as raw material in the manufacturing of biofuels. The majority of biomass used now comes from three sources: forestry, agriculture, and waste. This comprises conventional tree cutting into natural wood, sawmill wood deposits, and some timber handling industries, as well as rural vitality crops, rural build-ups, and garbage. Because bioenergy is a natural and economical source of energy storage, energy stored in it can be implemented at any moment [14,28,29]. Biomass, constituting 55% of renewable energy and over 6% of the global energy supply, ranks as the fourth most significant renewable energy source worldwide [30], following coal, oil, and natural gases [3] Biomass is produced by using photosynthetic vegetable matter. Microbes, agricultural produce, and lignocellulosic crops are all capable of producing energy for various forms of transportation fuels [31,32]. India has access to almost 5 quadrillion British Thermal Units (BTU), equating to 500 million metric tons of biomass, which represents 5% of all biomasses consumed in the United States in 2021 [2]. Biodiesel is an important component of renewable fuel. In the United States, there are 72 units for the manufacture of FAME (fatty acid methyl ester) biodiesel [33].
2.1. Biomass Sources for Energy
- ❖
- Wood and waste products from wood processing, such as wood chips and pellets, firewood made from timber, sawdust, and trash from pulp and paper factories.
- ❖
- Sugarcane, corn, soybeans, woody plants, crop residues, switchgrass, and algae are examples of agricultural crops and waste materials.
- ❖
- Municipal solid trash contains biogenic elements like cotton, paper, food, yard waste, and wool products.
2.2. Biofuel Productions and Its Uses
‘Bio’ refers to something living, while ‘fuel’ refers to something that releases energy. Together, these two terms make up the term “biofuel”. Biofuels are being marketed as a low-carbon substitute for fossil fuels since they may help to reduce GHG emissions and the resulting climate change impact from transportation [34]. Biofuels can be distinguished by their properties, which include the type of feedstock utilized, the conversion method, the technical specifications of the fuel, and the intended application. The terms “first” (made from edible crops and starch), “second” (made from no edible plant materials), “third (made from algae), “fourth(made form microorganisms)”, and “conventional and advanced” biofuels are frequently used. The manufacturing of biofuels varies depending on the natural resources used, production levels, production quantities, environmental circumstances, and consumer needs. Brans, residues of blubber animals, grass, and framing residue are all examples of naturally generated materials.
3. Classification of Biofuel on Generation Basis
3.1. First-Generation Fuel
Energy sources derived from starch, animal oil, and vegetable oils are included in this group of fuels. Corn and sugarcane are the biggest producers of biofuels. These crops were used in biorefineries in the United States and Brazil to manufacture biofuel or bioethanol [35]. One of the most significant crops for food for humans and animals is maize, which is planted for both grain and silage [36,37]. This could result in scarcity of food and debates on whether to choose gasoline over food [38]. Conventional or initial-generation biofuels take several forms that depend on the techniques used, such as bio alcohols, biodiesel, green diesels, and solid biofuels, which are suitable for usage as gasoline or in the generation of electricity. Figure 1 shows the process of biodiesel and bioethanol production from crops step by step. Bioethanol is fermented made from plant sugar (wheat, sugarcane, soybean, sugarbeet, corn, potato) and biodiesel produced from vegetable oil (soybean, mustard, sunflower, jatropha, coconut).
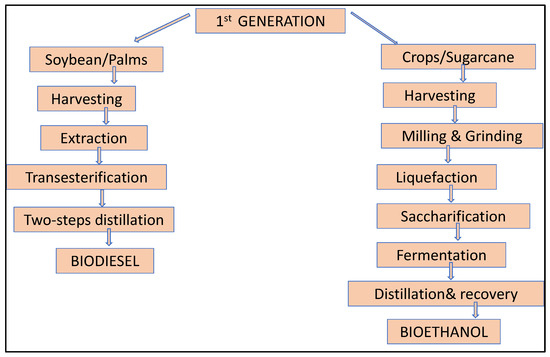
Figure 1.
Graphical representation of first-generation biofuel production.
3.2. Second-Generation Fuel
Carbohydrates can be derived from agricultural and non-edible plant parts [35]: hemicellulose, lignin, and cellulose (a non-digestible constituent of insoluble dietary fiber) are examples of chemical components. They have a lower density than cereal grains. Certain chemical methods were required to extract the lignin from the cellulose. Physical densification procedures such as cutting, milling, grinding, and pelleting are used to improve the denseness of biomass and reduce size [39]. Biofuels of the second generation are derived from a range of bushes, trees, and grasses. Consequently, the group of biomasses that does not encompass edible substances holds paramount significance. Several biofuels are available, including bio-SNG, ethanol, cellulose, and alcohol; these are used in the chemical industry and are designed for CI engines (Figure 2). Figure 3 shows the products of lignin.
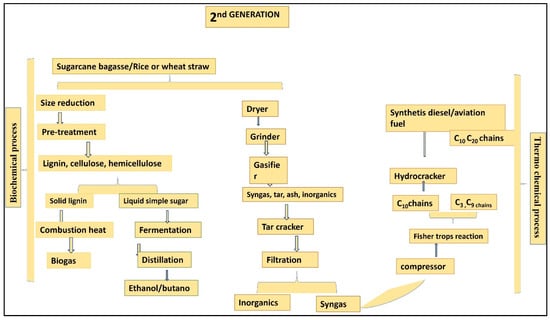
Figure 2.
Graphical representation of second-generation biofuel production.
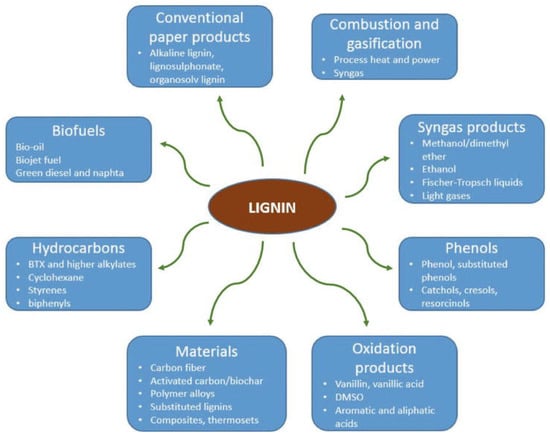
Figure 3.
Scheme of conventional and potential products from lignin.
3.3. Third-Generation Fuel
The word “algal fuel” primarily refers to algae-derived oil. Because of its high energy content, this oil can be used as a fuel substitute [40,41,42]. Microalgal oil and fuel are tens to hundreds of times more efficient than other crop kinds [41,43]. Microalgae [44], are a good source of food, oil, lipids, and fuel. Biodiesel, bioethanol, biohydrogen, and biogas can all be produced by algae [45]. Many approaches, including biophytolysis, dark fermentation, and photo fermentation, involving methanogenesis, acidogenesis, acetogenesis, and hydrolysis, are utilized to produce gasoline. This type of biofuel is mostly used for house heating and vehicular use (Figure 4). Algal species have a high concentration of carbohydrates, lipids, proteins, and other elements (including ash content) when measured on a dry weight basis. In order to produce several forms of biofuels, such as bio-oil and biodiesel, alternative molecules obtained through the adsorption method can be used and included. This process of biofuel manufacturing is widely accepted and commonly used [46].
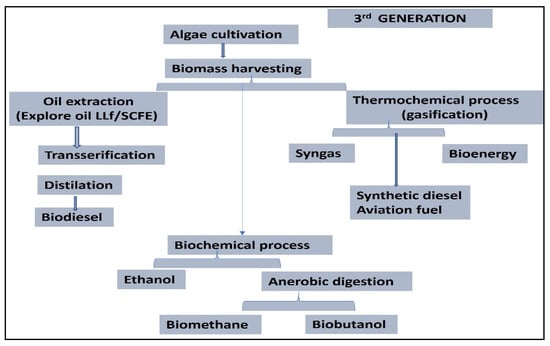
Figure 4.
Graphical representation of third-generation biofuel production.
3.4. Fourth-Generation Biofuels
The utilization of genetically modified organisms in biohydrogen production processes is a characteristic feature of fourth-generation feedstock [47]. The utilization of genetically modified biomass derived from second and third-generation sources falls within the category of fourth-generation feedstock (Figure 5). Genetic and metabolic alterations in microorganisms with the ability to produce biofuels streamline the processes required in harnessing and converting solar energy into the corresponding biofuel. Additionally, these modifications enable the extraction of carbon dioxide, mitigating emissions released into the environment [48,49,50]. Synthetic biology technologies for solar biofuels that absorb carbon are being developed. Cyanobacteria have rapid growth, CO2 fixation, and hereditary tractability. These prokaryotes are members of the bacterial kingdom and have membrane-entrapped structures. Biofuels are utilized in mobility as IC engine gasoline. The raw materials for fourth-generation biofuels will be almost infinite, cheap, and easily available. Solar-powered photosynthetic water splitting (water oxidation), ref. [51] has the potential to significantly increase the quantities of biofuels generated worldwide.
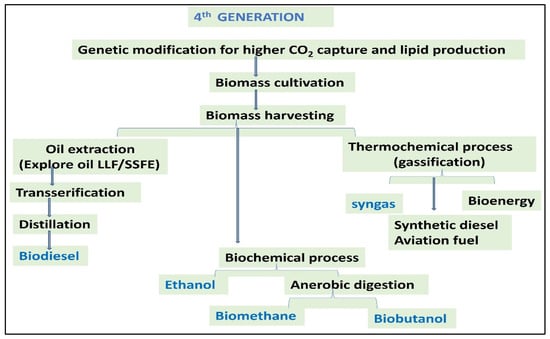
Figure 5.
Graphical representation of fourth-generation biofuel production.
How Fourth-Generation Biofuel Production Works
They no longer add C (C-neutral) to the environment. This method, however, eliminates C from the C-cycle.
- Algae, as an example, are genetically engineered to absorb enormous quantities of carbon; they are cultivated and extracted for bioenergy.
- The agricultural products are then converted into gasoline using second-generation technologies.
- Carbon is captured during the pre-combustion stage of gasoline production.
- The atmospheric carbon becomes geo-sequestered, which means it is stored in non-mineable coal reserves or exhausted natural gas and oil reservoirs. It will remain buried and protected for tens of thousands of decades.
Cyanobacteria are used to colonize a water-filled flat screen. The walls, like solar energy systems, are oriented with their faces toward the sun. Then, using CO2 and sunshine energy, cyanobacteria make a material that can be transformed into fuel [52].
4. Bioresources
Laboratory animals, plants, cells, genes, and microorganisms are all examples of “bioresources” that are used in research. The words “resource” and “biology” have the same meaning. They are also known as “biological resources” in other contexts. Many organisms share the same basic biological processes. Primary bioresources are created in forestry, agriculture, or aquaculture for a specific application-oriented function that facilitates the generation of food, significant commodities, or, finally, energy. The use of primary bioresources for energy generation is disputed due to its productivity constraints.
To begin, we need to understand the various products utilized in biofuel manufacturing. Terrestrial and marine resources are the two main groups.
4.1. Terrestrial Resources
Researchers in the USA have used vegetable oil for biodiesel, particularly soybean oil, which accounts for 57% of the total bioresources used. Others used are corn oil (14%), recycled waste (11%), canola oil (10%), and animal fat (8%) [53]. Linseed oil, jatropha oil, castor oil (Ricinus communis), and oilseeds of trees like neem (Azadirachta indica), stillingia oil (Chinese tallow seed oil), Karanja (Pongamia pinnata), rubber seed oil, and Silybummarianum oil are commonly used in other countries like India [54] as blending materials for non-renewable resources like diesel. Biomass materials from a variety of sources and types, including garbage and feedstocks such broiler chicken waste, mutton fat, algal oil, microbial oils, discardable cooking oil, pine and kapok oil, microalgae, and waste fish oil, can be converted into bioenergy.
4.1.1. Jatropha
The physical and chemical properties of jatropha oil may vary depending on the temperature, the genetic composition, and the maturity of the seeds. This may make it more challenging to utilize as a biodiesel feedstock since process adjustments may be required to account for the property changes [55].
Because jatropha is a wild plant that is commonly collected by low-income farmers in developing countries, the oil’s characteristics are expected to be variable. Oil from overripe fruit seeds, for example, or seeds maintained at high humidity levels, will contain a high amount of free fatty acids. Jatropha oil is ideal for biodiesel production due to its high cetane grade and low sulfur content. The oil content ranges from 18.4 to 42.3%. The majority of oil is found in the seed kernels, which have an oil content of 50–55%. Figure 6A,B shows the part of jatropha and partitions of seed oil.
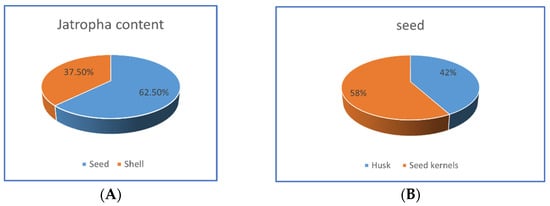
Figure 6.
Jatropha parts (A) and seed oil partitions (B).
Non-edible: Jatropha oil and seeds are poisonous. The seeds are regarded to be the most dangerous component of the plant, despite the fact that the entire plant contains toxins. A single seed can cause diarrhea, nausea, and stomach discomfort if consumed by people.
(i) Reduction in the fatty acid contained in the jatropha oil: According to the results of test carried out on jatropha oil, the oil has a high (21.6%) free fatty acid (FFA) content. Therefore, it had to be decreased.
Procedure: Jatropha oil was cooked to a temperature of 60 °C in a conical flask using its crude form. After being heated separately at (50 °C), concentrated H2SO4 (1% w/w) and methanol (30% v/v) were combined and added to the heated oil in the flask. After being agitated for an hour, the mixture was left to settle for another two hours [56].
(ii) Transesterification: The following is a step-by-step breakdown of the biodiesel production process. A measure of 10.5 mL of jatropha oil was metered into a 250 mL conical flask and heated to 50 °C. Methanol was poured into a soxhlet device, and the heater was turned on. The flask’s bottom was circular. This method purified the methanol that was present. The sodium hydroxide pellet was precisely weighed using a balance to give a reading of 0.25 g. A potassium methoxide solution was made in a 250 mL beaker with a 0.25 g (or a catalyst concentration of 0.5%) sodium hydroxide pellet and 63 mL (or a mole ratio of oil to methanol of 1:6) of methanol. After the solution had been vigorously agitated, the potassium hydroxide pellet was totally dissolved. The potassium methoxide solution was heated in an oven to 60 °C. The heated jatropha oil was then added to the potassium methoxide solution, which was vigorously stirred with a magnetic stirrer for 50 min. The mixture was then left 24 h to settle in a separating funnel. The lower layer of glycerol and soap was collected from the bottom of the separating funnel, while the biodiesel was put into a separate beaker. The biodiesel was then rinsed with warm water to remove any remaining glycerol and soap from the funnel. This was repeated until the biodiesel in the separating funnel was visible beneath the clear water. Following rinsing, the sample was dried on a hot plate, and any leftover water in the biodiesel was drained. The collected biodiesel volume was measured and recorded (Figure 7). The aforementioned activities were carried out with varying mole ratios of jatropha and methanol while keeping constant catalyst concentration, stirring duration, and temperature [56].
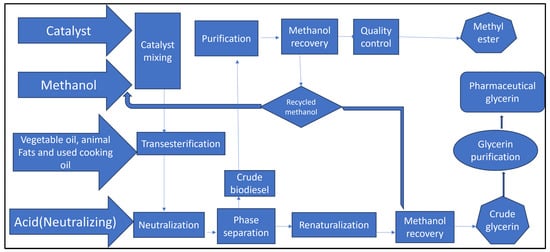
Figure 7.
Production of biodiesel from jatropha.
4.1.2. Agricultural Byproducts
Biofuel may be made from a variety of crops. It is estimated that more than 400 billion liters of bioethanol might be generated yearly from agricultural waste. Straw-powered automobiles may become obsolete as a result of current study. A recent study discovered five yeast strains capable of converting agricultural wastes such as straw, sawdust, and maize cobs into bioethanol, a well-known alcohol-based biofuel. Straw and other wastes may now be transformed into ethanol using time-consuming and inefficient procedures (University of East Anglia).
Ethanol is the most common biofuel produced in the United States. Ethanol and other alcohols are created by fermenting and distilling simple carbohydrates. As a result, any biological feedstock containing significant amounts of sugar or components that may be converted into sugar, such as starch or cellulose, can be utilized to produce ethanol. Sugar cane and sugar beets are examples of sugar-containing feedstocks. Corn contains starch, which may be easily converted to sugar. Cellulose, which comprises a significant component of trees, grass, and the bulk of agricultural and municipal wastes, may also be converted to sugar, although with greater difficulty than starch. The value of a biofuel is determined by its application. Although ethanol is primarily used as a gasoline alternative, it also has numerous other properties that make it valuable as a gasoline additive, such as the capacity to oxygenate and enhance octane.
4.1.3. Energy Plants
Renewable energy crops such as maize and sugarcane have been identified as important sources of biomass-based feedstocks for biofuel production. This South American country has been planting energy crops since the Middle Eastern oil embargo began in the 1970s. The names of some crops used for biofuel production are shown in Table 1 and their biofuel production capacities are presented in Table 2.

Table 1.
Crops used for biofuel production.

Table 2.
Biofuel yields using different energy crops as feedstock.
Corn: Corn is widely recognized as the dominant feedstock for ethanol-based biofuels. In a manner akin to the brewing process, the conversion of sugar-rich grain into ethanol takes place. The kernels undergo a crushing process, after which they are subsequently mixed with yeast and warm water. Ethanol production involves the fermentation of the mixture by yeast. Subsequently, ethanol is blended with gasoline to facilitate its utilization in pre-existing internal combustion engines.
Rapeseed: Throughout antiquity, the utilization of its oil has been employed for illuminating purposes and culinary applications. Currently, it functions as a notable provider of biodiesel. Canola is considered the most notable variety due to its comparatively reduced erucic acid level in comparison to other rapeseed varieties. This characteristic renders canola a healthier option for consumption by both people and animals.
Sugarcane: The Brazilian government incentivized farmers to cultivate sugarcane in response to a significant increase in oil costs. Similar to corn, sugarcane is utilized in the production of bioethanol. Brazil has made significant expenditures in this particular area, thereby achieving a competitive pricing level comparable to that of gasoline. It is noteworthy that, although the prevailing proportion of automobiles in Brazil presently employ flexible fuel engines, the predominant adoption of such engines occurred throughout the 1980s. The production cost of ethanol derived from sugarcane is six times lower compared to that of ethanol derived from corn. Nevertheless, following the sugarcane harvest, farmers engage in the practice of field burning, resulting in a notable escalation in the emission of greenhouse gases into the atmosphere. This practice, albeit to a limited extent, counteracts the carbon advantages associated with the utilization of bioethanol.
Palm oil: Palm oil, obtained from the fruit of palm trees, is recognized as a very energy-efficient biodiesel fuel within the current market. There is no requirement to alter diesel engines in order to enable their operation using palm oil. Biodiesel derived from palm oil has reduced noxiousness compared to conventional gasoline. Palm oil has played a significant role in facilitating the economic development of Malaysia and Indonesia. Nevertheless, the practice of cultivating palm trees for biodiesel in these countries has led to the daily incineration of several hectares of rainforest to make way for agricultural land. A multitude of plant and animal species face imminent peril, posing a significant threat to the fragile ecological balance.
Soybean: Soybeans possess the potential to serve as a viable fuel alternative, alongside their use in the production of tofu, sauce, crayons, and shampoos. It has been shown that soybeans are predominantly utilized in the production of biodiesel inside the United States. Soybean biodiesel, either in its pure form or when blended with conventional diesel fuels, has the potential to serve as a viable energy source for automobiles, trucks, and buses. Based on the findings of the National Academy of Sciences, it has been shown that soy diesel exhibits a higher energy output in comparison to maize ethanol. The quantity of biodiesel generated from one bushel of soybeans is around 5.68 L, which is equivalent to approximately 1.5 gallons. The oil content of alternative fuel sources, such as canola and sunflower seeds, exhibits a twofold increase compared to soybeans, with percentages of approximately 40% and 43%, respectively. In contrast, soybeans possess an oil content of approximately 20%.
Switchgrass: The most promising opportunity for reducing our reliance on fossil fuels lies with this particular plant. In comparison to corn, switchgrass possesses a cellulose variant that necessitates a lower amount of energy for the conversion to ethanol, as opposed to the processing of fossil fuels.
In contrast to maize ethanol, switchgrass cellulose ethanol has higher energy content. Despite the limited cultivation of this crop, researchers are now devising methodologies to harness its potential in forthcoming applications.
4.2. Marine Resources
Algae can be classified into two distinct categories: macroalgae, encompassing green, red, and brown algae, and microalgae, comprising blue-green algae, bacillariophyta, and dinoflagellates. Algae constitutes approximately 90% of the total population of marine plants.
4.2.1. Seaweed or Macroalgae
Seaweeds, also known as macroalgae, are utilized in the generation of sustainable energy due to their significant biomass, characterized by a high cellulose content and a low lignin content [2]. Algae are employed in the anaerobic digestion process for the purpose of methane production. Seaweed exhibits a substantial biomass, a notable growth rate, and minimal reliance on fresh water, land, fertilizer, or pesticides. The production of macroalgae does not necessitate deforestation or land clearance.
4.2.2. Microalgae
Microalgae possess the capacity to yield an energy output exceeding 9000 alt/ha/year and exhibit a lipid content ranging from 8 to 30% on a dry weight basis [64].
4.2.3. Cyanobacteria
Cyanobacteria are known to exert a substantial influence on the atmospheric carbon cycle due to their involvement in photosynthesis and their ability to inhabit freshwater and marine environments. The pyrolysis method can be utilized to extract oil from bio-resources [65]. These possess the capability to produce biofuel directly from CO2 or carbohydrate sources.
Different steps involved in biofuel production from microbial biomass shown in Figure 8.
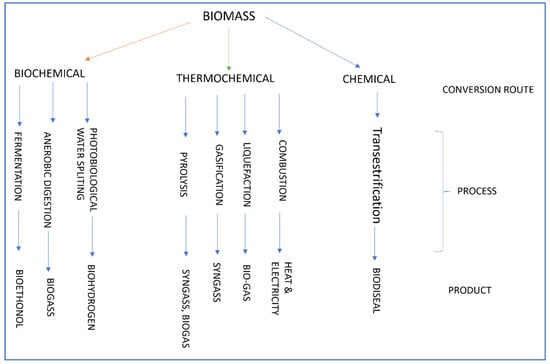
Figure 8.
Different methods for microbial biomass to energy production.
5. Further Classification of All Biofuels into Two Categories
5.1. Conventional Fuel
Conventional biofuels refer to those that are produced by proven technological processes such as fermentation, distillation, and transesterification. fuels belonging to the first generation are commonly classified within this category [34].
5.2. Advanced Fuel
The methodologies or pathways employed to produce biofuels are currently undergoing investigation and development, with ongoing efforts focused on research, pilot testing, and demonstration phases. The fuels can be classified into the second-, third-, and fourth-generation categories.
To address the increasing need for agricultural commodities, there exists a potential peril of additional deforestation and land exploitation, particularly in areas of significant biodiversity. This may also include the utilization of freshwater, fertilizers, and pesticides, which could potentially exert adverse effects on the surrounding ecosystem. The economic viability of certain biofuels remains a subject of debate within the context of the prevailing economic conditions, partially attributed to the relatively low cost of oil [47,66,67]. The manufacture of biofuels from microalgae necessitates a substantial amount of energy and currently lacks profitability [35]. The phrase “advanced biofuel” encompasses ethanol derived from cellulose, hemicellulose, lignin, sugar, starch (excluding corn), and other forms of waste biomass. These advanced biofuels have lifecycle greenhouse gas emissions that are a minimum of 50% lower than those associated with gasoline [68].
5.3. Alternative Biofuels
Developed and developing nations are actively pursuing alternative fuel sources as a viable long-term solution to the overuse of fossil fuels. The utilization of sustainable fuels has become imperative for the development of future services. Various alternative fuels, including emulsified or homogenized liquid fuel, gas turbine heavy fuels, slurry, and powdered coal, have the potential to replace conventional energy sources.
6. Products of Biofuel Process
6.1. Biodiesel
Biodiesel is produced through the conversion of renewable lipid sources, including animal fats, lignocellulosic biomass, and non-edible plants, into mono alkyl esters. Various types of crops, such as corn, sugarcane, vegetable oil, and wheat, as well as energy or non-edible crops such waste oils and lignocellulosic feedstock, are utilized in the production of biodiesel. The sustainability of biodiesel production relies on utilizing feedstocks that do not exert any adverse effects on the agri-food system [69]. The process of transesterification has the capability to generate biodiesel, which serves as a viable alternative to petroleum diesel and is derived from both vegetable and animal fats. Biodiesel is commonly blended with conventional oil or diesel in order to enhance fuel intake and facilitate cold starting, owing to its elevated density and viscosity. The utilization of machinery resulted in a reduction of approximately 78% in gas emissions arising from the amalgamation of biodiesel and conventional oil. The reduction in gaseous emissions is impacted by both the quality of the fuel and the blending ratio. Biodiesel exhibits superiority over diesel fuel in relation to sulfur concentration, flash point, aromatic content, lubricity, and biodegradability [70]. The substitution of petroleum diesel with biodiesel derived from virgin vegetable oil results in a reduction in carbon dioxide emissions and a decrease in petroleum use [71]. Biodiesel is composed of oxygen, which serves to augment the process of combustion and thus reduce the emission levels of hydrocarbons, carbon monoxide, and particulate matter. Oxygenated fuels, on the other hand, exhibit a propensity to elevate nitrogen oxide emissions [72,73,74,75].
6.2. Biobutanol
Biobutanol is a renewable biofuel that has gained significant attention in recent years. Biobutanol is frequently utilized as a substitute for conventional fuel due to its reduced volatility, heightened energy content, and diminished absorptive properties [76]. Gasoline can be utilized as a substitute without any modifications [77]. The production costs associated with lignocellulosic biomass are significant; nevertheless, its utilization has the potential to reduce these costs. Through a series of fermentation procedures, the waste deposit can be effectively converted into a valuable biofuel known as biobutanol. The anaerobic process of fermentation facilitates the conversion of acetone, butanol, and ethanol into biobutanol [78].
6.3. Bio Ethanol
Bioethanol, often known as ethanol, is a renewable fuel derived from biomass sources such as corn, sugarcane, and other plant materials. It is produced by alcohols, such as ethanol; these are synthesized through the process of fermenting and distilling simple carbohydrates. Bioethanol is a type of fuel derived from feedstock, that is commonly sourced from various plant materials such as wheat, sugar beet, corn, straw, and wood (Figure 9). The conversion of cellulose, a type of carbohydrate found in biomass, into bioethanol can be achieved by employing hydrolysis, fermentation, and distillation techniques [73]. Gasoline and bioethanol possess the capability to be either conjoined or employed in isolation. There was a significant reduction in the production cost of bioethanol derived from agricultural waste [79]. Due to the suboptimal vaporization characteristics of bioethanol under cold conditions, it is necessary to blend it with a small quantity of gasoline in order to mitigate the risk of engine stalling in mobile vehicles. As per the European Union (EU) quality standard EN(European norms) 228 Fdem [71], it is permissible to utilize bioethanol as a blend of up to 5% with conventional gasoline. The aforementioned blend is encompassed within the scope of car warranties and does not necessitate any alterations to the engine.
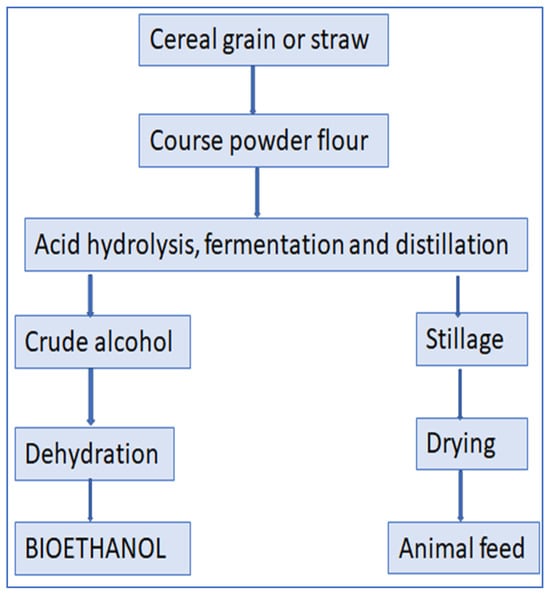
Figure 9.
Flow chart for the production bioethanol from cereal grain or straw.
Bioethanol is a viable alternative or supplement to gasoline as an additive. The economic conversion of various materials such as wood, straw, and domestic waste into bioethanol is a viable prospect. The enzymatic action of gluco-amylase facilitates the conversion of starch molecules into D-glucose. Following the process of enzymatic hydrolysis [80], fermentation, distillation, and dehydration, the result is the production of anhydrous bioethanol. Corn is the predominant feedstock utilized in the worldwide starch-to-bioethanol industry, characterized by its starch content ranging from 60% to 70%.
6.4. Biohydrogen
Biohydrogen is a form of hydrogen that is produced by biological processes, such as the fermentation of organic matter by microorganisms. It is considered to be a promising alternative to conventional hydrogen.
A variety of organic materials, such as agricultural leftovers, algal biomass, and rubbish, have the potential to serve as viable substrates for biohydrogen synthesis through thermochemical and biological processes, as well as for biogas reforming.
6.5. Bio-Oil and Biogas
Bio-oils are the primary designation for liquid fuels. Biogas production can be achieved using the process of anaerobic digestion, facilitated by certain microorganisms. Biogas is generated through the interaction between methane gas and carbon dioxide gas. Bio-oils refer to liquid or gaseous fuels that are derived through biochemical or thermochemical processes from biomass sources, including agricultural crops, municipal waste, and agricultural and forestry residues. The process of anaerobic digestion has the capability to transform the organic component of various biomass sources, such as sewage sludge, animal waste, and industrial effluents, into a combination of methane and carbon dioxide, sometimes referred to as “biogas”. Biogas is a fuel that possesses eco-friendly attributes, characterized by its cleanliness, affordability, and adaptability. In their study, investigated the production of bio-oil from macroalgae using a fixed-bed reactor [81]. The researchers achieved a yield of 47%, along with a co-product of 33% biochar. Employed microwave treatment as a method for the production of bio-oil from macroalgae. The researchers achieved a maximum yield of 18.4 weight percent.
6.6. Biomethanol
This substance is commonly known as “methanol”. In comparison to ethanol, methanol exhibits greater accessibility. Methanol is derived from either biogas or synthetic gas and is currently undergoing experimental evaluation as a potential fuel for internal combustion engines (Figure 10). The production of methanol is a costly chemical procedure. Currently, methanol production exclusively relies on waste biomass, specifically old wood or biowaste [82]. It can be observed that approximately 50% of the pyroligneous acid generated in the process of wood pyrolysis consists of methanol, acetone, phenols, and water [74,83]. Biomass represents a sustainable and replenishable resource that has significant promise as an inexhaustible fuel for the production of methanol. Bioenergy suppliers can be classified into two main categories: dedicated energy crop plantations; organic municipal trash and leftovers from the food and materials sectors.
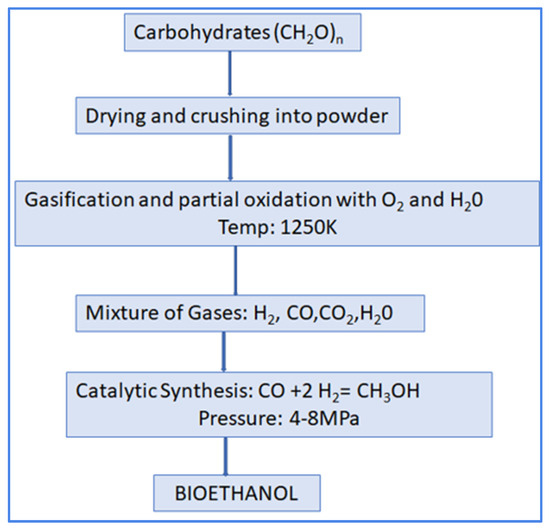
Figure 10.
Biomethanol from carbohydrates produced through gasification and partial oxidation with oxygen gas and water vapor.
The primary objective of the biotechnology research community is to utilize non-arable land for the cultivation of algae biomass, with a focus on enhancing productivity. This involves implementing efficient photosynthetic carbon dioxide (CO2) fixation mechanisms and effectively utilizing industrial CO2 emissions to enable the economic viability of biofuel production [84]. The biogas or methane production from anaerobic digestion of algal biomass has been the subject of substantial research and analysis [85]. Biohydrogen has superior performance when employed for the purpose of generating bioelectricity using fuel cells, rendering it a favored fuel alternative due to its absence of carbon emissions during combustion. The genetic engineering of algal hydrogenases has led to an increase in the yield of biohydrogen synthesis [86].
7. Biotechnological Improvement of Algal Biofuel
Algae, as a collective group, possess the capability to produce biofuel, albeit with variations in terms of lipid, starch, and cell composition. Certain individuals exhibit genetically enhanced levels of starch and fat accumulation, as well as possessing inherent abilities for biosynthesis [87]. The commercial production of biofuel precursors is constrained by the combination of high costs and poor production rates, primarily attributable to the accumulation of compounds under stressful conditions. This accumulation adversely affects the growth and output of biofuel precursors. There are three primary biotechnological approaches employed to enhance algal biofuel production via genetic modification of these microorganisms [88]. These strategies encompass augmenting the biosynthesis of lipids and starch, enhancing the capacity for carbon capture and lipid accumulation, and employing genetic engineering techniques to facilitate biohydrogen production.
7.1. Biotechnology for Enhancing Lipid, Starch, and Fatty Acids Synthesis
The upregulation of enzymes involves the biosynthesis of fatty acids and lipids [89,90,91,92], triglyceride (TAG) biosynthesis and assembly [93], and the enhancement of fatty alcohols [92,94], as well as the targeted suppression or inhibition of competitive pathways, such as starch biosynthesis or lipid catabolism [89]. Significant genetically modified (GM) algae strains encompass C. reinhardtii strains CC-4349 and Chlorella vulgaris mutant UV715. An elevation in the accumulation of hydrocarbons (specifically, free fatty acids, fatty alcohols, alkanes, and alkenes) was seen following the processes of knocking out, triparental conjugation, and heterologous expression. This increase was found to be eight-fold for UVM4 and 19-fold for PCC 6803.
7.2. Genetic Engineering for Carbon Capture and Lipid Accumulation Ability
Most algal strains commonly possess carbonic anhydrase (CA), an enzyme that has been extensively researched and proven to be efficient in facilitating the conversion between CO2 and bicarbonate. The state of California actively engages in the process of carbon dioxide sequestration. When compared to wild algae, transgenic algae exhibited a 2.2-fold increase in lipid accumulation and an increase in carbon fixation rates.
7.3. Biohydrogen Production through Genetic Engineering
The reduction of H+ to H2 is primarily facilitated by two enzymes, namely hydrogenases and nitrogenases [67]. Fe-hydrogenase is the enzyme that exhibits the largest hydrogen (H2) generation capacity, while also being sensitive to oxygen (O2).
8. Nanotechnology for Biofuel Production
Nanotechnology is a highly promising discipline that is significantly contributing to sustainable development by addressing various critical environmental challenges, including wastewater treatment [95,96], bio-sensing [97], and biofuel production [98,99]. The field of nanotechnology has opened new avenues and made significant advancements that hold great potential for addressing the production of bioethanol and biodiesel [18,100,101,102]. The incorporation of nanoparticles (NPs) into fuels has the potential to improve thermo-physical properties, such as thermal conductivity and mass diffusivity [103]. Several types of nanomaterials have been successfully utilized in the production of environmentally friendly biofuels derived from diverse sources of biomass.
8.1. Metal and Metal Oxide NPs
A diverse array of metal and metal–oxide nanoparticles (NPs), whether in their unmodified state or after undergoing functionalization, are extensively utilized in numerous applications about the production of bioethanol and biodiesel. The primary process involved in the manufacture of bioethanol is the enzymatic hydrolysis of cellulose and hemicellulose, which results in the breakdown of these complex polysaccharides into simpler sugars. This hydrolysis is facilitated by the action of specific enzymes, including cellulases, hemicellulases, and lipases [66,104]. Table 3 presents a compilation of several nanoparticles (NPs) that were utilized.

Table 3.
Different nanomaterials used for the production of biofuel.
8.2. Magnetic Nanomaterials
The study conducted to investigated the immobilization process of β-glucosidase A and cellobiohydrolase D onto magnetic nanoparticles (NPs) with the aim of producing bioethanol through the conversion of cellulosic biomass into sugar [119]. Research findings indicate that the immobilization of enzymes onto magnetic nanoparticles (NPs) can lead to improvements in the catalytic characteristics, thermal stability, and overall effectiveness of the enzymes [120]. The enzymes possess super-magnetic properties, which facilitate their easy recovery through the application of a magnetic field. Additionally, these enzymes can be recycled and reused [121]. The immobilization of cellulase onto zinc ferrite nanoparticles for the purpose of hydrolyzing pretreated biomass derived from Crotalaria juncea [12,122]. In study, examined the gasification–fermentation pathway as a means of producing bioethanol [117]. A significant increase of 213.5% was seen in the production of ethanol with the utilization of these catalysts.A technique to immobilize polymeric sulfonated ionic liquid onto magnetic porous Fe3O4/SiO2 core shell composites (Fe3O4/SiO2–PSIL). This immobilization method was utilized for the purpose of biodiesel generation using soybean oil and free fatty acids as substrates [123].
8.3. Acid-Functionalized Magnetic Nanoparticles
It plays a crucial role in the generation of biofuels. The findings was proposed that nanocatalysts functionalized with sulfamic acid exhibit higher levels of activity compared to those functionalized with sulfonic acid [124]. The utilization of acid functionalized nanoparticles (NPs) has been explored in the context of bioethanol production. For instance, silica-protected cobalt spinel ferrite NPs have been functionalized with various acid groups such as perfluoroalkylsulfonic acid (PFS), alkylsulfonic acid (AS), and butylcarboxylic acid (BCOOH).
8.4. Carbon Nanotubes (CNTs)
Theses possess several desirable characteristics, including a large surface area, minimal toxicity, chemical stability, mechanical robustness, excellent thermal and electrical conductivity, and cost-effectiveness, rendering them highly attractive [103,125]. Biodiesel exhibits elevated viscosity and density in comparison to conventional diesel fuel, resulting in an augmented brake-specific fuel consumption (BSFC) and consequent emission of smoke and nitrogen oxides (NOx) [126,127]. In order to address this problem, the utilization of nanomaterials as fuel additives was implemented with the aim of reducing the viscosity and density of the fuel mixture [126,128,129]. The introduction of multi-walled carbon nanotubes (CNTs) into biodiesel resulted in a decrease in emissions of nitrogen oxides (NOx), carbon monoxide (CO), and unburned hydrocarbons [130]. Similarly, observed that the incorporation of multi-walled CNT-OH additive into diesel–biodiesel–ethanol blends led to a significant reduction in brake-specific fuel consumption (BSFC) and emissions of hydrocarbons and CO [103]. Furthermore, this additive demonstrated improvements in engine brake power and torque.
9. Lanzajet Technology for Biofuel Production
The goal of this innovation was to cut down on emissions from the aviation industry by means of the manufacturing of sustainable aviation fuel (SAF). In the Atmos FUEL project, led by LanzaTech and Carbon Engineering, CO2 was extracted from the air and converted to SAF using the LanzaJet ATJ technology. Sustainable biocarbon, biochemicals, biopolymers, and drop-in for biofuels can be produced from lignocellulosic feedstocks such waste wood and biomass. Carbon engineering’s technology for atmospheric CO2 capture, LanzaTech’s technology for producing sustainable ethanol, and LanzaJet’s ATJ technology for creating SAF all contribute to the Atmos FUEL system [131].
10. Microwave Technology
The utilization of microwave energy in the processing of materials has the potential to provide benefits in terms of decreased processing time and energy conservation. This is due to the direct supply of energy to the materials through an electromagnetic field [132]. In addition, the utilization of microwave irradiation has garnered significant attention and widespread acceptance as a valuable technique for chemical reactions, particularly in the realm of organic synthesis. This is primarily attributed to its capacity to expedite reaction times and enhance product yield within a brief duration. Furthermore, microwave irradiation offers a safer and more convenient approach for heating reaction mixtures to achieve desired temperatures [133]. The transesterification process involves the utilization of monohydric alcohol in the presence of a catalyst to facilitate the conversion of crude oil into fatty acid alkyl esters, often known as biodiesel. The primary challenge impeding the commercialization of biodiesel is its operational expenditure. In order to address this issue, the utilization of microwave irradiation is implemented as an effective approach to decrease the reaction time and enhance the yield of biodiesel. This method is recognized for its expeditiousness and energy efficiency in comparison to the conventional transesterification technique employed in biodiesel production. The utilization of microwaves in the transesterification reaction can expedite the reaction rate through a thermal mechanism, while also facilitating the separation process [134]. Furthermore, the application of microwave electromagnetic radiation to reactant components such as triglycerides and alcohol results in a decrease in the energy required for the reaction to proceed. This reduction in activation energy might be attributed to the enhanced rotational movement of polar molecules [135].
11. Ultrasonic
The utilization of the ultrasonic technique has been widely acknowledged as an appropriate method for enhancing mass transfer between a solid and a liquid medium [136,137]. Ultrasonic or ultrasonic refers to sound waves that possess frequencies exceeding the upper limits of human audibility. It furnishes mechanical energy for the purpose of intense mixing, in addition to serving as the first activation energy for the transesterification process [136]. Consequently, this leads to an accelerated response time and an increased production of biodiesel. The occurrence known as cavitation possesses the capacity to induce both physical and chemical consequences on the reaction. A significant quantity of cavitation bubbles is created and progressively enlarged throughout multiple ultrasonic cycles. Upon collapse, the system experiences elevated temperature and pressure, resulting in intense intermingling between the immiscible reactants and rapid heating throughout the system. The introduction of ultrasonic waves into a liquid result in alternating cycles of expansion and compression phases [138]. In compression cycles, the molecules of a liquid are subjected to a positive pressure, causing them to be brought closer together. In contrast, the molecules experience separation due to the presence of negative pressure during expansion cycles [19]. Cavitation bubbles are generated in a series of cycles, and their dimensions undergo rapid expansion, increasing from ten to hundreds of times their original size, before violently collapsing within a timeframe of less than one microsecond [139]. Upon the occurrence of the collapse, it can be observed that each individual bubble functions as a localized area of intense activity. As a result, a significant amount of energy is produced, resulting in a rise in temperature and pressure, reaching levels of 5000 °C and 500 atm, respectively [140]. Moreover, the collapse of these bubbles has the potential to generate radicals, such as H+ and OH– in the case of water. These radicals can facilitate chemical interactions among the reactants.
12. Biofuel Production Process
- Pyrolysis is a thermochemical process that involves the decomposition of biomass in the absence of oxygen, resulting in the production of bio-oil and biochar.
- Hydrothermal liquefaction is a process that involves the conversion of wet biomass, such as algae, sewage, or a liquid slurry of feedstock, into bio-oil.
- Gasification is a process wherein biomass undergoes a purification and preparation procedure to convert it into a fuel. This involves subjecting the biomass to elevated temperatures in the presence of oxygen or steam, resulting in the production of hydrogen gas.
Low-temperature technologies
- Hydrolysis: To produce fuels or compounds that can be turned into fuels, biomass is first physically or chemically treated to break down the structure of plant cell walls. Plants are broken down to produce sugars. The efficiency with which cellulose is transformed into glucose, or sugar, may depend on the efficacy of pretreatments to alter the material’s structure and chemistry.
- Fermentation: Yeast performs a fermentation process, which is an anaerobic biological activity that converts sugars into alcohol [71]. Saccharomyces ceveresiae and other commercial yeasts are used to ferment sucrose. The invertase enzyme in yeast initiates glycogen and sucrose biosynthesis. Secondly, the yeast produces ethanol from fructose and glucose thanks to an enzyme called zymase.
- Gasification: To create gases such as carbon dioxide (CO2), hydrogen (H2), and carbon monoxide (CO), gasification can be used on organic carbonaceous resources. The gasification reaction involves several different thermal reactions, including drying, pyrolysis, combustion, and reduction. The gases produced in this process are known as syngas. Biomass gasification is commonplace in the energy industry due to the production of syngas (synthesis gas), hydrogen (H2), methane (CH4), and other valuable byproducts. The process requires heating organic materials to extremely high temperatures (600–1500 °C) in the presence of a measured amount of oxygen and/or steam. Gas engines can use the manufactured gas to create more H2 and methyl (CH3OH), or it can be processed into synthetic fuel using the Fischer–Tropsch method.
- Drying: The first step in this process is drying the biomass before moving on to the pyrolysis phase.
- Pyrolysis: Char, tar gases, and liquids are produced when the biomass raw material is treated with high temperatures (typically exceeding 240 °C) in an air-free environment.
- Combustion: Tar fumes or char from pyrolysis are typically used as the first catalyst. Drying, pyrolysis, and reduction are only some of the additional processes that can benefit from the heat recovered by heat recovery systems from the combustion process. Cracking occurs when heat causes huge, complicated particles to break down into smaller, simpler particles; for example, when tar molecules are split down into lighter, simpler molecules. This process is essential for optimum combustion, and it also has the added benefit of creating an enhanced and uncontaminated gas that might be used in internal combustion engines.
Reduction: This step is a chemical phenomenon that functions as the opposite of combustion, also referred to as the reaction zone. The process entails the removal of oxygen atoms from the byproducts of combustion, operating at high temperatures (ranging from 650 to 900 degrees Celsius), which leads to the production of combustible gases. The process of reduction occurs within the gasifier when streams of CO2 or water vapor pass through carbon that is highly reactive and present in hot charcoal. This interaction leads to a chemical reaction. As a result, the oxygen molecules found in both carbon dioxide (CO2) and water vapor (H2O) undergo elimination, resulting in a reorganization of the individual bonds within these particles. The equations illustrate the conversion of carbon dioxide (CO2) into carbon monoxide (CO) molecules in the specific process under consideration. Additionally, water vapor is changed into hydrogen (H2) and carbon monoxide (CO), both of which exhibit combustible characteristics.
Syngas is the principal byproduct of biomass gasification, and its composition is affected by the gasifying medium. Increasing the gasification temperature improves syngas output while reducing the formation of tar. This is because the gasification process, when carried out at lower temperatures, produces not just carbon monoxide (CO) and hydrogen (H2), but also heavier hydrocarbons. Longer periods of fluctuating occupation are typically associated with a worsening of tar degradation. Due to the drawbacks associated with excessive ash concentration, namely the promotion of slagging, using low-ash biomass is preferable in the production of syngas.
A practical method exists for transforming syngas into liquid hydrocarbon fuels like diesel and kerosene. In addition, several techniques, including Fischer–Tropsch synthesis, mixed-alcohol synthesis, and methanol-to-gasoline conversion, can be used to transform syngas into different gas fuels. Syngas can be processed in a variety of ways, resulting in the generation of biofuels like methanol, ethanol, and liquefied natural gas, to name a few bio-alcohols.
13. Sustainability and Circular Bioeconomy of Biofuel Production
The economic landscape of biofuels has experienced rapid growth since the beginning of the 21st century. There exist several rationales behind the recognition of biofuels as pertinent technology in both developed and developing nations. The function of energy production in driving agricultural development has been of notable significance in recent years. At present, the production of synthesis ethanol from ethylene and methanol derived from natural gas in the agricultural sector incurs significant costs. Nevertheless, in the presence of hydroelectricity, the economic viability of producing bioethanol and biomethanol from sugar juice can be achieved. The studies and evaluations highlight the importance of environmental consciousness and the diminishing availability of traditional energy resources such as coal, natural gas, and petroleum. The necessity of biofuel as the optimal choice in terms of environmental sustainability and energy efficiency has been highlighted in previous studies [2,20].
To facilitate the establishment of sustainable growing, harvesting, and consumption practices pertaining to macroalgae, it is crucial to consider the economic aspects associated with this matter [141,142]. The economic viability of biofuel production through algae farming relies on generating a significant profit that offsets the costs associated with labor, materials, and equipment expenses. Comprehensive investigation into the characteristics of algae is imperative due to its significant capacity to produce sustenance and pharmaceuticals. Nevertheless, the economic evaluation of this topic has received limited attention in the existing literature [15,143]. The financial performance of the algal biorefinery remains uncertain, as indicated in the study. The selection of algal species for organic feedstocks is influenced by varying climate and sea conditions. Algal farming necessitates labor, equipment, and supplies, which differ depending on the specific farming practices employed. The demand for algal products is influenced by factors such as the introduction of new products, prevailing market conditions, and the price of substitute goods. However, investing in algal farming entails high capital costs and is hindered by the absence of public policies. Processing technologies are limited by their immaturity, complexity, scalability, and cost. Contracting logistics are limited by asymmetric information, logistics and transportation, and quality requirements.
The price of utilizing Ulva-species-derived biofuel was USD 2.21 per kilogram, but the revenue generated from its components varied between USD 8 and USD 10.4 per kilogram [144]. utilizes analytical grade material as a point of reference [145], establishes a connection between this reference and the costs associated with marketable carrageenan [146].
A wide range of crops that are often used either for direct human consumption or as animal feed can also be used as feedstocks for biofuel production. The utilization of these crops for biofuel production has the potential to augment the agricultural land footprint, intensify the utilization of environmentally detrimental inputs, and elevate the cost of food. Furthermore, it is worth noting that the utilization of cellulosic feedstocks has the potential to create competition with food production in terms of resource allocation, namely in relation to land, water, and fertilizer. Because of this phenomenon, certain scholarly investigations argue that the manufacturing of biofuels may give rise to a variety of adverse alterations. Alterations in land-use patterns have the potential to elevate greenhouse gas (GHG) emissions by releasing carbon stocks from terrestrial sources into the atmosphere [147]. Soybeans cultivated in the Amazon region and oil palm farmed in Southeast Asia exemplify biofuel feedstocks that are cultivated on deforested tropical land and contribute substantially to greenhouse gas emissions [148]. The utilization of cellulosic feedstocks possesses the capacity to elevate crop prices, so incentivizing the expansion of agricultural activities into previously undeveloped areas can result in heightened emissions of greenhouse gases and losses in biodiversity [149].
The utilization of certain methods during the production and processing of biofuels has the potential to result in the emission of greenhouse gases (GHGs). The use of fertilizer results in the emission of nitrous oxide, a potent greenhouse gas. The predominant energy source utilized by most biorefineries is derived from fossil fuels. The literature shows that the timeframe of analysis plays a crucial role in determining the greenhouse gas (GHG) emissions associated with the production and consumption of biofuels. In certain cases, including the consideration of indirect land-use changes, these emissions may surpass those generated by fossil fuels.
14. Conclusions
One potential avenue for the utilization of biomass in developing nations is through the advancement of biomass technology, resulting in enhanced efficiency in biomass production and conversion processes. Hence, it is imperative that the biomass processing procedures employed in developing nations adhere to environmentally sustainable practices. Renewable biofuels derived from plants and photosynthetic microorganisms play a vital role in achieving a carbon-neutral bioeconomy. However, the advancement of sustainable biofuels necessitates several scientific developments. The objective of synthesizing high-value chemicals and biofuels in “synthetic factories” is to enhance the profitability of industrial operations.
Funding
This research received no external funding.
Data Availability Statement
Not applicable.
Acknowledgments
I would like to express my deep sense of gratitude and obligation to my University, CCS HAU, for giving me the opportunity for an international academic experience under NAHEP-IDP training program and to NAHEP-ICAR for supporting with the funds for the same. I am grateful to B. R. Kamboj, Worthy Vice-Chancellor, CCSHAU, Hisar for his overwhelming support in obtaining this opportunity. I am also thankful to K. D. Sharma, PI cum Dean PGS, CCSHAU, Hisar for his overall help and guidance. I express my indebtedness to my Mentor at Texas A&M University, Sergio Capareda, Head BETA Lab for his guidance, encouragement, suggestions, intellectual stimulation, and magnanimous help during the training period.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Escobar, J.C.; Lora, E.S.; Venturini, O.J.; Yanez, E.E.; Castillo, E.F.; Almazan, O. Biofuels: Environment, technology and food security. Renew. Sustain. Energy Rev. 2009, 13, 1275–1287. [Google Scholar] [CrossRef]
- Gaurav, N.; Sivasankari, S.; Kiran, G.S.; Ninawe, A.; Selvin, J. Utilization of bioresources for sustainable biofuels: A Review. Renew. Sustain. Energy Rev. 2017, 73, 205–214. [Google Scholar] [CrossRef]
- IEA. Global Energy Review: CO2 Emissions in 2021; IEA: Paris, France, 2022; License: CC BY 4.0; Available online: https://www.iea.org/reports/global-energy-review-co2-emissions-in-2021-2 (accessed on 15 May 2023).
- Mosnier, A.; Havlik, P.; Valin, H.; Baker, J.; Murray, B.; Feng, S.; Obersteiner, M.; McCarl, B.; Rose, S.; Schneider, U. The Net Global Effects of Alternative U.S. Biofuel Mandates: Fossil Fuel Displacement, Indirect Land Use Change, and the Role of Agricultural Productivity Growth. Energy Policy 2013, 57, 602–614. [Google Scholar] [CrossRef]
- Wang, X.; Li, C.; Chen, M.; Wang, J. Microwave-assisted pyrolysis of seaweed biomass for aromatics-containing bio-oil production. In Proceedings of the 7th International Conference on Energy Materials and Environment Engineering (ICEMEE 2021), Zhangjiajie, China, 23–25 April 2021. [Google Scholar]
- World Resources Institution (WRI). This Interactive Chart Shows Changes in the World’s Top 10 Emitters. 2020. Available online: https://www.wri.org/insights/interactive-chart-shows-changes-worlds-top-10-emitters (accessed on 11 May 2023).
- Ganzenmüller, R.; Pradhan, P.; Kropp, J.P. Sectoral performance analysis of national greenhouse gas emission inventories by means of neural networks. Sci. Total Environ. 2019, 656, 80–89. [Google Scholar] [CrossRef] [PubMed]
- Intergovernmental Panel on Climate Change (IPCC). Climate change 2013: The physical science basis. In Contribution of Working Group I to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change; Stocker, T.F., Qin, D., Plattner, G.-K., Tignor, M., Allen, S.K., Boschung, J., Nauels, A., Xia, Y., Bex, V., Midgley, P.M., Eds.; Cambridge University Press: Cambridge, UK; New York, NY, USA, 2013. [Google Scholar]
- FAO. World Food and Agriculture—Statistical Yearbook 2023; FAO: Rome, Italy, 2023. [Google Scholar] [CrossRef]
- FAO IFAD; UNICEF; WFP; WHO. The State of Food Security and Nutrition in the World 2018. Building Climate Resilience for Food Security and Nutrition; FAO: Rome, Italy, 2018. [Google Scholar]
- Cayuela, M.L.; Kuikman, P.; Bakker, R.; van Groenigen, J.W. Tracking C and N dynamics and stabilization in soil amended with wheat residue and its corresponding bioethanol by-product: A 13C/15 N study. GCB Bioenergy 2014, 6, 499–508. [Google Scholar] [CrossRef]
- Bera, B.; Bhattacharjee, S.; Sengupta, N.; Saha, S. Dynamics of deforestation and forest degradation hotspots applying geo-spatial techniques, apalchand forest in terai belt of himalayan foothills: Conservation priorities of forest ecosystem. Remote Sens. Appl. Soc. Environ. 2021, 22, 100510. [Google Scholar] [CrossRef]
- RFA. Ethanol Industry Outlook. 2020. Available online: https://ethanolrfa.org/wp-content/uploads/2020/02/2020-Outlook-Finalfor-Website.pdf (accessed on 15 May 2023).
- Singh, D.; Malik, K.; Sindhu, M.; Kumari, N.; Rani, V.; Mehta, S.; Malik, K.; Ranga, P.; Sharma, K.; Dhull, N.; et al. Biostimulation of Anaerobic Digestion Using Iron Oxide Nanoparticles (IONPs) for Increasing Biogas Production from Cattle Manure. Nanomaterials 2022, 12, 497. [Google Scholar] [CrossRef] [PubMed]
- Tamilarasan, K.; Kavitha, S.; Selvam, A.; Rajesh Banu, J.; Yeom, I.T.; Nguyen, D.D.; Saratale, G.D. Costeffective, low thermo-chemo disperser pretreatment for biogas production potential of marine macroalgae Chaetomorphaantennina. Energy 2018, 163, 533–545. [Google Scholar] [CrossRef]
- Tao, Z.; Li, C.; Li, J.; Ding, Z.; Xu, J.; Sun, X.; Zhao, M. Tillage and straw mulching impacts on grain yield and water use efficiency of spring maize in Northern Huang–Huai–Hai Valley. Crop J. 2015, 3, 445–450. [Google Scholar] [CrossRef]
- Koga, N.; Tsuji, H. Effects of reduced tillage, crop residue management and manure application practices on crop yields and soil carbon sequestration on an Andisol in northern Japan. Soil Sci. Plant Nutr. 2009, 55, 546–557. [Google Scholar] [CrossRef]
- Liu, X.; Singh, S.; Gibbemeyer, E.L.; Tam, B.E.; Urban, R.A.; Bakshi, B.R. The carbon-nitrogen nexus of transportation fuels J. Clean. Prod. 2018, 180, 790–803. [Google Scholar] [CrossRef]
- Vajnhandl, S.; Le Marechal, A.M. Ultrasound in textile dyeing and the decolouration/mineralization of textile dyes. Dye Pigm. 2005, 65, 89–101. [Google Scholar] [CrossRef]
- Demirbas, A. Biofuels sources, biofuel policy, biofuel economy and global biofuel projections. Energy Convers. Manag. 2008, 49, 2106–2116. [Google Scholar] [CrossRef]
- Kim, S.; Dale, B.E. Life cycle assessment of various cropping systems utilized for producing: Bioethanol and biodiesel. Biomass-Bioenergy 2005, 29, 426–439. [Google Scholar] [CrossRef]
- Kratzel, A.; Todt, D.; Steiner, S.; Gultom, M.; Nhu Thao, T.T.; Ebert, N.; Holwerda, M.; Steinmann, J.; Niemeyer, D.; Dijkman, R.; et al. Inactivation of Severe Acute Respiratory Syndrome Coronavirus 2 by WHO-Recommended Hand Rub Formulations and Alcohols. Emerg. Infect. Dis. 2020, 26, 1592–1595. [Google Scholar] [CrossRef] [PubMed]
- Karakashev, D.; Belinda, A.; Angelidaki, I. Anaerobic biotechnological approaches for production of liquid energy carriers from biomass. Biotechnol. Lett. 2007, 29, 1005–1012. [Google Scholar] [CrossRef]
- Ragauskas, J.A.; Williams, C.K.; Davison, B.H.; Britovsek, G.; Cairney, J.; Eckert, C.A.; Frederick, W.J., Jr.; Hallett, J.P.; Leak, D.J.; Liotta, C.L.; et al. The Path Forward for Biofuels and Biomaterials. Science 2006, 311, 484–489. [Google Scholar] [CrossRef]
- Octave, S.; Thomas, D. Biorefinery: Toward an industrial metabolism. Biochimie 2009, 91, 659–664. [Google Scholar] [CrossRef]
- Ubando, A.T.; Africa, A.D.M.; Maniquiz-Redillas, M.C.; Culaba, A.B.; Chen, W. Reduction of particulate matter and volatile organic compounds in biorefineries: A state-of-the-art review. J. Hazard. Mater. 2021, 403, 123955. [Google Scholar] [CrossRef]
- Xiong, W.; Morgan, J.A.; Ungerer, J.; Wang, B.; Maness, P.C.; Yu, J. The plasticity of cyanobacterial metabolism supports direct CO2 conversion to ethylene. Nat. Plants 2015, 1, 15053. [Google Scholar] [CrossRef]
- Arthe, R.; Rajesh, R.; Rajesh, E.M.; Rajendran, S.; Jeyachandran, S. Production of bioethanol from cellulosic cotton waste through microbial extracellular enzymatic hydrolysis and fermentation. Electron. J. Environ. Agric. Food Chem. 2008, 2008, 2984–2992. [Google Scholar]
- Castro, J.F.M.D. Biofuels—An Overview, Final Report, Prepared for: DGIS/DMW/IB 2007. Available online: https://np-net.pbworks.com/f/BZOS+(2007)+Biofuels+in+Africa_overview.pdf (accessed on 30 August 2016).
- Haykiri-Acma, H.; Yaman, S. Interaction between biomass and different rank coals during co-pyrolysis. Renew. Energy 2010, 35, 288–292. [Google Scholar] [CrossRef]
- Mielenz, J.R. Ethanol production from biomass: Technology and commercialization status. Curr. Opin. Microbiol. 2001, 4, 324–329. [Google Scholar] [CrossRef] [PubMed]
- Law, K.N.; Wanrosli, W.D.; Ghazali, A. Morphological and chemical nature of fiber strands of Oil Palm Empty-Fruit-Bunch (OPEFB). Bioresources 2007, 2, 351–362. [Google Scholar] [CrossRef]
- Gerveni, M.; Hubbs, T.; Irwin, S. Overview of the production capacity of US biodiesel plants. Fardoc Daily, 22 February 2023. [Google Scholar]
- Jeswani, K.H.; Chilvers, A.; Azapagic, A. Environmental sustainability of biofuels: A review. Proc. R. Soc. A 2023, 476, 2243. [Google Scholar] [CrossRef] [PubMed]
- Korenberg, C. The effect of ultraviolet-filtered light on the mechanical strength of fabrics. Tech. Res. Bull. 2007, 1, 23–27. [Google Scholar]
- Shweta; Satpal; Kumari, A.; Neelam; Sewhag, M.; Kharor, N.; Nagora, M. Performance of maize in drip irrigation system under semi-arid region. Forage Res. 2022, 48, 88–91. [Google Scholar]
- Shweta; Kavita; Neelam; Sehwag, M.; Satpal; Malik, K.; Singh, B. Evaluation of various maize based intercropping system. Forage Res. 2022, 48, 205–208. [Google Scholar]
- Wati, L.; Kumari, S.; Kundu, B.S. Paddy straw as substrate for ethanol production. Ind. J. Microbiol. 2007, 47, 26–29. [Google Scholar] [CrossRef]
- Besada, V.; Andrade, J.M.; Schultze, F.; Gonzalez, J.J. Heavy metals in edible seaweeds commercialised for human consumption. J. Mar. Syst. 2009, 75, 305–313. [Google Scholar] [CrossRef]
- Ishaque, M.; Kluepfel, D. Cellulase complex of a mesophilic Streptomyces strain. Can. J. Microbiol. 1980, 26, 183–189. [Google Scholar] [CrossRef]
- Mathur, S.; Waswani, H.; Singh, D.; Ranjan, R. Alternative fuels for Agriculture Sustainability: Carbon Foot print and economic Feasibility. Agriengineering 2022, 4, 993–1015. [Google Scholar] [CrossRef]
- Saha, B.C.; Bothast, R.J. Enzymes in lignocellulosic biomass conversion. ACS Symp. Ser. 1997, 666, 46–56. [Google Scholar]
- Kluepfel, D.; Shareck, F.; Mondou, F.; Morosoli, R. Characterization of cellulose and xylanase activities of Sreptomyces lividans. Appl. Microbiol. Biotechnol. 1986, 24, 230–234. [Google Scholar] [CrossRef]
- Hong, J.; Wang, Y.; Kumagai, H.; Tamaki, H. Construction of thermotolerant yeast expressing thermostable cellulase genes. J. Biotechnol. 2007, 130, 114–123. [Google Scholar] [CrossRef] [PubMed]
- Ban, S.; Lin, W.; Wu, F.; Luo, J. Algal-bacterial cooperation improves algal photolysis-mediated hydrogen production. Bioresour. Technol. 2018, 251, 350–357. [Google Scholar] [CrossRef] [PubMed]
- Ambaye, T.G.; Vaccari, M.; Bonilla-Petriciolet, A.; Prasad, S.; Van Hullebusch, E.D.; Rtimi, S. Emerging technologies for biofuel production: A critical review on recent progress, challenges and perspectives. J. Environ. Manag. 2021, 290, 112627. [Google Scholar] [CrossRef] [PubMed]
- Berla, B.M.; Saha, R.; Immethun, C.M.; Maranas, C.D.; Moon, T.S.; Pakrasi, H.B. Synthetic biology of cyanobacteria: Unique challenges and opportunities. Front. Microbiol. 2013, 4, 246. [Google Scholar] [CrossRef]
- Cavelius, P.; Engelhart-Straub, S.; Mehlmer, N.; Lercher, J.; Awad, D.; Brück, T. The potential of biofuels from first to fourth generation. PLoS Biol. 2023, 21, e3002063. [Google Scholar] [CrossRef]
- Hays, S.G.; Ducat, D.C. Engineering cyanobacteria as photosynthetic feedstock factories. Photosynth. Res. 2015, 123, 285–295. [Google Scholar] [CrossRef]
- Scaife, M.A.; Nguyen, G.T.D.T.; Rico, J.; Lambert, D.; Helliwell, K.E.; Smith, A.G. Establishing Chlamydomonas reinhardtii as an industrial biotechnology host. Plant J. 2015, 82, 532–546. [Google Scholar] [CrossRef] [PubMed]
- Inganäs, O.; Sundstrom, V. Solar energy for electricity and fuels. Ambio 2016, 45, 15–23. [Google Scholar] [CrossRef] [PubMed]
- Goria, K.; Kothari, R.; Singh, H.M.; Singh, A.; Tyagi, V. Biohydrogen: Potential applications, approaches, and hurdles to overcome. In Handbook of Biofuels; Academic Press: Cambridge, MA, USA, 2022; pp. 399–418. [Google Scholar] [CrossRef]
- Anonymous. U.S. Energy Information Administration. 2021. Available online: https://www.eia.gov/kids/energy-sources/biofuels/ (accessed on 10 May 2023).
- Usmani, R.A. Potential for energy and biofuel from biomass in India. Renew. Energy 2020, 155, 921–930. [Google Scholar] [CrossRef]
- Anonymous. Jatropha: Biodiesel and More. Farm Energy, 3 April 2019. [Google Scholar]
- Folaranmi, J. Production of Biodiesel (B100) from Jatropha Oil Using Sodium Hydroxide as Catalyst. J. Pet. Engineering. 2013, 2013, 956479. [Google Scholar] [CrossRef]
- Dhaliwal, S.S.; Oberoi, H.S.; Sandhu, S.K.; Nanda, D.; Kumar, D.; Uppal, S.K. Enhanced ethanol production from sugarcane juice by galactose adaptation of a newly isolated thermotolerant strain of Pichia kudriavzevii. Bioresour. Technol. 2011, 102, 5968–5975. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Shen, Y.; Wu, M.; Hou, J.; Jiao, C.; Li, Z.; Liu, X.; Bao, X. Engineering a wildtype diploid Saccharomyces cerevisiae strain for second-generation bioethanol production. Bioresour. Bioprocess. 2016, 3, 51. [Google Scholar] [CrossRef]
- Rochón, E.; Cebreiros, F.; Ferrari, M.D.; Lareo, C. Isopropanol-butanol production from sugarcane and sugarcane-sweet sorghum juices by Clostridium beijerinckii DSM 6423. Biomass Bioenergy 2019, 128, 105331. [Google Scholar] [CrossRef]
- Baima Ferreira Freitas, I.; Aparecida de Menezes, C.; Luiz Silva, E. An alternative for value aggregation to the sugarcane chain: Biohydrogen and volatile fatty acids production from sugarcane molasses in mesophilic expanded granular sludge bed reactors. Fuel 2020, 260, 116419. [Google Scholar] [CrossRef]
- Li, X.; Chen, S.; Yu, Y.; Wang, S.; Xu, Z.; Huang, H.; Jin, M. Ethanol production from mixtures of Distiller’s Dried Grains with Solubles (DDGS) and corn. Ind. Crops Prod. 2019, 129, 59–66. [Google Scholar] [CrossRef]
- Li, X.; Chen, S.; Huang, H.; Jin, M. In-situ corn fiber conversion improves ethanol yield in corn dry-mill process. Ind. Crops Prod. 2018, 113, 217–224. [Google Scholar] [CrossRef]
- Tiryaki, O.N.; Irmak, S. Evaluation of various corn variety kernels for hydrogen gas production by APR. Biomass Bioenergy 2020, 134, 105480. [Google Scholar] [CrossRef]
- Ramachandra, T.V.; Madhab, M.D.; Shilpi, S.; Joshi, N.V. Algal biofuel from urban wastewater in India: Scope and challenges. Renew. Sustain. Energy Rev. 2013, 21, 767–777. [Google Scholar] [CrossRef]
- Godvin Sharmila, V.; Dinesh Kumar, M.; Pugazhendi, A.; Bajhaiya, A.K.; Gugulothuf, P.; Banu, J.R. Biofuel production from Macroalgae: Present scenario and future scope. Bioengineered 2021, 12, 9216–9238. [Google Scholar] [CrossRef]
- Binhayeeding, N.; Yunu, T.; Pichid, N.; Klomklao, S.; Sangkharak, K. Immobilisation of Candida rugosa lipase on polyhydroxybutyrate via a combination of adsorption and cross-linking agents to enhance acylglycerol production. Process Biochem. 2020, 95, 174–185. [Google Scholar] [CrossRef]
- Bolatkhan, K.; Kossalbayev, B.D.; Zayadan, B.K.; Tomo, T.; Veziroglu, T.N.; Allakhverdiev, S.I. Hydrogen production from phototrophic microorganisms: Reality and perspectives. Int. J. Hydrogen Energy 2019, 44, 5799–5811. [Google Scholar] [CrossRef]
- Lewandrowski, J.; Rosenfeld, J.; Pape, D.; Hendrickson, T.; Jaglo, K. The greenhouse gas benefits of corn ethanol—Assessing recent evidence. Biofuels 2020, 11, 361–375. [Google Scholar] [CrossRef]
- Deng, C.; Lin, R.; Kang, X.; Wu, B.; O’Shea, R.; Murphy, J.D. Improving gaseous biofuel yield from seaweed through a cascading circular bioenergy system integrating anaerobic digestion and pyrolysis. Renew. Sustain. Energy Rev. 2020, 128, 109895. [Google Scholar] [CrossRef]
- Bala, B.K. Studies on biodiesels from transformation of vegetable oils for diesel engines. Energy Edu. Sci. Technol. 2005, 15, 1–45. [Google Scholar]
- Demirbas, A. Biofuels from Agricultural Biomass. Energy Sources Part A 2009, 31, 1573–1582. [Google Scholar] [CrossRef]
- Demirbas, A. Biodiesel production from vegetable oils via catalytic and non-catalytic supercritical methanol transesterification methods. Prog. Energy Combust. Sci. 2005, 31, 466–487. [Google Scholar] [CrossRef]
- Demirbas, A. Global biofuel strategies. Energy Edu. Sci. Technol. 2006, 17, 27–63. [Google Scholar]
- Demirbas, A.; Gullu, D. Acetic acid, methanol and acetone from lignocellulosics by pyrpolysis. Energy Edu. Sci. Technol. 1998, 1, 111–115. [Google Scholar]
- Demirbas, B. Biofuels for internal combustion engines. Energy Edu. Sci. Technol. 2009, 22, 117–132. [Google Scholar]
- Robak, K.; Balcerek, M. Review of second-generation bioethanol production from residual biomass. Food Technol. Biotechnol. 2018, 56, 174–187. [Google Scholar] [CrossRef]
- Gegg, P.; Wells, V. UK Macro-Algae biofuels: A strategic management review and future research agenda. JMSE 2017, 5, 32. [Google Scholar] [CrossRef]
- Giugni, D.; Giugni, V. Intellectual property: A powerful tool to develop biotech research. Microbiol. Biotechnol. 2010, 3, 493–506. [Google Scholar] [CrossRef]
- Xu, X.; Kim, J.Y.; Oh, Y.R.; Park, J.M. Production of biodiesel from carbon sources of macroalgae, Laminaria japonica. Bioresour. Technol. 2014, 169, 455–461. [Google Scholar] [CrossRef]
- Morone, A.; Chakrabarti, T.; Pandey, R.A. Assessment of alkaline peroxideassisted wet air oxidation pretreatment for rice straw and its effect on enzymatic hydrolysis. Cellulose 2017, 24, 4885–4898. [Google Scholar] [CrossRef]
- Suh, D.J.; Choi, J.H.; Woo, H.C. Pyrolysis of seaweeds for bio-oil and bio-char production. Chem. Eng. Trans. 2014, 37, 121–126. [Google Scholar]
- Vasudevan, P.; Sharma, S.; Kumar, A. Liquid fuel from biomass: An overview. J. Sci. Ind. Res. 2005, 64, 822–831. [Google Scholar]
- Gullu, D.; Demirbas, A. Biomass to methanol via pyrolysis process. Energy Convers. Manag. 2001, 42, 1349–1356. [Google Scholar] [CrossRef]
- Choi, H.I.; Lee, J.S.; Choi, J.W.; Shin, Y.S.; Sung, Y.J.; Hong, M.E.; Kwak, H.S.; Kim, C.Y.; Sim, S.J. Performance and potential appraisal of various microalgae as direct combustion fuel. Bioresour. Technol. 2019, 273, 341–349. [Google Scholar] [CrossRef] [PubMed]
- Jankowska, E.; Sahu, A.K.; Oleskowicz-Popiel, P. Biogas from microalgae: Review on microalgae’s cultivation, harvesting and pretreatment for anaerobic digestion. Renew. Sustain. Energy Rev. 2017, 75, 692–709. [Google Scholar] [CrossRef]
- Yang, D.-W.; Syn, J.-W.; Hsieh, C.-H.; Huang, C.-C.; Chien, L.-F. Genetically engineered hydrogenases promote biophotocatalysis-mediated H2 production in the green alga Chlorella sp. DT. Int. J. Hydrogen Energy 2019, 44, 2533–2545. [Google Scholar] [CrossRef]
- Sahoo, S.; Mahapatra, S.R.; Das, N.; Parida, B.K.; Rath, S.; Misra, N.; Suar, M. Functional elucidation of hypothetical proteins associated with lipid accumulation: Prioritizing genetic engineering targets for improved algal biofuel production. Algal Res. 2020, 47, 101887. [Google Scholar] [CrossRef]
- Khan, S.; Fu, P. Biotechnological perspectives on algae: A viable option for next generation biofuels. Curr. Opin. Biotechnol. 2020, 62, 146–152. [Google Scholar] [CrossRef]
- Kao, P.-H.; Ng, I.-S. CRISPRi mediated phosphoenolpyruvate carboxylase regulation to enhance the production of lipid in Chlamydomonas reinhardtii. Bioresour. Technol. 2017, 245, 1527–1537. [Google Scholar] [CrossRef]
- Takemura, T.; Imamura, S.; Tanaka, K. Identification of a chloroplast fatty acid exporter protein, CmFAX1, and triacylglycerol accumulation by its overexpression in the unicellular red alga Cyanidioschyzon merolae. Algal Res. 2019, 38, 101396. [Google Scholar] [CrossRef]
- Tan, K.W.M.; Lee, Y.K. Expression of the heterologous Dunaliella tertiolecta fatty acyl-ACP thioesterase leads to increased lipid production in Chlamydomonas reinhardtii. J. Biotechnol. 2017, 247, 60–67. [Google Scholar] [CrossRef]
- Yunus, I.S.; Jones, P.R. Photosynthesis-dependent biosynthesis of medium chain-length fatty acids and alcohols. Metab. Eng. 2018, 49, 59–68. [Google Scholar] [CrossRef]
- Fukuda, S.; Hirasawa, E.; Takemura, T.; Takahashi, S.; Chokshi, K.; Pancha, I.; Tanaka, K.; Imamura, S. Accelerated triacylglycerol production without growth inhibition by overexpression of a glycerol-3-phosphate acyltransferase in the unicellular red alga Cyanidioschyzon merolae. Sci. Rep. 2018, 8, 12410. [Google Scholar] [CrossRef] [PubMed]
- Kaczmarzyk, D.; Cengic, I.; Yao, L.; Hudson, E.P. Diversion of the long-chain acyl-ACP pool in Synechocystis to fatty alcohols through CRISPRi repression of the essential phosphate acyltransferase PlsX. Metab. Eng. 2018, 45, 59–66. [Google Scholar] [CrossRef] [PubMed]
- Aanchal, R.; Barman, S.; Basu, S. Complete removal of endocrine disrupting compound and toxic dye by visible light active porous g-C3N4/H-ZSM-5 nanocomposite. Chemosphere 2020, 241, 124981. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Basu, S. Highly reusable visible light active hierarchical porous WO3/SiO2 monolith in centimeter length scale for enhanced photocatalytic degradation of toxic pollutants. Sep. Purif. Technol. 2020, 231, 115916. [Google Scholar] [CrossRef]
- Sharma, S.; Kundu, A.; Basu, S.; Shetti, N.P.; Aminabhavi, T.M. Sustainable environmental management and related biofuel technologies. J. Environ. Manag. 2020, 273, 111096. [Google Scholar] [CrossRef]
- Ibrahim, M.L.; Nik Abdul Khalil, N.N.A.; Islam, A.; Rashid, U.; Ibrahim, S.F.; Sinar Mashuri, S.I.; Taufiq-Yap, Y.H. Preparation of Na2O supported CNTs nanocatalyst for efficient biodiesel production from waste-oil. Energy Convers. Manag. 2020, 205, 112445. [Google Scholar] [CrossRef]
- Sanusi, I.A.; Suinyuy, T.N.; Lateef, A.; Kana, G.E.B. Effect of nickel oxide nanoparticles on bioethanol production: Process optimization, kinetic and metabolic studies. Process Biochem. 2020, 92, 386–400. [Google Scholar] [CrossRef]
- Cherian, E.; Dharmendirakumar, M.; Baskar, G. Immobilization of cellulase onto MnO2 nanoparticles for bioethanol production by enhanced hydrolysis of agricultural waste. Chin. J. Catal. 2015, 36, 1223–1229. [Google Scholar] [CrossRef]
- Choo, M.-Y.; Oi, L.E.; Ling, T.C.; Ng, E.-P.; Lin, Y.-C.; Centi, G.; Juan, J.C. Deoxygenation of triolein to green diesel in the H2-free condition: Effect of transition metal oxide supported on zeolite Y. J. Anal. Appl. Pyrolysis 2020, 147, 104797. [Google Scholar] [CrossRef]
- Ingle, A.P.; Philippini, R.R.; Silverio da Silva, S. Pretreatment of sugarcane bagasse using two different acid-functionalized magnetic nanoparticles: A novel approach for high sugar recovery. Renew. Energy 2020, 150, 957–964. [Google Scholar] [CrossRef]
- Shekofteh, M.; Gundoshmian, T.M.; Jahanbakhshi, A.; Heidari-Maleni, A. Performance and emission characteristics of a diesel engine fueled with functionalized multi-wall carbon nanotubes (MWCNTs-OH) and diesel–biodiesel–bioethanol blends. Energy Rep. 2020, 6, 1438–1447. [Google Scholar] [CrossRef]
- Koti, S.; Govumoni, S.P.; Gentela, J.; Venkateswar Rao, L. Enhanced bioethanol production from wheat straw hemicellulose by mutant strains of pentose fermenting organisms Pichia stipitis and Candida shehatae. SpringerPlus 2016, 5, 1545. [Google Scholar] [CrossRef] [PubMed]
- Karimi, M. Immobilization of lipase onto mesoporous magnetic nanoparticles for enzymatic synthesis of biodiesel. Biocatal. Agric. Biotechnol. 2016, 8, 182–188. [Google Scholar] [CrossRef]
- Feyzi, M.; Norouzi, L. Preparation and kinetic study of magnetic Ca/Fe3O4@SiO2 nanocatalysts for biodiesel production. Renew. Energy 2016, 94, 579–586. [Google Scholar] [CrossRef]
- Miao, C.; Yang, L.; Wang, Z.; Luo, W.; Li, H.; Lv, P.; Yuan, Z. Lipase immobilization on amino-silane modified superparamagnetic Fe3O4 nanoparticles as biocatalyst for biodiesel production. Fuel 2018, 224, 774–782. [Google Scholar] [CrossRef]
- Xie, W.; Wan, F. Basic ionic liquid functionalized magnetically responsive Fe3O4@HKUST-1 composites used for biodiesel production. Fuel 2018, 220, 248–256. [Google Scholar] [CrossRef]
- Duman, F.; Sahin, U.; Atabani, A.E. Harvesting of blooming microalgae using green synthetized magnetic maghemite (γ-Fe2O3) nanoparticles for biofuel production. Fuel 2019, 256, 115935. [Google Scholar] [CrossRef]
- Sarno, M.; Iuliano, M. Highly active and stable Fe3O4/Au nanoparticles supporting lipase catalyst for biodiesel production from waste tomato. Appl. Surf. Sci. 2019, 474, 135–146. [Google Scholar] [CrossRef]
- Junior, E.G.S.; Justo, O.R.; Perez, V.H.; da Silva Melo, F.; Reyero, I.; Serrano-Lotina, A.; Mompean, F.J. Biodiesel synthesis using a novel monolithic catalyst with magnetic properties (K2CO3/γ-Al2O3/Sepiolite/γ-Fe2O3) by ethanolic route. Fuel 2020, 271, 117650. [Google Scholar] [CrossRef]
- Ferrero, G.O.; Sanchez Faba, E.M.; Rickert, A.A.; Eimer, G.A. Alternatives to rethink tomorrow: Biodiesel production from residual and non-edible oils using biocatalyst technology. Renew. Energy 2020, 150, 128–135. [Google Scholar] [CrossRef]
- Nematian, T.; Salehi, Z.; Shakeri, A. Conversion of bio-oil extracted from Chlorella vulgaris micro algae to biodiesel via modified superparamagnetic nano-biocatalyst. Renew. Energy 2020, 146, 1796–1804. [Google Scholar] [CrossRef]
- Santha, A.; Varghese, R.; Joy Prabu, H.; Johnson, I.; Magimai Antoni Raj, D.; John Sundaram, S. Production of sustainable biofuel from biogenic waste using CuO nanoparticles as heterogeneous catalyst. Mater. Today Proc. 2020, 36 Pt 2, 447–452. [Google Scholar] [CrossRef]
- Sahani, S.; Roy, T.; Sharma, Y.C. Smart waste management of waste cooking oil for large scale high quality biodiesel production using Sr-Ti mixed metal oxide as solid catalyst: Optimization and E-metrics studies. Waste Manag. 2020, 108, 189–201. [Google Scholar] [CrossRef]
- Kim, Y.-K.; Park, S.E.; Lee, H.; Yun, J.Y. Enhancement of bioethanol production in syngas fermentation with Clostridium ljungdahlii using nanoparticles. Bioresour. Technol. 2014, 159, 446–450. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.-K.; Lee, H. Use of magnetic nanoparticles to enhance bioethanol production in syngas fermentation. Bioresour. Technol. 2016, 204, 139–144. [Google Scholar] [CrossRef] [PubMed]
- Beniwal, A.; Saini, P.; Kokkiligadda, A.; Vij, S. Use of silicon dioxide nanoparticles for β-galactosidase immobilization and modulated ethanol production by coimmobilized K. marxianus and S. cerevisiae Deproteinized Cheese Whey. LWT 2018, 87, 553–561. [Google Scholar] [CrossRef]
- Song, Q.; Mao, Y.; Wilkins, M.; Segato, F.; Prade, R. Cellulase immobilization on superparamagnetic nanoparticles for reuse in cellulosic biomass conversion. AIMS Bioeng. 2016, 3, 264–276. [Google Scholar] [CrossRef]
- Zhang, H.L.; Baeyens, J.; Tan, T.W. The bubble-induced mixing in starch-toethanol fermenters. Chem. Eng. Res. Des. 2012, 90, 2122–2128. [Google Scholar] [CrossRef]
- Alftren, J.; Hobley, T.J. Covalent immobilization of β-glucosidase on magnetic particles for lignocellulose hydrolysis. Appl. Biochem. Biotechnol. 2013, 169, 2076–2087. [Google Scholar] [CrossRef]
- Manasa, P.; Saroj, P.; Korrapati, N. Immobilization of Cellulase Enzyme on Zinc Ferrite Nanoparticles in Increasing Enzymatic Hydrolysis on Ultrasound-Assisted Alkaline Pretreated Crotalaria Juncea Biomass. Indian J. Sci. Technol. 2017, 24, 1–7. [Google Scholar] [CrossRef]
- Xie, W.; Wang, H. Immobilized polymeric sulfonated ionic liquid on core-shell structured Fe3O4/SiO2 composites: A magnetically recyclable catalyst for simultaneous transesterification and esterifications of low-cost oils to biodiesel. Renew. Energy 2020, 145, 1709–1719. [Google Scholar] [CrossRef]
- Wang, H.; Covarrubias, J.; Prock, H.; Wu, X.; Wang, D.; Bossmann, S.H. Acidfunctionalized magnetic nanoparticle as heterogeneous catalysts for biodiesel synthesis. J. Phys. Chem. C 2015, 119, 26020–26028. [Google Scholar] [CrossRef]
- Guan, Q.; Li, Y.; Chen, Y.; Shi, Y.; Gu, J.; Li, B.; Miao, R.; Chen, Q.; Ning, P. Sulfonated multi-walled carbon nanotubes for biodiesel production through triglycerides transesterification. RSC Adv. 2017, 7, 7250–7258. [Google Scholar] [CrossRef]
- Prabakaran, B.; Udhoji, A. Experimental investigation into effects of addition of zinc oxide on performance, combustion and emission characteristics of dieselbiodiesel-ethanol blends in CI engine. Alexandria Eng. J. 2016, 55, 3355–3362. [Google Scholar] [CrossRef]
- Seraç, M.R.; Aydın, S.; Yılmaz, A.; Şevik, S. Evaluation of comparative combustion, performance, and emission of soybean-based alternative biodiesel fuel blends in a CI engine. Renew. Energy 2020, 148, 1065–1073. [Google Scholar] [CrossRef]
- Chacko, N.; Jeyaseelan, T. Comparative evaluation of graphene oxide and graphene nanoplatelets as fuel additives on the combustion and emission characteristics of a diesel engine fuelled with diesel and biodiesel blend. Fuel Process. Technol. 2020, 204, 106406. [Google Scholar] [CrossRef]
- Gad, M.S.; Jayaraj, S. A comparative study on the effect of nano-additives on the performance and emissions of a diesel engine run on Jatropha biodiesel. Fuel 2020, 267, 117168. [Google Scholar] [CrossRef]
- El-Seesy, A.I.; Abdel-Rahman, A.K.; Bady, M.; Ookawara, S. Performance, combustion, and emission characteristics of a diesel engine fueled by biodiesel-diesel mixtures with multi-walled carbon nanotubes additives. Energy Convers. Manag. 2017, 135, 373–393. [Google Scholar] [CrossRef]
- LanzaJet. LanzaJet Achieves Next Major Milestone with Innovative Sustainable Fuels Production Technology Fully Constructed at its Freedom Pines Fuels Facility. NEWS, 20 April 2023. [Google Scholar]
- Thostenson, E.T.; Chou, T.W. Microwave processing: Fundamentals and applications. Compos. A Appl. Sci. Manuf. 1999, 30, 1055–1071. [Google Scholar] [CrossRef]
- Amore, K.M.; Leadbeater, N.E. Microwave-promoted esterification reactions: Optimization and scale-up. Macromol. Rapid Commun. 2007, 28, 473–477. [Google Scholar] [CrossRef]
- Motasemi, F.; Ani, F.N. A review on microwave-assisted production of biodiesel. Renew. Sustain. Energy Rev. 2012, 16, 4719–4733. [Google Scholar] [CrossRef]
- Perreux, L.; Loupy, A. A tentative rationalization of microwave effects in organic synthesis according to the reaction medium, and mechanistic considerations. Tetrahedron 2001, 57, 9199–9223. [Google Scholar] [CrossRef]
- MMulet, A.; Cárcel, J.; Benedito, J.; Rosselló, C.; Simal, S. Ultrasonic Mass Transfer Enhancement in Food Processing. In Transport Phenomena in Food Processing; CRC Press: Boca Raton, FL, USA, 2002; ISBN 9780429118531. [Google Scholar]
- Stavarache, C.; Vinatoru, M.; Nishimura, R.; Maeda, Y. Fatty acids methyl esters from vegetable oil by means of ultrasonic energy. Ultrason. Sonochem. 2005, 12, 367–372. [Google Scholar] [CrossRef]
- Chowdhury, P.; Viraraghavan, T. Sonochemical degradation of chlorinated organic compounds, phenolic compounds and organic dyes—A review, Sci. Total Environ. 2009, 407, 2474–2492. [Google Scholar] [CrossRef] [PubMed]
- Pang, Y.L.; Abdullah, A.Z.; Bhatia, S. Review on sonochemical methods in the presence of catalysts and chemical additives for treatment of organic pollutants in wastewater. Desalination 2011, 277, 1–14. [Google Scholar] [CrossRef]
- Suslick, K.S. Sonochemistry. Science 1990, 247, 1439–1445. [Google Scholar] [CrossRef]
- Adams, J.M.M.; Schmidt, A.; Gallagher, J.A. The impact of sample preparation of the macroalgae Laminaria digitata on the production of the biofuels bioethanol and biomethane. J. Appl. Phycol. 2014, 27, 985–991. [Google Scholar] [CrossRef]
- Edward, M.; Edwards, S.; Egwu, U.; Sallis, P. Bio-methane potential test (BMP) using inert gas sampling bags with macroalgae feedstock. Biomass Bioenergy 2015, 83, 516–524. [Google Scholar] [CrossRef]
- Bae, Y.J.; Ryu, C.; Jeon, J.; Park, J.; Suh, D.J.; Suh, Y.; Chang, D.; Park, Y. The characteristics of bio-oil produced from the pyrolysis of three marine macroalgae. Bioresour. Technol. 2011, 102, 3512–3520. [Google Scholar] [CrossRef]
- Rajesh Rana, J.; Godvin Sharmila, V.; Kavitha, S.; Rajajothi, R.; Gunasekaran, M.; Angappane, S.; Kumar, G. TiO2—Chitosan thin film induced solar photocatalytic deflocculation of sludge for profitable bacterial pretreatment and biofuel production. Fuel. 2020, 273, 117741. [Google Scholar] [CrossRef]
- Sadhukhan, J.; Gadkari, S.; Martinez-Hernandez, E.; Ng, K.S.; Shemfe, M.; Torres-Garciac, E.; Lyncha, J. Novel macroalgae (seaweed) biorefinery systems for integrated chemical, protein, salt, nutrient and mineral extractions and environmental protection by green synthesis and life cycle sustainability assessments. Green Chem. 2019, 21, 2635–2655. [Google Scholar] [CrossRef]
- Prabhu, M.S.; Israel, A.; Palatnik, R.R.; Zilberman, D.; Golberg, A. Integrated biorefinery process for sustainable fractionation of Ulva ohnoi (Chlorophyta): Process optimization and revenue analysis. J. Appl. Phycol. 2020, 32, 2271–2282. [Google Scholar] [CrossRef]
- Searchinger, T.; Heimlich, R.; Houghton, R.A.; Dong, F.; Elobeid, A.; Fabiosa, J.; Tokgoz, S.; Hayes, D.; Yu, T.-H. Use of US croplands for biofuels increases greenhouse gases through emissions from land-use change. Science 2008, 319, 1238–1240. [Google Scholar] [CrossRef] [PubMed]
- Fargione, J.; Hill, J.; Tilman, D.; Polasky, S.; Hawthorne, P. Land clearing and the biofuel carbon debt. Science 2008, 319, 1235–1238. [Google Scholar] [CrossRef] [PubMed]
- Melillo, J.; Reilly, J.; Kickligher, D.; Gurgel, A.; Cronin, T.; Paltsev, S.; Felzer, B.; Wang, X.; Sokolov, A.; Schlosser, C.A. Indirect Emissions from Biofuels: How Important? Science 2009, 326, 1397–1399. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).






